Abstract
Although abscisic acid (ABA) is involved in a variety of plant growth and developmental processes, few genes that actually regulate the transduction of the ABA signal into a cellular response have been identified. In an attempt to determine negative regulators of ABA signaling, we identified mutants, designated enhanced response to ABA3 (era3), that increased the sensitivity of the seed to ABA. Biochemical and molecular analyses demonstrated that era3 mutants overaccumulate ABA, suggesting that era3 is a negative regulator of ABA synthesis. Subsequent genetic analysis of era3 alleles, however, showed that these are new alleles at the ETHYLENE INSENSITIVE2 locus. Other mutants defective in their response to ethylene also showed altered ABA sensitivity; from these results, we conclude that ethylene appears to be a negative regulator of ABA action during germination. In contrast, the ethylene response pathway positively regulates some aspects of ABA action that involve root growth in the absence of ethylene. We discuss the response of plants to ethylene and ABA in the context of how these two hormones could influence the same growth responses.
INTRODUCTION
Abscisic acid (ABA) modulates a wide variety of plant processes ranging from seed dormancy to leaf–water relations (reviewed in Zeevaart and Creelman, 1988). Over the past few years, mutational analysis of the ABA response in Arabidopsis has begun to uncover genes regulating the sensitivity of a plant cell to the hormone. Basically, genetic screens fall into two categories: screens that identify mutations conferring an ABA-insensitive phenotype and screens that identify mutations enhancing ABA sensitivity (Bonetta and McCourt, 1998; Leung and Giraudat, 1998). To date, studies of these mutants have led to the identification of two protein phosphatases (ABI1 [for ABA-insensitive] and ABI2), a protein farnesyltransferase (ERA1 [for enhanced response to ABA]), and two transcription factors (ABI3 and ABI4) involved in ABA action.
Recently, however, careful phenotypic analysis has determined that several of the hormone response mutants have altered sensitivities to more than one hormone. Mutations in the AXR2 gene of Arabidopsis, for example, confer cross-resistance to ABA, ethylene, and auxin (Wilson et al., 1990). However, because only dominant axr2 alleles exist and the molecular mechanism of AXR2 is not known, it is difficult to draw conclusions regarding the ability of this gene to confer ABA sensitivity. Mutations in the SAX1 gene, which is involved in brassinosteroid biosynthesis, confer increased ABA sensitivity to the seed, suggesting that the synthesis of one hormone can affect the sensitivity of the plant to other hormones (Ephritikhine et al., 1999).
Recently, the ETHYLENE-INSENSITIVE2 (EIN2) gene of Arabidopsis has been shown to be involved in multiple hormone responses, including the responses to ABA (Alonso et al., 1999). Originally identified as a loss-of-function mutation that confers a strong insensitivity to exogenous ethylene, molecular dissection of this gene has separated EIN2 ethylene-regulated responses from EIN2 jasmonate-regulated responses (Alonso et al., 1999). Although the relationship of ethylene and ABA has never been addressed by using the ein2 mutant, analysis of EIN2 with respect to the jasmonate and ethylene response pathways indicates that a single signaling component can act in two different hormone response pathways. These genetic studies support physiological data suggesting that overall sensitivity of a plant cell to a hormone is at least partially established by the interplay of several hormones (Trewavas, 1992). Therefore, assigning a hormone-sensitizing mutation to a specific hormone response pathway must be done with caution.
While characterizing a class of mutants (era3) with enhanced response to ABA, we determined that these represent new alleles of ein2. In addition, mutations in several genes that encode steps in the ethylene signal transduction pathway also confer altered responsiveness of seeds and roots to ABA. Although ein2 mutants produce hyperdormant seeds and overaccumulate ABA, the root growth of these mutants is insensitive to ABA. Therefore, ethylene apparently contributes to some aspects of a cell's sensitivity to ABA, and this role seems to depend on the developmental history of the tissue. Although physiological studies have described the antagonistic and synergistic effects of ethylene and ABA on plant growth and development, this study demonstrates the extensive cross-talk between ABA and ethylene signaling components (Ketring and Morgan, 1972; Abeles et al., 1992). We discuss the response of plants to ethylene and ABA in the context of how these two hormones could influence specific responses required for plant growth and development.
RESULTS
Isolation of era3 Mutants
The mutant line era3-1 was previously identified by screening for seed that could not germinate on media containing 0.3 μM ABA. This concentration ordinarily does not inhibit wild-type germination (Cutler et al., 1996). When crossed to the wild type, none of the 42 F1 seed showed enhanced sensitivity to ABA when germinating, and subsequent analysis of 382 F2 seed demonstrated that the phenotype of the era3-1 mutant is recessive and results from a single nuclear mutation (3:1;  2
2 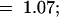 P > 0.90). After this analysis was conducted, a second fast-neutron-induced allele of era3 was identified through complementation analysis with era3-1 (data not shown). During the characterization of these two alleles, we learned of another era3 allele (Beaudoin et al., 2000, this issue); therefore, we designated our new allele era3-3.
P > 0.90). After this analysis was conducted, a second fast-neutron-induced allele of era3 was identified through complementation analysis with era3-1 (data not shown). During the characterization of these two alleles, we learned of another era3 allele (Beaudoin et al., 2000, this issue); therefore, we designated our new allele era3-3.
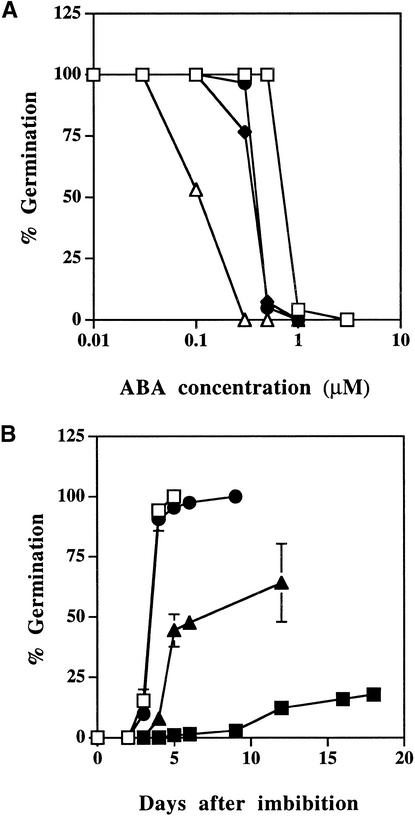
In Arabidopsis, mutants with reduced synthesis of or response to ABA have reduced dormancy, and era1 mutants, which have increased ABA responsiveness, showed increased dormancy (Bonetta and McCourt, 1998). Therefore, to clarify ABA sensitivity and dormancy requirements of the era3 alleles with respect to era1 and wild-type seed, ABA dose–response curves and dormancy factors of the era3 mutant were determined. Unlike the era1-2 null allele, seeds of both era3 mutant lines showed an age-dependent sensitivity to exogenous ABA (data not shown). Although seeds younger than 3 months were sensitive to 0.3 μM ABA during germination, older seeds required ABA concentrations >0.6 μM to severely inhibit germination (Figure 1A). Consistent with increased sensitivity to ABA during germination, era3-1 seeds also showed decreased germination efficiency under postimbibition conditions (Figure 1B). Normally, wild-type Arabidopsis seeds that have matured under dry conditions for a month do not require a chilling period to germinate. Under our experimental storage conditions, wild-type seeds showed good germination in the absence of a chilling period. In contrast, era3-1 seeds showed poor germination and required a chilling period of at least 4 days to alleviate dormancy. As seen with era1 mutants, the cold imbibition rescue of the hyperdormant phenotype in era3-1 is dose dependent (Cutler et al., 1996). The recessive nature of the era3 mutations together with their increased ABA sensitivity and dormancy suggest that ERA3 gene function is required to negatively regulate ABA-dependent seed dormancy.
Figure 1.
Germination of Wild-Type and era3 Seed.
(A) Germination in the presence of exogenous ABA. Open squares, wild type; filled diamonds, era3-1; filled circles, era3-3; open triangles, era1-2.
(B) Seed dormancy in response to increasing periods of chilling. Open squares, wild type; filled squares, era3-1 at 0 days of chilling at 4°C; filled triangles, era3-1 at 2 days of chilling at 4°C; filled circles, era3-1 at 4 days of chilling at 4°C.
Each point represents a germination test of 20 to 40 seed. Tests in (A) were performed in duplicate, and similar results were obtained in all cases. Tests in (B) were performed in triplicate. Vertical bars represent the standard errors. The percentage of germination was determined by dividing the number of seeds that germinated after 5 days of imbibition by the total number of seed.
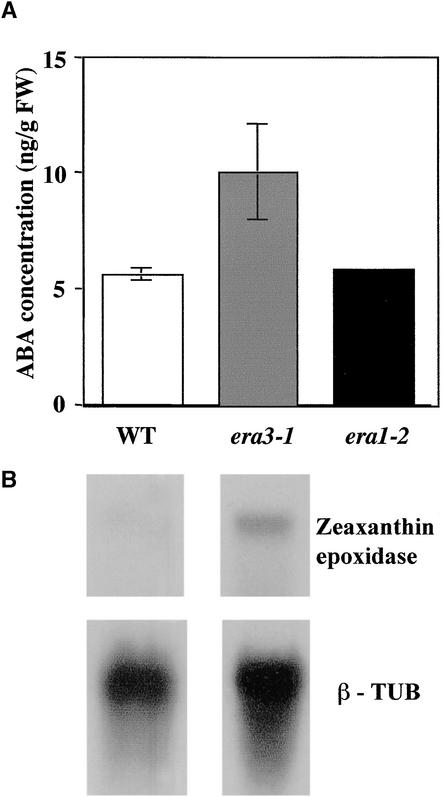
The increased sensitivity of era3 mutants to ABA may reflect abnormal ABA metabolism or altered ABA signal transduction. To differentiate between these possibilities, ABA concentrations were determined in wild-type, era1, and era3 plants. As shown in Figure 2A, the endogenous ABA concentration in era3 plants on a fresh weight basis averages twice that in either the wild type or the era1 mutant. Furthermore, transcript accumulation of zeaxanthin epoxidase, the first committed enzyme in ABA biosynthesis, was also increased compared with that of the wild type (Figure 2B). Together, these results suggest that era3 mutants overaccumulate ABA because of increased ABA biosynthesis.
Figure 2.
ABA Accumulation in Wild-Type and era3 Plants.
(A) Endogenous ABA concentrations in wild-type (WT), era3-1, and era1-2 plants. Wild-type and era3-1 values are the average of three independent measurements. era1-2 ABA content was determined only once. Averages and standard deviation are shown for WT and era3-1 mutants. FW, fresh weight.
(B) RNA transcript accumulation of zeaxanthin epoxidase (ABA1). After hybridization with the ABA1 probe, blots were stripped for β-tubulin (β-TUB) hybridization, which meant that absolute amounts could not be compared between hybridization experiments. Five micrograms of total RNA was loaded per lane.
era3 Mutants Identify New ein2 Alleles
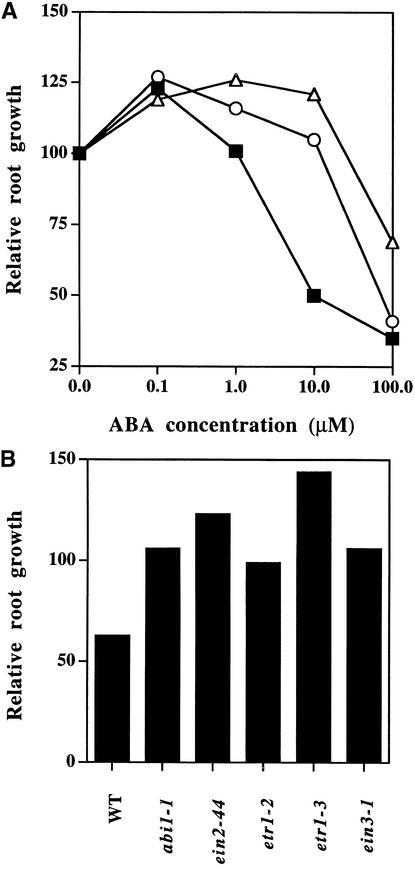
The ability of era3 mutants to hyperaccumulate ABA suggests that other ABA-regulated processes may be altered in this mutant background. Surprisingly, the roots of the era3-1 mutant showed less sensitivity to ABA than did those of the wild type (Figure 3A). When developmentally similar plants of both genotypes were transferred from minimal media to media with increasing concentrations of ABA, the era3 mutant lines showed insensitivity to ABA concentrations that ordinarily would reduce wild-type root growth by 50%. To further clarify root growth responses of era3 plants, we determined sensitivities to other hormones. Root growth of the mutants had less sensitivity than the wild type to cytokinin and the ethylene precursor 1-aminocyclopropane-1-carboxylate (ACC), whereas root growth on auxin was not altered (data not shown). The pattern of era3 root growth on media containing cytokinin and ACC is similar to that reported for ein2 mutants, and genetic mapping of era3-1 located this mutation to the region of chromosome 5 that spans the EIN2 gene (data not shown). Moreover, one ein2 allele failed to complement era3-1, suggesting that both mutations are allelic (Beaudoin et al., 2000; this issue). The recent cloning of the EIN2 gene has shown that this gene encodes a novel membrane-bound protein and that many loss-of-function alleles exist at this locus (Alonso et al., 1999). DNA gel blot analysis of era3-1 identified a molecular polymorphism at this locus (data not shown). Therefore, we believe era3-1 and era3-3 are new alleles of ein2, and we have designated our alleles ein2-44 and ein2-46, respectively.
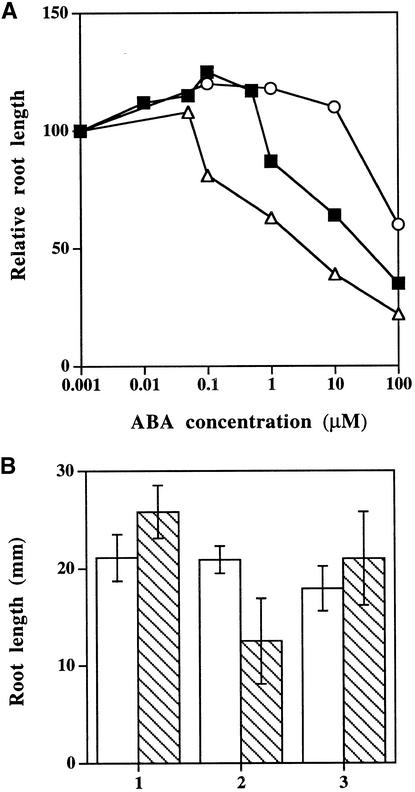
Figure 3.
Root Growth of Wild-Type and Ethylene Response Mutants Grown on Media Containing ABA.
(A) Root elongation of wild type (filled squares), ein2-44 (open triangles), and etr1-4 (open circles).
(B) Root elongation of wild type (WT), abi1-1, and several ethylene-insensitive mutants when grown on media containing 10 μM ABA.
Seven- to 10-day-old seedlings were placed on minimal media supplemented with increasing concentrations of ABA. Root elongation was measured after 4 days. Root growth on ABA was relative to the mean root elongation of the same genotype on minimal media. Each value represents the mean measurement for five to 15 seedlings. Experiments were conducted twice with wild-type and ein2 mutants and gave similar results, whereas experiments involving various etr1 alleles and ein3 were performed once.
Root Growth Response to ABA Requires Ethylene Signaling Components
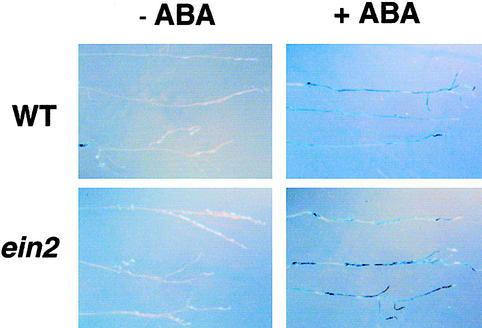
The extent of altered root sensitivity of ein2 mutants to ABA was further studied by monitoring transcript accumulation of the ABA-upregulated gene RAB18 in wild-type and ein2-44 roots. Using genetic crosses, we introduced the ein2-44 mutation into a transgenic plant containing a RAB18 promoter fragment fused to the β-glucuronidase (GUS) reporter gene. Histochemical staining for GUS activity was determined for plant roots exposed to ABA (Figure 4). When roots of the mutant and wild type were exposed to 10 μM ABA, blue precipitate could be detected along the length of the root, and both genotypes showed similar patterns of staining. The RAB18 expression in these lines was also determined by RNA gel blot analysis, and similar induction by ABA was observed in both wild-type and mutant samples (data not shown). Furthermore, RAB18 induction is ABA specific, because applying ACC did not induce the promoter construct (data not shown). Therefore, the response of the roots of ein2 plants to exogenous ABA is complex and depends on the process being assayed.
Figure 4.
ABA-Inducible Gene Expression in RAB18–GUS and ein2-44 RAB18–GUS Roots.
RAB18–GUS (WT) and ein2-44 RAB18–GUS (ein2-44) seedlings were treated with 0 (−ABA) or 10 μM (+ABA) ABA over a 24-hr period, and lines were stained to determine GUS activity for 12 hr at 37°C. Each point represents results for roots from three separate plants.
A role for EIN2 in determining some aspects of ABA response is perhaps not surprising because loss-of-function mutations in this ethylene response gene alter several hormone responses (Alonso et al., 1999). However, mutations in other ethylene response genes have not been reported to influence root growth in response to ABA. Molecular genetic analysis of ethylene action in Arabidopsis has identified a family of two-component receptor kinases (ETR1, ERS1, ERS2, ETR2, and EIN4), of which the ETR1 protein has been shown to bind ethylene (Bleecker et al., 1998). This family of receptors appears to act through the CTR1 (for constitutive triple response1) protein kinase, which in turn negatively regulates EIN2 (Hua and Meyerowitz, 1998; Johnson and Ecker, 1998). Possibly, EIN2 defines a unique node of cross-talk between ABA and ethylene signaling. Alternatively, normal ethylene signaling may be required for correct ABA sensitivity, which would mean that any mutation altering ethylene signal transduction would also show altered ABA sensitivity in growing roots and germinating seeds. To differentiate among these possibilities, we tested etr1 mutants showing reduced responsiveness to ethylene for root growth on ABA. As observed with ein2 mutants, an etr1-4 mutant that is defective in ethylene binding displays roots with reduced responsiveness to ABA (Figure 3A). Similar results were observed in plants carrying another etr1 mutation that interferes with ethylene binding (etr1-3) or an allele that binds ethylene but fails to transduce the hormone signal (etr1-2). Moreover, a mutation in EIN3, which is genetically downstream of EIN2, also conferred a reduced ABA responsiveness in roots (Figure 3B). Because these mutations, all of which confer an ethylene-insensitive phenotype, also reduced the responsiveness of roots to ABA, apparently a functional ethylene signaling pathway is required for normal ABA signaling in the root. Interestingly, when grown on ABA concentrations <1 μM, both wild-type and ethylene-insensitive mutant plants showed a 20% increase in root growth compared with plants grown on media to which no hormone had been added (Figure 3A). This result suggests that low concentrations of ABA stimulate Arabidopsis root growth and that this stimulation is independent of the ethylene signaling pathway. Thus, altered ethylene responsiveness affects only a subset of ABA responses in roots, and even these responses depend on the concentration of ABA used.
The dependence on ethylene signaling for normal ABA-induced root inhibition may reflect an increase in ethylene biosynthesis in response to high concentrations of exogenous ABA. For example, the insensitivity of ein2 roots to cytokinin results from the inability of this mutant to respond to increased ethylene rather than increased cytokinin (Cary et al., 1995). Hence, blocking ethylene biosynthesis or perception by using l-α-(2-aminoethoxyvinyl)-glycine (AVG) or Ag+ prevents cytokinin-induced root inhibition. To determine whether ABA uses a similar ethylene-mediated mode of action in the root, we measured ABA responsiveness of the roots of the wild type in the presence of AVG and Ag+. As expected, the ethylene perception inhibitor suppressed the inhibitory effects of high ABA concentrations on wild-type root (Figure 5A). In contrast, the ethylene biosynthetic inhibitor did not elicit an increase in ABA root insensitivity, suggesting that exogenous ABA does not induce ethylene synthesis. Moreover, AVG-treated roots showed slightly more sensitivity to ABA than did untreated roots, suggesting that ethylene may inhibit ABA responsiveness in roots. To determine the specificity of AVG under our experimental conditions, we measured the response of AVG-treated roots to ABA in the presence and absence of ACC (Figure 5B). ABA hypersensitivity observed by application of AVG was overcome by application of ACC, suggesting that the effect of AVG is to inhibit ethylene biosynthesis. To further clarify the role of ethylene on ABA sensitivity in roots, we tested eto1-1, a mutant that overproduces ethylene, for its responsiveness of roots to ABA. As expected, the roots of the eto1-1 mutant were highly insensitive to inhibitory concentrations of ABA, indicating that ethylene decreases ABA sensitivity in the roots (Figure 6A). Therefore, we concluded that inhibitory concentration of ABA does not appear to induce ethylene synthesis, but ethylene negatively regulates the sensitivity of roots to ABA.
Figure 5.
Root Growth of Wild-Type Seedlings in the Presence of Ethylene Biosynthetic (AVG) and Action Inhibitors (AgNO3).
(A) Seven-day-old wild-type seedlings were placed on minimal medium supplemented with no inhibitor (filled squares), 10 μM AgNO3 (open circles), or 2 μM AVG (open triangles). Root elongation was measured after 4 days. Relative growth is indicated in relationship to the mean root elongation of wild-type seedlings transferred to minimal medium without inhibitors. Each value represents the mean of measurements from at least 10 seedlings. Experiments were done in duplicate, and similar results were obtained in all cases.
(B) Seven-day-old wild-type seedlings were placed on medium free of ABA (open bars) or medium containing 1 μM ABA (striped bars). Treatment 1, no ACC or AVG supplement; treatment 2, added 1 μM AVG; treatment 3, added 2 μM ABG and 2 μM ACC. Root growth shown is the mean of the absolute root length of at least 10 seedlings. The experiment was performed in triplicate. Error bars represent sd.
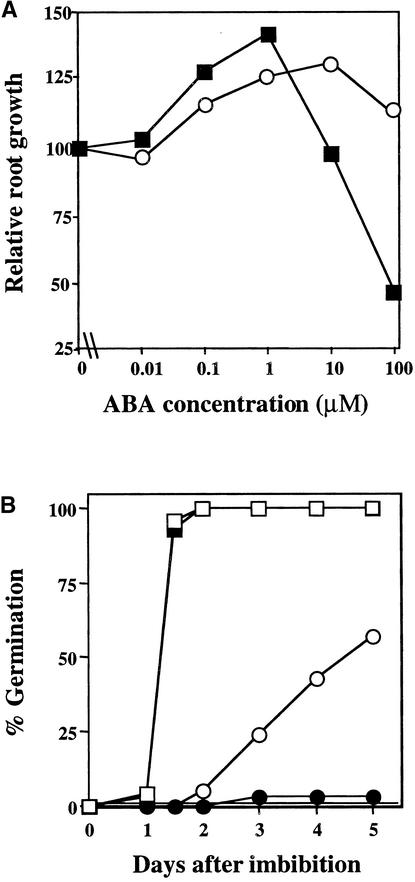
Figure 6.
Effects of Ethylene on Root Growth and Germination in the Presence of ABA.
(A) Relative root growth of wild-type (filled squares) and eto1-1 (open circles) seedlings in the presence of ABA. Experiments were performed as described earlier, and each point represents the mean value for five to 15 seedlings.
(B) Germination of wild-type seeds on minimal media in the presence (open squares) and absence (filled squares) of ACC and on media containing 3 μM ABA in the presence (open circles) and absence (filled circles) of ACC. Each point represents a germination test with ⩾20 seed. Each experiment was performed in duplicate, and similar results were obtained in all cases. The percentage of germination was determined by dividing the number of seeds that germinated after 5 days of imbibition by the total number of seed.
Ethylene Is a Negative Regulator of the ABA Response during Seed Germination
The observation that mutations in the EIN2 gene confer an increased sensitivity to ABA during germination suggests that other ethylene response mutants may also be defective in ABA response during germination. Bleecker et al. (1988) previously reported that the etr1-1 mutant germinates poorly in the absence of exogenous gibberellins. In contrast to wild-type seeds, etr1-4 seeds were able to germinate on minimal media, but they did not germinate on media containing 0.4 μM ABA, demonstrating that this mutant is also ABA supersensitive during germination (data not shown). To further test the interactions of ethylene and ABA during germination, we imbibed wild-type seeds on inhibitory concentrations of ABA in the presence or absence of ACC. Although ACC does not enhance germination in the absence of ABA, it does partially rescue ABA-induced inhibition of germination (Figure 6B).
This result predicts that mutations altering ethylene action should interact with mutations that perturb ABA responses. In particular, mutations that increase ethylene production or constitutively activate the ethylene response pathway may further decrease the ABA sensitivity of an ABA-insensitive mutant. With this in mind, we identified a collection of intergenic enhancers of abi1-1. From ∼60,000 M2 seeds, 40 mutants were identified that germinated on media containing 100 μM ABA, a concentration that would inhibit abi1-1 germination. Of these, three mutants showed vegetatively dwarfed growth and a hairy root phenotype similar to that reported for the ctr1 mutant (Kieber et al., 1993). Complementation analysis demonstrated these are indeed new alleles of ctr1, which we have designated ctr1-11, ctr1-12, and ctr1-13 (data not shown). The identification of ctr1 as an enhancer of the abi1-1 mutant is consistent with the notion that increasing ethylene signaling decreases the ABA sensitivity of the seed.
DISCUSSION
Although physiological studies have shown that different plant hormones can influence the same plant process, how these compounds affect plant function is unclear. We have shown that ethylene can influence several ABA-mediated physiological responses of Arabidopsis. First, we determined that ethylene affects only a subset of ABA responses. Second, although this study indicates that ethylene is a negative regulator of ABA action during seed germination and root growth, in the absence of ethylene, the ethylene signal transduction pathway appears to positively regulate the responsiveness of root to ABA. Finally, decreasing ethylene sensitivity increases endogenous ABA concentrations, and this interaction is accomplished, at least in part, by increasing mRNA accumulation during the first committed step in ABA biosynthesis. Thus, unlike several other hormone interactions involving ethylene, cross-talk between ABA and ethylene appears to occur at many levels and is dependent on the tissue and the process being assayed (Cary et al.,1995; Penninckx et al., 1998; Alonso et al., 1999).
Interactions between Ethylene and ABA Seed Responses
Mutations that decrease ethylene sensitivity increase the sensitivity of seeds to ABA. Conversely, a constitutive ethylene response or application of the ethylene precursor ACC decreases the ABA sensitivity of the seed. Together, these results suggest that ethylene is antagonistic to ABA during seed development. Physiological studies indicate a role for ethylene in promoting seed germination, and some evidence suggests ethylene influences this process by preventing ABA-induced dormancy (Ketring and Morgan, 1972; Abeles et al., 1992). Ethylene could negatively regulate ABA synthesis, as has been reported with deepwater rice (Kende et al., 1998), and the increased concentrations of ABA measured in ein2 plants indicate that ethylene sensitivity and ABA synthesis are coupled in Arabidopsis. Reducing the ethylene response could induce ABA synthesis, which in turn could increase the dormancy of the seed. Such a mechanism would explain why ethylene-insensitive mutants require prolonged ripening or chilling to germinate. However, that ein2 can suppress (Beaudoin et al., 2000, this issue) and that ctr1 can enhance the ABA-insensitive germination phenotype of abi1 suggest that ethylene must also influence the sensitivity of the seed to ABA.
During wild-type seed development, the embryo is exposed to endogenous ABA, which establishes a dormancy that persists after the ABA concentrations decline (Karssen et al., 1983). Possibly, ethylene interferes with the ability of ABA to establish seed dormancy. If this is true, then constitutive activation of the ethylene response in ctr1 embryos would decrease the ability of ABA to establish dormancy. This may explain why the ctr1 mutation is a genetic enhancer of the ABA-insensitive phenotype of abi1 in seeds. Alternatively, ethylene could act through a parallel and ABA-independent pathway to regulate seed dormancy negatively. In this case, the opposing influences of ABA and ethylene during seed development would eventually determine the extent of dormancy. However, although application of ACC can partially rescue inhibition of germination of mature wild-type seeds grown on ABA-containing medium, ethylene is not sufficient to stimulate germination in the absence of ABA. This observation suggests that ethylene alone does not positively regulate germination but most likely acts by interfering with the action of ABA.
Interactions between Ethylene and ABA Root Responses
In wild-type plants, application of low concentrations of ABA or ethylene encourages root growth, whereas higher concentrations inhibit root development (Davis and Zhang, 1991; Abeles et al., 1992). The similarity of root response to these two growth regulators argues that ABA and ethylene may affect the same cellular mechanisms. Increased sensitivity to ABA by roots exposed to AVG suggests that ethylene synthesis negatively regulates root responsiveness to ABA. Decreased ABA sensitivity observed in the roots of the ethylene overproduction mutant, eto1, strengthens this conclusion. Because the root length of the eto1 mutant is ∼50% of that of the wild type, one could argue that this shortness complicates the response of the root to exogenous ABA. However, under our experimental conditions, ABA can inhibit root growth of the ctr1 mutant, even though those roots are only 10% as long as the wild type (data not shown).
If increased ethylene synthesis interferes with the inhibitory effects of ABA on root growth, then why do ethylene-insensitive mutants show decreased ABA sensitivity? A reduction in ethylene synthesis or sensitivity would be expected to result in the same ABA response phenotype. Interestingly, studies using ethylene biosynthetic and response inhibitors to determine the interaction of ethylene in indole-3-acetic acid–induced elongation in light-grown Arabidopsis hypocotyls gave similar conflicting results (Smalle et al., 1997). One possible explanation is that ABA or some downstream ABA signaling component may act through the ethylene signaling pathway to regulate root growth (Figure 7A). The observation that the ethylene pathway is required for processes other than ethylene signaling is not unprecedented. For example, the Arabidopsis sugar response has been shown to be altered by various mutations in the ethylene response pathway (Zhou et al., 1998). Because dominant ETR1 mutations that interfere with the perception of ethylene confer an ABA-insensitive phenotype, the ABA signal might act at or upstream of the ETR receptors. If ethylene competes with the ABA signal, then increasing ethylene concentrations or decreasing ethylene responsiveness would result in an ABA-insensitive root phenotype (Figure 7B). Structural and biochemical studies of the ethylene receptor family raise the possibility that the histidine kinase activity associated with ETR1 may play roles other than ethylene signaling or may allow a subset of receptors to participate in additional pathways (Gamble et al., 1998).
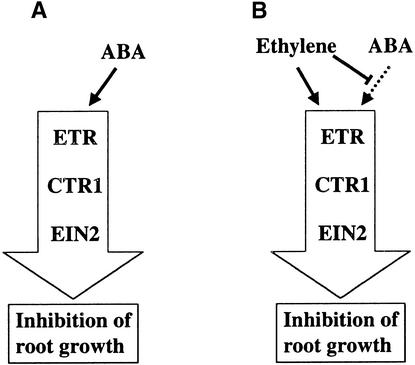
Figure 7.
Hypothetical Model for the Role of ABA and Ethylene in Regulating Root Growth in Arabidopsis.
(A) In the absence of ethylene, one way for ABA to inhibit root growth is by signaling through the ETR1 response pathway. Because this pathway is also regulated by ethylene, mutations in the pathway interfere with both ABA and ethylene signaling.
(B) In the presence of ethylene, ABA is unable to use this pathway; thus, mutations that increase ethylene synthesis confer an ABA-insensitive root phenotype.
Alternatively, ethylene might act in a response pathway parallel to that of the ABA response in roots. For example, several mutants have been reported to have altered responses to ethylene and auxin, but they appear to respond normally to ABA (Pickett et al., 1990; Luschnig et al., 1998). Because RAB18 expression in roots is induced by ABA, the responsiveness of roots of ein2 mutants argues there are ethylene-independent pathways for ABA signaling. Furthermore, ctr1 mutants have short roots, even in the presence of the abi1 mutation, which suggests that ethylene can act independently of ABA in inhibiting root growth. However, because we could not determine whether the ctr1 alleles used in this study are null and because the genetic basis of the dominant abi1-1 mutation is unclear, drawing conclusions about genetic relationships between these genes can be problematic. If both mutations are leaky, then they could be positioned in the same genetic pathway. Moreover, ethylene-insensitive mutants are insensitive to ABA or ethylene during root growth, whereas abi1 mutant roots are only insensitive to ABA (E. Nambara and P. McCourt, unpublished results). This result suggests that the ethylene response is downstream of the ABA signaling pathway, which is compatible with the observation that ein2 suppresses abi1 during germination. Moreover, the finding that the ctr1 mutant is an enhancer of abi1 could mean that the CTR1 kinase and ABI1 phosphatase may both influence EIN2 function in vivo. Although this scenario is not consistent with the ability of ABA to inhibit root growth in the absence of ethylene biosynthesis, the genetic interactions of these mutations suggest that ABA and ethylene signaling may be hierarchical. The demonstration that ethylene response genes can influence ABA biosynthesis and a subset of ABA responses makes it possible to study the interactions of both hormones. Using both ethylene and ABA response mutants should thus reveal a clearer picture of how these two hormone signals intersect.
METHODS
Plant Material and Growth Conditions
Wild-type and enhanced response to ABA3 (era3) plants (Arabidopsis thaliana) used in this study were of ecotype Columbia and carried a glabrous mutation. The era1-2 mutant line contains a complete deletion of the coding region of the ERA1 gene, and the phenotypes of these plants have been described previously (Cutler et al., 1996). The era3-1 and era3-3 mutants were isolated from fast-neutron-mutagenized M2 seed of ecotype Columbia (see Results). Plant media and growth conditions were described previously (McCourt and Keith, 1998).
Measurements of abscisic acid (ABA) sensitivity using germination as a criterion were conducted with seeds from plants 2 to 6 months after harvest. Seeds were surface-sterilized, sown on media containing various concentrations of ABA, chilled for 5 days, and incubated in continuous light. The percentage of germination was determined after 5 days of incubation. To quantify dormancy, we surface-sterilized 1-month-old seeds, sowed them on the control medium, chilled them for various periods, and incubated them in continuous light.
Germination tests of ethylene response mutants were conducted 5 days after chilling. Root growth was determined by germinating and culturing seeds on the control medium. Because of the slight delay in era3 germination because of their increased dormancy, seeds of the wild type and era3 mutants were imbibed for 11 and 13 days, respectively, so that the roots were at the same developmental age. After this, seedlings were transferred to plates containing various concentrations of ABA, 2 μM 1-aminocyclopropane-1-carboxylate (ACC), 10 μM AgNO3, or 2 μM l-α-(2-aminoethoxyvinyl)-glycine (AVG). Plates were placed in a vertical orientation, and root elongation was measured after 4 days of incubation.
Genetic Analysis
The era3-1 and era3-3 mutations were crossed with Landsberg erecta and then mapped by using simple sequence length polymorphism markers (Bell and Ecker, 1994). A complementation test was performed after reciprocal crosses between the era3-1 and era3-3 mutants. Germination of F1 seeds was tested in the presence of 0.5 μM ABA after 5 days of chilling.
Quantification of Endogenous ABA
Procedures for extraction, purification, and quantification of endogenous ABA have been previously described (Nambara et al., 1998). ABA content was measured in the aerial parts of 30-day-old plants grown under standard nonsterilized conditions.
RNA Gel Blot Analysis
Plants were grown under standard conditions for ∼2 weeks. RNA was isolated and analyzed by RNA gel blot hybridization, as previously described (Sambrook et al., 1989; Verwoerd et al., 1989). The ABA1 gene of Arabidopsis was ordered from the Arabidopsis Biological Resource Center (Columbus, OH) (accession number T45502) based on information derived from the sequence data of the ABA2 gene cloned from tobacco (Marin et al., 1996).
Construction of Transgenic RAB18 Lines and β-Glucuronidase Staining
In brief, the RAB18 upstream region was isolated by polymerase chain reaction amplification, and the fragment was digested with HindIII and BamHI. This fragment was ligated to the binary vector pBI101 (Clontech, Palo Alto, CA) digested with the same enzymes. Plasmid DNAs from two positive clones were independently transformed by electroporation (Bio-Rad, Hercules, CA) into Agrobacterium tumefaciens GV3101. Whole-plant transformation of wild-type Arabidopsis ecotype Columbia was as previously described by Clough and Bent (http://www.cropsci.uiuc.edu/~a-bent/protocol.html), and homozygous lines were identified by progeny testing of T2 transformants. Homozygous T3 seeds were backcrossed to wild-type plants. The plants in which the kanamycin resistance segregated as a single dominant trait were used in our experiments. The RAB18–β-glucuronidase (GUS) transgene was transferred into an era3 genetic background by genetic crosses, and the appropriate phenotypes were screened. A line homozygous for both markers was used for all experiments. GUS staining was done as described elsewhere (Jefferson et al., 1987).
Acknowledgments
We thank members of the McCourt laboratory for helpful discussion during this work and Dr. Jerome Giraudat for sharing unpublished results. We also thank Eric Schaller for supplying various ethylene response mutants for this study. This work was supported by a grant from the Natural Sciences and Engineering Research Council (NSERC) to P.M. and a Frontier grant (RIKEN) to Y.K. M.G. was partially supported by an NSERC predoctoral fellowship.
References
- Abeles, F.B., Morgan, P.W., and Saltveit, M.E., Jr. (1992). Ethylene in Plant Biology. (San Diego, CA: Academic Press).
- Alonso, J.M., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284, 2148–2152. [DOI] [PubMed] [Google Scholar]
- Beaudoin, N., Serizet, C., Gosti, F., and Giraudet, J. (2000). Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12, 1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, C.J., and Ecker, J.R. (1994). Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19, 137–144. [DOI] [PubMed] [Google Scholar]
- Bleecker, A.B., Estelle, M.A., Somerville, C., and Kende, H. (1988). Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241, 1086–1189. [DOI] [PubMed] [Google Scholar]
- Bleecker, A.B., Esch, J.J., Hall, A.E., Rodriguez, F.I., and Binder, B.M. (1998). The ethylene-receptor family from Arabidopsis: Structure and function. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 353, 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetta, D., and McCourt, P. (1998). Genetic analysis of ABA signal transduction pathways. Trends Plant Sci. 3, 231–235. [Google Scholar]
- Cary, A.J., Liu, W., and Howell, S.H. (1995). Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 107, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S., Ghassemian, M., Bonetta, D., Cooney, S., and McCourt, P. (1996). A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273, 1239–1241. [DOI] [PubMed] [Google Scholar]
- Davis, W.J., and Zhang, J. (1991). Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 55–76. [Google Scholar]
- Ephritikhine, G., Fellner, M., Vannini, C., Lapous, D., and Barbier-Brygoo, H. (1999). The sax1 dwarf mutant of Arabidopsis thaliana shows altered sensitivity of growth responses to abscisic acid, auxin, gibberellins and ethylene and is partially rescued by exogenous brassinosteroid. Plant J. 18, 303–314. [DOI] [PubMed] [Google Scholar]
- Gamble, R.L., Coonfield, M.L., and Schaller, G.E. (1998). Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc. Natl. Acad. Sci. USA 95, 7825–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, J., and Meyerowitz, E.M. (1998). Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94, 261–271. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). Nucleotide GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, P.R., and Ecker, J.R. (1998). The ethylene gas signal transduction pathway: A molecular perspective. Annu. Rev. Genet. 32, 227–254. [DOI] [PubMed] [Google Scholar]
- Karssen, C.M., Brinkhorst-van der Swan, D.L.C., Breekland, A.E., and Koornneef, M. (1983). Induction of dormancy during seed development by endogenous abscisic acid: Studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta 157, 158–165. [DOI] [PubMed] [Google Scholar]
- Kende, H., van der Knaap, E., and Cho, H.T. (1998). Deepwater rice: A model plant to study stem elongation. Plant Physiol. 118, 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketring, D.L., and Morgan, P.W. (1972). Physiology of oil seeds. IV. Role of endogenous ethylene and inhibitory regulators during natural and induced afterripening of dormant Virginia-type peanut seeds. Plant Physiol. 50, 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber, J.J., Rothenberg, M., Roman, G., Feldmann, K.A., and Ecker, J.R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72, 427–441. [DOI] [PubMed] [Google Scholar]
- Leung, J., and Giraudat, J. (1998). Abscisic acid signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 199–222. [DOI] [PubMed] [Google Scholar]
- Luschnig, C., Gaxiola, R.A., Grisafi, P., and Fink, G.R. (1998). EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis. Genes Dev. 12, 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin, E., Nussaume, L., Quesada, A., Gonneau, M., Sotta, B., Hugueney, P., Frey, A., and Marion-Poll, A. (1996). Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA1 locus of Arabidopsis thaliana. EMBO J. 15, 2331–2342. [PMC free article] [PubMed] [Google Scholar]
- McCourt, P., and Keith, K. (1998). Sterile techniques in Arabidopsis. Methods Mol. Biol. 82, 13–17. [DOI] [PubMed] [Google Scholar]
- Nambara, E., Kawaide, H., Kamiya, Y., and Naito, S. (1998). Characterization of an Arabidopsis thaliana mutant that has a defect in ABA accumulation: ABA-dependent and ABA-independent accumulation of free amino acids during dehydration. Plant Cell Physiol. 39, 853–858. [DOI] [PubMed] [Google Scholar]
- Penninckx, I.A., Thomma, B.P., Buchala, A., Metraux, J.P., and Broekaert, W.F. (1998). Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10, 2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett, F.B., Wilson, A.K., and Estelle, M. (1990). The aux1 mutant of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 94, 1462–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, 2nd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Smalle, J., Haegman, M., Kurepa, J., Van Montagu, M., and Straeten, D.V. (1997). Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc. Natl. Acad. Sci. USA 94, 2756–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas, A.J. (1992). Growth substances in context: A decade of sensitivity. Biochem. Soc. Trans. 20, 102–108. [DOI] [PubMed] [Google Scholar]
- Verwoerd, T.C., Dekker, B.M., and Hoekema, A. (1989). A small scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 17, 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, A.K., Pickett, F.B., Turner, J.C., and Estelle, M. (1990). A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol. Gen. Genet. 222, 377–383. [DOI] [PubMed] [Google Scholar]
- Zeevaart, J.A.D., and Creelman, R.A. (1988). Metabolism and physiology of abscisic acid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 439–473. [Google Scholar]
- Zhou, L., Jang, J.C., Jones, T.L., and Sheen, J. (1998). Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc. Natl. Acad. Sci. USA 95, 10294–10299. [DOI] [PMC free article] [PubMed] [Google Scholar]