Abstract
Clathrin-coated vesicles bud from selected cellular membranes to traffic-specific intracellular proteins. To study the dynamic properties of clathrin-coated membranes, we expressed clathrin heavy chain tagged with green fluorescent protein (GFP) in Dictyostelium cells. GFP-clathrin was functional and retained the native properties of clathrin: the chimeric protein formed classic clathrin lattices on cellular membranes and also rescued phenotypic defects of clathrin null cells. GFP-clathrin distributed into punctate loci found throughout the cytoplasm, on the plasma membrane, and concentrated to a perinuclear location. These clathrin-coated structures were remarkably motile and capable of rapid and bidirectional transport across the cell. We identified two local domains of the plasma membrane as sites for clathrin recruitment in motile cells. First, as cells translocated or changed shape and retracted their tails, clathrin was transiently concentrated on the membrane at the back of the cell tail. Second, as cells capped their cell surface receptors, clathrin was recruited locally to the membrane under the tight cap of cross-linked receptors. This suggests that local sites for clathrin polymerization on specific domains of the plasma membrane undergo rapid and dynamic regulation in motile cells.
INTRODUCTION
Most small membrane vesicles that transport molecules between organelles and the plasma membrane are initially encased in a protein “coat.” The clathrin coat, the first characterized proteinacious coat, surrounds membrane transport vesicles (for recent reviews, see Mukherjee et al., 1997; Schmid, 1997). These vesicles mediate endocytosis at the plasma membrane and the initial trafficking of lysosomal hydrolases from the trans-Golgi network to the lysosome. Clathrin itself is a multisubunit protein consisting of three heavy chains and three light chains. This three-legged protein complex, called a triskelion, has been visualized by negative staining and rotary shadowing. Triskelions assemble as polygonal lattice structures on membranes to mold coated pits that invaginate to form clathrin-coated vesicles. Both clathrin-coated pits on membranes and empty clathrin cage structures formed in vitro have been visualized by quick-freeze deep-etch and high-resolution electron microscopy, respectively. Although electron microscopy has uncovered much about the structure of clathrin, these static single images cannot reveal the dynamics of clathrin movement or behavior in live cells.
One cellular behavior that is particularly dynamic is cell motility. Models of cell motility have invoked a role for endocytosis, coupled with directed secretion, in driving cell movement and capping cell surface receptors (Bretscher, 1984). At present, little is known about the functional contribution of particular membrane proteins to motile processes. By virtue of its ability to selectively remodel discrete areas of the plasma membrane, clathrin could partner with the actin cytoskeleton to remodel the plasma membrane during cell motility functions. Indeed, this possibility has been suggested by structural studies that show clathrin distributed in close proximity to areas where the actin cytoskeleton is tethered to the plasma membrane (Aggeler and Werb, 1982; Maupin and Pollard, 1983). Clathrin-minus Dictyostelium cells display defects in motility events driven by the actin cytoskeleton such as cytokinesis (Niswonger and O'Halloran, 1997a) and cell translocation (Niswonger and O'Halloran, 1997b; Wessels, et al., 1999). If clathrin is responsible for removing discrete proteins and lipids, thereby remodeling the local membrane, then clathrin localization might change on discrete areas of the membrane during motility functions. To examine the dynamics and localization of clathrin in motile cells, we have imaged clathrin labeled with green fluorescent protein (GFP) in live Dictyostelium cells during cytokinesis, movement, streaming, capping, and phagocytosis.
MATERIALS AND METHODS
Strains, Growth, and Development
Dictyostelium discoideum strains were Ax2, an axenic wild-type strain, and 5E2, a clathrin-minus strain derived from Ax2 that carries blasticidin resistance (Niswonger and O'Halloran, 1997a). Cells were grown at 20°C in HL5 media (0.75% proteose peptone [Difco, Detroit, MI], 0.75% thiotone E peptone [Becton Dickinson, Cockeysville, MD], 0.5% Oxoid yeast extract [Unipath, Basingstoke, Hampshire, England], 1% glucose, 2.5 mM Na2HPO4, and 8.8 mM KH2PO4, pH 6.5) supplemented with penicillin-streptomycin (Life Technologies, Gaithersburg, MD) at 60 U/ml. Cells were either grown on plastic culture dishes or in suspension culture in shaking flasks on a gyratory shaker. For development of fruiting bodies, cells were plated on SM-5 agar plates on a lawn of bacteria (Escherichia coli B/r) and incubated at 20°C for 5 d (Niswonger and O'Halloran, 1997a).
Expression of GFP-Clathrin in Dictyostelium Cells
The GFP-clathrin expression plasmid p14A3dchcgfp was an integrating plasmid constructed from the plasmid pTZ19 containing the actin 15 promoter and the 2H3T terminator (Larochelle et al., 1997). The actin 15 promoter drives expression of the clathrin cDNA fused with the GFP variant, S65T-GFP, at its C terminus. Linkers of five alanines on both sides flanked the S65T-GFP. Wild-type AX2 cells and clathrin-minus cells were cotransformed with this GFP-clathrin expression construct and a second integrating plasmid that carried the G418 resistance gene, pTO103S (O'Halloran and Anderson, 1992a). Plasmids were introduced into cells by electroporation using a gene pulser (Bio-Rad, Richmond, CA) according to the method of Kuspa and Loomis (1992). Cells were plated in 96-well plates in HL5 media containing 10 μg/ml G418 (Life Technologies). Clonal cell lines were screened for GFP-clathrin expression by Western blot analysis. Cells (2 × 106 per lane) were lysed in boiling sample buffer and run on a 7.5% SDS-PAGE gel. The gel was transferred to nitrocellulose and blotted with a rabbit polyclonal antibody to Dictyostelium clathrin heavy chain (O'Halloran and Anderson, 1992b) followed with a horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G. The blot was developed according to standard methods (O'Halloran and Anderson, 1992b).
Capping, Phagocytosis Assays, and Endocytosis Assays
For capping experiments, cells were plated on coverslips and washed in PDF (11 mM K2HPO4, 13.2 mM KH2PO4, 20 mM KCl, 1 mM CaCl2, and 2.5 mM MgSO4, pH 6.4). Concanavalin A labeled with Texas Red (Molecular Probes, Eugene, OR) was added to cells at 0.5 mg/ml for 30 s. Cells were washed twice in PDF and incubated for various periods before imaging. For phagocytosis assays, Dictyostelium cells were plated on coverslips and rinsed with PDF buffer, and yeast cells (5 × 106 cells/ml; Molecular Probes) were added. Cells were immediately imaged with a confocal microscope. For endocytosis assays, log-phase cells were harvested and resuspended at 1 × 106 cells per ml HL5 (4 ml total) in small flasks. Rhodamine-dextran, Mr 70,000 (Molecular Probes), was added to a final concentration of 2 mg/ml. Cells were gently shaken at 20°C for 1 h. To stop endocytosis, the cells were washed twice with ice-cold HL5, resuspended in 4 ml of ice-cold HL5, and immediately analyzed on either a FACScan or FACstarPlus (Becton Dickinson) fluorescence-activated cell sorter. Eight thousand to 9000 cells were assessed for each sample, and the relative fluorescence of internalized rhodamine-dextran was determined. Background was determined by measuring the fluorescence of rhodamine-dextran internalized by parallel samples of cells incubated at 0°C; this background measurement was subtracted for each sample.
Cell Fixation and Microscopy
To image fixed cells, cells were allowed to adhere to coverslips for 15 min in a humid chamber, rinsed with PDF buffer, and then overlaid with a thin sheet of agarose (Fukui et al., 1987) before fixation in 1% formaldehyde/methanol at −10°C for 5 min. After fixation, the coverslips were washed in 17 mM phosphate buffer, pH 6.8, three times and mounted on slides. Cells were imaged using differential interference contrast microscopy and epifluorescence with a Zeiss (Thornwood, NY) Axiophot microscope, a Photometrics (Tucson, AZ) cooled charge-coupled device camera, and IP Lab software (Signal Analytics, Vienna, VA).
Electron Microscopy
Growing cells were ruptured, quick frozen, and prepared for electron microscopy as described by Heuser et al. (1993). Before rupture, cells were pretreated with 10 μmol of latrunculin (Molecular Probes) for 10 min. This treatment increases the number of coated pits on Dictyostelium membranes without changing their structural character (T.J. O'Halloran and J. Heuser, unpublished observation).
Confocal Microscopy
For live imaging, cells were grown in HL5 in a 60-mm Petri dish with a coverslip glued to a hole cut in the bottom of the dish. Before imaging, the HL-5 was replaced with PDF buffer. For imaging moving cells, cells were incubated in PDF buffer for 2–16 h before imaging to induce rapid polarized movement. Laser scanning confocal microscopy used a Zeiss Microsystem LSM microscope at an excitation wavelength of 488 nm and emission filter of 488/586 nm with attenuation set at 10%. Cells were scanned at various intervals, ranging from 2 to 15 s. Scan lengths ranged from 1 to 4 s. Confocal microscopy images were captured by Zeiss LSM-PC version 3.50. Videos were compiled using Quicktime 3.0 from confocal microscopy images. For still images, selected images were cropped, aligned, and adjusted for contrast in Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA).
RESULTS
Expression of Functional GFP-Clathrin in Dictyostelium Cells
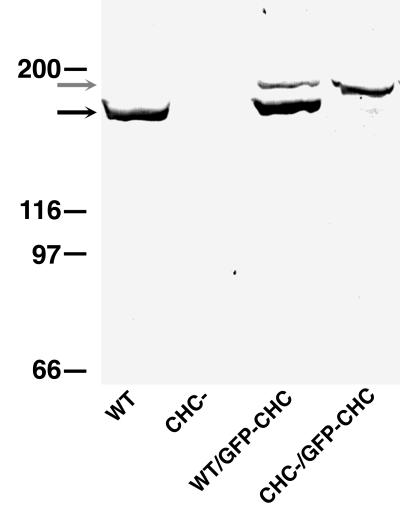
As a way to track the dynamic movement of clathrin traffic in living cells, we constructed a plasmid for expression of the Dictyostelium clathrin heavy chain tagged at its C terminus with GFP. The expression plasmid was transformed into both clathrin-minus and wild-type Dictyostelium cells, and transformants were selected. Western blots of these transformants probed with an anti-clathrin heavy chain serum demonstrated good expression of GFP-clathrin in both clathrin null and wild-type backgrounds (Figure 1). Possibly because of differences in plasmid copy number, levels of GFP-clathrin varied in different cell lines. Nonetheless, many cell lines achieved levels of GFP-clathrin equivalent to the endogenous levels of clathrin expressed by wild-type strains (Figure 1, compare the WT lane with the CHC-/GFP-CHC lane).
Figure 1.
Expression of GFP-clathrin in Dictyostelium cells. Wild-type (WT) and clathrin-minus (CHC−) Dictyostelium cells were transformed with the GFP-clathrin (GFP-CHC) expression plasmid. These strains were screened for GFP-clathrin expression by SDS-PAGE followed by Western blot analysis with a polyclonal antibody that recognizes wild-type clathrin (black arrow) and GFP-clathrin.
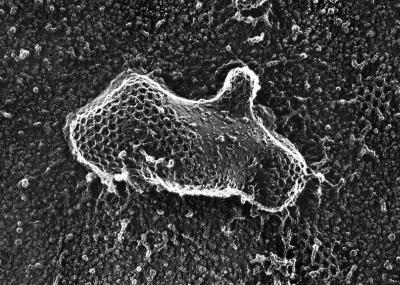
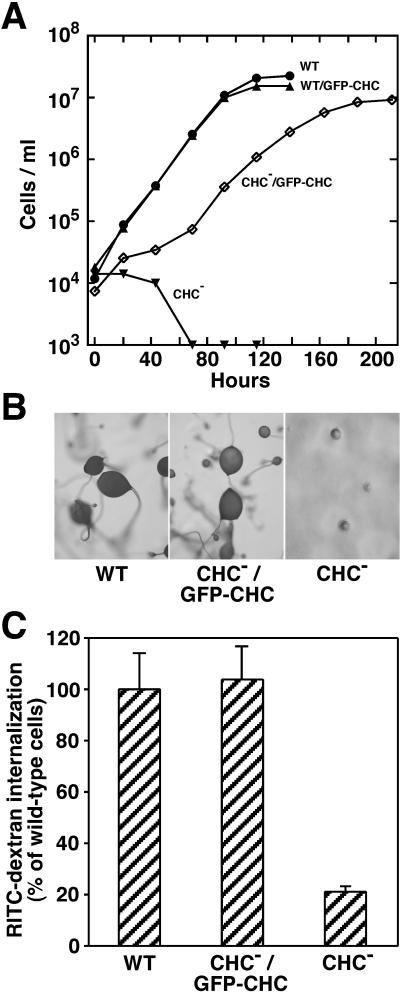
A potential concern with GFP-tagged proteins is the possibility that adding the 25-kDa GFP protein has altered the native properties of the tagged protein. Supporting the retention of native properties of GFP-clathrin was its ability to polymerize into lattices on intracellular membranes. Inspection of these coated pits by electron microscopy revealed polygonal lattices that were indistinguishable from those formed by wild-type clathrin (Figure 2). Additional support for native activity in GFP-clathrin was its ability to rescue several phenotypic deficiencies exhibited by clathrin-minus cells. Because clathrin-minus cells are defective in cytokinesis, they are completely unable to divide and grow in suspension culture (Niswonger and O'Halloran, 1997b). Expression of GFP-clathrin allowed these cells to grow in suspension culture (Figure 3A), demonstrating that their cytokinesis defect was rescued. GFP-clathrin also rescued developmental deficiencies displayed by clathrin null cells. Whereas clathrin-minus mutants are blocked early in development and fail to form multicellular fruiting bodies (O'Halloran and Anderson, 1992a; Niswonger and O'Halloran, 1997a), clathrin-minus cells expressing GFP-clathrin completed development and formed fruiting bodies indistinguishable from wild-type structures (Figure 3B). A third deficiency rescued by expression of GFP-clathrin in clathrin-minus mutants was endocytosis of a fluid-phase marker, a property that is dramatically reduced in clathrin-minus cells (Figure 3C). The rescue of these phenotypes provided strong evidence that the GFP-tagged clathrin functioned properly in cells and thus correctly reflected the dynamic behavior of clathrin.
Figure 2.
Coated pits of polymerized GFP-clathrin. Clathrin null cells expressing GFP-clathrin were prepared for electron microscopy using quick freeze and deep etch. An electron micrograph shows that the membranes of these cells display clathrin coats formed from GFP-clathrin that were indistinguishable in size and shape from the clathrin coats found in control wild-type cells. Micrograph kindly provided by Dr. John Heuser (Washington University Medical School, St. Louis, MO).
Figure 3.
Rescue of phenotypic defects by GFP-clathrin. (A) Rescue of cytokinesis. Cells from wild-type (WT) and clathrin-minus (CHC−) parental strains and cells expressing GFP-clathrin derived from these strains (WT/GFP-CHC and CHC−/GFP-CHC) were seeded at 1 × 104 cells/ml and grown in suspension culture on a shaking platform. Although CHC− cells failed completely to grow in suspension, CHC−/GFP-CHC cells divided and grew in suspension. (B) Rescue of development. Wild-type (WT), clathrin-minus (CHC−), and clathrin-minus cells expressing GFP-clathrin (CHC−/GFP-CHC) were seeded on a lawn of bacteria. After the bacterial food source was depleted, wild-type cells (WT) and cells expressing GFP-CHC (CHC−/GFP-CHC) developed into fruiting bodies; clathrin-minus (CHC−) cells formed only multicellular aggregates. (C) Rescue of endocytosis. Cells from wild-type (WT) and clathrin-minus (CHC−) parental strains and cells expressing GFP-clathrin (CHC−/GFP-CHC) were incubated with RITC-dextran, a fluid-phase marker, for 1 h. After washing to remove extracellular RITC-dextran, the fluorescence of internalized RITC-dextran was assessed for each sample by fluorescence-activated flow cytometry. The median fluorescence for each sample (8000–9000 cells) is reported as the percentage of median RITC-dextran fluorescence measured in wild-type cells.
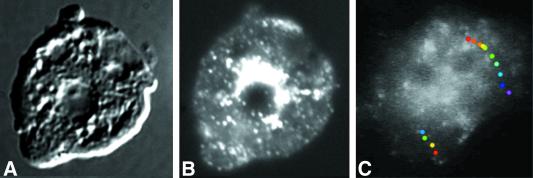
Inspection of cells expressing GFP-clathrin with fluorescence microscopy showed a distribution of clathrin in punctate loci scattered throughout the cell. In many cases, a bright patch of GFP-clathrin was apparent adjacent to the nucleus, possibly reflecting a perinuclear localization to the trans-Golgi (Figure 4B). The distribution to the plasma membrane, cytoplasm, and a perinuclear region is characteristic of the localization of clathrin vesicles revealed by immunofluorescence and electron microscopy in many kinds of cells (Anderson et al., 1977; Swanson et al., 1981; Robinson and Pearse, 1986; O'Halloran and Anderson, 1992a).
Figure 4.
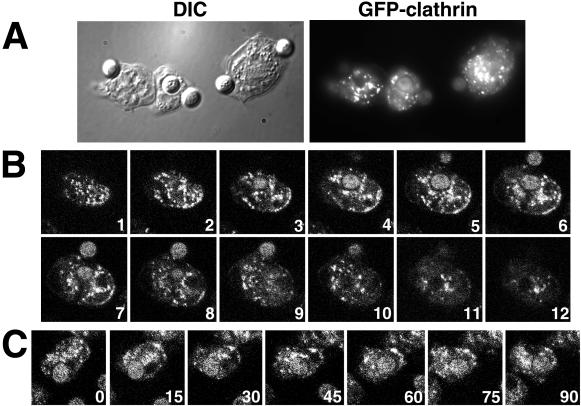
Dynamic distribution of GFP-clathrin. Cells expressing GFP-clathrin were fixed and imaged with differential interference contrast (A) and fluorescence microscopy (B). (C) Single frame from images collected at 1-s intervals of a living cell expressing GFP-clathrin. Although most fluorescent spots in this cell moved relatively short distances before disappearing, occasionally fluorescent loci moved relatively long distances. Superimposed on the frame are two paths of fluorescent spots that moved long distances, shown as a series of colored dots. Each dot marks the position of fluorescent spot at 1-s intervals, with the first position marked with a red dot and the last position marked with either a blue dot (the left track) or a purple dot (the right track). These were overlaid on the single image of the cell. See accompanying video. Video is 30 times real time.
Dynamic Visualization of Clathrin Traffic in Living Cells
To follow the localization and movement of clathrin, we used a low-light camera equipped with fluorescence optics to collect a timed series of images of GFP-clathrin living cells. These images showed fluorescent punctate dots, presumably clathrin-coated vesicles, that moved continuously around in the cytoplasm. The majority of these fluorescent loci persisted for ≤30 s, and moved for approximately ≤1 μm, indicating that they either “uncoated” or moved out of the plane of focus. Remarkably, occasional GFP-coated structures moved long distances and traversed approximately half the length of the cell before vanishing. These long-distance movements appeared to be on linear tracks on which the GFP–coated vesicles could exhibit bidirectional movement (Figure 4C). The subsequent disappearance of these structures could be due to the uncoating of GFP-clathrin from the membrane by an uncoating ATPase.
The ability to image functional clathrin in living cells allowed us to examine the dynamic distribution of clathrin during cell motility. We monitored the distribution of GFP-clathrin during four motility events: cell movement, the capping of cell surface receptors, phagocytosis, and cytokinesis.
Clathrin Dynamics in Live Dictyostelium Cells during Cytokinesis
We recently identified a requirement for clathrin in cytokinesis (Niswonger and O'Halloran, 1997b). Although clathrin-minus cells initially construct a cleavage furrow, ultimately the furrow becomes unstable, and cytokinesis fails (N. Gerald, C. Damer, and T.J. O'Halloran, unpublished results). One possible explanation for the requirement for clathrin in cytokinesis is that local endocytosis mediated by clathrin-coated vesicles is required for remodeling the membrane in preparation for construction of a functional furrow. To examine this possibility, we used confocal microscopy to image the membrane of dividing cells. A time-lapse series of confocal images of cells expressing GFP-clathrin showed a distribution of clathrin vesicles throughout the plasma membrane and also in the cytoplasm, similar to the distribution of clathrin found in interphase cells (Figure 4C).
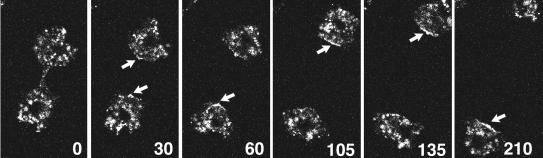
Although clathrin was not enriched in the cleavage furrow or other portions of the cell during cytokinesis, we observed an unexpected enrichment of GFP-clathrin at the back of the two daughter cells after they completed division and moved away from each other. An example of a cell nearing the completion of cytokinesis is shown in Figure 5. GFP-clathrin was observed at the tail of each daughter cell as the cells moved away from each other (arrows).
Figure 5.
Distribution of GFP-clathrin during cytokinesis. Clathrin-minus cells expressing GFP-clathrin were imaged every 15 s with a confocal microscope. Images shown are from 30-s intervals of one cell nearing completion of cytokinesis. GFP-clathrin accumulates at the posterior plasma membranes of the two daughter cells as they move away from each other (arrows). See accompanying video. Video is 120 times real time.
Clathrin Dynamics in Motile Dictyostelium Cells
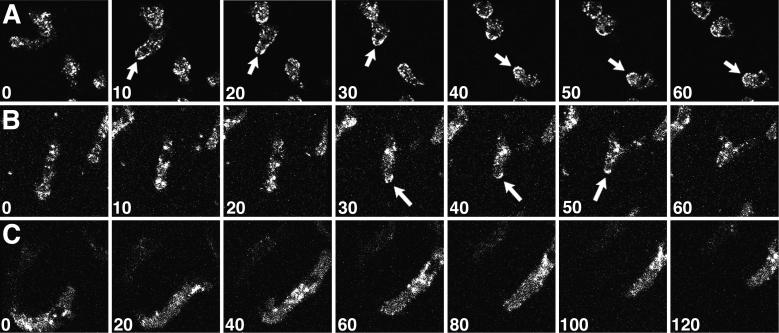
To determine whether the transient localization of clathrin on the plasma membrane of the tail of the cell was characteristic only of daughter cells separating during cytokinesis, we examined populations of Dictyostelium cells as they moved during interphase (Figure 6). In most translocating cells, punctate dots of GFP-clathrin moved around in the cell, apparently in a random pattern. Frequently, however, we observed a transient enrichment of GFP-clathrin to the posterior edge of the tail, similar to the localization to the tail observed in dividing cells (Figure 6, A and B, arrows). The accumulation of fluorescence on the C-shaped edge of the tail indicated that the GFP-clathrin was distributed on the plasma membrane, not in the adjacent cytoplasm.
Figure 6.
Clathrin localizes to the tail of moving cells. Confocal images were collected from fields of Dictyostelium cells expressing GFP-clathrin during cell locomotion; the numbers indicate the time interval (seconds) between frames. (A and B) During tail retraction, translocating cells displayed enriched GFP-clathrin at their posterior edges (arrows). (C) A cell translocating without tail retraction showed no enrichment of GFP-clathrin in its tail. See accompanying video. Video is 80 times real time.
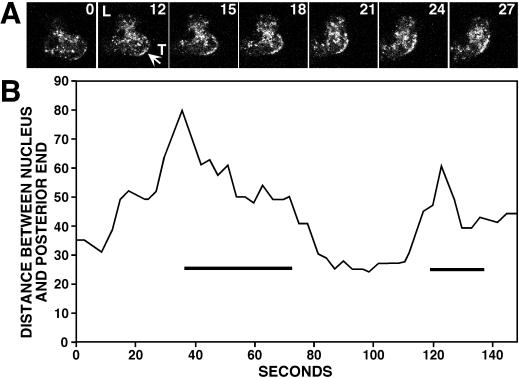
Although common, increased GFP-clathrin in the tail was not found in all moving cells. In many instances, cells translocated without showing an increase in GFP-clathrin at their tail (Figure 6C). Thus increased GFP-clathrin in the tail was not a prerequisite for cell translocation. Close examination of the subset of cells that exhibited the increase in GFP-clathrin at the posterior plasma membrane revealed a common behavior. GFP-clathrin appeared to localize to the rear of the cell only when cells retracted their tails while moving forward or changing direction. To test this, we measured the distance between the position of the nucleus of the cell and the edge of the tail in moving cells over time. As cells elongated and extended their leading edges forward, this distance increased; when cells subsequently retracted their tails, this distance decreased (Figure 7). The retraction phase of this cycle, ∼20–30 s, coincided with a transient increase in GFP-clathrin to the posterior plasma membrane. These results showed that clathrin does not generally localize on the tail plasma membrane of moving cells. Rather, clathrin associates with the back plasma membrane when this portion of the cell is rapidly retracted into the body of the cell.
Figure 7.
Clathrin localization to the posterior plasma membrane during tail retraction. (A) Time sequence taken of a translocating cell. The numbers indicate the time interval (seconds) between frames. GFP-clathrin concentrated at the posterior plasma membrane coincident with tail retraction (arrow). (B) The distance between the nucleus and the posterior end of a typical cell undergoing two cycles of lamellipodia extension and tail retraction was measured from confocal images taken every 3 s. GFP-clathrin localization to the posterior plasma membrane (bars) coincided with tail retraction.
Clathrin Dynamics in Cells Capping Cell Surface Receptors
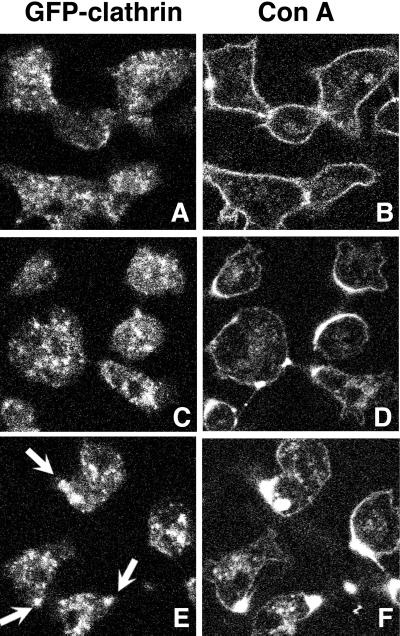
Many amoeboid cells, including Dictyostelium, gather cross-linked membrane receptors on their cell surfaces, a process called capping (Pasternak et al., 1989; Fukui et al., 1990; Jay and Elson, 1992). In some cells, clathrin coats localize underneath the capped ligand, suggesting an involvement for clathrin in the capping process (Salisbury et al., 1980). We therefore investigated the dynamic distribution of GFP-clathrin in capping Dictyostelium cells. We initiated capping of cell surface receptors using a fluorescently labeled lectin to cross-link cell surface receptors. Cells expressing GFP-clathrin were treated with Texas Red-concanavalin A (Con A) for 30 s and washed free of unbound lectin. GFP and Texas Red were visualized every 30 s for 10 min. Figure 8 shows cells at various times after the addition of Con A. No enrichment of clathrin on the plasma membrane was observed in the initial process of capping. GFP-clathrin did not accompany clusters of Con A during patch formation; nor did GFP-clathrin accompany the C-shaped ring of Con A that formed on one side of the cell. However, once the half-moon of capped ligand coalesced into a tight cap, GFP-clathrin colocalized with the cap (Figure 8, E and F). Subsequently, ∼15 min after ligand challenge, GFP-clathrin and labeled lectin were occasionally found together in small vesicles in the cell cytoplasm, suggesting that the capped ligand could be internalized through clathrin-mediated endocytosis (our unpublished results).
Figure 8.
GFP-clathrin localization during Con A capping. Clathrin-minus cells expressing GFP-clathrin were treated with Con A labeled with Texas Red and imaged 2 (A and B), 4.5 (C and D), and 6 (E and F) min after Con A treatment for GFP (A, C, and E) and Texas Red (B, D, and F). Initially GFP-clathrin did not colocalize with the C-shaped Texas Red cap formed early in capping (C and D), but once the cap coalesced into a compact spot, GFP-clathrin colocalized with the Texas Red-Con A cap (E, arrows).
Clathrin Dynamics during Phagocytosis
The process of internalization of cell surface receptors when bound and capped by ligand has similarities to phagocytosis, the process of internalization of solid particles. Like capping, phagocytosis involves ligand-stimulated internalization of the plasma membrane. To determine whether this analogous process triggered a similar association of clathrin with internalized plasma membrane, we examined phagocytosis. Figure 9 shows Dictyostelium cells engaged in the phagocytosis of yeast cells. In contrast with the internalization of membrane and receptors during capping, GFP-clathrin failed to colocalize with phagocytic cups. Indeed, a z-series of confocal images showed that GFP-clathrin was absent from the membrane engulfing the yeast and appeared excluded from the area surrounding the engulfed yeast. Thus these data suggested that clathrin does not associate with the membrane during the internalization of the plasma membrane during phagocytosis.
Figure 9.
GFP-clathrin localization during phagocytosis of yeast cells. Shown are Dictyostelium expressing GFP-clathrin in the process of internalizing yeast cells. (A) Dictyostelium cells fixed 10 min after the addition of yeast cells and imaged with differential interference contrast and fluorescence microscopy. (B) z-series of 1-μm confocal images taken of a fixed Dictyostelium cell. The numbers indicate the focal plane distance (micrometers) from the first image. The cell contains one partially and one fully engulfed yeast cell; both are visible in section 6. (C) Time series of confocal images of a Dictyostelium cell engulfing a yeast cell; the numbers indicate the interval (seconds) between frames.
DISCUSSION
Here we used GFP-clathrin to study the distribution of clathrin during dynamic behaviors in living cells. Importantly, expression in a Dictyostelium null background allowed us to assess the functional capacity of the GFP-tagged protein in living cells. The ability of GFP-clathrin to rescue the endocytic, cytokinesis, and developmental deficiencies of the clathrin-minus cells provided strong evidence for its ability to function in vivo. Indeed the lattice structures of coated pits assembled from GFP-clathrin were indistinguishable in appearance from lattices formed by wild-type clathrin heavy chain. In fact, we were initially puzzled that we could not detect a structure corresponding to the 25-kDa globular GFP protein in electron micrographs of lattices assembled from GFP-clathrin. However, a recent analysis of the molecular structure of purified clathrin triskelions assembled into lattices in vitro predicts that the C terminus of the clathrin trimer projects inward (Musacchio et al., 1999; Smith and Pearse, 1999). Our electron micrographs of lattices assembled from GFP-clathrin also support the idea that the GFP-containing domain at the C terminus of clathrin projects toward the membrane and is obscured by the overlying legs of the triskelion. This provides evidence from living cells that support the model for clathrin assembly obtained from purified proteins.
Throughout the years, the identification of cellular proteins that govern cell motility and their functions has been the focus of intensive study. The efforts of many have provided abundant evidence supporting an active role in motility for proteins associated with the actomyosin cytoskeleton. In contrast, less evidence has emerged for a similarly important and active role for specific proteins associated with the plasma membrane, although an active role for the membrane in driving cell motility has been proposed (Bretscher, 1984, 1989, 1992, 1996; Bretscher and Aguado-Velasco, 1998). It remained to be established whether the plasma membrane, and its complement of membrane proteins, is an active participant or a passive partner in cell motility.
When considering proteins that could actively remodel and change local domains of the plasma membrane, clathrin is an obvious candidate by virtue of its capacity to selectively endocytose proteins and lipids. Indeed throughout the years, several models have invoked an active role for clathrin coats in influencing the local domains of the plasma membrane as cells bend, change shape, and translocate in various motile cell behaviors (Salisbury et al., 1980; Bretscher, 1987; Bretscher and Aguado-Velasco, 1998). Our ability to monitor the localization of clathrin and cell shape simultaneously in living cells allowed us to address whether clathrin actively remodels the local membrane in cells during various motile behaviors. Our experiments fail to support a widespread role for clathrin in all of these events but suggest specific roles for clathrin in discrete locations during a subset of motility behaviors. Our studies are consistent with a recent proposal that the contribution of endocytosis to cell motility is driven by a non-clathrin mechanism (Aguado-Velasco and Bretscher, 1999).
Dynamic monitoring of clathrin localization during the course of cell division provided no evidence for a special role for clathrin-coated membranes during cleavage furrow construction, despite the severe defects in the furrow exhibited by clathrin null mutants. The cellular role of clathrin in cytokinesis remains to be determined, but these experiments rule out a model in which clathrin directly models the cleavage furrow. Instead, the defective cleavage furrow could result from deficits in intracellular membrane traffic in clathrin-minus cells. For example, protein sorting normally managed by clathrin traffic from the trans-Golgi Network or from recycling endosomes could be essential for delivery of the proper proteins to construct a robust cleavage furrow.
As cells moved away from each other after cytokinesis, and in other moving cells, we observed a pattern of clathrin localization on the tail of cells. This transient increase in clathrin occurred when the tail retracted from the substrate as the cell moved forward. Tail retraction is integral to cell translocation, a complex process that involves repeated cycles of lamellipodia extension, formation of attachments with the substratum, and cytoskeletal contraction. To continue forward movement, cells require mechanisms to release adhesions at their rear (Wessels et al., 1994; Lauffenburger and Horwitz, 1996; Cramer and Mitchison, 1997). Video tracking of integrins, membrane proteins that are involved in cell–substratum attachments, reveals that new adhesions tend to form at the leading edge of a motile cell and persist until they reach the rear (Regen and Horwitz, 1992). Some cell types use endocytosis to release their integrins from the substrate (Bretscher, 1984; Regen and Horwitz, 1992; Lawson and Maxfield, 1995; Palacek et al., 1996). Thus, the presence of clathrin in the tail of moving cells could facilitate the spatially regulated endocytosis of integrin-like receptors, internalizing these cell–substrate receptors to allow release of the tail as the cell moves forward. This possibility is especially intriguing because clathrin appears enriched in the plasma membrane of the tail just before its release.
A second possibility invokes a new role for clathrin lattices, a role in maintaining the polarized tail of a moving cell. By internalizing specific membrane proteins from the tail, clathrin could help maintain the identity and unique characteristics of the tail. This possibility predicts that clathrin-minus mutant Dictyostelium would be unable to maintain a discernible tail, an idea supported by a recent analysis of the movement and translocation of clathrin-minus Dictyostelium cells (Wessels et al., 2000). Without clathrin, mutant Dictyostelium lack a single dominant pseudopod and a trailing tail. Instead, clathrin-minus cells extrude multiple pseudopods and fail to adopt an elongate polarized morphology with a definitive tail (Wessels et al., 2000).
The cap of cross-linked receptors was a second locale of increased clathrin localization during cell motility. The recruitment of clathrin to this site may be important for the subsequent internalization of cross-linked receptors; although not blocked in capping, clathrin null cells are much less efficient than wild-type cells. The internalization of solid particles is similar to capping in many ways: both involve the signal-mediated internalization of large surface areas of membrane. However, recruitment of increased clathrin at the membrane is triggered solely by capping and not by phagocytosis. This indicates that the coalescence of receptors into a tight cap initiates a local and specific signal for increased clathrin polymerization at that site.
Cells can target exocytosis to the leading edge of moving cells (Bretscher and Aguado-Velasco, 1998); our results show that cells can also target the endocytic machinery of clathrin to specific domains of the plasma membrane. Although a study using GFP-clathrin showed that coated vesicles emanate from defined sites in cultured vertebrate cells (Gaidarov et al., 1999), we found no evidence for a similar restriction in Dictyostelium cells. This phenomenon may differ according to cell type. Sites for coated vesicle formation may rapidly change in highly motile cells such as Dictyostelium according to rapidly shifting functional domains of the plasma membrane. Presumably a signal for clathrin polymerization must be triggered in a spatially restricted manner, perhaps in the tails of receptors that arrive in particular domains (such as the back of the cell) or receptors at particular concentrations (such as the mass of aggregated receptors in a cap). To uncover these signals, it will be important to explore these experiments in other cell types where the identity and regulation of receptors such as integrins is well characterized. Because clathrin heavy chains are highly conserved in amino acid sequence across a wide range of species, addition of GFP to the C terminus of other clathrin heavy chains should also prove a useful tool, particularly in mammalian systems in which it is difficult to replace the endogenous clathrin heavy chain.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the O'Halloran and the De Lozanne laboratories, particularly Denis Larochelle for the DNA construct encoding GFP, and John Port for endocytosis experiments. We also thank John Heuser and Robin Roth (Washington University, St. Louis, MO) for generously providing electron micrographs and low-light images of GFP-expressing cells. This work was supported by National Institutes of Health grant GM-48624.
Footnotes
REFERENCES
- Aggeler J, Werb Z. Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J Cell Biol. 1982;94:613–623. doi: 10.1083/jcb.94.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado-Velasco C, Bretscher MS. Circulation of the plasma membrane in Dictyostelium. Mol Biol Cell. 1999;10:4419–4427. doi: 10.1091/mbc.10.12.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RGW, Brown MS, Goldstein JL. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977;10:351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Bretscher MS. Endocytosis: relation to capping and cell locomotion. Science. 1984;224:681–686. doi: 10.1126/science.6719108. [DOI] [PubMed] [Google Scholar]
- Bretscher MS. How animal cells move. Sci Am. 1987;257:72–90. doi: 10.1038/scientificamerican1287-72. [DOI] [PubMed] [Google Scholar]
- Bretscher MS. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 1989;8:1341–1348. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS. Cells can use their transferrin receptors for locomotion. EMBO J. 1992;11:383–389. doi: 10.1002/j.1460-2075.1992.tb05066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS. Moving membrane up to the front of migrating cells. Cell. 1996;85:465–467. doi: 10.1016/s0092-8674(00)81246-5. [DOI] [PubMed] [Google Scholar]
- Bretscher MS, Aguado-Velasco C. Membrane traffic during cell locomotion. Curr Opin Cell Biol. 1998;10:537–541. doi: 10.1016/s0955-0674(98)80070-7. [DOI] [PubMed] [Google Scholar]
- Cramer LP, Mitchison TJ. Investigation of the mechanism of retraction of the cell margin and rearward flow of nodules during mitotic cell rounding. Mol Biol Cell. 1997;8:109–119. doi: 10.1091/mbc.8.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y, De Lozanne A, Spudich JA. Structure and function of the cytoskeleton of a Dictyostelium myosin-defective mutant. J Cell Biol. 1990;110:367–378. doi: 10.1083/jcb.110.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y, Yumura S, Yumura TK. Agar-overlay immunofluorescence: high-resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol. 1987;28:347–356. doi: 10.1016/s0091-679x(08)61655-6. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Santini F, Warren R, Keen JH. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- Heuser J, Zhu Q, Clarke M. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J Cell Biol. 1993;121:1311–1327. doi: 10.1083/jcb.121.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay PY, Elson EL. Surface particle transport mechanism independent of myosin II in Dictyostelium. Nature. 1992;356:438–440. doi: 10.1038/356438a0. [DOI] [PubMed] [Google Scholar]
- Kuspa A, Loomis WF. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc Natl Acad Sci USA. 1992;89:8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle DA, Vithalani KK, De Lozanne A. Role of Dictyostelium racE in cytokinesis: mutational analysis and localization studies by use of green fluorescent protein. Mol Biol Cell. 1997;8:935–944. doi: 10.1091/mbc.8.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Maxfield FR. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- Maupin P, Pollard TD. Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J Cell Biol. 1983;96:51–62. doi: 10.1083/jcb.96.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Smith CJ, Roseman AM, Harrison SC, Kirchhausen T, Pearse BM. Functional organization of clathrin in coats: combining electron cryomicroscopy and X-ray crystallography. Mol Cell. 1999;3:761–770. doi: 10.1016/s1097-2765(01)80008-3. [DOI] [PubMed] [Google Scholar]
- Niswonger ML, O'Halloran TJ. Clathrin heavy chain is required for spore cell but not stalk cell differentiation in Dictyostelium discoideum. Development. 1997a;124:443–451. doi: 10.1242/dev.124.2.443. [DOI] [PubMed] [Google Scholar]
- Niswonger ML, O'Halloran TJ. A novel role for clathrin in cytokinesis. Proc Natl Acad Sci USA. 1997b;94:8575–8578. doi: 10.1073/pnas.94.16.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran T, Anderson RGW. Clathrin heavy chain is required for pinocytosis, the presence of large vacuoles and development in Dictyostelium. J Cell Biol. 1992a;118:1371–1378. doi: 10.1083/jcb.118.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran TJ, Anderson RGW. Characterization of the clathrin heavy chain from Dictyostelium discoideum. DNA Cell Biol. 1992b;11:321–330. doi: 10.1089/dna.1992.11.321. [DOI] [PubMed] [Google Scholar]
- Palacek SP, Schmidt CE, Lauffenburger DA, Horwitz AF. Integrin dynamics on the tail regions of migrating fibroblasts. J Cell Sci. 1996;109:941–952. doi: 10.1242/jcs.109.5.941. [DOI] [PubMed] [Google Scholar]
- Pasternak C, Spudich JA, Elson EL. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989;341:549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- Regen CM, Horwitz AF. Dynamics of beta 1 integrin-mediated adhesive contacts in motile fibroblasts. J Cell Biol. 1992;119:1347–1359. doi: 10.1083/jcb.119.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS, Pearse BMF. Immunofluorescent localization of 100K coated vesicle proteins. J Cell Biol. 1986;102:48–54. doi: 10.1083/jcb.102.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury JL, Condeelis JS, Satir P. Role of coated vesicles, microfilaments, and calmodulin in receptor-mediated endocytosis by cultured B lymphoblastoid cells. J Cell Biol. 1980;87:132–141. doi: 10.1083/jcb.87.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Pearse BM. Clathrin: anatomy of a coat protein. Trends Cell Biol. 1999;9:335–338. doi: 10.1016/s0962-8924(99)01631-1. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Taylor DL, Bonner JT. Coated vesicles in Dictyostelium discoideum. J Ultrastruct Res. 1981;75:243–249. doi: 10.1016/s0022-5320(81)80139-6. [DOI] [PubMed] [Google Scholar]
- Wessels D, Reynolds J, Johnson O, Voss E, Burns R, Daniels K, Garrard E, O'Halloran TJ, Soll DR. Clathrin plays a novel role in the regulation of cell polarity, pseudopod formation, uropod stability and motility in Dictyostelium. J Cell Sci. 2000;113:21–36. doi: 10.1242/jcs.113.1.21. [DOI] [PubMed] [Google Scholar]
- Wessels D, Vawter-Hugart H, Murray J, Soll DR. Three-dimensional dynamics of pseudopod formation and the regulation of turning during the motility cycle of Dictyostelium. Cell Motil Cytoskeleton. 1994;27:1–12. doi: 10.1002/cm.970270102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.