Abstract
The integrin cytoplasmic domain modulates cell proliferation, adhesion, migration, and intracellular signaling. The β1 integrin subunits, β1C and β1A, that contain variant cytoplasmic domains differentially affect cell proliferation; β1C inhibits proliferation, whereas β1A promotes it. We investigated the ability of β1C and β1A to modulate integrin-mediated signaling events that affect cell proliferation and survival in Chinese hamster ovary stable cell lines expressing either human β1C or human β1A. The different cytodomains of either β1C or β1A did not affect either association with the endogenous α2, αV, and α5 subunits or cell adhesion to fibronectin or TS2/16, a mAb to human β1. Upon engagement of endogenous and exogenous integrins by fibronectin, cells expressing β1C showed significantly inhibited extracellular signal–regulated kinase (ERK) 2 activation compared with β1A stable cell lines. In contrast, focal adhesion kinase phosphorylation and Protein Kinase B/AKT activity were not affected. Selective engagement of the exogenously expressed β1C by TS2/16 led to stimulation of Protein Kinase B/AKT phosphorylation but not of ERK2 activation; in contrast, β1A engagement induced activation of both proteins. We show that Ras activation was strongly reduced in β1C stable cell lines in response to fibronectin adhesion and that expression of constitutively active Ras, Ras 61 (L), rescued β1C-mediated down-regulation of ERK2 activation. Inhibition of cell proliferation in β1C stable cell lines was attributable to an inhibitory effect of β1C on the Ras/MAP kinase pathway because expression of activated MAPK kinase rescued β1C antiproliferative effect. These findings show that the β1C variant, by means of a unique signaling mechanism, selectively inhibits the MAP kinase pathway by preventing Ras activation without affecting either survival signals stimulated by integrins or cellular interactions with the extracellular matrix. These findings highlight a role for β1-specific cytodomain sequences in maintaining an intracellular balance of proliferation and survival signals.
INTRODUCTION
Integrins are a large family of heterodimeric transmembrane receptors composed of α and β subunits (Hynes, 1992). In addition to their role as adhesion receptors, integrins have been shown to regulate intracellular signaling pathways and cellular functions such as cell migration, proliferation, and survival (Schwartz et al., 1995; Bottazzi and Assoian, 1997; Frisch and Ruoslahti, 1997).
It is well established that the cytoplasmic domain of the β subunit is required for integrins to modulate many cellular functions and to trigger signaling events that result in protein phosphorylation (Hemler et al., 1995; Fornaro and Languino, 1997; Wei et al., 1998) and interactions with intracellular proteins (Hemler, 1998). Thus, mutations or deletions in the β1A subunit cytodomain have been shown to alter the ability of this integrin to trigger focal adhesion kinase (FAK) phosphorylation (Guan et al., 1991) and to interact with cytoskeletal proteins such as talin and filamin (Chen et al., 1995; Lewis and Schwartz, 1995; Pfaff et al., 1998).
The identification and characterization of a number of spliced variants of the integrin cytoplasmic domain in the β and α subgroups (Fornaro and Languino, 1997) have added a new level of complexity to integrin functions. Four different β1 isoforms have been identified (β1A, β1B, β1C, and β1D) and have been shown to differentially affect receptor localization, cell proliferation, cell adhesion and migration, interactions with intracellular proteins, and ultimately phosphorylation and activation of signaling molecules (Belkin et al., 1997; Fornaro and Languino, 1997; Belkin and Retta, 1998; Pfaff et al., 1998; Retta et al., 1998; Meredith et al., 1999).
The β1C integrin is an alternatively spliced variant of the β1 subfamily that contains a unique 48-amino acid sequence in its cytoplasmic domain (Languino and Ruoslahti, 1992). We and others have shown that either full-length β1C or its cytoplasmic domain inhibits prostate cancer epithelial cell (Fornaro et al., 1998; Meredith et al., 1999), endothelial cell (Meredith et al., 1999), and fibroblast (Fornaro et al., 1995; Meredith et al., 1995, 1999) proliferation. In vivo, β1C is expressed in nonproliferative, differentiated epithelium and is selectively down-regulated in prostatic adenocarcinoma, and its expression inversely correlates with markers of cell proliferation in breast carcinoma (Fornaro et al., 1996, 1998, 1999; Manzotti et al., 2000). However, the signaling pathways affected by β1C are still unknown.
FAK is a nonreceptor protein tyrosine kinase that has been shown to colocalize with integrins at focal contact sites (Guan et al., 1991). FAK becomes tyrosine phosphorylated in response to integrin engagement (Guan et al., 1991; Kornberg et al., 1991) and has been shown to prevent apoptosis (Frisch et al., 1996; Hungerford et al., 1996; Xu et al., 1996; Illic et al., 1998; Cary and Guan, 1999). Two recent reports have highlighted a new role for FAK in the modulation of cell cycle progression and in the inhibition of integrin-stimulated signaling events during mitosis (Zhao et al., 1998; Yamakita et al., 1999). The first study showed that FAK overexpression accelerates the G1/S phase transition, increases cyclin D1 levels, and decreases p21waf1 expression (Zhao et al., 1998). The second study demonstrated that FAK undergoes mitosis-specific serine phosphorylation accompanied by tyrosine dephosphorylation, which results in FAK/Cas/c-Src complex dissociation and inhibition of signal transduction pathways involving integrins (Yamakita et al., 1999).
In addition to stimulating FAK, integrins can also activate the phosphatidylinositol 3-kinase (PI 3-kinase) pathway (Keely et al., 1998). PI 3-kinases are a family of lipid kinases activated by a wide variety of extracellular stimuli. The lipid products of PI 3-kinases, specifically phosphatidylinositol(3,4)biphosphate and phosphatidylinositol(3,4,5)triphosphate, affect cell proliferation, survival, differentiation, and migration by targeting specific signaling molecules such as the serine/threonine protein kinase B, also known as AKT, and PKC (Jiang et al., 1999; Rameh and Cantley, 1999). Integrin-mediated adhesion to the extracellular matrix stimulates the production of phosphatidylinositol(3,4)biphosphate and phosphatidylinositol(3,4,5)triphosphate (Khwaja et al., 1997; King et al., 1997), the association of the p85 PI 3-kinase subunit with FAK (Chen and Guan, 1994), and AKT activation (Khwaja et al., 1997; King et al., 1997). AKT plays an important role in transducing survival signals in response to several growth factors and integrin engagement (Khwaja et al., 1997; Downward, 1998).
The small GTPase Ras is a critical component of signaling pathways that control cell proliferation, differentiation, and survival (Campbell et al., 1998; Rebollo and Martinez-A, 1999). The Ras/extracellular signal–regulated kinase (ERK) 1 and 2/MAP kinase pathway plays a pivotal role in modulating gene expression and cell cycle progression in response to mitogens (Robinson and Cobb, 1997; Guadagno and Ferrell, 1998; Brunet et al., 1999). Integrin clustering has been shown to stimulate Ras GTP loading (Clark and Hynes, 1996; Wary et al., 1996; King et al., 1997; Mainiero et al., 1997; Miranti et al., 1999) and to activate specific effectors of the Ras/MAP kinase signaling cascade such as Raf-1 and MAPK kinase (MEK) (Howe et al., 1998; Schlaepfer and Hunter, 1998). In several studies, the dominant negative N17 mutant of Ras has been shown to block extracellular matrix–mediated ERK2 activation (Clark and Hynes, 1996; Wary et al., 1996; King et al., 1997; Mainiero et al., 1997; Schlaepfer and Hunter, 1997; Wei et al., 1998), whereas in one report it had no effect (Chen et al., 1996b). The mechanisms of integrin-mediated activation of the MAP kinase cascade comprise three models (Howe et al., 1998). Two models include Src family kinases and Ras as critical links between integrin-mediated adhesion and MAP kinase activation. In the first model, integrin ligation leads to Src and FAK activation, Grb2 binding to FAK, and membrane localization of the guanine nucleotide exchange factor Sos, which then promotes Ras activation (Schlaepfer et al., 1994, 1998). In the second model, integrins activate the Ras/MAP kinase pathway via the tyrosine kinase Fyn and the adaptor protein Shc (Wary et al., 1996, 1998). A recent report has indicated that fibronectin-induced PKC activation plays a role in ERK2 activation upstream of Shc (Miranti et al., 1999). The third model proposes a Ras-independent activation of Raf and, thus, ERK2 by integrins (Chen et al., 1996b; Lin et al., 1997).
Using Chinese hamster ovary (CHO) stable cell lines expressing either human β1C or human β1A, we have analyzed the ability of β1C and β1A to modulate signaling pathways that control cell proliferation and survival. The β1C variant associates with the same α subunits as β1A and does not affect cell adhesion to β1 ligands. We show that β1C has an inhibitory effect on ERK2 activation mediated by fibronectin without affecting FAK phosphorylation and AKT activity. We also show that Ras activation stimulated by adhesion to fibronectin is inhibited in β1C transfectants and that constitutively active Ras and MEK rescue β1C-mediated down-regulation of ERK2 activation and inhibition of cell growth, respectively. This is the first description of a selective inhibitory role of the integrin cytoplasmic domain on the Ras/MAP kinase pathway. Moreover, AKT phosphorylation is observed in response to antibody-mediated engagement of human β1C and β1A, and ERK2 activation is supported by β1A but not by β1C ligation, indicating a different role for β1 variants in the activation of AKT and MAP kinase pathways. We suggest that by expressing variant β1 intracellular domains, cells may accomplish the delicate task of inhibiting proliferation without affecting either selective downstream survival signals (FAK and AKT) mediated by integrins or interactions with the extracellular environment.
MATERIALS AND METHODS
Reagents and Antibodies
Rabbit antibodies specific for the β1C subunit cytoplasmic domain were affinity-purified as described previously (Fornaro et al., 1996). The following antibodies were used: mouse mAbs P4C10 and TS2/16 to human β1 integrin (Life Technologies, Gaithersburg, MD, and American Type Culture Collection, Rockville, MD, respectively), 7E2 to hamster β1 integrin, PB1 to hamster α5β1 (a kind gift of Dr. R.L. Juliano, University of North Carolina, Chapel Hill, NC), E10 to phospho-ERK1 and 2 (New England Biolabs, Beverly, MA), 12CA5 to hemagglutinin (Boehringer Mannheim, Indianapolis, IN), and to pan Ras (Transduction Laboratories, Lexington, KY); rabbit affinity-purified antibodies to FAK Y397 (Biosource International, Camarillo, CA), to AKT (New England Biolabs), and to FAK and ERK1 and 2 (Santa Cruz Biotechnology, Santa Cruz, CA). Rabbit antisera to α5, αv, or α4 were provided by Dr. E. Ruoslahti (The Burnham Institute, La Jolla, CA), antiserum to α2 was provided by Dr. M.E. Hemler (Dana-Farber Cancer Institute, Boston, MA), and antiserum to β1C was described previously (Fornaro et al., 1996). Human plasma fibronectin and human vitronectin were purified as described (Engvall and Ruoslahti, 1977; Yatohgo et al., 1988). Poly-l-lysine and nonimmune rabbit and mouse immunoglobulin G were purchased from Sigma Chemical (St. Louis, MO).
Cells and Plasmids
To obtain stable cell lines expressing β1A in a tetracycline-regulated system, ClaI–XbaI fragment encoding full-length human β1A was isolated from Bluescript-β1A and subcloned into ClaI–SpeI sites in the pTet-Splice plasmid (a kind gift of Dr. D. Schatz, Yale University, New Haven, CT) to generate the pTet-β1A construct. The pTet-β1C construct has been described previously (Fornaro et al., 1999). CHO stable cell lines expressing either human β1C (clones 16.4, 16.28, and 16.30) or human β1A (clones 10.2, 10.18, and 10.23) integrins under the control of a tetracycline-regulated promoter were generated and maintained in growth medium containing 1 μg/ml tetracycline (Boehringer Mannheim) and 0.1 mg/ml G418 (Life Technologies) as described (Fornaro et al., 1999).
pMLC-1 plasmids containing hemagglutinin-tagged wild-type MEK (MEK WT) and constitutively active MEK (MEK EE) have been described previously (Bennett and Tonks, 1997). The pGEX-RBD plasmid encodes amino acids 1–149 of cRaf-1 fused to GST (Taylor and Shalloway, 1996). The pMT3-Ras 61 (L) encodes a c-rasH form containing a codon 61 mutation (Bennett et al., 1996).
β1C-CHO stable cell lines were transiently transfected by electroporation by using 10 μg of either MEK WT, MEK EE, Ras 61 (L), or vector alone as described (Fornaro et al., 1999). Cells were incubated for 48 h at 37°C in growth medium either in the absence or in the presence of 1 μg/ml tetracycline and serum-starved during the last 24 h of the 48-h culture before analysis of either cell proliferation or ERK2 activity as described below.
Flow Cytometry
Surface expression of exogenous human β1C and β1A integrins was achieved by withdrawal of tetracycline from the growth medium; in both cell transfectants, maximal and comparable β1C or β1A expression were consistently obtained 48 h after tetracycline removal. For each experiment, exogenous human β1 integrin expression was monitored by FACS with TS2/16 serum-free culture supernatant or 12CA5 as negative control antibody (Fornaro et al., 1999). Endogenous hamster β1 or α5β1 integrin expression was analyzed with either 5 μg/ml 7E2 or 1 μg/ml PB1, respectively (Fornaro et al., 1995).
Immunoprecipitation of β1C and β1A Integrins
CHO stable cell lines were cultured for 48 h in the absence of tetracycline to induce β1C or β1A integrin expression (Fornaro et al., 1999). Cells were detached with 0.05% trypsin/0.53 mM EDTA (Life Technologies) and surface iodinated as described previously (Bartfeld et al., 1993). Cells were lysed in 1% NP-40 (Calbiochem, La Jolla, CA), 0.5% sodium deoxycholate (Sigma), 0.1% SDS (American Bioanalytical, Natick, MA), 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1 mM PMSF (Life Technologies), 10 μg/ml aprotinin (Sigma), 10 μg/ml leupeptin (Calbiochem), and 10 μg/ml pepstatin (Sigma) for 30 min at 4°C. β1C and β1A integrins were immunoprecipitated with P4C10 and protein A–Sepharose (Sigma) as described (Fornaro et al., 1995). Immunocomplexes were dissociated with 20 mM Tris-HCl, pH 7.5, 2% SDS and boiled for 5 min. The dissociated material was then diluted 10-fold with lysis buffer and reprecipitated overnight at 4°C with 30 μl of rabbit serum specific for the β1C subunit cytoplasmic domain. Immunoprecipitates were recovered with protein A–Sepharose, washed four times with lysis buffer, and resuspended in loading buffer (2% SDS, 50 mM Tris-HCl, pH 6.8, 100 mM DTT [American Bioanalytical], 10% glycerol, and 0.1% bromphenol blue [Bio-Rad, Hercules, CA]). Proteins were separated by SDS-PAGE (7.5%) and visualized by autoradiography.
Immunoprecipitations of α Subunits Associated with β1C
CHO stable cell lines were cultured for 72 h in the absence of tetracycline; cells were then detached with 0.05% trypsin/0.53 mM EDTA and surface iodinated as described above. Cells were lysed in 1% Triton X-100 (American Bioanalytical), 150 mM NaCl, 20 mM Tris-HCl, pH 7.5, 1 mM MgCl2, 1 mM CaCl2, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin for 30 min at 4°C. β1C and β1A integrins were immunoprecipitated with P4C10 as described above. Immunoprecipitates were washed five times with lysis buffer, resuspended in 10 mM Tris-HCl, pH 7.5, 0.5% SDS, and incubated for 10 min at 70°C. The eluted material was diluted threefold with lysis buffer and reprecipitated with rabbit antiserum to α5, αV, α4, or α2 overnight at 4°C. Immunoprecipitates were recovered with protein A–Sepharose, washed three times with lysis buffer, and resuspended in loading buffer. Proteins were separated by SDS-PAGE (10%) and visualized by autoradiography.
Cell Adhesion Assay to β1 Ligands
Cell adhesion to fibronectin (10 μg/ml), 7E2 (1 μg/ml), TS2/16 (1:10 dilution of culture supernatant), mouse immunoglobulin G (1 μg/ml), and BSA (10 mg/ml; Sigma) was performed as described previously (Languino et al., 1993) with 25,000 51Cr-labeled cells (51Cr from DuPont–New England Nuclear, Wilmington, DE).
For analysis of FAK, AKT, and ERK2, CHO stable cell lines were cultured for 48 h either in the absence or in the presence of 1 μg/ml tetracycline, starved, and then detached as described above. Cells were held in suspension for 30–60 min at 37°C and either kept in suspension or plated on tissue culture plates coated with poly-l-lysine (5–10 μg/ml), fibronectin (10 μg/ml), TS2/16 (1:10 dilution of culture supernatant), or 7E2 (3 μg/ml) at 37°C for the indicated times. Where indicated, cells were incubated for 15 min at 37°C with 100 nM wortmannin (Calbiochem) before plating onto ligand-coated dishes. The cells were then washed twice with PBS (Life Technologies) and lysed in the appropriate ice-cold lysis buffer. The protein content in each lysate was quantitated with the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL).
In all instances, quantification of immunoreactive bands was performed by densitometric analysis; the values are given as fold increase on fibronectin, TS2/16, or 7E2 versus poly-l-lysine or suspension within each established cell line after normalization for protein loading. The values from several experiments are reported as means ± SEM.
FAK Analysis
CHO stable cell lines were lysed with 1% NP-40, 0.5% deoxycholate, 50 mM HEPES, pH 7.5, 150 mM NaCl, 100 mM sodium fluoride (Sigma), 1 mM sodium vanadate (Sigma), 5 mM Na4P2O7 (J.T. Baker, Phillipsburg, NJ), 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin for 30 min at 4°C, and insoluble material was removed by centrifugation at 14,000 × g for 15 min at 4°C.
FAK was immunoprecipitated from 500 μg of total cell lysate with 0.5 μg of C-20, an affinity-purified antibody to FAK. Immunocomplexes were collected with protein A–Sepharose, washed five times with lysis buffer, and resuspended in loading buffer. Proteins were separated by 10% SDS-PAGE, and FAK phosphorylation on Tyr397 was analyzed by immunoblotting with a rabbit affinity-purified antibody that recognizes FAK only when phosphorylated on Y397. FAK protein levels were analyzed by immunoblotting with C-20 rabbit affinity-purified antibody to FAK as described (Zheng et al., 1999).
AKT Analysis
CHO stable cell lines were lysed with 1% NP-40, 50 mM HEPES, pH 7.5, 150 mM NaCl, 2 mM EDTA, 50 mM sodium fluoride, 1 mM sodium vanadate, 1 mM Na4P2O7, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin for 30 min at 4°C. Analysis of AKT phosphorylation was performed by immunoblotting with phospho-specific antibody to Ser473 (New England Biolabs) according to the manufacturer's instructions.
AKT kinase activity was assayed according to Franke et al. (1995). Briefly, 50 μg of detergent cell extracts were cleared by centrifugation at 14,000 × g for 15 min at 4°C. AKT was immunoprecipitated with 0.1 μg of affinity-purified antibody to AKT. Immunocomplexes were collected with protein A–Sepharose and washed three times with lysis buffer, once with 20 mM HEPES, pH 7.5, and once with kinase buffer (20 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MnCl2, 10 mM MgCl2). The AKT kinase activity was assayed with kinase buffer containing 10 μCi of [γ-32P]ATP (3000 Ci/mmol; Amersham Life Sciences, Arlington Heights, IL), 5 μM ATP (Boehringer Mannheim), and 100 μg/ml histone H2B (Boehringer Mannheim) as a substrate for 20 min at 30°C. The reactions were terminated with loading buffer. Phosphorylated histone H2B was viewed by autoradiography.
ERK2 Analysis
CHO stable cell lines were lysed as described for analysis of AKT activation. Analysis of ERK2 phosphorylation by immunoblotting was performed with 0.5 μg/ml E10, a mAb that recognizes ERK2 only when phosphorylated at Thr202/Tyr204, according to the manufacturer's instructions (New England Biolabs). ERK2 activation was analyzed by in vitro kinase assay with myelin basic protein as described (Fornaro et al., 1999).
Assay for Detection of Activated Ras
Ras activation was analyzed as described previously (Taylor and Shalloway, 1996). Briefly, GST-RBD expression in transformed Escherichia coli DH5α was induced with 1 mM isopropyl-1-thio-β-d-galactopyranoside (American Bioanalytical) for 2 h at 37°C. The cells were then washed once with ice-cold 20 mM HEPES, pH 7.5, 150 mM NaCl and lysed by sonication in the following buffer: 20 mM HEPES, pH 7.5, 120 mM NaCl, 10% glycerol, 2 mM EDTA, 100 mg/ml lysozyme (American Bioanalytical), 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 μg/ml pepstatin. The lysate was clarified by centrifugation and incubated with glutathione Sepharose (Amersham Pharmacia Biotech, Piscataway, NJ) for 30 min at 4°C. The Sepharose beads were then washed six times with lysis buffer containing 0.5% NP-40 and stored in the same buffer at 4°C.
For affinity precipitation, cells were washed twice with ice-cold 20 mM HEPES, pH 7.5, 150 mM NaCl and lysed with the following buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% NP-40, 0.25% deoxycholate, 10% glycerol, 10 mM MgCl2, 25 mM sodium fluoride, 1 mM EDTA, 1 mM sodium vanadate, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin) for 30 min at 4°C. One milligram of whole cell lysate was incubated with GST-RBD bound to glutathione Sepharose for 30 min at 4°C. Bound proteins were washed three times with lysis buffer, eluted with loading buffer, and separated by SDS-PAGE (12%). Proteins were visualized by immunoblotting with 2 μg/ml anti-pan Ras mouse mAb according to the manufacturer's instructions (Transduction Laboratories).
Proliferation Assay
CHO stable cell lines were cultured for 48 h either in the absence or in the presence of 1 μg/ml tetracycline, starved during the last 24 h of the 48-h culture, and then detached with 0.05% trypsin/0.53 mM EDTA. Cells were resuspended in serum-free medium and plated (2,500–20,000 cells/well) on either 96- or 24-well plates coated with 1 μg/ml fibronectin for 1 h at 37°C. Attached cells were cultured for 72–96 h at 37°C in growth medium containing 5% FCS either in the absence or in the presence of 1 μg/ml tetracycline. Cells were washed, fixed with 3% paraformaldehyde, and stained overnight with 0.5% toluidine blue. Triplicate observations were performed. Two to 10 fields/well were randomly chosen and counted by microscopic examination. The results are expressed as number of cells per well. Group differences were compared with one-way analysis of variance.
RESULTS
Analysis of α Subunits Associated with β1C and of β1C-CHO Cell Adhesion
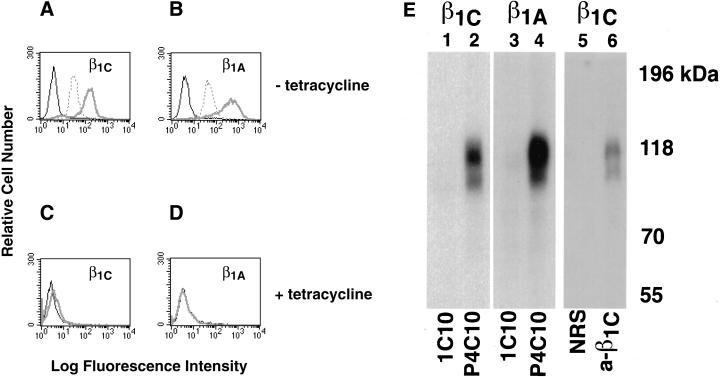
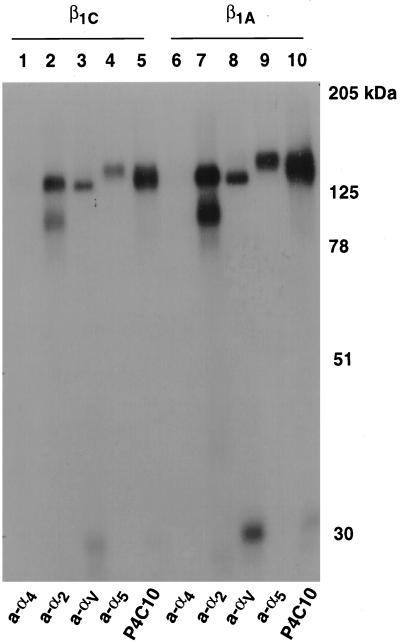
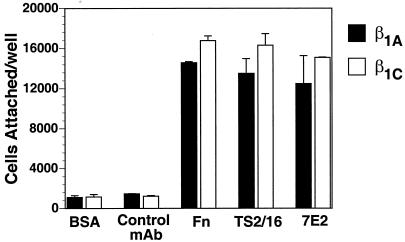
CHO stable cell lines expressing either human β1C or human β1A under the control of a tetracycline-regulated promoter were characterized for their ability to associate with α subunits and to adhere to integrin ligands. Exogenous expression of either β1C or β1A in CHO cells was analyzed by FACS with TS2/16 mAb to human β1 integrin; comparable levels of surface expression of β1C and β1A were consistently obtained in all the experiments 48 h after tetracycline removal (Figure 1, A and B). In parallel, the levels of endogenous β1 were evaluated in both β1C and β1A CHO stable cell lines by FACS with 7E2 mAb to hamster β1 integrin (Figure 1, A and B). Exogenous expression of either β1C or β1A was completely prevented by tetracycline (Figure 1, C and D). The expression of human β1C and β1A was also analyzed by immunoprecipitation from detergent cell extracts of 125I-labeled CHO cells. P4C10, a mAb to the human β1 extracellular domain, immunoprecipitated surface-expressed integrin complexes containing either β1C or β1A (Figure 1E, lanes 2 and 4). P4C10 immunocomplexes were reprecipitated with rabbit serum to the β1C cytodomain. These results confirm appropriate β1C cell surface expression (Figure 1E, lane 6). To characterize the α subunits associated with β1C, P4C10 immunocomplexes were reprecipitated with rabbit serum against α2, αV, or α5, which are known to be associated predominantly with β1 in CHO cells (Takada et al., 1992; Balzac et al., 1993). As shown in Figure 2, both exogenous β1C and β1A were associated with endogenous α2, αV, or α5 integrin subunits in CHO stable cell lines. The β1C- and β1A CHO stable cell lines also attached in a comparable manner to increasing concentrations of fibronectin, 7E2, or TS2/16; no differences were observed in the number of attached cells at 30, 90, or 120 min (Figure 3; our unpublished results).
Figure 1.
Surface expression of β1C and β1A in CHO cells. (A–D) β1C or β1A CHO stable cell lines were cultured for 48 h either in the absence (A and B) or in the presence (C and D) of 1 μg/ml tetracycline and analyzed by FACS with TS2/16 mAb to human β1 integrin, 7E2 mAb to hamster β1 integrin, or 12CA5 as a negative control, followed by FITC goat anti-mouse immunoglobulin G. Fluorescence intensity is expressed in arbitrary units. FACS analysis of a representative clone for each β1 variant is shown. Thick gray line, TS2/16; dotted line, 7E2; thin black line, 12CA5. (E) CHO stable cell lines were cultured as in A and B and surface-labeled with Na 125I; exogenous β1 integrins were immunoprecipitated with P4C10 mAb to human β1 integrin (lanes 2 and 4). The immunoprecipitated material was then eluted from protein A–Sepharose with 50 mM Tris-HCl, pH 7.5, 2% SDS and boiled for 5 min. The immunocomplexes were then reprecipitated with rabbit antiserum to the β1C cytoplasmic domain (lane 6) and separated on 7.5% SDS-PAGE. mAb 1C10 (lanes 1 and 3) or normal rabbit serum (lane 5) were used as negative controls. Lanes 1, 2, 5, and 6, β1C CHO; lanes 3 and 4, β1A CHO. Proteins were visualized by autoradiography. Prestained marker proteins (in kilodaltons) are shown.
Figure 2.
β1C associates with α5, αV, and α2 subunits. β1C or β1A CHO stable cell lines were cultured for 72 h in the absence of tetracycline and surface-labeled with iodine, and exogenous β1 integrins were immunoprecipitated with P4C10 (lanes 5 and 10). The immunoprecipitated material was then eluted from protein A–Sepharose with 10 mM Tris-HCl, pH 7.5, 0.5% SDS for 10 min at 70°C, reprecipitated with rabbit antiserum to α4 (lanes 1 and 6), α2 (lanes 2 and 7), αV (lanes 3 and 8), or α5 (lanes 4 and 9), and separated by 10% SDS-PAGE. Lanes 1–5, β1C CHO; lanes 6–10, β1A CHO. Proteins were detected by autoradiography. Prestained marker proteins (in kilodaltons) are shown.
Figure 3.
CHO cell adhesion is not affected by β1C expression. β1C or β1A CHO stable cell lines were cultured as described for Figure 1. Cells were detached and labeled with 51Cr in DMEM containing 10% FCS for 1 h at 37°C. Cells were then washed in serum-free medium, and 2.5 × 104 cells were allowed to adhere to fibronectin-coated (10 μg/ml), 7E2-coated (1 μg/ml), TS2/16-coated (1:10 dilution of culture supernatant), or negative control mAb-coated (1 μg/ml) or BSA-coated (10 mg/ml) wells at 37°C for 30 min. Attached cells were then washed and lysed, and radioactivity was measured by liquid scintillation counting. Duplicate observations with two separate clones for each β1 variant were performed in each experiment, and the experiments were repeated at least twice with similar results.
β1C Integrin Expression Does Not Affect FAK Phosphorylation or AKT Activation
To analyze the effect of β1C on integrin-mediated intracellular signaling pathways, we used the CHO stable cell lines described above (Figures 1 and 2). It has been shown that integrin ligation leads to tyrosine phosphorylation of intracellular proteins, including FAK (Schwartz et al., 1995).
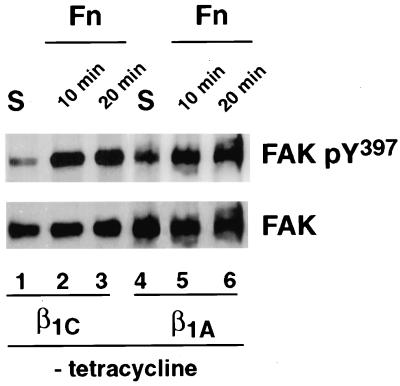
To examine whether FAK phosphorylation was differentially affected by β1C and β1A integrin variants, FAK was immunoprecipitated from detergent cell extracts prepared from either β1C or β1A stable cell lines. FAK phosphorylation was analyzed by immunoblotting with an antibody that recognizes FAK only when phosphorylated on Tyr397 (Sieg et al., 1999). As shown in Figure 4, cell adhesion to fibronectin induced FAK phosphorylation on Tyr397 in both β1C and β1A stable cell lines compared with cells in suspension (top panel). The results indicate that β1C integrin expression does not affect FAK phosphorylation mediated by adhesion to fibronectin.
Figure 4.
Expression of β1C integrin does not affect FAK activation. β1C or β1A CHO stable cell lines were cultured for 48 h in the absence of tetracycline and serum-starved during the last 24 h of the 48-h culture. The cells were detached and either held in suspension (S; lanes 1 and 4) or plated on tissue culture plates coated with fibronectin (Fn; lanes 2, 3, 5, and 6) for either 10 or 20 min at 37°C. FAK was immunoprecipitated from 500 μg of total cell lysate with 0.5 μg of affinity-purified antibody to FAK, and its phosphorylation was analyzed by immunoblotting with 0.2 μg/ml phospho-specific antibody to Tyr397. FAK protein levels were analyzed with 0.1 μg/ml affinity-purified antibody to FAK, and proteins were visualized by enhanced chemiluminescence. The experiments were repeated twice with consistent results.
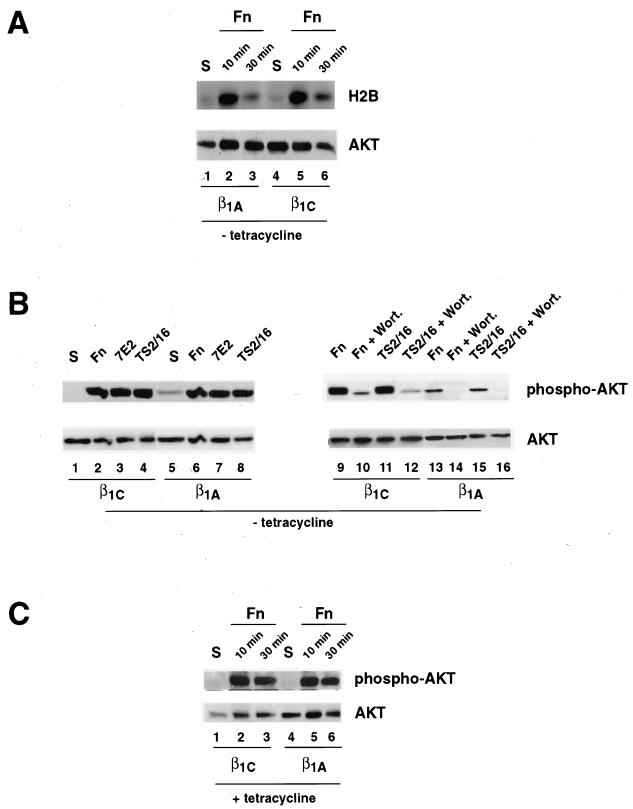
We then examined the ability of β1C and β1A integrins to activate AKT, a downstream effector of PI 3-kinase that promotes cell survival. AKT activity was first assayed on detergent cell extracts obtained from cells that attached to fibronectin for 10 or 30 min. As shown in Figure 5A, adhesion to fibronectin for 10 min induced comparable activation of AKT in both β1C and β1A stable cell lines as determined by in vitro kinase assay (top panel, lanes 2 and 5). However, upon adhesion to fibronectin for 30 min, a modest but consistent increase of AKT activation was observed in β1C versus β1A stable cell lines (top panel, lanes 3 and 6). Similar results were obtained by immunoblotting with a phospho-specific AKT antibody (Figure 5B); total lysates from cells that were either held in suspension or allowed to adhere for 30 min to fibronectin, TS2/16, or 7E2 were immunoblotted with phospho-specific antibody to Ser473. As shown in panel B, a marked increase in AKT serine phosphorylation was observed in β1C and β1A stable cell lines upon adhesion to fibronectin (top panel, lanes 2 and 6), 7E2 (top panel, lanes 3 and 7), or TS2/16 (top panel, lanes 4 and 8) compared with cells in suspension and with cells on poly-l-lysine (our unpublished results). These results indicate that both β1 variants activate AKT in CHO cells. Densitometric analysis performed on three separate experiments showed that cell adhesion to fibronectin, TS2/16, or 7E2 induced an increase in AKT Ser473 phosphorylation in β1C (6.3 ± 1.4-fold, 4.4 ± 0.8-fold, and 5.5 ± 1.9-fold increase, respectively) as well as in β1A (2.2 ± 0.5-fold, 2.1 ± 0.2-fold, and 2.7 ± 0.7-fold increase, respectively) stable cell lines (our unpublished results). No differences in AKT activation were detected upon adhesion to fibronectin between β1C and β1A stable cell lines cultured in the presence of tetracycline (Figure 5C, lanes 2, 3, 5, and 6) to prevent expression of exogenous β1 variants. AKT phosphorylation in response to engagement of either endogenous integrins or exogenous β1C and β1A variants by either fibronectin or TS2/16 was completely inhibited by wortmannin, a PI 3-kinase inhibitor (Figure 5B, top panel, lanes 10, 12, 14, and 16). These data show that β1C and β1A do not differentially affect PI 3-kinase/AKT pathway activation induced by fibronectin and that antibody-mediated engagement of β1C and β1A stimulates AKT phosphorylation.
Figure 5.
AKT activation in β1C and β1A transfectants. AKT activation was analyzed by in vitro kinase assay (A) and by immunoblotting (B and C). β1C or β1A CHO stable cell lines were cultured for 48 h either in the absence (A and B) or in the presence (C) of 1 μg/ml tetracycline and serum-starved during the last 24 h of the 48-h culture. The cells were detached and either held in suspension (S; A and C, lanes 1 and 4; B, lanes 1 and 5) or seeded on tissue culture plates coated with fibronectin (Fn; A and C, lanes 2, 3, 5, and 6; B, lanes 2, 6, 9, 10, 13, and 14), TS2/16 (B, lanes 4, 8, 11, 12, 15, and 16), or 7E2 (B, lanes 3 and 7) for either 10 min (A and C) or 30 min (A–C) at 37°C. Cells were also incubated with 100 nM wortmannin (Wort.) for 15 min at 4°C before plating on either fibronectin (B, lanes 10 and 14) or on TS2/16 (B, lanes 12 and 16). (A) AKT was immunoprecipitated from total cell lysate with 0.1 μg of affinity-purified antibody to AKT, and its kinase activity was analyzed by in vitro kinase assay with histone H2B (H2B) as a substrate. Phosphorylated H2B was visualized by autoradiography (top panel). (B and C) Detergent cell extracts were analyzed with 0.05 μg/ml phospho-specific antibody that recognizes AKT only when phosphorylated at Ser473 (top panels). The levels of AKT expression were examined with 0.1 μg/ml control AKT antibody (phosphorylation state independent; A–C, bottom panels). Proteins were visualized by enhanced chemiluminescence. The experiments were repeated twice with consistent results.
β1C Integrin Expression Inhibits MAP Kinase Activation Stimulated by Fibronectin
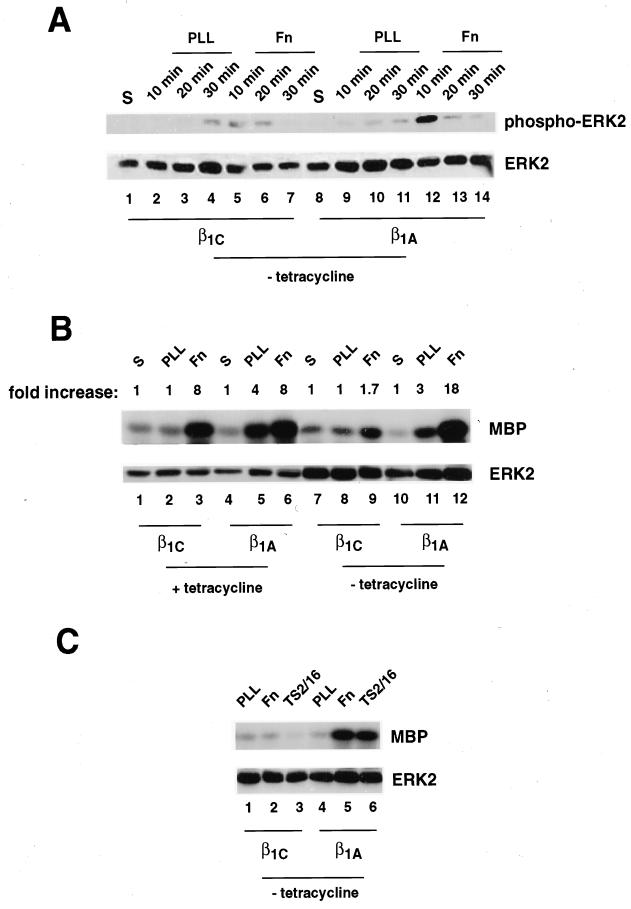
MAP kinase pathway activation by integrins is transient and is detectable soon after integrin engagement (maximum at 10 min in CHO cells; Figure 6A). We examined the ability of β1C and β1A to modulate ERK2 activation in CHO stable cell lines. Endogenous and exogenous integrins were engaged with fibronectin (Figure 6, A–C), whereas exogenous human β1C or exogenous human β1A integrins were engaged with TS2/16 (Figure 6C). The activation of ERK2 was analyzed by immunoblotting with E10 mAb, which recognizes the Thr202/Tyr204 phosphorylated form of ERK2 (Figure 6A, top panel), and by in vitro kinase assay (Figure 6, B and C, top panels); comparable amounts of ERK2 were used in the kinase assays (Figure 6, B and C, bottom panels). ERK2 activation was reduced significantly in β1C compared with β1A stable cell lines in response to integrin engagement by fibronectin as determined by immunoblotting (Figure 6A, top panel, lanes 5 and 12) and by in vitro kinase assay (Figure 6, B, top panel, lanes 9 and 12, and C, top panel, lanes 2 and 5). In the presence of tetracycline, adhesion to fibronectin mediated by endogenous integrins induced comparable ERK2 activation in both β1C and β1A stable cell lines (Figure 6B, top panel, lanes 3 and 6). Exogenous expression of β1 variants in CHO cells did not alter the expression levels of endogenous hamster β1 subunit or α5β1 integrin as assessed by FACS analysis (Figure 1, A and B; our unpublished results), indicating that the differences in ERK2 activation on fibronectin between β1C- and β1A-expressing cells were not due to changes in endogenous α5β1 integrin expression, the major fibronectin receptor in CHO cells.
Figure 6.
β1C prevents ERK2 activation mediated by fibronectin. β1C or β1A CHO stable cell lines were cultured as described for Figure 5. The cells were detached and either held in suspension (S; A, lanes 1 and 8; B, lanes 1, 4, 7, and 10) or seeded on tissue culture plates coated with poly-l-lysine (PLL; A, lanes 2–4 and 9–11; B, lanes 2, 5, 8, and 11; C, lanes 1 and 4), fibronectin (Fn; A, lanes 5–7 and 12–14; B, lanes 3, 6, 9, and 12; C, lanes 2 and 5), or TS2/16 (C, lanes 3 and 6) for either 10 min (A–C) or 20 or 30 min (A) at 37°C. Cells were lysed, and ERK2 activation was analyzed by immunoblotting (A) or by in vitro kinase assay (B and C). (A) Detergent cell extracts were analyzed with 0.5 μg/ml mAb E10, which recognizes ERK2 only when phosphorylated at Thr202/Tyr204 (top panel). (B and C) ERK2 was immunoprecipitated from 50 μg of total cell lysate with 0.5 μg of affinity-purified antibody to ERK2, and its kinase activity was analyzed by in vitro kinase assay with myelin basic protein (MBP) as a substrate. Phosphorylated MBP was visualized by autoradiography (top panels). The levels of expression of ERK2 were analyzed with 0.1 μg/ml rabbit affinity-purified antibody to ERK 2 (A–C, bottom panels). Proteins were visualized by enhanced chemiluminescence. In B, ERK2 activation is expressed as fold increase over the activity detected in cells held in suspension. The experiments were repeated at least twice with two separate clones for each variant with consistent results.
Ligation of β1C integrin by TS2/16 compared with poly-l-lysine did not induce activation of ERK2 as assessed by in vitro kinase assay (Figure 6C, top panel, lanes 1 and 3) or by immunoblotting with mAb E10 (our unpublished results). However, attachment of β1A stable cell lines to TS2/16 resulted in activation of ERK2 compared with poly-l-lysine (Figure 6C, top panel, lanes 4 and 6). These results show that β1C has an inhibitory effect on ERK2 activation mediated by fibronectin and, at variance with β1A, is not able to stimulate ERK2 activity. These results also show that ERK2 activity is inhibited in cells attached to fibronectin for 10 min when both FAK and AKT are activated.
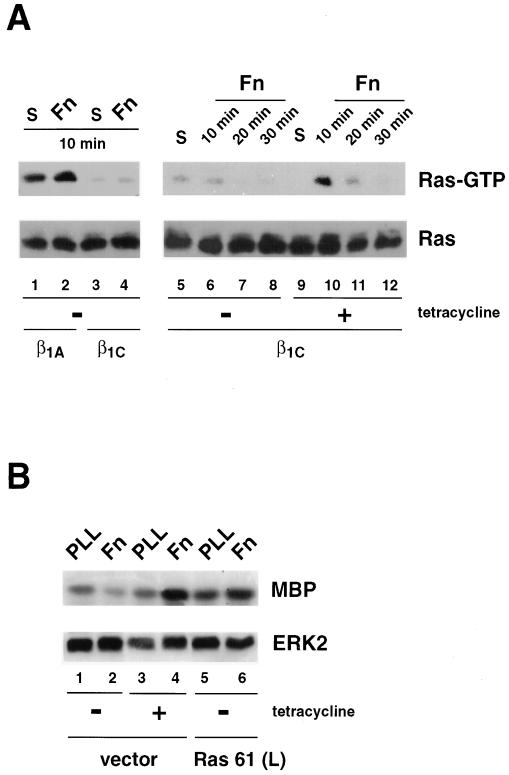
β1C Integrin Expression Inhibits Fibronectin-mediated Ras Activation
Several reports have shown the role of Ras as an important effector of integrin-mediated activation of the MAP kinase pathway (Schlaepfer et al., 1994, 1998; Clark and Hynes, 1996; Wary et al., 1996; King et al., 1997; Mainiero et al., 1997; Schlaepfer and Hunter, 1997; Wei et al., 1998). The data presented above indicate that β1C has an inhibitory effect on ERK2 activity. Therefore, to determine whether β1C mediated this effect at the level of Ras, Ras activation was assessed through its ability to bind the Ras-binding domain of Raf-1. This interaction has been shown to require GTP binding to Ras (Taylor and Shalloway, 1996). Adhesion of β1A cell transfectants to fibronectin as well as engagement of endogenous integrins by fibronectin in β1C stable cell lines cultured in the presence of tetracycline stimulated Ras activation (Figure 7A, top panel, lanes 1, 2, 9, and 10). Maximal activation of Ras in CHO cells in the presence of tetracycline was observed at 10 min (Figure 7A, top panel, lanes 10–12). In contrast, in the absence of tetracycline, β1C expression nearly abolished Ras activation mediated by fibronectin (Figure 7A, top panel, lanes 5–8). We investigated whether Ras could overcome the β1C inhibitory effect on fibronectin-mediated ERK2 activation by expressing a constitutively active form of Ras, Ras 61 (L). Transfection of β1C CHO stable cell lines with constitutively active Ras 61 (L) restored fibronectin-induced ERK2 activation to the levels observed in cells transfected with vector alone and cultured in the presence of tetracycline (Figure 7B, top panel, lanes 4 and 6). These data indicate that β1C inhibits the MAP kinase pathway by preventing Ras activation.
Figure 7.
β1C prevents Ras activation stimulated by fibronectin. (A) β1C or β1A CHO stable cell lines were cultured for 48 h either in the absence (lanes 1–8) or in the presence (lanes 9–12) of 1 μg/ml tetracycline and serum-starved during the last 24 h of the 48-h culture. The cells were detached and either held in suspension (S; lanes 1, 3, 5, and 9) or seeded on tissue culture plates coated with fibronectin (Fn; lanes 2, 4, 6–8, and 10–12) for 10 min (lanes 1–6, 9, and 10), 20 min (lanes 7 and 11), or 30 min (lanes 8 and 12) at 37°C. Cells were lysed, and Ras activation was analyzed by affinity precipitation with GST-RBD (top panels). Ras proteins were detected by immunoblotting with 2 μg/ml mAb to Ras (bottom panels). (B) β1C CHO stable cell lines were transiently transfected with constitutively activated Ras [Ras 61 (L); lanes 5 and 6] or vector alone (vector; lanes 1–4). Cells were cultured for 48 h either in the absence (lanes 1, 2, 5, and 6) or in the presence (lanes 3 and 4) of 1 μg/ml tetracycline and starved during the last 24 h of the 48-h culture. Transfected cells were then detached and plated on dishes coated with either poly-l-lysine (PLL; lanes 1, 3, and 5) or fibronectin (Fn; lanes 2, 4, and 6) for 10 min at 37°C. ERK2 in vitro kinase activity (top panel) and expression (bottom panel) were analyzed as described for Figure 6. In A and B (bottom panels), proteins were visualized by enhanced chemiluminescence. The experiments were repeated twice with consistent results.
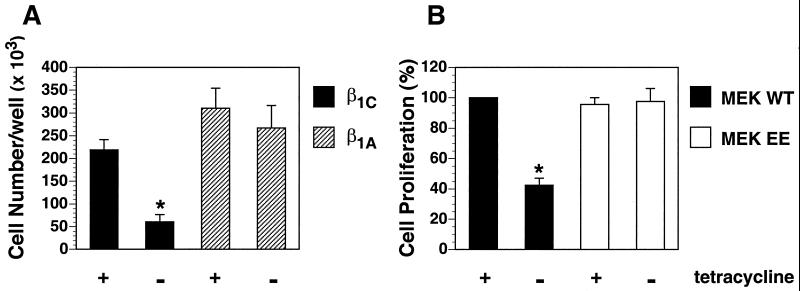
Inhibition of Cell Proliferation in β1C Transfectants Is Rescued by MEK
To evaluate whether down-regulation of ERK2 activity causes inhibition of cell proliferation in β1C transfectants, we transfected β1C CHO stable cell lines with either MEK WT or MEK EE. The levels of expression of both MEK WT and MEK EE were comparable as determined by immunoblotting with 12CA5 mAb to hemagglutinin (our unpublished results). As expected, β1C expression in CHO cells had an inhibitory effect on cell proliferation, whereas β1A did not affect cell proliferation in response to serum (Figure 8A). Transfection of β1C CHO stable cell lines with MEK EE restored cell proliferation to an extent similar to the level observed in cells cultured in the presence of tetracycline (Figure 8B). Thus, expression of constitutively active MEK rescues the inhibitory effect on cell proliferation exerted by β1C.
Figure 8.
Constitutively activated MEK rescues β1C inhibition of CHO cell proliferation. (A) β1C or β1A CHO stable cell lines were cultured as described for Figure 7A. Cells were detached, resuspended in serum-free medium, and plated (10,000 cells/well) on tissue culture plates coated with 1 μg/ml fibronectin for 1 h at 37°C. Attached cells were cultured for 96 h at 37°C in growth medium containing 5% FCS either in the absence or in the presence of 1 μg/ml tetracycline. Cells were washed, fixed with 3% paraformaldehyde, and stained overnight with 0.5% toluidine blue. Cell number was evaluated as described in MATERIALS AND METHODS. (B) β1C CHO stable cell lines were transiently transfected with either MEK WT or MEK EE. Cells were cultured for 48 h either in the absence or in the presence of 1 μg/ml tetracycline and starved during the last 24 h of the 48-h culture. Cells were detached, plated, and cultured for 72 h, and cell number was analyzed as described for A. Cell proliferation is expressed as percent relative to the value for MEK WT cultured in the presence of tetracycline. Shown is the average ± SEM from two separate experiments. Group differences were compared with one-way analysis of variance. In A, the differences in proliferation either between β1C CHO stable cell lines in the absence (*) and in the presence of tetracycline or between β1C CHO stable cell lines cultured in the absence of tetracycline (*) and β1A CHO stable cell lines cultured either in the presence or in the absence of tetracycline are statistically significant (p < 0.05). In B, the differences in cell proliferation either between MEK WT in the absence of tetracycline (*) and in the presence of tetracycline or between MEK WT in the absence of tetracycline (*) and MEK EE cultured either in the presence or in the absence of tetracycline are statistically significant (p < 0.05).
DISCUSSION
In this study, as indicated in the model shown in Figure 9, we demonstrate that β1C integrins inhibit ERK2 activation in response to cell adhesion to fibronectin by preventing Ras activation. It is also shown that β1C inhibits Ras and ERK2 activation without affecting either FAK phosphorylation or AKT activity. Engagement of β1C activates AKT but is not able to stimulate the MAP kinase pathway; this indicates that its unique cytodomain allows selective activation of the AKT kinase pathway in response to engagement of the common β1 extracellular domain. Furthermore, constitutively active MEK restored cell proliferation in β1C transfectants, suggesting that the negative effect of β1C on the Ras/ERK pathway causes inhibition of cell proliferation.
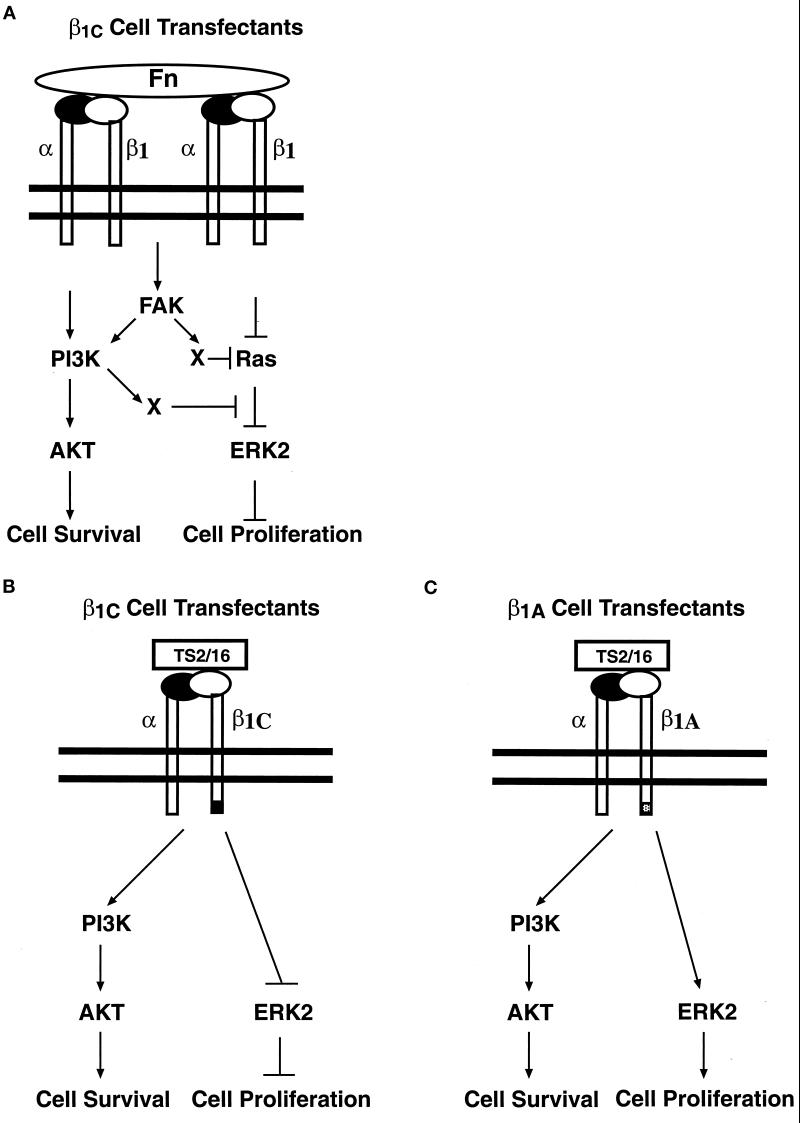
Figure 9.
Differential effect of β1C and β1A integrin cytoplasmic variants on FAK, AKT, and MAP kinase pathways. The schematic drawings illustrate the β1C effect on intracellular signaling pathways in response to exogenous and endogenous integrin engagement by fibronectin (Fn; A) or in response to either exogenous β1C (B) or exogenous β1A (C) ligation by TS2/16. The inhibitory effect of β1C on Ras/ERK2, but not on FAK and AKT pathways, is shown in A. The failure of β1C to induce ERK2 activation is shown in B. A previously described activation of the MAP kinase pathway by PI 3-kinase, downstream of Ras (King et al., 1997), is blocked in our model (A). It is also shown that AKT is activated in response to fibronectin (A), β1C (B), or β1A (C) engagement.
The aim of this investigation was to determine the roles of two integrin variants, β1C and β1A, in modulating specific signaling pathways that control cell proliferation and survival. Specifically, we studied MAP kinase, FAK, and AKT pathways. MAP kinase pathway involvement in mediating cell cycle progression and gene expression, as well as the ability of FAK and AKT to support cell survival and prevent anoikis, have been well documented (Frisch et al., 1996; Hungerford et al., 1996; Xu et al., 1996; Khwaja et al., 1997; Robinson and Cobb, 1997; Downward, 1998; Guadagno and Ferrell, 1998; Brunet et al., 1999; Cary and Guan, 1999). The mechanisms of integrin-mediated activation of the MAP kinase cascade comprise Ras-dependent and Ras-independent activation of ERK2 by integrins (Howe et al., 1998). Our results show that, in contrast to β1A, β1C has an inhibitory effect on Ras and ERK2 activation mediated by fibronectin. Selective inhibition of the Ras/MAP kinase pathway by β1C indicates that this integrin has the ability to either interfere with Ras membrane localization or inhibit positive regulators of Ras, or increase the activity of negative regulators of this molecule (Rebollo and Martinez-A, 1999). FAK has been shown to mediate Ras activation through Grb2/Sos binding (Schlaepfer and Hunter, 1998). However, in our system, we do not expect β1C to act through FAK because β1C inhibits ERK2 activity without affecting integrin signaling to FAK. This is the first description of a selective inhibitory role of the integrin cytoplasmic domain on a member of the MAP kinase family. In one instance, integrin down-regulation of FAK tyrosine phosphorylation and MAP kinase activity has been described (Sastry et al., 1999). Here we show that FAK phosphorylation and AKT activation can occur in the absence of ERK2 activation, indicating that β1C inhibits either a pathway downstream of FAK or AKT or a FAK- and AKT-independent pathway (Figure 9A). It has been described that PI 3-kinase is required for maximal fibronectin-mediated ERK2 activation and that it functions downstream of Ras (King et al., 1997); in our β1C-expressing cells, the PI 3-kinase/AKT pathway is active even though ERK2 is inhibited, suggesting that PI 3-kinase alone is not sufficient to activate ERK2 in the absence of Ras activation. It was reported recently that PKC inhibition selectively prevents ERK2 activation in response to integrin without affecting FAK tyrosine phosphorylation (Miranti et al., 1999). Thus, expression of β1C might down-regulate ERK2 activity in response to fibronectin adhesion via inhibition of PKC, which has been shown to act upstream of Ras (Miranti et al., 1999).
The β1C and β1A variants have a different subcellular distribution (Meredith et al., 1995); β1A localizes to focal contacts, whereas β1C remains diffuse on the cell surface. Thus, our results indicate that MAP kinase inhibition observed in β1C transfectants does not require β1C recruitment to focal adhesion complexes. In a previous report, we had attempted to study ERK2 activation in response to β1C or β1A engagement by TS2/16. However, we had not detected either β1C or β1A integrin-mediated ERK2 activation because of the low integrin levels and the low number of cells transfected in the transient expression system (Fornaro et al., 1999). Here, using stable cell lines that have higher levels of expression, we show the failure of β1C to activate ERK2, although we detect MAP kinase activation in response to β1A engagement (Figure 9B). In this study, it is also shown that AKT phosphorylation is observed in response to β1C engagement (Figure 9B). Therefore, specific domains in the extreme carboxy-terminal region of β1 are not required to activate the PI 3-kinase/AKT pathway. In our cell system as well as in the cell systems of others (King et al., 1997), AKT activation is PI 3-kinase dependent, because wortmannin completely prevents AKT serine phosphorylation in response to either endogenous or exogenous integrin engagement. Ras is a potent activator of PI 3-kinase, in addition to Raf and non-Raf pathways (Rebollo and Martinez-A, 1999); thus, in our experimental system, in which Ras is inhibited, stimulators of PI 3-kinase different from Ras are expected to be active. FAK is a potential candidate; PI 3-kinase is activated by FAK (Chen et al., 1996a). In our system, a causal effect of FAK activation on PI 3-kinase/AKT pathway stimulation, in response to either β1C or β1A engagement, remains to be investigated. Recent evidence points also to integrin-linked kinase (ILK) as a candidate effector for activation of AKT in response to integrin engagement, because ILK mediates PI 3-kinase–dependent AKT activation and binds the integrin β1 cytodomain (Hanningan et al., 1996; Delcommenne et al., 1998). However, ILK binds the integrin β1 cytodomain in a region that is not found in β1C (S. Dedhar, personal communication). Thus, although it is crucial for signaling pathways activated in response to β1A ligation, ILK is unlikely to play a role in the activation of AKT in β1C transfectants.
Cell adhesion to fibronectin or to β1 ligands is unaffected in response to β1C expression. Furthermore, the β1C variant associates with the same α subunits as β1A, indicating that up-regulation of β1C allows the cell to preserve the interaction with the extracellular matrix but, at the same time, to inhibit cell cycle progression. Therefore, we suggest that by expressing variant β1 intracellular domains, cells may accomplish the delicate task of inhibiting proliferation without affecting either selective downstream survival signals (FAK and AKT) mediated by integrins or interactions with the extracellular environment. This observation is very important because in vivo, β1C is expressed in nonproliferative and differentiated epithelium (Fornaro et al., 1998) and is selectively down-regulated in prostatic adenocarcinoma (Fornaro et al., 1996). Thus, the ability of β1C to sustain activation of signals that stimulate survival and differentiation (FAK and AKT) (Downward, 1998; Jiang et al., 1999) might be crucial to preventing apoptosis while blocking cell cycle progression and maintaining a differentiated phenotype. Failure to maintain a differentiated phenotype is believed to be an early event in cancer progression (Hunter, 1997), suggesting that loss of β1C might activate a cascade that contributes to a transformed phenotype.
We have shown previously that β1C expression increases p27kip1 protein levels (Fornaro et al., 1998). This cyclin kinase inhibitor is highly expressed in nonproliferative, quiescent cells, and its forced overexpression is sufficient to inhibit cell proliferation (Sherr and Roberts, 1995) and apoptosis (Hiromura et al., 1999). In prostate cancer, loss of p27kip1 is an adverse prognostic factor that correlates with poor patient survival (Catzavelos et al., 1997; Loda et al., 1997; Porter et al., 1997; Tsihlias et al., 1998; Yang et al., 1998). A report has indicated that oncogenic Ras-induced degradation of p27kip1 occurs through activation of the MAP kinase cascade (Kawada et al., 1997). Thus, it is conceivable that by blocking Ras activation, β1C expression, at variance with β1A expression, achieves the goal of inhibiting ERK2 activation and, consequently, p27kip1 degradation and cell proliferation.
ACKNOWLEDGMENTS
We thank Drs. M.E. Hemler, R.L. Juliano, and E. Ruoslahti for providing antibodies. We thank Dr. L. Moro for constructive suggestions on the manuscript. We are also grateful to R. Carbone for support in performing flow cytometric analysis and to N. Bennett for help with the preparation of the manuscript. This work was supported by National Institutes of Health grants CA-71870 and DK-52670 (to L.R.L.), by Army Prostate Cancer Research Program grant DAMD17-98-1-8506 (to L.R.L. and to M.F.), and by National Institutes of Health training grant T32DK0755622 (to C.A.S.).
Abbreviations used:
- AKT
Protein Kinase B/AKT
- CHO
Chinese hamster ovary
- ERK
extracellular signal–regulated kinase
- FAK
focal adhesion kinase
- ILK
integrin-linked kinase
- MEK
MAPK kinase
- MEK EE
constitutively active MEK
- MEK WT
wild-type MEK
- PI 3-kinase
phosphatidylinositol 3-kinase
REFERENCES
- Balzac F, Belkin AM, Koteliansky VE, Balabanov YV, Altruda F, Silengo L, Tarone G. Expression and functional analysis of a cytoplasmic domain variant of the β1 integrin subunit. J Cell Biol. 1993;121:171–178. doi: 10.1083/jcb.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfeld NS, Pasquale EB, Geltosky JE, Languino LR. The αvβ3 integrin associates with a 190-kDa protein that is phosphorylated on tyrosine in response to platelet-derived growth factor. J Biol Chem. 1993;268:17270–17276. [PubMed] [Google Scholar]
- Belkin AM, Retta SF. β1D integrin inhibits cell cycle progression in normal myoblasts and fibroblasts. J Biol Chem. 1998;273:15234–15240. doi: 10.1074/jbc.273.24.15234. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Retta SF, Pletjushkina OY, Balzac F, Silengo L, Fassler R, Koteliansky VE, Burridge K, Tarone G. Muscle β1D integrin reinforces the cytoskeleton-matrix link: modulation of integrin adhesive function by alternative splicing. J Cell Biol. 1997;139:1583–1595. doi: 10.1083/jcb.139.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AM, Hausdorff SF, O'Reilly AM, Freeman RMJ, Neel BG. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AM, Tonks NK. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- Bottazzi ME, Assoian RK. The extracellular matrix and mitogenic growth factors control G1 phase cyclins and cyclin-dependent kinase inhibitors. Trends Cell Biol. 1997;7:348–352. doi: 10.1016/S0962-8924(97)01114-8. [DOI] [PubMed] [Google Scholar]
- Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- Cary LA, Guan JL. Focal adhesion kinase in integrin-mediated signaling. Front Biosci. 1999;4:102–113. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- Catzavelos C, et al. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997;3:227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- Chen H-C, Appeddu PA, Isoda H, Guan J-L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996a;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- Chen H-C, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan J-L. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Chen H-C, Guan J-L. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Lin TH, Der CJ, Juliano LR. Integrin-mediated activation of MEK and mitogen activated protein kinase is independent of Ras. J Biol Chem. 1996b;271:18122–18127. doi: 10.1074/jbc.271.30.18122. [DOI] [PubMed] [Google Scholar]
- Clark E, Hynes R. Ras activation is necessary for integrin-mediated activation of extracellular signal-regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Languino LR. Alternatively spliced variants: a new view of the integrin cytoplasmic domain. Matrix Biol. 1997;16:185–193. doi: 10.1016/s0945-053x(97)90007-x. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Manzotti M, Tallini G, Slear AE, Bosari S, Ruoslahti E, Languino LR. β1C integrin in epithelial cells correlates with a nonproliferative phenotype: forced expression of β1C inhibits prostate epithelial cell proliferation. Am J Pathol. 1998;153:1079–1087. doi: 10.1016/s0002-9440(10)65652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Tallini G, Bofetiado CJM, Bosari S, Languino LR. Down-regulation of β1C integrin, an inhibitor of cell proliferation, in prostate carcinoma. Am J Pathol. 1996;149:765–773. [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Tallini G, Zheng DQ, Flanagan WM, Manzotti M, Languino LR. p27kip1 acts as a downstream effector of and is coexpressed with the β1C integrin in prostatic adenocarcinoma. J Clin Invest. 1999;103:321–329. doi: 10.1172/JCI4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Zheng DQ, Languino LR. The novel structural motif Gln795-Gln802 in the integrin β1C cytoplasmic domain regulates cell proliferation. J Biol Chem. 1995;270:24666–24669. doi: 10.1074/jbc.270.42.24666. [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the platelet-derived growth factor (PDGF)-activated phosphatidylinositol 3-kinase (PI 3-kinase) Cell. 1995;81:727–738. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chanhui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno TM, Ferrell JE. Requirement for MAPK activation for normal mitotic progression in Xenopus egg extracts. Science. 1998;282:1312–1314. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- Guan JL, Trevithick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanningan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Integrin associated proteins. Curr Opin Cell Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Weitzman JB, Pasqualini R, Kawaguchi S, Kassner PD, Berdichevsky FB. Structure, biochemical properties, and biological functions of integrin cytoplasmic domains. In: Takada Y, editor. Integrins: The Biological Problems. Boca Raton, FL: CRC Press; 1995. pp. 1–35. [Google Scholar]
- Hiromura K, Pippin JW, Fero ML, Roberts JM, Shankland SJ. Modulation of apoptosis by the cyclin-dependent kinase inhibitor p27kip1. J Clin Invest. 1999;103:597–604. doi: 10.1172/JCI5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Hungerford JE, Compton MT, Matter ML, Hoffstrom BG, Otey CA. Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J Cell Biol. 1996;135:1383–1390. doi: 10.1083/jcb.135.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Oncoprotein networks. Cell. 1997;88:333–346. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Illic D, Almeida EAC, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang BH, Aoki M, Zheng JZ, Li J, Vogt PK. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc Natl Acad Sci USA. 1999;96:2077–2081. doi: 10.1073/pnas.96.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada M, Yamagoe S, Murakami Y, Suzuki K, Mizuno S, Uehara Y. Induction of p27kip1 degradation and anchorage independence by Ras through the MAP kinase signaling pathway. Oncogene. 1997;15:629–637. doi: 10.1038/sj.onc.1201228. [DOI] [PubMed] [Google Scholar]
- Keely P, Parise L, Juliano R. Integrins and GTPases in tumor cell growth, motility and invasion. Trends Cell Biol. 1998;8:101–106. doi: 10.1016/s0962-8924(97)01219-1. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L, Earp H, Turner C, Prockop C, Juliano R. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering in β1 integrins. Proc Natl Acad Sci USA. 1991;88:8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino LR, Plescia J, Duperray A, Brian AA, Plow EF, Geltosky JE, Altieri DC. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1 dependent pathway. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- Languino LR, Ruoslahti E. An alternative form of the integrin β1 subunit with a variant cytoplasmic domain. J Biol Chem. 1992;267:7116–7120. [PubMed] [Google Scholar]
- Lewis JM, Schwartz MA. Mapping in vivo associations of cytoplasmic proteins with integrin β1 cytoplasmic domain mutants. Mol Biol Cell. 1995;6:151–160. doi: 10.1091/mbc.6.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Aplin AE, Shen Y, Chen Q, Schaller M, Romer L, Aukhil I, Juliano RL. Integrin-mediated activation of MAP kinase is independent of FAK evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol. 1997;136:1385–1395. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- Mainiero F, Murgia C, Wary KK, Curatola AM, Pepe A, Blumemberg M, Westwick JK, Der CJ, Giancotti FG. The coupling of α6β4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzotti M, Dell'Orto P, Maisonneuve P, Fornaro M, Languino LR, Viale G. Down-regulation of β1C integrin in breast carcinomas correlates with high proliferative fraction, high histological grade, and larger size. Am J Pathol. 2000;156:169–174. doi: 10.1016/s0002-9440(10)64716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith J, Jr, Takada Y, Fornaro M, Languino LR, Schwartz MA. Inhibition of cell cycle progression by the alternatively spliced integrin β1C. Science. 1995;269:1570–1572. doi: 10.1126/science.7545312. [DOI] [PubMed] [Google Scholar]
- Meredith JE, Jr, Kiosses WB, Takada Y, Schwartz MA. Mutational analysis of cell cycle inhibition by integrin β1C. J Biol Chem. 1999;274:8111–8116. doi: 10.1074/jbc.274.12.8111. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Ohno S, Brugge JS. Protein kinase C regulates integrin-induced activation of the extracellular regulated kinase pathway upstream of Shc. J Biol Chem. 1999;274:10571–10581. doi: 10.1074/jbc.274.15.10571. [DOI] [PubMed] [Google Scholar]
- Pfaff M, Liu S, Erle DJ, Ginsberg MH. Integrin β cytoplasmic domains differentially bind to cytoskeletal proteins. J Biol Chem. 1998;273:6104–6109. doi: 10.1074/jbc.273.11.6104. [DOI] [PubMed] [Google Scholar]
- Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–226. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- Rebollo A, Martinez-A C. Ras proteins: recent advances and new functions. Blood. 1999;94:2971–2980. [PubMed] [Google Scholar]
- Retta SF, Balzac F, Ferraris P, Belkin AM, Fassler R, Humphries MJ, De Leo G, Silengo L, Tarone G. β1-Integrin cytoplasmic subdomains involved in dominant negative function. Mol Biol Cell. 1998;9:715–731. doi: 10.1091/mbc.9.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Lakonishok M, Wu S, Truong TQ, Huttenlocher A, Turner CE, Horwitz AF. Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J Cell Biol. 1999;144:1295–1309. doi: 10.1083/jcb.144.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Integrin signaling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and Focal Adhesion Kinase- initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- Takada Y, Ylanne J, Mandelman D, Puzon W, Ginsberg MH. A point mutation of integrin β1 subunit blocks binding of α5β1 to fibronectin and invasion but not recruitment to adhesion plaques. J Cell Biol. 1992;119:913–921. doi: 10.1083/jcb.119.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- Tsihlias J, Kapusta LR, DeBoer G, Morava-Protzner I, Zbieranowski I, Bhattacharya N, Catzavelos GC, Koltz LH, Slingerland M. Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res. 1998;58:542–548. [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- Wei J, Shaw LM, Mercurio AM. Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the α6 integrin subunit. J Biol Chem. 1998;273:5903–5907. doi: 10.1074/jbc.273.10.5903. [DOI] [PubMed] [Google Scholar]
- Xu L-H, Owens LV, Sturge GC, Yang X, Liu ET, Craven RJ, Cance WG. Attenuation of the expression of the focal adhesion kinase induces apoptosis in tumor cells. Cell Growth Differ. 1996;7:413–418. [PubMed] [Google Scholar]
- Yamakita Y, Totsukawa G, Yamashiro S, Fry D, Zhang X, Hanks SK, Matsumura F. Dissociation of FAK/p130CAS/c-src complex during mitosis: role of mitosis-specific serine phosphorylation of FAK. J Cell Biol. 1999;144:315–324. doi: 10.1083/jcb.144.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RM, Naitoh J, Murphy M, Wang HJ, Phillipson J, deKernion JB, Loda M, Reiter RE. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol. 1998;159:941–945. [PubMed] [Google Scholar]
- Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via αVβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]