Abstract
In vitro homotypic fusion of yeast vacuoles occurs in three stages: priming, the Sec18 (NSF)-mediated changes that precede vacuole association; docking, the Ypt7 and SNARE-mediated pairing of vacuoles; and fusion, mediated by calmodulin/V0/t-SNARE interactions. Defects in catalysts of each stage result in fragmented (unfused) vacuoles. Strains with deletions in any of ERG genes 3–6, lacking normal ergosterol biosynthesis, have fragmented vacuoles. The ergosterol ligands filipin, nystatin and amphotericin B block the in vitro fusion of vacuoles from wild-type cells. Each of these inhibitors acts at the priming stage to inhibit Sec17p release from vacuoles. A reversible delay in Sec18p action prevents vacuoles from acquiring resistance to any of these three drugs, confirming that their action is on the normal fusion pathway. Ergosterol or cholesterol delivery to wild-type vacuoles stimulates their in vitro fusion, and the in vitro fusion of ergΔ vacuoles requires added sterol. The need for ergosterol for vacuole priming underscores the role of lipids in organizing the membrane elements of this complex reaction.
Keywords: ergosterol/membrane fusion/Sec17p/Sec18p/vacuoles
Introduction
The compartmentalization of eukaryotic cells is achieved through selective protein translocation into organelles, followed by a dynamic sorting among organelles during vesicular traffic. The fidelity of this traffic, and the low copy number of organelles, depend on accurate docking between membranes, and regulated fusion. This complex process is of basic importance to organelle identity, cell growth, neural transmission and protein secretion. Studies of membrane fusion, employing many organelles and organisms, have revealed common catalytic factors. These include SNAREs (Gerst, 1999), which are integral membrane proteins that can associate with each other through coiled-coil domains; Sec1p and homologs, which associate with specific SNAREs (Misura et al., 2000); Sec18p (NSF) and Sec17p (α-SNAP) chaperones, which disassemble SNARE complexes (Fasshauer et al., 1997); Rab-family GTPases and their effectors (Chavrier and Goud, 1999), which include nucleotide exchange factors and GTPase activating proteins; regulated channels for Ca2+ flux (Bennett, 1997); calcium signal effector proteins such as calmodulin (Peters and Mayer, 1998) and synaptotagmin (Desai et al., 2000); and phosphoinositol polyphosphatides (Huijbregts et al., 2000). The relationships between these factors, and how they work together to promote selective membrane fusion, are receiving intense study.
We are studying the homotypic fusion of yeast vacuoles, which requires all of the above elements (Wickner and Haas, 2000). The study of vacuole fusion is aided by the excellent state of knowledge of Saccharomyces cerevisiae genetics and genomics, by the genetic and biochemical definition of the Vps proteins (Rothman and Stevens, 1986; Banta et al., 1988), which catalyze protein delivery to the vacuole and also, in some cases, vacuole homotypic fusion, and by a rapid and colorimetric in vitro assay of vacuole fusion (Haas et al., 1994). Fusion occurs in three stages: (i) priming (the reactions that precede functional association of vacuoles), (ii) docking, which is productive vacuole association for (iii) fusion, which culminates in the mixing of luminal aqueous compartments and membrane constituents. During priming, salt, Sec18p, Sec17p and ATP disassemble complexes of SNAREs (Ungermann et al., 1998a) in cis (on the same vacuole), and alter the associations of a multisubunit HOPS (homotypic fusion and vacuole protein sorting) complex (Price et al., 2000; Sato et al., 2000; Seals et al., 2000). Priming and docking require phosphatidylinositol-4-phosphate and -4,5-bisphosphate (Mayer et al., 2000). Docking is initiated through reversible tethering by a functional complex of HOPS and Vam7p (a homolog of the neural SNAP25 protein) with Ypt7p (Price et al., 2000; Ungermann et al., 2000). Tethering leads to stable docking and trans-association of a few percent of the SNAREs (Ungermann et al., 1998b). This docking process requires the membrane electrochemical potential (Ungermann et al., 1999) and Rho GTPases (G.Eitzen, N.Thorngren and W.Wickner, submitted) as well as phosphatidylinositol-4- and -4,5-phosphatides, although it is not yet clear how these docking elements are functionally related. Docking triggers a calcium efflux from the vacuole (Peters and Mayer, 1988), which is GTPase gated (Eitzen et al., 2000). This calcium, in complex with calmodulin, binds to vacuole H+-ATPase V0 domains (Peters et al., 2001) and fosters their association in trans (on apposed vacuoles). Upon action of protein phosphatase 1 (Conradt et al., 1994; Peters et al., 1999), there is complete membrane fusion and luminal content mixing.
Yeast vacuoles normally undergo fission as well as fusion, and strains with deletions in genes encoding fusion catalysts are left with highly fragmented vacuoles (Banta et al., 1988; Wada et al., 1992). We have therefore searched for other essential catalysts of vacuole fusion by screening a library of yeast gene deletions (S.Seeley, M.Kato, W.Wickner and G.Eitzen, submitted) for those with fragmented vacuoles. We now report that deletion of genes encoding the enzymes of ergosterol biosynthesis causes striking vacuole fragmentation, and show that ergosterol is needed for the priming stage of the reaction.
Results
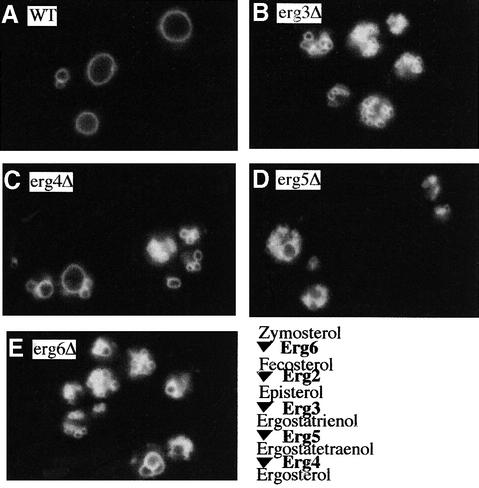
Many strains of S.cerevisiae have 1–3 vacuoles, but deletion of the ERG3, ERG4, ERG5 or ERG6 genes, encoding the enzymes of ergosterol biosynthesis (Lees et al., 1995), causes striking vacuole fragmentation (Figure 1). While <5% of wild-type (BY4742) cells had highly fragmented vacuoles, >80% of cells from erg6Δ, erg3Δ or erg5Δ strains had complete vacuole fragmentation, suggesting that ergosterol has a role in vacuole fusion. Deletion of the ERG4 gene, encoding the last step of ergosterol biosynthesis, caused only 40% vacuole fragmentation, indicating that the penultimate ergosterol precursor may be partially functional for vacuole fusion. This possible requirement for ergosterol was reflected by an in vitro fusion assay (Haas et al., 1994). For this assay, we employed vacuoles purified from two strains: one that lacks proteinase A and accumulates catalytically inactive pro-alkaline phosphatase, and the other with normal vacuolar proteases but with a deletion of the PHO8 gene, which encodes alkaline phosphatase. Fusion allows the protease from one vacuole to gain access to the prophosphatase of its fusion partner, converting it to the active form, which is assayed colorimetrically. The fusion seen with ERG5/pep4Δ and ERG5/pho8Δ vacuoles (Figure 2A, lanes 1 and 2) is diminished when one of the fusion partners is from an erg5Δ background (lanes 3 and 4), and is almost entirely lost when both vacuoles are from erg5Δ strains (lanes 5 and 6). These vacuoles had comparable levels of proteins such as Ypt7p and Vam3p, which are required for fusion, as well as the ‘reporter’ proteins proPho8p and Pep4p (Figure 2B).
Fig. 1. Vacuole appearance in wild-type and ergΔ strains. Wild-type strain BY4742 (A) and isogenic erg3–6Δ strains (B–E) were grown in 0.2 ml of YPD with 200 µg/ml G418 in 15 ml culture tubes at 30°C. After 16 h, 0.4 ml of YPD with 3 µM FM4-64 (Vida and Emr, 1995) were added, incubation was continued for 4 h at 30°C, and cells were photographed as reported (L.Wang, W.Wickner and A.Merz, submitted).
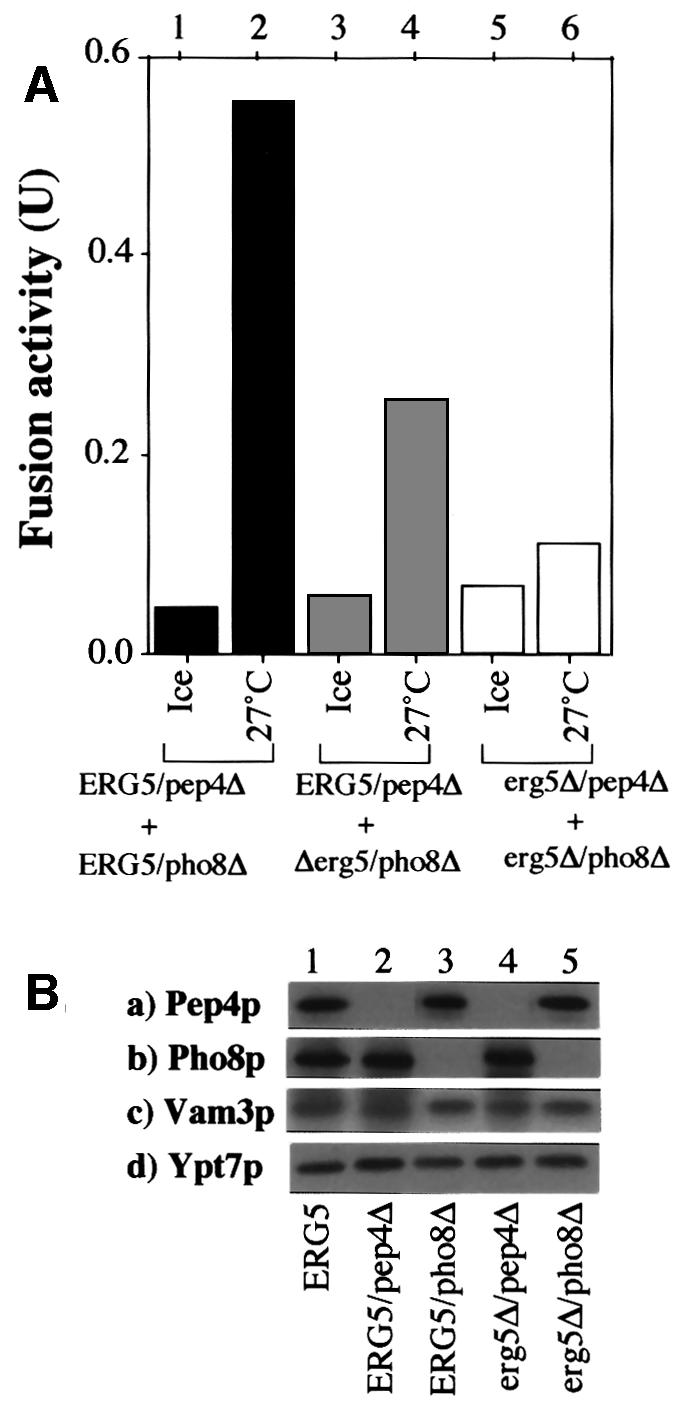
Fig. 2. (A) Fusion activity of vacuoles from ergΔ cells. Standard fusion reactions were performed with vacuoles from ERG5/pep4Δ, ERG5/Pho8Δ, erg5Δ/pep4Δ and erg5Δ/pho8Δ strains. (B) Vacuoles (3.75 µg) from each indicated strain were analyzed by immunoblotting.
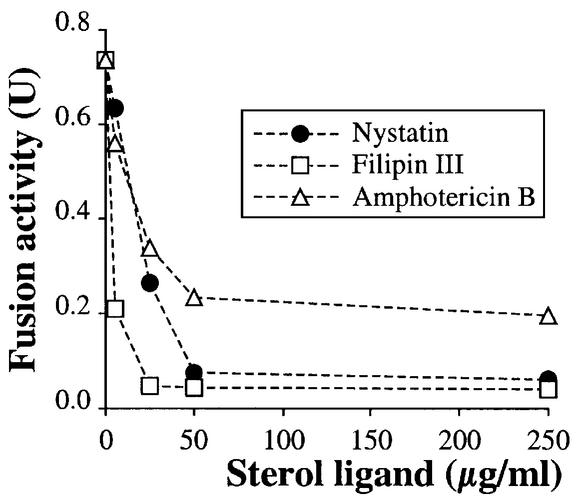
As a complementary test of the role of ergosterol in vacuole homotypic fusion, we assayed the effect of sterol-specific drugs on the in vitro fusion of vacuoles from wild-type (ERG) strains. With this assay, the addition of filipin, nystatin or amphotericin B, each known to be a specific sterol ligand (Kinsky, 1970), caused striking inhibition of the in vitro fusion reaction (Figure 3). Each of these drugs still allows ATP-driven acidification of vacuoles (data not shown), as assayed by quantitative quinacrine uptake (Ungermann et al., 1999), showing overall organelle integrity.
Fig. 3. Inhibition of the fusion of wild-type vacuoles by nystatin, filipin and amphotericin B. Standard reactions of vacuoles from BJ3505 and DKY6281 strains contained the indicated concentrations of nystatin, filipin or amphotericin B.
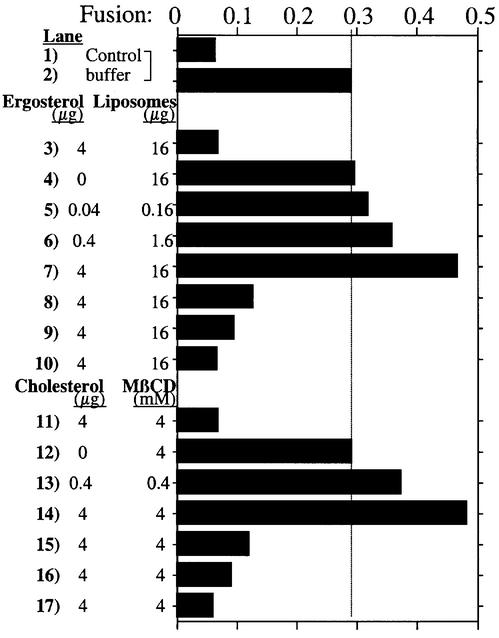
Added pure sterols can directly stimulate the in vitro fusion reaction. Vacuoles from erg5Δ strains, which are inactive for fusion (Figure 2A, lanes 5 and 6; Figure 4, lanes 1 and 2), are stimulated by the addition of sterol (Figure 4, lane 7). This stimulated fusion is on the normal pathway since it is sensitive to anti-Sec17p, anti-Vam3p and Gdi1p (lanes 8–10). Even the fusion of vacuoles from wild-type, ERG strains (Figure 5, lanes 1 and 2) can be stimulated by ergosterol (delivered to the vacuoles by liposomes; Figure 5, lanes 5–7) or cholesterol (delivered to the vacuole from a water-soluble complex with methyl-β-cyclodextrin; lanes 13 and 14), and the stimulated reaction is fully sensitive to normal reaction inhibitors (lanes 8–10 and 15–17). Without sterol, there was no effect on fusion of even the highest level of added liposomes (lane 4) or methyl-β-cyclodextrin (lane 12). Nystatin inhibition of vacuole fusion is also partially reversible by added sterol (data not shown). Taken together, the vacuole fragmentation seen in vivo in ergΔ strains, the sensitivity of in vitro fusion of vacuoles from ERG strains to ergosterol ligands, and the ability of added sterol to stimulate the fusion of ergΔ or wild-type vacuoles show that ergosterol is needed for this fusion reaction.
Fig. 4. Cholesterol promotes the fusion of vacuoles from erg5Δ strains. Vacuoles from erg5Δ/pep4Δ and erg5Δ/pho8Δ strains [5 µg each, 0.3 mg/ml in buffer A (20 mM PIPES–KOH pH 6.8, 200 mM sorbitol, 125 mM KCl, 5 mM MgCl2)] were mixed, centrifuged (10 000 g, 4°C, 2 min), and resuspended in 30 µl of buffer A on ice in the presence or absence of methyl-β-cyclodextrin with 4 µg of cholesterol. After incubation on ice for 60 min, the samples were again centrifuged (10 000 g, 4°C, 2 min), resuspended in fusion reaction buffer with calmodulin (183.3 µg/ml), LMA1 (11.5 µg/ml) and Sec18p (26.6 µg/ml), incubated at 27°C for 90 min, and fusion activity was measured.
Fig. 5. Ergosterol or cholesterol can promote the fusion of wild-type vacuoles. Mixed vacuoles from BJ3505 and DKY6281 strains (5 µg each, 0.3 mg/ml in fusion reaction buffer) were centrifuged (10 000 g, 4°C, 2 min), and resuspended in fusion reaction buffer (33 µl) with anti-Sec18 antibody (33.3 µg/ml) in the presence or absence of 0.16–16 µg of liposomes with 0.04–4 µg of ergosterol, respectively, or of methyl-β-cyclodextrin (MβCD; 0.4–4 mM) with 0.4–4 µg of cholesterol, respectively. After incubation at 27°C for 30 min, the samples were centrifuged (10 000 g, 4°C, 2 min), pellets were resuspended in fusion reaction buffer with calmodulin (183.3 µg/ml), LMA1 (11.5 µg/ml) and Sec18p (26.6 µg/ml), incubated at 27°C for 90 min in the presence or absence of anti-Sec17 antibody (66.7 µg/ml, lanes 8 and 15), anti-Vam3 antibody (66.7 µg/ml, lanes 9 and 16) or GTPγS (6.7 mM, lanes 10 and 17), and fusion activity was measured.
Priming requires ergosterol
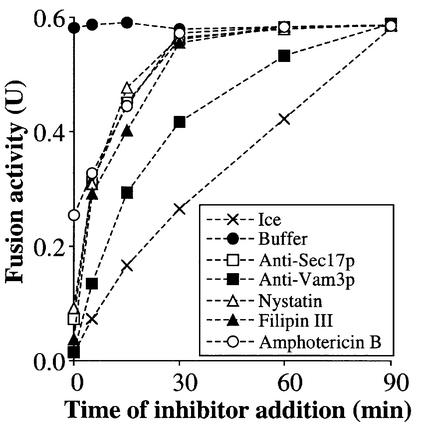
The requirement of ergosterol for vacuole fusion might reflect a role in general membrane integrity or bilayer function, which would then extend throughout the complex membrane fusion pathway, or be specific to a defined subreaction. To test this, aliquots of a large vacuole fusion reaction were mixed with inhibitors at different times during the in vitro fusion incubation, and the effect on the eventual fusion yield was measured (Figure 6). As controls, the addition of aliquots to tubes with buffer (filled circles) has no effect, while the transfer of aliquots to an ice bath (×) stops the reaction and is a measure of the fusion that has occurred by the time of transfer. As previously reported (Nichols et al., 1997), mixing an aliquot with antibody directed against the t-SNARE Vam3p (closed squares) from the start of the reaction causes complete fusion inhibition, but the reaction later becomes resistant to anti-Vam3p, reflecting completion of the docking step. The reaction becomes resistant to antibody directed against the priming co-chaperone Sec17p (open squares) earlier, reflecting the fact that priming precedes docking. The kinetics of the fusion reaction acquiring resistance to nystatin (open triangles), filipin (closed triangles) and amphotericin B (open circles) paralleled that of anti-Sec17p, suggesting that these ligands largely block priming.
Fig. 6. Nystatin, filipin and amphotericin B resistance is acquired with the same kinetics as resistance to anti-Sec18p antibody. Reactions (1350 µl) contained vacuoles from BJ3505 and DKY6281 strains in fusion reaction buffer. Aliquots (30 µl) were removed and placed on ice or added to inhibitors [anti-Sec17 antibody (66.7 µg/ml), anti-Vam3 antibody (66.7 µg/ml), nystatin (50 µg/ml), filipin (50 µg/ml) and amphotericin B (50 µg/ml)] at the indicated times. After 90 min, fusion activity was measured.
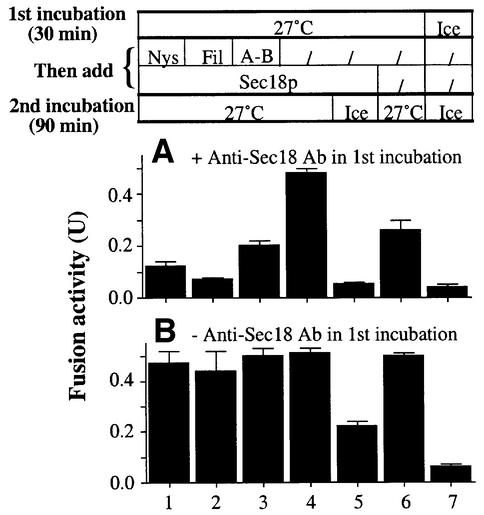
To determine whether the simultaneous resistance to anti-Sec17p and to sterol ligands is a kinetic coincidence of independent events, or whether the mechanisms that confer resistance to filipin, nystatin and amphotericin B are the same as those that catalyze fusion, we used antibody directed against Sec18p to specifically delay the onset of the priming cascade while incubating the vacuoles with ATP (Figure 7A). This first incubation period (30 min) otherwise suffices (Figures 6 and 7B, lanes 1–4) for the acquisition of drug resistance. Although antibody directed against Sec18p prevents fusion during this first incubation (Figure 7A, lane 5) or during a subsequent second incubation (lane 6), it is restored by the addition of excess Sec18p at the end of the first incubation (lane 4). During this first incubation, when priming was blocked by antibody directed against Sec18p, the vacuoles did not become resistant to nystatin, filipin or amphotericin B (lanes 1–3), showing that resistance is only acquired (Figures 6 and 7B) during the course of the Sec18p-triggered cascade of the normal fusion reaction. Thus, ergosterol is needed for priming and not merely for general membrane integrity.
Fig. 7. Resistance to nystatin, filipin or amphotericin B is not acquired until Sec18p has acted. Standard fusion reactions were started with (A) or without (B) 33.3 µg/ml anti-Sec18p antibody. After incubation for 30 min at 27°C, the samples received nystatin (250 µg/ml), filipin (50 µg/ml), amphotericin B (25 µg/ml) or buffer. After incubation for 5 min at 27°C, Sec18p (13.3 µg/ml) was added to reactivate the Sec18p pathway. A control sample received only buffer. After further incubation for 90 min at 27°C or on ice, fusion activity was measured. A standard reaction, kept on ice throughout the incubations, indicates the background.
One essential part of the priming reaction is the Sec18p-mediated release of Sec17p (Mayer et al., 1996). This release, which is assayed by the Sec17p recovered after the vacuoles are removed by centrifugation (Figure 8A), requires ATP and Sec18p (Figure 8B). Even in the presence of excess Sec18p, Sec17p release is strongly inhibited by nystatin (Figure 8C), filipin (Figure 8D), or amphotericin B (Figure 8E). Ergosterol is thus specifically needed for this priming reaction, which triggers the vacuole fusion cascade.
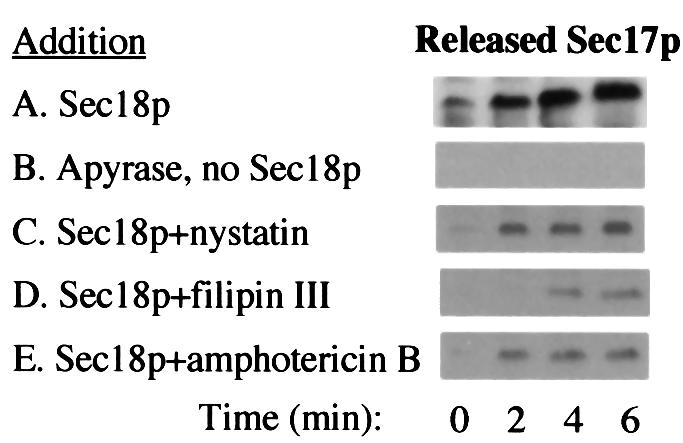
Fig. 8. Nystatin, filipin and amphotericin B inhibit Sec17p release from vacuoles. Samples equivalent to three standard fusion reactions were incubated at 27°C with Sec18p (13.3 µg/ml), apyrase (0.033 U/ml), anti-Sec18 antibody (66.7 µg/ml), nystatin (50 µg/ml), filipin (200 µg/ml) and amphotericin B (25 µg/ml), where indicated. After the incubation times, vacuoles were removed by centrifugation and samples of the supernatant were analyzed by immunoblotting with anti-Sec17p antibody.
Discussion
Ergosterol has an important role in vacuole homotypic fusion, both in vivo and in vitro. The fragmentation seen with ergΔ vacuoles (Figure 1) is a very reliable indicator of direct involvement in vacuole fusion. The first nine isolates in a screen for mutants with highly fragmented vacuoles (vam mutants) yielded the GTPase Ypt7p, the SNAREs Vam3p and Vam7p, and the six subunits of the HOPS complex, all directly involved in the fusion reaction. These ergΔ vacuoles have normal protein composition but are incapable of in vitro fusion (Figure 2). This is not simply due to the small size of the vacuoles, as small vam3Δ vacuoles fuse well with wild-type or nyvΔ vacuoles (Nichols et al., 1977; Ungermann et al., 1998b), and bem4Δ vacuoles, which are fragmented, show normal in vitro fusion (G.Eitzen and W.Wickner, unpublished). Most importantly, ergΔ vacuole fusion is at least partially restored by the re-addition of sterol (Figure 4), which even stimulates the fusion of wild-type vacuoles (Figure 5). Although the restoration of fusion to ergΔ vacuoles is incomplete, the incorporation of added sterol into vacuoles may not fully mimic normal sterol content and asymmetry. Three drugs that are specific for sterols block the in vitro vacuole fusion reaction (Figure 3) at the priming stage (Figures 6 and 7) and prevent the release of Sec17p (Figure 8), a defined subreaction of priming (Mayer et al., 1996). While these drugs may have other effects, their major effect on vacuole fusion occurs in the priming reaction (Figure 6). It is the later docking reaction that requires a membrane potential, a sensitive indicator of membrane integrity (Ungermann et al., 1999). Although before now priming has been characterized by its protein rearrangements (Mayer et al., 1996; Ungermann et al., 1998a, 1999; Xu et al., 1998), the need for both ergosterol and phosphatidylinositol-4,5-bisphosphate (Mayer et al., 2000) for priming suggests that membrane lipids play a vital role as well.
It is suprising that ergosterol is important for the vacuole, as it represents only a minor portion of the membrane lipid. Yeast glycosphingolipids and ergosterol are progressively enriched along the secretory pathway, reaching their highest levels in the plasma membrane (Zinser et al., 1991, 1993). Other organelles, such as the vacuole, exhibit a low ratio of ergosterol to phospholipids and have low levels of sphingolipids. The need for sphingolipids for the sorting of glycosylphosphatidylinositol-anchored Gas1p (but not for carboxypeptidase Y) from the endoplasmic reticulum to the Golgi (Hovarth et al., 1994; Skrzypek et al., 1997; Sutterlin et al., 1997), and for ergosterol for vacuole fusion, as reported here, shows that lipids and proteins may often fulfill vital functions at cellular locations where they are not most abundant.
Lipids are thought to fulfill several roles in membrane trafficking. These include modulating the physical properties of membranes, forming a separate phase or ‘raft’, and serving as a scaffold for binding specific sets of proteins. Ergosterol and other sterols are known to alter membrane fluidity. If Sec18p-mediated Sec17p release is coupled to movement of the membrane anchors of Sec17p-associated SNAREs, then ergosterol might affect Sec17p release through governing SNARE mobility. Indeed, the Sec18p-mediated release of Sec17p from vacuole membranes is blocked by antibody directed against Vam3p, the vacuolar t-SNARE (Sato and Wickner, 1998), and it has recently been reported (Lang et al., 2001) that SNAREs can be found concentrated in cholesterol-rich clusters, which are important for membrane fusion. Vacuolar sterol might also participate in forming a separate phase, or ‘raft’, in the vacuole membrane, enriched in proteins and lipids that partake in the fusion pathway. Triton X-100 extracts of vacuole membranes have a 65S particle, which bears several key proteins of the fusion cascade (Seals et al., 2000), but almost no Sec17p or Sec18p (D.Seals, A.Merz and W.Wickner, unpublished). Cholesterol-rich protein rafts have been heavily studied in the secretory pathway, both in mammalian cells and in yeast (Ikonen and Simons, 1998; Bagnat et al., 2000), but have not yet been implicated in Sec18p/NSF-mediated priming per se. A third possible role for lipids in trafficking is as a scaffold for membrane association of specific proteins. Just as proteins bearing FYVE motifs recognize phosphatidylinositol-3-phosphatides, and this recognition is essential to allow these proteins to function (Burd and Emr, 1998), it is conceivable that ergosterol might provide a binding site for certain vacuolar peripheral membrane proteins.
Sterols have been reported to govern traffic and membrane fusion in other systems. Sterols are required for the formation of ‘rafts’ of distinct lipid and protein composition, which have well studied roles in the trafficking of these lipids and proteins to target membranes. In addition, Mycoplasma fusion with Sendai or influenza virus requires cholesterol (Citovsky et al., 1988). This fusion reaction, however, begins on the extra-cytoplasmic faces of two membranes, and thus does not entail the actions of Sec18/NSF and Sec17p/α-SNAP.
Sec18p is an ATP-driven chaperone that releases Sec17p from the vacuoles, causing disassembly of cis-SNARE complexes. These proteins, as well as the ternary SNARE complex, are of known structure (Sutton et al., 1998; Rice and Brünger, 1999; Yu et al., 1999), and it has been shown that pure Sec18p/NSF and Sec17p/ α-SNAP can disassemble the ternary complex of pure SNAREs (Fasshauer et al., 1997). These model studies largely employ recombinant SNAREs without their membrane anchors, or full-length SNAREs with their membrane anchors, which were solubilized with the aid of detergents. cis-SNARE complexes may not be simply trimeric, but may also include Sec17p (Ungermann et al., 1998a), the HOPS complex, which itself includes Vps33p (a Sec1p homolog with affinity for the t-SNARE Vam3p), and other components as well. The functional role of ergosterol in Sec18p-driven Sec17p release from the vacuole may become clearer as the complete molecular constituents and structure of the cis-SNARE complex and its bound Sec17p are studied further.
Materials and methods
The yeast strains BJ3505 and DKY have been described (Haas et al., 1994). Deletion mutants of ERG3, ERG4, ERG5 or ERG6 genes in BY4742 yeast were purchased from Research Genetics. Deletions of PEP4 and PHO8 were made in these strains as described (Peters and Mayer, 1998). Reagents were as described (Mayer et al., 1996; Eitzen et al., 2000). Nystatin, filipin III and amphotericin B were from Sigma, and were dissolved in dimethylsulfoxide at 150, 30 and 30 mg/ml, and diluted prior to addition to the assay with PS buffer (10 mM PIPES–KOH pH 6.8, 200 mM sorbitol). Ergosterol, cholesterol, l-d-phosphatidylcholine and methyl-β-cyclodextrin were purchased from Sigma. The assay for the release of Sec17p was described previously (Mayer et al., 1996).
A standard fusion reaction contained 3 µg of each vacuole type, isolated from strains BJ3505 and DKY6281, in a total volume of 30–35 µl of reaction buffer (10 mM PIPES–KOH pH 6.8, 200 mM sorbitol, 125 mM KCl, 5 mM MgCl2, 10 µM coenzyme A, 1 mM Mg-ATP, 40 mM creatine phosphate, 0.5 mg/ml creatine kinase, 6.6 ng/ml leupeptin, 16.6 ng/ml pepstatin, 16.6 µM o-phenanthroline, 3.3 µM Pefabloc SC). After 90 min at 27°C, alkaline phosphatase activity was determined (Haas et al., 1994).
Vacuoles were supplemented with ergosterol from liposomes (Cruz et al., 2000) and with cholesterol from its complex with methyl- β-cyclodextrin as described (Christian et al., 1997; Cruz et al., 2000).
Acknowledgments
Acknowledgements
We thank Li Wang for help with fluorescence microscopy and Y.Kato for expert technical assistance. This work was supported by grants from the National Institute of General Medical Sciences and the Uehara Memorial Foundation.
References
- Bagnat M., Keranen,S., Shevchenko,A., Shevchenko,A. and Simons,K. (2000) Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl Acad. Sci. USA, 97, 3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta L.M., Robinson,J.S., Klionsky,D.J. and Emr,S.D. (1988) Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol., 107, 1369–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M.K. (1997) Ca2+ and the regulation of neurotransmitter secretion. Curr. Opin. Neurobiol., 7, 316–322. [DOI] [PubMed] [Google Scholar]
- Burd C.G. and Emr,S.D. (1998) Phosphatidylinositol (3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell, 2, 157–162. [DOI] [PubMed] [Google Scholar]
- Chavrier P. and Goud,B. (1999) The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol., 11, 466–475. [DOI] [PubMed] [Google Scholar]
- Christian A.E., Haynes,M.P., Phillips,M.C. and Rothblat,G.H. (1997) Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res., 38, 2264–2272. [PubMed] [Google Scholar]
- Citovsky V., Rottem,S., Nussbaum,O., Laster,Y., Rott,R. and Loyter,A. (1988) Animal viruses are able to fuse with prokaryotic cells. Fusion between Sendai or influenza virions and Mycoplasma. J. Biol. Chem., 263, 461–467. [PubMed] [Google Scholar]
- Conradt B., Haas,A. and Wickner,W. (1994) Determination of four biochemically distinct, sequential stages during vacuole inheritance in vitro. J. Cell Biol., 126, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J.C., Sugii,S., Yu,C. and Chang,T.Y. (2000) Role of Niemann–Pick type C1 protein in intracellular trafficking of low density lipoprotein-derived cholesterol. J. Biol. Chem., 275, 4013–4021. [DOI] [PubMed] [Google Scholar]
- Desai R.C., Vyas,B., Earles,C.A., Littleton,J.T., Kowalchyck,J.A., Martin,T.A. and Chapman,E.R. (2000) The C2B domain of synaptotagmin is a Ca2+-sensing module essential for exocytosis. J. Cell Biol., 150, 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen G., Will,E., Gallwitz,D., Haas,A. and Wickner,W. (2000) Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J., 19, 6713–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D., Otto,H., Eliason,W.K., Jahn,R. and Brünger,A.T. (1997) Structural changes are associated with soluble N-ethylmaleimide sensitive fusion protein attachment protein receptor complex formation. J. Biol. Chem., 272, 28036–28041. [DOI] [PubMed] [Google Scholar]
- Gerst J.E. (1999) SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol. Life Sci., 55, 707–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A., Conradt,B. and Wickner,W. (1994) G-protein ligands inhibit in vitro reactions of vacuole inheritance. J. Cell Biol., 126, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovarth A., Sutterlin,C., Manning-Krieg,U., Movva,N.R. and Riezmann,H. (1994) Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J., 13, 3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts R.P.H., Topalof,L. and Bankaitis,V.A. (2000) Lipid metabolism and regulation of membrane trafficking. Traffic, 1, 195–202. [DOI] [PubMed] [Google Scholar]
- Ikonen E. and Simons,K. (1998) Protein and lipid sorting from the trans-Golgi network to the plasma membrane in polarized cells. Semin. Cell Dev. Biol., 9, 503–509. [DOI] [PubMed] [Google Scholar]
- Kinsky S.C. (1970) Antibiotic interaction with model membranes. Annu. Rev. Pharmacol., 10, 119–142. [DOI] [PubMed] [Google Scholar]
- Lang T., Bruns,D., Wenzel,D., Riedel,D., Holroyd,P., Thiele,C. and Jahn,R. (2001) SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J., 20, 2202–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees N.D., Skaggs,B., Kirsch,D.R. and Bard,M. (1995) Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae—a review. Lipids, 30, 221–226. [DOI] [PubMed] [Google Scholar]
- Mayer A., Wickner,W. and Haas,A. (1996) Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell, 85, 83–94. [DOI] [PubMed] [Google Scholar]
- Mayer A., Scheglmann,D., Dove,S., Glatz,A., Wickner,W. and Haas,A. (2000) Phosphatidylinositol-(4,5)-bisphosphate regulates two steps of homotypic vacuole fusion. Mol. Biol. Cell, 11, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misura K.M.S., Scheller,R.H. and Wels,W.I. (2000) Three-dimensional structure of the neuronal-Sec1–syntaxin 1a complex. Nature, 404, 355–362. [DOI] [PubMed] [Google Scholar]
- Nichols B.J., Ungermann,C., Pelham,H.R.B. and Wickner,W. (1997) Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature, 387, 199–202. [DOI] [PubMed] [Google Scholar]
- Peters C. and Mayer,A. (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature, 396, 575–580. [DOI] [PubMed] [Google Scholar]
- Peters C., Andrews,P.D., Stark,M.J.R., Cesaro-Tadic,S., Glatz,A., Podtelejnikov,A., Mann,M. and Mayer,A. (1999) Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science, 285, 1084–1087. [DOI] [PubMed] [Google Scholar]
- Peters C., Bayer,M.J., Buhler,S., Andersen,J.S., Mann,M. and Mayer,A. (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature, 409, 581–587. [DOI] [PubMed] [Google Scholar]
- Price A., Seals,D., Wickner,W. and Ungermann,C. (2000) The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J. Cell Biol., 148, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L.M. and Brünger,A.T. (1999) Crystal structure of the vesicular transport protein Sec17: implications for SNAP function in SNARE complex disassembly. Mol. Cell, 4, 85–95. [DOI] [PubMed] [Google Scholar]
- Rothman J.H. and Stevens,T.H. (1986) Protein sorting in yeast: mutants defective in vacuole biogenesis localize vacuolar proteins into the late secretory pathway. Cell, 47, 1041–1051. [DOI] [PubMed] [Google Scholar]
- Sato K. and Wickner,W. (1998) Functional reconstitution of Ypt7p GTPase and a purified vacuole SNARE complex. Science, 281, 700–702. [DOI] [PubMed] [Google Scholar]
- Sato T.K., Rehling,P., Peterson,M.R. and Emr,S.D. (2000) Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell, 6, 661–671. [DOI] [PubMed] [Google Scholar]
- Seals D., Eitzen,G., Margolis,N., Wickner,W. and Price,A. (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl Acad. Sci. USA, 97, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypek M., Lester,R.L. and Dickson,R.C. (1997) Suppressor gene analysis reveals an essential role for sphingolipids in transport of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae. J. Bacteriol., 179, 1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterlin C., Doering,T.L., Schimoller,F., Schroder,S. and Riezman,H. (1997) Specific requirements for the ER to Golgi transport of GPI-anchored proteins in yeast. J. Cell Sci., 110, 2703–2714. [DOI] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer,D., Jahn,R. and Brünger,A.T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4Å resolution. Nature, 395, 347–353. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Nichols,B.J., Pelham,H.R. and Wickner,W. (1998a) A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J. Cell Biol., 140, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Sato,K. and Wickner,W. (1998b) Defining the functions of trans-SNARE pairs. Nature, 396, 543–548. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Wickner,W. and Xu,Z. (1999) Vacuole acidification is required for trans-SNARE pairing, LMA1 release and homotypic fusion. Proc. Natl Acad. Sci. USA, 96, 11194–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Price,A. and Wickner,W. (2000) A new role for a SNARE protein as a regulator of the Ypt7/Rab-dependent stage of docking. Proc. Natl Acad. Sci. USA, 97, 8889–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida T.A. and Emr,S.D. (1995) A new vital stain for monitoring vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol., 128, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y., Ohsumi,Y. and Anraku,Y. (1992) Genes for directing vacuole morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J. Biol. Chem., 267, 18665–18670. [PubMed] [Google Scholar]
- Wickner W. and Haas,A. (2000) Yeast vacuole fusion: a window on organelle trafficking mechanisms. Annu. Rev. Biochem., 69, 247–275. [DOI] [PubMed] [Google Scholar]
- Xu Z., Sato,K. and Wickner,W. (1998) LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex and is released during fusion. Cell, 93, 1125–1134. [DOI] [PubMed] [Google Scholar]
- Yu R.C., Jahn,R. and Brünger,A.T. (1999) NSF N-terminal domain crystal structure: models of NSF function. Mol. Cell, 4, 97–107. [DOI] [PubMed] [Google Scholar]
- Zinser E., Sperka-Gottlieb,C.D., Fasch,E.V., Kohlwein,S.D., Paltauf,F. and Daum,G. (1991) Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J. Bacteriol., 173, 2026–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser E., Paltauf,F. and Daum,G. (1993) Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol., 175, 2853–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]