Abstract
Regulation of translation elongation, membrane insertion, and assembly of the chloroplast-encoded D1 protein of photosystem II (PSII) was studied using a chloroplast translation system in organello. Translation elongation of D1 protein was found to be regulated by (1) a redox component that can be activated not only by photosynthetic electron transfer but also by reduction with DTT; (2) the trans-thylakoid proton gradient, which is absolutely required for elongation of D1 nascent chains on the thylakoid membrane; and (3) the thiol reactants N-ethylmaleimide (NEM) and iodosobenzoic acid (IBZ), which inhibit translation elongation with concomitant accumulation of distinct D1 pausing intermediates. These results demonstrate that D1 translation elongation and membrane insertion are tightly coupled and highly regulated processes in that proper insertion is a prerequisite for translation elongation of D1. Cotranslational and post-translational assembly steps of D1 into PSII reaction center and core complexes occurred independently of photosynthetic electron transfer or trans-thylakoid proton gradient but were strongly affected by the thiol reactants DTT, NEM, and IBZ. These compounds reduced the stability of the early PSII assembly intermediates, hampered the C-terminal processing of the precursor of D1, and prevented the post-translational reassociation of CP43, indicating a strong dependence of the D1 assembly steps on proper redox conditions and the formation of disulfide bonds.
INTRODUCTION
Photosystem II (PSII) is a multiprotein thylakoid membrane complex that catalyzes oxidation of water and reduction of plastoquinone. An intriguing feature of PSII is its vulnerability to light, which induces inactivation of PSII and subsequent damage and degradation of the D1 reaction center protein. The basic concept of the rapid light-dependent turnover of the D1 protein in PSII is relatively well documented (Prasil et al., 1992; Aro et al., 1993). However, little is known about regulation of the PSII repair process—either at the level of protein synthesis, insertion, and concomitant assembly of the D1 protein or during the later post-translational assembly steps that lead to functional PSII. We demonstrated recently that the D1 protein integrates and assembles with other PSII polypeptides, particularly the D2 protein, during translation elongation (Zhang et al., 1999). The D2 protein in the stroma-exposed membranes seems to serve as a receptor for elongating D1 protein (Adir et al., 1990; van Wijk et al., 1997); moreover, interaction with D2 becomes tighter with increasing length of the D1 nascent chain (Zhang et al., 1999).
Post-translational membrane insertion and translocation of nuclear-encoded thylakoid proteins have received much attention and are known to follow at least three distinct routes (Robinson et al., 1998; Schnell, 1998; Keegstra and Cline, 1999). Insertion and assembly of chloroplast-encoded proteins into the thylakoid membrane, however, are far less understood (Wollmann, 1998; Wollman et al., 1999). Membrane proteins such as D1 and cytochrome f are generally thought to insert cotranslationally into the thylakoid membrane. Studies in vitro have shown that cytochrome f can also insert post-translationally into the thylakoid membrane, a translocation that requires ATP and SecA (Nohara et al., 1996; Mould et al., 1997). Mutations in the cytochrome f signal sequence that prevented the translocation of the protein not only blocked the accumulation of cytochrome f but also interfered with the translocation of D1 and light-harvesting complex II (LHCII; Smith and Kohorn, 1994). These results point to the possibility of a common translocation pathway for D1, cytochrome f, and LHCII.
Chloroplast gene expression is strongly regulated by light in plants and in the unicellular algae Chlamydomonas reinhardtii. Many experimental studies have been focused on the light-dependent translation of psbA mRNA, which encodes the D1 protein (Gillham et al., 1994; Rochaix, 1996; Bruick and Mayfield, 1999). Translation initiation of the D1 protein in Chlamydomonas is activated by binding regulatory proteins to the 5′ end of the psbA mRNA, and activation of these RNA binding proteins may be mediated by the chloroplast redox state and ADP-dependent phosphorylation induced by light (Danon and Mayfield, 1994a, 1994b; Kim and Mayfield, 1997). Translation elongation of D1 is also strictly light regulated in higher plants (Kim et al., 1991, 1994; Taniguchi et al., 1993; Kuroda et al., 1996; Kettunen et al., 1997; Mühlbauer and Eichacker, 1998). D1 translation takes place on polyribosomes associated with the thylakoid membrane, and ribosomal pausing occurs at distinct sites during the elongation of the protein. Such ribosomal pausing has been postulated to allow cotranslational protein folding and efficient assembly of PSII (Kim et al., 1991; Zhang et al., 1999).
The D1 protein is synthesized as a precursor (pD1) with a C-terminal extension (Reisfeld et al., 1982) and processed to the mature form by the action of a lumenal peptidase (Bowyer et al., 1992). The functional importance of the C-terminal extension of pD1 remains an issue of debate because the mutants that lack the C-terminal extension still maintain photosynthetic capacity at wild-type levels (Lers et al., 1992). However, processing of the C-terminal extension is absolutely essential for the activation of the oxygen-evolving machinery of PSII (Metz and Seibert, 1984; Diner et al., 1988).
Although the control of D1 protein translation elongation on the thylakoid membrane has been studied to some extent, the regulatory interplay between translation elongation, membrane insertion, and the assembly of the D1 protein into PSII is unresolved. Also, the regulatory events involved in the post-translational assembly of PSII, such as reassociation of CP43 and C-terminal processing of pD1 during the repair process, are poorly understood. To simultaneously address D1 protein elongation, insertion, and assembly, we have adopted the experimental system of translation in vitro in intact chloroplasts and studied the regulation of elongation of D1 nascent chains and their cotranslational interaction with other PSII proteins in ribosome pellets. The post-translational regulation of PSII assembly was investigated by sucrose density fractionation analysis of PSII subcomplexes in the thylakoid membrane.
RESULTS
Optimization of Chloroplast Translation
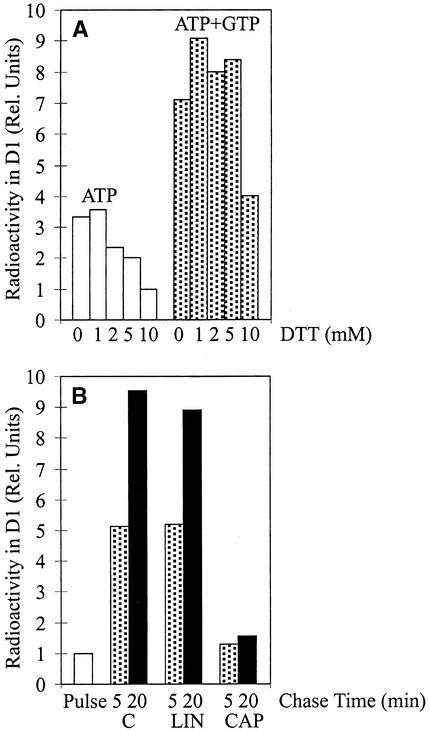
Translation mixtures generally used in chloroplast protein synthesis include 10 mM Mg-ATP and 10 mM DTT (dithiothreitol [DTTred]) (Mullet et al., 1986). However, the incorporation of 35S-methionine into the precursor and mature D1 protein (full-length D1) was two- to threefold greater if 2 mM GTP was also included in the translation mixture (Figure 1A). This indicates that the amount of endogenous GTP in chloroplasts is suboptimal for protein translation in our translation system. Using different concentrations of DTTred (in the range of 0 to 10 mM) during chloroplast translations, we observed that synthesis of full-length D1 was not stimulated by DTTred (Figure 1A). Therefore, at variance with earlier experiments, 2 mM GTP was routinely included in the translation mixture and DTTred was omitted, unless otherwise stated.
Figure 1.
D1 Protein Translation in Intact Chloroplasts.
(A) Optimization of D1 synthesis. Intact chloroplasts were pulse labeled in the light for 10 min. Reactions were performed in the presence of 10 mM ATP or 10 mM ATP and 2 mM GTP and several concentrations of DTTred (DTT; 0, 1, 2, 5, and 10 mM). The relative amount (Rel. Units) of radioactivity incorporated into the precursor and mature D1 protein after 10 min of pulse labeling in the presence of 10 mM DTTred and 10 mM ATP, which are the conditions usually used for translation in vitro in intact chloroplasts (Mullet et al., 1986), was set as 1.
(B) Translation elongation of the D1 protein during the chase. Intact chloroplasts were pulse labeled for 2.5 min and chased for 5 or 20 min in the presence of lincomycin (100 μg mL−1) or chloramphenicol (100 μg mL−1), which were added to the chase buffer immediately after the pulse. The relative amount of radioactivity incorporated into the precursor and mature D1 protein after 2.5 min of pulse labeling was set as 1. C, control; CAP, chloramphenicol; LIN, lincomycin.
Control of D1 Elongation
Because of the possibility that both translation initiation and elongation contributed to the accumulation of radiolabeled precursor and mature D1 protein during the 10-min pulse labeling (Figure 1A), the elongating D1 protein in intact chloroplasts was chased in the presence of unlabeled methionine added immediately after a short pulse of 2.5 min to monitor the efficiency of translation elongation only (Figure 1B). Indeed, the presence of lincomycin, an inhibitor of translation initiation, had no effect on the incorporation of radioactivity into full-length D1 protein, whereas chloramphenicol, an inhibitor of translation elongation, completely blocked the synthesis of D1.
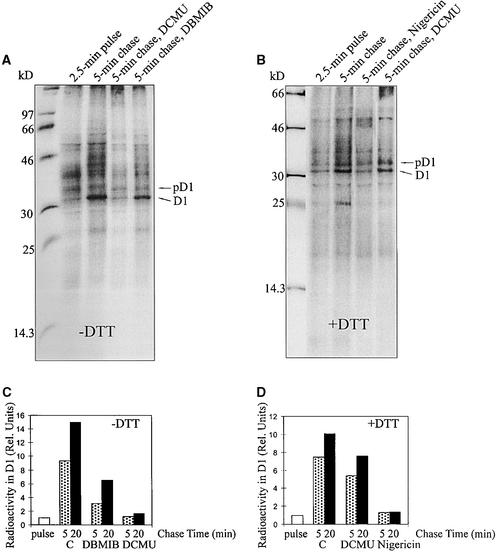
As a next step, we studied the effects of the electron transfer inhibitors 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB) on elongation of the D1 protein. As shown in Figures 2A and 2C, addition of DCMU at the beginning of the 5-min chase period completely inhibited translation elongation of the D1 protein; addition of DBMIB, an inhibitor of cytochrome b6f complex, also retarded the elongation of D1. However, addition of DTTred in a low concentration (5 mM) partially abolished the inhibitory effect of DCMU in the light (Figures 2B and 2D), pointing to a possible redox component essential for translation elongation. Nigericin (Figures 2B and 2D) and carbonyl cyanide m-chlorophenylhydrazone (data not shown), both uncouplers of the trans-thylakoid proton gradient, also inhibited the elongation of the D1 protein, but this inhibition could not be restored with DTTred (Figure 2B).
Figure 2.
Effects of Photosynthetic Electron Transfer Inhibitors and Uncouplers on D1 Translation Elongation.
Intact chloroplasts were incubated for 5 min at 23°C and 50 μE m−2 sec−1 in a translation mixture in the absence (−DTT) ([A] and [C]) or the presence (+DTT) ([B] and [D]) of 5 mM DTTred. After 2.5 min of pulse labeling in intact chloroplasts, nigericin (1 μM), DCMU (10 μM), or DBMIB (2 μM) together with unlabeled methionine (10 mM) was added to the chase buffer.
(A) and (B) Autoradiography of 35S-labeled translation products in the thylakoid membrane. The molecular mass markers in kilodaltons are indicated at left.
(C) and (D) Quantification of 35S-methionine incorporation into the precursor and mature D1 protein. The relative amount (Rel. Units) of radioactivity incorporated into the precursor and mature D1 protein after 2.5 min of pulse labeling was set as 1. C, control (i.e., only 10 mM unlabeled methionine was added in the beginning of the chase).
The involvement of possible thiol redox regulation of translation elongation was studied by applying the thiol reactants N-ethylmaleimide (NEM, which alkylates free thiol groups), iodosobenzoic acid (IBZ, a strong oxidant that induces unnatural disulfide bonds), and DTTred (which reduces disulfide bonds) during the chase. Indeed, all three thiol reagents inhibited the accumulation of radiolabel in the D1 protein but to various extents (Figure 3A). Some radiolabeled bands of molecular masses greater than that of D1, particularly those seen in Figure 3A when thiol reactants were present during the chase, possibly represent tRNA–D1 nascent chain complexes that remain associated with the membranes during the isolation of thylakoid preparations. A band of ∼40 kD, for example, was also present in the pulse sample but nearly disappeared during the 5-min chase period, with a concomitant accumulation of full-length D1 in the thylakoid membrane (Figure 3A).
Figure 3.
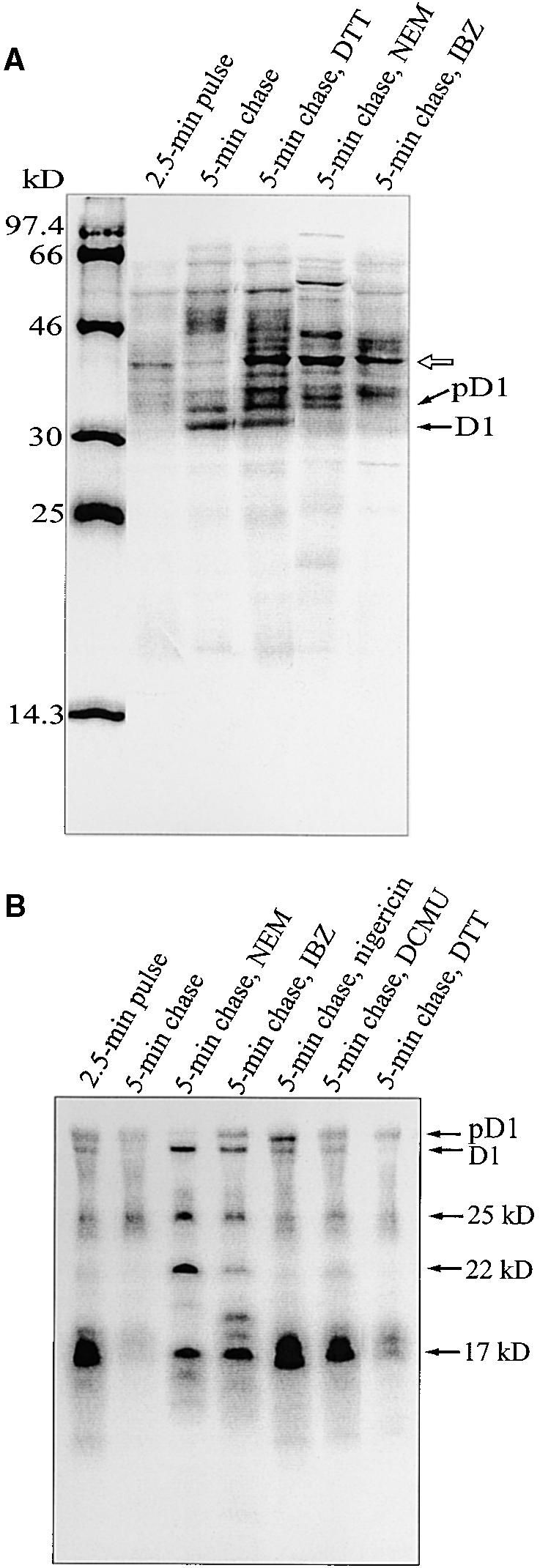
Regulation of Translation Elongation.
(A) Autoradiography of labeled thylakoid membrane proteins. After 2.5 min of pulse labeling in intact chloroplasts, the accumulation of label in the D1 protein was chased in the light for 5 min in the presence of 10 mM unlabeled methionine and NEM (5 mM), IBZ (10 mM), or DTTred (DTT; 20 mM). The molecular mass markers in kilodaltons are indicated at left. The open arrow indicates a possible tRNA–D1 nascent chain complex.
(B) Immunoprecipitation of labeled D1 intermediates from RNCs. After 2.5 min of pulse labeling in intact chloroplasts, followed by 5 min of chase in the presence of NEM (5 mM), IBZ (10 mM), DTTred (DTT; 20 mM), DCMU (10 μM), or nigericin (1 μM), the thylakoid-bound polysomes were isolated and solubilized with SDS, and immunoprecipitation was performed by adding an excess of N-terminal D1 antiserum. The precipitated products were separated by SDS-PAGE and visualized by autoradiography. The D1 intermediates of 17, 22, and 25 kD are indicated at right. Minor amounts of D1 and pD1 were always found in RNCs.
Chase of the D1 Intermediates in Ribosome Pellets
To analyze the elongation process of D1 in more detail, we isolated thylakoid-bound ribosome nascent chain complexes (RNCs) and quantitatively immunoprecipitated the D1 nascent chains with an excess of D1 antibody. As presented in Figure 3B, after 2.5 min of pulse labeling, the D1 intermediates of 17, 22, and 25 kD were present in RNCs. By the end of a 5-min chase, most of the radioactivity had disappeared from the D1 intermediates (particularly that of 17 kD) in RNCs (Figure 3B) and was instead accumulated into the full-length D1 protein that had been released from ribosomes and was detected in the thylakoid membrane fraction (Figure 3A). However, when nigericin or DCMU was added at the beginning of the 5-min chase, the pattern of labeled D1 intermediates in RNCs at the end of the 5-min chase was very similar to that of the 2.5-min pulse sample (Figure 3B). Indeed, elongation of D1 was almost completely blocked, and only negligible amounts of labeled full-length D1 accumulated in the thylakoid membrane (Figure 3A). It is worth noting that the D1 nascent chains were not degraded, although translation elongation was blocked (Figure 3B). Lesser amounts of nascent D1 chains were observed after a 5-min chase in the presence of DTTred (Figure 3B); simultaneously, only partial inhibition of accumulation of radiolabeled full-length D1 protein was observed (Figure 3A). The addition of NEM or IBZ had a pronounced effect on D1 elongation; they only partially allowed the elongation of the 17-kD D1 intermediate during the chase and also retarded the elongation of the 22- and 25-kD intermediates (Figure 3B). In the presence of IBZ, an additional 19-kD D1 intermediate accumulated (Figure 3B).
Analysis of Polysome-Associated psbA mRNA
To verify that DCMU, nigericin, and the thiol reactants did not destabilize the thylakoid-bound polysomes or induce release of psbA mRNA, we studied the amount of psbA mRNA associated with thylakoid-bound polysomes in the presence of these different chemicals. As shown in Figure 4, the amount of psbA mRNA actively associated with thylakoid-bound polysomes was very similar in all samples after 2.5-min pulse labeling followed by a 5-min chase in intact chloroplasts. This apparent constant amount of psbA mRNA in thylakoid-bound ribosomes indicates that the chemicals do not release psbA mRNA from thylakoid-bound polysomes and that possibly multiple rounds of translation initiations occur in isolated chloroplasts.
Figure 4.
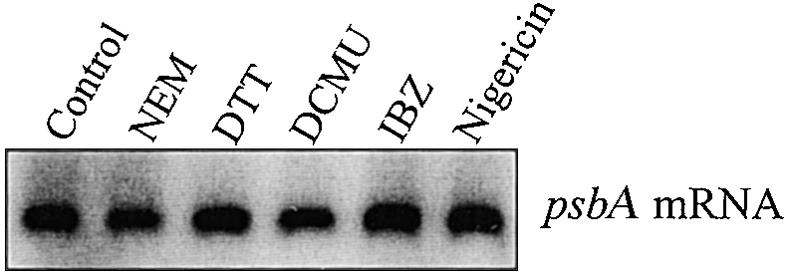
RNA Gel Blot Analysis of psbA mRNA Associated with Thylakoid-Bound Polysomes.
Thylakoid-bound polysomes were isolated after 2.5 min of pulse labeling followed by a 5-min chase in intact chloroplasts in the presence of the following reagents: NEM (5 mM), IBZ (10 mM), DTTred (DTT; 20 mM), DCMU (10 μM), or nigericin (1 μM). The polysome-associated RNA was isolated by extraction with phenol/chloroform and then probed with a random prime-labeled Synechocystis sp 6803 psbA2 gene.
Interaction of D1 Nascent Chains with D2
In our recent study (Zhang et al., 1999), we showed that already during translation elongation, the D1 nascent chain of 25 kD forms a tight interaction with the D2 protein. Here, we used protein cross-linking techniques to study the nature of the interaction of D1 nascent chains with D2. RNCs were isolated after 2.5 min of pulse labeling and subsequently treated with the cross-linking agent BMH (bismaleimide hexane; the homobifunctional sulfhydryl cross-linker). In RNCs without cross-linking, the D1 intermediates of 17, 22, and 25 kD were efficiently precipitated with the N-terminal D1 antibody (Figure 5). The 25-kD labeled polypeptide, precipitated with the D2 antibody, has also been shown to be a D1 intermediate (Zhang et al., 1999). After cross-linking with BMH, several labeled high molecular mass cross-linked products, including a 50-kD product migrating in front of IgG heavy chain (Figure 5), were precipitated with the D1 antibody. The appearance of high molecular mass cross-linked products occurred concomitantly with a loss of labeled D1 intermediates, particularly the 25-kD intermediate. Importantly, the cross-linked 50-kD product and products of higher molecular mass could also be precipitated with the D2 antibody, with a concomitant loss of the 25-kD labeled band. Most likely, therefore, the 50-kD cross-linked product probably arose from the cross-linking of the 25-kD D1 nascent chain with D2. Some of the 50-kD product was seen even without cross-linking, apparently the result of a tight interaction of the 25-kD D1 intermediate and the D2 protein. Precipitated cross-linked products of higher molecular mass (indicated by arrows in Figure 5) probably contained D1 intermediates along with the D2 protein and also other PSII proteins.
Figure 5.
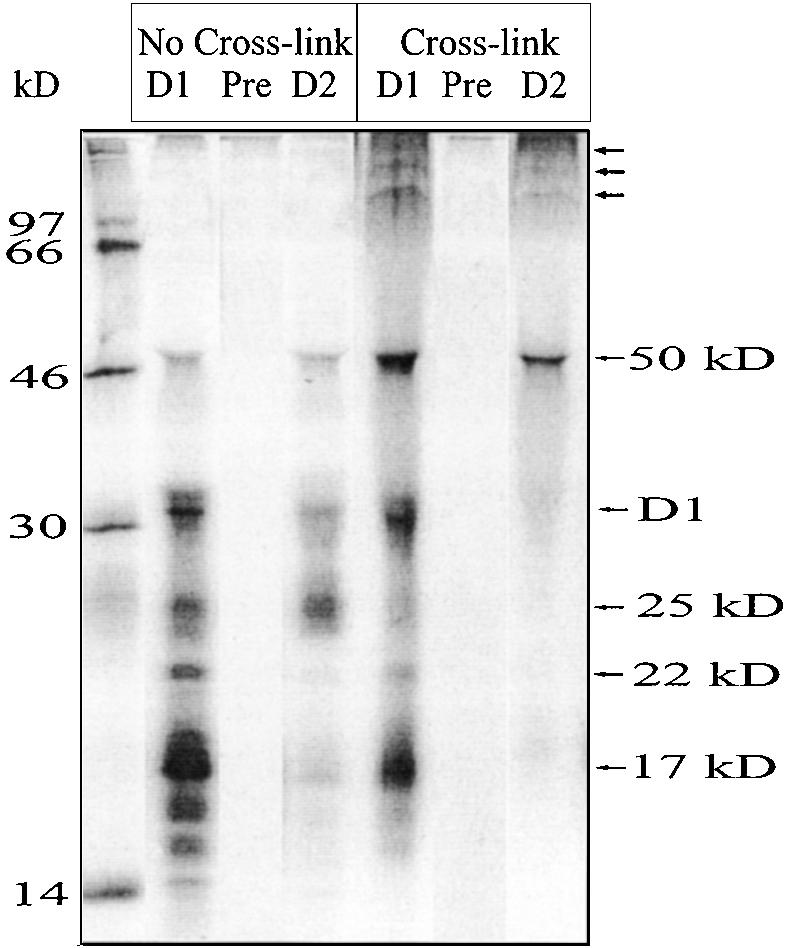
Interaction of D1 Nascent Chains with the D2 Protein.
After 2.5 min of pulse labeling in intact chloroplasts, thylakoid-bound ribosomes were isolated and incubated with the homobifunctional sulfhydryl cross-linker BMH. After cross-linking, the samples were denatured with SDS and immunoprecipitated with antisera raised against the N-terminal residues (58 to 86) of the D1 protein (lanes labeled D1) or against the residues (230 to 245) of the D2 protein (lanes labeled D2). Lanes labeled Pre indicate immunoprecipitation with preserum (similar results were obtained with both the D1 and D2 presera). The precipitated products were separated by SDS-PAGE and visualized by autoradiography. The molecular mass markers in kilodaltons are indicated at left.
Stability of the Early Assembly Steps of PSII
We next investigated whether the stability of the early D1 interactions with D2 and other PSII components is dependent on the energy and redox state of the thylakoid membrane. To this end, we monitored the amount of newly synthesized unassembled precursor and mature D1 protein present in sucrose gradient fractions 12 to 16 (see Zhang et al., 1999) after 5- and 20-min chases in the presence of electron transfer inhibitors, uncouplers, or thiol reactants. Although many of these chemicals severely hampered the elongation of D1 (Figures 2 and 3), some full-length D1 was always synthesized (e.g., already during the pulse), thus making it possible to study the assembly processes. Longer exposure times were required for autoradiograms in these experiments than in the control experiments. Also, polysomes with possible tRNA–D1 nascent chain complexes, as seen in Figure 3A, were pelleted and thus not recovered in the sucrose fractions collected for analysis of the assembly process of the newly synthesized proteins into PSII complexes.
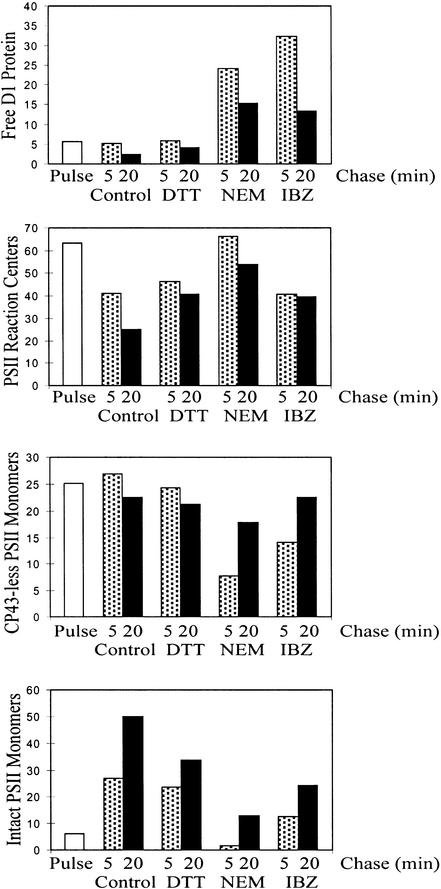
In the control experiments, only 5% of the labeled precursor and mature D1 protein was found unassembled after a 5-min chase and ∼2% after a 20-min chase (Figures 6 and 7). Moreover, DCMU, DBMIB, and nigericin did not exert any destabilizing effect on the early assembly stages of the D1 protein (data not shown). On the contrary, cotranslational stable assembly of the D1 protein with D2 was severely hampered by the thiol reactants NEM (Figures 6 and 7) and IBZ (Figure 7), revealing after the 5-min chase ∼24 and 32% of labeled precursor and mature D1 remaining as unassembled proteins, respectively. After a 20-min chase, ∼15 and 13% of labeled precursor and mature D1 were still unassembled when the chase was performed in the presence of NEM and IBZ, respectively. This suggests that formation of one or more S–S bonds plays an important role in the early assembly steps of the D1 protein. This could occur at the level of D1–D2 interaction or elsewhere in the insertion/assembly machinery.
Figure 6.
Thiol Reactants Hamper Incorporation of Newly Synthesized D1 Protein into PSII Subcomplexes.
Intact chloroplasts were radiolabeled for 2.5 min and subsequently chased for 5 and 20 min in the light with no additions or in the presence (+) of 5 mM NEM or 20 mM DTTred (DTT). After translation, thylakoids were solubilized, and different PSII subcomplexes were separated in the sucrose gradients. The gradients were fractionated into 20 equal fractions, and the proteins in each fraction were separated by SDS-PAGE and visualized by autoradiography. According to earlier assignments (Zhang et al., 1999), unassembled PSII proteins were found in fractions 12 to 16, PSII reaction centers (RC) in fractions 10 and 11, PSII core complexes lacking CP43 in fraction 9, and intact PSII cores (including CP43) in fractions 7 and 8.
Figure 7.
Quantification of Incorporation of Newly Synthesized D1 Protein into PSII Subcomplexes.
Accumulation of radioactivity in the precursor and mature D1 protein in different PSII subpopulations was quantified after 5- and 20-min chases. Each chase was conducted with no additional chemicals (Control) or in the presence of DTTred (DTT), NEM, or IBZ, and PSII subcomplexes were separated, as given in the legend to Figure 6. Relative amounts of radioactivity in unassembled D1 protein (free D1 protein), PSII reaction centers, CP43-less PSII monomers, and intact PSII monomers (including CP43) are indicated. The data are expressed as the percentage of accumulated total labeled precursor and mature D1 protein.
Analysis of Post-Translational Assembly Steps of PSII
Although the initial assembly of the D1 protein into PSII is cotranslational, the formation of functional PSII complexes also depends on several post-translational assembly steps, including reassociation of CP43 and C-terminal processing of the precursor D1 protein. After a 2.5-min pulse in the control sample (Figures 6 and 7), ∼63% of labeled D1 protein was found in the reaction center complexes (fractions 10 and 11), ∼25% in fraction 9 (which is highly enriched in PSII core complexes devoid of CP43), and 6% in PSII core complexes with associated CP43 (fractions 7 and 8). During the chase period, the incorporation of labeled precursor and mature D1 protein into the PSII core (fractions 7 to 9) gradually increased, with a concomitant loss of radiolabeled D1 in the PSII reaction center complexes (Figures 6 and 7). Although D1 translation elongation was almost completed within the 5-min chase period (Figure 3B), the post-translational assembly steps continued for a much longer period. After a 20-min chase, most of the labeled D1 protein (73%) had incorporated into PSII core monomers (fractions 7 to 9), and only 25% of labeled D1 protein was found in reaction center complexes (fractions 10 and 11; Figures 6 and 7). Use of a longer chase time, however, did not further improve the assembly of PSII.
The addition of the electron transfer inhibitors DCMU or DBMIB or the uncouplers nigericin or gramicidin at the beginning of the chase period had no effects on the post-translational assembly steps of PSII (data not shown). pD1 translated during the pulse (before the addition of nigericin or DCMU in the chase) was processed properly, and reassociation of CP43 occurred independently of the presence of photosynthetic electron transfer or the proton gradient across the thylakoid membrane.
Thiol reactants DTTred, NEM, and IBZ distinctively slowed the post-translational assembly of PSII (Figures 6 and 7). DTTred had less of an effect than did NEM or IBZ, whereas oxidized DTT (DTTox; trans-4,5-dihydroxy-1,2-dithiane) enhanced the assembly process (data not shown). The presence of a high concentration of DTTred (20 mM) in the chase partially blocked the assembly from the PSII reaction center to the PSII core complexes. Approximately 48% of newly synthesized D1 protein was incorporated into PSII cores after a 5-min chase in the presence of DTTred and ∼55% after a 20-min chase. After a 5-min chase in the presence of NEM (Figures 6 and 7), however, little of the newly synthesized D1 protein (10%) was incorporated into PSII core complexes. In the sucrose density fractions 7 and 8, containing intact PSII core complexes with CP43 (Zhang et al., 1999), the newly synthesized D1 protein was always present in its processed form. In the presence of NEM, only minimal amounts of this intact core complex (2% after a 5-min chase and 13% after a 20-min chase) were formed (Figures 6 and 7), indicating that CP43 reassociation was strongly hampered. Processing of the precursor form of the D1 protein was likewise strongly retarded in the presence of NEM or DTTred (Figure 6). The effect of IBZ on PSII assembly was similar to that of NEM (Figure 7). Both the assembly of the newly synthesized D1 into PSII core complexes and the processing of pD1 (data not shown) were severely slowed under these conditions.
Newly Synthesized Chlorophyll or Carotenoids Are Not Required for D1 Elongation and Assembly into PSII during the Repair Process
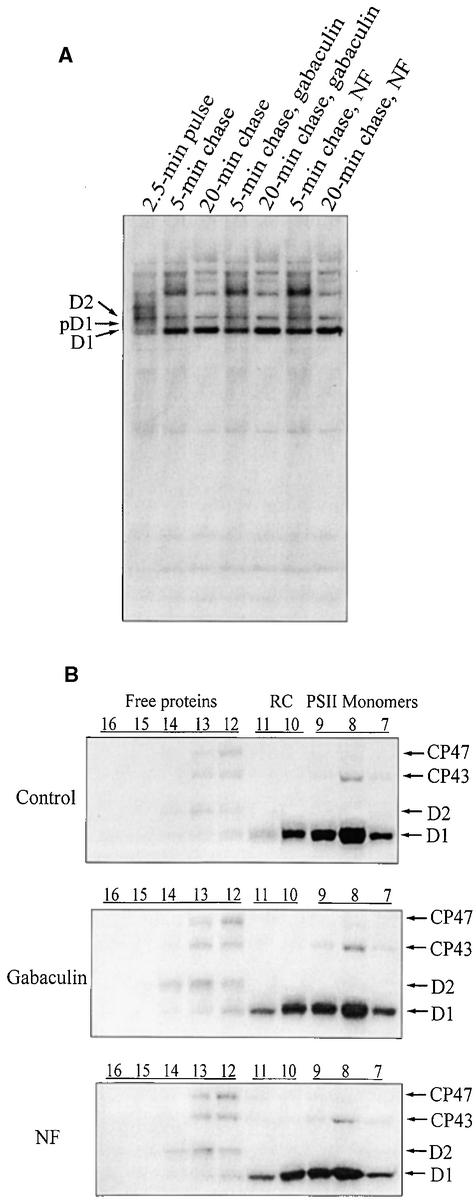
Finally, we tested whether newly synthesized chlorophyll or carotenoid was required for elongation and assembly of the D1 protein during the PSII repair process in mature chloroplasts. To this end, we added the chlorophyll biosynthesis inhibitor gabaculin and the carotenoid biosynthesis inhibitor norflurazon to the chloroplast translation system. As shown in Figure 8A, accumulation of the full-length D1 protein (precursor and mature) in the thylakoid membrane was not affected by the presence of either gabaculin or norflurazon. Similarly, the assembly of D1 into PSII within the 20-min chase occurred essentially in the same manner as discussed above (Figures 6 and 7), whether gabaculin or norflurazon was present or not (Figure 8B). Therefore, newly synthesized chlorophyll or carotenoid clearly is not required for D1 translation elongation and assembly into PSII during the repair process.
Figure 8.
Effect of Pigment Synthesis Inhibitors on D1 Translation Elongation and Assembly into PSII during the Repair Process.
(A) Autoradiography of labeled thylakoid membrane proteins. After 2.5 min of pulse labeling in intact chloroplasts, gabaculin (1 mM) or norflurazon (NF; 0.1 mM) was added, and the accumulation of label in the precursor and mature D1 protein was chased for 5 and 20 min in the light. After translation, the thylakoids were isolated and subjected to SDS-PAGE and autoradiography.
(B) Incorporation of 35S-methionine into PSII subcomplexes. To analyze the assembly process of PSII, the thylakoids were isolated after translations in vitro as in (A) and solubilized, and PSII subcomplexes were separated in the sucrose gradients. The gradients were fractionated into 20 equal fractions, and the proteins of each fraction were separated by SDS-PAGE and visualized by autoradiography. For more details, see the legend to Figure 6.
DISCUSSION
We have used the chloroplast translation/assembly system to address several central mechanistic aspects underlying the biogenesis of the D1 protein. In this article, we have focused on the regulation of the cotranslational PSII reassembly involving translation elongation and membrane insertion of D1 as well as regulation of the subsequent post-translational assembly steps of PSII.
Regulation of Translation Elongation and Insertion of D1 into the Thylakoid Membrane
Synthesis of several chloroplast-encoded proteins, and particularly polytopic membrane proteins, are generally thought to occur on thylakoid-bound polysomes. Therefore, the energy and redox state of the thylakoid membrane are likely to play a role in membrane protein elongation, insertion, and assembly. Indeed, eliminating the trans-thylakoid pH gradient with nigericin or blocking the electron transfer from PSII to the plastoquinone pool with DCMU, even in the presence of light, ATP, and GTP, nearly completely inhibits D1 protein elongation (Figure 2). Inhibition of translation elongation by DCMU could be partially restored by addition of DTTred (Figure 2) or, as reported earlier (Kuroda et al., 1996; Mühlbauer and Eichacker, 1998), by reduction of plastoquinone and activation of photosystem I (PSI) electron transfer. However, such restoration of translation elongation was not achieved in the presence of nigericin (Figures 2B and 2D). These results suggest the existence of two distinct regulation steps in D1 protein translation elongation—one mediated by one or more redox components and the other by the trans-thylakoid proton gradient.
The regulatory role of the redox state of the plastoquinone pool has been implicated in several processes in the chloroplast. However, direct involvement of the plastoquinone redox state in regulating translation elongation is unlikely because both DCMU (which keeps the plastoquinone pool oxidized) and DBMIB (which keeps the plastoquinone pool reduced) inhibited D1 protein synthesis in the absence of DTTred (Figure 2). Therefore, functioning of the complete electron transfer chain in intact chloroplasts seems to activate a redox component downstream of PSI, and this is necessary for translation elongation.
Inhibition of D1 synthesis by dissipation of the trans-thylakoid proton gradient in the presence of nigericin is probably not the result of a direct effect on D1 translation elongation per se. This can be deduced from the fact that accurate and efficient D1 protein translation initiation as well as elongation occurs in different translation systems in vitro, such as a wheat germ translation system (Taniguchi et al., 1995) and a homologous chloroplast translation system (Hirose and Sugiura, 1996; R. Nilsson and K.J. van Wijk, unpublished results) in the absence of thylakoid membranes. Moreover, nigericin added to a cell-free homologous chloroplast translation system does not substantially inhibit translation elongation (Houben et al., 1999). On the contrary, as shown by analysis of RNCs with immunoprecipitation (Figure 3B), nigericin immediately blocked D1 translation elongation on the thylakoid membrane when cotranslational insertion of elongating D1 into the thylakoid membrane was involved. Thus, the control of translation elongation by the pH gradient is most likely directly related to the translocation and insertion of the D1 nascent chains into the membrane.
Both the pH gradient and the thylakoid proteins Hcf106 and Tha4 are requirements for post-translational pH-dependent protein translocation across the thylakoid membrane (Settles et al., 1997; Mori et al., 1999; Walker et al., 1999). The pH gradient also stimulates the translocation of several nuclear-encoded substrates in combination with the SecA- or signal recognition particle–dependent pathway (Robinson et al., 1998; Schnell, 1998; Keegstra and Cline, 1999). Thus, the pH gradient plays a central role in insertion and translocation of both nuclear- and chloroplast-encoded thylakoid proteins. In the cotranslational insertion of proteins into Escherichia coli periplasmic membrane, the ribosome nascent chains are generally considered to dock onto the SecYEG translocon (Schatz and Dobberstein, 1996). Moreover, reconstituted translocation with purified E. coli SecY and SecE translocon is strongly stimulated by a pH gradient (Brundage et al., 1990). CpSecY and cpSecE, chloroplast homologs of the bacterial SecY and SecE translocon proteins, are present in the thylakoid membrane (Laidler et al., 1995; Schuenenmann et al., 1999). Furthermore, the cpSecY null mutant exhibits a severely decreased thylakoid membrane network (Roy and Barkan, 1998), indicating a central role for cpSecY in thylakoid biogenesis. However, the involvement of cpSecY as a translocon component in the cotranslational membrane insertion of thylakoid proteins remains to be elucidated.
The inhibitory effect of thiol reactants on D1 protein synthesis (Figure 3A) could be the result of a modification of some general factors involved in protein elongation, such as ribosome components, elongation factors, or chloroplast tRNA aminoacyl synthases. In that case, however, modification of these factors should induce a general inhibition of protein translation elongation, whereas we observed several distinct pausing sites of psbA mRNA during the chase in the presence of NEM and IBZ (Figure 3B). Inhibition of D1 elongation by preventing the ligation of cofactors is also unlikely, given that ligation of cofactors is not essential for D1 elongation but only increases the stability of D1 against proteolytic degradation (Kim et al., 1994). Moreover, because most of the D1 cofactors are likely to reside in the D–E loop (Xiong et al., 1998), the pauses, if caused by inhibition of ligation, should mainly be seen at 25 kD, which was not the case in our experiments (Figure 3B). More probably, the distinct D1 pausing intermediates observed in the presence of NEM and IBZ arise from modification of thiol/disulfide groups involved in protein insertion, as has been shown for protein translocation in the endoplasmic reticulum (Nicchitta and Blobel, 1989). Because D1 protein translation elongation, insertion, and assembly are tightly linked processes, improper insertion or interaction with D2 probably blocks certain specific elongation sites, and the elongating D1 protein is trapped under such conditions.
Regulation of Cotranslational and Post-Translational Assembly of PSII
Cross-linking studies with BMH (Figure 5) showed that the cysteine residues in elongating D1 nascent chains are located close to the cysteine residues in D2 and also suggested that the formation of a disulfide bond might be important for the stability of D1/D2 early assembly intermediates. In accord with these results, we found that the thiol-modifying agent NEM and the strong oxidant IBZ markedly prevented the stable, initial assembly of D1 protein with the D2 protein (see Figures 6 and 7). Conceivably, formation of a disulfide bond between one of the two cysteine residues (C-18 or C-125, most probably C-125) in the D1 protein and a cysteine residue in the D2 protein is essential for stable integration and cotranslational assembly of D1 during repair of PSII.
Formation of S–S bridges are also important for post-translational assembly steps of PSII, particularly for the reassociation of CP43. Indeed, a high concentration of DTTred, which keeps thiol groups reduced, retarded this assembly step (see Figures 6 and 7), whereas DTTox, which enhances the formation of disulfide bridges, had a stimulatory effect (data not shown). NEM as well as IBZ exerted even stronger negative effects on the reassembly of CP43 than did DTTred—presumably because NEM alkylates the free thiol groups, and IBZ, as a strong oxidant, can induce unnatural S–S bridges. Clearly, CP43 is a very dynamic component in PSII. Its dissociation and reassociation, which constantly occur during the repair of PSII centers in vivo (Barbato et al., 1992; Baena-Gonzalez et al., 1999), seem to depend on formation of disulfide bonds. This also suggests that a strict compartmentation of thiol-reducing and thiol-oxidizing conditions is maintained in chloroplasts. Despite reducing conditions in the stroma, more oxidizing conditions seem to be favored inside the thylakoid membrane. Such compartmentation has been well demonstrated in the endoplasmic reticulum (Braakman et al., 1992; Hwang et al., 1992; Huppa and Ploegh, 1998).
The distinct correlation between the processing of pD1 and the reassembly of CP43 into intact PSII core complexes is shown in Figure 6. From our results, however, we cannot distinguish whether the pD1 processing is a prerequisite for assembly of CP43 or vice versa. From a functional point of view, perhaps such strict regulation of the assembly and processing steps could guarantee the correct timing for ligation of the Mn cluster, thereby protecting the sensitive reactivation process of PSII from photoinactivation (Rova et al., 1998). Under all conditions, when the assembly of PSII was distorted by applying thiol reactants, the processing of pD1 was also severely slowed (Figure 6). This thiol-redox regulation of the C-terminal processing activity of the lumenal protease probably has significance in coordination of PSII function and protein assembly.
In summary, the results presented in this study elucidate several important regulatory steps in the biogenesis of the D1 protein in mature chloroplasts. We show that besides translation initiation, translation elongation of D1 is also regulated by redox status. Membrane insertion is probably regulated by the transmembrane proton gradient, whereas translation elongation per se requires a redox component, which can be induced by functional electron transfer or replaced by DTTred. Several cotranslational and post-translational assembly steps of PSII are either destabilized or completely blocked by the thiol reactants. This finding, together with the results from cross-linking experiments, provides evidence that thiol redox regulation and the formation of disulfide bridges play an important role in the early interaction of the D1 and D2 proteins and in the C-terminal processing of pD1 as well as in the reassociation of CP43 during the assembly of PSII.
METHODS
Isolation of Intact Chloroplasts, in Vitro Translation, and Sucrose Gradient Analysis
Isolation of intact chloroplasts was performed essentially according to Zhang et al. (1999). Spinach (Spinacea oleracea) was grown hydroponically at 23°C in a 10-hr-light/14-hr-dark cycle. For all experiments, we used fully developed leaves that had been harvested after 1 hr in light. Immediately after harvest, spinach leaves were briefly homogenized in 330 mM sorbitol, 5 mM ascorbate, 0.05% BSA, 2 mM EDTA, 1 mM MgCl2, and 50 mM Hepes-KOH, pH 7.6; filtered through Miracloth (Calbiochem-Novabiochem Co., Darmstadt, Germany); and centrifuged for 1 min at 1000g. The pellets were resuspended in medium A (330 mM sorbitol and 50 mM Hepes-KOH, pH 8.0), loaded on the top of Percoll (Amersham Pharmacia Biotech AB, Uppsala, Sweden) step gradients (40 and 70% in medium A), and centrifuged for 5 min at 4500g at 4°C. Chloroplasts in 70% Percoll were diluted with medium A, centrifuged for 2 min at 1300g, and washed once with medium A.
Translations in vitro in isolated chloroplasts were performed essentially according to van Wijk et al. (1995), with the modifications indicated in Zhang et al. (1999). After a 5-min preincubation (0.5 mg mL−1 chlorophyll) at 23°C in the light (50 μE m−2 sec−1), carrier-free 35S-labeled methionine was added in a final concentration of 0.5 μCi μL−1 and the chloroplasts were pulse labeled for 2.5 min, followed by an additional 5- or 20-min chase in the presence of 10 mM unlabeled methionine and various other reagents, as indicated in the figure legends. The translation was stopped by adding a 10-fold volume of ice-cold lysis buffer (7 mM magnesium acetate, 118 mM potassium acetate, and 46 mM Hepes-KOH, pH 7.6). Subsequently, the thylakoids were washed twice in medium B (5 mM MgCl2, 10 mM NaCl, and 25 mM Mes-NaOH, pH 6.0). All solutions used in this study contained a cocktail of protease inhibitors: antipain (2 μg mL−1), leupeptin (2 μg mL−1), and pefabloc (100 μg mL−1).
Thylakoids (0.5 mg mL−1 chlorophyll) were solubilized for 5 min on ice in medium B containing 1% (w/v) n-dodecyl β-d-maltoside. The suspension was then loaded onto an 11-mL linear sucrose gradient of 5 to 35% sucrose in 5 mM MgCl2, 10 mM NaCl, 0.5 M betaine, 0.03% (w/v) n-dodecyl β-d-maltoside, and 25 mM Mes-NaOH, pH 5.7, made with a Gradient Master (model 106; Biocomp Instruments, Inc., New Brunswick, Canada) and centrifuged for 26 hr at 180,000g at 4°C. After centrifugation, the sucrose gradients were divided into 20 aliquots by using a Piston Gradient Fractionator (model 151; Biocomp Instruments).
The thylakoid membrane and sucrose gradient fractions were analyzed by SDS-PAGE according to Zhang et al. (1999), using 15% acrylamide gels with 6 M urea. For autoradiography, gels were stained, dried, and exposed to film. Quantification of the relative amount of 35S-methionine incorporation into the precursor and mature D1 protein after SDS-PAGE was performed by scanning and analyzing the autoradiograms with the software program IMAGE (AIS 3.0, rev 1.4; Imaging Research Inc., Ontario, Canada). Protein-specific antibodies were used to identify the photosystem II (PSII) proteins.
Isolation of Thylakoid-Bound Polysomes (Ribosome Nascent Chain Complexes) and Immunoprecipitation
The thylakoid-bound polysomes (ribosome nascent chain complexes [RNCs]) were isolated as described by Zhang et al. (1999). Polysomes solubilized in 1% SDS, 15 mM DTT, and 50 mM Tris-HCl, pH 7.5, were subsequently diluted with 4 volumes of 50 mM Tris-HCl, pH 7.5, containing 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100. Saturating amounts of antiserum raised against N-terminal residues 58 to 86 of the D1 protein were added, incubated at room temperature for 2 hr, mixed with BSA-saturated protein A–Sepharose CL-4B, and incubated for another hour. The Sepharose beads were washed four times with 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100, and once with 50 mM Tris-HCl, pH 7.5; the bound antigen was released in 1% SDS and 50 mM Tris-HCl, pH 7.5. The immunoprecipitated proteins were separated by SDS-PAGE. For autoradiography, gels were stained, dried, and exposed to film.
Protein Cross-Linking
After 2.5 min of pulse labeling, thylakoid-bound polysomes were isolated; suspended in 250 mM sucrose, 50 mM Hepes-KOH, pH 7.4, 5 mM magnesium acetate, and 50 mM potassium acetate; and centrifuged for 2 min at 5000g to remove any aggregated material. Cross-linking reactions were performed at 25°C for 30 min by adding bismaleimide hexane (BMH) to a final concentration of 1 mM. After incubation, the cross-linker was quenched by addition of 30 mM DTT and kept at 25°C for 10 min.
Isolation of Polysomal RNA and RNA Gel Blot Analysis
To analyze the amount of psbA mRNA associated with thylakoid-bound polysomes in the pulse-chase experiments, the chloroplasts (100 μg) were lysed and thylakoid-bound polysomes were isolated as described by Zhang et al. (1999). The polyrRNA was extracted with phenol/chloroform and precipitated with 2.5 volumes of ethanol and 0.1 volume of 10 M lithium chloride at −20°C. RNA gel blot analysis was performed with a random prime-labeled Synechocystis sp 6803 psbA2 gene as described by Tyystjärvi et al. (1996).
Acknowledgments
We are grateful to Dr. Eira Kanervo for critical reading of the manuscript. This work was supported by grants from the Academy of Finland, the European Community (ERB IC 15-CT98-0126), Nordisk Kontaktorgan för Jordbruksforskning, and the Carl Trygger Foundation.
References
- Adir, N., Shochat, S., and Ohad, I. (1990). Light-dependent D1 protein synthesis and translocation is regulated by reaction center II: Reaction center II serves as an acceptor for the D1 precursor. J. Biol. Chem. 265, 12563–12568. [PubMed] [Google Scholar]
- Aro, E.-M., Virgin, I., and Andersson, B. (1993). Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143, 113–143. [DOI] [PubMed] [Google Scholar]
- Baena-Gonzalez, E., Barbato, R., and Aro, E.-M. (1999). Role of phosphorylation in the repair cycle and oligomeric structure of photosystem II. Planta 208, 196–204. [Google Scholar]
- Barbato, R., Friso, G., Rigoni, F., Dalla-Vecchia, F., and Giacometti, G.M. (1992). Structural changes and lateral redistribution of photosystem II during donor side photoinhibition of thylakoids. J. Cell Biol. 119, 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer, J.R., Packer, J.C.L., McCormack, B.A., Whitelegge, J.P., Robinson, C., and Tayor, M. (1992). Carboxyl-terminal processing of the D1 protein and photoactivation of water splitting in photosystem II. J. Biol. Chem. 267, 5424–5433. [PubMed] [Google Scholar]
- Braakman, I., Helenius, J., and Helenius, A. (1992). Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 11, 1717–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick, R.K., and Mayfield, S.P. (1999). Light-activated translation of chloroplast mRNAs. Trends Plant Sci. 4, 190–195. [DOI] [PubMed] [Google Scholar]
- Brundage, L., Hendrick, J.P., Schiehel, E., Driessen, A.J.M., and Wicker, W. (1990). The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell 62, 649–657. [DOI] [PubMed] [Google Scholar]
- Danon, A., and Mayfield, S.P. (1994. a). ADP-dependent phosphorylation regulates RNA-binding in vitro: Implication in light-mediated translation. EMBO J. 13, 2227–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon, A., and Mayfield, S.P. (1994. b). Light-regulated translation of chloroplasts messenger RNAs through redox potential. Science 266, 1717–1719. [DOI] [PubMed] [Google Scholar]
- Diner, B.A., Ries, D.F., Cohen, B.N., and Metz, J.G. (1988). COOH-terminal processing of polypeptide D1 of the photosystem II reaction center of Scenedesmus obliquus is necessary for the assembly of the oxygen-evolving complex. J. Biol. Chem. 263, 8972–8980. [PubMed] [Google Scholar]
- Gillham, N.W., Boynton, J.E., and Hauser, C.R. (1994). Translational regulation of gene expression in chloroplast and mitochondria. Annu. Rev. Genet. 28, 71–93. [DOI] [PubMed] [Google Scholar]
- Hirose, T., and Sugiura, M. (1996). Cis-acting elements and trans-acting factors for accurate translation of chloroplast psbA mRNAs: Development of an in vitro translation system from tobacco chloroplasts. EMBO J. 17, 1687–1695. [PMC free article] [PubMed] [Google Scholar]
- Houben, E., de Gier, J., and van Wijk, K.J. (1999). Insertion of leader peptidase into the thylakoid membrane during synthesis in a chloroplast translation system. Plant Cell 11, 1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppa, J.B., and Ploegh, H.L. (1998). The eS-sence of –SH in the ER. Cell 92, 145–148. [DOI] [PubMed] [Google Scholar]
- Hwang, C., Sinckey, A.J., and Lodish, H.F. (1992). Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257, 1496–1502. [DOI] [PubMed] [Google Scholar]
- Keegstra, K., and Cline, K. (1999). Protein import and routing system of chloroplasts. Plant Cell 11, 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen, R., Pursiheimo, S., Rintamäki, E., van Wijk, K.J., and Aro, E.-M. (1997). Transcriptional and translational adjustments of psbA gene expression in mature chloroplasts during photoinhibition and subsequent repair of photosystem II. Eur. J. Biochem. 247, 441–448. [DOI] [PubMed] [Google Scholar]
- Kim, J., and Mayfield, S.P. (1997). Protein disulfide isomerase as a regulator of chloroplast translational activation. Science 278, 1954–1957. [DOI] [PubMed] [Google Scholar]
- Kim, J., Klein, P.G., and Mullet, J.E. (1991). Ribosomes pause at specific sites during synthesis of membrane-bound chloroplast reaction center protein D1. J. Biol. Chem. 266, 14931–14938. [PubMed] [Google Scholar]
- Kim, J., Eichacker, L.A., Rudiger, W., and Mullet, J.E. (1994). Chlorophyll regulates accumulation of the plastid-encoded chlorophyll proteins P700 and D1 by increasing apoprotein stability. Plant Physiol. 104, 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda, H., Kobashi, K., Kaseyama, H., and Satoh, K. (1996). Possible involvement of a low redox potential component(s) downstream of photosystem I in the translational regulation of the D1 subunit of the photosystem II reaction center in isolated pea chloroplasts. Plant Cell Physiol. 37, 754–761. [Google Scholar]
- Laidler, V., Chaddock, A.M., Knott, T.C., Walker, D., and Robinson, C. (1995). A SecY homolog in Arabidopsis thaliana. J. Biol. Chem. 270, 17664–17667. [DOI] [PubMed] [Google Scholar]
- Lers, A., Heifetz, P.B., Boynton, J.E., Gillham, N.W., and Osmond, C.B. (1992). The carboxyl-terminal extension of the D1 protein of photosystem II is not required for optimal photosynthetic performance under CO2- and light-saturated growth conditions. J. Biol. Chem. 267, 17494–17497. [PubMed] [Google Scholar]
- Metz, J.G., and Seibert, M. (1984). Presence in photosystem II core complexes of a 34-kilodalton polypeptide required for water photolysis. Plant Physiol. 76, 829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, H., Summer, E.J., Ma, X., and Cline, K. (1999). Component specificity for the thylakoid Sec and ΔpH-dependent protein transport pathways. J. Cell Biol. 146, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould, R.M., Knoght, J.S., Bogsch, E., and Gray, J.C. (1997). Azide-sensitive thylakoid membrane insertion of chimeric cytochrome f polypeptides imported by isolated pea chloroplasts. Plant J. 11, 1051–1057. [DOI] [PubMed] [Google Scholar]
- Mühlbauer, S.K., and Eichacker, L.A. (1998). Light-dependent formation of the photosynthetic proton gradient regulates translation elongation in chloroplasts. J. Biol. Chem. 273, 20935–20940. [DOI] [PubMed] [Google Scholar]
- Mullet, J.E., Klein, R.R., and Grossman, A.R. (1986). Optimization of protein synthesis in isolated higher plant chloroplast: Identification of paused translation intermediates. Eur. J. Biochem. 155, 331–338. [DOI] [PubMed] [Google Scholar]
- Nicchitta, C.V., and Blobel, G. (1989). Nascent secretory chain binding and translocation are distinct processes: Differentiation by chemical alkylation. J. Cell Biol. 108, 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara, T., Asai, T., Nakai, M., Sugiura, M., and Endo, T. (1996). Cytochrome f encoded by the chloroplast genome is imported into thylakoids via the Sec A–dependent pathway. Biochem. Biophys. Res. Commun. 224, 474–478. [DOI] [PubMed] [Google Scholar]
- Prasil, O., Adir, N., and Ohad, I. (1992). Dynamics of photosystem II: Mechanisms of photoinhibition and recovery process. In The Photosystems: Structure, Function and Molecular Biology, Vol. 11, J. Barber, ed (Amsterdam: Elsevier Science Publishers), pp. 295–348.
- Reisfeld, A., Mattoo, A.K., and Edelman, M. (1982). Processing of a chloroplast-translated membrane protein in vivo: Analysis of the rapidly synthesized 32000-dalton shield protein and its precursor in Spirodela oligorrhiza. Eur. J. Biochem. 124, 125–129. [DOI] [PubMed] [Google Scholar]
- Robinson, C., Hynds, P.J., Robinson, D., and Matt, A. (1998). Multiple pathways for the targeting of thylakoid proteins in chloroplasts. Plant Mol. Biol. 38, 209–221. [PubMed] [Google Scholar]
- Rochaix, J.D. (1996). Post-transcriptional regulation of chloroplast gene expression in Chlamydomonas reinhardtii. Plant Mol. Biol. 32, 327–341. [DOI] [PubMed] [Google Scholar]
- Rova, M., Namedov, F., Magnuson, A., Fredriksson, P.O., and Styring, S. (1998). Coupled activation of donor and the acceptor side of photosystem II during photoactivation of the oxygen evolving cluster. Biochemistry 37, 11039–11045. [DOI] [PubMed] [Google Scholar]
- Roy, M., and Barkan, A. (1998). A SecY homologue is required for the elaboration of the chloroplast thylakoid membrane and for normal chloroplast gene expression. J. Cell Biol. 141, 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz, G., and Dobberstein, B. (1996). Common principles of protein translocation across membranes. Science 271, 1519–1526. [DOI] [PubMed] [Google Scholar]
- Schnell, D.J. (1998). Protein targeting to the thylakoid membrane. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 97–106. [DOI] [PubMed] [Google Scholar]
- Schuenenmann, D., Amin, P., Hartmann, E., and Hoffman, N.E. (1999). Chloroplast SecY is complexed to SecE and involved in the translocation of the 33-kDa but not the 23-kDa subunit of oxygen-evolving complex. J. Biol. Chem. 274, 12177–12182. [DOI] [PubMed] [Google Scholar]
- Settles, A.M., Yonetani, A., Baron, A., Bush, D.R., Cline, K., and Martienssen, R. (1997). Sec-independent protein translocation by the maize Hcf106 protein. Science 278, 1467–1470. [DOI] [PubMed] [Google Scholar]
- Smith, T.A., and Kohorn, B.D. (1994). Mutations in a signal sequence for the thylakoid membrane identify multiple protein transport pathways and nuclear suppressors. J. Cell Biol. 126, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi, F., Yamamoto, Y., and Satoh, K. (1995). Recognition of the structure around the site of cleavage by the carboxyl-terminal processing protease for D1 precursor protein of the photosystem II reaction center. J. Biol. Chem. 270, 10711–10716. [DOI] [PubMed] [Google Scholar]
- Taniguchi, M., Kuroda, H., and Satoh, K. (1993). ATP-dependent protein synthesis in isolated pea chloroplasts. FEBS Lett. 317, 57–61. [DOI] [PubMed] [Google Scholar]
- Tyystjärvi, T., Mulo, P., Mäenpää, P., and Aro, E.-M. (1996). D1 polypeptide degradation may regulate psbA gene expression at transcriptional and translational levels in Synechocystis sp. PCC 6803. Photosynth. Res. 47, 111–120. [DOI] [PubMed] [Google Scholar]
- van Wijk, K.J., Bingsmark, S., Aro, E.-M., and Andersson, B. (1995). In vitro synthesis and assembly of photosystem II core proteins. J. Biol. Chem. 270, 25685–25695. [DOI] [PubMed] [Google Scholar]
- van Wijk, K.J., Roobol-Boza, M., Kettunen, R., Andersson, B., and Aro, E.-M. (1997). Synthesis and assembly of the D1 protein into photosystem II: Processing of C-terminus and identification of the initial assembly partners and complexes during photosystem II repair. Biochemistry 36, 6178–6186. [DOI] [PubMed] [Google Scholar]
- Walker, M.B., Roy, L.M., Coleman, E., Voelker, R., and Barkan, A. (1999). The maize tha4 gene functions in Sec-independent protein transport in chloroplasts and is related to hcf106, tatA and tatB. J. Cell Biol. 147, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann, F.-A. (1998). The structure, function and biogenesis of cytochrome b6/f complexes. In Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas, J.-D. Rochaix, M. Goldschmidt-Clemount, and S. Merchant, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 459–476.
- Wollman, F.-A., Minai, L., and Nechustai, R. (1999). The biogenesis and assembly of photosynthetic proteins in thylakoid membranes. Biochim. Biophys. Acta 1141, 21–85. [DOI] [PubMed] [Google Scholar]
- Xiong, J., Subramaniam, S., and Govindjee. (1998). A knowledge-based three-dimensional model of the photosystem II reaction center of Chlamydomonas reinhardtii. Photosynth. Res. 56, 229–254. [Google Scholar]
- Zhang, L.X., Paakkarinen, V., van Wijk, K.J., and Aro, E.-M. (1999). Co-translational assembly of the D1 protein into photosystem II. J. Biol. Chem 274, 16062–16067. [DOI] [PubMed] [Google Scholar]