Abstract
The tomato Mi gene confers resistance against root-knot nematodes and potato aphids. Chimeric constructs of the functional gene, Mi-1.2, with a homolog, Mi-1.1, were produced, and their phenotypes were examined in Agrobacterium rhizogenes–transformed roots. Exchange of the leucine-rich repeat (LRR) region of Mi-1.1 into Mi-1.2 resulted in the loss of ability to confer nematode resistance, as did substitution of a 6–amino acid sequence from the Mi-1.1 LRR into Mi-1.2. Introduction of the Mi-1.2 LRR-encoding region into Mi-1.1 resulted in a lethal phenotype, as did substitution of the fragment encoding the N-terminal 161 amino acids of Mi-1.1 into Mi-1.2. Transient expression of the latter two chimeric constructs in Nicotiana benthamiana leaves produced localized cell death. The cell death caused by the N-terminal exchange was suppressed by coinfiltration with a construct expressing the N-terminal 161 amino acids of Mi-1.2. The phenotypes of these and other constructs indicate that the LRR region of Mi-1.2 has a role in signaling localized cell death and that the N-terminal 161 amino acids have a role in regulating this death.
INTRODUCTION
Disease resistance in plants is often characterized by a gene-for-gene relationship that requires a specific plant resistance (R) gene and a corresponding pathogen avirulence (avr) gene (Flor, 1955). Recognition initiates a cascade of defense responses, often including a hypersensitive response (HR) consisting of localized cell death at the infection site. Recently, R genes that mediate resistance to viruses, bacteria, fungi, and nematodes have been cloned from several plant species (Baker et al., 1997; Hammond-Kosack and Jones, 1997). Most encode proteins that carry a structural motif with a repeating pattern of 20 to 30 amino acids called a leucine-rich repeat (LRR). LRR-containing R genes can be subdivided into two broad classes: those in which the predicted gene product contains an N-terminal, extracellular LRR and a membrane anchor; and those in which the R gene product is predicted to be cytoplasmic. Cytoplasmically located R gene products are characterized by the presence of a conserved region containing a nucleotide binding site (NBS) and a C-terminal LRR region.
Root-knot nematodes (genus Meloidogyne) are parasitic roundworms that damage >1000 different food and fiber crops around the world (Williamson and Hussey, 1996). In a compatible interaction, second-stage nematode juveniles (J2s) penetrate the host, generally near root tips, then migrate intercellularly to the region of cell differentiation. In response to signals from the nematode, plant cells adjacent to the head of the nematode enlarge to form large, multinucleate, metabolically active cells that serve as the source of nutrients for the developing, endoparasitic form of the nematode. Concurrent hyperplasia and hypertrophy in the surrounding host tissues lead to the formation of the galls or root knots characteristic of Meloidogyne spp infection.
Tomato is an excellent host for root-knot nematodes. However, many modern tomato varieties are resistant to these nematodes because of the presence of the gene Mi (Roberts et al., 1986). This single gene confers resistance against the three major root-knot nematode species that infect tomato: Meloidogyne incognita, M. javanica, and M. arenaria. The resistance conferred by Mi is characterized by the development of a localized region of dead plant cells around the anterior of the invading nematode within a few days of infection (Dropkin, 1969). Microscopic observation indicates that nematode J2s do not elicit an extensive HR while migrating through the root tissue but do so while attempting to establish a feeding site (Paulson and Webster, 1972). This timing suggests that cell penetration by the nematode stylet may be required to elicit the response.
The Mi gene was isolated by a positional cloning approach (Milligan et al., 1998). Two candidate genes, Mi-1.1 and Mi-1.2, were identified in the 65-kb region to which Mi had been localized. The predicted proteins encoded by these genes are 91% identical in amino acid sequence and belong to the family of plant resistance genes characterized by the presence of a NBS and a LRR region, as shown in Figure 1 (Hammond-Kosack and Jones, 1997). Complementation studies revealed that Mi-1.2, but not Mi-1.1, was sufficient to confer resistance to M. javanica and thus corresponded to Mi. Transgenic plants with Mi-1.2 were also found to be resistant to the potato aphid, Macrosiphum euphorbiae, indicating that this gene confers resistance to potato aphids as well as to root-knot nematodes (Rossi et al., 1998).
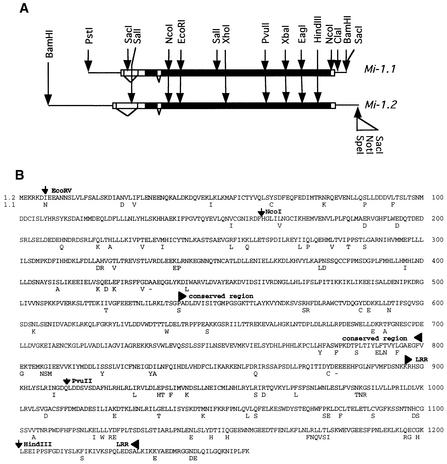
Figure 1.
Comparison of Mi-1.1 and Mi-1.2.
(A) The horizontal lines represent the 7.1- and 8.9-kb genomic fragments corresponding to Mi-1.1 and Mi-1.2, respectively, used to produce the chimeric constructs. The wide bars represent the transcribed region, with the black fill indicating the protein-encoding region and the white fill representing untranslated regions. Introns are indicated by angled lines. Restriction sites used for constructing exchanges are indicated by arrows.
(B) The deduced amino acid sequence of Mi-1.2 and amino acids of Mi-1.1 that differ between the two proteins. The locations of restriction enzyme sites used to construct the chimeric genes and the position of the conserved region containing the NBS and the LRR region are indicated.
Mi encodes a large 1257–amino acid protein, Mi, that most resembles Prf, the protein encoded by the tomato gene Prf that is required for Pto-mediated resistance to Pseudomonas syringae (Salmeron et al., 1996). The Mi protein lacks a cleavable signal sequence and thus is thought to be localized to the cytoplasm. This suggests that nematode recognition by the host, if it is mediated by Mi, occurs within the cell. A central region of 260 amino acids containing the NBS is highly conserved in this family of resistance genes. The C-terminal region of Mi is made up of 14 highly imperfect copies of a LRR motif, containing on average 24 amino acids per repeat. Evidence is accumulating that in other resistance genes, the LRR region carries determinants for specificity of pathogen recognition (Ellis et al., 1999). Compared with other NBS-LRR proteins, Mi and Prf encode long N-terminal extensions. The N-terminal region of Mi does not strongly resemble other sequences found in protein databases.
Because Mi-1.1 does not confer nematode resistance, it provides an excellent substrate for exchanging DNA with Mi-1.2 to localize the sequences required for specific functions. However, the production of transgenic plants by Agrobacterium tumefaciens–mediated transformation and the assay of these plants for nematode resistance are time-consuming processes. Approximately 1 year is required before the phenotype of the introduced sequences can be determined. To circumvent this time lag, we have used an Agrobacterium rhizogenes–based transformation assay (Tepfer, 1990). This bacterium transfers to plant cells not only the T-DNA of the binary vector but also a second T-DNA region that results in transformed roots with a highly branched or “hairy” phenotype (Simpson et al., 1986). We present evidence here that the response of the A. rhizogenes–transformed roots to nematodes mirrors that of standard roots with regard to Mi phenotype. This system has allowed us to examine a series of Mi exchange constructs, several of which appear to produce a lethal phenotype. We also used A. rhizogenes containing these constructs to infiltrate Nicotiana benthamiana leaves, in which transient expression confirmed the cell death phenotype. Comparisons of the phenotypes of the chimeric constructs indicate that the LRR region of Mi-1.2 plays a role in the transmission of a signal for cell death that cannot be replaced by the corresponding region from Mi-1.1 and that the N-terminal 161 amino acids of Mi-1.2 are important for regulation of the signal transduction.
RESULTS
Mi-1.2 Confers Nematode Resistance in A. rhizogenes–Transformed Roots
Because the phenotype of roots transformed by A. rhizogenes is altered from that of standard roots, we examined whether A. rhizogenes–transformed roots of resistant and susceptible tomato would respond as expected to root-knot nematode infection. Cotyledons from the nearly isogenic tomato lines Motelle (Mi/Mi) and Moneymaker (mi/mi) were infected with A. rhizogenes strain ATCC 15834. After 7 to 10 days, multiple roots developed from the surface of the cotyledons. One root tip from each cotyledon (1 cm long) was then excised and transferred to plates. Two weeks after infection with M. javanica eggs, transformed hairy roots from Moneymaker cotyledons showed swollen root tips, as was expected in a susceptible interaction, whereas roots from Motelle cotyledons did not. Enlarged nematodes typical of the susceptible response were seen inside the swollen Moneymaker root tips. In contrast, undeveloped nematodes associated with localized cell death in the host were found in transformed Motelle roots, indicating an incompatible interaction.
Moneymaker tomato cotyledons were infected with A. rhizogenes strain ATCC 15834 containing the binary vector pTFS-40 harboring either Mi-1.1 or Mi-1.2. pTFS-40 carries an intron-containing gene encoding β-glucuronidase, which permits testing for T-DNA transfer by staining the transgenic roots for β-glucuronidase activity. Approximately 75% of the transformants selected on kanamycin medium stained blue in the presence of X-Gluc. Transformants containing Mi-1.1 or Mi-1.2 were tested for resistance against M. javanica, M. incognita, and M. arenaria. For Mi-1.2, 12 of 18 independent transformants were resistant to each of the three nematode species. None of the 17 independent transformants with Mi-1.1 were resistant, which is consistent with the results from transgenic tomatoes. With A. rhizogenes transformation, the response of roots to nematodes could be determined by 6 weeks after transformation.
Chimeric Constructs between Mi-1.1 and Mi-1.2 Can Produce a Lethal Phenotype
The predicted proteins encoded by Mi-1.1 and Mi-1.2 were 91% identical (Figure 1B). We localized the region or regions in Mi-1.2 that are important for determining resistance by making a series of chimeric constructs between Mi-1.1 and Mi-1.2. Three restriction enzyme cleavage sites were used to produce the constructs Mi-DS1 to Mi-DS5 (shown in Figure 2). A NcoI site common to both Mi-1.1 and Mi-1.2 is located 161 amino acids downstream of the translational start site, and a PvuII site is present at amino acid 910 for Mi-1.1 and amino acid 912 for Mi-1.2 (Figure 1). A HindIII site was generated at amino acid 1198 in Mi-1.1 to correspond to a HindIII site already present at the corresponding position in Mi-1.2. A construct (Mi-DS1) that included 2.8 kb of Mi-1.2 containing the 5′ flanking sequence plus the first 161 amino acids of the coding region fused at the NcoI site with the remainder of Mi-1.1 was not able to confer nematode resistance in A. rhizogenes–transformed roots (Figure 2). Substituting the PvuII-HindIII fragment of Mi-1.1 into Mi-1.2 (Mi-DS2) also resulted in loss of resistance. In this construct, the entire LRR region was exchanged except for two amino acid differences between the putative LRR regions of Mi-1.1 and Mi-1.2 (Figure 1). These results suggested that the sequences of the Mi-1.2 LRR that differ from those in Mi-1.1 are essential for nematode resistance.
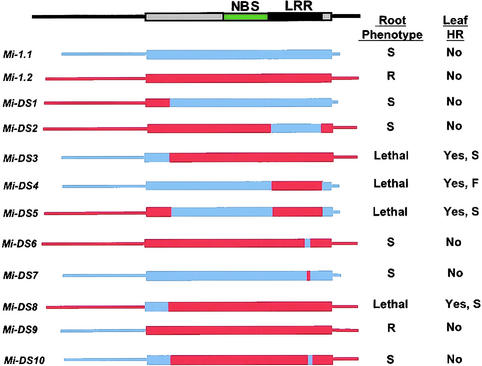
Figure 2.
Mi-1 Gene Chimeric Constructs and Their Phenotypes.
The diagram at the top shows the locations of the conserved region (green) with the NBS and the LRR region (black). The position of the protein-coding region is shown as a wide bar. Mi-1.1 is shown in blue, Mi-1.2 in red. The names of the sequence exchange constructs are shown at left. At right, the first column shows the phenotype of A. rhizogenes–transformed root cultures after introduction of each construct. Response to nematode infection is indicated by S for a susceptible response, R for resistant response; Lethal indicates that no viable, transformed roots were obtained. The second column shows the phenotypes after A. rhizogenes infiltration of N. benthamiana leaves. No indicates no visible cell death (browning); Yes indicates tissue collapse and cell death. F indicates a fast response, in which the localized cell death was complete by 3 days after infiltration; S indicates a slower response that was apparent only after 4 to 5 days.
Attempts to transform susceptible tomato with a construct (Mi-DS3) containing the 2.1-kb region of Mi-1.1, which included the 5′ flanking sequence plus the first 161 amino acids fused with the remainder of Mi-1.2, recovered no viable transformed roots. Constructs in which the Mi-1.2 LRR was substituted into Mi-1.1 to produce Mi-DS4 and in which the Mi-1.1 5′ region of Mi-DS4 was replaced with the Mi-1.2 5′ region to produce Mi-DS5 also resulted in no recovery of viable transformed roots. These root transformation experiments were repeated with two other A. rhizogenes strains, A4RS and LBA 9402. Again, no viable roots were obtained with Mi-DS3, Mi-DS4, or Mi-DS5. Attempts to recover transformed plants by using A. tumefaciens strain LBA 4404 harboring Mi-DS3, Mi-DS4, or Mi-DS5 were also unsuccessful (M. Rossi and V.M. Williamson, unpublished data). These results suggested that the constructs Mi-DS3, Mi-DS4, and Mi-DS5 have a lethal phenotype in tomato. Alternatively, because stable integration of T-DNAs from the Ti-binary vector is required for kanamycin resistance, the lack of viable transformed roots possibly reflected failure of the NPT-II gene to integrate or to be expressed in some of the transformants.
To test the apparent lethal phenotype of some of the chimeric Mi-1.1/1.2 constructs in another way, we infiltrated N. benthamiana leaves with A. rhizogenes containing constructs Mi-DS1 through Mi-DS5. As shown in Figure 3A, Mi-DS3, Mi-DS4, and Mi-DS5 produced cell death on leaves, whereas none of the constructs that produced viable transgenic roots displayed a death response in the infiltrated leaves. A reproducible difference in the time to appearance of the dead tissue was seen among constructs. Localized cell death and tissue collapse resulting from Mi-DS4 were complete by 3 days after infiltration, whereas those due to Mi-DS3 and Mi-DS5 were apparent only after 4 to 5 days. Reverse transcription–polymerase chain reaction (PCR) using Mi-1.1– and Mi-1.2–specific primers confirmed expression of the transcripts corresponding to the introduced constructs Mi-DS1–DS5 in N. benthamiana leaves 24 hr after Agrobacterium infiltration.
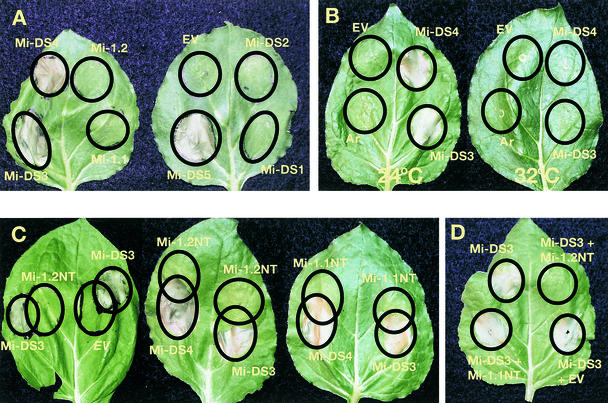
Figure 3.
Phenotype of Transiently Expressed Constructs in N. benthamiana Leaves.
(A) Leaves infiltrated with A. rhizogenes containing Mi-1 constructs after 4 days at room temperature. The circles indicate the boundaries of the infiltrated areas. Note areas of death in circles infiltrated with A. rhizogenes carrying Mi-DS3, Mi-DS4, and Mi-DS5. The empty vector (EV) is pTFS-40.
(B) Leaves were incubated at room temperature (24°C) or 32°C for 6 days after inoculation. The empty vector (EV) is pTFS40. Ar, infiltration with untransformed A. rhizogenes strain A4RS.
(C) Leaves were infiltrated to produce overlapping circles. Circles marked Mi-1.2NT and Mi-1.1NT were infiltrated with constructs expressing the N-terminal 161 amino acids of Mi-1.2 or Mi-1.1 under transcriptional control of the CaMV 35S promoter. The empty vector (EV) is 35SP-pCAMBIA2201.
(D) Leaves were infiltrated with a mixture of equal numbers of cells carrying the indicated constructs.
Nematode resistance mediated by the Mi-1 gene is effective only at temperatures below 32°C (Dropkin, 1969). The loss of resistance at the higher temperature correlates with loss of the characteristic nematode-associated cell necrosis. To determine whether the localized cell death caused by Mi-DS3 and Mi-DS4 infiltration was temperature sensitive, we incubated duplicate plants with infiltrated leaves at 24°C and 32°C. After 6 days, the infiltrated areas of the plants grown at 24°C were dead, but no cell death was evident in the infiltrated area of the plants grown at 32°C (Figure 3B). However, when N. benthamiana plants were incubated for 3 days at 32°C and then were shifted back to 24°C, Mi-DS4, but not Mi-DS3, produced localized cell death after 6 days (data not shown). Control plants maintained at 32°C for the same period of time did not show cell death for any of the infiltrated regions.
Six–Amino Acid Region Is Required by Mi-1.2 LRR for Resistance and for Death
Results of nematode resistance assays with roots transformed with Mi-DS1 and Mi-DS2 indicated that the amino acids in Mi-1.2 LRR that differ from those in Mi-1.1 LRR are essential for nematode resistance. Alignment of the amino acid sequences of the predicted gene products for Mi-1.1 and Mi-1.2 showed that the greatest difference occurred at a span of six consecutive amino acids (Mi-1.2 amino acids 1172 to 1177; Figure 1). Substitution from Mi-1.1 into Mi-1.2 of the DNA encoding these six amino acids to produce Mi-DS6 resulted in the loss of ability to confer nematode resistance in transformed roots. The same six amino acids from Mi-1.1 were exchanged into Mi-DS3 to create Mi-DS10. Mi-DS10 produced no nematode resistance and no localized cell death on N. benthamiana leaves. Substitution of the DNA encoding the corresponding six amino acids from Mi-1.2 into Mi-1.1 to create Mi-DS7 did not result in recovery of the nematode resistance phenotype in transformed roots and did not produce localized cell death in N. benthamiana leaves. These results indicate that the six–amino acid sequence in the Mi-1.2 LRR (LRLLTL) is essential, but not sufficient, for both the nematode resistance in the A. rhizogenes–transformed roots and the cell death response in the N. benthamiana transient expression assay.
Exchange of 5′ Proximal Regions
The lethal construct Mi-DS3 carries both the N-terminal 161 amino acids and the flanking 5′ region of Mi-1.1. To determine which of these was responsible for the lethal phenotype, an EcoRV site was introduced after amino acid 6 in both Mi-1.1 and Mi-1.2. This alteration changed amino acid 7 of Mi-1.1 from asparagine to isoleucine but did not change the encoded protein of Mi-1.2. The next 155 amino acids in the sequence from Mi-1.1 were swapped into Mi-1.2 to create Mi-DS8. Conversely, the corresponding 155 amino acids from Mi-1.2 were exchanged into Mi-DS3 to produce Mi-DS9. Attempts to produce transformed roots with Mi-DS8 did not yield viable roots, but this construct did produce cell death after infiltration into N. benthamiana leaves. The timing of the appearance of visible tissue collapse was the same as that for Mi-DS3. In contrast, Mi-DS9 produced viable hairy roots in tomato that were nematode resistant and produced no cell death when infiltrated into N. benthamiana leaves.
Mi-1.2 N Terminus Represses Localized Cell Death Mediated by Mi-DS-3 in Trans
Because Mi-DS3 and Mi-DS8 did not produce viable hairy roots and did induce a localized cell death on N. benthamiana leaves after transient expression, we hypothesized that the Mi-1.2 N-terminal 161 amino acids play a role in regulating cell death. To investigate this possibility, we developed a construct expressing the Mi-1.2 N-terminal 161–amino acid sequence under control of the cauliflower mosaic virus (CaMV) 35S promoter. This construct did not produce localized cell death when infiltrated into N. benthamiana leaves. To compare the plant's response to the transient expression of the cell death–inducing construct Mi-DS3 with its response to the coexpression of Mi-DS3 with the Mi-1.2 N-terminal 161–amino acid construct, we infiltrated A. rhizogenes strains with each of these constructs to produce overlapping circles. The area of overlap did not show the cell collapse and browning that Mi-DS3 alone produced (Figure 3C). The same blocking of cell death was seen in replicate experiments on six different N. benthamiana leaves. In control experiments, when Mi-DS3 was infiltrated in overlapping circles with the empty binary vector or with a construct expressing the Mi-1.1 N-terminal 161 amino acids under control of the 35S promoter, the overlapping areas showed cell death (Figure 3C). In contrast, neither the Mi-1.1 nor Mi-1.2 N-terminal 161–amino acid fragment blocked the cell death induced by Mi-DS4 in an overlapping circle experiment (Figure 3C). Coinfiltration of equal-volume mixtures of A. rhizogenes cells carrying Mi-DS3 and those carrying the Mi-1.2 N-terminal 161–amino acid—expressing construct did not show necrosis, whereas cotransformation with Mi-DS3 and the empty binary vector or the Mi-1.1 NT construct resulted in cell death (Figure 3D). In contrast, coinfiltration of neither the Mi-1.1 nor the Mi-1.2 N terminus with Mi-DS4 repressed cell death. The difference in phenotypes produced by Mi-DS3 and Mi-DS4 indicates that the amino acids of Mi-1.2 between 162 and 911 that differ from those in Mi-1.1 are also important in cell death regulation.
DISCUSSION
Sequence Exchanges Suggest Mi Has a Role in Signaling Cell Death
The ability to observe useful phenotypes in A. rhizogenes–transformed roots and in N. benthamiana leaves transiently expressing the same constructs has allowed us to analyze several exchanges between the Mi gene, Mi-1.2, and its closely related homolog, Mi-1.1. The LRR region was targeted for the initial exchange experiments because LRRs have been shown to mediate protein–protein interactions in other systems (Kobe and Deisenhofer, 1994; Jones and Jones, 1996). Substitution of the Mi-1.1 LRR region into Mi-1.2 resulted in loss of resistance. The LRR region that was exchanged contains 40 amino acids that differ between Mi-1.1 and Mi-1.2. The longest contiguous stretch of differences is a six–amino acid stretch from positions 1172 to 1178 of Mi-1.2. Substituting the corresponding six–amino acid sequence from Mi-1.1 results in loss of resistance. Probably other changes would also result in loss of function, given that single amino acid changes in the LRR region of several NBS-LRR resistance genes have been reported to result in loss of resistance (Bent et al., 1994; Mindrinos et al., 1994; Grant et al., 1995; Warren et al., 1998).
All sequence exchanges in which the Mi-1.2 LRR was coupled with other parts of the Mi-1.1 coding region produced a lethal phenotype when expressed in transformed tomato roots or transiently expressed in infiltrated N. benthamiana leaves. Substitution of the previously mentioned six–amino acid sequence of Mi-1.1 into the Mi-1.2 LRR of construct Mi-DS3 to produce Mi-DS10 resulted in loss of the constitutively lethal phenotype. These experiments indicate that the Mi-1.2 LRR region is necessary for signaling cell death and that sequences that differ between the Mi-1.1 and Mi-1.2 LRR regions are responsible. However, exchange of the six amino acids of the Mi-1.2 LRR into Mi-1.1 is not sufficient to restore signaling of cell death.
A role in signal transduction has been previously postulated for the LRR region of the protein encoded by the Arabidopsis R gene RPS-5 (Warren et al., 1998). In this case, a single amino acid change in the LRR region of RPS-5 not only resulted in loss of resistance mediated by this gene but also compromised the resistance mediated by several other genes in Arabidopsis, suggesting that this mutation affected interaction of the LRR region with a signal transduction element that is common to several R genes. For the flax rust resistance gene L, experiments have implicated the LRR region as carrying determinants for specificity of pathogen recognition (Ellis et al., 1999). Alleles that differ only in the LRR region have different pathogen-specificity phenotypes, and some in vitro exchanges have produced specificities that correlate with the LRR region. However, other L alleles with different pathogen specificities differ only in the N-terminal region, indicating that the N terminus can also play a role in determining specificity. Our experiments suggest that the LRR region has a role in signaling localized cell death. Possibly, the LRR region has roles in both signaling and pathogen recognition.
Mi-1.1 and Mi-1.2 contain two introns, one upstream of the ATG and a second 42 nucleotides downstream from the start codon. Exchange of the 5′ noncoding region, including the first intron, did not change the resistance phenotype (cf. Mi-DS3 with Mi-DS8 and Mi-1.2 with Mi-DS9 in Figure 2). The second intron, 75 nucleotides long, is 97% identical between Mi-1.1 and Mi-1.2. Because both copies of the gene are transcribed, most likely differences in the encoded protein sequence rather than in untranslated regions of the gene are responsible for the phenotypic differences.
In contrast to the relatively short N-terminal region of most other cloned R genes of the NBS-LRR type, Mi encodes an N-terminal extension, which encodes a novel protein sequence. An exception is the tomato gene Prf, the product of which is required for resistance mediated by the kinase-encoding R gene Pto. Prf also encodes an N-terminal extension having some similarity in sequence to Mi-1.2 (44% similarity for amino acids 317 to 471 of Mi-1.2). Exchange of the N-terminal 161 amino acids of Mi-1.1 into Mi-1.2 results in a lethal phenotype. This result, together with additional exchanges, suggests that the corresponding region of Mi-1.2 functions as or interacts with a repressor of the cell death signaled by the remainder of the Mi-1.2 protein. The regulatory function cannot be performed by the corresponding region of Mi-1.1, even though these regions differ by only eight amino acids (Figure 1). Coexpression of the N-terminal 161 amino acids of Mi-1.2 in a transient assay repressed the death mediated by Mi-DS3, which supports the possibility that the N terminus has a role in regulating the signal transduction. Failure to repress cell death mediated by Mi-DS4 can be explained if sequences C-terminal to the first 161 amino acids (between amino acids 162 and 911) that are present in Mi-1.2 but not Mi-1.1 are also required for repressing the death. The more rapid death of leaf cells in response to transient expression of Mi-DS4 than that in response to Mi-DS3 and Mi-DS5 supports a role for this second region. Interestingly, secondary structure computer programs predict two coiled-coil regions in the N terminus of Mi, one located at amino acid positions 24 to 58 and the other at positions 315 to 360 (Cohen and Perry, 1986; K. Fort and V.M. Williamson, unpublished data). It is tempting to speculate that one or both of these coiled coils are involved in protein–protein interactions that confer the negative regulation. The assays we have developed here will be useful for investigating the functions of the regions between amino acids 162 and 911 in more detail.
Model for Resistance Signaling
Our findings suggest a model in which the Mi LRR region has a role in transduction of the signal(s) leading to cell death. In the model shown in Figure 4, the N terminus of Mi-1.2 represses the ability of the LRR region to transmit the signal. When the nematode is present, a product of the nematode (an elicitor or Avr gene product) interacts directly or indirectly with the N terminus or the LRR region of Mi-1.2 to release the repression of cell death. Alternatively, a host product induced or modified by the presence of the nematode could interact with Mi-1.2. For example, Prf-dependent HR is produced by gain-of-function mutations in the gene encoding the kinase Pto (Rathjen et al., 1999). In either scenario, activation of localized host cell death prevents the development of a feeding cell and results in failure of the nematode to acquire sufficient nutrients for development. This model is consistent with the phenotypes of all our exchange constructs if the N-terminal 161 amino acids of Mi-1.1 cannot repress the signal transduction mediated by the Mi-1.2 LRR and if the Mi-1.1 LRR cannot trigger the signal transduction. The differences in time taken to produce death among constructs suggest that sequences between amino acids 162 and 911 (Figure 4, NT2 and NBS) are also involved in the repression.
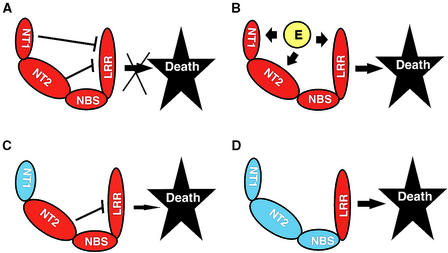
Figure 4.
Model of Mi as a Regulatory Protein.
In this model, the LRR region is at least partially responsible for signaling cell death. Mi is represented as four “sausage-link” shapes; Mi-1.1 components are blue, Mi-1.2 components red. Links represent NT1 (N-terminal amino acids 1 to 161), NT2 (amino acids 162 to 540), NBS, and LRR regions.
(A) NT1, NT2, and possibly the NBS region repress the cell death signaled by the LRR region. Repression is indicated by T bars.
(B) An elicitor (E) from the nematode or from the plant (in response to the nematode) interacts, directly or indirectly, with Mi-1.2 and prevents the repression of the signaling. Thus, in the presence of the nematode, localized cell death is triggered.
(C) The NT1 region of Mi-1.1 cannot repress the signaling of death. However, cell death is slow because of some repression from NT2 or NBS.
(D) The Mi-1.1 NT1, NT2, and NBS regions do not repress the signaling of cell death by the Mi-1.2 LRR.
In our model, both or either of the LRR and N-terminal regions could mediate the specificity of pathogen recognition. The relative importance of each region may vary among R genes, as has been suggested in results from Ellis et al. (1999) for flax rust resistance genes. Some NBS-LRR genes, including the L and N genes, are characterized by an N-terminal Toll/Interleukin-1 Receptor domain, whereas others do not carry this domain but have a sequence resembling a leucine zipper preceding the NBS region. There is evidence that the downstream signaling differs between these two groups (Glazebrook, 1999).
Clues from Animal Apoptosis Regulators
There are intriguing similarities between plant NBS-LRR resistance proteins and a group of regulatory proteins in animal cell death pathways, including CED-4 in Caenorhabditis elegans and Apaf-1 in humans (Ellis and Jones, 1998; van der Biezen and Jones, 1998; Aravind et al., 1999). These proteins function as activators of the apoptotic proteases. A central region of the animal genes contains a NBS region, sometimes called an apoptotic ATPase domain, that strongly resembles the NBS region of plant R genes. An even more extensive structural similarity exists between R genes and the human gene Nod1, which encodes a LRR region that follows the NBS region (Yu et al., 1998). Many of the animal proteins, but not plant R proteins, carry a caspase recruitment domain (CARD) that physically interacts with specific procaspases. Recent studies have shown that the ATPase domain is required for oligomerization of CED-4, which brings into physical proximity monomers of a procaspase, leading to their activation and initiation of a caspase cascade and cell death (Yang et al., 1998). The N termini of Mi and other resistance proteins bear no resemblance to CARD sequences; nevertheless, it is tempting to speculate that R genes also act as regulatory molecules (van der Biezen and Jones, 1998) and that proteins binding to the N-terminal or C-terminal regions are activated by proximity mediated by NBS-domain oligomerization. In this scenario, Mi is a key control molecule in signaling an active defense response.
At this point, it is not certain whether the death initiated by our chimeric constructs in plant leaves or roots is a result of the same signaling pathway as the HR triggered by the nematode infection, although the similar temperature sensitivity of the two processes suggests that this is the case. Also, in some plant/pathogen systems, HR does not appear to be required for gene-for-gene resistance (Yu et al., 1998; Richael and Gilchrist, 1999). However, the components of the defense pathway activated may differ among NBS-LRR genes, and a localized cell death that prohibits sufficient feeding by the obligatorily parasitic nematode seems a reasonable resistance strategy. LRR regions are often the most variable region among closely related R genes, and comparison of their sequences suggests that these genes are undergoing diversifying selection (McDowell et al., 1998; Michelmore and Meyers, 1998). Perhaps some of the variability in the LRR region modulates the defense response pathway signaled and also plays a role in determining specificity. At present, it has not been shown that Mi-mediated aphid resistance is characterized by localized cell death. Perhaps the defense pathway triggered by aphids in leaves is different from that triggered by nematodes in roots. Investigation of the mechanism by which specific genes modulate resistance to specific pathogens is currently an exciting area, and we are just beginning to understand how this complex defense response is so precisely regulated and is so effective. The assays presented here, coupled with additional sequence exchanges, should facilitate further understanding of its operation.
METHODS
Plasmid Construction
The 7.1-kb PstI-BamHI fragment (Figure 1) containing Mi-1.1 was subcloned into pLitmus 29 (New England Biolabs, Beverly, MA). An 8.9-kb BamHI-SpeI fragment containing Mi-1.2 (Milligan et al., 1998) was subcloned from pSM141 into pBluescript KS (Stratagene, La Jolla, CA).
A common NcoI site located 161 amino acids downstream of the ATG in Mi-1.1 and Mi-1.2 was chosen for production of chimeric genes. The Mi-1.1 NcoI-EagI fragment was introduced into the Mi-1.2 NcoI-NotI sites. The resulting plasmid was further digested with EcoRI and SacI and then fused with the Mi-1.1 EcoRI-SacI fragment to create insert Mi-DS1. To create construct Mi-DS3, we generated a BamHI site at the PstI site of Mi-1.1 by amplifying Mi-1.1 with primers 5′-GTCGGATCCGATATCGGTATTATTTTGAGATTGGATTAATG-3′ (to add the BamHI site) and 5′-CATTCACTATCAACCCATGGAAATCTCTTATG-3′ (to span the NcoI site). The resulting 2.8-kb fragment was digested with BamHI and NcoI and was introduced into Mi-1.2 that had been digested by the same enzymes.
To exchange the leucine-rich repeat (LRR) regions, a HindIII site was introduced at amino acid 1189 in Mi-1.1. The XbaI-BamHI fragment of Mi-1.1 was subcloned into pBluescript KS and mutagenized by the polymerase chain reaction (PCR) using two mutant primers (5′-CTGCGGGGATGTCATAAGCTTGAGGAGATTCCACCTAG-3′ and 5′-CTAGGTGGAATCTCCTCAAGCTTATGACATCCCCGCAG-3′) and T3 and T7 primers. The mutagenized XbaI-BamHI fragment, now containing a HindIII site, was subcloned into the SalI-BamHI fragment of Mi-1.1 in pBluescript KS. The SalI-BamHI fragment was then subcloned into the EcoRI-BamHI fragment of Mi-1.1 in pLitmus 28 (New England Biolabs) lacking a PvuII site. The XhoI-SacI fragment of Mi-1.2 was also subcloned into pLitmus 28 lacking a PvuII site. The PvuII-HindIII fragments of Mi-1.1 and Mi-1.2 were then exchanged. Plasmid Mi-DS4 was created by subcloning the SalI-BamHI fragment into Mi-1.1. Plasmid Mi-DS2 was created by subcloning the XhoI-SacI fragment into Mi-1.2.
To replace six of the LRR amino acids in Mi-1.2 with those of Mi-1.1, we subcloned the XbaI-HindIII fragment of Mi-1.2 into pBluescript KS. Mutagenesis was performed by PCR using the primers 5′-TTCAATCAAGTTTCGATATCCAAGTGGGAGGTTGGAGAG-3′ and 5′-CTTGGATATCGAAACTTGATTGAAGTTCAAAAATTTGAGATTCTCAAAAG-3′. The mutagenized DNA fragment was then introduced into pBluescript KS containing the Mi-1.2 XhoI-SpeI fragment. The Mi-1.2 XhoI-SpeI fragment was then subcloned back into Mi-1.2 to create plasmid Mi-DS6. To replace the six LRR amino acids encoded in Mi-1.1 with those of Mi-1.2, we subcloned the EagI-BamHI fragment of Mi-1.1 into pBluescript KS. Mutagenesis was performed by PCR using the primers 5′-GTAGTCTTAAGTTCAAAAATTTGAGAT–TCTCAAAGGTG-3′ and 5′-TTGAACTTAAGACTACTGACTCTTTCC-AAGTGGGAGGTTGGAG-3′. The Mi-1.1 EagI-BamHI fragment of Mi-1.1 was then subcloned back into Mi-1.1 to create Mi-DS7.
To create plasmid Mi-DS10, we subcloned the NcoI-NotI fragment of Mi-DS6 into the NcoI-NotI sites of Mi-DS3. To create plasmid Mi-DS5, we subcloned the NcoI-ClaI fragment of Mi-DS4 (filled in at the ClaI site) into the NcoI-NotI site of Mi-1.2 (filled in at the NotI site). To exchange the Mi-1.1 and Mi-1.2 N-terminal coding regions, we subcloned the Mi-1.1 SacI-NcoI and Mi-1.2 SalI-NcoI fragments into pBluescript KS containing a NcoI site in its polylinker. An EcoRV site was created after amino acid 6 in both Mi-1.1 and Mi-1.2 by PCR using primers 5′-GGAAAAACGAAAAGATATCGAAGAAGCAAACAACTC-3′ and 5′-GAGTTGTTTGCTTCTTCGATATCTTTTCGTTTTTCC-3′. The Mi-1.1 and Mi-1.2 EcoRV-NcoI fragments were exchanged, and the resulting constructs were reintroduced into Mi-1.2 and Mi-DS3 to create Mi-DS8 and Mi-DS9.
To express the Mi-1.2 N-terminal 161 amino acids under the control of the cauliflower mosaic virus (CaMV) 35S promoter, a cassette containing the CaMV 35S promoter with a nopaline synthase terminator was first digested as a HindIII-EcoRI fragment and then moved into the binary vector pCAMBIA2201 (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK), which was digested by the same enzymes, to create 35SP-pCAMBIA2201. The DNA encoding the Mi-1.1 and Mi-1.2 N-terminal 161–amino acid regions were amplified with primers (Mi-1.1, 5′-GTGGGATCCTCA-TGGAAAAACGAAAAGATAATG-3′ and 5′-GTGGAGCTCTATCAA-CCCATGGAAATCTCTATG-3′; and Mi-1.2, 5′-GTGGGATCCTCA-TGGAAAAACGAAAAGATATTG-3′ and 5′-GTGGAGCTCTATCAA-CCCATGGAAATCTCTATG-3′) as BamHI-SacI fragments and then introduced into 35SP-pCAMBIA2201.
Plant Transformation
The binary vector pTFS-40 (British Sugar, Norwich, UK) containing the desired Mi domain swap clones was transferred into Agrobacterium rhizogenes ATCC 15834 and LBA 9402 (pRi 1855) by electrotransformation (Shen and Forde, 1989). The same Mi domain swap constructs were also transferred into A. rhizogenes A4RS (Jouanin et al., 1986) by triparental mating (Bevan, 1984). Individual cotyledons were excised from 8- to 10-day-old tomato seedlings and immersed in an A. rhizogenes suspension for 5 min. The excised cotyledons were placed on a sterilized filter paper to remove excess bacteria. The inoculated cotyledons were placed on Murashige and Skoog medium (Murashige and Skoog, 1962) containing 2% sucrose and 0.8% agar. After 3 days of preincubation, the cotyledons were transferred into an 0.8% agar in Murashige and Skoog medium containing 2% sucrose, 50 mg/L kanamycin, and 250 mg/L cefatoxime. After 7 to 10 days of incubation at 25°C in the light, roots emerged on the surface of the cotyledons.
Nematode Assays
Meloidogyne javanica and M. incognita strains VW4 and VW6, respectively, were used (Milligan et al., 1998). A greenhouse culture of M. arenaria (from the Department of Nematology, University of California, Davis) was used. For experiments in soil, we inoculated transgenic tomato plants with nematodes according to the procedure described by Yaghoobi et al. (1995). For experiments on agar plates, nematode egg masses were surface sterilized with 10% bleach, vortex mixed for 15 min, and then washed three times with water. Hairy roots were transferred to Gamborg's B5 medium (Sigma) containing 2% sucrose and 2% gelrite. Approximately 1000 surface-sterilized eggs were pipetted around the root tips per plate. The plates were kept at 23°C in the dark for 2 weeks. Nematodes within the roots were stained red with acid fuchsin and scored as resistant or susceptible, as previously described (Ho et al., 1992).
Transient Agrobacterium-Mediated Expression
Mi-1.1, Mi-1.2, and domain swaps between Mi-1.1 and Mi-1.2 were transiently expressed in Nicotiana benthamiana tissue as described elsewhere (Rathjen et al., 1999; Tai et al., 1999), except that A. rhizogenes strain A4RS was used. For the high-temperature experiment, plants were inoculated with A. rhizogenes grown at 32°C for 2 days and incubated in a 32°C growth chamber for specified times.
RNA Isolation and Reverse Transcription–PCR Amplification
Total RNA was isolated from N. benthamiana leaves 24 hr after A. rhizogenes infiltration as described by Ausubel et al. (1987) and treated with DNaseI to remove residual genomic DNA. cDNA was synthesized using the RT-PCR kit from Gibco BRL (Gaithersburg, MD) according to the manufacturer's instructions. For Mi-1.1, primers C1/2 (5′-CAGTGAAGTGGAAGTGATGA-3′) and C1S1 (5′-CCCAGCAAAGTACAATCTAC-3′) were used to amplify a 1.5-kb fragment. For Mi-1.2, primers C1/2 and C2S4 (5′-CTAAGAGGAATCTCATCACAGG-3′) were used to amplify a 1.6-kb fragment. The C1/2 primer corresponds to the coding region and is common to both Mi-1.1 and Mi-1.2. Primer sets C1S1 and C2S4 correspond to the 3′ untranslated regions of Mi-1.1 and Mi-1.2, respectively. To test for possible amplification of contaminating DNA, we performed PCR with RNA as the template but without addition of reverse transcriptase. Also, DNAs from tomato cultivars Motelle and Moneymaker were used as positive and negative controls, respectively.
DNA Sequence and Analysis
Cycle-sequencing reactions were performed with a Thermosequenase kit (Amersham Life Sciences) and run on a sequencer (model 4200; Licor, Lincoln, NE; or model 377; Applied Biosystems, Foster City, CA). In all cases, both DNA strands were sequenced. DNA sequence data were edited and compiled with the Sequencher 3.0 program (GeneCodes, Ann Arbor, MI).
Acknowledgments
We thank John Gardner, Richard Michelmore, John Rathjen, Jim Lincoln, Jeff Chang, Paul Feldstein, Jafar Yaghoobi, Kevin Fort, and other colleagues at the Center for Engineering Plants for Resistance Against Pathogens (CEPRAP) for their help and advice. We also thank Kris Lambert for help with figures, Isgouhi Kaloshian and Oscar Martinez for suggestions on methods, and David Tepfer for kindly providing A. rhizogenes strains LBA9402 and A4RS. This research was supported by National Science Foundation (NSF) Cooperative Agreement No. BIR-8920216 to CEPRAP, an NSF Science and Technology Center.
References
- Aravind, L., Dixit, V.M., and Koonin, E.V. (1999). The domains of death: Evolution of the apoptosis machinery. Trends Biochem. Sci. 24, 47–53. [DOI] [PubMed] [Google Scholar]
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.G., and Struhl, K., eds (1987). Current Protocols in Molecular Biology. (New York: Greene and Wiley).
- Baker, B., Zambryski, P., Staskawicz, B., and Dinesh-Kumar, S. (1997). Signaling in plant–microbe interactions. Science 276, 726–733. [DOI] [PubMed] [Google Scholar]
- Bent, A.F., Kunkel, B.N., Dahlbeck, D., Brown, K.L., Schmidt, R., Giraudat, J., Leung, J., and Staskawicz, B.L. (1994). RPS2 of Arabidopsis thaliana: A leucine-rich repeat class of plant disease resistance genes. Science 265, 1856–1860. [DOI] [PubMed] [Google Scholar]
- Bevan, M.W. (1984). Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 12, 8711–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, C., and Perry, D. (1986). α-Helical coiled coils—A widespread motif in proteins. Trends Biochem. Sci. 11, 245–248. [Google Scholar]
- Dropkin, V.H. (1969). The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne: Reversal by temperature. Phytopathology 59, 1632–1637. [Google Scholar]
- Ellis, J., and Jones, D. (1998). Structure and function of proteins controlling strain-specific pathogen resistance in plants. Curr. Opin. Plant Biol. 1, 288–293. [DOI] [PubMed] [Google Scholar]
- Ellis, J., Lawrence, G., Luck, J., and Dodds, P. (1999). Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. Plant Cell 11, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor, H.H. (1955). Host–parasite interaction in flax rust—Its genetic and other implications. Phytopathology 45, 680–685. [Google Scholar]
- Glazebrook, J. (1999). Genes controlling expression of defense responses in Arabidopsis. Curr. Opin. Plant Biol. 2, 280–286. [DOI] [PubMed] [Google Scholar]
- Grant, M.R., Godiard, L., Straube, E., Ashfield, T., Lewald, J., Sattler, A., Innes, R.W., and Dangl, J.L. (1995). Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269, 843–846. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack, K.E., and Jones, J.D.G. (1997). Plant disease resistance genes. Annu. Rev. Plant Physiol. 48, 575–607. [DOI] [PubMed] [Google Scholar]
- Ho, J.-Y., Weide, R., Ma, H.M., Wordragen, M.F., Lambert, K.N., Koornneef, M., Zabel, P., and Williamson, V.M. (1992). The root-knot nematode resistance gene (Mi) in tomato: Construction of a molecular linkage map and identification of dominant cDNA markers in resistant genotypes. Plant J. 2, 971–982. [PubMed] [Google Scholar]
- Jones, D.A., and Jones, J.D.G. (1996). The roles of leucine-rich repeats in plant defences. Adv. Bot. Res. Adv. Plant Pathol. 24, 90–167. [Google Scholar]
- Jouanin, L., Tourneur, J., and Casse-Delbart, F. (1986). Restriction maps and homologies of the three plasmids of Agrobacterium rhizogenes strain A4. Plasmid 16, 124–134. [DOI] [PubMed] [Google Scholar]
- Kobe, B., and Deisenhofer, J. (1994). The leucine-rich repeat: A versatile binding motif. Trends Biochem. Sci. 19, 415–421. [DOI] [PubMed] [Google Scholar]
- McDowell, J.M., Dhandaydham, M., Long, T.A., Aarts, M.G.M., Goff, S., Holub, E.B., and Dangl, J.L. (1998). Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of Arabidopsis. Plant Cell 10, 1861–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelmore, R.W., and Meyers, B.C. (1998). Clusters of resistance genes in plants evolve by a divergent selection and a birth-and-death process. Genome Res. 8, 1113–1130. [DOI] [PubMed] [Google Scholar]
- Milligan, S., Bodeau, J., Yaghoobi, J., Kaloshian, I., Zabel, P., and Williamson, V. (1998). The root-knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10, 1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindrinos, M., Katagiri, F., Yu, G.-L., and Ausubel, F.M. (1994). The A. thaliana disease-resistant gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell 78, 1089–1099. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Paulson, R.E., and Webster, J.M. (1972). Ultrastructure of the hypersensitive reaction in roots of tomato, Lycopersicon esculentum L., to infection by the root-knot nematode, Meloidogyne incognita. Physiol. Plant Pathol. 2, 227–234. [Google Scholar]
- Rathjen, J.P., Chang, J.H., Staskawicz, B.J., and Michelmore, R.W. (1999). Constitutively active Pto induces a Prf-dependent hypersensitive response in the absence of avrPto. EMBO J. 18, 3232–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richael, C., and Gilchrist, D. (1999). The hypersensitive response: A case of hold or fold. Physiol. Mol. Plant Pathol. 55, 5–12. [Google Scholar]
- Roberts, P.A., May, D., and Matthews, W.C. (1986). Root-knot nematode resistance in processing tomatoes. Calif. Agric. 40, 24–26. [Google Scholar]
- Rossi, M., Goggin, F., Milligan, S.B., Kaloshian, I., Ullman, D., and Williamson, V.M. (1998). The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc. Natl. Acad. Sci. USA 95, 9750–9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron, J.M., Oldroyd, G.E.D., Rommens, C.M.T., Scofield, S.R., Kim, H.S., Lavelle, D.T., Dahlbeck, D., and Staskawicz, B.J. (1996). Tomato Prf is a member of the leucine-rich repeat class of plant disease resistance genes and lies embedded within the Pto kinase gene cluster. Cell 86, 123–133. [DOI] [PubMed] [Google Scholar]
- Shen, W.-J., and Forde, B.G. (1989). Efficient transformation of Agrobacterium spp. by high voltage electroporation. Nucleic Acids Res. 17, 8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, R.B., Spielmann, A., Margossian, L., and McKnight, T.D. (1986). A disarmed binary vector from Agrobacterium tumefaciens functions in Agrobacterium rhizogenes. Plant Mol. Biol. 6, 403–415. [DOI] [PubMed] [Google Scholar]
- Tai, T.H., Dahlbeck, D., Clark, E.T., Gajiwala, P., Pasion, R., Whalen, M.C., Stall, R.E., and Staskawicz, B.J. (1999). Expression of the Bs2 pepper gene confers resistance to bacterial spot disease in tomato. Proc. Natl. Acad. Sci. USA 96, 14153–14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepfer, D. (1990). Genetic transformation using Agrobacterium rhizogenes. Physiol. Plant. 79, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Biezen, E., and Jones, J. (1998). The NB-ARC domain: A novel signaling motif shared by plant resistance gene products and regulators of cell death in animals. Curr. Biol. 8, 226–227. [DOI] [PubMed] [Google Scholar]
- Warren, R.F., Henk, A., Mowery, P., Holub, E., and Innes, R.W. (1998). A mutation within the leucine-rich repeat domain of the Arabidopsis disease resistance gene RPS5 partially suppresses multiple bacterial and downy mildew resistance genes. Plant Cell 10, 1439–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, V.M., and Hussey, R.S. (1996). Nematode pathogenesis and resistance in plants. Plant Cell 8, 1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghoobi, J., Kaloshian, I., Wen, Y., and Williamson, V.M. (1995). Mapping a new nematode resistance locus in Lycopersicon peruvianum. Theor. Appl. Genet. 91, 457–464. [DOI] [PubMed] [Google Scholar]
- Yang, X., Chang, H.Y., and Baltimore, D. (1998). Essential role of CED-4 oligomerization in CED-3 activation and apoptosis. Science 281, 1355–1357. [DOI] [PubMed] [Google Scholar]
- Yu, I.C., Parker, J., and Bent, A.F. (1998). Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc. Natl. Acad. Sci. USA 95, 7819–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]