Abstract
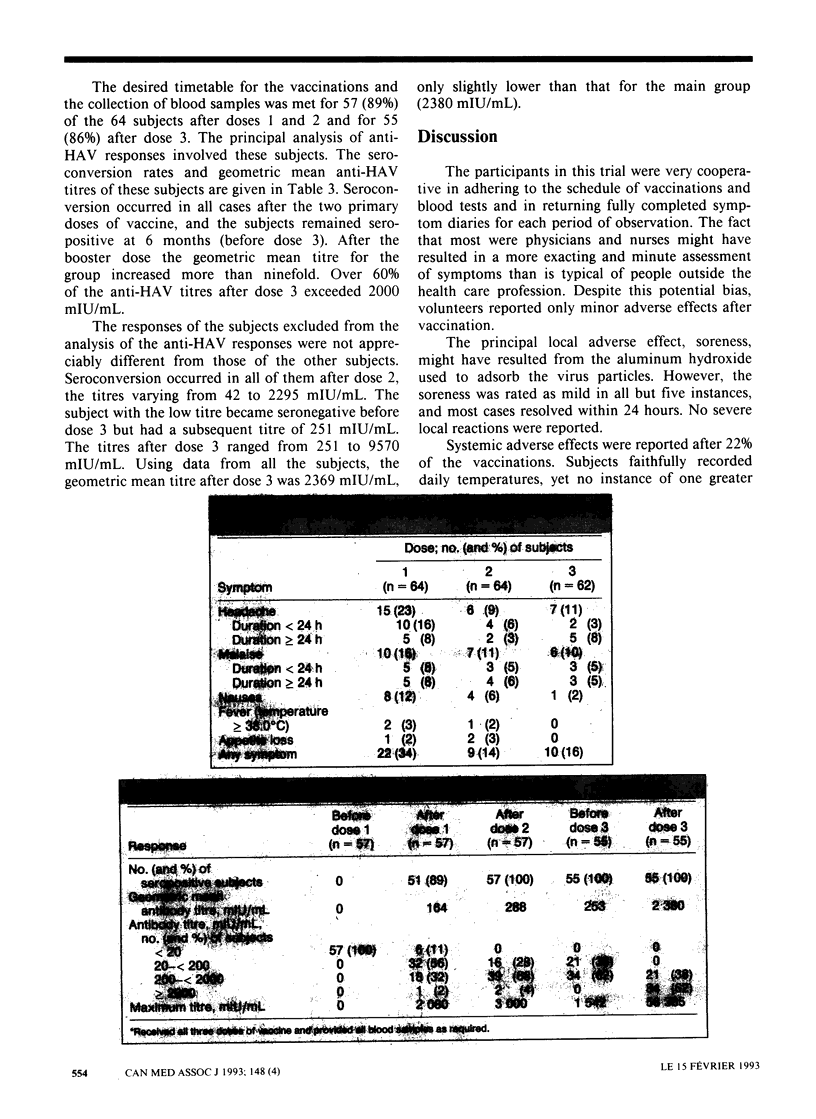
OBJECTIVE: To assess the side effects and immune responses after three serial doses of a new inactivated hepatitis A vaccine in people 40 years of age or more. DESIGN: Open, noncomparative trial. SETTING: A hospital, a regional laboratory and public health units in British Columbia. PARTICIPANTS: A volunteer sample of 64 healthy adults aged 40 to 61 years who were seronegative for hepatitis A virus (HAV). All were staff or associates of the health facilities. Exclusion criteria included elevated serum alanine and aspartate aminotransferase levels, a history of liver disease and recent travel to areas of high risk for HAV infection. INTERVENTION: A formalin-inactivated, alum-adsorbed vaccine containing 720 ELISA (enzyme-linked immunosorbent assay) units of antigen from HAV strain HM175 per 1.0-mL dose was injected intramuscularly into the delgoid area. The second and third doses were given 1 and 6 months later respectively. MAIN OUTCOME MEASURES: A detailed diary of any adverse effects for 3 days after each dose. HAV antibody levels in blood samples taken before and 30 days after each dose. RESULTS: All subjects completed the planned series of vaccinations and blood tests; symptom diaries were returned after 190 (99%) of 192 vaccinations. Local symptoms, most often soreness, were reported after 46% of the vaccinations but were mild and usually resolved within 24 hours. A temperature of more than 38.0 degrees C was never reported. Seroconversion occurred in all cases after the two primary doses, and the subjects were still seropositive at 6 months. After the booster dose the geometric mean titre was 2380 mIU/mL, all values being 200 mIU/mL or greater. CONCLUSION: In healthy adults 40 years of age or more the HAV vaccine was well tolerated and highly immunogenic. Final antibody levels were much higher than reported in people passively immunized against HAV with immune serum globulin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André F. E., Hepburn A., D'Hondt E. Inactivated candidate vaccines for hepatitis A. Prog Med Virol. 1990;37:72–95. [PubMed] [Google Scholar]
- Conrad M. E., Lemon S. M. Prevention of endemic icteric viral hepatitis by administration of immune serum gamma globulin. J Infect Dis. 1987 Jul;156(1):56–63. doi: 10.1093/infdis/156.1.56. [DOI] [PubMed] [Google Scholar]
- Corey L., Holmes K. K. Sexual transmission of hepatitis A in homosexual men: incidence and mechanism. N Engl J Med. 1980 Feb 21;302(8):435–438. doi: 10.1056/NEJM198002213020804. [DOI] [PubMed] [Google Scholar]
- Flehmig B., Haage A., Pfisterer M. Immunogenicity of a hepatitis A virus vaccine. J Med Virol. 1987 May;22(1):7–16. doi: 10.1002/jmv.1890220103. [DOI] [PubMed] [Google Scholar]
- Francis D. P., Hadler S. C., Prendergast T. J., Peterson E., Ginsberg M. M., Lookabaugh C., Holmes J. R., Maynard J. E. Occurrence of hepatitis A, B, and non-A/non-B in the United States. CDC sentinel county hepatitis study I. Am J Med. 1984 Jan;76(1):69–74. doi: 10.1016/0002-9343(84)90752-6. [DOI] [PubMed] [Google Scholar]
- Hadler S. C., Erben J. J., Francis D. P., Webster H. M., Maynard J. E. Risk factors for hepatitis A in day-care centers. J Infect Dis. 1982 Feb;145(2):255–261. doi: 10.1093/infdis/145.2.255. [DOI] [PubMed] [Google Scholar]
- Lemon S. M. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985 Oct 24;313(17):1059–1067. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Hilleman M. R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979 Feb;160(2):213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Hughes J. V., Miller W. J., Giesa P. A., Banker F. S., Emini E. A. An inactivated hepatitis A viral vaccine of cell culture origin. J Med Virol. 1986 May;19(1):23–31. doi: 10.1002/jmv.1890190105. [DOI] [PubMed] [Google Scholar]
- Widell A., Hansson B. G., Moestrup T., Nordenfelt E. Increased occurrence of hepatitis A with cyclic outbreaks among drug addicts in a Swedish community. Infection. 1983 Jul-Aug;11(4):198–200. doi: 10.1007/BF01641196. [DOI] [PubMed] [Google Scholar]
- Winokur P. L., Stapleton J. T. Immunoglobulin prophylaxis for hepatitis A. Clin Infect Dis. 1992 Feb;14(2):580–586. doi: 10.1093/clinids/14.2.580. [DOI] [PubMed] [Google Scholar]
- Zajac B. A., West D. J., McAleer W. J., Scolnick E. M. Overview of clinical studies with hepatitis B vaccine made by recombinant DNA. J Infect. 1986 Jul;13 (Suppl A):39–45. doi: 10.1016/s0163-4453(86)92668-x. [DOI] [PubMed] [Google Scholar]


