Abstract
The compound leaf primordium of pea represents a marginal blastozone that initiates organ primordia, in an acropetal manner, from its growing distal region. The UNIFOLIATA (UNI) gene is important in marginal blastozone maintenance because loss or reduction of its function results in uni mutant leaves of reduced complexity. In this study, we show that UNI is expressed in the leaf blastozone over the period in which organ primordia are initiated and is downregulated at the time of leaf primordium determination. Prolonged UNI expression was associated with increased blastozone activity in the complex leaves of afila (af), cochleata (coch), and afila tendril-less (af tl) mutant plants. Our analysis suggests that UNI expression is negatively regulated by COCH in stipule primordia, by AF in proximal leaflet primordia, and by AF and TL in distal and terminal tendril primordia. We propose that the control of UNI expression by AF, TL, and COCH is important in the regulation of blastozone activity and pattern formation in the compound leaf primordium of the pea.
INTRODUCTION
The genetic analysis of compound leaf development has concentrated on the model organisms pea and tomato. These two species are distantly related, pea (Fabales) being in the eurosid I group of eudicots and tomato (Solanales) in the euasterid I group (Angiosperm Phylogeny Group, 1998). The leaves of these two species, however, undergo different morphogeneses: pea leaves initiate organs acropetally (Meicenheimer et al., 1983), whereas tomato leaves do so in a basipetal fashion (Dengler, 1984). Recently, genes have been identified that influence indeterminacy in tomato and pea leaf primordia and thus control aspects of their leaf architecture. In tomato, two class 1 KNOTTED1-like homeobox (KNOX) genes, TKN1 and TKN2, members of a gene family important in shoot apical meristem (SAM) maintenance and function, promote more ramified leaf forms when overexpressed in transgenic plants (Hareven et al., 1996; Parnis et al., 1997; Janssen et al., 1998). In pea, the gene UNIFOLIATA (UNI; Eriksson, 1929) is important in regulating compound leaf architecture such that the leaves of uni plants are reduced to a more simplified form. UNI is thought to promote compound architectures by maintaining a period of indeterminacy in a developing leaf primordium (Hofer et al., 1997). In addition to its effect on leaves, the uni mutation also perturbs floral development, transforming wild-type flowers into proliferating floral structures of mainly sepalloid and carpelloid organs (Hofer et al., 1997). The pleiotropic effects of the uni mutation suggest that UNI plays an important role in patterning both leaves and flowers in pea. In tomato, the mutation that probably corresponds to uni has recently been identified as falsiflora (fa), which is also pleiotropic in effect. The inflorescences of fa plants are converted into ramified, leafy structures without flowers, and the mutant leaves have fewer small, lateral leaflets than do wild-type leaves (Molinero-Rosales et al., 1999).
The UNI and FA genes are homologs of the floral meristem identity genes FLORICAULA (FLO; Coen et al., 1990) and LEAFY (LFY; Weigel et al., 1992) from Antirrhinum and Arabidopsis, respectively (Hofer et al., 1997; Molinero-Rosales et al., 1999). Other potential homologs of FLO/LFY have been identified in monocotyledonous (Colombo et al., 1998; Kyozuka et al., 1998) and dicotyledonous (Anthony et al., 1993; Rottman et al., 1993; Kelly et al., 1995; Pouteau et al., 1997; Souer et al., 1998; Molinero-Rosales et al., 1999) angiosperm species, in basal angiosperms and gnetales (Frohlich and Meyerowitz, 1997), and in the gymnosperm pine (Mouradov et al., 1998). Of the dicots studied to date, FLO/LFY transcripts were detected in the leaf primordia of tobacco, Arabidopsis, pea, Impatiens, tomato, and petunia (Kelly et al., 1995; Blázquez et al., 1997; Hofer et al., 1997; Pouteau et al., 1997; Pnueli et al., 1998; Souer et al., 1998; Molinero-Rosales et al., 1999), but mutant leaf phenotypes have been described only for those species with compound leaves: pea and tomato. Hofer et al. (1997) proposed a common function for UNI in regulating indeterminacy during both leaf and flower development. An opposite role in specifying lateral organ determinacy was suggested for the tobacco homolog NICOTIANA FLO/LFY (NFL; Kelly et al., 1995), although no loss-of-function tobacco mutant has been identified to support this role in leaf development. There are no reported morphological variations in the leaves of lfy or the corresponding petunia mutant, aberrant leaf and flower (alf), and neither LFY nor ALF is thought to play a positive role in leaf development (Weigel et al., 1992; Souer et al., 1998). Vegetative tissues that accumulated LFY in Arabidopsis were viewed as primordia with the potential to adopt an alternative floral fate. It was suggested that when LFY reached a critical value, these lateral primordia would become competent to respond to floral initiation signals (Blázquez et al., 1997).
Here, we examine the role of UNI in pea leaf development by studying its expression in several mutant backgrounds. Previous genetic evidence has shown that UNI interacts with the AFILA (AF) and TENDRIL-LESS (TL) genes to control aspects of pea leaf architecture (Sharma, 1981; Marx, 1986, 1987; Hofer and Ellis, 1996, 1998). The reduction in overall leaf complexity seen in tl, af, and af tl mutant plants when combined with the uni mutation (Marx, 1987; Hofer and Ellis, 1996) suggests that interactions between UNI, AF, and TL are important in regulating the branching potential of a pea compound leaf. In this article, we demonstrate that the AF, TL, and COCHLEATA (COCH) genes negatively regulate UNI expression. We propose that these interactions influence the organogenic potential of the primordium and are fundamental in determining the compound leaf architecture of the pea.
RESULTS
Pea Leaf Development
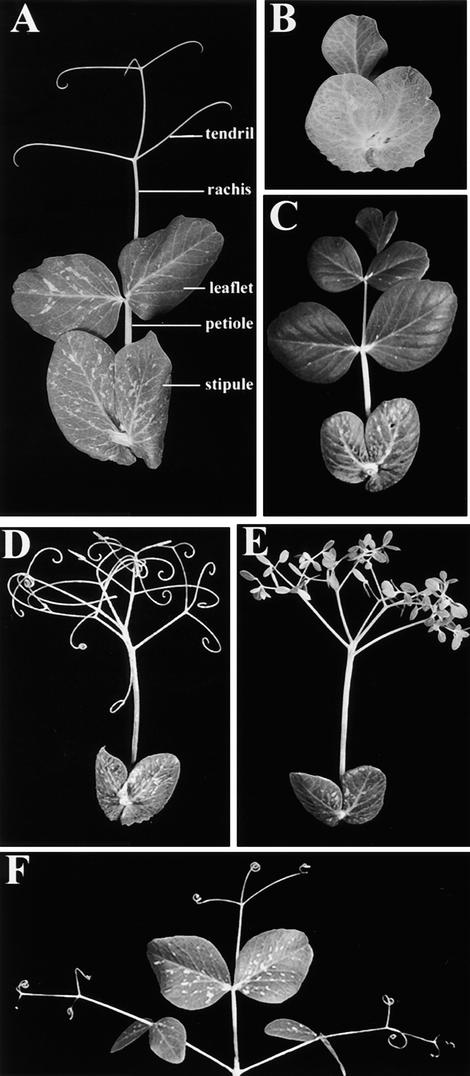
The mature wild-type pea leaf shown in Figure 1A is compound pinnate, consisting of a basal pair of foliaceous stipules, a pair of proximal leaflets, two pairs of distal tendrils, and a terminal tendril. Stipule, leaflet, and tendril primordia are initiated in an acropetal manner on the compound leaf primordium (Meicenheimer et al., 1983), which is termed a marginal blastozone (Hagemann and Gleissberg, 1996). The recessive uni mutation (Eriksson, 1929) reduces the complexity of the pea compound leaf. The single laminate form for which the mutation is named is shown in Figure 1B; however, leaves on a uni mutant plant may also be lobed, bi-, and trifoliate. The semidominant tl mutation (de Vilmorin and Bateson, 1911) replaces distal and terminal tendrils with leaflets (Figure 1C), whereas the recessive af mutation (Kujala, 1953; Goldenberg, 1965) replaces leaflets with branching rachides bearing tendrils (Figure 1D). In the af tl double mutant, branching rachides at proximal, distal, and terminal positions terminate in small leaflets (Figure 1E).
Figure 1.
Morphology of Wild-Type and Mutant Pea Leaves.
(A) A wild-type (JI 1194) compound leaf showing a basal pair of stipules, a petiole and a blade comprising a pair of proximal leaflets, two distal pairs of tendrils, and a terminal tendril, all borne on a rachis.
(B) A uni (JI 2171) mutant leaf with a basal pair of stipules and the leaf blade reduced to a unifoliate form.
(C) A tl (JI 1197) mutant leaf with a basal pair of stipules and leaflets at all positions on the leaf blade.
(D) An af (JI 1195) mutant leaf with a basal pair of stipules and all positions on the leaf blade occupied by branching rachides bearing tendrils.
(E) An af tl (JI 1199) double mutant leaf with a basal pair of stipules and all positions on the leaf blade occupied by branching rachides terminating in small leaflets.
(F) A coch (JI 2165) mutant leaf showing that each structure occupying the position of a stipule mimicks the organization of the leaf blade with a pair of leaflets, pairs of tendrils, and a terminal tendril.
The coch mutation (Wellensiek, 1959) can increase the complexity of pea leaves at the stipule position. On plants homozygous for the coch-5137 allele, stipules between nodes 8 and 15 often mimic the morphology of the blade, with fully organized leaflets and tendrils (Figure 1F). We refer to these lateral structures as compound stipules because they arise at the stipule position, but note that they lack stipules themselves. Between nodes 8 and 15, both stipules may be compound, both may be reduced to a simple petiolate form, or the pair can be a combination of one compound and one reduced stipule (Blixt, 1967; Gourlay, 1999).
Effects of the uni Mutation on Leaf Development
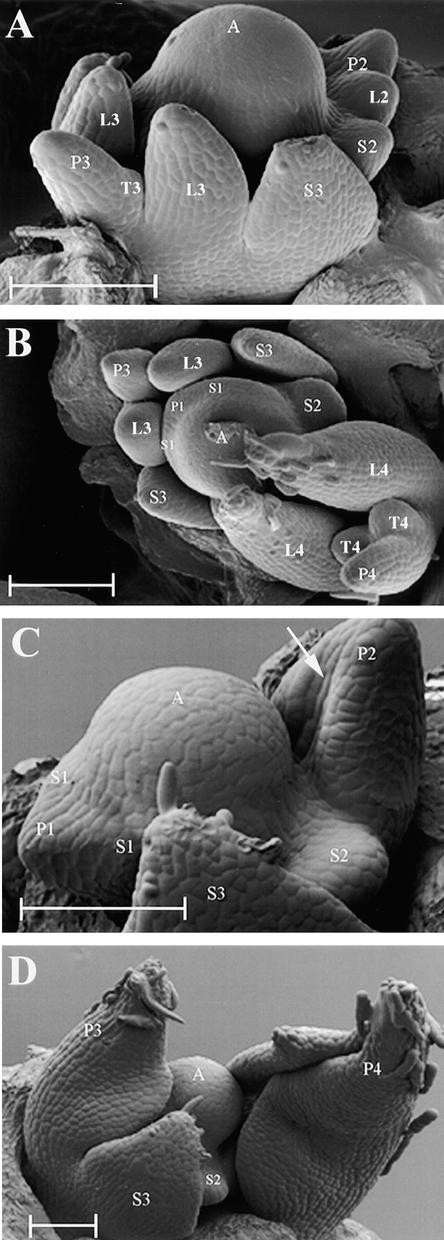
To examine the effects of the uni mutation on leaf primordium initiation and early development, scanning electron microscopy (SEM) analysis was performed on the dissected apices of 2-week-old wild-type and uni mutant JI 2171 plants, as shown in Figure 2. Leaf primordia arose laterally from the SAM at ∼180° to each other in a sequential manner on wild-type plants (Figure 2A). By the end of plastochron 1 (P1), a pair of stipule primordia (S1) had emerged and were visible as small bumps on either side of the leaf primordium, or marginal blastozone (Figure 2B). The proximal leaflet primordia were next to emerge and could be seen clearly at P2 (Figure 2A). During P3, the distal tendril primordia were initiated. The proximal leaflet and stipule primordia had flattened, were beginning to grow in toward the apex, and had begun to initiate epidermal hairs (Figure 2A). Whereas the leaflet primordia had begun to fold during P3, the stipule primordia (S3) had not. No more organs were initiated from the blastozone, which became determined during P4 and formed a terminal tendril (Figure 2B).
Figure 2.
SEM Showing the Effects of the uni Mutation on Leaf Primordium Initiation and Early Development.
(A) and (B) Two-week-old wild-type (JI 2171) vegetative shoot apices.
(C) and (D) Two-week-old sibling uni mutant (JI 2171) vegetative shoot apices. The white arrow in (C) indicates a central crease marking the beginning of lamina folding of a single, terminal leaflet.
A, shoot apex; P1 to P4, plastochron 1 to plastochron 4 of leaf development; S1 to S3, stipule primordia present on P1 to P3 marginal blastozones; L2 to L4, proximal leaflet primordia present on P2 to P4 marginal blastozones; T3 and T4, tendril primordia present on P3 and P4 marginal blastozones.  .
.
In the JI 2171 uni mutant, disruption to normal leaf development occurred early after leaf primordium initiation (Figure 2C). The blastozone emerged laterally on the SAM, and stipule primordia (S1) emerged late in P1, as seen in wild-type plants (Figure 2B). The stipules (S1 to S3) appeared to develop normally and at a similar rate as those of the wild type, but no further pairs of leaflet or tendril primordia were initiated (Figures 2C and 2D). At P2, the blastozone showed signs of differentiation into a terminal, unifoliate leaflet with a central crease marking the beginning of lamina folding (Figure 2C). This reduction of organogenic potential indicated that in plants of this age, UNI was required during P2 to maintain the developing wild-type marginal blastozone. During P3 and P4, the terminal unifoliate leaflet expanded, began to fold, and initiated epidermal hairs at its tip (Figure 2D).
Analysis of Wild-Type, tl, af, and af tl Compound Leaf Development
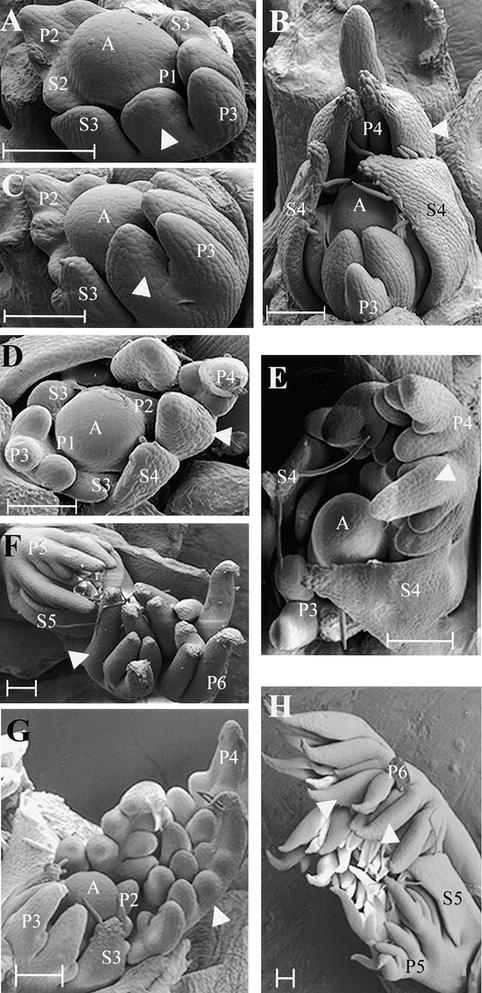
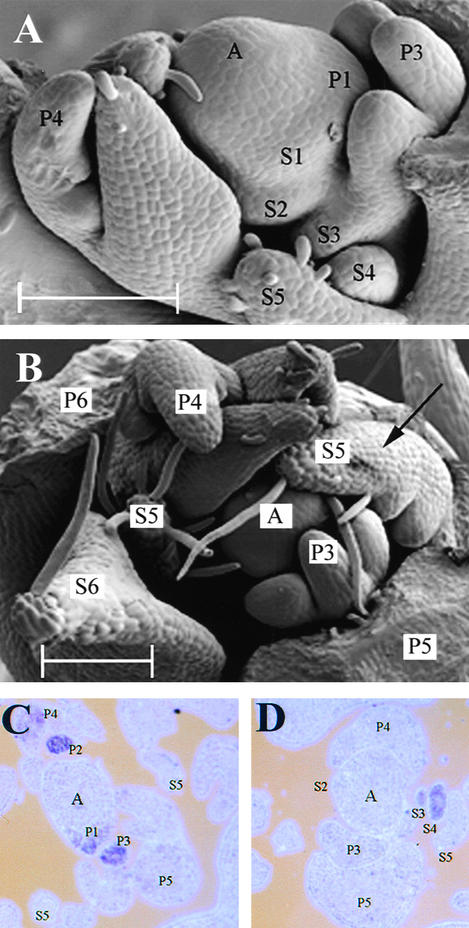
Leaf primordium initiation and early development in wild-type, tl, af, and af tl pea leaves have been described in detail previously by several authors (Meicenheimer et al., 1983; Gould et al., 1986; Villani and DeMason, 1997, 1999a). A brief SEM analysis of near-isogenic lines of these genotypes—JI 1194, JI 1197, JI 1195, and JI 1199, respectively—is presented in Figure 3 to facilitate the identification of those structures labeled in subsequent in situ hybridization sections. All plants included in the SEM analysis were 2 weeks old and had five fully expanded leaves; the leaf primordium being initiated from the SAM on these plants at the time it was dissected corresponded to node 13 on a mature plant, counting the first scale leaf as node 1.
Figure 3.
SEM Showing the Effects of the tl, af, and af tl Mutations on Early Leaf Development.
(A) and (B) Two-week-old wild-type (JI 1194) vegetative shoot apices. White arrowheads indicate leaflet primordia. A, vegetative shoot apex; P1 to P4, plastochron 1 to plastochron 4 of leaf development; S2 to S4, stipule primordia present on P2 to P4 marginal blastozones.
(C) and (D) Two-week-old tl mutant (JI 1197) vegetative shoot apices. White arrowheads indicate leaflet primordia. A, vegetative shoot apex; P1 to P4, plastochron 1 to plastochron 4 of leaf development; S3 and S4, stipule primordia present on P3 and P4 marginal blastozones.
(E) and (F) Two-week-old af mutant (JI 1195) vegetative shoot apices. White arrowhead in (E) indicates a secondary blastozone, initiating tertiary primordia acropetally. These tertiary primordia will develop into tendrils, indicated by a white arrowhead in (F). A, vegetative shoot apex; P3 to P6, plastochron 3 to plastochron 6 of leaf development; S4 and S5, stipule primordia present on P4 and P5 primary marginal blastozones.
(G) and (H) Two-week-old af tl double mutant (JI 1199) vegetative shoot apices. White arrowhead in (G) indicates a secondary blastozone initiating tertiary blastozones acropetally. The distal tip of a secondary blastozone, marked by the P6 label in (H), has been removed. Quaternary leaflet primordia, derived from tertiary blastozones, are indicated with white arrowheads in (H). A, vegetative shoot apex; P2 to P6, plastochron 2 to plastochron 6 of leaf development; S3 and S5, stipule primordia present on P3 and P5 primary marginal blastozones.
 .
.
Stipule primordium initiation and development in all four lines appeared similar to that described for wild-type JI 2171 above. No differences in proximal organ development were observed over the first two plastochrons, in agreement with a previous report (Meicenheimer et al., 1983). On wild-type and tl mutant samples, primordia initiated during P2 formed a proximal pair of leaflets that during P3, expanded and began to bend inward toward the SAM (Figures 3A and 3C). Expansion continued during P4, and a central fold in the leaflets became clearly visible (Figures 3B and 3D). Late in P3, the second pair of organ primordia began to emerge from the marginal blastozone of the wild type and tl mutants (Figures 3B and 3D). On 2-week-old plants, these organ primordia typically formed tendrils in wild-type plants and leaflets in tl mutants. At P4, the blastozone did not usually initiate any further lateral organ primordia and became determinate, forming a terminal tendril or a terminal leaflet on wild-type and tl leaves, respectively.
Leaf development on af and af tl mutant shoots was similar to that of wild-type shoots during P1 and P2; however, differences in lateral primordium development became apparent during P3, as has been reported previously (Meicenheimer et al., 1983; Villani and DeMason, 1999a). Lateral primordia initiated during P2 did not form determinate organs as in wild-type and tl leaves. Instead, they initiated further primordia during P3 in a manner similar to that of the primary leaf marginal blastozone (Figures 3E and 3G) and are hereafter denoted as secondary marginal blastozones. This observed increase in organogenic potential in af and af tl leaves compared with the wild type and tl showed that AF function in the suppression of secondary blastozone activity was first manifested in lateral primordia at P3. During P3, both primary and secondary blastozones of af and af tl mutants initiated primordia acropetally. The rate of acropetal organ initiation observed on af and af tl primary blastozones appeared similar to that observed on wild-type and tl plants in that all were initiating their second pair of lateral organs at P3 (cf. Figures 3B and 3G). During P4, the primary blastozones of af and af tl leaves developed an additional distal pair of lateral primordia not seen on wild-type and tl plants. This showed that the function of AF in the suppression of primary blastozone activity was first manifested at P4.
Differences in the development of af and af tl leaves were observed during P4. On af leaves, lateral primordia initiated from the primary and secondary blastozones during P3 and P4 did not branch further, were seen to elongate during P5, and formed tendrils during P6 (Figure 3F). In contrast, primordia arising from similar positions on af tl mutant leaves remained organogenic and formed additional branches, which were denoted as tertiary blastozones (Figure 3H). This showed that the function of TL in the suppression of tertiary blastozone activity in af secondary blastozones was first manifested during P5. The quaternary branching that results from tertiary blastozone activity in af tl leaves accounts for the greater complexity of these leaves than the af leaves when both are fully developed (cf. the number of organs in Figures 1D and 1E). The quaternary primordia of af tl leaves developed into small leaflets (Figure 1E), which started to form late in P5 and were clearly visible as folded laminae at P6 (Figure 3H).
In summary, our SEM analysis showed that in a wild-type leaf, the function of UNI in the maintenance of the marginal blastozone was first evident during P2, whereas the function of AF in the suppression of blastozone activity in lateral primordia and in the primary blastozone was first evident at P3 and P4, respectively. The function of TL in the suppression of blastozone activity in the tertiary lateral primordia of af mutant leaves was first apparent during P5. Our observations of changes during leaf ontogeny were in broad agreement with those made in earlier studies that compared wild-type and mutant leaf development (Meicenheimer et al., 1983; Gould et al., 1986; Villani and DeMason, 1997, 1999a). Because previous reports had provided genetic evidence to suggest that UNI, AF, and TL interact to pattern the pea leaf (Marx, 1987; Hofer and Ellis, 1996, 1998), we examined UNI expression in the wild type, tl, af, and af tl mutants to determine whether there were any differences in UNI transcript accumulation in these different genetic backgrounds.
UNI Expression in Vegetative Apices
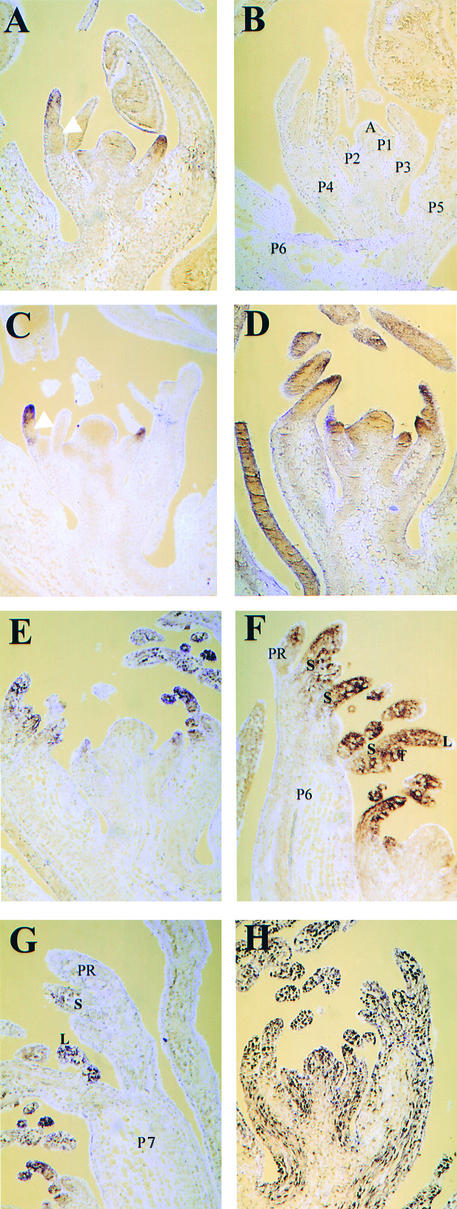
Figure 4 shows frontal longitudinal sections of vegetative apices hybridized in situ to a digoxigenin-labeled UNI RNA probe. In all four lines, UNI was expressed in marginal blastozones but not in the main shoot axis or SAM. In wild-type leaves, transcripts were first detected at P1, had accumulated by P2, and then accumulated distally in the marginal blastozone up to P4. At P5, UNI expression appeared to be downregulated, and transcripts were not detected above background (Figure 4A). No signal was detected in control sections probed with UNI sense RNA probes (Figure 4B). The duration of UNI expression in tl mutant leaves appeared similar to that in the wild type. Transcripts were first detected at P1, accumulated distally up to P4, and were downregulated at P5 (Figure 4C). In af mutant sections, UNI expression was detected distally in the primary blastozone up to P5, one plastochron longer than expression in wild-type and tl primordia (Figure 4D). The leaves of the af tl double mutant showed even more prolonged UNI expression in the primary blastozone, up to P6 (Figures 4E and 4F); by P7, however, expression was not detectable above background in the primary blastozone (Figure 4G). At P6 and P7, UNI was still strongly expressed in secondary and tertiary blastozones and developing leaflets (Figures 4F and 4G). In contrast to the pattern of UNI expression, HISTONE H3 (HH3) transcripts were detected in a widespread pattern in af tl sections (Figure 4H). Because HH3 transcription is likely to be more abundant in actively dividing cells (Robertson et al., 1997), this probe provides a control to verify that signal above background from the UNI antisense RNA probe is not a consequence of high cell density in areas of active division.
Figure 4.
Localization of UNI Transcripts in Wild-Type, tl, af, and af tl Mutant Samples by RNA in Situ Hybridization on Frontal Longitudinal Sections.
Sections were taken from 2-week-old seedlings.
(A) and (B) Wild type (JI 1194).
(C) tl mutant (JI 1197).
(D) af mutant (JI 1195).
(E) to (H) af tl double mutant (JI 1199).
Sections shown in (A) and (C) to (G) were hybridized with an antisense digoxigenin-labeled UNI probe, section shown in (B) was hybridized with a sense digoxigenin-labeled UNI probe, and section shown in (H) was hybridized with an antisense digoxigenin-labeled HH3 probe. The plastochronic ages indicated in (B) apply to sections (A) to (E) and (H), where (A) and (E) are in midplastochron, (C) and (D) are very early in a plastochron, and (B) and (H) are late in a plastochron. White arrowheads in (A) and (C) indicate the positions of lateral organs that emerged in front of the plane of section. The section in (D) is lateral to P1 on the shoot apex, reducing the view of the P2 and P3 blastozones. A, vegetative shoot apex; L, leaflet; P1 to P7, plastochronic age of leaf primordia; PR, primary marginal blastozone; S, secondary blastozone; T, tertiary blastozone.
The differences in expression patterns observed between these lines in longitudinal sections showed that interactions between the UNI, AF, and TL genes do occur. The prolongation of UNI expression to P5 in af mutant sections suggested that UNI gene expression is suppressed by AF late in P4 and during P5 in wild-type primary marginal blastozones. The prolongation of UNI expression to P6 in the primary blastozone of af tl primordia suggested that UNI is suppressed by TL in af primary blastozones late in P5 and during P6. The similar patterns of expression seen in wild-type and tl mutant sections, however, suggested that TL does not regulate UNI transcript accumulation in primary blastozones during P1 to P4 in a wild-type background.
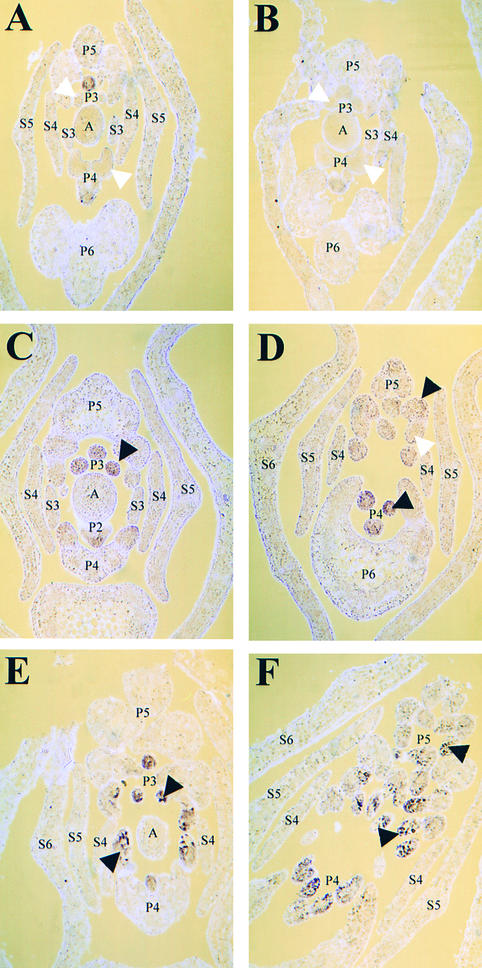
UNI expression in primary blastozones was most clearly visible in the frontal longitudinal sections examined above. UNI transcripts were detected in the secondary blastozones of af and af tl leaves but not in the determinate lateral organ primordia of wild-type and tl leaves. Clear zones, in which UNI transcripts were less abundant, were visible on wild-type and tl P4 blastozones. These demarcated the position of lateral primordia (Figures 4A and 4C). To show UNI expression patterns in lateral primordia more clearly, we used transverse sections, as seen in Figure 5. The sections were taken ∼40 μm below (Figures 5A to 5C and 5E) and ∼70 μm above (Figures 5D and 5F) the shoot apex.
Figure 5.
Localization of UNI Transcripts in Wild-Type, tl, af, and af tl Mutant Samples by RNA in Situ Hybridization on Transverse Sections.
Sections taken from 2-week-old seedlings hybridized with an antisense digoxigenin-labeled UNI probe.
(A) Wild type (JI 1194).
(B) tl mutant (JI 1197).
(C) and (D) af mutant (JI 1195).
(E) and (F) af tl double mutant (JI 1199).
White arrowheads in (A) and (B) indicate lateral primordia on P3 and P4 marginal blastozones where transcripts were not detected. The black arrowheads in (C) to (E) indicate secondary lateral blastozones on P3, P4, and P5 primary blastozones where UNI transcripts were detected. White arrowhead in (D) indicates a tertiary primordium in which UNI expression was downregulated compared with the expression in an adjacent secondary blastozone (marked with a black arrowhead). Black arrowheads in (F) indicate tertiary blastozones where UNI transcripts were detected. (A) to (C) and (E) were taken ∼40 μm below the shoot apex; (D) and (F) were taken ∼70 μm above the shoot apex. A, vegetative shoot apex; P2 to P5, plastochronic age of leaf primordia; S3 to S6, stipule primordia present on P3 to P6 marginal blastozones.
No marked differences in UNI expression were detected between wild-type and tl mutant samples. Transcripts were detected up to P4 and accumulated distally in the marginal blastozone, as shown in the longitudinal sections (cf. Figures 4A and 4C with Figures 5A and 5B). UNI expression was not detected in lateral organ primordia or in the SAM of these samples (Figures 5A and 5B).
Differences in UNI expression were more clearly apparent between wild-type and af single or af tl double mutant samples in transverse sections. In af mutants during P2, transcripts accumulated distally in the primary blastozone but were barely detectable in the first-initiated lateral primordia (Figure 5C). This expression pattern was similar to that of the wild type at this stage. SEM analysis demonstrated that P2 wild-type and af marginal blastozones are similar in appearance, but during P3 the first-initiated lateral primordia of af mutant leaves behave differently, like blastozones, because they initiate further organ primordia instead of becoming determinate organs. In accordance with their morphological similarity to the primary marginal blastozone, these secondary blastozones expressed UNI at P3 (Figure 5C). By P4, the af primordium shown had initiated a second pair of secondary blastozones, and these also showed UNI expression (Figure 5D). Signal was detected faintly in the primary and secondary blastozones of af mutants at P5, but expression was downregulated in the lateral organ primordia that arose from them (Figure 5D). As was demonstrated by SEM and discussed above (Figure 3F), these lateral primordia initiated from the primary and secondary blastozones are determinate and develop into tendrils during subsequent plastochrons.
In summary so far, UNI expression was detected in the secondary blastozone primordia of an af mutant but was not detected in lateral organ primordia at the same position on wild-type leaves. This result, in combination with SEM analysis, suggested that both UNI transcription and blastozone activity were suppressed by AF in the lateral organ primordia of wild-type leaves after P2 (during P3 to P5). These transverse sections also confirmed results obtained from longitudinal sections, which showed that UNI was expressed in the primary marginal blastozone at P5 in af mutant primordia compared with expression at P4 in the wild-type primordia. Thus, these results suggest that UNI expression is also suppressed by AF in the primary blastozone of wild-type leaves after P4 (during P5).
The UNI expression pattern appeared similar in transverse sections of af single mutants and af tl double mutants up to P4. At P3 and P4, UNI expression was observed in the primary and secondary marginal blastozones of af tl leaf primordia (Figure 5E). At P5, UNI expression was detected in the primary, secondary, and tertiary blastozones of af tl leaf primordia (Figure 5F), whereas UNI expression was not clearly detectable in the tertiary organ primordia of af mutant leaves at P5 (Figure 5D). This suggested that UNI expression is suppressed by TL in the lateral tendril primordia of af mutant leaves during P5. As was seen in longitudinal section, UNI expression was prolonged in an af tl primary blastozone compared with that in an af primary blastozone, suggesting suppression by TL after P5 in an af mutant background. Finally, these data suggest that AF suppresses UNI in the lateral organ primordia of tl mutant leaves, because UNI expression was prolonged in lateral primordia of the af tl double mutant.
Stipule Development and UNI Expression in coch Mutant Plants
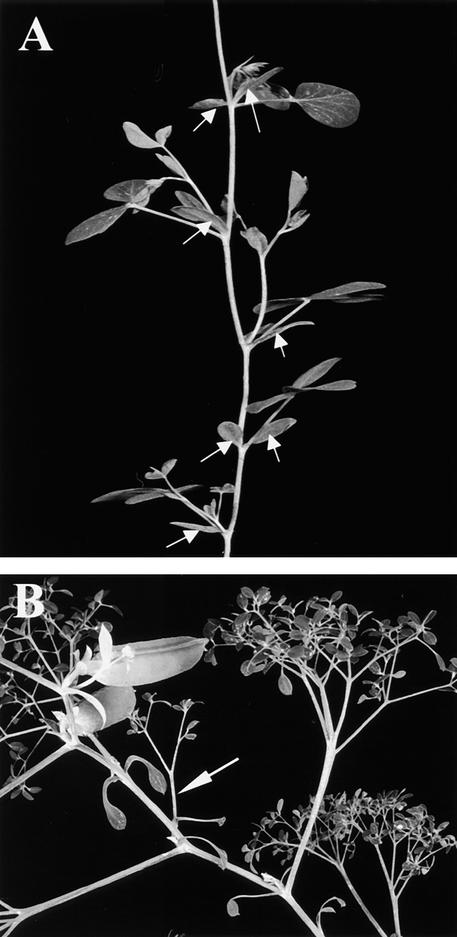
Our results from SEM combined with longitudinal and transverse sections showed that UNI expression was correlated with blastozone activity. To test this correlation further, we performed in situ hybridization with coch plants to determine whether UNI expression could be detected in the stipule primordia at a time when they exhibited blastozone activity and gave rise to compound stipule architectures. Previous work has shown that compound stipules form in plants homozygous for the coch-5137 allele between nodes 8 and 15 (Blixt, 1967; Gourlay, 1999). To demonstrate this and to aid in identification of the structures in section, we performed SEM analysis of stipule development in 2-week-old coch-5137 plants (JI 2165).
Leaflets and tendrils in the coch mutant samples were initiated in a manner similar to that of their corresponding wild type, variety Weitor (Blixt, 1967). Figure 6A shows that one pair of leaflets, two pairs of tendrils, and a terminal tendril had initiated normally on P4 coch leaves. Early stipule development is also shown (Figure 6A). Stipule primordia (labeled S1 and S2) emerged during P1 and remained indistinguishable from their corresponding wild-type primordia at P2. Wild-type stipules broadened and flattened during P3, P4, and P5, whereas the coch stipule primordia observed did not; they appeared to expand at a markedly slower rate. For example, P3 stipule primordia of all wild-type lines examined to date, including variety Weitor, were comparable in size to their adjacent P3 leaflet primordia (see Figures 2A and 3A; Gourlay, 1999); however, coch stipule primordia at P3 (labeled S3 in Figure 6A) were much smaller than their adjacent P3 leaflet primordia. These observations showed that COCH promotes normal stipule growth and development and that this activity was first evident at P3. Later stages of stipule development on coch mutant plants are shown in Figure 6B, when compound stipules were observed. One compound stipule with two pairs of lateral organ primordia (S5 in Figure 6B) is visible on a P5 marginal blastozone. Note that the other member of the pair of S5 stipules does not appear to be compound; therefore, the stipules at this position would be predicted to resemble those examined by in situ hybridization in Figure 6C.
Figure 6.
SEM Showing Effects of the coch Mutation on Early Stipule Development and Localization of UNI Transcripts by RNA in Situ Hybridization.
(A) and (B) Two-week-old coch mutant (JI 2165) vegetative shoot apices. Black arrow in (B) indicates a compound stipule (S5) with two pairs of lateral organ primordia.
(C) and (D) Transverse sections taken from a 2-week-old coch seedling hybridized with an antisense digoxigenin-labeled UNI probe. (C) was taken ∼40 μm below the shoot apex, and (D) was taken ∼100 μm below the shoot apex, at a slightly oblique angle.
A, vegetative shoot apex; P1 to P6, plastochronic age of leaf primordia; S1 to S6, stipule primordia present on P1 to P6 marginal blastozones.  .
.
In coch leaves, UNI expression was detected in primary marginal blastozones up to P4 (Figure 6C), as was previously seen in wild-type (JI 1194) leaves (Figures 4A and 5A). This part of the expression pattern was in accordance with the fact that patterning in the leaf blade is not altered by the coch mutation; only stipule development is affected. Unlike the case in wild-type plants, UNI expression was detected in the developing stipule primordia of coch mutant plants; in the example shown, transcripts accumulated on one side only, in S3 and S4 (Figure 6D). This represented ectopic UNI expression, because transcripts were not detected in wild-type S3 and S4 stipules (data not shown for the corresponding wild-type variety Weitor; a comparable wild-type expression pattern is shown in Figure 5A). No signal was detected in S1 and S2 coch mutant stipule primordia or in those older than S4. The detection of ectopic UNI expression in the stipule primordia of 2-week-old JI 2165 plants coincides with a time when compound architectures arise at this position. At other points in ontogeny, such as from node 16 onward, when compound stipules are not formed on coch-5137 plants (Gourlay, 1999), UNI expression was not detected in stipule primordia (data not shown).
Gene Expression in uni Mutants
The presence of a rachis and additional pairs of leaflets or tendrils on some double and triple uni mutant combinations indicated that UNI gene function was not always correlated with extended marginal blastozone activity. It was described previously that uni af and uni af tl leaves can be pentafoliate and thus more complex than uni leaves, which are trifoliate at their maximum complexity (Hofer and Ellis, 1998). An example of a uni af tl triple mutant leaf with a rachis and two pairs of leaflets is shown in Figure 7A. The complexity of this leaf suggests that in the absence of AF and UNI gene activities, a redundant UNI-like function permits the formation of a rachis bearing more than one pair of leaflets. This has been referred to previously as a function of “gene X,” and candidates for the identity of this gene have been discussed (Hofer and Ellis, 1998). Among those considered was KNOTTED1 (KN1), which is expressed in the vegetative shoot apex of maize but is downregulated at sites of leaf initiation (Smith et al., 1992; Jackson et al., 1994). KNOX homologs are expressed in tomato compound leaf primordia, and overexpression of KNOX genes has been shown to result in more ramified leaves, suggesting that KNOX genes can influence pattern formation in leaves and shoots (Hareven et al., 1996; Parnis et al., 1997; Janssen et al., 1998). It therefore seemed possible that in the absence of UNI, ectopic expression of a pea KNOX homolog in pea leaf primordia might result in the formation of additional pairs of lateral organs.
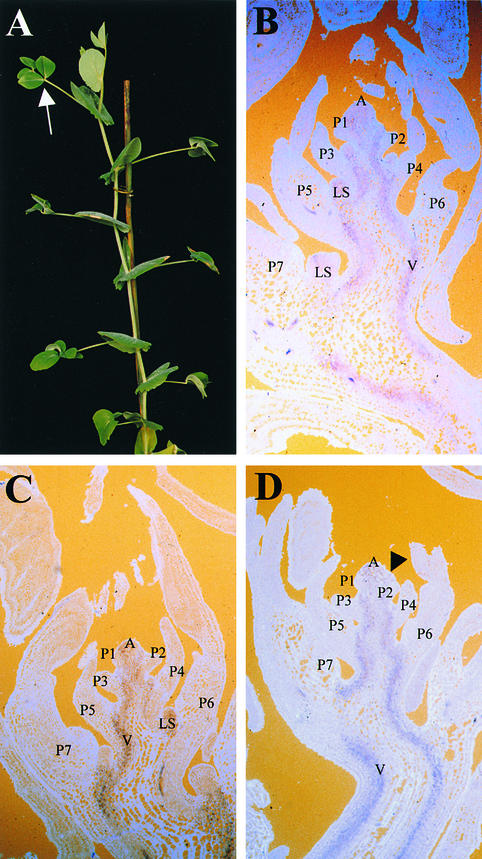
Figure 7.
Morphology of a uni af tl Triple Mutant and Localization of PSKN1 Transcripts by RNA in Situ Hybridization.
(A) Nodes 5 and above of a uni af tl plant before flowering. The white arrow indicates a leaf at node 12 with two pairs of leaflets, borne on a rachis (obscured from view).
(B) Frontal longitudinal section of a 3-week-old uni af tl (XM 7175) shoot apex hybridized to an antisense PSKN1 probe.
(C) Frontal longitudinal section of a 3-week-old uni (JI 2171) shoot apex hybridized to an antisense PSKN1 probe.
(D) Frontal longitudinal section of a 3-week-old wild-type (JI 1194) shoot apex hybridized to an antisense PSKN1 probe. The black arrowhead indicates an inflorescence meristem developing in the axil of the P2 marginal blastozone.
A, shoot apex; LS, lateral shoot; P1 to P7, plastochron 1 to plastochron 7 of leaf development; V, developing vasculature of the main stem.
To investigate this possibility, we isolated a pea class I KNOX homolog (Hofer et al., 2000) and examined its expression pattern in uni af tl triple mutant leaves by RNA in situ hybridization. The expression patterns of PSKN1 in uni af tl (Figure 7B), uni (Figure 7C), and wild-type shoot apices (Figure 7D) were similar. In all cases, PSKN1 gene expression was confined to the shoot apex and developing vasculature in the main stem and was downregulated in leaf primordia, in a pattern similar to that observed in maize (Smith et al., 1992; Jackson et al., 1994). No signal was obtained when a sense PSKN1 probe was used as a control (data not shown). Given these gene expression patterns, we concluded that PSKN1 was unlikely to confer a redundant UNI-like function in pea marginal blastozones.
DISCUSSION
The term blastozone was proposed to designate regions of the shoot competent for organogenesis. Leaves are derived from marginal blastozones; shoot axes are derived from apical blastozones (Hagemann and Gleissberg, 1996). In this article, we focus on UNI function in the pea leaf, in the context of a marginal blastozone, in which it may perform a role different from that proposed for LFY in the transition to flowering (Blázquez and Weigel, 2000). A wild-type pea compound leaf primordium exhibits a prolonged period as a blastozone, compared with a primordium initiated at an equivalent node on a uni mutant plant, because the wild-type primordium possesses a greater growth potential and capacity for organogenesis. Previously, we described the wild-type pea leaf as developing from a transiently indeterminate primordium (Hofer et al., 1997), although the term indeterminacy is usually used to refer to the quality of prolonged growth and organogenesis that is observed in a shoot (Steeves and Sussex, 1989). We use the term marginal blastozone in this paper to help avoid any possible ambiguity in descriptions of compound leaves as opposed to shoots. We showed that UNI expression is negatively regulated by COCH, AF, and TL and that UNI expression in pea leaf primordia is correlated with maintenance of marginal blastozones. We suggest that these interactions are fundamental in establishing a wild-type pea leaf architecture through the regulation of marginal blastozone activity.
UNI Maintains the Wild-Type Leaf as a Marginal Blastozone
Blastozone activity is maintained in the distal portion of a developing wild-type pea leaf and is lost in lateral primordia as the cells are organized, or become determined, into stipule, leaflet, or tendril pathways. Cells at the apex of the marginal blastozone also eventually lose their organogenic capacity and form a terminal tendril (Meicenheimer et al., 1983). Our SEM analysis confirmed that in 2-week-old wild-type plants, all compound leaf organ primordia were initiated during the first three plastochrons and that the marginal blastozone became determined during the fourth plastochron (Figures 3A and 3B). This is also in agreement with tissue culture experiments suggesting that a pea leaf primordium becomes determined over four plastochrons of growth (Gould et al., 1994). Our SEM analysis on uni plants demonstrated that they had a reduction in the number of plastochrons, from four to two, over which the marginal blastozone is maintained (Figures 2A to 2D). This reduction in organogenic potential was accompanied by the early expression of processes associated with determinate growth and differentiation such as lamina expansion and folding and epidermal hair formation (Figures 2B and 2D). This suggests that UNI promotes blastozone activity and inhibits lamina-forming processes in the wild-type compound leaf primordium. In accordance with this role, UNI transcripts were found to accumulate in the distal region of the marginal blastozone of wild-type leaves over the first four plastochrons of development (Figure 4A) but were not detected in determinate organ primordia, that is, in stipule, leaflet, and tendril primordia (Figure 5A). Presumably, therefore, suppression of UNI transcription in lateral primordia is an important event in ensuring formation of determinate organs.
A recently proposed model for compound leaf development in pea is based on the phenotypes of double and triple mutants at the loci uni, af, and tl (Hofer and Ellis, 1998). In this model, the compound leaf is divided into four domains, defined by interactions between UNI, AF, TL, and COCH. The model also suggests that UNI may be inhibited by COCH in domain 1 (stipule and petiole), by AF in domain 2 (proximal leaflets and rachis), by AF and TL in domain 3 (distal tendril pairs and rachis), and by TL in domain 4 (a trefoil of tendrils), thus preventing stipule-, leaflet-, and tendril-fated cells from adopting a central rachis, or blastozone, fate. The molecular evidence we present here is summarized in Figure 8 and is in general agreement with this model.
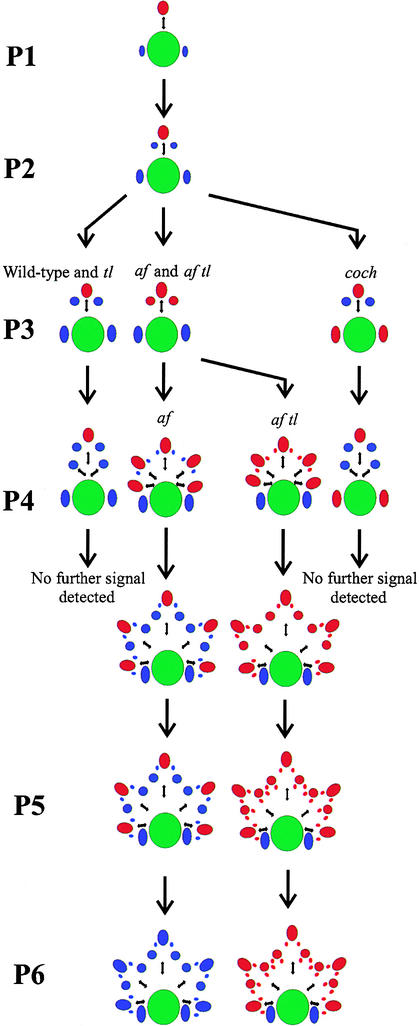
Figure 8.
Summary Diagram Showing UNI Expression in Vegetative Shoot Apices of Wild-Type, tl, af, af tl, and coch Mutant Seedlings.
Representations of transverse sections through the shoot apex are followed through the early stages of leaf development (arrows). The main shoot axis is shown as a large green circle, blastozones in which UNI expression was detected are shown as red ovals, and organs in which UNI expression was not detected are shown as blue ovals. Small black double-headed arrows represent the rachis and rachides. The plastochronic age of each marginal blastozone is shown at left (P1 to P6). During P1 and P2, the morphology and pattern of UNI expression in all the genotypes studied was the same (top center), but during P3, both the morphology and the expression patterns changed. In the far left-hand column, UNI expression was detected in the primary marginal blastozones of wild-type and tl mutant seedlings, up to and during P4. Lateral primordia that arose from these showed no signal and formed determinate organs (stipules, leaflets, and tendrils). In the second column from the left, UNI transcripts accumulated in primary blastozones and in the secondary blastozones that arose during P3 in both af and af tl mutant leaves. Both of these mutants initiated tertiary branches late in P3 and during P4 (second and third columns). In af mutant leaves, the tertiary branches were determinate tendril primordia showing no detectable UNI expression (second column), whereas af tl tertiary blastozones showed UNI signal, remained indeterminate, and exhibited quaternary branching during P5 (third column). In af tl mutant leaves, UNI transcripts were detected in the primary marginal blastozone up to P6. In the far right-hand column, UNI expression was detected in the primary marginal blastozones of coch mutant seedlings, up to and during P4, but not in the lateral leaflet or tendril organ primordia that arose from these. During P3 and P4, UNI expression was detected in stipule primordia that behaved like blastozones and initiated lateral leaflet and tendril organ primordia (these lateral primordia are not shown). After this time, no further signal was detected.
Interactions between UNI and COCH
UNI expression was detected in the stipule primordia of coch mutant plants at a time when compound stipule architectures were predicted to form (Figures 6B and 6D). This represents ectopic expression, because wild-type stipules do not accumulate UNI transcripts. Ectopic UNI expression was also observed in compound stipule primordia of coch af double mutants at a time when these plants were developing compound stipules bearing unbranched tendrils (Gourlay, 1999). We hypothesize that ectopic UNI expression confers a blastozone identity on stipule primordia, allowing compound architectures to form. The timing of UNI expression is critical to this hypothesis, however, and causality is difficult to establish in the absence of appropriate transgenic studies. If ectopic UNI expression causes a change in stipule primordium fate, then UNI gene expression would be expected to precede detectable changes in morphology. However, we were able to detect ectopic expression only at P3, when morphological changes were already apparent. Therefore, the misexpression of UNI may be a consequence of the change in morphology, not its cause. The uni mutant phenotype provides indirect support in favor of our hypothesis. By demonstrating that UNI function is required to maintain the marginal blastozone, we have inferred that ectopic UNI function in stipule primordia maintains them as blastozones (as in coch) and lack of expression fails to maintain the primordia as blastozones (as in the wild type).
Further evidence to support our hypothesis comes from the fact that compound stipules are not found on coch uni double mutant plants. Although the full description of this double mutant phenotype is beyond the scope of this paper, Figure 9A shows five nodes on a representative coch uni plant that began to flower at node 15. Maximum leaf complexity in pea is usually found at or just below the first node of flowering (Makasheva, 1983), and compound stipules are formed between nodes 8 and 15 on coch (JI 2165) plants. The stipules at nodes 11 to 15 of the coch uni plant shown were all simple laminae (Figure 9A). This epistasis with respect to stipule development further indicates that UNI function is required for the development of compound stipule architectures.
Figure 9.
Stipule Morphology in Mutant Backgrounds.
(A) A coch uni double mutant. Stipules at five nodes are indicated with white arrows. Node 11 is at the bottom of the panel; node 15, with an axial flower, is at the top.
(B) An st af tl (JI 1201) plant with a compound stipule opposite a simple stipule at node 18. The compound stipule is indicated with a white arrow.
The detection of ectopic UNI transcripts in coch mutant stipule primordia at the time when compound architectures form and the lack of compound stipules in coch uni double mutant plants suggest that COCH normally inhibits UNI in wild-type stipule primordia. There is no evidence for a direct interaction, but this inhibition would prevent a blastozone fate in stipule primordia, as discussed above, and is in agreement with the domain 1 interaction suggested by Hofer and Ellis (1998). However, because neither ectopic UNI expression nor compound stipule architectures are observed at every node on coch mutant plants, UNI apparently is suppressed in stipule primordia by factors other than COCH. The STIPULES REDUCED (ST) gene, which is involved in expansion of the stipule (Pellew and Sverdrup, 1923), may be an additional factor that suppresses UNI in stipule primordia. We have observed compound stipule architectures at some nodes on st af tl (JI 1201) triple mutant plants, whereas we have never seen compound stipules on af tl double mutant plants (see Figure 1E). Figure 9B shows an example of an st af tl triple mutant plant with a compound stipule opposite a simple stipule at node 18. If a simple model proposes that COCH and ST both act redundantly to repress UNI, and hence blastozone activity, in stipule-fated cells, then the stipules of coch st double mutants would be predicted to be compound. However, such a simple model does not hold, because coch st plants fail to form stipule laminae at all (Blixt, 1967; Marx, 1987; Gourlay, 1999). This novel phenotype suggests that COCH and ST act in the same pathway. Further investigation is required to ascertain whether ST repression of blastozone activity in stipules is also dependent on the absence of AF or TL.
Interactions between UNI and AF
An antagonistic relationship between AF and TL has been suggested previously (Marx, 1987), such that AF inhibits TL in the proximal part of the leaf to allow leaflet formation and that TL inhibits AF in the distal part of the leaf, leading to the formation of distal and terminal tendrils. This model predicts that the loss of TL function would lead to leaflet formation at distal and terminal positions; that is, a tl mutant leaf, and loss of AF function would allow tendrils to form at proximal positions. It does not, however, account for the branching rachides found at proximal positions on af mutant leaves, nor does it provide a satisfactory explanation for the supercompound architecture of the leaf of an af tl double mutant. The UNI expression data presented here and summarized in Figure 8 provide an explanation for the tl, af, and af tl mutant leaf architectures and suggest an alternative model for the development of the pea leaf.
Organ primordia initiated from the marginal blastozone during P2 on af single mutant and af tl double mutant leaves are morphologically indistinguishable from those of wild-type plants and tl mutants at this stage (Meicenheimer et al., 1983; Gould et al., 1986; Villani and DeMason, 1997, 1999). Differences between these four genotypes first occur during P3, when these first-initiated organ primordia (which would form determinate leaflets in wild-type and tl mutant leaves) behave like blastozones in af and af tl leaves (Figures 3E and 3G) and initiate tertiary primordia. At this time, UNI expression is detectable in both af and af tl rachide primordia but not in the determinate leaflet primordia of wild-type or tl plants (cf. P3 in Figures 5A and 5B with P3 in Figures 5C and 5E). Therefore, in the absence of AF function, ectopic UNI expression in the first-initiated organ primordia of af and af tl mutant leaves was associated with a change in developmental fate, from a determinate leaflet primordium to that of a blastozone. This suggests that AF normally inhibits UNI in the proximal leaflet primordia of wild-type and tl leaves. Despite the lack of evidence for a direct interaction, this proposed inhibition is in agreement with the domain 2 interaction in the model suggested by Hofer and Ellis (1998).
Previous authors have suggested that the AF gene either directs (Gould et al., 1994) or promotes (Lu et al., 1996; Villani and DeMason, 1997, 1999a; Hofer and Ellis, 1998) lamina formation in a wild-type pea leaf. Clearly, AF is not required for leaflet formation per se, because leaflets form on af tl, af uni (Marx, 1987), and af tl uni plants (Hofer and Ellis, 1996). However, AF clearly is required for leaflets to form at proximal positions on a wild-type leaf (with a functional UNI gene) because on af and af tl mutants these are replaced by branching rachides. We propose that AF normally suppresses UNI expression in proximal primordia positions and that the branched af and af tl mutant phenotypes occur as a result of ectopic UNI expression, which confers blastozone identity on proximal leaflet primordia. Again, the timing of UNI gene expression in the leaflet primordia is critical in determining whether it causes, or is a consequence of, changed primordial fates. We were unable to detect differences in UNI expression in wild-type and af blastozones during P2, before any morphological differences were apparent. Therefore, these in situ hybridization studies alone do not support the hypothesis that UNI gene activity confers a blastozone fate. The uni mutant phenotype does, however, support this hypothesis indirectly. We have inferred from the uni mutant phenotype that UNI expression not only is associated with a blastozone fate but also is part of its cause. This hypothesis remains to be confirmed by UNI overexpression studies.
Further support comes from the uni af double mutant leaf, which has a variable phenotype ranging from unifoliate to pentafoliate (Hofer and Ellis, 1998). The first-initiated lateral organs may be tendrils or leaflets, but ramified rachides have never been observed at this position. Similarly, uni af tl triple mutant leaves can be pentafoliate at their maximum complexity, but the lateral organs are always simple leaflets, never secondary blastozones. This epistasis of uni over af at lateral leaflet positions supports the hypothesis that UNI is required for blastozone activity in the lateral primordia of af leaves. When another aspect of lateral organ development—leaflet shape—is considered, a novel phenotype clearly is associated with uni af and uni af tl leaflets that is absent in uni leaves. Most uni af and uni af tl leaflets are not planar; their midveins are curved in the dorsoventral plane (as can be seen at lower nodes in Figure 7A). This novel phenotype occurs after leaflet identity has been established and suggests that UNI and AF act nonindependently later in leaflet development.
Class I KNOX Gene Expression
To address the surprising finding that uni af and uni af tl leaves can be more complex than uni leaves (Hofer and Ellis, 1998), we examined the expression pattern of a class I KNOX homolog. We thought it possible that misexpression of a KNOX gene in uni af marginal blastozones could result in a more complex leaf, given that overexpression of genes of this class confers ramification on tomato compound leaves (Hareven et al., 1996; Parnis et al., 1997; Janssen et al., 1998). This possibility was also considered in light of dominant knox mutants in maize with distal leaf tissue transformed into more proximal tissue types (Freeling, 1992). Continuation of blastozone activity in uni af and uni af tl leaves could be accounted for as a proximal transformation, with the shoot meristem viewed as the ultimate proximal cell group (Muehlbauer et al., 1997). In a previous model, we suggested that such a transformation could occur as a result of an additional activity (“gene X”; Hofer and Ellis, 1998).
Our in situ hybridization analysis (Figure 9) showed no differences in PSKN1 expression between wild-type, uni, and uni af tl leaf primordia. We concluded that it was unlikely that ectopic PSKN1 gene expression corresponded to gene X activity in uni af tl marginal blastozones. However, the uni af tl leaf primordia at the particular nodes we examined could have been constrained in their complexity and therefore might not have reflected the expression pattern in leaf primordia that would go on to develop a rachis. The possibility also exists that PSKN1 influences patterning in uni af tl blastozones from a distance, given the demonstration that KN1 protein is mobile (Lucas et al., 1995). Finally, other pea KNOX homologs may be expressed ectopically in the absence of UNI; therefore, we cannot rule out KNOX homologs as candidates for gene X.
Interactions between UNI and TL
The epistasis of uni over tl (Marx, 1987) suggests that these two genes act in the same pathway; however, the role of the wild-type TL gene in the determination of leaf pattern is uncertain because the tl mutation is semidominant. On a heterozygous plant (TL/tl), the tendrils are straplike and considered to be intermediate between leaflets and tendrils (Marx, 1987; Villani and DeMason, 1999b). Perhaps the wild-type TL gene plays no role in lamina or tendril formation, but the altered activity of the gene in the tl mutant allows it to have an influence on leaf patterning that it would not normally exert. In attempting to understand the function of the TL gene, we can consider legume leaf form more broadly. The wild-type tendrilled pea leaf is a relatively rare form that may have arisen after a loss or gain of function in the leaf relative to other legumes. Therefore, one interpretation of the tl mutation is that it represents the restoration of a typical legume leaf function.
The effects of TL on UNI expression were seen in the af tl double mutant. Our expression data showed that UNI expression was extended to P6 in the primary blastozone of an af tl double mutant leaf primordium, compared with P5 in an af mutant leaf primordium. Furthermore, UNI expression in the tertiary primordia of af leaves that would go on to form tendrils (Figure 3F) was not above background values at P5 (Figure 5D), whereas the tertiary blastozones of af tl mutant leaves expressed UNI at P5 (Figure 5F) and would remain indeterminate to initiate quaternary primordia (Figure 3H). In this proposed role as a repressor of UNI expression, TL would be functionally redundant with AF, which would explain why UNI expression was not observed to extend laterally or distally in a tl mutant leaf compared with wild-type expression. Perhaps TL suppression of UNI transcription is so weak in a wild-type background, and AF suppression of UNI is sufficiently more important, that differences in UNI expression between wild-type and tl plants are not detectable by in situ hybridization.
af tl Leaflets Have a Novel Phenotype
The af tl leaf is more ramified at its extremities than the af leaf, and this ramification appears to be more than a simple additive phenotype. For example, there are between 20 and 30 tendrils on the af leaf shown in Figure 1D. The replacement of tendrils by leaflets on an equivalent af tl leaf would be expected to result in ∼30 leaflets if the phenotype were additive, but as Figure 1E shows, >60 leaflets can be counted. This novel, supercompound phenotype suggests that AF and TL can act together in the suppression of blastozone activity.
We showed that UNI expression was correlated with blastozone activity in compound stipules and rachides. A contradictory result was the detection of UNI transcripts in the miniature leaflets of the developing af tl leaf. UNI expression was observed even though the leaflets had ceased organogenesis and had begun to differentiate, which demonstrates that UNI expression alone is insufficient to confer blastozone activity.
With respect to UNI expression, these miniature leaflets appeared to differ from the larger leaflets found on wild-type and tl leaves. Regulation of wax deposition in af tl leaflets has already been noted to be distinctive compared with that in the wild type and tl (Marx, 1987). The wachslos (wlo) mutation normally suppresses wax deposition on the adaxial surfaces of leaflets. In af genetic backgrounds, such as af tl wlo, the miniature leaflets have waxy adaxial surfaces, which suggests that the af mutation counteracts the effect of the wlo mutation (Marx, 1987). An alternative interpretation is that laminae in an af mutant background may not be equivalent to wild-type leaflets and therefore may not be subject to the same regulation (Murfet and Reid, 1993). In our model of leaf pattern determination in pea, we would need to invoke a UNI transcript-independent mechanism in af tl leaflets to explain the determination and differentiation of these laminae despite the maintenance of UNI expression. Further refinement of the model and a deeper understanding of these complex genetic interactions await molecular characterization of the AF and TL genes.
METHODS
Plant Material
All plant lines were obtained from the John Innes germ plasm collection. The lines JI 1195 (af/af), JI 1197 (tl/tl), JI 1199 (af/af, tl/tl), and JI 1201 (st/st, af/af, tl/tl) are part of a near-isogenic series with JI 1194 as the corresponding wild-type line (Marx, 1974, 1987). JI 2165 carries the coch mutation and was generated by ethyl methanesulfonate mutagenesis of the Weitor (JI 379) variety (Blixt, 1967). JI 2171 is the type line for the uni mutation, and XM 7175 carries a deletion allele of uni in an af tl genetic background (Hofer et al., 1997). Plants were potted individually in John Innes No. 1 potting mix plus 30% grit and grown in greenhouses under a 16-hr photoperiod supplemented with 360-W sodium lamps.
In Situ Hybridization
Sense and antisense digoxigenin-labeled UNI, HH3, and PSKN1 probes were prepared from full-length cDNA clones transcribed by using T3 or T7 RNA polymerase. Probes were hybridized to 8-μm longitudinal or transverse sections cut from wax-embedded samples, as previously described (Coen et al., 1990). Sections were counterstained with 0.1% (w/v) calcofluor white fluorescent brightener and viewed by light microscopy with epifluorescent illumination.
Scanning Electron Microscopy
Samples were dissected to reveal the shoot apical meristem (SAM), frozen in liquid nitrogen, sublimated under vacuum at −95°C for 2 min, and sputter-coated in platinum at 10 mA for 2 min. Samples were viewed under vacuum in a 3-kV electron beam by using a field emission gun (FEG) scanning electron microscope (model XL 30; Philips Electronics N.V., Eindhoven, The Netherlands).
Acknowledgments
We thank Mike Ambrose, the curator of the John Innes Pisum germ plasm collection, Richard Gould and Miriam Balcam for horticultural services, Andrew Davis for photography, and Kim Findlay for advice on electron microscopy. We thank members of the laboratory for stimulating discussions and Tony Michael (Institute of Food Research, Norwich, UK) for the gift of the HH3 probe. C.W.G. was supported by a John Innes Foundation studentship. J.M.I.H was supported by Grant No. AR0125 from the Ministry of Agriculture, Fisheries and Food.
References
- Angiosperm Phylogeny Group. (1998). An ordinal classification for the families of flowering plants. Ann. Mo. Bot. Gard. 85, 531–553. [Google Scholar]
- Anthony, R.G., James, P.E., and Jordan, B.R. (1993). Cloning and sequence analysis of a flo/lfy homologue isolated from cauliflower (Brassica oleracea L. var. botrytis). Plant Mol. Biol. 22, 1163–1166. [DOI] [PubMed] [Google Scholar]
- Blázquez, M.A., and Weigel, D. (2000). Integration of floral inductive signals in Arabidopsis. Nature 404, 889–892. [DOI] [PubMed] [Google Scholar]
- Blázquez, M.A., Soowal, L.N., Lee, I., and Weigel, D. (1997). LEAFY expression and flower initiation in Arabidopsis. Development 124, 3835–3844. [DOI] [PubMed] [Google Scholar]
- Blixt, S. (1967). Linkage studies in Pisum VII. The manifestation of the genes cri, and coch, and the double-recessive in Pisum. Agric. Hort. Genet. 25, 131–144. [Google Scholar]
- Coen, E.S., Romero, J.M., Doyle, S., Elliot, R., Murphy, G., and Carpenter, R. (1990). floricaula: A homeotic gene required for flower development in Antirrhinum majus. Cell 63, 1311–1322. [DOI] [PubMed] [Google Scholar]
- Colombo, L., Marziani, G., Masiero, S., Wittich, P.E., Schmidt, R.J., Sari Gorla, M., and Enrico Pé, M. (1998). BRANCHED SILKLESS mediates the transition from spikelet to floral meristem during Zea mays ear development. Plant J. 16, 355–363. [Google Scholar]
- Dengler, N.G. (1984). Comparison of leaf development in normal (+/+), entire (e/e), and lanceolate (La/+) plants of tomato, Lycopersicon esculentum ‘Ailsa Craig’. Bot. Gaz. 145, 66–77. [Google Scholar]
- de Vilmorin, P., and Bateson, W. (1911). A case of gametic coupling in Pisum. Proc. R. Soc. Lond. Ser. B 84, 9–11. [Google Scholar]
- Eriksson, G. (1929). Erbkomplexe des Rotklees und der Erbsen. Z. Pflanzen. 84, 735–744. [Google Scholar]
- Freeling, M. (1992). A conceptual framework for maize leaf development. Dev. Biol. 153, 44–58. [DOI] [PubMed] [Google Scholar]
- Frohlich, M.W., and Meyerowitz, E.M. (1997). The search for flower homeotic gene homologs in basal angiosperms and gnetales: A potential new source of data on the evolutionary origin of flowers. Int. J. Plant Sci. 158, S131–S142. [Google Scholar]
- Goldenberg, J.B. (1965). afila, a new mutation in pea (Pisum sativum L.). Biol. Genet. 1, 27–31. [Google Scholar]
- Gould, K.S., Cutter, E.G., and Young, J.P.W. (1986). Morphogenesis of the compound leaf in three genotypes of the pea, Pisum sativum. Can. J. Bot. 64, 1268–1276. [Google Scholar]
- Gould, K.S., Cutter, E.G., and Young, J.P.W. (1994). The determination of pea leaves, leaflets, and tendrils. Am. J. Bot. 81, 352–360. [Google Scholar]
- Gourlay, C.W. (1999). An Analysis of Genes Involved in Pea Compound Leaf Development. Ph.D. Dissertation (Norwich, UK: University of East Anglia).
- Hagemann, W., and Gleissberg, S. (1996). Organogenetic capacity of leaves: The significance of marginal blastozones in angiosperms. Plant Syst. Evol. 199, 121–152. [Google Scholar]
- Hareven, D., Gutfinger, T., Parnis, A., Eshed, Y., and Lifschitz, E. (1996). The making of a compound leaf: Genetic manipulation of leaf architecture in tomato. Cell 84, 735–744. [DOI] [PubMed] [Google Scholar]
- Hofer, J.M.I., and Ellis, T.H.N. (1996). The effect of Uni on leaf shape. Pisum Genet. 28, 21–23. [Google Scholar]
- Hofer, J.M.I., and Ellis, T.H.N. (1998). The genetic control of patterning in pea leaves. Trends Plant Sci. 3, 439–444. [Google Scholar]
- Hofer, J., Turner, L., Hellens, R., Ambrose, M., Matthews, P., Michael, A., and Ellis, N. (1997). UNIFOLIATA regulates leaf and flower morphogenesis in pea. Curr. Biol. 7, 581–587. [DOI] [PubMed] [Google Scholar]
- Hofer, J., Gourlay, C., Michael, A., and Ellis, T.H.N. (2000). Expression of a class 1 knotted1-like homeobox gene is downregulated in pea compound leaf primordia. Plant Mol. Biol. (in press). [DOI] [PubMed]
- Jackson, D., Veit, B., and Hake, S. (1994). Expression of maize KNOTTED1-related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120, 405–413. [Google Scholar]
- Janssen, B.J., Lund, L., and Sinha, N. (1998). Overexpression of a homeobox gene, LeT6, reveals indeterminate features in the tomato compound leaf. Plant Physiol. 117, 771–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, A.J., Bonnlander, M.B., and Meeks-Wagner, D.R. (1995). NFL, the tobacco homolog of FLORICAULA and LEAFY, is transcriptionally expressed in both vegetative and floral meristems. Plant Cell 7, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala, V. (1953). Felderbse beiwelcher die ganze Blattspreite in Ranken umgewandelt ist. Arch. Soc. Zool. Bot. Fenn. 8, 44–55. [Google Scholar]
- Kyozuka, J., Konishi, S., Nemoto, K., Izawa, T., and Shimamoto, K. (1998). Down-regulation of RFL, the FLO/LFY homolog of rice, accompanied with panicle branch initiation. Proc. Natl. Acad. Sci. USA 95, 1979–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, B., Villani, P.J., Watson, J.C., DeMason, D.A., and Cooke, T.J. (1996). The control of pinna morphology in wildtype and mutant leaves of the garden pea (Pisum sativum L.). Int. J. Plant Sci. 157, 659–673. [Google Scholar]
- Lucas, W.J., Bouché-Pillon, S., Jackson, D.P., Nguyen, L., Baker, L., Ding, B., and Hake, S. (1995). Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science 270, 1980–1983. [DOI] [PubMed] [Google Scholar]
- Makasheva, R.K. (1983). Morphology and classification of cultivated peas. In The Pea, V.S. Kothekar, ed, B.K. Sharma, trans (New Delhi: Oxonian Press), pp. 20–77.
- Marx, G.A. (1974). Stocks available. Pisum Genet. 6, 60. [Google Scholar]
- Marx, G.A. (1986). Tendrilled acacia (tac): An allele at the Uni locus. Pisum Genet. 18, 49–52. [Google Scholar]
- Marx, G.A. (1987). A suite of mutants that modify pattern formation in pea leaves. Plant Mol. Biol. Rep. 5, 311–335. [Google Scholar]
- Meicenheimer, R.D., Muehlbauer, F.J., Hindman, J.L., and Gritton, E.T. (1983). Meristem characteristics of genetically modified pea (Pisum sativum) leaf primordia. Can. J. Bot. 61, 3430–3437. [Google Scholar]
- Molinero-Rosales, N., Jamilena, M., Zurita, S., Gómez, P., Capel, J., and Lozano, R. (1999). FALSIFLORA, the tomato orthologue of FLORICAULA and LEAFY, controls flowering time and floral meristem identity. Plant J. 20, 685–693. [DOI] [PubMed] [Google Scholar]
- Mouradov, A., Glassick, T., Hamdorf, B., Murphy, L., Fowler, B., Marla, S., and Teasdale, R.D. (1998). NEEDLY, a Pinus radiata ortholog of FLORICAULA/LEAFY genes, expressed in both reproductive and vegetative meristems. Proc. Natl. Acad. Sci. USA 95, 6537–6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlbauer, G.J., Fowler, J.E., and Freeling, M. (1997). Sectors expressing the homeobox gene liguleless3 implicate a time-dependent mechanism for cell fate acquisition along the proximal–distal axis of the maize leaf. Development 124, 5097–5106. [DOI] [PubMed] [Google Scholar]
- Murfet, I.C., and Reid, J.B. (1993). Developmental mutants. In Peas: Genetics, Molecular Biology and Biotechnology, R. Casey and D.R. Davies, eds (Wallingford, UK: CAB International), pp. 165–216.
- Parnis, A., Cohen, O., Gutfinger, T., Hareven, D., Zamir, D., and Lifschitz, E. (1997). The dominant development mutants of tomato, mouse-ear and curl, are associated with distinct modes of abnormal transcriptional regulation of a knotted gene. Plant Cell 9, 2143–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellew, C., and Sverdrup, A. (1923). New observations on the genetics of peas. J. Genet. 13, 125–131. [Google Scholar]
- Pnueli, L., Carmel-Goren, L., Hareven, D., Gutfinger, T., Alvarez, J., Ganal, M., Zamir, D., and Lifschitz, E. (1998). The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 125, 1979–1989. [DOI] [PubMed] [Google Scholar]
- Pouteau, S., Nicholls, D., Tooke, F., Coen, E., and Battey, N. (1997). The induction and maintenance of flowering in Impatiens. Development 124, 3343–3351. [DOI] [PubMed] [Google Scholar]
- Robertson, A.J., Kapros, T., and Waterborg, J. (1997). A cell cycle–regulated Histone H3 gene of alfalfa with an atypical promoter structure. DNA Sequence 7, 209–216. [DOI] [PubMed] [Google Scholar]
- Rottman, W.H., Boes, T.K., and Strauss, S.H. (1993). Structure and expression of a LEAFY homologue from Populus. Cell Biochem. Suppl. 17B, 23. [Google Scholar]
- Sharma, B. (1981). Genetic pathway of foliage development in Pisum sativum. Pulse Crop Newsl. 1, 56–57. [Google Scholar]
- Smith, L.G., Greene, B., Veit, B., and Hake, S. (1992). A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development 116, 21–30. [DOI] [PubMed] [Google Scholar]
- Souer, E., van der Krol, A., Kloos, D., Spelt, C., Bliek, M., Mol, J., and Koes, R. (1998). Genetic control of branching pattern and floral identity during Petunia inflorescence development. Development 125, 733–742. [DOI] [PubMed] [Google Scholar]
- Steeves, T.A., and Sussex, I.M. (1989). Organogenesis in the shoot: Later stages of leaf development. In Patterns in Plant Development, 2nd ed (Cambridge, UK: Cambridge University Press), pp. 147–175.
- Villani, P.J., and DeMason, D.A. (1997). Roles of the af and tl genes in pea leaf morphogenesis: Characterisation of the double mutant (af/af,tl/tl). Am. J. Bot. 84, 1323–1336. [PubMed] [Google Scholar]
- Villani, P.J., and DeMason, D.A. (1999. a). The Af gene regulates timing and direction of major developmental events during leaf morphogenesis in garden pea (Pisum sativum). Ann. Bot. 83, 117–128. [Google Scholar]
- Villani, P.J., and DeMason, D.A. (1999. b). Roles of the Af and Tl genes in pea leaf morphogenesis: Leaf morphology and pinna anatomy of the heterozygotes. Can. J. Bot. 77, 611–622. [Google Scholar]
- Weigel, D., Alvarez, J., Smyth, D.R., Yanofsky, M.F., and Meyerowitz, E.M. (1992). LEAFY controls floral meristem identity in Arabidopsis. Cell 69, 843–859. [DOI] [PubMed] [Google Scholar]
- Wellensiek, S.J. (1959). Neutronic mutations in peas. Euphytica 8, 209–215. [Google Scholar]