Abstract
Rice contains several MADS box genes. It has been demonstrated previously that one of these genes, OsMADS1 (for Oryza sativa MADS box gene1), is expressed preferentially in flowers and causes early flowering when ectopically expressed in tobacco plants. In this study, we demonstrated that ectopic expression of OsMADS1 in rice also results in early flowering. To further investigate the role of OsMADS1 during rice flower development, we generated transgenic rice plants expressing altered OsMADS1 genes that contain missense mutations in the MADS domain. There was no visible alteration in the transgenic plants during the vegetative stage. However, transgenic panicles typically exhibited phenotypic alterations, including spikelets consisting of elongated leafy paleae and lemmas that exhibit a feature of open hull, two pairs of leafy palea-like and lemma-like lodicules, a decrease in stamen number, and an increase in the number of carpels. In addition, some spikelets generated an additional floret from the same rachilla. These characteristics are very similar to those of leafy hull sterile1 (lhs1). The map position of OsMADS1 is closely linked to that of lhs1 on chromosome 3. Examination of lhs1 revealed that it contains two missense mutations in the OsMADS1 MADS domain. A genetic complementation experiment showed that the 11.9-kb genomic DNA fragment containing the wild-type OsMADS1 gene rescued the mutant phenotypes. In addition, ectopic expression of the OsMADS1 gene isolated from the lhs1 line resulted in lhs1-conferred phenotypes. These lines of evidence demonstrate that OsMADS1 is the lhs1 gene.
INTRODUCTION
In response to floral induction, the inflorescence meristem becomes committed to flowering. LEAFY (LFY) and APETALA1 (AP1) in Arabidopsis and FLORICAULA (FLO) and SQUAMOSA (SQUA) in Antirrhinum are responsible for promoting the specification of floral meristem identity (reviewed in Ma, 1994). The genes required for specifying the fate of floral organ primordia include AP1, AP2, AGAMOUS (AG), PISTILATA (PI), and AP3 in Arabidopsis and SQUA, PLENA (PLE), GLOBOSA (GLO), and DEFICIENS (DEF) in Antirrhinum (reviewed in Weigel and Meyerowitz, 1994). Excluding AP2, these floral homeotic genes encode MADS box proteins that are highly conserved transcription factors in plants, animals, yeast, and fungi and that are regulated by the floral meristem identity gene LFY (Parcy et al., 1998; Wagner et al., 1999).
Several other MADS box genes have more subtle functions associated with floral meristem and floral organ identity. Expression of AG-LIKE2 (AGL2), AGL4, and AGL9 of Arabidopsis begins after the onset of expression of floral meristem identity genes but before the activation of floral organ identity genes (Flanagan and Ma, 1994; Savidge et al., 1995; Mandel and Yanofsky, 1998). DEFH72 and DEFH200 of Antirrhinum appear to function in mediating interactions between the meristem and organ identity genes through direct interaction with PLE (Davies et al., 1996). FLORAL BINDING PROTEIN2 (FBP2) of petunia and TOMATO GENE5 (TM5) of tomato control organ identity as well as determinacy of the floral meristem (Angenent et al., 1994; Pnueli et al., 1994). Bonhomme et al. (1997) proposed that SaMADS D in Sinapis alba may act in inflorescence meristem identity and interact with genes specifying floral organ identity. However, the function of many MADS box genes of the AP1 and AGL9 group (Purugganan et al., 1995) remains unknown.
The identification of regulatory genes expressed in cereal spikelets has resulted in a greater understanding of the molecular basis of flower development. Two AG orthologs of maize, ZAG1 and ZMM2, have each evolved separate but partially overlapping activities (Mena et al., 1996). Silky1, a maize ortholog of AP3, has been cloned by direct transposon tagging (Schmidt and Ambrose, 1998). INDETERMINATE SPIKELET1, a member of the AP2 gene family, is required for determining the spikelet meristem fate and thereby limits the number of floral meristems produced in maize (Chuck et al., 1998).
Several MADS box genes that play important roles in controlling flower development in rice also have been studied (Chung et al., 1994, 1995; Kang et al., 1995, 1997; Greco et al., 1997; Kang and An, 1997; Lopez-Dee et al., 1999; Moon et al., 1999a, 1999b). Using antisense experiments, Kang et al. (1998) previously demonstrated that rice MADS box genes OsMADS3 (for Oryza sativa MADS box gene3) and OsMADS4 are the putative orthologs of AG and PI, respectively. Based on its expression pattern, amino acid sequence similarity, and interaction with OsMADS4 in yeast, OsMADS16 has been proposed as a homolog of AP3 (Moon et al., 1999a).
Mutants are useful for the functional analysis of a given gene. When genetic mutants are not readily available, inactivation of a gene function by cosuppression or by using an antisense strategy has been undertaken to elucidate what role the gene plays (Angenent et al., 1993, 1994; Pnueli et al., 1994; Mizukami and Ma, 1995; Kang et al., 1998). Alternatively, for identifying proteins that are parts of a complex, one can generate dominant-negative forms such that the altered protein can inhibit the normal function of the coexisting endogenous one. In Arabidopsis, an AG protein lacking a C-terminal region inhibits normal AG function, generating a phenotype similar to that of ag1 mutant (Mizukami et al., 1996). Finally, ectopic expression of a gene often provides valuable information regarding its function (Mizukami and Ma, 1992; Jack et al., 1994; Weigel and Nilsson, 1995). In this study, we generated transgenic plants expressing mutant forms of OsMADS1, a rice MADS box gene, to get a clue about possible functions of the gene. Through analysis of phenotype of the transgenic plants, we found that inhibition of OsMADS1 function results in the phenotype similar to that of leafy hull sterile1 (lhs1) (Kinoshita et al., 1976). Moreover, wild-type OsMADS1 rescued the mutant phenotype, demonstrating that lhs1 is a homeotic mutation of OsMADS1. Finally, by examining the lhs1 spikelets, we conclude that this MADS box gene plays important roles in determining floral meristem identity and in floral organ development.
RESULTS
Expression of the C-Terminal Truncated OsMADS1 Gene in Rice Plants
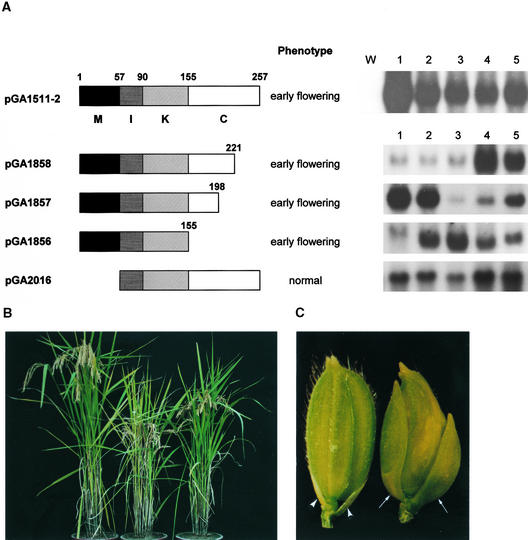
The functional role of OsMADS1 was investigated by overexpression of the wild or mutant forms of the gene in rice plants. We generated transgenic rice plants expressing OsMADS1 constitutively under the control of the rice actin1 (act1) promoter (Figure 1A, pGA1511-2). Most of the primary transgenic plants exhibited severe dwarfism, and their panicles were partly embedded in leaf sheaths (Figure 1B, center), compared with the control plants transformed with the binary vector pGA1671 (Figure 1B, left). Transgenic plants flowered 5 to10 days earlier than did the wild-type controls and exhibited remarkably shorter panicles. RNA gel blot analysis revealed that much more transgene was expressed than wild type (Figure 1A, right). In addition, we observed that two glumes overgrew in transgenic plants that were strongly expressing the transgene, mimicking the palea and lemma (Figure 1C). These results show that overexpression of the OsMADS1 gene promotes determination of the floral meristem and formation of paleae and lemmas. It had been observed previously that gene expression begins in floral meristems at an early stage of flower development and is strongly expressed in paleae and lemmas at a later stage (Chung et al., 1994).
Figure 1.
Construction of Truncated OsMADS1 and Phenotype of Transgenic Plants.
(A) Schematic representations of truncated OsMADS1 proteins, phenotype, and RNA gel blot analyses of the transgenic plants. Shown at left is the OsMADS1 protein represented by four regions: M, MADS domain; I, I region; K, K domain; C, C-terminal region. The numbers indicate the boundaries of the regions and the positions of the truncations. In the center is the phenotype of the T2 transgenic rice plants that express the construct given at left. Shown at right are RNA gel blot data from the independent transgenic plants, which were derived from transformation of the constructs shown at left. Equal amounts of total RNA loading were examined by using ethidium bromide staining of 25S and 18S rRNAs (data not shown). In pGA1511-2 transgenic lines, line 1 flowered ∼10 days earlier than wild-type or control plants carrying the binary vector pGA1671; the other lines flowered ∼5 days earlier than controls. In pGA1858, pGA1857, and pGA1856 transgenic lines, lines 1858-4, 1858-5, 1857-1, 1857-2, 1857-5, 1856-2, 1856-3, 1856-4, and 1856-5 flowered ∼5 days earlier than controls, and other lines did not show early flowering. Five micrograms of total RNA from prepared leaves was loaded in all other lanes. The C-terminal region of OsMADS1 was used as a gene-specific probe (Chung et al., 1994), except that RNAs from pGA1856 lines were hybridized with the K region of OsMADS1. W, wild-type plants; numbers, independent transgenic lines.
(B) Phenotypes of transgenic plants expressing full-length OsMADS1 or the C-terminal truncated form. Shown at left is a control plant transformed with the binary vector pGA1671; at center is the transgenic plant with pGA1511-2 (line 1511-2-1); at right is the transgenic plant with pGA1856 (line 1856-2).
(C) Spikelets of the wild type (left) and transgenic line 1511-2-1 (right). Normal glumes are short and inconspicuous (arrowheads), whereas the transgenic spikelet shows overgrowth of glumes (arrows) that resemble paleae and lemmas. This phenotype was observed in most transgenic plants expressing the full-length or the C-terminal–truncated OsMADS1.
To understand the function of OsMADS1, three mutants (pGA1856, pGA1857, and pGA1858) lacking the C-terminal region and a mutant (pGA2016) lacking the MADS domain were constructed (Figure 1A). The truncated proteins were expressed in rice plants by using the act1 promoter. Interestingly, transgenic rice plants expressing the C-terminal deletion of the gene had the early-flowering and dwarf phenotypes (Figure 1B, right), although to a lesser extent than the transgenic plants expressing the wild-type OsMADS1 (Figure 1B, center). There was no obvious alteration of floral organs, except for an occasional elongation of glumes, as was observed for transgenic plants expressing the wild-type OsMADS1 (Figure 1C). RNA gel blot analyses revealed that all transgenic plants showing early flowering expressed the introduced genes abundantly, indicating that the altered phenotype most likely reflected the expression of OsMADS1 (Figure 1A). On the other hand, ectopic expression of the OsMADS1 mutant lacking the MADS domain (pGA2016) showed no phenotypic alteration, probably because of a lack of dimerization and DNA binding. As was previously demonstrated, AG genes lacking the MADS domain fail to dimerize and bind DNA (Mizukami et al., 1996). These results indicate that whereas the MADS box region is essential, the C-terminal region is not needed for inducing the early-flowering and dwarf phenotypes.
Expression of OsMADS1 Containing Missense Mutations in the MADS Box
Not only is the MADS domain region required for DNA binding, but in some MADS domain proteins the region is also involved in either homo- or heterodimerization (reviewed in Shore and Sharrocks, 1995). Therefore, we postulated that mutations in this region would interfere with the function of MADS genes. To test the hypothesis, seven mutants in the MADS box region of OsMADS1 were generated (Figure 2). In two constructs (pGA1701 and pGA1860), the mutations were in a region corresponding to DNA binding residues of a human MADS box gene, SRF (Norman et al., 1988). Three constructs (pGA1702, pGA1703, and pGA1861) carried mutations in amino acid sequences corresponding to the serum response factor (SRF) residues that were involved in dimerization. The remaining two constructs (pGA1862 and pGA1863) were made by introducing missense mutations into amino acid sequences corresponding to the SRF residues involved in both DNA contacting and dimerization. These mutant genes were placed under the control of the act1 promoter and were introduced into rice plants by means of an Agrobacterium-mediated transformation. During the vegetative growth stage, transgenic plants grew normally and were indistinguishable from wild-type plants. There was no significant reduction in plant height or flowering time, indicating that the mutant OsMADS1 did not behave like the wild-type or the C-terminal truncated forms of the gene.
Figure 2.
MADS Domain Sequences of the Site-Directed OsMADS1 Mutants.
At left are the amino acids that differ from those of the wild-type MADS domain sequence. The SRF MADS domain region and the amino acid residues involved in DNA contacting or dimerization are shown. The asterisk indicates two amino acid residues generated in the place of Leu8. At right are the binary vectors, which consist of the actin act1 promoter and the mutant OsMADS1.
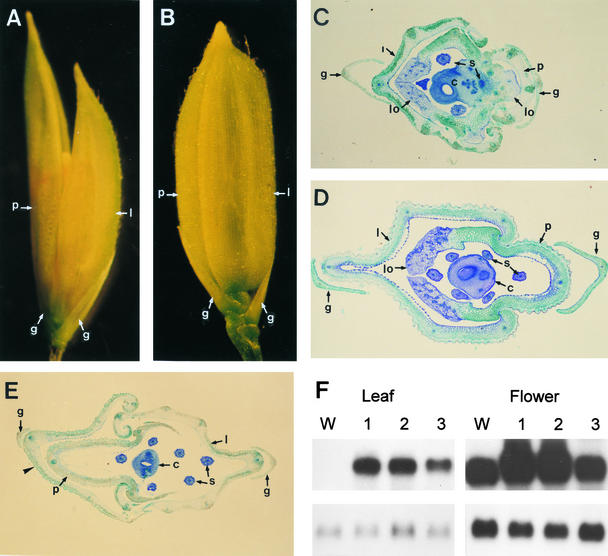
Rather than the early-flowering and dwarf phenotypes, most of the transgenic plants produced abnormal spikelets that carried elongated papery leafy paleae and lemmas (Figure 3A). Whereas wild-type spikelets are enclosed within the palea and lemma, the mutants had an open hull because of a leafy characteristic of the palea and lemma (Figures 3A and 3B). The inner whorls of wild-type spikelets comprise a pair of lodicules, six stamens, and a carpel (Figure 3D). Abnormal structures observed in the inner whorls of mutant spikelets included two pairs of leafy palea- and lemma-like lodicules, fewer stamens (Figures 3A and 3C), and an occasional additional carpel (data not shown). Some flowers produced an additional palea- and lemma-like leafy structure (Figure 3E). These mutant phenotypes were not observed, however, for plants overexpressing either the wild-type or the C-terminal truncated OsMADS1 gene product. RNA gel blot analyses with leaves and flowers of transgenic plants revealed that the transcripts were abundantly expressed in all lines showing the mutant phenotype (Figure 3F). Transgenic plants with the severe mutant phenotype accumulated a high amount of the transgene transcript. A few normal-looking plants weakly expressed the transgene (data not shown). Although we could not distinguish between the transgene transcript and the wild-type transcript in flowers, a marked increase in the amount of transcript in spikelets should reflect high expression of the transgene.
Figure 3.
Phenotypes of Transgenic Plants Expressing the OsAMDS1 Mutant.
(A) A spikelet from a plant of transgenic line 1703-1 transformed with pGA1703. The spikelet has a phenotype similar to lhs1, showing an open flower with the elongated leafy palea and lemma.
(B) A spikelet of a wild-type plant.
(C) A cross-sectioned spikelet from a plant of line 1703-1. The spikelet consists of normal glumes, abnormal palea and lemma, two pairs of leafy palea- and lemma-like lodicules, four stamens, and a carpel.
(D) A cross-sectioned wild-type spikelet. The spikelet has glumes, palea and lemma, a pair of lodicules, six stamens, and a carpel.
(E) A spikelet from the line 1703-3. The spikelet has an additional palea- and lemma-like structure (arrowhead). Original palea and lemma developed abnormally. Other organs—lodicules, stamens, and a carpel—are almost identical to the wild type.
(F) RNA gel blot analysis of the OsMADS1 transcript in the leaves and flowers of the 1703 transgenic lines. Five micrograms of total RNA from leaves (left) and 10 μg from flowers (right) were used for RNA gel blot analysis. At top, the amount of OsMADS1 transcript expressed was measured using the gene-specific probe for OsMADS1. Because of the similar sizes of the endogenous and transgenic OsMADS1 transcripts, the signals shown are the sum of the two. Lanes W, wild type; lanes 1, line 1703-1; lanes 2, line 1703-2; lanes 3, line 1703-3. At bottom are the controls. The same filters were washed and rehybridized with the rice α-tubulin gene OsTubA1 (Jeon et al., 2000).
c, carpel; g, normal glume; l, abnormal lemma; lo, leafy palea- and lemma-like lodicules; p, abnormal palea; s, stamen.
Chromosomal Mapping of OsMADS1
Several mutants in rice display abnormal flower development (Yoshimura et al., 1997). To elucidate whether any of the mutations occur in OsMADS1, we determined the location of the gene on a genetic map by using an F11 recombinant inbred population of rice. The result revealed that OsMADS1 is located between RG100 and RZ313 on chromosome 3 (data not shown). Interestingly, this region also includes lhs1 (Kinoshita et al., 1976; Khush and Librojo, 1985; Yoshimura et al., 1997), which results in mutant phenotypes that resemble the panicles of the transgenic plants that express OsMADS1, which carries missense mutations in the MADS box region.
Identification of the OsMADS1 Mutation in lhs1
Because the locus of lhs1 is closely associated with that of OsMADS1, we examined whether the lhs1 mutant line carried any alteration in the OsMADS1 gene. The coding regions of the OsMADS1 gene were isolated from the lhs1 mutant line by using polymerase chain reaction primers located within introns. Sequence analysis of the amplified fragments revealed that the nucleotides C and G at positions 70 and 80, respectively, in the coding region (Chung et al., 1994) were changed to T and A, respectively. Consequently, the arginine of codon 24 and the glycine of codon 27 were replaced with cysteine and aspartic acid, respectively, in the lhs1 mutation (Figure 4). Alignment of the MADS domains of various MADS box genes from rice and Arabidopsis shows that the amino acids in the region in which the lhs1 mutation occurred are conserved in all MADS genes, as previously observed (reviewed in Shore and Sharrocks, 1995). Pellegrini et al. (1995) have reported that the Arg24 is involved in both DNA contacting and dimerization and that the Gly27 is located at the DNA contacting position. The results presented in Figure 3 show that replacing one to three amino acids in the MADS domain alters the floral organ development, the alterations being quite similar to those for lhs1. Therefore, the homeotic alterations in the lhs1 flowers are likely to have resulted from the mutations in the OsMADS1 gene.
Figure 4.
Amino Acid Changes Corresponding to Nucleotide Changes in the lhs1 Allele.
The amino acid sequence is in the MADS box domain. Boldface letters indicate the changes in the lhs1 allele.
Point mutations in the MADS domain were also found in homeotic mutants of Arabidopsis and Antirrhinum. These include ap1-2, cauliflower-2 (cal-2), cal-3, and pi-3 in Arabidopsis and defa-nicotianoides in Antirrhinum (Mandel et al., 1992; Schwarz-Sommer et al., 1992; Goto and Meyerowitz, 1994; Kempin et al., 1995). In particular, the mutant ap1-2, cal-3, and defa-nicotianoides alleles result from a missense mutation of Gly27 to Asp, the same alteration as in lhs1 (Mandel et al., 1992; Schwarz-Sommer et al., 1992; Kempin et al., 1995). These results support the importance of the 27th amino acid residue for MADS box gene function.
Genetic Complementation of lhs1 by OsMADS1
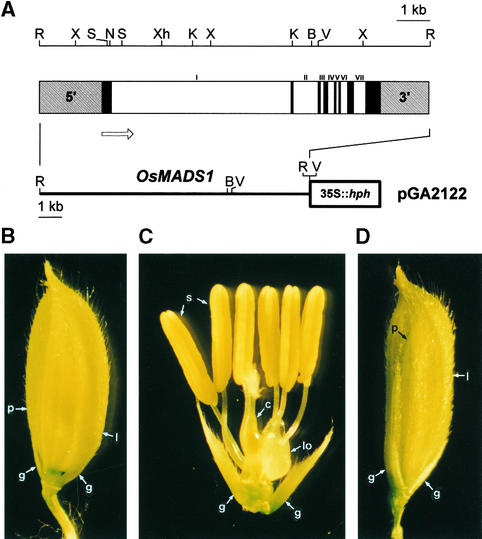
To investigate whether the phenotype of the lhs mutant was indeed attributable to the alterations of OsMADS1, we performed a complementation experiment, using the wild-type OsMADS1 clone isolated from a genomic library. Restriction mapping and DNA gel blot analysis of the genomic clone showed that the 11,851-bp EcoRI fragment carried the entire OsMADS1 gene (Figure 5A, top). The fragment consisted of a 1917-bp 5′ upstream sequence, eight exons, seven introns, and a 1484-bp 3′ region (Figure 5A, center). The first intron was 5524 bp long and located in codon 62. The other introns were much shorter, ranging between 90 and 771 bp. All introns contained the consensus GT and AG sequences at the 5′ and 3′ ends, respectively. Neither the 5′ upstream region, the 3′ downstream region, nor the introns contained any open reading frames of notable length, thus indicating that the 11,851-bp EcoRI fragment carries only the OsMADS1 gene.
Figure 5.
Structure of the OsMADS1 Genomic Clone, the Vector Used for Genetic Complementation, and Phenotypes of the Transgenic Plants.
(A) A restriction map of the OsMADS1 genomic clone is shown at top. B, BamHI; K, KpnI; N, NotI; R, EcoRI; S, SacI; V, EcoRV; X, XbaI; Xh, XhoI. At center is the genomic structure of OsMADS1. The 11,851-bp EcoRI fragment contains the entire OsMADS1 gene, which consists of seven introns (white bars) and eight exons (black bars) (GenBank accession number AF204063). The length of each intron is, in order from left, 5524, 771, 120, 207, 90, 218, and 378 bp. The numbers on top of the DNA fragment indicate the introns. The promoter and terminator regions are shown in the diagonally striped rectangles. The open arrow indicates the direction of transcription. At bottom is the binary vector pGA2122 containing the OsMADS1 genomic clone. The vector also harbors the hygromycin phosphotransferase (hph) gene under the control of the 35S promoter for selection of transgenic plants.
(B) A spikelet from a transgenic plant from line 2122-4. The structure of palea and lemma in plant 2122-4 is indistinguishable from that of a wild-type spikelet.
(C) A dissected spikelet from a plant of line 2122-4 showing inner floral organs. The spikelet consists of a pair of lodicules, six stamens, and a carpel, which are identical to those of the wild type.
(D) A spikelet from a transgenic plant from line 2122-6. The palea is partially recovered.
c, carpel; g, normal glume; l, abnormal lemma; lo, leafy palea- and lemma-like lodicules; p, abnormal palea; s, stamen.
The genomic DNA fragment that included the entire wild-type OsMADS1 gene along with the large intron was cloned into the binary vector pGA1182 (Figure 5A, bottom). A large intron is often present after the MADS box region, such as in AG of Arabidopsis and PLE and FARINELLI of Antirrhinum. Insertion of T-DNA or a transposon in the intron of these genes resulted in loss of gene function (Yanofsky et al., 1990; Bradley et al., 1993; Davies et al., 1999). The binary vector pGA2122, carrying the entire OsMADS1 gene, was introduced into the Agrobacterium strain LBA4404 and used for transformation of the lhs1 plants. Four of nine independently transformed plants generated spikelets that were completely recovered from the mutant phenotype (Figures 5B and 5C). Another four lines showed a partial recovery (Figure 5D). Control transgenic plants transformed with the vector pGA1182 did not show any complementation of the lhs1-conferred phenotype (data not shown). Genomic DNA gel blot analysis revealed that all four of the transgenic lines that completely rescued the mutant phenotype contained both the wild-type OsMADS1 gene and the lhs1 allele (data not shown). These results strongly support the hypothesis that the phenotype of the lhs1 mutant is caused by mutations in the OsMADS1 gene.
Spikelets of the lhs1 Mutant
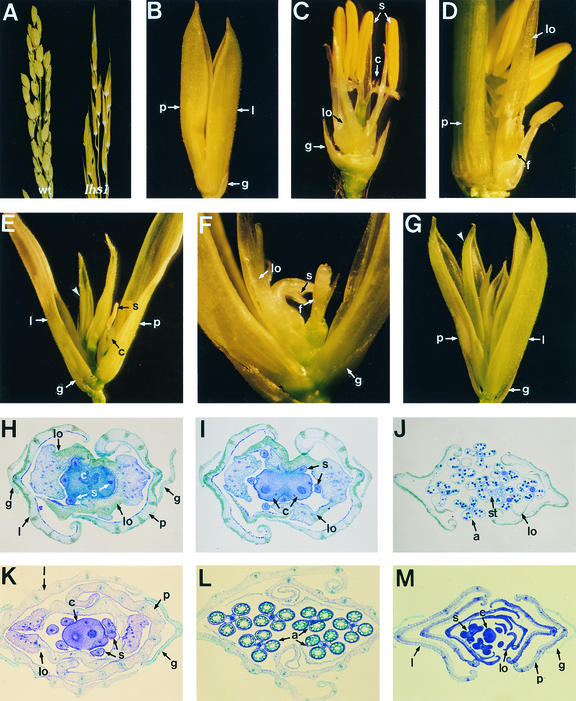
To examine the functional role of OsMADS1 during flower development, we analyzed panicle development in the lhs1 mutant (Figure 6). We observed that spikelets of the lhs1 mutant contained leafy paleae and lemmas that resulted in open flowers (Figures 6A and 6B). A pair of lodicules became leafy, resembling the palea and lemma. The number of stamens was reduced to four, on average (Figures 6C and 6H), although occasionally an additional carpel developed (Figure 6I). In some lhs1 spikelets, a new abnormal flower was formed in a whorl of stamens from the same rachilla (Figures 6D and 6E) or from a stigma of a carpel (Figure 6F), indicating that the lhs1 spikelets had incomplete floral meristem determination. Flowers with eight stamens were rarely observed, possibly because of the generation of a new flower in a spikelet (Figure 6J). In all spikelets, glumes developed normally (Figures 6A to 6C), and most anthers in lhs1 flowers produced normal pollen (Figure 6J).
Figure 6.
Spikelets from lhs1 and Transgenic Plants.
(A) Panicles from wild-type (left) and lhs1 (right) plants.
(B) A spikelet from the lhs1 mutant. The palea and lemma are overdeveloped in comparison with those of the wild type (see Figure 3B). The spikelet is open because of abnormal growth of leafy palea and lemma.
(C) A dissected lhs1 spikelet in which a palea and lemma were ripped off.
(D) An lhs1 spikelet generating a new floret.
(E) An lhs1 spikelet generating a new flower (arrowhead) consisting of leafy palea and lemma.
(F) An lhs1 spikelet generating a new flower in sequence.
(G) A transgenic spikelet from transgenic line 2145-9 generating multiple paleae and lemmas (arrowhead).
(H) A cross-section of an lhs1 spikelet. The spikelet consists of normal glumes, abnormal leafy palea and lemma, two pairs of leafy palea- and lemma-like lodicules, four stamens, and a carpel.
(I) A cross-section of an lhs1 spikelet. The spikelet consists of leafy palea and lemma, abnormal lodicules, six stamens, and two carpels.
(J) A cross-section of an lhs1 spikelet. The spikelet consists of two pairs of lodicules, eight stamens, and two carpels.
(K) A cross-section of a spikelet from a plant of transgenic line 2145-8.
(L) A cross-section of anthers of a spikelet from a plant of line 2145-8.
(M) A cross-section of a spikelet from a plant of line 2145-9, showing multiple paleae and lemmas.
a, anther; c, carpel; f, flower; g, glume; l, lemma; lo, lodicule; p, palea; s, stamen; st, stigma.
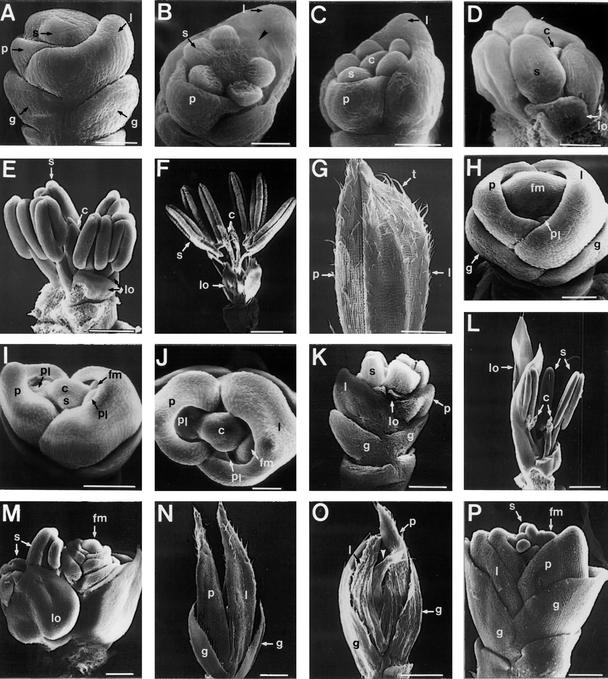
We compared the morphology of wild-type flowers with that of lhs1 flowers at various developmental stages, using scanning electron microscopy (Figure 7). In the floral primordium of a wild-type flower, the glumes, palea, and lemma developed first (Figure 7A), after which the whorls of lodicules and stamens became apparent (Figure 7B). While the stamen primordia were continuing to grow, the remaining tissue of the central meristem elongated and gave rise to the carpel primordium (Figure 7C). Anther and stigma precursors and lodicules then became distinct (Figure 7D). At a late stage of flower development, anther locules and filaments differentiated (Figure 7E). At this point, unlike Arabidopsis petals, the second whorl of the lodicules did not elongate (Smyth et al., 1990). The stigmatic papillae of a carpel were then developed (Figure 7F). Figure 7G shows a mature flower of the wild-type spikelet before anthesis. Long trichomes appeared more abundantly on veins of palea and lemma.
Figure 7.
Scanning Electron Microscopy of Wild-Type, lhs1, and Transgenic Spikelets.
Scanning electron microscopy of spikelets of wild-type ([A] to [G]), lhs ([H] to [O]), and transgenic line 2145-9 (P) rice.
(A) In outer whorls, glumes and palea and lemma primordia are formed. The central floral meristem starts initiating stamen primordia. 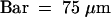 .
.
(B) Six developing stamens are visible. One stamen primordium is developing late (arrowhead). Removal of the lemma reveals that lodicule primordia form at this stage (data not shown). 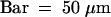 .
.
(C) The carpel primordium is apparent. 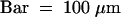 .
.
(D) A dissected spikelet in which palea and lemma were ripped off. A pair of lodicules is formed between stamens and a lemma. 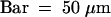 .
.
(E) In stamens, filaments and anthers are apparently differentiated. 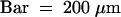 .
.
(F) The mature spikelet in which palea and lemma were ripped off. 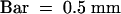 .
.
(G) The palea and lemma of a mature spikelet. 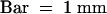 .
.
(H) Glume and palea and lemma primordia are formed in outer whorls of a lhs spikelet. In the inner whorls, a new palea- and lemma-like structure is visible. 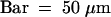 .
.
(I) Palea- and lemma-like structures and a floral meristem are formed in the inner whorls of the lhs1 spikelet. The central meristem is differentiated to stamens and a carpel. 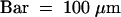 .
.
(J) Top view of (I). 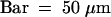 .
.
(K) The lhs spikelet carries five stamens. Lodicules are formed at the base of the stamens. 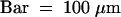 .
.
(L) The spikelet consists of leafy lodicules, three stamens, and two carpels. Palea, lemma, and right lodicule were removed. 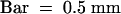 .
.
(M) The spikelet consists of leafy lodicules, two stamens, and a new flower. Palea and lemma were removed to reveal inner organs. 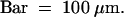
(N) A mature spikelet. Because of the leafy characteristic of the palea and lemma, the spikelet is open. 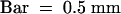 .
.
(O) The spikelet exhibits successive formation of paleae and lemmas (arrowhead). 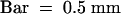 .
.
(P) Early development of the spikelet from a plant of transgenic line 2145-9. The young spikelet consisted of successive leafy paleae and lemmas and floral primordia. 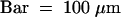 .
.
c, carpel; fm, floral primordium; g, glume; l, lemma; lo, lodicule; p, palea; pl, palea- and lemma-like structure; s, stamen; t, trichome.
The lhs1 spikelets were distinguishable from those of the wild type when floral primordia began to develop (Figure 7H). At this stage, a new palea and lemma structure was seen before the formation of stamen and carpel primordia. The palea and lemma began to overdevelop at an early stage of flower development (cf. Figures 7H to 7J with 7A to 7C). Stamen and carpel primordia were formed irregularly (Figures 7I and 7J), with the number of stamens and carpels varying from two to eight and one or two, respectively, in lhs1 spikelets (Figures 7K to 7M). At the later stage, when stamens began to elongate, the lhs1 spikelets formed open hulls (Figure 7K), and the first whorl primordia eventually became the leaflike structures (Figure 7N). As anthers and filaments differentiated, lodicules were developed to leafy structures (Figure 7L). In some lhs1 spikelets, a new flower appeared between a stamen and a palea and lemma (Figure 7M). Because the floral primordium initiated later, the new flower appeared to be younger than the primary flower. In leafy hulls of mature spikelets, trichomes were underdeveloped (Figure 7N) compared with those in the wild type (Figures 7G). Some flowers carried several leafy paleae and lemmas within a spikelet (Figure 7O).
To examine whether overexpression of lhs1 acted as a dominant-negative form, the OsMADS1 cDNA clone from the lhs1 mutant was placed under control of the maize ubiquitin promoter that showed strong activity in monocots (reviewed in McElroy and Brettell, 1994), and the construct was introduced to a wild-type rice plant. Six of the seven transgenic lines showed phenotypes that were similar to that of lhs1. Of these, four lines showed a mild phenotype of lhs1 (Figures 6K and 6L). The structure of the spikelets in the four lines was similar to those shown in Figures 6B, 6C, and 6H. In two lines, 2145-9 and 2145-11, some of the flowers developed more than two floral primordia within a spikelet (Figure 7P). Consequently, the number of leafy paleae and lemmas was increased, and new leafy palea and lemma formed successively in the spikelet (Figures 6G and 6M), indicating that the flowers have partial inflorescence characteristics.
Kinoshita et al. (1976) previously reported that lhs1 in the winter season produces spikelets composed of many leafy paleae and lemmas without a carpel and stamens. It will be interesting to determine whether the expression level of lhs1 or of a dimerization partner that interacts with LHS1 changes during winter. These results suggest that mutations in the MADS domain of OsMADS1 act as a dominant-negative form when they are strongly expressed. We did not observe the phenotypes of dwarfism and early flowering that appeared when wild-type OsMADS1 was expressed constitutively in rice plants, which indicates that the lhs1 allele acts specifically in flower tissues in a dominant-negative fashion.
DISCUSSION
In this study we have elucidated a role of the OsMADS1 gene by expressing mutant forms of the gene. Missense mutations in the MADS domain of OsMADS1 caused abnormal spikelets similar to those caused by lhs1 in transgenic rice plants. This phenotype was not observed for transgenic plants expressing either the wild-type OsMADS1 or the forms that lacked the MADS box or the C terminus. This result, together with the fact that several mutations in the MADS domain all resulted in similar phenotypes, suggests that the abnormal spikelet development is caused by formation of a defective complex between mutant OsMADS1 and wild-type OsMADS1 or other MADS proteins. This defective complex may not properly recognize its target sequence, thereby resulting in a lack of proper function. Considering that these OsMADS1 mutants did not induce early flowering and dwarfism, it is also possible that the complex binds to a false target sequence or to another protein that is not a normal partner in wild-type spikelets.
The lhs1 mutation has been reported to be a recessive allele (Khush and Librojo, 1985). However, the transgenic plants expressing the mutated OsMADS1 gene displayed phenotypes of abnormal spikelet in the primary transgenic lines, indicating that the mutant OsMADS1 functions as a dominant allele. This discrepancy might reflect a difference in the expression level of the mutant protein. In LHS1/lhs1 plants, the amount of the mutant OsMADS1 protein produced is expected to be equal to that of the wild-type OsMADS1 protein. This amount of mutant protein was probably insufficient to have had negative effects on other proteins. However, in transgenic plants displaying the mutant phenotype, the amount of mutant protein must have been much higher than that of the wild-type protein because a strong promoter was used for expression of the transgene.
The mutant phenotypes do not appear to have been caused by cosuppression of the endogenous OsMADS1 gene. RNA gel blot analysis demonstrated that the amount of total OsMADS1 mRNA in flowers of transgenic plants was more than that in wild-type flowers. OsMADS1 mRNA was also detected in high abundance in the leaves of transgenic plants. In addition, the altered phenotype was observed at a high frequency in the transgenic lines. Ordinarily, the frequency of cosuppression is not this high (Angenent et al., 1993, 1994). Therefore, the missense mutant forms in the MADS domain of OsMADS1 apparently behave as a dominant-negative form. Krizek et al. (1999) have reported that overexpression of AP3 or AG lacking the N-terminal region of the MADS domain caused the mutant phenotype, although the frequency of the mutant phenotype was not as great as the frequency at which the lhs1 phenotype occurred, as seen in the expression of the missense mutant forms. Probably a lack of DNA binding ability in the abnormal dimers caused a defect of the gene function.
Transgenic plants overexpressing the C-terminal deletion mutants of OsMADS1 had the weak early-flowering and dwarf phenotypes, which are typical in transgenic plants expressing the wild-type OsMADS1. Therefore, the C-terminal region does not appear to be required for inducing early flowering. Several pieces of evidence demonstrate the importance of the C terminus of plant MADS box genes. Three ap1 alleles and a ca1 allele have been reported to result from mutations in the C-terminal region (Kempin et al., 1995; Egea-Cortines et al., 1999). Recent studies with the yeast system have demonstrated that three MADS box proteins—SQUA, DEF, and GLO—form ternary complexes by way of their C termini (Egea-Cortines et al., 1999). We have also demonstrated with yeast and mammalian cells that the C terminus of OsMADS1 contains a transcription activation motif (J. Lim, Y.-H. Moon, G. An, and S.K. Jang, manuscript submitted for publication). The results suggest that the C terminus of a MADS box protein is important for specific functions of these proteins.
Because deletion of the C-terminal region from OsMADS1 did not interfere with its role for inducing early flowering, the early-flowering phenotype resulting from ectopic expression of OsMADS1 might show a negative effect of the gene product. Perhaps OsMADS1 negatively regulates other gene products that are required for the duration of vegetative growth in the shoot apex. If that is the case, then the OsMADS1 C terminus is not needed for regulation. In the yeast two-hybrid system, OsMADS1 that lacks a C terminus forms a heterodimer with other MADS domain proteins, namely, OsMADS6, OsMADS14, OsMADS15, and OsMADS17 (Moon et al., 1999b; S. Jang and G. An, unpublished data). Therefore, ectopic OsMADS1 might interact with a MADS domain protein that acts as a floral repressor, such as FLOWERING LOCUS C (Michaels and Amasino, 1999), thereby inducing early flowering. It is also possible that the formation of a complex by several proteins is needed to suppress vegetative growth and induce reproductive growth. Mutants lacking the C terminus of OsMADS1 would be invaluable in unveiling the role of the C terminus of the gene.
We have demonstrated, on the basis of several lines of evidence, that lhs1 is the result of mutations in OsMADS1. First, the location of lhs is mapped near OsMADS1 on chromosome 3. Second, the phenotype of the lhs1 mutant is almost identical to those of transgenic plants expressing OsMADS1 with missense mutations in the MADS box region. Third, the lhs1 mutant allele carries mutations in the MADS box region of OsMADS1. Fourth, introduction of the wild-type OsMADS1 gene rescues the phenotype of the lhs mutant. Fifth, overexpression of the lhs1 allele in wild-type rice plants induces the phenotype of the lhs1 mutant.
lhs1 is a single recessive gene and has been characterized by leaflike transformation of paleae and lemmas (Khush and Librojo, 1985). In the weak phenotype of the lhs1 mutant, the spikelet consists of leafy palea and lemma, two pairs of palea- and lemma-like structures, fewer stamens, and more carpels. In plants with the strong phenotype, the lhs1 mutation results in generation of new flowers within the spikelet. Scanning electron microscopy shows that floral meristems of lhs1 spikelets are irregularly differentiated into floral organs or a new flower. A normal pattern of lodicules, stamens, and carpel development is seldom present in lhs1 spikelets. This suggests that OsMADS1 plays a pivotal role in floral meristem determination during the early development of rice flowers. Morphological changes in paleae and lemmas might also result from defects of the OsMADS1 function at later stages, because the gene is expressed abundantly in mature paleae and lemmas (Chung et al., 1994). Apparently, OsMADS1 also affects the development of lodicules. The abnormal lodicules in lhs1 flowers might be the result of downregulation of other MADS box genes that are associated with lodicules. We also do not exclude the possibility that abnormal lodicules may be the product of the incomplete paleae and lemmas that replaced lodicules in the second whorl.
As previously stated, OsMADS1 interacts with other MADS domain proteins of the AP1/AGL9 group in a yeast two-hybrid system. Therefore, the phenotype of the lhs1 mutant might result from formation of nonfunctional dimers or multimers between LHS1 and other MADS domain proteins that are essential for flower development. Khush and Librojo (1985) reported that overdeveloped palea and naked seed rice are allelic to lhs1. Characterization of these mutants would be useful in further understanding the function of OsMADS1.
On the basis of sequence homology, OsMADS1 can be classified in the AP1/AGL9 group (Purugganan et al., 1995). Inactivation of the tomato TM5 and petunia FBP2 resulted in a defect of the inner three whorls and development of additional whorls of organs or new flowers in the center of flowers (Angenent et al., 1994; Pnueli et al., 1994). The results suggest that TM5 and FBP2 control organ identity as well as determinacy of the floral meristem. In situ hybridization experiments with AGL9 suggest that the gene may function early in flower development to mediate between expression of floral meristem identity genes and activation of organ identity genes; later, it may control the development of petals, stamens, and carpels (Mandel and Yanofsky, 1998). The present study suggests that OsMADS1 shares some similarity with TM5 and FBP2 with respect to determination of the floral meristem.
Unlike the dicot MADS box genes, OsMADS1 appears to play an additional role during the development of the palea and lemma at the late stage of flower development. Infrequently, more complex flowers evolved, probably because of an early function of OsMADS1 in the formation of the floral meristem. We often observed a new flower replacing the whorl of stamens, mimicking the phenotype of the Arabidopsis ap1. However, it is premature to conclude that OsMADS1 is the functional AP1 homolog of rice. The phenotype of the lhs1 mutant includes a decrease in the number of stamens, which has not been reported for ap1. We have isolated two rice MADS box genes, OsMADS14 and OsMADS15, the sequences of which are most homologous to ZAP1, an AP1 homolog in maize (Moon et al., 1999b; J. Lim, Y.-H. Moon, G. An, and S.K. Jang, submittedmanuscript for publication). Recently, Cacharrón et al. (1999) reported two MADS box genes from maize, ZMM8 and ZMM14, that probably are orthologous to OsMADS1. Expression patterns of both maize genes suggest that they may be involved in determining the spikelet meristem and in distinguishing the upper from the lower floret in the maize spikelet. Theissen et al. (1996) grouped OsMADS1 with two MADS box genes of maize, ZMM3 and ZMM8, on the basis of sequence similarity. Several MADS box genes in rice can be classified in the AP1 group. Studying these MADS box genes will help us to understand the regulatory mechanisms involved in spikelet development in monocot plants.
METHODS
Plant Materials
leafy hull sterile1 (lhs1) was recovered by Toshiro Kinoshita (Hokkaido University, Japan) and was made available by H.J. Koh (Seoul National University, Korea). A japonica cultivar, Dongjin, was used for rice transformation.
Mutagenesis and Vector Constructions
The C-terminal deletion constructs of Oryza sativa MADS box gene1 (OsMADS1) were produced by polymerase chain reaction with the OsMADS1 cDNA, which was cloned in pBluescript SK− (Stratagene, La Jolla, CA) as a template. T3 was used as the forward primer. The reverse primers, which were used for generation of C-terminal–deleted mutants, are as follows: 5′-TATTCCTCGAGGCTGTTGCTACTTGCTCTTCAG-3′ (pGA1856); 5′-TATTCCTCGAGGATGAGGCTAATCAGCAAGAAC-3′ (pGA1857); and 5′-TATTCCTCGAGG-GTGATGTTACCCAATCTGCAGGG-3′ (pGA1858).
The bases complementary to the stop codon are underlined. The MADS box deletion construct pGA2016 was generated with the T7 primer and 5′-GCTCTAGACCATGTCCAGCTCATCATG-3′; here, the underlined bases encode a new translation initiation codon. Missense mutations were generated by site-directed mutagenesis. Single-stranded DNA was isolated from an Escherichia coli RZ1032 harboring the OsMADS1 MADS box region, which was subcloned into pBluescript SK−. Mutagenesis was performed according to the method of Kunkel et al. (1991). The primers for mutagenesis are as follows: 5′-GAACGGCCTGCTCGAGGAGGCCTACGAGCT-3′ (pGA1701); 5′-TACGAGCTCTCCCTCCAGTTCGACGCCGAGGTCG-3′ (pGA1702); 5′-GGAATTCGCCAAGCGGATCGAG-3′ (pGA1703); 5′-ACGTTCGACGAGCGCAGGAAC-3′ (pGA1860); 5′-AAGACCTACCAGCAGTCCCTC-3′ (pGA1861); 5′-TCAGCCGGCAGGTGGAATTCGCCAAGCGCAG-3′ (pGA1862); and 5′-GCCAAGCTCGAG-AACGGC-3′ (pGA1863). The bases corresponding to the mutated amino acid residues are underlined. All amplified fragments were sequenced to verify that the desired mutations had been produced and that there were no other changes in the remaining MADS box region.
The mutated genes were cloned into XbaI and XhoI sites between the actin act1 promoter (McElroy et al., 1990) and the T7 terminator of the binary vector pGA1671. The control plasmid pGA1511-2 was constructed by insertion of the wild-type OsMADS1 cDNA into pGA1671.
The lhs1 cDNA was constructed by replacing the NaeI-NsiI fragment of the wild-type OsMADS1 cDNA (Chung et al., 1994) with the same fragment of the lhs1 allele. The constructed cDNA, which encoded the entire lhs1-coding region, was placed under the control of the maize ubiquitin promoter and the nopaline synthase terminator by using pGA1611 (Kang et al., 1998). The chimeric molecule was named pGA2145.
Isolation of the OsMADS1 Genomic Clone and Genetic Complementation of lhs1
A rice genomic library constructed in the λ DASH vector with IR36 DNA (kindly provided by S. Kay, Scripps Institute, La Jolla, CA) was used for isolation of a genomic clone, as described by Sambrook et al. (1989). Phage DNA was prepared by the method of Chisholm (1989). Subcloning and DNA sequencing were performed as described previously (Sambrook et al., 1989). The pGA2122 plasmid was constructed by cloning of the EcoRI genomic fragment containing the entire OsMADS1 gene into the binary vector pGA1182, which contained the hygromycin phosphotransferase gene as a selection marker.
Production and Growth of Transgenic Rice Plants
Rice transformation was performed by the Agrobacterium-mediated cocultivation methods previously described (Jeon et al., 1999). All transgenic rice plants were generated on 40 mg/L hygromycin B–containing medium. The regenerated plants were grown in a greenhouse kept typically at 30°C during the day and 20°C at night. The light/dark cycle in the greenhouse was 14/10 hr.
DNA and RNA Gel Blot Analyses
Genomic DNA was isolated from mature leaves at the heading stage, as described previously (Dellaporta et al., 1983). Five micrograms of genomic DNA was digested with EcoRV and BamHI, separated on a 0.7% agarose gel, blotted onto a nylon membrane, and hybridized with a 32P-labeled probe. Total RNAs were isolated from leaves and flowers at the heading stage by the RNA isolation kit (Tri Reagent; MRC Inc., Cincinnati, OH). The isolated total RNAs were fractionated on a 1.3% agarose gel, blotted onto a nylon membrane, and hybridized with a 32P-labeled probe. All procedures of blot analysis were performed as described previously (Kang et al., 1998).
Mapping Procedures
An F11 recombinant inbred population, consisting of 164 lines derived from a cross between Milyang 23 and Gihobyeo, was used to construct a molecular map. All mapping procedures were performed as previously reported (Kang et al., 1997; Cho et al., 1998).
Microscopic Analysis
Rice flowers were fixed in a fixative solution of 50% ethanol, 0.9 M glacial acetic acid, and 3.7% formaldehyde for 15 hr at 4°C, dehydrated with ethanol, infiltrated with xylene, and embedded in paraffin (Paraplast X-tra; Oxford Labware, St. Louis, MO). Twelve-micrometer-thick sections were transferred onto gelatin-coated glass slides, deparaffinized in xylene, and rehydrated in a graded ethanol and water series. The sections were stained in 0.1% toluidine blue O (Sigma), dehydrated with ethanol, infiltrated with xylene, and covered permanently. Light microscopy was performed with a Nikon labophoto-2.
For scanning electron microscopy, the fixed samples were washed with a sodium phosphate buffer, pH 6.8, dehydrated through an ethanol series, and incubated in an ethanol–isoamyl acetate (1:3 [v/v]) mixture for 1 hr. The samples were then dried, mounted on scanning electron microscopy stubs, and coated with gold. The mounted specimens were observed with a scanning electron microscope (model S-4300; Hitachi, Ibaraki-ken, Japan) at an accelerating voltage of 15 kV.
Acknowledgments
We thank Hee Jong Koh and Toshiro Kinoshita for providing the lhs1 seeds and Susan McCouch and Takuji Sasaki for providing rice molecular markers. We thank Ray Wu for providing the rice act1 promoter and Gi-Hwan Yi for sharing the Dongjin seeds with us. We also thank Chahm An for critical reading of the manuscript; Gurdev Khush, Ilha Lee, and Hee Jong Koh for helpful discussions; and Woong-Suk Yang for technical assistance. This work was supported in part by a grant from the National Research Laboratory Program of Korea Institute of Science and Technology Evaluation and Planning.
References
- Angenent, G.C., Franken, J., Busscher, M., Colombo, L., and van Tunen, A.J. (1993). Petal and stamen formation in petunia is regulated by the homeotic gene fbp1. Plant J. 4, 101–112. [DOI] [PubMed] [Google Scholar]
- Angenent, G.C., Franken, J., Busscher, M., Weiss, D., and van Tunen, A.J. (1994). Co-suppression of the petunia homeotic gene fbp2 affects the identity of the generative meristem. Plant J. 5, 33–44. [DOI] [PubMed] [Google Scholar]
- Bonhomme, F., Sommer, H., Bernier, G., and Jacqmard, A. (1997). Characterization of SaMADS D from Sinapis alba suggests a dual function of the gene in inflorescence development and floral organogenesis. Plant Mol. Biol. 34, 573–582. [DOI] [PubMed] [Google Scholar]
- Bradley, D., Carpenter, R., Sommer, H., Hartley, N., and Coen, E. (1993). Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the plena locus of Antirrhinum. Cell 72, 85–95. [DOI] [PubMed] [Google Scholar]
- Cacharrón, J., Saedler, H., and Theissen, G. (1999). Expression of MADS box genes ZMM8 and ZMM14 during inflorescence development of Zea mays discriminates between the upper and the lower floret of each spikelet. Dev. Genes Evol. 209, 411–420. [DOI] [PubMed] [Google Scholar]
- Chisholm, D. (1989). A convenient moderate-scale procedure for obtaining DNA from bacteriophage λ. BioTechniques 7, 21–23. [PubMed] [Google Scholar]
- Cho, Y.-G., McCouch, S.R., Kuiper, M., Kang, M.R., Pot, J., Groenen, J.T.M., and Eun, M.Y. (1998). Integrated map of AFLP, SSLP and RFLP markers using a recombinant inbred population of rice (Oryza sativa L.). Theor. Appl. Genet. 97, 370–380. [Google Scholar]
- Chuck, G., Meeley, R.B., and Hake, S. (1998). The control of maize spikelet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev. 12, 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, Y.-Y., Kim, S.-R., Finkel, D., Yanofsky, M.F., and An, G. (1994). Early flowering and reduced apical dominance result from ectopic expression of a rice MADS box gene. Plant Mol. Biol. 26, 657–665. [DOI] [PubMed] [Google Scholar]
- Chung, Y.-Y., Kim, S.-R., Kang, H.-G., Noh, Y.S., Park, M.C., Finkel, D., and An, G. (1995). Characterization of two rice MADS box genes homologous to GLOBOSA. Plant Sci. 109, 45–56. [Google Scholar]
- Davies, B., Egea-Cortines, M., de Andrade Silva, E., Saedler, H., and Sommer, H. (1996). Multiple interactions amongst floral homeotic MADS box proteins. EMBO J. 15, 4330–4343. [PMC free article] [PubMed] [Google Scholar]
- Davies, B., Motte, P., Keck, E., Saedler, H., Sommer, H., and Schwarz-Sommer, Z. (1999). PLENA and FARINELLI: Redundancy and regulatory interactions between two Antirrhinum MADS-box factors controlling flower development. EMBO J. 18, 4023–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellaporta, S.L., Wood, J., and Hicks, J.B. (1983). A plant DNA minipreparation: Version 2. Plant Mol. Biol. Rep. 1, 19–22. [Google Scholar]
- Egea-Cortines, M., Saedler, H., and Sommer, H. (1999). Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus. EMBO J. 18, 5370–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan, C.A., and Ma, H. (1994). Spatially and temporally regulated expression of the MADS-box gene AGL2 in wild-type and mutant Arabidopsis flowers. Plant Mol. Biol. 26, 581–595. [DOI] [PubMed] [Google Scholar]
- Goto, K., and Meyerowitz, E.M. (1994). Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev. 8, 1548–1560. [DOI] [PubMed] [Google Scholar]
- Greco, R., Stagi, L., Colombo, L., Angenent, G.C., Sari-Gorla, M., and Pè, M.E. (1997). MADS box genes expressed in developing inflorescences of rice and sorghum. Mol. Gen. Genet. 253, 615–623. [DOI] [PubMed] [Google Scholar]
- Jack, T., Fox, G.L., and Meyerowitz, E.M. (1994). Arabidopsis homeotic gene APETALA3 ectopic expression: Transcriptional and posttranscriptional regulation determine floral organ identity. Cell 76, 703–716. [DOI] [PubMed] [Google Scholar]
- Jeon, J.-S., Chung, Y.-Y., Lee, S., Yi, G.-H., Oh, B.-G., and An, G. (1999). Isolation and characterization of an anther-specific gene, RA8, from rice (Oryza sativa L.). Plant Mol. Biol. 39, 35–44. [DOI] [PubMed] [Google Scholar]
- Jeon, J.-S., Lee, S., Jung, K.-H., Jun, S.-H., Kim, C.H., and An, G. (2000). Tissue-preferential expression of a rice α-tubulin gene, OsTubA1, mediated by the first intron. Plant Physiol., in press. [DOI] [PMC free article] [PubMed]
- Kang, H.-G., and An, G. (1997). Isolation and characterization of a rice MADS box gene belonging to the AGL2 gene family. Mol. Cells 7, 45–51. [PubMed] [Google Scholar]
- Kang, H.-G., Noh, Y.-S., Chung, Y.-Y., Costa, M.A., An, K., and An, G. (1995). Phenotypic alterations of petal and sepal by ectopic expression of a rice MADS box gene in tobacco. Plant Mol. Biol. 29, 1–10. [DOI] [PubMed] [Google Scholar]
- Kang, H.-G., Jang, S., Chung, J.-E., Cho, Y.-G., and An, G. (1997). Characterization of two rice MADS box genes that control flowering time. Mol. Cells 7, 559–566. [PubMed] [Google Scholar]
- Kang, H.-G., Jeon, J.-S., Lee, S., and An, G. (1998). Identification of class B and class C floral organ identity genes from rice plants. Plant Mol. Biol. 38, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Kempin, S.A., Savidge, B., and Yanofsky, M.F. (1995). Molecular basis of the cauliflower phenotype in Arabidopsis. Science 267, 522–525. [DOI] [PubMed] [Google Scholar]
- Khush, G.S., and Librojo, A.L. (1985). Naked seed rice (NSR) is allelic to op and lhs. Rice Genet. News. 2, 71. [Google Scholar]
- Kinoshita, T., Hidano, Y., and Takahashi, M. (1976). A mutant ‘long hull sterile’ found out in the rice variety, ‘Sorachi’—Genetical studies on rice plant. LXVII. Mem. Fac. Agric. Hokkaido Univ. 10, 247–268. [Google Scholar]
- Krizek, B.A., Riechmann, J.L., and Meyerowitz, E.M. (1999). Use of the APETALA1 promoter to assay the in vivo function of chimeric MADS box genes. Sex. Plant Reprod. 12, 14–26. [Google Scholar]
- Kunkel, T.A., Bebenek, K., and McClary, J. (1991). Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 204, 125–139. [DOI] [PubMed] [Google Scholar]
- Lopez-Dee, Z.P., Wittich, P., Pè, M.E., Rigola, D., Del-Buono, I., Sari-Gorla, M., Kater, M.M., and Colombo, L. (1999). OsMADS13, a novel rice MADS-box gene expressed during ovule development. Dev. Genet. 25, 237–244. [DOI] [PubMed] [Google Scholar]
- Ma, H. (1994). The unfolding drama of flower development: Recent results from genetic and molecular analyses. Genes Dev. 8, 745–756. [DOI] [PubMed] [Google Scholar]
- Mandel, M.A., and Yanofsky, M.F. (1998). The Arabidopsis AGL9 MADS box gene is expressed in young flower primordia. Sex. Plant Reprod. 11, 22–28. [Google Scholar]
- Mandel, M.A., Gustafson-Brown, C., Savidge, B., and Yanofsky, M.F. (1992). Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360, 273–277. [DOI] [PubMed] [Google Scholar]
- McElroy, D., and Brettell, R.I.S. (1994). Foreign gene expression in transgenic cereals. Trends Biotechnol. 12, 62–68. [Google Scholar]
- McElroy, D., Zhang, W., Cao, J., and Wu, R. (1990). Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2, 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena, M., Ambrose, B.A., Meeley, R.B., Briggs, S.P., Yanofsky, M.F., and Schmidt, R.J. (1996). Diversification of C-function activity in maize flower development. Science 274, 1537–1540. [DOI] [PubMed] [Google Scholar]
- Michaels, S.D., and Amasino, R.M. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami, Y., and Ma, H. (1992). Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell 71, 119–131. [DOI] [PubMed] [Google Scholar]
- Mizukami, Y., and Ma, H. (1995). Separation of AG function in floral meristem determinacy from that in reproductive organ identity by expressing antisense AG RNA. Plant Mol. Biol. 28, 767–784. [DOI] [PubMed] [Google Scholar]
- Mizukami, Y., Huang, H., Tudor, M., Hu, Y., and Ma, H. (1996). Functional domains of the floral regulator AGAMOUS: Characterization of the DNA binding domain and analysis of dominant negative mutations. Plant Cell 8, 831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, Y.-H., Jung, J.-Y., Kang, H.-G., and An, G. (1999. a). Identification of a rice APETALA3 homologue by yeast two-hybrid screening. Plant Mol. Biol. 40, 167–177. [DOI] [PubMed] [Google Scholar]
- Moon, Y.-H., Kang, H.-G., Jung, J.-Y., Jeon, J.-S., Sung, S.-K., and An, G. (1999. b). Determination of the motif responsible for interaction between the rice APETALA1/AGAMOUS-LIKE9 family proteins using a yeast two-hybrid system. Plant Physiol. 120, 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, C., Runswick, M., Pollock, R., and Treisman, R. (1988). Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell 55, 989–1003. [DOI] [PubMed] [Google Scholar]
- Parcy, F., Nilsson, O., Busch, M.A., Lee, I., and Weigel, D. (1998). A genetic framework for floral patterning. Nature 395, 561–566. [DOI] [PubMed] [Google Scholar]
- Pellegrini, L., Tan, S., and Richmond, T.J. (1995). Structure of serum response factor core bound to DNA. Nature 376, 490–498. [DOI] [PubMed] [Google Scholar]
- Pnueli, L., Hareven, D., Broday, L., Hurwitz, C., and Lifschitz, E. (1994). The TM5 MADS box gene mediates organ differentiation in the three inner whorls of tomato flowers. Plant Cell 6, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purugganan, M.D., Rounsley, S.D., Schmidt, R.J., and Yanofsky, M.F. (1995). Molecular evolution of flower development: Diversification of the plant MADS-box regulatory gene family. Genetics 140, 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, 2nd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Savidge, B., Rounsley, S.D., and Yanofsky, M.F. (1995). Temporal relationship between the transcription of two Arabidopsis MADS box genes and the floral organ identity genes. Plant Cell 7, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, R.J., and Ambrose, B.A. (1998). The blooming of grass flower development. Curr. Opin. Plant Biol. 1, 60–67. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer, Z., Hue, I., Huijser, P., Flor, P.J., Hansen, R., Tetens, F., Lonnig, W.E., Saedler, H., and Sommer, H. (1992). Characterization of the Antirrhinum floral homeotic MADS-box gene deficiens: Evidence for DNA binding and autoregulation of its persistent expression throughout flower development. EMBO J. 11, 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore, P., and Sharrocks, A.D. (1995). The MADS-box family of transcription factors. Eur. J. Biochem. 229, 1–13. [DOI] [PubMed] [Google Scholar]
- Smyth, D.R., Bowman, J.L., and Meyerowitz, E.M. (1990). Early flower development in Arabidopsis. Plant Cell 2, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen, G., Kim, J.T., and Saedler, H. (1996). Classification and phylogeny of the MADS-box gene families in the morphological evolution of eukaryotes. J. Mol. Evol. 43, 484–516. [DOI] [PubMed] [Google Scholar]
- Wagner, D., Sablowski, R.W., and Meyerowitz, E.M. (1999). Transcriptional activation of APETALA1 by LEAFY. Science 285, 582–584. [DOI] [PubMed] [Google Scholar]
- Weigel, D., and Meyerowitz, E.M. (1994). The ABCs of floral homeotic genes. Cell 78, 203–209. [DOI] [PubMed] [Google Scholar]
- Weigel, D., and Nilsson, O. (1995). A developmental switch sufficient for flower initiation in diverse plants. Nature 377, 495–500. [DOI] [PubMed] [Google Scholar]
- Yanofsky, M.F., Ma, H., Bowman, J.L., Drews, G.N., Feldmann, K.A., and Meyerowitz, E.M. (1990). The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346, 35–39. [DOI] [PubMed] [Google Scholar]
- Yoshimura, A., Ideta, O., and Iwata, N. (1997). Linkage map of phenotype and RFLP markers in rice. Plant Mol. Biol. 35, 49–60. [PubMed] [Google Scholar]