Abstract
Differentiation of fungal conidia of phytopathogens into the infection structure, appressorium, requires contact with a hard surface and host signals. The molecular signaling involved in the induction of this differentiation is poorly understood. We report the cloning of a mitogen-activated protein kinase kinase (MEK), CgMEK, from Colletotrichum gloeosporioides and its role in the induction of these developmental processes involved in pathogenesis. Disruption of CgMEK1 resulted in the loss of its ability to form appressoria in response to the host's signals and a loss of virulence. Results of confocal microscopic examination of germinating conidia of the gene-disrupted mutants were similar to those for wild-type conidia treated with an MEK inhibitor, suggesting that CgMEK1 is involved in two developmental processes in the differentiation into appressorium: (1) polarized cell division, with the preferential increase in F-actin in one of the daughter nuclei after nuclear division and the formation of septum; and (2) differentiation of the germ tube into an appressorium. CgMEK1 is required for the differentiation.
INTRODUCTION
Many phytopathogenic fungi are known to require hard surface contact of conidia before they can differentiate into infection structures called appressoria (Emmett and Parbery, 1975; Xiao et al., 1997). Physical or chemical signals from the host are also known to be required for this differentiation (Grover, 1971; Lapp and Skoropad, 1978; Parbery and Blakeman, 1978; Staples and Hoch, 1987; Kolattukudy et al., 1995). Anthracnose disease, caused by the Colletotrichum (Gloeosporium) or the Glomerella group, is very common and destructive of numerous crop and ornamental plants worldwide. The conidia of Colletotrichum gloeosporioides, a causal agent of anthracnose disease on fruits, need a hard surface contact for 2 hr before they can differentiate into appressoria in response to host signals such as surface wax and the ripening hormone ethylene (Flaishman and Kolattukudy, 1994; Flaishman et al., 1995; Hwang and Kolattukudy, 1995). Protein phosphorylation is involved in this signal-induced process of appressorium formation because inhibition of protein phosphorylation by kinase inhibitors severely inhibits the appressorium formation induced by ethylene and host wax. The proteins phosphorylated in response to treatment with ethylene and wax include those similar in size to the mitogen-activated protein kinase, MAP kinase (MAPK; Flaishman et al., 1995).
MAPK signaling pathways play a pivotal role in sensing extracellular signals and relaying the signals to control gene expression. (Nishida and Gotoh, 1993; Herskowitz, 1995). They involve three sequentially acting kinases—MAPK, MAPK kinase (MEK), and MEK kinase (MEKK) (Marshall, 1994)—the primary structures of which are remarkably conserved among eukaryotes from Saccharomyces cerevisiae to Homo sapiens. MAPK pathways are involved in a variety of developmental processes in yeast and filamentous fungi (Madhani and Fink, 1998a, 1998b). MAPKs have been cloned from phytopathogenic fungi (Li et al., 1997; Hamer and Talbot, 1998; Lev et al., 1999). Transformants carrying a defective mapk gene, pmk1, in Magnaporthe grisea failed to produce appressoria and thus lost virulence (Xu and Hamer, 1996). MAPKs also are reported to be required for full virulence of the maize pathogens Cochliobolus heterostrophus (Lev et al., 1999) and Ustilago maydis (Mayorga and Gold, 1999). Another MAPK, MPS1, which is involved in cell wall integrity, has been cloned from M. grisea. The MPS1 gene–disrupted mutants formed appressoria but showed decreased pathogenicity (Xu et al., 1998). These observations suggested that MAPKs have diverse functions in fungal pathogenesis. However, little is known about the identity and role of the kinases that activate the MAPKs in phytopathogens or about the signaling involved in the induction of cytokinesis and appressorium formation by hard surface contact and chemicals from the host.
We report here the cloning of the cDNA and gene for MEK, designated CgMEK1, from C. gloeosporioides and demonstrate that disruption of this gene blocks hard surface–induced cytokinesis at the stage immediately following nuclear division. Thus, in the CgMEK1-disrupted mutants, the preferential increase in F-actin associated with one of the daughter nuclei and septum formation does not occur. This loss of polarity prevented germination and hyphal development. Instead, the CgMEK1 mutants exhibited a fission- or budding-type activity leading to the formation of oval cells. The apparent loss of polarity could be overcome with host signals (e.g., ethylene) or nutrients and thus allow germination. However, CgMEK1 disruption totally blocked the differentiation of the germ tube into an appressorium. Treating the wild-type conidia with MEK inhibitor caused blockage in developmental changes that mimicked those caused by CgMEK1 disruption. Thus, the MAPK cascade is involved in at least two processes—one involving a polarized increase in F-actin in response to hard surface contact, septum formation, and germination, and the other involving differentiation of the germ tube into an appressorium. Our results suggest that the MAPK involved in the first process can also be phosphorylated by an MEK different from MEK1. However, MEK1 is essential for activating the MAPK involved in the differentiation into appressoria. The MEK1 mutant lost pathogenicity on its natural host, avocado fruits.
RESULTS
Isolation of the cDNA and Gene for CgMEK1
When degenerate oligonucleotides corresponding to the conserved regions VII and VIII of the catalytic domains of the MEK were used for polymerase chain reaction (PCR) with single-stranded cDNA fragments from ethylene-treated conidia as template, the expected 90-bp product was obtained. The upstream catalytic region of the MEK was obtained as a 900-bp product by PCR with a gene-specific antisense primer synthesized on the basis of the sequence of this 90-bp cDNA fragment and the universal M13 sense primer, and using a cDNA library from ethylene-treated conidia as template. The peptide sequence deduced from the nucleotide sequence of the 900-bp product was 65, 48, 48, and 51% identical to the corresponding kinase domains of the fungal MEKs FUZ7 (Banuett and Herskowitz, 1994) from U. maydis and STE7 (Teague et al., 1986), PBS2 (Boguslawski and Polazzi, 1987), and MKK2 (Irie et al., 1993) from S. cerevisiae, respectively. Thus, this cDNA fragment represents an authentic CgMEK. The 900-bp insert was then used to screen cDNA and genomic libraries of C. gloeosporioides. A 2-kb cDNA was isolated from a cDNA library and sequenced (GenBank accession number AF169644). Using the same probe, we isolated a genomic clone from a genomic DNA library and sequenced a 3.1-kb segment containing the entire CgMEK1 gene (GenBank accession number AF169643).
Comparison of the cDNA and genomic sequences of CgMEK1 revealed that the open reading frame in CgMEK1 is composed of four exons interrupted by three introns. The motif algorithm showed that the 5′ flanking region of this gene has a putative cis-element for mating factor α (MATα2), a stress response element, and elements for heat shock factors. The open reading frame would encode a protein with an estimated molecular mass of 55.5 kD and pI 8.7. Alignment of CgMEK1 to optimize homology with the predicted amino acid sequences of FUZ7, STE7, PBS2, and MKK2 is shown in Figure 1. CgMEK1 shares 51, 44, 40, and 35% identity with FUZ7, STE7, PBS2, and MKK2, respectively. CgMEK1 contains the conserved catalytically important Asp in the subdomain VIb and the 11 conserved kinase domains. These results show that the clones represent an authentic MEK from C. gloeosporioides.
Figure 1.
Alignment of the Predicted Kinase Domain of CgMEK1 and Other Fungal MEKs.
FUZ7 from U. maydis (Banuett and Herskowitz, 1994), and STE7 (Teague et al., 1986), PBS2 (Boguslawski and Polazzi, 1987), and MKK2 (Irie et al., 1993) from S. cerevisiae. The 11 subdomains are shown in roman numerals. The catalytically conserved Asp (D) in the subdomain VIb is marked by a solid circle.
Disruption of CgMEK1
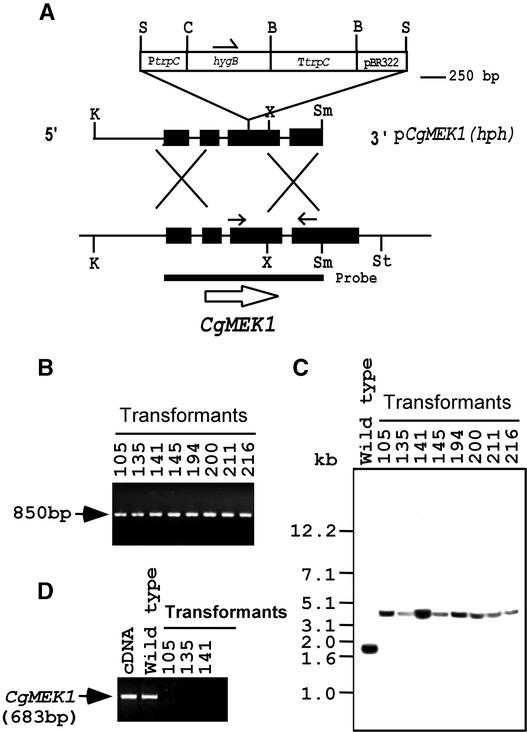
A gene disruption was performed to test for the possible involvement of CgMEK1 in the early developmental stages that lead to appressorium formation by the conidia. As shown in Figure 2A, pCgMEK1(hph), a gene disruption vector, was constructed by inserting a 2.4-kb hygromycin resistance gene into a 1.9-kb genomic fragment of CgMEK1 in such a way that the hygromycin resistance gene was in the third exon of CgMEK1, which encodes the catalytic domain VII. pCgMEK1(hph) was used to transform C. gloeosporioides, and transformants were selected by growth on hygromycin. Transformants were then screened by PCR to detect the junction representing insertion of the disruption construct into the genome by homologous recombination, as indicated in Methods. Of 337 transformants, 11 gave the expected 850-bp junction PCR product, and eight of these are shown in Figure 2B. Preliminary DNA gel blot analysis indicated that three of them also have ectopic integration(s) (data not shown), but eight transformants showed no indication of ectopic integration. The genomic DNA gel blot analysis from these transformants showed that the 1.9-kb band obtained by KpnI and SmaI digestion of the DNA from the wild type was replaced by a 4.3-kb band in the mutants, thus representing insertion of the hygromycin resistance gene in the mutants (Figure 2C). To test for expression of the CgMEK1 gene by the mutants, we subjected the total RNA extracted from conidia of the wild type and CgMEK1-disrupted mutants to reverse transcription (RT)–PCR with gene-specific primers from the coding region. The 683-bp product expected from the native CgMEK1 gene was not formed from the RNA of the gene-disrupted mutants, whereas the wild type yielded this product (Figure 2D).
Figure 2.
Disruption of CgMEK1.
(A) CgMEK1 replacement vector, pCgMEK1(hph), and CgMEK1 gene. Black boxes, exons of CgMEK1; thin lines, introns. The scale is shown at right. Sense and antisense primers used for RT-PCR are indicated by arrows. B, BamHI; C, ClaI; K, KpnI; PtrpC, promoter of trpC; S, SalI; Sm, SmaI; St, SstI; TtrpC, terminator of trpC; X, XhoI.
(B) Spore PCR to screen for CgMEK1 mutants. A junction PCR was performed using spores as the template source, as described in Methods. Of the 11 mutants, eight are shown.
(C) Genomic DNA gel blot analysis. Genomic DNA isolated from the eight mutants shown in (B) and digested with KpnI and SmaI was probed with the SmaI-digested cDNA fragment.
(D) RT-PCR analysis showing the formation of the expected transcripts from the wild type but not from the mutants. Total RNA isolated from conidia treated with ethylene on a hard surface was used.
Transformants Carrying Disrupted CgMEK1 Are Unresponsive to Host Signals, Ethylene, and Surface Wax
The conidiation of the CgMEK1 mutants on potato dextrose agar plates was similar to that of the wild-type C. gloeosporioides (data not shown). When the germination and appressorium-forming abilities of conidia of CgMEK1 mutants were compared with those of the wild type on a hard surface in the presence of either ethylene or host wax, marked differences between the mutants and the wild type were apparent (Table 1). Treating the conidia of CgMEK1 mutants with ethylene or wax resulted in 20% germination, whereas >90% of wild-type conidia germinated under the same conditions. The CgMEK1 mutant conidia that germinated did not differentiate into appressoria, whereas most (94%) wild-type conidia that germinated produced appressoria under the same conditions. In rich media (e.g., 0.5% yeast extract or potato dextrose broth), however, ∼95% of the CgMEK1 mutant conidia germinated, as did the wild-type conidia. These results suggest that the blockage of germination in the CgMEK1 mutant might be overcome with nutrients but that the differentiation of the germ tube into an appressorium requires CgMEK1.
Table 1.
Germination and Appressorium Formation of Conidia of the Wild Type and CgMEK1 Mutanta
| Water (%)
|
Ethephon (%)
|
Wax (%)
|
Yeast Extract (%)
|
||||
|---|---|---|---|---|---|---|---|
| Appressorium Formation |
Germination | Appressorium Formation |
Germination | Appressorium Formation |
Germination | Germination | |
| Wild type | 5.3 ± 2.5 | 10.4 ± 0.5 | 89.3 ± 1.5 | 97.0 ± 0.6 | 85.4 ± 5.4 | 93.2 ± 1.3 | 96.4 ± 1.1 |
| Δ CgMEK1 | 0 | 8.4 ± 5.4 | 0 | 20.7 ± 5.7 | 0 | 16.2 ± 9.5 | 94.8 ± 2.7 |
Five thousand conidia from mutant 105 were incubated in water containing 10 μM ethephon, 0.1 mg/mL wax, or 0.5% yeast extract for 18 hr. Percentage of germination includes all conidia that germinated, including those that formed appresoria. CgMEK1 mutants 135 and 141 gave results similar to those for mutant 105.
CgMEK1 Is Involved in Regulation of Cell Division in Conidia and Formation of Appressoria
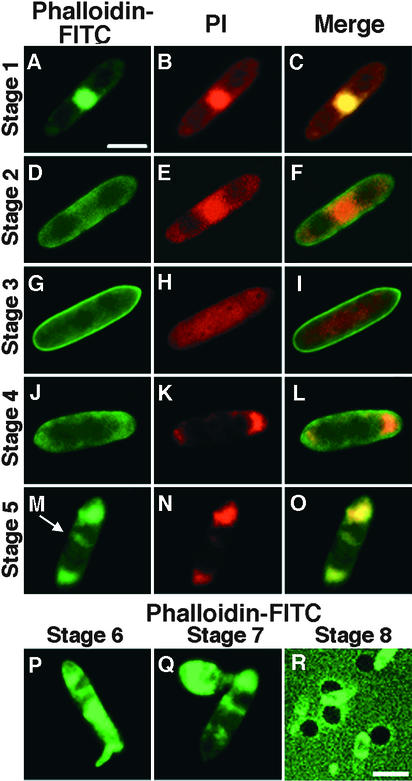
The cytochemical and morphological changes that occur during germination of C. gloeosporioides conidia were examined using confocal and light microscopy. Localization and rearrangement of the F-actin filaments that control cytoskeletal structures were visualized by staining with fluorescein isothiocyanate (FITC)–conjugated phalloidin that specifically binds to polymerized actin (F-actin) but not to G-actin (Wulf et al., 1979). The localization of F-actin was indicated by green fluorescence. Simultaneously, the conidia were stained with propidium iodide (PI), which indicates the location of DNA by red fluorescence. The cytochemical and morphological changes of conidia observed during germination and formation of appressoria on hard surfaces could be divided into eight stages.
In the conidia at the beginning (stage 1), phalloidin-FITC signals and PI signals were co-localized in the nucleus at the center of the cells seen in Figures 3A to 3C. Within 30 min on the hard surface, delocalization of F-actin from the nucleus (stage 2; Figures 3D to 3F) and subsequent gradual dispersal of faint PI signal throughout the cells (stage 3; Figures 3G to 3I) were seen. The PI signals began to concentrate at both ends of the cells, indicating nuclear division, and the F-actin associated preferentially with one of the nuclei (stage 4; Figures 3J to 3L). The number of conidia reaching this stage peaked at ∼60 min of incubation. Phalloidin-FITC signals also became more concentrated at both ends of the cells, and at this stage, an F-actin–rich septum structure dividing the conidia into two cells was observed, indicating the completion of cytokinesis (stage 5; Figures 3M to 3O) within 2 to 4 hr of incubation on the hard surface. In addition, both the PI and phalloidin-FITC signals became more intense in one of the two daughter cells in each conidium, and the germ tube began to emerge specifically from this daughter cell (stage 6; Figure 3P). A single germ tube emerged from this part of each conidium. Conidia started to germinate ∼4 hr after contact with a hard surface, and the number of germinating conidia reached maximum in another 2 hr of incubation. Formation of F-actin–rich appressoria at the end of germ tubes was observed between 6 and 10 hr of incubation (stage 7; Figure 3Q). Finally, the appressoria became so highly melanized that no fluorescence could be detected from them (stage 8). When the slides were exposed to intense light for excitation, melanized appressoria could be visualized as dark spots (Figure 3R); however, this heavy melanization was restricted to the appressoria only. The number of conidia that reached this stage peaked between 8 and 16 hr of incubation.
Figure 3.
Confocal Microscopic Analysis of Germinating Wild-Type Conidia.
Conidia at low population density (10 per μL) were incubated on glass slides for 0.5 to 12 hr in the absence of chemical signals from the host. Images were visualized by phalloidin-FITC signals in (A), (D), (G), (J), (M), and (P) to (R); by PI signals in (B), (E), (H), (K), and (N); and by merged images in (C), (F), (I), (L), and (O). Arrow marks the actin-rich septum structure. 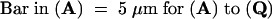 ;
;  .
.
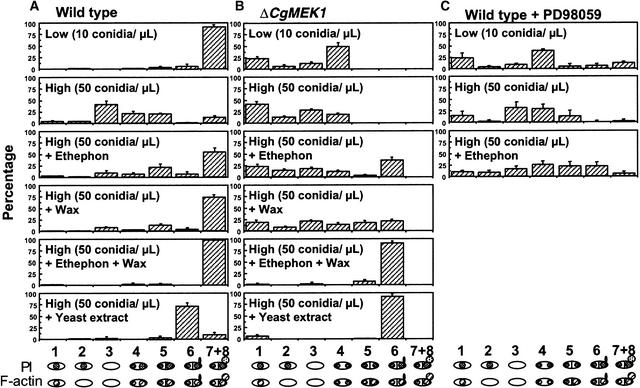
The progression through the eight stages of conidial germination and differentiation when in contact with a hard surface for 12 hr was analyzed quantitatively, as shown in Figure 4A. When the population density of conidia was low (10 conidia per μL), wild-type conidia completed all stages, through germination and appressorium formation, within 12 hr, and >90% of conidia formed highly melanized appressoria. When the population density of conidia was increased to 50 per μL, the progression of germination and appressorium formation was greatly delayed. Under this condition, in which self-inhibition of germination was occurring, adding ethephon (an ethylene-generating agent) or host wax from avocado fruits to the conidial suspension enhanced both germination and appressorium formation, reversing the effect of the self-inhibitor (Figure 4A). When both avocado wax and ethephon were added, formation of melanized appressoria was hastened, and under these conditions, >95% of conidia showed highly melanized appressoria. Adding yeast extract induced germination of conidia with branching of the germ tubes and formation of multiple germ tubes per conidium. However, this treatment did not induce appressorium formation.
Figure 4.
Quantitative Analysis of Cytochemical and Morphological Changes That Occur during Incubation of Conidia on a Hard Surface.
Wild type (A), CgMEK1mutants (B), and wild type plus PD98059 (C) conidia were incubated on glass slides for 12 hr with (+) or without added chemicals corresponding to host signals (1 μg/mL avocado wax and/or 10 μM ethephon) or yeast extract (0.5% [w/v]). Symbols and numbers at bottom indicate the stages of conidium germination and appressorium formation defined in the text.
The effects of CgMEK1 disruption on cytochemical and morphological changes occurring during germination of conidia at low population density on a hard surface were examined for 12 hr (Figures 4B and 5A to 5I). F-actin in the conidia of CgMEK1 mutant 105 appeared more tightly packed in the nuclei than did that seen in the wild type at stage 1 (Figures 5A to 5C). At stages 2 and 3, the mutant conidia looked almost identical to those observed in the wild type (data not shown). After nuclear division, equal amounts of F-actin were found at both ends of the mutant conidia, and the F-actin–rich septum structure was not produced; examination using light microscopy confirmed the absence of septum formation in the absence of host chemicals (data not shown). Instead, the middle of the conidial cell showed constriction (Figures 5D to 5F), as has been seen with the pseudohyphal growth pattern in S. cerevisiae (Madhani and Fink, 1998a), and the majority of the conidia remained arrested at this stage for at least 12 hr. Subsequently (by 24 hr), budding or fission-type cell division occurred in ∼20% of the cells (Figures 5G to 5I). Mutant 135 gave results identical to those obtained with mutant 105.
Figure 5.
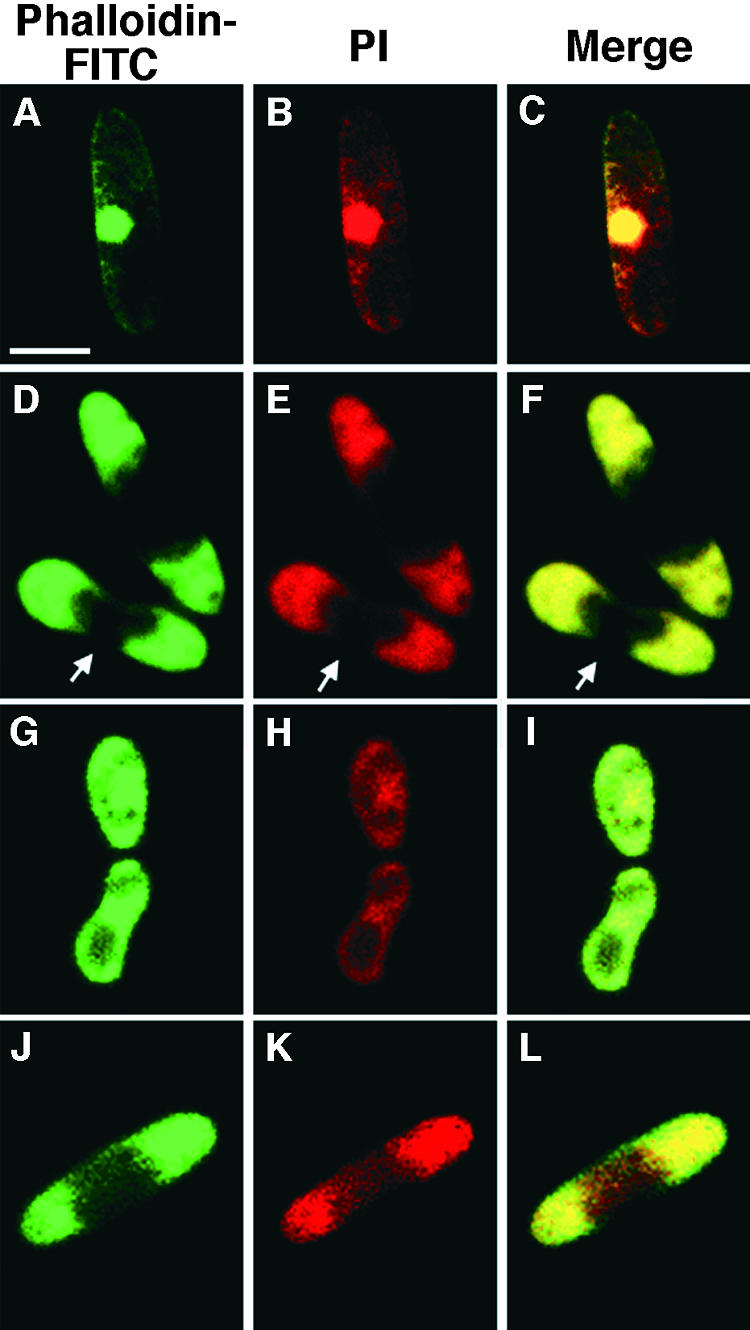
Confocal Microscopic Analysis of Germinating CgMEK1 Mutant Conidia and MEK Inhibitor–Treated Wild-Type Conidia.
(A) to (I) CgMEK1 mutant conidia were incubated on glass slides for 0 hr ([A] to [C]), 12 hr ([D] to [F]), or 24 hr ([G] to [I]) at a low population density (10 conidia per μL) in the absence of host chemical signals.
(J) to (L) Wild-type conidia, also at 10 conidia per μL, were incubated on glass slides for 12 hr in the presence of 10 μM PD98059.
Images were visualized by phalloidin-FITC signals (left), PI signals (middle), and merged images (right). Arrows indicate the constriction of the conidium. 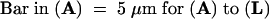 .
.
At a higher population density of conidia (50 per μL), progression in the cytochemical and morphological changes in the CgMEK1-disrupted conidia was greatly delayed, as was observed in the wild-type conidia (Figure 4B). Addition of avocado wax or ethephon stimulated cell division and germination, but appressorium formation was not initiated. The germ tubes of CgMEK1-disrupted conidia that formed in response to the addition of wax and ethephon were longer than those observed in the wild-type conidia. When the conidia were treated with both avocado wax and ethephon, >90% germinated but were arrested at stage 6, with none forming appressoria. In contrast, >95% of the wild type formed appressoria (Figure 4). Addition of yeast extract also overcame the blockage of development in stage 4, and most of the mutant conidia so treated proceeded through unequal distribution of F-actin, cell division, germination, and elongation of germ tubes (stage 6); however, they did not form appressoria (Figure 4) at any conidial population density (data not shown). Disruption of CgMEK1 resulted in complete inhibition of differentiation of the germ tubes (observed in both mutants 105 and 135).
An MEK Inhibitor Inhibits Hard Surface–Induced Cytokinesis and Septum Formation and Ethylene-Induced Appressoria Formation
Treating wild-type conidia at the low population density with PD98059, an inhibitor of MEK (Alessi et al., 1995), inhibited further development after the nuclear division and left the conidia blocked in stage 4 (Figure 4C) without preferential accumulation of F-actin at one end of the conidium. The cytochemical appearance of the conidia treated with the inhibitor arrested at this stage (Figures 5J to 5L) was very similar to that of the CgMEK1 mutant (Figures 5D to 5F). Adding ethylene overcame the inhibitory effects of PD98059 on cytokinesis and germination of conidia, but formation of appressoria was still effectively inhibited (Figure 4C). These results strongly suggest that MEK activity plays a dual role: one in polarized cell division and germination, and the other in the differentiation of the germ tube into an appressorium. The role of CgMEK1 in the former can be replaced by another CgMEK that is activated by the chemical signals from the host and is not sensitive to inhibition by PD98059, but appressorium formation requires CgMEK1, which is inhibited by this MEK inhibitor. Thus, the consequence of treatment with MEK inhibitor mimicked that resulting from CgMEK1 disruption, strongly suggesting that the observed consequences of CgMEK1 disruption are due to the absence of this MEK and not to other defects.
Protein Phosphorylation in the Early Phase of Germination and Appressorium Formation Induced by a Hard Surface and a Host Signal Is Suppressed by CgMEK1 Disruption
Protein phosphorylation has been shown to be involved in the early phase of germination and appressorium formation induced by ethylene and host wax in C. gloeosporioides (Flaishman et al., 1995; Kim et al., 1998). To test for the possible involvement of CgMEK1 in this phosphorylation, we examined in vivo protein phosphorylation enhanced by ethylene treatment of the conidia on a hard surface. Conidia of the wild type and the CgMEK1 mutant, prelabeled with 32P-phosphate for 3 hr, were placed on a glass plate and treated with ethylene for 2 hr. The protein phosphorylation pattern of the wild type was similar to that previously observed (Flaishman et al., 1995); this pattern was altered, however, by CgMEK1 disruption (Figure 6). Quantitation of the phosphorylation showed that the amounts of phosphorus-32 in the 29- and 43-kD proteins in the CgMEK1 mutant were 40 and 45%, respectively, less than those in the wild type. In contrast, the phosphorus-32 content of the other labeled proteins (at 22-, 20-, and 18-kD) in the CgMEK1 mutant was similar to that found in the wild type.
Figure 6.
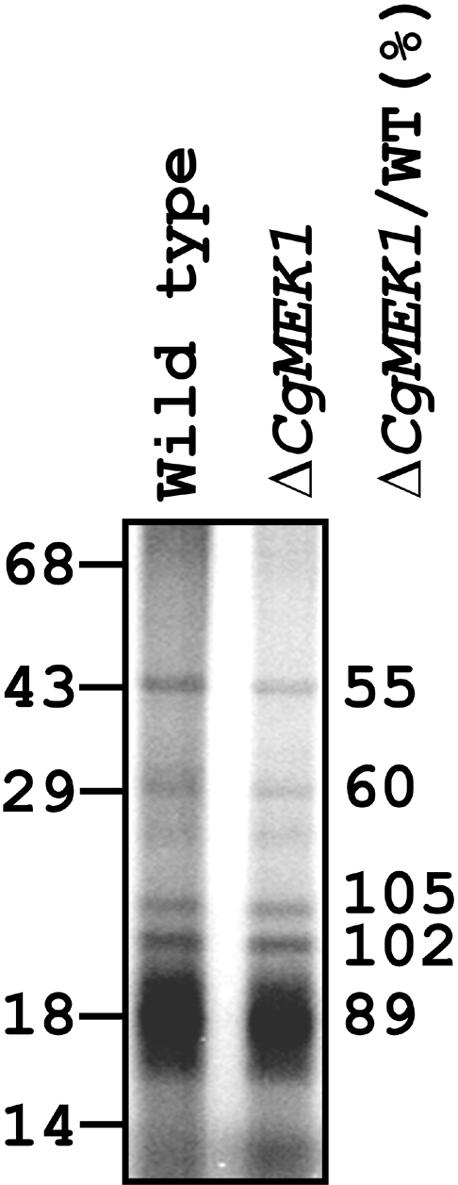
Phosphorylation of Proteins in the Spores of the Wild Type and CgMEK1 Mutants of C. gloeosporioides by Ethylene Treatment on a Hard Surface.
Spores were labeled with carrier-free inorganic 32P-phosphate for 3 hr and then plated onto Pyrex (Corning, Inc., Corning, NY) glass plates with ethephon (10 μM) for 2 hr. After SDS-PAGE of the proteins, the gel was dried and the radioactivity was detected and quantified with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). WT, wild type. Numbers at left and right indicate molecular weight and 32P labeling in the mutant as percent of the wild type, respectively.
Tests for Pathogenicity of the CgMEK1 Mutant on Avocado Fruits
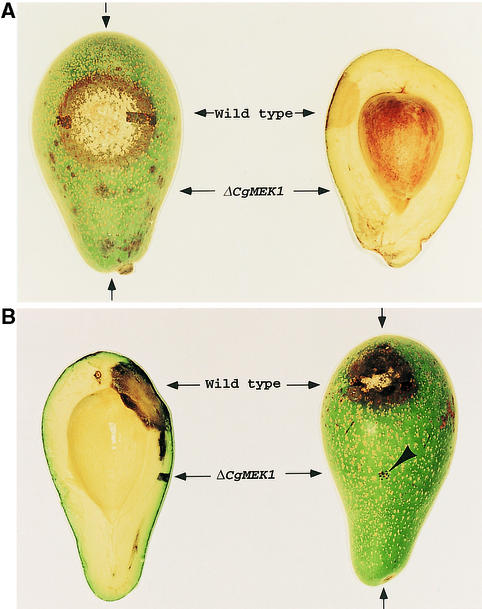
To test whether the gene-disrupted mutant is pathogenic on the natural host of C. gloeosporioides, we placed equal numbers (7500) of conidia from the CgMEK1 mutant and the wild type on avocado fruits and then incubated them under high humidity. The surface region on which wild-type conidia were placed showed dense mycelial growth in 6 days, whereas the surface region on which the conidia of the CgMEK1 mutant were placed appeared only slightly dark and had no evident signs of fungal growth (Figure 7A, left). To examine the penetration of infection by the pathogen and its CgMEK1 mutant into the host, we cut the fruit longitudinally across the lesions. The region at which wild-type conidia were placed showed clear symptoms of deep penetration of infection, almost reaching the seed (Figure 7A, right). However, the region at which the conidia of the CgMEK1 mutant were placed showed no infection, revealing that CgMEK1 disruption caused a loss in virulence against its natural host.
Figure 7.
Tests for Pathogenicity of the Wild Type and the CgMEK1 Mutant of C. gloeosporioides on Intact or Wounded Avocado Fruit.
(A) Cross-section of an avocado fruit (right) cut through the inoculated area (left).
(B) Cross-section of a needle-pricked avocado fruit (left) cut through the inoculated area (right).
Arrows at top and bottom indicate where avocado was cut. Arrowhead indicates pinpricked areas.
To test whether the gene-disrupted mutants can infect through wounds, we inoculated equal numbers (104) of conidia from the wild type and CgMEK1 mutants on a segment of the avocado fruits in which 10 pinpricks were made. The fruits were incubated in a high-humidity chamber, and the infection process was monitored. The regions inoculated with the wild-type conidia began to show the infection symptoms in 3 days, whereas the regions inoculated with the mutant conidia showed no clear external infection symptoms even after 10 days (Figure 7B, right). Longitudinal sectioning showed that the infection by the mutant was confined to the pricked regions, with very little invasive spread of the lesion into the host beyond that region (Figure 7B, left).
DISCUSSION
MAPK is involved in the signal transduction required for pathogenesis by Candida albicans and several phytopathogenic filamentous fungi (Hamer and Talbot, 1998; Madhani and Fink, 1998a, 1998b; Lev et al., 1999; Mayorga and Gold, 1999). Induction of germination and appressorium formation by host signals in C. gloeosporioides appeared to involve phosphorylation of certain proteins, suggesting the involvement of MEK in this process. To test for this possibility, we cloned an MEK from this organism and examined the consequences of disrupting this gene. Based on the results obtained with CgMEK1 mutants and the effects of an MEK inhibitor, we propose the ways in which MAPK may be involved in regulating cell division, germination, and appressorium formation in C. gloeosporioides conidia and the ways in which these processes are modulated by hard surface contact, self-inhibitors, host signals, and nutrients (Figure 8).
Figure 8.
Modulation of Processes by Hard Surface Contact, Self-Inhibitors, Host Signals, and Nutrients.
Shown are the effects of hard surface contact, self-inhibitors, host chemical signals, and nutrients on MAPK pathways that regulate discrete steps in germination and appressorium formation by C. gloeosporioides conidia. These roles are postulated based on our results.
Hard surface contact induces dispersal of F-actin and nuclear division, as we have observed. A MAPK is involved in bringing about a preferential accumulation of F-actin in one of the daughter nuclei and subsequent septum formation to complete the cell division followed by germination. In the absence of nutrients or host signals, the polar distribution of F-actin and subsequent events do not occur in CgMEK1 mutants, indicating that CgMEK1 can phosphorylate the MAPK (MAPKx) involved in this process. In the presence of host signals such as surface wax, ethylene, or nutrients (e.g., yeast extract), the CgMEK1 mutant undergoes normal cell division and germination but not appressorium formation. These chemicals probably function by activating an MEK (CgMEKx) that is distinctly different from CgMEK1. Because these chemicals do not induce appressorium formation in the CgMEK1 mutants, this differentiation process shows an absolute requirement for CgMEK1. Nutrients are postulated to activate CgMEKx, but not CgMEK1, because the nutrients cause cell division and germination but not appressorium formation. CgMEKx can phosphorylate MAPKx, which is involved in regulating cell division and germination, but not MAPK1, which is selectively involved in regulating appressorium formation. Otherwise, host signals and nutrients would have been able to induce appressorium formation in the CgMEK1 mutant, but they do not. Because hard surface contact, in the absence of self-inhibitors, can induce cell division, germination, and appressorium formation, we conclude that CgMEK1 activated by hard surface contact can phosphorylate both MAPKx and MAPK1. Our observation that in the presence of ethylene, CgMEK1 disruption causes only a 40 to 45% decrease in phosphorylation of the protein of MAPK size is consistent with the involvement of another MEK, CgMEKx, postulated here. Among the three sequentially activating kinase families in the MAPK cascade, MEK has been reported to have the fewest members, and these members are highly specific for their MAPK substrates (Garrington and Johnson, 1999).
Our current data and results from other systems (Cook et al., 1997; Madhani et al., 1997; Gustin et al., 1998; Madhani and Fink, 1998a, 1998b) suggest that MAPK signaling plays diverse roles in the determination of cell polarity in various fungi. Studies of appressorium-forming conidia have shown that polar growth of conidia is required for germination; a loss in the polarity in the germ tube growth results in the differentiation into appressoria (Hoch and Staples, 1991). Our data show that MAPK signaling is involved at stages during which actin-based cytoskeletal rearrangements occur, such as cell division and germination that involve polarity, and also at the stage of germ tube differentiation that involves cessation of polarized growth of the end of the germ tube. A MAPK was found to be required for filamentous growth and full virulence of U. maydis (Mayorga and Gold, 1999).
The involvement of MAPK signaling in polarized cell growth has been extensively studied in S. cerevisiae (Madhani and Fink, 1998a, 1998b). MAPK cascades such as Ste11p-Ste7p-Fus3p and Ste11p-Ste7p-Kss1p are required for mating and filamentation, respectively, showing that different MAPKs take part in pathway-specific developmental events, even though they share common upstream signaling components (Gustin et al., 1998). For example, STE7 deletion, or a mutation of kss1p that makes it unable to be phosphorylated, inhibits pseudohyphal development in yeast, whereas deletion of kss1p does not (Cook et al., 1997; Madhani et al., 1997; Bardwell et al., 1998). Therefore, it has been postulated that unphosphorylated Kss1p is inhibitory to filamentous growth and that Ste7p-catalyzed phosphorylation of Kss1p relieves this inhibition (Cook et al., 1997; Madhani et al., 1997). Similarly, in the present fungal pathogen, MAPKx in the unphosphorylated form may inhibit cytokinesis. Consistent with this view, we observed that the hard surface–induced cytokinesis was not inhibited by a MAPK inhibitor, SB209190, in the wild-type conidia of C. gloeosporioides at low population density—although it did effectively inhibit appressorium formation, probably by its inhibition of MAPK1 (data not shown). Thus, although the kinase activity of MAPKx may not be involved in cytokinesis and germination, relief of its inhibitory activity by phosphorylation by an MEK is necessary for cytokinesis and germination (Figure 7). This relief is provided by phosphorylation by either CgMEK1 or CgMEKx, which is activated by host chemicals or nutrients. PD98059 is known to be a relatively specific MEK inhibitor because it does not inhibit MAPK or MEKK or many other protein Ser/Thr kinases (Alessi et al., 1995). The postulated CgMEKx must be insensitive to PD98059, as has been observed for some of the mammalian MEKs (Pang et al., 1995; Kumar et al., 1998), whereas CgMEK1 is sensitive to this inhibitor, as is mammalian MEK1/2. Therefore, the inhibition of cytokinesis and germination by this MEK inhibitor, but not the inhibition of appressorium formation, was overcome with the chemical signals from the host, exactly as was seen with the CgMEK1 mutant. Thus, the roles postulated for MEKs on the basis of the results obtained with the CgMEK1 mutant (Figure 8) are strongly supported by the observation that the results obtained with an MEK inhibitor mimicked those obtained with the mutant. The validity of our analogy of the postulated MAPKx with Kss1p remains to be tested.
Phosphorylation of proteins other than MAPK is also involved in germination and appressorium formation. The phosphorylation of an 18-kD protein was little affected by CgMEK1 disruption. The high degree of phosphorylation of this protein suggests that it might be involved in an early stage in appressorium formation. In budding yeast, low molecular mass proteins such as Cdc42 are required to orient the actin cytoskeleton to form a bud during cytokinesis and to form mating projections. Phosphorylation of such proteins in chemotropic growth during the mating process does not involve MAPK signaling (Schrick et al., 1997), which is consistent with our finding that phosphorylation of the 18-kD protein is not much affected by CgMEK1 disruption. Multiple signal transduction pathways involving different kinases have been proposed to be involved in the induction of the developmental processes that lead to appressorium formation by hydrophobic hard surface contact and by chemical stimuli such as cAMP and plant wax components in M. grisea (Choi and Dean, 1997; Thines et al., 1997; Kronstad et al., 1998). However, the proteins that are phosphorylated by the various kinases have not been identified in fungal pathogens.
Because fungal conidia contain chemicals that inhibit germination and appressorium formation until they are well dispersed in a favorable environment (Macko, 1981; Tsurushima et al., 1995; Hegde and Kolattukudy, 1997; Liu and Kolattukudy, 1999), a high population density of conidia delays the entire process of germination and formation of appressoria (depending on the population density). However, little is known about the site of action of self-inhibitors in any fungus. Confocal microscopic examination showed that under high conidial population density conditions, dispersal of nuclear F-actin, nuclear division, and cell division—the earliest observable cytochemical events—were delayed or prevented in C. gloeosporioides. This observation is consistent with our previous finding that self-inhibitors suppress the earliest detectable gene expression (calmodulin gene) associated with conidial germination and appressorium formation triggered by hard surface contact (Liu and Kolattukudy, 1999). However, addition of host chemicals such as wax and ethylene reversed this inhibition and induced germination and appressorium formation. These observations are consistent with the notion that the role of self-inhibitors is to keep the conidia dormant until they reach an environment favorable to growth, and contact with the host would signal such a favorable environment.
If C. gloeosporioides infection requires appressorium formation, disruption of a gene required for this differentiation process would be expected to cause a loss in virulence. In fact, the CgMEK1 mutant clearly showed loss of virulence on its natural host. If the involvement of CgMEK1 is confined to the initial penetration into the host, then the mutant should be able to infect a host with a mechanically breached outer surface. However, infection by the mutant on pinpricked avocado fruits was confined to the wound region, with little further invasion of the host tissue, whereas the wild type readily infected the pricked fruit and extensively invaded the host tissue. This CgMEK1 is probably involved in the invasive growth beyond the initial penetration. A gene, Cap20, expressed in association with appressoria, was found to be expressed at the infection front deep inside the fruit (Hwang et al., 1995), and appressorium formation in the interior of plants has been previously reported (Freeman and Rodriguez, 1993). Colletotrichum lindemuthianum mycelia placed on mechanically produced wounds on bean hypocotyl could not invade the host, suggesting that the requirement of appressorium formation for the invasion of the host involves processes beyond the penetration of the outer barrier of the host (Bailey et al., 1992). Despite great progress in the use of resistance genes to engineer fungal resistance into plants, use of antifungal chemicals remains an important method of protecting plants. Molecular signaling pathways involved in pathogenic processes are targets exploited in the pharmaceutical industry. Similarly, molecular signals involved in the early phases of plant–fungus interaction may provide suitable targets for developing effective new antifungal treatments to protect plants.
METHODS
Materials and Bacterial Strains
Conidia from Colletotrichum gloeosporioides, isolated from avocados (Dr. Dov Prusky, Volcani Centre, Bet Dagan, Israel), were obtained from 5- to 7-day-old cultures (Hwang and Kolattukudy, 1995). Avocado fruits (Fuerte) were from Dr. John A. Menge (University of California, Riverside). All plasmids were propagated in Escherichia coli DH5α (Life Technologies, Rockville, MD). PD98059 was from Calbiochem (San Diego, CA), and Fluoromount-G mounting medium was from Southern Biotechnology (Birmingham, AL). Other chemicals were from Sigma (St. Louis, MO) or Amresco (Solon, OH), and labeled nucleotides were from Amersham (Arlington Heights, IL). Duralon-UV membranes were from Stratagene (La Jolla, CA). Restriction and modification enzymes and Taq DNA polymerase were from Life Technologies.
Polymerase Chain Reaction Amplification of cDNA Fragments Encoding Segments of CgMEK1
Degenerate oligonucleotides for the conserved catalytic domains VII (KL/ICDFG) and VIII (YMS/APER) (Hanks and Quinn, 1991) of the mitogen-activated protein kinase kinases (MEKs) were used for polymerase chain reaction (PCR): sense, 5′-AAA(G)ITITGT(C)GAT(C)TTT-(C)GGNGT-3′; and antisense, 5′-NCGT(C)TCNGGIIICATA(G)TA-3′. Single-stranded cDNA fragment templates (10 ng per reaction) used for PCR were constructed with an oligo(dT) primer and total RNA isolated from ethylene-treated conidia of C. gloeosporioides (2 hr in a liquid culture, shaken at 200 rpm, at ambient temperature). PCRs were performed in a total volume of 50 μL containing 200 μM of each dNTP, 1 μg of each primer, and 2.5 units of Taq DNA polymerase in buffer from the manufacturer (Life Technologies). The PCR was performed in a programmable thermal controller (MJ Research, Watertown, MA) for 40 cycles, each consisting of denaturation at 94°C for 1 min, annealing at 42°C for 30 sec., and polymerization at 72°C for 1 min. The PCR products were separated on 1.5% agarose gel, and the products of ∼90 bp were further isolated with a Mermaid kit (Bio 101, Vista, CA) and subcloned into a pCR2.1 vector (Invitrogen, Carlsbad, CA). Clones were sequenced with an automated sequencer from Applied Biosystems (Foster City, CA). The protein homology search was conducted with a BLAST program from the National Center for Biotechnology Information (Altschul et al., 1997). The one that shared the most sequence identity with MEK was used for further research.
Isolation of CgMEK1 cDNA Clone
Based on the nucleotide sequence of the 90-bp segment of the putative MEK, an antisense primer, 5′-GTCGATGTTCCAACGAAG-3′, was synthesized. This primer and an M13 sense primer were used for PCR with a cDNA library prepared with RNA isolated from the ethylene-treated conidia mentioned above as the template. The resulting 900-bp product was subcloned into pBluescript plasmid, yielding pCgMEK1-1. The insert of pCgMEK1-1 was used to screen a cDNA library constructed in a λZAP vector. The insert from a hybridizing clone was excised according to the instructions from Stratagene to yield the pBluescript plasmid pCgMEK1, which was then sequenced.
Determination of CgMEK1 Genomic Sequence
Screening a genomic library of C. gloeosporioides constructed in a λGEMII vector with the pCgMEK1-1 yielded λCgMEK1. After single phage was obtained, phage DNA was purified with a Qiagen (Valencia, CA) Lambda kit. A 9.0-kb fragment produced by SstI digestion was subcloned into a pCRII vector, yielding pCRII/EG5. A 3.1-kb segment of this insert, containing CgMEK1, was then sequenced.
Construction of a Disruption Vector and Fungal Transformation
The subcloning of a 1.9-kb fragment produced by KpnI and SmaI digestion of a genomic clone yielded pKS−/EG5 (1.9 kb). A SalI site was inserted into the coding region in the genomic fragment of CgMEK1 in pKS−/EG5 (1.9 kb) by inverse PCR with a sense primer, 5′-TGGAGAACTGATCAACTC-3′, and an antisense primer, 5′-GGCCGGGTCGACGACACGCCGAAATCGCAC-3′. The PCR fragment was treated with T4 DNA kinase, purified with a Geneclean kit (Bio 101), and self-ligated to generate pKS−/EG5(SalI). This construct was digested with SalI and dephosphorylated with calf intestinal alkaline phosphatase (Boehringer Mannheim). The SalI fragment carrying a hygromycin resistance gene (hph) was released from pCSN43 (Staben et al., 1989) and cloned into the SalI-digested pKS−/EG5(SalI) to generate the final construct pCgMEK1(hph) for CgMEK1 gene replacement. Transformation of C. gloeosporioides with this construct and selection of mutants were performed as described by Hwang et al. (1995).
Spore PCR to Identify CgMEK1 Mutants
Stable transformants selected with hygromycin (200 μg/mL) were sporulated on potato dextrose agar plates containing 1% avocado fruit pulp and no hygromycin. To test for gene disruption, we performed a junction PCR directly, using spores as the template source; an antisense primer from a segment of the CgMEK1 gene outside that used in constructing the replacement vector, 5′-CCTTGGGAATCGAATTGG-3′; and a sense primer from the hph gene, 5′-AGG-AGTCGCATAAGGGAG-3′. PCR products were electrophoresed on a 1% agarose gel, and those showing the expected 850-bp products were selected for further studies.
DNA Gel Blot Analysis of CgMEK1 Mutants and Wild Type
Genomic DNAs of the wild type and putative CgMEK1 mutants were prepared as described (Kämper et al., 1994), and 2 μg of DNA was completely digested with KpnI and SmaI. The digests were fractionated by electrophoresis on an 0.8% agarose gel, transferred to a Nytran nylon membrane (Nytran Plus; Schleicher and Schuell, Keene, NH) and hybridized at 65°C overnight to a 1.3-kb cDNA fragment, prepared by SmaI digestion of pCgMEK1, and 32P-labeled with a Rediprime kit (Amersham Pharmacia Biotech, Buckinghamshire, UK) After hybridization, the membranes were washed for 20 min at ambient temperature in 2 × SSPE (1 × SSPE is 0.15 M NaCl, 10 mM sodium phosphate, and 1 mM EDTA, pH 7.4) containing 0.1% SDS. Additional washing was performed with 0.1 × SSPE containing 0.1% SDS at 65°C for 20 min. The membranes were then exposed to x-ray film at −80°C.
Reverse Transcription–PCR Examination of Production of CgMEK1 Transcripts by the Wild Type and CgMEK1 Mutants of C. gloeosporioides
Conidia from the wild type and CgMEK1 mutants of C. gloeosporioides were treated with ethephon (10 μM) on a glass surface, the total RNA was isolated according to the methods described previously (Kim et al., 1998), and 5 μg of RNA was transcribed by SuperScript II H− reverse transcriptase (Bethesda Research Laboratories), using oligo(dT) to produce the first-strand cDNA in a 40-μL reaction mixture. A 1-μL aliquot of this mixture was used for PCR in a 50-μL reaction mixture with the sense primer 5′-CATCACATCATGCACCGAG-3′ and the antisense primer 5′-CTGGCGGGTTCGCGCGGG-3′. The PCR procedure consisted of an initial denaturation step at 94°C for 3 min and 25 cycles of the following steps: denaturation at 94°C for 30 sec, annealing at 56°C for 40 sec, and extension at 72°C for 1 min. A final elongation step was performed at 72°C for 5 min. The PCR product was analyzed on a 1% agarose gel.
Tests for Germination and Appressorium Formation of the Wild Type and CgMEK1 Mutants of C. gloeosporioides
Germination and appressorium formation of wild-type C. gloeosporioides and CgMEK1 mutants were tested on a cover glass surface in the presence of ethephon (10 μM), avocado wax (100 μg/mL), or yeast extract (0.5%) as described previously (Podila et al., 1993; Kim et al., 1998).
Confocal Microscopic Analysis
The conidia germinated on glass slides for different periods of time were fixed with 4% paraformaldehyde, pH 7.0, at room temperature for 15 min and washed twice in 20 mM phosphate buffer, pH 7.0. The conidia were then incubated with 0.05 mg/mL phalloidin–fluorescein isothiocyanate (FITC; Sigma) and 5 μg/mL propidium iodide (PI) for 40 min at room temperature in a humidified chamber. The glass slides were washed three times in 20 mM phosphate buffer, pH 7.0, for a total of 30 min, cover-slipped with Fluoromount-G mounting media (Southern Biotechnology), and visualized with a Bio-Rad MRC 1024 confocal microscope. The FITC- and PI-dependent fluorescence signals were detected using 480- and 560-nm excitation filters, respectively.
Conidia showing cytochemical and morphological changes during germination and differentiation of germ tubes into appressoria were scored under a confocal microscope. Typical data from triplicate samples are shown in Figure 5. In each replicate, ∼150 to 200 conidia were examined.
Inhibitor Treatments
Conidia were incubated with 10 μM PD98059 or 30 μM SB202190 for 12 hr on glass slides. Cytochemical and morphological changes in the conidia were examined by confocal microscopy as above.
Pathogenicity Tests
Pathogenicity of the wild-type pathogen and the gene-disrupted mutant was tested on avocado fruits as described previously (Hwang et al., 1995) with minor modifications. On each surface-sterilized fruit, we placed 7500 conidia per cm2 in 200 μL of water. To test for infection on wounded avocado fruits, we pricked each fruit 10 times with a needle (26G5/8) within a 1-cm2 surface area and then placed 10,000 conidia per cm2 in 200 μL of water on the wound. The inoculated fruits were incubated at room temperature under high humidity for 6 to 10 days in a closed chamber. When the fruits inoculated with the wild type showed lesions in the area at which the spore suspension had been placed, the fruits were longitudinally cut across the infection sites and photographed with a Nikon camera (model FM2) at shutter speed 1/1 without filter under fluorescent light.
Protein Phosphorylation
Protein phosphorylation was examined as described by Kim et al. (1998).
Acknowledgments
This work was supported in part by National Science Foundation grants (Nos. IBN-9816868 and IBN-9318544). We thank Drs. John A. Menge and Elinor Pond for generously providing us with avocado fruits and Linda Rogers and Todd Walls for assistance in preparing the manuscript.
References
- Alessi, D.R., Cuenda, A., Cohen, P., Dudley, D.T., and Saltiel, A.R. (1995). PD 09809 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270, 27489–27494. [DOI] [PubMed] [Google Scholar]
- Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, J. (1997). Gapper BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, J.A., O'Connell, R.J., Pring, R.J., and Nash, C. (1992). Infection strategies of Colletotrichum species. In Colletotrichum: Biology, Pathology, and Control, J.A. Bailey and M.J. Jeger, eds (Oxon, UK: CAB International), pp. 88–120.
- Banuett, F., and Herskowitz, I. (1994). Identification of Fuz7, a Ustilago maydis MEK/MAPKK homolog required for a locus-dependent and -independent steps in the fungal life cycle. Genes Dev. 8, 1367–1378. [DOI] [PubMed] [Google Scholar]
- Bardwell, L., Cook, J.G., Voora, D., Baggott, D.M., Martinez, A.R., and Thorner, J. (1998). Repression of yeast Ste12 transcription factor by direct binding of unphosphorylated Kss1 MAPK and its regulation by the Ste7 MEK. Genes Dev. 12, 2887–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslawski, G., and Polazzi, J.O. (1987). Complete nucleotide sequence of a gene conferring polymyxin B resistance on yeast: Similarity of the predicted polypeptide to protein kinases. Proc. Natl. Acad. Sci. USA 84, 5848–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, W.B., and Dean, R.A. (1997). The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell 9, 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, J.G., Bardwell, L., and Thorner, J. (1997). Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signalling pathway. Nature 390, 85–88. [DOI] [PubMed] [Google Scholar]
- Emmett, R.W., and Parbery, D.G. (1975). Appressoria. Annu. Rev. Phytopathol. 13, 147–167. [Google Scholar]
- Flaishman, M.A., and Kolattukudy, P.E. (1994). Timing of fungal invasion using host's ripening hormone as a signal. Proc. Natl. Acad. Sci. USA 91, 6579–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaishman, M.A., Hwang, C.-H., and Kolattukudy, P.E. (1995). Involvement of protein phosphorylation in the induction of appressorium formation in Colletotrichum gloeosporioides by its host surface wax and ethylene. Physiol. Mol. Plant Pathol. 47, 103–117. [Google Scholar]
- Freeman, S., and Rodriguez, R.J. (1993). Genetic conversion of a fungal pathogen to a nonpathogenic, endophytic mutualist. Science 260, 75–78. [DOI] [PubMed] [Google Scholar]
- Garrington, T.P., and Johnson, G.L. (1999). Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11, 211–218. [DOI] [PubMed] [Google Scholar]
- Grover, G.K. (1971). Participation of host exudate chemicals in appressorium formation by Colletotrichum piperatum. In Ecology of Leaf Surface Microorganisms, T.F. Preece and C.H. Dickinson, eds (London: Academic Press), pp. 509–518.
- Gustin, M.C., Albertyn, J., Alexander, M., and Davenport, K. (1998). MAP kinase pathway in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62, 1226–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, J.E., and Talbot, N.J. (1998). Infection-related development in the rice blast fungus Magnaporthe grisea. Curr. Opin. Microbiol. 1, 693–697. [DOI] [PubMed] [Google Scholar]
- Hanks, S.K., and Quinn, A.M. (1991). Protein kinase catalytic domain sequence database: Identification of conserved features of primary structure and classification of family members. Methods Enzymol. 200, 38–62. [DOI] [PubMed] [Google Scholar]
- Hegde, Y., and Kolattukudy, P.E. (1997). Cuticular waxes relieve self- inhibition of germination and appressorium formation by the conidia of Magnaporthe grisea. Physiol. Mol. Plant Pathol. 51, 75–84. [Google Scholar]
- Herskowitz, I. (1995). MAP kinase pathways in yeast: For mating and more. Cell 80, 187–197. [DOI] [PubMed] [Google Scholar]
- Hoch, H.C., and Staples, R.C. (1991). Signaling for infection structure formation in fungi. In The Fungal Spore and Disease Initiation in Plants and Animals, G.T. Cole and H.C. Hoch, eds (New York: Plenum Press), pp. 25–46.
- Hwang, C.-S., and Kolattukudy, P.E. (1995). Isolation and characterization of genes expressed uniquely during appressorium formation by Colletotrichum gloeosporioides conidia induced by the host surface wax. Mol. Gen. Genet. 247, 282–294. [DOI] [PubMed] [Google Scholar]
- Hwang, C.-S., Flaishman, M.A., and Kolattukudy, P.E. (1995). Cloning of a gene expressed during appressorium formation by Colletotrichum gloeosporioides and a marked decrease in virulence by disruption of this gene. Plant Cell 7, 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie, K., Takase, M., Lee, K.S., Levin, D.E., Araki, H., Matsumoto, K., and Oshima, Y. (1993). MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell Biol. 13, 3076–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämper, J.T., Kämper, U., Rogers, L.M., and Kolattukudy, P.E. (1994). Identification of regulatory elements in the cutinase promoter from Fusarium solani f. sp. pisi. J. Biol. Chem. 269, 9195–9204. [PubMed] [Google Scholar]
- Kim, Y.-K., Li, D., and Kolattukudy, P.E. (1998). Induction of Ca2+-calmodulin signaling by hard-surface contact primes Colletotrichum gloeosporioides conidia to germinate and form appressoria. J. Bacteriol. 180, 5144–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy, P.E., Rogers, L.M., Li, D., Hwang, C.-S., and Flaishman, M.A. (1995). Surface signaling in pathogenesis. Proc. Natl. Acad. Sci. USA 92, 4080–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad, J., De Maria, A.D., Funnell, D., Laidlaw, R.D., Lee, N., de Sa, M.M., and Ramesh, M. (1998). Signaling via cAMP in fungi: Interconnections with mitogen-activated protein kinase pathways. Arch. Microbiol. 170, 395–404. [DOI] [PubMed] [Google Scholar]
- Kumar, A., Middleton, A., Chambers, T.C., and Mehta, K.D. (1998). Differential roles of extracellular signal-regulated kinase-1/2 and p38MAPK in interleukin-1β and in HepG2 cells. J. Biol. Chem. 273, 15742–15748. [DOI] [PubMed] [Google Scholar]
- Lapp, M.S., and Skoropad, W.P. (1978). Location of appressoria of Colletotrichum graminicola on natural and artificial barley leaf surfaces. Trans. Br. Mycol. Soc. 70, 225–228. [Google Scholar]
- Lev, S., Sharon, A., Hadar, R., Ma, H., and Horwitz, B.A. (1999). A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: Diverse roles for mitogen-activated protein kinase homologs in foliar pathogens. Proc. Natl. Acad. Sci. USA 96, 13542–13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D., Rogers, L.M., and Kolattukudy, P.E. (1997). Cloning and expression of cDNA encoding a mitogen-activated protein kinase from a phytopathogenic filamentous fungus. Gene 195, 161–166. [DOI] [PubMed] [Google Scholar]
- Liu, Z.-M., and Kolattukudy, P.E. (1999). Early expression of calmodulin gene, which precedes appressorium formation in Magnaporthe grisea, is inhibited by self-inhibitors and requires surface attachment. J. Bacteriol. 181, 3571–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macko, V. (1981). Inhibitors and stimulants of spore germination and infection structure formation in fungi. In The Fungal Spore Morphogenetic Controls, G. Turian and H.R. Holh, eds (New York: Academic Press), pp. 565–584.
- Madhani, H.D., and Fink, G.R. (1998. a). The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 8, 348–353. [DOI] [PubMed] [Google Scholar]
- Madhani, H.D., and Fink, G.R. (1998. b). The riddle of MAP kinase signaling specificity. Trends Genet. 14, 151–155. [DOI] [PubMed] [Google Scholar]
- Madhani, H.D., Styles, C.A., and Fink, G.R. (1997). MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell 91, 673–674. [DOI] [PubMed] [Google Scholar]
- Marshall, C.J. (1994). MAP kinase kinase kinase, MAP kinase kinase, and MAP kinase. Curr. Opin. Genet. Dev. 4, 82–89. [DOI] [PubMed] [Google Scholar]
- Mayorga, M.E., and Gold, S.E. (1999). A MAP kinase encoded by the ubc3 gene of Ustilago maydis is required for filamentous growth and full virulence. Mol. Microbiol. 34, 485–497. [DOI] [PubMed] [Google Scholar]
- Nishida, E., and Gotoh, Y. (1993). The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 18, 128–131. [DOI] [PubMed] [Google Scholar]
- Pang, L., Sawada, T., Decker, S.J., and Saltid, A.R.. (1995). Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J. Biol. Chem. 270, 13585–13588. [DOI] [PubMed] [Google Scholar]
- Parbery, D.G., and Blakeman, J.P. (1978). Effect of substances associated with leaf surfaces on appressorium formation by Colletotrichum acutatum. Trans. Br. Mycol. Soc. 70, 7–19. [Google Scholar]
- Podila, G.K., Rogers, L.M., and Kolattukudy, P.E. (1993). Chemical signals from avocado surface wax trigger germination and appressorium formation in Colletotrichum gloeosporioides. Plant Physiol. 103, 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrick, K., Garvik, B., and Hartwell, L.H. (1997). Mating in Saccharomyces cerevisiae: The role of the pheromone signal transduction pathway in the chemotropic response to pheromone. Genetics 147, 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staben, C., Jensen, B., Singer, M., Pollock, J., Schechtman, M., Kinsey, J., and Selker, E. (1989). Use of a bacterial Hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet. Newsl. 36, 79–81. [Google Scholar]
- Staples, R.C., and Hoch, H.C. (1987). Infection structures—Form and function. Exp. Mycol. 11, 163–169. [Google Scholar]
- Teague, M.A., Chaleff, D.T., and Errede, B. (1986). Nucleotide sequence of the yeast regulatory gene STE7 predicts a protein homologous to protein kinases. Proc. Natl. Acad. Sci. USA 83, 7371–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines, E., Elibert, F., Sterner, O., and Anke, H. (1997). Glisprenin A, an inhibitor of the signal transduction pathway leading to appressorium formation in germinating conidia of Magnaporthe grisea on hydrophobic surfaces. FEMS Microbiol. Lett. 151, 219–224. [Google Scholar]
- Tsurushima, T., Ueno, T., Fukami, H., Irie, H., and Inoue, M. (1995). Germination self-inhibitors from Colletotrichum gloeosporioides f. sp. jussiaea. Mol. Plant-Microbe Interact. 8, 652–657. [Google Scholar]
- Wulf, E., Deboben, A., Bautz, F.A., Faulstich, H., and Wieland, T.H. (1979). Fluorescent phalloidin, a tool for the visualization of cellular actin. Proc. Natl. Acad. Sci. USA 76, 4498–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, J.-Z., Watanabe, T., Sekido, S., Choi, W.-B., Kamakura, T., and Yamaguchi, L. (1997). An anti-hydrotactic response and solid surface recognition of germ tubes of the rice blast fungus, Magnaporthe grisea. Biosci. Biotechnol. Biochem. 61, 1225–1229. [Google Scholar]
- Xu, J.-R., and Hamer, J.E. (1996). MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 10, 2696–2706. [DOI] [PubMed] [Google Scholar]
- Xu, J.-R., Staiger, C.J., and Hamer, J.E. (1998). Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc. Natl. Acad. Sci. USA 95, 12713–12718. [DOI] [PMC free article] [PubMed] [Google Scholar]