Abstract
Magnaporthe grisea produces an infection structure called an appressorium, which is used to breach the plant cuticle by mechanical force. Appressoria generate hydrostatic turgor by accumulating molar concentrations of glycerol. To investigate the genetic control and biochemical mechanism for turgor generation, we assayed glycerol biosynthetic enzymes during appressorium development, and the movement of storage reserves was monitored in developmental mutants. Enzymatic activities for glycerol generation from carbohydrate sources were present in appressoria but did not increase during development. In contrast, triacylglycerol lipase activity increased during appressorium maturation. Rapid glycogen degradation occurred during conidial germination, followed by accumulation in incipient appressoria and dissolution before turgor generation. Lipid droplets also moved to the incipient appressorium and coalesced into a central vacuole before degrading at the onset of turgor generation. Glycogen and lipid mobilization did not occur in a Δpmk1 mutant, which lacked the mitogen-activated protein kinase (MAPK) required for appressorium differentiation, and was retarded markedly in a ΔcpkA mutant, which lacks the catalytic subunit of cAMP-dependent protein kinase A (PKA). Glycogen and lipid degradation were very rapid in a Δmac1 sum1-99 mutant, which carries a mutation in the regulatory subunit of PKA, occurring before appressorium morphogenesis was complete. Mass transfer of storage carbohydrate and lipid reserves to the appressorium therefore occurs under control of the PMK1 MAPK pathway. Turgor generation then proceeds by compartmentalization and rapid degradation of lipid and glycogen reserves under control of the CPKA/SUM1-encoded PKA holoenzyme.
INTRODUCTION
The first barrier to penetration and proliferation of pathogenic microorganisms is the plant cuticle. To breach the plant cuticle, fungi can either utilize coordinately regulated cell wall–degrading enzymes (Walton, 1994; Tonukari et al., 2000) or generate enormous invasive forces to break the cuticle mechanically (Bechinger et al., 1999). The rice blast fungus Magnaporthe grisea is amenable to genetic analysis and has therefore emerged as an excellent model for investigating plant infection by fungi (Talbot, 1995; Howard and Valent, 1996). M. grisea causes a serious disease of cultivated rice and infects rice leaves by producing a specialized structure called an appressorium. The appressorium, which is melanin-pigmented in M. grisea, is a dome-shaped cell that differentiates from the end of a short germ tube after the attachment and germination of a spore on the leaf surface. Penetration of the rice leaf results primarily from the exertion of mechanical force against the plant cuticle. The appressorium generates enormous cellular turgor, estimated to be as much as 8 MPa, which allows the fungus to send a narrow penetration peg through the rice leaf cuticle (Howard et al., 1991). How such high pressure can be generated within a living cell and then translated into mechanical force is an intriguing problem. Glycerol accumulates in the appressorium to very high concentrations, >3.0 M, and is believed to generate hydrostatic pressure by drawing water into the cell (de Jong et al., 1997; Money, 1997). Glycerol is retained in the appressorium because the melanin layer in the appressorium wall retards its efflux during turgor generation (de Jong et al., 1997). Melanin-deficient mutants therefore do not generate appressorial turgor and are nonpathogenic (Howard and Ferrari, 1989; Chumley and Valent, 1990).
How glycerol is synthesized in such large amounts in the M. grisea appressorium is not known, but the process must involve extensive mobilization of storage reserves from the conidium and de novo synthesis of the polyol in the appressorium. Appressoria form in water on the leaf surface and do not require exogenous nutrients to function. The source of glycerol is therefore present in the spore before it germinates on the leaf surface. The genetic means by which turgor is controlled in M. grisea appressoria also remains unclear. Recently, we showed that the conserved eukaryotic mechanism for control of cellular turgor, the high-osmolarity glycerol pathway, operates in M. grisea but does not regulate appressorium turgor (Dixon et al., 1999). A mitogen-activated protein kinase (MAPK)–encoding gene, OSM1, was identified that could complement yeast Δhog1 mutants and was required for mycelial growth under hyperosmotic conditions. M. grisea Δosm1 mutants were fully pathogenic, however, and generated the same turgor pressure as an isogenic wild-type strain (Dixon et al., 1999). The genetic control of appressorium turgor is therefore mediated by some other mechanism, perhaps involving regulatory components that already have been shown to be required for initial development of appressoria.
The formation of appressoria by M. grisea involves two distinct developmental phases. The first is a recognition phase. The conidium germinates to produce a short germ tube, which then detects signals that induce appressorium morphogenesis (Bourett and Howard, 1990; Howard and Valent, 1996). The second maturation phase of development involves formation of the appressorium, cell wall differentiation, and deposition of the melanin layer that precedes turgor generation. The regulatory pathway for appressorium formation in M. grisea requires generation of a cAMP signal (Lee and Dean, 1993). A novel receptor protein has been identified that takes part in this process (DeZwaan et al., 1999), and a heterotrimeric G-protein is probably also involved in transmission of inductive signals (Liu and Dean, 1997). Generation of intracellular cAMP is catalyzed by the MAC1-encoded adenylate cyclase (Choi and Dean, 1997; Adachi and Hamer, 1998); Δmac1 mutants are unable to form appressoria and are nonpathogenic. The Δmac1 mutation is unstable in certain strain backgrounds, however, because of bypass suppressor mutations in the SUM1 gene that encodes the regulatory subunit of cAMP-dependent protein kinase A (PKA). The mutant Δmac1 sum1-99 shows cAMP-independent PKA activity because of a mutation in one of the cAMP binding domains of the subunit. This leads to partially constitutive cAMP signaling and restoration of appressorium development. Interestingly, Δmac1 sum1-99 mutants are still reduced in pathogenicity (Adachi and Hamer, 1998). PKA signaling may therefore be independently required for control of appressorium function in a manner that cannot be restored in Δmac1 sum1-99 mutants. Consistent with this, mutation of the CPKA gene—which encodes a catalytic subunit of PKA—affects appressorium function, producing small, nonfunctional infection cells (Mitchell and Dean, 1995; Xu et al., 1997).
Downstream of the cAMP signal for appressorium formation, a MAPK cascade operates. PMK1 encodes a MAPK that is functionally related to the FUS3 gene from budding yeast (Xu and Hamer, 1996). Δpmk1 mutants do not produce appressoria and are nonpathogenic even when introduced directly into plant tissue by injection. Furthermore, Δpmk1 mutants are not complemented by exogenous cAMP but do undergo the early hooking stages of appressorium formation in its presence. Components of this cAMP and MAPK signaling pathway appear to be conserved among diverse pathogenic fungi (Gao and Nuss, 1996; Kronstad, 1997; Lev et al., 1999; Takano et al., 2000).
In this study, we have investigated the genetic control and biochemical mechanism for appressorium turgor generation by M. grisea. Using a combination of cell biological and biochemical analyses, we have studied appressorium function and storage carbohydrate mobilization in regulatory mutants blocked at different stages of appressorium development. We present evidence indicating that transfer of storage carbohydrate and lipid reserves to the appressorium occurs under control of the PMK1 MAPK pathway. Appressorium turgor generation then proceeds and appears to depend on breakdown of lipid and glycogen under control of the CPKA/SUM1-encoded PKA.
RESULTS
Glycerol Biosynthesis in the Appressorium of M. grisea
The synthesis of glycerol in eukaryotic cells can occur via several routes. We set out to explore the potential pathway for glycerol synthesis in M. grisea appressoria by identifying enzymatic activities likely to be involved in its production. Glycerol production from carbohydrate sources in yeast involves glycerol-3-phosphate dehydrogenase (GPD; EC 1.1.1.8) activity. This enzyme catalyzes reduction of the glycolytic intermediate dihydroxyacetone phosphate to glycerol-3-phosphate in an NADH-dependent reaction (Ansell et al., 1997). GPD is then converted to glycerol by two specific glycerol-3-phosphatases encoded by the genes HOR1 and HOR2 (Hirayama et al., 1995; Norbeck et al., 1996). GPD exists in three forms in Saccharomyces cerevisiae. Two are cytosolic enzymes encoded by GPD1 and GPD2 (Ansell et al., 1997) and provide alternate routes for cytosolic glycerol production in yeast. The third GPD is found in the inner mitochondrial membrane and is encoded by the GUT2 gene. This enzyme catalyzes an FAD-dependent oxidation of glycerol-3-phosphate for subsequent metabolism through glycolysis (Rönnow and Kiellanbrandt, 1993). Glycerol can also be produced from dihydroxyacetone by an NADPH-dependent dihydroxyacetone reductase and from glyceraldehyde by an NADPH-dependent glyceraldehyde reductase (EC 1.1.1.77). In the filamentous fungus Aspergillus nidulans, both reactions are catalyzed by a single enzyme, an NADPH-dependent glycerol dehydrogenase (GD) (Redkar et al., 1995); this enzyme may also exist in budding yeast (Norbeck and Blomberg, 1997).
We decided to assay each of the glycerol-generating enzyme activities in M. grisea. To do this, we first subjected M. grisea mycelium to hyperosmotic stress. Previously, we had shown that M. grisea responds to osmotic stress by accumulating a number of polyols, including glycerol (Dixon et al., 1999). We reasoned that enzymes involved in glycerol synthesis would therefore be readily detectable in this tissue. In parallel experiments, we also assayed each enzyme in protein extracts made directly from germinated conidia undergoing appressorium development. The results are shown in Table 1. We found that only a little GPD activity was present in M. grisea mycelium and that it did not alter in magnitude in response to hyperosmotic stress. Similar amounts of the enzyme were found in developing appressoria. Dihydroxyacetone reductase and glyceraldehyde reductase activities were also found in M. grisea mycelium and were induced (by approximately threefold) during hyperosmotic stress. In developing appressoria, both of these latter enzyme activities were present and remained active even after 48 hr, when appressoria were fully mature. We conclude that glycerol synthesis from carbohydrate sources is probably mediated in M. grisea appressoria not only by NADH-dependent GPD activity but also by NADPH-dependent reduction of dihydroxyacetone and glyceraldehyde.
Table 1.
Glycerol Biosynthetic Activities in Mycelium and Developing Appressoria of M. grisea
| Substrate | Specific Activity (mU mg−1 Protein)
|
Enzyme Activity | ||||
|---|---|---|---|---|---|---|
| Myceliuma
|
Developing Appressoriab
|
|||||
| CM | OS (24 hr) | OS (48 hr) | 24 hr | 48 hr | ||
| DHAP + NADH | 5.71 ± 2.14 | 4.93 ± 1.7 | 6.60 ± 4.3 | 6.13 ± 2.33 | 5.77 ± 2.54 | NAD-dependent glycerol-3-phosphate dehydrogenase |
| DHA + NADPH | 9.20 ± 4.3 | 34.00 ± 4.3 | 21.90 ± 5.63 | 9.53 ± 3.27 | 11.20 ± 4.13 | NADPH-dependent dihydroxyacetone reductasec |
| GAD + NADPH | 18.70 ± 6.22 | 50.00 ± 9.67 | 32.40 ± 9.85 | 15.40 ± 4.88 | 13.30 ± 6.67 | NADPH-dependent glyceraldehyde reductasec |
M. grisea mycelium was grown in complete medium and subjected to hyperosmotic stress (OS) by transfer to 0.4 M NaCl for 24 or 48 hr. Total protein was extracted and enzymatic assays were performed as described in Methods.
Conidia were allowed to germinate in hydrophobic plastic Petri dishes and form appressoria. Total protein was extracted at 24- and 48-hr time points.
In A. nidulans, both dihydroxyacetone reductase and glyceraldehyde reductase activities are the result of the action of a single enzyme NADP-dependent glycerol dehydrogenase (Redkar et al., 1995).
CM, complete medium; DHA, dihydroxyacetone; DHAP, dihydroxyacetone phosphate; GAD, glyceraldehyde.
Glycogen Degradation Occurs before Appressorium Turgor Generation
Having established that biosynthetic activities for glycerol generation were present in appressoria, we decided to investigate the likely carbohydrate sources for glycerol production. M. grisea conidia contain several storage carbohydrates, including mannitol, trehalose, and glycogen (Dixon et al., 1999). Previous ultrastructural studies of M. grisea have shown that glycogen rosettes are abundant in the appressorial cytoplasm (Bourett and Howard, 1990), but their numbers decrease rapidly during cell maturation. We therefore examined the cellular distribution of glycogen during appressorium formation, as shown in Figure 1. In the wild-type M. grisea isolate Guy11, glycogen was present in abundance in conidia but was rapidly degraded during germination of spores on a hydrophobic surface. By 4 hr after germination, glycogen was largely absent from the conidium, germ tube, and incipient appressorium. During development of appressoria, glycogen was deposited within the appressorium and then rapidly degraded at the onset of melanization. The cellular localization and mobilization of glycogen are therefore consistent with a role for the carbohydrate in appressorium function. Consequently, we decided to examine the distribution of glycogen in regulatory mutants of M. grisea that are known to affect the formation and function of appressoria. The mutants selected allowed us to assess the relative importance of the cAMP signaling pathway and MAPK cascades—previously implicated in appressorium morphogenesis—in carbohydrate reserve mobilization. Full descriptions of all mutant strains used in the study are given in Table 2. Conidia from the Δpmk1 MAPK mutant nn95, the ΔcpkA mutant DH51, and the Δmac1 sum1-99 mutant DA-99 were incubated on hydrophobic surfaces to allow infection-related development and then were examined cytologically for glycogen deposition. The results are shown in Figure 2; a quantitative analysis of our observations is given in Table 3. Glycogen was abundant in conidia of the Δpmk1 mutant nn95 but was not degraded effectively during germination. After 8 hr, large amounts of glycogen were still present in conidia (Figure 2A), and even after 12 hr, 85.3 ± 4.7% of conidia contained glycogen compared with 2.0 ± 1.0% of conidia from the isogenic wild-type strain Guy11 (Table 3). Glycogen degradation during germination and subsequent deposition in differentiating germ tubes therefore do not occur in the absence of the PMK1-encoded MAPK.
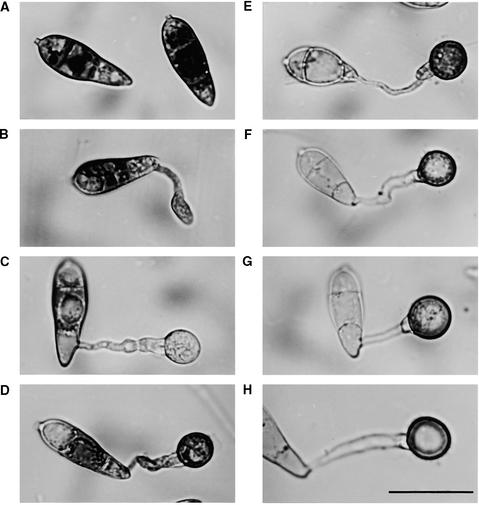
Figure 1.
Cellular Distribution of Glycogen during Appressorium Morphogenesis by M. grisea.
Conidia from the wild-type M. grisea strain Guy11 were allowed to germinate in water on hydrophobic plastic cover slips (see Methods) and form appressoria. Sample cover slips were removed every 2 hr and incubated in a glycogen staining solution containing 60 mg of KI and 10 mg of I2 per milliliter of distilled water (Weber et al., 1998). Yellowish-brown glycogen deposits became visible immediately in bright-field optics with a Nikon Optiphot-2 microscope.
(A) to (H) Dense deposits of glycogen were visible in ungerminated conidia (0 hr) (A) but were greatly reduced in the germinating conidial cell and its germ tube after 2 hr (B) and in the nascent appressorium after 4 hr (C). After formation of the basal septum, iodine staining reappeared for a time after 6 (D) and 8 hr (E). Glycogen disappeared again during appressorium maturation after 12 (F), 24 (G), and 48 hr (H).
Note that the process of appressorium formation in M. grisea occurs considerably faster on plastic cover slips than on onion epidermis (see Figure 4). Bar in (H) = 20 μm for (A) to (H).
Table 2.
M. grisea Strains Used in This Study
| Strain | Genotype | Mutant Phenotype | Reference |
|---|---|---|---|
| Guy11 | Wild-type rice pathogen | Silué et al., 1992 | |
| nn95 | Δpmk1 | Unable to elaborate appressoria. Non-pathogenic, even when introduced into plant tissue by infiltration. PMK1 encodes a MAP kinase that can act as a functional homolog of FUS3 from budding yeast |
Xu and Hamer, 1996 |
| DF51 | ΔcpkA | Non-pathogenic. Makes small non-functional appressoria. CPKA encodes a catalytic subunit of cAMP-dependent protein kinase A. |
Mitchell and Dean, 1995 Xu et al., 1997 |
| DA-99 | Δmac1 sum1-99 | Bypass suppressor of Δmac1 adenylate cyclase mutant. Restores appressorium formation, but not full pathogenicity. Carries mutation in a cAMP-binding site of SUM1-encoded regulatory subunit of PKA |
Adachi and Hamer, 1998 |
| 6-R-10 | buf1 | Melanin biosynthesis mutant. Unable to penetrate rice cuticle. BUF1 encodes polyhydroxynaphthalene reductase |
Chumley and Valent, 1990 Howard and Valent, 1996 |
Figure 2.
Cellular Distribution of Glycogen in Developmental Mutants of M. grisea.
Conidia from the regulatory mutants of M. grisea were allowed to germinate in water on hydrophobic plastic cover slips and form appressoria. Sample cover slips were removed every 2 hr and stained for the presence of glycogen (see Methods and legend to Figure 1). Conidia and developing appressoria are shown at 0 and 8 hr.
(A) and (D) Δpmk1 mutant nn95.
(B) and (E) ΔcpkA mutant DF51.
(C) and (F) Δmac1 sum1-99 mutant DA-99.
The mobilization of glycogen was severely impaired in the Δpmk1 mutant with substantial amounts of glycogen still present in conidia after 8 hr. In the ΔcpkA mutant, glycogen mobilization was retarded and glycogen was not accumulated in the developing appressorium after 8 hr. The Δmac1 sum1-99 mutant produced conidia with depleted glycogen contents, particularly when harvested from cultures >10 days old. Glycogen degradation in the appressorium occurred more rapidly than in Guy11 and was complete in 8 to 10 hr. Bar in (F) = 10 μm for (A) to (F).
Table 3.
Translocation of Glycogen from Conidia during Infection-Related Development by M. grisea
| Strain | Time | Conidia Containing Glycogena (%) |
|---|---|---|
| Guy11 | 0 | 95.33 ± 2.08 |
| 2 | 90.67 ± 5.13 | |
| 4 | 83.00 ± 3.61 | |
| 6 | 76.67 ± 9.29 | |
| 8 | 25.67 ± 9.64 | |
| 12 | 2.00 ± 1.00 | |
| Δmac-1 sum1-99 (DA-99) | 0 | 87.67 ± 3.51 |
| 2 | 85.67 ± 5.51 | |
| 4 | 24.67 ± 12.50 | |
| 6 | 5.00 ± 2.65 | |
| 8 | 2.33 ± 0.58 | |
| 12 | 1.33 ± 1.53 | |
| ΔcpkA (DF51) | 0 | 94.00 ± 2.65 |
| 2 | 92.67 ± 4.62 | |
| 4 | 87.00 ± 4.36 | |
| 6 | 73.00 ± 10.54 | |
| 8 | 47.67 ± 12.06 | |
| 12 | 17.00 ± 9.17 | |
| Δpmk1 (nn95) | 0 | 93.00 ± 5.00 |
| 2 | 90.33 ± 1.53 | |
| 4 | 88.33 ± 3.79 | |
| 6 | 89.00 ± 3.46 | |
| 8 | 86.00 ± 4.58 | |
| 12 | 85.33 ± 4.73 |
Conidia were allowed to germinate on hydrophobic plastic cover slips and form appressoria. Samples were removed at intervals and stained for the presence of glycogen, as described in Methods. The value recorded is the mean ±sd from observing a sample of 300 terminating conidia each in three independent replications of the experiments.
The role of the cAMP signaling pathway was examined by observing conidia from the ΔcpkA mutant DH51 and the Δmac1 sum1-99 mutant DA-99. In the ΔcpkA mutant DH51, glycogen degradation during germination was delayed (Figure 2B), and 47.7% ± 12.1% of conidia contained glycogen after 8 hr compared with 25.7% ± 9.6% from Guy11. The strains are not isogenic, but similar results were found when a 4091-5-8 wild-type strain of M. grisea (isogenic to DF51) was examined (data not shown). In the Δmac1 sum1-99 mutant DA-99, glycogen degradation during germination was extremely rapid, and conidia were often depleted of glycogen even at the initial harvest. Glycogen degradation was effectively complete in conidia from DA-99 after 6 hr (only 5.0% ± 2.6% of conidia retained any glycogen at this time). The catalytic subunit of PKA is thus required for effective glycogen degradation during conidial germination and appressorium maturation. In contrast, activating this holoenzyme by mutation of the regulatory subunit accelerates glycogen degradation so that it precedes completion of appressorium formation.
Triacylglycerol Lipase Activity Is Induced during Appressorium Maturation
Our cytological studies clearly implicated glycogen breakdown in glycerol synthesis, but we were surprised that GPD and GD enzyme activities were not more markedly induced during appressorium maturation. We therefore decided to investigate alternative sources for glycerol synthesis that might be used in addition to glycogen. The most efficient mechanism for producing glycerol is from triacylglycerol because that allows ATP generation by fatty acid oxidation after lipolysis; moreover, whether glycerol could be produced solely from carbohydrate sources has been questioned (Davis et al., 2000). To determine whether triacylglycerol is a potential source for appressorial glycerol, we first assayed protein extracts from developing appressoria for the presence of triacylglycerol lipase (EC 3.1.1.3) activity. Such activity was abundant in developing appressoria, as shown in Figure 3A. Importantly, the triacylglycerol lipase activity was rapidly induced at the onset of appressorium formation and remained high throughout maturation and generation of turgor.
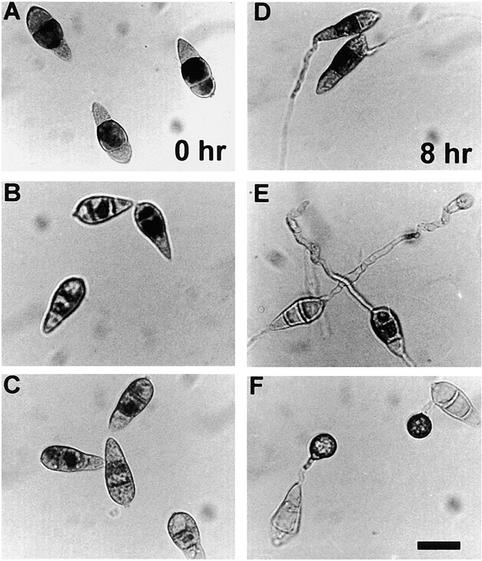
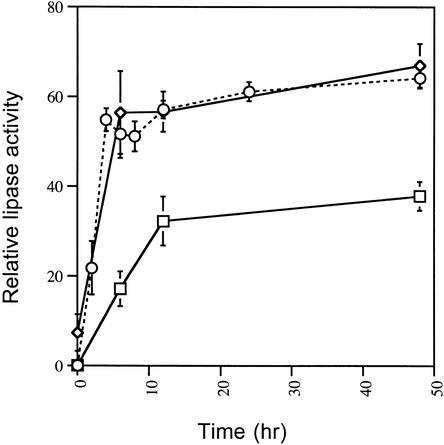
Figure 3.
Triacylglycerol Lipase Activity in Conidia and Developing Appressoria of M. grisea.
Conidia were allowed to form appressoria on the hydrophobic surface of Petri dishes, and protein was extracted as described in Methods. Triacylglycerol lipase activity was assayed by measuring the liberation of oleic acid from the substrate triolein, which was determined by densitometric analysis of the liberated oleic acid resolved by thin-layer chromatography. Enzymatic activity is expressed as relative lipase activity compared with the activity expressed by 2.5 units of purified triacylglycerol lipase from Rhizopus arrhizus (Roche Molecular Biochemicals). Each data point is the mean activity determined from three independent replications of the experiment. The error bars show the standard deviation. Triacylglycerol lipase activity is shown from the wild-type M. grisea strain Guy11 (diamonds), from the ΔcpkA mutant DF51 (squares), and the Δmac1 sum1-99 mutant DA-99 (circles with dotted line).
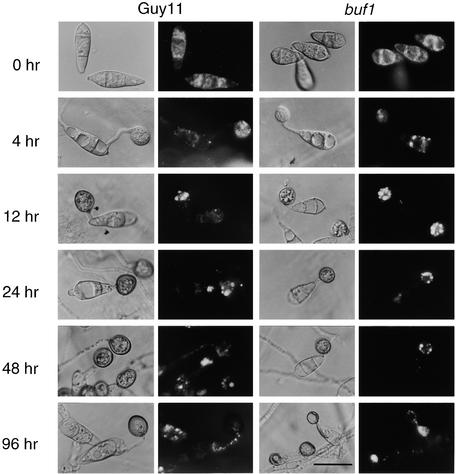
To understand the potential role of triacylglycerol stores in appressorium function, we examined the distribution of lipid bodies in infection structures by Nile Red staining and epifluorescence microscopy. Lipid droplets were abundant in conidia of the wild-type M. grisea strain Guy11, as shown in Figure 4. Importantly, their abundance was identical whether the conidia were harvested from plants or from plate cultures, indicating that their presence was not merely a consequence of artificial axenic culture on rich media (data not shown). We examined both a Guy11 strain and a buf1 mutant, 6-R-10 (Chumley and Valent, 1990; Romao and Hamer, 1992), by microscopy. The buf1 mutant was added because the presence of lipid was sometimes masked by the thick melanin layer in the appressorium wall of wild-type M. grisea. Buf1 mutants appear to synthesize glycerol normally but do not develop turgor because they lack this melanin layer, which normally retards the efflux of appressorial glycerol (Howard et al., 1991; de Jong et al., 1997; Money, 1997). Conidia from either Guy11 or the buf1 mutant were inoculated onto onion epidermal strips, and appressoria were allowed to form. The plant surface was used because the appressoria can penetrate it readily, which allows the process of appressorium-mediated infection to be viewed to completion (Chida and Sisler, 1987). In both Guy11 and the buf1 mutant, lipid droplets were translocated into the nascent appressorium within 4 hr. When this process was complete, the appressorial cytoplasm became isolated from that of the germ tube by the formation of a basal septum (Figure 4, 12 hr). Soon afterward, the lipid droplets within the developing appressorium began to enlarge. At the onset of appressorium formation, large lipid deposits were observed within appressoria, where they coalesced and were taken up into large vacuoles. Lipid degradation then occurred rapidly during appressorium maturation, and fully melanized appressoria (formed 24 to 48 hr after germination) were almost entirely devoid of lipid droplets.
Figure 4.
Cellular Distribution of Lipid Droplets during Appressorium Morphogenesis by M. grisea.
Conidia were allowed to germinate on onion epidermis in water drops and form appressoria. Sample preparations were removed at various intervals during a 96-hr period and stained for the presence of triacylglycerol by using Nile Red, as described in Methods. Photographs were taken at 0, 4, 12, 24, 48, and 96 hr after inoculation; for each time, a Hoffman modulation contrast image (left panel) and an epifluorescence image of Nile Red–stained material (right panel) are presented. Shown are the results for preparations of the wild-type strain Guy 11 and the buf1 mutant 6-R-10. Because melanin masked the epifluorescence somewhat, a nonmelanized mutant was examined for better quality resolution of lipid bodies. In both strains, numerous small lipid droplets were seen in ungerminated conidia (0 hr) and in nascent appressoria (4 hr), whereas later (12, 24, and 48 hr), fewer but larger lipid droplets were observed coalescing into vacuolar structures. Lipid bodies were degraded at the onset of turgor generation and cuticle penetration (24 to 96 hr after inoculation). Bar = 20 μm for all images.
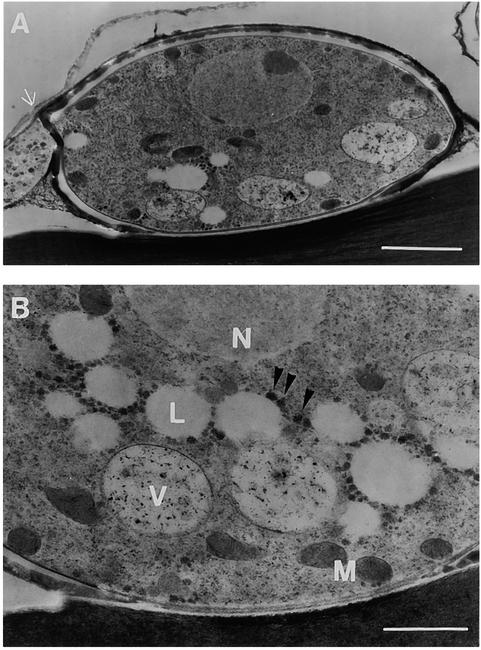
The movement of lipid bodies during appressorium maturation was confirmed by ultrastructural analysis (Figure 5). Appressorium sections were prepared by freeze-substitution fixation 24 hr after inoculation, by which time the appressoria had formed their basal septum and begun to deposit an electron-dense melanin layer (Figure 5A). The cytoplasm of maturing appressoria contained several small vacuoles, lipid droplets, and numerous glycogen granules in addition to other organelles, such as mitochondria (Figure 5B). Lipid droplets were typically electron translucent, presumably from leaching of lipid material during the prolonged substitution phase (Howard and Aist, 1979). Glycogen granules (Bourett and Howard, 1990) were often located close to the lipid droplets (Figure 5).
Figure 5.
Ultrastructure of an Appressorium of M. grisea.
Appressoria from the wild-type strain Guy11 were fixed by freeze-substitution 24 hr after inoculation of conidia onto onion epidermis.
(A) Median section of a whole appressorium formed on the surface of onion epidermis. The basal septum (arrow) is visible.
(B) Ultrastructural detail of another appressorium. Several electron-light lipid droplets (L), dark crystalline glycogen granules (arrowheads), and vacuoles with heterogenous contents (V) are visible, in addition to a nucleus (N) and mitochondria (M).
Bar in (A) = 2 μm; bar in (B) = 1 μm.
PMK1-Dependent Mobilization and CPKA-Dependent Degradation of Triacylglycerol during Appressorium Development
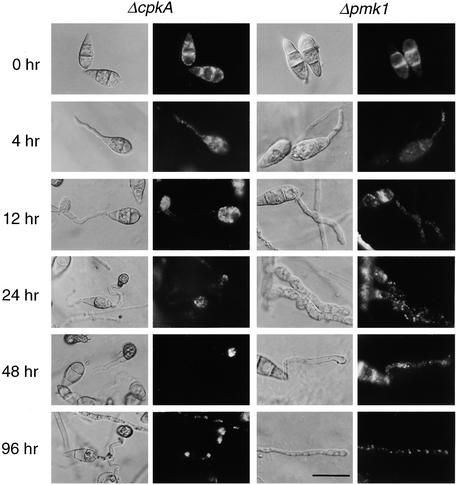
To determine whether lipid mobilization and degradation were subject to genetic control by the cAMP and MAPK signaling pathways, the translocation and degradation of lipid droplets were observed in each mutant background. The results are given in Figure 6, and a quantitative analysis of lipid translocation in each mutant is presented in Figure 7. The Δpmk1 mutant nn95 fails to form appressoria (Xu and Hamer, 1996) and instead forms an undifferentiated germ tube on onion epidermis. Its conidia contained amounts of lipid droplets similar to those of the isogenic wild-type strain Guy11 and these degraded slowly during germination. Lipid droplets translocated from the conidium more slowly than in Guy11 (Figure 6) and became evenly distributed throughout the conidium and germ tube. Within 48 hr, the germ tube tip of Δpmk1 mutants became highly vacuolated, indicating that apical extension growth had stalled. We conclude that the translocation of lipid droplets to the germ tube tip before appressorium differentiation cannot occur in the absence of the PMK1-encoded MAPK.
Figure 6.
Cellular Distribution of Lipid Droplets during Infection-Related Development by ΔcpkA and Δpmk1 Mutants of M. grisea.
Conidia were allowed to germinate on onion epidermis in water drops and were removed at intervals during a 96-hr period and stained with Nile Red for the presence of triacylglycerol (see Methods). Photographs were taken 0, 4, 12, 24, 48, and 96 hr after inoculation; for each time, a Hoffman modulation contrast image (left panel) and an epifluorescence image of Nile Red–stained material (right panel) are presented. In the ΔcpkA mutant DF51, lipid mobilization to the developing appressorium occurred as in wild-type strains of M. grisea except that appressorium formation was delayed until 12 hr after inoculation. The appressoria of ΔcpkA were small and sometimes misshapen. Lipid droplets enlarged during appressorium development (at 48 and 96 hr), but no degradation was observed during this experiment. In the Δpmk1 mutant nn95, no appressoria were formed; instead, lipid droplets were found evenly distributed into the developing germ tubes, which ultimately developed vacuolates (at 48 and 96 hr) and stopped growing. Bar = 20 μm for all images.
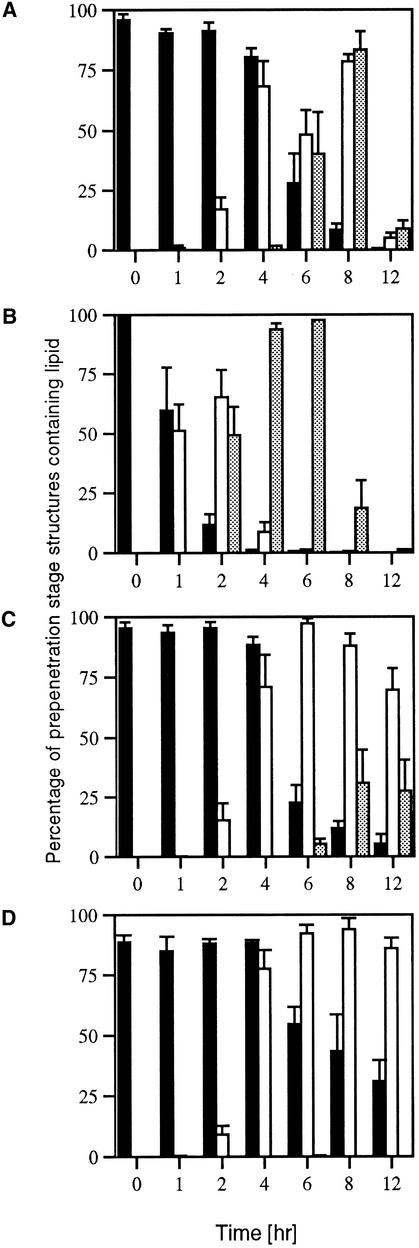
Figure 7.
Quantitative Analysis of Lipid Distribution during Infection-Related Development by M. grisea.
Conidia were allowed to germinate in water drops on the surface of onion epidermis and to undergo infection-related development. Samples were removed at intervals over a 12-hr period and stained for the presence of triacylglycerol by using Nile Red. The percentage of fungal structures that contained lipid bodies at a given time was recorded from a sample of 300 germinated conidia. The bar charts show the mean and standard deviation from three independent replications of the experiment. Solid black bars represent conidia, open bars represent germ tubes, and gray bars represent appressoria.
(A) Wild-type M. grisea strain Guy11.
(B) Δmac1 sum1-99 mutant DA-99.
(C) ΔcpkA mutant DF51.
(D) Δpmk1 mutant nn95.
The role of cAMP signaling in lipid metabolism was studied in two ways. First, we measured triacylglycerol lipase activity in appressoria from both the ΔcpkA mutant and the Δmac1 sum1-99 mutant. Triacylglycerol lipase activity in the ΔcpkA mutant DF51 was only 50% of that of the wild-type strain Guy11 (Figure 3B). The induction of enzyme activity was also delayed, such that maximal enzyme activity was not reached until 12 hr after inoculation, compared with 4 hr after inoculation in Guy11. In contrast, the Δmac1 sum1-99 mutant DA-99 showed some triacylglycerol lipase activity even in ungerminated conidia, and the enzyme was rapidly induced to the same amounts as in the wild-type Guy11 (Figure 3B). These contrasting effects on lipase induction were consistent with the distribution of lipid bodies in each mutant. In the Δcpka mutant, appressorium formation was delayed until 12 hr after inoculation (Figure 6), and the appressoria formed were smaller than those of Guy 11 (Mitchell and Dean, 1995; Xu et al., 1997). Lipid droplets translocated to the germ tube tip and the developing appressorium normally, but in contrast to Guy11, lipid degradation did not occur during maturation, and several large lipid droplets were observed in each appressorium even after 96 hr (Figure 6). The translocation of lipid and its deposition were substantially retarded in ΔcpkA compared with the wild type, as shown in Figure 7C. Less than 30% of ΔcpkA appressoria, for example, contained lipid after 8 hr, whereas >80% of those in Guy11 did (Figure 7).
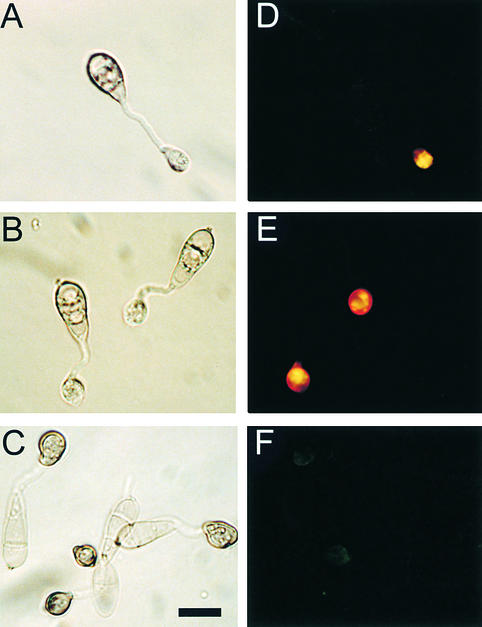
Lipid droplets in conidia of the Δmac1 sum1-99 mutant DA-99 rapidly translocated to the germ tube within 4 hr of germination (Figures 7B and 8A). Lipid droplets coalesced to form large lipid bodies and vacuolar structures within 8 hr (Figure 8B) and were completely degraded before melanization and maturation of the appressorium (Figure 8C). Lipid degradation in the Δmac1 sum1-99 mutant was uniformly completed before 12 hr after inoculation (Figure 7B). We conclude that the catalytic subunit of PKA is required for effective lipolysis during appressorium generation of turgor and that in a Δmac1 sum1-99 mutant, partially constitutive PKA activity results in completion of lipolysis before complete cellular differentiation.
Figure 8.
Cellular Distribution of Lipid Droplets during Infection-Related Development by the Δmac1 sum1-99 Mutant DA-99.
Conidia were allowed to germinate in water drops on the surface of plastic cover slips and undergo infection-related development. Samples were removed at intervals over a 12-hr period and stained for the presence of triacylglycerol with Nile Red; for each interval, a Hoffman modulation contrast image (left panel) and an epifluorescence image of Nile Red–stained material are presented.
(A) and (D) At 4 hr after inoculation of conidia, lipid droplets have already migrated to the incipient appressorium and begun to coalesce.
(B) and (E) At 8 hr after inoculation of conidia, lipid droplets are beginning to be degraded, before melanization of the appressorium.
(C) and (F) At 12 hr after inoculation of conidia, lipid droplets have been almost completely degraded.
Bar in (C) = 10 μm for (A) to (F).
Virulence of Δmac1 sum1-99 Is Related to the Age and Storage Reserves of Conidia
The regulatory PKA subunit mutant Δmac1 sum1-99 was originally isolated as a bypass suppressor of Δmac1 that restored its ability to make appressoria. The pathogenicity of Δmac1 sum1-99 mutants was not restored completely, however, thus indicating that the mutant was still defective in pathogenicity-related functions (Adachi and Hamer, 1998). In view of the marked effects of the Δmac1 sum1-99 mutations on glycogen and lipid mobilization, and because of the triacylglycerol lipase activity found in ungerminated conidia, we decided to analyze the pathogenicity phenotype of this mutant in more detail. Plate cultures of the Δmac1 sum1-99 mutant DA-99 were therefore produced, and conidia were harvested at various times after the initial inoculation. Conidial suspensions were adjusted to a uniform concentration of 104 conidia mL–1 and sprayed onto seedlings of the susceptible rice cultivar CO-39. Disease symptoms were scored after 96 hr; representative leaves are shown in Figure 9. The number of disease lesions per infected leaf decreased from 44.33 ± 7.37 for seedlings sprayed with conidia from 7-day-old cultures to 4.33 ± 1.53 when conidia from a 15-day-old plate were used (results are for a sample size of 40 plants and three independent experiments). The concentrations of glycogen and lipid in conidia were severely depleted in conidia from older cultures (the conidia shown in Figure 2C, for example, were taken from 12-day-old plate cultures), indicating that breakdown of reserve carbohydrates and lipid occurs prematurely in Δmac1 sum1-99, attenuating its ability to carry out appressorium-mediated infection.
Figure 9.
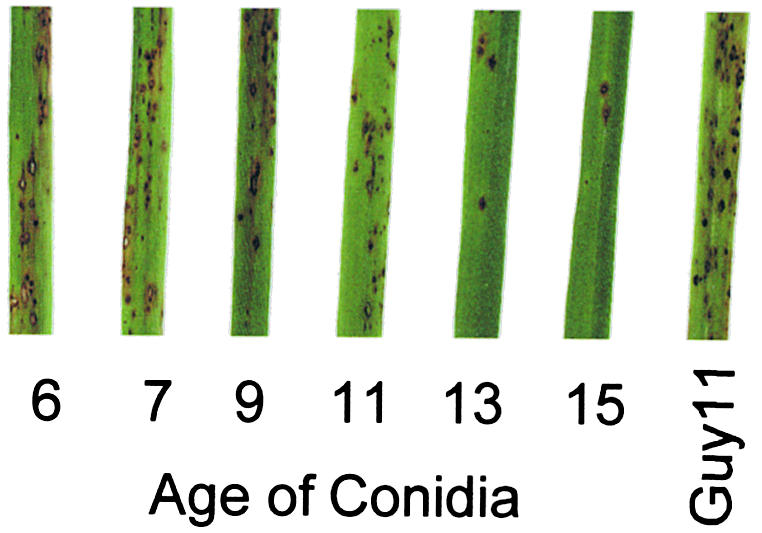
Pathogenicity of the Δmac1 sum1-99 Mutant DA-99 Is Related to the Age and Storage Reserve Status of Conidia.
Conidia from the Δmac1 sum1-99 mutant DA-99 were harvested from plate cultures that had been incubated for 6, 7, 9, 11, 13, and 15 days. Conidial suspensions were diluted to a uniform concentration of 104 mL–1 and sprayed onto 14-day-old rice seedlings of the blast-susceptible cultivar CO-39. The disease was allowed to progress for 96 hr, when disease lesions had not yet coalesced, and representative leaves were removed and photographed. An identical conidial suspension from the wild-type strain Guy11 was used in the positive control experiment. The severity of disease lesions decreased with increasing conidial age in the Δmac1 sum1-99 mutant.
DISCUSSION
Our aim in this investigation was to define the key enzymatic steps required for glycerol production in appressoria and to determine how the process of turgor generation is genetically controlled. The major enzymatic activity for glycerol accumulation in budding yeast during hyperosmotic stress is NADH-dependent GPD encoded by the GPD1 gene (Albertyn et al., 1994). GPD activity in yeast is also generated by activity of the GPD2 gene, which appears to be responsible for redox regulation in the cytosol (Ansell et al., 1997). The enzymatic conversion of dihydroxyacetone phosphate to glycerol-3-phosphate is coupled to the reoxidation of NADH and therefore provides a route for replenishing NAD+. GPD1 is transcriptionally activated during hyperosmotic stress and is under control of the high-osmolarity glycerol pathway (Albertyn et al., 1994; Gustin et al., 1998). We found GPD activity in M. grisea mycelium, but it was not induced by hyperosmotic stress. Instead, the activities of NADPH-dependent dihydroxyacetone reductase and glyceraldehyde reductase were increased, suggesting that glycerol production during hyperosmotic stress in M. grisea may be more similar mechanistically to the process in A. nidulans than that in yeast (Redkar et al., 1995). This is consistent with our previous findings that hyperosmotic stress in M. grisea leads predominantly to arabitol generation, which is regulated by the OSM1 MAPK, and OSM1-independent production of smaller amounts of glycerol (Dixon et al., 1999). In A. nidulans, dihydroxyacetone reductase and glyceraldehyde reductase activities are the result of a single NADPH-dependent GD (Schuurink et al., 1990). We found similar activities of both dihydroxyacetone reductase and glyceraldehyde reductase in crude protein extracts of M. grisea mycelium, indicating that a single enzyme might be present in the rice blast fungus. At this stage, however, we cannot preclude that our crude extract contains more than one ketose or aldose reductase enzyme capable of catalyzing these reactions, or that conditions for assaying the GPD2-type isoform of GPD (Ansell et al., 1997) were achieved in our assay. In appressorium extracts, GPD and both GD activities were present and sustained during appressorium turgor generation. Enzymatic activities for the generation of glycerol from carbohydrate sources are thus present in appressoria at amounts similar to those in mycelium. These enzyme activities are, however, also likely to be involved in glycerol production for lipid biosynthesis and membrane biogenesis and therefore might not be the principal route for generating the osmotically active glycerol required for turgor generation.
Triacylglycerol lipase activity was found in protein extracts taken from conidia undergoing appressorium differentiation and was induced markedly during their formation and maturation. Triacylglycerol lipases previously have been characterized in fungi for commercial purposes, but investigations have concentrated almost exclusively on extracellular lipases (Toida et al., 1998). The breakdown of fat is an efficient means of producing glycerol rapidly and would also lead to production of fatty acids for oxidation and ATP generation. Production of acetyl CoA from β-oxidation of fatty acids would provide another route for glycerol production by way of the glyoxylate cycle and gluconeogenesis, resulting in glycerol production catalyzed by GD or GPD. A pathogenicity gene PTH2 recently described encodes an M. grisea homolog of carnitine acetyltranferase, an enzyme required for activated fatty acids to traverse the mitochondrial membrane for oxidation (Sweigard et al., 1998). Perhaps the lack of pathogenicity of this mutant is due to an inability to process fatty acids generated by lipolysis during appressorium formation, resulting in either glycerol depletion or toxic accumulation of fatty acids. Another consequence of triacylglycerol degradation is the probable release of secondary messengers such as diacylglycerol. Diacylglycerol is known to stimulate appressorium formation in M. grisea on noninductive (hydrophilic) surfaces (Thines et al., 1997) and can also restore appressorium formation to a Δpth11 mutant (DeZwaan et al., 1999). The PTH11-encoded receptor is thought to react to surface hydrophobicity; generation of cAMP downstream of PTH11 therefore might stimulate not only morphogenesis but also lipid degradation and release of diacylglycerol to further stimulate cellular differentiation.
Glycerol production from solely carbohydrate or lipid sources is theoretically possible in the M. grisea appressorium, although whether sufficient reserves of each type are present is unclear and will await more precise quantification. Perhaps, however, both glycogen and lipid contribute to glycerol production, given the enormous concentrations required and the need to ensure redox balance within the cell. The potential for the glyoxylate cycle to contribute to glycerol production also highlights why lipase, GPD, and GD activities might all be present in the appressorium.
In our microscopy studies, transport and compartmentalization of glycogen and triacylglycerol to the developing appressorium were observed, followed by rapid dissolution of these reserves during cell maturation. Glycogen degraded rapidly during germination and thus is probably one of the principal energy sources during germ tube extension—in addition to the mannitol and trehalose also present within M. grisea conidia (Talbot, 1995; Dixon et al., 1999; A.J. Foster and N.J. Talbot, unpublished observations). Ultrastructural studies confirmed that glycogen was present as rosettes in the cytoplasm and disappeared rapidly preceding turgor generation, as previously reported (Bourett and Howard, 1990). Movement of lipid droplets to the developing appressorium, meanwhile, was also striking, the larger fat globules coalescing before being taken up for degradation into a large central vacuole. The site of lipid degradation corresponded to large vacuoles found within appressoria, which could be readily visualized with the vacuolar stain Neutral Red and the lytic marker enzyme acid phosphatase (R.W.S. Weber, E. Thines, and N.J. Talbot, unpublished observations). We think it likely, therefore, that lipolysis occurs within the central appressorial vacuole, which is prominent in mature appressoria that have elaborated penetration pegs (Bourett and Howard, 1990). Transport of lipid reserves and their vacuolar degradation have been reported in the maize pathogen Colletotrichum graminicola during conidial germination (Schadeck et al., 1998a, 1998b). Because C. graminicola uses a mechanical infection mechanism similar to that of M. grisea (Bechinger et al., 1999), perhaps the considerable invasive forces generated by appressoria of C. graminicola are also produced by a glycerol-dependent mechanism.
Genetic Regulation of Appressorium Turgor in M. grisea
Because mobilization of conidial glycogen and triacylglycerol during appressorium formation depended on the presence of the PMK1-encoded MAPK, we conclude that the mass transfer of these reserves is part of the appressorium differentiation process. The lack of glycogen degradation during conidial germination observed in a Δpmk1 mutant indicates that even the initial stages of germination are affected by the absence of PMK1, so that the developmental sequence for appressorium morphogenesis must be triggered early during germ tube extension.
The importance of cAMP signaling to appressorium function is clarified by the observation that dissolution of glycogen and triacylglycerol is retarded markedly in a ΔcpkA mutant that lacks a catalytic subunit of PKA. The regulation of lipases and glycogen-degrading enzymes by direct PKA phosphorylation is known in mammalian systems (Severson et al., 1981; Stralfors and Belfrage, 1983; Carling and Hardie, 1989; Palmer et al., 1990), and PKA is a central regulator of carbohydrate mobilization in yeast (Thevelein, 1994). In M. grisea, cAMP signaling apparently has been adapted to break down storage reserves within the correct cellular compartment and at the precise time required for the appressorium-mediated plant infection process, as shown in Figure 10. This implies that ΔcpkA mutants are nonpathogenic primarily because of insufficient or retarded turgor generation, which prevents cuticle penetration. To test this, incipient cytorrhysis experiments have measured appressorium turgor (Howard et al., 1991) in a ΔcpkA mutant; preliminary observations show a retardation and measurable reduction in turgor compared with an isogenic wild-type strain (P.V. Balhadère and N.J. Talbot, unpublished observations). The Δmac1 sum1-99 mutant has been useful in investigating the role of cAMP signaling in appressorium turgor. This mutant is a bypass suppressor of Δmac1 (Adachi and Hamer, 1998), the result of a mutation that changes a leucine to an arginine residue in one of the cAMP binding sites of the regulatory subunit of PKA. The result of this mutation is that PKA activity is partially constitutive; consistent with this, cAMP-independent PKA activity has been recorded in mycelial extracts (Adachi and Hamer, 1998). We found that glycogen breakdown and lipolysis were greatly accelerated in the Δmac1 sum1-99 mutant, indicating a PKA-mediated activation of glycogen-degrading enzymes (glycogen phosphorylase, for example) in the mutant in the absence of a cAMP signal. Similarly, triacylglycerol lipase activity was accelerated and could be found in conidia of the Δmac1 sum1-99 mutant even before germination. Adachi and Hamer (1998), however, reported that CPKA-encoded PKA activity could not be measured in conidial germlings of the Δmac1 sum1-99 mutant. They concluded that although the Δmac1 sum1-99 mutation leads to cAMP-independent PKA activity in mycelium, it may inhibit PKA activity in conidial germlings. The assay they used to measure PKA activity, however, utilized the synthetic PKA substrate kemptide, which raises the possibility that the lack of PKA activity measured in conidial germlings is the result of competitive inhibition of the assay by the natural substrates for CPKA-encoded PKA—which are likely to be present in abundance during appressorium development. We think that scenario more likely than an inhibition of PKA in this mutant, based on the converse effects on storage carbohydrates and lipids observed in the ΔcpkA and Δmac1 sum1-99 mutants. This does not alter the major conclusion of Adachi and Hamer (1998) that additional cAMP-Sum1–regulated activities are probably required for appressorium morphogenesis; instead, it proposes a role for the SUM1/CPKA-encoded PKA holoenzyme in appressorium function and an explanation for the reduced pathogenicity of Δmac1 sum1-99.
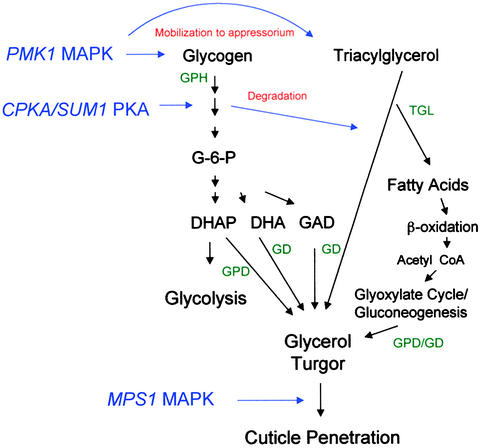
Figure 10.
Outline Model for the Genetic Control of Appressorium Turgor Generation by the Rice Blast Fungus M. grisea.
In this model, the mobilization of glycogen and triacylglycerol reserves to the developing appressorium occurs under control of the PMK1 MAPK pathway. Glycerol generation is achieved by degradation of triacylglycerol by triacylglycerol lipase, which is regulated by CPKA/SUM1-encoded PKA. Fatty acids are processed by β-oxidation and may be used to produce glycerol by way of the glyoxylate cycle and gluconeogenesis. Glycogen degradation, which is similarly regulated within the appressorium at the onset of turgor generation, is used to fuel glycolysis during glycerol accumulation and may contribute to glycerol production by the metabolism of dihydroxyacetone 3-phosphate (DHAP), dihydroxyacetone (DHA), or glyceraldehyde (GAD). Glycerol accumulation results in hydrostatic turgor and is translated into the force required for cuticle penetration by reorientation of the cytoskeleton, localized dissolution of the cell wall, and formation of penetration pegs. These processes are likely to be regulated by the action of PKA and stimulation of a signal transduction pathway involving the MPS1 MAPK (Xu et al., 1998). In the model shown, genetic control points are shown in blue and putative enzymatic activities are given in green. GPH, glycogen phosphorylase (EC 2.4.1.1); TGL, triacylglycerol lipase (EC 3.1.1.3); GPD, NADH-dependent glycerol-3-phosphate dehydrogenase (EC 1.1.1.8); GD, NADPH-dependent glycerol dehydrogenase (EC 1.1.1.77). Multiple arrows indicate several intermediate steps in pathway not shown.
We investigated the pathogenicity phenotype of the Δmac1 sum1-99 mutant after noticing the rapid lipolysis and glycogen breakdown in conidia. The triacylglycerol lipase activity in ungerminated conidia suggested to us that conidial age might affect the ability to complete appressorium-mediated infection in Δmac1 sum1-99 mutants. The reduced pathogenicity phenotype of the Δmac1 sum1-99 mutant DA-99 was identical to that previously reported by Adachi and Hamer (1998) when mature 12- to 14-day-old conidia were used in infections (∼40% of the normal numbers of lesions). But inoculating rice seedlings with younger conidia caused more severe disease symptoms, and conidia from 6-day-old plate cultures caused symptoms that were almost indistinguishable from the wild type. These results indicate that the correct spatial and temporal regulation of glycogen mobilization and lipolysis is essential for appressorium-mediated infection of rice plants by M. grisea. It also highlights the potential for investigating glycogen and lipid metabolic pathways for selecting new targets for disease control.
METHODS
Strains of Magnaporthe grisea
Wild-type and mutant strains of M. grisea are stored in the laboratory of N.J. Talbot at the University of Exeter. The Δcpka strain DF51, the Δpmk1 mutant nn95, and the Δmac1 sum1-99 mutant DA-99 were kindly donated by Dr. J.E. Hamer (Purdue University, West Lafayette, IN). Long-term storage of the strains was performed by growing the fungus through sterile filter-paper discs, followed by desiccation for 48 hr and storage at −20°C.
Assays for Infection-Related Morphogenesis and Pathogenicity
M. grisea conidia were obtained by harvesting plate cultures of the fungus grown on complete medium (Talbot et al., 1993) in a 14-hr-light and 10-hr-dark cycle at 24°C for 10 to 12 days. Conidia were harvested by scraping sporulating cultures with a glass rod in sterile distilled water, followed by centrifugation at 1000g for 10 min and resuspension in distilled water to a concentration of ∼105 spores μL−1. For biochemical studies, drops of this standard inoculum were applied to the bottom of plastic Petri dishes and incubated at 27°C for as long as 48 hr. Formation of abundant appressoria was stimulated under these conditions by surface hydrophobicity (Hamer et al., 1988; Lee and Dean, 1994). Similarly, for light microscopy, individual drops of standard inoculum were placed on plastic microscope cover slips (PGC Scientific, Frederick, MD) and incubated in a damp chamber at 24°C. Alternatively, individual drops of inoculum suspension were placed on 1-cm2 sections of adaxial onion epidermis excised from the outer fleshy leaves of onion bulbs and supported by a glass slide (Chida and Sisler, 1987). As before, incubation was at 24°C in a damp chamber, and samples were removed at intervals for observation. For pathogenicity assays, 14-day-old rice seedlings were infected with suspensions of M. grisea conidia prepared in 0.2% gelatin at a concentration of 104 conidia mL−1. Two-week-old seedlings of the susceptible rice cultivar CO-39 were sprayed with the suspension by using an artist's airbrush (Badger Co., Franklin Park, IL). Plants were incubated in plastic bags for 48 hr to maintain high humidity and then transferred to controlled environment chambers at 24°C and 84% relative humidity, with 900 μE m−2 sec−1 tungsten illumination and 14-hr light periods. Plants were incubated for 96 to 144 hr for full disease symptoms to become apparent. The first disease symptoms were observed 96 hr after seedling inoculation.
Preparation of Protein Extracts from M. grisea Appressoria
To extract protein from ungerminated conidia, we placed drops of conidial suspension at 105 conidia mL−1 directly into liquid nitrogen and ground them to a fine powder with a mortar and pestle. The material was resuspended in extraction buffer (final concentration 20 mM Tris-HCl, pH 7.5, and 2 mM EDTA), and an aliquot of protease inhibitor cocktail CompleteTM (Roche Diagnostics, Lewes, East Sussex, UK) was added according to the manufacturer's instructions. For protein extractions from developing appressoria, conidia were applied to the hydrophobic surface of Petri dishes and incubated at 24°C for as long as 48 hr. After the attached structures were washed three times with double-distilled water, liquid nitrogen was added to the Petri dishes, and the germinating conidia, germ tubes, and appressoria were harvested by scraping the surface of the Petri dish with a plastic cover slip. The harvested material was then transferred to a pestle and mortar and ground to a fine powder in the presence of liquid N2 before being resuspended in extraction buffer, as described above. Cellular debris was then removed by centrifugation at 13,000g for 15 min at 4°C. The protein concentration of extracts was determined by using a Pierce BCA protein assay kit (Pierce Chemical Co., Rockford, IL) with BSA as the standard.
Enzyme Assays
Glycerol-3-phosphate dehydrogenase (GPD) was assayed at room temperature by an adaptation of the method of Redkar et al. (1995). Briefly, the 1-mL reaction reagent contained 20 mM imidazole-HCl, pH 7.0, 1 mM DTT, 1 mM MgCl2, 0.67 mM dihydroxyacetone phosphate, and either 0.09 or 0.2 mM NADH. Glycerol dehydrogenase (GD) was assayed at room temperature in a 1-mL reaction reagent containing 50 mM glycine/NaOH, pH 9.6, 5 mM of either dihydroxyactone or dl-glyceraldehyde, and either 0.09 or 0.2 mM NADPH. Oxidation of NADH or NADPH was monitored with an Ultraspec 200 spectrophotometer (Pharmacia Biotechnology) at 340 nm for 2 min. Enzyme determinations were conducted with at least three protein concentrations, and the rate of product formation was shown to be proportional to the amount of enzyme added to the reaction. The reactions were performed at alkaline pH to avoid oxidation of NADH/NADPH by any oxidase present in crude appressorial extracts. An absorptivity of 6.3 × 103 M−1 cm−1 for NADH/NADPH was used in calculating the enzyme activity. One unit of enzyme is defined as the amount required to form 1 μmol of NAD+/NADP+ per minute.
Triacylglycerol lipase activity was analyzed by thin-layer chromatography (TLC), with triolein (1,2,3,-tri[cis-9-octadecanoyl] glycerol; Sigma) as a substrate. A 25-mg aliquot of triolein was added to 3 mL of sodium acetate buffer, pH 5.5, and 50 μg of appressorium protein extract. The mixture was incubated at 37°C for 30 min with constant stirring at 200 rpm. A 2-mL aliquot of cyclohexane (Sigma) was then added to the mixture, followed by another 5 min of incubation at 37°C. A 1-mL aliquot of the upper, organic phase was removed, dried under vacuum, and resuspended in 100 μL of methanol. Triacylglycerol lipase activity was monitored by measuring the liberation of oleic acid from the glyceryl trioleate substrate, as determined by the size of the oleic acid spots resolved by TLC. A 10-μL aliquot of the methanol suspension was applied to silica gel 60 F254 plates (Riedel de Haen, Seelze, Germany). The chromatograms were developed in hexane:ethyl acetate:acetic acid (80:20:1), and spots were visualized by incubation in I2 vapor. As a positive control, 1, 2.5, and 10 units of an extracellular lipase from the fungus Rhizopus arrhizus (Roche Molecular Biochemicals, Mannheim, Germany) were used in independent assays under identical reaction conditions. Densitometric analysis of TLC separations was performed by using the public domain NIH Image program developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/.
Cytological Analysis
Lipid droplets in germinating conidia and appressoria were visualized by staining with a Nile Red solution (Greenspan et al., 1985; Weber et al., 1999) consisting of 50 mM Tris/maleate buffer, pH 7.5, with 20 mg mL−1 polyvinylpyrrolidone and 2.5 μg mL−1 Nile Red Oxazone (9-diethylamino-5H-benzo[α]phenoxazine-5-one; Sigma). Cytological analysis was performed with freshly harvested conidia, conidia incubated on strips of onion epidermis, or conidia incubated on plastic cover slips. Guy11 conidia were also harvested directly from infected rice plants for analysis. In all cases, suitable material was mounted directly in the Nile Red staining solution. Within a few seconds, lipid droplets began to fluoresce when viewed with a Nikon Optiphot-2 microscope with the EFD-3 episcopic fluorescence attachment and the B-2A filter (excitation at 450 to 490 nm, 505 nm dichroic mirror, 520 nm barrier filter). No fluorescence was observed when Nile Red was omitted from the staining solution. Glycogen staining was achieved by mounting plastic cover slips with conidial inoculum directly in a staining solution consisting of 60 mg of KI and 10 mg of I2 per milliliter in distilled water (Weber et al., 1998). Yellowish-brown glycogen deposits became visible immediately in bright-field optics with a Nikon Optiphot-2 microscope. No blue starch deposits were observed in M. grisea, and no glycogen deposits were visible when I2 was omitted from the staining solution.
Transmission Electron Microscopy
For ultrastructural investigations by transmission electron microscopy, material was prepared by freeze-substitution fixation. Conidia of Guy11 were inoculated onto onion epidermis and allowed to form appressoria, as described above. After 24 hr, small squares (2 to 3 mm2) of epidermis bearing appressoria of M. grisea were frozen by plunging into Freon-23 cooled to −180°C with liquid nitrogen and then were transferred to the substitution fluid consisting of 1% (w/v) OsO4 in anhydrous acetone (Howard and O'Donnell, 1987). The samples were incubated in the substitution fluid for 48 hr at −80°C and then for 1 hr each at −20°C and 0°C, followed by three washes in anhydrous acetone at room temperature and infiltration in Spurr's resin (Spurr, 1969) for 24 hr each in four- and twofold dilutions in acetone and in pure resin. Samples were then polymerized by heating to 70°C for 7 hr. Thin sections were contrasted for 10 min in 1% (w/v) uranyl acetate in ethanol and for 3 min in lead citrate (Reynolds, 1963) and were viewed at 80 kV with a transmission electron microscope (model JFM 100-S; JEOL, Tokyo, Japan).
Acknowledgments
We thank Gavin E. Wakley for advice regarding electron microscopy and Dr. Nick Smirnoff for critically reading the manuscript. This work was supported by grants to N.J.T. from the Biotechnology and Biological Sciences Research Council (BBSRC; Grant No. 9/P08629) and the European Union Cereal Pathogens Network (CEREPAT). Work on rice blast in N.J.T.'s laboratory is authorized by the Plant Health division of the Ministry of Agriculture, Fisheries and Food (License No. PHF 43/2823/9/1999).
References
- Adachi, K., and Hamer, J.E. (1998). Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell 10, 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertyn, J., Hohmann, S., Thevelein, J.M., and Prior, B.A. (1994). GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 14, 4135–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell, R., Granath, K., Hohmann, S., Thevelein, J., and Adler, L. (1997). The two isoenzymes for yeast NAD-dependent glycerol 3-phosphate dehydrogenase, encoded by GPD1 and GPD2, have distinct roles in osmoadaptation and redox regulation. EMBO J. 16, 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechinger, C., Giebel, K.-F., Schnell, M., Leiderer, P., Deising, H.B., and Bastmeyer, M. (1999). Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus. Science 285, 1896–1899. [DOI] [PubMed] [Google Scholar]
- Bourett, T.M., and Howard, R.J. (1990). In vitro development of penetration structures in the rice blast fungus Magnaporthe grisea. Can. J. Bot. 68, 329–342. [Google Scholar]
- Carling, D., and Hardie, D.G. (1989). The substrate and sequence specificity of the cAMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim. Biophys. Acta 1012, 81–86. [DOI] [PubMed] [Google Scholar]
- Chida, T., and Sisler, H.D. (1987). Restoration of appressorial penetration ability by melanin precursors in Pyricularia oryzae treated with antipenetrants and in melanin-deficient mutants. J. Pestic. Sci. 12, 49–55. [Google Scholar]
- Choi, W., and Dean, R.A. (1997). The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell 9, 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley, F.G., and Valent, B. (1990). Genetic analysis of melanin deficient, nonpathogenic mutants of Magnaporthe grisea. Mol. Plant-Microbe Interact. 3, 135–143. [Google Scholar]
- Davis, D.J., Burlak, C., and Money, N.P. (2000). Biochemical and biomechanical aspects of appressorial development of Magnaporthe grisea. In Advances in Rice Blast Research, D. Tharreau, M.-H. Lebrun, N.J. Talbot, and J.-L. Notteghem, eds (Berlin: Springer-Verlag), pp. 248–256.
- de Jong, J.C., McCormack, B.J., Smirnoff, N., and Talbot, N.J. (1997). Glycerol generates turgor in rice blast. Nature 389, 244–245. [Google Scholar]
- DeZwaan, T.M., Carroll, A.M., Valent, B., and Sweigard, J.A. (1999). Magnaporthe grisea Pth11p is a novel plasma membrane protein that mediates appressorium differentiation in response to inductive surface cues. Plant Cell 11, 2013–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, K.P., Xu, J.-R., Smirnoff, N., and Talbot, N.J. (1999). Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by the rice blast fungus Magnaporthe grisea. Plant Cell 11, 2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, S., and Nuss, D.L. (1996). Distinct roles for two G protein α subunits in fungal virulence, morphology, and reproduction revealed by targeted gene disruption. Proc. Natl. Acad. Sci. USA 93, 14122–14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan, P., Mayer, E.P., and Fowler, S.D. (1985). Nile Red: A selective fluorescent stain for lipid droplets. J. Cell Biol. 100, 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin, M.C., Albertyn, J., Alexander, M., and Davenport, K. (1998). MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62, 1264–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, J.E., Howard, R.J., and Chumley, F.G. (1988). A mechanism for surface attachment in spores of a plant pathogenic fungus. Science 239, 288–290. [DOI] [PubMed] [Google Scholar]
- Hirayama, T., Maeda, T., Saito, H., and Shonozaki, K. (1995). Cloning and characterization of seven cDNAs for hyperosmolarity-responsiveness (HOR) genes of Saccharomyces cerevisiae. Mol. Gen. Genet. 249, 127–138. [DOI] [PubMed] [Google Scholar]
- Howard, R.J., and Aist, J.R. (1979). Hyphal tip cell ultrastructure of the fungus Fusarium: Improved preservation by freeze-substitution. J. Ultrastruct. Res. 6, 224–234. [DOI] [PubMed] [Google Scholar]
- Howard, R.J., and O'Donnell, K.L. (1987). Freeze-substitution of fungi for cytological analysis. Exp. Mycol. 11, 250–269. [Google Scholar]
- Howard, R.J., and Ferrari, M.A. (1989). Role of melanin in appressorium formation. Exp. Mycol. 13, 403–418. [Google Scholar]
- Howard, R.J., and Valent, B. (1996). Breaking and entering—Host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu. Rev. Microbiol. 50, 491–512. [DOI] [PubMed] [Google Scholar]
- Howard, R.J., Ferrari, M.A., Roach, D.H., and Money, N.P. (1991). Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc. Natl. Acad. Sci. USA 88, 11281–11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad, J.W. (1997). Virulence and cAMP in smuts, blast, and blight. Trends Plant Sci. 2, 193–199. [Google Scholar]
- Lee, Y.-H., and Dean, R.A. (1993). cAMP regulates infection structure formation in the plant pathogenic fungus Magnaporthe grisea. Plant Cell 5, 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.-H., and Dean, R.A. (1994). Hydrophobicity of contact surface induces appressorium formation in Magnaporthe grisea. FEMS Microbiol. Lett. 115, 71–75. [Google Scholar]
- Lev, S., Sharon, A., Hadar, R., Ma, H., and Horwitz, B.A. (1999). A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: Diverse roles for mitogen-activated protein kinase homologs in foliar pathogens. Proc. Natl. Acad. Sci. USA 96, 13542–13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., and Dean, R.A. (1997). G protein α-subunit genes control growth, development and pathogenicity of Magnaporthe grisea. Mol. Plant-Microbe Interact. 10, 1075–1086. [DOI] [PubMed] [Google Scholar]
- Mitchell, T.K., and Dean, R.A. (1995). The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast fungus Magnaporthe grisea. Plant Cell 7, 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money, N.P. (1997). Mechanism linking cellular pigmentation and pathogenicity in rice blast disease. Fungal Genet. Biol. 22, 151–152. [DOI] [PubMed] [Google Scholar]
- Norbeck, J., and Blomberg, A. (1997). Metabolic and regulatory changes associated with growth of Saccharomyces cerevisiae in 1.4 M NaCl. Evidence for osmotic induction of glycerol dissimilation via the dihydroxyacetone pathway. J. Biol. Chem. 28, 5544–5554. [DOI] [PubMed] [Google Scholar]
- Norbeck, J., Pahlman, A.K., Akhtar, N., Blomberg, A., and Adler, L. (1996). Purification and characterization of two isoenzymes of dl-glycerol-3-phosphatase from Saccharomyces cerevisiae. Identification of the corresponding GPP1 and GPP2 genes and evidence for osmotic regulation of Gpp2p expression by the osmosensing mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 271, 13875–13881. [DOI] [PubMed] [Google Scholar]
- Palmer, W.K., Oscai, L.B., Bectel, P.J., and Fisher, G.A. (1990). Dibutyryl cAMP-induced increases in triacylglycerol lipase activity in developing L8 myotube cultures. Can. J. Physiol. Pharmacol. 68, 689–693. [DOI] [PubMed] [Google Scholar]
- Redkar, R.J., Locy, R.D., and Singh, N.K. (1995). Biosynthetic pathways of glycerol accumulation under salt stress in Aspergillus nidulans. Exp. Mycol. 19, 241–246. [DOI] [PubMed] [Google Scholar]
- Reynolds, E.S. (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romao, J., and Hamer, J.E. (1992). Genetic organisation of a repeated DNA sequence family in the rice blast fungus. Proc. Natl. Acad. Sci. USA 76, 5269–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnow, B., and Kiellanbrandt, M.C. (1993). GUT2, a gene for mitochondrial glycerol-3-phosphate dehydrogenase of Saccharomyces cerevisiae. Yeast 9, 1121–1130. [DOI] [PubMed] [Google Scholar]
- Schadeck, R.J.G., Leite, B., and Buchi, D.F. (1998. a). Lipid mobilization and acid phosphatase activity in lytic compartments during conidium dormancy and appressorium formation of Colletotrichum graminicola. Cell Struct. Funct. 23, 333–340. [DOI] [PubMed] [Google Scholar]
- Schadeck, R.J.G., Buchi, D.F., and Leite, B. (1998. b). Ultrastructural aspects of Colletotrichum graminicola conidium germination, appressorium formation and penetration on cellophane membranes: Focus on lipid reserves. J. Submicrosc. Cytol. Pathol. 30, 555–561. [Google Scholar]
- Schuurink, R., Busink, R., Hondman, D.H.A., Witteveeen, C.F.B., and Visser, J. (1990). Purification and properties of NADP+-dependent glycerol dehydrogenase from Aspergillus nidulans. J. Gen. Microbiol. 137, 629–636. [DOI] [PubMed] [Google Scholar]
- Severson, D.L., Fletcher, T., Groves, G., Hurley, B., and Sloan, S. (1981). Hydrolysis of triolein, cholesterol oleate, and 4-methylumbelliferyl stearate by acid and neutral ester hydrolases (lipases) from pigeon adipose tissue: Effect of cAMP-dependent protein kinase. Can. J. Biochem. 59, 418–429. [DOI] [PubMed] [Google Scholar]
- Spurr, A.R. (1969). A low epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43. [DOI] [PubMed] [Google Scholar]
- Stralfors, P., and Belfrage, P. (1983). Phosphorylation of hormone-sensitive lipase by cyclic AMP–dependent protein kinase. J. Biol. Chem. 258, 15146–15152. [PubMed] [Google Scholar]
- Sweigard, J.A., Carroll, A.M., Farrall, L., Chumley, F.G., and Valent, B. (1998). Magnaporthe grisea genes obtained through insertional mutagenesis. Mol. Plant-Microbe Interact. 11, 404–412. [DOI] [PubMed] [Google Scholar]
- Takano, Y., Kikuchi, T., Kubo, Y., Hamer, J.E., Mise, K., and Furusawa, I. (2000). The Colletotrichum lagenarium MAP kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol. Plant-Microbe Interact. 13, 374–383. [DOI] [PubMed] [Google Scholar]
- Talbot, N.J. (1995). Having a blast: Exploring the pathogenicity of Magnaporthe grisea. Trends Microbiol. 3, 9–16. [DOI] [PubMed] [Google Scholar]
- Talbot, N.J., Ebbole, D.J., and Hamer, J.E. (1993). Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell 5, 1575–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein, J.M. (1994). Signal transduction in yeast. Yeast 10, 1753–1790. [DOI] [PubMed] [Google Scholar]
- Thines, E., Eilbert, F., Sterner, O., and Anke, H. (1997). Signal transduction leading to appressorium formation in germinating conidia of Magnaporthe grisea: Effects of second messengers diacylglycerols, ceramindes and sphingomyelin. FEMS Microbiol. Lett. 156, 91–94. [Google Scholar]
- Toida, J., Arikawa, Y., Kondou, K., Fukuzawa, M., and Sekiguchi, J. (1998). Purification and characterization of triacylglycerol lipase from Aspergillus oryzae. Biosci. Biotechnol. Biochem. 62, 759–763. [DOI] [PubMed] [Google Scholar]
- Tonukari, N.J., Scott-Craig, J.S., and Walton, J.D. (2000). The Cochliobolus carbonum SNF1 gene is required for cell wall–degrading enzyme expression and virulence on maize. Plant Cell 12, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton, J.D. (1994). Deconstructing the cell wall. Plant Physiol. 104, 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, R.W.S., Pitt, D., and Webster, J. (1998). Teaching techniques for mycology. 3. Amylase secretion by Aspergillus oryzae. Mycologist 12, 8–9. [Google Scholar]
- Weber, R.W.S., Wakley, G.E., and Pitt, D. (1999). Histochemical and ultrastructural characterization of vacuoles and spherosomes as components of the lytic system in hyphae of the fungus Botrytis cinerea. Histochem. J. 31, 293–301. [DOI] [PubMed] [Google Scholar]
- Xu, J.-R., and Hamer, J.E. (1996). MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 10, 2696–2706. [DOI] [PubMed] [Google Scholar]
- Xu, J.-R., Urban, M., Sweigard, J.A., and Hamer, J.E. (1997). The CPKA gene of Magnaporthe grisea is essential for appressorial penetration. Mol. Plant-Microbe Interact. 10, 187–194. [Google Scholar]
- Xu, J.-R., Staiger, C.J., and Hamer, J.E. (1998). Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc. Natl. Acad. Sci. USA 95, 12713–12718. [DOI] [PMC free article] [PubMed] [Google Scholar]