Abstract
To learn more about the structure of the DNA terminus at Tetrahymena thermophila telomeres, we have devised a ligation-mediated primer extension protocol to accurately measure the length of the G-strand overhang. We show that overhang length and the identity of the 3′-terminal nucleotide are tightly regulated. The majority of overhangs terminate in the sequence 5′-TTGGGGT and >80% are either 14–15 or 20–21 nucleotides in length. No significant changes in overhang length were detected as cells traversed the cell cycle. However, changes in length distribution were observed when cells exited the cell cycle, indicating an altered balance between DNA synthesis and degradation or end protection. We also provide evidence that rDNA molecules have overhangs on both telomeres. Full-length rDNA could be cloned by a strategy that depends on overhangs being present at both ends. Moreover, analysis of leading strand telomeres revealed that a significant fraction have overhangs ≥5 nucleotides. Our results indicate that generation of the terminal telomeric DNA structure is highly regulated and requires several distinct DNA-processing events.
Keywords: DNA replication/telomerase/telomere/Tetrahymena
Introduction
A major question in telomere biology concerns how the extreme terminus of the telomeric DNA is packaged to render it accessible to telomerase at certain stages in the cell cycle but inaccessible to other DNA-modifying activities such as nucleases and the DNA repair machinery (Greider, 1996; Griffith et al., 1999; Lundblad, 2000). Ultimately, the mechanism for packaging the DNA terminus depends on its structure. If the telomeric DNA ends in a single-stranded overhang, the overhang may be protected by single-stranded DNA binding proteins, as has been observed in the ciliated protozoa Oxytricha and Euplotes (Price, 1995; Horvath et al., 1998). Alternatively, it may be incorporated into a folded structure, such as the ‘t-loop’, which has been observed at some mammalian and protist telomeres (Griffith et al., 1999; Murti and Prescott, 1999; Munoz-Jordan et al., 2001). t-loops are lariat-like structures that are formed on the end of the chromosome when the overhang on the 3′ G-rich strand invades the duplex region of the telomeric tract. If the telomeric DNA is blunt ended, the two strands are likely to be capped by terminus binding protein(s).
Replication of telomeric DNA by the cellular replication machinery is predicted to generate structurally asymmetric telomeres with a blunt end on the leading strand telomere and an overhang on the G-rich strand of the lagging strand telomere (Lingner et al., 1995; Wellinger et al., 1996). Maintenance of such an asymmetric structure throughout the cell cycle would result in the two telomeres being packaged differently. It would also mean that only the lagging strand telomere could be extended by telomerase because telomerase requires single-stranded DNA as a substrate and is incapable of extending a blunt-ended duplex (Lingner and Cech, 1996). At present, there is considerable debate over whether such structurally asymmetric telomeres exist in nature or whether the leading strand telomere is always modified to generate a G-strand overhang (Makarov et al., 1997; Wright et al., 1997; Riha et al., 2000). Although telomeric DNA structure has been examined in a number of organisms, in each case critical information is missing about the structure of the leading or lagging strand telomere, and how this structure changes during the cell cycle. Hence, the structure of the terminal complex and how telomerase deals with this structure to add new repeats remain unclear.
In Saccharomyces cerevisiae, trypanosomes and the ciliates Euplotes and Oxytricha, the leading strand telomeres are processed to generate G-strand overhangs. Overhangs are thus present on both telomeres during at least part of the cell cycle (Klobutcher et al., 1981; Wellinger et al., 1993; Price, 1995; Munoz-Jordan et al., 2001). In yeast, overhangs of ∼50–100 nucleotides (nt) appear transiently in late S phase, but then disappear (Wellinger et al., 1993). These ‘long’ overhangs can form in the absence of telomerase, and thus are likely to be made by the combined action of nucleases that resect the C-strand, and telomerase, which extends the G-strand (Dionne and Wellinger, 1996; Wellinger et al., 1996). At present, it is not clear what structure is generated when the long overhangs disappear. It is generally assumed that the long overhangs are trimmed to shorter overhangs, but this remains to be proven.
Vertebrate telomeres have G-strand overhangs of ∼150–300 nt that are present throughout the cell cycle (Makarov et al., 1997; McElligott and Wellinger, 1997; Wright et al., 1997; Huffman et al., 2000). Formation of t-loops by these overhangs provides an elegant way to protect the DNA termini, but yet make them available to telomerase during S phase, as the loops would most likely be destroyed by passage of a replication fork. Thus, one might expect long (150–300 nt) overhangs to be present on both leading and lagging strand telomeres. However, attempts to examine G-strand overhang distribution on mammalian telomeres have led to conflicting results, with one study suggesting that such overhangs are present on only the lagging strand telomeres and another study suggesting that they are present on all telomeres (Makarov et al., 1997; Wright et al., 1997, 1999).
Here we present studies with the ciliate Tetrahymena thermophila that provide a comprehensive picture of leading and lagging strand telomere structure, and how this structure changes during the cell cycle. In Tetrahymena, the rDNA is an extrachromosomal palindrome that is amplified to a copy number of ∼10 000 molecules per macronucleus (Kapler, 1993; Coyne et al., 1996). The copy number of the other ∼200 macronuclear chromosomes is ∼45. This large number of telomeres facilitates structural analysis of both rDNA and non-rDNA telomeres. Tetrahymena macronuclear telomeres are composed of ∼300 bp of T2G4⋅A2C4 repeats (Blackburn and Gall, 1978; Larson et al., 1987). Although most telomeres are packaged into non-nucleosomal DNA–protein complexes (Blackburn and Chiou, 1981; Cohen and Blackburn, 1998), the telomere-binding proteins remain to be identified (Sheng et al., 1995). Previous studies demonstrated that the rDNA telomeres have G-strand overhangs that are in the 14–16 nt range (Henderson and Blackburn, 1989), but did not address whether overhang length changes during the cell cycle or whether overhangs exist on one or both ends of the rDNA.
We have devised a method that has allowed us to measure accurately the length of the overhangs on both rDNA and non-rDNA telomeres, and have used this procedure to follow changes in overhang length throughout the cell cycle. We show that overhang length is quite tightly regulated and the majority of telomeres end in the sequence 5′-GGGGT. Overhang length does not change significantly as cells traverse the cell cycle, but does change when they become starved. We also show that Tetrahymena have G-strand overhangs on both leading and lagging strand telomeres. Our results demonstrate that generation of the terminal DNA structure is a highly regulated process that involves two specific processing steps: one to generate the correct 3′-terminal nucleotide and one to generate an overhang at the leading strand telomere.
Results
Measurement of G-overhang length by ligation-mediated primer extension
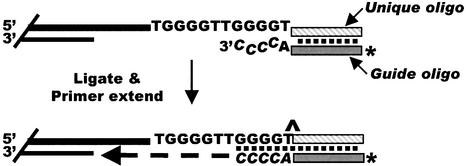
We have devised a method to accurately measure G-strand overhang length, which relies on primer extension from a unique sequence oligonucleotide that has been ligated to the end of the overhang (Figure 1). The unique sequence oligonucleotide that is to be ligated to the overhang is annealed to a complementary guide oligonucleotide that has an additional 5 nt of telomeric sequence at the 3′-terminus. The resulting 5 nt overhang acts as an adapter to guide ligation to the telomere. Following ligation, excess unligated unique oligonucleotide/guide oligonucleotide duplex is removed using a Qiagen PCR purification column. The guide oligonucleotide molecules that remain firmly anchored at the end of the G-strand overhang, by means of the ligated unique oligonucleotide, are then primer extended to the junction between the overhang and the double-stranded telomeric DNA using T4 DNA polymerase plus dATP and dCTP. Since T4 polymerase lacks strand displacement and 5′→3′ exonuclease activity, the final length of the extended guide oligonucleotide reflects the length of the overhang.
Fig. 1. Strategy for ligation-mediated primer extension: the duplex formed between the unique oligonucleotide and the 32P-labeled guide oligonucleotide is ligated to the 3′ G-strand overhang. The guide oligonucleotide is extended with T4 polymerase and the primer extension products are separated on a 12% sequencing gel. An asterisk marks the 32P label; ∧ marks the site of ligation.
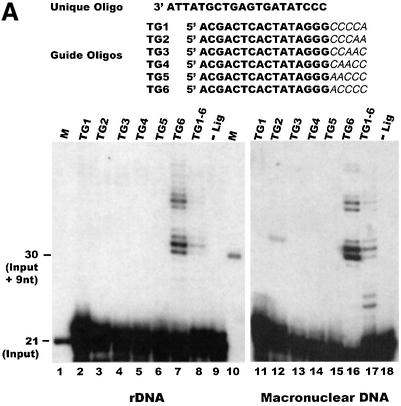
We tested the utility of this method with both total macronuclear DNA and purified rDNA from Tetrahymena (the rDNA minichromosomes have more telomeres per microgram of DNA). Since we did not know the identity of the terminal nucleotide on the macronuclear telomeres, we used six different 32P-labeled guide oligonucleotides. These six guide oligonucleotides complemented each permutation of the T2G4 telomeric sequence, thus ensuring that the adapter portion of at least one guide oligonucleotide would be the exact complement of the terminal 5 nt of the G-strand overhang. As shown in Figure 2A, the guide oligonucleotide TG6, which terminated in the sequence 5′-ACCCC (lanes 7 and 16), gave a large amount of extension product in reactions using both the purified rDNA minichromosomes and total macronuclear DNA. The amount of product generated with the other guide oligonucleotides was either undetectable or much reduced (see below for further discussion). No extension product was obtained if ligase was omitted from the ligation step (Figure 2A, lanes 9 and 18). This demonstrates that the guide oligonucleotide was not extended unless it was anchored to the template DNA via the ligated unique oligonucleotide. Thus, primer extension must occur from the extreme 3′ end rather than part way along the G-strand overhang.
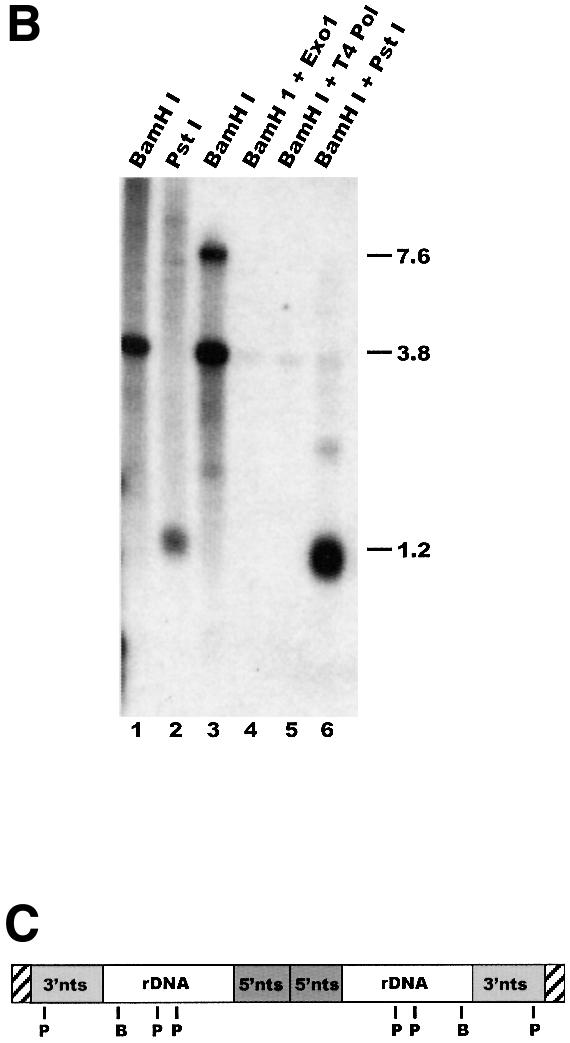
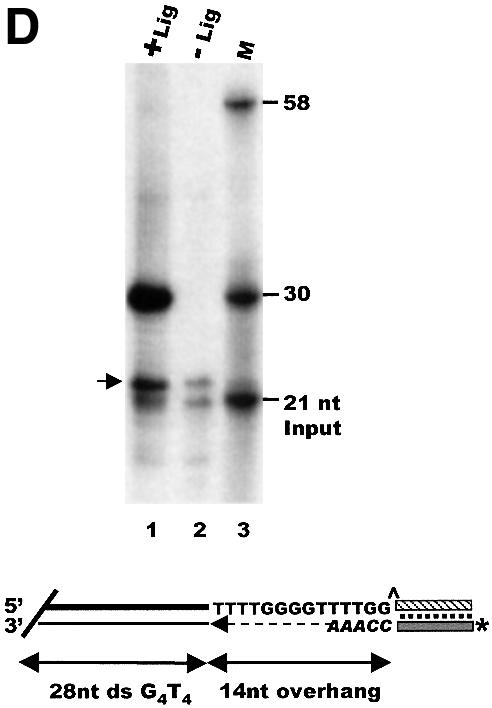
Fig. 2. Measurement of G-overhang length by ligation-mediated primer extension. (A) Extension products obtained using T.thermophila DNA and various guide oligonucleotides: lane 1, input 21 nt guide oligonucleotide; lane 10, 30 nt marker showing the expected size of the input guide oligonucleotide + 9 nt extension product (a 14 nt overhang); lanes 2–9, purified rDNA minichromosomes; lanes 11–18, total macronuclear DNA; lanes 2 and 11, guide oligonucleotide TG1; lanes 3 and 12, TG2; lanes 4 and 13, TG3; lanes 5 and 14, TG4; lanes 6 and 15, TG5; lanes 7 and 16, TG6; lanes 8 and 17, mix of TG1–TG6; lanes 9 and 18, mix of TG1–TG6, no DNA ligase. (B) Ligation occurs specifically to the telomeric G-strand overhang: alkaline agarose gel showing restriction fragments labeled by ligation of 32P-labeled unique oligonucleotide/TG6 duplex to total macronuclear DNA (lanes 1 and 2) or purified rDNA telomeres (lanes 3–6): lane 1, ligation followed by BamHI digestion; lane 2, ligation followed by PstI digestion; lane 3, BamHI digestion prior to ligation; lane 4, Exo1 then BamHI digestion and ligation; lane 5, T4 DNA polymerase then BamHI digestion and ligation; lane 6, samples from lane 3 were digested with PstI to release the 1.2 kb telomeric fragment. (C) Organization of Tetrahymena rDNA: hatched boxes represent telomeres; shaded boxes labeled 3′nts and 5′nts represent the 5′ and 3′ non-transcribed spacer regions; open boxes represent the rDNA transcription units. B and P mark BamHI and PstI sites. (D) Measurement of overhang length using Euplotes crassus DNA: lane 1, primer extension products; lane 2, minus ligase control; lane 3, marker oligonucleotides. The 21 nt marker corresponds to the input guide oligonucleotide, the 30 and 58 nt markers correspond to the expected product if the guide oligonucleotide is extended only to the end of the 14 nt G-strand overhang or all the way through the duplex region of the telomere. The arrow marks excess guide oligonucleotide to which a single untemplated nucleotide was added by the T4 polymerase. A diagram showing the organization of Euplotes telomeres and the products expected from ligation-mediated primer extension is shown below.
To determine whether ligation occurred specifically at telomeres rather than at random chromosome breaks, we next examined the DNA fragments to which the unique oligonucleotide had been ligated. Duplexes of 32P-labeled unique oligonucleotide and unlabeled TG6 guide oligonucleotide were ligated to total macronuclear DNA, and the DNA was digested with BamHI or PstI to release the rDNA telomeres as either 3.8 or 1.2 kb restriction fragments. This treatment resulted in heavy labeling of the rDNA telomeric restriction fragments, but not of the internal 14 kb BamHI or the 0.95, 4.3 and 8 kb PstI fragments (Figure 2B, lanes 1 and 2 and C). The rDNA was visible as discrete bands because of its abundance relative to other macronuclear chromosomes. Ligation to the telomeric restriction fragments was dependent on their having a 3′ overhang and could be abolished by prior treatment with a 3′→5′ exonuclease. When rDNA telomeres were released by BamHI digestion, gel purified, and ligated with the unique oligonucleotide/TG6 duplex (lane 3), a strong band of 3.8 kb was observed, as expected (note that the upper band of 7.6 kb in lane 3 is caused by self-ligation of the telomeric BamHI fragments). However, this band disappeared if the rDNA was treated with ExoI or T4 polymerase prior to BamHI digestion (lanes 4 and 5).
In a final control to check that the length of the primer extension product represented the length of the G-strand overhang accurately, we used our procedure to measure G-overhang length on telomeres from the ciliate Euplotes. Euplotes telomeres have exactly 28 bp of double-stranded G4T4⋅C4A4 repeats and a 14 nt overhang with the sequence 5′-T4G4T4G2 (Klobutcher et al., 1981). As shown in Figure 2D, when T4 DNA polymerase was used to extend the anchored guide oligonucleotide in the presence of only dCTP and dATP, a product of 30 nt was obtained. This is what would be expected for a 14 nt overhang, as the guide oligonucleotide was 21 nt with a 5 nt adapter sequence. Thus, 9 nt would have to be added to reach the end of a 14 nt overhang. If the T4 polymerase had strand displacement activity and were able to read through into the double-stranded region of the telomere, a product of 58 nt would be expected (30 nt from the guide oligonucleotide and overhang plus 28 nt from the double-stranded telomeric DNA). However, the 58 nt product was barely detectable, indicating that primer extension terminated efficiently at the junction with the double-stranded DNA.
Several features of the ligation-mediated primer extension protocol seem to contribute to its specificity and precision. First, the 5 nt telomeric adapter sequence on the guide oligonucleotide results in specific ligation of the unique sequence oligonucleotide to the G-strand overhang. Ligation is very inefficient if this adapter sequence is <5 nt (data not shown). The ligated unique sequence oligonucleotide allows stable base pairing of the guide oligonucleotide to the terminus of the telomeric DNA so that excess unligated oligonucleotide can be removed. This prevents primer extension from starting part way along the overhang. Use of a DNA polymerase that lacks strand displacement or 5′→3′ exonuclease activity means that primer extension stops quite precisely at the junction between the overhang and the double-stranded telomeric tract. As a result, the length of the extension products reflects the overhang length to within 1–2 nt (see below).
Identity of the terminal nucleotide
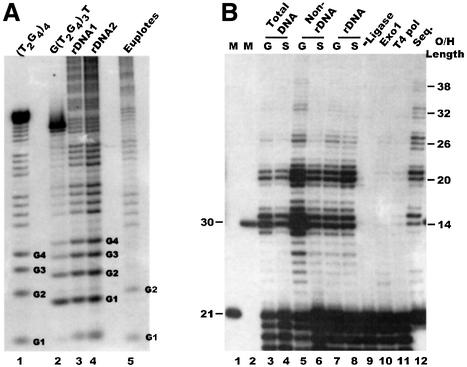
We were somewhat surprised to find that the majority of macronuclear telomeres could be ligated to the unique oligonucleotide/TG6 duplex, as this means that the G-strand overhangs terminate in the sequence 5′-TTGGGGT. Previous studies using osmium tetroxide footprinting of 3′ end-labeled rDNA suggested that the overhangs terminate in the sequence 5′-TTGGGG (Henderson and Blackburn, 1989). However, the first few nucleotides are poorly resolved by this technique, so it is difficult to identify the most 3′ nucleotide. To resolve this issue, we 3′ end-labeled rDNA and two control oligonucleotides that had the sequences 5′-(T2G4)4 or 5′ G(T2G4)3T, respectively, treated them with dimethylsulfate, and then performed a G-specific cleavage reaction. As shown in Figure 3A, the products from the oligonucleotide that terminated in 5′-TTGGGGT co-migrated with the 3′-terminal cleavage products from the rDNA, while the products generated from the oligonucleotide that terminated in 5′-TTGGGG were offset by one nucleotide. This co-migration of cleavage products from the rDNA and 5′-G(T2G4)3T oligonucleotide indicates that most macronuclear telomeres terminate with the sequence 5′-TTGGGGT, and supports the results obtained with ligation-mediated primer extension.
Fig. 3. Structure of Tetrahymena G-strand overhangs. (A) Identity of the terminal nucleotide. 3′ end-labeled DNA was cleaved at DMS-modified G-residues: lane 1, control oligonucleotide 5′ (T2G4)4; lane 2, control oligonucleotide 5′-G(T2G4)3T; lanes 3 and 4, rDNA from two different Tetrahymena clones; lane 5, Euplotes DNA. (B) Overhang length at rDNA and non-rDNA telomeres: lane 1, input guide oligonucleotide TG6; lane 2, marker oligonucleotide showing the expected size of the input oligonucleotide + 9 nt extension product; lanes 3–11, primer extension products obtained with T4 DNA polymerase; lane 12, products obtained with Sequenase; lanes 3 and 4, total DNA from exponentially growing and starved cells; lanes 5 and 6, non-rDNA from growing and starved cells; lanes 7 and 8, rDNA from growing and starved cells; lanes 9–12, rDNA from growing cells; lane 9, control reaction without ligase; lanes 10 and 11, Exo1 and T4 polymerase digestion prior to ligation. The overhang lengths corresponding to specific reaction products are indicated on the right.
Although primer extension reactions performed with purified rDNA only gave significant amounts of reaction product with the TG6 guide oligonucleotide, some preparations of total macronuclear DNA gave rise to product with both the TG6 and the TG2 guide oligonucleotides (Figure 2A). The main product obtained with the TG2 oligonucleotide corresponded to a 15 nt overhang with the sequence 5′-(GGGGTT)2GGG. This unusual overhang was clone specific as it was only present in some of the progeny when Tetrahymena strains B2086.2 and CU428.2 were mated. It also appeared to be present on only a fraction of the non-rDNA telomeres.
Length of the G-strand overhang
When ligation-mediated primer extension was performed with both rDNA and non-rDNA from various strains of growing and starved Tetrahymena cells (Figures 2A and 3B and data not shown), a very reproducible pattern of extension products was observed. The main products always differed in length by multiples of 6 nt, and the most abundant products corresponded to overhangs of 14 or 15 nt and 20 or 21 nt. This well defined product distribution indicates that overhang length is quite tightly regulated. Since the purified rDNA minichromosomes and macronuclear DNA from which the rDNA had been removed gave essentially identical extension products (Figure 3B, compare lanes 5 and 6 with 7 and 8), it appears that the structure of the rDNA telomeres is representative of macronuclear telomeres in general. The 6 nt periodicity in overhang length parallels both the 6 nt variation in the length of telomeric restriction fragments reported previously (Miller and Collins, 2000) and the periodicity of the extension products obtained from an in vitro reaction with Tetrahymena telomerase. Although the 6 nt periodicity suggests that regulation of overhang length is linked to repeat addition by telomerase, other factors must also be involved as the G-overhangs terminate with the sequence 5′-TTGGGGT, whereas the most prominent products generated by Tetrahymena telomerase in vitro result from the addition of 5′-GGGGTTG (Greider, 1995).
As shown in Figures 2A and 3B, the main extension products reproducibly appear as doublets (i.e. 14 and 15 nt, 20 and 21 nt, etc.). This phenomenon could be caused by heterogeneity in the length of the overhangs so that the duplex portion of the telomeric tract starts with the sequence C3(A2C4)n⋅G3(T2G4)n at some telomeres and C2(A2C4)n⋅G2(T2G4)n at others. Alternatively, natural breathing of the duplex during the extension reaction could allow the T4 polymerase to read 1 nt into the duplex region of the telomeric DNA. Based on experiments using different reaction conditions, we suspect that both factors contribute to doublet formation. Control experiments demonstrated that the extension pattern was not caused by template-dependent pausing of the T4 polymerase. The enzyme is quite processive and the small amount of product caused by pausing has a different pattern (data not shown).
Molecules that are smaller than the input guide oligonucleotide are apparent in both Figures 2A and 3B. These appear to result from degradation of the residual unligated guide oligonucleotide by the 3′→5′ nuclease activity associated with T4 polymerase. The smaller products are not observed if more of the unligated oligonucleotide is removed by applying the sample to Qiagen PCR purification columns after both the ligation and primer extension steps (Figure 4A) or if the extension reaction is performed with Sequenase (T7 polymerase), a DNA polymerase that has no 3′→5′ exonuclease activity (Figure 3B, lane 12). Since the T4 exonuclease activity is considerably slower than the polymerase, the exonuclease activity does not affect the final length of the extension products. As expected, the Sequenase reaction gives a higher proportion of longer products than the T4 DNA polymerase because of its strand displacement activity.
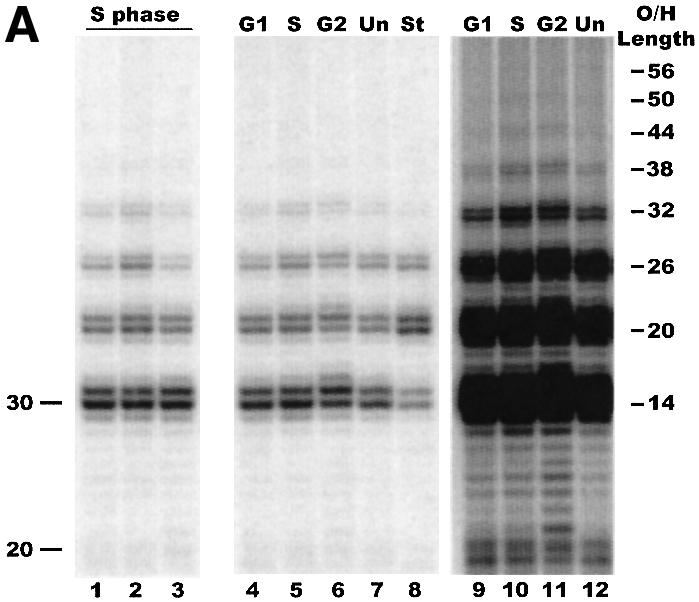
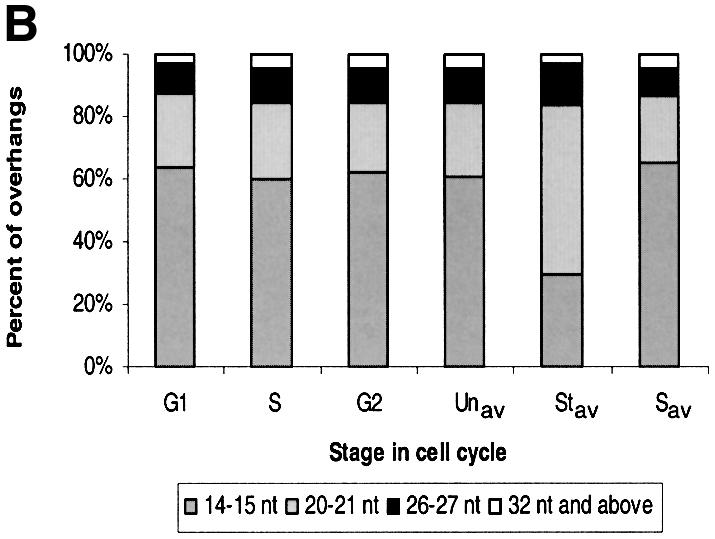
Fig. 4. Cell cycle-related changes in G-overhang length. (A) Primer extension products obtained using cells synchronized by centrifugal elutriation: lanes 1–3, DNA isolated from three different batches of S phase cells; lanes 4–6, DNA isolated from a single batch of elutriated cells after 30 min growth (lane 4), 60 min growth (lane 5) and 90 min growth (lane 6); lane 7, DNA from unsynchronized exponentially growing cells; lane 8, DNA from starved cells; lanes 9–12, longer exposure of lanes 4–7. Positions of markers are shown on the left. The overhang lengths corresponding to specific reaction products are indicated on the right. (B) Distribution of G-overhangs throughout the cell cycle. G1, S and G2, the reaction products in lanes 4–6 of (A) were quantified by PhosphorImager analysis and the total amount of reaction product in each pair of bands (i.e. the 14 and 15 nt products, the 20 and 21 nt products, etc.) was normalized to the total amount of product in that lane: G1, cells in G1 and early S phase (50% BrdU staining); S phase, 95% BrdU staining; G2, cells in G2 and late S (40% BrdU staining); Unav, Stav, Sav, the reaction products were quantified from four different batches of unsynchronized growing cells, starved cells and cells synchronized in S phase.
Cell cycle regulation of overhang length
Given that yeast telomeres undergo major cell cycle-related changes in G-overhang length (Wellinger et al., 1993), we next determined whether this also occurs at Tetrahymena telomeres. Cells at defined stages in the cell cycle were obtained by using centrifugal elutriation to collect cells that were in macronuclear G1 and then allowing them to grow until they reached S phase or G2 (Tang et al., 1997). The extent of synchrony and timing of S phase were monitored by growing the cells in the presence of bromodeoxyuridine (BrdU) for 30 min time intervals after elutriation and subsequently examining BrdU uptake by indirect immunofluorescence. The bulk of the cells entered S phase 30–60 min after elutriation (data not shown). At the 30 min time point, only 50% of the macronuclei showed BrdU incorporation and the overall intensity of staining was low; this rose to 95% at the 60 min time point and the intensity of staining became much stronger. By 90 min, the frequency of incorporation had decreased to 40% and the intensity of staining had also decreased. The low frequency of micronuclear staining in the G1 and S phase cultures provided additional evidence that the level of synchrony was high.
Interestingly, when we analyzed the G-overhangs from the elutriated cells we were unable to detect any obvious cell cycle-related changes in overhang length (Figure 4A). The pattern of extension products obtained with DNA isolated from several preparations of S phase cells was not significantly different from that obtained from unsynchronized exponentially growing cells (compare lanes 1–3 and 5 with 7). A similar pattern of extension products was also observed when DNA was isolated 30, 60 and 90 min after elutriation from a single batch of synchronized cells (Figure 4A, lanes 4–6, and B, G1, S, G2). Control reactions using oligonucleotides that had 20, 56 and 74 nt of T2G4 repeat showed that although ligation and primer extension are less efficient with longer substrates, we should have been able to see overhangs in the 50–80 nt range if they were present on a significant fraction of the telomeres (data not shown). Thus, regulation of overhang length in Tetrahymena appears to be different from that observed in yeast, where long G-strand overhangs are readily detectable in late S phase.
Unexpectedly, we did observe a difference in the distribution of overhang lengths with telomeres isolated from starved versus exponentially growing cells (Figure 4A, lanes 7 and 8). This difference was barely visible when the cells were starved for only 12 h (Figure 3B), but became quite apparent when the period of starvation was increased to 24 h (Figure 4A, lanes 7 and 8). PhosphorImager analysis of the primer extension products generated from multiple experiments revealed that the fraction of telomeres with the shortest (14–15 nt) and longest (>36 nt) overhangs decreased, while the fraction with 20–21 nt overhangs more than doubled (Figure 4B, Unav, Stav, Sav). This finding indicates that the balance between synthesis and degradation of the G- and C-strands must change as cells exit the cell cycle.
Are overhangs present on both telomeres?
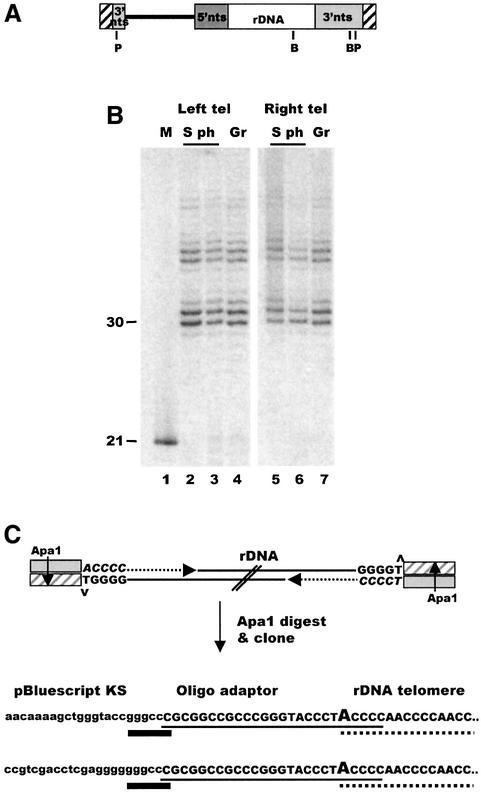
To address whether Tetrahymena chromosomes have overhangs on both or only one telomere, we decided to make use of rDNA molecules engineered to be non-palindromic (Figure 5A). Like the natural rDNA, Tetrahymena cells maintain these 14 kb molecules as stable linear minichromosomes that have telomeres on each end (Yao et al., 1990; Yasuda and Yao, 1991). The advantages of the non-palindromic rDNA are that it is relatively small (see below) and the two telomeres give rise to different sized restriction fragments. We could thus separate the individual telomeres prior to examining overhang length by ligation-mediated primer extension. Figure 5B shows the primer extension products obtained using either the 1.4 kb BamHI fragment that comes from the natural rDNA telomere (Figure 5A, right telomere) or the 10 kb BamHI fragment that contains both plasmid DNA and natural rDNA subtelomeric sequence (Figure 5A, left telomere). Both telomeres gave rise to the same pattern of extension products regardless of when in the cell cycle the DNA was isolated (Figure 5B, data not shown). Thus, G-overhangs of similar length were present on both the right and left rDNA telomeres.
Fig. 5. Are G overhangs present on both rDNA telomeres? (A) Organization of the 14 kb non-palindromic rDNA. The minichromosome carries a single rDNA transcription unit (unshaded box) with an extra BamHI site and a portion of the cloning vector (black line) used to engineer the molecule to be defective in palindrome formation. Hatched boxes represent telomeres, shaded boxes are the 3′ and 5′ non-transcribed spacer regions, B and P mark BamHI and PstI sites. (B) Primer extension products obtained from the left and right telomere of the non-palindromic rDNA: lane 1, input oligonucleotide; lanes 2–4, extension products from the left telomere; lanes 5–7, products from the right telomere; lanes 2, 3, 5 and 6, S phase cells; lanes 4 and 7, growing cells. (C) Strategy for cloning rDNA molecules with overhangs on both ends. Unique oligonucleotide/guide oligonucleotide duplexes were ligated to the 3′ overhangs on purified rDNA and the guide oligonucleotide extended with T7 polymerase. Following ApaI digestion, molecules with two sticky ends were cloned into pBluescript. The sequences shown below were obtained from the cloned rDNA molecules using the M13 reverse primer (upper sequence) and the M13 universal primer (lower sequence). The heavy line marks the ApaI site at the junction with the pBluescript vector sequence; the lighter line marks the oligonucleotide adapter; the dotted line marks the start of G-strand overhang on the rDNA telomere; the capitalized A is complementary to the 3′-terminal T on the overhang.
Although the above experiment demonstrates that overhangs can be present at either telomere, it does not address whether overhangs are present on both telomeres of a single molecule. To examine this possibility, we attempted to clone the 14 kb non-palindromic rDNA molecule following ligation of a unique oligonucleotide/TG6 duplex carrying a restriction site to the G-overhang (Figure 5C). If both ends of the rDNA molecules have overhangs, they should be relatively easy to clone, as subsequent restriction digestion of the ApaI site in the ligated duplex would yield a sticky end on each end of the molecule. The above cloning strategy gave rise to two clones that contained full-length 14 kb non-palindromic rDNA molecules. Sequencing demonstrated that the clones had the expected linker and telomeric DNA sequence (i.e. the telomeric G-strand terminated with the sequence 5′-GGGGT) at each vector/insert junction (Figure 5C). Thus, it appears that some rDNA molecules have G-overhangs on both ends. However, it was not clear whether the low number of clones containing full-length rDNA molecules was caused by the relative rarity of molecules with overhangs on both telomeres, or merely reflected the instability of clones containing the large rDNA molecule.
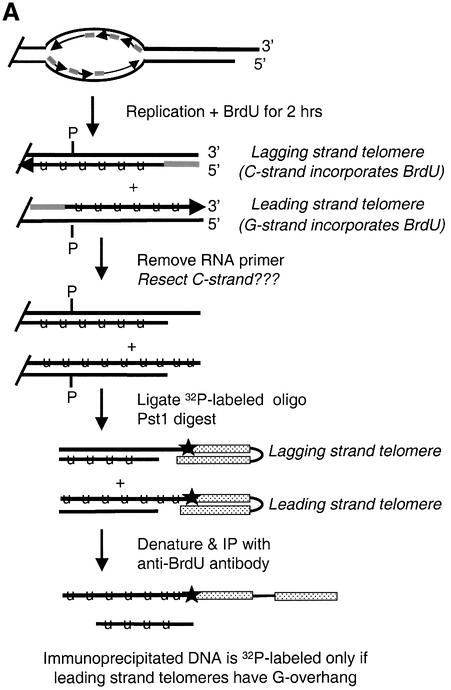
To address this question, we performed a more direct assay to look for overhangs on leading strand telomeres (the telomeres predicted to be blunt ended). Our approach was to grow cells in BrdU for one round of DNA replication so as to label the G-strands on only the leading strand telomeres (Wright et al., 1997). We then ligated 32P-labeled unique oligonucleotide to those telomeres that had G-overhangs, released the telomeric restriction fragments by PstI digestion, and isolated the G-strands of leading strand telomeres by immunoprecipitation with BrdU antibodies (Figure 6A). 32P should only precipitate with the leading strand telomeric DNA if the leading strand telomeres had G-strand overhangs.
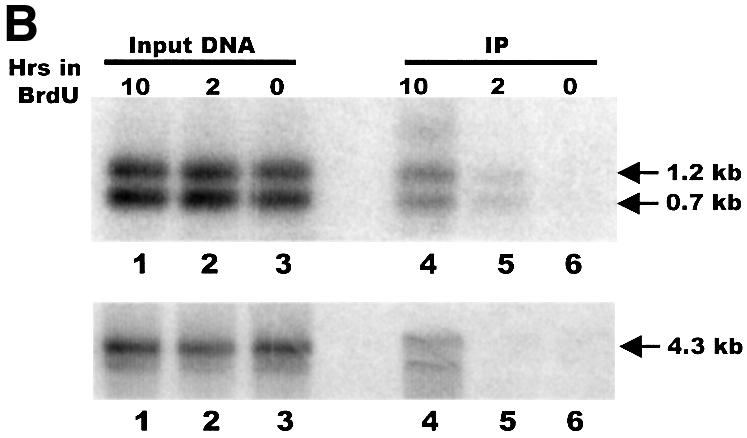
Fig. 6. Leading strand telomeres have G-overhangs. (A) Strategy for detecting leading strand telomeres with G-overhangs. The ligation step was performed as previously described except that the unique oligonucleotide/guide oligonucleotide duplex was replaced by a single 5′ end-labeled foldback oligonucleotide. This foldback oligonucleotide had the same 5′-ACCCC adapter sequence as the TG6 guide oligonucleotide. Lines marked with U represent BrdU-labeled DNA, the gray lines represent RNA primers, P marks PstI sites, the star marks the 32P label. (B) DNA immunoprecipitated with BrdU antibody. Samples of input and immunoprecipitated DNA were separated on a 1% agarose gel and blotted to nylon membrane. Upper panel: the membrane was directly exposed to a PhosphorImager screen to identify telomeric restriction fragments labeled by ligation to the 32P-labeled foldback oligonucleotide. Lanes 1–3, input DNA that had been labeled with BrdU for 10 h (lane 1), 2 h (lane 2) or 0 h (lane 3). Lanes 4–6, immunoprecipitates from samples labeled with BrdU for 10 h (lane 4), 2 h (lane 5) or 0 h (lane 6). The arrows mark the restriction fragments from the left (0.7 kb) and the right (1.2 kb) telomere. Lower panel: the membrane was hybridized to a probe for a 4.3 kb non-telomeric rDNA restriction fragment. Lanes 1–6 are the same as in the upper panel.
Cultures of Tetrahymena were grown in BrdU for just under one cell cycle (∼2 h) to allow incorporation of BrdU into only the newly replicated daughter strands, or for 3–4 cell cycles (10–12 h) to allow incorporation into both strands. The rDNA was isolated and a unique oligonucleotide was ligated to the G-strand overhangs as before. The rDNA was digested with PstI, denatured, and the BrdU-labeled DNA immunoprecipitated. An excess of cold rDNA was added prior to the denaturation step to minimize re-annealing of the BrdU-labeled G- and C-strands. When the immunoprecipitation products were separated on an agarose gel, blotted to nylon membrane and directly exposed to a PhosphorImager screen, we found that almost no 32P was precipitated with samples that lacked BrdU (Figure 6B, lane 6, upper panel). However, a significant amount of 32P-labeled DNA was precipitated from the samples that had incorporated BrdU for one round of DNA replication (lane 5). This 32P-labeled DNA corresponded in size to the left (0.7 kb) and right (1.2 kb) telomeric restriction fragments. Thus, it appeared that at least some of the leading strand telomeres had G-strand overhangs of sufficient length (≥5 nt) to allow ligation of the labeled unique oligonucleotide.
As might be expected, substantially more 32P was precipitated with the samples that had incorporated BrdU during multiple rounds of replication. The longer period of BrdU uptake should have resulted in BrdU incorporation into both DNA strands, and hence immunoprecipitation of leading and lagging strand telomeres. This overall higher level of BrdU incorporation, and hence immunoprecipitation, in the 10 h samples was confirmed by subsequent Southern hybridization using a 4 kb non-telomeric restriction fragment as the probe (Figure 6B, lower panel). Because we do not know the final concentration of BrdU per cell or the efficiency of the immunoprecipitation with different levels of BrdU incorporation, it was not possible to estimate what proportion of leading strand telomeres have G-strand overhangs. However, the amount of 32P-labeled leading strand DNA relative to total DNA that was immunoprecipitated was comparable for the 2 and 10 h samples. Thus, it appears that overhangs may be present on a substantial fraction of the leading strand telomeres.
Discussion
In this paper we describe a novel ligation-mediated primer extension protocol that makes it possible to measure the length of telomeric G-strand overhangs very accurately. This procedure has allowed us to characterize the structure of Tetrahymena telomeres throughout the cell cycle, and to demonstrate that overhangs are present on both leading and lagging strand telomeres. Our studies demonstrate that the nucleoprotein complexes present at Tetrahymena telomeres contain very specific DNA structures and provide insight into how these structures are generated. It should be possible to use the ligation-mediated primer extension protocol to obtain detailed information about the telomeric structure of other organisms that have a regular telomeric repeat sequence. For organisms that have fewer telomeres than Tetrahymena, the sensitivity could be increased by enriching for telomeric restriction fragments prior to the ligation step.
Generation of the terminal nucleotide
Using the ligation-mediated primer extension protocol has allowed us to define the terminal nucleotide on T.thermophila macronuclear telomeres. Interestingly, the great majority of rDNA telomeres and most non-rDNA telomeres end with the sequence 5′-TGGGGT instead of the 5′-GGGTTG sequence that would be expected if the terminus were generated as a result of telomerase dissociating during the translocation step (Greider and Blackburn, 1989; Greider, 1996). This implies that in vivo telomerase either dissociates before the whole RNA template is copied, or that it dissociates at the end of the template but the 3′ end of the telomeric DNA is then processed. One possibility is that the natural DNA terminus is created by the nuclease activity that appears to be part of the telomerase holoenzyme (Collins and Greider, 1993; Collins, 1999). However, since the 5′-GGGGT terminus is not generated in vitro, the nuclease activity would have to be subject to in vivo regulation. It is intriguing that a fraction of the non-rDNA telomeres from certain Tetrahymena clones terminate with the sequence 5′-TTGGG. This genetically determined variation in non-rDNA telomeres points to a very specific mechanism for regulating 3′-terminus formation.
Regulation of overhang length
The length of the G-strand overhang on Tetrahymena telomeres is clearly well regulated as >80% of the overhangs are 14–15 or 20–21 nt in length, and all the telomeres have overhangs that differ in length by multiples of six. This well defined length distribution suggests that overhang length is regulated by a counting activity that keeps track of the number of single-stranded telomeric repeats and regulates further repeat addition or removal. The interesting changes in overhang length that take place when cells enter G0 provide further insight into how length regulation may be achieved. In cycling cells, ∼60% of the overhangs are 14–15 nt, ∼20% are 20–21 nt and ∼10% are 26–27 nt. However, as the cells become starved, the fraction of overhangs with 14–15 nt falls to ∼25%, while the fraction with 20–21 nt increases to 60%, and the fraction with 26–27 nt increases slightly. The specific conversion of 14–15 nt overhangs to 20–21 nt overhangs upon starvation must involve either removal of a single repeat from the C-strand by a 5′→3′ nuclease or addition of a single repeat to the G-strand by telomerase or telomerase in association with a 3′→5′ nuclease. One way that this type of tight regulation might be achieved is through the binding of a G-overhang capping protein that has a preference for a specific length of T2G4 sequence. The convergence of the shortest and longest overhangs to 20–21 nt, or less frequently 26–27 nt, suggests that 20–21 and maybe 26–27 nt overhangs are somehow optimal during periods of stress. This could be because they provide the best substrate for the overhang-binding protein(s). When cells are cycling rapidly, formation of a stable nucleoprotein complex may be less important.
It is striking that we were unable to detect long G-strand overhangs on Tetrahymena telomeres in late S phase. Although Tetrahymena cannot be blocked efficiently at specific stages in the cell cycle, centrifugal elutriation allowed us to obtain cultures traversing the cell cycle with a high level of synchrony. Thus, if long overhangs analogous to those observed in yeast were present for significant periods of time during S phase or G2, they should have been detected by our ligation-mediated primer extension protocol. However, we cannot rule out very transient changes in overhang structure, as it was not possible to obtain absolutely synchronous cultures. In addition, the ligation-mediated primer extension may not be sensitive enough to detect long overhangs if they are present on only a small fraction of the telomeres. Until recently, it was thought that the generation of long overhangs in yeast might be required to make the telomeric DNA a good telomerase substrate. However, recent experiments have shown that telomerase can use an overhang of 4 nt for repeat addition (Diede and Gottschling, 1999). Thus, the long overhangs that are generated in yeast during S phase may merely be a consequence of the replication machinery dislodging protective telomere proteins (e.g. Cdc13p) and hence allowing access of nucleases to the telomeric DNA. Our results support this latter scenario as telomerase is clearly able to maintain Tetrahymena telomeres despite the apparent absence of long G-strand overhangs.
Leading versus lagging strand telomeres
Our results demonstrate that Tetrahymena chromosomes have G-strand overhangs on both the leading and lagging strand telomeres. Since leading strand synthesis is predicted to generate a blunt-ended duplex, the leading strand telomere must therefore be subject to a specific processing step following passage of the replication machinery. This processing step could involve resection of the C-strand by a nuclease or extension of the G-strand by telomerase. However, for the G-strand to be extended by telomerase, the duplex would first have to be melted by some form of helicase activity to make the 3′-terminus accessible to the telomerase RNA template. Regardless of whether the leading strand telomere is generated by telomerase or a nuclease, the processing step is clearly well regulated as the G-strand overhang is ≥14 nt and ≤27 nt at 90% of all macronuclear telomeres. Whether or not the leading and lagging strand telomeres have overhangs of the same length is unclear. It could be that in cycling cells overhangs of 14 or 15 nt are generated by removal of the RNA primer during lagging strand synthesis, while the longer overhangs are generated by processing of the leading strand telomere. We tried to address this point experimentally by examining the primer extension products associated with immunoprecipitated leading strand telomeres, but heavy DNA losses prevented successful analysis.
Telomere architecture
Our finding that overhangs exist on both leading and lagging strand telomeres indicates that the two telomeres of Tetrahymena macronuclear chromosomes are likely to be packaged into architecturally similar structures. One intriguing question concerns whether these structures involve the formation of t-loops. To date, t-loops have only been observed at the telomeres of organisms with much longer telomeric tracts and G-overhangs of 75–500 nt (Griffith et al., 1999; Murti and Prescott, 1999; Munoz-Jordan et al., 2001). Thus, it is not clear whether stable t-loops could form from an ∼300 bp telomeric tract and a 14–27 nt G-overhang. In mammalian cells, t-loop formation is mediated by telomere-binding proteins (Griffith et al., 1999), so it is possible that, in Tetrahymena, equivalent proteins play an even more essential role in t-loop stabilization. Alternatively, protection of the DNA terminus might be mediated solely by proteins that bind directly to the G-strand overhang. The very short telomeres of hypotrichous ciliates are protected by proteins that are tailored to recognize the 14 or 16 nt 3′ G2/4T4G4T4 overhang (Price, 1995), so a similar mechanism of end protection may have evolved for organisms such as yeast and Tetrahymena, which have medium length (∼300 bp) telomeres. In yeast, protection would probably be accomplished by the G-strand binding protein Cdc13 in complex with StnI (Pennock et al., 2001). While overhang-binding proteins have not yet been isolated from Tetrahymena, our results with G0 cells suggest they would have a preference for an overhang of 20 or 26 nt with the sequence 3′-TG4T2G4T2.
Materials and methods
Tetrahymena growth and transformation
Tetrahymena thermophila cell lines CU427.2 [Chx/Chx (VI, cy-s)], CU428.2 [Mpr/Mpr (VII, mp-s)] and B2086.2 were grown at 30°C in 1× or 2× PPYS medium (2% protease peptone, 0.2% yeast extract, 0.003% sequestrin). Clones containing the 14 kb rDNA minichromosome were generated by exconjugant transfection using the rDNA vector D500, which is defective in palindrome formation (Yao et al., 1990; Yasuda and Yao, 1991). Cell lines CU428 and B2086 were starved for 16 h in 10 mM Tris–HCl pH 7.5, then mixed and transformed by particle bombardment (Cassidy-Hanley et al., 1997) 10 h later. The exconjugants were transferred to fresh medium and transformants were selected by adding paramomycin to 250 µg/ml 6 h later. Clones that contained a high copy number of the non-palindromic rDNA were identified by Southern blotting.
DNA isolation
DNA was isolated from exponentially growing and starved cultures as described by Brehm and Cech (1983). The rDNA was isolated by extraction with a hot phenol/cresol mix (Spangler and Blackburn, 1985), followed by gel purification. Alternatively, the telomeric restriction fragments were gel purified directly from total macronuclear DNA following BamHI or PstI digestion. Macronuclear DNA that lacked rDNA was obtained by gel purification of DNA that migrated more slowly than the rDNA.
Measurement of G-overhang length by ligation-mediated primer extension
Duplexes of the unique and guide oligonucleotides were prepared by heating 0.25 pmol of unlabeled phosphorylated unique oligonucleotide and 0.25 pmol of 32P 5′ end-labeled guide oligonucleotide to 75°C, and slow cooling to room temperature. The 0.25 pmol of duplex were ligated to 1 µg of AluI–HinfI-digested total macronuclear DNA or to 5–25 ng rDNA telomeric restriction fragment obtained by gel purification following BamHI or PstI digestion. Ligation was performed at 16°C overnight with 40 U of T4 DNA ligase. Most of the unligated duplex was removed using a Qiagen PCR purification column. Primer extension was performed at 30°C for 90 min in 50 µl reactions containing 40 µl of column eluate, 0.5 mM dATP, 0.5 mM dCTP, 50 mg/ml bovine serum albumin (BSA), 10 mM Tris pH 7.9, 50 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol (DTT) and 4 U of T4 DNA polymerase. The samples were then extracted with phenol/chloroform or re-applied to a Qiagen PCR purification column to remove the remaining unligated oligonucleotide. Following ethanol precipitation and denaturation, the primer extension products were separated on 12% polyacrylamide sequencing gels.
3′ end-labeling and guanine-specific cleavage
Control oligonucleotides and purified rDNA were 3′ end-labeled with terminal transferase and [32P]ddATP for 1 h at 37°C. Each 10 µl reaction contained 100 fmol of DNA ends, 25 mM Tris pH 6.6, 200 mM potassium cacodylate, 250 µg/µl BSA, 1.5 mM CoCl2, 0.5 µl ddATP (6000 Ci/mM) and 15 U of terminal transferase. The 3′ end-labeled DNA was gel purified, and the guanine-specific modification and cleavage performed as previously described (Vermeesch and Price, 1994). One microliter of a 1/100 dilution of dimethyl sulfate (DMS) was added to 19 µl of 3′ end-labeled DNA, and left for 10 min at 25°C. Pyrrolidine (20 µl; 2 M) was added to stop the reaction, and the DNA was cleaved at methylated guanines by heating to 90°C for 15 min.
Centrifugal elutriation
Elutriation of Tetrahymena cells in macronuclear G1 was performed essentially as described for Paramecium (Tang et al., 1997), except that the pump and rotor speeds were optimized for the smaller Tetrahymena cells and the different tubing. One liter of exponentially growing culture (2.0 × 105 cells/ml) was loaded into a 40 ml elutriation chamber and then washed with fresh medium using a pump speed of 105 ml/min and a rotor speed of 1000 r.p.m. The cells were elutriated by raising the pump speed to 160 ml/min and collecting 100 ml fractions. The second and third fractions were saved as they contained the bulk of the G1 cells. Subsequent BrdU labeling indicated that >95% of the elutriated cells were in G1.
BrdU labeling and detection
Cells were grown in 100 mg/ml BrdU for 15 or 30 min intervals. They were then washed with 10 mM Tris pH 7.5, fixed in 70% ethanol and air-dried onto polylysine-coated slides. The slides were treated with 2 M HCl for 30 min at room temperature and washed with Tris-buffered saline, 0.1% BSA. Incorporated BrdU was detected by incubation with anti-BrdU antibody (Amersham-Pharmacia) for 1 h at room temperature, followed by 30 min incubation with fluorescein isothiocyanate-conjugated secondary antibody.
Cloning full-length rDNA minichromosomes
Two partially complementary oligonucleotides, Tdup1KNA (5′-AGGGTACCCGGGCGGCCGCGGGCCCGTACCCC) and Tdup2ANK (5′-ACGGGCCCGCGGCCGCCCGGGTACCCTACCCC), were annealed to form duplexes that contained a unique ApaI site and had a similar terminal structure to the unique oligonucleotide/TG6 duplex. This ApaI duplex was ligated to G-strand overhangs on the 14 kb rDNA as described above. The oligonucleotides were primer extended with T7 DNA polymerase, and the extension products were covalently joined to the 5′ telomeric strand by a second round of ligation. The DNA was then digested with ApaI, ligated to ApaI-digested pBluescript KS and transformed into XL-10Gold ultracompetent cells (Stratagene). Clones containing full-length rDNA molecules were identified by colony hybridization and restriction mapping. The insert/vector junctions were then sequenced.
Separation of leading and lagging strand telomeres
Cultures of Tetrahymena harboring the 14 kb non-palindromic rDNA were grown in the presence of 120 mM BrdU for a single cell division (∼2 h), or in 60 mM BrdU for 3–4 cell divisions (10 h). The rDNA was gel purified, ligated to the 5′-end-labeled foldback oligonucleotide Napatel (5′-ATCTCGGGCCCGCGGCCGCCTAATAGGCGGCCGCGGGCCCGAGATACCCC) as described above, and digested with PstI to release the leading and lagging strand telomeres. The newly replicated DNA strands were separated from the parental strands by immunoprecipitation with antibody to BrdU (Sigma). Protein G beads were prepared by incubating 10 ml of beads with 10 ml of antibody for 1 h at room temperature in 200 µl of 20 mM Tris pH 8.6, 100 mM H3BO3, 120 mM NaCl, 100 mM KCl, 1.5 mM MgCl2, 0.1 mM EDTA, 5 mM β-mercaptoethanol, and then washing the beads three times with phosphate-buffered saline (PBS)/1% Triton X-100. A 20-fold excess of non-BrdU-labeled rDNA was added to the BrdU-labeled DNA (to prevent subsequent re-annealing of the BrdU-labeled restriction fragments), the sample was denatured by boiling for 3 min, immediately chilled on ice, and added to the antibody-bound protein G beads suspended in PBS/1% Triton X-100. The 70 µl reaction was incubated for 30 min at room temperature, the beads were then washed six times with PBS/1% Triton X-100, and the bound DNA eluted by boiling for 5 min in 10 mM Tris pH 8.0, 1 mM EDTA, 1% SDS. The unbound DNA fraction was deproteinized before the bound and unbound fractions were extracted with phenol/chloroform, ethanol precipitated, separated on a 1% agarose gel and transferred to nylon membrane.
Acknowledgments
Acknowledgements
We thank Peter Bruns for the Tetrahymena cell lines CU428 and B2086, Meng-Chao Yao for the non-palindromic rDNA vector, and Jeff Kapler for his advice on growing Tetrahymena and rDNA isolation. We thank Eric Cole for his help with the centrifugal elutriation and Jeff Payne for allowing us to use his centrifuge. This work was supported by a National Institutes of Health grant (RO1 GM41803).
References
- Arezi B. and Kuchta,R.D. (2000) Eukaryotic DNA primase. Trends Biochem. Sci., 25, 572–576. [DOI] [PubMed] [Google Scholar]
- Blackburn E.H. and Chiou,S.S. (1981) Non-nucleosomal packaging of a tandemly repeated DNA sequence at termini of extrachromosomal DNA coding for rRNA in Tetrahymena. Proc. Natl Acad. Sci. USA, 78, 2263–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E.H. and Gall,J.G. (1978) A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J. Mol. Biol., 120, 33–53. [DOI] [PubMed] [Google Scholar]
- Brehm S.L. and Cech,T.R. (1983) Fate of an intervening sequence ribonucleic acid: excision and cyclization of the Tetrahymena ribosomal ribonucleic acid intervening sequence in vivo. Biochemistry, 22, 2390–2397. [DOI] [PubMed] [Google Scholar]
- Cassidy-Hanley D., Bowen,J., Lee,J.H., Cole,E., VerPlank,L.A., Gaertig,J., Gorovsky,M.A. and Bruns,P.J. (1997) Germline and somatic transformation of mating Tetrahymena thermophila by particle bombardment. Genetics, 146, 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. and Blackburn,E.H. (1998) Two types of telomeric chromatin in Tetrahymena thermophila. J. Mol. Biol., 280, 327–344. [DOI] [PubMed] [Google Scholar]
- Collins K. (1999) Ciliate telomerase biochemistry. Annu. Rev. Biochem., 68, 187–218. [DOI] [PubMed] [Google Scholar]
- Collins K. and Greider,C.W. (1993) Tetrahymena telomerase catalyzes nucleolytic cleavage and nonprocessive elongation. Genes Dev., 7, 1364–1376. [DOI] [PubMed] [Google Scholar]
- Coyne R.S., Chalker,D.L. and Yao,M.C. (1996) Genome downsizing during ciliate development: nuclear division of labor through chromosome restructuring. Annu. Rev. Genet., 30, 557–578. [DOI] [PubMed] [Google Scholar]
- Diede S.J. and Gottschling,D.E. (1999) Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerases α and δ. Cell, 99, 723–733. [DOI] [PubMed] [Google Scholar]
- Dionne I. and Wellinger,R.J. (1996) Cell cycle-regulated generation of single-stranded G-rich DNA in the absence of telomerase. Proc. Natl Acad. Sci. USA, 93, 13902–13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. (1995) Telomerase biochemistry and regulation. In Blackburn,E. and Greider,C. (eds), Telomeres. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Greider C.W. (1996) Telomere length regulation. Annu. Rev. Biochem., 65, 337–365. [DOI] [PubMed] [Google Scholar]
- Greider C.W. and Blackburn,E.H. (1989) A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature, 337, 331–337. [DOI] [PubMed] [Google Scholar]
- Griffith J.D., Comeau,L., Rosenfield,S., Stansel,R.M., Bianchi,A., Moss,H. and de Lange,T. (1999) Mammalian telomeres end in a large duplex loop. Cell, 97, 503–514. [DOI] [PubMed] [Google Scholar]
- Henderson E.R. and Blackburn,E.H. (1989) An overhanging 3′ terminus is a conserved feature of telomeres. Mol. Cell. Biol., 9, 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath M.P., Schweiker,V.L., Bevilacqua,J.M., Ruggles,J.A. and Schultz,S.C. (1998) Crystal structure of the Oxytricha nova telomere end binding protein complexed with single strand DNA. Cell, 95, 963–974. [DOI] [PubMed] [Google Scholar]
- Huffman K.E., Levene,S.D., Tesmer,V.M., Shay,J.W. and Wright,W.E. (2000) Telomere shortening is proportional to the size of the 3′ G-rich telomeric overhang. J. Biol. Chem., 275, 19719–19722. [DOI] [PubMed] [Google Scholar]
- Kapler G.M. (1993) Developmentally regulated processing and replication of the Tetrahymena rDNA minichromosome. Curr. Opin. Genet. Dev., 3, 730–735. [DOI] [PubMed] [Google Scholar]
- Klobutcher L.A., Swanton,M.T., Donini,P. and Prescott,D.M. (1981) All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3′ terminus. Proc. Natl Acad. Sci. USA, 78, 3015–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D.D., Spangler,E.A. and Blackburn,E.H. (1987) Dynamics of telomere length variation in Tetrahymena thermophila. Cell, 50, 477–483. [DOI] [PubMed] [Google Scholar]
- Lingner J. and Cech,T.R. (1996) Purification of telomerase from Euplotes aediculatus: requirement of a primer 3′ overhang. Proc. Natl Acad. Sci. USA, 93, 10712–10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingner J., Cooper,J.P. and Cech,T.R. (1995) Telomerase and DNA end replication: no longer a lagging strand problem? Science, 269, 1533–1534. [DOI] [PubMed] [Google Scholar]
- Lundblad V. (2000) DNA ends: maintenance of chromosome termini versus repair of double strand breaks. Mutat. Res., 451, 227–240. [DOI] [PubMed] [Google Scholar]
- Makarov V.L., Hirose,Y. and Langmore,J.P. (1997) Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell, 88, 657–666. [DOI] [PubMed] [Google Scholar]
- McElligott R. and Wellinger,R.J. (1997) The terminal DNA structure of mammalian chromosomes. EMBO J., 16, 3705–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.M. and Collins,K. (2000) The Tetrahymena p80/p95 complex is required for proper telomere length maintenance and micronuclear genome stability. Mol. Cell, 6, 827–837. [DOI] [PubMed] [Google Scholar]
- Munoz-Jordan J.L., Cross,G.A., de Lange,T. and Griffith,J.D. (2001) t-loops at trypanosome telomeres. EMBO J., 20, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murti K.G. and Prescott,D.M. (1999) Telomeres of polytene chromosomes in a ciliated protozoan terminate in duplex DNA loops. Proc. Natl Acad. Sci. USA, 96, 14436–14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock E., Buckley,K. and Lundblad,V. (2001) Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell, 104, 387–396. [DOI] [PubMed] [Google Scholar]
- Price C.M. (1995) Telomere-binding proteins of ciliated protozoa. In Eckstein,F. and Lilley,D. (eds), Nucleic Acids and Molecular Biology. Vol. 9. Springer-Verlag, Berlin, Germany, pp. 299–307.
- Riha K., McKnight,T.D., Fajkus,J., Vyskot,B. and Shippen,D.E. (2000) Analysis of the G-overhang structures on plant telomeres: evidence for two distinct telomere architectures. Plant J., 23, 633–641. [DOI] [PubMed] [Google Scholar]
- Sheng H., Hou,Z., Schierer,T., Dobbs,D.L. and Henderson,E. (1995) Identification and characterization of a putative telomere end-binding protein from Tetrahymena thermophila. Mol. Cell. Biol., 15, 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler E.A. and Blackburn,E.H. (1985) The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J. Biol. Chem., 260, 6334–6340. [PubMed] [Google Scholar]
- Tang L., Adl,S.M. and Berger,J.D. (1997) A CDC2-related kinase is associated with macronuclear DNA synthesis in Paramecium tetraurelia. J. Eukaryot. Microbiol., 44, 269–275. [Google Scholar]
- Wellinger R.J., Wolf,A.J. and Zakian,V.A. (1993) Saccharomyces telomeres acquire single-strand TG13 tails late in S phase. Cell, 72, 51–60. [DOI] [PubMed] [Google Scholar]
- Vermeesch J.R. and Price,C.M. (1994) Telomeric DNA sequence and structure following de novo telomere synthesis in Euplotes crassus. Mol. Cell. Biol., 14, 554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W.E., Tesmer,V.M., Huffman,K.E., Levene,S.D. and Shay,J.W. (1997) Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes Dev., 11, 2801–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W.E., Tesmer,V.M., Liao,M.L. and Shay,J.W. (1999) Normal human telomeres are not late replicating. Exp. Cell Res., 251, 492–499. [DOI] [PubMed] [Google Scholar]
- Yao M.C., Yao,C.H. and Monks,B. (1990) The controlling sequence for site-specific chromosome breakage in Tetrahymena. Cell, 63, 763–772. [DOI] [PubMed] [Google Scholar]
- Yasuda L.F. and Yao,M.C. (1991) Short inverted repeats at a free end signal large palindromic DNA formation in Tetrahymena. Cell, 67, 505–516. [DOI] [PubMed] [Google Scholar]