Abstract
The carpel is the female reproductive organ of flowering plants. In Arabidopsis, congenital fusion of two carpels leads to the formation of an enclosed gynoecium. The margins of the two fused carpels are meristematic in nature and give rise to placentas, ovules, septa, abaxial repla, and the majority of the stylar and stigmatic tissues. Thus, understanding how the marginal tissues are specified and identifying genes that direct their development may provide important insight into higher plant reproductive development. In this study, we show that LEUNIG and AINTEGUMENTA are two critical regulators of marginal tissue development. Double mutants of leunig aintegumenta fail to develop placentas, ovules, septa, stigma, and style. This effect is specific to the leunig aintegumenta double mutant and is not found in other double mutant combinations such as leunig apetala2 or aintegumenta apetala2. Additional analyses indicate that the absence of marginal tissues in leunig aintegumenta double mutants is not mediated by ectopic AGAMOUS. We propose that LEUNIG and AINTEGUMENTA act together to control the expression of common target genes that regulate cell proliferation associated with marginal tissue development.
INTRODUCTION
Carpels, the female reproductive organ in flowering plants, are formed in the center of the flower, internally to stamens, petals, and sepals. The primary function of carpels is to generate and protect the developing ovules that, when fertilized, develop into seeds. Carpel identity has been shown to be specified by two separate pathways (reviewed in Bowman et al., 1999). One pathway is mediated by the C class floral homeotic gene AGAMOUS (AG), and the other pathway is mediated by the SPATULA (SPT) and CRABS CLAW (CRC) genes. Both pathways are negatively regulated by the A class genes such as APETALA2 (AP2) and LEUNIG (LUG) (Bowman et al., 1991a; Drews et al., 1991; Liu and Meyerowitz, 1995; Alvarez and Smyth, 1999; Bowman and Smyth, 1999), thus restricting the development of carpel identity to organs in the center of a flower.
Subsequent to the determination of carpel identity, regional specification and differentiation of carpel tissues take place. Analyses of ETTIN (ETT) and CRC suggested the existence of apical–basal and abaxial–adaxial boundaries in carpel primordia (Sessions and Zambryski, 1995; Sessions, 1997; Sessions et al., 1997; Alvarez and Smyth, 1999; Bowman and Smyth, 1999; Eshed et al., 1999). In ett mutants, the alteration of carpel tissue distribution with respect to the apical–basal and abaxial–adaxial boundaries suggests that ETT plays a role in specifying or responding to regional domains. In the case of crc, the isolation of its enhancers such as GYMNOS (GYM) establishes a role of CRC in specifying the abaxial region of the carpel that is not readily apparent from crc single mutants (Eshed et al., 1999).
In Arabidopsis, congenital fusion of two carpels leads to the formation of an enclosed gynoecium. The margins of the two fused carpels are medially situated with respect to the inflorescence. This medially situated and congenitally fused margin of the gynoecium exhibits a distinct developmental program. At early stages, when the gynoecial primordium is developing as a cylinder, the medially situated marginal tissue is active in cell division and gives rise to a ridge in the adaxial side of the gynoecium. This so-called medial ridge is meristematic in nature and apparently gives rise to placentas, ovules, and septa (Bowman et al., 1999). Hence, the medial ridge is of utmost importance for the female fertility of the plant. In addition, the medially situated and congenitally fused margin generates abaxial repla and contributes, at least partially if not mostly, to the formation of stigma and style. Hence, for simplicity and clarity, we use the term “medial ridge–derived tissues” to refer to the placentas, ovules, and septa and the term “marginal tissues” to refer to all of the medial ridge–derived tissues plus abaxial repla, stigma, and style. However, the laterally situated tissue of the gynoecium gives rise to the carpel walls (i.e., carpel valves) that protect the ovules within. Although lineage analyses are not yet available, the subdivision of a gynoecium into medial and lateral domains is supported by the expression of several genes that are specifically expressed either in the medial or in the lateral domain. For example, FRUITFULL (FUL) RNA is detected in the lateral domain and subsequently in carpel walls, whereas other AGAMOUS-LIKE (AGL) genes such as AGL1 and AGL5 are detected in the medial domain and subsequently in ovules, abaxial repla, and septa (Ma et al., 1991; Savidge et al., 1995; Flanagan et al., 1996; Gu et al., 1998; Bowman et al., 1999). Thus, the medial and lateral domains are distinguished by the patterns of both gene expression and function.
Although no mutation in Arabidopsis has been identified that specifically abolishes the medial or the lateral domain, numerous mutations have been isolated that cause various abnormalities in gynoecia, such as a reduced fusion between the two carpels and reduced septa, stigmatic, or transmitting tissues (reviewed in Bowman et al., 1999). Of particular interest are aintegumenta (ant) and lug mutants, both of which exhibit defects in carpel fusion (Komaki et al., 1988; Liu and Meyerowitz, 1995; Elliott et al., 1996; Klucher et al., 1996). In ant mutants, in addition to defects in carpel fusion, floral organ number in the first three whorls is reduced, and sepals, petals, and leaves are narrower than those in the wild-type plants (Elliott et al., 1996; Klucher et al., 1996). These defects can be attributed to smaller floral meristems (Krizek, 1999) and fewer cells in each of the lateral organs (Mizukami and Fischer, 2000). ant mutants are also female-sterile because of their severe defect in ovule integument initiation (Elliott et al., 1996; Klucher et al., 1996). ANT mRNA was detected in primordia of cotyledons, floral organs, placentas, ovules, and integuments of the ovules (Elliott et al., 1996; Long and Barton, 1998). Thus, both the mutant phenotype and the expression pattern suggest a role of ANT in promoting cell proliferation during the development of organ primordium.
Like ant mutants, lug mutants exhibit unfused carpels, narrow leaves and floral organs; fewer floral organs; and reduced female fertility (Liu and Meyerowitz, 1995). lug flowers exhibit homeotic transformations in which sepals are transformed into carpels or stamens and petals are transformed into stamens. The defect of lug in floral organ identity and floral organ number was previously shown to be caused by ectopic expression of AG mRNA. The reduced female fertility, on the other hand, was shown to be caused by abnormally overproliferating inner integument and a lack of embryo sac (Roe et al., 1997; Schneitz et al., 1997; Z. Liu and V.P. Klink, unpublished results).
Although homeotic transformation is rare in ant mutant flowers, the similarity between lug and ant mutants in other aspects of their phenotypes indicates that LUG and ANT may play similar roles during Arabidopsis development. In this study, we found that lug ant double mutants exhibit a strong synergistic interaction in the development of gynoecial marginal tissue. This interaction is specific to lug ant double mutants and is not found in other double mutant combinations between genes that encode repressors of AG expression. Our analyses provide important insight into LUG and ANT function as well as into the mechanism of gynoecium marginal tissue development.
RESULTS
Wild-Type Gynoecium Development
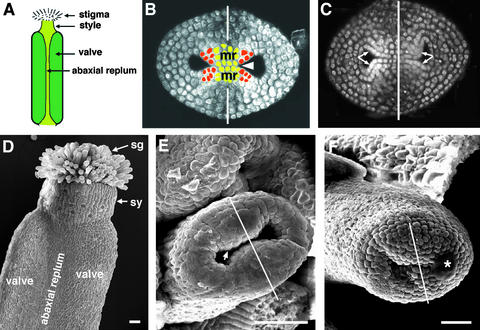
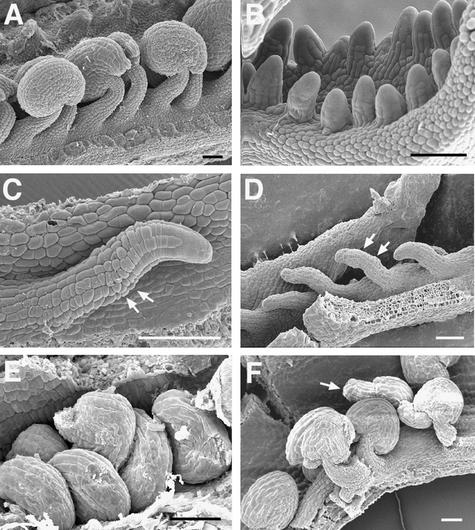
Figure 1 shows wild-type Arabidopsis gynoecium development. A gynoecium consists of two fused carpels and is capped by a single style and stigma (Figures 1A and 1D; Hill and Lord, 1989; Okada et al., 1989; Smyth et al., 1990; Gasser and Robinson-Beers, 1993; Sessions and Zambryski, 1995; Bowman et al., 1999). The two fused carpels are located laterally with respect to the inflorescence axis. These two carpels are congenitally fused, so the gynoecium initially develops as a slotted cylinder (Figures 1B, 1C, and 1E). At stage 9, as the cylinder starts to close at the apex, the apical epidermal cells differentiate into stigmatic papillary cells. These stigmatic cells first appear at the medial surface (Figure 1F) and then differentiate over the entire apical surface of the gynoecium. At stage 11, the style starts to differentiate just beneath the stigma (Figure 1D).
Figure 1.
Gynoecium Organization in Wild-Type Arabidopsis (Landsberg erecta).
(A) A diagram of a mature gynoecium. Two laterally situated carpel valves (dark green) are fused together. Abaxial replum is the joining area between the two carpel valves. The gynoecium is topped with a single style and stigma.
(B) An optical cross-section of a wild-type gynoecium (early stage 7). The staging throughout this study is based on Smyth et al. (1990). The tissue was stained with a DNA-staining dye, propidium iodide. The white line indicates the medial plane. The two inward outgrowths are the medial ridges (mr), which are initiated at the medial position. The medial ridges grow and fuse together to form a single septum (arrowhead). Cells of medial ridges are artificially colored with red and light green. The four red areas indicate the four placentas. Because lineage analyses are not yet available, the medial ridges are marked with approximations.
(C) An optical cross-section of a wild-type gynoecium at stages 7 to 8. Ovule primordia are evident in each of the four placentae indicated by the four arrows. The stronger propidium iodide stain in ovule primordia reflects greater cell division activity.
(D) A scanning electron microscopic (SEM) image of a mature wild-type gynoecium. The stigma (sg), style (sy), the two carpel valves, and the abaxial replum are indicated.
(E) An SEM image of a wild-type stage 7 gynoecium. The line indicates the medial plane, where medial ridges emerge (arrow).
(F) An SEM image of a wild-type stage 10 gynoecium. The line indicates the medial plane. The stigmatic papillar cells are starting to differentiate prominently at the medial apical surface. The asterisk indicates a dust contaminant during sample preparation.
 .
.
Inside the gynoecium cylinder, the medially situated marginal tissues give rise to two ridges that grow toward each other (Figures 1B, 1C, and 1E). These two medial ridges (Bowman et al., 1999) meet and then fuse together, resulting in a single septum (Figure 1B) that separates the gynoecium cylinder into a bilocular chamber. In addition, these two medial ridges give rise to four placentas and subsequently four rows of ovules (Figures 1B and 1C).
Abnormal Gynoecium Development in lug and ant Single Mutants
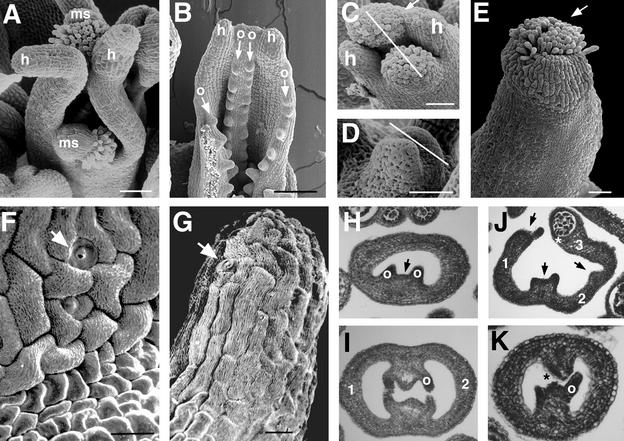
Figure 2 illustrates gynoecium development in lug and ant single mutants. In lug single mutant gynoecia, abnormality is observed in both the lateral and the medial domains. First, the carpel valves grow excessively tall, forming hornlike protrusions at the apex (Figures 2A and 2B). These horns are not topped with stigmatic papillae, however; they are topped with cells that resemble cells of the style (cf. Figures 2F and 2G). Second, the postgenital fusion between the two medial ridges does not occur (Figures 2B and 2I); despite this failure in ridge fusion, however, ovules are formed from the placentas (Figures 2B and 2I). Hence, proper medial ridge fusion is not required for ovule formation. Third, the lateral and the medial domains are often unfused—either partially unfused near the apex (Figure 2C) or almost completely unfused (Figure 2A)—resulting in two horn-bearing carpel valves and two stigma/style/ovule–bearing medial structures (Figure 2A). Lack of valve fusion also does not affect the ability of the medial ridge to develop ovules (Figures 2B and 2J). The lack of congenital fusion can occur early (Figure 2D), when the two carpel valve primordia initiate and outgrow the two medial structures, or it can occur later in development (Figure 2C). The absence of stigma and a reduction in stylar tissue on top of the carpel valves whenever the carpel valves fail to fuse with medial structures (Figure 2A) suggest that the medial domain may play an important role in directing stigma/style formation. In addition to the abnormality in lateral and medial tissue development, the number of carpels in lug single mutants ranges from one to three (Figures 2H to 2J). In lug-1 mutants, 25% of the gynoecia have one or 1.5 carpels, 71% of the gynoecia have two carpels, and 3.7% of the gynoecia have three carpels (Table 1). In lug-3, 75% of the gynoecia have one to 1.5 carpels, whereas only 25% of the gynoecia have two carpels (Table 1).
Figure 2.
Gynoecium Development in lug and ant Single Mutants.
(A) A lug-3 gynoecium at stage 11 or 12. The two carpel valves and the two medial structures (ms) are not fused with each other. The valves are topped with horns (h), whereas the medial structures are topped with style and stigmatic papillae.
(B) A lug-1 gynoecium unfused near the apex, revealing the developing ovules (o and arrows), despite failure in fusion. h, horn.
(C) A lug-1 gynoecium. The line indicates the medial plane. Stigmatic papillae are prominently developing from the two medial swellings. One valve at the right is still fused to the medial swelling (arrow) and is developing some stigmatic papillae. h, horn.
(D) A stage 8 lug-2 mutant gynoecium. This gynoecium is developing more slowly than the rest of the flower. The line indicates the medial plane. The two laterally situated protrusions (possibly protruding horns) represent the two carpel valves. Congenital fusion may be failing at this early stage.
(E) A stage 12 ant-9 gynoecium, showing fewer and shorter stigmatic papillae (arrow) than the wild type (Figure 1D).
(F) A close-up image of the wild-type style, which consists of stomata cells (arrow) and cells exhibiting fine surface grooves.
(G) A close-up of the tip of a lug-1 horn. The horn is topped with cells that harbor fine surface grooves and with stomata (arrow). These cells resemble the cells of the style in (F).
(H) A cross-section of a one-carpel lug-1 gynoecium. There is only one medial ridge (arrow) and one carpel valve. Note the reduced septal tissue and the two ovule primordia (o).
(I) A cross-section of a lug-1 gynoecium at stage 10. Postgenital fusion of the two medial ridges failed to occur. The two carpel valves are labeled 1 and 2. One of the four ovules (o) is indicated.
(J) A cross-section of a three-carpel lug-1 gynoecium. 1, 2, and 3 indicate the three carpel valves. The three arrows indicate the three medial ridges. Carpel 3 is partially transformed into a stamen, with an anther locule labeled with an asterisk.
(K) A cross-section of an ant-9 gynoecium at stage 10. The medial ridges fail to fuse with each other (*). One ovule (o) is indicated.
 ;
;  .
.
Table 1.
Number of Carpels per Gynoecium in the Wild Type and Mutants (%)a
| Carpels/Gynoecium | Wild Type | lug-1 | lug-3 | ant-9 | lug-1 ant-9 | lug-3 ant-9 |
|---|---|---|---|---|---|---|
| 1–1.5 | 0 | 25 | 75 | 12.5 | 0 | 19.4 |
| 2 | 100 | 71 | 25 | 83 | 89 | 80.5 |
| 3 | 0 | 3.7 | 0 | 4.1 | 10.5 | 0 |
| (29) | (28) | (12) | (24) | (19) | (36) |
Numbers within parentheses indicate the number of gynoecia examined.
ant-9 single mutant gynoecia also develop abnormally, albeit to a lesser extent. The stigmatic papillae are fewer and shorter than in the wild type (Figure 2E; Elliott et al., 1996). ant-9 medial ridges are frequently unfused to each other (Figure 2K; Klucher et al., 1996), and ant mutant carpels sometimes do not fuse near the apex (Elliott et al., 1996). Small horns are sometimes observed in ant single mutants (data not shown). Like lug single mutants, ant-9 mutant gynoecia also consist of one to 1.5, two, and three carpels at 12.5%, 83%, and 4.1%, respectively (Table 1).
The number of ovules initiated in lug-1, lug-3, and ant-9 single mutants is markedly decreased, as shown in Table 2. The average number of ovules per carpel in the wild type is 26.4 ± 1.3, whereas ant-9 single mutants average 14.8 ± 4.4 ovules per carpel. lug-1 and lug-3 mutants also show fewer ovules, averaging 15.4 ± 4.2 and 14.9 ± 3.1, respectively (Table 2). Although 100% of lug-1, lug-3, and ant-9 plants form stigma, style, and septa (Table 2), these marginal tissues are not necessarily normal, as described above. Hence both lug and ant single mutants exhibit various types and degrees of defects in gynoecium development.
Table 2.
Formation of Marginal Tissues in the Wild Type and Mutants
| Characteristic | Wild Type | lug-1 | lug-3 | ant-9 | lug-1 ant-9 | lug-3 ant-9 |
|---|---|---|---|---|---|---|
| Ovules/carpela | 26.4 ± 1.3 (13) | 15.4 ± 4.2 (11) | 14.9 ± 3.1 (11) | 14.8 ± 4.4 (13) | 0.0 ± 0 (40) | 0.0 ± 0 (67) |
| Gynoecia that form stigmab,c | 100% (22) | 100% (11) | 100% (29) | 100% (17) | 0% (8) | 0% (16) |
| Gynoecia that form styleb,c | 100% (13) | 100% (11) | 100% (14) | 100% (9) | 0% (8) | 0% (8) |
| Gynoecia that form septab,d | 100% (29) | 100% (20) | 100% (18) | 100% (16) | 47% (17) | 0% (28) |
Nomarski microscopy of xylene-cleared gynoecia was used to count the number of ovules per carpel. Numbers in parentheses indicate the number of carpels examined.
Numbers within parentheses indicate the number of gynoecia examined.
Scanning electron microscopy analyses were used to visualize stigma and style.
Light microscopy of tissue cross-sections was used to visualize septum formation.
Gynoecium Development and Ovule Initiation in lug ant Double Mutants
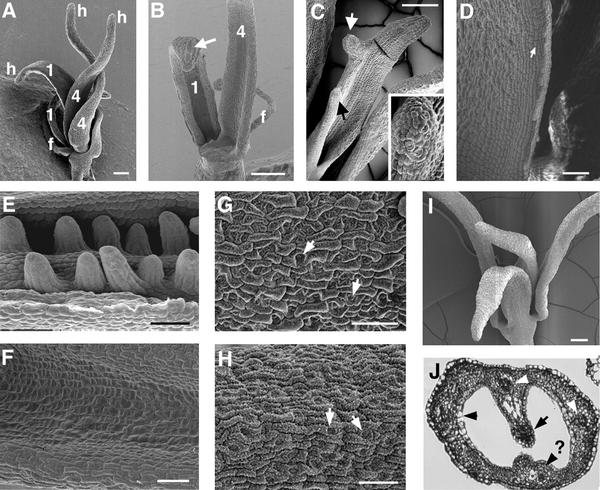
We constructed double mutants of lug and ant to test for any redundant functions not revealed in either single mutant. Both lug-1 ant-9 and lug-3 ant-9 double mutants were constructed. lug-1 is an intermediate-strength allele, whereas lug-3 is a strong allele (Liu and Meyerowitz, 1995). The lug-1 mutation is caused by a single base pair substitution that alters a splicing acceptor site (Conner and Liu, 2000). lug-3 is caused by a nonsense mutation that results in early termination of the protein and is likely a null (Conner and Liu, 2000). ant-9 is a strong allele caused by a transposon Activator (Ac) insertion into the second intron (Elliott et al., 1996). Figures 3 and 4 illustrate the defects observed in lug-1 ant-9 and lug-3 ant-9 double mutants. We found that in lug-3 ant-9 double mutants, the fourth-whorl gynoecium consisted of just two horn-bearing carpel valves (Figures 3A and 4E). Each carpel valve had a lateral vascular bundle (data not shown), and none of the lug-3 ant-9 carpels examined (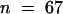 ) formed any ovule (cf. Figures 3E and 3F; Table 2). Occasionally (five times in 26), nub-shaped protrusions were found at the edge of the lug-3 ant-9 carpel valves (Figure 3C). The epidermal morphology of these nubs resembled that of horns rather than ovules (Figure 3C inset). Hence, neither the fourth-whorl nor the first-whorl carpels in lug-3 ant-9 double mutants formed any ovule, septum, or stigma (Figures 3A to 3C, 3F, and 4E, and Table 2). Except for stylelike cells at the tip of the horns and on nubs, no style was present (Figures 3A to 3C and Table 2).
) formed any ovule (cf. Figures 3E and 3F; Table 2). Occasionally (five times in 26), nub-shaped protrusions were found at the edge of the lug-3 ant-9 carpel valves (Figure 3C). The epidermal morphology of these nubs resembled that of horns rather than ovules (Figure 3C inset). Hence, neither the fourth-whorl nor the first-whorl carpels in lug-3 ant-9 double mutants formed any ovule, septum, or stigma (Figures 3A to 3C, 3F, and 4E, and Table 2). Except for stylelike cells at the tip of the horns and on nubs, no style was present (Figures 3A to 3C and Table 2).
Figure 3.
Gynoecium Marginal Tissues Are Absent in lug ant Double Mutants.
(A) The flower of a lug-3 ant-9 double mutant having a total of five floral organs. The filament (f) represents a third-whorl organ. The other four organs all have characteristics of carpel valves, such as horns (h) and epidermal cell morphology. Two of these organs (both labeled 1) are first- whorl organs, and the other two organs (labeled 4) are fourth-whorl organs. Ovule, septum, stigma, and style are absent.
(B) A dissected lug-3 ant-9 double mutant flower. Shown is the adaxial (inner) surface of a fourth-whorl (4) and a first-whorl (1) organ. A stamen filament (f) is visible. The first-whorl organ exhibits features of sepals as well as carpels; the long cell (arrow) on the abaxial surface is characteristic of sepals, whereas the thickening of the edge is characteristic of carpels. Ovule, stigma, and style were not detected in the first-whorl organs or in the fourth-whorl organs.
(C) A lug-3 ant-9 double mutant fourth-whorl carpel. Nublike protrusions (arrows) at the edge of the valve display surface morphology characteristic of horns. The inset shows a magnification (×4) of the nub (indicated by the black arrow).
(D) A close-up of the outer edge of an unfused lug-3 ant-9 fourth-whorl carpel. The rectangular cells (arrow) are characteristic of cells at the edge of carpel valves.
(E) A close-up of the inner surface of a lug-1 gynoecium. Two rows of ovules are initiated from the placenta.
(F) A close-up of the inner surface of a mature fourth-whorl organ in a lug-3 ant-9 double mutant. Note the absence of any ovule primordium.
(G) A close-up of the abaxial epidermal surface of a wild-type Ler carpel valve. Immature stomata cells (arrows) and epicuticular wax are visible.
(H) A close-up of the abaxial epidermal surface of a lug-3 ant-9 fourth-whorl organ. The immature stomata cells (arrows) and epicuticular wax (small white dots over the entire surface) are characteristic of carpel valves and are similar to the wild type, as shown in (G). The more developed stomata and the more prominent wax than those in (G) reflect the older age of this flower.
(I) A lug-1 ant-9 double mutant flower. None of the floral organs bears any marginal tissues.
(J) A cross-section of a stage 13 gynoecium of a lug-1 ant-9 double mutant. No ovule is formed, although some septal tissue is present. Three vascular bundles are visible (arrowheads). The fourth (indicated by an arrowhead and a question mark) is less developed. A transmitting tract is also present (arrow).
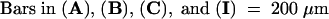 ;
; 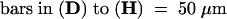 .
.
Figure 4.
Floral Defects Are Enhanced in lug ant Double Mutants.
(A) A wild-type flower.
(B) A lug-3 flower. Floral organs are narrower than those in the wild type.
(C) A lug-3 gynoecium with characteristic horns (arrows). The arrowhead indicates some stigmatic tissue.
(D) An ant-9 flower.
(E) A lug-3 ant-9 double mutant flower. There are three staminoid/carpelloid first-whorl sepals, two filaments (arrowheads), and three third-whorl stamens. No petal is formed. The two fourth-whorl organs do not fuse, are topped with horns (arrows), and are devoid of any marginal tissues.
(F) A lug-3 inflorescence.
(G) An ant-9 inflorescence.
(H) A lug-3 ant-9 inflorescence. Note the many carpelloid first-whorl and fourth-whorl organs with horns (arrows). Floral organs are narrower than either lug-3 or ant-9 single mutants.
lug-1 single mutants exhibit a phenotype less severe than lug-3, and lug-1 ant-9 double mutant gynoecia also exhibit a less severe phenotype. In the gynoecia of lug-1 ant-9 double mutants, marginal tissues were in most cases absent (Figure 3I). However, 47% of lug-1 ant-9 gynoecia (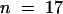 ) developed smaller than normal medial ridges, and these smaller medial ridges gave rise to partially formed septal tissues (including transmitting tract) and a medial vascular bundle (Figure 3J). Nevertheless, none of the lug-1 ant-9 first and fourth-whorl carpels could develop ovules, or stigma, or style (except for the stylarlike cells at the tip of horns) (Table 2). Thus, the ovule-forming ability of the placenta and the development of style and stigma are more sensitive than septa to the simultaneous loss of LUG and ANT.
) developed smaller than normal medial ridges, and these smaller medial ridges gave rise to partially formed septal tissues (including transmitting tract) and a medial vascular bundle (Figure 3J). Nevertheless, none of the lug-1 ant-9 first and fourth-whorl carpels could develop ovules, or stigma, or style (except for the stylarlike cells at the tip of horns) (Table 2). Thus, the ovule-forming ability of the placenta and the development of style and stigma are more sensitive than septa to the simultaneous loss of LUG and ANT.
Scanning electron microscopy analyses of the epidermal morphology of the sterile fourth-whorl organs indicate that they are carpel valves (Figures 3D, 3G, and 3H). Therefore, the lack of ovules and stigma/style in the lug ant double mutants probably does not result from homeotic transformation of carpels into leaves or other sterile organs. Specifically, the abaxial epidermis in the mutant carpel valve consists of a patchwork of clusters of four to five cells that surround an immature unopened stomata and is thus indistinguishable from the abaxial epidermis of a wild-type valve (cf. Figures 3G and 3H). Additionally, rectangular cells, characteristic of the edge of carpel valves, develop on the edge of the fourth- whorl organs in lug-3 ant-9 double mutants (Figure 3D). The cellular morphology of the adaxial surface of lug ant double mutant carpel valves and wild-type carpel valves is also similar (data not shown). The hornlike structures at the top of these sterile organs (Figure 3A) are consistent with their carpel valve identity, given that horns are found only in lug carpels or carpelloid sepals. Furthermore, the partially formed septal tissue and transmitting tract in the lug-1 ant-9 double mutant gynoecia (Figure 3J) support their carpel identity.
In addition to defects in the gynoecium, lug ant double mutants exhibit enhanced defects in whorls 1 to 3 of the flowers. In lug-3 single mutant flowers (Figures 4B and 4F; Liu and Meyerowitz, 1995), the sepals at medial positions are staminoid/carpelloid, whereas the sepals at lateral positions are relatively normal. Second-whorl petals can be absent or staminoid. Third-whorl stamens are fewer in number, with only two or three stamens usually found. ant-9 single mutants also display fewer floral organs in whorls 1 to 3 (Elliott et al., 1996; Klucher et al., 1996). Under our growth conditions and in the Landsberg erecta (Ler) background, ant-9 flowers typically develop four narrow sepals, four narrow petals, and four stamens, and homeotic transformations are rare (Figures 4D and 4G; Elliott et al., 1996). We found that lug-3 ant-9 double mutants have narrower floral organs than either of the single mutants (Figures 4E and 4H). As shown in Figures 3A and 4E, the number of first-whorl organs decreased, with most flowers developing three instead of four first-whorl organs. Moreover, these first-whorl organs are usually carpelloid with horns. The second-whorl organs are completely absent. The third whorl usually consists of one to four abnormal stamens. Occasionally, filaments are found in the first or the third whorl (Figures 3A, 3B, and 4E). The fourth whorl, as described earlier, consists of one, 1.5, or two carpel valves topped with horns (Table 1). Thus, like lug ap2 and ant ap2 (Liu and Meyerowitz, 1995; Elliott et al., 1996), lug ant double mutants exhibit a synergistic effect on the number and identity of the floral organs.
AG RNA Expression in lug-1, ant-9, and lug-1 ant-9 Mutants
AG is a key regulatory gene for flower development with at least two important functions: the specification of carpel and stamen identity, and the control of floral determinacy (Bowman et al., 1989, 1991a). AG encodes a transcription factor of the MADS box family (Yanofsky et al., 1990). In wild-type plants, AG RNA is absent in the inflorescence meristem as well as in stage 1, stage 2, and early stage 3 floral meristems. AG mRNA is first detected in the center of a midstage 3 floral meristem and later is restricted to stamen and carpel primordia (Figure 5A; Drews et al., 1991). This expression of AG is sufficient to specify stamen and carpel identity. In addition, AG is expressed in the medial ridge, ovule primordia, the chalazal region of ovules, and later in the endothelium (Figures 5B and 5C; Bowman et al., 1991b; Reiser et al., 1995).
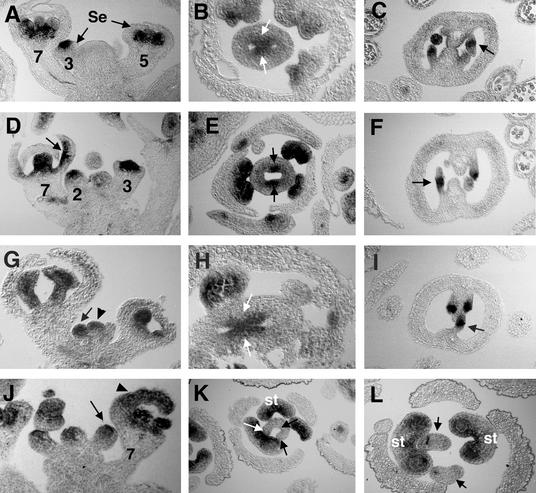
Figure 5.
AG mRNA Expression in the Wild Type, lug-1 and ant-9 Single Mutants, and lug-1 ant-9 Double Mutants.
(A) to (C) AG expression in the wild type (Ler). (A) AG is expressed in the center of a stage 3 flower and in the stamen and carpel primordia of flowers at later stages. Numbers indicate the stages of flowers, based on Smyth et al. (1990). Se, sepals. (B) AG is expressed in the medial ridge (arrows) but is absent in carpel valve in this stage 8 flower. (C) AG is expressed in the chalazal region (arrow) of the developing ovules. Integuments are initiated from this region.
(D) to (F) AG mRNA expression in lug-1 mutants. (D) AG is ectopically expressed in the inner surface of a sepal in a stage 7 lug-1 flower (arrow). AG is expressed precociously in a late stage 2 flower. (E) AG is expressed in the medial ridge in a stage 7 lug-1 flower. Note the reduced AG expression as well as the reduced medial ridges (arrows). (F) AG is expressed in the chalazal area (arrow) of developing lug-1 ovules.
(G) to (I) AG expression in ant-9 mutants. (G) AG is precociously expressed in a midstage 2 ant-9 floral meristem (arrow). The stage of this floral meristem was verified by examining adjacent sections. The meristem (arrowhead) at the center is a floral meristem rather than an inflorescence meristem. No ectopic expression of AG was found in this section. (H) AG is expressed in the medial ridge (arrows) of an ant-9 gynoecium. (I) AG is expressed in the chalazal area (arrow) of developing ovules in ant-9.
(J) to (L) AG mRNA expression in lug-1 ant-9 double mutants. (J) AG is precociously expressed in a stage 2 floral meristem (arrow) in lug-1 ant-9 double mutants. Ectopic AG expression is detected in the developing sepal of a stage 7 flower (arrowhead). (K) AG expression is absent in the gynoecium of a stage 7 lug-1 ant-9 double mutant flower. Arrows indicate the areas in which AG expression and medial ridge formation would have occurred in wild-type flowers. Nevertheless, AG expression is readily detected in the three developing stamens (st) of the same lug-1 ant-9 flower. (L) Another lug-1 ant-9 flower, showing two carpel valves (arrows) completely unfused to each other. No ovule is formed; neither are any medial ridge-derived tissues. The two carpel valves are surrounded by two stamens (st), which are surrounded by three sepals.
Because the defects in transformation of floral organ identity and organ number in lug mutants were caused by ectopic and precocious expression of AG (Liu and Meyerowitz, 1995), we tested whether abnormal AG expression was also responsible for the flower and gynoecial defects seen in the lug ant double mutants. Specifically, in situ hybridization was used to test directly whether AG RNA was expressed precociously, ectopically, or both in young floral meristems of lug-1 ant-9 double mutants. Further, the expression of AG in gynoecia was examined to test if a higher level of AG expression in the medial ridge, or an earlier AG expression preceding medial ridge initiation, or an ectopic AG expression in the entire gynoecium (including lateral and medial domains) could be responsible for the defects of marginal tissue development in lug ant double mutants.
Precocious and ectopic AG mRNA expression was observed in floral meristems and floral organ primordia of lug-1 single mutants (Figure 5D; Liu and Meyerowitz, 1995). AG mRNA was detected precociously in stage 2 floral meristems and ectopically in sepal primordia of lug mutants. However, in the medial ridge of lug-1 mutants, the expression of AG was decreased, as was the size of the medial ridge (Figure 5E). Finally, AG mRNA expression was unaffected in the chalazal region of the ovule in lug-1 mutants (Figure 5F). In ant-9 mutants, AG mRNA was also expressed precociously in stage 2 floral meristems (Figure 5G). In developing sepals, ectopic AG expression was occasionally but infrequently observed (data not shown). In the medial ridge and the chalazal region of the ovule in ant-9 mutants, the expression of AG mRNA was similar to that in the wild type (Figures 5H and 5I). In floral meristems and floral organ primordia of lug-1 ant-9 double mutants, both precocious and ectopic AG mRNA expression was observed (Figure 5J). In the lug-1 ant-9 double mutant gynoecia examined in our in situ hybridization experiments, AG mRNA was not detected in the anticipated medial ridge areas (Figures 5K and 5L). Thus, AG mRNA expression conveniently marks the medial ridge tissue in the wild type, and its absence in the lug-1 ant-9 gynoecia correlates with the absence of the medial ridge.
In the wild-type gynoecium at stage 5 and later, expression of AG mRNA subsides, becomes completely absent from carpel valves, and is restricted to the medial ridge (Figure 5B). This subsiding AG expression was similarly observed in lug-1 and ant-9 single mutants as well as in lug-1 ant-9 double mutants (Figures 5B, 5E, 5H, and 5K), indicating that the loss of medial ridge in lug ant double mutants was not caused by prolonged or increased AG expression in the gynoecium.
Ovule Formation in Double Mutants of Negative Regulators of AG
Because our genetic analyses revealed a redundant role in marginal tissue development for LUG and ANT (both negative regulators of AG mRNA expression in floral meristems and floral organ primordia), we examined medial ridge–derived tissues in double mutants of additional genes that are negative regulators of AG. The known AG repressors include LUG, ANT, AP2, and CURLY LEAF (CLF). AP2 is a class A floral homeotic gene involved in both the negative regulation of AG and the specification of sepal and petal identity (Bowman et al., 1991a; Jofuku et al., 1994). CLF is required for the negative regulation of AG in vegetative tissues and for maintaining proper AG expression at late stages of flower development (Goodrich et al., 1997). We examined ovule and placenta formation in lug-2 ap2-2, ant-9 ap2-2, and lug-8 clf-2 double mutants. As shown in Figure 6, unlike lug ant-9 double mutants, the lug-2 ap2-2, ap2-2 ant-9, and lug-8 clf-2 double mutants all formed placentas and initiated ovule development. Furthermore, stigmatic and stylar tissues were detected, although they were reduced (data not shown). Thus, the absence of ovules and other marginal tissues is unique to lug ant double mutants and is not a property of all double mutants of negative regulators of AG.
Figure 6.
Ovule Formation in Double Mutants of Negative Regulators of AG.
(A) ap2-2 ovules at anthesis.
(B) A close-up of a lug-2 ap2-2 double mutant carpel. Although the carpel is unfused, two rows of ovule primordia have initiated.
(C) An ant-9 single mutant ovule. The ovule does not develop any integument. Arrows indicate the anticipated integument initiation site.
(D) A close-up of the ant-9 ap2-2 double mutant carpel. Like ant-9 single mutants, ap2-2 ant-9 double mutants form ovules. These ovules do not have any integument. Arrows indicate the anticipated integument initiation site.
(E) clf-2 single mutant seeds.
(F) Developing ovules in lug-8 clf-2 double mutants. Some of these ovules have a protruding inner integument (arrow), similar to ovules of lug single mutants (Roe et al., 1997; Schneitz et al., 1997).
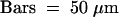 .
.
DISCUSSION
LUG and ANT Are Critical Regulators of Marginal Tissue Development
We found that the ability to form ovules, septa, stigma, and style is only weakly affected in lug or ant single mutants but is strongly affected in the lug ant double mutants. Strong lug ant double mutations completely abolish the formation of placentas, ovules, septa, and stigma. Although stylelike cells are found at the tip of horns, the lug ant double mutants also lack a style. Our finding indicates that LUG and ANT play a critical role in the development of gynoecium marginal tissues. The strong synergistic interaction between lug and ant in gynoecium marginal tissues is not observed in other double mutants, including ap2-2 lug-2, ap2-2 ant-9, and clf-2 lug-8 (Figure 6). Hence, LUG and ANT are unique among this group of AG repressors, and this effect must reflect a specific requirement of these two genes in the development of these tissues.
The Mechanism of LUG and ANT Action in Medial Ridge Development Differs from That in Floral Organ Identity Determination
Previous studies indicated that in developing floral meristems and floral organ primordia, ectopic and precocious AG expression contributes to both homeotic transformation of floral organs and the reduction of floral organ number in lug mutants (Liu and Meyerowitz, 1995). In this study, we show that AG expression starts much earlier in ant-9 mutant floral meristems than in the wild type. This precocious AG expression in ant-9 may be at least partially responsible for the reduced floral organ numbers observed in ant mutant flowers. Thus, one function of ANT may be to prevent AG expression in very young floral meristems, to allow for sufficient cell proliferation and thereby regulate the number of cells in a floral meristem. The rare occurrence of ectopic AG expression in ant-9 floral organ primordia explains the infrequent homeotic transformation observed in ant mutant flowers.
The defect in marginal tissue development, particularly in the medial ridge development, observed in ant single and ant lug double mutants, is consistent with a previously proposed role of ANT in regulating cell proliferation associated with organ primordial outgrowth. More recently, overexpression of ANT in 35S::ANT transgenes was shown to cause enlarged embryos and bigger lateral organs in Arabidopsis and tobacco (Krizek, 1999; Mizukami and Fischer, 2000). Having observed that the increased cell number in the bigger organs of 35S::ANT transgenic plants resulted from an extended period of cell proliferation, Mizukami and Fischer (2000) proposed that ANT regulates cell proliferation by maintaining the meristematic competence of cells during organogenesis. In addition, the importance of ANT in medial ridge formation is further supported by the recent work on crc gym ant triple mutants (Eshed et al., 1999) in which wild-type ANT activity is required for ectopic ovule formation on the abaxial surface of the crc gym mutant gynoecium. Because ANT encodes a protein with two DNA binding AP2 domains (Elliott et al., 1996; Klucher et al., 1996), it probably regulates the transcription of target genes with roles in cell proliferation associated with medial ridge development.
In contrast, the role of LUG in cell proliferation control is not well established. The narrower and smaller floral organs and leaves in lug single mutants are similar to ant single mutants and may thus reflect a role of lug in cell proliferation that is separate from its role in floral organ identity specification. The strong synergistic interaction between lug and ant in the medial ridge further supports a role of LUG in cell proliferation. We propose that LUG, a protein with WD repeats (Conner and Liu, 2000), may directly interact with the ANT protein to regulate the expression of common target genes in the medial ridge. Removing wild-type activities of both genes may considerably reduce the expression of these target genes and diminish cell proliferation activity in the medial ridge.
Several lines of evidence indicate that the absence of medial ridge formation and marginal tissues in lug ant double mutants is not mediated by ectopic AG. First, medial ridge formation still occurs in double mutants of lug-2 ap2-2, ant-9 ap2-2, and clf-2 lug-8 (Figure 6), which exhibit similar homeotic transformation in floral organ identity and a decrease in organ number as a result of increased ectopic AG expression. Second, transgenic plants that ectopically express AG under the control of a cauliflower mosaic virus 35S promoter can still initiate ovules in first-whorl as well as fourth-whorl carpels (Mandel et al., 1992; Mizukami and Ma, 1992; Ray et al., 1994), although these ovules are abnormal or carpel-like. Third, in situ examination of AG mRNA expression (Figure 5) revealed neither ectopic AG expression nor any abnormally prolonged AG expression in the gynoecial primordia, medial ridge, or the ovules of lug-1 single, ant-9 single, or lug-1 ant-9 double mutants. Finally, because stigma and ovule still form in ag ap2 double mutants (Bowman et al., 1991a; Alvarez and Smyth, 1999), AG activity is not essential for the development of these tissues.
Development of Medial and Lateral Domains Is Relatively Independent
Our study shows that eliminating the medial domain (as shown by a lack of any marginal tissues) does not drastically affect lateral domain development. Furthermore, the medially derived marginal tissues and the laterally derived carpel valves can develop and differentiate in the absence of fusion between them, suggesting that medial and lateral domains may develop relatively independently of each other.
In support of this notion, we note that ett mutants have a gynoecium defect almost complementary to that of lug ant double mutants. ett-1 mutations cause an almost complete loss of valve tissue in the gynoecium (Sessions and Zambryski, 1995). Flowers of ett tousled (tsl) double mutants appear to have a stronger defect, in which the gynoecium consists of only a small mound of ovule-bearing tissue developing in the center of the flower lacking all carpel valve tissues (Roe et al., 1997). Perhaps the formation of these two types of gynoecium tissues—lateral carpel valve as opposed to marginal tissues—requires two different sets of genes: ETT/TSL and LUG/ANT, respectively. The former may promote carpel valve, whereas the latter may promote the marginal tissues. The interaction and fusion between these two tissues may further modulate gynoecium morphogenesis. Thus, in the absence of normal interaction, as in the case of the lug single mutants, hornlike protrusions may occur, and in the case of ett, the stigmatic tissue may grow on the outer surface of the gynoecium (Sessions and Zambryski, 1995).
LUG and ANT Can Function as Either Positive or Negative Regulators, Depending on the Tissue Context
Our study indicates that LUG and ANT promote medial ridge and marginal tissue development. This is in contrast to their roles as negative regulators of AG and, hence, of carpel identity. Thus, LUG and ANT may function as either a positive or a negative regulator, depending on the tissue context. This notion is best illustrated for LUG in the study of CRC (Alvarez and Smyth, 1999; Bowman and Smyth, 1999). CRC controls several aspects of carpel development and encodes a zinc finger and a helix-loop-helix domain. CRC mRNA is expressed in two distinct domains in gynoecium: the abaxial epidermal cell layer, and four internal groups of cells adjacent to the placental tissue (Bowman and Smyth, 1999). LUG influences the pattern of CRC expression in two opposite ways—by repressing CRC in the outer whorls of the flower and by promoting CRC expression in the four internal expression domains of the gynoecium (Bowman and Smyth, 1999). Perhaps LUG could interact with different partners to affect transcription in opposite ways. Such a mechanism might modulate its activity differently in flowers versus gynoecia.
Because LUG promotes CRC internal domain expression (Bowman and Smyth, 1999), CRC is a candidate target gene of LUG involved in medial ridge development. Like lug mutations, the crc-1 null mutation causes a reduction of ovules. However, crc-2, a CRC promoter mutation that specifically abolishes the abaxial epidermal expression but leaves the internal expression domains intact, does not produce fewer ovules (Bowman et al., 1999; Eshed et al., 1999). Hence, the internal CRC expression domain is likely to promote the production of ovules, and the reduction of ovules in lug mutants probably reflects the loss of CRC internal expression domains. Nevertheless, CRC cannot be the sole factor in mediating the effect of lug on medial ridge development because crc ant double mutants do not exhibit the same defect as lug ant in medial ridge development (Eshed et al., 1999). Thus, LUG must regulate other genes in addition to CRC that are critical for medial ridge development.
Additional genes that participate in the development of gynoecium marginal tissue include SPT, TSL, and PERIANTHIA (PAN). spt single mutant carpels lack a transmitting tract and exhibit defects in the postgenital fusion of the septum, whereas crc spt double mutant gynoecia exhibit a decrease in stigmatic papillae, style, ovules, and a complete loss of the septal tissue (Alvarez and Smyth, 1999; Bowman et al., 1999). lug tsl and pan tsl double mutants have also been noted to exhibit reduced marginal tissues (Roe et al., 1997). Nevertheless, none of these double mutants exhibits a defect in the marginal tissue development that is as striking and as complete as the one observed in lug ant double mutants. Thus, TSL, PAN, CRC, and SPT may all participate in the regulation of the development of gynoecium marginal tissue, acting either downstream of or together with LUG/ANT. In addition, genes expressed specifically in the medial ridge or marginal tissues could be candidate targets of LUG/ANT regulation—including AGL1/AGL5, AGL 11, SHOOTMERISTEMLESS (STM), and several class I knotted-like homeobox proteins (Hake et al., 1995; Rousley et al., 1995; Savidge et al., 1995; Flanagan et al., 1996; Long et al., 1996). In situ examination of the expression patterns of these candidate target genes in lug ant double mutants may help confirm their function and shed light on the mechanism of ANT/LUG action in gynoecial marginal tissue development.
METHODS
Plant Growth and Genetics
Seeds (Arabidopsis thaliana) were sown in Metro-Mix 200 soil (E.C. Geiger, Harleysville, PA). Biological larvicide Gnatrol (Penn State Seeds, Dallas, PA) was added to the water used to moisten the soil before sowing. The planted seeds were incubated at 4 to 10°C for 3 days and then were placed under constant cool white fluorescent lights at 20°C; the plants were fertilized ∼14 days after germination with Miracle-Gro plant food.
Because lug, ant, and ap2 all map to chromosome 4, and because ant-9 is completely sterile, the double mutants between lug and ant or between lug and ap2 were constructed as follows. lug-1 and lug-3 single mutant carpels were each pollinated by the ap2-2+/+ ant-9 transheterozygote plants. The wild-type F1 progeny segregated in F2 as either 1/4 lug, 1/4 ant-9, and 1/2 wild type (class I) or 1/4 lug, 1/4 ap2-2, and 1/2 wild type (class II). Seeds from 80 individual lug-1 mutants (class I) were collected and planted, and eight of these 80 lug-1 individuals segregated as 1/4 lug-1 ant-9 double mutants in F3. Similarly, seeds of 25 lug-3 F2 individuals (class I) were collected, and four of these 25 segregated as 1/4 lug-3 ant-9 double mutants in F3. The lug-1 ap2-2 double mutants were obtained by screening a large number of F2 progeny of lug-1+/+ ap2-2. The ant-9 ap2-2 /+ ap2-2 and the ant-9+/+ ap2-2 seeds were kindly provided by Dr. David Smyth, and the lug-8 clf-2 seeds were kindly provided by Dr. Justin Goodrich. In our experiments, ant-9 mutants exhibited a less severe floral defect than previously reported in ant-9 of the C24 ecotype (Elliott et al., 1996), possibly because of the Landsberg erecta (Ler) background introduced into the ant-9 used in our studies.
Microscopic Analyses
For scanning electron microscopy, the carpels, siliques, and flowers were first dissected under a stereo microscope and then fixed, coated, and photographed as described previously (Bowman et al., 1989, 1991a). Images were directly captured with the SEMICAPS software (SEMICAPS Inc., Santa Clara, CA) and the AMRAY 1000A scanning electron microscope. For the light microscopy histological sections, samples were fixed, stained, and sectioned using the in situ protocol (Drews et al., 1991). Tissues in 8-μm-thick sections were dewaxed in 100% xylene, hydrated in ethanol series, and stained with toluidine blue. The stained tissues were dehydrated and mounted with Cytoseal 60 mounting medium (Stephens Scientific, Riverdale, NJ) and visualized and photographed under a Zeiss Axioplan2 microscope. For confocal microscopy, tissues were fixed, stained, and dissected according to Running et al. (1995) and were visualized with a Bio-Rad MRC 1024 confocal microscope.
In Situ Hybridization
The floral tissue (entire inflorescence) was fixed as previously described (Drews et al., 1991), except that fixation time was 1 hr. The antisense AG probe was transcribed from the plasmid pCIT656 (Yanofsky et al., 1990). The antisense probe was synthesized using the Epicenter Technologies AmpliScribe T7 transcription kit (Madison, WI) according to manufacturer's protocol except that 5 μL of 10 mM digoxigenin-11-UTP and cold UTP were added in 1:1 ratio. The probe was hybridized to the 8-μm-thick sections of the tissue, which were then processed essentially as described by Lincoln et al. (1994) and Long and Barton (1998).
NOTE ADDED IN PROOF
For the molecular analyses of LEUNIG, see J. Conner and Z. Liu, Proceedings of the National Academy of Sciences of the United States of America, volume 97, in press.
Acknowledgments
We thank David Smyth for the ant-9 and ant-9 ap2-2 seeds, Justin Goodrich for the clf-2 lug-8 seeds, Jai-Young Song and Joann Conner for help with the in situ hybridization experiments, Eric Baehrecke for use of the Zeiss Axioplan2 microscope, and Tim Maugel for assistance with scanning electron microscopy. We thank Drs. Eric Baehrecke, Joann Conner, Todd Cooke, and anonymous reviewers for critical comments on the manuscript. We also thank Dr. Elizabeth Zimmer and the National Museum of Natural History Laboratory of Molecular Systematics for providing a Smithsonian Visiting Student Summer Fellowship to V.P.K. This work was supported by a National Institutes of Health postdoctoral fellowship (GM20426-01) to R.G.F. and the United States Department of Agriculture (Grant Nos. 96-35304-3712 and 98-35304-6714) to Z.L.
References
- Alvarez, J., and Smyth, D.R. (1999). CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development 126, 2377–2386. [DOI] [PubMed] [Google Scholar]
- Bowman, J.L., and Smyth, D.R. (1999). CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development 126, 2387–2396. [DOI] [PubMed] [Google Scholar]
- Bowman, J.L., Smyth, D.R., and Meyerowitz, E.M. (1989). Genes directing flower development in Arabidopsis. Plant Cell 1, 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, J.L., Smyth, D.R., and Meyerowitz, E.M. (1991. a). Genetic interactions among floral homeotic genes of Arabidopsis. Development 112, 1–20. [DOI] [PubMed] [Google Scholar]
- Bowman, J.L., Drews, G.N., and Meyerowitz, E.M. (1991. b). Expression of Arabidopsis floral homeotic gene AGAMOUS is restricted to specific cell types late in flower development. Plant Cell 3, 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, J.L., Baum, S.F., Eshed, Y., Putterill, J., and Alvarez, J. (1999). Molecular genetics of gynoecium development in Arabidopsis. Curr. Top. Dev. Biol. 45, 155–205. [DOI] [PubMed] [Google Scholar]
- Conner, J.A., and Liu, Z. (2000). LEUNIG, a putative transcriptional co-repressor that regulates AGAMOUS expression during flower development. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- Drews, G.N., Bowman, J.L., and Meyerowitz, E.M. (1991). Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 65, 991–1002. [DOI] [PubMed] [Google Scholar]
- Elliott, R.C., Betzner, A.S., Huttner, E., Oakes, M.P., Tucker, W.Q.J., Gerente, D., Perez, P., and Smyth, D.R. (1996). AINTEGUMENTA and APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed, Y., Baum, S.F., and Bowman, J.L. (1999). Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell 99, 199–209. [DOI] [PubMed] [Google Scholar]
- Flanagan, C.A., Hu, Y., and Ma, H. (1996). Specific expression of AGL1 MADS-box gene suggests regulatory functions in Arabidopsis gynoecium and ovule development. Plant J. 10, 343–353. [DOI] [PubMed] [Google Scholar]
- Gasser, C.S., and Robinson-Beers, K. (1993). Pistil development. Plant Cell 5, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich, J., Puangsomlee, P., Martin, M., Long, D., Meyerowitz, E.M., and Coupland, G. (1997). A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature 386, 44–51. [DOI] [PubMed] [Google Scholar]
- Gu, Q., Ferrandiz, C., Yanofsky, M.F., and Martienssen, R. (1998). The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 125, 1509–1517. [DOI] [PubMed] [Google Scholar]
- Hake, S., Char, B.R., Chuck, G., Foster, T., Long, J., and Jackson, D. (1995). Homeobox genes in the functioning of plant meristems. Philos. Trans. R. Soc. Lond. 350, 45–51. [DOI] [PubMed] [Google Scholar]
- Hill, J.P., and Lord, E.M. (1989). Floral development in Arabidopsis thaliana: A comparison of the wild type and the homeotic pistillata mutant. Can. J. Bot. 67, 2922–2936. [Google Scholar]
- Jofuku, K.D., den Boer, B., Van Montagu, M., and Okamuro, J.K. (1994). Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6, 1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher, K.M., Chow, H., Reiser, L., and Fischer, R.L. (1996). The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. Plant Cell 8, 137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaki, M.K., Okada, K., Nishino, E., and Shimura, Y. (1988). Isolation and characterization of novel mutants of Arabidopsis thaliana defective in flower development. Development 104, 195–203. [Google Scholar]
- Krizek, B.A. (1999). Ectopic expression of AINTEGUMENTA in Arabidopsis plants results in increased growth of floral organs. Dev. Genet. 25, 224–236. [DOI] [PubMed] [Google Scholar]
- Lincoln, C., Long, J., Yamaguchi, J., Serikawa, K., and Hake, S. (1994). A knotted-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell 6, 1859–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z., and Meyerowitz, E.M. (1995). LEUNIG regulates AGAMOUS expression in Arabidopsis flowers. Development 121, 975–991. [DOI] [PubMed] [Google Scholar]
- Long, J.A., and Barton, M.K. (1998). The development of apical embryonic pattern in Arabidopsis. Development 125, 3027–3035. [DOI] [PubMed] [Google Scholar]
- Long, J.A., Moan, E.I., Medford, J.I., and Barton, M.K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379, 66–69. [DOI] [PubMed] [Google Scholar]
- Ma, H., Yanofsky, M.F., and Meyerowitz, E.M. (1991). AGL1–AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Genes Dev. 5, 484–495. [DOI] [PubMed] [Google Scholar]
- Mandel, M.A., Bowman, J.L., Kempin, S.A., Ma, H., Meyerowitz, E.M., and Yanofsky, M.F. (1992). Manipulation of flower structure in transgenic tobacco. Cell 71, 133–143. [DOI] [PubMed] [Google Scholar]
- Mizukami, Y., and Fischer, R.L. (2000). Plant organ size control: AINTEGUMENTA regulate growth and cell numbers during organogenesis. Proc. Natl. Acad. Sci. USA 97, 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami, Y., and Ma, H. (1992). Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell 71, 119–131. [DOI] [PubMed] [Google Scholar]
- Okada, K., Komaki, M.K., and Shimura, Y. (1989). Mutational analysis of pistil structure and development of Arabidopsis thaliana. Cell Differ. Dev. 28, 27–38. [DOI] [PubMed] [Google Scholar]
- Ray, A., Robinson-Beers, K., Ray, S., Baker, S.C., Lang, J.D., Preuss, D., Milligan, S.B., and Gasser, C.S. (1994). Arabidopsis floral homeotic gene BELL (BEL1) controls ovule development through negative regulation of AGAMOUS gene (AG). Proc. Natl. Acad. Sci. USA 91, 5761–5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser, L., Modrusan, Z., Margossian, L., Samach, A., Ohad, N., Haughn, G.W., and Fischer, R.L. (1995). The BELL1 gene encodes a homeodomain protein involved in pattern formation in the Arabidopsis ovule primordium. Cell 83, 735–742. [DOI] [PubMed] [Google Scholar]
- Roe, J., Nemhauser, J.L., and Zambryski, P.C. (1997). TOUSLED participates in apical tissue formation during gynoecium development in Arabidopsis. Plant Cell 9, 335–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousley, S.D., Ditta, G.S., and Yanofsky, M.F. (1995). Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 7, 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Running, M.P., Clark, S.E., and Meyerowitz, E.M. (1995). Confocal microscopy of the shoot apex. Methods Cell Biol. 49, 217–229. [DOI] [PubMed] [Google Scholar]
- Savidge, B., Rousley, S.D., and Yanofsky, M.F. (1995). Temporal relationships between the transcription of two Arabidopsis MADS box genes and floral organ identity genes. Plant Cell 7, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneitz, K., Hulskamp, M., Kopczak, S., and Pruitt, R. (1997). Dissection of sexual organ ontogenesis: A genetic analysis of ovule development in Arabidopsis thaliana. Development 124, 1367–1376. [DOI] [PubMed] [Google Scholar]
- Sessions, R.A. (1997). Arabidopsis (Brassicaceae) flower development and gynoecium patterning in wild type and ettin mutants. Am. J. Bot. 84, 1179–1191. [PubMed] [Google Scholar]
- Sessions, R.A., and Zambryski, P.C. (1995). Arabidopsis gynoecium structure in the wild type and in ettin mutants. Development 121, 1519–1532. [DOI] [PubMed] [Google Scholar]
- Sessions, R.A., Nemhauser, J.L., McColl, A., Roe, J.L., Feldmann, K.A., and Zambryski, P.C. (1997). Ettin patterns the Arabidopsis floral meristem and reproductive organs. Development 124, 4481–4491. [DOI] [PubMed] [Google Scholar]
- Smyth, D.R., Bowman, J.L., and Meyerowitz, E.M. (1990). Early flower development in Arabidopsis. Plant Cell 2, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky, M.F., Ma, H., Bowman, J.L., Drews, G.N., Feldmann, K.A., and Meyerowitz, E.M. (1990). The protein encoded by the Arabidopsis homeotic gene AGAMOUS resembles transcription factors. Nature 346, 35–40. [DOI] [PubMed] [Google Scholar]