Abstract
Pollen tube cells adhere to the wall surface of the stylar transmitting tract epidermis in lily. This adhesion has been proposed as essential for the proper delivery of the sperm cells to the ovule. An in vitro adhesion bioassay has been used to isolate two stylar molecules required for lily pollen tube adhesion. The first molecule was determined to be a small, cysteine-rich protein with some sequence similarity to lipid transfer proteins and now called stigma/stylar cysteine-rich adhesin (SCA). The second, larger, molecule has now been purified from style fragments and characterized. Chemical composition, specific enzyme degradations, and immunolabeling data support the idea that this molecule required for pollen tube adhesion is a pectic polysaccharide. In vitro binding assays revealed that this lily stylar adhesive pectin and SCA are able to bind to each other in a pH-dependent manner.
INTRODUCTION
The plant cell wall has long been considered structurally inert, its role thought to be limited primarily to protection and support. More recently, a parallel has been made between the plant cell wall and the extracellular matrix of animal cells, despite their structural and biochemical differences. Studies investigating the structure, composition, and function of the cell wall during plant development and response to the environment describe the plant cell wall, or extracellular matrix, as a cellular compartment important in cell communication (Carpita and Gibeaut, 1993; Roberts, 1994; Schindler, 1998). A well-documented case of this sort is the recognition and rejection of self-pollen in the self-incompatibility reaction, which involves molecules from pollen and the stigma/style that intersect in the extracellular matrix (Williams et al., 1994; Schopfer et al., 1999).
Adhesion may be an essential event in cell–cell communication in plants, much as it is in animals (Lord et al., 1996). Several types of adhesion can occur in plant cells: between wall and substratum, between two walls, and between wall and plasma membrane. To date, a large number of surface molecules (adhesins) capable of binding to a receptor have been isolated from bacteria and fungi that colonize plant or animal tissues (Ofek and Doyle, 1994; Cormack et al., 1999). In algae, several types of adhesion molecules have been reported, such as algal-cell adhesion molecule, a homolog of animal adhesion proteins (Huber and Sumper, 1994), proteoglycans (Wetherbee et al., 1998; Wustman et al., 1998), and various peroxidases, polyphenols, and acidic polysaccharides (Fowler and Quatrano, 1997; Vreeland et al., 1998). Lectins have been reported as recognition and binding proteins produced by the plant and interacting with bacterial cells in nodulation, for example (Hirsch, 1999). In the case of intercellular adhesion in plants, pectins in the middle lamella have been implicated as attachment molecules between cells, as judged by staining and immunolocalization (Liners et al., 1994; Knox, 1997; Willats et al., 1999), biochemical data (Fry, 1988; Satoh, 1998), and some genetic studies (Sinha and Lynch, 1998). In plant reproduction, several molecules with properties of adhesives have been isolated, including TTS, an arabinogalactan protein from styles (Cheung et al., 1995), and the extensin-like Pex proteins from pollen (Rubinstein et al., 1995). Recently, lipophilic molecules have been proposed to mediate the pollen–stigma adhesion in Arabidopsis (Zinkl et al., 1999).
In several species, including lily and Arabidopsis, adhesion of pollen tubes to the transmitting tract epidermis of the style has been most clearly observed after cryofixation of pollinated styles (Janson et al., 1994; Jauh and Lord, 1995; Lennon et al., 1998; Park et al., 2000). Pollen tubes grown in vivo adhere to each other as well, an event not seen in vitro. Adhesion has been proposed to be essential for the proper delivery of the tube cell to the ovary (Lord et al., 1996). In an in vitro adhesion assay developed for lily, pregerminated pollen tubes adhered to an in vitro stylar matrix within 2 hr (Jauh et al., 1997). Recently, Park et al. (2000) reported that at least two molecules from the style were required for lily pollen tube adhesion, one small and the other large. Neither molecule accounted for pollen tube adhesion on its own. The small molecule is a 9-kD basic protein named stigma/stylar cysteine-rich adhesin (SCA), with some sequence similarity to plant lipid transfer proteins, including eight conserved cysteine residues. SCA has been localized to the transmitting tract of lily and is not detected in pollen tubes unless they are grown in vivo (Park et al., 2000).
Understanding the mechanism of the adhesion event among SCA, the large stylar molecule, and the pollen tubes requires identification of the large molecule. In this article, we describe the use of the in vitro adhesion bioassay to isolate this second component involved in lily pollen tube adhesion. Purified from style fragments, the molecule was characterized by chemical analysis, specific enzyme degradation, and immunolabeling. We show that the molecule required for lily pollen tube adhesion in combination with SCA is a complex acidic polysaccharide belonging to the pectin family (rhamnogalacturonan and low-esterified [<50% degree of random esterification] homogalacturonan regions). This molecule is recognized by the monoclonal antibody JIM5, which is specific for low-esterified pectins. Immunolocalization with JIM5 at the transmitting tract epidermis surface supports its role in pollen tube adhesion. We provide direct evidence that pectins are involved in cell adhesion but only in the presence of another, smaller, protein. In addition, we show that lily stylar pectin and SCA are able to bind to each other, a feature that begins to explain their mechanism of action in the adhesion assay.
RESULTS
Isolation of the Stylar Large Molecule Necessary for in Vitro Pollen Tube Adhesion
Style fragments were sequentially extracted, and each fraction was tested for its ability to induce pollen tube adhesion when combined with SCA. Table 1 demonstrates that none of the style wall extracts were adhesive on their own but that several caused significant pollen tube adhesion on the in vitro stylar matrix when combined with SCA. The citrate and imidazole fractions were the most adhesive. Fractions extracted with the calcium chelator trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid (CDTA) or with sodium carbonate had some adhesive activity but less than that of the imidazole extract. All four extracts were further investigated, but the imidazole extraction procedure was selected because it was the most effective in the assay and yielded more material than citrate extraction did. In addition, the imidazole buffer used for the extraction was easily removed by dialysis. Table 2 describes the purification steps of the adhesive imidazole extract. As previously noted, none of the fractions allowed pollen tube adhesion when used on their own in the assay. The adhesive fraction of the imidazole extract was isolated in the fraction precipitated between 40 and 60% ethanol content and then in the fraction eluted by anion-exchange chromatography between 300 and 400 mM NaCl. Adhesion assays performed with the purest SCA isolated from the stigma exudate (SCA[SE]) were more effective than were the ones conducted with SCA partially purified from a stigma extract (SCA[SG]). However, the greater amount of protein (as measured by protein content) in the SCA(SG) allowed for an equivalent number of pollen tubes to adhere on the nitrocellulose membrane, as did those from SCA(SE) (data not shown). Figure 1 shows the further purification of the imidazole fraction by size-exclusion chromatography and the separation of the eluate into 10 pooled fractions according to their total sugar and uronic acid contents. Fraction C was the most adhesive when combined with SCA (Table 2) and caused adherence of a very large number (401 ± 53) of pollen tubes to the coated nitrocellulose membrane (Figure 2A) and to each other (Figure 2B). Using the elution profiles of the dextran standards for comparison, we estimated the size of the fraction C molecule as 1.5 MD.
Table 1.
Use of an Adhesion Assay to Isolate the Large Molecule from the Lily Style
| Fractions | No. of Adhered Pollen Tubesa |
|---|---|
| SCA | 6 ± 3 (7) |
| Citrate soluble | 8 ± 2 (4) |
| Citrate soluble + SCA | 184 ± 34 (4) |
| CDTA soluble | 15 ± 6 (3) |
| CDTA soluble + SCA | 105 ± 41 (7) |
| Imidazole soluble | 1 ± 1 (4) |
| Imidazole soluble + SCA | 227 ± 52 (6) |
| Na2CO3 soluble | 0 (3) |
| Na2CO3 soluble + SCA | 112 ± 23 (3) |
| KOH solubleb | 0 (3) |
| KOH soluble + SCA | 35 ± 21 (3) |
| KOH insoluble | 1 ± 1 (3) |
| KOH insoluble + SCA | 23 ± 11 (3) |
The number of pollen tubes adhered to nitrocellulose membrane coated with 75 μg of the indicated style fraction or 75 μg of the indicated style fraction combined with 10 μg of SCA partially purified from SCA(SG). The numbers in parentheses indicate the number of replicates.
The five KOH extracts were combined in equal amounts (15 μg of each extract).
Table 2.
Purification of the Stylar Adhesive Molecule using an Adhesion Assay with SCA
| No. of Adhered Pollen Tubes
|
||
|---|---|---|
| Fractionsa | + SCA(SG)b | + SCA(SE)c |
| Imidazole extract | 227 ± 52 (6) | 328 ± 50 (3) |
| Ethanol precipitation of imidazole extract | ||
| Ethanol 20 to 40% insoluble | 77 ± 50 (2) | —d |
| Ethanol 40 to 60% insoluble | 236 ± 86 (8) | 334 ± 26 (3) |
| Ethanol 60 to 80% insoluble | 101 ± 30 (2) | — |
| Q Sepharose of ethanol 40 to 60% insoluble | ||
| Unbound | 35 ± 34 (3) | — |
| 100 mM NaCl eluted | 13 ± 13 (3) | — |
| 200 mM NaCl eluted | 10 ± 6 (3) | — |
| 300 mM NaCl eluted | 58 ± 40 (3) | — |
| 400 mM NaCl eluted | 220 ± 29 (4) | — |
| 500 mM NaCl eluted | 39 ± 24 (3) | — |
| 2 M NaCl eluted | 46 ± 26 (4) | — |
| Sepharose CL-4B of the 400 mM NaCl elutede | ||
| Fraction A | — | 320 ± 16 (2) |
| Fraction B | — | 261 ± 29 (2) |
| Fraction C | — | 401 ± 53 (5) |
| Fraction D | — | 204 ± 66 (2) |
| Fraction E | — | 286 ± 38 (2) |
| Fraction F | — | 20 ± 11 (2) |
| Fraction G | — | 298 ± 82 (2) |
| Fraction H | — | 37 ± 24 (2) |
| Fraction I | — | 13 ± 5 (2) |
| Fraction J | — | 1 ± 1 (2) |
None of the imidazole extracts (75 μg) noted in this table allowed pollen tube adhesion when used on their own (i.e., <20 adhered pollen tubes with at least three replicates for each extract).
Number of pollen tubes adhered to a nitrocellulose membrane coated with 75 μg of imidazole extract combined with 10 μg of SCA partially purified SCA(SG). The numbers in parentheses indicate the number of replicates.
Number of pollen tubes adhered to a nitrocellulose membrane coated with 50 μg of imidazole extract combined with 5 μg of SCA isolated from SCA(SE). The numbers in parentheses indicate the number of replicates.
(—), not determined.
Fractions of the size exclusion Sepharose CL-4B column are identified as shown in Figure 1.
Figure 1.
Gel-Filtration Analysis of the Imidazole Stylar Extract.
Elution profiles were obtained by detection of the uronic acid (mHDP assay) and the carbohydrate (phenol sulfuric assay) contents. Samples (2 mg) were separated on a Sepharose CL-4B column equilibrated with 100 mM imidazole-HCl, pH 7.5. Fractions (2 mL) were pooled as indicated by dotted lines and arranged from A to J. The elution of the dextran molecular mass standards (2000, 600, 170, and 75 kD) is indicated by the arrowheads. Before application to this column, the stylar material was extracted from the phenol/acetic acid/water–insoluble stylar fragments with imidazole buffer, precipitated between 40 and 60% ethanol, and eluted from the Q Sepharose column between 300 and 400 mM NaCl.
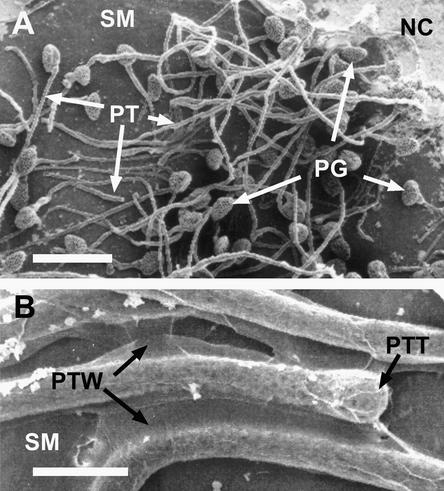
Figure 2.
Scanning Electron Microscopy of Lily Pollen Tubes Adhered to an in Vitro Stylar Matrix.
(A) Pollen tubes adhere massively via the pollen tube tips to the nitrocellulose membrane coated with 50 μg of fraction C of the imidazole extract and 5 μg of SCA isolated from SCA(SE).
(B) Close-up of (A). Note adhesion between the walls of the pollen tubes. Shrinkage of the tube cell cytoplasm is an artifact of fixation.
NC, nitrocellulose membrane; PG, pollen grain; PT, pollen tube; PTT, pollen tube tip; PTW, pollen tube wall; SM, stylar matrix.
 ;
;  .
.
Large Molecule Required for in Vitro Pollen Tube Adhesion Is a Pectin
Chemical compositions of the adhesive imidazole fractions are given in Table 3. The different purification steps yielded a fraction composed mostly of carbohydrates with a very low amount of protein. A net increase in the galacturonic acid content was obtained during the purification steps. The purest fraction, C, was a galacturonic acid–enriched polymer containing some rhamnosyl, arabinosyl, galactosyl, and glucuronosyl residues and traces of xylosyl and other residues. The ratio of rhamnose to galacturonic acid was 0.09. Fractions B, D, and E isolated by size-exclusion chromatography were chemically similar to fraction C and were also composed mainly of galacturonic acid (68 to 71 mol%) with the same other glycosyl residues. The ratio of rhamnose to galacturonic acid ranged from 0.09 to 0.13 (data not shown).
Table 3.
Chemical Composition of the Stylar Adhesive Fractions from the Imidazole Extraction
| Yield Recovery and Composition |
Crudea | Ethanol 40 to 60% Insoluble |
Q Sepharose Elution 300 to 400 mM NaCl |
Size Exclusion Fraction C |
|---|---|---|---|---|
| Yield (mg dry weight) | 644 | 325 | 88 | 7 |
| Total protein % (w/w) | 3.5 | 3.3 | 0.8 | 0.5 |
| Total carbohydrate % (w/w) | 86.8 | 94.6 | 96.1 | 98.6 |
| Glycosyl residue (mol%) | ||||
| Arabinosyl | 10.5 | 10.1 | 7.9 | 4.6 |
| Rhamnosyl | 4.9 | 3.9 | 5.1 | 6.6 |
| Fucosyl | 0.5 | 0.2 | 0.3 | 0.8 |
| Xylosyl | 3.9 | 1.2 | 0.9 | 1.5 |
| Mannosyl | 6.8 | 0.5 | 0.3 | 0.8 |
| Galactosyl | 12.4 | 13.8 | 10.3 | 4 |
| Glucosyl | 6.7 | 1 | 0.8 | 0.8 |
| Glucuronosyl | 13.7 | 6.4 | 5.9 | 7.6 |
| Galacturonosyl | 40.9 | 63 | 68.5 | 73.3 |
Crude imidazole extract was fractionated by an ethanol precipitation series (20% increment), anion-exchange chromatography (Q Sepharose eluted with NaCl series of 100 mM increment), and size-exclusion chromatography (Sepharose CL-4B).
Enzymatic pretreatment of fraction C with proteinase K did not reduce the number of adhered pollen tubes in the assay in comparison with the controls, as shown in Table 4. This result indicated that the active component of this fraction was not the small amount of protein present in the fraction. On the other hand, pretreatment of SCA with proteinase K markedly disrupted pollen tube adhesion in the assay. In contrast, specific degradation of fraction C with endopolygalacturonase 2 (PG2) considerably decreased the number of adhered pollen tubes in comparison with that of the controls. Commercial polygalacturonic acid was more fully degraded by PG2 than was fraction C, as evidenced by the amount of reducing sugar released (Table 4).
Table 4.
Adhesion of Lily Pollen Tubes on a Matrix Coated with Active Fractions from the Imidazole Extraction and SCA after Their Treatments with Enzymes
| Fraction and Enzymatic Treatment | No. of Adhered Pollen Tubesa |
|---|---|
| Proteinase K treatmentb | |
| Imidazole extractc + SCA(SG) | 236 ± 86 (8) |
| + SCA(SG) (pK treated)d | 1 ± 1 (2) |
| + SCA(SG) (boiled pK) | 195 ± 43 (2) |
| Imidazole extract (pK treated) + SCA(SG) | 215 ± 34 (2) |
| (pK treated) + SCA(SG) (pK treated) | 13 ± 8 (2) |
| PG2 treatmente | |
| Fraction Cf + SCA(SE) | 401 ± 53 (5) |
| Fraction C (PG2 treated)g + SCA(SE) | 51 ± 16 (3) |
| Fraction C (boiled PG2) + SCA(SE) | 311 ± 43 (2) |
Numbers in parentheses indicate the number of replicates.
Adhesion assay using 75 μg of imidazole extract combined with 10 μg of SCA partially purified from SCA(SG).
Crude imidazole extract precipitated between 40 and 60% ethanol.
Imidazole extract and SCA were separately incubated with proteinase K (pK treated) or boiled proteinase K (boiled pK) and combined for the adhesion assay.
Adhesion assay using 50 μg of imidazole extract (Fraction C) combined with 5 μg of SCA isolated from SCA(SE).
Fraction C of the size-exclusion column as identified in Figure 1.
Fraction C was incubated with PG2 or with boiled PG2 and combined with SCA. The ratio of the amount of reducing sugars at the end and at the start of the PG2 treatment was 9. In the same conditions, the ratio obtained with the commercial polygalacturonic acid was 40.
Immunoblot labeling of fraction C with monoclonal antibodies showed a very strong reaction with JIM5 (which recognizes low-esterified pectins) and a medium reaction with JIM7 (which recognizes esterified pectin), as displayed in Figure 3. This fraction did not react with the (β-d-Glc)3 Yariv phenylglycoside, a diagnostic tool for detecting arabinogalactan proteins (Komalavilas et al., 1991). When fraction C was treated with endopolygalacturonase, the JIM5 labeling was substantially reduced.
Figure 3.
Immunoblot Labeling of Fraction C before and after Treatment with PG2.
JIM5 recognizes low-esterified homogalacturonan, JIM7 recognizes esterified pectins, and (β-d-Glc)3 Yariv phenylglycoside [(β-D-Glc)3] is a colored reagent used as a diagnostic stain for arabinogalactan proteins.
Together, these data (specific extraction, chemical composition, specific enzymatic degradation, and immunolabeling) demonstrate that fraction C, isolated from the style and required in our assay for lily pollen tube adhesion, is a pectic polysaccharide with probably a low degree of esterification in the homogalacturonan region.
In Vitro Pollen Tube Adhesion Depends on Concentration and pH of Matrix Components
Optimization of the adhesion assay using the lily stylar pectin (fraction C of the imidazole extract) and SCA(SE) is shown in Figure 4. A combination of 50 μg of fraction C (Figure 4A) with 5 μg of SCA (data not shown) was necessary and sufficient to yield a large number of pollen tubes that adhered to the matrix and to each other. Moreover, as illustrated in Figure 4B, the pH of SCA and fraction C before their application to the nitrocellulose membrane affected the amount of pollen tube adhesion. Adhesion of pollen tubes to the in vitro stylar matrix was greatest between pH 5.0 to 8.0, with an optimum at pH 5.0 to 6.0, which corresponds to the pH of the lily stylar exudate (data not shown) and to the growth medium for lily pollen tubes. Adhesion was noticeably disrupted at pHs <5.0 and >8.0. The pI of SCA is 8.7 (Park et al., 2000).
Figure 4.
Concentration and pH Characteristics of the Lily Stylar Pectin (Fraction C of the Imidazole Extract) and SCA-Mediated Adhesion of Lily Pollen Tubes to an in Vitro Stylar Matrix.
(A) Dosage effect of fraction C on pollen tube adhesion. Fraction C was obtained by precipitation of the crude imidazole stylar extract at an ethanol content between 40 and 60%, elution from the Q Sepharose column at between 300 and 400 mM NaCl, and gel filtration on the Sepharose CL-4B. Fraction C was combined with 5 μg of SCA(SE).
(B) pH effect of the two solutions (50 μg of fraction C and 5 μg of SCA(SE) on lily pollen tube adhesion. The pH of each solution was adjusted with 50 mM citrate or 50 mM Tris-HCl buffers before combination and application to the nitrocellulose membrane.
Bars indicate the standard deviation from the mean for three replicates.
Low-Esterified Pectins Are Located on the Transmitting Tract Epidermal Surface
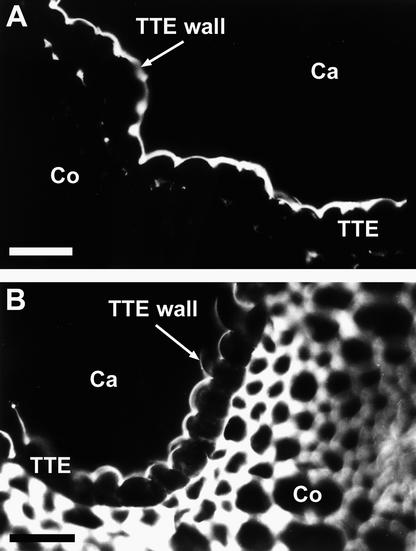
Figure 5 reveals the immunolocalization of pectins in a lily-style cross-section by use of JIM5 and JIM7. Figure 5A demonstrates a strong labeling with JIM5 (low-esterified pectins) in the extracellular matrix surface of the transmitting tract epidermis, which is the area of the style where pollen tubes adhere on their progress to the ovary. A low amount of labeling was apparent in the stylar cortex after a longer exposure time (data not shown). In contrast, Figure 5B shows that esterified pectins, detected with JIM7, were located throughout the style as well as on the transmitting tract epidermis.
Figure 5.
Immunolocalization of Labeled Monoclonal Antibodies to Low-Esterified (JIM5) and Esterified (JIM7) Homogalacturonans in Cross-Sections of Cryofixed Lily Styles.
(A) JIM5 strongly labeled the outer surface of the transmitting tract epidermis.
(B) JIM7 labeled the walls of all the stylar cells.
Ca, stylar canal; Co, stylar cortex; TTE, transmitting tract epidermis.
 .
.
Lily Stylar Pectins and SCA Cannot Be Replaced by Other Components in the Adhesion Assay
Table 5 reveals that substitution of other commercial polymers such as BSA, dextran, gum arabic, alginic acid, or esterified pectin for the lily stylar pectins (fraction C) did not allow pollen tube adhesion when combined with SCA. These compounds were chosen to mimic various features of the fraction C molecule such as charge and size. Substantial pollen tube adhesion was obtained with the polygalacturonate from citrus, but the number of pollen tubes adhering was approximately one-fourth of that with fraction C. Substituting small, basic proteins (cytochrome c or poly-d-lysine) for SCA in the assay did not produce pollen tube adhesion. When poly-d-lysine was used, many pollen grains adhered to the in vitro stylar matrix but the pollen tubes did not.
Table 5.
Adhesion Assays Using Various Commercial Polymers to Substitute for the Lily Stylar Pectin (Fraction C) or SCA
| Fractions | No. of Adhered Pollen Tubesa |
|---|---|
| Fraction C + SCA(SE) | 401 ± 53 (5) |
| BSA + SCA(SG) | 0 (8) |
| Alginic acid + SCA(SE) | 0 (4) |
| Gum arabic + SCA(SG) | 5 ± 2 (6) |
| Dextran + SCA(SE) | 0 (4) |
| PGAb + SCA(SE) | 105 ± 34 (5) |
| PEc + SCA(SE) | 15 ± 3 (4) |
| Fraction C + cytochrome c | 10 ± 5 (9) |
| Fraction C + poly-d-lysine | 9 ± 2 (4) |
Number of pollen tubes adhered to nitrocellulose membrane coated with 50 to 200 μg of the fractions combined with 5 to 20 μg of SCA partially purified from SCA(SG) or isolated from SCA(SE) or with 5 to 100 μg of cytochrome c or poly-d-lysine. The numbers in parentheses indicate the number of replicates.
Polygalacturonic acid.
Pectin esterified with a degree of esterification of 93%.
Lily Stylar Pectin and SCA Bind to Each Other in a pH-Dependent Manner
When SCA was combined with polymers of different characteristics (e.g., neutral, charged, or high molecular mass) and then size-fractionated as shown in Figure 6, it bound to several compounds, including the lily stylar pectin. SCA and dextran did not bind together, but SCA and alginic acid did. However, only the lily stylar pectin plus SCA allowed for pollen tube adhesion (see Table 5). Interestingly, if lily stylar pectin and SCA were combined in a solution at pH 10.0, which is greater than the pI of SCA (8.7), approximately one-third of the SCA did not bind to the lily stylar pectin (see band in the filtrate in Figure 5E) and pollen tube adhesion was markedly reduced (see Figure 3C data for pH 10.0). This suggests that charge interactions between the pectin and SCA are necessary but not sufficient to induce pollen tube adhesion.
Figure 6.
Ability of SCA to Bind Different Polymers at Several pHs.
SDS-PAGE of SCA (5 μg) isolated from SCA(SE) after combination with different carbohydrate polymers (50 μg) and size-filtration with a Centricon filter (molecular mass cutoff of 100 kD). A, fraction C plus SCA, pH 6.0; B, dextran plus SCA, pH 6.0; C, SCA, pH 6.0 (control); D, alginic acid plus SCA, pH 6.0; E, fraction C plus SCA, pH 10.0; F, Centricon filtrate; R, Centricon retentate; SCA, stigma/stylar cysteine-rich adhesin isolated from SCA(SE). Arrow indicates the molecular mass standard (6.7 kD).
DISCUSSION
Composition of Pectins and Their Isolation
Pectins are complex wall macromolecules composed of homogalacturonan that can be methyl- and acetyl-esterified, rhamnogalacturonan 1, rhamnogalacturonan 2, and the less common xylogalacturonan (Albersheim et al., 1996; Carpita et al., 1996). These polysaccharides can be extracted from the primary cell wall by chemical or enzymatic treatments. Homogalacturonan is a linear polymer of repeated units of (1,4)-α-d-galacturonic acid that can be cross-linked with calcium, depending on the extent of esterification, and can form a gel in the wall (Goldberg et al., 1996). Rhamnogalacturonan 2 has the same homopolymer backbone as the homogalacturonan but is highly substituted with 10 different glycosyl residues, including such unusual sugars as aceric acid (reviewed in Mohnen, 1999). Rhamnogalacturonan 1 has been described as a large subunit composed of the repeating disaccharide backbone of (1,4)-α-d-galacturonic acid-(1,2)-α-l-rhamnose with a wide variety of side chains attached to the rhamnosyl residues, ranging from monomers to polysaccharides of galactan, arabinan, and arabinogalactan (reviewed in O'Neill et al., 1990).
Our results suggest that the lily stylar pectin is composed of “smooth regions” of low-esterified homogalacturonan and regions of rhamnogalacturonan with arabinosyl or galactosyl substitutions. This suggestion is based on our data for JIM5 reactivity for low-esterified pectins (Knox et al., 1990), the results from endopolygalacturonase treatment, and the chemical composition. The question of whether rhamnogalacturonan is linked to homogalacturonan remains open (Mohnen, 1999). Some evidence indicates that the three classes of molecules (homogalacturonan and rhamnogalacturonan 1 and 2) are covalently linked to each other, given that endopolygalacturonase treatment releases oligouronides and the two rhamnogalacturonan types (Fry, 1988; Albersheim et al., 1996). Jarvis (1984) reported that rhamnogalacturonan 1 could contain homogalacturonan. To date it has been established that two rhamnogalacturonan 1 side chains can be cross-linked by diferulic acid residues (Fry, 1988) or that two rhamnogalacturonan 2 molecules can be covalently cross-linked through borate diester bonds and reinforced by calcium (Kobayashi et al., 1999). According to the classification of pectins by Schols and Voragen (1996), which is based in part on the ratio of rhamnose to galacturonic acid, our adhesive stylar pectin is a rhamnogalacturonan.
Our preliminary results showed that imidazole was more effective than the widely used calcium chelator CDTA in the pectin extraction method, as judged in terms of pollen tube adhesion in the bioassay. Perhaps the CDTA was not readily removed by dialysis and remained associated with pectins, as reported by Mort et al. (1991). A reliable substitute for the CDTA in the extraction of highly negatively charged polymers was the imidazole buffer used by Nothnagel et al. (1983) and suggested by Mort et al. (1991) in a technical review on pectins, even though imidazole buffer only weakly chelates calcium (Goldberg et al., 1996). Moreover, Mort et al. (1991) demonstrated that the CDTA and imidazole extractions released mostly acidic polymers of similar composition and low esterification levels. Our results also demonstrated that the imidazole fraction was enriched in low-esterified pectin. In addition, our pectin extraction method of hand-dissected open styles instead of the blended or ground wall material generally used allowed us to isolate fractions enriched in transmitting tract epidermis surface pectins and to reduce contamination from the esterified pectins that make up the primary wall of the style cortex.
Evidence for the Role of Pectins in Adhesion
Many reports have suggested that pectins are involved in intercellular adhesion, on the basis of biochemical data such as cellular dissociation, using calcium chelators (Goldberg et al., 1996) and endopolygalacturonases (Mohnen, 1999), and on the basis of their localizations with a gold-labeled endopolygalacturonase (Roland and Vian, 1981) and various monoclonal antibodies (Knox, 1997). Indeed, the development of monoclonal antibodies against pectin epitopes has improved our understanding of pectin maturation, regulation, and localization. Localization of the different classes of pectins within the wall appears to depend on species, organ, tissue, and cell type, with the pectin network being temporally and spatially regulated (Roberts, 1990). The development of tissues often involves a loss of cell adhesion. The most studied phenomenon is probably fruit ripening and the loss of wall adherence by solubilization of the middle lamella (Watson et al., 1994). Many reports based on immunolocalization data (reviewed in Knox, 1997) have suggested that low-esterified homogalacturonan, present in the middle lamella close to the cell corners, is responsible for cell adhesion, probably by calcium cross-linking. Recently, an antibody produced from a phage display library specific for blocks of 30 galacturonic acids has been developed and found to be localized in the regions of cell-to-cell contact in Arabidopsis suspension culture cells (Willats et al., 1999). In addition, low-esterified pectins have been implicated in tissue fusion in the maize adherent1 mutant, which shows abnormal cell and organ fusions (Sinha and Lynch, 1998). Recently, Sieber et al. (2000) have shown an Arabidopsis mutant with altered cuticle in which pectic polysaccharides were present in the junctions of fused organs. In the quartet mutants of Arabidopsis, failure of pectin degradation in the pollen mother cell wall was associated with the failure of microspore separation during pollen development (Rhee and Somerville, 1998).
There are inconsistencies in the literature on pectins and cell adhesion. As noted by Knox (1997), low-esterified homogalacturonans detected with JIM5 and 2F4, an antibody that recognizes a calcium-dependent conformation of homogalacturonan (Liners et al., 1989), were more often associated with cell separation at middle lamellae, not cell adhesion. In a study of friable and compact sugar beet calli, Liners et al. (1994) suggested that cell adhesion was mainly the result of the presence of methyl-esterified pectins with negligible calcium binding and that cell dissociation was associated with an increase in the acetylation of pectins. Bush and McCann (1999) suggested that the degree of pectin esterification might be a regulating factor in cell–cell adhesion. Moore et al. (1986) showed that rhamnogalacturonan 1 in sycamore suspension culture was also restricted to the middle lamella, especially in the junctions between cells. Comparing embryogenic and nonembryogenic carrot calli, Kikuchi et al. (1996) demonstrated that the size of cell clusters correlated with the ratio of the neutral sugar arabinose to galactose substitutions, suggesting the significance of the neutral side chains of pectin in cell attachment. Moreover, loss of galactose and arabinose has been also reported during the disadherence of walls during fruit ripening (Gross and Sams, 1984; Redgwell et al., 1997). All these biochemical and immunolocalization data suggest that pectins are good candidates for cell adhesion, but no functional assays for adhesion have been developed to test this hypothesis. Our results from such a functional adhesion assay clearly demonstrate that pectins are required for the adhesion of lily pollen tubes to an in vitro stylar matrix. The loss of adhesion after the endopolygalacturonase treatment best confirms this. Whether low-esterified pectins with calcium cross-linking, esterified pectins, neutral side chains of rhamnogalacturonan, or various combinations of these are directly or indirectly involved in this adhesion is still unknown. The contribution of calcium to adhesion in the lily pollen tube bioassay is difficult to assess because it is present in the medium and is necessary for pollen tube growth, a prerequisite for adhesion. An unforeseen component of adhesion in our system is a protein involved in the mechanism. Indeed, lily stylar pectin alone does not allow adhesion of pollen tubes but acts only in combination with the small, basic, cysteine-rich protein SCA. There have been no previous reports of wall proteins combining with pectins to function in cell adhesion.
Pectin Binding Proteins
In addition to the structural polysaccharides and wall enzymes, a wide range of proteins and glycoproteins exist in the wall (reviewed in Cassab, 1998; Kohorn, 2000). Extensins can be cross-linked in the wall to pectins by way of rhamnogalacturonan (Qi et al., 1995), and expansins bind tightly to cellulose and contain cellulose binding domains (Cosgrove, 1999). Penel and Greppin (1996) have shown that anionic and cationic isoperoxidases can bind specifically to a calcium–homogalacturonan gel column. They speculate that this reflects the presence of basic amino acids in the protein such as arginine or lysine. Other proteins or proteoglycans located at the cell wall/plasma membrane interface have also been reported to bind to pectins. Members of the wall-associated kinases family can covalently link to pectin (Kohorn, 2000). Arabinogalactan proteins can be anchored to the plasma membrane through a glycosylphosphatidylinositol anchor (Youl et al., 1998; Svetek et al., 1999), and arabinogalactan proteins have been reported to bind or copurify with pectin (Baldwin et al., 1993; Kido et al., 1996; reviewed in Nothnagel, 1997). The interaction between lily SCA and pectin does not appear to be mediated by cross-linkages but more probably occurs through ionic interactions, as with many wall enzymes and some lectins (Fry, 1986). Indeed, an increase of the pH over the isoelectric point of SCA releases part of the SCA from the pectin, greatly reducing pollen tube adhesion. Charge interactions may be important for proper adhesion but are insufficient to explain pollen tube adhesion because SCA binds as well to alginic acid, another negatively charged polymer—a combination that does not allow for pollen tube adhesion. Reports have shown that cysteine-rich domains of proteins can mediate specific carbohydrate binding (Allen, 1983; Fiete et al., 1998), as do lectins through “deep or shallow pockets” in their three-dimensional structure (Rini, 1995). In binding to pollen tubes of lily (Park et al., 2000), SCA may be acting as an adhesin between the pectin matrices of the pollen tubes and the stylar transmitting tract epidermal cells.
Pectins and Pectin-Degrading Enzymes in Pollen Tubes
Pectins (low esterified and esterified) are a major component of pollen tube walls (reviewed in Knox, 1997; Taylor and Hepler, 1997), including those in lily (Jauh and Lord, 1996). Rhamnogalacturonan 2 has also been detected in the lily pollen tube wall (Matoh et al., 1998). During pollen germination and tube growth, genes encoding polygalacturonases, pectin esterases, and pectate lyases are highly expressed (for details, see reviews in Taylor and Hepler, 1997; Wakeley et al., 1998). All three enzymes have been localized in the pollen tube (Taylor and Hepler, 1997). In addition, very high polygalacturonase activity has been reported in pollen (Pressey, 1991). The role of these enzymes in the cell biology of pollen is unclear. Polygalacturonase secreted by the tube cell may facilitate the penetration of the style by degrading stylar pectins, or it may also provide wall precursors from the style for tube growth; it may even act on the pollen tube wall itself to facilitate growth (Hadfield and Bennett, 1998). Lily pollen tubes disadhere from the stylar matrix back from their tips, perhaps through the action of polygalacturonases breaking adhesions. These enzymes produced by the pollen could be modifying the stylar matrix and contributing to the adhesion itself. Polygalacturonase may produce oligogalacturonides that could act as signal molecules (Hadfield and Bennett, 1998) as oligosaccharins do (Fry et al., 1993; John et al., 1997). The pollen tube may be acting like a fungal hypha in this respect by eliciting a response from the style. The fact that proper pollen tube adhesion in lily requires the combination of pectin and SCA adds yet another putative role for these enzymes, that is, degradation of the stylar matrix to release SCA from pectin.
In pollination, adhesion and guidance of the pollen tubes to the ovary appear to be linked (Lord et al., 1996). A parallel adhesion and guidance system operates in animals, where a small secreted protein, netrin, interacts with a large matrix molecule, laminin, to guide neuron outgrowth. Netrin binds a receptor in the neuron while also binding laminin in the matrix acting as a bridge between the two; accordingly, the neuron tracks this path of extracellular matrix molecules (Hedgecock and Norris, 1997). In lily, the mechanism of action of pectin, SCA, and the pollen tubes in the adhesion process may be similar, but many questions remain unanswered. What is the receptor of the SCA/pectin complex in the pollen tube? What is the binding site/mechanism of SCA and the pectin? Do the pollen tubes play an active role in adhesion or disadhesion by releasing enzymes able to degrade the matrix? We are currently investigating the fine structure of the adhesion sites on pollen tubes and transmitting tract epidermal cells and are looking for the component in the tube cell that binds the stylar matrix. Further characterization of the adhesive pectic polysaccharide from lily styles is necessary to determine the mechanism of adhesion in this two-component system.
METHODS
Plant Material
Lily (Lilium longiflorum Thumb cv Nelly White) flowers were collected 1 to 2 days after anthesis. Ovaries were removed, stigmas and styles separated, and the styles longitudinally bisected. The style fragments and stigmas were washed separately with 1 mM sodium phosphate buffer, pH 5.5, containing 1 mM DTT and 1 mM sodium bisulfite on ice for 2 hr to yield the style and stigma exudates. The washed style fragments were collected by filtration on nylon mesh, freeze-dried, and stored at −20°C for later use. The washed stigmas were stored at −80°C. The style and stigma exudates were used to isolate the stigma/stylar cysteine-rich adhesin (SCA) as in Park et al. (2000). The purified SCA obtained from the exudates (SCA[SE]) was used for critical experiments. Routine assays were performed with partially purified SCA obtained from an extract of washed stigmas (SCA[SG]). In this method, the washed stigmas were blended in an extraction buffer of 1 mM sodium phosphate buffer, pH 5.5, 1 mM DTT, and 1 mM sodium bisulfite. Debris was removed by centrifugation. The extract was then partially purified on a cation-exchange column and processed as in Park et al. (2000). SDS-PAGE and immunoblotting with a polyclonal antibody raised against lily SCA (Park et al., 2000) revealed that this partially purified fraction (SCA[SG]) was enriched in SCA but also contained several other minor proteins.
Sequential Extractions of Style Fragments
The washed style fragments (50 g dry weight; ∼1700 flowers) were agitated for 30 min at 70°C with 700 mL of phenol/acetic acid/water (4.5:2:0.5 [w/v/v]) reagent. The extract was filtered through GF/A glass fiber filter (Whatman, Maidstone, UK) to yield a phenol/acetic acid/water–soluble extract (filtrate) and insoluble fragments (retentate).
The soluble phenol/acetic acid/water extract was cooled on ice, mixed with 30 mL of 10% (w/v) ammonium formate and 2.4 liters of acetone, and allowed to precipitate overnight. The pellet was recovered by centrifugation at 10,000g for 30 min at 4°C and washed three times with 80% (v/v) acetone. The pellet was resuspended twice in 75 mL of 50 mM sodium citrate buffer, pH 5.5, on ice for 2 hr, and the insoluble material was removed by centrifugation. The two citrate-soluble fractions (which accounted for 3% [by weight] of the total material precipitated with acetone) were pooled, dialyzed (using tubing with a molecular mass cutoff of 3.5 kD; Spectrum Laboratories, Laguna Hills, CA) against distilled water, and lyophilized.
The phenol/acetic acid/water–insoluble stylar fragments were washed three times with water and three times with acetone. Cell wall components were sequentially extracted as described by Selvendran and Ryden (1990), with modifications as mentioned. Briefly, the washed stylar fragments were treated for 12 hr at room temperature with 50 mM trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid (CDTA), pH 6.5, or 500 mM imidazole-HCl, pH 7.0 (Nothnagel et al., 1983), containing 0.02% sodium azide (CDTA-soluble or imidazole-soluble extracts). The insoluble residue of the CDTA or imidazole-HCl extractions was washed three times with distilled water and treated with 50 mM sodium carbonate for 12 hr at 4°C and then again at 20°C for 2 hr. The resulting two fractions obtained after the temperature treatments were pooled to yield a sodium carbonate–soluble extract. The residue was washed with distilled water and treated for 90 min under nitrogen with a sequence of five KOH solutions ranging from 0.5 to 4 M as described by Selvendran and Ryden (1990). The remaining insoluble material was washed three times with distilled water and freeze-dried (KOH-insoluble extract). In each step, soluble materials were acidified to pH 5.0 with acetic acid, dialyzed exhaustively against distilled water, and freeze-dried.
In Vitro Adhesion Assay of Lily Pollen Tubes
The different wall extracts (75 μg) were tested for their adhesive properties on their own or with the addition of 5 μg of SCA(SE) or 10 μg of SCA(SG).
The in vitro adhesion assays of lily pollen tubes on an artificial matrix were performed as described by Park et al. (2000). Pollen from six anthers was suspended in 20 mL of germination medium, which resulted in an estimated 125,000 ± 17,000 pollen tubes. Several 28-mm2 nitrocellulose membranes (MSI, Westboro, MA) were placed in a Petri dish, and the 20 mL of pollen suspension was then added. If all of the pollen tubes floating above a nitrocellulose membrane were to adhere, there would have been 604 ± 82 pollen tubes per membrane. The actual number of pollen tubes that adhered to the 28-mm2 surface was counted with a stereomicroscope after the membrane was stained with Coomassie blue (Sigma).
Gum arabic from Acacia, esterified pectin (93% esterification) from citrus, polygalacturonic acid sodium salt from citrus, alginic acid sodium salt (low viscosity) from kelp, dextran (2000 kD), poly-d-lysine, cytochrome c from bovine heart, and BSA were obtained from Sigma. These standards were tested for their adhesive properties at concentrations ranging from 5 to 200 μg as a substitute for one of the two components. Each wall extract or standard used for the adhesion assay was prepared at 1 mg mL−1 in distilled water unless otherwise indicated.
The pH of the two extracts (fraction C and SCA) was modified before the two molecules were combined in preparing the adhesion assay. The pH range investigated was 2.0 to 12.0, as set with 50 mM sodium citrate and 50 mM Tris-HCl buffers.
Scanning Electron Microscopy of in Vitro Pollen Tube Adhesion Assay
The assay was prepared with 5 μg of SCA(SE) and 50 μg of fraction C from the imidazole extraction. Processing for scanning electron microscopy was as described in Park et al. (2000). Specimens were examined with a Philips XL-30FEG scanning electron microscope (Philips Electron Optics N.V., Eindhoven, The Netherlands) at 10 kV.
Fractionation of the Adhesive Stylar Extracts
Crude stylar extracts that were active in the adhesion assay were sequentially precipitated at 4°C overnight by adding 95% ethanol to obtain final ethanol concentrations of 20, 40, 60, and 80% (v/v). Pellets were recovered by centrifugation (10,000g for 15 min), washed three times with their corresponding ethanol concentration, dissolved in distilled water, dialyzed against distilled water, and freeze-dried.
The fractions active in the adhesion assay were dissolved with 100 mM imidazole-HCl, pH 7.0, at 2 mg mL−1. The samples were applied to a 50 × 1.5-cm anion-exchange column of DEAE-Sepharose CL-6B or Q Sepharose fast flow (both from Sigma) equilibrated in the same buffer. After washing with 100 mL of buffer to elute the unbound components, 100-mL NaCl solutions (100, 200, 300, 400, and 500 mM and 2 M NaCl) were applied stepwise, and the eluted fractions were collected separately. Fractions were dialyzed exhaustively against distilled water and freeze-dried.
The extracts fractionated by anion-exchange chromatography and revealing in vitro adhesive activities were further purified by size exclusion chromatography. Gel filtration was performed on a 120 × 1-cm column of Sepharose CL-4B equilibrated and eluted with 100 mM imidazole-HCl, pH 7.0, at 0.5 mL min−1. One to three mg of material was loaded onto the column for each run. Fractions (2 mL) were collected, pooled, dialyzed, and freeze-dried. Five dextran standards, of 2000 (Sigma), 600, 170, 75, and 40 kD (Polysciences, Warrington, PA), were also applied for molecular mass calibration of the column. Recoveries from anion-exchange and gel-filtration columns were consistently >90% (w/w).
Analysis of the Adhesive Stylar Extracts
Carbohydrate, uronic acid, and protein content were estimated using the phenol sulfuric acid method (Dubois et al., 1956) with galactose as a standard, using the m-hydroxydiphenyl method (Blumenkrantz and Asboe-Hansen, 1973) with galacturonic acid as a standard, and using the Bio-Rad (Hercules, CA) protein assay with BSA as a standard, respectively. Uronic acids contributed ∼12% of the color of neutral sugars in the phenol sulfuric assay. Total carbohydrate was estimated by summing the concentrations obtained from the m-hydroxydiphenyl assay and the phenol sulfuric assay after correcting for uronic acid interference.
Glycosyl composition analysis was performed by gas–liquid chromatography of the trimethylsilyl derivatives of the methyl glycosides, as described in Komalavilas et al. (1991) and Park et al. (2000).
Immuno dot-blot assays were performed after blotting 10 μg of extract (from a solution of 1 mg mL−1 in distilled water) under vacuum onto nitrocellulose membrane. Blots were processed according to Jauh and Lord (1996), except that the primary monoclonal antibodies (gift of Paul Knox, University of Leeds, UK) were diluted 1:50. The monoclonal antibodies were JIM5 (for low-esterified homogalacturonans, degree of esterification [DE] < 50%) and JIM7 (for esterified homogalacturonans, DE > 35%) (Knox et al., 1990). Antibody binding was revealed with a goat anti–rat IgG (whole molecule) antibody conjugated with alkaline phosphatase (Sigma) diluted 1:1000. Arabinogalactan proteins were also detected by dot-blot, using the (β-d-Glc)3 Yariv phenylglycoside as described in Komalavilas et al. (1991).
Proteins were separated on 12.5% SDS–polyacrylamide gels (Laemmli, 1970) and stained with Coomassie blue. Prestained molecular mass markers (Bio-Rad) ranged from 211 to 6.7 kD.
Enzymatic Treatments of the Adhesive Stylar Extracts and SCA
Extracts (1 mg mL−1) were incubated for 2 hr at 32°C with 200 μL of proteinase K solution (1% w/v; Sigma) prepared in 10 mM Tris-HCl, pH 7.5. The enzymatic reaction was stopped in a boiling water bath for 15 min. The control consisted of adding previously boiled proteinase K to the extracts. Protein degradation was followed by SDS-PAGE as described above.
Extracts (1 mg mL−1) were treated with a purified endopolygalacturonase (PG2; gift of Dean DellaPenna, University of Nevada, Reno) according to Watson et al. (1994), except that 75 mM NaCl was used instead of 150 mM NaCl. The enzymatic reaction was stopped by incubation in a boiling water bath for 5 min. For the control, denatured PG2 (boiled for 5 min) was added to the extracts. Polygalacturonase activity was monitored by measuring the amount of reducing sugars released during the enzymatic reaction with the p-hydroxybenzoic acid hydrazide method (Lever, 1972). The digestion of commercial polygalacturonic acid with PG2 was performed simultaneously and used as a control.
Immunolabeling of the Style with JIM5 and JIM7
Fresh style sections were cut on a cryostat as described by Jauh and Lord (1995) and labeled with JIM5 and JIM7 according to Jauh and Lord (1996) by using a secondary anti–rat antibody (IgG whole molecule) conjugated to fluorescein isothiocyanate (Sigma). Style sections were observed under Nomarski DIC optics or fluorescence illumination on a Nikon (Melville, NY) Microphot FXA microscope equipped with a fluorescein isothiocyanate (absorption 485 to 520 nm; emission 520 to 560 nm) filter set.
In Vitro Binding Assays
SCA(SE) (5 μg) and 50 μg of a carbohydrate polymer (fraction C from the imidazole extract, dextran, or alginic acid) were combined at pH 6.0 or 10.0. After 15 min of incubation at room temperature, the mixtures (55 μL) were diluted with 1 mL of 100 mM Tris-HCl buffer, pH 6.0 or 10.0, and passed through a Centricon filter (molecular mass cutoff of 100 kD; Millipore, Bedford, MA). After being rinsed four times with 1 mL of buffer each, the retentate and the filtrate were collected and concentrated by vacuum centrifugation. The dry residues (retentates and filtrates) were resuspended with an identical volume of SDS buffer (50 μL) to allow quantitative comparisons, and 10 μL of each suspension was loaded on 12.5% SDS–polyacrylamide gels. For a control, SCA (5 μg) only was passed through the 100-kD Centricon filter.
Acknowledgments
We are grateful to Paul Knox (University of Leeds, UK) for the gift of monoclonal antibodies (JIM5 and JIM7) and Dean DellaPenna (University of Nevada, Reno) for the pure endopolygalacturonase (PG2). We also thank Guang Yuh Jauh (Academia Sinica, Taiwan) for the stylar immunolocalizations and Kathleen Eckard and Madhav Yadav for their technical support. This work was supported by a National Science Foundation grant (No. IBN-9603826) to E.M.L.
References
- Albersheim, P., Darvill, A.G., O'Neill, M.A., Schols, H.A., and Voragen, A.G.J. (1996). An hypothesis: The same six polysaccharides are components of the primary cell walls of all higher plants. In Pectins and Pectinases, J. Visser and A.G.J. Voragen, eds (Amsterdam: Elsevier Science BV), pp. 47–55.
- Allen, A.K. (1983). Potato lectin, a glycoprotein with two domains. In Chemical Taxonomy, Molecular Biology, and Function of Plant Lectins (New York: Alan R. Liss, Inc.), pp. 71–85.
- Baldwin, T.C., McCann, M.C., and Roberts, K. (1993). A novel hydroxyproline-deficient arabinogalactan protein secreted by suspension-cultured cells of Daucus carota. Purification and partial characterization. Plant Physiol. 103, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz, N., and Asboe-Hansen, G. (1973). New method for quantitative determination of uronic acids. Anal. Biochem. 54, 484–489. [DOI] [PubMed] [Google Scholar]
- Bush, M.S., and McCann, M.C. (1999). Pectic epitopes are differentially distributed in the cell walls of potato (Solanum tuberosum) tubers. Physiol. Plant. 107, 201–213. [Google Scholar]
- Carpita, N.C., and Gibeaut, D.M. (1993). Structural models of primary cell walls of flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3, 1–30. [DOI] [PubMed] [Google Scholar]
- Carpita, N.C., McCann, M., and Griffing, L.R. (1996). The plant extracellular matrix: News from the cell's frontier. Plant Cell 8, 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassab, G.I. (1998). Plant cell wall proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 281–309. [DOI] [PubMed] [Google Scholar]
- Cheung, A.Y., Wang, H., and Wu, H.M. (1995). A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82, 383–393. [DOI] [PubMed] [Google Scholar]
- Cormack, B.P., Ghori, N., and Falkow, S. (1999). An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285, 578–582. [DOI] [PubMed] [Google Scholar]
- Cosgrove, D.J. (1999). Enzymes and other agents that enhance cell wall extensibility. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 391–417. [DOI] [PubMed] [Google Scholar]
- Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. [Google Scholar]
- Fiete, D.J., Beranek, M.C., and Baenziger, J.U. (1998). A cysteine-rich domain of the “mannose” receptor mediates GalNAc-4-SO4 binding. Proc. Natl. Acad. Sci. USA 95, 2089–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, J.E., and Quatrano, R.S. (1997). Plant cell morphogenesis: Plasma membrane interactions with the cytoskeleton and cell wall. Annu. Rev. Cell Dev. Biol. 13, 697–743. [DOI] [PubMed] [Google Scholar]
- Fry, S.C. (1986). Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu. Rev. Plant Physiol. 37, 165–186. [Google Scholar]
- Fry, S.C. (1988). The Growing Plant Cell Wall: Chemical and Metabolic Analysis. (London: Longman Scientific and Technical).
- Fry, S.C., Aldington, S., Hetherington, P.R., and Aitken, J. (1993). Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiol. 103, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, R., Morvan, C., Jauneau, A., and Jarvis, M.C. (1996). Methyl-esterification, de-esterification and gelation of pectins in the primary cell wall. In Pectins and Pectinases, J. Visser and A.G.J. Voragen, eds (Amsterdam: Elsevier Science BV), pp. 151–172.
- Gross, K.C., and Sams, C.E. (1984). Changes in cell wall neutral sugar composition during fruit ripening: A species survey. Phytochemistry 23, 2457–2461. [Google Scholar]
- Hadfield, K.A., and Bennett, A.B. (1998). Polygalacturonases: Many genes in search of a function. Plant Physiol. 117, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock, E.M., and Norris, C.R. (1997). Netrins evoke mixed reactions in motile cells. Trends Genet. 13, 251–253. [DOI] [PubMed] [Google Scholar]
- Hirsch, A.M. (1999). Role of lectins (and rhizobial exopolysaccharides) in legume nodulation. Curr. Opin. Plant Biol. 2, 320–326. [DOI] [PubMed] [Google Scholar]
- Huber, O., and Sumper, M. (1994). Algal-CAMs: Isoforms of a cell adhesion molecule in embryos of the alga Volvox with homology to Drosophila fasciclin I. EMBO J. 13, 4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson, J., Reinders, M.C., Valkering, A.G.M., Van Tuyl, J.M., and Keijzer, C.J. (1994). Pistil exudate production and pollen tube growth in Lilium longiflorum Thunb. Ann. Bot. 73, 437–446. [Google Scholar]
- Jarvis, M.C. (1984). Structure and properties of pectic gels in plant cell walls. Plant Cell Environ. 7, 153–164. [Google Scholar]
- Jauh, G.Y., and Lord, E.M. (1995). Movement of the tube cell in lily style in the absence of the pollen grain and the spent pollen tube. Sex. Plant Reprod. 8, 168–172. [Google Scholar]
- Jauh, G.Y., and Lord, E.M. (1996). Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta 199, 251–261. [Google Scholar]
- Jauh, G.Y., Eckard, K.J., Nothnagel, E.A., and Lord, E.M. (1997). Adhesion of lily pollen tubes on an artificial matrix. Sex. Plant Reprod. 10, 173–180. [Google Scholar]
- John, M., Rohrig, H., Schmidt, J., Walden, R., and Schell, J. (1997). Cell signalling by oligosaccharides. Trends Plant Sci. 2, 111–115. [Google Scholar]
- Kido, S., Yasufuku, H., and Azuma, J.I. (1996). Isolation and characterization of arabinogalactan proteins released by cellulase digestion of cabbage leaves. J. Agric. Food Chem. 44, 3432–3436. [Google Scholar]
- Kikuchi, A., Edashige, Y., Ishii, T., Fujii, T., and Satoh, S. (1996). Variations in the structure of neutral sugar chains in the pectic polysaccharides of morphologically different carrot calli and correlations with the size of cell clusters. Planta 198, 634–639. [DOI] [PubMed] [Google Scholar]
- Knox, P.J. (1997). The use of antibodies to study the architecture and developmental regulation of plant cell walls. Int. Rev. Cytol. 171, 79–120. [DOI] [PubMed] [Google Scholar]
- Knox, P.J., Linstead, P.J., King, J., Cooper, C., and Roberts, K. (1990). Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181, 512–521. [DOI] [PubMed] [Google Scholar]
- Kobayashi, M., Nakagawa, H., Asaka, T., and Matoh, T. (1999). Borate-rhamnogalacturonan II bonding reinforced by Ca2+ retains pectic polysaccharides in higher-plant cell walls. Plant Physiol. 119, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn, B.D. (2000). Update on plasma membrane/cell wall adhesion. Plant Physiol., in press. [DOI] [PMC free article] [PubMed]
- Komalavilas, P., Zhu, J.-K., and Nothnagel, E.A. (1991). Arabinogalactan-proteins from the suspension culture medium and plasma membrane of rose cells. J. Biol. Chem. 266, 15956–15965. [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lennon, K.A., Roy, S.J., Hepler, P.K., and Lord, E.M. (1998). The structure of the transmitting tissue of Arabidopsis thaliana (L.) and the path of pollen tube growth. Sex. Plant Reprod. 11, 49–59. [Google Scholar]
- Lever, M. (1972). A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 47, 273–279. [DOI] [PubMed] [Google Scholar]
- Liners, F., Letesson, J.J., Didembourg, C., and Van Cutsem, P. (1989). Monoclonal antibodies against pectin. Recognition of a conformation induced by calcium. Plant Physiol. 91, 1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liners, F., Gaspar, T., and Van Cutsem, P. (1994). Acetyl- and methyl-esterification of pectins of friable and compact sugar-beet calli: Consequences for intercellular adhesion. Planta 192, 545–556. [Google Scholar]
- Lord, E.M., Walling, L.L., and Jauh, G.Y. (1996). Cell adhesion in plants and its role in pollination. In Membranes: Specialized Functions in Plants, M. Smallwood, J.P. Knox, and D.J. Bowles, eds (Oxford, UK: BIOS Scientific Publishers), pp. 21–37.
- Matoh, T., Takasaki, M., Takabe, K., and Kobayashi, M. (1998). Immunocytochemistry of rhamnogalacturonan II in cell walls of higher plants. Plant Cell Physiol. 39, 483–491. [Google Scholar]
- Mohnen, D. (1999). Biosynthesis of pectins and galactomannans. In Comprehensive Natural Products Chemistry, Vol. 3: Carbohydrates and Their Derivatives Including Tannins, Cellulose and Related Lignins, D. Baron and K. Nakanishi, eds (Amsterdam: Elsevier Science), pp. 497–527.
- Moore, P.J., Darvill, A.G., Albersheim, P., and Staehelin, L.A. (1986). Immunogold localization of xyloglucan and rhamnogalacturonan I in the cell walls of suspension-cultured sycamore cells. Plant Physiol. 82, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort, A.J., Moerschbacher, B.M., Pierce, M.L., and Maness, N.O. (1991). Problems encountered during the extraction, purification, and chromatography of pectic fragments, and some solutions to them. Carbohydr. Res. 215, 219–227. [Google Scholar]
- Nothnagel, E.A. (1997). Proteoglycans and related components in plant cells. Int. Rev. Cytol. 174, 195–291. [DOI] [PubMed] [Google Scholar]
- Nothnagel, E.A., McNeil, M., Albersheim, P., and Dell, A. (1983). Host–pathogen interactions. XXII. A galacturonic acid oligosaccharide from plant cell walls elicits phytoalexins. Plant Physiol. 71, 916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek, I., and Doyle, R.J. (1994). Bacterial Adhesion to Cells and Tissues. (London: Chapman and Hall).
- O'Neill, M., Albersheim, P., and Darvill, A.G. (1990). The pectic polysaccharides of primary cell walls. In Methods in Plant Biochemistry, Carbohydrates, P.M. Dey, and J.B. Harborne, eds (London: Academic Press), pp. 415–441.
- Park, S.-Y., Jauh, G.-Y., Mollet, J.-C., Eckard, K.J., Nothnagel, E.A., Walling, L.L., and Lord, E.M. (2000). A lipid transfer-like protein is necessary for lily pollen tube adhesion to an in vitro stylar matrix. Plant Cell 12, 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penel, C., and Greppin, H. (1996). Pectin binding proteins: Characterization of the binding and comparison with heparin. Plant Physiol. Biochem. 34, 479–488. [Google Scholar]
- Pressey, R. (1991). Polygalacturonase in tree pollens. Phytochemistry 26, 1867–1870. [Google Scholar]
- Qi, X.Y., Beherens, B.X., West, P.R., and Mort, A.J. (1995). Solubilization and partial characterization of extensin fragments from cell walls of cotton suspension cultures: Evidence for a covalent cross-link between extensin and pectin. Plant Physiol. 108, 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgwell, R.J., Fischer, M., Kendal, E., and MacRae, E.A. (1997). Galactose loss and fruit ripening: High-molecular-weight arabinogalactans in the pectic polysaccharides of fruit cell walls. Planta 203, 174–181. [Google Scholar]
- Rhee, S.Y., and Somerville, C.R. (1998). Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant J. 15, 79–88. [DOI] [PubMed] [Google Scholar]
- Rini, J.M. (1995). Lectin structure. Annu. Rev. Biophys. Biomol. Struct. 24, 551–577. [DOI] [PubMed] [Google Scholar]
- Roberts, K. (1990). Structure at the plant cell surface. Curr. Opin. Cell Biol. 2, 920–928. [DOI] [PubMed] [Google Scholar]
- Roberts, K. (1994). The plant extracellular matrix: In a new expansive mood. Curr. Opin. Cell Biol. 6, 1020–1027. [DOI] [PubMed] [Google Scholar]
- Roland, J.C., and Vian, B. (1981). Use of purified endopolygalacturonase for topochemical study of elongating cell walls at the ultrastructural level. J. Cell Sci. 48, 333–343. [DOI] [PubMed] [Google Scholar]
- Rubinstein, A., Broadwater, A., Lowrey, K., and Bedinger, P. (1995). Pex1, a pollen-specific gene with an extensin-like domain. Proc. Natl. Acad. Sci. USA 92, 3086–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, S. (1998). Functions of the cell wall in the interaction of plant cells: Analysis using carrot cultured cells. Plant Cell Physiol. 39, 361–368. [Google Scholar]
- Schindler, T.M. (1998). The new view of the primary cell wall. Z. Pflanzenernaehr. Bodenkd. 161, 485–498. [Google Scholar]
- Schols, H.A., and Voragen, A.G.J. (1996). Complex pectins: Structure elucidation using enzymes. In Pectins and Pectinases, J. Visser and A.G.J. Voragen, eds (Amsterdam: Elsevier Science BV), pp. 3–19.
- Schopfer, C.R., Nasrallah, M.E., and Nasrallah, J.B. (1999). The male determinant of self-incompatibility in Brassica. Science 286, 1697–1700. [DOI] [PubMed] [Google Scholar]
- Selvendran, R.R., and Ryden, P. (1990). Isolation and analysis of plant cell walls. In Methods in Plant Biochemistry, Carbohydrate, P.M. Dey and J.B. Harborne, eds (New York: Academic Press), pp. 549–579.
- Sieber, P., Schorderet, M., Ryser, U., Buchala, A., Kolattukudy, P., Metraux, J.-P., and Nawrath, C. (2000). Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell 12, 721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, N., and Lynch, M. (1998). Fused organs in the adherent1 mutation in maize show altered epidermal walls with no perturbations in tissue identities. Planta 206, 184–195. [Google Scholar]
- Svetek, J., Yadav, M.P., and Nothnagel, E.A. (1999). Presence of a glycosylphosphatidylinositol lipid anchor on rose arabinogalactan proteins. J. Biol. Chem. 27, 14724–14733. [DOI] [PubMed] [Google Scholar]
- Taylor, L.P., and Hepler, P.K. (1997). Pollen germination and tube growth. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 461–491. [DOI] [PubMed] [Google Scholar]
- Vreeland, V., Waite, J.H., and Epstein, L. (1998). Polyphenols and oxidases in substratum adhesion by marine algae and mussels. J. Phycol. 34, 1–8. [Google Scholar]
- Wakeley, P.R., Rogers, H.J., Rozycka, M., Greenland, A.J., and Hussey, P.J. (1998). A maize pectin methylesterase-like gene, ZmC5, specifically expressed in pollen. Plant Mol. Biol. 37, 187–192. [DOI] [PubMed] [Google Scholar]
- Watson, C.F., Zheng, L., and DellaPenna, D. (1994). Reduction of tomato polygalacturonase β subunit expression affects pectin solubilization and degradation during fruit ripening. Plant Cell 6, 1623–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherbee, R., Lind, J.L., Burke, J., and Quatrano, R.S. (1998). The first kiss: Establishment and control of initial adhesion by raphid diatoms. J. Phycol. 34, 9–15. [Google Scholar]
- Willats, W.G.T., Gilmartin, P.M., Mikkelsen, J.D., and Knox, J.P. (1999). Cell wall antibodies without immunization: Generation and use of de-esterified homogalacturonan block-specific antibodies from a naive phage display library. Plant J. 18, 57–65. [DOI] [PubMed] [Google Scholar]
- Williams, E.G., Clarke, A.E., and Knox, R.B. (1994). Genetic Control of Self-Incompatibility and Reproductive Development in Flowering Plants. (Dordrecht, The Netherlands: Kluwer Academic Publishers).
- Wustman, B.A., Lind, J., Wetherbee, R., and Gretz, M.R. (1998). Extracellular matrix assembly in diatoms (Bacillariophyceae). III. Organization of fucoglucoronogalactans within the adhesive stalks of Achnanthes longipes. Plant Physiol. 116, 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youl, J., Bacic, A., and Oxley, D. (1998). Arabinogalactan-proteins from Nicotiana alata and Pyrus communis contain glycosylphosphatidylinositol membrane anchors. Proc. Natl. Acad. Sci. USA 95, 7921–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkl, G.M., Zwiebel, B.I., Grier, D.G., and Preuss, D. (1999). Pollen–stigma adhesion in Arabidopsis: A species-specific interaction mediated by lipophilic molecules in the pollen exine. Development 126, 5431–5440. [DOI] [PubMed] [Google Scholar]