Abstract
The ookinete surface proteins (P25 and P28) are proven antimalarial transmission-blocking vaccine targets, yet their biological functions are unknown. By using single (Sko) and double gene knock-out (Dko) Plasmodium berghei parasites, we show that P25 and P28 share multiple functions during ookinete/oocyst development. In the midgut of mosquitoes, the formation of ookinetes lacking both proteins (Dko parasites) is significantly inhibited due to decreased protection against lethal factors, including protease attack. In addition, Dko ookinetes have a much reduced capacity to traverse the midgut epithelium and to transform into the oocyst stage. P25 and P28 are partially redundant in these functions, since the efficiency of ookinete/oocyst development is only mildly compromised in parasites lacking either P25 or P28 (Sko parasites) compared with that of Dko parasites. The fact that Sko parasites are efficiently transmitted by the mosquito is a compelling reason for including both target antigens in transmission-blocking vaccines.
Keywords: knock-out/ookinete/P25/P28/Plasmodium
Introduction
Transmission of malaria parasites from humans to mosquitoes requires the ingestion by the mosquito vector of the sexual stages (gametocytes) of the parasite that circulate in the blood of the human host. In the mosquito midgut, these gametocytes transform into gametes. Following fertilization, the zygote differentiates within 18–24 h into a motile ookinete that ‘escapes’ from the midgut by invading and traversing the midgut epithelium. The ookinete differentiates into the oocyst on the outside of the midgut between the basal plasma membrane of the epithelium and the collagenous basal lamina (BL). Within the oocyst, the sporozoites are formed, which are the infectious stages that can infect a new host.
The ookinete plasma membrane has some 10–15 proteins on its surface, of which two predominate: P25 and P28 (for a review see Kaslow, 1996). These proteins have been described and are remarkably similar in human, bird and rodent malaria parasites of the genus Plasmodium (Kumar and Carter, 1985; Vermeulen et al., 1985; Kaslow et al., 1988, 1989; Duffy et al., 1993; Paton et al., 1993; Duffy and Kaslow, 1997; Tsuboi et al., 1997a,b, 1998). Both proteins are characterized by a secretory N-terminal signal sequence followed by three or four epidermal growth factor (EGF) domains and a glycosylphosphatidylinositol (GPI) anchor (Alejo-Blanco et al., 1999). Synthesis of both proteins begins between 0.5 and 2 h after the formation of the female gametes, and the proteins are still present in young oocysts (Fries et al., 1990; Vermeulen et al., 1985; Kumar and Carter, 1985; Paton et al., 1993; Ranawaka et al., 1994b).
P25 and P28 are targets of effective transmission-blocking antibodies, which can inhibit the development of oocysts by >99% (Kaslow, 1996). These antibodies interfere both with ookinete maturation and oocyst formation, but it is not known which functions P25 and P28 fulfil during the development of the ookinete into the oocyst. Surface proteins containing EGF domains are widely distributed and can serve a broad range of receptor/ligand functions, including roles in the regulation of development (Blobel et al., 1992; Schier and Shen, 2000), the regulation of digestive enzyme secretion (Dembinski et al., 1982) and signal transduction (Zhang et al., 1998). The possible roles of P25 and P28 could include a structural role in the surface membrane of the ookinete, a function in the interaction of the ookinete with the midgut environment of the vector, such as in the protection against lethal factors in the mosquito (Yeates and Steiger, 1981; Grotendorst et al., 1986; Grotendorst and Carter, 1987), or a function in the recognition, attachment or penetration of the midgut epithelium. These latter processes would include interaction with the peritrophic matrix, the midgut epithelium (Sieber et al., 1991) and the basal lamina, where laminin is incriminated as a ligand for ookinete binding (Adini and Warburg, 1999; Vlachou et al., 2001).
In this study we investigated the function of P25 and P28 of Plasmodium berghei through analysis of the phenotype of knock-out (ko) parasites lacking these proteins as a result of genetic modifications using well established methodologies (de Koning-Ward et al., 2000). We found that P25 and P28 play roles in ookinete survival in the midgut, penetration of the epithelium and transformation of the ookinete into the oocyst. The demonstration that P25 and P28 are partially redundant in respect of these multiple roles has implications for the design of transmission-blocking vaccines.
Results
Generation of P.berghei P25 or P28 single and double ko parasites
The genes encoding P25 and P28 can vary in length, and therefore have been named with different numbers in different Plasmodium species. However, they are unmistakable at the sequence level, and to facilitate cross-species comparisons we propose that the orthologues be named consistently. The p28 gene of P.berghei has been described before as Pbs21 (Paton et al., 1993) and the p25 gene as Pbs25 (Tsuboi et al., 1997a). It had been demonstrated in Plasmodium falciparum that p25 and p28 are closely linked on chromosome 10 (Duffy and Kaslow, 1997). Using PCR we demonstrated that these genes are adjacent and separated by a short intergenic region in the avian parasite Plasmodium gallinaceum (2.0 kb), P.falciparum (1.65 kb) and P.berghei (1.4 kb; data not shown and Figure 1A). There was no evidence that the intergenic region contained a third open reading frame (ORF) (data not shown).
Fig. 1. Generation of P.berghei parasites lacking either or both P25 or P28 proteins and analysis of expression of these proteins in ko parasites. (A) Schematic diagram of the p25-p28 locus and the replacement vectors used to disrupt p25 (25Sko), p28 (28Sko) and both genes (28/25Dko). All vectors contain the TgDHFR/TS selection cassette (grey arrow) flanked by p25/p28 sequences. Correct integration of the vectors results in the disruption of the genes as shown. In the wt locus, the ORFs are separated by a 1.4 kb intergenic region. The transcription start sites (bent arrows) of both genes are indicated in addition to the direction of transcription (arrow from Margos et al., 1998). In the disrupted loci, the sizes of appropriate restriction fragments used for Southern analysis (see B) are shown. For Southern analysis of the correct integration events, three probes (hatched lines) were used: probe A, recognizing the ORF of p28; probe B, hybridizing to the TgDHFR/TS gene; and probe C, hybridizing to the 3′UTR of the PbDHFR/TS gene, which flanks the TgDHFR/TS gene in the selection cassette. H, HindIII; C, ClaI; P, PstI; B, BclI; Bs, BsmI; S, SmaI; H2, HincII; E, EcoRI; N, NdeI. (B) Southern analysis of digested genomic DNA of wt and ko parasites demonstrates correct integration of the replacement vectors in the different ko parasites. The size of the restriction fragments of the disrupted genes and the probes that recognize these fragments are shown in (A). Probe C recognizes both the integrated vector and the endogenous DHFR/TS of P.berghei (the 14 kb H3/NdeI fragment and the 1.4 kb BclI fragment). (C) RNA blot analysis of ko and wt parasites. RNA was isolated from purified gametocytes and hybridized with probes against the ORFs of p28 (probe A) and p25 (probe D). After disruption of p28, no transcription of p28 was detected. After disruption of the p25 gene, aberrant sized p25 mRNA species are produced that are slightly larger and smaller than the mRNA (1.4 kb) of the undisrupted gene. (D) Protein blot analysis of expression of P25 and P28 in mature ookinetes. Parasites were reacted with polyclonal anti-ookinete immune serum (lanes 2–5), which recognizes both P25 and P28, and with a Mab (13.1) (lane 1) against P28. Lane 5 is overexposed to demonstrate the complete absence of both proteins in the Dko parasites. (E) Expression of P25 and P28 in mature ookinetes, as shown by immune fluorescence analysis of acetone-fixed parasites. Parasites were labelled with Mab 13.1 to antigen P28 (M28), a monospecific anti-Pbs25 polyclonal antiserum (M25) (Rodriguez et al., 2000) and a polyclonal anti-ookinete immune serum (P25/28).
The close linkage of these genes enabled the design of a DNA vector to knock out both genes simultaneously using a single selectable marker (Figure 1A), resulting in the selection of ko parasites lacking both proteins (25/28Dko mutant parasites). In addition, two DNA vectors were made to individually disrupt p25 or p28 (25Sko and 28Sko parasites, respectively; Figure 1A). All vectors contained the pyrimethamine-resistant Toxoplasma gondii DHFR/TS as a selectable marker and were designed to disrupt the target genes by double cross-over replacement as described previously (Ménard and Janse, 1997) (Figure 1A). Transformation of parasites and selection of resistant, transformed parasites was performed as described earlier (de Koning-Ward et al., 2000). Parasites were transfected with each vector in two independent experiments and we selected two 25/28Dko clones, two 25Sko clones and two 28Sko clones for further analysis. Correct disruption of the genes by integration of the vectors was confirmed by PCR (data not shown) and Southern analysis of genomic DNA (Figure 1B). In wild-type (wt) parasites, both genes are transcribed in female gametocytes. In the Dko and 28Sko parasites, no mRNA from p28 was present, as expected. Interestingly, in both Dko and 25Sko parasites, aberrantly sized mRNA species of the disrupted p25 are present (Figure 1C). Aberrant mRNA species are sometimes seen with this design of insertion vector and are due to transcriptional run through into the promoter region of the selectable marker, but this transcript generally does not result in a productive mRNA species (Figure 1A). The 28Sko and 25Sko parasites produced normal amounts of mRNA of the non-targeted p25 and p28 genes, respectively, compared with wt (Figure 1C and data not shown). Analysis of the presence of the proteins in ookinetes of the ko parasites by immunoblotting and immunofluorescence demonstrated the complete absence of the proteins encoded by the disrupted genes in the Sko and Dko parasites (Figure 1D and E). Despite the possibility that truncated proteins could be synthesized from the aberrant sized mRNA species, no truncated protein products were detected. Production of P25 and P28 by the non-disrupted genes in the Sko parasites appeared to be comparable to wt (Figure 1D).
P25 and P28 play a role in ookinete survival in the mosquito midgut
Asexual blood stage development and production of gametocytes of Sko and Dko parasites were not different from wt parasites (data not shown). We analysed ookinete development both in vitro and in the midgut of Anopheles stephensi mosquitoes. In vitro ookinete maturation and numbers of mature ookinetes (stage V/VI) of the Sko parasites were not significantly different from wt (Table I). Only the Dko parasites produced fewer mature ookinetes (mean of 63% compared with wt; Table I). In the cultures of Dko parasites, an increased number of immature ookinetes (stage IV) were observed and mature ookinetes often showed a ‘swollen’ appearance (Figure 2D). Based on these observations and the fact that P25 and P28 may represent as much as 25% of the total proteins of the surface membrane, we examined whether the membrane integrity of Dko parasites was significantly compromised. However, no obvious morphological changes in plasma membrane structure were found in Dko ookinetes by transmission electron microscopy and freeze–fracture examination (data not shown). Moreover, the Trypan blue exclusion test on Sko and Dko parasites did not indicate that the integrity of the surface membrane was affected (data not shown), and expression of the ookinete-specific protein CTRP was unaffected (Figure 2F and G). One notable characteristic of ookinetes of both Sko and Dko is their failure to form the large aggregates in vitro readily displayed by wt ookinetes (Figure 2A, B and D; Table I).
Table I. Characteristics of ookinete and oocyst formation of parasites lacking P25 (25Sko) or P28 (28Sko) or lacking both proteins (25/28Dko) compared with wild-type (wt) parasites.
| wt | 25Sko | 28Sko | 25/28Dko | |
|---|---|---|---|---|
| Relative ookinete production in vitroa | 100 | 90.9 ± 13.1 | 94 ± 9.7 | 63 ± 7.9 |
| (P <0.01*) | ||||
| Percentage of ookinetes in clustersb | 79.7 ± 8.9 | 25.0 ± 5.0 | 10.6 ± 0.4 | 13.5 ± 2.1 |
| P <0.001** | P <0.001** | (P <0.001**) | ||
| Relative sensitivity to trypsinc | ||||
| control | 100 | 100 | 100 | 100 |
| trypsin type I | 142.1 ± 52.6 | 50.6 ± 45.0 | 124.2 ± 17.5 | 50.5 ± 3.5 |
| (P = 0.17*) | ||||
| trypsin type II | 68.6 ± 17.0 | 70.4 ± 54.4 | 153.7 ± 54.9 | 64.5 ± 16.3 |
| (P = 0.07*) | ||||
| Relative ookinete production in vivoa | 100 | 115.9 ± 2.8 | 120.0 ± 10.9 | 7.7 ± 10.2 |
| Relative oocyst productiond | ||||
| after host feeding | 100.0 | 56.6 ± 11.9 | 68.3 ± 13.1 | 0.05 ± 0.01 |
| P <0.001** | P <0.05** | P <0.001** | ||
| after feeding in vitro formed ookinetes | 100.0 | 36.4 ± 5.4 | 30.4 ± 7.4 | 0.5 ± 0.6 |
| P <0.001** | P = 0.05** | P <0.001** | ||
| Oocyst formation from ookinetese | ||||
| fed via membrane | 163.2 ± 53.0 | ND | ND | 0.26 ± 0.26 |
| (P <0.001*) | ||||
| microinjected in haemocoel | 2.7 ± 3.2 | ND | ND | 0.04 ± 0.04 |
| P <0.001* | (P <0.005*) |
aTotal number of ookinetes (stages IV–VI) expressed as a percentage of wt (mean ± SEM).
bClustering is defined as the presence of two or more ookinetes (stages IV–VI) in close contact in Giemsa-stained cell monolayers (mean ± SEM).
cSurvival of each parasite type (mean percentage ± SEM) is compared with its own control value defined as 100%.
dArithmetic mean number of oocysts per midgut, expressed as a percentage of wt infection ± SEM
detected on guts 10 days after infection.
eArithmetic mean number of oocysts per midgut, expressed as a percentage of wt infection ± SEM
detected on guts 10 days after infection.
*Unpaired t-test; **Mann–Whitney test.
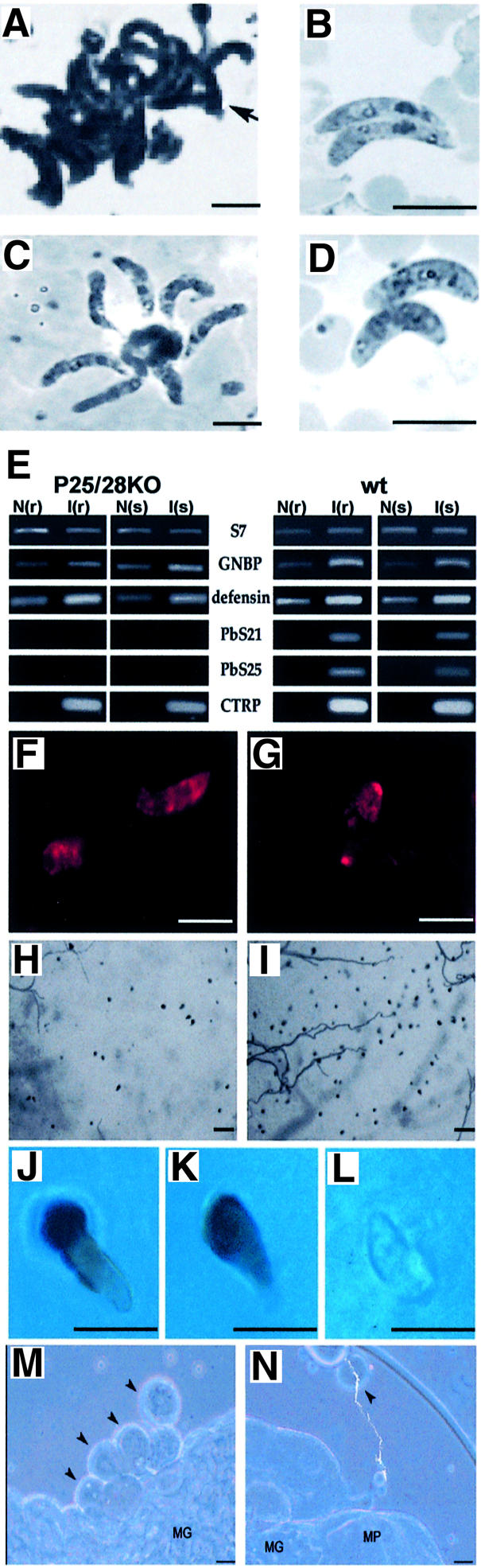
Fig. 2. Characteristics of the phenotype of Dko and wt ookinetes during ookinete maturation, penetration of the midgut epithelium and development into oocysts. (A–D) Light microscopic morphology of in vitro cultured ookinetes in Giemsa-stained smears. (A) wt ookinetes form characteristic clusters, which are absent in Sko and Dko ookinete cultures (Table I). (B and D) Dko parasites (D) have in general a ‘swollen’ appearance (both in smears and in live preparations) compared with wt (B) and Sko ookinetes. However, no changes in membrane integrity could be demonstrated by electron microscopy in these ookinetes as a result of the lack of P25 and P28 (results not shown). (C) P25ko ookinetes attached in a typical ‘floret’ to mononuclear cells in culture after trypsin treatment. Scale bar = 10 µm. (E) RT–PCR analysis of the expression of the immune response genes GNPB and defensin in A.gambiae in refractory (r) and susceptible (s) lines 24 h after feeding on uninfected (N) or infected (I) mice (see Materials and methods). The immune responses against infection with 25/28Dko and wt are compared. The clear immune response in both the refractory and susceptible strain after infection with Dko parasites demonstrates that in both strains ookinetes are able to penetrate the midgut wall. RT–PCR analyses of mRNA of genes encoding mosquito ribosomal protein S7, P25, P28 and CTRP are used as controls. (F and G) Dko ookinetes within the midgut epithelium stained with antibodies recognizing CTRP in the susceptible (F) and refractory (G) strain of A.gambiae. Scale bar = 10 µm. (H and I) Melanized, penetrated ookinetes on the surface (haemocoel side) of the midgut epithelium of the refractory strain of A.gambiae. (H) 25/28Dko; (I) wt. Scale bar = 50 µm. (J–L) Melanized wt (J) and Dko (K) ookinetes emerging from midgut epithelia of the refractory strain of A.gambiae and a non-melanized Dko ookinete, which are typically found deep within the midgut epithelia of refractory A.gambiae. Scale bar = 10 µm. (M and N) wt (M) and Dko (N) oocysts (10 days old) isolated from midguts of A.stephensi that are infected by micro-injection of ookinetes into the haemocoel. In wt infected mosquitoes, oocysts are often found in clumps attached to the midgut basal lamina, whereas these clumps are lacking in Dko parasites and oocysts are often found free in the haemocoel. Scale bar = 50 µm.
Ookinete production in the mosquito midgut by the Sko parasites was not significantly different from that of wt parasites (Table I). However, in vivo ookinete production of Dko parasites was strongly inhibited (mean efficiency of only 7.7% compared with wt). The large difference in efficiency of Dko ookinete production between in vitro and in vivo indicates that additional factors in the mosquito midgut negatively influence Dko ookinete development. Since ookinetes are susceptible to damage by mosquito midgut proteases (Yeates and Steiger, 1981), we tested the in vitro susceptibility of ko ookinetes to trypsin. Although we observed large differences in the susceptibility of ookinetes between different experiments, the Dko parasites appear to be significantly more susceptible to trypsin compared with wt and Sko parasites (Table I).
P25 and P28 have partially redundant functions
We investigated the possibility that P25 and P28 play a critical role in ookinete passage through the midgut epithelium. The standard laboratory model for investigation of P.berghei transmission is A.stephensi, where reproducibility and intensity (number of oocysts) of infection permit statistical analysis of data (Medley et al., 1993). Mosquitoes can be infected either through feeding on infected rodents or through membrane feeding of in vitro formed ookinetes. We first tested the ability of Sko and Dko parasites to produce oocysts when mosquitoes were fed on infected mice. P25Sko and P28Sko parasites produced significant numbers of oocysts, whereas oocyst production in mosquitoes infected with Dko parasites was inhibited by >99% (Table I). The much stronger inhibition of oocyst development of Dko parasites in comparison with the Sko parasites was also observed after infection of mosquitoes through membrane feeding of equal numbers of ookinetes (Table I). The formation of significant numbers of oocysts in both Sko parasites that give rise to infectious sporozoites (see below) indicates that P25 and P28 have partially redundant functions. In all experiments, the efficiency of oocyst formation of Sko parasites was significantly lower (30.4–68.3% compared with wt) than that of ookinete formation (90.9–120% compared with wt). These observations strongly suggest that P25 and P28 also play a role during conversion of the mature ookinete to the oocyst, but did not pinpoint whether penetration of ookinetes or the later conversion of ookinetes into oocysts is affected in the ko parasites.
Ookinetes lacking P25 and P28 can traverse the midgut epithelium
In order to investigate the process of penetration of the epithelium by ookinetes lacking P25 and P28, we infected the mosquito species Anopheles gambiae with wt and Dko parasites. Anopheles gambiae was used because in this species innate immune responses have been defined which can be used as markers for the process of crossing the epithelium by P.berghei ookinetes (Dimopoulos et al., 1997, 1998). In addition, a refractory strain of A.gambiae encapsulates and melanizes ookinetes in the BL region of the epithelium after crossing the epithelium and as they emerge into the extracellular space (Paskewitz et al., 1988). Unlike normal ookinetes, these melanized ookinetes can easily be detected. After feeding of A.gambiae on mice infected with Dko parasites, ookinete numbers were usually very low, which was not unexpected because of the low efficiency of in vivo Dko ookinete formation (see above) and the generally lower efficiency of ookinete formation in A.gambiae compared with A.stephensi. In the few experiments in which significant numbers of Dko ookinetes were formed, an immune response was elicited 20 h after feeding, which was comparable to mosquitoes fed with wt parasites (Figure 2E). This result indicated that Dko ookinetes can cross the epithelium. Moreover, (melanized) Dko ookinetes were found in the refractory strain in the BL region on the haemocoel side of the midgut epithelium, proving that ookinetes lacking both P25 and P28 can cross the epithelium in this mosquito species. Although melanized Dko ookinetes were usually rare, they resembled the melanized wt parasites in their pear-shaped appearance (Figure 2H, I, J, K and L), and ookinetes were seen to round up, indicating the onset of oocyst formation. Furthermore, these characteristics of penetrated ookinetes were all absent in mosquitoes infected with CTRP ko ookinetes, which have been shown to be unable to enter the cells of the midgut epithelium (Dessens et al., 1999; Yuda et al., 1999; Templeton et al., 2000). These results demonstrate that P25 and P28 do not play a critical role in the recognition and attachment of ookinetes to the luminal face of the midgut epithelium. However, the low numbers of (melanized) penetrated Dko ookinetes compared with wt indicate that penetration of the epithelium by Dko parasites is impaired (see also below and Discussion). The penetration by Dko ookinetes of the midgut epithelium was also confirmed in A.stephensi by staining of ookinetes penetrating the midgut by antibodies against the ookinete-specific CTRP protein (Dessens et al., 1999) (Figure 2F and G and data not shown).
Transformation of ookinetes into oocysts in the haemocoel is impaired in parasites lacking P25 and P28
Since P25 and P28 are still present in the young oocyst of wt parasites, it is possible that the strong reduction in oocyst numbers of Dko parasites is not only due to the less efficient ookinete development and penetration, but might also be the result of impairment of early transformation of Dko ookinetes into oocysts after traversing the midgut wall. To bypass the process of penetration of the midgut epithelium, we therefore compared oocyst development from Dko and wt ookinetes that were injected directly into the haemocoel of A.stephensi. Micro-injected ookinetes of both wt and Dko were able to develop into oocysts (Figure 2M and N), but with an efficiency much lower than when ookinetes were membrane fed to mosquitoes (Table I). Micro-injected Dko ookinetes transformed into oocysts much less successfully than wt ookinetes. However, the ratio of Dko/wt oocysts was higher when injected and membrane-fed ookinetes were compared (1.5 × 10–2 versus 1.6 × 10–3, respectively; Table I). Interestingly, wt oocysts resulting from micro-injected ookinetes were almost invariably found in clumps attached to the wall of the midgut and Malpighian tubules (Figure 2M), whereas the Dko oocysts were not clumped and were frequently found free in the haemocoel (Figure 2N). The higher efficiency of transformation of micro-injected Dko ookinetes into oocysts compared with that of wt ookinetes indicated that P25 and P28 play no significant role in the early development of oocysts.
Irrespective of whether oocysts in A.stephensi were derived from feeding on infected blood directly from the host, from in vitro cultured ookinetes via membranes, or from the micro-injection of in vitro cultured ookinetes, the numbers of sporozoites of Sko and Dko parasites in the salivary glands paralleled the number of oocysts present on the midgut (data not shown). The infectivity of the Sko and Dko sporozoites was demonstrated by feeding infected mosquitoes on uninfected mice. Blood infections were recorded in 80–100% of mice infected with the Sko clones and in 12–25% of those infected with the Dko clones, i.e. with efficiencies directly comparable to the number of sporozoites present in the salivary glands (data not shown). Sko and Dko parasites were genetically stable after transmission through the mosquito and maintained the genome alteration caused by the disruption of the p25/p28 locus (data not shown).
Discussion
Conserved organization of the genes encoding P25 and P28 in Plasmodium
The genome of Plasmodium species of humans, rodents and birds contains genes that encode for proteins homologous to the P25 and P28 proteins that were first described in P.falciparum. The genes have probably arisen by a duplication event resulting in a tandemwise linked, head-to-tail organization of the genes. Interestingly, this genome organization has been conserved in human, rodent and bird malaria (our unpublished observations). Tight linkage of tandemwise organized, closely related genes has been found for more surface proteins of the sexual stages of Plasmodium, such as P48/45 and p47 (van Dijk et al., 2001), Pfs230 and a paralogous gene (Gardner et al., 1998). The close linkage of the P25 and P28 genes, in the absence of other genes in the intergenic region, facilitated the simultaneous disruption of two genes that encode paralogous proteins.
The functions of P25 and P28 of P.berghei are partially and mutually redundant
P25 and P28 are abundant proteins present on the surface of zygotes, ookinetes and young oocysts. Sko parasites lacking either P25 or P28 were transmitted efficiently by mosquitoes. Furthermore, oocyst production by Sko parasites was only mildly compromised, although reduced compared with wt, indicating a redundancy in function of P25 and P28. The fact that parasites that lack both proteins were strongly affected in their transmission capacity indicates that other proteins are unable to compensate for the functions of P25 and P28. The redundancy in function is not a feature of all paralogous gene pairs described for surface proteins of the sexual stages of Plasmodium. For example, disruption of p48/45 of the tandemwise arranged p48/45 and p47 resulted in an almost complete inhibition of sexual development despite normal levels of p47 transcription (van Dijk et al., 2001). Therefore, although redundancy in protein function has implications for vaccine design (see below), it is not a general phenomenon of the duplicated gene pairs in the genome of Plasmodium.
P25 and P28 of P.berghei have multiple functions during ookinete/oocyst development
Although ookinete development of the Dko parasites is less efficient than that of wt parasites, we found that these parasites were able to produce significant numbers of mature ookinetes under in vitro conditions. P25 and P28 may represent as much as 25% of the total ookinete surface protein (Dearsly, 1989), and as GPI-anchored proteins may well be concentrated in cholesterol and glycosphingolipid-enriched microdomains of the plasma membrane (Severs and Robenek, 1983; Wunderlich et al., 1991; Friedrichson and Kurzchalia, 1998). It was, therefore, unexpected that neither electron microscope nor cell viability studies suggested that the gross architecture of the membrane was severely compromised and that the mature Dko ookinetes could penetrate the peritrophic membrane of the midgut wall. The marked reduction in Dko ookinete numbers in the mosquito in comparison with in vitro ookinete production strongly suggests that P25 and P28 confer protection to the developing ookinete against the ‘hostile’ environment of the mosquito midgut. Factors known to be deleterious to ookinete survival include vertebrate-derived complement and phagocytes, which are active in the blood meal (Sinden and Smalley, 1976; Lensen et al., 1997), and proteases (Yeates and Steiger, 1981). The high in vitro susceptibility of the Dko parasites to trypsin suggests that these surface proteins can confer protection against such lethal factors. An interesting observation was that following exposure of P25ko ookinetes to trypsin, they were found to attach in typical ‘florets’ to mononuclear cells in culture (Figure 2C). This phenomenon was never observed in trypsin-treated wt ookinetes, but suggests that proteases would render ookinetes more susceptible to attack by phagocytes. Phagocytic attack is known to occur in mosquitoes infected with P.falciparum (Sinden and Smalley, 1976; Lensen et al., 1997).
In addition to a role in the formation and survival of ookinetes, our studies clearly establish the existence of later role(s) of the P25/P28 proteins in crossing the epithelium, and in the interaction with the basal lamina and consequent triggering of oocyst development. The observation that micro-injected Dko ookinetes are surprisingly efficient in forming oocysts indicates a function during crossing of the epithelium. They do so at a frequency of 1.5 × 10–2 relative to micro-injected wt ookinetes, whereas the corresponding frequency for membrane-fed Dko ookinetes is ∼10-fold lower (1.6 × 10–3 relative to the wt). Furthermore, Dko ookinetes are seen in transit through the epithelium at generally low and variable numbers compared with wt. Interestingly, in the refractory strain of A.gambiae we observed a significant number of unmelanized ookinetes deeper within the epithelium. They had a gelatinous appearance (Figure 2J, K and L), possibly reflecting an early stage of encapsulation before melanization. Unmelanized wt ookinetes were not detected in this location at the same time point. This observation is consistent with the possibility that the Dko ookinetes are retarded within the epithelium and, as a consequence, are killed by epithelial defence reactions [in keeping with the so-called ‘time bomb’ model of ookinete invasion described by Han et al. (2000)]. It should be noted that wt ookinetes shed P28 proteins within the cytoplasm of the epithelial cells that they traverse (Han et al., 2000). This may possibly down-regulate the epithelial defences in wt parasites. The micro-injection data also support a late function for P25/28 after crossing of the midgut epithelium, since micro-injected Dko ookinetes are single, not aggregated and frequently unattached to the midgut, whereas their wt counterparts are typically clustered and attached to the haemocoel surface of the midgut and Malpighian tubules. This observation suggests that P25 and P28 may be important in binding to the BL, a step that might trigger development towards the oocyst stage. This interpretation is consistent with the fact that P25 from P.berghei (Vlachou et al., 2001) and P28 from the avian parasite P.gallinaceum (Adini and Warburg, 1999) bind directly to laminin—a prominent component of the midgut BL. In summary, our data indicate that the P25/28 proteins play a role in both the development/survival of ookinetes within the midgut and in traversing the midgut epithelium and early transformation into the oocyst stage.
None of our assays has revealed a functional difference between p25 and p28 Sko parasites; this implies that the encoded proteins are redundant. Interestingly, functional redundancy has been described for the various GPI-anchored, cysteine-rich surface proteins of the SAG multigene family in the related apicomplexan parasite T.gondii (Mineo et al., 1993). Like P25 and P28, antibodies to SAG1 inhibited parasite interaction with host tissues, yet SAG1-deficient parasites were invasive, as were SAG3-negative parasites. The close parallels between these observations and the data presented here suggest that it might be common for host–parasite interactions to be mediated by multiple proteins whose individual functions may overlap (Manger et al., 1998). This would provide a more reliable system for parasite survival and may further serve to increase the potential vector range of the parasite. The reduced efficiency of mosquito infection by both Sko parasites compared with wt parasites indicates that the gene duplication may also represent a gene dosage phenomenon, as described for elongation factor 1-α genes (Vinkenoog et al., 1998). Another explanation may be the postulated phenomenon of complementary degenerative mutations, whereby duplicated genes become less fit than the original progenitor gene, and therefore fix and remain active yet partially redundant (Force et al., 1999). It is also possible that the apparent redundancy in P25/P28 function might stem from the existence of alternative pathways for ookinete interaction with vector cells, a property known to be associated with erythrocyte invasion by merozoites (Reed et al., 2000).
The functions of P25 and P28 and mechanisms of transmission-blocking immunity
Being both highly immunogenic and invariant in the field, P25/28 are prioritized malaria vaccine candidates. Anti-P25/28 immunity attacks the parasite in the mosquito and is antibody mediated (Kaslow, 1998). A P28 monoclonal antibody (Mab) inhibited parasite development by three mechanisms (Ranawaka et al., 1993, 1994a,b). First, zygotes/ookinetes were killed in the midgut by antibody-dependent cell-mediated killing; secondly, ookinetes were prevented from differentiating into oocysts partly as a consequence of the antibody preventing interaction of the ookinete with the midgut epithelium; and thirdly, the development of young oocysts was inhibited by IgG. The first two mechanisms of action may correspond to the protective role in the bloodmeal, and the traversal of the midgut epithelium postulated as functions for P25 and P28 in the present study.
The redundancy in function between P25 and P28 has raised an important issue in the rational formulation of transmission-blocking vaccines. Because neither P25 nor P28 is individually essential for parasite survival, whereas loss of both proteins results in a loss of viability of >99%, we feel it would be prudent to target both molecules simultaneously in a vaccine. This would reduce the probability that spontaneous single-gene null mutants at either locus would lead to the survival of vaccine-insensitive, yet viable, parasites. Moreover, potent immunogenicity has been described between Pfs25 and Pfs28 following simultaneous immunization as a hybrid recombinant protein (Gozar et al., 1998). Together, these two criteria provide compelling reasons against using either protein alone in a vaccine formulation.
Materials and methods
Generation of P25Sko, P28Sko and P25/28Dko parasites and genotype analysis
Three different vectors were made to disrupt p25 and p28 (Figure 1A). These vectors contain the TgDHFR/TS selection cassette flanked by sequences of the p25/p28 locus. For the P28Sko vector, a 902 bp PstI fragment, including the p28 promoter and coding sequences, was excised from plasmid pBSPbs21, which comprised a 2.0 kb BclI fragment of the p28 locus. This fragment was cloned into the PstI site of plasmid pDb-Db (Tomas et al., 1998), creating p215 Db-Db. A BsmI–EcoRI 1072 bp fragment containing coding and downstream sequences of p28, obtained from pBSPbs21, was inserted in the EcoRV–EcoRI site of p215Db-Db. In this way, 10 bp of the p28 ORF (nucleotides 902–912) are missing in the disruption plasmid. The NheI–SpeI fragment was used to disrupt p28. For the P25Sko vector, a 615 bp fragment containing the first 440 bp of p25 was amplified by PCR, introducing flanking ClaI–HindIII (5′) and PstI (3′) sites, and cloned into pDb-Db, creating p255D-D. A 746 bp fragment of the p25/28 intergenic region was amplified by PCR, introducing EcoRV (5′) and EcoRI (3′) sites, and cloned into the same sites in p255D-D, creating p255Db-Db25Ig, which would truncate p25 at nucleotide 440. The HindIII–EcoRI fragment was used to disrupt p25. For the P25/28Dko vector, the 1072 bp P28 3′ fragment was cloned into the EcoRV–EcoRI site of p255Db-Db, creating p255Db-Db213. The ClaI–SpeI fragment was used to disrupt both p25 and p28. Plasmodium berghei parasites (ANKA strain, clone 15cy1) were transfected and cloned as described before (Ménard and Janse, 1997). Disruption of the p25 and p28 genes was analysed by PCR, DNA and RNA blot analyses (Ménard and Janse, 1997).
Expression of P25 and P28 in wt and ko parasites
Western blots were made using ∼2–5 × 104 ammonium chloride-enriched ookinetes per lane. Primary antibodies were UPC10 (Sigma) as a control IgG2a, anti-Pbs21 Mab 13.1 diluted 1:10 000 (Spano et al., 1996), or anti-ookinete-homogenate immune serum (which recognizes both Pbs21 and Pbs25) diluted 1:1000 (Rodriguez et al., 2000). Reactions were visualized using peroxidase- or alkaline phosphatase-conjugated anti-mouse antibody according to the manufacturer’s instructions (Bio-Rad, UK). Polyclonal anti-ookinete antiserum, anti-Pbs25 antiserum or anti-Pbs21 Mab 13.1 (Rodriguez et al., 2000) was used diluted 1:1000 as primary antibody in immunofluorescence analysis. Fluorescein isothiocyanate (FITC)-labelled goat anti-mouse, diluted 1:100, was used as the second antibody. Antigen expression was examined in acetone-fixed ookinete blood films or in live ookinete suspensions. To determine whether ookinetes had penetrated through the midgut epithelium, whole mosquito midguts were dissected in phosphate-buffered saline (PBS) and suspended in appropriate FITC-conjugated primary antiserum containing 0.1% propidium iodide. After 10 min, the preparation was transferred to PBS and observed in a confocal laser-scanning microscope (Bio-Rad MRC 600).
Phenotype analysis of ko parasites
In vitro cultivation of ookinetes. Ookinetes were cultured in vitro as described by Sinden (1996) and Janse et al. (1985). For each ko parasite line, ookinete numbers were determined in duplicate in two independent cultures. Ookinete maturation was determined according to the classification of Janse et al. (1985). The integrity of ookinete membranes was assayed according to the method of Trypan blue exclusion described by Celis and Celis (1994). Clustering of mature ookinetes was estimated at 20 h in Giemsa-stained smears by light microscopy.
Susceptibility of ookinetes to trypsin. Trypsin (2.8 µg/µl), dissolved in ookinete culture medium, was added to ookinete cultures in 24-well plates 20 h after the start of the cultures. The trypsin concentration is based on the peak trypsin concentration measured in midguts 20 h after a blood meal (Gass and Yeates, 1979). Trypsin type I (Sigma T-8003) at 9820 U/mg and trypsin type II-S (Sigma T-7409) at 1420 BAEE U/mg were used. Four hours after incubation of the mature ookinetes with trypsin, ookinetes were counted as described above.
In vivo ookinete and oocyst development. Anopheles stephensi (strain SD500) were fed on infected mice or via membrane feeding with in vitro cultured ookinetes as described by Sinden (1996). Ookinete development in midguts was determined 20 h after feeding. The contents of a minimum of three groups of five individual midguts were dissected into 2 µl of PBS and ookinetes counted as described above. Oocyst numbers were determined on days 10–12 after feeding in 30–100 mosquitoes in three replicated experiments (Sinden, 1996). Sporozoites were counted in salivary glands on day 18 from five individual mosquitoes per group (Sinden, 1996). For micro-injection of in vitro cultured ookinetes, 800 ookinetes (in 1 µl) were injected into the haemocoel of four groups (20 per group) of susceptible A.stephensi (Weathersby, 1952). Midguts were dissected as above and examined along with the Malpighian tubules for attached oocysts. The haemocoelomic fluid was examined for free oocysts.
Penetration of ookinetes through the midgut epithelium. We infected A.gambiae (susceptible strain 4A r/r and refractory strain L 3-5; Dimopoulos et al., 1997) by feeding on infected mice. To detect ookinetes on the haemocoel side of the epithelium, midguts were dissected at 20–24 h post-infection in PBS containing 1% paraformaldehyde (PFA). After removal of the blood meal, the midguts were washed five times in PBS and then fixed in 4% PFA for 30 min. Fixed midguts were washed four times in PBS and then permeabilized in 0.1% Triton X-100 in PBS for 30 min and washed four times with PBS. The midguts were incubated with primary polyclonal anti-CTRP serum for 20 h in 0.2% bovine serum albumin in PBS, then washed four times in PBS and incubated with a secondary CY-5-conjugated antibody (goat anti-rabbit) in PBS. After staining, the midguts were washed four times in PBS, mounted on microscope slides and examined with a confocal laser-scanning microscope to localize ookinetes in the midgut epithelium.
To study the immune response against penetrated ookinetes, we investigated the expression of defensin and GNBP (Dimopoulos et al., 1997). In addition, we determined the expression of three ookinete proteins, P25, P28 and CTRP (Dessens et al., 1999), by RT–PCR. RNA extraction, cDNA synthesis and RT–PCR expression analysis of immune response genes defensin and GNBP in infected and naive A.gambiae were performed as described previously using the ribosomal S7 gene as a normalization standard (Dimopoulos et al., 1997). The PCR cycle number was constant for a particular sequence in the analysed samples as follows: S7, 24; defensin, 26; GNBP, 32; CTRP, 45; P28, 45; P25, 45. The primers used were as follows: S7A, 5′-GGCGATCATCATCTACGT-3′ and S7B, 5′-GTAGCTGCTGCAAACTTCGG-3′; GNBPA, 5′-GCAACGAGAATCTGTACC-3′ and GNBPB, 5′-TAACCACCAGCAACGAGG-3′; defensin A, 5′-CTGTGCCTTCCTAGAGCAT-3′ and defensin B, 5′-CACACCCTCTTCCCAGGAT-3′; Pbs21A, 5′-CTATATGTATAAACCAGGG-3′ and Pbs21B, 5′-ACTGATTATTGACATTCCG-3′; Pbs25A, 5′-TGTGTTCCAGAAACTTGCC-3′ and Pbs25B, 5′-GAATTAGTGAAAATTATCC-3′; CTRPA, 5′-AGACAAGGATGCCTACACC-3′ and CTRPB, 5′-AACCTTCACTACTATCACC-3′.
To study melanization of penetrated ookinetes, dissected midguts of the susceptible 4A r/r and refractory L 3-5 strains of A.gambiae were analysed at days 7 and 10 after feeding by bright field microscopy 10 days later, as described previously (Sinden, 1996).
Acknowledgments
Acknowledgements
The authors would like to thank Dr Inga Siden-Kiamos and colleagues for their free exchange of data, and Prof. P.Lachman for fruitful discussions. Work at Imperial College was funded by the Wellcome Trust, the European Union TMR Programme (R.S., M.K., J.M.), Concyacet (M.C.R.), the European Union Framework 5 Programme (A.L.B.), the Greek government (A.H.) and GlaxoWellcome plc (G.A.B.). Work in Leiden has been funded in part by the INCO-DC programme of the DGXII Department of the European Commission (Contract Number ERBIC18CT960052) and the Life Sciences Foundation (ALW), which is subsidized by the Netherlands Organisation for Scientific Research (NWO). A.M.T. was co-financed by a post-doctoral grant from Programa Praxis (JNICT), by Centro de Malaria e Outras Doencas Tropicais (co-ordinator Dr V.A.do Rosario) and by Instituto de Ciencias Biomedicas de Abel Salazar da Universidade do Porto, Portugal. Work in Heidelberg was funded by the European Union TMR Programme.
References
- Adini A. and Warburg,A. (1999) Interaction of Plasmodium gallinaceum ookinetes and oocysts with extracellular matrix proteins. Parasitology, 119, 331–336. [DOI] [PubMed] [Google Scholar]
- Alejo-Blanco A.R., Paez,A., Gerold,P., Dearlsy,A.L., Margos,G., Schwarz,R.T., Barker,G., Rodriguez,M.C. and Sinden,R.E. (1999) The biosynthesis and post-translational modification of Pbs21, an ookinete surface protein of Plasmodium berghei. Mol. Biochem. Parasitol., 98, 163–173. [DOI] [PubMed] [Google Scholar]
- Blobel C.P., Wolfsberg,T.G., Turck,C.W., Myles,D.G., Primakoff,P. and White,J.M. (1992) A potential fusion peptide and an integrin ligand domain in a protein active in sperm–egg fusion. Nature, 356, 248–252. [DOI] [PubMed] [Google Scholar]
- Celis A. and Celis,J. (1994) General procedures for tissue culture. In Celis,J. (ed.), Cell Biology: A Laboratory Handbook. Vol. 1. Academic Press, San Diego, CA, pp. 1–12.
- Dearsly A.L. (1989) Sexual and sporogonic development of Plasmodium berghei. PhD Thesis, Imperial College, University of London, London, UK.
- de Koning-Ward T.F., Janse,C.J. and Waters,A.P. (2000) The development of genetic tools for dissecting the biology of malaria parasites. Adv. Microbiol., 20, 157–185. [DOI] [PubMed] [Google Scholar]
- Dembinski A., Gregory,H., Konturek,S.J. and Polanski,M. (1982) Trophic action of epidermal growth factor on the pancreas and gastroduodenal mucosa in rats. J. Physiol. (Lond)., 325, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessens J.T., Beetsma,A.L., Dimopoulos,G., Wengelnik,K., Crisanti,A., Kafatos,F.C. and Sinden,R.E. (1999) CTRP is essential for mosquito infection by malaria ookinetes. EMBO J., 18, 6221–6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G., Richman,A., Muller,H.M. and Kafatos,F.C. (1997) Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc. Natl Acad. Sci. USA, 94, 11508–11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G., Seeley,D., Wolf,A. and Kafatos,F.C. (1998) Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. EMBO J., 17, 6115–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P.E. and Kaslow,D.C. (1997) A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccine. Infect. Immun., 65, 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P.E., Pimenta,P. and Kaslow,D.C. (1993) Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J. Exp. Med., 177, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force A., Lynch,M., Pickett,F.B., Amores,A., Yan,Y.L. and Postlethwait,J. (1999) Preservation of duplicate genes by complementary, degenerative mutations. Genetics, 151, 1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichson T. and Kurzchalia,T.V. (1998) Microdomains of GPI-anchored proteins in living cells revealed by cross-linking. Nature, 394, 802–805. [DOI] [PubMed] [Google Scholar]
- Fries H.C.W., Lamers,M.B.A.C., Vandeursen,J., Ponnudurai,T. and Meuwissen,J.H.T.E. (1990) Biosynthesis of the 25-kDa protein in the macrogametes/zygotes of Plasmodium falciparum. Exp. Parasitol., 71, 229–235. [DOI] [PubMed] [Google Scholar]
- Gardner M.J. et al. (1998) Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science, 282, 1126–1132. [DOI] [PubMed] [Google Scholar]
- Gass R.F. and Yeates,R.A. (1979) In vitro damage of cultured ookinetes of Plasmodium gallinaceum by digestive proteinases from susceptible Aedes aegypti. Acta Trop., 36, 243–252. [PubMed] [Google Scholar]
- Gozar M.M.G., Price,V.L. and Kaslow,D.C. (1998) Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect. Immun., 66, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst C.A. and Carter,R. (1987) Complement effects on the infectivity of Plasmodium gallinaeceum to Aedes aegypti mosquitoes. II. Changes in sensitivity to complement-like factors during zygote development. J. Parasitol., 73, 980–984. [PubMed] [Google Scholar]
- Grotendorst C.A., Carter,R., Rosenberg,R. and Koontz,L.C. (1986) Complement effects on the infectivity of Plasmodium gallinaeceum to Aedes aegypti mosquitoes. I. Resistance of zygotes to the alternative pathway of complement. J. Immunol., 136, 4270–4274. [PubMed] [Google Scholar]
- Han Y.S., Thompson,J., Kafatos,F.C. and Barillas-Mury,C. (2000) Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J., 19, 6030–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse C.J., Rouwenhorst,R.J., Van der Klooster,P.F., Van der Kaay,H.J. and Overdulve,J.P. (1985) Development of Plasmodium berghei ookinetes in the midgut of Anopheles atroparvus mosquitoes and in vitro. Parasitology, 91, 219–225. [DOI] [PubMed] [Google Scholar]
- Kaslow D.C. (1996) Transmission blocking vaccines. In Hofman,S.L. (ed.), Malaria Vaccine Development. ASM Press, Washington, DC, pp. 181–228.
- Kaslow D.C., Quakyi,I.A., Syin,C., Raum,M.G., Keister,D.B., Coligan,J.E., McCutchan,T.F. and Miller,L.H. (1988) A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature, 333, 74–76. [DOI] [PubMed] [Google Scholar]
- Kaslow D.C., Syin,C., McCutchan,T.F. and Miller,L.H. (1989) Comparison of the primary structure of the 25 kDa ookinete surface antigens of Plasmodium falciparum and Plasmodium gallinaceum reveals six conserved regions. Mol. Biochem. Parasitol., 33, 283–287. [DOI] [PubMed] [Google Scholar]
- Kumar N. and Carter,R. (1985) Biosynthesis of two stage-specific membrane proteins during transformation of Plasmodium gallinaceum zygotes into ookinetes. Mol. Biochem. Parasitol., 14, 127–139. [DOI] [PubMed] [Google Scholar]
- Lensen A.H.W., Bolmer-van de Vegte,M., van Gemert,G.J., Eling,W.M.C. and Sauerwein,R.W. (1997) Leucocytes in Plasmodium falciparum-infected blood meal reduce transmission of malaria to Anopheles mosquitoes. Infect. Immun., 65, 3834–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger I.D., Hehl,A.B. and Boothroyd,J.C. (1998) The surface of Toxoplasma tachyzoites is dominated by a family of glycosyl phosphatidylinositol-anchored antigens related to SAG1. Infect. Immun., 66, 2237–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G., van Dijk,M.R., Ramesar,J., Janse,C.J., Waters,A.P. and Sinden,R.E. (1998) Transgenic expression of a mosquito-stage malarial protein, Pbs21, in blood stages of transformed Plasmodium berghei and induction of an immune response upon infection. Infect. Immun., 66, 3884–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley G.F., Sinden,R.E., Fleck,S., Billingsley,P.F., Tirawanchai,N. and Rodriguez,M.H. (1993) Heterogeneity in patterns of malarial oocyst infections in the mosquito vector. Parasitology, 106, 441–449. [DOI] [PubMed] [Google Scholar]
- Ménard R. and Janse,C.J. (1997) Gene targeting in malaria parasites. Methods, 13, 134–147. [DOI] [PubMed] [Google Scholar]
- Mineo J.R., McLeod,R., Mack,D., Smith,J., Khan,I.A., Ely,K.H. and Kasper,L.H. (1993) Antibodies to Toxoplasma gondii major surface protein (SAG1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J. Immunol., 150, 3951–3964. [PubMed] [Google Scholar]
- Paskewitz S.M., Brown,M.R., Lea,A.O. and Collins,F.H. (1988) Ultrastructure of the encapsulation of Plasmodium cynomolgi (B strain) on the midgut of a refractory strain of Anopheles gambiae. J. Parasitol., 74, 432–439. [PubMed] [Google Scholar]
- Paton M.G., Barker,G.C., Matsuoka,H., Ramesar,J., Janse,C.J., Waters,A.P. and Sinden,R.E. (1993) Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol. Biochem. Parasitol., 59, 263–275. [DOI] [PubMed] [Google Scholar]
- Ranawaka G.R.R., Alejo-Blanco,R. and Sinden,R.E. (1993) Studies on the effect of transmission-blocking antibody ingested in primary and secondary blood feeds upon the development of Plasmodium berghei in the mosquito vector. Parasitology, 107, 225–231. [DOI] [PubMed] [Google Scholar]
- Ranawaka G.R.R., Alejo-Blanco,A.R. and Sinden,R.E. (1994a) Characterization of the effector mechanisms of a transmission-blocking antibody upon differentiation of Plasmodium berghei gametocytes into ookinetes in vitro. Parasitology, 109, 11–17. [DOI] [PubMed] [Google Scholar]
- Ranawaka G.R.R., Fleck,S.L., Alejo-Blanco,A.R. and Sinden,R.E. (1994b) Characterization of the modes of action of anti-Pbs21 malaria transmission-blocking immunity: ookinete to oocyst differentiation in vivo. Parasitology, 109, 403–411. [DOI] [PubMed] [Google Scholar]
- Reed M.B., Caruana,S.R., Batchelor,A.H., Thompson,J.K., Crabb,B.S. and Cowman,A.F. (2000) Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. Proc. Natl Acad. Sci. USA, 97, 7509–7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M.C., Dessens,J., Gerold,P., Sinden,R.E. and Margos,G. (2000) Characterisation and expression of Pbs25, a sexual and sporogonic stage specific protein of Plasmodium berghei. Mol. Biochem. Parasitol., 110, 147–159. [DOI] [PubMed] [Google Scholar]
- Schier A.F. and Shen,M.M. (2000) Nodal signalling in vertebrate development. Nature, 403, 385–389. [DOI] [PubMed] [Google Scholar]
- Severs N.J. and Robenek,H. (1983) Detection of microdomains in biomembranes. An appraisal of recent developments in freeze– fracture cytochemistry. Biochim. Biophys. Acta, 737, 373–408. [DOI] [PubMed] [Google Scholar]
- Sieber K.P., Huber,M., Kaslow,D., Banks,S.M., Torii,M., Aikawa,M. and Miller,L.H. (1991) The peritrophic membrane as a barrier: its penetration by Plasmodium gallinaceum and the effect of a monoclonal antibody to ookinetes. Exp. Parasitol., 72, 145–156. [DOI] [PubMed] [Google Scholar]
- Sinden R.E. (1996) Infection of mosquitoes with rodent malaria. In Crampton,J.M., Beard,C.B. and Louis,C. (eds), Molecular Biology of Insect Disease Vectors: A Methods Manual. Chapman and Hall, London, UK, pp. 67–91.
- Sinden R.E. and Smalley,M.E. (1976) Gametocytes of Plasmodium falciparum: phagocytosis by leucocytes in vivo and in vitro. Trans. R. Soc. Trop. Med. Hyg., 70, 344–345. [DOI] [PubMed] [Google Scholar]
- Spano F., Matsuoka,H., Ozawa,R., Chinzei,Y. and Sinden,R.E. (1996) Epitope mapping on the ookinete surface antigen Pbs21 of Plasmodium berghei: identification of the site of binding of transmission-blocking monoclonal antibody 13.1. Parassitologia, 38, 559–563. [PubMed] [Google Scholar]
- Templeton T.J., Kaslow,D.C. and Fidock,D.A. (2000) Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol. Microbiol., 36, 1–9. [DOI] [PubMed] [Google Scholar]
- Tomas A.M., van der Wel,A.M., Thomas,A.W., Janse,C.J. and Waters,A.P. (1998) Transfection systems for animal models of malaria. Parasitol. Today, 14, 245–249. [DOI] [PubMed] [Google Scholar]
- Tsuboi T., Cao,Y.M., Kaslow,D.C., Shiwaku,K. and Torii,M. (1997a) Primary structure of a novel ookinete surface protein from Plasmodium berghei. Mol. Biochem. Parasitol., 85, 131–134. [DOI] [PubMed] [Google Scholar]
- Tsuboi T., Ya-Ming,C., Hitsumoto,Y., Yanagi,T., Kanbara,H. and Torii,M. (1997b) Two antigens on zygotes and ookinetes of Plasmodium yoelii and Plasmodium berghei that are distinct targets of transmission-blocking immunity. Infect. Immun., 65, 2260–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T., Kaslow,D.C., Gozar,M.M., Tachibana,M., Cao,Y.M. and Torii,M. (1998) Sequence polymorphism in two novel Plasmodium vivax ookinete surface proteins, Pvs25 and Pvs28, that are malaria transmission-blocking vaccine candidates. Mol. Med., 4, 772–782. [PMC free article] [PubMed] [Google Scholar]
- van Dijk M.R. et al. (2001) A central role for P48/45 in malaria parasite male gamete fertility. Cell, 104, 153–164. [DOI] [PubMed] [Google Scholar]
- Vermeulen A.N., Ponnudurai,T., Beckers,P.J., Verhave,J.P., Smits,M.A. and Meuwissen,J.H. (1985) Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med., 162, 1460–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkenoog R., Sperança,M.A., Williamson,D., van Breemen,O., Thomas,A.W., Ramesar,J., Janse,C.J., del Portillo,H.A. and Waters,A.P. (1998) Malaria parasites contain two identical copies of an elongation factor-1α gene. Mol. Biochem. Parasitol., 94, 1–12. [DOI] [PubMed] [Google Scholar]
- Vlachou D., Lycett,G., Siden-Kiamos,I., Blass,C., Sinden,R.E. and Louis,C. (2001) Anopheles gambiae laminin interacts with the P25 surface protein of Plasmodium berghei ookinetes. Mol. Biochem. Parasitol., 112, 229–237. [DOI] [PubMed] [Google Scholar]
- Weathersby A.B. (1952) The role of the stomach wall in the exogenous development of Plasmodium gallinaceum as studied by means of haemocoel injections of susceptible and refractory mosquitoes. J. Infect. Dis., 91, 198–205. [DOI] [PubMed] [Google Scholar]
- Wunderlich F., Fiebig,S., Vial,H. and Kleinig,H. (1991) Distinct lipid compositions of parasite and host cell plasma membranes from Plasmodium chabaudi-infected erythrocytes. Mol. Biochem. Parasitol., 44, 271–277. [DOI] [PubMed] [Google Scholar]
- Yeates R.A. and Steiger,S. (1981) Ultrastructural damage of in vitro cultured ookinetes of Plasmodium gallinaceum (Brumpt) by purified proteinases of susceptible Aedes aegypti (L.). Z. Parasitenkd., 66, 93–97. [DOI] [PubMed] [Google Scholar]
- Yuda M., Sakaida,H. and Chinzei,Y. (1999) Targeted disruption of the Plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J. Exp. Med., 190, 1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Talbot,W.S. and Schier,A.F. (1998) Positional cloning identifies zebra fish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell, 92, 241–251. [DOI] [PubMed] [Google Scholar]



