Abstract
UV irradiation stimulates expression of the gene encoding the key enzyme chalcone synthase (CHS), which leads to the generation of protective flavonoids in parsley cell cultures. CHS transcripts increase after 3 to 4 hr, and early genes are involved in the signal transduction to the CHS promoter. By using the fluorescent differential display technique in a large-scale screening, several early UV light–induced genes were isolated. Of these, a novel glutathione S-transferase (PcGST1) is induced within 2 hr and precedes CHS expression. Overexpression of PcGST1 in transformed cell lines containing a CHS promoter/luciferase reporter (CHS-LUC) affected the onset of LUC transcription. Supplementing these cell lines with glutathione immediately stimulated CHS-LUC expression within 2 hr in dark-incubated cells and resulted in a biphasic induction profile in UV-irradiated cells. Our data indicate the involvement of glutathione and PcGST1 in early events of a UV light–dependent signal transduction pathway to CHS. In this context, the oxidative status of a cell acts as a central regulating element.
INTRODUCTION
During photomorphogenesis, the expression of numerous genes is controlled by several photoreceptors that absorb in the visible and UV range of sunlight. In addition to the family of photoreversible phytochromes, several photoreceptors absorbing blue and UV-A light have recently been isolated from higher plants (Furuya and Schaefer, 1996; Cashmore et al., 1999). Although phytochrome-mediated responses have been extensively studied, only a few elements involved in signal transduction have been isolated so far (Neff et al., 2000). UV-B irradiation of higher plants also specifically induces various responses that are attributed to the action of a hypothesized UV-B photoreceptor (Jordan, 1996). Of these responses, the induction of phenylpropanoid and flavonoid glycoside biosynthetic pathways and the consequential accumulation of UV-protective flavonoids in UV-irradiated cells of a parsley suspension culture have been analyzed in detail (Hahlbrock and Scheel, 1989). As a key enzyme of flavonoid biosynthesis, chalcone synthase (CHS) is transcriptionally stimulated by UV light in this cell culture but also in leaves of adult parsley plants (Chappell and Hahlbrock, 1984; Frohnmeyer et al., 1992). CHS mRNA is strongly and exclusively induced by irradiation with low fluences of short-wavelength light with an apparent lag phase of a few hours (Chappell and Hahlbrock, 1984). This CHS induction is independent of the formation of dimerized DNA produced by irradiation with high fluences of UV-B (Frohnmeyer et al., 1999). Other external signals are ineffective in stimulating CHS expression, rendering the parsley cell culture a suitable system for the study of UV-dependent signal transduction.
The parsley CHS promoter contains four cis-acting elements that mediate the light response (Schulze-Lefert et al., 1989). Several trans-acting factors binding to the CHS promoter have been isolated (Weisshaar et al., 1991; Feldbruegge et al., 1997; Kircher et al., 1998), but their involvement in the light-mediated activation of CHS expression has not been clarified. Using pharmacological effectors, investigators have characterized several upstream components such as calcium, calmodulin, and serine/threonine kinases as elements mediating the UV-dependent CHS expression in parsley (Frohnmeyer et al., 1997, 1999). These components also affect the UV-induced CHS expression in Arabidopsis and soybean cell cultures, pointing to the existence of a conserved signaling cascade to the CHS promoter (Christie and Jenkins, 1996; Frohnmeyer et al., 1998; Long and Jenkins, 1998). In these cell cultures, the UV-induced CHS transcription additionally depends on intact protein synthesis, indicating the participation of early UV-induced gene products in this signal transduction (Christie and Jenkins, 1996; Frohnmeyer et al., 1998; Kircher et al., 1998).
To isolate such light-induced early genes, we performed a large-scale analysis by using fluorescent differential display (FDD) (Liang and Pardee, 1992), which has been successfully adapted to plant tissues (Uchida et al., 1998; Kuno et al., 2000). We analyzed the composition of genes stimulated within the first 2 hr under light regimes specifically leading to CHS induction and identified a novel UV-induced glutathione S-transferase (PcGST1). To investigate the role of PcGST1 in processes leading to CHS activation in vivo, we established stable transformation of parsley cell cultures with an effector and a reporter plasmid. Constitutive expression of PcGST1 in a parsley cell line harboring a CHS promoter–controlled luciferase reporter gene (CHS-LUC) caused an earlier increase of UV-induced reporter gene activity and was accompanied by a substantial time shift of maximal LUC activity. Supplementing these cell lines with reduced glutathione (GSH), a substrate of GSTs, led to the immediate onset of LUC expression even without a light stimulus. These results indicate that PcGST1 and GSH are functionally involved in UV-induced signal transduction in parsley.
RESULTS
Identification of Early UV-B–Stimulated Genes by Differential Display
FDD was chosen to screen for UV-induced genes, which are expressed before onset of CHS transcription. Being highly UV responsive, protoplasts isolated from a parsley cell culture were irradiated with a 4-min UV-B pulse (using a 305-nm cutoff filter) and then incubated in the dark or with continuous UV-A light, blue light, red light, or far-red light. Cells were harvested after 2 hr, and RNA was prepared from two independent experiments. All samples were subjected to FDD analysis, and the detected fragments were monitored in parallel to compare directly their expression pattern. By this approach, the concurrent determination of differentially expressed genes enabled us to distinguish between genes preceding CHS expression (after UV-A and UV-B irradiation) and genes that are stimulated by long-wavelength light (blue, red, and far red) but are not related to CHS expression.
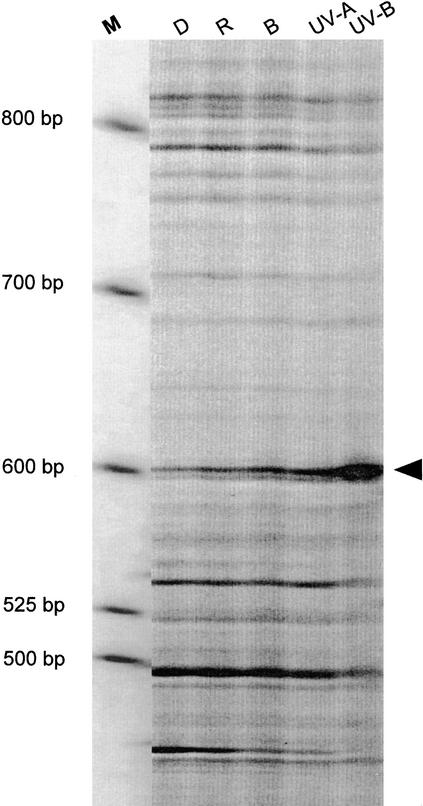
A large-scale screening with 280 different combinations of primer pairs led to the detection of ∼25,000 constitutive expressed bands. Three fragments were upregulated exclusively under conditions (UV-A and UV-B) stimulating CHS expression. Figure 1 illustrates the most pronounced increase of one fragment, which specifically accumulates after UV-B pulse treatment, whereas irradiation with longer wavelengths was ineffective. Besides these, an unexpectedly large number of fragments were induced by red and far-red light (12) or by red and UV-A light (6), confirming that independent phytochrome-mediated gene expression exists in parsley cell cultures (Poppe et al., 1994).
Figure 1.
Representative Differential Display Gel with a UV-B–Induced Fragment Amplified Using 3′-dG(dT)15dC as the Anchor Primer and CAGGCCCTTC as the Specific Primer.
Parsley cells were irradiated with continuous red (R), blue (B), or UV-A light or with a UV-B pulse or were kept in darkness (D) until harvest after 2 hr. The arrowhead indicates the position of the differentially expressed gene encoding PcGST1. M, molecular size marker, in base pairs.
PcGST1 Belongs to the Class of Type III GSTs and Has Substantial Enzymatic Activity
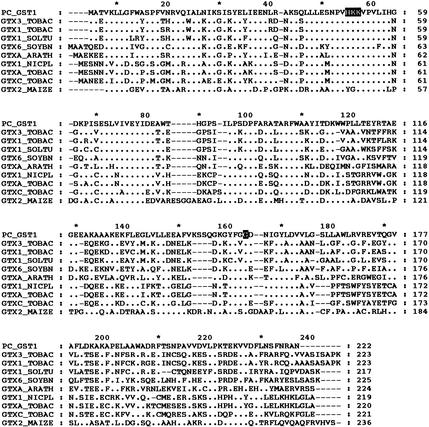
One UV-induced cDNA fragment detected by FDD (Figure 1) was further analyzed for single-strand cDNA polymorphism, and the inserts with the correct patterns were chosen for sequencing. Extension of the FDD fragment from a cDNA library of UV-irradiated parsley cells resulted in isolation of a 836-bp insert that contained a full-length cDNA with a 157-bp 3′ untranslated region (GenBank accession number AF177944). As shown in Figure 2, an open reading frame predicted a protein of 222 amino acids for which searches of the Swiss-Prot database revealed a 38 to 42% identity to type III GSTs from higher plants. The deduced amino acid sequence from the isolated parsley cDNA, termed PcGST1, was conserved mainly in the N-terminal region encoding a glutathione binding site (Figure 2). Besides this homology, the appearance and position of one intron in the genomic sequence matched those of other type III GSTs (data not shown).
Figure 2.
Amino Acid Alignment of PcGST1 with Type III GSTs from Various Plants.
Comparison of PcGST1 with the deduced amino acid sequences of GTX3_, GTX1_, GTXA_, and GTXC_TOBAC from tobacco (Takahashi et al., 1991; Takahashi and Nagata, 1992; Boot et al., 1993); GTX6_SOYBN from soybean (GH2/4, Hsp26; Hagen et al., 1988); GTX1_SOLTU from tomato (Taylor et al., 1990); GTXA_ARATH from Arabidopsis (GenBank accession number 46421); GTX1_NICPL from tobacco (Dominov et al., 1992); and GTX2_MAIZE from maize (Bz2; Nash et al., 1990). Sequence identities to PcGST1 are between 38 and 42%. Conserved amino acids of type III GSTs (Droog, 1997) are presented as white letters. Amino acids conserved in type III GSTs and PcGST1 are indicated by dots. Dashes represent sequence gaps to allow for maximum alignment.
The enzymatic activity of recombinant PcGST1 was investigated after synthesis in Escherichia coli. The 25-kD protein revealed considerable transferase activity (114 units/mg protein) for 1-chloro-2,4-dinitrobenzene substrate, whereas the phenylpropanoid p-coumaric acid was not accepted as a substrate. In contrast to the transferase activity, the protein showed negligible glutathione peroxidase activity (0.4 unit/mg protein). These substrate specificities are comparable with those of other type III GSTs (Dixon et al., 1998) and provide evidence that PcGST1 can act as a transferase.
PcGST1 Expression Is Regulated by UV-B Light and Auxins
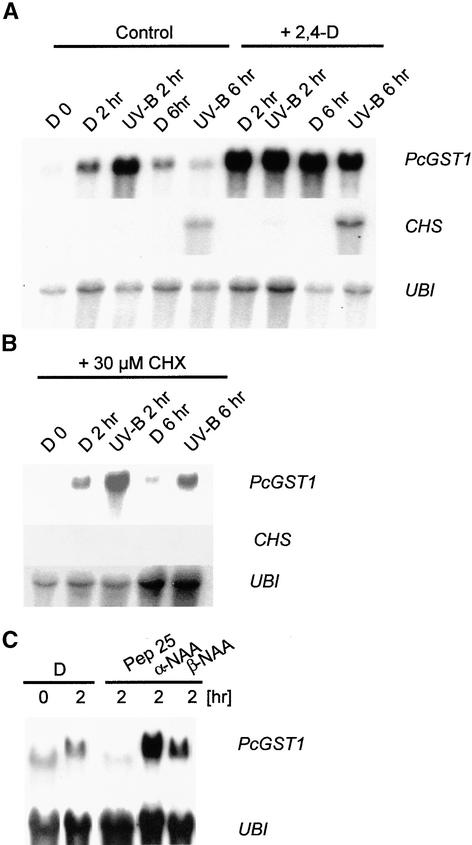
Most members of type III GSTs are transcriptionally induced by multiple stimuli, including fungal infection, heat shock, heavy metals, and hormone treatment (summarized in Marrs, 1996). In contrast, fast stimulation by UV light has not been reported. To determine whether other external signals also activate PcGST1, we performed an RNA gel blot analysis, as depicted in Figure 3. Additionally, CHS and PcGST1 mRNAs were quantified by phosphoimaging analysis, and relative expression of GST/UBI and CHS/UBI (stimulated/nonstimulated) was calculated as n-fold induction values. Parsley cells were soaked on filter paper and subsequently treated with various stimuli such as UV-B light, elicitors, or the auxin analog 2,4-dichlorophenoxyacetic acid (2,4-D). UV-B pulse irradiation and subsequent transfer to darkness resulted in a strong stimulation of PcGST1 mRNA and 35-fold increased levels were detectable 2 hr after light treatment. The PcGST1 mRNA accumulation was transient and returned to basal values (1.4-fold increase) within 6 hr, whereas CHS mRNA accumulated 39-fold at this time (Figure 3A). PcGST1 was also increased fivefold after 2 hr in dark-incubated cells, possibly reflecting a partial activation by the mechanical stress of the handling procedure (Figure 3A). Irradiation of the cells with longer wavelengths did not activate PcGST1 or CHS expression, thus confirming the data from FDD analysis (data not shown).
Figure 3.
RNA Gel Blot Analysis of the Steady State Amounts of PcGST1 and CHS Transcripts and Ubiquitin Concentrations in Response to UV-B Light, Hormone, and Elicitor Treatment.
(A) Detection of transcripts in response to exposure to UV-B light, treatment with 2,4-D, or both. Parsley cells were kept in darkness (D) or irradiated with a pulse of UV-B light through a 305-nm cutoff filter and kept in the dark until harvest after 2 hr (UV-B 2 hr) or 6 hr (UV-B 6 hr). Simultaneously, 4.5 μM 2,4-D was added to dark-incubated cells, which were then either irradiated with UV-B light or kept in darkness until harvest. RNA gel blot analysis was performed with 20 μg of total RNA, as described in Methods. Filters were probed with cDNA inserts for PcGST1, CHS, and ubiquitin (UBI) for loading control analysis.
(B) Detection of transcripts in the absence of intact protein synthesis. Shown is PcGST1 and CHS mRNA accumulation of cells after 30-min pretreatment with 30 μM cycloheximide (CHX), inhibiting protein synthesis and subsequent dark incubation or UV-B irradiation, as described in (A).
(C) Detection of transcripts in response to treatment with the synthetic elicitor Pep25 or the auxin analogs α-NAA and β-NAA. Shown is PcGST1 mRNA accumulation after treatment of dark-incubated cells with 175 nM Pep25, 10 μM α-NAA, or 10 μM β-NAA. All cells were kept in darkness until harvest after 2 hr.
The fast transcriptional stimulation of several plant GSTs also occurs in the presence of protein synthesis inhibitors, defining these as early genes (Abel and Theologis, 1996). The rapid UV-induction of PcGST1 was therefore monitored after inhibition of the translational machinery with cycloheximide. PcGST1 mRNA accumulation under these conditions was similar to that found in untreated cells, and expression was maximal after 2 hr (Figure 3B). Again, a weak induction of the gene could be detected already in dark-incubated cells for the reasons discussed above. In contrast to PcGST1, however, accumulation of CHS mRNA was completely repressed after cycloheximide treatment (Figure 3B).
Preliminary experiments indicated that PcGST1 expression was also induced after transfer of 5-day-old cultured cells to fresh medium containing 2,4-D as the only growth factor (data not shown). Treatment of parsley cells with 4.5 μM 2,4-D (representing the internal concentration of B5 medium) resulted in a stimulation of PcGST1 mRNA irrespective of subsequent light treatment or dark incubation (Figure 3A). CHS mRNA accumulation was strictly dependent on UV treatment but was markedly higher (75-fold increase) in cells supplemented with 2,4-D (Figure 3A). The auxin homolog α-naphthylene acetic acid (α-NAA) was also capable of stimulating PcGST1 expression, whereas a weaker auxin homolog (β-NAA) resulted in only slight induction (Figure 3C). To investigate whether fungal elicitors affect PcGST1 expression, cells were treated with Pep25 polypeptide, an active elicitor component of Phytophthera sojae that causes a multicomponent defense response in parsley cell culture (Nuernberger et al., 1994). As shown in Figure 3C, PcGST1 expression is not induced but repressed by Pep25, which therefore excludes the direct participation of PcGST1 in the activation of the pathogen defense response.
CHS-LUC Expression Reflects Endogenous CHS Contents in Transgenic Parsley Lines
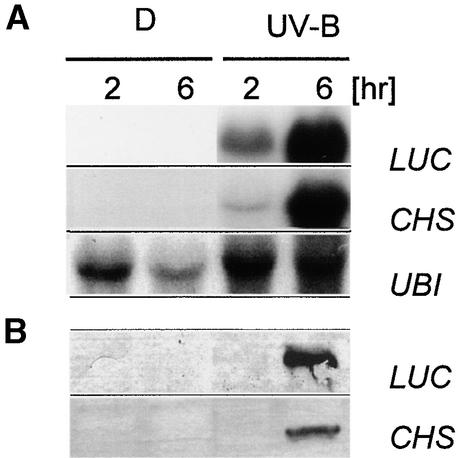
The early expression of PcGST1 under light regimes that stimulate subsequent CHS transcription and flavonoid biosynthesis raises the question as to whether a functional link exists between these genes. As a prerequisite for such studies, we established the stable transformation of parsley cells and compared the UV responsiveness of reporter gene activity with the expression of endogenous CHS. Transformed lines were created by particle bombardment, and a LUC reporter gene under control of the parsley 615-bp full-length CHS promoter (CHS-LUC) was introduced into parsley cells. Hygromycin-resistant calli were selected, and stimulation of the reporter gene by UV-B was determined in vivo with a charge-coupled device camera (Frohnmeyer et al., 1999). One cell line revealed high responsiveness to UV light and was chosen as a background line for further experiments. As shown in Figure 4A, stimulation with UV light caused comparable expression of LUC and endogenous CHS mRNA after 6 hr, whereas neither transcript was detectable in dark-incubated cells. This pattern was verified at the protein level (Figure 4B), indicating that LUC activity in this line reflects endogenous CHS concentrations.
Figure 4.
UV-B–Induced Steady State Transcript Amounts and Protein Accumulation of LUC, CHS, and Ubiquitin in Transformed Parsley Cell Lines.
(A) RNA gel blot analyses of transcript amounts of LUC, CHS, and ubiquitin (UBI) in response to exposure to UV-B light pulses. A stably transformed parsley line harboring a CHS promoter–LUC reporter fusion was kept in darkness (D) or irradiated with a UV-B light pulse through a 305-nm cutoff filter and kept in the dark until harvest after 2 or 6 hr. RNA gel blot analysis was performed as in Figure 3, except that one additional probe for LUC was used.
(B) Immunodetection of CHS and LUC proteins in response to UV-B light pulses. Cells were treated as described in (A), and 15 μg of protein per lane was resolved by SDS-PAGE. CHS and LUC were detected on gel blots by using specific antibodies.
Coexpression of PcGST1 Causes Earlier UV-Dependent CHS-LUC Induction
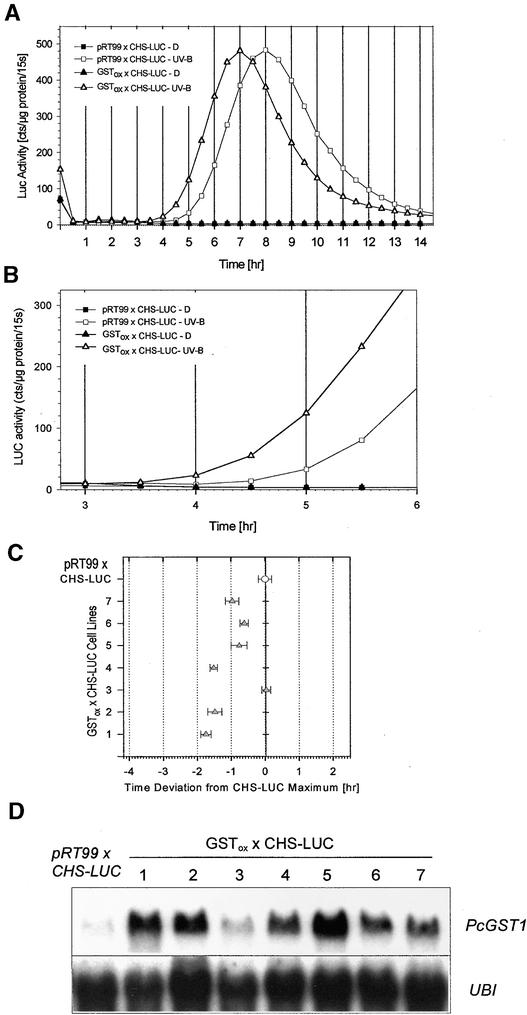
The influence of PcGST1 on UV-induced CHS expression was further analyzed in double-transformed cell lines. For this, a cauliflower mosaic virus 35S promoter–driven PcGST1 cDNA effector construct (GSTox) was integrated by particle bombardment into the described background line containing CHS-LUC (Figure 4). As a control, the background line was transformed with the empty effector plasmid pRT99. The resulting double-transformed lines (GSTox × CHS-LUC, hereafter referred to as PcGST1ox line and pRT99 × CHS-LUC, hereafter referred to as control or background line) were further selected as described in Methods. Seven resistant calli were analyzed, and their UV responsiveness with respect to CHS induction was compared with that of several control lines. A representative kinetic of CHS-LUC expression of one control line and of a PcGST1ox line is shown in Figure 5A. Luciferin was added to dark-incubated and UV-B–irradiated cells, and LUC activity was monitored every 30 min for 14 hr. The reporter enzyme activity reflects actual transcription rates under these conditions and allows detection of real-time gene expression events in vivo (Millar et al., 1992). LUC activity remained low in dark-incubated control and GSTox cells. However, reporter gene activity started to increase 4.5 hr after UV-B pulse treatment in the pRT99-containing background line, reaching a maximum after 8 hr and returning to basal values within 14 hr (Figure 5A). This expression pattern could be reproduced with negligible deviation in three different control lines. GSTox cells showed a similar expression pattern, but LUC activity was increasing already at 3.5 hr after stimulation with UV light (Figure 5B), which was manifested by an earlier maximal LUC activity (Figure 5A). The temporal shift of maximal LUC activity was verified in six of the seven transformed PcGST1ox lines, as determined in three independent experiments (Figure 5C). The maximal LUC activity in PcGST1ox lines appeared between 45 min and 2 hr earlier than in the background cell line (Figure 5C) and correlated with the amount of PcGST1 expression for each PcGST1ox line (Figure 5D). One reason for this correlation might be that the PcGST1 protein is already present when PcGST1ox cell lines are stimulated with UV light, and this situation causes a shortcut in the signaling to CHS, which is reflected by a decrease of the initial lag phase. In wild-type cells, the synthesis of PcGST1 protein starts only after UV treatment and causes delayed CHS expression.
Figure 5.
CHS-LUC Expression after UV-B Treatment and Effect of PcGST1.
(A) LUC activity of the CHS-LUC background line (pRT99 × CHS-LUC) compared with a double-transformed PcGST1 overexpressing line (GSTox × CHS-LUC). Cells of both lines were incubated in darkness (D) or irradiated with a 7-min pulse of UV-B light and subsequently transferred to darkness. Luciferin (1 mM) was added to the cells immediately after irradiation, and the actual counts for 15 sec (15s) were determined every 30 min during a 14-hr period as a measure of LUC activity. The protein concentration of each probe was determined after LUC measurement. Cts represent the amount of detected photons per time unit. LUC activity is expressed as the mean of three sample replicates from three independent experiments. Standard deviation was <15% in all cases.
(B) Section of (A) illustrating the early increase of LUC activity in pRT99 × CHS-LUC and GSTox × CHS-LUC lines after UV stimulation.
(C) LUC activity of the CHS-LUC background line (pRT99 × CHS-LUC) compared with that of seven different double-transformed lines that in addition harbor cauliflower mosaic virus 35S promoter–controlled PcGST1 cDNA (GSTox × CHS-LUC 1 to 7). Cells were treated as described in (A). The time of maximal CHS-LUC expression in the control cells was set as 0, and the deviation of maximal LUC activity from each line (1 to 7), determined in three independent experiments, was plotted in relation to it.
(D) RNA gel blot analysis of the steady state transcript amounts of PcGST1 and ubiquitin (UBI) in the background line (pRT99 × CHS-LUC) and in dark-incubated, double-transformed lines expressing PcGST1 cDNA (GSTox × CHS-LUC 1 to 7). RNA gel blot analysis was performed as given for Figure 3.
PcGST1 and GSH Are Sufficient for Immediate CHS Stimulation
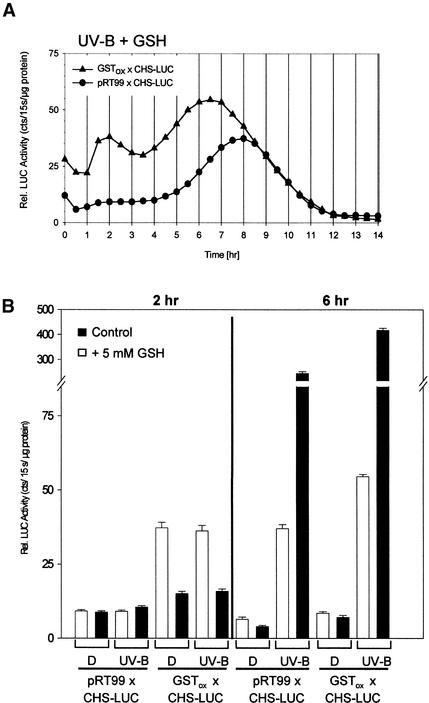
Constitutive synthesis of PcGST1 affected the timing of UV-induced CHS-LUC expression, but an initial lag phase remained (Figures 5A and 5B). To address whether the GST substrate GSH is a limiting parameter, further kinetic studies were performed. In vivo LUC activity was determined in UV-irradiated control and PcGST1ox lines after the culture medium was supplemented with GSH. As shown in Figure 6A, CHS-LUC expression was immediately stimulated in PcGST1ox line 1 (described in Figures 5C and 5D) and reached a first maximum within 2 hr. Under these conditions LUC activity increased biphasically, with a second maximum appearing after 6 to 7 hr. A similar expression pattern was achieved with PcGST1ox lines 2 and 7 (Figures 5C and 5D). In the UV-irradiated control line pRT99, in contrast, CHS-LUC expression remained low during the early period, and the induction profile was not affected by GSH (Figure 6A). Supplementation with GSH caused the immediate increase of LUC activity even in dark-incubated cells of PcGST1ox line 1 (Figure 6B). Within 2 hr, a fourfold stimulation of reporter gene activity appeared under these conditions, whereas LUC activity did not increase in cells of the control line (Figures 6A and 6B). These data indicate that CHS-LUC expression can be immediately activated without the stimulus of UV light if GSH and PcGST1 concentrations are increased. In contrast, GSH supplementation negatively affected CHS-LUC expression 6 hr after UV irradiation in control and PcGST1ox lines and dampened the strong increase of reporter gene activity (Figure 6B). GSH obviously promotes CHS-LUC expression in the presence of PcGST1 at early stages but apparently generally inhibits an increase of reporter gene activity irrespective of PcGST1 at later stages. The influence of the antioxidant on early and late signaling events to CHS is thus controversial.
Figure 6.
LUC Activity of pRT99 × CHS-LUC Background and GSTox × CHS-LUC Lines and the Influence of GSH.
(A) Long-term kinetic CHS-LUC expression in UV-irradiated cells after addition of GSH (5 mM) to the cell culture medium (UV-B + GSH). Conditions were as described in Figure 5A.
(B) Influence of GSH on CHS-LUC expression in dark-incubated or UV-B light–irradiated lines. Cells of a background line and of a PcGST1-overexpressing line were incubated for 30 min in culture medium (Control) or in medium supplemented with GSH (+5 mM GSH). Cells were kept in darkness or irradiated with a 7-min pulse of UV-B light and further incubated in darkness in the presence of luciferin (1 mM). After 2 and 6 hr, LUC activity and protein concentration were determined from each probe. In each case, relative (Rel.) LUC activity was measured in three different cell lines (lines 1, 2, and 7) for a total of nine determinations for each data point. Bars represent the standard deviation.
The influence of supplemented GSH on internal glutathione concentrations in dark-incubated cells was investigated by determining the concentrations of reduced and oxidized GSH (GSSG). In parallel, CHS-LUC expression was monitored under these same conditions. As shown in Tables 1 and 2, addition of GSH resulted in a strong, fivefold increase of intracellular GSH after 2 hr in the control and PcGST1ox lines. The total GSH amount was generally less in PcGST1ox lines, reflecting a greater consumption of this metabolite by the increased amounts of PcGST1. GSSG concentrations were notably greater in PcGST1ox lines but strongly decreased after supplementation with GSH (Table 2). Again, LUC activity increased only in dark-incubated, GSH-supplemented PcGST1ox lines (Table 2). Taken together, these findings show that increased LUC activity in the absence of a UV light stimulus can be induced if PcGST1 is expressed and if a high redox pool of GSH (corresponding to a low percentage of GSSG) is present in parsley cell cultures.
Table 1.
Levels of Total GSH, Percentage of GSSG, and Corresponding LUC Activity in Control (pRT99) and GSTox Lines after 2-hr Incubation in Darkness
| GSH + GSSGa(nmol/g fresh weight) | GSSG (%) |
Relative LUC Activitya(cts/sec/μg protein) | |
|---|---|---|---|
| CHS-LUC × pRT99 | 129 | 0.4 | 8 |
| CHS-LUC × GSTox | 89 | 7.4 | 14 |
The data represent the average results of two or three experiments. Variation between replicates was <10%.
Table 2.
Levels of Total GSH, Percentage of GSSG, and the Corresponding LUC Activity in Control (pRT99) and GSTox Lines Incubated in 5 mM GSH-Containing Medium (as Described in Table 1)
| GSH + GSSGa(nmol/g fresh weight) | GSSG (%) |
Relative LUC Activitya(cts/sec/μg protein) | |
|---|---|---|---|
| CHS-LUC × pRT99 | 663 | 0.8 | 9 |
| CHS-LUC × GSTox | 447 | 0.5 | 40 |
The data represent the average results of two or three experiments. Variation between replicates was <10%.
DISCUSSION
UV-mediated signaling to CHS includes early genes, stimulated during the initial lag phase before onset of CHS transcription, as was found in parsley, soybean, and Arabidopsis (Christie and Jenkins, 1996; Frohnmeyer et al., 1998; Kircher et al., 1998). Using differential display, we isolated early UV-induced genes and analyzed the influence of PcGST1 on subsequent CHS expression. Four key observations suggest that GSH and PcGST1 are involved in a signal cascade that transmits the light stimulus to the CHS promoter: (1) PcGST1 from parsley cell suspension cultures is expressed within 2 hr after a UV-B pulse. The transient accumulation of PcGST1 transcripts needs no intact de novo protein synthesis and is stimulated only by wavelengths that also lead to subsequent CHS expression. (2) A functional analysis in transformed cell lines revealed that CHS-LUC reporter gene activity reflects endogenous CHS transcription. The onset of UV-induced LUC expression appeared after a lag phase of 4 to 5 hr, representing the time frame necessary to establish the complete signaling network to the CHS promoter. This initial period was chosen as a parameter to monitor the influence of GSH and PcGST1 on the signal transduction to CHS. (3) Coexpression of PcGST1 in CHS-LUC lines caused a 1- to 2-hr earlier increase of LUC activity. In this situation, the maximal LUC activity appeared earlier than in control lines, although no kinetic differences of LUC expression were detected between these lines. The remaining lag phase in PcGST1ox lines indicates that PcGST1 influences the timing of CHS-LUC induction but is not sufficient for its light-independent stimulation. (4) Supplementing UV-stimulated and dark-incubated PcGST1ox lines with GSH removed the initial lag phase and caused immediate activation of CHS-LUC expression; meanwhile, the activity of the reporter gene remained low in pRT99-transformed control cells. The importance of these results for UV signaling to CHS and possible functions of GSH and GST action during these processes are discussed in the following sections.
Besides other stress factors, high fluences of light, including UV irradiation, can generate oxidative stress through reactive oxygen species (ROS) in plants (Green and Fluhr, 1995), leading to the production of oxidative damage. GSTs with GSH peroxidase activity participate in the detoxification of products created by oxidative damage and thereby protect cells against such stress (Dixon et al., 1998). This protection mechanism is often accelerated by increased amounts of GSH (May et al., 1998). Although these observations describe a possible scenario of GST and GSH action in cells that have been treated with high fluences of UV light, several findings disprove the possibility that such a mechanism mediates CHS induction. First, parsley and Arabidopsis cells already respond to low-fluence UV light by transcriptional stimulation of CHS, but the generation of ROS is not detectable under these light conditions (Christie and Jenkins, 1996; Frohnmeyer et al., 1997). Second, increased concentrations of PcGST1 and GSH already stimulate CHS expression in dark-incubated cells, whereas the generation of ROS is excluded under these conditions. Third, treatment of tobacco plants with ROS-promoting agents causes PR1 stimulation, whereas flavonoid biosynthesis and CHS expression remain unaffected (Green and Fluhr, 1995). In addition, generation of ROS has been also reported after incubation of parsley cell cultures with fungal elicitors or synthetic peptides such as Pep25 (Nuernberger et al., 1994); however, this treatment represses UV-induced CHS (Lozoya et al., 1991) and also expression of PcGST1. From these data, the attractive hypothesis that GSH and PcGST1 act directly through the removal of oxidative stress products is unlikely and points to a different function during UV-dependent signal transduction.
PcGST1 belongs to plant type III GSTs, as revealed by sequence homology and genomic intron–exon patterns (Droog, 1997). Genes of this subclass were originally identified in a variety of species after induction by various different treatments—particularly by auxins, but also by pathogen infection, heavy metals, or heat shock (Marrs, 1996). PcGST1 is the first member of this class found to be rapidly and transiently induced by UV-B. Strong but continuous stimulation of PcGST1 was also found after treating parsley cells with auxins. Although the hormone-dependent PcGST1 stimulation points to the enzyme's additional function of conjugating 2,4-D and consequential detoxification besides its role in responding to UV light stimulation, interestingly, CHS expression is also increased after 2,4-D supplementation.
The function of PcGST1 during UV signaling, however, is unclear, and the identification of the substrate in vivo needs further investigation. Similarly, although many GSTs have been isolated from plants, their functions have been clarified in only a few cases. Coexpression of GTXC (ParC) (Takahashi and Nagata, 1992) increased the resistance of tobacco plants to low temperature and high salt concentrations (Roxas et al., 1997). These data provide direct evidence for a link between stress protection and GST activity, although the molecular reasons for this effect remain unknown. In contrast, the substrate of another type III GST has been identified in maize. Bz2 catalyzes the last step of anthocyanin biosynthesis and facilitates the transport of cyanidin-3-glucoside into the vacuole by an unknown mechanism (Marrs et al., 1995). Although low in sequence homology, An9 from Petunia hybrida complements a maize bz2 mutant and has a comparable function during anthocyanin biosynthesis (Alfenito et al., 1998). The involvement of PcGST1 during late steps of flavonoid biosynthesis in parsley is unlikely because its expression pattern does not match the late accumulation of flavonoid end products 24 to 48 hr after treatment with UV light (Hahlbrock, 1981; Dangl et al., 1987). In agreement with this, the enzyme accepts no phenylpropanoids, at least in vitro.
UV light irradiation also stimulates a supply pathway that provides substrates for subsequent flavonoid biosynthesis in parsley cell cultures. Members of this pathway are already synthesized in dark-incubated cells, and their expression is additionally stimulated in parallel to PAL and CHS by light treatment. One member of this pathway, acyl CoA oxidase, reveals a similar induction pattern as PcGST1 but can also be stimulated by fungal elicitors (Logemann et al., 2000). As yet, no function of GSTs within this supply pathway has been described, and PcGST1 expression is repressed after elicitor treatment of parsley cells. These findings argue against the involvement of GSTs in the supply pathway to flavonoid biosynthesis. Another early UV-induced gene encodes CPRF1, a transcription factor with the capacity for in vitro binding to the CHS promoter (Weisshaar et al., 1991). In contrast to PcGST1, coexpression of CPRF1 does not stimulate but instead inhibits CHS expression (Feldbruegge et al., 1994), indicating a different role for CPRF1 during UV signal transduction.
Our data provide evidence for a novel function of GSTs involved in the UV-mediated signal transduction to CHS. In dark-incubated cells, increased amounts of the protein and of its substrate are essential for CHS stimulation. Interestingly, GSH and GST activity are already known as key elements during elicitor-stimulated CHS expression in cultured bean suspension cells. Although CHS is not UV-inducible in this system, it represents a key element during the hypersensitive response. Elicitor stimulation induces GST and CHS expression, and this effect can be partly mimicked by GSH supplementation (Dron et al., 1988; Wingate et al., 1988; Levine et al., 1994). Although UV light but not elicitors activates PcGST1 or CHS in parsley, remarkable parallels are found in the signal transduction to CHS in both systems.
The molecular mechanisms underlying the activation of CHS expression in parsley include several possibilities. GSH and GSTs possibly affect CHS transcription by changing the redox state of antioxidant pools, as indicated by the increase of redox state of glutathione in PcGST1ox lines supplemented with external GSH. Such changes can alter the expression of nuclear genes (Creissen et al., 1999) and involve redox-modulated regulatory factors (Link, 1996). For example, binding of the FOS-JUN transcription factors to DNA in mammalian cells is regulated by the reduction and oxidation of conserved cystidine residues in the DNA binding domain (Abate et al., 1990). Besides these redox-modulated mechanisms, our working hypothesis for PcGST1 action during UV light signaling includes another possibility. Perhaps the protein can directly modify a repressor and thereby stimulate CHS expression. Further work is in progress to identify substrates and binding partners of PcGST1 in vivo.
METHODS
Plant Cell Culture, Protoplast Preparation, and Transient Transformation
Cell suspension cultures of parsley (Petroselinum crispum) were maintained in modified B5 medium in the dark, and one-tenth of its volume was subcultured weekly (Frohnmeyer et al., 1997). Protoplast preparation and transient transformation by electroporation were performed as described previously (Dangl et al., 1987; Renelt et al., 1993). Transgenic chalcone synthase–luciferase (CHS-LUC) cell lines were kept in a solid phase of modified B5 medium supplemented with 0.8% agarose and 30 mg/L hygromycin. Calli were grown for several months under these conditions, and stable integration of the plasmids was verified by DNA gel blot analysis. Every 2 weeks, one-tenth of the calli were transferred to new plates.
Light Sources and Experimental Conditions
Short-wavelength UV-B irradiation for periods ranging from 30 sec to 7 min was obtained from a Philips TL 40 W/12 fluorescent tube (λmax 310 nm, half-bandwidth 40 nm, 17.6 μmol m−2 sec−1). This light was filtered through transmission cutoff filters that reduced the fluence rate to 13 μmol m−2 sec−1 (>305 nm). UV-A light (15 μmol m−2 sec−1), blue light (18 μmol m−2 sec−1), and far-red light 13 μmol m−2 sec−1) were as described previously (Schaefer, 1978; Frohnmeyer et al., 1992). For the isolation of early genes, protoplasts were freshly prepared and irradiated with continuous light (UV-A, blue, red, or far red) or with 4-min UV-B pulses and subsequent dark incubation until harvest after 2 hr. For experiments with growth factors (4.5 μM 2,4-D, 10 μM α-naphthylene acetic acid [α-NAA], 10 μM β-naphthylene acetic acid [β-NAA]), the elicitor Pep25 (175 nM), and reduced glutathione (5 mM), the substances were dissolved in culture medium.
Fluorescent Differential Display and Cloning of PcGST1
Total RNA from parsley suspension culture and derived protoplasts was prepared as previously described (Frohnmeyer et al., 1992) and treated with DNase (RQ1; Gibco BRL, Gaithersburg, MD). After phenol/chloroform extraction, the RNA was precipitated with ethanol, dissolved in Tris–EDTA buffer (10 mM Tris-HCL, pH 7.5; 1 mM EDTA), and subjected to differential display analysis (Uchida et al., 1998). For this, first-strand cDNAs were synthesized from 2.5 μg of total RNA with a Texas Red–labeled 3′-dG(dT)15dG or 3′-dG(dT)15dC primers by using a Superscript Preamplification System (Gibco BRL, Karlsruhe, Germany) and the reaction components were diluted to 200 μL by the 2.5 μM primer solution. Subsequent amplification was performed in a 96-well thermal cycler (GeneAmp PCR System 9600; Perkin-Elmer, Norwalk, CT) containing 1 nmol of dNTP, a mixture of 0.5 units of Ampli-Taq polymerase (Perkin-Elmer) and 0.5 units of Taq polymerase (Nippon Gene, Toyama, Japan), 10 pmol of arbitrary primer (10 mer primer; OPERON Technologies, Alameda, CA), and 2 μL of cDNA solution that contained 5 pmol of Texas Red–labeled 3′-dG(dT)15dG or 3′-dG(dT)15dC primers in a 20-μL polymerase chain reaction (PCR) solution. The conditions for PCR were one cycle of 94°C for 3 min, 40°C for 5 min, and 72°C for 5 min, then 24 cycles of 94°C for 15 sec, 40°C for 2 min, and 72°C for 1 min, and a final extension for 5 min at 72°C. After addition of loading buffer, each PCR product was concentrated by evaporation at 60°C for 25 min and denatured at 80°C for 3 min. Aliquots of each sample were separated on a 6% Long Ranger Pharmacia gel system (FMC BioProducts, Rockland, ME) containing 6.1 M urea, and 1.2 × TBE (60 mM Tris-HCL, pH 8.3; 60 mM boric acid, 1.2 mM EDTA), and the fragments were visualized by an automated DNA sequencer (SQ-5500; Hitachi Ltd., Tokyo, Japan). To isolate the band of interest, the fingerprinting pattern was scanned by the fluorescent image analyzer (FMBIO-100; TaKaRa Shuzo, Tokyo, Japan). Positive bands were cut out from the gel and reamplified by using the same PCR conditions as were used for fluorescent differential display (FDD) (Ito et al., 1994). Amplified cDNA fragments were cloned into pT7 Blue T-vector (Novagen, Madison, WI) and reamplified by using Texas Red–labeled 3′-dG(dT)15dG or 3′-dG(dT)15dC and the appropriate arbitrary primer. The correct sizes of reamplified fragments were routinely verified on agarose gels and on gel systems used for FDD. Clones with identical sizes were sequenced with a sequencing kit (Amersham) and a DNA sequencer (SQ-5500; Hitachi Ltd.). The size and induction pattern of positive fragments was verified by RNA gel blot analysis. Cloning of full-length PcGST1 cDNA was performed by using a cDNA library constructed from RNA of parsley cells that had been irradiated for 2 hr with UV light. This sequence has been submitted to the GenBank database under accession number AF177944.
Synthesis of PcGST1 in Escherichia coli
The PcGST1 cDNA was introduced into the expression vector PQE30 (Qiagen, Hilden, Germany), and E. coli strain M15pREP4 was transformed with the resulting plasmid. Single-step purification of the fusion protein was performed as described in the manufacturer's protocol. The expression and all purification steps were monitored by protein staining of each fraction after SDS-PAGE. Assays for glutathione S-transferase (GST) were performed with the universal substrate 1-chloro-2,4-dinitrobenzene or p-coumaric acid, and activity was determined spectroscopically by the change in absorbance at 340 nm (Habig et al., 1974). Peroxidase activity was assayed with cumene hydroperoxide as the substrate. The reaction was measured spectrophotometrically by monitoring the oxidation of NADPH at 340 nm (Simmons et al., 1989).
RNA Gel Blot Analysis and Immunodetection
Total RNA (20 μg) extracted from cell suspension culture was separated in agarose–formaldehyde gels and blotted onto UV Duralon membranes (Stratagene, La Jolla, CA), as described (Frohnmeyer et al., 1997). A full-length cDNA fragment of PcGST1 subcloned into Bluescript KSII was excised by EcoRI digestion. This fragment, together with CHS and UBI4 cDNA (Reimold et al., 1983; Kawalleck et al., 1993), was used for preparing random-primed probes. After washing with 2 × SSC, 1 × SSC, and 0. 2 × SSC (1 × SSC is 0.15 M NaCl, 0.015 M sodium citrate) at 60 to 64°C, the filters were autoradiographed at −80°C for 6 to 24 hr by using an intensifying screen (BioMax TranScreen; Kodak, Rochester, NY). Blots from three experiments were quantitated with a Fuji BAS1000 bioimaging ana-lyzer (Raytest, Paris, France) and using PCBASD software (Raytest, Straubenhardt, Germany). Total protein determinations, separation of protein extracts by SDS-PAGE, protein blotting, and immunodetection were performed according to Frohnmeyer et al. (1992). Polyclonal antibodies raised against LUC were used at 1:1000 dilution and purchased from Rockland (Gilbertsville, PA).
Expression of PcGST1 in Parsley Cell Cultures and Detection of LUC Enzyme Activity
Construction of the CHS-LUC reporter in a plasmid conferring hygromycin resistance has been described (Frohnmeyer et al., 1999). The PcGST1 open reading frame was introduced into the EcoRI site of pRT99neo (Toepfer et al., 1988). This vector contains a neomycin phosphotransferase gene conferring G418 resistance, which was used as a second selection for double-transformed cell lines with a uniform CHS-LUC background. CHS-LUC cells were continuously selected on hygromycin-containing solid medium. For transformation by particle inflow gun (Finer et al., 1992), calli were dissolved in liquid medium and used after 3 days. 35S/PcGST1 was bound to gold particles by CaCl2/ethanol precipitation (2 μg of DNA/0.5 mg of gold; 1.5 to 3 μm in diameter; Sigma) in the presence of spermidine, and cells were bombarded as described (Frohnmeyer et al., 1999). Double-transformed cells were selected on solid medium containing 7.5 mg/L G418 and 7.5 mg/L hygromycin and grown for 4 to 6 weeks until calli became visible. Further selection of these calli was performed on solid medium containing 15 mg/L G418 and 15 mg/L hygromycin for a few months. Calli were then dissolved in liquid medium and cultivated for several weeks, as described for wild-type cells (Frohnmeyer et al., 1997). Experiments were performed with cells 3 to 5 days after passage to new medium.
LUC assays were performed as described (Frohnmeyer et al., 1999) after pulverization of cell cultures or protoplasts in LucI buffer (100 mM KH2PO4, pH 7.8, and 1 mM DTE) and centrifugation for 10 min at 14,000g. The samples were normalized to the amount of soluble protein present, as determined by the Bradford assay (Bio-Rad). Steady state LUC expression was continuously monitored in vivo in a Berthold (Straubenhardt, Germany) Microlumat LB 96P by using the repeated mode after supplementing the cells with luciferin (1 mM).
Analysis of Glutathione
Parsley cell culture was ground in 2 mL of HClO4 and centrifuged at 10,000g for 5 min; the supernatant used for determination of total glutathione (GSH) and oxidized GSH (GSSG) was as described by Griffith (1980).
Acknowledgments
We thank Dierk Scheel for the generous gift of synthetic Pep25 elicitor and Karsten Jaekel for assistance during the determination of GSH concentrations and GST activity. This work was funded by Grant No. FR936/1 from Deutsche Forschungsgemeinschaft to H.F. and in part by a Hitachi Advanced Research Laboratory (HARL) grant to M.F. and H.F. L.L. was supported by a graduate fellowship from Landesgraduiertenfoerderung.
References
- Abate, C., Patel, L., Rauscher, F., and Curran, T. (1990). Redox regulation of Fos and Jun DNA binding activity in vitro. Science 249, 1157–1161. [DOI] [PubMed] [Google Scholar]
- Abel, S., and Theologis, A. (1996). Early genes and auxin action. Plant Physiol. 111, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfenito, M.R., Souer, E., Goodman, C.D., Buell, R., Mol, J., and Walbot, V. (1998). Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell 10, 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot, K., van der Zaal, B., Velterop, J., Quint, A., Mennes, A., Hooykaas, P., and Libbenga, K. (1993). Further characterization of expression of auxin-induced genes in tobacco (Nicotiana tabacum) cell suspension cultures. Plant Physiol. 102, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore, A., Jarillo, J., Wu, Y.-J., and Liu, D. (1999). Cryptochromes: Blue light receptors for plants and animals. Science 284, 760–765. [DOI] [PubMed] [Google Scholar]
- Chappell, J., and Hahlbrock, K. (1984). Transcription of plant defense genes in response to UV light or fungal elicitor. Nature 311, 76–78. [Google Scholar]
- Christie, J., and Jenkins, G. (1996). Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 8, 1555–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creissen, G., Firmin, J., Fryer, M., Kular, B., Leyland, N., Reynolds, H., Pastori, G., Wellburn, F., Baker, N., Wellburn, A., and Mullineaux, P. (1999). Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 11, 1277–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl, J.L., Hauffe, K.D., Lipphardt, S., Hahlbrock, K., and Scheel, D. (1987). Parsley protoplasts retain differential responsiveness to UV light and fungal elicitor. EMBO J. 6, 2551–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, D., Cummins, I., Cole, D., and Edwards, R. (1998). Glutathione-mediated detoxification systems in plants. Curr. Opin. Plant Biol. 1, 258–266. [DOI] [PubMed] [Google Scholar]
- Dominov, J., Stenzler, L., Lee, S., Schwarz, J., Leisner, S., and Howell, S. (1992). Cytokinins and auxins control the expression of a gene in Nicotiana plumbaginifolia by feedback regulation. Plant Cell 4, 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron, M., Clouse, S.D., Dixon, R.A., Lawton, M.A., and Lamb, C.J. (1988). Glutathione and fungal elicitor regulation of a plant defense gene promoter in electroporated protoplasts. Proc. Natl. Acad. Sci. USA 85, 6738–6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droog, F. (1997). Plant glutathione-S-transferases, a tale of theta and tau. J. Plant Growth Regul. 16, 95–107. [Google Scholar]
- Feldbruegge, M., Sprenger, M., Dinkelbach, M., Yazaki, K., Harter, K., and Weisshaar, B. (1994). Functional analysis of a light-responsive plant bZIP transcriptional regulator. Plant Cell 6, 1607–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldbruegge, M., Sprenger, M., Hahlbrock, K., and Weisshaar, B. (1997). PcMYB1, a novel plant protein containing a DNA-binding domain with one MYB-repeat, interacts in vivo with a light-regulatory promoter unit. Plant J. 11, 1079–1093. [DOI] [PubMed] [Google Scholar]
- Finer, J.J., Jones, M.W., and McMullen, M.D. (1992). Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep. 11, 323–328. [DOI] [PubMed] [Google Scholar]
- Frohnmeyer, H., Ehmann, B., Kretsch, T., Rocholl, M., Harter, K., Nagatani, A., Furuya, M., Batschauer, A., Hahlbrock, K., and Schaefer, E. (1992). Differential usage of photoreceptors for chalcone synthase gene expression during plant development. Plant J. 2, 899–906. [Google Scholar]
- Frohnmeyer, H., Bowler, C., and Schaefer, E. (1997). Evidence for some signal transduction elements involved in UV-light dependent responses in parsley protoplasts. J. Exp. Bot. 48, 739–750. [Google Scholar]
- Frohnmeyer, H., Bowler, C., Xhu, G., Yamagata, H., Schaefer, E., and Chua, N.-H. (1998). Different roles for calcium and calmodulin in phytochrome- and UV-regulated expression of chalcone synthase. Plant J. 13, 763–772. [Google Scholar]
- Frohnmeyer, H., Loyall, L., Blatt, M., and Grabov, A. (1999). Millisecond UV-B irradiation evokes prolonged elevation of cytosolic-free Ca2+ and stimulates gene expression in transgenic parsley cell cultures. Plant J. 20, 109–117. [DOI] [PubMed] [Google Scholar]
- Furuya, M., and Schaefer, E. (1996). Photoperception and signaling of induction reactions by different phytochromes. Trends Plant Sci. 1, 301–307. [Google Scholar]
- Green, R., and Fluhr, R. (1995). UV-B–induced PR-1 accumulation is mediated by active oxygen species. Plant Cell 7, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith, O.W. (1980). Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 106, 207–212. [DOI] [PubMed] [Google Scholar]
- Habig, W., Pabst, M., and Jacoby, W. (1974). Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139. [PubMed] [Google Scholar]
- Hagen, G., Uhrhammer, N., and Guilefoyle, T. (1988). Regulation of expression of an auxin-induced soybean sequence by cadmium. J. Biol. Chem. 263, 6442–6446. [PubMed] [Google Scholar]
- Hahlbrock, K. (1981). Flavonoids. In The Biochemistry of Plants, Vol. 7, P.K. Stumpf and E.E. Conn, eds (New York: Academic Press), pp. 425–456.
- Hahlbrock, K., and Scheel, D. (1989). Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 347–369. [Google Scholar]
- Ito, T., Kito, K., Adati, N., Mitsui, Y., Hagiwara, H., and Sakaki, Y. (1994). Fluorescent differential display: Arbitrarily primed RT-PCR fingerprinting on an automated DNA sequencer. FEBS Lett. 351, 231–236. [DOI] [PubMed] [Google Scholar]
- Jordan, B. (1996). The effect of ultraviolet-B radiation on plants: A molecular perspective. Adv. Bot. Res. 22, 98–162. [Google Scholar]
- Kawalleck, P., Somssich, I., Feldbruegge, M., Hahlbrock, K., and Weisshaar, B. (1993). Polyubiquitin gene expression and structural properties of the UBI4–2 gene in Petroselinum crispum. Plant Mol. Biol. 21, 673–684. [DOI] [PubMed] [Google Scholar]
- Kircher, S., Weisshaar, B., Hahlbrock, K., Schaefer, E., and Frohnmeyer, H. (1998). Comparative analysis of a plant bZIP transcriptional regulator family: Heterodimerisation, light-dependent expression and nuclear transport of CPRF1, CPRF2 and CPRF4 from parsley. Mol. Gen. Genet. 257, 595–605. [DOI] [PubMed] [Google Scholar]
- Kuno, N., Muramatsu, T., Hamazoto, F., and Furuya, M. (2000). Identification by large-scale screening of phytochrome-regulated genes in etiolated seedlings of Arabidopsis using a fluoresecent differential display technique. Plant Physiol. 122, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, A., Tenhaken, R., Dixon, R., and Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79, 583–593. [DOI] [PubMed] [Google Scholar]
- Liang, P., and Pardee, A.B. (1992). Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 257, 967–971. [DOI] [PubMed] [Google Scholar]
- Link, G. (1996). Green life: Control of chloroplast gene transcription. Bioessays 18, 465–471. [Google Scholar]
- Logemann, E., Tavernaro, A., Schulz, W., Somssich, I.E., and Hahlbrock, K. (2000). UV light selectively coinduces supply pathways from primary metabolism and flavonoid secondary product formation in parsley. Proc. Natl. Acad. Sci. USA 97, 1903–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, J.C., and Jenkins, G.I. (1998). Involvement of plasma membrane redox activity and calcium homeostasis in the UV-B and UV-A/blue light induction of gene expression in Arabidopsis. Plant Cell 10, 2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoya, R., Block, A., Lois, R., Hahlbrock, K., and Scheel, D. (1991). Transcriptional repression of light-induced flavonoid synthesis by elicitor treatment of cultured parsley cells. Plant J. 1, 227–234. [Google Scholar]
- Marrs, K.A. (1996). The functions and regulation of glutathione- S-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 127–158. [DOI] [PubMed] [Google Scholar]
- Marrs, K.A., Alfenito, M.R., Lloyd, A.M., and Walbot, V. (1995). A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene bronze-2. Nature 375, 397–400. [DOI] [PubMed] [Google Scholar]
- May, M., Vernoux, T., Leaver, C., van Montagu, M., and Inze, D. (1998). Glutathione homeostasis in plants: Implications for environmental sensing and plant development. J. Exp. Bot. 49, 649–667. [Google Scholar]
- Millar, A.J., Short, S.R., Chua, N.-H., and Kay, S.A. (1992). A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell 4, 1075–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash, J., Luersen, K., and Walbot, V. (1990). Bronze-2 gene of maize: Reconstruction of a wild-type allele and analysis of transcription and splicing. Plant Cell 2, 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, M., Fankhauser, C., and Chory, J. (2000). Light: An indicator of time and place. Genes Dev. 14, 257–271. [PubMed] [Google Scholar]
- Nuernberger, T., Nennstiel, D., Jabs, T., Sacks, W.R., Hahlbrock, K., and Scheel, D. (1994). High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 78, 449–460. [DOI] [PubMed] [Google Scholar]
- Poppe, C., Ehmann, B., Frohnmeyer, H., Furuya, M., and Schaefer, E. (1994). Regulation of phytochrome A mRNA abundance in parsley seedlings and cell suspension cultures. Plant Mol. Biol. 26, 481–486. [DOI] [PubMed] [Google Scholar]
- Reimold, U., Kroeger, M., Kreuzaler, F., and Hahlbrock, K. (1983). Coding and 3′ noncoding nucleotide sequence of chalcone synthase mRNA and assignment of amino acid sequence of the enzyme. EMBO J. 2, 1801–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renelt, A., Colling, C., Hahlbrock, K., Nuernberger, T., Parker, J., Sacks, W., and Scheel, D. (1993). Studies on elicitor recognition and signal transduction in plant defense. J. Exp. Bot. 44, 257–268. [Google Scholar]
- Roxas, V., Smith, R., Jr., Allen, E., and Allen, R. (1997). Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Natl. Biotechnol. 15, 988–991. [DOI] [PubMed] [Google Scholar]
- Schaefer, E. (1978). Kunstlicht und Pflanzenzucht. In Optische Strahlungsquellen, H. Albrecht, ed (Grafenau: Lexica Verlag), pp. 249–266.
- Schulze-Lefert, P., Dangl, J.L., Becker-André, M., Hahlbrock, K., and Schulz, W. (1989). Inducible in vivo footprints define sequence necessary for UV light activation of the parsley chalcone synthase gene. EMBO J. 8, 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, T., Jamall, I., and Lockshin, R. (1989). Selenium-independent glutathione-peroxidase activity associated with glutathione S-transferase from housefly Musca domestica. Comp. Biochem. Physiol. 94B, 323–327. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y., and Nagata, T. (1992). parB: An auxin-regulated gene encoding glutathione S-transferase. Proc. Natl. Acad. Sci. USA 92, 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y., Kusaba, M., Hiroka, Y., and Nagata, T. (1991). Characterization of the auxin-regulated par gene from tobacco mesophyll protoplasts. Plant J. 1, 324–332. [DOI] [PubMed] [Google Scholar]
- Taylor, J., Fritzemaier, K., Hauser, I., Kombrink, E., Rohwer, F., Schroeder, J., Strittmatter, G., and Hahlbrock, K. (1990). Structural analysis and activation by fungal infection of a gene encoding a pathogenesis-related protein in potato. Mol. Plant-Microbe Interact. 3, 72–77. [PubMed] [Google Scholar]
- Toepfer, R., Schell, J., and Steinbiss, H. (1988). Versatile cloning vectors for transient gene expression and direct gene transfer in plant cells. Nucleic Acids Res. 16, 8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, K., Muramatsu, T., Jamet, E., and Furuya, M. (1998). Control of expression of a gene encoding an extensin by phytochrome and a blue light receptor in spores of Adiantum capillus-veneris L. Plant J. 15, 813–819. [DOI] [PubMed] [Google Scholar]
- Weisshaar, B., Block, A., Armstrong, G., Herrmann, A., Schulze-Lefert, P., and Hahlbrock, K. (1991). Regulatory elements required for light-mediated expression of the Petroselinum crispum chalcone synthase gene. EMBO J. 10, 1777–1786. [PubMed] [Google Scholar]
- Wingate, P.M., Lawton, M.A., and Lamb, C. (1988). Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol. 87, 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]