Abstract
The internal light-regulatory element (iLRE) of ferredoxin (Fed-1) mRNA, comprising the 5′ leader and at least the first 13 codons of the open reading frame, controls transcript abundance after illumination of the plant in a translation-dependent manner. We have characterized the RNA binding activities associated with the Fed-1 iLRE and have identified one activity as the heat shock protein HSP101, a protein shown to bind the 5′ leader of tobacco mosaic virus. HSP101 was sufficient and necessary to mediate a high level of translational activity from a Fed-1 iLRE–containing mRNA in yeast. Moreover, the Fed-1 iLRE substantially enhanced translation of reporter mRNAs in plant protoplasts expressing HSP101. Expression of HSP101 was subject to developmental regulation in leaves in that expression was highest in young leaves. These data suggest that Fed-1 mRNA may use the HSP101 regulatory mechanism as a means of ensuring a high level of translation required for the light-mediated regulation of Fed-1 mRNA stability.
INTRODUCTION
The plant heat shock protein HSP101 belongs to a class of heat shock proteins that is conserved in bacteria, yeast, and plants and functions to confer thermotolerance (Sanchez and Lindquist, 1990; Lee et al., 1994; Schirmer et al., 1994; Wells et al., 1998). In plants, the expression of HSP101 is subject to developmental regulation (Singla et al., 1997), suggesting that the protein may play a developmental role beyond its known thermotolerance function. Recently, HSP101 was identified as the trans-acting factor responsible for mediating the translational enhancement associated with the 5′ leader (called Ω) from tobacco mosaic virus (TMV) (Wells et al., 1998). HSP101 was shown to function as an RNA binding protein that binds to a poly(CAA) region within Ω that is responsible for the translational enhancement. The translational regulatory function of HSP101 could be recapitulated in yeast expressing an Ω-containing reporter mRNA. Moreover, genetic analysis in yeast expressing HSP101 demonstrated that the HSP101-mediated translational enhancement specifically required two initiation factors (eIF): eIF3 and eIF4G (Wells et al., 1998). These observations suggest that the genomic mRNA of TMV evolved to access a cellular regulatory mechanism that uses HSP101 as a means to optimize recruitment of 40S ribosomal subunits and, by so doing, to optimize translation of the viral mRNA. The discovery of a translational regulatory function for HSP101 raises the question of which cellular mRNAs might serve as clients for this regulation.
In pea, the nuclear-encoded photosynthetic electron transport protein ferredoxin 1 is encoded by the single-copy, intronless Fed-1 gene (Elliott et al., 1989) and is transported into the chloroplast to facilitate electron transfer from photosystem 1 to NADP+. Expression from Fed-1 is controlled by an internal light-regulatory element (iLRE) present within the transcript that regulates the stability of the mRNA (Petracek et al., 1998). The 5′ untranslated leader and the first 47 codons of the Fed-1 coding region are sufficient and necessary to direct full light regulation, even when fused to reporter mRNAs in transgenic tobacco (Dickey et al., 1992, 1998). Light regulation is observed even with the coding region component of the Fed-1 iLRE shortened to the first 13 codons (Dickey et al., 1998). Active translation of Fed-1 mRNA is required for light regulation of its stability (Dickey et al., 1994), and most mutations that abolish light regulation also reduce the association of Fed-1 mRNA with polyribosomes (Dickey et al., 1998). However, at least one mutation that affects Fed-1 light regulation does not disrupt translation or its light-regulated polyribosome association (Dickey et al., 1998), suggesting that despite a requirement for active translation in the control of Fed-1 mRNA stability, additional components may be required for the light-mediated regulation of transcript abundance.
As a first step toward identifying the trans-acting factors involved in the translation of Fed-1 mRNA, we characterized the RNA binding activities associated with the Fed-1 iLRE and examined the functional consequence that one trans-acting factor, identified as HSP101, has on Fed-1 mRNA translation. Using mapping studies, we found that the Fed-1 5′ untranslated leader is essential for binding HSP101. Translational enhancement directed by the Fed-1 iLRE could be recapitulated in yeast expressing HSP101. The Fed-1 iLRE also functioned as a translational enhancer in plant protoplasts expressing HSP101. In addition to its thermal induction, expression of HSP101 was detected in moderate amounts in leaves, the greatest expression being observed in young leaves. These data suggest that Fed-1 mRNA may serve as a client mRNA for regulation by HSP101 to achieve a high level of translation. Such a mechanism would ensure the active translation of Fed-1 mRNA that is necessary for the light-mediated control of its stability.
RESULTS
The Fed-1 iLRE Functions as a Binding Site for One or More Specific RNA Binding Activities
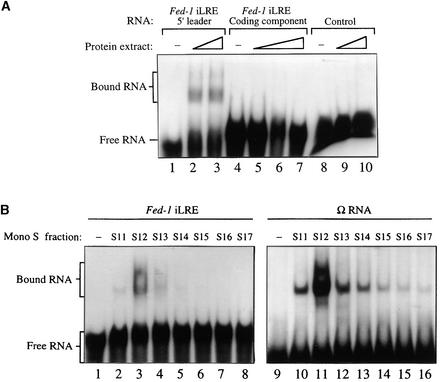
To examine whether the Fed-1 iLRE is recognized by a specific trans-acting factor, we initially performed RNA binding reactions with in vitro–synthesized, radiolabeled Fed-1 iLRE and crude plant extract, and the resulting complexes were resolved by gel shift analysis. Because the Fed-1 iLRE contains two regions of CA-rich sequences similar to the poly(CAA) element in Ω that has been identified as the high-affinity binding site for HSP101 (Tanguay and Gallie, 1996), the trans-acting factor responsible for the translational enhancement associated with this viral leader (Gallie and Walbot, 1992), the binding reaction utilized wheat embryo extract, which contains a high HSP101 content with low amounts of protease and RNase activities. Moreover, although the Fed-1 iLRE used in this analysis originates from pea, the observation that it functions in tobacco and Arabidopsis (Vorst et al., 1993; Bovy et al., 1995; Dickey et al., 1998) suggests conservation of this regulatory mechanism among plants. Complex formation after adding the complete Fed-1 iLRE RNA (i.e., the 5′ leader plus 47 codons of the Fed-1 coding region) to the binding assay was observed using gel shift analysis (data not shown). The large size of the RNA as well as intermolecular base pairing between molecules of Fed-1 iLRE RNA precluded good resolution of the protein–RNA complexes; therefore, the 5′ leader of the Fed-1 iLRE was tested separately from the coding region component in the binding assay. Specific binding to the Fed-1 5′ leader was observed under highly stringent conditions and led to the formation of at least two complexes: a faint complex of reduced mobility and a faster migrating, more prominent complex that appeared to consist of two bands (as shown in Figure 1A, lanes 2 and 3). No binding was detected to the coding region component of the Fed-1 iLRE when it was separated from the 5′ leader (Figure 1A, lanes 5 to 7) or to a control RNA of random sequence (Figure 1A, lanes 9 and 10).
Figure 1.
The Fed-1 5′ Leader Component of the iLRE Serves as a Specific Recognition Site for Trans-Acting Factor Binding.
(A) The radiolabeled Fed-1 5′ leader RNA and the coding region component of the iLRE were tested individually in binding reactions with crude wheat germ extract, and the resulting complexes were resolved by using gel shift analysis. A 58-nucleotide RNA of random sequence (Tanguay and Gallie, 1996) was used in the binding reaction as a control RNA. Increasing amounts of crude extract used in the binding reactions are represented by the ramps above the appropriate lanes.
(B) Complex formation, resulting from RNA binding reactions between fractions obtained from Mono S chromatography containing HSP101 and either the radiolabeled Fed-1 iLRE (left) or Ω RNA (right), was compared using gel shift analysis.
The position and number of complexes formed between the 5′ leader of the Fed-1 iLRE after gel shift analysis resembled those observed when Ω, the TMV 5′ leader, was used in identical binding reactions (Leathers et al., 1993). To determine whether the activity responsible for binding Ω also bound the Fed-1 iLRE, we compared complex formation with each RNA by using fractions obtained in the final step of HSP101 purification with Mono S chromatography, as previously described (Tanguay and Gallie, 1996). The fractions containing HSP101 activity that bound to the minimal Fed-1 iLRE (Fed-1 5′ leader plus seven codons of the Fed-1 open reading region) corresponded to those that also bound Ω RNA; for example, a single fraction containing the highest Ω binding activity also bound the Fed-1 iLRE and resulted in the formation of similar complexes (Figure 1B, cf. lanes 3 and 11). Complex formation with the Fed-1 iLRE was less than that observed for Ω, suggesting that the affinity of HSP101 for Ω was greater than it was for the Fed-1 iLRE.
HSP101 Mediates High Translational Activity from the Fed-1 iLRE
HSP101 functions as a trans-acting, translational regulator that enhances protein synthesis when an mRNA contains a high-affinity HSP101 binding site within the 5′ leader, as is found with Ω, the 5′ leader of TMV, a well-characterized translational enhancer (Gallie et al., 1987a, 1987b, 1989; Gallie and Walbot, 1992; Wells et al., 1998). The ability of HSP101 to mediate translational enhancement from Ω could be recapitulated in yeast expressing either wheat or tobacco HSP101, where typically the translation of Ω-containing mRNA exceeded that of control mRNA by at least 10-fold (Wells et al., 1998). The requirement for HSP101 was absolute: Ω failed to enhance translation in the absence of HSP101. The degree to which HSP101 mediated translational enhancement from an mRNA in yeast correlated with its affinity for the 5′ leader: The high level of enhancement afforded by HSP101 from an Ω-containing mRNA correlated with the high affinity of HSP101 for the sequence, whereas HSP101 did not regulate translation of an mRNA containing the tobacco etch virus 5′ leader, in agreement with its lower affinity for this sequence (Wells et al., 1998).
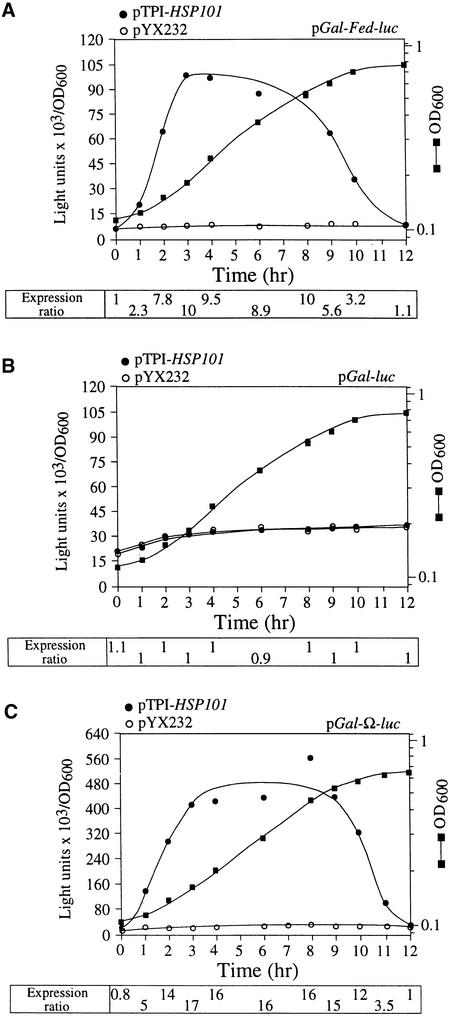
To determine whether Fed-1 mRNA utilized the HSP101 regulatory mechanism in vivo, we examined whether HSP101 could enhance translation of a luciferase (luc) reporter mRNA containing the Fed-1 iLRE, using the same yeast assay used in the analysis of the Ω leader (Wells et al., 1998). The tobacco HSP101 cDNA was placed under the control of the constitutively active triose phosphate isomerase (TPI) promoter in yeast expression vector pYX232, which resulted in pTPI–HSP101. luc, with or without the complete Fed-1 iLRE, was placed under the control of the galactose-regulated GAL1 promoter to yield pGAL–Fed–luc or pGAL–luc, respectively. A combination of pGAL–Fed–luc and either pTPI–HSP101 or pYX232 was introduced into yeast strain SL304A, an hsp104 null mutant. SL304A containing pGAL–luc and either pTPI–HSP101 or pYX232 served as negative controls. Luciferase assays were performed to measure the expression of pGAL–Fed–luc and pGAL–luc in the presence or absence of HSP101. Expression from each luciferase construct during growth was measured as light units per optical density unit of yeast so that luciferase expression would be normalized to the same number of cells regardless of the growth phase. Expression from the Fed-1 iLRE–containing luc construct was less than that from the luc control construct because the fusion of the N-terminal 47 amino acids of ferredoxin to luciferase reduced its enzyme activity (see the following section for further analysis). To quantify the extent of the HSP101 translational regulatory function, we calculated the expression ratio of translation from pGAL–Fed–luc, pGAL–luc, or pGAL–Ω–luc in the presence (pTPI–HSP101) or absence (pYX232) of HSP101 expression; it is included below the respective graphs in Figure 2. The expression ratio therefore is a measure of the HSP101-mediated enhancement, independent of any differences in absolute expression attributable to differences in strains, media conditions, or growth phase. Because stationary cells were used to initiate the experiment, a characteristic lag phase in cell growth (observed in the optical density of the growth curves shown in Figure 2) was observed during the first hour after transfer to fresh medium. Expression from the Fed–luc construct was substantially more (up to 10-fold) in yeast expressing HSP101 than it was from the same mRNA in yeast lacking HSP101 (Figure 2A). Moreover, expression from the control luc mRNA construct remained unaffected by the presence or absence of HSP101 (Figure 2B). The preferential translation of Fed–luc mRNA, which was initiated as the cells left stationary phase, persisted at an increased rate until the midexponential phase, whereupon its preferential translation progressively decreased as cell growth slowed. That is, the behavior during the late-exponential/early stationary phase of growth was identical to the pattern of translational enhancement observed when Ω was present as the leader sequence (Figure 2C), in good agreement with our previous observations (Wells et al., 1998). These results demonstrate that HSP101 is sufficient and necessary to mediate translational enhancement from the Fed-1 iLRE.
Figure 2.
HSP101 Enhances Expression of an mRNA Containing the Fed-1 iLRE in Yeast.
(A) and (B) Expression of pGAL–Fed–luc or the control pGAL–luc construct, respectively, was determined using yeast strain SL304A transformed with pTPI–HSP101 (filled circles) or pYX232 (open circles) to examine the regulatory role of HSP101 during translation.
(C) Expression of pGAL–Ω–luc was examined in SL304A transformed with pTPI–HSP101 (filled circles) or pYX232 (open circles). The mRNA served as a positive control for HSP101-mediated regulation of translation from luc containing Ω as the 5′ leader. Transformants were first grown to late-exponential stage (after which the expression from pGAL–Fed–luc in yeast expressing HSP101 was the same as that in yeast from which HSP101 was absent) and then inoculated into synthetic galactose medium at time zero.
Luciferase expression was measured at various times during the growth cycle and normalized to the optical density (filled squares, right scale in [A] to [C]) during growth. The expression ratio from each construct in yeast expressing HSP101 compared with its expression in yeast not containing HSP101 is indicated below each graph.
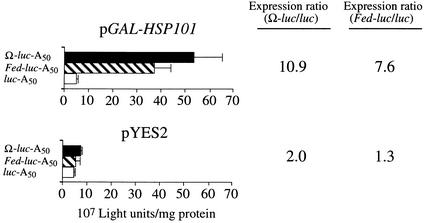
To demonstrate that the enhancement afforded by the Fed-1 iLRE in yeast expressing HSP101 was not due to nuclear events during gene expression, we synthesized Fed–luc (containing the Fed-1 5′ leader), Ω–luc, and control mRNAs in vitro as capped and poly(A)+ mRNAs (containing a poly[A]50 tail) and used RNA electroporation to deliver the mRNAs to exponentially growing yeast expressing HSP101. The same mRNAs were delivered to yeast not expressing HSP101 to serve as controls. Figure 3 demonstrates that translation from the control luc mRNA in yeast expressing HSP101 did not differ substantially from that observed in the absence of HSP101 expression, confirming that HSP101 does not enhance the translational activity of mRNAs in general, that is, in mRNAs without a high-affinity binding site. The expression from the Ω–luc mRNA construct was enhanced 10.9-fold relative to the luc control mRNA in yeast that expressed HSP101 (Figure 3), in good agreement with the previous observations for exogenously delivered Ω–luc mRNA (Wells et al., 1998). As with Ω–luc mRNA, the expression from the Fed–luc mRNA construct was enhanced (7.6-fold) relative to the luc control mRNA in yeast expressing HSP101 (Figure 3). The extent of enhancement from the Fed–luc mRNA construct was somewhat less than that observed for Ω–luc mRNA, which correlates with the lower affinity that HSP101 exhibits for the Fed-1 iLRE relative to Ω, as was observed in the binding reactions shown in Figure 1B. The extent of enhancement conferred by the 5′ leader of Fed-1 (relative to the control luc construct) as shown in Figure 3 is also somewhat less than that conferred by the complete Fed-1 iLRE (relative to the control luc construct), as observed in Figure 2A, suggesting that the Fed-1 iLRE is required for full translational function. These data support the conclusion that HSP101 enhances translation of an mRNA that contains the Fed-1 5′ leader or iLRE.
Figure 3.
HSP101 Enhances Translation from Exogenously Delivered Fed–luc–A50 mRNA in Yeast.
In vitro–synthesized luciferase mRNA constructs terminating in a poly(A)50 tail were electroporated into SL304A (containing either pGAL1–HSP101, i.e., HSP101 under the control of the GAL1 promoter, or pYES2, as indicated above each set of histograms); luciferase expression was measured after the completion of translation. The expression ratio is shown at right. Fed–luc–A50 mRNA was tested relative to luc–A50 mRNA as a negative control, and Ω–luc–A50 mRNA was used as a positive control. The error bars represent the standard error.
Fed-1 iLRE Functions as a Translational Enhancer in Plants
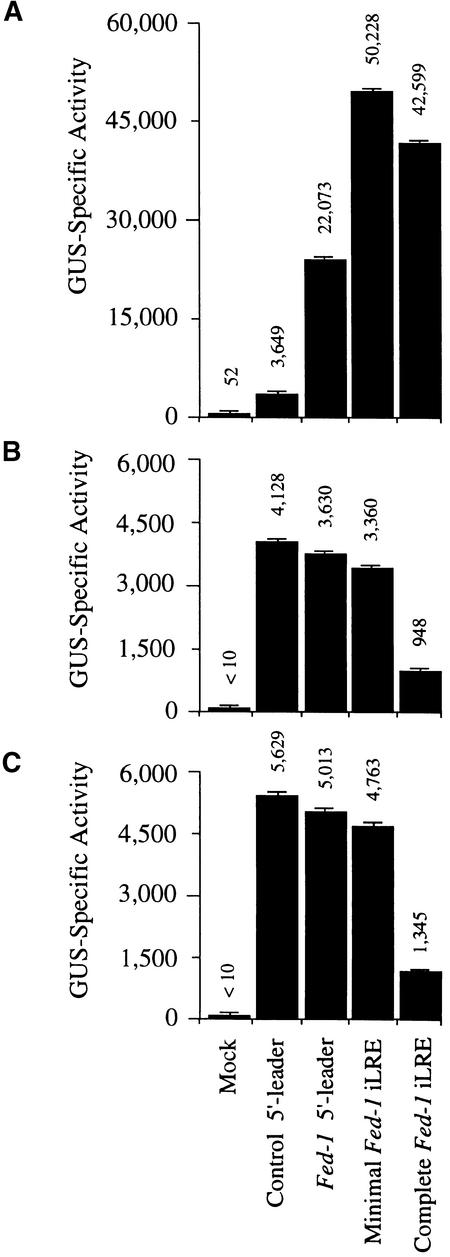
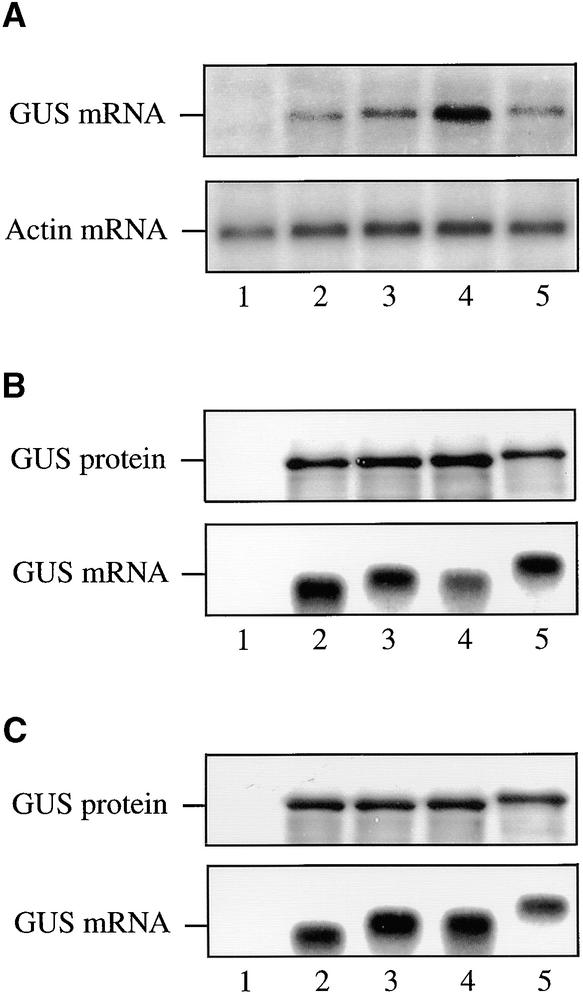
Because HSP101 mediates translational enhancement of an mRNA containing the Fed-1 iLRE in yeast, we examined whether the Fed-1 iLRE could function as a translational enhancer in plants in which HSP101 ordinarily is expressed. The Fed-1 iLRE was introduced upstream of the uidA coding region that encodes β-glucuronidase (GUS), and the construct was placed under the control of the 35S promoter. In addition to the complete Fed-1 iLRE (containing the 5′ leader plus 47 codons of the Fed-1 coding region), the minimal Fed-1 iLRE (containing the 5′ leader plus five codons) was also introduced upstream of the uidA coding region to make a translational fusion. Constructs containing the Fed-1 5′ leader or the 30-nucleotide vector control leader under the control of the 35S promoter were also made. 5 μg of each plasmid construct was delivered to carrot protoplasts, and after a 15-hr incubation, the resulting amount of GUS expressed from each construct was measured by assaying for GUS activity, as shown in Figure 4A. The presence of the Fed-1 5′ leader enhanced expression by 6.1-fold relative to the control, whereas the minimal Fed-1 iLRE increased expression by 13.8-fold, and the complete Fed-1 iLRE increased expression by 11.7-fold (Figure 4A). The increased expression from the construct containing the Fed-1 5′ leader was not a consequence of an increase in the amount of mRNA at steady state (Figure 5A , cf. lanes 3 and 2, GUS mRNA), which suggests that the Fed-1 5′ leader increases the rate of protein synthesis. The presence of the minimal Fed-1 iLRE increased expression an additional twofold over that observed for the construct containing the Fed-1 5′ leader alone (Figure 4A), which correlated with a reproducible twofold increase in the steady state amount of mRNA (Figure 5A, cf. lanes 4 and 3, GUS mRNA), suggesting that the first five codons of the Fed-1 iLRE may be required to regulate RNA stability positively. No increase in the steady state amount of RNA was observed for the construct containing the complete Fed-1 iLRE (Figure 5A, lane 5, GUS mRNA), which suggests that the additional sequence in the complete Fed-1 iLRE may negatively regulate mRNA stability. Despite a steady state RNA level less than that observed for the minimal Fed-1 iLRE construct, expression from the complete Fed-1 iLRE construct was almost as much as that from the minimal Fed-1 iLRE construct (Figure 4A).
Figure 4.
The Fed-1 iLRE Functions as a Translational Enhancer in Plant Protoplasts.
(A) Expression levels of uidA reporter gene constructs containing the Fed-1 5′ leader, minimal Fed-1 iLRE (Fed-1 5′ leader plus five codons of the Fed-1 coding region), complete Fed-1 iLRE (Fed-1 5′ leader plus 47 codons of the Fed-1 coding region), or a control leader were compared in vivo for carrot protoplasts as 35S promoter–based plasmid constructs.
(B) and (C) Same as in (A), except that expression levels were compared after translation of in vitro–synthesized mRNAs in a wheat germ or rabbit reticulocyte lysate, respectively. The fusion of the N-terminal 47–amino acid sequence of the Fed-1 gene product to GUS (i.e., in the complete Fed-1 iLRE construct) reduced the specific activity of the reporter, as was observed in the in vitro translations. Each experiment was repeated at least three times.
Mock represents delivery of plasmid vector alone. The error bars represent the standard error.
Figure 5.
The Fed-1 iLRE Controls mRNA Stability in Transiently Transformed Protoplasts.
(A) The steady state level of RNA expressed in carrot protoplasts from each 35S promoter–based uidA reporter construct used for Figure 4A was determined by RNA gel blot analysis. The steady state level of actin mRNA was also determined and served as an internal control.
(B) and (C) For the in vitro translation assays using wheat germ (B) or rabbit reticulocyte lysate (C), the amount of GUS protein (top) and RNA (bottom) was determined by SDS-PAGE analysis of the 35S-Met–labeled protein and RNA gel blot analysis, respectively. Protein expression from in vitro–synthesized mRNAs was determined after 120 min of translation.
Lanes 1, mock (plasmid vector alone); lanes 2, uidA containing a 30-nucleotide vector control leader; lanes 3, uidA containing the Fed-1 5′ leader; lanes 4, the minimal Fed-1 iLRE translationally fused to the uidA coding region; and lanes 5, the complete Fed-1 iLRE translationally fused to the uidA coding region. Each experiment was repeated at least three times.
To determine whether the presence of the N-terminal five or 47 codons of the Fed-1 coding region in the uidA construct might affect GUS enzyme activity, we placed the control and Fed-1–uidA constructs under the control of the T7 promoter to synthesize and translate the mRNAs in vitro and then to quantify the amount and activity of GUS expressed. Translation of the control and Fed-1–uidA constructs in wheat germ or in rabbit reticulocyte lysate yielded equivalent amounts of GUS protein (Figures 5B and 5C, GUS protein, respectively), and the stability of the RNA constructs in vitro did not differ substantially (Figures 5B and 5C, GUS mRNA, respectively). As expected, the fusion protein expressed from the complete Fed-1 iLRE–uidA construct (in which 47 additional amino acids are N-terminally fused to GUS) was larger than the wild-type GUS protein (cf. Figures 5B and 5C, lanes 5 and 2, GUS protein). The fusion protein resulting from the minimal Fed-1 iLRE–uidA construct (containing only five additional amino acids fused to GUS) was not sufficiently larger than wild-type GUS protein (Figures 5B and 5C, lanes 4, GUS mRNA) to have an easily observable increase in molecular mass. That the same amount of protein was expressed from the control, the Fed-1 5′ leader, and the iLRE-containing constructs in the in vitro systems suggests that the Fed-1 5′ leader and iLRE do not function to increase translation in vitro. A similar amount of GUS activity was observed for the control and the Fed-1 5′ leader constructs when translated in wheat germ or rabbit reticulocyte lysate (cf. the Fed-1 5′ leader construct with the control construct in Figures 4B and 4C, respectively), which is consistent with each construct expressing similar amounts of protein, as observed in Figures 5B and 5C. A similar amount of GUS activity was also observed for the minimal Fed-1 iLRE–uidA construct (cf. the minimal Fed-1 iLRE construct with the control construct in Figures 4B and 4C), suggesting that the presence of the five additional amino acids at the N terminus did not affect the activity of GUS. However, activity from the GUS fusion expressed from the complete Fed-1 iLRE–uidA construct was reduced 4.4- and 4.2-fold in wheat germ and rabbit reticulocyte lysate, respectively, relative to the control construct (cf. the complete Fed-1 iLRE construct with the control construct in Figures 4B and 4C), suggesting that fusion of the 47 N-terminal amino acids to the GUS protein reduced the activity of the enzyme. These data demonstrate that the increase in GUS expression observed in vivo for those fusion constructs (i.e., the minimal Fed-1 iLRE–uidA and complete Fed-1 iLRE–uidA constructs) was not a result of an increase in the enzymatic activity of the fusion protein. Moreover, the reduced activity of the GUS fusion protein expressed from the complete Fed-1 iLRE–uidA construct in vitro in Figures 4B and 4C suggests that the degree of translational enhancement conferred by the complete Fed-1 iLRE in vivo (as determined by the measurement of GUS activity in Figure 4A) is underestimated by approximately fourfold; in other words, the complete Fed-1 iLRE enhanced expression more than did the minimal Fed-1 iLRE.
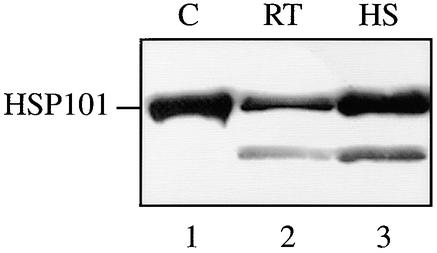
If HSP101 is responsible for the translational enhancement associated with the Fed-1 5′ leader or iLRE in plants, then HSP101 must be expressed in the carrot cells used in the in vivo analysis shown in Figure 4A. Protein gel blot analysis for HSP101, shown in Figure 6, confirmed that this was indeed the case for the carrot protoplasts used and that the level of HSP101 expression was moderately high because a heat shock induced HSP101 expression by only two- to threefold.
Figure 6.
Expression of HSP101 in Carrot Suspension Cells.
Carrot suspension cells used for the experiments described in Figures 4 and 7 were analyzed for the amount of HSP101 expression by protein gel blot analysis. Ten micrograms of total protein from control and heat-stressed (at 41°C for 1 hr) cells was resolved on a 10% SDS–polyacrylamide gel, transferred to a nitrocellulose membrane, and probed with anti-HSP101 antibodies. Wheat germ extract (lane 1) was included as a positive control for HSP101. A degradation product of HSP101 is present in lanes 2 and 3. C, wheat germ extract; RT, room temperature protoplasts; HS, heat-treated protoplasts.
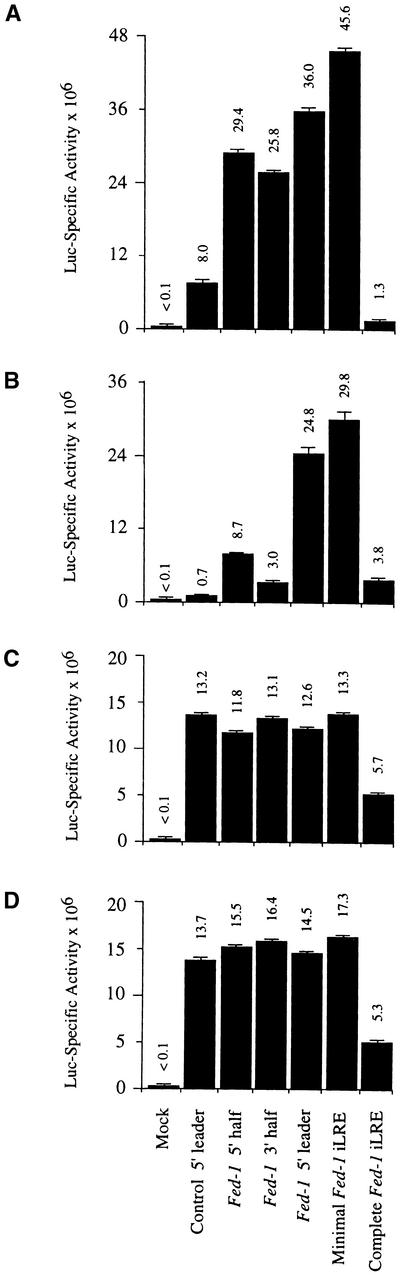
To dissect the regulatory effect of the Fed-1 iLRE on translation and mRNA stability and examine whether the effect of the Fed-1 iLRE is independent of the reporter gene used, control and Fed-1 iLRE–containing luciferase mRNA constructs were synthesized in vitro and delivered to carrot protoplasts. The leader was also divided in half, and each half was introduced individually upstream of the luc coding region. Figure 7A shows that the 5′ and 3′ halves of the Fed-1 5′ leader increased luciferase expression relative to the control by 3.7- and 3.2-fold, respectively. Combining the 5′ and 3′ halves to form the Fed-1 5′ leader resulted in a 4.5-fold increase in luciferase expression (Figure 7A). The observation that each half of the Fed-1 5′ leader increased expression almost as much as did their combined effect in the full Fed-1 5′ leader suggests a possible functional redundancy between the two halves of the leader. The presence of the minimal Fed-1 iLRE (containing the 5′ leader plus five codons) increased luciferase expression relative to the control construct by 5.7-fold (Figure 7A). When the complete Fed-1 iLRE was tested as a translational fusion with the luciferase coding region, expression was substantially less than that from the control construct (Figure 7A).
Figure 7.
The 5′ and 3′ Halves of the Fed-1 iLRE Are Partially Redundant in Promoting Translation in Plant Protoplasts.
(A) and (B) Expression levels of luc reporter gene constructs containing the Fed-1 5′ half, Fed-1 3′ half, full-length Fed-1 5′ leader, minimal Fed-1 iLRE (Fed-1 5′ leader plus five codons of the Fed-1 coding region), the complete Fed-1 iLRE (Fed-1 5′ leader plus 47 codons of the Fed-1 coding region), the vector control leader, or Mock (plasmid vector alone) were compared in vivo for control (A) and heat-shocked (B) carrot protoplasts after the delivery of in vitro–synthesized mRNAs. The heat treatment in (B) was for 1 hr at 40°C followed by 1 hr at 24°C.
(C) and (D) Expression of the constructs given above was compared for control and heat-shocked protoplasts after their in vitro translation in wheat germ (C) or rabbit reticulocyte (D) lysate.
The error bars represent the standard error.
After heat stress in plants, a global repression of translation is imposed from which only a few transcripts, most notably, heat shock mRNAs, escape (Key et al., 1981; Gallie et al., 1995). The 5′ leader of heat shock mRNAs is responsible for their continued translational competence after a heat stress (Pitto et al., 1992). The TMV 5′ leader (Ω) functions like a heat shock 5′ leader in that its presence enables an mRNA to escape the heat shock–mediated translational repression (Pitto et al., 1992), behavior consistent with the function of HSP101 in mediating its translational enhancement (Wells et al., 1998). If HSP101 mediates the translational regulation associated with the Fed-1 iLRE in vivo, then the presence of the Fed-1 iLRE in a transcript should enable the mRNA to escape the translational repression that would ordinarily be imposed by a heat shock in the absence of the regulatory element. To examine this possibility, we delivered the same luc mRNAs that were delivered to carrot protoplasts in Figure 7A to carrot cells treated at 40°C for 1 hr and allowed them to recover at 24°C for 1 hr before isolating the protoplasts and measuring the resulting amount of luciferase expressed after incubation (Figure 7B). Expression from the control luc mRNA construct was repressed by more than an order of magnitude in carrot cells subjected to heat shock relative to expression from the same mRNA in non-heat-shocked carrot cells (cf. expression from the control 5′ leader construct in Figures 7B and Figure 7A). In contrast, expression from the luc mRNA constructs containing the Fed-1 5′ leader or the minimal Fed-1 iLRE decreased only minimally relative to the expression from these same mRNAs in control cells, and expression from the luc mRNA construct containing the complete Fed-1 iLRE actually increased threefold in the heat-shocked cells (cf. expression levels in Figures 7B and 7A). Thus, the presence of the Fed-1 iLRE, like Ω, enables an mRNA to escape translational thermorepression. Interestingly, expression from the luc mRNA construct containing the 5′ half of the Fed-1 5′ leader better escaped translational repression in the heat-shocked protoplasts than did the construct containing the 3′ half of the Fed-1 5′ leader, thus indicating a difference between the two halves of the leader. The observation that the Fed-1 iLRE can confer translational competence to an mRNA under heat shock conditions is consistent with the notion that HSP101 may be responsible for the translational regulation associated with the Fed-1 iLRE in vivo.
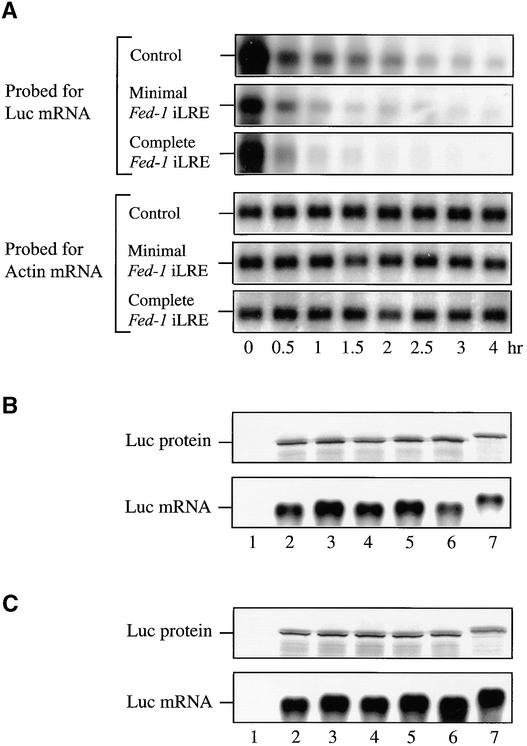
Similar to the observations made with the corresponding GUS fusion construct, expression from the luc mRNA construct containing the complete Fed-1 iLRE was significantly lower than that observed for the other constructs (Figures 7A and 7B). To determine whether this reduction resulted from a reduction in luciferase enzyme activity, as had been observed above with the GUS fusion construct, all of the luc mRNA constructs were translated in vitro in wheat germ (Figure 8B) or rabbit reticulocyte (Figure 8C) lysate. Each luc mRNA construct was translated to a similar extent in vitro in each lysate, and as expected, a larger protein product was observed when the complete Fed-1 iLRE was translationally fused to the luciferase coding region (Figures 8B and 8C, lanes 7, Luc protein). The amounts of luciferase activity resulting from the translation of each mRNA were also similar in wheat germ or rabbit reticulocyte lysate (Figures 7C and 7D, respectively), except for the construct containing the complete Fed-1 iLRE, for which expression was reduced by 2.3- and 2.6-fold, respectively, relative to the control luc mRNA. This reduction accounted for only part of the low activity resulting from this construct in vivo.
Figure 8.
Determination of the Stability of Fed-1 iLRE–Containing mRNA Constructs in Transiently Transformed Protoplasts.
(A) The stability of mRNAs from the constructs given in Figure 7A containing the vector control leader, the minimal Fed-1 iLRE, or the complete Fed-1 iLRE was determined by RNA gel blot analysis after delivery of the constructs into carrot protoplasts at the times indicated below each lane. The amount of actin mRNA, which served as an internal control, was also determined.
(B) and (C) For the in vitro translation assays using wheat germ (B) or rabbit reticulocyte lysate (C), the amount of luciferase protein (top) and mRNA (bottom) was determined by SDS-PAGE analysis of the 35S-Met−labeled protein and RNA gel blot analysis, respectively. Protein expression from the in vitro–synthesized mRNAs was determined after 120 min of translation. Lanes 1, no RNA control; lanes 2, luc containing a 30-nucleotide vector control leader; lanes 3, luc containing the 5′ half of the Fed-1 5′ leader; lanes 4, luc containing the 3′ half of the Fed-1 5′ leader; lanes 5, luc containing the Fed-1 5′ leader; lanes 6, the minimal Fed-1 iLRE translationally fused to the luc coding region; and lanes 7, the complete Fed-1 iLRE translationally fused to the luc coding region. Each experiment was repeated at least three times.
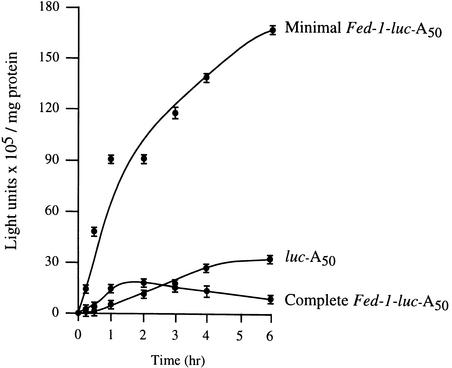
To determine whether the increased expression observed in vivo for the minimal Fed-1 iLRE and the decreased expression observed in vivo for the complete Fed-1 iLRE (beyond what would be predicted from the effect of the N-terminal fusion on luciferase enzyme activity) was a result of altered mRNA stability or translational efficiency, we measured the half-life and extent of translation for the constructs containing the minimal Fed-1 iLRE, complete Fed-1 iLRE, or the control leader in vivo. After mRNA delivery to carrot protoplasts, the physical mRNA half-life could be determined by measuring the rate of RNA decay in aliquots of cells harvested at various times (Figure 8A). The complete Fed-1 iLRE–luc mRNA decayed more rapidly (calculated half-life [t0.5] of 45 min) than did either the minimal Fed-1 iLRE–luc (t0.5 86 min) or control (t0.5 68 min) constructs (Figure 8A), suggesting that the reduced expression from the complete Fed-1 iLRE construct shown in Figure 7A resulted in part from reduced RNA stability. The translational efficiency of each mRNA was determined from the luciferase expression curves (shown in Figure 9) obtained after mRNA delivery. The minimal Fed-1 iLRE–luc mRNA construct was more rapidly recruited for translation than was the control; expression from the construct was ∼43-fold more than that from the control only 25 min after mRNA delivery (Figure 9). Likewise, expression from the luc mRNA containing the complete Fed-1 iLRE was greater (by 3.25-fold) than that from the control mRNA at this same interval, even without normalizing for the inhibitory effect of the 47–amino acid Fed-1 N-terminal fusion on luciferase enzyme activity. As Table 1 shows for both Fed-1 iLRE–containing constructs, the rate of translation, as determined from the initial slope of each expression curve shown in Figure 9, was greater than that for the control (i.e., the minimal and complete Fed-1 iLRE–luc constructs were 10.2- and 2.8-fold greater, respectively), indicating that they were translated more efficiently. The minimal Fed-1 iLRE–luc construct was translationally active for >6 hr, whereas the control construct was translationally active for ∼4 to 6 hr (Figure 9), supporting the observation made with the corresponding uidA constructs (see Figure 5A) and the luciferase physical measurements of t0.5 (Figure 8A) that a moderate increase in mRNA stability is conferred by the minimal Fed-1 iLRE. The complete Fed-1 iLRE–luc construct was translationally active for only 75 min, an observation confirming the physical t0.5 data that indicate that the complete Fed-1 iLRE–luc construct is less stable than the control. As a result of its shorter half-life, expression from the luc mRNA containing the complete Fed-1 iLRE ceased early. Consequently, the lower expression associated with the presence of the complete Fed-1 iLRE is in part a result of reduced mRNA stability and in part an indication of the inhibitory effect of the Fed-1 N-terminal fusion on luciferase enzyme activity. Because the minimal and complete Fed-1 iLREs direct a more rapid initial rate of translation than does the control, particularly when the differences in mRNA stability are taken into account, we can conclude that both the minimal and complete Fed-1 iLREs function to increase translational efficiency. Moreover, these data indicate that the 5′ and 3′ halves of the Fed-1 5′ leader exhibit some redundancy with regard to translational enhancement.
Figure 9.
The Fed-1 iLRE Increases Translational Efficiency in Plants.
Expression of luc reporter gene constructs containing the minimal Fed-1 iLRE (Fed-1 5′ leader plus five codons of the Fed-1 coding region), the complete Fed-1 iLRE (Fed-1 5′ leader plus 47 codons of the Fed-1 coding region), or the vector control leader was determined over time in vivo in carrot protoplasts after the delivery of in vitro–synthesized mRNAs. Aliquots of cells electroporated with each construct were collected at various times after RNA delivery and assayed for luciferase activity, which was plotted as a function of the cell incubation time. The translational efficiency for each of the constructs was measured from the maximum slope of each curve (see Table 1). The results are representative of three independent experiments. The error bars represent the standard error.
Table 1.
Effect of the Fed-1 iLRE on Translational Efficiency
| mRNA | Translational Efficiencya(light units/min/mg Protein) | Relative Rate of Translation |
|---|---|---|
| luc-A50 | 1,083 | 1.0 |
| Minimal Fed-1–luc–A50 | 11,000 | 10.2 |
| Complete Fed-1–luc–A50 | 3,067b | 2.8 |
Data calculated from the initial slopes of the curves shown in Figure 9.
Value does not take into account the reduced mRNA stability or inhibition of luciferase enzyme activity associated with this construct.
HSP101 Is Expressed in Moderate Constitutive Amounts under Nonstressed Conditions
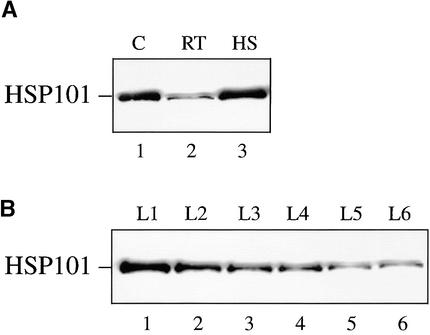
If HSP101 mediates high translational activity from Fed-1 mRNA during light regulation, it would need to be expressed in leaves, where Fed-1 expression and light regulation occur. As observed for other heat shock proteins, the expression of HSP101 is under developmental regulation such that it is highly expressed in developing seed tissues (Singla et al., 1997; T. Young, J. Ling, R.L. Tanguay, and D.R. Gallie, unpublished data). Although expression of HSP101 was observed in carrot protoplasts (Figure 6), this may have been a consequence of establishing the culture as a cell suspension. To determine whether HSP101 is expressed in unstressed leaves, we grew carrot and tobacco under nonstress conditions (i.e., at 24°C). Expression of HSP101 was observed at a moderate constitutive amount in nonstressed carrot leaves (Figure 10A, lane 2), and as expected, thermal stress at 41°C induced its expression (lane 3). HSP101 was also expressed in tobacco, its expression being greatest in young leaves and decreasing as the leaves expanded to their mature size (Figure 10B). These data demonstrate that HSP101 is expressed in moderate constitutive amounts in nonstressed leaves and at a developmental stage during which the bulk of protein synthesis occurs.
Figure 10.
Expression of HSP101 in Carrot and Tobacco Leaves under Nonstressed Conditions.
Carrot and tobacco plants were grown at 24°C, and 10 μg of total leaf protein was resolved on a 10% SDS–polyacrylamide gel, transferred to a nitrocellulose membrane, and probed with anti-HSP101 antibodies.
(A) Control and heat-stressed (41°C for 1 hr) carrot leaves were analyzed. C, wheat germ extract; RT, room temperature leaves; HS, heat-treated leaves.
(B) HSP101 expression was determined for consecutively older leaves of tobacco plants beginning with leaves 1 inch long through those with fully expanded leaves; for example, L1 is the youngest and L6 is the first fully expanded leaf.
DISCUSSION
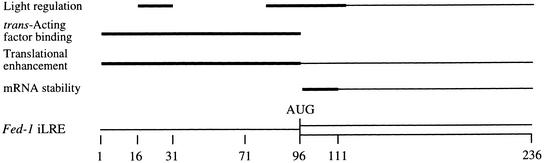
The light-mediated control of Fed-1 mRNA stability is an example of a regulatory mechanism in which the amount of translational activity functions as an integral component in the regulation of mRNA turnover. Previous work had demonstrated that most mutations that abolish Fed-1 light regulation also reduce the association of Fed-1 mRNA with polyribosomes (Dickey et al., 1998). Because a high level of translation is required for Fed-1 mRNA light regulation, and because of the sequence similarities between the Fed-1 iLRE and Ω, the 5′ leader of TMV mRNA, we examined whether the Fed-1 iLRE might utilize the HSP101 translational regulatory mechanism responsible for mediating the translational enhancement conferred by Ω. The observations made in this study indicate that the 5′ leader component rather than the coding region component of the Fed-1 iLRE binds HSP101, as illustrated in Figure 11. Moreover, our observations suggest that the Fed-1 iLRE directs enhanced translation in yeast and plants through a mechanism requiring HSP101.
Figure 11.
Functional Map of the Fed-1 iLRE.
The complete Fed-1 iLRE is shown at bottom; the 5′ leader is indicated as a line, and the coding region component of the iLRE is indicated by an open box. Above the appropriate regions of the iLRE are the elements involved in the light regulation of Fed-1 mRNA stability (from Dickey et al., 1998), trans-acting factor binding, translational enhancement, and mRNA stability. Regions of primary importance are indicated with a thick line, and those that might play an accessory role are indicated with a thin line.
Although the sequence preferences of HSP101 have not been fully characterized, the HSP101 binding site within Ω was mapped to a poly(CAA) region. In this respect, we find it interesting that two CA-rich sequences present in the Fed-1 iLRE exhibit only an auxiliary function in binding HSP101 (D.R. Wells and D.R. Gallie, unpublished observations). However, one striking similarity between the Fed-1 5′ leader and Ω is their low guanosine content (6 and 1.5%, respectively) and a high adenosine–uridine content (70.5 and 77.6%, respectively). Some flexibility in the binding requirements of HSP101 would be expected if the protein has evolved to regulate expression from multiple mRNAs and does so on a competitive basis. Identification of additional client mRNAs will be necessary to determine the range of HSP101 binding preferences.
Despite evidence suggesting that HSP101 binds to the Fed-1 iLRE, HSP101 may not be the only protein responsible for the interaction with Fed-1 iLRE. Additional trans-acting factors may be required because a high level of translation from Fed-1 mRNA in vivo is not in itself sufficient to confer the light regulation of its stability; for example, replacing the Fed-1 5′ leader with Ω abolished its light regulation, but the mRNA remained polysome associated (Dickey et al., 1998). Consequently, if HSP101 is responsible for the high level of translation from Fed-1 mRNA in vivo, one or more factors may be required to control the stability of the mRNA in response to light. However, the binding data do support HSP101 as a likely candidate in mediating the in vivo translational regulation conferred by the Fed-1 iLRE.
The functional requirement of HSP101 in mediating the translational enhancement from the Fed-1 iLRE was demonstrated in yeast by using an assay previously developed for the analysis of the translational regulatory mechanism associated with Ω (Wells et al., 1998). Expression of HSP101 in yeast enhanced the translation of luciferase reporter mRNA in the presence of the Fed-1 iLRE but not in its absence. Because the Fed-1 iLRE failed to enhance the translation of luciferase reporter mRNA in the absence of HSP101 expression, the Fed-1 iLRE itself does not enhance translation. In good agreement with the HSP101-mediated regulation of Ω-containing constructs, we observed that HSP101 enhanced translation from a Fed-1 iLRE–containing mRNA under conditions of rapid fermentative growth but not under limiting growth conditions. The loss of HSP101 function during conditions of limited nutrient availability was correlated with a loss in its RNA binding activity (Wells et al., 1998) and suggests that HSP101 binding is a prerequisite for the translational regulation in vivo.
The observations obtained from the yeast studies were supported by the results obtained in carrot cells, in which the 5′ leader component of Fed-1 iLRE functioned as a translational enhancer (see Figure 11), demonstrating that the Fed-1 iLRE directs a high amount of translation in vivo in plants in addition to conferring light regulation. Definitive evidence demonstrating that HSP101 is responsible for mediating the in vivo translational enhancement exhibited by the Fed-1 iLRE in plants (as it is responsible in yeast) will require studies with mutants deficient in HSP101 expression. However, expression from a transcript containing the complete Fed-1 iLRE did increase after heat shock, as did HSP101 expression—observations consistent with the notion that HSP101 may be responsible for mediating the translation regulation associated with the Fed-1 iLRE in vivo. A prerequisite for such a HSP101-mediated mechanism in vivo is the expression of HSP101 in leaves under nonstressed conditions. HSP101 expression was observed at moderate constitutive amounts in nonstressed carrot and tobacco leaves, particularly in young leaves, in which protein synthesis is most active (see Figure 10).
The Fed-1 iLRE did not increase expression when mRNAs containing the element were translated in wheat germ lysate containing HSP101. Our previous results demonstrated that the HSP101-mediated enhancement conferred by Ω in wheat germ lysate was only twofold (Tanguay and Gallie, 1996). Because of the abundance of the translation initiation factors and the absence of endogenous mRNAs in wheat germ lysate, translation in the lysate does not reflect the competitive conditions that prevail in vivo. As a consequence, those elements of an mRNA that are essential for efficient translation in vivo, such as a 5′ cap structure or a poly(A) tail, increase translation in wheat germ lysate by only a fewfold or less. Therefore, under the noncompetitive conditions that prevail in wheat germ lysate, the HSP101-mediated recruitment of translational machinery to an mRNA would be expected to afford little competitive advantage.
In addition to the 5′ leader, the coding region component of the Fed-1 iLRE also affected expression. Inclusion of the N-terminal five codons (i.e., in the minimal Fed-1 iLRE) or 47 codons (i.e., in the complete Fed-1 iLRE) increased reporter gene expression in protoplasts (see Figures 4 and 7) through increasing the translational efficiency of the mRNAs (see Figure 9). The minimal Fed-1 iLRE (the Fed-1 5′ leader plus five codons) also reproducibly directed a higher steady state amount of mRNA than that observed for the construct containing the Fed-1 5′ leader alone—a finding that accounts for part of the increased expression from reporter gene constructs containing this sequence (see Figure 9). This observation suggests that the sequence downstream of the initiation codon representing the N-terminal five codons of the Fed-1 coding region may be involved in positive regulation of mRNA stability (see Figure 11) and correlates with the observation that alteration of the sequence downstream of the Fed-1 initiation codon decreased or abolished the light regulation of Fed-1 mRNA stability (Dickey et al., 1998). Interestingly, the complete Fed-1 iLRE (containing the 5′ leader plus 47 codons of the Fed-1 coding region) reduced luciferase mRNA stability measurably, suggesting that this downstream sequence may regulate mRNA stability negatively. The decreased mRNA stability of the construct containing the complete Fed-1 iLRE was greater when mRNAs (i.e., luc) rather than DNA-based (i.e., uidA) constructs were delivered to plant protoplasts. One explanation for this may be that the association of exogenously delivered mRNAs with RNA binding proteins involved in the regulation of RNA stability may differ from that of endogenous mRNAs, which, in turn, may influence the effect of the minimal and complete Fed-1 iLREs on reporter mRNA stability.
How then might Fed-1 transcript stability be regulated? The t0.5 for Fed-1 mRNA in dark-treated plants is just 50% (i.e., 1.2 hr) of that measured for the same mRNA in light-treated plants (i.e., 2.4 hr) (Petracek et al., 1998). The observation that most mutations that abolish Fed-1 light regulation result in an increase in Fed-1 mRNA abundance specifically in dark-treated plants suggests that Fed-1 mRNA is an inherently stable mRNA that is subject to active turnover in the dark. Suspension cells, however, such as those used in this study, are not equivalent to light- or dark-treated plants; the cultured cells used are not photosynthetic and therefore are not light responsive, and the Fed-1 mRNA remains fully polysome associated in cultured cells (Petracek et al., 2000). HSP101-mediated translational enhancement, on the other hand, is observed in both plants and suspension cells (Gallie et al., 1989; Dowson Day et al., 1993). In addition to the reduction in Fed-1 mRNA abundance, dark treatment of leaves also results in a loss in the active translation of Fed-1 mRNA and its association with polysomes. The loss of Fed-1 association with polysomes in leaves is not a result of a light-mediated reduction of the translational regulatory function of HSP101; substituting Ω, the HSP101-regulated 5′ leader of TMV, for the Fed-1 5′ leader caused the transcript to remain associated with polysomes even in the dark (Dickey et al., 1998).
One possible model for the translational regulation of Fed-1 transcript stability is that after dark treatment, either the binding of HSP101 to the Fed-1 iLRE or its activity once bound may be modulated by additional regulatory proteins. Dark-induced inhibition of HSP101 binding or function by other trans-acting factors would provide the means to reduce the translational activity of Fed-1 mRNA, which, in turn, could lead to an increase in the susceptibility of this mRNA to attack by a specific nuclease that would reduce the t0.5 observed for this mRNA. The fact that no light regulation of mRNA stability was observed when Ω was substituted for the Fed-1 leader supports the notion that other factors are required to mediate the light regulation of Fed-1 mRNA in dark-treated plants. The identification, therefore, of HSP101 as the potential factor responsible for regulating the translational activity of Fed-1 mRNA represents the first step in elucidating the light regulation of Fed-1 mRNA stability.
METHODS
Plasmid Constructs and in Vitro RNA Synthesis
The 35S promoter–based uidA constructs and T7-based constructs containing the Fed-1 5′ leader, minimal internal light-regulating element (iLRE), or complete iLRE were described previously (Dickey et al., 1992), as were the T7-based constructs containing Ω or control sequences (Leathers et al., 1993). The DNA concentration was quantified spectrophotometrically after linearization and adjusted to 0.5 mg/mL. In vitro transcription was performed as previously described (Yisraeli and Melton, 1989), with 40 mM Tris-HCl, pH 7.5, containing 6 mM MgCl2, 100 μg/mL BSA, 0.5 mM each of ATP, CTP, UTP, and GTP, 10 mM DTT, 0.3 unit/μL RNasin (Promega), and 0.5 unit/μL T7 RNA polymerase. Radiolabeled probes were made as uncapped RNAs by using either 32P-ATP or 32P-CTP, and the full-length transcripts were resolved and electroeluted from 4% polyacrylamide gels. Quantitation of RNA yields were determined spectrophotometrically. Capped β-glucuronidase (GUS) or luciferase (luc) mRNA constructs were synthesized under the same conditions, except that 1 mM m7GpppG was added to the transcription reaction in which the GTP concentration had been reduced to 0.167 mM.
The tobacco HSP101 cDNA was introduced into the BamHI/NotI sites of pYES2 (Invitrogen Corp., Carlsbad, CA) in which HSP101 expression is under the control of the GAL1 promoter. The cDNA was also introduced into the BamHI site of pYX232 (Novagen, Inc., Madison, WI), in which HSP101 expression is under the control of the triose phosphate isomerase (TPI) promoter. luc or Ω–luc was introduced into the HindIII/BamHI sites of pYES2. SL304A was transformed with plasmid constructs by using the polyethylene glycol/LiCl method, as described elsewhere (Hill et al., 1991; Gietz et al., 1992).
RNA Gel Shift Assay
For the binding reactions, 1 ng of radiolabeled RNA and crude extract or fractions enriched for HSP101 were used in a 14-μL reaction volume containing 10 mM Tris, pH 7.5, 35 mM KCl, 1.0 mM MgCl2, 5% glycerol, 1 mM DTT, 0.7 mg/mL total yeast RNA, and 0.5 unit/μL RNasin. After 15 min of incubation at 4°C, heparin was added to a concentration of 5 mg/mL, and the mixture was incubated for an additional 10 min. The RNA–protein complexes were resolved on native 3.5% polyacrylamide gels, dried, and analyzed by autoradiography. The extent of complex formation was quantitated by using PhosphorImage analysis.
Translational Enhancement Assay in Yeast
For the analysis of the Fed-1 iLRE function during yeast growth, SL304A (leu2-3,112 trp1-1 ura3-1 ade2-1 his3-11,15 lys2 Δcan1-100 hsp104::LEU2) (Schirmer et al., 1994), a Δhsp104 yeast mutant (generously provided by Dr. Susan Lindquist, University of Chicago), was transformed with pTPI–HSP101 (tobacco HSP101 in pYX232) and pGAL1–Ω–luc or pGAL1–luc and grown in synthetic medium containing the appropriate supplements. Overnight yeast cultures grown in either synthetic medium were diluted to the indicated optical density into fresh medium, and samples were taken at various intervals during their subsequent growth for luciferase assays.
mRNA Delivery
For mRNA delivery to yeast spheroplasts by electroporation, SL304A containing pGAL–HSP101 was grown to midlogarithmic phase in synthetic galactose (2%) medium, electroporated as described (Everett and Gallie, 1992), and incubated for 4 hr before being harvested for analysis. Luciferase assays were performed as described elsewhere (Tanguay and Gallie, 1996). Six replicate samples of each mRNA delivered to yeast were assayed, each in duplicate.
Protoplasts were isolated from carrot (RCWC) cell suspension by digestion with 0.25% CELF cellulase (Worthington Biochemical Corp., Freehold, NJ), 1% cytolase, 0.05% pectolyase Y23, 0.5% BSA, and 7 mM β-mercaptoethanol in protoplast isolation buffer (12 mM sodium acetate, pH 5.8, 50 mM CaCl2, and 0.25 M mannitol) for 90 to 120 min. Protoplasts were washed with protoplast isolation buffer followed by electroporation buffer (10 mM Hepes, pH 7.2, 130 mM KCl, 10 mM NaCl, 4 mM CaCl2, and 0.2 M mannitol), and then they were resuspended in electroporation buffer to ∼106 cells/mL. For the heat shock experiment, carrot suspension cells were treated at 40°C for 1 hr and allowed to recover at 24°C for 1 hr before isolating the protoplasts as described above. Equal amounts of mRNAs (∼2.5 μg) were added to 400 μL of cell suspension immediately before electroporation (250 μF, 300 V, 0.2-mm-gap electrode) with an IBI GeneZapper (New Haven, CT). The electroporated cells were incubated in protoplast growth media (Murashige and Skoog salts [Murashige and Skoog, 1962], pH 5.8, 30 g/L sucrose, 100 mg/L myo-inositol, 0.1 mg/L 2,4-dichlorophenoxyacetic acid, 1.3 mg/L niacin, 0.25 mg/L thiamine, 0.25 mg/L pyridoxine, and 0.25 mg/L calcium pantothenate) supplemented with 20% (v/v) of culture in medium (protoplast growth medium conditioned with carrot cells for 3 days) overnight before assaying for reporter gene activity. For each experiment, an mRNA was delivered to triplicate samples of protoplasts, and each sample was assayed in duplicate. Each experiment was repeated at least three times.
In Vitro Translation
Equal amounts of mRNA were translated by using wheat germ and rabbit reticulocyte lysate, as described by the manufacturer (Promega), and radiolabeled methionine. After translation, 3-μL aliquots were analyzed for protein yield following resolution on SDS–polyacrylamide gels and exposure to film. Aliquots were also assayed for GUS or luciferase activity, as described below. Each mRNA construct was translated in triplicate, and each in vitro translation was assayed in duplicate.
Luciferase and GUS Assays
Carrot protoplast extracts or in vitro translation lysates were added to luciferase assay buffer (25 mM Tris, pH 8.0, 5 mM MgCl2, and 0.1 mM EDTA supplemented with 33.3 mM DTT, 270 μM CoA, and 500 μM ATP), and after injection of 0.5 mM luciferin into the reaction mixture, they were assayed for luciferase activity with a Monolight 2010 Luminometer (Analytical Luminescence Laboratory, San Diego, CA).
GUS activity was assayed in a 100-μL reaction mixture, as described elsewhere (Gallie et al., 1989), with 1 mM 4-methylumbelliferyl-β-d-glucuronide as the substrate. After incubation for 30 min at 37°C, the reaction was terminated by addition of 900 μL of 0.2 M NaCO2. The amount of the fluorescent product in each assay was measured with a TKO 100 fluorometer (Hoefer Scientific Inc., San Francisco, CA), using excitation at 365 nm and emission at 455 nm.
RNA Gel Blot Analysis
Total RNA was extracted from protoplasts into which DNA or RNA constructs had been introduced, as described elsewhere (Chomczynski and Sacchi, 1987). For experiments to determine physical mRNA half-life, poly(A)+ mRNA was isolated by using binding to oligo dT resin. The RNA was displayed on a denaturing formaldehyde–agarose gel, transferred to nylon membrane, and probed with in vitro–synthesized, radiolabeled anti-luc or anti-uidA RNA. For wheat germ and rabbit reticulocyte lysate programmed with in vitro–synthesized mRNA, equivalent aliquots were loaded on a denaturing gel and processed similarly.
Acknowledgments
This work was supported by Grant Nos. NRICGP 96-35301-3144,A1 and 98-35100-6150 from the U.S. Department of Agriculture to D.R.G. and National Science Foundation Grant No. NSF546157 to W.F.T. and L.F.D.
References
- Bovy, A., Van Den Berg, C., De Vrieze, G., Thompson, W.F., Weisbeek, P., and Smeekens, S. (1995). Light-regulated expression of the Arabidopsis thaliana ferredoxin gene requires sequences upstream and downstream of the transcription initiation site. Plant Mol. Biol. 27, 27–39. [DOI] [PubMed] [Google Scholar]
- Chomczynski, P., and Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 162, 156–159. [DOI] [PubMed] [Google Scholar]
- Dickey, L.F., Gallo-Meagher, M., and Thompson, W.F. (1992). Light regulatory sequences are located within the 5′ portion of the Fed-1 message sequence. EMBO J. 11, 2311–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey, L.F., Nguyen, T.-T., Allen, G.C., and Thompson, W.F. (1994). Light modulation of ferredoxin mRNA abundance requires an open reading frame. Plant Cell 6, 1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey, L.F., Petracek, M.E., Nguyen, T.-T., Hansen, E.R., and Thompson, W.F. (1998). Light regulation of Fed-1 mRNA requires an element in the 5′ untranslated region and correlates with differential polyribosome association. Plant Cell 10, 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson Day, M.J., Ashurst, J.L., Mathias, S.F., Watts, J.W., Wilson, T.M.A., and Dixon, R.A. (1993). Plant viral leaders influence expression of a reporter gene in tobacco. Plant Mol. Biol. 23, 97–109. [DOI] [PubMed] [Google Scholar]
- Elliott, R., Pedersen, T.J., Fristensky, B., White, M.J., Dickey, L.F., and Thompson, W.F. (1989). Characterization of a single copy gene encoding ferredoxin 1 from pea. Plant Cell 1, 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett, J.G., and Gallie, D.R. (1992). RNA delivery in Saccharomyces cerevisiae using electroporation. Yeast 8, 1007–1014. [DOI] [PubMed] [Google Scholar]
- Gallie, D.R., and Walbot, V. (1992). Identification of the motifs within the tobacco mosaic virus 5′-leader responsible for enhancing translation. Nucleic Acids Res. 20, 4631–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie, D.R., Sleat, D.E., Watts, J.W., Turner, P.C., and Wilson, T.M.A. (1987. a). The 5′-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res. 15, 3257–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie, D.R., Sleat, D.E., Watts, J.W., Turner, P.C., and Wilson, T.M.A. (1987. b). A comparison of eukaryotic viral 5′-leader sequences as enhancers of mRNA expression in vivo. Nucleic Acids Res. 15, 8693–8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie, D.R., Lucas, W.J., and Walbot, V. (1989). Visualizing mRNA expression in plant protoplasts: Factors influencing efficient mRNA uptake and translation. Plant Cell 1, 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie, D.R., Caldwell, C., and Pitto, L. (1995). Heat shock disrupts cap and poly(A) tail function during translation and increases mRNA stability of introduced reporter mRNA. Plant Physiol. 108, 1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, D., St. Jean, A., Woods, R.A., and Schiestl, R.H. (1992). Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20, 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, J., Donald, K.A., and Griffiths, D.E. (1991). DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 19, 5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key, J.L., Lin, C.Y., and Chen, Y.M. (1981). Heat shock proteins of higher plants. Proc. Natl. Acad. Sci. USA 78, 3526–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathers, V., Tanguay, R., Kobayashi, M., and Gallie, D.R. (1993). A phylogenetically conserved sequence within viral 3′ untranslated RNA pseudoknots regulates translation. Mol. Cell. Biol. 13, 5331–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.-R.J., Nagao, R.T., and Key, J.L. (1994). A soybean 101-kD heat shock protein complements a yeast HSP104 deletion mutant in acquiring thermotolerance. Plant Cell 6, 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. [Google Scholar]
- Petracek, M.E., Dickey, L.F., Nguyen, T.-T., Gatz, C., Sowinski, D.A., Allen, G.C., and Thompson, W.F. (1998). Ferredoxin-1 mRNA is destabilized by changes in photosynthetic electron transport. Proc. Natl. Acad. Sci. USA 95, 9009–9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracek, M.E., Nguyen, T., Thompson, W.F., and Dickey, L.F. (2000). Premature termination codons destabilize ferredoxin-1 mRNA when ferredoxin-1 is translated. Plant J. 21, 563–570. [DOI] [PubMed] [Google Scholar]
- Pitto, L., Gallie, D.R., and Walbot, V. (1992). Functional analysis of sequence required for post-transcriptional regulation of the maize HSP70 gene in monocots and dicots. Plant Physiol. 100, 1827–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, Y., and Lindquist, S. (1990). HSP104 required for induced thermotolerance. Science 248, 1112–1115. [DOI] [PubMed] [Google Scholar]
- Schirmer, E.C., Lindquist, S., and Vierling, E. (1994). An Arabidopsis heat shock protein complements a thermotolerance defect in yeast. Plant Cell 6, 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla, S.L., Pareek, A., and Grover, A. (1997). Yeast HSP104 homologue rice HSP110 is developmentally- and stress-regulated. Plant Sci. 125, 211–219. [Google Scholar]
- Tanguay, R.L., and Gallie, D.R. (1996). Isolation and characterization of the 102-kilodalton RNA-binding protein that binds to the 5′ and 3′ translational enhancers of tobacco mosaic virus RNA. J. Biol. Chem. 271, 14316–14322. [DOI] [PubMed] [Google Scholar]
- Vorst, O., van Dam, F., Weisbeek, P., and Smeekens, S. (1993). Light-regulated expression of the Arabidopsis thaliana ferredoxin A gene involves both transcriptional and post-transcriptional processes. Plant J. 3, 793–803. [DOI] [PubMed] [Google Scholar]
- Wells, D.R., Tanguay, R.L., Le, H., and Gallie, D.R. (1998). HSP101 functions as a specific translational regulatory protein whose activity is regulated by nutrient status. Genes Dev. 12, 3236–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisraeli, J.K., and Melton, D.A. (1989). Synthesis of long capped transcripts in vitro by SP6 and T7 RNA polymerases. Methods Enzymol. 180, 42–50. [DOI] [PubMed] [Google Scholar]