Abstract
Plants respond to insect feeding with a number of defense mechanisms. Using maize genotypes derived from Antiquan germ plasm that are resistant to Lepidoptera, we have demonstrated that a unique 33-kD cysteine proteinase accumulates in the whorl in response to larval feeding. The abundance of the proteinase increased dramatically at the site of larval feeding after 1 hr of infestation and continued to accumulate for as long as 7 days. The 33-kD cysteine proteinase was most abundant in the yellow-green portion of the whorl—the normal site of larval feeding and the tissue that has the greatest inhibitory effect on larval growth in bioassays. The proteinase was expressed in response to wounding and was found in senescent leaves. It may be a marker of programmed cell death. The gene coding for the proteinase, mir1, has been transformed into Black Mexican Sweet callus. When larvae were reared on callus expressing the proteinase, their growth was inhibited ∼60 to 80%. The expression of a cysteine proteinase, instead of a cysteine proteinase inhibitor, may be a novel insect defense mechanism in plants.
INTRODUCTION
Over the past 25 years, maize inbreds resistant to feeding by larvae of numerous lepidopteran species have been developed from Antiguan germ plasm (Williams and Davis, 1982; Williams et al., 1990a). Inbreds derived from this germ plasm (Mp704 and Mp708) are resistant to feeding by fall armyworm (Spodoptera frugiperda), southwestern corn borer (Diatraea grandiosella), European corn borer (Ostinia nubilalis), sugarcane borer (D. saccharalis), tobacco budworm (Heliothis virescens), corn earworm (Helicoverpa zea), and other Lepidoptera. Fall armyworm larvae feed extensively on whorl leaf tissue, often resulting in crop losses. Genetic and quantitative trait loci analyses indicate that resistance to these Lepidoptera is a quantitative trait regulated by several genes (Williams et al., 1989; Khairallah et al., 1998). Traits such as high hemicellulose content, low protein content, and leaf toughness appear to be correlated with reduced larval growth (Williams et al., 1998). No studies have indicated conclusively that secondary products contribute to the resistance, but two-dimensional gel electrophoresis has indicated that the presence of 36- and 21-kD proteins in the whorl may be predictive of resistance (Callahan et al., 1992).
Bioassays in which fall armyworm larvae are reared on lyophilized whorl tissues indicate that larvae reared on resistant material weigh ∼50% less than those reared on susceptible material (Williams et al., 1990b). Larvae reared on lyophilized whorl tissue from resistant genotypes are smaller, grow more slowly, and pupate later than those reared on similar material from susceptible genotypes (Chang et al., 2000). The major effect of this germplasm is to slow larval growth and development and to increase the amount of time larvae are vulnerable to predators and parasites.
The same phenotype, a 50% reduction in larval growth, is apparent when larvae are reared on nonfriable callus initiated from mature embryos of resistant genotypes (Williams et al., 1985, 1987). Therefore, attempts have been made to determine if there are differences in the proteins present in callus initiated from several resistant and susceptible inbreds. A 33-kD cysteine proteinase was identified in nonfriable callus initiated from resistant (Mp704 and Mp708) but not susceptible (Tx601 and Ab24E) inbreds (Jiang et al., 1995). Several lines of evidence suggested that this proteinase was involved in the ability of callus from Mp704 and Mp708 to retard larval growth. First, there was a negative correlation between larval weight and the relative concentration of the 33-kD cysteine proteinase when larvae were reared on callus initiated from F2 progeny of Mp704 × Tx601 (Jiang et al., 1995). Second, when the morphology of the callus from the resistant inbred Mp704 changed from nonfriable to friable, the 33-kD cysteine proteinase was not detected. Larvae reared on the friable callus were substantially larger than those reared on nonfriable callus (Jiang et al., 1995). Third, the 33-kD cysteine proteinase purified from Mp704 had sevenfold greater specific activity and 15-fold more enzymatic efficiency than an immunologically related 36-kD cysteine proteinase purified from callus of the susceptible inbred Ab24E (Jiang et al., 1995).
The 33-kD cysteine proteinase is encoded by mir1, which was isolated from a cDNA library prepared from Mp708 callus (Pechan et al., 1999). The derived amino acid sequence indicates that the cysteine proteinase is synthesized as a preproprotein that must be post-translationally processed to form the 33-kD enzyme. Although mir1 is similar to other genes encoding putative cysteine proteinases, including two other cDNA clones isolated from Mp708 callus, mir2 and mir3, the last 281 bp on the 3′ end differentiate it from others in the database (Pechan et al., 1999). Of these, 75 bp are in the coding sequence. A database search conducted with the 25 C-terminal amino acids found no matches.
mir1 is a single-copy gene that maps to chromosome 6, bin 6.02, on the maize genome. In quantitative trait loci analysis of related germ plasm, several unidentified traits related to southwestern corn borer resistance have been mapped near this region (Khairallah et al., 1998), but not to bin 6.02, where mir1 is found.
We postulated that the 33-kD cysteine proteinase inhibits the growth of fall armyworm larvae when they are reared on callus initiated from the resistant inbreds, Mp704 and Mp708, and speculated that its presence may be related to the resistance found in the plant. Initially, we were unable to detect the proteinase in the whorl tissue, the site of larval feeding. If the 33-kD cysteine proteinase contributes to the resistance found in these genotypes, then it should be present or induced at the larval feeding site. The results of this study demonstrate that a low concentration of the 33-kD cysteine proteinase was constitutively present in the whorl and that it accumulated greatly in response to larval feeding. In addition, larvae reared on Black Mexican Sweet (BMS) callus that had been transformed with mir1 and was expressing active 33-kD cysteine proteinase were markedly smaller than those reared on control callus. These results suggest that these corn inbreds have a novel insect defense mechanism that includes the upregulation of a unique cysteine proteinase in response to feeding by lepidopteran larvae.
RESULTS
Presence of the 33-kD Proteinase in Excised Whorl Tissues
Excised yellow-green whorl tissues from a susceptible (SC229 × Tx601) and a resistant (Mp704 × Mp707) hybrid were infested with armyworm larvae to determine whether feeding would result in the accumulation of the 33-kD cysteine proteinase. Monoclonal antibody prepared against the purified and enzymatically active 33-kD cysteine was used for immunoblot analysis (L. Ye and D.S. Luthe, unpublished data). Twenty-four hours after infestation with fall armyworm larvae, the 33-kD cysteine proteinase was detected in both uninfested and infested whorl tissue from the resistant hybrid (Figure 1). The relatively large amount of the proteinase in the uninfested tissue may have resulted from the wounding that occurred when the whorl tissue was harvested and incubated for 24 hr. The abundance of the 33-kD cysteine proteinase in uninfested tissue varied among the experiments using excised whorls, but it was generally equivalent to that in the infested tissues. It was not detected, however, in uninfested or infested whorls of the susceptible hybrid (Figure 1). This provided the first evidence that the 33-kD cysteine proteinase was expressed in vegetative tissue from resistant genotypes. However, we did not know whether the 33-kD cysteine proteinase was constitutively present in the whorl, induced by larval feeding, or induced by mechanical damage when the whorl tissue was collected and incubated.
Figure 1.
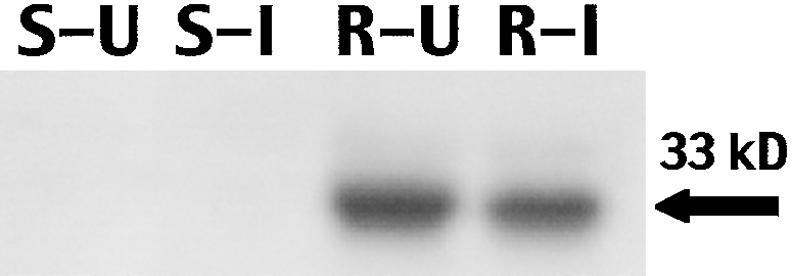
Expression of the 33-kD Cysteine Proteinase in Excised Whorls.
Immunoblot analysis of proteins extracted from excised whorls of SC229 × Tx601 (susceptible [S]) and Mp704 × Mp708 (resistant [R]) maize hybrids that were infested (I) or uninfested (U) with one fall armyworm larva (5 days old) for 24 hr. The arrow indicates the position of the 33-kD cysteine proteinase.
Accumulation of the 33-kD Cysteine Proteinase in the Whorl in Response to Larval Feeding
To differentiate among the possibilities listed above, 5-week-old intact plants, not excised whorls, were infested with three 5-day-old fall armyworm (Figure 2A) or southwestern corn borer (Figure 2B) larvae. Samples were taken from infested plants at the site of larval feeding in the yellow-green region of the whorl and also in the green region of the whorl 2 to 3 cm distal to the feeding site. Control samples were taken from the midwhorl (yellow-green) region of uninfested plants. When interpreting the following data, keep in mind that the amount of total protein in the lane containing the positive control from Mp708 callus (Figure 2C) was ∼1% as much as the amount placed in the lanes containing the whorl samples. The use of the hybrid Mp704 × Mp707 may account for the presence of two immunoreacting bands observed in these blots. Two proteins differing slightly in molecular mass but with similar isoelectric points were apparent when callus protein from this hybrid was analyzed by two-dimensional gel electrophoresis (B. Jiang and D.S. Luthe, unpublished data). It is also possible that the larger band is unprocessed preprotein.
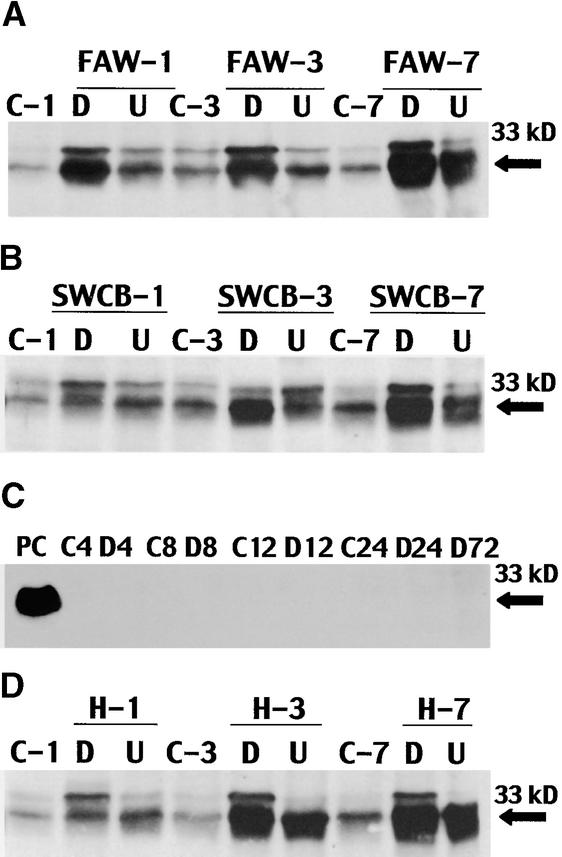
Figure 2.
Expression of 33-kD Cysteine Proteinase in Whorls of Intact Plants in Response to Larval Feeding and Wounding.
Immunoblot analysis of proteins extracted from whorls infested with three 5-day-old larvae or mechanically wounded. Samples were collected from uninfested plants (control [C]), at the site of larval feeding (damaged [D]), and 2 to 3 cm distal from the feeding site (undamaged [U]). The arrow indicates the position of the 33-kD cysteine proteinase.
(A) Response of the resistant hybrid Mp704 × Mp707 to feeding by fall armyworm (FAW) larvae. Samples were collected 1, 3, and 7 days after infestation.
(B) Response of the resistant hybrid Mp704 × Mp707 to feeding by southwestern corn borer (SWCB) larvae. Samples were collected 1, 3, and 7 days after infestation.
(C) Response of the susceptible hybrid Tx601 inbred to feeding by fall armyworm larvae. Samples were taken from damaged or uninfested control plants 4, 8, 12, 24, and 72 hr after infestation. The positive control (PC) was an extract of Mp08 callus.
(D) Response of the resistant hybrid Mp704 × Mp707 to mechanical damage. Plants were damaged with a hemostat (H), and samples were collected 1, 3, and 7 days after wounding.
The immunoblot (Figures 2A, 2B, and 2D) indicated that 33-kD cysteine proteinase was present in low amounts in uninfested plants. However, 1 day after infestation, the amount of the 33-kD cysteine proteinase increased greatly at the site of larval feeding in the infested plant. It also accumulated, though to a lesser extent, distal to the feeding site. The accumulation distal to the feeding site may be the result of a systemic signal, but this has not been confirmed. Accumulation of the 33-kD cysteine proteinase at the site of larval feeding was apparent by 1 day after infestation and continued to accumulate for 7 days. The accumulation pattern was similar for plants infested with either fall armyworm (Figure 2A) or southwestern corn borer larvae (Figure 2B). When the susceptible control, Tx601, was analyzed (Figure 2C), 33-kD cysteine proteinase was detected in neither uninfested nor infested whorls at any time, even as long as 72 hr after infestation.
Accumulation of the 33-kD Cysteine Proteinase in the Whorl in Response to Mechanical Wounding
In addition to larval feeding, the 33-kD proteinase was induced in the whorl of Mp704 × Mp707 by mechanical wounding (Figure 2D). When whorls were severely wounded by crushing the tissue with a hemostat, accumulation was similar to that obtained after the larval feeding. The 33-kD cysteine proteinase was most abundant at the site of wounding but also accumulated distal to the wound site. The 33-kD cysteine proteinase also accumulated when the whorls were cut with a knife, but the intensity of the bands was less (data not shown). The susceptible genotypes tested, however, showed no wound-induced accumulation of the 33-kD cysteine proteinase.
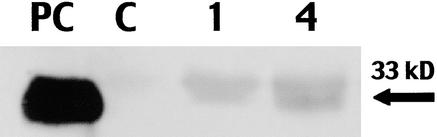
To determine how rapidly 33-kD cysteine proteinase accumulated after feeding, fall armyworm larvae were placed in the whorl, and samples were collected from the feeding site 1 and 4 hr after infestation (Figure 3). Immunoblot analysis indicated that accumulation of the proteinase at the feeding site was apparent 1 hr after infestation. We have not determined whether the rapid accumulation of the 33-kD cysteine proteinase is regulated at the transcriptional or post-transcriptional level.
Figure 3.
Temporal Accumulation of the 33-kD Cysteine Proteinase in Whorls of the Resistant Hybrid Mp704 × Mp707 Infested with Fall Armyworm Larvae.
Immunoblot analysis of proteins extract from Mp708 callus (PC), uninfested whorls (C), and whorls infested with fall armyworm larvae for 1 and 4 hr. The arrow indicates the position of the 33-kD cysteine proteinase.
Developmental Stage of the Whorl Affects Larval Growth
There is a gradual increase in chlorophyll concentration in the whorl as it develops (Figures 4A and 4D). The immature base of the whorl is white to light yellow (∼5 μg of chlorophyll g−1 fresh weight), the middle region is yellow-green (∼11 μg of chlorophyll g−1 fresh weight), and the upper region is green (∼25 μg of chlorophyll g−1 fresh weight). Although fall armyworm larvae will feed on the green tissue, the yellow-green region within the whorl is the preferred feeding site. Previous studies (Davis et al., 1999) indicated that fall armyworm larvae reared on the yellow-green region of the resistant hybrid Mp704 × Mp708 were markedly smaller (22.1 mg) than those reared on the same region of the susceptible hybrid Ab24E ×Sc229 (135.3 mg). Larvae reared on the yellow region of both resistant and susceptible hybrids were large and nearly equivalent in size (251.1 and 275.1 mg, respectively). Larvae reared on the green tissue of resistant hybrids were smaller (55.1 mg) than those reared on susceptible hybrids (155.4 mg), but the differences tended to be less than those observed for larvae reared on the yellow-green tissue. The relative size of larvae reared on these three regions of Mp704 × Mp707 is shown in Figure 4D. To determine whether there was any difference in the amount of the 33-kD cysteine proteinase present during whorl development, whorl sections that ranged from yellow to green were collected from the susceptible hybrid (SC229 × Tx601; Figure 4B) and resistant hybrid (Mp704 × Mp707; Figure 4C) and tested for the presence of the 33-kD cysteine proteinase. On the day before sampling, field-grown plants were infested with 30 neonate larvae; because the neonate larvae are very small, there was no obvious damage to the whorl during this time. No 33-kD cysteine proteinase had accumulated in any region of the whorl from the susceptible hybrid (Figure 4B). In the resistant hybrid (Figure 4C), 33-kD cysteine proteinase was not detected in the yellow region but was most abundant in the middle, yellow-green region, the region that has greatest inhibitory effect on larval growth. The green region contained slightly less of the proteinase than the yellow-green region. The lower abundance of the proteinase in the green region probably reflects that the synthesis of the proteinase occurred in the yellow-green region and ceased as the leaf matured. Thus, the abundance of the 33-kD cysteine proteinase in the whorl appears to be correlated with the ability of the different developmental regions to inhibit larval growth.
Figure 4.
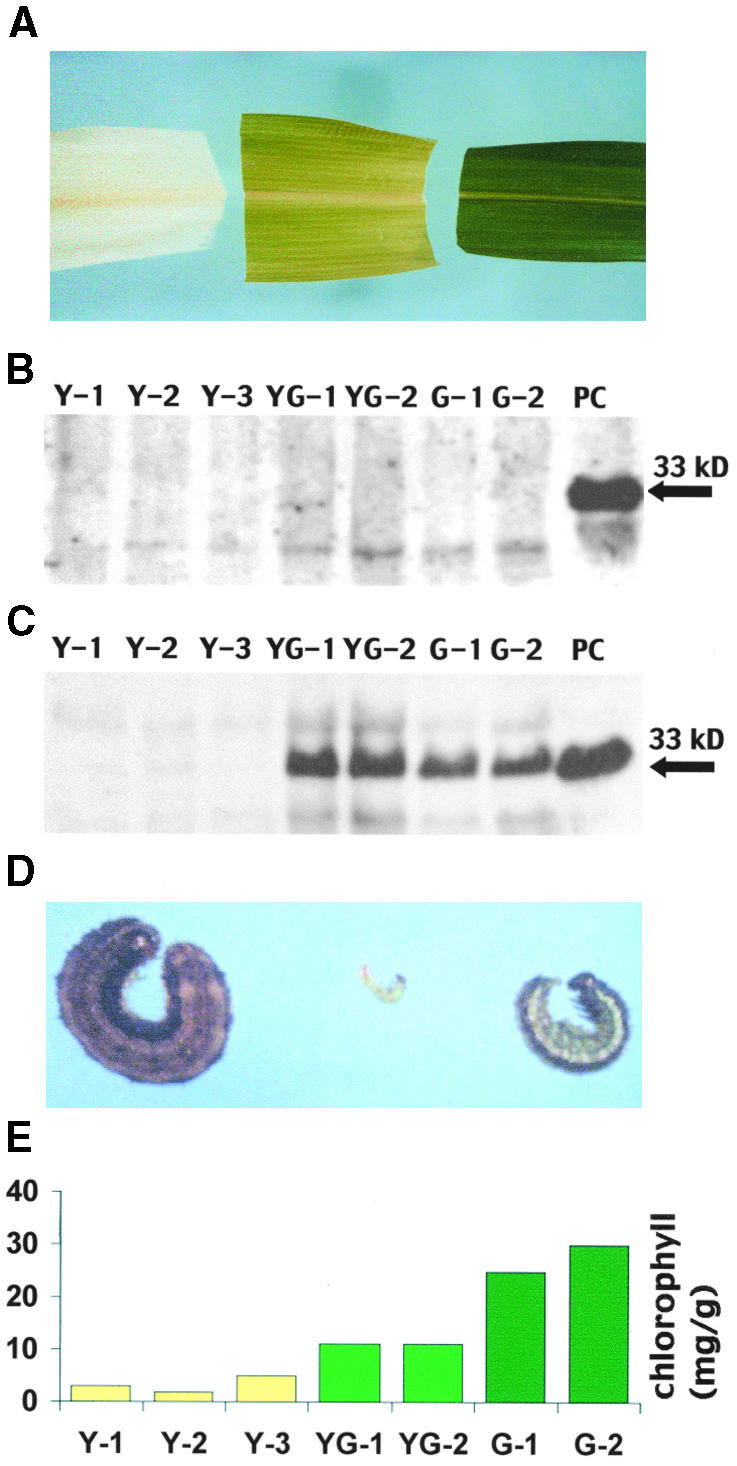
Distribution of the 33-kD Cysteine Proteinase within the Developing Whorl and Correlation with Larval Weight and Chlorophyll Content.
(A) Yellow, yellow-green, and green whorl segments used for immunoblot analysis. Sequential samples were cut from the yellow, yellow-green, and green whorl regions 1 day after infestation of intact plants with 30 neonate fall armyworm larvae.
(B) Analysis of the susceptible hybrid. Immunoblot analysis of protein isolated from yellow (Y-1, Y-2, and Y-3), yellow-green (YG-1 and YG-2), and green (G-1 and G-2) whorl regions of the susceptible hybrid SC229 × Tx601 and Mp708 callus (positive control [PC]). The arrow indicates the position of the 33-kD cysteine proteinase.
(C) Analysis of the resistant hybrid. Immunoblot analysis of protein isolated from yellow, yellow-green, and green whorl regions of the resistant hybrid Mp704 × Mp707 and Mp708 callus (PC). The arrow indicates the position of the 33-kD cysteine proteinase.
(D) Larval size. Relative size of fall armyworm larvae reared (from left to right) on excised yellow, yellow-green, and green whorl regions of Mp704 × Mp707.
(E) Chlorophyll concentration during whorl development. Chlorophyll concentrations (μg/g fresh weight) were determined for the yellow, yellow-green, and green whorl regions of Mp704 × Mp707.
Accumulation of the 33-kD Cysteine Proteinase during Seed Germination and Senescence
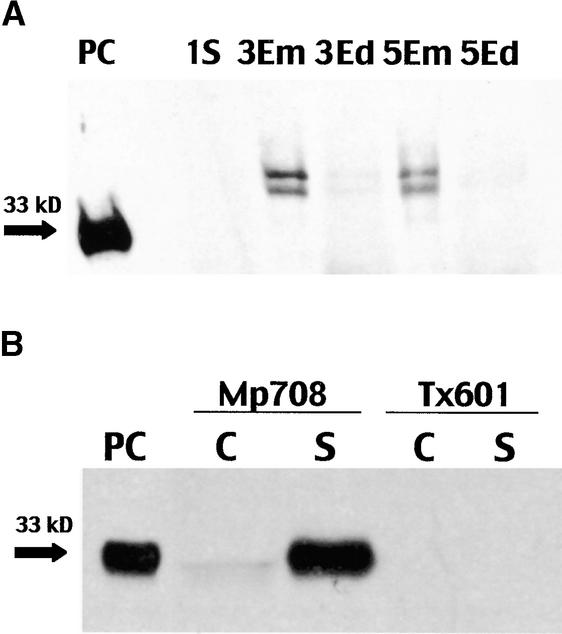
Cysteine proteinases are typically expressed during seed germination and leaf senescence. To determine if this was also the case for the 33-kD cysteine proteinase, both germinating seeds and senescent leaves were analyzed for the presence of the enzyme. The 33-kD proteinase was not present in the kernel after 1 day of germination (Figure 5A), nor was it present in the endosperm or embryo of seeds germinated for 3 or 5 days. Because proteinase activity in maize endosperm generally reaches its greatest values between 4 and 6 days after germination (de Barros and Larkins, 1990; Mitsuhashi and Oaks, 1994), the 33-kD cysteine proteinase should have been detectable by this time. Two larger proteins with apparent molecular masses of 41 and 38 kD that cross-reacted with the monoclonal antibody to the 33-kD cysteine proteinase were present in 3- and 5-day-old embryos but not in the endosperm. Hence, the 33-kD cysteine proteinase does not appear to be one of the proteinases that is synthesized by the aleurone and secreted into the endosperm during germination.
Figure 5.
Expression of the 33-kD Cysteine Proteinase in Samples Prepared from Germinating Seeds of Mp708 and Senescent Leaves from Mp708 and Tx601.
(A) Germinating seeds. Immunoblot analysis of Mp708 proteins isolated from whole seeds germinated for 1 day (1S), embryos excised from seed germinated for 3 and 5 days (3Em and 5Em), and endosperm excised from seed germinated for 3 and 5 days (3Ed and 5Ed). The positive control (PC) was a protein extract from Mp708 callus. The arrow marks the position of the 33-kD cysteine proteinase.
(B) Senescent leaves. Immunoblot analysis of proteins isolated from green (C) and senescent (S) leaves of Mp708 and Tx601. The positive control was a protein extract from Mp708 callus. The arrow marks the position of the 33-kD cysteine proteinase.
To test for expression of the proteinase during senescence, we removed a lower leaf undergoing senescence from Mp708 and Tx601 and analyzed it for the presence of the 33-kD cysteine proteinase. The proteinase was abundant in the senescent leaf of Mp708 but was not detected in Tx601 (Figure 5B).
Transformed BMS Cells Expressing the 33-kD Cysteine Proteinase Inhibit Larval Growth
Densitometry of bands on two-dimensional gels of protein extracts of nonfriable callus from Mp708 and other resistant genotypes indicated that the 33-kD cysteine proteinase accounted for ∼1% of the protein on the gels. Nonfriable callus from these genotypes appears to “overexpress” the 33-kD proteinase. This overexpression may account for the 50% inhibition of growth observed when larvae are reared on this callus. We attempted to mimic this phenotype by transforming BMS suspension culture cells with mir1, which codes for the 33-kD cysteine proteinase. Previous experiments indicated that BMS did not contain a proteinase that cross-reacted with the monoclonal antibody to the 33-kD cysteine proteinase and that larvae could be successfully reared on callus derived from BMS cells.
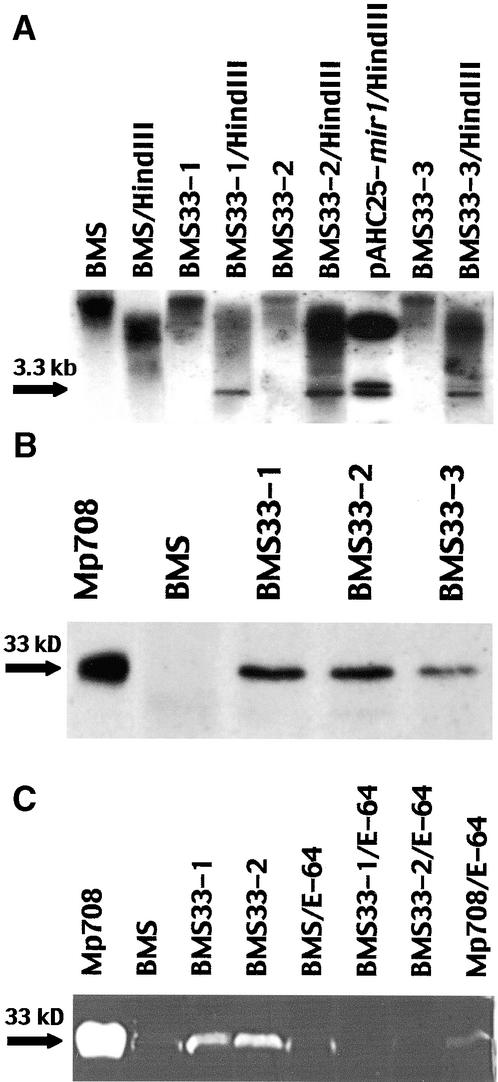
Three lines were transformed with mir1:BMS33-1, BMS33-2, and BMS33-3. DNA gel blot analysis indicated that the mir1 construct was present in the three transformed lines but not in BMS (Figure 6A). Immunoblot analysis (Figure 6B) confirmed that the 33-kD cysteine proteinase was expressed in the transformed lines. Activity staining on gels containing gelatin indicated that a protein with proteolytic activity corresponding to the size of the 33-kD cysteine proteinase was present in the transformed lines BMS33-1 and BMS33-2 (Figure 6C), but there was no detectable proteolytic activity in BMS33-3. The proteolytic activity in BMS33-1, BMS33-2, and Mp708 callus was inhibited by the thiol-proteinase inhibitor E64.
Figure 6.
Expression of 33-kD Cysteine Proteinase in BMS Callus Transformed with mir1.
(A) DNA gel blot analysis. Lanes contain DNA isolated from BMS and from three BMS lines transformed with mir1 (BMS, BMS33-1, BMS33-2, and BMS33-3) or DNA from the same lines digested with HindIII (BMS/HindIII, BMS33-1/HindIII, BMS33-2/HindIII, and BMS33- 3/HindIII). pAHC25-mir1/HindIII is the transformation vector cut with HindIII. DNA gel blots were probed with mir1; the arrow marks the 3.3-kb band that hybridized with mir1.
(B) Immunoblot analysis. Lanes contain protein isolated from Mp708 callus, nontransformed BMS callus, and three BMS lines transformed with mir1 (BMS33-1, BMS33-2, and BMS33-3). The arrow indicates the position of the 33-kD cysteine proteinase.
(C) Activity of gel analysis. Extracts of Mp708 callus, BMS callus, and transformed BMS lines (BMS3-1 and BMS33-2) were analyzed on activity gels containing gelatin. Samples (BMS/E-64, BMS33-1/E-64, BMS33-2/E-64, and Mp708/E-64) were incubated with the cysteine proteinase inhibitor E64 before electrophoresis. The arrow indicates the position of the 33-kD cysteine proteinase.
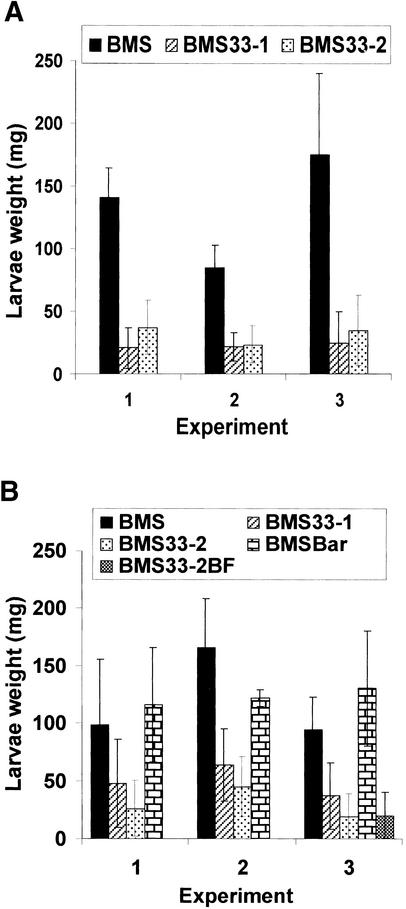
Several larval bioassays were conducted with the transformed and nontransformed BMS callus (Figure 7A). In experiments 1 and 2, the growth for larvae reared on BMS33-1 and BMS33-2 averaged ∼81 and 74% less, respectively, than that of larvae reared on nontransformed BMS callus. The effect of the BMS33-1 and BMS33-2 on the growth of tobacco budworm larvae also was tested (Figure 7A, experiment 3). In this experiment, BMS33-1 and BMS33-2 inhibited larval growth by 85 and 80%, respectively, compared with those feeding on nontransformed BMS callus. Limitations on callus availability have prevented testing the effect of transformed callus on other Lepidoptera species.
Figure 7.
Larval Feeding Bioassays on BMS Callus Transformed with mir1 and Expressing the 33-kD Cysteine Proteinase.
(A) Nontransformed and mir1-transformed BMS callus. Fall armyworm (experiments 1 and 2) or tobacco budworm (experiment 3) larvae were reared on nontransformed BMS or BMS transformed with mir1 (BMS33-1 and BMS33-2). Error bars represent standard deviations.
(B) Vector-transformed and mir1-transformed BMS. Fall armyworm larvae were reared on BMS, BMS33-1, BMS33-2, BMS transformed with expression vector only (BMSBar) (experiments 1 and 2), and BMS33-2 maintained on phosphinotricin BF medium (BMS33-2BF). Error bars represent standard deviations.
Because transformed callus was maintained on medium containing phosphinotricin (Basta) and the control callus was not, there was the possibility that the herbicide taken up by the callus was inhibiting larval growth. To test this, we reared larvae on callus transformed with the vector-only control (BMSBar), which also was maintained on the herbicide (Figure 7B). In experiment 1 (Figure 7B), the weight of larvae reared on BMS33-1 and BMS33-2 was inhibited by 50 and 77%, respectively, compared with that of those reared on the BMSBar control. In experiment 2, the inhibition of growth on BMS33-1 and BMS33-2 was 50 and 63%, respectively, of that on BMSBar. For experiments 1 and 3, there was sufficient transformed callus to increase the number of fall armyworm larvae in the bioassay, such that at least 20 larvae were reared for each treatment. Relative to the vector-only control, the growth of larvae reared in BMS33-1 and BMS33-2 was inhibited by ∼72 and 85%, respectively. In addition, a portion of the BMS33-2 callus was maintained in medium without the herbicide (phosphinotricin [Basta]-free, or BF). Larvae reared on BMS33-2 or BMS33-2 (BF) were nearly equivalent in size. The growth of larvae reared on BMS33-2 (BF) was inhibited 79% compared with those on the nontransformed BMS callus. This confirms that the phosphinotricin used for selection of transformed lines was not responsible for the inhibition of larval growth. These experiments indicate that the presence of mir1 and the expression of the 33-kD cysteine proteinase in BMS callus inhibit larval growth in a manner similar to callus initiated from resistant inbreds.
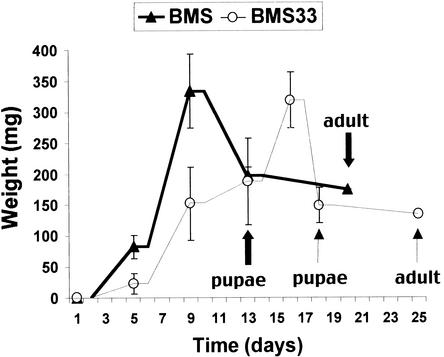
In a typical bioassay, larvae are reared on callus for 5 to 7 days. To determine the long-term effect of the transformed callus on larval growth and development, larvae were reared on transformed and nontransformed BMS callus until they pupated and adult moths emerged (Figure 8). The 10 larvae reared on nontransformed BMS callus reached their maximum weight on day 9 of the experiment. Because there was an insufficient amount of nontransformed BMS callus to maintain the larvae, seven died from lack of food. However, three larvae survived and pupated on day 13. On day 20, one moth emerged from this group of pupae. Larvae reared on transformed BMS callus (data from BMS33-1 and BMS33-2 were combined in Figure 7B) reached their maximum weight on day 16, pupated on day 17, and emerged as moths on day 25. Of 35 larvae reared on transformed callus, 14 pupated, and nine emerged as moths. There was no difference in the maximum weight of larvae reared on transformed and nontransformed callus, but those reared on callus expressing the 33-kD cysteine proteinase attained this weight 7 days later than did those reared on nontransformed callus. Pupation and moth emergence were also delayed for these larvae. The weight of pupae and moths was slightly less for those reared on the transformed callus. The small number of larvae reared on nontransformed BMS precluded statistical analysis of these data.
Figure 8.
Effect of BMS Callus Transformed with mir1 on Fall Armyworm Growth and Development.
Fall armyworm larvae were reared on nontransformed BMS callus or BMS callus transformed with mir1 (BMS33) until they pupated and emerged as moths. Results for BMS33-1 and BMS33-2 were pooled for this experiment. The wide and narrow arrows mark pupation and adult emergence for larvae reared on nontransformed BMS callus and BMS33, respectively. Error bars represent standard deviations.
DISCUSSION
We have demonstrated that feeding by fall armyworm and southwestern corn borer larvae resulted in the accumulation of a unique 33-kD cysteine proteinase in the whorls of corn genotypes that are resistant to feeding by several lepidopteran species. Ectopic expression of mir1, which encodes the 33-kD cysteine proteinase, in BMS callus inhibited larval growth. The 33-kD cysteine proteinase appears to be unique because there is no identity with other proteins in the database of the 25 C-terminal amino acids. Callus initiated from two susceptible inbreds, Ab24E and SC229, contains 36- and 34.5-kD cysteine proteinases, respectively, that cross-react with monoclonal antibody to the 33-kD cysteine proteinase (Y.-m. Chang and D.S. Luthe, unpublished data). In the hybrid SC229 × Ab24E, neither of these proteinases was induced in the whorl by larval feeding (T. Pechan and D.S. Luthe, unpublished data). These data suggest that both the structure of the 33-kD cysteine proteinase and the regulation of its expression may be unique.
Although numerous reports have indicated that cysteine proteinase inhibitors are induced by insect feeding (Ryan, 1990), to the best of our knowledge, there have been no reports of the enzyme, instead of the inhibitor, accumulating in response to an herbivore. In the past, it has been suggested that acid hydrolases in vacuoles deter insect feeding (Boller, 1986). For example, high concentrations of bromelian in stems and leaves of pineapple may have a protective function (Boller, 1986). More recently, other types of proteinases (or their cDNAs) have been shown to be induced by insect feeding, mechanical wounding, or pathogen attack. These include leucine aminopeptidase (Pautot et al., 1993; Chao et al., 1999), a subtilisin-like proteinase (Jorda et al., 1999), carboxypeptidase (Metha et al., 1996), and aspartic peptidase (Schaller and Ryan, 1996) in tomato and cysteine proteinase in tobacco (Linthorst et al., 1993).
Other than larval feeding, wounding, and senescence, we do not know what factors cause the accumulation of the 33-kD cysteine proteinase in whorls or callus. However, the 33-kD cysteine proteinase could be involved in the programmed cell death (PCD) response. Cysteine proteinases are expressed during PCD in plants (Beers, 1997; Hadfield and Bennett, 1997; Mittler et al., 1997; Morel and Dangl, 1997; Pennell and Lamb, 1997; Buckner et al., 1998; Groover and Jones, 1999), but those cysteine proteinases differ from the caspases that trigger PCD in animals (Buckner et al., 1998). No plant analogs of the caspases have been identified (Buckner et al., 1998), and it is not known if the cysteine proteinases synthesized by plants during PCD “regulate” or “execute” cell death (Groover and Jones, 1999). In soybean cells, a specific cysteine proteinase accumulates when PCD is induced by active oxygen species (Solomon et al., 1999). Ectopic expression of a soybean cysteine proteinase inhibitor in these cells blocked PCD when they were exposed to either active oxygen species or avirulent Pseudomonas syringae (Solomon et al., 1999).
Three examples of PCD in plants may be related to expression of the 33-kD cysteine proteinase in the insect-resistant maize genotypes: degeneration of the aleurone layer during seed germination (Wang et al., 1996; Bethke et al., 1999), leaf senescence (Lohman et al., 1994; Jones et al., 1995; Drake et al., 1996; Griffiths et al., 1997; Guerrero et al., 1998), and somatic embryogenesis in cell cultures (Pennell and Lamb, 1997). Although the 33-kD cysteine proteinase is not expressed during seed germination (Figure 5A), it is expressed in senescent leaves (Figure 5B) and in nonfriable callus (Jiang et al., 1995). In the case of nonfriable callus, we speculate that it is undergoing PCD and that the 33-kD cysteine proteinase is overexpressed as part of this process. Friable callus that looks “healthier” and is faster growing does not express the 33-kD cysteine proteinase and does not inhibit larval growth (Jiang et al., 1995). However, no difference in morphology was observed when mir1 was ectopically expressed in BMS callus. Recently, nitrogen deficiency in rice suspension culture has been reported to induce a cysteine proteinase (Ho et al., 2000). Because both seed germination and senescence require nitrogen remobilization, it is not surprising that cysteine proteinase expression increases under conditions of low nitrogen.
The 33-kD cysteine proteinase appears to be differentially expressed during whorl development (Figure 4), suggesting that a developmental component is involved in its accumulation. In soybean, the expression of a cysteine proteinase is both temporally and developmentally regulated in the pods and leaves (Kalinski et al., 1997). In expanding soybean leaves, expression of cysteine proteinase mRNA increases as the leaf matures and makes the transition from sink to source (Kalinski et al., 1997). A similar event may be occurring as the whorl matures from yellow to yellow-green and undergoes a similar transition.
We do not know how the 33-kD cysteine proteinase inhibits larval growth. The proteinase may directly harm the larvae, or it may catalyze the production of a toxic molecule from a substrate present in either the plant or insect. Or perhaps it may act like “enhancin,” a Tricoplusia ni granulovirus protein (Lepore et al., 1996). This metalloproteinase degrades proteins in the peritropic membrane of the insect, and the increased permeability of this membrane enhances the susceptibility of the larvae to nucleopolyhedrovirus. To attempt to understand the mechanism of growth retardation, we have examined the effect of yellow and yellow-green whorl tissue from resistant and susceptible genotypes on insect physiological indices (Chang et al., 2000). These indices measure consumption of diet, growth rate, apparent digestibility, and the efficiency of converting ingested and digested diet into body mass (Waldbauer, 1968). When larvae were reared on yellow-green tissue from resistant genotypes, they had a slower growth rate, which apparently resulted from their decreased ability to convert ingested and digested food into body mass (Chang et al., 2000).
Although the specific effect of the 33-kD cysteine proteinase on insect physiology is unknown, expression of the enzyme in BMS cells appears to be deleterious to the growth of fall armyworm and tobacco budworm larvae. Because the insect resistance trait in this germplasm appears to be controlled by several genes, possibly mir1 is one of the genes contributing to this phenotype. The accumulation of the proteinase in the yellow-green region of the whorl in response to larval feeding suggests that the enzyme may help defend the plant against insect attack. This suggestion is supported by data indicating that this region of the whorl has the greatest inhibitory effect on larval growth. The accumulation of the unique 33-kD cysteine proteinase in response to larval feeding may be part of a novel insect defense mechanism in plants.
METHODS
Plant Materials and Insect Rearing
Maize (Zea mays) lines used in this study were the resistant genotypes Mp704, Mp708, and Mp704 × Mp707. The susceptible genotypes used were Tx601 and SC229 × Tx601. The two hybrids SC229 × Tx601 and Mp704 × Mp708 are unrelated. The inbred Mp708 was selected for resistance to leaf-feeding by southwestern corn borer (Diatraea grandiosella) and fall armyworm (Spodoptera frugiperda) from progeny of a cross between Tx601 × Mp704. Mp707 was derived from the population MpSWB-4 (Williams and Davis, 1984; Williams et al., 1990a). Maize was grown in field plots at the Plant Science Research Center, Mississippi Agricultural and Forestry Experiment Station (Mississippi State University), or in pots placed outside. Fall armyworm or southwestern corn borer were reared at the U.S. Department of Agriculture–Agricultural Research Service Corn Host Plant Resistance Research Unit insect-rearing laboratory. Unless otherwise noted, larvae used for infestations were 5 days old or approximately third instar.
Infestation of Plants and Immunoblot Analysis
For the first immunoblot analysis (Figure 1), whorls were cut from field-grown plants ∼5 weeks after planting and taken to the laboratory, where yellow-green whorl sections (∼5 cm wide) were excised. Whorl sections were placed in Petri dishes containing Murashige and Skoog medium (Murashige and Skoog, 1962) and infested with five fall armyworm neonate larvae. Whorl sections were kept in a growth chamber at 28°C with 12-hr-dark/12-hr-light cycles for 24 hr. Control samples were excised whorl sections incubated under identical conditions but without larvae.
To eliminate the effect of wounding, we infested intact corn plants grown in pots for ∼5 weeks by carefully placing fall armyworm or southwestern corn borer larvae (three larvae per plant) in the whorl. Alternatively, plants were mechanically wounded by crushing the whorl five times with a hemostat. This resulted in severe damage, but the plants were still structurally intact. Control samples were taken from plants grown in a separate pot. Whorl tissues were collected from the site of insect feeding (or mechanical wounding) and from tissue 2 to 3 cm distal from the damaged site in the green region of the whorl. Whorl samples (500 mg) were immediately frozen in liquid nitrogen, homogenized, and extracted with 1 mL of SDS-PAGE sample buffer (Laemmli, 1970). Samples were prepared for SDS-PAGE as previously described (Laemmli, 1970). Except for lanes containing extracts from Mp708 callus, aliquots containing equal amounts of protein (100 μg per lane) were loaded in each lane. Protein concentrations were determined by Bio-Rad protein assay with BSA as a standard. The amount of Mp708 callus protein was 1 μg, only 1% of that of the whorl extracts. For all immunoblots, a duplicate SDS–polyacrylamide gel was run and stained with Coomassie Brilliant Blue R 250 to confirm that equal amounts of protein were loaded in each lane. Proteins separated by SDS-PAGE were blotted onto nitrocellulose membrane by using the ABN Polyblot system (American Bionetics, Hayward, CA). Immunoreacting proteins were detected by electrochemiluminescence (ECL system; Amersham), according to the manufacturer's instructions. Monoclonal antibody against 33-kD maize cysteine proteinase was prepared according to Goding (1980).
To determine the distribution of the protease during whorl development, sequential sections (2.5 cm each) were cut from the yellow (Y-1, Y-2, and Y-3), yellow-green (YG-1 and YG-2), and green (G-1 and G-2) regions of whorls from intact, field-grown plants 1 day after infestation with 30 neonate larvae. Three plants were used for each experiment, and each experiment was repeated three times. Samples were analyzed by immunoblot analysis as described above. The chlorophyll content was determined for each segment.
Black Mexican Sweet Transformation
The plant transformation vector pAHC25 (Christensen and Quail, 1996) was used to introduce mir1 (Pechan et al., 1999) into Black Mexican Sweet (BMS) maize suspension cells by microprojectile bombardment using the PDS1000/He biolistic system (Bio-Rad). Cells were selected and maintained on Murashige and Skoog medium containing 3 mg/L phosphinotricin. Transformed cells were confirmed by DNA gel blot analysis, immunoblot, and in-gel proteinase activity assays (Michaud et al., 1993).
Larval-Feeding Bioassays
Insect-feeding bioassays were conducted using methods described by Williams et al. (1985)(1987). Bioassays were conducted in a 30-mL plastic diet cup containing ∼2 g of BMS callus placed on an agar plug. To reduce fungal contamination, the callus was coated with a 1.2% agar solution containing 50 μg/mL gentamicin and 5 mm sorbic acid. One neonate larva was placed in each cup. Cups were incubated at 28°C in a growth chamber with 12-hr-light/12-hr-dark cycles. A typical bioassay contained at least 10 cups per treatment. Larvae were weighed after 5 to 7 days, depending on the experiment. In one experiment, they were transferred to fresh callus until pupation and adult eclosion.
Acknowledgments
This research was supported by the U.S. Department of Agriculture (USDA)–Agricultural Research Service, Mississippi Agricultural and Forestry Experiment Station (MAFES), and the USDA National Research Initiative Competitive Grant Program Award No. 98 35302-6819 to D.S.L. This article was approved for publication as No. J9625 of the Mississippi Agricultural and Forestry Experiment Station.
References
- Beers, E.P. (1997). Programmed cell death during plant growth and development. Cell Death Differ. 4, 649–661. [DOI] [PubMed] [Google Scholar]
- Bethke, P.C., Lonsdale, J.E., Fath, A., and Jones, R.L. (1999). Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell 11, 1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller, T. (1986). Roles of proteolytic enzymes in interactions of plants with other organisms. In Plant Proteolytic Enzymes, Vol. 2, M.J. Dalling, ed (Boca Raton, FL: CRC Press), pp. 67–95.
- Buckner, B., Janick-Buckner, D., Gray, J., and Johal, G.S. (1998). Cell-death mechanisms in maize. Trends Plant Sci. 3, 218–223. [Google Scholar]
- Callahan, F.E., Davis, F.M., and Williams, W.P. (1992). Steady-state polypeptide profiles of whorl tissue from Lepidoptera resistant and susceptible corn inbred lines. Crop Sci. 32, 1203–1207. [Google Scholar]
- Chang, Y.-M., Luthe, D.S., Davis, F.M., and Williams, W.P. (2000). Influence of whorl region from resistant and susceptible corn ge-notypes on fall armyworm (Lepidoptera: Noctuidae) growth and development. J. Econ. Entomol. 93, 478–483. [DOI] [PubMed] [Google Scholar]
- Chao, W.S., Gu, Y.-Q, Patout, V., Bray, E.A., and Walling, L.L. (1999). Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity and the wound signals systemin, methyl jasmonate and abscisic acid. Plant Physiol. 120, 979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, A.H., and Quail, P.H. (1996). Ubiquitin promoter-based vectors for high-level expression of selectable and screenable marker genes in monocotyledonous plants. Transgenic Res. 5, 213–218. [DOI] [PubMed] [Google Scholar]
- Davis, F.M., Williams, W.P., Chang, Y.M., Baker, G.T., and Hedin, P.A. (1999). Differential growth of fall armyworm larvae (Lepidoptera: Noctuidae) reared on three phenotypic regions of whorl leaves from a resistant and a susceptible maize hybrid. Florida Entomol. 82, 248–254. [Google Scholar]
- de Barros, E.G., and Larkins, B.A. (1990). Purification and characterization of zein-degrading proteases from endosperm of germinating maize seeds. Plant Physiol. 94, 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake, R., John, I., Farrell, A., Cooper, W., Schuch, W., and Grierson, D. (1996). Isolation and analysis of DNAs encoding tomato cysteine proteinases expressed during leaf senescence. Plant Mol. Biol. 30, 755–767. [DOI] [PubMed] [Google Scholar]
- Goding, J.W. (1980). Antibody production by hybridomas. J. Immunol. Methods 39, 285–308. [DOI] [PubMed] [Google Scholar]
- Griffiths, C.M., Hosken, S.E., Oliver, D., Chojecki, J., and Thomas, H. (1997). Sequencing, expression pattern and RFLP mapping of a senescence-enhanced cDNA from Zea mays with high homology to oryzain γ and aleurain. Plant Mol. Biol. 34, 815–821. [DOI] [PubMed] [Google Scholar]
- Groover, A., and Jones, A.M. (1999). Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol. 119, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero, C., de la Calle, M., Reid, M.S., and Valpuesta, V. (1998). Analysis of the expression of two thiolprotease genes from daylily (Hemerocallis spp.) during flower senescence. Plant Mol. Biol. 36, 565–571. [DOI] [PubMed] [Google Scholar]
- Hadfield, K.A., and Bennett, A.B. (1997). Programmed senescence of plant organs. Cell Death Differ. 4, 662–670. [DOI] [PubMed] [Google Scholar]
- Ho, S.-L., Tong, W.-F., and Yu, S.-M. (2000). Multiple mode regulation of a cysteine proteinase gene expression in rice. Plant Physiol. 122, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, B.-H., Siregar, U., Willeford, K.O., Luthe, D.S., and Williams, W.P. (1995). Association of a 33-kD cysteine proteinase found in corn callus with the inhibition of fall armyworm larval growth. Plant Physiol. 108, 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M.L., Larsen, P.B., and Woodson, W.R. (1995). Ethylene-regulated expression of a carnation cysteine proteinase during flower petal senescence. Plant Mol. Biol. 28, 505–512. [DOI] [PubMed] [Google Scholar]
- Jorda, L., Coego, A., Conejero, V., and Vera, P. (1999). A genomic cluster containing four differentially regulated subtilisin-like processing protease genes is in tomato plants. J. Biol. Chem. 274, 2360–2365. [DOI] [PubMed] [Google Scholar]
- Kalinski, A., Rowley, D., Dwivedi, R.S., and Herman, E.M. (1997). The expression and accumulation of soybean vegetative-cell thiol protease is temporally and developmentally regulated. Plant Physiol. Biochem. 35, 795–802. [Google Scholar]
- Khairallah, M.M., Bohn, M., Jiang, C., Deutsch, J.A., Jewell, D.C., Mihm, J.A., Melchinger, A.E., Gonzalez-de-leon, D., and Hoisington, D.A. (1998). Molecular mapping of QTL for southwestern corn borer resistance, plant height and flowering in tropical maize. Plant Breed. 117, 309–318. [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lepore, L.S., Roelvink, P.R., and Granados, R.R. (1996). Enhancin, the granulosis virus protein that facilitates nucleopolyhedrovirus (NPV) infections, is a metalloprotease. J. Invertebr. Pathol. 68, 131–140. [DOI] [PubMed] [Google Scholar]
- Linthorst, H.J.M., van der Does, C., Brederode, F.T., and Bol, J.F. (1993). Circadian expression and induction by wounding of tobacco genes for cysteine proteinase. Plant Mol. Biol. 21, 685–694. [DOI] [PubMed] [Google Scholar]
- Lohman, K.N., Gan, S., John, M.C., and Amasino, R.M. (1994). Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiol. Plant. 92, 320–328. [Google Scholar]
- Metha, R.A., Warmbardt, R.D., and Matto, A.K. (1996). Tomato fruit carboxypeptidase: Properties, induction upon wounding, and immunocytochemical localization. Plant Physiol. 110, 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud, D., Faye, L., and Yelle, S. (1993). Electrophoretic analysis of plant cysteine and serine proteinases using gelatin containing polyacrylamide gels and class-specific proteinase inhibitors. Electrophoresis 14, 94–98. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi, W., and Oaks, A. (1994). Development of endopeptidase activities in maize (Zea mays L.) endosperms. Plant Physiol. 104, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler, R., Del Pozo, O., Meisel, L., and Lam, E. (1997). Pathogen-induced programmed cell death in plants, a possible defense mechanism. Dev. Genet. 21, 279–289. [DOI] [PubMed] [Google Scholar]
- Morel, J.-B., and Dangl, J.L. (1997). The hypersensitive response and the induction of cell death in plants. Cell Death Differ. 4, 671–683. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Pautot, V., Holzer, F.M., Reisch, B., and Walling, L.L. (1993). Leucine aminopeptidase: An inducible component of the defense response in Lycopersicon esculentum (tomato). Proc. Natl. Acad. Sci. USA 90, 9906–9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechan, T., Jiang, B.-H., Steckler, D.S., Ye, L., Lin, L., Luthe, D.S., and Williams, W.P. (1999). cDNA clones encoding cysteine proteinases from corn (Zea mays L.) callus. Plant Mol. Biol. 40, 111–119. [DOI] [PubMed] [Google Scholar]
- Pennell, R.I., and Lamb, C. (1997). Programmed cell death in plants. Plant Cell 9, 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, C.A. (1990). Protease inhibitors in plants: Gene for improving defenses against insects and pathogens. Annu. Rev. Phytopathol. 28, 425–429. [Google Scholar]
- Schaller, A., and Ryan, C.A. (1996). Molecular cloning of a tomato leaf cDNA encoding an aspartic protease, a systemic wound response protein. Plant Mol. Biol. 31, 1073–1077. [DOI] [PubMed] [Google Scholar]
- Solomon, M., Belenghi, B., Delledonne, M., Menachem, E., and Levine, A. (1999). The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11, 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbauer, G.P. (1968). The consumption and utilization of food by insects. Adv. Insect Physiol. 5, 229–288. [Google Scholar]
- Wang, M., Oppejijk, B.J., Lu, X., Van Duijn, B., and Schilperoot, R.A. (1996). Apoptosis in barley aleurone during germination and its inhibition by abscisic acid. Plant Mol. Biol. 32, 1125–1134. [DOI] [PubMed] [Google Scholar]
- Williams, W.P., and Davis, F.M. (1982). Registration of Mp704 germplasm line of maize. Crop Sci. 22, 1269–1270. [Google Scholar]
- Williams, W.P., and Davis, F.M. (1984). Registration of Mp705, Mp706, and Mp707 germplasm lines of maize. Crop Sci. 24, 1217. [Google Scholar]
- Williams, W.P., Buckley, P.M., and Davis, F.M. (1985). Larval growth and behavior of the fall armyworm on callus initiated from susceptible and resistant corn hybrids. J. Econ. Entomol. 78, 951–954. [Google Scholar]
- Williams, W.P., Buckley, P.M., and Davis, F.M. (1987). Tissue culture and its use in investigations of insect resistance of maize. Agric. Ecosyst. Environ. 18, 185–190. [Google Scholar]
- Williams, W.P., Buckley, P.M., and Davis F.M. (1989). Combining ability for resistance in corn to fall armyworm and southwestern corn borer. Crop Sci. 29, 913–915. [Google Scholar]
- Williams, W.P., Davis, F.M., and Windham, G.L. (1990. a). Registration of Mp708 germplasm line of maize. Crop Sci. 30, 757. [Google Scholar]
- Williams, W.P., Buckley, P.M., Hedin, P.A., and Davis, F.M. (1990. b). Laboratory bioassay for resistance in corn to fall armyworm and southwestern corn borer. J. Econ. Entomol. 83, 1578–1581. [Google Scholar]
- Williams, W.P., Davis, F.M., Buckley, P.M., Hedin, P.A., Baker, G.T., and Luthe, D.S. (1998). Factors associated with resistance to fall armyworm (Lepidoptera: Noctuidae) and southwestern corn borer (Lepidoptera: Crambidae) in corn at different vegetative stages. J. Econ. Entomol. 91, 1471–1480. [Google Scholar]