Abstract
The nature of kinesin interactions with membrane-bound organelles and mechanisms for regulation of kinesin-based motility have both been surprisingly difficult to define. Most kinesin is recovered in supernatants with standard protocols for purification of motor proteins, but kinesin recovered on membrane-bound organelles is tightly bound. Partitioning of kinesin between vesicle and cytosolic fractions is highly sensitive to buffer composition. Addition of either N-ethylmaleimide or EDTA to homogenization buffers significantly increased the fraction of kinesin bound to organelles. Given that an antibody against kinesin light chain tandem repeats also releases kinesin from vesicles, these observations indicated that specific cytoplasmic factors may regulate kinesin release from membranes. Kinesin light tandem repeats contain DnaJ-like motifs, so the effects of hsp70 chaperones were evaluated. Hsc70 released kinesin from vesicles in an MgATP-dependent and N-ethylmaleimide-sensitive manner. Recombinant kinesin light chains inhibited kinesin release by hsc70 and stimulated the hsc70 ATPase. Hsc70 actions may provide a mechanism to regulate kinesin function by releasing kinesin from cargo in specific subcellular domains, thereby effecting delivery of axonally transported materials.
INTRODUCTION
Translocation of organelles along microtubules by the kinesin heterotetramer requires several discrete activities (Brady, 1995), all of which may be sites for regulating kinesin-based processes. Although hydrolysis of ATP, binding to microtubules, and generation of force have received considerable attention to date, relatively little information is available about interactions between kinesin and membrane-bound organelles (MBOs). Curiously, although kinesin is generally thought to mediate transport of MBOs along microtubules, it is typically purified from soluble fractions of tissue extracts. In subcellular fractionation studies, ∼70% of cellular kinesin was recovered in soluble fractions (Hollenbeck, 1989). However, the presence of a significant pool of soluble kinesin inside cells is problematic, because kinesin isolated in this way is a microtubule-activated ATPase. Soluble kinesin in cells might be expected to deplete cellular ATP through this ATPase activity or to interfere with MBO-associated motors sterically by competing for microtubule-binding sites. One possibility is that a mechanism exists to limit the activity of soluble kinesin in vivo. Several groups have provided evidence that conformational changes in soluble kinesin bring the tail of kinesin in contact with its head and inhibits microtubule-activated ATPase activity (Hackney et al., 1992; Verhey et al., 1998; Coy et al., 1999; Friedman and Vale, 1999). However, this inhibition by folding of soluble kinesin is typically seen in low-ionic-strength buffers and is not significant in buffers with intracellular ionic strength and pH (Hirokawa et al., 1989). Alternatively, levels of soluble kinesin in vivo might be substantially lower than fractionation studies imply.
Several lines of evidence indicate that most kinesin is bound to MBOs in intact cells (Brady, 1995; Brady and Sperry, 1995). Kinesin immunoreactivity is associated with detergent-soluble, punctate structures in close proximity to microtubules, with little evidence of an unbound fraction (Pfister et al., 1989b). When a nonhydrolyzable analogue of ATP, adenylyl 5′-imidodiphosphate (AMP-PNP), is perfused into squid axoplasm, all MBOs stop moving and remain attached to microtubules through kinesin links (Brady, 1985; Lasek and Brady, 1985), indicating both that kinesin is associated with these MBOs and that kinesin attachment to MBOs is relatively stable. Similarly, pulse labeling of kinesin in axonal transport demonstrated that kinesin travels in fast axonal transport with MBO with little or no kinesin detectable in slow axonal transport with soluble, cytoplasmic proteins (Elluru et al., 1995). Finally, kinesin still bound to MBOs after the initial fractionation is highly resistant to in vitro treatments, including high salt and carbonate, that release most peripheral membrane proteins (Leopold et al., 1992; Schnapp et al., 1992).
Such considerations suggest that regulation of kinesin binding to MBOs could be an important physiological pathway for regulating kinesin-based transport of MBOs. Although little is known about regulation of kinesin binding to membranes, the tightness of binding suggested that release of kinesin from MBOs might involve an energy-dependent step, presumably mediated by one or more enzymatic activities. Active removal of kinesin from membranes could be increased during homogenization because of disruption of intracellular compartments. Such disruptions may activate cellular processes that are kept under tight control in the intact cell and thereby may increase soluble fractions of kinesin in tissue extracts. Two types of experiments were undertaken to test the hypothesis that membrane-bound kinesin is released during homogenization and extraction of tissues. First, whole-cell detergent extraction was combined with immunofluorescence to compare the behavior of kinesin with proteins known to be soluble in vivo. If the majority of cellular kinesin is in a soluble cytoplasmic pool, then the soluble kinesin fraction should behave like soluble marker proteins. Conditions that effectively extract all soluble cytoplasmic markers had minimal effect on kinesin immunolocalization. Second, the fraction of tissue kinesin that remained bound to vesicles was assayed after homogenization in the presence of various enzyme inhibitors. If kinesin release is an active process, then inhibition of the relevant enzymatic activities should inhibit release. Homogenization with buffers containing millimolar levels of either a sulfhydryl modifying agent (N-ethylmaleimide [NEM]) or a chelator of divalent cations (EDTA) significantly increased the percent of cellular kinesin in membrane fractions.
Previously, an antibody directed against the tandem repeat domain of kinesin light chains (KLCs) was found to release kinesin from MBOs. This led to reexamination of sequences in the tandem repeat domain. Identification of motifs characteristic of the J-domain in DnaJ, a cofactor for hsp70 chaperones (Cyr et al., 1994; Kelley, 1998), suggested a role for members of this chaperone family. Incubation of MBOs with purified hsc70 indicated that hsc70 released kinesin from MBOs in a nucleotide-dependent and NEM-sensitive manner. These results suggest that kinesin in vivo is tightly bound to MBO, but activation of hsc70 during homogenization releases a large fraction of bound kinesin into the supernatant. Consistent with this, treatment of permeabilized, unfixed cells with purified hsc70 led to a reduction in kinesin immunoreactivity. The hsc70 pathway may serve as a mechanism for regulating kinesin function by releasing kinesin from MBOs in specific subcellular domains, much as hsc70 is thought to remove clathrin coats from coated vesicles (Ungewickell et al., 1995; Holstein et al., 1996; Ungewickell et al., 1997), and other hsp70 family members mediate a variety of protein–membrane interactions (Horst et al., 1997; Terada et al., 1997; Zimmermann, 1998).
MATERIALS AND METHODS
The following cell lines were used: BHK21 wt (kidney cell line), BHK21 stably transfected with green fluorescent protein (GFP) under the control of the cytomegalovirus promoter (a gift from Z. Min), and 3T3 (mouse fibroblast cell line). Four chamber culture slides (Becton Dickinson, Franklin Lakes, NJ) were coated with poly-l-lysine (0.8 mg/ml in water; Sigma, St. Louis, MO) for 1 h at room temperature and then washed three times with water. Forty-eight hours before extraction, cells were plated at densities of 2500–5000 cells/cm2 in Dulbecco's modified Eagle's medium supplemented with 5% FBS and a mixture of antibiotics. Immediately before detergent treatment, cells were rinsed twice with 37°C PHEM buffer (60 mM 1,4-piperazinediethanesulfonic acid, 25 mM HEPES, 10 mM EGTA, and 2 mM MgCl2, pH 7.4) to remove serum and debris. Cells were then extracted for 4 min at 37°C with various concentrations of either Triton X-100 (0.01–1%; Sigma), or digitonin (0.05–0.2%; Calbiochem, La Jolla, CA) freshly made in PHEM supplemented with 1 mM GTP, 10 μM Taxol, and protease inhibitors (20 mM AEBSF, 200 mg/ml aprotinin, 100 mM pepstatin, and 1 mM leupeptin). Digitonin extraction buffers were also supplemented with 5 mM EDTA (Mackall et al., 1979). Unextracted controls were incubated in supplemented PHEM for 4 min before fixation. Cells treated with hsc70 were first extracted with 0.01% Triton X-100 for 1 min in PHEM (as above). Then the solution was replaced with a fresh aliquot of 0.01% Triton X-100 containing PHEM supplemented with 200 μM ATP, 10 mM MgCl2, and purified bovine hsc70, and cells were extracted for an additional 4 min.
At the end of detergent incubations, cells were fixed in 2% paraformaldehyde and 0.01% glutaraldehyde in PHEM for 10 min at 37°C. Fixed cells were rinsed in PBS and then extracted in 0.6% Triton X-100 and PBS for 20 min, except that GFP-transfected cells were extracted in 0.2% Triton X-100 and PBS for 10 min. Before application of primary antibodies, cells were incubated in blocking solution (2.5% gelatin, 2.5% BSA, and 0.01% Na azide in PBS) for 1 h at room temperature. Primary and secondary antibodies were diluted in blocking solution. Primary antibodies were incubated at 4°C for ∼16 h, whereas secondary antibodies were incubated for 45 min at room temperature. We used the following antibodies: rabbit anti-tubulin polyclonal antibody (Sigma T-3626) at 1:200; H2 anti-kinesin mouse monoclonal antibody at 5–20 μg/ml; goat anti-rabbit immunoglobulin G-Texas Red (Molecular Probes, Eugene, OR) at a dilution of 1:500; and goat-anti-mouse Oregon Green 488 at a dilution of 1:500 (Molecular Probes). After incubation with antibodies, cells were washed in PBS. After additional washes in 50 mM Tris, pH 8.0, slides were incubated with either a 1:400 dilution of To-Pro3 (Molecular Probes) for 15 min or a 1 μg/ml dilution of Hoechst dye (Aldrich Chemicals, St. Louis, MO) for 2 min and then rinsed with 50 mM Tris, pH 8.0. Slides were coversliped with ProLong antifade (Molecular Probes) or Gel/Mount (Biomedia, Foster City, CA). GFP, Texas Red immunofluorescence, and To-Pro3 or Hoechst fluorescence were visualized on a Zeiss (Thornwood, NY) Axiovert microscope using either a 40× (numerical aperture, 1.4) or a 63× (numerical aperture, 1.3) objective. Images were acquired either with a Bio-Rad (Hercules, CA) confocal laser scanning microscope or with a cooled charge-coupled device camera (ORCA; Hamamatsu, Hamamatsu City, Japan) controlled by Openlab Software (Improvision, Lexington, MA). Images were processed for presentation in Adobe Photoshop (Adobe Systems, Mountain View, CA). All images shown in the same panel were altered for contrast identically.
Microsomal vesicles were purified by homogenizing fresh bovine brains in 5 volumes of homogenization buffer (HB; 300 mM sucrose, 10 mM HEPES, pH 7.4, 5 mM MgCl2, and protease inhibitor mixture [1 mM -4-(2-aminoethyl)benzenesulfonyl fluoride and 10 μg/ml leupeptin, pepstatin, and aprotinin]). As indicated, HB was used without additions, with NEM (0.1–5 mM), or with EDTA (5 mM) added to buffer before homogenization. For NEM experiments, the suspension was centrifuged 15 min at 39,800 × g. This 39,800 × g pellet (V1) was resuspended in homogenization buffer by 10 passages through a 25-gauge hypodermic needle to disperse vesicles for further analysis. The 39,800 × g supernatant was centrifuged 40 min at 120,000 × g. The supernatant was layered onto 600 mM sucrose in 10 mM HEPES, pH 7.4, and centrifuged for 2 h at 260,000 × g. The 260,000 × g pellet (V2) was resuspended in homogenization buffer as described above. Vesicle samples were either processed for immunoblots or used for release assays. For quantitative immunoblots, the supernatant (S) and vesicle fractions (V1 and V2) were probed for the presence of kinesin with the H2 antibody as described previously (Pfister et al., 1989b). Primary antibodies were detected by 125I-protein A, and the amount of kinesin in different fractions was quantitated with a Molecular Dynamics (Sunnyvale, CA) PhosphorImager after overnight exposure of screens.
For quantitative and qualitative comparisons between NEM and EDTA treatments, this protocol was modified to generate a single vesicle fraction that retained a lower percentage of kinesin. Brains were homogenized in a buffer containing 320 mM sucrose and 10 mM HEPES, pH 7.4, and homogenates were centrifuged at 11,000 × g maximum for 8 min to eliminate debris, nuclei, and mitochondria. Three milliliters of each supernatant were taken from each tube and centrifuged for 1 h at 200,000 × g max in a Beckman Instruments (Palo Alto, CA) TLA.100.3 ultracentrifuge rotor. After recovering the soluble fraction, the 200,000 × g pellets were resuspended by brief sonication in 1.5 ml of HB. Protein concentration was measured by the Coommasie blue assay (Pierce, Rockford, IL). Equal amounts (50 μg) of protein from each fraction were separated by SDS-PAGE and transferred to a ZetaProbe nylon membrane (Bio-Rad) 16 h at 25 V in 10 mM CAPS buffer, pH 11. Chemiluminescent assays and quantitative inmunoblots were performed as described previously (Stenoien and Brady, 1997). The amounts of kinesin, hsc70, synaptophysin, and other proteins were quantitated with a Molecular Dynamics PhosphorImager after overnight exposure of screens. Monoclonal antibodies against hsc70 and synaptophysin were purchased from StressGen (Victoria, BC). Secondary antibodies were from Jackson ImmunoResearch (West Grove, PA).
The effects of hsc70 on the kinesin bound to microsomal vesicles (V2) were evaluated by incubating at a concentration of 1 mg/ml total vesicle protein with or without hsc70 for 30 min at 37°C in release buffer (HB plus 75 mM KCl). Hsc70 was used at concentration of 10 μg/ml for a molar ratio of 2:1 for hsc70:kinesin. After centrifugation over 600 mM sucrose in 10 mM HEPES, pH 7.4, at 260,000 × g, the supernatants and pellets were probed for the presence of kinesin using the H2 antibody on quantitative immunoblots. Antibodies were detected using 125I-labeled protein A and analyzed by PhosphorImager. The effects of NEM on hsc70-mediated release of endogenous kinesin were determined by addition of NEM (2 mM) to V2 vesicles in vitro either at the same time as hsc70 (time 0) or at different intervals after hsc70. To control for nonspecific effects of the NEM vehicle, the action of NEM on free sulfhydryl groups was blocked by simultaneous addition of dithiothreitol (DTT; 2 mM).
To evaluate the ability of different KLC constructs to affect hsc70-mediated release of kinesin from vesicles, varying concentrations of recombinant proteins were added to purified microsomal vesicles (1 mg/ml total vesicle protein) with hsc70 at a concentration of 10 μg/ml (a molar ratio of 2:1 for hsc70:kinesin) for 30 min at 37°C. Four different constructs were generated: glutathione S-transferase (GST; no insert), GST-LCA (full-length KLC1), GST-TR1 (two tandem repeats), and GST-TR2 (four tandem repeats). The molar ratios for fusion protein to hsc70 ranged from 0.25 to 8:1. To determine whether different KLC constructs differed in their ability to bind hsc70, activation of hsc70 ATPase was assayed. Bovine brain hsc70 (1 μM) was incubated in a volume of 10 μl containing 0.2 mM [γ-32P]ATP with or without 2 μM GST-LCA or GST-TR1 or GST alone for 1 h at 37°C in ATPase assay buffer (75 mM KCl, 5 mM MgCl2, 10 mM HEPES, pH 7.4, and protease inhibitor mixture). At given time intervals, an aliquot (0.5–0.7 μl) was withdrawn and spotted on polyethyleneimine-cellulose plates (Aldrich, Milwaukee, WI). After developing in 0.5 M LiCl and 1 M formic acid, the spots on the chromatograms corresponding to [32P]phosphate and [γ-32p]ATP were excised, and the amount of radioactivity was measured. The ATP hydrolyzed was expressed as the percent radioactivity in phosphate as a fraction of total radioactivity calculated from the sum of radioactivity in phosphate and ATP.
RESULTS
Differential detergent extraction was used to evaluate the extractability of kinesin in cultured cells. Brief extraction of cells with 0.015% digitonin creates holes in the plasma membrane with minimal effects on intracellular organelles (Mackall et al., 1979; Womack et al., 1983; Ramsby and Makowski, 1998). Cultured cells extracted under these conditions release 90–100% of traditional cytosolic markers such as lactate dehydrogenase and carbonic anhydrase, but mitochondrial, lysosomal, and endoplasmic reticulum markers are retained (Ramsby et al., 1994). The proteins released by digitonin treatment include myosin II, calpastatin and other proteins >300 kDa in molecular mass (Weigel et al., 1983; Ramsby et al., 1994). In contrast, extraction of cells with Triton X-100 solubilizes many plasma membrane and intracellular organelle membrane proteins in a time- and concentration-dependent manner (Ramsby and Makowski, 1998).
The behavior of kinesin during detergent extractions was compared with that of a protein known to be soluble in vivo. BHK21 cells constitutively expressing GFP were fixed directly or extracted before fixation with either Triton X-100 or digitonin (Figure 1). When fixed without extraction GFP was retained in the cell, but even the mildest detergent treatments led to rapid loss of cytoplasmic GFP, leaving only a small residual fraction in nuclei. Comparison between GFP and kinesin distributions in unextracted cells suggested that these two proteins did not colocalize. GFP permeated the cell, matching well to cell boundaries and thickness, but kinesin immunoreactivity appeared to be more restricted, perhaps enriched in selected cellular domains.
Figure 1.
Soluble GFP but not kinesin is released from detergent-permeablilized cells. Fluorescent images of unextracted (A), 0.015% digitonin-extracted (B), and 0.1% Triton X-100-extracted (C) wild-type BHK21 cells (H2) or BHK21cells stably expressing GFP are shown. After fixation, cells were processed for immunofluorescence with a mouse anti-KHC antibody (H2) or GFP fluorescence was directly visualized (GFP; green, left column). All cells were also stained with an anti-tubulin antibody (red) and with the nuclear marker To-Pro3 (blue; middle column). Merged images are overlays of pseudocolored green–red–blue images (right column). Thin colored lines separate images of cells from different fields. The pattern of kinesin immunoreactivity differs from GFP fluorescence even in unextracted cells. These differences are more obvious after detergent extraction. Mild detergent extraction (digitonin) removes most of the GFP, except for a residual nuclear fraction. In contrast, significant kinesin immunoreactivity remains visible throughout the cell even after harsher detergent extraction (Triton X-100), indicating that most kinesin is not soluble.
Digitonin extraction before fixation revealed more striking differences between GFP and kinesin localization (Figure 1). Virtually all GFP was extracted from cytoplasmic domains within 4 min, leaving only a weak signal in the nucleus. In contrast, the bulk of the kinesin remained as discrete structures that were often closely apposed to microtubules in double-label studies. Significant punctate kinesin immunoreactivity remains even after more stringent extractions using Triton X-100 under conditions in which intracellular organelles begin to be extracted (Ramsby and Makowski, 1998). Although kinesin immunoreactivity appeared reduced with Triton X-100 treatment (Figure 1), much kinesin remained as punctate structures. Longer extractions and higher concentrations of Triton X-100 that disrupt internal membranes substantially reduced kinesin immunoreactivity ( Morfin et al., 2000; Pfister et al., 1989b). Triton X-100 extraction also eliminated essentially all cytoplasmic GFP but was no more effective than digitonin in removing nuclear GFP. Although immunofluorescence methods are semiquantitative, they demonstrated that most kinesin immunoreactivity is resistant to treatments that release cytosolic proteins.
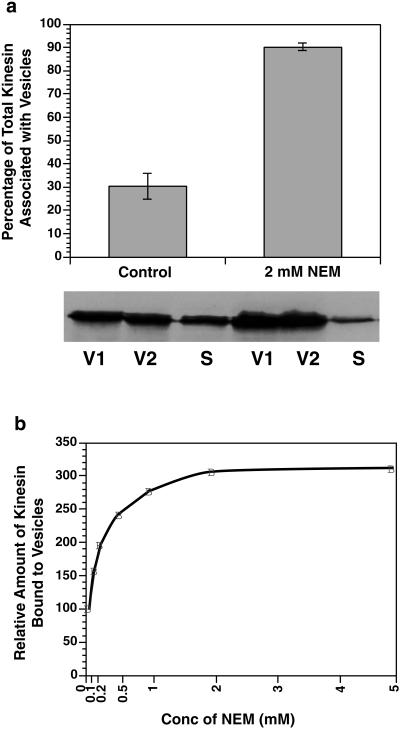
A more quantitative measure of kinesin bound to MBOs can be obtained by subcellular fractionation. Kinesin levels were evaluated by quantitative immunoblots of two purified organelle fractions (V1 and V2) and the supernatant (S) using standard cell fractionation methods. Consistent with previous reports (Hollenbeck, 1989), ∼70% of cellular kinesin was present in the supernatant with control buffers (Figure 2a). A wide range of buffer conditions and enzyme inhibitors were evaluated for an ability to affect the partitioning of kinesin between membrane and soluble fractions. Two treatments significantly increased the amount of kinesin in membrane fractions: NEM, a sulfhydryl modifying agent, and EDTA, a chelator of divalent cations. Both were effective at millimolar concentrations when added to homogenization buffers.
Figure 2.
Effects of NEM on kinesin release from vesicles during homogenization. (a) Microsomal vesicles were purified by homogenizing fresh bovine brains either with or without 2 mM NEM. Three fractions were defined: the 39,800 × g pellet (V1), the 260,000 × g pellet (V2), and the 260,000 × g supernatant (S). The supernatant and vesicle fractions were probed for the presence of kinesin using the H2 antibody on quantitative immunoblots as described previously (Pfister et al., 1989b). The presence of 2 mM NEM during homogenization minimized kinesin release from vesicles during homogenization. Error bars represent ±SEM; n = 3. (b) The concentration dependence of NEM effects on the amount of kinesin in V2 was assayed by varying the concentrations of NEM added to homogenization buffer from 0.1 to 5.0 mM before the homogenization. Kinesin was quantitated as described above. NEM at concentrations of 1–2 mM maximally inhibited kinesin release from vesicles.
Addition of NEM to homogenization buffer before extraction alkylates any free sulfhydryls that become accessible during homogenization and inhibits enzyme activities requiring free sulfhydryls. Millimolar NEM in the homogenization buffer increased the amount of total protein in both V1 and V2 fractions only ∼5%. In contrast, the amount of kinesin associated with V1 and V2 membrane fractions increased approximately threefold, going from ∼0.5% to ∼1.5% of total protein. Under these conditions, >90% of recovered kinesin partitioned with vesicle fractions after NEM treatment (Figure 2), compared with 30% of total kinesin in vesicle fractions under standard conditions. Kinesin associated with MBOs after NEM treatment remained bound through sucrose gradient fractionation, indicating that increased membrane-bound kinesin with NEM treatment was not due to aggregation of soluble kinesin. Consistent with this, in vitro studies on inhibition of kinesin ATPase, microtubule binding and gliding assays by NEM, showed that NEM-treated kinesin does not aggregate but remains soluble (Pfister et al., 1989a; Sickles et al., 1996). These experiments indicate that NEM inhibits kinesin release from MBOs during homogenization and suggested that at least one NEM-sensitive pathway in cytoplasmic extracts releases kinesin from MBOs during homogenization.
A comparable increase of kinesin in membrane fractions was produced by addition of 5 mM EDTA to homogenization buffers. In Figure 3a, the upper histogram shows that NEM, EDTA, or the combination of NEM and EDTA treatments had little effect on the total amount of protein in cytosolic and sonicated membrane fractions. Similarly, none of these treatments altered the distribution of either hsc70, a predominantly cytoplasmic protein, or synaptophysin, an integral membrane protein (Figure 3b). However, the lower histogram shows that treatment with either NEM (51%) or EDTA (54%) more than doubled the amount of kinesin in membrane fractions compared with control buffers (26%). Adding both NEM and EDTA to homogenization buffer increased the amount of kinesin in membrane fractions to nearly 80% (Figure 3). The fact that NEM and EDTA effects on kinesin binding to membranes were at least partially additive suggests that multiple pathways may exist to release kinesin from MBOs and become activated during standard homogenization protocols.
Figure 3.
NEM and EDTA increase the amount of kinesin in membrane fractions after subcellular fractionation. (a) Bar graphs showing partitioning of total protein and kinesin in cytosolic versus membrane fractions. Changes in the partitioning of kinesin in the absence (control) or presence of NEM, EDTA, or NEM and EDTA combined are shown. Average kinesin levels were calculated from results of two quantitative immunoblots. Bars indicate the range of variation. (b) Representative immunoblots showing the partitioning of kinesin, hsc70, and the integral membrane protein p38 in control and treated samples. The partitioning of total protein, hsc70, and p38 between cytosolic and membrane fractions was unaltered by the various treatments. However, all treatments dramatically shifted kinesin from cytosolic to membrane fractions.
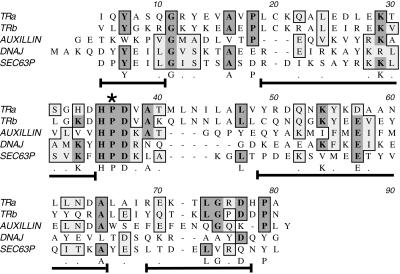
The ability of NEM and EDTA to inhibit release of kinesin from MBOs suggested an active process but did not identify the cellular components responsible. Immunochemical studies had implicated KLCs in kinesin binding to vesicles (Hirokawa et al., 1989; Stenoien and Brady, 1997), so the KLC primary sequence was examined for motifs that might be involved in regulation of membrane binding. The central domain of KLC contains five imperfect tandem repeats of 42 amino acids each (Cyr et al., 1991). These repeats have a high degree of similarity to each other and are conserved to an unusually high degree (>95% identity) across species (Brady, 1995; Stenoien and Brady, 1997). Our previous work had implicated these tandem repeat domains in kinesin binding to MBOs, because an antibody (KLC-ALL) directed against the repeat domain released kinesin from isolated vesicles in vitro and inhibited fast axonal transport. As a result, KLC tandem repeat domains were thought to mediate protein–protein or protein–lipid bilayer interactions needed for kinesin binding to MBOs (Stenoien and Brady, 1997).
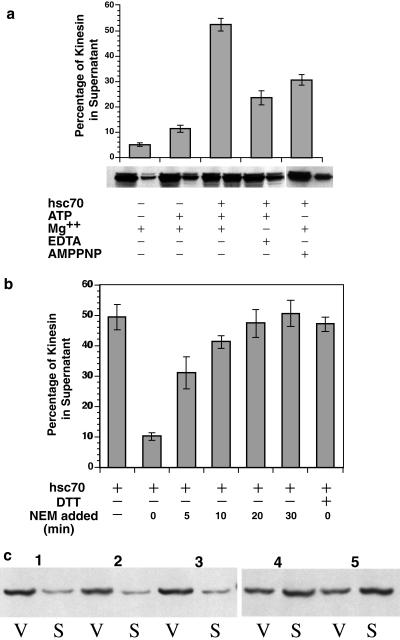
Analysis of KLC sequences (Figure 4) revealed that tandem repeat domains contain conserved sequences characteristic of the “J-domain” motif first described in DnaJ (Tsai and Douglas, 1996). The predicted structure of the tandem repeat domains (Cyr et al., 1991) was also consistent with a J-domain function (Hill et al., 1995; Kelley, 1998). The hallmarks of a J-domain are a tripeptide HPD sequence between two alpha helical-rich stretches (Figure 4), creating a finger-like structure that can interact with members of the hsp70 chaperone family. A variety of proteins have been found to contain J-domains, several of which mediate processes involving protein–membrane interactions and molecular chaperones (Cyr et al., 1994; Kelley, 1998). This suggested that an hsp70 chaperone might bind to KLC. The action of hsp70 proteins on kinesin–MBO interactions was tested by evaluating the in vitro ability of hsc70 to release kinesin from purified vesicle fractions (Figure 5). Purified V2 vesicles were incubated with or without hsc70 for 30 min at 37°C. Only 5% of vesicle-associated kinesin was released into the supernatant by incubation with buffer alone. In contrast, ∼50% of bound kinesin was released from vesicle surfaces by in vitro incubations with hsc70 and ATP (Figure 5a). This is likely to be an underestimate of kinesin releasable by hsc70, because vesicle fractions used for these assays were purified by standard cell fractionation methods. Kinesin release by hsc70 during homogenization is likely to be more extensive, because much endogenous membrane-bound kinesin was lost during purification (Figures 2 and 3). The effect of ATP alone was also assayed, because ATP is required for hsc70 function. Approximately 12% of kinesin was released into the supernatant of the sample after incubation with buffer plus ATP in the absence of added hsc70 (Figure 5). This ATP-dependent release probably resulted from hsc70 contamination of vesicle fractions, because a small amount of hsc70 was consistently detectable in immunoblots of the vesicle fractions (see Figure 3). Moreover, immunoblots with anti-hsc70 antibody showed release of endogenous hsc70 from samples only in the presence of added ATP (our unpublished data).
Figure 4.
Structure and sequence homologies between KLC tandem repeats and J-domains. Alignment of KLC tandem repeat sequences with a prokaryote J-domain sequence from Escherichia coli DnaJ and two eukaryotic J-domains from auxilin and Sec63P is shown (see DISCUSSION for more details). The invariant motifs are an essential HPD sequence (star) and the arrangement of short, flanking stretches of alpha helix (brackets under aligned sequences). The position of the alpha helical stretches is based on the nuclear magnetic resonance structure of the J-domains in DnaJ (Hill et al., 1995). Sequences in these helical stretches are more variable across family members, although some residues appear to be either conserved (letters under the sequence) or homologous (dots under sequence). Shading shows conserved residues in multiple J-domain sequences. This alignment shows that the primary sequences of tandem repeats 1–2 and 3–4 are consistent with a J-domain function.
Figure 5.
Release of endogenous kinesin from vesicles by Hsc70. V2 vesicles (purified without the use of NEM as described in the text) were resuspended and incubated at a concentration of 1 mg/ml total protein with or without hsc70 for 30 min at 37°C in release buffer (HB plus 75 mM KCl). Hsc70 was used at a concentration of 10 μg/ml for a molar ratio for hsc70:kinesin of 2:1. After incubation, V2 vesicles were recentrifuged at 260,000 × g, and then supernatants and pellets were probed for the presence of kinesin using the H2 antibody on quantitative immunoblots as described above. (a) Purified hsc70 released 50% of the endogenous kinesin remaining on V2 vesicles in a nucleotide-dependent manner. The reaction required Mg-ATP and was blocked by a nonhydrolyzable ATP analogue (AMP-PNP) and EDTA. The amount of kinesin released by hsc70 in this assay was comparable with that released by an antibody against the KLC tandem repeat domain (Stenoien and Brady, 1997). (b) When 2 mM NEM is added to vesicles in vitro at the same time as the hsc70 (time 0), hsc70-mediated release is blocked. The inhibition of hsc70-mediated kinesin release by NEM was eliminated by simultaneous addition of DTT (2 mM). The ability of NEM to inhibit hsc70-mediated release permitted definition of a time course for kinesin release. This could be estimated by adding NEM (2 mM) at the indicated times after the start of incubation, where the start of incubation is the addition of hsc70. When the reaction is allowed to proceed for 5 min before addition of NEM, substantial amounts of kinesin were released. Release of kinesin by hsc70 was essentially complete between 10 and 20 min, so addition of NEM at times >10 min had no effect on the amount of kinesin released by hsc70. (c) The target for NEM modification is a vesicle component, not hsc70 itself. To determine the site of action for NEM, the amount of kinesin remaining bound to vesicles (V) was compared with the amount of kinesin found in the soluble fraction (S) for five different conditions. When vesicles were pretreated with 5 mM NEM, the amount of kinesin released from vesicles by untreated hsc70 (VS pair 2) was similar to that seen without hsc70 (VS pair 1) or with NEM present during the hsc70 incubation (VS pair 3). In contrast, hsc70 pretreated with 5 mM NEM (VS pair 4) exhibited an ability to release kinesin comparable with that of untreated hsc70 (VS pair 5). For pretreatments with NEM, unreacted NEM was neutralized by addition of excess DTT before assay. Error bars represent ±SEM; n = 3 experiments unless otherwise specified.
The roles of divalent cations and ATP hydrolysis in release of vesicle-bound kinesin by hsc70 were assessed because hsc70 function requires Mg++-dependent hydrolysis of ATP (Wilbanks et al., 1994). Addition of millimolar EDTA in the absence of added Mg++ significantly reduced the amount of kinesin released by hsc70/ATP to ∼20% (Figure 5a). This was consistent with observations that EDTA increased the amount of kinesin retained by MBOs during homogenization (Figure 3). Under the conditions of the assay, EDTA did not completely inhibit, probably as a result of residual divalent cations present in the vesicle fraction and added ATP. Efficiency of release was also reduced by incubation with either AMP-PNP or adenosine 5′-[β,γ-methylene]triphosphate, two nonhydrolyzable analogues of ATP. Both ATP analogues reduced hsc70-mediated release of kinesin from vesicles to ∼30% (Figure 5a). Neither of these analogues bind tightly to hsc70 but act as weak competitive inhibitors in the presence of residual ATP from vesicle fractions. Together, these results indicate that binding and hydrolysis of MgATP is required for hsc70-mediated release of kinesin from MBOs.
Release of membrane-bound kinesin by hsc70 was also sensitive to NEM in vitro (Figure 5b). When 2 mM NEM and hsc70 were added at the same time (time 0), kinesin release by hsc70 was effectively blocked. This block required alkylation of a sulfhydryl by NEM because addition of comparable amounts of NEM inactivated by DTT at time 0 had no effect on release of kinesin by hsc70. However, addition of NEM to samples at various times after addition of hsc70 generated a time course for kinesin release (Figure 5b). Adding NEM >20 min after hsc70 did not affect the amount of kinesin released into supernatant, but clear decrements in kinesin release resulted if NEM was added ≤10 min after starting hsc70 incubation. If vesicle fractions were pretreated with 5 mM NEM and excess NEM was neutralized by addition of excess DTT before addition of hsc70, hsc70 was no longer able to release kinesin from vesicle fractions (Figure 5b). In contrast, a similar pretreatment of hsc70 with NEM had no effect on its ability to release kinesin from vesicles. Thus, the NEM-sensitive component required for hsc70 release of kinesin must be an MBO-associated component.
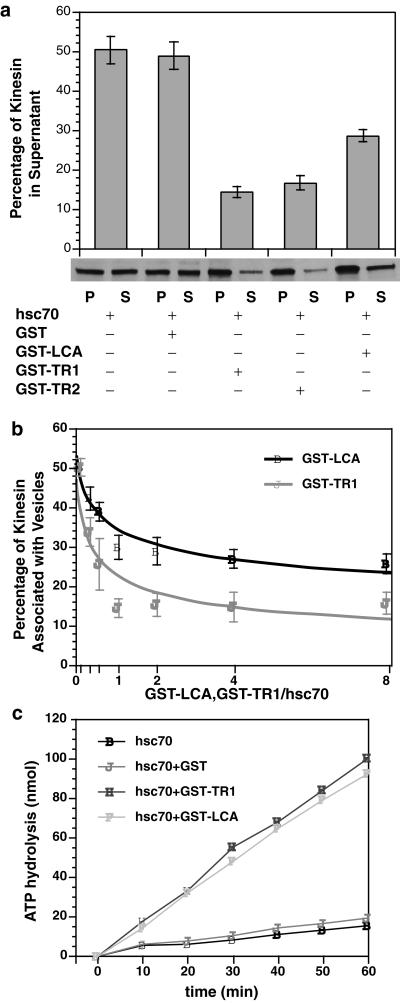
To characterize interactions between KLC and hsc70 further, fusion proteins containing different KLC constructs were added to the kinesin release assay. Four different bacterially expressed GST fusion proteins were used: 1) GST-LCA contains full-length KLC A fused to GST; 2) GST-TR1 contains KLC tandem repeat amino acids 238–321 fused to GST; 3) GST-TR2 contains KLC tandem repeat amino acids 238–488 fused to GST; and 4) GST alone was used as a control in these assays. GST-LCA, GST-TR1, and GST-TR2 all inhibited release of kinesin by hsc70, but GST alone was ineffective (Figure 6a). GST-TR1 and GST-TR2 were more effective than GST-LCA in inhibiting kinesin release by hsc70 (Figure 6a). Differences in degree of inhibition between GST-LCA and GST-TR1/TR2 raised the possibility of negative cooperativity between tandem repeats and other KLC domains. When the abilities of GST-LCA and GST-TR1 to inhibit kinesin release was tested at various molar ratios (Figure 6b), both GST-LCA and GST-TR1 approached saturation at a 1:1 molecular ratio to hsc70. However, GST-TR1 was more effective at inhibiting kinesin release than GST-LCA at all molar ratios. GST-LCA never reduced kinesin release by hsc70 to less than half-maximal, whereas GST-TR1 almost completely inhibited hsc70-mediated kinesin release.
Figure 6.
Fusion proteins containing KLC or KLC tandem repeats inhibited the ability of hsc70 to release kinesin from vesicles. (a) Hsc70-mediated release of kinesin from V2 vesicle fractions was assayed as described above. Four different constructs were generated: GST, GST-LCA, GST-TR1, and GST-TR2. At a molar ratio of 1:1 for fusion protein:hsc70, GST-LCA, GST-TR1, and GST-TR2 constructs inhibited release of kinesin by hsc70. In contrast, GST alone was inactive in these assays. Constructs containing only KLC tandem repeat domains (TR1 and TR2) were more effective than ones also containing other KLC domains (GST-LCA). Recombinant KLC and tandem repeat fusion proteins differ in their relative efficacy to inhibit hsc70-mediated release of kinesin. (b) The lesser ability of GST-LCA to inhibit hsc70-mediated release relative to GST-TR1/TR2 might reflect differences in the amount of active protein or an effect of domains in KLC other than the tandem repeats. If the amount of active protein differs, increased molar ratios of KLC to hsc70 should reduce observed differences. However, at all molar ratios tested, GST-TR1 was more effective at inhibiting kinesin release by hsc70 than GST-LCA. The effects of GST-LCA appeared to plateau at ∼30%. (c) To determine whether GST-LCA and GST-TR1 differed in their interactions with hsc70, the ability of different constructs to activate hsc70 ATPase activity was evaluated. Both GST-LCA and GST-TR1 stimulated hsc70 ATPase activity to a similar extent, indicating a comparable ability to bind and activate hsc70 ATPase. GST alone was inactive. Therefore, differences in the ability of LCA and TR1 fusion proteins to inhibit hsc70-mediated release of kinesin from vesicles were not due to differences in their ability to interact with hsc70, so KLC domains outside the tandem repeats must affect the ability of hsc70 to release kinesin from MBOs. Error bars represent ±SEM; n = 3 experiments unless otherwise specified.
Conformational differences between GST-LCA and GST-TR1/TR2 that affected interactions with hsc70 could also explain this difference. The ability of a J-domain to bind hsc70 can be evaluated by assaying its stimulation of hsc70 ATPase activity (Cyr et al., 1994; Tsai and Douglas, 1996; Greene et al., 1998). GST alone was inactive in hsc70 ATPase assays, but both GST-LCA and GST-TR1 stimulated hsc70 ATPase activity to a similar extent (Figure 6c). This indicates that tandem repeats and full-length KLC fusion proteins were comparable in their ability to bind and activate hsc70 ATPase. Taken together, these results suggest that KLC J-domain motifs interact directly with hsc70. Differences between KLC and tandem repeat domain inhibition of hsc70-mediated kinesin release from vesicles may reflect interaction of other domains in KLC with membrane components.
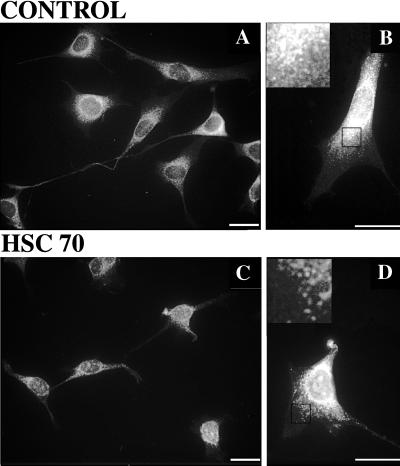
Finally, the ability of exogenous hsc70 to release kinesin from membranes in situ was examined (Figure 7). Cells were permeabilized by incubation with 0.01% Triton X-100 for 4 min in the presence or absence of exogenous hsc70 before fixation. Brief incubation with hsc70 produced a consistent reduction in kinesin immunoreactivity (Figure 7, a and b). Kinesin immunoreactivity in processes was most severely affected, but there was also depletion of kinesin in cell bodies relative to control cells. The fact that not all kinesin was extracted by a short hsc70 incubation is consistent with the biochemical studies (see Figure 5) but may also represent either a fraction of kinesin that is resistant to hsc70 release or one that requires an additional cofactor not preserved under these conditions.
Figure 7.
Exogenous hsc70 releases kinesin from gently permeabilized cells. Kinesin immunofluorescence in untreated (A and B) and hsc70-treated (C and D) cells is shown. Images A and C were acquired with a 40×, objective and B and D were acquired with a 63× objective; calibration bars are 20 μm in each case. Insets in B and D were digitally enlarged (4×) from the areas demarcated by black boxes. Kinesin inmunoreactivity declined after hsc70 treatment. Comparing A and C, the amount of kinesin in processes and in the perinuclear halo is reduced. Comparing B and D, this erosion of kinesin immunoreactivity in the perinuclear halo is more readily visulalized. The effect is most obvious near the cell center (B and D, insets), where the density of punctuate structures is reduced, resulting in a thinner perinuclear stain. Longer treatments will remove more kinesin immunoreactivity.
DISCUSSION
The large amount of kinesin present in soluble fractions of tissue extracts had always been at odds with its putative role as a motor for MBOs. Moreover, homogenization data were not consistent with evidence that kinesin was bound very tightly to vesicles (Leopold et al., 1992; Schnapp et al., 1992) or with in vivo studies that detected only vesicle-associated kinesin (Pfister et al., 1989b; Elluru et al., 1995). Similarly, gentle permeabilization of cells releases a smaller fraction of cellular kinesin than homogenization. These observations suggested that some membrane-associated kinesin might be actively released during subcellular fractionations. When a variety of enzyme inhibitors were evaluated, two chemically distinct treatments substantially increased the amount of kinesin associated with MBOs in homogenates: NEM and EDTA. Subcellular fractionation with these two agents suggest that virtually all cellular kinesin is associated with MBOs, so that the removal, and possibly the addition, of kinesin to vesicles are likely to be active, highly regulated processes.
The action of these agents is consistent with other elements of membrane trafficking in cells. Previous studies showed that alkylation of free sulfhydryls by NEM inhibits a number of cellular processes associated with membranes, including vesicle fusion (Rothman, 1994; Hay and Scheller, 1997) and posttranslational translocation of precursor proteins into the endoplasmic reticulum (Chirico et al., 1988), mitochondria (Murakami et al., 1988), or nucleus (Shi and Thomas, 1992). Several distinct NEM-sensitive factors appear to act at different stages of intracellular transport pathways. At least two NEM-sensitive components are involved in endosomal fusion (Rodriguez et al., 1994). A membrane-associated component is sensitive to low levels of NEM (1 mM), whereas a second component is cytosolic and requires higher NEM concentrations for inhibition (≥3 mM). NEM concentrations in the range of 1–2 mM had maximal effects on the amount of membrane-associated kinesin, a concentration comparable with that reported with the membrane-associated NEM-sensitive component in endosomal fusion (Rodriguez et al., 1994). Consistent with this, pretreatment of vesicles with NEM but not pretreatment of hsc70 blocked the ability of hsc70 to release kinesin. These results suggest that at least one membrane-associated component sensitive to low concentrations of NEM is required for release of kinesin from MBOs during homogenization. The identity of this membrane-associated component is not yet known. Both kinesin heavy chain (KHC) and KLC can be modified by NEM (Pfister et al., 1989a) and are potential candidates. However, washing MBOs with 1 M NaCl blocks hsc70 release of kinesin but has little effect on kinesin binding to MBOs. This suggests that a vesicle component other than kinesin is required for hsc70-mediated release, and this would also be a potential NEM target.
KLCs have long been thought to participate in binding of kinesin motors to MBOs (Hirokawa et al., 1989; Cyr et al., 1991). This interpretation is reinforced by changes in KHC distribution associated with knocking out one of the KLC genes in mouse (Rahman et al., 1999), but the molecular basis of that interaction was not well understood. Based on actions of an antibody against KLC, the KLC tandem repeat domain was proposed to interact with receptor molecules on MBOs (Stenoien and Brady, 1997). The presence of two conserved HPD sequences characteristic of the J-domain in DnaJ-like proteins (Tsai and Douglas, 1996) and similarities between the structure predicted for KLC tandem repeats and known J-domain structures (Hill et al., 1995) suggested that KLC might contain functional J-domains. J-domain motifs are found in a variety of polypeptides that mediate cellular processes in cooperation with hsp70 chaperone family members (Cyr et al., 1994). Hsp70-like proteins function as molecular chaperones to mediate or facilitate a variety of biological processes that include folding and unfolding of proteins, formation or dissociation of protein complexes, and translocation of proteins across membranes (Hartl, 1996; Schatz and Dobberstein, 1996). Significantly, many hsp70-mediated processes are also NEM sensitive (Chirico et al., 1988; Murakami et al., 1988; Shi and Thomas, 1992), although hsp70 itself is not sensitive to NEM. The homologies between KLC tandem repeats and J-domain sequences suggested that a member of the Hsp70 chaperone protein family might catalyze removal of kinesin from vesicles. Incubating vesicles with purified hsc70 and MgATP selectively removed tightly bound kinesin from membrane surfaces. A requirement for MgATP would explain the effects of EDTA in stabilizing kinesin association with membranes during fractionations, whereas a requirement for NEM-sensitive cofactors is consistent with NEM stabilization of kinesin binding.
The tight binding of kinesin to MBOs probably involves multiple organelle binding domains involving both heavy and light chains of kinesin. KLC domains such as the heterogeneous carboxyl and conserved amino termini may play other roles, such as targeting of kinesin to a specific type of organelle or mediating interactions between KLC and KHC (Cyr et al., 1991; Stenoien and Brady, 1997). The presence of other domains with different functions would explain why recombinant KLC and tandem repeat fusion proteins differ in their ability to inhibit hsc70-mediated release of kinesin from MBOs.
The KLC tandem repeat domain itself may have two functions: as a binding domain for membrane components and as a DnaJ-like cochaperone that recruits hsp70 to MBO surfaces for selective removal of kinesin. Consistent with this idea, KLC tandem repeats exhibit homology not only with J-domains but also with a tetratricopeptide repeat motif (Gindhart et al., 1998), a motif thought to be involved in protein–protein interactions. The two functions for KLC may be analogous to dual roles played by other DnaJ-like proteins.
For example, both auxilin and Sec63 regulate binding of peptide substrates to hsp70 and stimulate hsp70 ATPase activity. Auxilin was recently identified as a cofactor that binds hsc70 via a J-domain and recruits hsc70 to the clathrin-coated vesicle via its clathrin binding domain (Ungewickell et al., 1995). When the two domains of auxilin are expressed separately, the ability to recruit hsc70 to coated vesicles is lost, even though each domain retains its specific binding activity (Holstein et al., 1996; Ungewickell et al., 1997). Similarly, Sec63 is an integral membrane protein in Saccharomyces cerevisiae with a lumenal DnaJ-like region that mediates interaction with Bip, an hsp70 family member, and promotes protein translocation into the endoplasmic reticulum. A soluble Sec63p lumenal domain inhibited efficient precursor import into proteoliposomes (Ann and Randy, 1997) but retained its ability to activate hsc70.
The activities of KLC, auxilin, and Sec63P suggest that the J-domain of DnaJ-like proteins serves to regulate kinetics of peptide substrate binding and hsp70 ATPase activity, but associated domains may be required for recruiting hsp70s to specific locations. As a result, both KLC and tandem repeat fusion proteins exhibit comparable abilities to interact with hsc70, but tandem repeats alone are more efficient at inhibiting hsc70-mediated release of kinesin. Additional KLC domains present in the full-length GST-LCA may retain the ability to bind membranes containing endogenous kinesin. This effectively increases the local concentration of hsc70 near bound kinesin and facilitates release of kinesin, albeit less efficiently than if LCA were associated with a KHC. In contrast, GST-TR1/TR2 act as soluble dominant negative factors that bind hsc70 but have lost the ability to recruit hsc70 to kinesin on a vesicle surface.
The complex organization of neurons and many other eukaryotic cells requires specialized mechanisms to ensure that proteins are efficiently transported to appropriate destinations. This implies the existence of specific targeting mechanisms, but once an organelle has reached the correct destination, mechanisms must exist to prevent continued translocation. Previous investigations on regulation of kinesin motors assumed that posttranslational modifications would be involved (Bloom et al., 1993; Lee and Hollenbeck, 1995; Okada et al., 1995). Unfortunately, despite extensive study, no biochemical switch has been found to turn off kinesin motor activity. Although such pathways may exist, an alternative mechanism to prevent an MBO from translocating farther is removal of kinesin from MBOs. Cytoplasmic hsc70 was found to release kinesin from vesicles in a nucleotide-dependent and NEM-sensitive manner in vitro. These results suggest that members of the hsp70 chaperone family might have a role in regulating delivery of axonally transported materials in vivo to the proper sites by releasing motor proteins from their cargoes in specific subcellular regions.
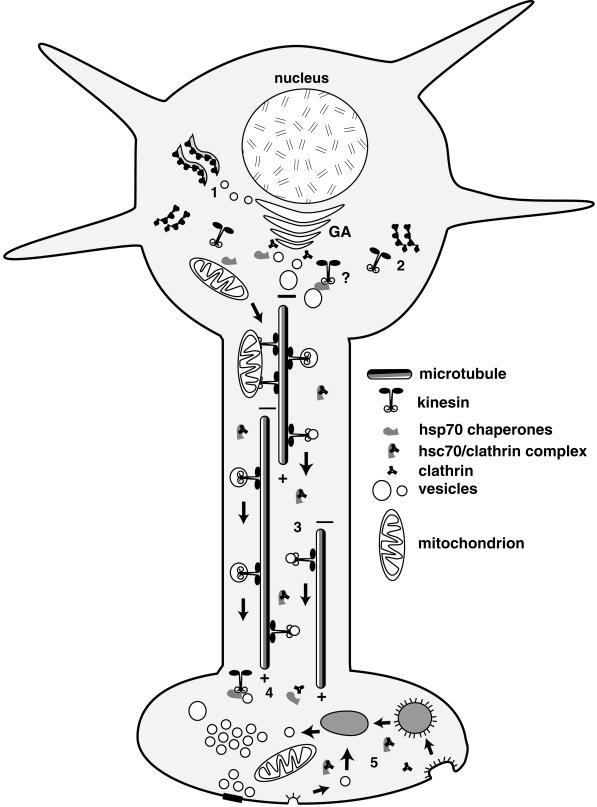
An attractive corollary to this hypothesis is that a reciprocal relationship may exist between clathrin-coated vesicles and kinesin transport vesicles (Figure 8). For example, cellular locations in neurons with high levels of coated vesicle assembly and disassembly are places either where kinesin is thought to be added to an MBO (i.e., post-Golgi formation of transport vesicles) or where kinesin is removed from an MBO (i.e., presynaptic terminals) (Maycox et al., 1992). Studies on axonal transport of clathrin and hsc70 are consistent with this idea (Garner and Lasek, 1981; Gower and Tytell, 1987; de Waegh and Brady, 1989; Black et al., 1991). Very little clathrin is detectable moving with MBOs in fast axonal transport (Black et al., 1991). Instead, the bulk of clathrin and associated coated vesicle proteins move as part of slow axonal transport (Garner and Lasek, 1981; Gower and Tytell, 1987; Black et al., 1991). Similarly, hsc70 moves as part of slow axonal transport (de Waegh and Brady, 1989), and coated vesicle proteins coimmunoprecipitate with hsc70 (Black et al., 1991). Coated vesicle proteins and hsc70 must form a stable complex in axons that is dissociated in presynaptic terminals or other domains with active recycling of membrane proteins (see Figure 8). Axonal hsc70 bound to clathrin coat proteins would not be available to act on kinesin, but hsc70 in presynaptic terminals with active membrane recycling would be able to act on kinesin. Such a model for hsc70 action provides a mechanistic link between kinesin binding to MBOs and other stages of vesicle trafficking. Regardless, the highly conserved amino acid sequence of KLC tandem repeat domains establishes that this motif has an essential function. The ability of hsc70 to bind tandem repeats and release kinesin from vesicles suggests that control of kinesin binding to membranes represents such an essential function.
Figure 8.
Model for hsc70 regulation of kinesin-mediated axonal transport. The molecular chaperone hsc70 may regulate kinesin binding in both the cell body and in subcellular domains targeted for delivery of material in fast axonal transport. To package materials for movement in fast axonal transport, membrane proteins must be synthesized on rough endoplasmic reticulum (1) and packaged in the Golgi apparatus (GA). Kinesin is not associated with Golgi membranes (Pfister et al., 1989b; Leopold et al., 1992) but is enriched on transport vesicles near the trans-Golgi face (see, for example, Figures 1 and 7). Kinesin motors are synthesized on free polysomes (2) and must be added to transport vesicles post-Golgi. This pathway has not been characterized, but the role of chaperones in delivering proteins synthesized on free polysomes to organelles such as the mitochondrion and the enrichment of hsc70 near the trans-Golgi face suggest a role for hsc70 adding kinesin to MBOs in the cell body. Vesicles with kinesin motors are moved in fast axonal transport toward the plus ends of microtubules (3) away from the cell body. In the axon, hsc70 is complexed with cytoplasmic proteins such as clathrin (Black et al., 1991). Upon reaching a presynaptic terminal, kinesin is removed from MBO surfaces (4) by available hsc70 that is released from cytoplasmic complexes with clathrin or other proteins. This frees clathrin to play a role in vesicle recycling (5), in which hsc70 may also be important for removal of clathrin coats from coated vesicles that recycle synaptic vesicle components or repackage membrane proteins for return to the cell body by retrograde transport mediated by dynein. Cytoplasmic kinesin appears to be rapidly degraded in terminal regions after release from membranes, because it is not enriched in terminal regions or returned by retrograde transport (Li et al., 1999).
ACKNOWLEDGMENTS
We thank H. Ross Payne, Robin Wray, Zhao Min, and Pilar Coffee for technical assistance during these studies. Preparation of this manuscript was supported in part by grants from the National Institute of Neurological Disease and Stroke (NS-23868 and NS-23320), the National Institute of Aging (AG-12646), the National Aeronautics and Space Administration (NAG2-962), and the Welch Foundation (1237).
REFERENCES
- Ann KC, Randy S. The lumenal domain of sec63p stimulates the ATPase activity of Bip and mediates Bip recruitment to the translocon in Saccharomyces cerevisiae. J Cell Biol. 1997;137:1483–1493. doi: 10.1083/jcb.137.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Chestnut MH, Pleasure IT, Keen JH. Stable clathrin: uncoating protein (HSC70) complexes in intact neurons and their axonal transport. J Neurosci. 1991;11:1163–1172. doi: 10.1523/JNEUROSCI.11-05-01163.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom GS, Richards BW, Leopold PL, Ritchey DM, Brady ST. GTPγS inhibits organelle transport along axonal microtubules. J Cell Biol. 1993;120:467–476. doi: 10.1083/jcb.120.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ST. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Brady ST. A kinesin medley: biochemical and functional heterogeneity. Trends Cell Biol. 1995;5:159–164. doi: 10.1016/s0962-8924(00)88980-1. [DOI] [PubMed] [Google Scholar]
- Brady ST, Sperry AO. Biochemical and functional diversity of microtubule motors in the nervous system. Curr Opin Neurobiol. 1995;5:551–558. doi: 10.1016/0959-4388(95)80058-1. [DOI] [PubMed] [Google Scholar]
- Chirico WJ, Waters MG, Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988;332:805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Coy DL, Hancock WO, Wagenbach M, Howard J. Kinesin's tail domain is an inhibitory regulator of the motor domain. Nat Cell Biol. 1999;1:288–292. doi: 10.1038/13001. [DOI] [PubMed] [Google Scholar]
- Cyr DM, Langer T, Douglas MG. DNAJ-like proteins: molecular chaperones and specific regulators of HSP70. Trends Biochem Sci. 1994;19:176–181. doi: 10.1016/0968-0004(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Cyr JL, Pfister KK, Bloom GS, Slaughter CA, Brady ST. Molecular genetics of kinesin light chains: generation of isoforms by alternative splicing. Proc Natl Acad Sci USA. 1991;88:10114–10118. doi: 10.1073/pnas.88.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waegh SM, Brady ST. Axonal transport of the clathrin uncoating ATPase (HSC70): a role for HSC70 in modulation of coated vesicle assembly in vivo. J Neurosci Res. 1989;23:433–440. doi: 10.1002/jnr.490230409. [DOI] [PubMed] [Google Scholar]
- Elluru R, Bloom GS, Brady ST. Fast axonal transport of kinesin in the rat visual system: functionality of the kinesin heavy chain isoforms. Mol Biol Cell. 1995;6:21–40. doi: 10.1091/mbc.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, Vale RD. Single-molecule analysis of kinesin motility reveals regulation by the cargo-binding tail domain. Nat Cell Biol. 1999;1:293–297. doi: 10.1038/13008. [DOI] [PubMed] [Google Scholar]
- Garner JA, Lasek RJ. Clathrin is axonally transported as part of slow component b: the microfilament complex. J Cell Biol. 1981;88:172–178. doi: 10.1083/jcb.88.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart JG, Jr, Desai CJ, Beushausen S, Zinn K, Goldstein LS. Kinesin light chains are essential for axonal transport in Drosophila. J Cell Biol. 1998;141:443–454. doi: 10.1083/jcb.141.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower D, Tytell M. Axonal transport of clathrin associated proteins. Brain Res. 1987;407:1–8. doi: 10.1016/0006-8993(87)91213-3. [DOI] [PubMed] [Google Scholar]
- Greene MK, Maskos K, Landry SJ. Role of the J-domain in the cooperation of Hsp40 with Hsp70. Proc Natl Acad Sci USA. 1998;95:6108–6113. doi: 10.1073/pnas.95.11.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney DD, Levitt JD, Suhan J. Kinesin undergoes a 9S to 6S conformational transition. J Biol Chem. 1992;267:8696–8701. [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hay JC, Scheller RH. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- Hill RB, Flanagan JM, Prestegard JH. 1H and 15N magnetic resonance assignments, secondary structure, and tertiary fold of Escherichia coli DnaJ(1–78) Biochemistry. 1995;34:5587–5596. doi: 10.1021/bi00016a033. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Pfister KK, Yorifuji H, Wagner MC, Brady ST, Bloom GS. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989;56:867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ. The distribution, abundance, and subcellular localization of kinesin. J Cell Biol. 1989;108:2335–2342. doi: 10.1083/jcb.108.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein SE, Ungewickell H, Ungewickell E. Mechanism of clathrin basket dissociation: separate functions of protein domains of the DnaJ homologue auxilin. J Cell Biol. 1996;135:925–937. doi: 10.1083/jcb.135.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst M, Oppliger W, Rospert S, Schonfeld HJ, Schatz G, Azem A. Sequential action of two hsp70 complexes during protein import into mitochondria. EMBO J. 1997;16:1842–1849. doi: 10.1093/emboj/16.8.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WL. The J-domain family and the recruitment of chaperone power. Trends Biochem Sci. 1998;23:222–227. doi: 10.1016/s0968-0004(98)01215-8. [DOI] [PubMed] [Google Scholar]
- Lasek RJ, Brady ST. Attachment of transported vesicles to microtubules in axoplasm is facilitated by AMP-PNP. Nature. 1985;316:645–647. doi: 10.1038/316645a0. [DOI] [PubMed] [Google Scholar]
- Lee K-D, Hollenbeck PJ. Phosphorylation of kinesin in vivo correlates with organelle association and neurite outgrowth. J Biol Chem. 1995;270:5600–5605. doi: 10.1074/jbc.270.10.5600. [DOI] [PubMed] [Google Scholar]
- Leopold PL, McDowall AW, Pfister KK, Bloom GS, Brady ST. Association of kinesin with characterized membrane-bounded organelles. Cell Motil Cytoskeleton. 1992;23:19–33. doi: 10.1002/cm.970230104. [DOI] [PubMed] [Google Scholar]
- Li J-Y, Pfister KK, Brady ST, Dahlström A. Axonal transport and distribution of immunologically distinct kinesin heavy chains in rat neurons. J Neurosci Res. 1999;58:226–241. [PubMed] [Google Scholar]
- Mackall J, Meredith M, Lane MD. A mild procedure for the rapid release of cytoplasmic enzymes from cultured animal cells. Anal Biochem. 1979;95:270–274. doi: 10.1016/0003-2697(79)90216-1. [DOI] [PubMed] [Google Scholar]
- Maycox PR, Link E, Reetz A, Morris SA, Jahn R. Clathrin-coated vesicles in nervous tissue are involved primarily in synaptic vesicle recycling. J Cell Biol. 1992;118:1379–1388. doi: 10.1083/jcb.118.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Tsai G, Brady S T. Assay of membrane-associated kinesin. In: Vernos I, editor. Kinesin Protocols. Clifton, NJ: Humana Press; 2000. (in press). [Google Scholar]
- Murakami H, Pain D, Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988;107:2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Sato-Yoshitake R, Hirokawa N. The activation of protein kinase A pathway selectively inhibits anterograde axonal transport of vesicles but not mitochondria transport or retrograde transport in vivo. J Neurosci. 1995;15:3053–3064. doi: 10.1523/JNEUROSCI.15-04-03053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister KK, Wagner MC, Bloom GS, Brady ST. Modification of the microtubule-binding and ATPase activities of kinesin by N-ethylmaleimide (NEM) suggests a role for sulfhydryls in fast axonal transport. Biochemistry. 1989a;28:9006–9012. doi: 10.1021/bi00449a008. [DOI] [PubMed] [Google Scholar]
- Pfister KK, Wagner MC, Stenoien D, Bloom GS, Brady ST. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989b;108:1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Kamal A, Roberts EA, Goldstein LS. Defective kinesin heavy chain behavior in mouse kinesin light chain mutants. J Cell Biol. 1999;146:1277–1288. doi: 10.1083/jcb.146.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsby ML, Makowski GS. Differential detergent fractionation of eukaryotic cells. Analysis by two dimensional gel electrophoresis. In: Link AJ, editor. 2-D Proteome Analysis Protocols. Totowa, NJ: Humana Press; 1998. pp. 53–65. [DOI] [PubMed] [Google Scholar]
- Ramsby ML, Makowski GS, Khairallah EA. Differential detergent fractionation of isolated hepatocytes: biochemical, immunochemical, and two dimensional gel electrophoresis characterization of cytoskeletal and noncytoskeletal compartments. Electrophoresis. 1994;15:265–277. doi: 10.1002/elps.1150150146. [DOI] [PubMed] [Google Scholar]
- Rodriguez L, Stirling CJ, Woodman PG. Multiple N-ethylmaleimide-sensitive components are required for endosomal vesicle fusion. Mol Biol Cell. 1994;5:773–783. doi: 10.1091/mbc.5.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- Schnapp BJ, Reese TS, Bechtold R. Kinesin is bound with high affinity to squid axon organelles that move to the plus-end of microtubules. J Cell Biol. 1992;119:389–399. doi: 10.1083/jcb.119.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Thomas JO. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Biol Cell. 1992;12:2186–2192. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickles DW, Brady ST, Testino A, Wrenn RW. Direct effect of the neurotoxicant acrylamide on kinesin-based microtubule motility. J Neurosci Res. 1996;46:7–17. doi: 10.1002/(SICI)1097-4547(19961001)46:1<7::AID-JNR2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Stenoien DS, Brady ST. Immunochemical analysis of kinesin light chain function. Mol Biol Cell. 1997;8:675–689. doi: 10.1091/mbc.8.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada K, Kanazawa M, Bukau B, Mori M. The human DnaJ homologue dj2 facilitates mitochondrial protein import and luciferase refolding. J Cell Biol. 1997;139:1089–1095. doi: 10.1083/jcb.139.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Douglas MG. A conserved HPD sequence of the J-domain is necessary for YDJ1 stimulation of Hsp70 ATPase activity at a site distinct from substrate binding. J Biol Chem. 1996;271:9347–9354. doi: 10.1074/jbc.271.16.9347. [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SE. Functional interaction of the auxilin J domain with the nucleotide- and substrate-binding modules of Hsc70. J Biol Chem. 1997;272:19594–19600. doi: 10.1074/jbc.272.31.19594. [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SEH, Lindner R, Prasad K, Barouch W, Martin B, Green LE, Eisenberg E. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Lizotte DL, Abramson T, Barenboim L, Schnapp BJ, Rapoport TA. Light chain-dependent regulation of Kinesin's interaction with microtubules. J Cell Biol. 1998;143:1053–1066. doi: 10.1083/jcb.143.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel PH, Ray DA, Oka JA. Quantitation of intracellular membrane-bound enzymes and receptors in digitonin-permeabilized cells. Anal Biochem. 1983;133:437–449. doi: 10.1016/0003-2697(83)90106-9. [DOI] [PubMed] [Google Scholar]
- Wilbanks SM, DeLuca-Flaherty C, McKay DB. Structural basis of the 70-kilodalton heat shock cognate protein ATP hydrolytic activity. I. Kinetic analyses of active site mutants. J Biol Chem. 1994;269:12893–12898. [PubMed] [Google Scholar]
- Womack MD, Kendall DA, MacDonald RC. Detergent effects on enzyme activity and solubilization of lipid bilayer membranes. Biochim Biophys Acta. 1983;733:210–215. doi: 10.1016/0005-2736(83)90524-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. The role of molecular chaperones in protein transport into the mammalian endoplasmic reticulum. Biol Chem. 1998;379:275–282. [PubMed] [Google Scholar]