Abstract
Current evidence is inconclusive regarding the point of signaling convergence downstream from different members of the phytochrome family. In transgenic Arabidopsis, the activity of a reporter enzyme under the control of the −453 to +67 fragment of an Lhcb1*2 promoter shows very low fluence responses (VLFRs) and high-irradiance responses (HIRs) mediated by phytochrome A and low-fluence responses (LFRs) mediated by phytochrome B. A 5′ deletion of the promoter to −134 abolished the HIR without affecting VLFR or LFR. In transgenic tobacco, VLFR and LFR were observed for the −176 to −31 or −134 to −31 fragments of Lhcb1*2 fused to 35S cauliflower mosaic virus minimal promoters, but only the largest fragment showed HIR. We propose that sustained activation of phytochrome A with far-red light initiates a signaling cascade that deviates from phytochrome B signaling and transient phytochrome A signaling and that this divergence extends as far as the Lhcb1*2 promoter.
INTRODUCTION
Living organisms rely on receptor molecules to perceive environmental and internal signals. Very often, receptors have evolved into families of various sizes that are specialized in the perception of variants of certain stimuli. Receptor families in plants do not appear to be as large as those in other systems (Matsunami and Buck, 1997). Nonetheless, in the model plant system Arabidopsis, there are five members in the ethylene receptor family (Solano and Ecker, 1998), five members in the family of phytochrome photoreceptors (Quail et al., 1995), and two members in the cryptochrome family of blue light photoreceptors (Cashmore et al., 1999).
It is unknown whether divergent transduction pathways have coevolved with the divergent members of receptor families in plants. Ethylene signaling appears to converge immediately after the receptors in the protein kinase CTR (Solano and Ecker, 1998). Little is known about the mechanisms of signaling downstream from cryptochromes, but phytochromes provide an excellent system for investigating whether different receptors have their own signaling cascade.
The most abundant and physiologically important members of the phytochrome family are phytochrome A (phyA) and phyB (Quail et al., 1995; Fankhauser and Chory, 1997). Both are cytoplasmic in the dark and partially migrate to the nucleus upon light absorption (Kircher et al., 1999; Yamaguchi et al., 1999). Maximum activation of phyA and phyB occurs under far-red (FR) light and red (R) light, respectively (McCormac et al., 1993; Whitelam et al., 1993). This is reflected by the complementary roles of phyA and phyB in the perception of the natural light environment (Yanovsky et al., 1995). Both phyA and phyB control the expression of several genes during the transition from heterotrophy to autotrophy, which is initiated when etiolated (dark-grown) seedlings are exposed to light (Kuno et al., 2000).
Current evidence is inconclusive regarding the point of convergence of phyA and phyB signaling (see Quail [1998] for further discussion). The genetic approach has yielded mutants that selectively affect phyA-mediated (Whitelam et al., 1993; Hoecker et al., 1998, 1999; Hudson et al., 1999) or phyB-mediated (Ahmad and Cashmore, 1996; Wagner et al., 1997) responses as well as mutants that affect both (Ahmad and Cashmore, 1996; Genoud et al., 1998). Although some of the relevant genes have been sequenced, whether their products fulfill an intrinsic signaling role or a structural/regulatory role is not known (e.g., the genes could be involved in specific translocation of phyA or phyB to the nucleus). Thus, genetic evidence can be interpreted in favor of either immediate or delayed convergence of phyA and phyB signaling. Some proteins identified by means of the two-hybrid system can bind both phyA and phyB (Ni et al., 1998), giving credence to the immediate convergence theory. The kinetics of signal input versus output are more easily accounted for by hypothesizing different signaling cascades for phyA and phyB (Casal et al., 1998a). phyA, but not phyB, enhances the expression of the chalcone synthase gene (Batschauer et al., 1996). Conversely, phyB, but not phyA, controls the amplitude of the circadian rhythm of Lhcb1*1 in Arabidopsis (Anderson et al., 1997). However, different signaling intensities of phyA and phyB acting through a common pathway could also account for these selective effects. Anderson et al. (1997) have proposed convergence of phyA and phyB signaling initiated by a single pulse of R light at or before interaction of the signals with the factor CGF-1/GT-1 that binds GATA motifs in the −74 to −42 fragment of the Lhcb1*1 promoter in Arabidopsis. On the basis of the genetic data (Yanovsky et al., 1997, 2000) and the synergistic or antagonistic nature of the interactions between phyA and phyB under different light conditions (Cerdán et al., 1999), we hypothesize that phyA itself initiates two different signaling cascades, each of which differs from that initiated by phyB, and that this could account for the different response modes of phytochromes (Casal et al., 1998b).
The activity of the Lhcb1*2 promoter of tobacco responds to light signals perceived by phyA and phyB in transgenic tobacco or Arabidopsis (Cerdán et al., 1997, 1999). If phyA and phyB signaling cascades converge before the signals reach the target gene, then it should be impossible to discriminate between them by promoter deletion.
RESULTS
Modes of Action of PhyA and PhyB
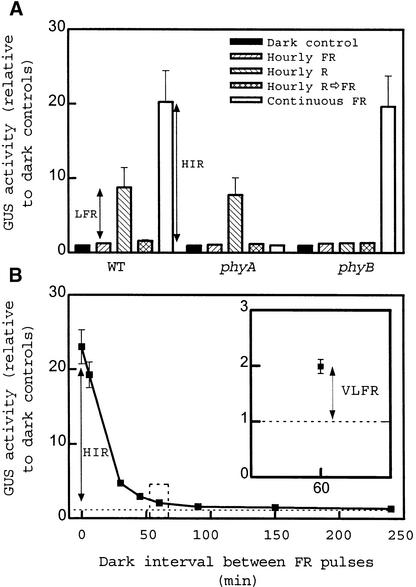
Transgenic seedlings of Arabidopsis were grown in the dark for 2 days and transferred to different light conditions for 24 hr before harvest. The activity of the reporter enzyme β-glucuronidase (GUS) driven by the −752 to +67 region of the tobacco Lhcb1*2 promoter was increased by hourly pulses (lasting 3 min) of R light (Figure 1A); however, this promotion was cancelled by subsequent FR light pulses back to the amount of activity induced by hourly FR light pulses given alone. The R/FR light–reversible, low-fluence response (LFR) was mediated by phyB. In Arabidopsis ecotype Landsberg erecta, the phyA mutant shows enhanced LFR by eliminating the negative regulation of phyB signaling by phyA, but this is not the case in the Columbia ecotype (Cerdán et al., 1999). The promotion of GUS activity above that of dark controls, caused by hourly FR light pulses perceived by phyA, that is, the so-called very low fluence response (VLFR), was small but statistically significant (P < 0.005) (the Columbia ecotype shows reduced VLFR; Yanovsky et al., 1997; Cerdán et al., 1999). At equal total fluence (i.e., 5% of the fluence rate), continuous FR light caused a much stronger effect than did hourly FR light, and this difference is the high-irradiance response (HIR) mediated by phyA.
Figure 1.
Three Modes of Phytochrome-Mediated Responses Control the Expression of Lhcb1*2: VLFR, LFR, and HIR.
(A) The LFR and the HIR. GUS activity driven by the −752 to +67 Lhcb1*2 promoter of tobacco fused to gusA in transgenic seedlings of Arabidopsis exposed to hourly pulses of FR light, hourly pulses of R light, hourly pulses of R light immediately followed by FR light, or continuous FR light. Seedlings of the wild type (WT) and of the phyA-211 and phyB-9 mutants are included. Data are means ±se of four or five replicate samples. Double-headed arrows show the magnitude of LFR and HIR.
(B) The VLFR and the HIR. GUS activity driven by the −752 to +67 Lhcb1*2 promoter of tobacco fused to gusA in transgenic seedlings of Arabidopsis exposed to repeated pulses of FR light separated by intervals of darkness of the durations indicated. Data are means ±se of four replicate samples. Inset, detail of the effect of hourly pulses of FR light. Double-headed arrows show the magnitude of VLFR and HIR.
Etiolated seedlings were also exposed to 3-min pulses of FR light separated by intervals of darkness of variable duration (Figure 1B). Even one pulse every 4 hr increased GUS activity above that of the dark controls (P < 0.001). In absolute terms, activity hardly increased when the frequency was increased to one pulse per hour; a dramatic rise occurred, however, when the darkness interval lasted <30 min. The HIR requires continuous FR light or very frequent FR light pulses (Mancinelli, 1994; Shinomura et al., 2000). Thus, VLFR and HIR are two quasidiscrete phases of response to phyA that are initiated even under transient excitation or only under sustained excitation, respectively, of phyA.
Deletion of the Lhcb1*2 Promoter in Transgenic Arabidopsis
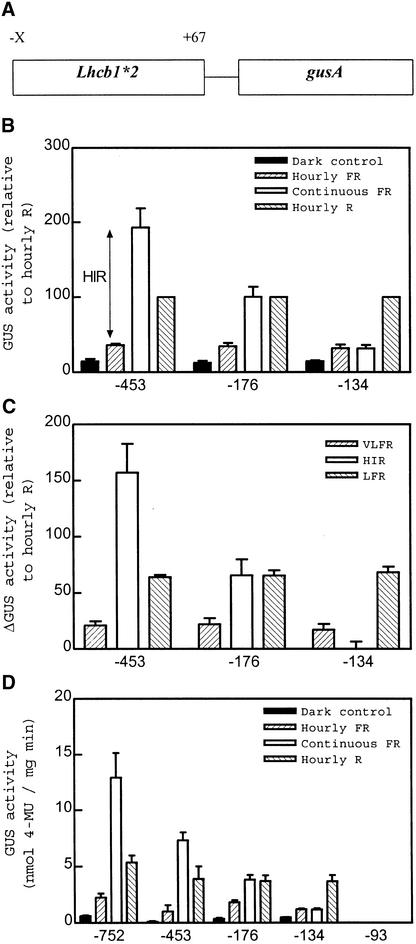
Progressive 5′ deletion was used to investigate whether phytochrome signaling under VLFR, LFR, and HIR conditions would all target the same region of the Lhcb1*2 promoter in transgenic Arabidopsis (Figure 2). Data are presented as the average of at least six independent transgenic lines (each line is a replicate) to avoid artifacts of copy number and position effects. When these lines were analyzed individually, the absolute amounts of GUS expression varied, but not the pattern of response to the different light treatments. For this reason, we related the data to the GUS activities under R light pulses. Absolute values of single representative lines are also shown to demonstrate that the pattern is not a result of the averaging procedure.
Figure 2.
Uncoupled Decrease in HIR Compared with VLFR and LFR after Deletion of a Lhcb1*2 Promoter in Transgenic Arabidopsis Plants.
(A) Schematic representation of the constructs used in the present set of experiments. X represents the variable 5′ ends, as indicated in (B) to (D).
(B) Promoter deletion from −453 to −134 eliminates the HIR. Etiolated seedlings were exposed to hourly pulses of FR light, hourly pulses of R light, or continuous FR light. Data are means ±se of six to 10 independently transformed Arabidopsis lines. For each line, activity was normalized to the values observed after treatment with R light pulses.
(C) Comparative effects on VLFR, LFR, and HIR. VLFR, LFR, and HIR were calculated from the data in (B) (±se of the difference). The VLFR was calculated as the difference between the activities under hourly FR light pulses and those of the dark controls, the HIR was calculated as the difference between the responses to continuous FR light and those to hourly pulses of FR light, and the LFR was calculated as the difference between the responses to hourly pulses of R light and those to hourly pulses of FR light.
(D) Data corresponding to single representative transgenic lines (average ±se of three replicate samples). 4-MU, 4-methylumbelliferone.
GUS activities in darkness as well as under hourly pulses of R or FR light were virtually unaffected by deletions that leave a −134 to +67 promoter fragment (Figures 2B and 2D). However, GUS activity showed a gradual decrease in seedlings exposed to continuous FR light. Continuous FR light was more effective than hourly R light pulses in the −453 to +67 fragment but less effective than pulsed R light in the −134 to +67 fragment. Thus, VLFR and LFR were stable for the −453, −176, and −134 promoters, whereas HIR decreased between −453 and −176 and was completely absent in the −134 fragment (Figure 2C). The −93 to +67 fragment showed no detectable VLFR, LFR, or HIR (Figure 2D).
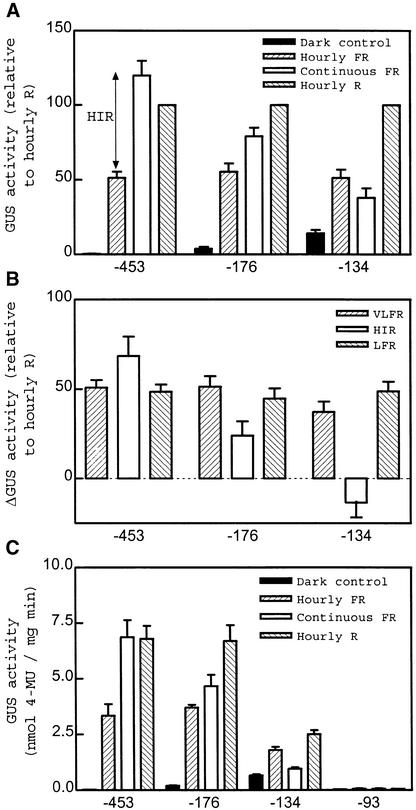
Deletion of the Lhcb1*2 Promoter in Tobacco
Because we were using the Lhcb1*2 promoter from tobacco as a molecular marker, we decided to use transgenic tobacco seedlings grown for 4 days in darkness and subsequently, before harvest, transferred for 48 hr to the different light or dark conditions tested. In the −453 to +67 fragment, VLFR, LFR, and HIR had a similar magnitude (Figure 3). The HIR decreased when the promoter was deleted between −453 and −176 and was abolished by deletion to −134. In contrast, VLFR and LFR were not affected by deletion between –453 and –176 and showed a relatively small decrease with further deletion to −134 (Figure 3). Even when single −176 and −134 lines were chosen, based on similar absolute LFR, only the larger promoter fragment showed HIR (data not shown). No significant VLFR, LFR, or HIR was observed for the −93 to +67 promoter (Figure 3C).
Figure 3.
Uncoupled Decrease in HIR Compared with VLFR and LFR after Deletion of a Lhcb1*2 Promoter in Transgenic Tobacco Seedlings.
(A) Promoter deletion from −453 to −134 eliminates the HIR. Etiolated seedlings were exposed to hourly pulses of FR light, hourly pulses of R light, or continuous FR light. Data are means ±se of six to eight independently transformed tobacco lines. For each line, activity was normalized to the values observed under R light pulses.
(B) Comparative effects on VLFR, LFR, and HIR, calculated as described in Figure 2C.
(C) Data corresponding to single representative transgenic lines (average of three replicate samples ±se). 4-MU, 4-methylumbelliferone.
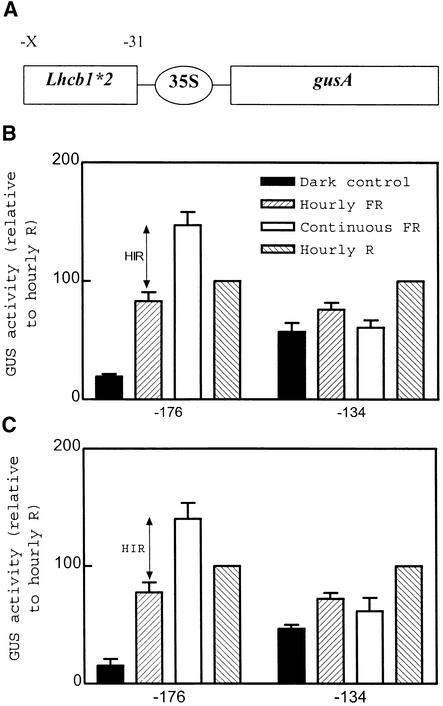
The −176 to −134 Fragment Is Also Necessary for HIR in the Context of Truncated Cauliflower Mosaic Virus 35S Promoters
The selective disappearance of the HIR as a result of deletion of the 42-bp region between −176 and −134 was also investigated in the context of truncated cauliflower mosaic virus (CaMV) 35S minimal promoters that per se are unable to respond to phytochromes (Cerdán et al., 1997). For these “gain of function” experiments, the −176 to −31 and the −134 to −31 fragments were fused to Δ90 and Δ46 minimal promoters. By using these constructions, we could also warrant that the effects were transcriptional, not mediated (at least not fully mediated) by the 5′ noncoding region of Lhcb1*2. The −176 to −31 fragment of the Lhcb1*2 promoter conferred VLFR, LFR, and HIR to both minimal promoters (Figure 4). A 42-bp deletion (which left a −134 to −31 promoter fragment) completely abolished the HIR without eliminating the VLFR and LFR (Figure 4). The 42-bp deletion also caused a marked decrease in the overall ability of the promoter to respond to light. Thus, GUS activity in darkness increased when expressed relative to the activity in seedlings exposed to R light pulses. However, in absolute terms, the GUS activity of dark-grown seedlings did not increase substantially in the presence of promoter deletions (data not shown).
Figure 4.
Dissection of the HIR in Gain-of-Function Experiments.
(A) Schematic representation of the constructs used in the present set of experiments. X represents the variable 5′ ends, as indicated in (B) and (C); the ellipse corresponds to the 35S minimal promoters, which are −90 in (B) and −46 in (C).
(B) and (C) The −176 to −31 but not the −134 to −31 fragment of the Lhcb1*2 promoter confers HIR to a minimum 35S CaMV promoter. Seedlings transformed with the constructs described in (A) were exposed to pulses of FR light, pulses of R light, or continuous FR light. Data are means ±se of five to seven independently transformed lines. For each line, activity was normalized to the values observed after treatment with R light pulses.
Elements Downstream of −134 Are Necessary but Not Sufficient for Full HIR
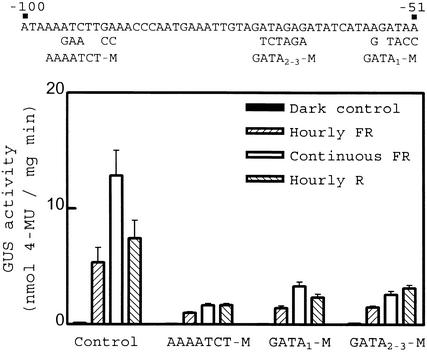
The tobacco Lhcb1*2 promoter contains an AAAATCT sequence between −98 and −92 that is similar to the binding site of the Myb-related factor CCA1 (Wang et al., 1997), which is involved in the circadian regulation of transcription (Wang and Tobin, 1998). The promoter also bears a tandem of three GATA boxes between −55 and −52 (GATA1), −65 and −62 (GATA2), and −71 and −68 (GATA3). The factor CGF-1/GT-1 binds to GATA motifs and is important for acute responses to a pulse of R light mediated by phyA and phyB (Anderson et al., 1997). We built up substitutions of AAAATCT (AAAATCT-M), GATA3 (GATA3-M), and GATA2 and GATA3 (GATA2-3-M), in the context of the −453 to +67 Lhcb1*2 fragment. Each of these constructions showed decreases in VLFR, LFR, and HIR (Figure 5). This observation indicates that the region between −134 and −31 contains elements that are necessary but not sufficient for HIR. Moreover, it demonstrates that not all the reductions in the ability to respond to light result in the selective decrease in HIR.
Figure 5.
Substitutions Affecting AAAATCT or GATA Motifs Reduce VLFR, LFR, and HIR.
Tobacco seedlings transformed with the −453 to +67 fragment of the homologous Lhcb1*2 promoter bearing the substitutions indicated at top were exposed to hourly pulses of FR light, hourly pulses of R light, or continuous FR light. Data are means ±se of five to eight independently transformed lines. 4-MU, 4-methylumbelliferone.
DISCUSSION
Three photobiological modes have been described for phytochrome-mediated responses (reviewed in Casal et al., 1998b), including the expression of Lhcb1*2 in transgenic tobacco and Arabidopsis seedlings (Cerdán et al., 1997, 1999): the VLFR of phyA, the LFR of phyB (and eventually phyC, phyD and/or phyE), and the HIR of phyA. In the −453 to +67 fragment of the Lhcb1*2 promoter and in the −176 to −31 fragment of the Lhcb1*2 promoter fused to a truncated (−46 to +8 or −90 to +8) CaMV 35S minimal promoter, HIR was greater than or equal to LFR and VLFR. If increasing deletion were merely a matter of reducing the number of binding sites available for factors common to the three response modes, then HIR could not fall below the levels of LFR and VLFR. However, in the −134 to +67 fragment of the Lhcb1*2 promoter and in the −134 to −31 fragment of the Lhcb1*2 promoter fused to a truncated CaMV 35S minimal promoter, HIR was actually absent (i.e., less than the amounts of LFR and VLFR) (Figures 2 to 4). Promoter deletion discriminated against HIR compared with LFR and VLFR. Thus, phyA signaling that yields HIR must divert from both phyB signaling and phyA signaling to yield VLFR (Figure 6). Divergence of phyA and phyB signaling pathways goes as far as a gene promoter.
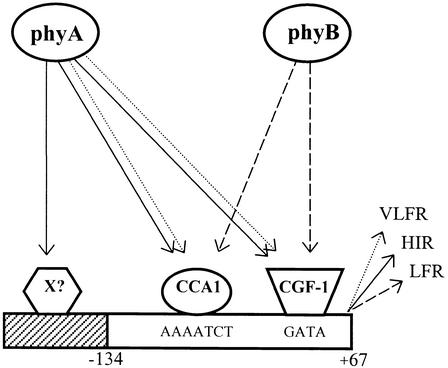
Figure 6.
Simplified Model Describing Putative Phytochrome Signal Transduction Pathways Reaching the Lhcb1*2 Promoter.
Sustained activation of phyA initiates a signaling cascade (solid lines) that partially deviates from the transduction chains initiated either by transient excitation of phyA (dotted line) or by phyB (dashed line). X represents an unidentified factor binding the promoter region specifically necessary for HIR.
The nature of the HIR is a long-standing question in photomorphogenesis. Most models have considered that the FR light–absorbing form of phytochromes is active under light pulses, whereas some additional active form of phytochrome operates under HIR conditions (Mancinelli, 1994; Shinomura et al., 2000). Present results show that qualitative differences between continuous FR light and infrequent light pulses go beyond the phyA molecule itself and can reach the target genes. This is consistent with previous observations showing that VLF1 and VLF2 loci (polymorphic between ecotypes Landsberg erecta and Columbia) affect the VLFR of phyA but not the HIR (Yanovsky et al., 1997). In contrast, fhy3-1, a mutant of Arabidopsis involved in phyA signaling, retains almost normal VLFR but is severely affected in HIR (Yanovsky et al., 2000).
Examples in other systems show that kinases are able to initiate divergent signaling depending on the duration of their activation. In the pheochromocytoma cell line PC12, treatment with nerve growth factor leads to activation of mitogen-activated protein (MAP) kinase for several hours and cell differentiation, whereas treatment with epidermal growth factor causes only transient activation of MAP kinases and cell proliferation but no differentiation (Marshall, 1995). In yeast, Ste11p (the kinase of the kinase that phosphorylates MAP kinase) is only transiently activated by high solute concentration and initiates acclimation to high osmolarity but not mating. Chronic stimulation in mutants lacking a negative feedback leads to the activation of the mating pathway (O'Rourke and Herskowitz, 1998; Sprague, 1998). phyA has kinase activity (Yeh and Lagarias, 1998) and is able to phosphorylate endogenous substrates (Fankhauser et al., 1999). Thus, phyA could be a kinase able to initiate divergent signaling, depending on the duration and intensity of the light treatment, the VLFR pathway initiated under intermittent irradiation, and the HIR pathway initiated under prolonged irradiation at relatively high fluence rates.
A detailed characterization of the promoter region specifically required for HIR is beyond the scope of this article. However, the −176 to −134 region contains a TGGA motif, for which the distance to the CAAT box (49 to 52 bp) is conserved in the different Lhcb genes that show HIR (data not shown). In addition, full HIR requires elements downstream of −134, as indicated by substitutions affecting the GATA or AAAATCT motifs predicted to bind CGF-1/GT-1 (Anderson et al., 1997) and CCA1 (Wang et al., 1997), respectively (Figure 5). Thus, HIR could involve transcriptional synergy such that factors that bind to different DNA regions cooperate by increasing complex stability (Chi et al., 1995; Carey, 1998).
Sustained activation of phyA signaling thus targets a region of the Lhcb1*2 promoter that is not required for the responses mediated by either phyB signaling or transient phyA signaling. Other regions of the promoter, such as AAAATCT and GATA boxes, are required by all three modes of response of the phytochromes. These observations pose the question as to whether the nuclear factors PIF3 (Ni et al., 1998, 1999) and COP1 (Von Arnim and Deng, 1996; Osterlund and Deng, 1998; Osterlund et al., 1999), which affect both phyA- and phyB-mediated responses, actually target regions common to VLFR, LFR, and HIR or, alternatively, are selectively involved in some of these modes of action of phytochromes.
Phytochrome evolution has resulted in different isovariants such as phyA and phyB, for which divergent abilities of perception can readily be observed under laboratory (McCormac et al., 1993; Whitelam et al., 1993) and natural radiation conditions (Yanovsky et al., 1995). Current results show that phyA and phyB can follow different pathways to reach a target gene. The multiplicity of photoreceptors increases the variety of signals that can be perceived. The multiplicity of transduction pathways enhances the ability to modulate the system of perception. Different pathways can be regulated by specific modulators and can generate interactions. Interactions between phyA and phyB are antagonistic or synergistic, depending on whether phyA signaling is by the VLFR or the HIR mode, respectively (Cerdán et al., 1999). The general consensus gained from other systems is that complex, highly networked signaling systems are more versatile than simple systems in terms of signal input–signal output possibilities and are more robustly buffered against environmental changes that have no apparent informational value (Meagher et al., 1999).
METHODS
Construction of Fusions Containing Different Fragments of the Lhcb1*2 Promoter
The 5′ deletions from −752 and −453 to +67 of the tobacco Lhcb1*2 promoter fused to the coding region of gusA in the binary plasmid pBI101.2 have been described previously (Cerdán et al., 1997). The −176, −134, and −93 deletions were obtained by polymerase chain reaction (PCR) with specific primers bearing a HindIII tail at their 5′ end: 5′-CCCAAGCTTGGTTGGACCACAGTAG-3′ for the −176 deletion, 5′-CCCAAGCTTACAAAGTGCCA-3′ for the −134 deletion, and 5′-CCGAAGCTTGAAACCC-3′ for the −93 deletion. The template was a pUC19-derived plasmid bearing the −453 (HindIII) to +67 (BamHI) fragment of the Lhcb1*2 promoter. Deletion fragments with 3′ endpoints at +67 were obtained by PCR with the 5′-specific primers described above in combination with a vector-specific primer. The amplified fragments were cut with HindIII and BamHI and subcloned in the pBI101.2 vector (Jefferson et al., 1987). Deletion fragments with 3′ endpoints at −31 were obtained by using the same 5v-specific primers in combination with a 3v-specific primer (5′-CCC-AAGCTTGGGATTGATTGAAAGAG-3′) that also contained the 5v tail with a HindIII site. Amplified fragments were cut with HindIII and subcloned in pBIΔ46 and pBIΔ90 plasmids containing, respectively, the −46 and −90 35S minimal promoters fused to the coding region of gusA. The pBIΔ46 and pBIΔ90 plasmids were kindly provided by Drs. Luis Herrera-Estrella and Gerardo Argüello-Astorga (CINVESTAV, Irapuato, Mexico).
GATA or AAATCT boxes were mutated in the −453 to +67 fragment of Lhcb1*2. For each site, two fragments were amplified by PCR with a promoter-specific primer (bearing 5′ base substitutions and a restriction site), a vector-specific primer, and the template described above for the 5v deletions. The fragments were cut with restriction enzymes, ligated in a tripartite reaction into a pUC19-derived plasmid, and transferred to pBI101.2 (between the HindIII and BamHI sites). All the constructs were confirmed by dideoxynucleotide sequencing.
Plant Material
The wild-type and phyA-211 transgenic lines of Arabidopsis thaliana ecotype Columbia, transformed with the −752 to +67 Lhcb1*2 promoter fragment fused to the gusA coding region, have been described previously (Cerdán et al., 1999). The phyB-9 line carrying the same transgene was obtained by crossing the phyB-9 mutant with the wild-type transgenic line, selecting for phyB-9 phenotype and kanamycin resistance in the F2 generation, and testing lack of segregation in the next generation (Casal et al., 1998a; Cerdán et al., 1999).
Deletion derivatives of the Lhcb1*2 promoter were introduced into A. thaliana ecotype Columbia by way of Agrobacterium tumefaciens, using the floral dip method (Clough and Bent, 1998). Several independent transgenic lines were obtained for each construct. The T2 generation was tested for kanamycin resistance, and those lines showing segregation for at least one insert were selected for subsequent experiments. The nature and the integrity of the transgene were tested by PCR with vector-specific primers.
Several independent lines of transgenic Nicotiana tabacum, cultivar SR1, were obtained for each construct by using the leaf disc method (Horsch et al., 1985). These lines were tested for kanamycin resistance. The nature and integrity of the transgene were confirmed by using gusA-specific probes in DNA gel blots and vector-specific primers in PCR.
Approximately 100 seeds for Arabidopsis and 50 seeds for tobacco were sown in clear plastic boxes (40 × 33 × 15 mm height) containing 3 mL of 0.8% agar. For the experiments with Arabidopsis, the boxes were incubated in darkness at 7°C for 3 days, given a red (R) light pulse to promote seed germination, and incubated in the dark (25°C) for 2 days before light treatments. For the experiments with tobacco, the boxes were exposed to white light for 24 hr immediately after sowing and subsequently transferred to darkness (25°C) for 4 days before beginning the light treatments.
Light Treatments
Arabidopsis and tobacco seedlings were exposed to 24- and 48-hr light treatments (25°C), respectively, before harvest. Continuous far-red (FR) light (6 μmol m−2 sec−1) and hourly pulses of FR light (3 min at 120 μmol m−2 sec−1) were provided by incandescent lamps in combination with a water filter, a red acetate filter, and six 2-mm-thick blue acrylic filters (Paolini 2031; La Casa del Acetato, Buenos Aires, Argentina). Hourly pulses of R light (maximum emission 662 nm, 30 μmol m−2 sec−1) were provided by light-emitting diodes. In the experiment in which pulses of FR light were given at variable time intervals, the duration (3 min) and fluence rate (20 μmol m−2 sec−1) of the pulses were kept constant. The calculated proportion of phytochrome in the FR light–absorbing form (Casal et al., 1991) was 10% for the FR sources and 87% for the R light source.
Measurements of β-Glucuronidase Activity
For the measurements of β-glucuronidase (GUS) activity, the plants were harvested under dim green light, homogenized in 50 μL of ice-cooled extraction buffer, and microcentrifuged at 4°C. The supernatant was stored at −80°C (usually for <1 week). GUS activity was measured according to Jefferson et al. (1987), using 4-methylumbelliferyl-β-d-glucuronide (Sigma) as substrate. The standard curves were prepared with 4-methylumbelliferone (Sigma). Protein content was measured according to Lowry et al. (1951).
Acknowledgments
This work was financially supported by FONCYT (Grant No. PICT 08-00115-02089 to J.J.C.), CONICET (Grant Nos. PICT0295 to R.J.S. and PIP 0888/98 to J.J.C.), Universidad de Buenos Aires (Grant No. TG59 to J.J.C.), and Fundación Antorchas (Grant No. A-13622/1-40 to J.J.C.).
References
- Ahmad, M., and Cashmore, A.R. (1996). The pef mutants of Arabidopsis thaliana define lesions early in the phytochrome signaling pathway. Plant J. 10, 1103–1110. [DOI] [PubMed] [Google Scholar]
- Anderson, S.L., Somers, D.E., Millar, A.J., Hanson, K., Chory, J., and Kay, S.A. (1997). Attenuation of phytochrome A and B signaling pathways by the Arabidopsis circadian clock. Plant Cell 9, 1727–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschauer, A., Rocholl, M., Kaiser, T., Nagatani, A., Furuya, M., and Schäfer, E. (1996). Blue and UV-A light–regulated CHS expression in Arabidopsis independent of phytochrome A and phytochrome B. Plant J. 9, 63–69. [Google Scholar]
- Carey, M. (1998). The enhanceosome and transcriptional synergy. Cell 92, 5–8. [DOI] [PubMed] [Google Scholar]
- Casal, J.J., Sánchez, R.A., Di Benedetto, A.H., and De Miguel, L.C. (1991). Light promotion of seed germination in Datura ferox is mediated by a highly stable pool of phytochrome. Photochem. Photobiol. 53, 249–254. [Google Scholar]
- Casal, J.J., Cerdán, P.D., Staneloni, R.J., and Cattaneo, L. (1998. a). Different phototransduction kinetics of phytochrome A and phytochrome B in Arabidopsis thaliana. Plant Physiol. 116, 1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal, J.J., Sánchez, R.A., and Botto, J.F. (1998. b). Modes of action of phytochromes. J. Exp. Bot. 49, 127–138. [Google Scholar]
- Cashmore, A.R., Jarillo, J.A., Wu, Y.J., and Liu, D. (1999). Cryptochromes: Blue light receptors for plants and animals. Science 284, 760–765. [DOI] [PubMed] [Google Scholar]
- Cerdán, P.D., Staneloni, R.J., Casal, J.J., and Sánchez, R.A. (1997). A 146 bp fragment of the tobacco Lhcb1*2 promoter confers very-low-fluence, low-fluence and high-irradiance responses of phytochrome to a minimal CaMV 35S promoter. Plant Mol. Biol. 33, 245–255. [DOI] [PubMed] [Google Scholar]
- Cerdán, P.D., Yanovsky, M.J., Reymundo, F.C., Nagatani, A., Staneloni, R.J., Whitelam, G.C., and Casal, J.J. (1999). Regulation of phytochrome B signaling by phytochrome A and FHY1 in Arabidopsis thaliana. Plant J. 18, 499–507. [DOI] [PubMed] [Google Scholar]
- Chi, T., Lieberman, P., Ellwood, K., and Carey, M. (1995). A general mechanism for transcriptional synergy by eukaryotic activators. Nature 377, 254–257. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Fankhauser, C., and Chory, J. (1997). Light control of plant development. Annu. Rev. Cell Dev. Biol. 13, 203–229. [DOI] [PubMed] [Google Scholar]
- Fankhauser, C., Yeh, K.C., Lagarias, J.C., Zhang, H., Elich, T.D., and Chory, J. (1999). PKS1, a substrate phosphorylated by phytochrome, that modulates light signaling in Arabidopsis. Science 284, 1539–1541. [DOI] [PubMed] [Google Scholar]
- Genoud, T., Millar, A.J., Nishizawa, N., Kay, S.A., Schäfer, E., Nagatani, A., and Chua, N.H. (1998). An Arabidopsis mutant hypersensitive to red and far-red light signals. Plant Cell 10, 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker, U., Xu, Y., and Quail, P.H. (1998). SPA1: A new genetic locus involved in phytochrome A–specific signal transduction. Plant Cell 10, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker, U., Tepperman, J.M., and Quail, P.H. (1999). SPA1, a WD-repeat protein specific to phytochrome A signal transduction. Science 284, 496–499. [DOI] [PubMed] [Google Scholar]
- Horsch, R.B., Fry, J.E., Hoffman, N.L., Eichholtz, D., Rogers, S.G., and Fraley, R.T. (1985). A simple and general method for transferring genes into plants. Science 227, 1229–1231. [DOI] [PubMed] [Google Scholar]
- Hudson, M., Ringli, C., Boylan, M.T., and Quail, P.H. (1999). The FAR1 locus encodes a novel nuclear protein specific to phytochrome A signaling. Genes Dev. 13, 2017–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher, S., Kozma-Bognar, L., Kim, L., Adam, E., Harter, K., Schäfer, E., and Nagy, F. (1999). Light quality–dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11, 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno, N., Muramatsu, T., Hamazato, F., and Furuya, M. (2000). Identification by large-scale screening of phytochrome-regulated genes in etiolated seedlings of Arabidopsis using a fluorescent differential display technique. Plant Physiol. 122, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- Mancinelli, A.L. (1994). The physiology of phytochrome action. In Photomorphogenesis in Plants, R.E. Kendrick and G.H.M. Kronenberg, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 211–269.
- Marshall, C.J. (1995). Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185. [DOI] [PubMed] [Google Scholar]
- Matsunami, H., and Buck, L.B. (1997). A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell 90, 775–784. [DOI] [PubMed] [Google Scholar]
- McCormac, A.C., Wagner, D., Boylan, M., Quail, P.H., Smith, H., and Whitelam, G.C. (1993). Photoresponses of transgenic Arabidopsis seedlings expressing introduced phytochrome B–encoding cDNAs: Evidence that phytochrome A and phytochrome B have distinct photoregulatory functions. Plant J. 4, 19–27. [Google Scholar]
- Meagher, R.B., McKinney, E.C., and Kandasamy, M.K. (1999). Isovariant dynamics expand and buffer the responses of complex systems: The diverse plant actin gene family. Plant Cell 11, 995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1998). PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95, 657–667. [DOI] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1999). Binding of phytochrome B to its nuclear signaling partner PIF3 is reversibly induced by light. Nature 400, 781–784. [DOI] [PubMed] [Google Scholar]
- O'Rourke, S.M., and Herskowitz, I. (1998). The Hog1 MAPK prevents cross-talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev. 12, 2874–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund, M.T., and Deng, X.W. (1998). Multiple photoreceptors mediate the light-induced reduction of GUS-COP1 from Arabidopsis hypocotyl nuclei. Plant J. 16, 201–208. [DOI] [PubMed] [Google Scholar]
- Osterlund, M.T., Ang, L.H., and Deng, X.W. (1999). The role of COP1 in repression of Arabidopsis photomorphogenic development. Trends Cell Biol. 9, 113–118. [DOI] [PubMed] [Google Scholar]
- Quail, P.H. (1998). The phytochrome family: Dissection of functional roles and signaling pathways among family members. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1399–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail, P.H., Boylan, M.T., Parks, B.M., Short, T.W., Xu, Y., and Wagner, D. (1995). Phytochromes: Photosensory perception and signal transduction. Science 268, 675–680. [DOI] [PubMed] [Google Scholar]
- Shinomura, T., Uchida, K., and Furuya, M. (2000). Elementary processes of photoperception by phytochrome A for high-irradiance response of hypocotyl elongation in Arabidopsis. Plant Physiol. 122, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano, R., and Ecker, J.R. (1998). Ethylene gas: Perception, signaling and response. Curr. Opin. Plant Biol. 1, 393–398. [DOI] [PubMed] [Google Scholar]
- Sprague, J.F. (1998). Control of MAP kinase signaling specificity or how not to go HOG wild. Genes Dev. 12, 2817–2820. [DOI] [PubMed] [Google Scholar]
- Von Arnim, A., and Deng, X.W. (1996). A role for transcriptional repression during light control of plant development. Bioessays 18, 905–910. [DOI] [PubMed] [Google Scholar]
- Wagner, D., Hoecker, U., and Quail, P.H. (1997). RED1 is necessary for phytochrome B–mediated red light–specific signal transduction in Arabidopsis. Plant Cell 9, 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.Y., and Tobin, E.M. (1998). Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93, 1207–1217. [DOI] [PubMed] [Google Scholar]
- Wang, Z.Y., Kenigsbuch, D., Sun, L., Harel, E., Ong, M.S., and Tobin, E.M. (1997). A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell 9, 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam, G.C., Johnson, E., Peng, J., Carol, P., Anderson, M.L., Cowl, J.S., and Harberd, N.P. (1993). Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5, 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S.A., and Nagatani, A. (1999). Light-dependent translocation of a phytochrome B–GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 145, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky, M.J., Casal, J.J., and Whitelam, G.C. (1995). Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: Weak deetiolation of the phyA mutant under dense canopies. Plant Cell Environ. 18, 788–794. [Google Scholar]
- Yanovsky, M.J., Casal, J.J., and Luppi, J.P. (1997). The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia, dissect two branches of phytochrome A signal transduction that correspond to very-low-fluence and high-irradiance responses. Plant J. 12, 659–667. [DOI] [PubMed] [Google Scholar]
- Yanovsky, M.J., Whitelam, G.C., and Casal, J.J. (2000). fhy3-1 retains inductive responses of phytochrome A. Plant Physiol. 123, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, K.C., and Lagarias, J.C. (1998). Eukaryotic phytochromes: Light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc. Natl. Acad. Sci. USA 95, 13976–13981. [DOI] [PMC free article] [PubMed] [Google Scholar]