Abstract
The biological role of small membrane proteins of the new FXYD family is largely unknown. The best characterized FXYD protein is the γ-subunit of the Na,K-ATPase (NKA) that modulates the Na,K-pump function in the kidney. Here, we report that, similarly to γa and γb splice variants, the FXYD protein CHIF (corticosteroid-induced factor) is a type I membrane protein which is associated with NKA in renal tissue, and modulates the Na,K-pump transport when expressed in Xenopus oocytes. In contrast to γa and γb, which both decrease the apparent Na+ affinity of the Na,K-pump, CHIF significantly increases the Na+ affinity and decreases the apparent K+ affinity due to an increased Na+ competition at external binding sites. The extracytoplasmic FXYD motif is required for stable γ-subunit and CHIF interaction with NKA, while cytoplasmic, positively charged residues are necessary for the γ-subunit’s association efficiency and for CHIF’s functional effects. These data document that CHIF is a new tissue-specific regulator of NKA which probably plays a crucial role in aldosterone-responsive tissues responsible for the maintenance of body Na+ and K+ homeostasis.
Keywords: CHIF/FXYD/Na,K-ATPase γ-subunit/Xenopus oocyte
Introduction
Recently, a new family of small membrane proteins, the FXYD protein family, has been defined (Sweadner and Rael, 2000), which includes the so-called γ-subunit of the Na,K-ATPase (NKA), phospholemman (Moorman et al., 1995), corticosteroid hormone-induced factor (CHIF; Attali et al., 1995), mammary tumor marker 8 (MAT-8; Morrison et al., 1995), RIC (related to ion channel; Fu and Kamps, 1997) and two as yet unknown proteins FXYD 6 and 7. The proteins of the FXYD family have a signature sequence which encompasses the single transmembrane domain and adjacent regions and includes the FXYD motif. The N-terminus was shown to be exposed to the extracytoplasmic side in the γ-subunit (Béguin et al., 1997; Therien et al., 1997) and in phospholemman (Palmer et al., 1991). Despite a similar membrane topology, the FXYD proteins are structurally clearly distinct from other small single-span membrane proteins such as Isk which regulates K+ channels (Suessbrich and Busch, 1999) or phospholamban which regulates the sarcoplasmic Ca2+-ATPase (Simmerman and Jones, 1998). Members of the FXYD family are widely distributed in mammalian tissues, possibly with a prominent expression in tissues that perform fluid and electrolyte transport or are electrically excitable (Sweadner and Rael, 2000). Although evidence exists that FXYD proteins may be regulators of ion transporters or may form ion channels under certain conditions (Attali et al., 1995; Moorman et al., 1995; Jones et al., 1997; Minor et al., 1998), their authentic biological function is still unknown. The only exception is the γ-subunit, which was shown to be associated with the renal NKA (Forbush et al., 1978; Mercer et al., 1993; Béguin et al., 1997) and to modulate its functional properties (Béguin et al., 1997; Therien et al., 1997, 1999; Arystarkhova et al., 1999; Pu et al., 2001).
NKA belongs to the P-type ATPase family and is a ubiquitous plasma membrane enzyme that uses the energy of ATP hydrolysis to maintain the transmembrane Na+ and K+ gradients of animal cells. The Na+ gradients provide the energy for many secondary transport systems necessary to maintain basal cellular homeostasis as well as to support specialized cellular functions. For instance, in renal epithelial cells, NKA is confined to the basolateral membrane and thus becomes the driving force for net Na+ reabsorption which is essential for body Na+ homeostasis and thus blood pressure. The minimal functional enzyme unit comprises a catalytic α-subunit containing the cation, the ATP and the phosphate-binding sites, and a glycosylated β-subunit, which is necessary for the structural and functional maturation of the α-subunit (for a review see Geering, 2000). Vertebrates have four α and three β isoforms which produce isozymes with different transport properties (Crambert et al., 2000).
In view of the crucial importance of NKA in many physiological and pathophysiological processes, the identification of tissue-specific regulatory mechanisms becomes an important issue. NKA is subjected to short- and long-term regulation by a variety of hormones and neurotransmitters (Féraille and Doucet, 2001). Short-term regulation of NKA may involve protein kinase-mediated phosphorylation and results in the modulation of the cell surface expression and/or the transport kinetics of the enzyme. On the other hand, long-term regulation, mediated by mineralocorticoid and thyroid hormones, involves changes in protein synthesis. Finally, NKA may be regulated through the interaction with other membrane or cytoplasmic proteins, as illustrated by the modulatory effect of the γ-subunit on renal NKA.
The existence of a small protein associated with renal NKA was first postulated by Forbush et al. (1978) and later confirmed by the molecular cloning of the mammalian γ-subunit (Mercer et al., 1993). In the kidney, the two recently identified splice variants, γa and γb (Küster et al., 2000; Sweadner and Rael, 2000), are expressed predominantly in the basolateral membrane of medullary thick ascending limb (mTAL). γa is also present in the macula densa and principal cells of the cortical collecting duct (CCD) and γb in the cortical TAL (Pu et al., 2001). γa and γb may be subjected to cell-specific co- or post-translational modifications (Arystarkhova et al., 1999; Küster et al., 2000).
We first described that γ-subunits indeed modulate the transport activity of NKA by changing the voltage sensitivity of K+ activation when expressed in Xenopus oocytes (Béguin et al., 1997). Other modulatory effects of γ-subunits on NKA activity have since been reported, including an increase in the affinity for ATP (Therien et al., 1997, 1999), an increase in the K+ antagonism of cytoplasmic Na+ activation by the association of both γa and γb (Pu et al., 2001) and a decrease in the apparent affinities for both Na+ and K+ (Arystarkhova et al., 1999). Significantly, a recent study has reported that a heterozygous mutation in the γa-subunit gene is linked to cases of human primary hypomagnesemia (Meij et al., 2000). The mutation of a conserved glycine residue in the transmembrane domain apparently leads to defective routing of the γ-subunit and Na,K-pumps to the plasma membrane.
CHIF (Attali et al., 1995) is another member of the FXYD family, which is expressed exclusively in epithelial cells and which displays a >50% sequence similarity with γ-subunits. CHIF is abundant in the basolateral membranes of surface cells in the distal colon and of principal cells in the medullary portions of renal collecting ducts (CDs) (Shi et al., 2001). Dexamethasone, aldosterone and low Na+ intake were shown to increase the abundance of CHIF mRNA in the colon (Attali et al., 1995; Capurro et al., 1996; Wald et al., 1997; Brennan and Fuller, 1999) whereas low Na+ intake increased the CHIF protein level in the kidney and the colon (Shi et al., 2001). Although expression of CHIF in Xenopus oocytes can induce a K+-specific conductance, possibly through regulation of endogenous oocyte K+ channels (Attali et al., 1995), the real physiological function of CHIF is unknown.
In view of the presence of both CHIF and γ-subunits in the basolateral membrane of epithelial cells, the similar hormonal regulation of CHIF and NKA, and the recent observation that CHIF co-immunoprecipitates with NKA in colon membranes (Garty et al., 2001), we tested in this study whether CHIF may be a ‘γ-like’, cell type-specific regulator of NKA. Our results show that CHIF is a type I membrane protein, which associates with NKA, but not with colonic H,K-ATPase (HKA), another P-type ATPase. The FXYD motif is important for stable interaction of CHIF and γ-subunits with NKA. When expressed in Xenopus oocytes, CHIF modulates NKA transport properties but in a different way from γa- or γb-subunits. Altogether, our study identifies CHIF as a new modulator of NKA which increases the enzyme’s Na+ affinity in tissues implicated in Na+ conservation.
Results
Association of CHIF and different γ-variants with NKA in Xenopus oocytes and in renal tissue
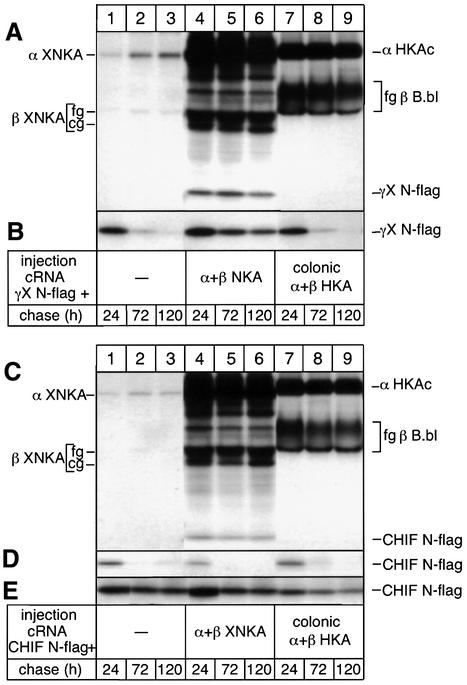
The specific association of CHIF and γ-subunits with NKA was first investigated in Xenopus oocytes expressing Xenopus NKA or colonic HKA α- and β-subunits together with N-terminally epitope-flagged, rat CHIF (CHIF N-flag) or Xenopus γ-subunits (γX N-flag). Oocytes were metabolically labeled, subjected to various chase periods and membrane fractions were immunoprecipitated under non-denaturing conditions. The γ-subunit, which is degraded when expressed alone (Figure 1B, lanes 1–3), becomes stabilized after co-expression with NKA (lanes 4–6) but not after co-expression with colonic HKA (lanes 7–9), and can be co-immunoprecipitated with NKA α (Figure 1A, lanes 4–6) but not with colonic HKA α (lanes 7–9) subunit antibodies after prolonged chase periods. These results confirm the specific interaction of the γ-subunit with NKA (Béguin et al., 1997). Similarly to the γ-subunit, CHIF was co-immunoprecipitated with NKA α (Figure 1C, lanes 4–6) but not with HKA α (Figure 1A and C, lanes 7–9) subunits, indicating that CHIF associates specifically and stably with NKA but not with another closely related, P-type ATPase which, like CHIF, is expressed in colon and kidney (Jaisser and Beggah, 1999). Based on results of radioimmunolabeling assays to intact oocytes, we have shown previously that the γ-subunit is associated in equimolar amounts with cell surface-expressed Na,K-pumps (Béguin et al., 1997). Considering that CHIF has two and the Xenopus γ-subunit has four methionines, the intensity of the CHIF signal co-immunoprecipitated with NKA suggests that this is also the case for CHIF.
Fig. 1. Specific association of CHIF and the γ-subunit with NKA. Oocytes were injected with N-terminally, epitope-flagged Xenopus γ-subunit (γX N-flag) (A and B) or rat CHIF (CHIF N-flag) (C–E) cRNAs (0.35 ng) alone or together with Xenopus NKA α1 (7 ng) and β1 (0.5 ng) or colonic HKA α (9 ng) and Bufo bladder β (1.5 ng) cRNAs. Oocytes were labeled for 24 h with [35S]methionine and, after the indicated chase periods, microsomes were prepared and immunoprecipitated under non-denaturing conditions with an NKA (A) or a HKA (C) α antibody or with a Flag antibody (B and D). Samples from microsomes shown in (E) were loaded directly on gels without immunoprecipitation. The positions of Xenopus α (α XNKA), colonic α (α HKAc), core (cg) and fully glycosylated (fg) Xenopus β (β XNKA) and Bufo bladder β (β HKA B.bl) subunits are indicated. Shown are fluorograms of 5–13.7% SDS–polyacrylamide gradient gels. A representative example of three similar experiments is shown.
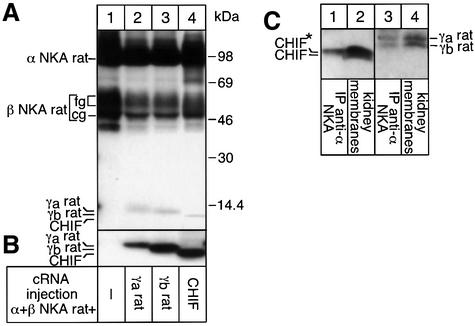
Similarly to CHIF N-flag and γX N-flag, wild-type CHIF and the two rat γa and γb variants, which differ only in a short stretch of N-terminal amino acids (Küster et al., 2000; Sweadner and Real, 2000), could stably interact with NKA after expression in oocytes (Figure 2A, lanes 2–4). To verify the physiological relevance of the interaction of CHIF with NKA, we tested whether not only γ-subunit-NKA but also CHIF–NKA complexes exist in renal tissue. Western blot analysis of kidney membranes indeed revealed the presence of CHIF, γa and γb variants (Figure 2C, lanes 2 and 4), which could all be co-immunoprecipitated with an NKA α antibody (lanes 1 and 3). Interestingly, CHIF may undergo co- or post-translational processing since, on Tricine gels, it was resolved as a doublet (lane 2) and the putatively modified CHIF species was co-immunoprecipited with an NKA α antibody (lane 1).
Fig. 2. CHIF and two γ splice variants associate with NKA in oocytes and in renal tissue. Oocytes were injected with CHIF, γa or γb cRNAs (0.5 ng) together with rat NKA α (9 ng) and β (1.3 ng) cRNAs, labeled for 48 h with [3H]leucine and subjected to a 24 h chase. Microsomes were prepared and subjected to immunoprecipitations under non-denaturing condition with an α antibody before gel migration (A) or directly loaded on gels (B). (C) Western blot analysis of kidney microsomes, which were either loaded directly on gels (lanes 2 and 4) or subjected to non-denaturing immunoprecipitations with an NKA α antibody before gel migration (lanes 1 and 3). After protein transfer to nitrocellulose membranes, the same membrane was probed first with a CHIF antibody (lanes 1 and 2) and then with a rat γ-subunit antibody (lanes 3 and 4). In (A), the migration of protein makers of known molecular mass is indicated.
Biosynthesis and processing of CHIF and γ-subunits
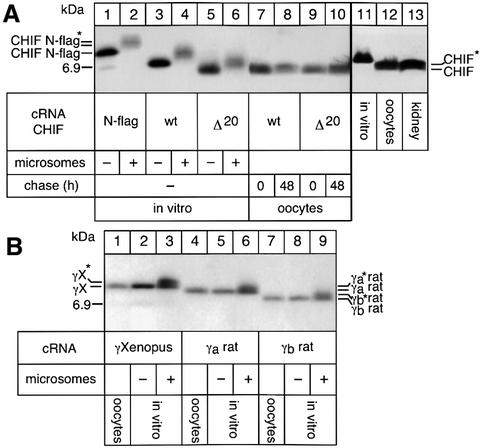
In contrast to γX N-flag (Figure 1A, lanes 1–3), CHIF N-flag was not sensitive to degradation when expressed alone in oocytes and could be detected for up to a 120 h chase period (Figure 1E, lanes 1–3). On the other hand, no CHIF N-flag was revealed in immunoprecipitations with Flag antibodies after prolonged chase periods (Figure 1D), suggesting that the N-terminus of CHIF is removed. Since no shift in gel migration of CHIF was detectable on SDS–polyacrylamide gels (Figure 1E), we compared the migration of CHIF expressed in vitro, in oocytes or in renal tissue on high resolution SDS–Tricine gels. In the absence of microsomes, in vitro synthesized N-flag CHIF, wild-type and truncated CHIF showed the expected, progressive reduction in their molecular mass (Figure 3A, lanes 1, 3 and 5). The molecular mass of wild-type CHIF, synthesized in oocytes (lanes 7, 8 and 12) or detected in renal tissue (lane 13), was ∼1.4 kDa lower than that of wild-type CHIF synthesized in vitro in the absence of membranes (lanes 3 and 11). The reduction in size of CHIF probably reflects the elimination of the ∼20 hydrophobic amino acids present at the N-terminus of CHIF (Attali et al., 1995). Indeed, the molecular mass of CHIF synthesized in vivo corresponded to that of a truncated mutant lacking the first 20 amino acids (lanes 5, 9 and 10). Together with the observation that CHIF N-flag synthesized in vitro in the presence of microsomes was recognized by Flag antibodies (lane 2), these results indicate that CHIF has a cleavable signal peptide, which is removed in oocytes and in native tissue but not in the in vitro translation system. In addition to co- or post-translational cleavage, CHIF appears to be subjected to other modifications since it migrated as a double or fuzzy band when it was synthesized in a reticulocyte lysate supplemented with microsomes (lanes 2, 4 and 6), after expression in oocytes (lanes 7, 8 and 12) and in its native form present in renal tissue (lane 13).
Fig. 3. Biosynthesis and processing of CHIF and γa- and γb-subunits in a reticulocyte lysate, in Xenopus oocytes and in renal tissue. (A) Processing of CHIF N-flag, wild-type CHIF and truncated CHIF in which the first 20 N-terminal amino acids were deleted (Δ20). [35S]methionine-labeled CHIF synthesized in a reticulocyte lysate in the absence (lanes 1, 3 and 5) or presence (lanes 2, 4 and 6) of canine pancreatic microsomes, or in oocytes subjected to a 24 h pulse and a 48 h chase period (lanes 7–10) were immunoprecipitated with a CHIF antibody. Lanes 11–13 show western blot analysis of CHIF synthesized in vitro, expressed in oocytes or detected in renal tissue by using a CHIF antibody. (B) Processing of Xenopus γ-subunits and rat γa and γb splice variants. [3H]leucine-labeled γ-subunits synthesized in a reticulocyte lysate in the absence (lanes 2, 5 and 8) or presence (lanes 3, 6 and 9) of pancreatic microsomes, or in oocytes after a 48 h pulse and a 24 h chase period (lanes 1, 4 and 7) were immunopre cipitated with Xenopus or rat γ antibodies. Presented are fluorograms of 16.5% SDS–Tricine gels. Modified CHIF N-flag, CHIF and γ-subunits are indicated by an asterisk. The position of migration of a 6.9 kDa protein marker is indicated. In (A) and (B), a representative example of three similar experiments is shown.
In contrast to CHIF, γa- and γb-subunit variants have no cleavable signal peptide, as suggested by the similar molecular mass of the peptides synthesized in oocytes (Figure 3B, lanes 1, 4 and 7) and in vitro in the absence of microsomes (lanes 2, 5 and 8). In addition, unlike CHIF, only γ variants synthesized in vitro in the presence of microsomes (lanes 3, 6 and 9), but not those synthesized in oocytes (lanes 1, 4 and 7), migrated as a doublet, suggestive of a post-translational modification. As determined by mutational analysis, neither the common FXYD motif nor C-terminal positively charged amino acids were required for the putative modifications of CHIF or γ-subunits (data not shown). Altogether, these data indicate that CHIF and γ-subunits are subjected to distinct co- or post-translational modifications, and CHIF but not γ-subunits are subjected to signal peptide cleavage.
Structural requirements for the stable interaction of γ-subunits and CHIF with NKA
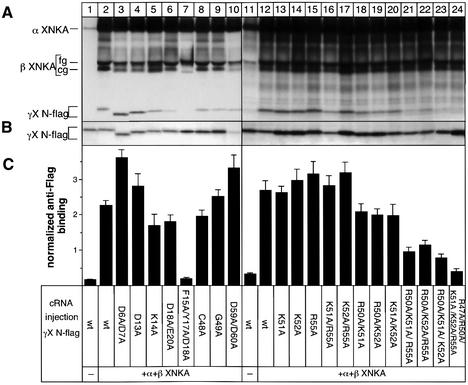
To identify the structural requirements which are necessary for specific, long-term interaction of γ-subunits and CHIF with NKA, we generated a series of γX N-flag (Figure 4) or CHIF (Figure 6A) mutants, expressed them in oocytes together with NKA and tested their association efficiency by anti-Flag radioimmunolabeling of intact oocytes and/or by non-denaturating immunoprecipitations.
Fig. 4. Mutations introduced into γX N-flag. Shown are the amino acid sequences of wild-type γX with the flag epitope at the N-terminus (1) and of γ mutants with alanine substitutions in the N- and C- termini (2–21). A summary of the results shown in Figure 5 on the efficiency of the association of the γ mutants with NKA is given.
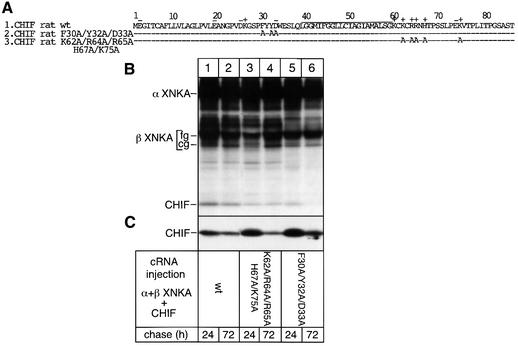
Fig. 6. Effect of CHIF mutations on the efficiency of association with NKA. (A) Amino acid sequence of rat wild-type CHIF and of CHIF mutants with alanine substitutions of N- or C- terminal amino acids. (B) Oocytes were injected with wild-type or mutant CHIF cRNAs (0.5 ng) together with Xenopus NKA α (7 ng) and β (0.5 ng) cRNAs. After injection, oocytes were metabolically labeled for 24 h and subjected to chase periods of 24 and 72 h. Microsomes were prepared and subjected to non-denaturing immunoprecipitations with an NKA α antibody (B) or subjected directly to SDS–PAGE (C). A representative example of three similar experiments is shown.
Wild-type γX N-flag and all mutants were expressed at similar levels in oocytes (Figure 5B). As previously described (Béguin et al., 1997), only NKA-associated γ-subunits (Figure 5A and C, lane 2) but not γ-subunits alone (Figure 5A and C, lane 1) were expressed at the cell surface and could be detected by radioimmunolabeling of intact oocytes. In the extracytoplasmic γ N-terminus, mutations of negatively and positively charged residues had only a slight or no effect on the co-immunoprecipitation of the γ-subunit with NKA (Figure 5A, lanes 3–6) and on anti-Flag γ binding to intact oocytes (Figure 5C, lanes 3–6). On the other hand, mutation of the FXYD protein family motif completely abolished association of the γ-subunit with NKA (Figure 5A and C, lane 7). Introduction of single or multiple mutations into the cytoplasmic γ C-terminus revealed a role for positively charged amino acids (Figure 5A and C, lanes 12–24), but not for cysteine, glycine or negatively charged residues (Figure 5A and B, lanes 8–10) in the efficient association with NKA. Single mutations of K51, K52 or R55 (Figure 5A and C, lanes 13–15) or double mutations of K51 or K52 and R55 (Figure 5A and C, lanes 16 and 17) had no effect on the γ-subunit’s association with NKA. On the other hand, double mutations of R50 and K51 or K52 (Figure 5C, lanes 18 and 19) or of K51 and K52 (lane 20) reduced anti-Flag γ binding by 30–40% compared with oocytes expressing the wild-type γ-subunit (lane 12). The association efficiency of the γ-subunit was reduced further in oocytes expressing γ mutants with triple mutations in R50, K51, K52 and/or R55 (Figure 5A and C, lanes 21–23) and was completely abolished in oocytes expressing the γ mutant R47A/R50A/K51A/K52A/K55A (5A mutant) (Figure 5A and C, lane 24). Correct Ccyt–Nout orientation of the 5A mutant was verified by a proteinase K assay (Geering et al., 1996) on homogenates of oocytes expressing C-terminally myc-tagged wild-type and mutant γ-subunits. Proteinase K-treated C-myc wild-type as well as mutant γ-subunits were recognized by a γ antibody directed against the N-terminus, but no longer by a C-myc antibody, indicating that both wild-type and mutant γ-subunits have a similar membrane orientation permitting cleavage of the cytoplasmically oriented C-terminus (data not shown). Altogether, these data indicate that not only the FXYD motif in the N-terminus of the γ-subunit but also the positively charged residues in the C-terminus play a role in the stable association with NKA.
Fig. 5. Effects of γ mutations on the efficiency of association with NKA. Oocytes were injected with wild-type or mutant γX N-flag cRNA (0.5 ng) together with Xenopus NKA α (7 ng) and β (0.5 ng) cRNAs. After injection, oocytes were metabolically labeled for 24 h and subjected to a 48 h chase period. Microsomes were prepared and immunoprecipitations were performed under non-denaturing conditions with an NKA α antibody (A) or with a Flag antibody (B). Mutations of negatively charged residues in the γ N-terminus produced abnormal gel migration (lanes 3 and 4). (C) Normalized anti-Flag binding to γX N-flag in intact oocytes. Anti-Flag binding was performed 3 days after cRNA injection and normalized for differences in cell surface expression of Na,K-pumps in different oocytes, as assessed by maximal pump current measurement. Shown are the means ± SE of results obtained from 20–30 oocytes. For a description of the mutants, see Figure 4.
CHIF mutants analogous to the γ mutants (Figure 6A) were prepared and tested for their ability to interact stably with NKA by co-immunoprecipitation experiments. In contrast to analogous γ mutants, the CHIF 5A mutant (lane 3) and the FXYD mutant (lane 5) were associated with NKA after a 24 h chase period. Interaction was maintained over a 72 h chase period with the C-terminal 5A mutant (lane 4) but not with the N-terminal FXYD mutant (lane 6). Thus, CHIF and γ-subunits may not have the same structural requirements for NKA interaction, but the FXYD motif appears to be important in both CHIF and the γ-subunit for long-term, stable NKA interactions.
Modulation of NKA function by CHIF and γ splice variants
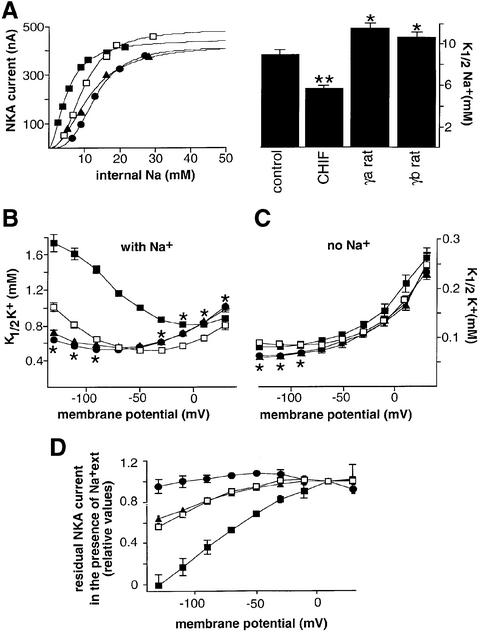
The potential functional role of CHIF was assessed and compared with that of the two rat γ splice variants by electrophysiological measurements of NKA transport functions in Xenopus oocytes. We first tested whether the apparent Na+ affinity (K1/2Na+) of NKA is modified in the presence of CHIF, γa- or γb-subunits (Figure 7A). Whereas the maximal Na,K-pump current did not change (see Table I), CHIF significantly increased, and both γ splice variants reduced, the Na,K-pump’s apparent Na+-affinity compared with that of NKA expressed alone. Significantly, at physiologically low intracellular Na+ concentrations (e.g. 5 mM), the Na,K-pump transport activity was ∼4 times higher for NKA associated with CHIF than for NKA lacking CHIF or for NKA associated with γ splice variants (Figure 7A).
Fig. 7. CHIF modulates NKA in a manner different from the two γ splice variants. Oocytes were injected with rat CHIF, γa or γb (1.5 ng) cRNAs together with rat NKA α1 (9 ng) and β1 (1.3 ng) cRNAs and incubated for 3 days. For the recording of the apparent Na+ affinity, cRNAs of the rat epithelial Na+ channel subunits (α, β, γ, 0.3 ng each) were also injected. (A) On the left, representative examples of Na+ activation curves of Na,K-pump currents, determined in oocytes expressing NKA alone (open squares) or together with CHIF (closed squares), γa (closed circles) or γb (closed triangles), are shown. The curves were fitted with a floating Hill coefficient. On the right, apparent Na+ affinities (K1/2Na+) of NKA alone (control), or expressed with CHIF, γa or γb are shown using a fixed Hill coefficient of 2.25 (see Material and methods). *P <0.05, **P <0.01 compared with control. (B and C) Voltage dependence of external K+ activation (K1/2K+) of Na,K-pumps in the absence and presence of 90 mM external Na+. The parameters of the Hill equation were fitted to the steady-state currents induced by increasing concentrations of K+ (see Materials and methods). Symbols as in (A). *P <0.05 between NKA alone and NKA associated with γa- or γb-subunits. For clarity, the asterisk is omitted for the CHIF effect (B). (D) Sensitivity of NKA associated with CHIF, γa or γb to external Na+. K-activated and ouabain-sensitive Na,K-pump currents were determined at different membrane potentials. Shown are the residual NKA currents measured in the presence of 90 mM external Na+ compared with those measured in the absence of external Na+ and arbitarily set to 1 at 10 mV. For all recordings, data are means ± SE of 30–40 oocytes from 4–8 different batches.
Table I. NKA properties in the presence of CHIF, γa or γb.
| α1 +α2 (NKA) | Maximal pump current (nA) | K1/2Na+ (mM) |
K1/2K+ (mM) |
Ouabain (Kd) (nM) | |
|---|---|---|---|---|---|
| With Na+ | Without Na+ | ||||
| NKA | 511 ± 44 | 9.0 ± 0.5 | 0.52 ± 0.01 | 0.096 ± 0.006 | 8.1 ± 0.5 |
| NKA + CHIF | 440 ± 24 | 5.8 ± 0.3* | 1.00 ± 0.02* | 0.105 ± 0.005 | 7.6 ± 0.3 |
| NKA + γa | 371 ± 20 | 11.6 ± 0.6* | 0.55 ± 0.01 | 0.092 ± 0.004 | 7.6 ± 0.4 |
| NKA + γb | 422 ± 15 | 10.6 ± 0.4* | 0.56 ± 0.01 | 0.085 + 0.005 | 7.3 ± 0.7 |
*Data are significantly different from those for NKA (P <0.05).
We next compared the influence of CHIF and γ-subunits on the activation or inhibition of the Na,K-pump activity by extracellular K+ or Na+. In the absence of extracellular Na+, a situation in which the K+ activation kinetics most directly reflect the intrinsic affinity of the extracellular binding site for K+, CHIF had no detectable effect on the activation of the Na,K-pump current by extracellular K+ (K1/2K+), while γa- and γb-subunits induced a 1.5- to 2-fold increase in the high negative membrane potential range (Figure 7C). The results obtained in this study with γa and γb differ from our previous findings, which showed that rat γ-subunits increased the K1/2K+ over the whole potential range in the absence of Na+ (Béguin et al., 1997). The discrepancy may be explained by the previous use of a rat γ-subunit containing a wrong N-terminal sequence initially published by Mercer et al. (1993) and later corrected by the same group (Minor et al., 1998).
The effects of CHIF and γ-subunits on the K+ activation of Na,K-pumps were very different in the presence of extracellular Na+ (Figure 7B), a condition in which the kinetics of activation by K+ depend not only on the intrinsic affinity for K+ but also on the competition between extracellular Na+ and K+ ions for binding to the E2 conformation of the enzyme. In the presence of extracellular Na+, the presence of CHIF induced a large increase in K1/2K+ in the negative potential range. The presence of γa- or γb-subunits resulted in changes that were similar between these two variants but opposite to those observed with CHIF. With γa or γb, the K1/2K+ values were larger in the +30 to –30 membrane potential range and smaller in the high negative range.
Altogether, these results suggest that the main effect of CHIF and γ-subunits is to influence the transport properties of NKA by affecting steps involved in Na+ transport. This seems to be the only effect of CHIF, while γa and γb have a small additional effect on K+ transport. The influence of CHIF and γ-subunits on the Na+ translocating steps was substantiated further by the observation that CHIF-associated NKA exhibited a higher sensitivity to the voltage-dependent inhibition by extracellular Na+, leading to an important inhibition of the Na,K-pump current in the presence of Na+ at high negative membrane potentials (Figure 7D). On the other hand, NKA associated with the γa-subunit was insensitive to the presence of external Na+, whereas NKA associated with γb had a similar sensitivity to that of NKA expressed alone (Figure 7D). A summary of the functional effects of CHIF and the two γ variants on the transport properties of NKA at physiological resting potentials (–50 mV) is shown in Table I.
Since the γ-subunit was shown to bind photoaffinity-labeled ouabain derivatives (Forbush et al., 1978), we finally investigated the intrinsic ouabain affinity of human α1–β1 complexes associated with rat CHIF or with the two human γ splice variants. No difference in Kd values for ouabain was observed for either of the complexes (see Table I).
Structural requirements in CHIF for the modulatory effect on NKA
To gain insight into the structural requirements for the functional effects of CHIF on NKA, we studied the transport properties and the cell surface expression of NKA expressed in oocytes with the 5A mutant. Oocytes expressing NKA alone or together with wild-type or mutant CHIF showed comparable levels of NKA at the cell surface as assessed by anti-Flag radioimmunolabeling of the NKA β-subunit (Figure 8B, lanes 1–3). On the other hand, whereas NKA associated with CHIF had similar maximal Na,K-pump currents and exhibited the expected decrease in its K+ affinity, NKA associated with the 5A mutant showed a drastic reduction in the Na,K-pump current (Figure 8A, lane 3) and an increase in the K+ affinity (Figure 8C, compare lanes 2 and 3). Furthermore, ouabain binding to oocytes expressing NKA with the 5A mutant was significantly reduced compared with oocytes expressing wild-type CHIF (NKA–wild-type CHIF, 61 ± 6 fmol/oocyte; NKA–5A mutant, 12 ± 1 fmol/oocyte; n = 20, P <0.01). These results indicate that the positively charged amino acids in the cytoplasmic C-terminus of CHIF are important determinants for the functional effect of CHIF on NKA and that their elimination produces dominant CHIF mutants which inhibit NKA function.
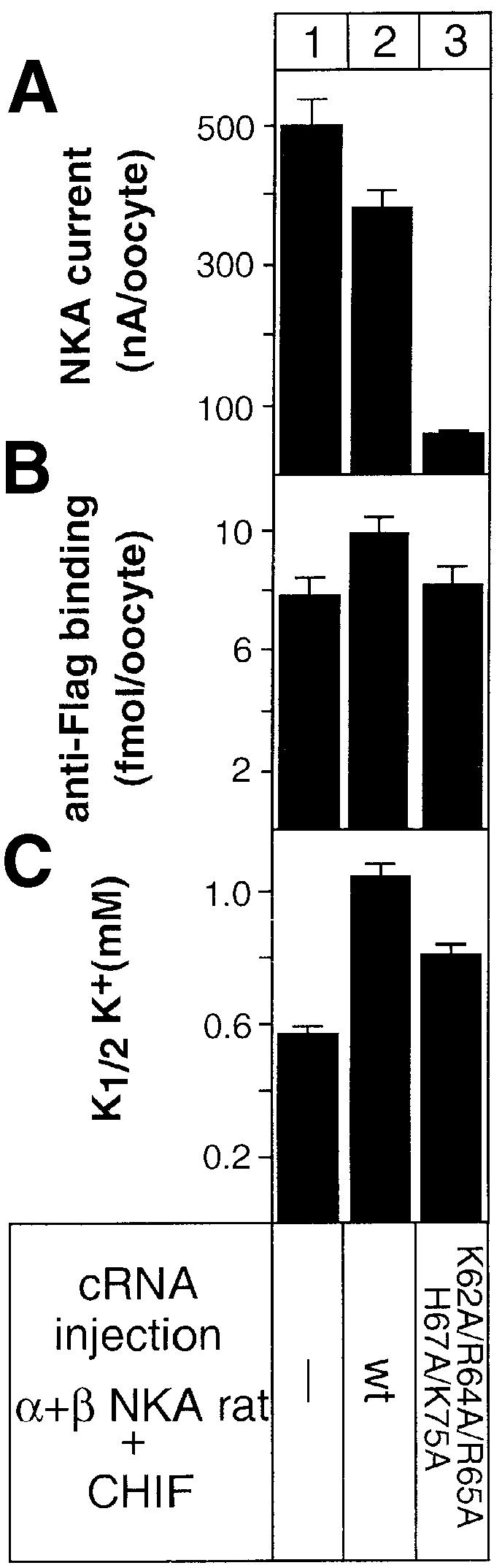
Fig. 8. Effect of mutations in CHIF on the modulation of the NKA activity. Oocytes were injected with wild-type or mutant CHIF cRNA (1.5 ng) together with rat NKA α (9 ng) and β (1.3 ng) (A and C) or together with rat NKA α (9 ng) and Xenopus Flag epitope-tagged β cRNA (1.5 ng) (B). Maximal pump currents (A) and apparent K+ affinities (K1/2K+) (C) were recorded at –50 mV. Cell surface expression of NKA associated with wild-type and mutant CHIF was probed by radioimmunolabeling of intact oocytes with a Flag antibody (B). Data are means ± SE from 10–15 oocytes.
Discussion
CHIF is a small transmembrane protein with a so far unknown function which, together with the γ-subunit of NKA, belongs to the FXYD protein family (Sweadner and Rael, 2000). In the present study, we characterized the co- and post-translational processing of CHIF, and demonstrated its specific association with NKA and the effects of this association on several functional characteristics of this ion pump. Taken together with data showing tissue-specific and regulated expression of CHIF, our data identify CHIF as a bona fide NKA regulatory subunit, analogous to but clearly different from the γ-subunit.
CHIF undergoes signal peptide cleavage and is subjected to post-translational modifications
Certain FXYD proteins, such as phospholemman, RIC and CHIF, but not γ-subunits or FXYD 7, have a hydrophobic domain at the N-terminus suggesting the presence of a putative signal peptide. For phosholemman, it was shown that the signal peptide is cleaved resulting in an Nout–Ccyt orientation of the peptide in the membrane (Palmer et al., 1991) similar to that demonstrated for the γ-subunit lacking a signal peptide (Béguin et al., 1997; this study). Recently, it was reported that the putative signal peptide of CHIF, synthesized in a reticulocyte lysate supplemented with microsomes, was not removed, resulting in a hypothetical membrane topology of CHIF with two membrane segments and the N- and C-termini exposed to the cytoplasmic side (Shi et al., 2001). In this study, we demonstrate that the signal peptide of CHIF is indeed not removed during in vitro synthesis, but is cleaved in oocytes and in native renal tissue permitting a type I membrane topology of CHIF characteristic of other members of the FXYD protein family. Interestingly, cleavage of the signal peptide of CHIF, expressed in oocytes, is a slow process, which may explain the lack of signal peptide cleavage in the in vitro translation system where the time and location for processing are limited. It is not known whether signal peptide cleavage of CHIF occurs during synthesis by a conventional signal peptidase, or rather post-translationally in the endoplasmic reticulum or another cellular compartment by a so far unknown cleavage enzyme.
Similarly to the γ-subunit, native CHIF detected in renal tissue migrates as a doublet on SDS–Tricine gels, indicative of co- or post-translational modifications. In γ-subunits, tissue-specific modifications may be important in the modulatory effect of the γ-subunit on NKA (Arystarkhova et al., 1999). The nature and the functional effect of CHIF modifications remain to be determined, but it is likely that they are different from those in γ-subunits since CHIF but not γ-subunits undergoes modifications in Xenopus oocytes.
CHIF is a tissue-specific subunit of NKA similar to the γ-subunit
In the present study, we show that CHIF interacts specifically with NKA after co-expression in Xenopus oocytes and is associated with NKA in renal tissue, similarly to the γ-subunit. The notion that CHIF is a real subunit of NKA is supported further by its co-localization with NKA in the basolateral membrane of renal CD principal cells and of distal colon surface cells (Shi et al., 2001) and by its ability to co-immunoprecipitate with NKA in colon membranes (Garty et al., 2001) but not with colonic HKA (this study).
The structural requirements for stable NKA interaction may not be identical in CHIF and the γ-subunit but, in both, the family-specific FXYD motif located in the extracytoplasmic N-terminus appears to play a role in the stable association with NKA. This result, together with the observation that a phospholemman-like protein also co-purifies with shark rectal gland NKA (Mahmmoud et al., 2000), raises the possibility that other members of the FXYD protein family could interact with NKA.
The structural determinants which permit the specific interaction of CHIF and γ-subunits with NKA but not with other P-type ATPases are not known. Possible interaction sites may be located in the C-terminal domain of the NKA α-subunit encompassing M8–M10, which is the least conserved domain in different P-type ATPases and which was shown to be released selectively from the membrane, together with the γ-subunit, upon thermal denaturation (Donnet et al., 2001).
The functional effects of CHIF on NKA are distinct from those of γ-subunits
Comparative studies on the effects of CHIF and the γ splice variants on the transport properties of NKA in intact cells reveal that CHIF modulates NKA function in a different manner from the γ-subunit, and that the two γ splice variants have similar modulatory effects on the NKA transport function.
Electrophysiological measurements of the Na,K-pump current show that association of CHIF with NKA increases the apparent affinity for both intra- and extracellular Na+ binding. This suggests that CHIF association produces a modification of the Na+-binding site or an increase in the stability of an Na+ occlusion conformation. The increase in the affinity for extracellular Na+ also results in a stronger voltage-dependent competition between external Na+ and K+ ions for their binding sites on the E2 conformation of the enzyme and, in consequence, in a voltage-dependent decrease in the apparent affinity for extracellular K+.
The functional effects of CHIF may be mediated at least partially by the cytoplasmic domain of CHIF via charged amino acid residues. A CHIF mutant, lacking most of the C-terminal positively charged amino acids, indeed associates with NKA, but the NKA–CHIF complex has a low turnover and loses its ability to bind ouabain, suggesting that it is strongly shifted to the E1 conformation.
In contrast to CHIF, γ-subunits decrease the apparent affinity for intracellular Na+ and increase the apparent affinity for external K+ at high negative membrane potentials, independently of external Na+. On the other hand, at less negative membrane potential, γ-subunits decrease the apparent K+ affinity and this effect is observed only in the presence of external Na+, suggesting a shift in the NKA E1–E2 conformational equilibrium towards E1. The possibility that γ-subunits could have several concomitant and independent effects on NKA function is supported by other published data. It was indeed reported that γ-subunits mediate a parallel decrease in the Na+ and K+ activation of ATPase activity (Arystarkhova et al., 1999). Also, γ-subunits not only increase the ATP sensitivity of NKA, which is consistent with a shift towards the E1 conformation (Therien et al., 1997, 1999), but also increase the K+ antagonism of intracellular Na+ binding, which suggests an additional effect of γ-subunits on intrinsic binding of K+ at cytoplasmic sites (Pu et al., 2001).
In agreement with a recent report (Pu et al., 2001) that showed that both γ splice variants affected the catalytic properties of NKA without a detectable difference, our results indicate that γa and γb also have similar effects on the transport function of NKA. Although we observed some discrete differences in the effects of γa and γb on the sensitivity of the NKA transport activity to external Na+, the physiological relevance of this observation remains unclear.
The physiological relevance of CHIF and γ-subunits as modulators of NKA
CHIF is expressed mainly in kidney and colon (Attali et al., 1995) and γ-subunits in kidney (Mercer et al., 1993; Béguin et al., 1997). Interestingly, in the kidney nephron, CHIF is expressed exclusively in the CD (Shi et al., 2001) and γ-subunits in the TAL, in the macula densa and in the CCD (Pu et al., 2001). This nearly exclusive expression of CHIF and γ-subunits in different segments of the renal tubule suggests that the two peptides may have functionally distinct effects adapted to nephron segment-specific physiological needs.
A physiologically, particularly relevant effect of CHIF and γ-subunits described in this study concerns the modulation of the Na+ affinity of NKA. In this context, it is interesting to note that NKA shows different Na+ affinities in different nephron segments (Barlet-Bas et al., 1990). In the CCD, the K1/2Na+ is 3 mM while, in the proximal convoluted tubule and the cTAL, the K1/2Na+ is ∼10 mM. Since isozymes other than α1–β1 NKA isozymes have not been detected in the kidney, which could explain the different NKA Na+ affinities, it is tempting to speculate that the selective presence of auxiliary subunits such as CHIF and γ-subunits determines the Na+ affinity of NKA along the nephron. This conclusion is supported by the good correlation between (i) the Na+ affinity of NKA measured in situ in different nephron segments and that determined for NKA expressed in oocytes in the presence of CHIF or γ-subunits and (ii) the Na+ affinity of CHIF- or γ-subunit-associated NKA and the distribution of CHIF and γ-subunits along the nephron.
In view of the crucial importance of NKA in the reabsorptive and secretory capacity of the kidney, small changes in its ion affinities, as observed in γ-subunit- or CHIF-associated NKA, may be necessary and sufficient for a fine regulation of the transepithelial ion and fluid transport. Association of NKA with γ-subunits may indeed be favorable in nephron segments, such as the cTAL, which show a high rate of Na+ reabsorption that requires a high density of NKA, preferentially with a reduced Na+ affinity, to cope with and to extrude efficiently the important cellular Na+ load. In contrast, association of NKA with CHIF, which increases its Na+ affinity, may be useful in principal cells of nephron segments such as the CD or in surface cells of the colon which are ultimate sites for fluid and electrolyte conservation. The CCD, for example, plays a major role in K+ homeostasis and is the target for mineralocorticoid hormones which regulate Na+ reabsorption when Na+ conservation is required (Féraille and Doucet, 2001). Under conditions of Na+ depletion, luminal Na+ in the CD is decreased to very low values and the presence of a Na,K-pump with a high Na+-affinity will maintain low intracellular Na+ concentrations and a high negative apical membrane potential favoring efficient Na+ reabsorption. Moreover, small changes in the intracellular Na+ concentration, in the range of physiologically low Na+ concentrations, due to early actions of aldosterone on apical Na+ entry, would considerably improve Na+ reabsorption in the presence of Na,K-pumps associated with CHIF. Finally, it is noteworthy that CHIF expression is increased by a low Na+ diet (and hence increased aldosterone secretion) (Shi et al., 2001) permitting association with Na,K-pump units, newly synthesized during the late phase of aldosterone action. Since K+ secretion in the distal nephron is tightly coupled to Na+ reabsorption and NKA activity (Giebisch, 1998), association of CHIF with NKA in the CD may also be favorable to K+ secretion in conditions of a high K+, mineralocorticoid-activated state.
In summary, our data indicate that CHIF is a type I membrane protein which interacts specifically with NKA and modulates its transport properties in the opposite way to γ-subunits by increasing its apparent Na+ affinity. It is likely that this regulatory mechanism of NKA plays a crucial role in aldosterone-responsive tissues responsible for the maintenance of body Na+ and K+ homeostasis.
Materials and methods
Site-directed mutagenesis
Point mutations were introduced into the N flag epitope-tagged Xenopus γ-subunit cDNA (Béguin et al., 1997) and into rat CHIF cDNA by the PCR-based method. The mutations were confirmed by DNA sequencing. Rat CHIF was epitope tagged using the same procedure as previously described (Béguin et al., 1997). Human γa was purchased from IMAGE Consortium (No. 2814684). Rat and human γb were generated by PCR using γa cDNA as a template. DNAs of Xenopus NKA α1 and β1, rat NKA α1 and β1 (kindly provided by J.Lingrel), rat colonic HKA α (kindly provided by G.Shull), Bufo marinus bladder β-subunits (kindly provided by F.Jaisser), rat CHIF (Attali et al., 1995) and of rat and human γa and γb were subcloned into the pSD5 vector.
Expression of cRNAs in Xenopus oocytes
Stage V–VI oocytes were obtained from Xenopus laevis as described previously (Geering et al., 1996). In vitro synthesized cRNAs, encoding Xenopus or rat NKA α-, β- and γ-subunits, rat colonic HKA α-subunits, Bufo bladder β-subunits, rat CHIF or rat epithelial Na+ channel α-, β- and γ-subunits (gift from B.C.Rossier), were injected into oocytes in different combinations as described in the Figure legends. To study protein expression and association, oocytes were incubated in modified Barth’s medium (MBS) containing either 0.6 mCi/ml [35S]methionine or 1 mCi/ml [3,4,5H]leucine for 24 h and subjected to 24–120 h chase periods in MBS containing 10 mM cold methionine or leucine. After various pulse–chase periods, digitonin extracts or microsomal fractions were prepared as described (Geering et al., 1996).
Immunoprecipitation and western blot analysis
Previously characterized antibodies were used to immunoprecipitate rat and Xenopus NKA α-subunits (Béguin et al., 1997), Xenopus and rat γ-subunits (Béguin et al., 1997), CHIF (Shi et al., 2001) and colonic HKA α-subunits (gift from F.Jaisser) under denaturing or non-denaturing conditions (Geering et al., 1996). Immunopreciptates were resolved on SDS–polyacrylamide gels or SDS–Tricine gels (prepared according to the manufacturer’s instructions) and revealed by fluorography. Western blot analysis was performed as previously described (Shi et al., 2001).
Preparation of kidney microsomes
Medulla and papilla portions were dissected from rat kidney, cut into small pieces and homogenized with a Teflon–glass homogenizer in a buffer containing 30 mM dl-histidine, 5 mM EDTA, 250 mM sucrose, 200 mM phenylmethylsulfonyl fluoride (PMSF), 180 mM Tris–HCl, pH 7.4. Microsomes were prepared by differential centrifugation, freeze–thawed twice and sonicated for 10 s. Microsomes were solubilized for 5 h at 4°C in a buffer containing 0.5% CHAPS, 20 mM Tris–HCl pH 7.4, 100 mM NaCl, 1 mM PMSF and 5 µg/ml each of leupeptin, pepstatin and antipain.
In vitro translation
In vitro translation of CHIF, Xenopus and rat γ-subunits, in the presence or absence of canine pancreatic microsomal membrane, was performed according to the manufacturer’s instructions (Promega).
Radioimmunolabeling of Xenopus oocytes and [3H]ouabain binding
Radioimmunolabeling was performed as previously described (Béguin et al., 1997) on intact oocytes expressing either epitope-flagged Xenopus γ- or β-subunits. [3H]ouabain binding on intact oocytes and on oocyte microsomes was performed as previously described (Crambert et al., 2000).
Electrophysiology
Electrophysiological measurements were performed 3 days after cRNA injection of ouabain-resistant, rat NKA by using the two-electrode voltage clamp technique in the presence of 1 µM ouabain, which inhibits endogenous, oocyte Na,K-pumps. To determine the apparent K+ affinity, oocytes were loaded with Na+ as described (Jaisser et al., 1994). The kinetics of Na,K-pump current activation by K+ were examined over a –130 to +30 mV potential range with a series of ten 200 ms voltage steps in the presence or absence of external Na+ as previously described (Béguin et al., 1997). These rapid voltage steps prevented the activation of K+-induced currents by CHIF in the positive potential range (Attali et al., 1995). The maximal current (Imax) and the apparent K+ affinity (K1/2K+) at different potentials were obtained by fitting the Hill equation to the data. Hill coefficients of 1.6 and 1.0 were used for experiments in the presence or absence of external Na+, respectively (Jaisser et al., 1994).
Measurements of the apparent Na+ affinity of NKA in intact cells were performed as described previously by co-expressing rat NKA α1 and β cRNAs together with rat epithelial Na+ channel α, β and γ cRNAs (Hasler et al., 1998). The Hill equation was fitted initially to the experimental data by using a floating Hill coefficient which ranged between 1.8 and 2.7. The maximal current (Imax) and the apparent Na+ affinity (K1/2 Na+) were then obtained by using the Hill equation with a fixed Hill coefficient of 2.25.
Statistical analysis was performed by paired and unpaired Student’s t-tests and the results are expressed as means ± SE.
Acknowledgments
Acknowledgements
We thank Sophie Roy and Danièle Schaer for technical assistance, F.Jaisser for the HKA α-antibody, and B.C.Rossier, G.Shull, G.Sachs and J.Lingrel for cDNA probes. This work was supported by grants from the Swiss National Fund for Scientific Research No. 31-53721.98 and from the Roche Research Foundation.
References
- Arystarkhova E., Wetzel,R.K., Asinovski,N.K. and Sweadner,K.J. (1999) The γ subunit modulates Na(+) and K(+) affinity of the renal Na,K-ATPase. J. Biol. Chem., 274, 33183–33185. [DOI] [PubMed] [Google Scholar]
- Attali B., Latter,H., Rachamim,N. and Garty,H. (1995) A corticosteroid-induced gene expressing an ‘IsK-like’ K+ channel activity in Xenopus oocytes. Proc. Natl Acad. Sci. USA, 92, 6092–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlet-Bas C., Cheval,L., Khadouri,C., Marsy,S. and Doucet,A. (1990) Differences in the Na affinity of Na+-K+-ATPase along the rabbit nephron: modulation by K. Am. J. Physiol., 259, F246–F250. [DOI] [PubMed] [Google Scholar]
- Béguin P., Wang,X.Y., Firsov,D., Puoti,A., Claeys,D., Horisberger,J.D. and Geering,K. (1997) The γ subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J., 16, 4250–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F.E. and Fuller,P.J. (1999) Acute regulation by corticosteroids of channel-inducing factor gene messenger ribonucleic acid in the distal colon. Endocrinology, 140, 1213–1218. [DOI] [PubMed] [Google Scholar]
- Capurro C., Coutry,N., Bonvalet,J.P., Escoubet,B., Garty,H. and Farman,N. (1996) Cellular localization and regulation of CHIF in kidney and colon. Am. J. Physiol., 40, C753–C762. [DOI] [PubMed] [Google Scholar]
- Crambert G., Hasler,U., Beggah,A.T., Yu,C., Modyanov,N.N., Horisberger,J.D., Lelievre,L. and Geering,K. (2000) Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. J. Biol. Chem., 275, 1976–1986. [DOI] [PubMed] [Google Scholar]
- Donnet C., Arystarkhova,E. and Sweadner,K.J. (2001) Thermal denaturation of the Na,K-ATPase provides evidence for α–α oligomeric interaction and γ subunit association with the C-terminal domain. J. Biol. Chem., 276, 7357–7365. [DOI] [PubMed] [Google Scholar]
- Féraille E. and Doucet,A. (2001) Sodium–potassium-adenosinetriphos phatase-dependent sodium transport in the kidney: hormonal control. Physiol. Rev., 81, 345–418. [DOI] [PubMed] [Google Scholar]
- Forbush B. III, Kaplan,J.H. and Hoffman,J.F. (1978) Characterization of a new photoaffinity derivative of ouabain: labeling of the large polypeptide and of a proteolipid component of the Na,K-ATPase. Biochemistry, 17, 3667–3676. [DOI] [PubMed] [Google Scholar]
- Fu X. and Kamps,M. (1997) E2a-Pbx1 induces aberrant expression of tissue-specific and developmentally regulated genes when expressed in NIH 3T3 fibroblasts. Mol. Cell. Biol., 17, 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H., Cluzeaud,F., Farman,N. and Karlish,S.J.D. (2001) A tissue-specific interaction between Na,K-ATPase and CHIF. Biophys. J., 80, 501a. [Google Scholar]
- Geering K. (2000) Topogenic motifs in P-type ATPases. J. Membr. Biol., 174, 181–190. [DOI] [PubMed] [Google Scholar]
- Geering K., Beggah,A., Good,P., Girardet,S., Roy,S., Schaer,D. and Jaunin,P. (1996) Oligomerization and maturation of Na,K-ATPase: functional interaction of the cytoplasmic NH2 terminus of the β subunit with the α subunit. J. Cell Biol., 133, 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebisch G. (1998) Renal potassium transport: mechanisms and regulation. Am. J. Physiol., 274, F817–F833. [DOI] [PubMed] [Google Scholar]
- Hasler U., Wang,X., Crambert,G., Beguin,P., Jaisser,F., Horisberger,J.D. and Geering,K. (1998) Role of β-subunit domains in the assembly, stable expression, intracellular routing and functional properties of Na,K-ATPase. J. Biol. Chem., 273, 30826–30835. [DOI] [PubMed] [Google Scholar]
- Jaisser F. and Beggah,A.T. (1999) The nongastric H+-K+-ATPases: molecular and functional properties. Am. J. Physiol., 276, F812–F824. [DOI] [PubMed] [Google Scholar]
- Jaisser F., Jaunin,P., Geering,K., Rossier,B.C. and Horisberger,J.D. (1994) Modulation of the Na,K-pump function by β subunit isoforms. J. Gen. Physiol., 103, 605–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.H., Davies,T.C. and Kidder,G.M. (1997) Embryonic expression of the putative γ subunit of the sodium pump is required for acquisition of fluid transport capacity during mouse blastocyst development. J. Cell Biol., 139, 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küster B., Shainskaya,A., Pu,H.X., Goldshleger,R., Blostein,R., Mann,M. and Karlish,S.J. (2000) A new variant of the γ subunit of renal Na,K-ATPase. Identification by mass spectrometry, antibody binding and expression in cultured cells. J. Biol. Chem., 275, 18441–18446. [DOI] [PubMed] [Google Scholar]
- Mahmmoud Y.A., Vorum,H. and Cornelius,F. (2000) Identification of a phospholemman-like protein from shark rectal glands. Evidence for indirect regulation of Na,K-ATPase by protein kinase C via a novel member of the FXYD family. J. Biol. Chem., 275, 35969–35977. [DOI] [PubMed] [Google Scholar]
- Meij I.C. et al. (2000) Dominant isolated renal magnesium loss is caused by misrouting of the Na(+),K(+)-ATPase γ subunit. Nature Genet., 26, 265–266. [DOI] [PubMed] [Google Scholar]
- Mercer R.W., Biemesderfer,D., Bliss,D.P., Collins,J.H. and Forbush,B.,III (1993) Molecular cloning and immunological characterization of the γ polypeptide, a small protein associated with the Na, K-ATPase. J. Cell Biol., 121, 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor N.T., Sha,Q., Nichols,C.G. and Mercer,R.W. (1998) The γ subunit of the Na,K-ATPase induces cation channel activity. Proc. Natl Acad. Sci. USA, 95, 6521–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman J.R. et al. (1995) Unitary anion currents through phospho lemman channel molecules. Nature, 377, 737–740. [DOI] [PubMed] [Google Scholar]
- Morrison B.W., Moorman,J.R., Kowdley,G.C., Kobayashi,Y.M., Jones,L.R. and Leder,P. (1995) Mat-8, a novel phospholemman-like protein expressed in human breast tumors, induces a chloride conductance in Xenopus oocytes. J. Biol. Chem., 270, 2176–2182. [DOI] [PubMed] [Google Scholar]
- Palmer C.J., Scott,B.T. and Jones,L.R. (1991) Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J. Biol. Chem., 266, 11126–11130. [PubMed] [Google Scholar]
- Pu H.X., Cluzeaud,F., Goldshlegger,R., Karlish,S.J.D., Farman,N. and Blostein,R. (2001) Functional role and immunocytochemical localization of the γa and γb forms of the Na,K-ATPase γ subunit. J. Biol. Chem., in press. [DOI] [PubMed] [Google Scholar]
- Shi H., Levy-Holzman,R., Cluzeaud,F., Farman,N. and Garty,H. (2001) Membrane topology and immunolocalization of CHIF in kidney and intestine. Am. J. Physiol., 280, F505–F512. [DOI] [PubMed] [Google Scholar]
- Simmerman H.K. and Jones,L.R. (1998) Phospholamban: protein structure, mechanism of action and role in cardiac function. Physiol. Rev., 78, 921–947. [DOI] [PubMed] [Google Scholar]
- Suessbrich H. and Busch,A.E. (1999) The IKs channel: coassembly of IsK (minK) and KvLQT1 proteins. Rev. Physiol. Biochem. Pharmacol., 137, 191–226. [DOI] [PubMed] [Google Scholar]
- Sweadner K.J. and Rael,E. (2000) The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence and expression. Genomics, 68, 41–56. [DOI] [PubMed] [Google Scholar]
- Therien A.G., Goldshleger,R., Karlish,S.J.D. and Blostein,R. (1997) Tissue-specific distribution and modulatory role of the γ subunit of the Na,K-ATPase. J. Biol. Chem., 272, 32628–32634. [DOI] [PubMed] [Google Scholar]
- Therien A.G., Karlish,S.J. and Blostein,R. (1999) Expression and functional role of the γ subunit of the Na,K-ATPase in mammalian cells. J. Biol. Chem., 274, 12252–12256. [DOI] [PubMed] [Google Scholar]
- Wald H., Popvtzer,M.M. and Garty,H. (1997) Differential regulation of CHIF mRNA by potassium intake and aldosterone. Am. J. Physiol. 272, F617–F623. [DOI] [PubMed] [Google Scholar]