Abstract
A key step for nitrate assimilation in photosynthetic eukaryotes occurs within chloroplasts, where nitrite is reduced to ammonium, which is incorporated into carbon skeletons. The Nar1 gene from Chlamydomonas reinhardtii is clustered with five other genes for nitrate assimilation, all of them regulated by nitrate. Sequence analysis of genomic DNA and cDNA of Nar1 and comparative studies of strains having or lacking Nar1 have been performed. The deduced amino acid sequence indicates that Nar1 encodes a chloroplast membrane protein with substantial identity to putative formate and nitrite transporters in bacteria. Use of antibodies against NAR1 has corroborated its location in the plastidic membrane. Characterization of strains having or lacking this gene suggests that NAR1 is involved in nitrite transport in plastids, which is critical for cell survival under limiting nitrate conditions, and controls the amount of nitrate incorporated by the cells under limiting CO2 conditions.
INTRODUCTION
In photosynthetic eukaryotes, nitrate assimilation involves two consecutive reduction steps, which are delimited intracellularly by two membrane barriers—the plasma membrane and the chloroplast membrane. The first reduction step occurs in the cytosol and is catalyzed by the nitrate reductase (NR) enzyme. The second reduction step occurs in the chloroplast, where nitrite reductase (NiR) catalyzes the reduction to ammonium of the nitrite formed by the action of NR. Finally, ammonium is incorporated into carbon skeletons by the glutamine synthetase–glutamate synthase cycle (Hoff et al., 1994; Crawford, 1995).
Intensive efforts have been devoted to identifying and characterizing nitrate transporters involved in the plasma membrane barrier for two reasons. First, nitrate entry to the cell requires specific transporters (Tanner and Caspari, 1996). Second, in contrast to NR and NiR, which are encoded by single-copy genes in many organisms (Johnstone et al., 1990; Zhou and Kleinhofs, 1996; Fernández et al., 1998), transporter systems are redundant and seem to play a key role in plant nutrition by regulating nitrate assimilation according to the environmental and nutritional conditions (Crawford and Glass, 1998). These nitrate transporter genes have been classified into two families, Nrt1 and Nrt2 (Crawford and Glass, 1998). The Nrt1 family was originally proposed to encode low-affinity nitrate transporters (LANTs) (Tsay et al., 1993), and some members of this family transport basic amino acids as well with a similar efficiency (Zhou et al., 1998). Recently, the Arabidopsis Chl1 gene (the first Nrt1 gene identified) has been reported to produce a system with a dual function, having both high- and low-affinity nitrate transport activity (Wang et al., 1998; Liu et al., 1999). The Nrt2 family encodes high-affinity nitrate transporters (HANTs), and its members have been identified in fungi (Unkles et al., 1991), algae (Quesada et al., 1994), and yeast (Pérez et al., 1997). Nrt2 genes have also been cloned from plants (Trueman et al., 1996; Quesada et al., 1997; Amarasinghe et al., 1998).
In the unicellular green alga Chlamydomonas reinhardtii, four high-affinity nitrate/nitrite transporters (HANT/HANiT) have been characterized. System I corresponds to a bispecific HANT/HANiT encoded by Nrt2;1/Nar2; system II corresponds to a monospecific HANT encoded by Nrt2;2/Nar2 (Quesada et al., 1994; Galván et al., 1996); system III is a bispecific HANiT and LANT, probably encoded by Nrt2;3 (Quesada et al., 1998a; Rexach et al., 1999; Navarro et al., 2000); and system IV is a bispecific HANT/HANiT, probably encoded by Nrt2;4 (Rexach et al., 1999; Navarro et al., 2000). These four systems are involved in the entry of nitrate/nitrite into the cell and thus help regulate the pathway by providing nitrate/nitrite to the cells.
The transport step of nitrite into the chloroplast is not well understood, and information at the molecular level is lacking. Some authors suggest that this is a regulated process mediated by a saturable nitrite transporter (Brunswick and Cresswell 1988a, 1988b; Krämer et al., 1988), but others propose that there is no need for such a plastidic transporter because the nitrite could efficiently diffuse as nitrous acid (Shingles et al., 1996).
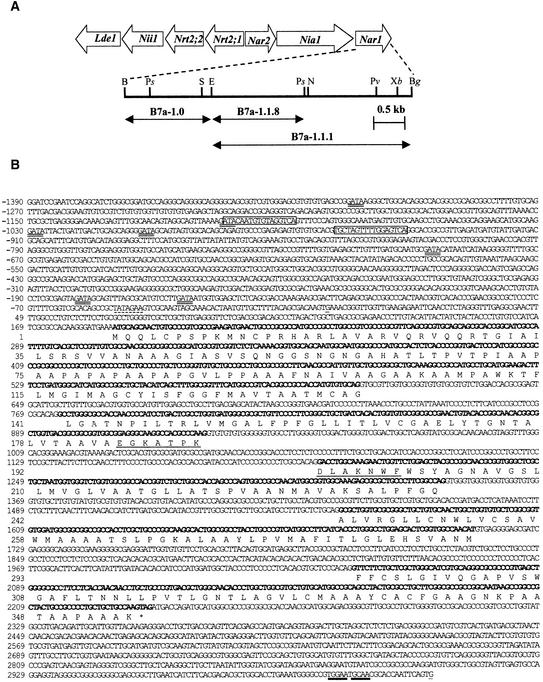
In Chlamydomonas, six genes related to nitrate assimilation are clustered within a 45-kb genome region in linkage group IX, the same region in which the Nar1 gene is located at the 3′ end of the NR gene Nia1 (Figure 1A) (Quesada et al., 1993, 1998b). Nar1 seems to have a role in nitrate assimilation, because it shows coordinated regulation with other genes for nitrate assimilation in Chlamydomonas: induction by nitrate, repression by ammonium, and control by the positive regulatory gene Nit2 (Quesada et al., 1993, 1998b).
Figure 1.
The Nitrate Gene Cluster from Chlamydomonas and DNA Sequence of the Nar1 Gene.
(A) Scheme of the Chlamydomonas nitrate assimilation gene cluster, showing details within the Nar1 region for the different subclones (arrows), and the physical map. B, BamHI; Bg, BglII; E, EcoRI; N, NotI; Ps, PstI; Pv, PvuII; S, SstI; and Xb, XbaI.
(B) Genomic sequence (GenBank accession number AF149738), exons deduced from the cDNA sequence (GenBank accession number AF149737) in bold, and deduced amino acid sequence are shown. Underlined G (position +1) indicates the cDNA 5′ end. A putative TATA box is indicated with a dashed line. The putative nitrate-dependent transcription motifs are boxed. GATA boxes are double underlined. Two contiguous noncanonical polyadenylation sequences in Chlamydomonas are underlined with thick lines. Positions 2097 to 2119, corresponding to the primer used in the RACE polymerase chain reaction, are shown in italic. The 14-mer oligopeptide used to raise the specific NAR1 antibody is underlined.
Here, we show that Nar1 encodes an integral membrane protein located in the chloroplast, that it is essential for cell growth under limiting nitrate availability, and that it allows for regulation of nitrogen assimilation, depending on the carbon supply to the cells. We also demonstrate that nitrite transport to the chloroplast is a regulated process in which NAR1 plays an important role.
RESULTS
Analysis of the Nar1 Genomic and cDNA Sequences
The Nar1 genomic fragment B7a-1.1.8 (Figure 1A) was used as a probe to screen a Chlamydomonas cDNA library. One clone with a 1.7-kb insert was isolated and sequenced. Because this clone was shorter that the 1.8 kb predicted for a full-length Nar1 cDNA, a 5′ rapid amplification of cDNA ends (RACE) polymerase chain reaction was performed to isolate the 5′ end cDNA sequence. The genomic region BamHI-BglII containing Nar1 was also sequenced and compared with the sequence of the cDNA (Figure 1B). The open reading frame in the cDNA clone encoded a polypeptide of 355 residues containing a putative chloroplast transit peptide sequence (Franzén et al., 1990) covering the first 92 residues. The genomic sequence was interrupted by four introns (Figure 1B), with sequences at their 5′ and 3′ ends, (G,T)/GTGNG and CAG/G, respectively, matching the typical splicing sequences from eukaryotes (Breathnach and Chambon, 1981). The promoter region, which would cover 1390 bp, contains a putative TATA box (position −47 from the beginning of the cDNA), two nitrate-dependent transcription motifs corresponding to an AT-rich sequence followed by (G,A)(C,G)TCA (Hwang et al., 1997), and several GATA sequences, two pairs of which were separated by 20 to 22 bp (Rastogi et al., 1997).
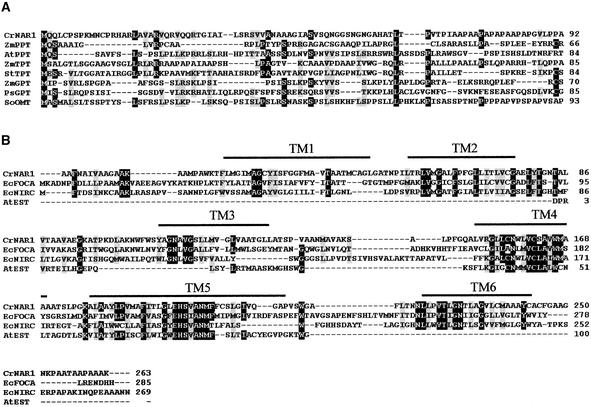
The putative transit peptide of NAR1 shows some features of typical transit peptides in Chlamydomonas (Franzén et al., 1990) and a small region conserved along with the long transit peptide sequences of glucose 6-phosphate/phosphate, phosphoenolpyruvate/phosphate, triose phosphate/phosphate, and the oxoglutarate/malate translocators from chloroplast envelope membranes (Figure 2). These transit peptide sequences, which are structurally different from those of imported stromal or thylakoid proteins, consist of ∼70 to 90 amino acids, with a slight conservation among them, and the processed mature proteins have an N-terminal alanine (Brink et al., 1995; Weber et al., 1995; Kammerer et al., 1998). The putative mature NAR1 protein (263 amino acids) shows a high identity with putative formate transporters from Escherichia coli (32%) (GenBank accession number P21501; Suppmann and Sawers, 1994) and Methanobacterium phormicium (31%) (GenBank accession number P35839; White and Ferry, 1992) as well as with putative nitrite transporters NIRC from E. coli (26%) (GenBank accession number P11097; Peakman et al., 1990) and Salmonella typhimurium (28%) (GenBank accession number P25926; Wu et al., 1991). A partial expressed sequence tag from Arabidopsis, with unknown function, showed some identity to NAR1 (30%) (GenBank accession number N37972; Newman et al., 1994). These bacterial proteins and NAR1 show a very similar hydropathy profile (Kyte and Doolittle, 1982), with six predicted membrane-spanning segments (TMPRED) (PSORTII program; http:/psort.nibb.ac.jp) within which there appears to be the highest conservation among the sequences, especially in transmembrane domains 4 and 5 (Figure 2).
Figure 2.
Alignment of Sequences from NAR1, Chloroplast Envelope Membrane Transit Peptides, and Bacterial Anion Transporters.
(A) Alignment between chloroplast transit peptides from NAR1 and the envelope membrane proteins phosphoenolpiruvate/phosphate translocators from maize (ZmPPT; GenBank accession number U66404) and Arabidopsis (AtPPT; U66321), the triose phosphate/phosphate translocators from maize (ZmTPT; Z26595) and Solanum tuberosum (StTPT; X67045), the glucose 6-phosphate/phosphate translocators from maize (ZmGPT; AF020813) and P. sativum (PsGPT; AF020814), and the oxolutarate/malate translocator from spinach (SoOMT; Q41364). Identical and similar amino acids are shaded in black and gray, respectively. Dashes indicate artificially introduced gaps to allow optimal alignment. Numbers at right indicate position of the last amino acid in the line of the respective protein.
(B) Alignment between the Chlamydomonas NAR1 mature protein and E. coli FOCA (EcFOCA; P21501), E. coli NIRC (EcNIRC; P11097), and an Arabidopsis expressed sequence tag (AtEST; N37972). Other details are as given in (A).
Immunodetection of NAR1
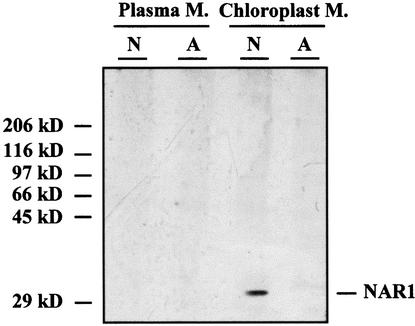
To verify the localization of NAR1 in the chloroplast membranes, we raised antibodies against an antigenic NAR1 oligopeptide (Figure 1B) and used them to immunodetect NAR1 in a protein gel blot from Chlamydomonas membrane preparations (Figure 3). No immunoprecipitate bands were obtained from plasma or chloroplast membranes from ammonium-grown cells. A protein band of ∼30 kD was immunodetected only from chloroplast membranes from cells that had been induced with nitrate; the size is compatible with that expected from the sequence data for the mature NAR1 protein. The expression pattern of the protein also matches the data for expression of Nar1 transcripts, which are induced in nitrate-grown and repressed in ammonium-grown cells (Quesada et al., 1993). Antibodies against NRT2;1 were used as a control in both membrane preparations. In this case, a 55-kD band immunodetected in plasma membranes from nitrate-induced cells was absent both in plasma membranes from ammonium-grown cells and in chloroplast membranes from nitrate-induced cells (M. Pozuelo, E. Fernández, and A. Galván, unpublished results).
Figure 3.
Protein Gel Blot of Plasma and Chloroplast Membrane Proteins from Chlamydomonas.
Cells were grown in ammonium (A) or induced in nitrate media (N). Plasma and chloroplast membranes (M.) were isolated and then analyzed for immunodetection of NAR1 as indicated in Methods. Molecular size markers are indicated at left.
Construction and Characterization of Chlamydomonas Strains Having or Lacking Nar1
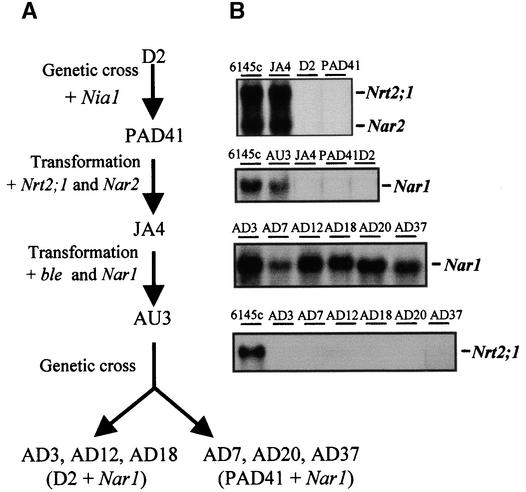
Although Chlamydomonas strains lacking the Nar1 gene (i.e., the D2 strain) were available, the function of NAR1 in nitrate assimilation appeared elusive, given that this strain grows in nitrite-containing media and efficiently assimilates nitrite (Quesada et al., 1993; Rexach et al., 1999). Accordingly, to gain information on the role of Nar1 in nitrate assimilation, we developed the following strategy. Chlamydomonas strains having or not having functional genes for NR and the HANT (Nar2, Nrt2;1) and either possessing or lacking Nar1 were isolated (see scheme illustrated in Figure 4A). The starting strain D2 is a mutant carrying a 15-kb deletion on the nitrate gene cluster, thus lacking Nar1, Nia1, Nar2, Nrt2;1, and Nrt2;2 (Quesada et al., 1993). First, a functional NR gene was introduced by genetic cross in a D2 genetic background to obtain the strain PAD41. That is, strain PAD41 is a segregant from the genetic cross between strains D2 and T7, which is a transformant of the NR mutant 305 that carries an unlinked copy of the Nia1 gene (Kindle et al., 1989). The selection of these NR+ strains, which are unable to grow in nitrate-containing media, was performed by measuring NR activity in the segregants. Second, strain PAD41 was transformed with plasmid pT2CO-5, which carried functional genes for HANT (Nrt2;1 and Nar2); the transformants were selected from nitrate growth tests (Quesada et al., 1994; Galván et al., 1996), which allowed the isolation of strain JA4. Third, the Nar1 gene was transferred to strain JA4 by transformation with a plasmid (pBleNar1) carrying the Nar1 gene and the bleomycin resistance marker (Stevens et al., 1996). Several antibiotic-resistant transformants were obtained, but only one, strain AU3, showed an intact copy of Nar1. Finally, a genetic backcross between strains AU3 and D2 allowed us to obtain segregant strains that were Nar1+ but lacked the HANT gene Nrt2;1 and either lacked (strains AD3, AD12, and AD18) or had (AD7, AD20, and AD37) the Nia1 gene. RNA gel blots of nitrate-induced cells were performed to demonstrate the expression of Nrt2;1, Nar2, and Nar1 (Figure 4B).
Figure 4.
Strategy for Constructing Chlamydomonas Strains Able to Assimilate Nitrate or Nitrite and Either Having or Lacking a Functional Nar1 Gene.
(A) The starting Chlamydomonas strain D2 is a deletion mutant (ΔNar1, Nia1, Nar2, Nrt2;1, and Nrt2;2) in which a functional NR gene, Nia1, was introduced by genetic cross, resulting in strain PAD41. Strain JA4 is a transformant of PAD41 with plasmid pT2-CO5, carrying functional genes for HANT (Nrt2;1 and Nar2). Strain AU3 was isolated from transformants of JA4 with plasmid pBleNar1, carrying the Nar1 gene and the bleomycin resistance marker. The genetic cross between strain AU3 and D2 segregated Nar1+ strains lacking the HANT gene Nrt2;1 and either lacking (strains AD3, AD12, and AD18) or having (AD7, AD20, and AD37) the Nia1 gene.
(B) Total RNA was isolated from nitrate-induced cells, and expression of Nrt2;1, Nar2, and Nar1 was analyzed in RNA gel blots with 15 μg of RNA per lane and using the corresponding gene probes.
This strategy allowed us to study the Nar1 function by comparing strains Nar1+ and Nar1−, and those containing NR and HANT genes and thus able to assimilate nitrate (strain AU3 Nar1+ versus strain JA4 Nar1−). Similarly, Nar1 function was studied in strains that assimilated only nitrite and lacked NR and HANT genes (strains AD3, AD12, and AD18 Nar1+ versus D2 Nar1−) or that contained the NR gene but lacked HANT genes (strains AD7, AD20, and AD37 Nar1+ versus PAD41).
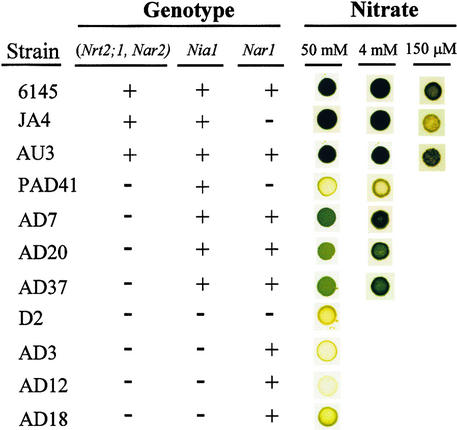
The phenotype for growth on solid media containing 50 or 4 mM nitrate was first examined (Figure 5). Strains JA4 and AU3, which have both HANT (Nrt2;1 and Nar2) and NR, grew properly on these media. Thus, Nar1 seems to be dispensable for growth in nitrate, because its absence in strain JA4 did not result in any particular phenotype. However, when intracellular nitrate reduction was limited, that is, in strains lacking Nrt2;1 and Nar2 genes and having NR (PAD41 versus AD7, AD20, and AD37), the presence of NAR1 seemed to be essential for growth (Figure 5). This growth was slow but substantial with respect to strain PAD41. These results show that NAR1 is required for growth in nitrate-containing media when only small amounts of nitrate can enter the cells. Limiting nitrate concentrations were also supplied to strains AU3 and JA4 by way of 150 μM nitrate-containing media (Figure 5). Strain AU3, which is Nar1+, grew markedly better than did strain JA4 (Nar1−) under this condition.
Figure 5.
Phenotype for Nitrate Growth in Solid Media of Constructed Chlamydomonas Strains with Different Genotypes for Nitrate Assimilation.
Approximately 103 cells were inoculated onto solid minimal media containing 50 mM, 4 mM, or 150 μM nitrate as the only nitrogen source. The figure shows the green in the colonies after 7 days for strains 6145c, JA4, and AU3 and after 20 days for all other strains.
Nitrite Transport Activity by Intact Chloroplast and Whole Cells
The deduced amino acid sequence of NAR1, its immunodetection in chloroplast membranes, and nitrate growth tests suggested that NAR1 is related in some way to the nitrite transport step to the chloroplast. To explore this hypothesis, we determined nitrite transport activities in both intact chloroplasts and whole cells from the constructed strains and compared data from strains with a similar genetic background except for the presence or absence of Nar1.
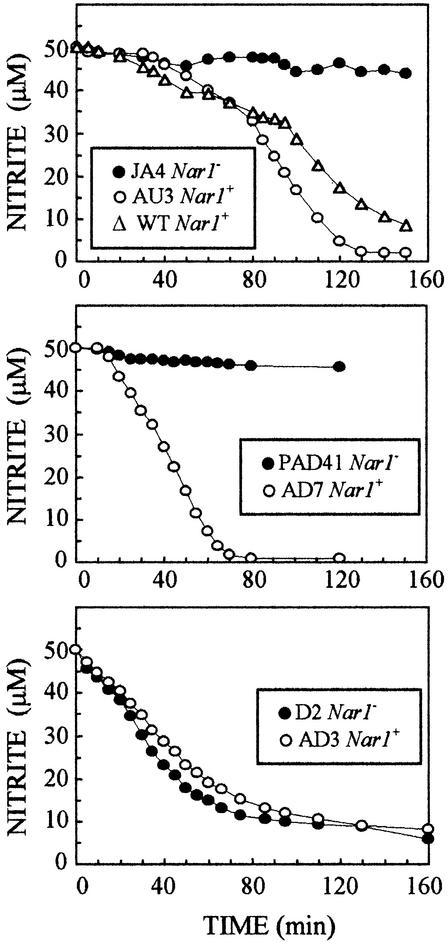
Intact chloroplasts were isolated from cells previously induced for 3 hr in medium that contained 4 mM nitrate and was bubbled with 4% CO2. Nitrite transport activity was determined at pH 8.0, to avoid a possible diffusion component, in medium that contained 50 μM nitrite and was bubbled with 4% CO2 (Figure 6). Under these conditions, chloroplasts from the Nar1+ strains AU3 and AD7 showed much greater nitrite transport activities than did the corresponding Nar1− strains JA4 and PAD41. Chloroplasts from the wild type were also analyzed as an additional control for a Nar1+ strain. In this case, nitrite transport activity was similar to that from strain AU3 (Figure 6). NiR activity was also determined in chloroplast preparations (Table 1). No differences in NiR activities were found that might account for the observed plastidic nitrite transport activities in Nar1+ and Nar1− strains, because NiR activity was even higher in those Nar1− strains. These results suggested that NiR activity was not the limiting factor for differences on plastidic nitrite transport if a diffusion component were operative; they also suggested that NAR1, under our experimental conditions, was responsible for or allowed the expression of a plastidic nitrite transporter. The Ks value for this plastidic nitrite transport activity was estimated at ∼5 μM NO2− for the wild type and for strains AU3 and AD7 (Table 1).
Figure 6.
Nitrite Transport Activity by Intact Chloroplasts from Chlamydomonas Mutant Strains Nar1+/−.
Intact chloroplasts were isolated from nitrate-induced cells, as indicated in Methods. Equal amounts of chloroplasts, based on chlorophyll and NiR activity, were transferred to isotonic media, pH 8.0, containing 50 μM NaNO2. Nitrite content in the media was determined at the indicated times, as detailed in Methods.
Table 1.
NiR Activity and Estimated Ks for Nitrite Transport Activity from Intact Chloroplasts
| Strains |
Ks for Nitrite Transport Activitya (μM) |
NiR Activity (mU/mg Chl)b |
|---|---|---|
| JA4 (Nar1−) | NDc | 1163 ± 381 (n = 3) |
| AU3 (Nar1+) | 4.8 ± 3.8 (n = 3) | 778 ± 29 (n = 3) |
| WT (Nar1+) | 5.5 ± 1.6 (n = 3) | 579 ± 37 (n = 3) |
| PAD41 (Nar1−) | ND | 857 ± 182 (n = 3) |
| AD7 (Nar1+) | 8 ± 4 (n = 3) | 715 ± 122 (n = 3) |
| D2 (Nar1−)d | 7 | 956 |
| AD3 (Nar1+)d | 3 | 842 |
Ks was determined from the progress curves (Galván et al., 1996). Statistical values represent the mean ±sd from three independent experiments.
mU, nmol nitrite hr −1; Chl, chlorophyll.
ND, not determinable under our experimental conditions.
Data for strains D2 and AD3 correspond to a representative experiment from two independent ones.
When strains D2 (Nar1−) and AD3 (Nar1+) were analyzed for plastidic nitrite transport, both strains showed a similar, efficient activity (Figure 6). These strains have in common the absence of both NR activity and HANT.
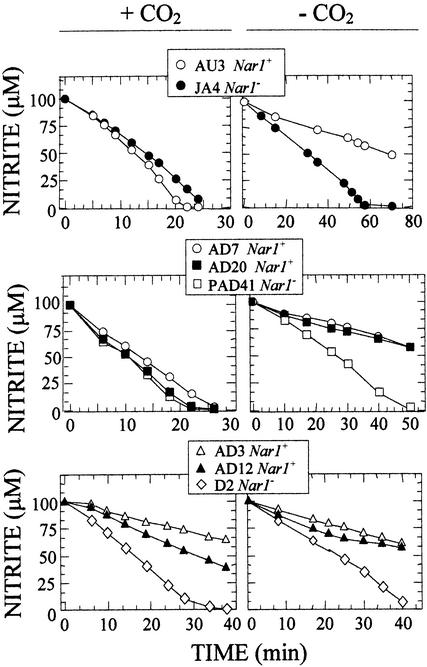
Nitrite transport activities by whole cells were also determined after induction in the above conditions, that is, a 4 mM nitrate–containing medium being bubbled with 4% CO2. Then cells were transferred to a medium containing 100 μM nitrite and bubbled either with 4% CO2-enriched air or without CO2 enrichment. As shown in Figure 7, at the higher CO2 content, no difference in nitrite transport activity was observed between JA4 and AU3 or among PAD41, AD7, and AD20. Curiously, when cells were deprived of CO2 (Figure 7), nitrite transport activity was less in the Nar1+ strains than in the Nar1− ones. We interpreted these results as showing that NAR1 permits the cells to respond to the absence of CO2 and in this way also to control nitrite transport in response to low carbon. Again, this effect was not observed in strains lacking NR, in which nitrite uptake activity was also greater in strain D2 (Nar1−) than in its corresponding partner strains, AD3 and AD12, whether under high or limiting CO2.
Figure 7.
Effect of CO2 Deprivation on Nitrite Transport Activity by Whole Cells from Chlamydomonas Mutant Strains Nar1+/−.
Cells from the indicated strains were induced in nitrate media bubbled with 4% CO2-enriched air for 3 hr and transferred to fresh media containing 100 μM nitrite. Nitrite uptake activity was then determined under either 4% CO2-enriched air (+CO2) or air without added CO2 (−CO2).
If NAR1 were a plastidic nitrite transporter in Chlamydomonas, then others also should exist because strains lacking the Nar1 gene efficiently assimilate nitrite. To answer this question, we performed the following experiments.
First, expression of transcripts from possible Nar1 analogs in the deletion mutant D2 was checked by RNA gel blot. Total RNA was isolated from strain D2, either grown in ammonium or induced in nitrate, and was hybridized at low stringency with the Nar1 cDNA as a probe. The specific Nar1 1.8-kb transcript was absent in strain D2. However, this strain expressed a transcript of ∼3 kb that was repressed in ammonium (Figure 8). This result suggested the presence of an Nar1 analog in Chlamydomonas, which was also supported by DNA gel blot analysis at low stringency. In that experiment, bands appeared that indicated hybridization to the Nar1 cDNA probe both in the wild type and in the mutant D2 (data not shown).
Figure 8.
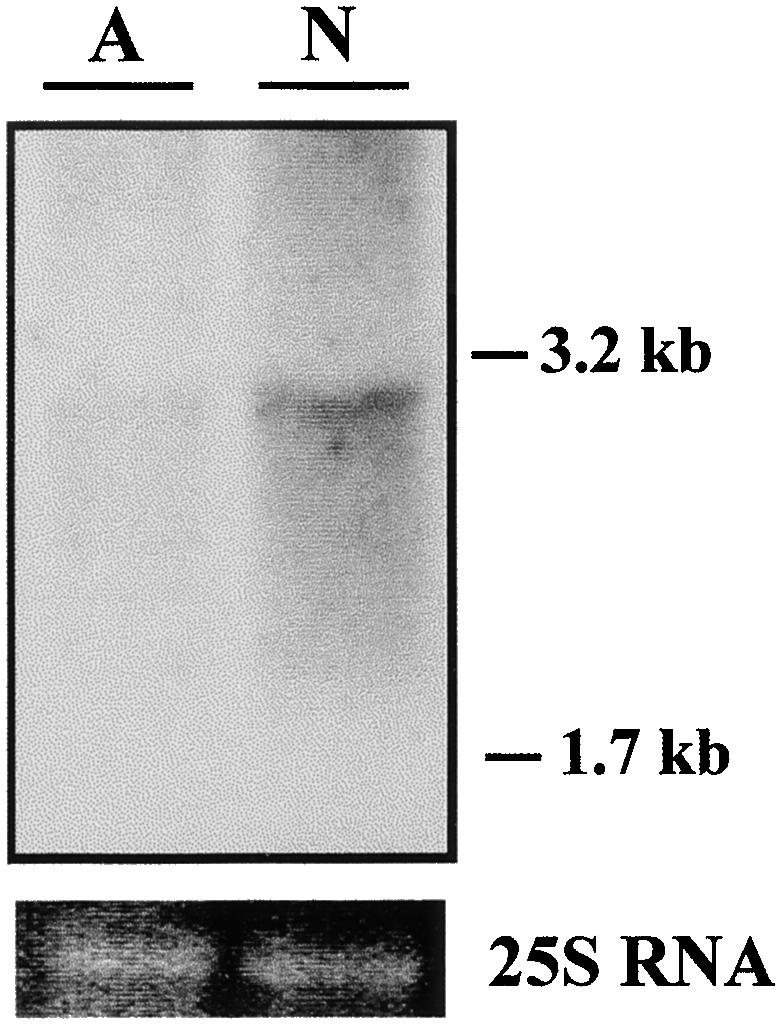
Detection of Gene Transcripts Hybridizing to Nar1 Probes in Mutant Strain D2.
RNA gel blot analysis of mutant D2 was performed with 15 μg of total RNA per lane, using the Nar1 cDNA as a probe under low-stringency conditions. RNA was isolated from cells grown in ammonium (A) or induced in nitrate media (N).
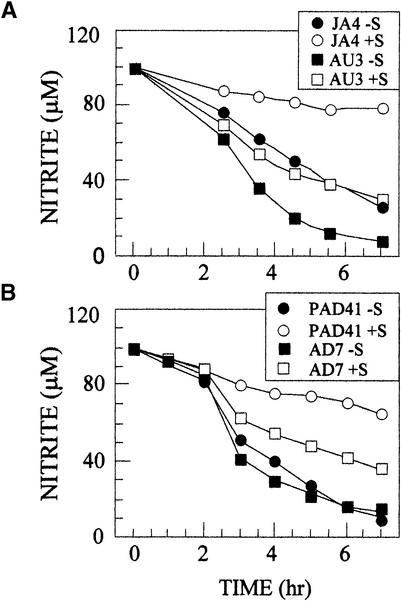
Second, the proposal that some components of nitrite transporters could be encoded by plastidic genes (Krämer et al., 1988) was analyzed by studying the effect of streptomycin, an inhibitor of ribosome 70S–dependent protein synthesis. Cells from strains Nar1+ and Nar1− were induced for nitrite transport activity in the absence of CO2 and in the presence or absence of streptomycin (Figure 9). The streptomycin did not affect the expression of NiR activity (data not shown), but nitrite transport activity diminished markedly in the Nar1− strains (JA4 and PAD41) with respect to the corresponding Nar1+ strains (AU3 and AD7). These data suggest that plastidic factors are required for most of the nitrite transport observed in Nar1-deficient mutants, and when expression of this plastidic factor–dependent transport activity is inhibited, NAR1 accounts for the observed nitrite transport.
Figure 9.
Effect of Streptomycin on the Expression of Nitrite Uptake Activity in Strains Nar1+/−.
(A) Ammonium-grown cells from the indicated strains, JA4 and AU3, were transferred to 100 μM nitrite media in either the presence (open symbols) or the absence (closed symbols) of 100 μg/mL streptomycin (S) under limiting CO2. At the indicated times, the amount of nitrite in the media was determined.
(B) Cells from strains PAD41 and AD7 were treated as indicated in (A).
Nitrate Assimilation by Strains JA4 (Nar1−) and AU3 (Nar1+)
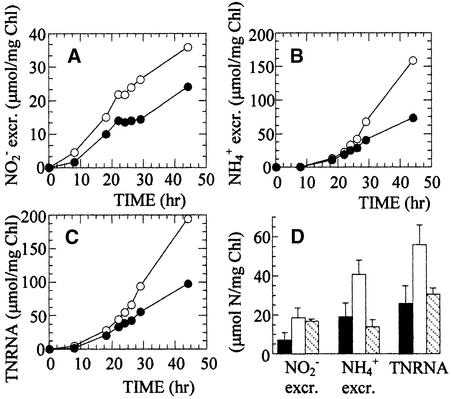
The effect of Nar1 on the efficiency of nitrate assimilation in response to CO2 deprivation was studied further by using strains AU3 and JA4, which can assimilate nitrate properly (Figure 10). Cells were induced in nitrate-containing media, to ensure Nar1 expression, and then transferred to fresh media containing 4 mM nitrate and bubbled without CO2. In these conditions, Chlamydomonas is known to excrete nitrite and ammonium from nitrate (Azuara and Aparicio, 1983), and indeed, excretion of nitrite (Figures 10A and 10D) and ammonium (Figures 10B and 10D) was observed in both strains. However, the Nar1− strain JA4 excreted more nitrite to the media and more ammonium than its corresponding partner strain AU3, which is Nar1+. The sum of nitrite plus ammonium excreted was referred to as total nitrate reduced and nonassimilated (TNRNA), which in strain JA4 was double that in strain AU3 (Figures 10C and 10D). This pattern for nitrite, ammonium excretion, and TNRNA was analyzed in the wild-type strain as another control of a Nar1+ strain. As shown in Figure 10D, ammonium excretion and TNRNA were comparable in the wild type and strain AU3 and considerably greater than in strain JA4.
Figure 10.
Excretion of Nitrite and Ammonium by Nitrate-Assimilating Cells of Strains AU3 and JA4 after Deprivation of CO2.
(A) and (B) Cells from strains JA4 (closed symbols) and AU3 (open symbols) were incubated in minimal media containing 4 mM nitrate, and the amounts of nitrite (A) and ammonium (B) excreted in the media were determined.
(C) TNRNA represents the sum of nitrite plus ammonium derived from nitrate and excreted into the medium.
(D) Results for 24-hr excretion amounts from four independent experiments. Closed bars, JA4; open bars, AU3; striped bars; wild-type strain. Error bars indicate sd.
DISCUSSION
Nar1 encodes an integral protein of the chloroplast membranes that is critical for cell survival under limiting nitrate conditions and the control of nitrate assimilation according to the availability of carbon. The localization of NAR1 in the chloroplast membranes is supported by DNA sequencing analysis, which predicts a protein with six transmembrane-spanning segments containing a typical chloroplast signal peptide, and the immunodetection of NAR1 in the chloroplast membranes. The predicted amino acid sequence for NAR1 showed a significant homology with the bacterial integral membrane proteins FOCA (Suppmann and Sawers, 1994) and NIRC (Peakman et al., 1990) and with an expressed sequence tag from Arabidopsis of unknown function (Newman et al., 1994). FOCA is proposed to be a formate transporter, and in fact, FOCA mutants have been shown to be defective in formate transport (Suppmann and Sawers, 1994). Although NIRC is proposed to be a nitrite transporter, no phenotype has been found for NIRC mutants (Cole, 1996).
According to the results presented here, NAR1 is involved in some way in the nitrite transport to the chloroplast. Two hypotheses can be presented for the specific function of NAR1. In the first, NAR1 is only a regulator; in the second, NAR1 has a dual function as both a transporter and a regulator. We favor this second hypothesis for the following reasons. That NAR1 could be a transporter is supported by several data. First, NAR1 is required for growth under limiting nitrate conditions. When nitrate cannot enter the cells efficiently—either because HANT systems are lacking or because the cells have HANT but nitrate is limiting at micromolar concentrations in the medium—a plastidic high-affinity nitrite transporter, for example, NAR1, would make efficient the nitrite entry into the chloroplast and allow the cells to grow. Second, nitrite transport activity is detected in isolated intact chloroplasts from wild-type and Nar1+ strains AU3 and AD7 under conditions in which this activity is almost negligible in the chloroplast from Nar1− strains JA4 and PAD41. Third, when expression of nitrite transport systems dependent on plastidic protein biosynthesis is blocked, NAR1 allows an efficient nitrite transport activity.
In addition, NAR1 controls the amount of nitrite entering the chloroplast. This conclusion is supported by two facts: NAR1 permits the cells, which are actively consuming nitrite, to respond to a signal of carbon deficiency by rapidly decreasing the nitrite consumption rate; and NAR1 also permits the amount of TNRNA to decrease substantially when carbon is limited. TNRNA corresponds to the sum of nitrite excreted (the amount of nitrate reduced into the cytosol that is not assimilated into the chloroplast) plus ammonium excreted (the amount of nitrite reduced into the chloroplast that cannot be incorporated into carbon skeletons). The excretion of ammonium from nitrate is an important phenotype difference between the Nar1− strain JA4 and the Nar1+ strain AU3 and the wild type. These data suggest that NAR1 is preventing a wasteful nitrate reduction to ammonium and that the control step occurs at the level of chloroplast nitrite transport.
How could a nitrite transporter such as NAR1 control nitrate assimilation according to the nitrogen and carbon status? In Chlamydomonas, apparently four nitrate/nitrite transporters operate at the plasma membrane, each showing different substrate affinities and regulation patterns (Galván et al., 1996; Rexach et al., 1999). Systems I (NRT2;1/NAR2, a bispecific HANT/HANiT), II (NRT2;2/NAR2, a specific HANT), and III (NRT2;3, a specific HANiT) operate optimally at high CO2; their activity is blocked by ammonium (Galván et al., 1996; Rexach et al., 1999; Navarro et al., 2000). System IV (NRT2;4, a bispecific HANT/HANiT) operates optimally under limiting CO2, and its activity is not blocked by ammonium but rather is inhibited by chloride (Rexach et al., 1999; Navarro et al., 2000). Here, we have shown that nitrite transport to the chloroplast is also a regulated process in which NAR1 and other hypothetical transporters participate. These transporters in the chloroplast membrane could have different substrate affinities or capacities for transporting nitrite, could be subject to different forms of regulation by ammonium or CO2, or could exhibit some combination of these differences. The coordination between plasma membrane and plastidic transporters would ensure an optimal nitrate assimilation according to the availability of carbon and nitrogen. The number of reports showing that transporters work as sensors or regulators is increasing. For example, in Saccharomyces cerevisiae, MEP2 is a member of the ammonium permeases and serves as both a high-affinity ammonium transporter and a sensor (Lorenz and Heitman, 1998). A member of the sugar transporter family has also been proposed to have a dual function as a transporter and a sensor in yeast and plants (reviewed in Lalonde et al., 1999).
Whether nitrite enters into plant chloroplasts by a diffusion mechanism or by a mediated transport is an open question (Brunswick and Cresswell, 1988a, 1988b; Shingles et al., 1996), and no nitrite transporter into the chloroplast has been studied at the molecular level until now. The existence of such nitrite transporters could be essential for controlling the amount of nitrite entering the chloroplast and avoiding intracellular accumulation of a toxic compound such as nitrite. Our results indicate that nitrite transport to the chloroplast is a regulated process and not the result of diffusion related to a sink effect of the NiR activity. Therefore, in strains containing NR and Nar1 (wild type, AU3, and AD7), a plastidic nitrite transport activity is induced, in contrast to strains with NR but lacking Nar1 (JA4 and PAD41); however, the NiR activity is not a mirror of the transport activity. The Chlamydomonas NAR1 seems to be a first member of plastidic nitrite transporters homologous to the bacterial FOCA and NIRC. However, this picture is more complex because other chloroplast nitrite transporters seem to exist in Chlamydomonas. Several findings point to this: (1) in Nar1 deletion mutants, at least two plastidic nitrite transport activities are differentially expressed and regulated (V. Mariscal, J. Rexach, E. Fernández, and A. Galván, unpublished results); and (2) in Nar1 deletion mutants, the existence of a Nar1 analog is supported by the detection, with the Nar1 cDNA probe, of a 3-kb transcript regulated by nitrate along with a nitrite transport activity dependent on plastidic protein synthesis. Whether or not a component of nitrite transport is encoded by a chloroplast gene remains to be solved. Alternatively, other anion transporters could also transport nitrite. Recently, a transporter encoded by a plastidic gene has been shown to transport bicarbonate in Chlamydomonas (Rolland et al., 1997), and formate, bicarbonate, and nitrite are chemically similar molecules.
In Chlamydomonas, the NR enzyme plays an important role in regulating nitrate/nitrite transporters (Galván et al., 1992; Quesada and Fernández, 1994; Navarro et al., 1996). In addition, a physical link between Nia1 and Nar1 genes exists in Chlamydomonas so that the Nar1 promoter region (1.5 kb of BamHI-EcoRI) is also the terminating region of the NR gene. The phenotype assigned to the Nar1 gene in Chlamydomonas strains having a functional NR was not maintained in strains that lacked NR. The reason for this might be related to the differences in nitrate/nitrite signaling related to the activity of the NR enzyme (Navarro et al., 1996) and requires further research.
METHODS
Strains and Growth Conditions
Chlamydomonas reinhardtii wild-type 6145c (mt−) and strains D2 (mt− Δ[Nrt2;2, Nrt2;1, Nar2, Nia1, and Nar1]), 305cw15 (mt+ Nia1), and T7 (mt+ Nia1::pMN24 Nia1) have been described elsewhere (Harris, 1989; Quesada et al., 1993, 1994).
Cells were grown at 25°C under continuous light and 4% (v/v) CO2-enriched air in minimal medium containing 7.5 mM ammonium chloride (Harris, 1989). Cells were collected at their midexponential phase of growth by centrifugation (4000g for 5 min), washed twice with 50 mM potassium phosphate, pH 7.0, and induced for 3 hr in 4 mM nitrate-containing medium. Cultures were bubbled either with 4% CO2-enriched air or with air washed through a solution saturated with KOH.
Genetic Procedures
Genetic crosses were performed by the random spore plating method (Levine and Ebersold, 1960). Segregants were analyzed for their ability to grow on media containing ammonium, nitrate, or nitrite and for the presence or absence of nitrate reductase (NR) activity.
Isolation of Intact Chloroplasts
Ammonium-grown cells were synchronized during three light–dark cycles (12-hr-light/12-hr-dark) and then induced in 4 mM nitrate media bubbled with 4% CO2-enriched air and under light. Intact chloroplasts were isolated according to standard protocols (Mendiola-Morgenthaler et al., 1985; Mason et al., 1991). Treatment with autolysin released the cell wall, after which the cells were broken by passing through a 27-gauge stainless steel needle (Mason et al., 1991). Intact chloroplasts were collected from the 60 to 40% (v/v) Percoll interface (Mendiola-Morgenthaler et al., 1985). The yield of intact chloroplasts, estimated on the basis of both chlorophyll and nitrite reductase (NiR) activity recovery, was 11.1% ± 3.3% and 14.8% ± 4%, respectively (mean ±sd of five independent preparations). This yield is in accordance with previous reports (Mason et al., 1991).
Nitrite Transport Activity
Cells grown in ammonium or induced in nitrate media were transferred at a cell concentration of 10 to 30 μg of chlorophyll per milliliter into fresh media containing 100 μM nitrite. Samples were taken at the times indicated, and the nitrite concentration in the media was measured.
Nitrite transport from intact chloroplasts was determined as before except that an isotonic medium containing 300 mM sorbitol and 50 mM Hepes-KOH, pH 8.0, was used. The nitrite concentration in the medium was 50 μM.
NR and NiR Assays and Analytical Methods
NR (Fernández et al., 1989) and NiR (Galván et al., 1991) were assayed as previously reported. Nitrite was determined as described by Snell and Snell (1949), ammonium as described by Conway (1962), protein as described by Smith et al. (1985), and chlorophyll as described by Arnon (1949).
Screening of a cDNA Library
A 1.7-kb Nar1 cDNA was isolated by screening ∼2 × 105 plaques from an amplified cDNA library of Chlamydomonas in λEXlox, kindly provided by P.A. Lefebvre (University of Minnesota, St. Paul). A radioactively labeled genomic clone B7a-1.1.8 (Figure 1A) was used as a probe, according to previously reported methods (Sambrook et al., 1989; Quesada et al., 1994). The rapid amplification of cDNA ends (RACE) polymerase chain reaction was performed using a Nar1 primer (5′-TTCCTCACCAACAACCTGCTGCCCG-3′) corresponding to positions 2097 to 2119 (Figure 1B), poly(A)+ RNA isolated from the wild-type strain induced in nitrate-containing media (Schloss et al., 1984), and AP2 as a second primer (Marathon cDNA Amplification Kit; Clontech Laboratories, Inc., Palo Alto, CA), according to the manufacturer's instructions.
Sequencing of DNA and Analysis of Sequences
DNA sequencing was performed with fluorescence-labeled terminators for an automated sequencer (model ABI 310; Applied Biosystems, Perkin-Elmer Co., Foster City, CA), according to the manufacturer's instructions. Sequences were analyzed using the Wisconsin GCG Group DNA sequence analysis software (Devereux et al., 1984), the TMpred tool (Hofmann and Stoffel, 1993; http:/www.ch.embnet.org/software/TMPRED_form.html), and PSORTII (http:/psort.nibb.ac.jp).
Isolation of DNA and RNA from Chlamydomonas and Hybridization Analysis
Isolation of genomic DNA and the DNA gel blot analysis were performed according to previously described methods (Ranum et al., 1988; Sambrook et al., 1989). Conditions for hybridization were as described by Schloss et al. (1984), and washes were performed at 65°C, with 0.2 × SSC solution (1 × SSC is 0.15 M NaCl and 0.015 M sodium citrate) containing 0.2% SDS. Isolation of total RNA was performed as previously reported (Schloss et al., 1984). RNA was fractionated on 1.6% agarose gels containing 17.5% formaldehyde and transferred onto nylon membranes (Nytran-N2; Schleicher and Schuell) with saline buffer (10 × SSC). Conditions for hybridization were as reported (Schloss et al., 1984) at 42°C and 50% formamide. Washes were performed at 65°C with 0.2 × SSC solution containing 0.2% SDS. When low stringency is indicated, hybridizations were performed at 58°C without formamide and washes were at 60°C, twice with 2 × SSC containing 0.5% SDS and twice with 0.5 × SSC containing 0.5% SDS.
Radioactive probes were labeled by the random primer method (Feinberg and Vogelstein, 1984) for both RNA and DNA gel blot analysis. The probes used were B6a-6, a 3-kb EcoRI-SmaI fragment hybridizing to the 5′ ends of both genes Nrt2;1 and Nar2; B7a-1.1.8, a PstI-EcoRI fragment containing a Nar1 gene region (Quesada et al., 1993); and the Nar1 cDNA.
Isolation of Membranes and Immunodetection of NAR1
The Chlamydomonas strain 305cw15 was grown in minimum medium with 8 mM ammonium or was induced with 4 mM nitrate for 5 hr (Harris, 1989). This strain, which lacks cell walls, was used to prepare plasma membranes according to the two-phase (polyethylene glycol 3350/dextran T500) method (Dolle, 1988). Chloroplast membranes were obtained by lysing intact chloroplasts in a hypotonic medium (50 mM Hepes-KOH, pH 7.5, and 2 mM MgCl2) (Mendiola-Morgenthaler et al., 1985). After centrifugation at 600g for 3 min, the pellet was discarded. Membranes from the supernatant were collected by centrifugation at 100,000g for 1 hr. The chloroplast plasma membrane proteins (50 μg/lane) were fractionated by SDS-PAGE in 12% polyacrylamide gels, transferred to nitrocellulose filters, and hybridized with antibodies against a NAR1 polypeptide (EGKATPKDLAKNWF) that corresponds to residues 98 to 111 from the mature protein (Harlow and Lane, 1988).
Transformation of Chlamydomonas
Transformation of Chlamydomonas strains was done using the glass bead method (Kindle et al., 1989) and 1 μg of DNA. The plasmid DNA pT2-CO5 contains the Nrt2;1 and Nar2 genes, and the transformants receiving this plasmid DNA were selected in nitrate-containing media (Quesada et al., 1994). The plasmid pBleNar1, which contained both the bleomycin resistance marker and Nar1, was constructed from plasmid pSP109, which contains the bleomycin resistance marker (Stevens et al., 1996) and pB7a (Quesada et al., 1993). The Nar1 gene was released from pB7a by digestion with BamHI and cloned in pSP109 into a BamHI site. Transformants receiving this plasmid were selected in ammonium medium containing 40 μg/mL zeomycine (Stevens et al., 1996).
Acknowledgments
We thank Maribel Macías for technical assistance, Dr. Faustino Merchán for help with computers, and Inés Molina and Conchi Santos for secretarial assistance. This work was supported in part by the European Union Biotechnology program as part of the Project of Technological Priority 1997–2000 (Grant No. BIO4CT962231), the Dirección General de Investigación Científica y Técnica (Spain) (Grant Nos. PB95-0554-CO-01 and PB98-1022-CO2-02), and the Junta de Andalucía (PAI, Grant No. CVI-0128).
References
- Amarasinghe, B.H.R.R., De Bruxelles, G.L., Braddon, M., Onyeocha, I., Forde, B.G., and Udvardi, M.K. (1998). Regulation of Gmnrt2 expression and nitrate transport activity in roots of soybean (Glycine-Max). Planta 206, 44–52. [DOI] [PubMed] [Google Scholar]
- Arnon, D.I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuara, M.P., and Aparicio, P. (1983). In vivo blue-light activation of Chlamydomonas reinhardtii nitrate reductase. Plant Physiol. 71, 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach, R., and Chambon, P. (1981). Organization and expression of eukaryotic split genes coding for proteins. Annu. Rev. Biochem. 50, 349–384. [DOI] [PubMed] [Google Scholar]
- Brink, S., Fischer, K., Klosgen, R.B., and Flugge, U.I. (1995). Sorting of nuclear-encoded chloroplast membrane protein to the envelope and the thylakoid membrane. J. Biol. Chem. 270, 20808–20815. [DOI] [PubMed] [Google Scholar]
- Brunswick, P., and Cresswell, C.F. (1988. a). Nitrite uptake into intact pea chloroplasts. I. Kinetics and relationship with nitrite assimilation. Plant Physiol. 86, 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick, P., and Cresswell, C.F. (1988. b). Nitrite uptake into intact pea chloroplasts. II. Influence of electron transport regulators, uncouplers, ATPase and anion uptake inhibitors and protein binding reagents. Plant Physiol. 86, 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J.A. (1996). Nitrate reduction to ammonium by enteric bacteria: Redundancy, or a strategy for survival during oxygen starvation? FEMS Microbiol. Lett. 136, 1–11. [DOI] [PubMed] [Google Scholar]
- Conway, D.J. (1962). Microdiffusion Analysis and Volumetric Error, 5th ed. (London: Crosby Lockwood), pp. 105–110.
- Crawford, N.M. (1995). Nitrate: Nutrient and signal for plant growth. Plant Cell 7, 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, N.M., and Glass, A.D.M. (1998). Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 3, 389–395. [Google Scholar]
- Devereux, J., Haeberly, P., and Smithies, O. (1984). A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle, R. (1988). Isolation of plasma membrane and binding of the Ca2+ antagonist ninodipine in C. reinhardtii. Physiol. Plant. 73, 7–14. [Google Scholar]
- Feinberg, A.P., and Vogelstein, B. (1984). A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 137, 266–267. [DOI] [PubMed] [Google Scholar]
- Fernández, E., Schnell, R., Ranum, L.P.W., Hussey, S.C., Silflow, C.D., and Lefebvre, P.A. (1989). Isolation and characterization of the nitrate reductase structural gene in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 86, 6449–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández, E., Galván, A., and Quesada, A. (1998). Nitrogen assimilation and its regulation. In Molecular Biology of Chlamydomonas: Chloroplast and Mitochondria, J.D. Rochaix, M. Goldschmidt-Clermont, and S. Merchant, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 637–659.
- Franzén, L.G., Rochaix, J.D., and von Heijne, G. (1990). Chloroplast transit peptides from the green alga Chlamydomonas reinhardtii share features with both mitochondrial and higher plant chloroplast presequences. FEBS Lett. 260, 165–168. [DOI] [PubMed] [Google Scholar]
- Galván, A., Córdoba, F., Cárdenas, J., and Fernández, E. (1991). Regulation of nitrite uptake and nitrite reductase expression in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1074, 6–11. [DOI] [PubMed] [Google Scholar]
- Galván, A., Cárdenas, J., and Fernández, E. (1992). Nitrate reductase regulates expression of nitrite uptake and nitrite reductase activities in Chlamydomonas reinhardtii. Plant Physiol. 98, 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván, A., Quesada, A., and Fernández, E. (1996). Nitrate and nitrite are transported by different specific transport systems and by a bispecific transporter in Chlamydomonas reinhardtii. J. Biol. Chem. 271, 2088–2092. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Harris, E. (1989). The Chlamydomonas Sourcebook. (New York: Academic Press).
- Hoff, T., Truong, H.N., and Caboche, M. (1994). The use of mutants and transgenic plants to study nitrate assimilation. Plant Cell Environ. 17, 489–506. [Google Scholar]
- Hofmann, K., and Stoffel, W. (1993). Tmbase—A database of membrane scanning protein segments. Biol. Chem. Hoppe-Seyler 347, 166. [Google Scholar]
- Hwang, C.F., Lin, Y., D'Souza, T., and Cheng, C.L.. (1997). Sequences necessary for nitrate-dependent transcription of Arabidopsis nitrate reductase genes. Plant Physiol. 113, 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone, I.L., McCabe, P.C., Greaves, P., Gurr, S.J., Cole, G.E., Brow, M.A.D., Unkles, S.E., Clutterbuck, A.J., Kinghorn, J.R., and Innis, M.A. (1990). Isolation and characterization of the crna-niiA-niaD gene cluster for nitrate assimilation in Aspergillus nidulans. Gene 90, 181–192. [DOI] [PubMed] [Google Scholar]
- Kammerer, B., Fischer, K., Hilpert, B., Schubert, S., Gutensohn, M., Weber, A., and Függe, U.I. (1998). Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: The glucose 6-phosphate/phosphate antiporter. Plant Cell 10, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle, K.L., Schnell, R.A., Fernández, E., and Lefebvre, P.A. (1989). Stable nuclear transformation of Chlamydomonas reinhardtii using the Chlamydomonas gene for nitrate reductase. J. Cell Biol. 109, 2589–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer, E., Tischner, R., and Schmidt, A. (1988). Regulation of assimilatory nitrate reduction at the level of nitrite in Chlorella fusca. Planta 176, 28–35. [DOI] [PubMed] [Google Scholar]
- Kyte, J., and Doolittle, R.F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132. [DOI] [PubMed] [Google Scholar]
- Lalonde, S., Boles, E., Hellman, H., Barker, L., Patrick, J.W., and Frommer, W.B. (1999). The dual function of sugar carriers: Transport and sugar sensing. Plant Cell 11, 707–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, R.P., and Ebersold, W.T. (1960). The genetics and cytology of Chlamydomonas. Annu. Rev. Microbiol. 14, 197–216. [DOI] [PubMed] [Google Scholar]
- Liu, K.H., Huang, C.Y., and Tsay, Y.F. (1999). CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, M.C., and Heitman, J. (1998). The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 17, 1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, C.B., Matthews, S., Bricker, T.M., and Moroney, J.V. (1991). Simplified procedure for the isolation of intact chloroplast from Chlamydomonas reinhardtii. Plant Physiol. 97, 1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola-Morgenthaler, L., Eichenberger, W., and Boschetti, A. (1985). Isolation of chloroplast envelopes from Chlamydomonas. Lipid and polypeptide composition. Plant Sci. 41, 97–104. [Google Scholar]
- Navarro, M.T., Prieto, R., Fernández, E., and Galván, A. (1996). Constitutive expression of nitrate reductase changes the regulation of nitrate and nitrite transporters in Chlamydomonas reinhardtii. Plant J. 9, 819–827. [Google Scholar]
- Navarro, M.T., Guerra, E., Fernández, E., and Galván, A. (2000). Nitrite reductase mutants as an approach to understanding nitrate assimilation in Chlamydomonas reinhardtii. Plant Physiol. 122, 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, T., de Bruijn, F.J., Green, P., Keegstra, K., Kende, H., McIntosh, L., Ohlrogge, J., Raikhel, N., Somerville, S., Thomashow, M., Retzel, E., and Somerville, C. (1994). Genes galore: A summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 106, 1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakman, T., Crouzet, J., Mayaux, J., Busby, S., Mohan, S., Harborne, N., Wooton, J., Nicolson, R., and Cole, J. (1990). Nucleotide sequence, organisation and structural analysis of the products of genes in the nirB-cysG region of the Escherichia coli chromosome. Eur. J. Biochem. 191, 315–323. [DOI] [PubMed] [Google Scholar]
- Pérez, M.D., González, C., Avila, J., Brito, N., and Siverio, J.M. (1997). The YNT1 gene encoding the nitrate transporter in the yeast Hansenula polymorpha is clustered with genes YNI1 and YNR1 encoding nitrite reductase and nitrate reductase, and its disruption causes inability to grow in nitrate. Biochem. J. 321, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada, A., and Fernández, E. (1994). Expression of nitrate assimilation related genes in Chlamydomonas reinhardtii. Plant Mol. Biol. 24, 185–194. [DOI] [PubMed] [Google Scholar]
- Quesada, A., Galván, A., Schnell, R., Lefebvre, P.A., and Fernández, E. (1993). Five nitrate assimilation related loci are clustered in Chlamydomonas reinhardtii. Mol. Gen. Genet. 240, 387–394. [DOI] [PubMed] [Google Scholar]
- Quesada, A., Galván, A., and Fernández, E. (1994). Identification of nitrate transporter genes in Chlamydomonas reinhardtii. Plant J. 5, 407–419. [DOI] [PubMed] [Google Scholar]
- Quesada, A., Krapp, A., Trueman, L., Daniel-Vedele, F., Fernández, E., Forde, B.G., and Caboche, M. (1997). PCR-identification of a Nicotiana plumbaginifolia cDNA homologous to the high affinity nitrate transporters of the crnA family. Plant Mol. Biol. 34, 265–274. [DOI] [PubMed] [Google Scholar]
- Quesada, A., Hidalgo, J., and Fernández, E. (1998. a). Three Ntr2 genes are differentially regulated in Chlamydomonas reinhardtii. Mol. Gen. Genet. 258, 373–377. [DOI] [PubMed] [Google Scholar]
- Quesada, A., Gómez, I., and Fernández, E. (1998. b). Clustering of the nitrite reductase gene and a light-regulated gene with nitrate assimilation loci in Chlamydomonas reinhardtii. Planta 206, 259–265. [DOI] [PubMed] [Google Scholar]
- Ranum, L.P.W., Thompson, M.D., Schloss, J.A., Lefebvre, P.A., and Silflow, C.D. (1988). Mapping flagellar genes in Chlamydomonas using restriction fragment length polymorphisms. Genetics 120, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi, R., Bate, N.J., Sivasankar, S., and Rothstein, S.J. (1997). Footprinting of the spinach nitrite reductase gene promoter reveals the preservation of nitrate regulatory elements in fungi and higher plants. Plant Mol. Biol. 34, 465–476. [DOI] [PubMed] [Google Scholar]
- Rexach, J., Montero, B., Fernández, E., and Galván, A. (1999). Differential regulation of the high affinity nitrite transport systems III and IV in Chlamydomonas reinhardtii. J. Biol. Chem. 274, 27801–27806. [DOI] [PubMed] [Google Scholar]
- Rolland, N., Amoroso, G., Sültemeyer, D., Joyard, J., and Rochaix, J.D. (1997). Disruption of the plastid ycf10 open reading frame affects uptake of inorganic carbon in the chloroplast of Chlamydomonas. EMBO J. 16, 6713–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schloss, J.A., Silflow, C.D., and Rosenbaum, J.L. (1984). mRNA abundance changes during flagellar regeneration in Chlamydomonas reinhardtii. Mol. Cell Biol. 4, 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingles, R., Roh, M.H., and McCarty, R.E. (1996). Nitrite transport in chloroplast inner envelope vesicles. I. Direct measurement of proton-linked transport. Plant Physiol. 112, 1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, P.K., Krohn, R.I., Hermanson, G.T., Mallia, A.K., Gartner, F.H., Provenzano, M.D., Fujimoto, E.K., Goeke, B.J., and Klenk, D.C. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85. [DOI] [PubMed] [Google Scholar]
- Snell, F.D., and Snell, C.T. (1949). Nitrates. In Colorimetric Methods of Analysis, Vol. 2, F.D. Snell, and C.T. Snell, eds (New York: Van Nostrand), pp. 802–807.
- Stevens, D.R., Rochaix, J.D., and Purton, S. (1996). The bacterial phleomycin resistance gene ble as a dominant selectable marker in Chlamydomonas. Mol. Gen. Genet. 251, 23–30. [DOI] [PubMed] [Google Scholar]
- Suppmann, B., and Sawers, G. (1994). Isolation and characterization of hypophosphite resistant mutants of Escherichia coli: Identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol. Microbiol. 11, 965–982. [DOI] [PubMed] [Google Scholar]
- Tanner, W., and Caspari, T. (1996). Membrane-transport carriers. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 595–626. [DOI] [PubMed] [Google Scholar]
- Trueman, L.J., Onyeocha, I., and Forde, B.G. (1996). Recent advances in the molecular biology of a family of eukaryotic high affinity nitrate transporters. Plant Physiol. Biochem. 34, 621–627. [Google Scholar]
- Tsay, Y.F., Schroeder, J., Feldman, K.A., and Crawford, N.M. (1993). A herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72, 705–713. [DOI] [PubMed] [Google Scholar]
- Unkles, S.E., Hawker, K.L., Grieve, C., Campbell, E.I., Montague, P., and Kinghorn, J.R. (1991). CrnA encodes a nitrate transporter in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 88, 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R., Liu, D., and Crawford, N.M. (1998). The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proc. Natl. Acad. Sci. USA 95, 15134–15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, A., Menzlaff, E., Arbinger, B., Gutensohn, M., Eckerskorn, C., and Flügge, U.I. (1995). The 2-oxoglutarate/malate translocator of chloroplast envelope membranes: Molecular cloning of a transporter containing a 12-helix motif and expression of the functional protein in yeast cells. Biochemistry 34, 2621–2627. [DOI] [PubMed] [Google Scholar]
- White, W.B., and Ferry, J.G. (1992). Identification of formate dehydrogenase–specific mRNA species and nucleotide sequence of the fdhC gene of Methanobacterium formicicum. J. Bacteriol. 174, 4997–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J.Y., Siegle, L.M., and Kredich, N.M. (1991). High-level expression of Escherichia coli NADPH-sulfite reductase: Requirement for a cloned cysG plasmid to overcome limiting siroheme cofactor. J. Bacteriol. 173, 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J., and Kleinhofs, A. (1996). Molecular evolution of nitrate reductase genes. J. Mol. Evol. 42, 432–442. [DOI] [PubMed] [Google Scholar]
- Zhou, J.J., Theodoulou, F.L., Muldin, I., Ingemarsson, B., and Miller, A.J. (1998). Cloning and functional characterization of a Brassica napus transporter that is able to transport nitrate and histidine. J. Biol. Chem. 273, 12017–12023. [DOI] [PubMed] [Google Scholar]