Abstract
We screened for mutations that either enhanced or suppressed the abscisic acid (ABA)–resistant seed germination phenotype of the Arabidopsis abi1-1 mutant. Alleles of the constitutive ethylene response mutant ctr1 and ethylene-insensitive mutant ein2 were recovered as enhancer and suppressor mutations, respectively. Using these and other ethylene response mutants, we showed that the ethylene signaling cascade defined by the ETR1, CTR1, and EIN2 genes inhibits ABA signaling in seeds. Furthermore, epistasis analysis between ethylene- and ABA-insensitive mutations indicated that endogenous ethylene promotes seed germination by decreasing sensitivity to endogenous ABA. In marked contrast to the situation in seeds, ein2 and etr1-1 roots were resistant to both ABA and ethylene. Our data indicate that ABA inhibition of root growth requires a functional ethylene signaling cascade, although this inhibition is apparently not mediated by an increase in ethylene biosynthesis. These results are discussed in the context of the other hormonal regulations controlling seed germination and root growth.
INTRODUCTION
Abscisic acid (ABA) regulates various aspects of plant growth and development, including seed maturation and dormancy, as well as adaptation to abiotic environmental stresses (Zeevaart and Creelman, 1988; Davies and Jones, 1991). Substantial progress has been made in the characterization of ABA signaling pathways (Busk and Pagès, 1997; Bonetta and McCourt, 1998; Leung and Giraudat, 1998; MacRobbie, 1998). In particular, mutational analyses in Arabidopsis have led to the identification of several genes that control ABA responsiveness. These genetic screens were based primarily on the inhibition of seed germination by applied ABA. The ABA-insensitive (abi) mutants abi1 to abi5 are able to germinate in the presence of ABA concentrations that are inhibitory to the wild type (Koornneef et al., 1984; Ooms et al., 1993; Finkelstein, 1994; Nambara et al., 1995). In contrast, germination of the era1 (enhanced response to ABA) to era3 mutant seed is prevented by low concentrations of ABA that ordinarily permit germination of wild-type seed (Cutler et al., 1996). As judged from their impact on seed dormancy, these two sets of mutations also alter the regulation of seed germination by endogenous ABA. Like ABA-deficient mutants (Karssen et al., 1983; Léon-Kloosterziel et al., 1996a), the ABA-insensitive mutants abi1 to abi3 display marked reductions in seed dormancy (Koornneef et al., 1984; Ooms et al., 1993; Nambara et al., 1995). Conversely, the ABA-supersensitive era1 mutation confers enhanced seed dormancy (Cutler et al., 1996).
The abi3, abi4, and abi5 mutants exhibit additional defects in various aspects of seed maturation (Koornneef et al., 1984; Finkelstein and Somerville, 1990; Ooms et al., 1993; Finkelstein, 1994; Parcy et al., 1994; Nambara et al., 1995). The ABI3 and ABI4 genes have been cloned and encode putative transcriptional regulators. ABI3 is orthologous to the maize Viviparous 1 protein (McCarty et al., 1991; Giraudat et al., 1992). ABI4 contains an APETALA2-like DNA binding domain (Finkelstein et al., 1998).
The abi1, abi2, and era1 mutations clearly affect ABA responses in vegetative tissues as well. The era1 mutation enhances sensitivity to ABA in stomatal guard cells and hence reduces transpirational water loss during drought (Pei et al., 1998). The ERA1 gene encodes the β subunit of the protein farnesyl transferase, a negative regulator of ABA action (Cutler et al., 1996). The ABI1 and ABI2 genes encode homologous protein serine/threonine phosphatases 2C (Leung et al., 1994, 1997; Meyer et al., 1994; Rodriguez et al., 1998). The abi1-1 and abi2-1 mutants carry the same amino acid substitution at equivalent positions in the ABI1 and ABI2 proteins, respectively (Leung et al., 1997; Rodriguez et al., 1998). These two mutations are dominant and lead to largely overlapping sets of ABA-insensitive phenotypes, including reduced seed dormancy, defective stomatal regulation, altered ABA regulation of gene expression, and ABA-resistant seed germination and seedling growth (Koornneef et al., 1984; Finkelstein and Somerville, 1990; Leung et al., 1997; Allen et al., 1999). In contrast to the ABA-resistant abi1-1 mutant, loss-of-function alleles of ABI1 are supersensitive to ABA, indicating that the ABI1 phosphatase (and, by homology, possibly ABI2 as well) is a negative regulator of ABA responses (Gosti et al., 1999).
More recently, genetic screens based on ABA-regulated reporter genes in vegetative tissues have been developed. The ade1 (ABA-deregulated gene expression) mutation enhances gene expression in response to ABA but not to cold (Foster and Chua, 1999). The hos5 (high expression of osmotically responsive genes) mutant displays an increased sensitivity of gene expression to ABA and osmotic stress but not to cold (Xiong et al., 1999). The ade1 and hos5 mutations have little effect on seed germination.
To identify additional loci controlling sensitivity to ABA, we screened for mutations that either enhance or suppress the ABA-resistant seed germination phenotype of abi1-1. Remarkably, in each of these two screens we recovered alleles of mutants that were previously known to affect responsiveness to another plant hormone, ethylene. Using these and other ethylene response mutants, we demonstrate the existence of extensive interactions between the ABA and ethylene signaling cascades. These interactions, however, differ markedly in seed and in root. The ethylene pathway regulates seed dormancy negatively by inhibiting ABA signaling. In contrast, these two hormonal cascades act synergistically in inhibiting root growth.
RESULTS
Isolation of ctr1 Mutants as Phenotypic Enhancers of abi1-1
Seeds homozygous for the abi1-1 mutation were treated with ethyl methanesulfonate (Gosti et al., 1999), and the M2 population was screened for mutants that showed enhancement of the ABA-resistant seed germination phenotype M2 seeds were plated on agar medium containing 100 μM ABA, a concentration that inhibits germination of abi1-1. After 5 days at 21°C, germinated seedlings with green cotyledons or an elongated root with hairs were selected as candidate enhancers. These individuals were propagated in soil, and their phenotype was retested in the next (M3) generation. From an estimated total of 4000 M1 plants and 185,000 M2 seeds screened, we finally retained 34 independent lines (originating from distinct pools of M1 plants) that were markedly more resistant than abi1-1 to ABA inhibition of seed germination.
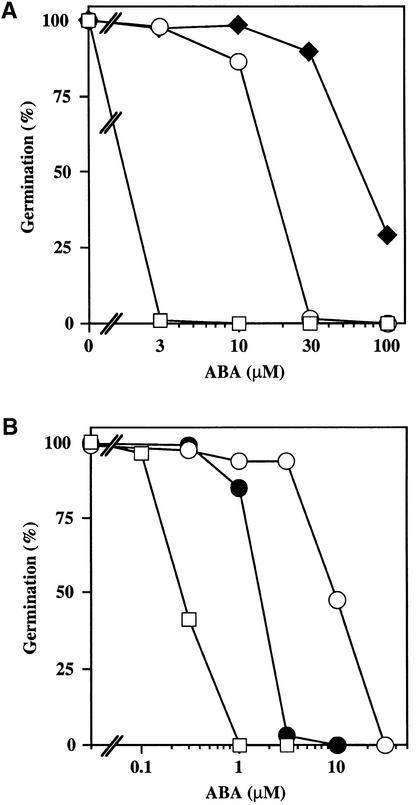
Among these enhancer lines, 16 displayed a characteristic morphological phenotype. Light-grown seedlings had small cotyledons and a short hairy root (Figure 1A), and the adult plants were severely stunted with dark green leaves (not shown). Six of these dwarf enhancer lines were randomly selected for genetic analysis. In germination assays, these enhancer lines could be reliably distinguished from abi1-1 by their resistance to 30 μM ABA (Figure 2A). Backcrosses to abi1-1 showed that in all six lines analyzed, the enhancer phenotype (germination on 30 μM ABA) and the dwarf phenotype were recessive. In complementation tests, the F1 progeny displayed both phenotypes, indicating that the six lines are allelic to each other and that these enhancer mutations are also responsible for the dwarf phenotype. We analyzed one of these enhancer lines (isolation number H3) in more detail.
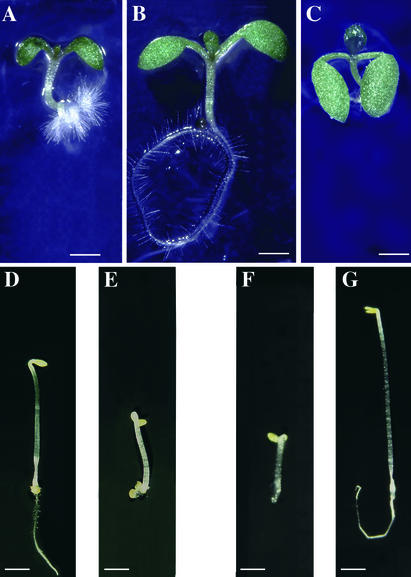
Figure 1.
Morphological Phenotypes of Mutants.
(A) Eight-day-old seedling of mutant line H3 (subsequently renamed abi1-1 ctr1-10) grown with a 16-hr-light photoperiod.
(B) Eight-day-old abi1-1 seedling grown with a 16-hr-light photoperiod.
(C) Eight-day-old seedling of mutant line 84B (subsequently renamed abi1-1 ein2-45) grown with a 16-hr-light photoperiod. The root is out of focus but was similar to that of abi1-1.
(D) Three-day-old abi1-1 seedling grown in the dark.
(E) Three-day-old seedling of mutant line H3 (subsequently renamed abi1-1 ctr1-10) grown in the dark.
(F) Three-day-old Landsberg erecta (Ler) seedling grown in the dark on medium supplemented with 10 μM 1-aminocylcopropane-1-carboxylic acid (ACC).
(G) Three-day-old seedling of mutant line 84B/1 (subsequently renamed ein2-45) grown in the dark on medium supplemented with 10 μM ACC.
 .
.
Figure 2.
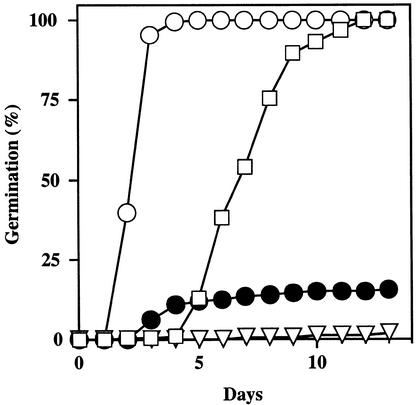
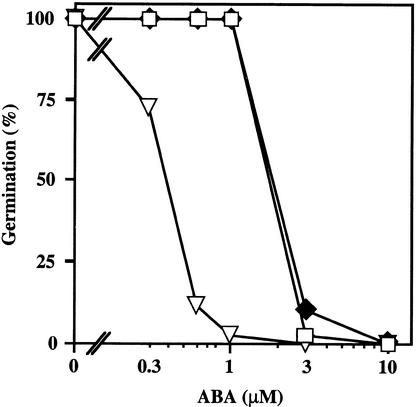
ABA Dose Response for Germination Inhibition.
(A) Seeds of Ler wild type (open squares), abi1-1 (open circles), and H3 (subsequently renamed abi1-1 ctr1-10; filled diamonds) were plated on medium supplemented with the indicated concentrations of ABA, chilled for 4 days at 4°C in darkness, and incubated for 5 days at 21°C with a 16-hr-light photoperiod. The number of germinated seeds (with green cotyledons, elongated root with hairs, or both) was expressed as the percentage of the total number of seeds plated (100 to 200).
(B) Seeds of Ler wild type (open squares), abi1-1 (open circles), and 84B (subsequently renamed abi1-1 ein2-45; filled circles) were plated on medium supplemented with the indicated concentrations of ABA, chilled for 4 days at 4°C in darkness, and incubated for 3 days at 21°C with a 16-hr-light photoperiod. The number of germinated seeds (with green cotyledons) was expressed as the percentage of the total number of seeds plated (100 to 200).
The morphological characteristics described above for light-grown H3 seedlings and adult plants were very similar to those reported for the constitutive ethylene response mutant ctr1 (Kieber et al., 1993). The F1 progeny of a complementation cross between H3 and the recessive ctr1-1 mutant displayed the same characteristic dwarf phenotype as the parents, indicating that the enhancer mutation contained in line H3 is indeed a ctr1 mutant allele. This allele was named ctr1-10, and line H3 thus corresponds to the abi1-1 ctr1-10 double mutant. When exposed to ethylene, dark-grown Arabidopsis seedlings develop a characteristic triple response: inhibition of hypocotyl and root elongation, radial swelling of the hypocotyl, and exaggeration of the apical hook (Guzman and Ecker, 1990). Dark-grown H3 seedlings, like ctr1-1 (Kieber et al., 1993), displayed a constitutive triple response in the absence of exogenous ethylene (Figure 1E), whereas abi1-1 seedlings had a wild-type etiolated morphology (Figure 1D). The CTR1 gene encodes a Raf-like protein kinase that is a negative regulator of ethylene signaling (Kieber et al., 1993). DNA sequence analysis showed that the ctr1-10 mutation is a G-to-A transition at nucleotide 4511 in the CTR1 gene (GenBank accession number L08790), which converts amino acid Asp-676 to Asn in the CTR1 kinase. Because Asp-676 corresponds to an invariant residue of kinase domains, the ctr1-10 mutation probably disrupts the catalytic activity of CTR1, as was predicted for all other ctr1 mutant alleles (Kieber et al., 1993). For subsequent studies, line H3 was outcrossed to the Landsberg erecta (Ler) wild type, and the ctr1-10 mutation was isolated in a wild-type ABI1 background.
Isolation of an ein2 Mutant as Phenotypic Suppressor of abi1-1
We described previously a screen for phenotypic suppressors of abi1-1 (Gosti et al., 1999). In brief, seeds homozygous for the abi1-1 mutation were treated with ethyl methanesulfonate, and the M2 population was screened for mutants that showed a reversion of the ABA-resistant seed germination phenotype of abi1-1. Mutant line 84B is one of the extragenic suppressors isolated in that screen. Line 84B, which showed a partial reversion of the resistance of abi1-1 to the ABA inhibition of seed germination, could be conveniently distinguished from abi1-1 by its sensitivity to 3 μM ABA (Figure 2B). In addition, the cotyledons of 1-week-old 84B seedlings grown on ABA-free medium curled downward (Figure 1C) and were markedly bigger than both abi1-1 (Figure 1B) and the Ler wild type (not shown). 84B plants also had rosette leaves that were larger than those of abi1-1 and the Ler wild type (not shown). Backcrossing 84B to abi1-1 showed that the suppressor phenotype (sensitivity to 3 μM ABA) and the cotyledon phenotype were both monogenic and recessive. Furthermore, these phenotypes cosegregated with each other in the 79 F2 individuals analyzed, indicating that both phenotypes most likely result from the same mutation.
To separate this suppressor mutation from the abi1-1 mutation, 84B was outcrossed to the Ler wild type. We selected an F2 line (84B/1) that was homozygous for the wild-type ABI1 allele (as determined by polymerase chain reaction analysis; Leung et al., 1997) and had larger cotyledons and rosette leaves than did Ler. Ethylene-insensitive mutants of Arabidopsis grown in air have bigger rosette leaves than does the wild type (Bleecker et al., 1988; Guzman and Ecker, 1990). Furthermore, we mapped the 84B/1 mutation to the top of chromosome 5 in a region that contains the ethylene-insensitive EIN2 locus (data not shown). Hence, we investigated whether 84B/1 was allelic to the ein2-1 mutant (Guzman and Ecker, 1990). Like the 84B/1 plants, 1-week-old ein2-1 seedlings grown in the light had enlarged and downward-curling cotyledons (data not shown). Dark-grown ein2-1 seedlings fail to develop the ethylene-evoked triple response (Guzman and Ecker, 1990). Similarly, 84B/1 seedlings, when germinated in the dark on medium supplemented with the immediate ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC), did not display the triple response (Figure 1G), whereas the wild-type control did (Figure 1F). As in ein2-1, this ACC-insensitive phenotype was recessive in 84B/1, and the F1 progeny of a complementation cross between 84B/1 and ein2-1 was unresponsive to ACC. These data demonstrate that 84B/1 is a novel ein2 mutant allele, which we call ein2-45 (hence, the initial suppressor line 84B corresponds to the abi1-1 ein2-45 double mutant). The EIN2 gene encodes a novel integral membrane protein of unknown molecular function (Alonso et al., 1999). DNA sequencing revealed that the ein2-45 mutation is a G-to-A transition at nucleotide 5195 of the EIN2 gene (GenBank accession number AF141202), which converts amino acid Gly-1138 to Glu in the hydrophilic C-terminal domain of the EIN2 protein.
Seeds of Ethylene-Insensitive Mutants Are Supersensitive to ABA
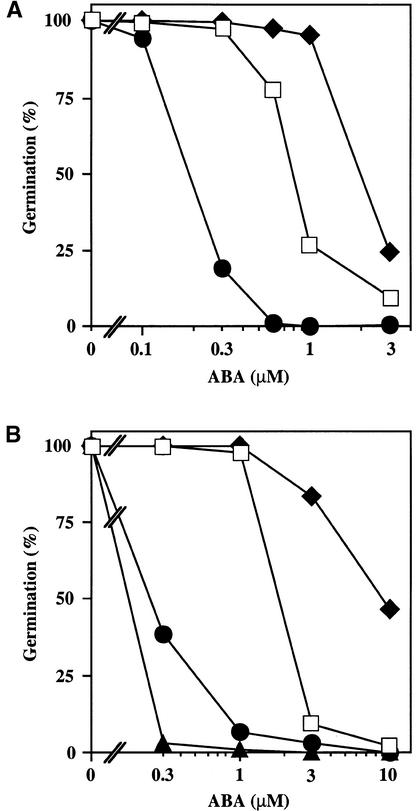
As described above, the ABA-resistant phenotype of abi1-1 seeds is enhanced and suppressed by the ctr1-10 and ein2-45 mutations, respectively. These mutations also modify the ABA sensitivity of seed germination in the absence of the abi1-1 mutation. Figure 3A shows that ctr1-10 seeds were less sensitive to ABA than were Ler wild-type seeds, and, conversely, that ein2-45 seeds were supersensitive to ABA. These results do not seem to be biased by the fact that ctr1-10 and ein2-45 were initially selected in screens based on ABA sensitivity. Indeed, similar results were obtained when we tested the distinct ctr1-1 and ein2-1 alleles, which have been isolated solely on the basis of their ethylene-related phenotypes (Guzman and Ecker, 1990; Kieber et al., 1993). Compared with the wild-type Columbia (Col) strain from which these mutants are derived, ABA sensitivity of seed germination was decreased in ctr1-1 but enhanced in ein2-1 (Figure 3B). Moreover, a complementation test between ein2-45 and era3-1 showed that the latter mutant, which was selected by Cutler et al. (1996) because of its enhanced sensitivity to ABA during seed germination, is also allelic to ein2 (data not shown). The dominant ethylene-insensitive etr1-1 mutation inhibits ethylene binding to the ETR1 receptor (Bleecker et al., 1988; Schaller and Bleecker, 1995), and hence the step of the ethylene signaling cascade it affects differs from that affected by ein2. Nevertheless, like ein2, etr1-1 seeds were markedly more sensitive to ABA than were wild-type Col seeds (Figure 3B).
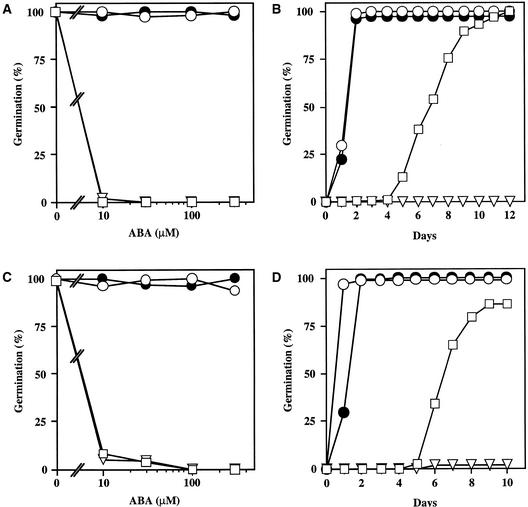
Figure 3.
ABA Dose Response for Germination Inhibition.
Seeds were plated on medium supplemented with the indicated concentrations of ABA, chilled for 4 days at 4°C in darkness, and incubated at 21°C with a 16-hr-light photoperiod. The number of germinated seeds (with fully emerged radicle tip) was expressed as the percentage of the total number of seeds plated (100 to 200).
(A) Seeds of Ler wild type (open squares), ctr1-10 (filled diamonds), and ein2-45 (filled circles) were incubated for 2 days at 21°C.
(B) Seeds of the Col wild type (open squares), ctr1-1 (filled diamonds), ein2-1 (filled circles), and etr1-1 (filled triangles) were incubated for 3 days at 21°C.
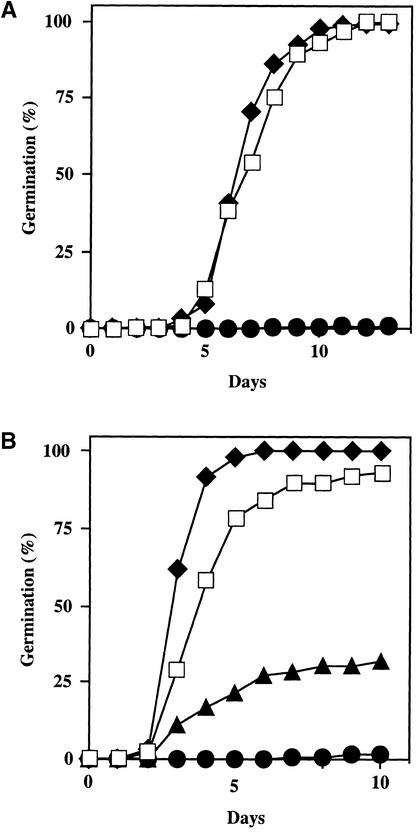
Endogenous ABA plays a major role in promoting Arabidopsis seed dormancy. Seed dormancy is diminished in various ABA-deficient or ABA-insensitive mutants (Koornneef et al., 1984; Ooms et al., 1993; Léon-Kloosterziel et al., 1996a) but is enhanced in the ABA-supersensitive era1 mutant (Cutler et al., 1996). Because ethylene response mutations altered sensitivity to ABA in the germination assay described above, we analyzed the effect of these mutations on seed dormancy. Freshly harvested seeds of the ctr1-1 mutant germinated only slightly faster than did wild-type Col seeds (Figure 4B), and the ctr1-10 response did not differ substantially from that of the Ler wild type (Figure 4A). Hence, under our experimental conditions, ctr1 mutations had at most a limited effect on seed dormancy. In contrast, the ethylene-insensitive ein2 and etr1-1 mutants displayed markedly enhanced seed dormancy. When assayed without any pretreatment, freshly harvested seeds of ein2-45 (Figure 4A), ein2-1, and etr1-1 (Figure 4B) had a much lower germination rate than did seeds of the corresponding wild type. For etr1-1, this result is consistent with the previous observation by Bleecker et al. (1988). However, after a cold treatment (stratification) that breaks dormancy, ein2 and etr1-1 mutant seeds displayed full germination.
Figure 4.
Seed Dormancy.
Freshly harvested seeds were plated on ABA-free medium and incubated directly (without cold pretreatment) at 21°C with a 16-hr-light photoperiod. The number of germinated seeds (with fully emerged radicle tip) at a given time was expressed as the percentage of the total number of seeds plated (100 to 200). In simultaneous experiments in which seeds from the same batches were first chilled for 4 days at 4°C in darkness to break dormancy, all genotypes displayed 100% germination after 5 days at 21°C (data not shown).
(A) Seeds of the Ler wild type (open squares), ctr1-10 (filled diamonds), and ein2-45 (filled circles).
(B) Seeds of the Col wild type (open squares), ctr1-1 (filled diamonds), ein2-1 (filled circles), and etr1-1 (filled triangles).
These results suggested that the ein2 and etr1-1 mutations may increase seed dormancy by enhancing the sensitivity to endogenous ABA. This model predicts that the effect of ein2 and etr1-1 on seed dormancy should be masked by mutations that inactivate the ABA signaling cascade. Such was not the case when the abi1-1 and ein2-45 mutations were combined because abi1-1 ein2-45 double mutant seeds were more dormant than wild-type seeds (Figure 5). However, as judged from the ABA dose required to achieve 50% inhibition of seed germination, the ABA sensitivity in abi1-1 seeds is reduced from wild-type sensitivity by only ∼10-fold (Figure 2; Koornneef et al., 1984; Finkelstein and Somerville, 1990). We thus tested mutants with seeds even more extremely insensitive to ABA. Both the abi3-4 mutant and the abi1-1 abi3-1 double mutant display >1000-fold reductions in ABA sensitivity at germination (Figures 6A and 6C; Finkelstein and Somerville, 1990; Ooms et al., 1993). Remarkably, the abi3-4 ein2-45 double mutant and the abi1-1 abi3-1 ein2-45 triple mutant were as nondormant as the abi3-4 and abi1-1 abi3-1 parents (Figures 6B and 6D). As discussed above, these results are consistent with the hypothesis that the EIN2 cascade regulates negatively the degree of seed dormancy by inhibiting ABA action.
Figure 5.
Seed Dormancy.
Freshly harvested seeds of the Ler wild type (open squares), abi1-1 (open circles), ein2-45 (open triangles), and abi1-1 ein2-45 (filled circles) were plated on ABA-free medium and incubated directly (without cold pretreatment) at 21°C with a 16-hr-light photoperiod. The number of germinated seeds (with fully emerged radicle tip) at a given time was expressed as the percentage of the total number of seeds plated (100 to 200). In simultaneous experiments in which seeds from the same batches were first chilled for 4 days at 4°C in darkness to break dormancy, all genotypes displayed 100% germination after 5 days at 21°C (data not shown).
Figure 6.
Effect of the ein2-45 Mutation on the Seed Phenotypes of Severely ABA-Insensitive Mutants.
(A) and (C) ABA dose response for germination inhibition. Seeds were plated on medium supplemented with the indicated concentrations of ABA, chilled for 3 days at 4°C in darkness, and incubated for 3 days at 21°C with a 16-hr-light photoperiod. The number of germinated seeds (with fully emerged radicle tip) was expressed as the percentage of the total number of seeds plated (70 to 100). Data from seeds of the Ler wild type (open squares), abi3-4 (open circles), ein2-45 (open triangles), and abi3-4 ein2-45 (filled circles) are shown in (A). Data from seeds of the Ler wild type (open squares), abi1-1 abi3-1 (open circles), ein2-45 (open triangles), and abi1-1 abi3-1 ein2-45 (filled circles) are shown in (C).
(B) and (D) Seed dormancy. Freshly harvested seeds were plated on ABA-free medium and incubated directly (without cold pretreatment) at 21°C with a 16-hr-light photoperiod. The number of germinated seeds (with fully emerged radicle tip) at a given time was expressed as the percentage of the total number of seeds plated (100 to 200). In simultaneous experiments in which seeds from the same batches were first chilled for 4 days at 4°C in darkness to break dormancy, all genotypes displayed 100% germination after 5 days at 21°C (data not shown). Data from seeds of the Ler wild type (open squares), abi3-4 (open circles), ein2-45 (open triangles), and abi3-4 ein2-45 (filled circles) are shown in (B). Data from seeds of the Ler wild type (open squares), abi1-1 abi3-1 (open circles), ein2-45 (open triangles), and abi1-1 abi3-1 ein2-45 (filled circles) are shown in (D).
Root Growth of Ethylene-Insensitive Mutants Is Resistant to ABA Inhibition
We investigated whether, besides increasing ABA sensitivity in seeds, ein2 mutations also affect ABA responses in vegetative tissues. Detached leaves of ein2-45 and wild-type Ler displayed similar kinetics of water loss, suggesting that ein2-45 does not alter ABA regulation of stomatal aperture (data not shown). Likewise, induction of the RAB18 (Lang and Palva, 1992) and RD29A (Yamaguchi-Shinozaki and Shinozaki, 1993) mRNAs by exogenous ABA was not substantially modified in ein2-45 (data not shown). In contrast, the ein2-45 mutation markedly decreased the sensitivity of root growth to inhibition by ABA. Root growth in ein2-45 was essentially as ABA resistant as in abi1-1 (Figure 7). This effect was not specific to the ein2-45 allele, however, because ein2-1 as well as etr1-1 roots displayed less sensitivity to ABA than did wild-type Col (Figure 8A). As expected, the ethylene-insensitive ein2 and etr1-1 mutants were also resistant to inhibition of root growth by applied ACC (Figure 8B). In contrast, the ABA-insensitive abi1-1 mutant had a wild-type sensitivity to ACC (Figure 8B).
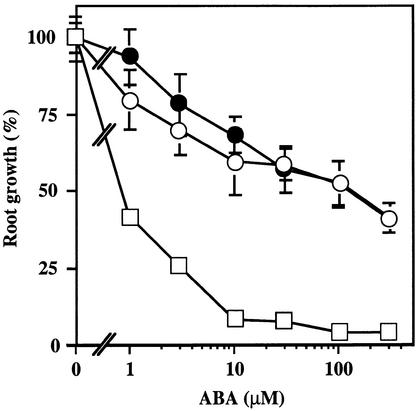
Figure 7.
ABA Dose Response for Root Growth Inhibition.
Seeds of the Ler wild type (open squares), abi1-1 (open circles), and ein2-45 (filled circles) were germinated and grown for 8 days on ABA-free medium. These seedlings were then incubated vertically on medium supplemented with the indicated concentrations of ABA, and their root length was scored after 5 days. Root growth of ABA-treated seedlings was expressed as a percentage relative to controls incubated on ABA-free medium. Values shown are mean ±sd from samples consisting of 15 to 20 seedlings each.
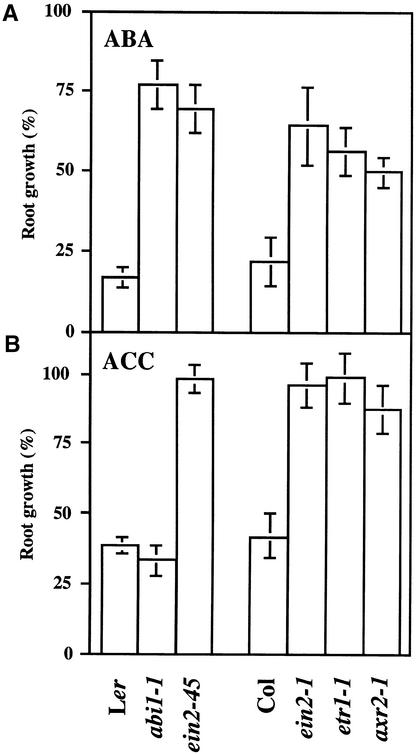
Figure 8.
Sensitivity of Root Growth to Applied ABA and ACC.
(A) and (B) Seeds of the indicated genotypes were germinated and grown for 6 days on medium containing no additional supplements. These seedlings were then incubated vertically on medium supplemented with 100 μM ABA (A) or 0.2 μM ACC (B), and their root length was scored after 4 days. Root growth of ABA- or ACC-treated seedlings was expressed as a percentage relative to controls incubated on normal medium. Values shown are mean ±sd from samples consisting of 15 to 20 seedlings each.
We investigated whether ABA inhibition of root growth might be mediated by an ABA-induced increase in ethylene biosynthesis. Under the experimental conditions of our root growth bioassay, treating wild-type plantlets with as much as 100 μM ABA failed to induce the ethylene-responsive GST2 mRNA (Zhou and Goldsbrough, 1993), whereas this transcript did accumulate in response to as little as 0.2 μM ACC (data not shown). Moreover, ethylene production was similar in plantlets treated with 100 μM ABA (7.9 ± 4.2 pL hr−1 seedling−1) and in untreated controls (7.7 ± 3.8 pL hr−1 seedling−1), whereas, as expected, ethylene production was enhanced in plantlets exposed to 10 μM ACC (100.2 ± 8.3 pL hr−1 seedling−1). Hence, the present data indicate that ABA inhibition of root growth requires a functional ethylene signaling cascade; however, this inhibition is apparently not mediated by an increase in ethylene biosynthesis.
The only mutant previously described as being cross-resistant to ethylene and ABA is the axr2-1 mutant. This dominant mutation was originally recovered in a screen for auxin-resistant seedlings and was subsequently shown to decrease the sensitivity of root growth to inhibition by auxin, ethylene, and ABA (Wilson et al., 1990). Like ein2 and etr1-1, axr2-1 roots were indeed resistant to both ABA and ACC (Figure 8). However, in marked contrast to ein2 and etr1-1, germination of axr2-1 seeds was not supersensitive to ABA (Figure 9). This observation is consistent with the view that the axr2-1 mutant has a primary defect in the action of auxin rather than that of ethylene (Wilson et al., 1990; Timpte et al., 1994).
Figure 9.
ABA Dose Response for Germination Inhibition.
Seeds of the Col wild type (open squares), ein2-1 (open triangles), and axr2-1 (filled diamonds) were plated on medium supplemented with the indicated concentrations of ABA, chilled for 4 days at 4°C in darkness, and incubated for 5 days at 21°C with a 16-hr-light photoperiod. The number of germinated seeds was expressed as the percentage of the total number of seeds plated (70 to 100).
DISCUSSION
Mutational and molecular analyses in Arabidopsis have identified several of the elements in the ethylene signal transduction pathway (Johnson and Ecker, 1998). Ethylene is perceived by a family of receptors related to ETR1, and ethylene binding inhibits the signaling activities of these receptors (Hua and Meyerowitz, 1998). In the absence of ethylene, the receptors activate CTR1, which regulates negatively the downstream signaling components, including EIN2 (Kieber et al., 1993; Clark et al., 1998; Alonso et al., 1999). We isolated the ein2-45 mutation as a suppressor of the ABA-resistant seed germination phenotype of abi1-1. Furthermore, in the wild-type ABI1 background, ein2-45 mutant seeds were also supersensitive to ABA. This phenotype is not specific to the ein2-45 allele because we found that the ein2-1 mutation similarly enhances ABA sensitivity of seed germination and that the ABA-supersensitive era3-1 mutant described by Cutler et al. (1996) is also an ein2 mutant allele. Conversely, like ein2-1 (Guzman and Ecker, 1990), ein2-45 and era3-1 mutants were insensitive to ethylene, as shown by their failure to develop the characteristic triple response evoked by ethylene (or ACC) in dark-grown seedlings. These data show that, in addition to its role as a positive regulator of ethylene signaling, EIN2 is a negative regulator of ABA sensitivity in seeds.
ein2 mutants have also been recovered in screens for mutants resistant to cytokinins (Cary et al., 1995) or inhibitors of auxin transport (Fujita and Syono, 1996) and in screens for delayed leaf senescence (Oh et al., 1997). None of the other ethylene-insensitive loci was recovered in these screens. Furthermore, expression of the EIN2 C-terminal domain in ein2 mutant plants restored responsiveness to jasmonic acid and paraquat-induced oxygen radicals but was not sufficient to induce the triple response (Alonso et al., 1999). Hence, EIN2 has been proposed to lie at the crossroads of multiple hormone response pathways (Alonso et al., 1999). However, EIN2 is not the sole ethylene signaling element that controls ABA responsiveness in seeds. As did the ein2 mutations, the ethylene-insensitive etr1-1 mutation in the ETR1 receptor enhanced ABA sensitivity in seeds. Conversely, seeds of the constitutive ethylene response mutants ctr1 were less sensitive than the wild type to ABA. Hence, our results indicate that the entire pathway defined by ETR1, CTR1, and EIN2 impinges on ABA transduction in seeds.
It is currently unclear whether the ETR1–EIN2 cascade mediates ethylene signaling exclusively. For instance, various observations suggest that ETR1 (Hua and Meyerowitz, 1998), and possibly a whole subset of the ethylene receptors (Gamble et al., 1998), may have additional, ethylene-independent functions. Nevertheless, the simplest interpretation of the present data is that the enhanced dormancy of etr1-1 and ein2 seeds actually results from impaired ethylene signaling, and hence that endogenous ethylene regulates negatively the dormancy of Arabidopsis seeds. Ethylene has long been suspected to play a role in seed dormancy, but direct evidence has been scarce (Abeles et al., 1992; Kepczynski and Kepczynska, 1997). Results of studies with biosynthesis inhibitors were ambiguous because in many cases these inhibitors markedly lowered ethylene production without affecting seed germination (Kepczynski and Kepczynska, 1997). However, studies with 2,5-norbornadiene, a competitive antagonist of ethylene, indicated that endogenous ethylene is necessary for the germination of dormant seeds from Chenopodium album (lamb's-quarters) (Machabée and Saini, 1991) and Amaranthus retroflexus (Kepczynski et al., 1997). Similarly, our genetic data indicate that endogenous ethylene regulates negatively the degree of seed dormancy in Arabidopsis. Under our experimental conditions, endogenous ethylene content in the wild type was at or close to saturating values for its action on seed dormancy because constitutive activation of the ethylene pathway in the ctr1 mutants or treating wild-type seeds with 10 μM ACC (data not shown) barely decreased dormancy.
Seed dormancy mutants of Arabidopsis can be classified into two groups depending on whether or not they are affected in ABA action (biosynthesis or sensitivity) (Léon-Kloosterziel et al., 1996b). As discussed above, the etr1-1 and ein2 mutations markedly enhanced the sensitivity of seed germination to exogenous ABA. Furthermore, epistasis analyses between ein2-45 and severely ABA-insensitive mutants indicated that ethylene regulates negatively the degree of seed dormancy by decreasing the sensitivity to endogenous ABA. The correlation between dormancy and ABA sensitivity, however, was not perfect in all the genotypes analyzed here. etr1-1 seeds were somewhat less dormant but were more sensitive to exogenous ABA than ein2-1 seeds. Conversely, abi1-1 ein2-45 double-mutant seeds were more dormant but less sensitive to exogenous ABA than were wild-type Ler seeds. It is unclear whether these few discrepancies suggest that ethylene has in addition some ABA-independent effect on seed dormancy or whether they simply result from the fact that inhibition of seed germination by exogenous ABA mimics only imperfectly the action of endogenous ABA on seed dormancy (Steber et al., 1998).
Our results support that ethylene regulates seed dormancy largely by counteracting the effect of ABA (Figure 10A). In this respect, there are many similarities between the roles of ethylene and gibberellin (GA). GA is absolutely required for the germination of wild-type Arabidopsis seed because GA-deficient mutants such as ga1 fail to germinate (unless supplied with exogenous GA) (Koornneef and van der Veen, 1980). In contrast to the ga1 single mutant, however, double mutants that combine ga1 with ABA-deficient or ABA-insensitive mutations do not require exogenous GA to germinate (Koornneef et al., 1982; Nambara et al., 1992; Steber et al., 1998). Finally, the GA-deficient ga1 and GA-insensitive sly1 mutations increase the sensitivity of seed germination to ABA inhibition. ga1 and sly1 mutants were isolated by Steber et al. (1998) as suppressors of abi1-1 in a screen very similar to the one in which we recovered ein2-45. Hence, both endogenous GA and ethylene appear to control seed germination by decreasing ABA responsiveness (Figure 10A). GA and ethylene can replace each other to a certain extent because germination of the ga1 mutant could be induced by exogenous ethylene (Karssen et al., 1989), and conversely, a GA treatment stimulated germination of the etr1-1 mutant (Bleecker et al., 1988). The roles of endogenous GA and ethylene are unlikely to be completely redundant, however, because mutations that impair the action of either of these two hormones lead to tangible alterations in seed germination.
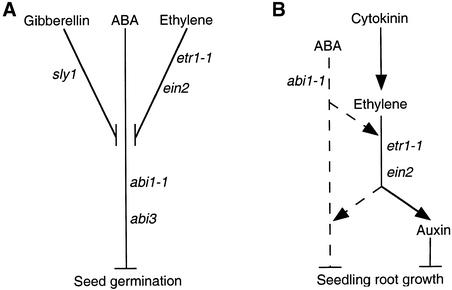
Figure 10.
Schematic Representations of the Interactions between the ABA and Ethylene Signaling Cascades.
(A) Regulation of seed germination. Ethylene and GA signaling cascades promote seed germination by decreasing sensitivity to endogenous ABA in imbibed seeds. The abi1-1 and abi3 mutants are ABA insensitive, the sly1 mutant is GA insensitive, and the etr1-1 and ein2 mutants are ethylene insensitive. The positions of the intersections between the ABA, ethylene, and GA pathways are speculative.
(B) Inhibition of seedling root growth. Inhibition of root growth in response to increased amounts of ethylene is largely mediated through internal accumulation of auxin. Cytokinin inhibits root growth by stimulating ethylene biosynthesis. ABA inhibition of root growth is apparently not mediated by an increase in ethylene biosynthesis but requires an active ethylene signaling cascade. Either the basal activity of the ethylene pathway is required to synergize ABA action or ABA stimulates the ethylene signaling cascade.
Primary seed dormancy is established on the mother plant during the late stages of seed development (seed maturation). Studies on ABA-deficient mutants clearly demonstrated that embryonic ABA is required for the onset of dormancy during Arabidopsis seed maturation (Karssen et al., 1983; Léon-Kloosterziel et al., 1996a). Because ABA content markedly decreases at the end of seed maturation, Karssen et al. (1983)(1989) postulated that after the onset of dormancy, endogenous ABA may not be required for its maintenance. However, recent experiments in barley (Wang et al., 1995) and in Nicotiana plumbaginifolia (Grappin et al., 2000) indicate that the maintenance of dormancy in imbibed seeds is actually an active process involving de novo ABA synthesis. Ethylene and GA do not seem to regulate the induction of dormancy during seed maturation and are thought to act mainly during imbibition to break dormancy and trigger germination (Karssen et al., 1989; Nambara et al., 1991; Abeles et al., 1992; Kepczynski and Kepczynska, 1997). The observation that seeds of ethylene-insensitive (this study) and GA-insensitive (Steber et al., 1998) mutants are supersensitive to exogenous ABA suggests that ethylene and GA may counteract directly the action of ABA in dormancy maintenance by inhibiting ABA signaling in imbibed seeds.
Whereas etr1-1 and ein2 seeds were supersensitive to ABA, however, the roots of these mutants were cross-resistant to ethylene and ABA. Treatment with exogenous ethylene (or ACC), or constitutive activation of the ethylene cascade by the ctr1 mutation, inhibits the growth of seedling roots. Ethylene inhibits auxin transport in both shoots and roots, and evidence suggests that ethylene inhibition of root growth is largely mediated through internal auxin accumulation (Timpte et al., 1995; Fujita and Syono, 1996; Luschnig et al., 1998; Marchant et al., 1999). In particular, the ethylene resistance of axr2 (Wilson et al., 1990), aux1 (Pickett et al., 1990), axr1 (Timpte et al., 1995), and eir1 (Luschnig et al., 1998) mutant roots seems to be a secondary consequence of the auxin insensitivity of these mutants (Figure 10B). Roots of the ethylene-insensitive mutants ein1 (allelic to etr1) and ckr1 (allelic to ein2) are resistant to cytokinin (Su and Howell, 1992; Cary et al., 1995) because cytokinin inhibits root growth by stimulating ethylene biosynthesis (Cary et al., 1995; Vogel et al., 1998) (Figure 10B). Whereas etr1-1 and ein2 roots were resistant to both ABA and ethylene, abi1-1 root was resistant to ABA but had normal sensitivity to ethylene. This suggested that ethylene acts “downstream” of ABA. However, ABA-treated seedlings did not display any detectable change in ethylene production or in abundance of the ethylene-responsive GST2 mRNA. This is consistent with previous studies also indicating that ABA has at most only small effects on ethylene production (Abeles et al., 1992; Woeste et al., 1999). Hence, unlike cytokinin, ABA inhibition of root growth is apparently not mediated by an ABA-induced stimulation of ethylene production. Nevertheless, the decreased ABA sensitivity of etr1-1 and ein2 roots shows that ethylene signaling is required for efficient ABA inhibition of root growth. This implies either that ABA can stimulate the ethylene signaling pathway or that the seedling's content of endogenous ethylene at rest has a synergistic effect on root growth inhibition by ABA (Figure 10B). Whether ABA inhibition of root growth requires auxin action, however, is currently unclear. Roots of the axr2-1 mutant are cross-resistant to auxin, ethylene, and ABA (Wilson et al., 1990). In contrast, aux1 and eir1 roots are resistant to auxin and ethylene but are as sensitive as the wild type to ABA (Pickett et al., 1990; Luschnig et al., 1998).
This study revealed the existence of important interactions between ABA and ethylene signaling cascades in seeds and in roots. These interactions are antagonistic in the control of seed dormancy but synergistic in inhibiting root growth. Furthermore, in each of these two processes, the interaction between ABA and ethylene pathways is embedded in a specific and more complex web of multihormonal regulations. These results illustrate that hormone signaling cascades interact in elaborate networks highly dependent on the organ and the developmental stage of the plant. The signaling elements at the points at which these various pathways intersect and the mechanisms underlying the integration of the corresponding signals remain to be identified.
METHODS
Plant Material and Growth Conditions
The Arabidopsis thaliana mutants abi1-1 (Koornneef et al., 1984), abi3-1 (Koornneef et al., 1984), and abi3-4 (Ooms et al., 1993) are in the Landsberg erecta (Ler) ecotype and were obtained from Maarten Koornneef (Agricultural University, Wageningen, The Netherlands). The mutants ein2-1 (Guzman and Ecker, 1990), etr1-1 (Bleecker et al., 1988), ctr1-1 (Kieber et al., 1993), and axr2-1 (Wilson et al., 1990) are in the ecotype Columbia (Col) and were obtained from the Nottingham Arabidopsis Stock Centre (Nottingham, UK).
Plants were grown in a greenhouse (22°C with a 16-hr-light photoperiod) on soil irrigated with mineral nutrients. Seeds used for comparative studies were derived from plants grown and harvested simultaneously. For in vitro experiments, seeds were surface-sterilized and plated on medium containing Murashige and Skoog (1962) inorganic salts at half concentration, 100 mg/L myo-inositol, 1 mg/L thiamine-HCl, 0.5 mg/L nicotinic acid, 0.5 mg/L pyridoxine-HCl, 1% sucrose, 2.5 mM MES-KOH, and 0.8% agar, pH 5.7. Except in dormancy tests, after the seeds were plated on agar medium, they were incubated for 3 to 4 days at 4°C in darkness to break dormancy before being transferred to 21°C. Unless otherwise indicated, plates were incubated at 21°C with a 16-hr-light photoperiod. When indicated, abscisic acid (ABA) (mixed isomers; Sigma) or 1-aminocyclopropane-1-carboxylic acid (ACC; Sigma) was added to the medium. ACC stock solutions were prepared in water. ABA stock solutions were prepared in methanol, and equivalent volumes of methanol were added to the ABA-free control plates.
Screen for Suppressors and Enhancers of abi1-1
The screen for suppressors of abi1-1 has been described previously (Gosti et al., 1999). The suppressor line 84B (abi1-1 ein2-45) was backcrossed twice to abi1-1 before detailed analysis. The screen for phenotypic enhancers of abi1-1 was performed with the same M2 population as for the suppressor screen (Gosti et al., 1999). Approximately 1000 seeds from each of the 185 independent M2 pools (each pool corresponding to ∼15 viable M1 plants) were surface-sterilized and plated on agar plates containing 100 μM ABA. Seeds were kept for 4 days at 4°C in darkness to break dormancy and then were transferred to 21°C with a 16-hr-light photoperiod for 5 days. Germinated seeds that had developed green cotyledons or an elongated root with hairs (or both) were selected. These candidates were transferred to ABA-free medium for 1 week before being propagated in soil. M3 seeds were retested for ABA resistance at germination. Enhancers originating from the same pool of M2 seeds were assumed to be siblings, and only one representative was retained for further analysis. The enhancer line H3 (abi1-1 ctr1-10) was backcrossed to abi1-1 before detailed analysis.
DNA Sequencing
The original CTR1 (Kieber et al., 1993) and EIN2 (Alonso et al., 1999) sequences were from the Col ecotype, whereas the ctr1-10 and ein2-45 mutants are in the Ler ecotype. To identify the ctr1-10 mutation, we obtained overlapping genomic fragments encompassing the entire CTR1 gene from both the Ler wild type and ctr1-10 DNA by polymerase chain reaction with specific primers, and the amplified products were sequenced directly by using appropriate primers. Comparing the sequences revealed that the ctr1-10 mutation correlated with a single nucleotide change in the coding region. Moreover, the CTR1 gene contains only silent nucleotide differences between the Col and Ler wild-type ecotypes. A similar strategy was followed to identify the ein2-45 mutation. In addition to various silent nucleotide differences, the EIN2 gene contains two nonsilent differences between the Col and Ler wild-type ecotypes: Tyr-160 in Col is replaced by Cys in Ler, and Thr-1288 in Col is replaced by Pro in Ler.
Genetic Mapping
The suppressor mutation in line 84B/1 was mapped by using simple sequence length polymorphisms (Bell and Ecker, 1994) and cleaved amplified polymorphic sequences (CAPS) (Konieczny and Ausubel, 1993), markers that detect polymorphism between the Ler and Col Arabidopsis strains.
Construction of Double and Triple Mutants
The abi1-1 abi3-1 and abi3-4 ein2-45 double mutants were selected in the F2 progeny of crosses between the two corresponding homozygous parents. The abi1-1 abi3-1 ein2-45 triple mutant was selected in the F2 progeny of a cross between abi1-1 abi3-1 and 84B (abi1-1 ein2-45). The ein2-45 mutation was tracked through its inhibiting the formation of the triple response in dark-grown seedlings treated with 10 μM ACC. The abi1-1 and abi3-1 genotypes were verified by using polymerase chain reaction markers. A CAPS marker that distinguishes the wild-type ABI1 allele from the abi1-1 allele has been described previously (Leung et al., 1997). A dCAPs marker (Neff et al., 1998), which could distinguish ABI3 from abi3-1, was generated. The oligonucleotide primers 5′-TCCTTCCGAGGTGACCCA-CGT-3′ and 5′-CGGAAGATTATACATTTGCAATGG-3′ amplify a DNA fragment that contains a PshA1 restriction site in ABI3 but not in abi3-1. Finally, the abi3-4 mutation was followed on the basis of its associated green seed phenotype (Ooms et al., 1993).
Measurement of Ethylene Production
Ethylene production was measured on seedlings grown at 21°C with a 16-hr-light photoperiod. After growing for 6 days on germination medium, wild-type Ler seedlings were transferred to 12 × 12-cm Petri plates (∼200 seedlings per plate) containing 45 mL of either normal medium or medium supplemented with either 100 μM ABA or 10 μM ACC. The lids of these Petri plates were equipped with a septum. Three days after transfer the plates were sealed, and 1-mL air samples were collected 1 hr later for analysis. Ethylene was measured with a gas chromatograph fitted with a flame ionization detector.
Acknowledgments
We thank Maarten Koornneef for providing the abi mutants; Peter McCourt for providing the era mutants; Peter Goldsbrough for providing the GST2 cDNA; Christiane Valon for help with the enhancers screen; Alain Latché and Simone Albert for the ethylene measurements; David Bouchez and Christine Camilleri for advice on markers for simple sequence length polymorphisms; Marcel Jacquin for technical assistance with plant culture; Liliane Troussard for technical assistance with DNA sequencing; and Nathalie Mansion for photographic work. We also thank Joe Ecker for sharing data before publication, Herman Höfte for helpful discussion, and François Parcy and Jeffrey Leung for valuable comments on this manuscript. This work was supported by the Centre National de la Recherche Scientifique, the European Community BIOTECH program (Grant No. BIO4-CT96-0062), and a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche (Grant No. 97-5-11773) to C.S.
References
- Abeles, F.B., Morgan, P.W., and Saltveit, M.E., Jr. (1992). Ethylene in Plant Biology. (San Diego, CA: Academic Press).
- Allen, G.J., Kuchitsu, K., Chu, S.P., Murata, Y., and Schroeder, J.I. (1999). Arabidopsis abi1–1 and abi2–1 phosphatase mutations reduce abscisic acid–induced cytoplasmic calcium rises in guard cells. Plant Cell 11, 1785–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, J.M., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284, 2148–2152. [DOI] [PubMed] [Google Scholar]
- Bell, C.J., and Ecker, J.R. (1994). Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19, 137–144. [DOI] [PubMed] [Google Scholar]
- Bleecker, A.B., Estelle, M.A., Somerville, C., and Kende, H. (1988). Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241, 1086–1089. [DOI] [PubMed] [Google Scholar]
- Bonetta, D., and McCourt, P. (1998). Genetic analysis of ABA signal transduction pathways. Trends Plant Sci. 3, 231–235. [Google Scholar]
- Busk, P.K., and Pagès, M. (1997). Regulation of abscisic acid–induced transcription. Plant Mol. Biol. 37, 425–435. [DOI] [PubMed] [Google Scholar]
- Cary, A.J., Liu, W., and Howell, S.H. (1995). Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 107, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, K.L., Larsen, P.B., Wang, X., and Chang, C. (1998). Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc. Natl. Acad. Sci. USA 95, 5401–5406. Erratum. Proc. Natl. Acad. Sci. USA 95, 9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S., Ghassemian, M., Bonetta, D., Cooney, S., and McCourt, P. (1996). A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273, 1239–1241. [DOI] [PubMed] [Google Scholar]
- Davies, W.J., and Jones, H.G. (1991). Abscisic Acid Physiology and Biochemistry. (Oxford, UK: BIOS Scientific Publishers).
- Finkelstein, R.R. (1994). Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J. 5, 765–771. [Google Scholar]
- Finkelstein, R.R., and Somerville, C.R. (1990). Three classes of abscisic acid (ABA)–insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol. 94, 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein, R.R., Wang, M.L., Lynch, T.J., Rao, S., and Goodman, H.M. (1998). The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein. Plant Cell 10, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, R., and Chua, N.H. (1999). An Arabidopsis mutant with deregulated ABA gene expression: Implications for negative regulator function. Plant J. 17, 363–372. [DOI] [PubMed] [Google Scholar]
- Fujita, H., and Syono, K. (1996). Genetic analysis of the effects of polar auxin transport inhibitors on root growth in Arabidopsis thaliana. Plant Cell Physiol. 37, 1094–1101. [DOI] [PubMed] [Google Scholar]
- Gamble, R.L., Coonfield, M.L., and Schaller, G.E. (1998). Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc. Natl. Acad. Sci. USA 95, 7825–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat, J., Hauge, B.M., Valon, C., Smalle, J., Parcy, F., and Goodman, H.M. (1992). Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4, 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti, F., Beaudoin, N., Serizet, C., Webb, A.A.R., Vartanian, N., and Giraudat, J. (1999). ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11, 1897–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grappin, P., Bouinot, D., Sotta, B., Miginiac, E., and Jullien, M. (2000). Control of seed dormancy in Nicotiana plumbaginifolia: Post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 210, 279–285. [DOI] [PubMed] [Google Scholar]
- Guzman, P., and Ecker, J.R. (1990). Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, J., and Meyerowitz, E.M. (1998). Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94, 261–271. [DOI] [PubMed] [Google Scholar]
- Johnson, P.R., and Ecker, J.R. (1998). The ethylene gas signal transduction pathway: A molecular perspective. Annu. Rev. Genet. 32, 227–254. [DOI] [PubMed] [Google Scholar]
- Karssen, C.M., Brinkhorst-van der Swan, D.L.C., Breekland, A.E., and Koornneef, M. (1983). Induction of dormancy during seed development by endogenous abscisic acid: Studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta 157, 158–165. [DOI] [PubMed] [Google Scholar]
- Karssen, C.M., Zagorski, S., Kepczynski, J., and Groot, S.P.C. (1989). Key role of endogenous gibberellins in the control of seed germination. Ann. Bot. 63, 71–80. [Google Scholar]
- Kepczynski, J., and Kepczynska, E. (1997). Ethylene in seed dormancy and germination. Physiol. Plant. 101, 720–726. [Google Scholar]
- Kepczynski, J., Bihun, M., and Kepczynska, E. (1997). Ethylene involvement in the dormancy and germination of Amaranthus seeds. In Biology and Biotechnology of the Plant Hormone Ethylene, A.K. Kanelis, C. Chang, H. Kende, and D. Grierson, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 113–122.
- Kieber, J.J., Rothenberg, M., Roman, G., Feldmann, K.A., and Ecker, J.R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72, 427–441. [DOI] [PubMed] [Google Scholar]
- Konieczny, A., and Ausubel, F.M. (1993). A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 4, 403–410. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., and van der Veen, J.H. (1980). Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genet. 58, 257–263. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., Jorna, M.L., Brinkhorst-van der Swan, D.L.C., and Karssen, C.M. (1982). The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genet. 61, 385–393. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., Reuling, G., and Karssen, C.M. (1984). The isolation and characterization of abscisic acid–insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 61, 377–383. [Google Scholar]
- Lang, V., and Palva, E.T. (1992). The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol. Biol. 20, 951–962. [DOI] [PubMed] [Google Scholar]
- Léon-Kloosterziel, K.M., Alvarez Gil, M., Ruijs, G.J., Jacobsen, S.E., Olszewski, N.E., Schwartz, S.H., Zeevaart, J.A.D., and Koornneef, M. (1996. a). Isolation and characterization of abscisic acid–deficient Arabidopsis mutants at two new loci. Plant J. 10, 655–661. [DOI] [PubMed] [Google Scholar]
- Léon-Kloosterziel, K.M., van de Bunt, G.A., Zeevaart, J.A.D., and Koornneef, M. (1996. b). Arabidopsis mutants with reduced seed dormancy. Plant Physiol. 110, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, J., and Giraudat, J. (1998). Abscisic acid signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 199–222. [DOI] [PubMed] [Google Scholar]
- Leung, J., Bouvier-Durand, M., Morris, P.-C., Guerrier, D., Chefdor, F., and Giraudat, J. (1994). Arabidopsis ABA-response gene ABI1: Features of a calcium-modulated protein phosphatase. Science 264, 1448–1452. [DOI] [PubMed] [Google Scholar]
- Leung, J., Merlot, S., and Giraudat, J. (1997). The Arabidopsis ABSCISIC ACID–INSENSITIVE 2 (ABI2) and ABI1 genes encode redundant protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig, C., Gaxiola, R.A., Grisafi, P., and Fink, G.R. (1998). EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 12, 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machabée, S., and Saini, H.S. (1991). Differences in the requirement for endogenous ethylene during germination of dormant and non-dormant seeds of Chenopodium album L. J. Plant Physiol. 138, 97–101. [Google Scholar]
- MacRobbie, E.A. (1998). Signal transduction and ion channels in guard cells. Philos. Trans. R. Soc. Lond. B 353, 1475–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant, A., Kargul, J., May, S.T., Muller, P., Delbarre, A., Perrot-Rechenmann, C., and Bennett, M.J. (1999). AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 18, 2066–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty, D.R., Hattori, T., Carson, C.B., Vasil, V., Lazar, M., and Vasil, I.K. (1991). The viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell 66, 895–905. [DOI] [PubMed] [Google Scholar]
- Meyer, K., Leube, M.P., and Grill, E. (1994). A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264, 1452–1455. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nambara, E., Akazawa, T., and McCourt, P. (1991). Effects of the gibberellin biosynthetic inhibitor uniconazol on mutants of Arabidopsis. Plant Physiol. 97, 736–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara, E., Naito, S., and McCourt, P. (1992). A mutant of Arabidopsis which is defective in seed development and storage protein accumulation is a new abi3 allele. Plant J. 2, 435–441. [Google Scholar]
- Nambara, E., Keith, K., McCourt, P., and Naito, S. (1995). A regulatory role for the ABI3 gene in the establishment of embryo maturation in Arabidopsis thaliana. Development 121, 629–636. [Google Scholar]
- Neff, M.M., Neff, J.D., Chory, J., and Pepper, A.E. (1998). dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: Experimental applications in Arabidopsis thaliana genetics. Plant J. 14, 387–392. [DOI] [PubMed] [Google Scholar]
- Oh, S.A., Park, J.-H., Lee, G.I., Paek, K.H., Park, S.K., and Nam, H.G. (1997). Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J. 12, 527–535. [DOI] [PubMed] [Google Scholar]
- Ooms, J.J.J., Léon-Kloosterziel, K.M., Bartels, D., Koornneef, M., and Karssen, C.M. (1993). Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana. A comparative study using abscisic acid–insensitive abi3 mutants. Plant Physiol. 102, 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy, F., Valon, C., Raynal, M., Gaubier-Comella, P., Delseny, M., and Giraudat, J. (1994). Regulation of gene expression programs during Arabidopsis seed development: Roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6, 1567–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.-M., Ghassemian, M., Kwak, C.M., McCourt, P., and Schroeder, J.I. (1998). Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282, 287–290. [DOI] [PubMed] [Google Scholar]
- Pickett, F.B., Wilson, A.K., and Estelle, M. (1990). The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 94, 1462–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, P.L., Benning, G., and Grill, E. (1998). ABI2, a second protein phosphatase 2C involved in abscisic acid signal transduction in Arabidopsis. FEBS Lett. 421, 185–190. [DOI] [PubMed] [Google Scholar]
- Schaller, G.E., and Bleecker, A.B. (1995). Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270, 1809–1811. [DOI] [PubMed] [Google Scholar]
- Steber, C.M., Cooney, S.E., and McCourt, P. (1998). Isolation of the GA-response mutant sly1 as a suppressor of ABI1-1 in Arabidopsis thaliana. Genetics 149, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, W., and Howell, S.H. (1992). A single genetic locus, ckr1, defines Arabidopsis mutants in which root growth is resistant to low concentrations of cytokinin. Plant Physiol. 99, 1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpte, C., Wilson, A.K., and Estelle, M. (1994). The axr2-1 mutation of Arabidopsis thaliana is a gain-of-function mutation that disrupts an early step in auxin response. Genetics 138, 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpte, C., Lincoln, C., Pickett, F.B., Turner, J., and Estelle, M. (1995). The AXR1 and AUX1 genes of Arabidopsis function in separate auxin-response pathways. Plant J. 8, 561–569. [DOI] [PubMed] [Google Scholar]
- Vogel, J.P., Woeste, K.E., Theologis, A., and Kieber, J.J. (1998). Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc. Natl. Acad. Sci. USA 95, 4766–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M., Heimovaara-Dijkstra, S., and Van Duijn, B. (1995). Modulation of germination of embryos isolated from dormant and nondormant barley grains by manipulation of endogenous abscisic acid. Planta 195, 586–592. [Google Scholar]
- Wilson, A.K., Pickett, F.B., Turner, J.C., and Estelle, M. (1990). A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol. Gen. Genet. 222, 377–383. [DOI] [PubMed] [Google Scholar]
- Woeste, K.E., Vogel, J.P., and Kieber, J.J. (1999). Factors regulating ethylene biosynthesis in etiolated Arabidopsis thaliana seedlings. Physiol. Plant. 105, 478–484. [Google Scholar]
- Xiong, L., Ishitani, M., Lee, H., and Zhu, J.K. (1999). HOS5—A negative regulator of osmotic stress–induced gene expression in Arabidopsis thaliana. Plant J. 19, 569–578. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki, K., and Shinozaki, K. (1993). Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol. Gen. Genet. 236, 331–340. [DOI] [PubMed] [Google Scholar]
- Zeevaart, J.A.D., and Creelman, R.A. (1988). Metabolism and physiology of abscisic acid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 439–473. [Google Scholar]
- Zhou, J., and Goldsbrough, P.B. (1993). An Arabidopsis gene with homology to glutathione S-transferases is regulated by ethylene. Plant Mol. Biol. 22, 517–523. [DOI] [PubMed] [Google Scholar]