Abstract
The present study examined whether the ability of mutant p53 to block apoptosis depended on its transcriptional activity. A core domain mutant p53 (143 Val to Ala), in which two N-terminal residues (22 and 23) essential for transactivation were also mutated (Leu to Glu and Trp to Ser, respectively), was examined. While p53 containing only the core mutation efficiently interfered with drug-induced apoptosis, further modification at the N-terminus abolished this blocking activity. Furthermore, expression of c-myc, a suggested target for core mutant p53 transactivation, was elevated in the core mutant p53-expressing cells, but was abolished in the presence of the transcription-deficient p53 core mutant. In addition, wild-type p53, mutated in the N-terminus (residues 22 and 23), was unable to induce apoptosis by itself. Nevertheless, it synergized with drugs in the induction of apoptosis. This suggests that the integrity of the N-terminus is essential for both the activity of wild-type p53 in apoptosis and for mutant p53-mediated block of drug-induced apoptosis. This supports the notion that core p53 mutants act via a gain of function mechanism.
Keywords: apoptosis/c-myc/mutant p53/transcription activity
Introduction
The p53 tumor suppressor gene plays a central role in the regulation of the cell cycle. Once activated by genotoxic stress, wild-type p53 may induce a variety of cellular processes including growth arrest and apoptosis (Ko and Prives, 1996; El-Deiry, 1998; Gottlieb and Oren, 1998). Numerous stimuli can cause p53-dependent apoptosis, including DNA damage (Maltzman and Czyzyk, 1984; Kastan et al., 1991), activation of dominant oncogenes (Serrano et al., 1997) and hypoxia (Graeber et al., 1996).
p53-induced apoptosis has been shown by several studies to be dependent on the sequence-specific transactivation (SST) function of the p53 protein (Sabbatini et al., 1995; Attardi et al., 1996; Yonish-Rouach et al., 1995; Chao et al., 2000; Jimenez et al., 2000). Other reports have shown that p53-mediated apoptosis may also occur through SST-independent mechanisms (Caelles et al., 1994; Wagner et al., 1994; Haupt et al., 1995; Roemer and Mueller-Lantzsch, 1996; Ding et al., 2000; Kokontis et al., 2001). The contribution of each of these pathways to p53-induced apoptosis, and the interactions between the two, are poorly understood. Nevertheless, the physiological and clinical importance of the p53 apoptotic pathway have been clearly demonstrated. p53-induced apoptosis acts to protect the organism from cancer development by eliminating potential tumor precursor cells. Additionally, p53-dependent apoptosis can affect the outcome of cancer therapy. Experiments comparing transformed murine fibroblasts from normal and p53-deficient mice demonstrated that disruption of p53 function reduces apoptosis induced by anticancer agents (Lowe et al., 1993). Subsequent studies using an in vivo model have provided further support for this notion (Lowe et al., 1994).
Given the major role of p53 as a tumor suppressor, it is not surprising that mutations in the p53 gene are the most frequent genetic alterations in human cancer (Hussain and Harris, 1998). Most p53 mutations are clustered within the DNA-binding domain of the protein, resulting in mutant proteins expressed at high levels (Rotter, 1983). Typically, a mutation in one of the p53 alleles leads to inactivation of the remaining wild-type allele by a dominant-negative mechanism. Transdominance of p53 mutants over the wild-type p53 form and repression of wild-type p53 activity were demonstrated in various experimental systems (Kern et al., 1992; Shaulian et al., 1992; Srivastava et al., 1993; Unger et al., 1993; Chene, 1998). Addition ally, it appears that at least some mutant p53 proteins may exert oncogenic effects by a gain of function mechanism, independent of the inhibition of wild-type p53. Supporting this model are observations that expression of mutant p53 protein in p53-null transformed cells increases tumorigenicity (Wolf et al., 1984; Dittmer et al., 1993; Hsiao et al., 1994; Lanyi et al., 1998), mutation frequency (Iwamoto et al., 1996) and metastatic potential (Crook and Vousden, 1992; Hsiao et al., 1994). Resistance to chemotherapy is a major obstacle to cancer treatment. The relationship between mutant p53 expression and the resistance of tumors to apoptosis is not clear. While several studies have demonstrated that expression of mutant p53 results in chemoresistance, others have reported an opposite correlation (reviewed in Hickman and Dive, 1999).
In our previous studies we have found that in M1/2, a p53 non-producer murine myeloid cell line, wild-type p53 synergizes with p53-independent apoptosis induced by removal of growth factors, while the expression of the p53 135 (Ala to Val) mutant interferes with this apoptotic pathway (Peled et al., 1996). We reported that the same murine mutant, expressed in M1/2 cells, can protect against p53-independent apoptosis mediated by γ-irradiation and several chemotherapeutic agents (Li et al., 1998).
To further expand these observations, we introduced into the M1/2 cell line human p53 mutants, frequently expressed in human tumors, and examined their ability to suppress the induction of apoptosis by chemotherapeutic agents. We found that these mutant proteins confer anti-apoptotic function against cisplatin, α-amanitin and etoposide, but do not interfere with doxorubicin-, DRB (5,6-dichloro-1-β-d-ribofuranosylbenzimidazole)- or actino mycin D-induced apoptosis. The observation that the p53 N-terminal domain is rarely modified in human tumors (Hussain and Harris, 2000), coupled with the characterization of this domain as the transcription activation domain of wild-type (Unger et al., 1992; Subler et al., 1994) and mutant p53 (Lin et al., 1995; Frazier et al., 1998), prompted us to investigate whether the transcriptional activity of mutant p53 is required for the anti-apoptotic function of this protein. For this purpose we generated cell lines that express N-terminally modified p53 core mutant and compared their apoptosis blocking activity with that mediated by cell lines expressing the p53 core mutant. We found that transcriptional activation is necessary for the interference of mutant p53 with drug-induced apoptosis. As it is expected that mutant p53 will transactivate target genes other then those transactivated by wild-type p53, we focused our analysis on the expression of c-myc, which is suggested to be a target gene for mutant p53 (Frazier et al., 1998). We found that cells express higher levels of c-myc in the presence of mutant p53, yet this elevation in c-myc is abolished when mutant p53 is transcriptionally inactive and unable to induce drug resistance. In addition, we addressed the need for transcription in wild-type p53-induced apoptosis by using the same modification. We show that p53-dependent apoptosis may occur without the involvement of SST, but unlike apoptosis mediated by SST-competent p53, SST-independent apoptosis occurs only upon genotoxic stress.
Results
In our previous studies we have observed that expression of the murine mutant p53 protein, p53 135 (Ala to Val), protects cells from apoptosis induced by several chemotherapeutic agents (Li et al., 1998). Our present experiments were aimed at determining whether a similar phenomenon is also exhibited by human p53 mutants. Using a retroviral infection procedure (see Materials and methods), we established a series of stable clones expressing human p53 mutant proteins, derived from M1/2, a myeloid p53 non-producer parental cell line. The clones generated expressed various levels of either p53 143 (Val to Ala), which is known to be a temperature-sensitive (ts) mutation (Zhang et al., 1994), or p53 248 (Arg to Trp) or p53 273 (Arg to His), which are the most frequent mutations in p53 found in human cancer cells.
Analysis of the effect of the p53 143 (Val to Ala) mutant protein on drug-induced apoptosis
Cell cycle and apoptosis analysis of clones expressing the p53 143 (Val to Ala) protein, following a shift from 37 to 32°C, confirmed that the p53 protein expressed behaves in a ts manner. However, the induction of apoptosis in these clones at 32°C was slower, compared with that observed previously with established clones expressing the mouse ts protein p53 135 (Ala to Val) (data not shown). We analyzed the effect of p53 143 (Val to Ala) protein expression on p53-independent apoptosis induced by anti cancer agents. Two representative clones were selected, p53ts-143-103 and p53ts-143-106, expressing lower and higher levels of the p53 143 (Val to Ala) protein, respectively (see Figure 1A). Cells were treated with increasing concentrations of various drugs and maintained for 24 h either at 37°C, the permissive temperature for the mutant conformation of p53 143 (Val to Ala), or at 32°C, the permissive temperature for the wild-type p53 conformation. Apoptosis was mostly analyzed by the fluorescence-activated cell sorter (FACS)-based acridine orange (AO) DNA denaturability assay. This assay is based on the differential denaturability of interphase, mitotic and apoptotic DNA at low pH. Chromatin degradation, which is specific for apoptosis, renders the DNA sensitive to denaturation (Darzynkiewicz et al., 1994). This is a very reliable and reproducible assay for apoptosis. Some of the experiments were also performed in parallel by the propidium iodide (PI) exclusion assay. Both assays gave similar results. It should be mentioned that since the M1/2 cell line and its derived clones are dependent on conditioned medium (CM) (Peled et al., 1996), each experiment was performed with the same CM batch. Variations observed in the percentage of apoptosis in different experiments are probably due to the use of different CM batches.
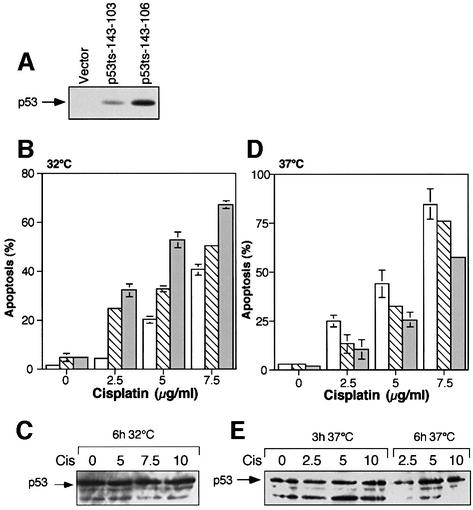
Fig. 1. Cisplatin-induced apoptosis in p53 143 (Val to Ala) expressing M1/2 cells. (A) A quantitative western blot analysis of M1/2-derived clones expressing p53 143 (Val to Ala). Vector, a clone expressing the empty vector. (B) Wild-type p53 synergizes with cisplatin-induced apoptosis. A comparison of apoptosis induction in M1/2-derived clones expressing the p53 143 (Val to Ala) protein or the empty vector after cisplatin treatment and incubation at 32°C. Open bars represent a clone generated by infection with empty vector, hatched bars represent clone p53ts-143-103 and gray bars represent clone p53ts-143-106. (C) p53 143 (Val to Ala) protein expression was quantitated in clone p53ts-143-106 after 6 h of cisplatin treatment at 32°C. Cis, cisplatin. (D) Mutant p53 143 (Val to Ala) protein expression interferes with cisplatin-induced apoptosis. M1/2-derived clones expressing p53 143 (Val to Ala) protein were incubated with cisplatin at 37°C for 24 h and harvested for FACS. Open bars represent a clone generated by infection with empty vector, hatched bars represent clone p53ts-143-103 and gray bars represent clone p53ts-143-106. (E) Cells treated as in (D) were harvested for western blot analysis after 3 and 6 h of treatment. Western blotting was performed with p53ts-143-106 cells. Cis, cisplatin.
Cisplatin-mediated apoptosis
Cisplatin causes DNA damage by intra- and interstrand cross-links in the DNA, which may induce cell cycle arrest and apoptosis (Zamble and Lippard, 1995). Figure 1 depicts the apoptotic patterns observed for cisplatin treatments. Upon a shift to 32°C, p53 143 (Val to Ala)-expressing cells underwent low levels of apoptosis in the absence of any treatment, due to the expression of p53 in the wild-type conformation. However, following treatment with cisplatin, the fraction of cells undergoing apoptosis was enhanced significantly (Figure 1B). This is in agreement with our previous observation, in which a synergy between murine wild-type p53-mediated apoptosis and cisplatin-mediated apoptosis was evident (Li et al., 1998). A similar set of samples was utilized to compare the levels of p53 143 (Val to Ala) protein before and after cisplatin treatment. As can be seen in Figure 1C, drug treatment at 32°C did not affect the levels of the p53 143 (Val to Ala) protein.
We next analyzed the effect of p53 143 (Val to Ala) protein expression at 37°C (the permissive temperature for the mutant conformation) on cisplatin-mediated apoptosis. Twenty-four hours after cisplatin treatment, cells were fixed and subjected to the AO assay. FACS analysis revealed that p53 143 (Val to Ala) expressing clones experienced lower levels of apoptosis, compared with that of the vector expressing clone (Figure 1D). Furthermore, the degree of protection from cisplatin-induced apoptosis seemed to correlate directly with mutant p53 protein levels (see Figure 1A). To further confirm this pattern of apoptosis, we exposed cells in parallel to the PI assay. Twenty-four hours after treatment of the various clones with 10 µg of cisplatin, cells were subjected to PI labeling. We found that clones p53ts-143-103 and p53ts-143-106 exhibited 33 and 43% less apoptosis, respectively, compared with the empty vector expressing clone. These data are consistent with the cisplatin resistance that we had previously observed in murine mutant p53 135 (Ala to Val) expressing cells (Li et al., 1998). We examined mutant p53 protein levels to ensure its presence after cisplatin treatment, and found that expression not only was not reduced but was slightly increased (Figure 1E).
α-amanitin-mediated apoptosis
We then utilized α-amanitin, a specific inhibitor of RNA polymerase II (Cochet-Meilhac and Chambon, 1974; De Mercoyrol et al., 1989), which has been shown to induce apoptosis in certain cell lines (Leist et al., 1994; Andera and Wasylyk, 1997; Koumenis and Giaccia, 1997; Ljungman et al., 1999). Figure 2A depicts the levels of apoptosis in control and p53 143 (Val to Ala) expressing clones, after 24 h at 32°C with or without α-amanitin. As with cisplatin, a clear cooperation was observed between wild-type p53 expression and α-amanitin-induced apoptosis.
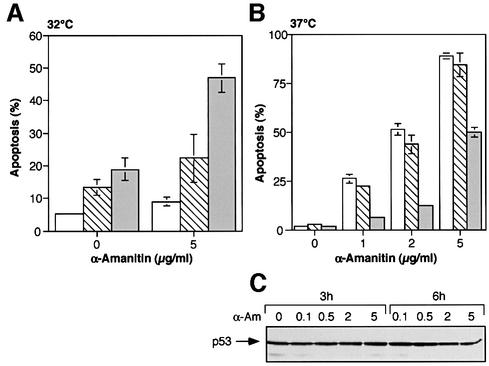
Fig. 2. α-amanitin-induced apoptosis in p53 143 (Val to Ala) expressing M1/2 cells. (A) Synergism between wild-type p53 and α-amanitin in the induction of apoptosis. Representation of apoptosis of p53 143 (Val to Ala) expressing cells at 32°C, after exposure to 5 µg of α-amanitin. Open bars represent a clone generated by infection with empty vector, hatched bars represent clone p53ts-143-103 and gray bars represent clone p53ts-143-106. (B) Mutant p53 143 (Val to Ala) protein expression interferes with α-amanitin-induced apoptosis. Measurement of apoptosis in p53 143 (Val to Ala) expressing cells after a 24 h treatment with α-amanitin at 37°C. Open bars represent a clone generated by infection with empty vector, hatched bars represent clone p53ts-143-103 and gray bars represent clone p53ts-143-106. (C) p53 protein expression of p53ts-143-106 cells grown at 37°C and treated with α-amanitin (α-Am).
Upon treatment with increasing concentrations of α-amanitin at 37°C, the clone that expresses high levels of mutant p53 143 (Val to Ala) protein, p53ts-143-106, exhibited lower levels of apoptosis, compared with the control clone (Figure 2B). Thus, expression of the mutant protein p53 143 (Val to Ala) can also protect the cells from α-amanitin-induced apoptosis. Exposure to α-amanitin did not change the levels of the p53 143 (Val to Ala) protein. A typical western blot using the p53 143 (Val to Ala) expressing clone p53ts-143-106 is represented in Figure 2C. It should be mentioned that the difference in the levels of apoptosis in the presence of 5 µg of α-amanitin at 32 versus 37°C is most likely the result of the different temperatures, and of the use of different CM batches in the individual experiments.
Doxorubicin-mediated apoptosis
Doxorubicin is frequently used in the treatment of a variety of human malignancies (Hortobagyi, 1997). Suggested mechanisms for the cytotoxic effect of doxorubicin include intercalation into DNA, inhibition of enzymes such as topoisomerase II and the generation of free radicals. This drug has also been shown to act as an apoptosis inducer (Gewirtz, 1999). To measure the effects of p53 in the wild-type conformation on doxorubicin-mediated apoptosis, cells maintained at 37°C were treated with increasing concentrations of doxorubicin and shifted to 32°C for 24 h. As can be seen in Figure 3A, wild-type p53 and doxorubicin synergize to induce apoptosis.
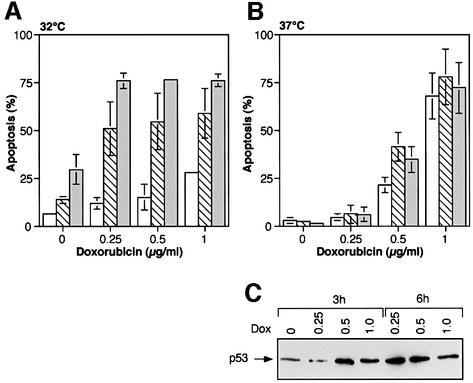
Fig. 3. Doxorubicin-induced apoptosis in p53 143 (Val to Ala) expressing M1/2 cells. (A) Wild-type p53 protein expression cooperates with doxorubicin in the induction of apoptosis. FACS analysis of M1/2 clones expressing p53 143 (Val to Ala) protein after a 24 h exposure to increasing concentrations of doxorubicin at 32°C. Open bars represent a clone generated by infection with empty vector, hatched bars represent clone p53ts-143-103 and gray bars represent clone p53ts-143-106. (B) Expression of mutant p53 143 (Val to Ala) protein does not affect doxorubicin-mediated apoptosis. Graphical representation of the percentage of apoptosis in p53 143 (Val to Ala) expressing clones following doxorubicin treatment at 37°C. Open bars represent a clone generated by infection with empty vector, hatched bars represent clone p53ts-143-103 and gray bars represent clone p53ts-143-106. (C) Western blot analysis for p53ts-143-106 cells grown at 37°C and treated for 3 and 6 h with doxorubicin (Dox).
At 37°C (the permissive temperature for the mutant conformation) the percentage of apoptosis in p53 143 (Val to Ala) expressing clones following doxorubicin treatment was similar to, or even higher than the control clone (Figure 3B). To exclude the possibility that the drug treatment merely abolished expression of mutant p53 protein in these cells, we analyzed mutant p53 protein levels before and after doxorubicin treatment. Western blot analysis of mutant p53 protein levels in the p53 143 (Val to Ala) expressing clone p53ts-143-106 revealed that protein expression was elevated upon doxorubicin treatment (Figure 3C). Hence, the lack of mutant p53-mediated suppression of apoptosis was not due to a reduction in its protein level. In agreement with our observations with cisplatin and α-amanitin, wild-type p53 synergizes with doxorubicin-induced apoptosis. Unlike cisplatin and α-amanitin, where mutant p53 blocked apoptosis, in the case of treatment with doxorubicin, such blocking activity was not evident.
Actinomycin D-induced apoptosis
We next investigated the effect of p53 expression on apoptosis induced by the intercalating agent actino mycin D. Intercalation of actinomycin D into DNA creates a physical block to the various RNA polymerases, thus inhibiting transcription (Pratt and Ruddon, 1979). Actinomycin D has been shown to induce apoptotic cell death, probably as an outcome of transcription inhibition. At 32°C, the permissive temperature for the wild-type conformation, p53 expression and actinomycin D clearly cooperate in the induction of apoptosis (Figure 4A). The p53 143 (Val to Ala) protein level was elevated by the exposure to the drug (Figure 4B).
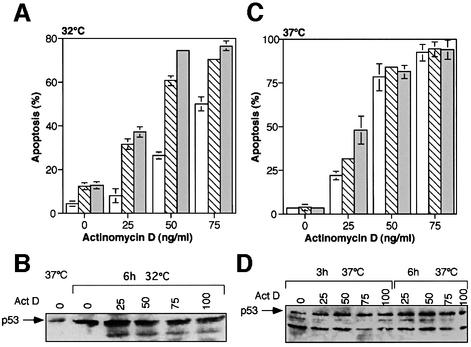
Fig. 4. Actinomycin D-induced apoptosis in p53 143 (Val to Ala) expressing M1/2 cells. (A) Cooperation between wild-type p53 and actinomycin D in the induction of apoptosis. Results of FACS analysis of M1/2 p53 143 (Val to Ala) expressing clones following treatment with actinomycin D and a temperature shift to 32°C. Open bars represent a clone generated by infection with empty vector, hatched bars represent clone p53ts-143-103 and gray bars represent clone p53ts-143-106. (B) Western blot analysis for p53 for p53ts-143-106 cells exposed to the same treatments as in (A) for 6 h. Act D, actinomycin D. (C) Expression of mutant p53 143 (Val to Ala) protein does not affect actinomycin D-mediated apoptosis. Percentage of apoptotic cells of the M1/2-derived clones following a 24 h exposure to actinomycin D. Open bars represent a clone generated by infection with empty vector, hatched bars represent clone p53ts-143-103 and gray bars represent clone p53ts-143-106. (D) Western blot analysis of p53ts-143-106 cells for the same treatments as in (C). Act D, actinomycin D.
As we have previously demonstrated for the murine p53 135 (Ala to Val) mutant (Li et al., 1998), the expression of p53 143 (Val to Ala) at 37°C does not interfere with actinomycin D-induced apoptosis (see Figure 4C). This lack of protective effect against actinomycin D-induced apoptosis by mutant p53 is not due to a reduction in the levels of the mutant p53 protein, as actinomycin D treatments result in the stabilization of mutant p53 143 (Val to Ala) (Figure 4D). Thus, p53 143 (Val to Ala) expression does not affect actinomycin D-induced apoptosis.
M1/2 clones expressing the human p53 248 (Arg to Trp) mutant or p53 273 (Arg to His) mutant were examined for sensitivity to the same array of drugs as p53 143 (Val to Ala). We also investigated the response of M1/2 clones expressing the different mutations to etoposide, a topoisomerase II inhibitor (Hande, 1998), and to DRB, a specific inhibitor of RNA polymerase II function (Dubois et al., 1994; Yankulov et al., 1995). The results obtained with the various p53 mutations and the different drugs tested are summarized in Table I. As can be seen, similar patterns of protection were observed with all p53 mutations. The various p53 core domain mutants interfered with cisplatin-, α-amanitin- and etoposide-induced apoptosis, but exerted no blocking activity on doxorubicin-, actinomycin D- or DRB-mediated apoptosis.
Table I. Specific interference with apoptosis-inducing agents by various human p53 mutations.
| Mutation | Cisplatin | α-amanitin | Actinomycin D | Doxorubicin | Etoposide | DRB |
|---|---|---|---|---|---|---|
| 143Val → Ala | + | + | – | – | + | – |
| 248Arg → Trp | + | + | – | – | + | – |
| 273Arg → His | + | + | – | – | + | – |
–, no blocking activity; +, protective effect.
The effect of loss of transcriptional competence on the apoptotic activity of p53
We next investigated the mechanism that underlies the protective effect of mutant p53 against cisplatin-, α-amanitin- and etoposide-induced apoptosis. The observation that the p53 N-terminal domain is rarely modified in human tumors (Hussain and Harris, 2000), coupled with the characterization of this domain as the transcription activation domain of p53 (Unger et al., 1992; Subler et al., 1994), prompted us to investigate the possibility that transcription activation by mutant p53 is required for the rescue from apoptosis. It has been shown previously that a double mutation changing Leu to Gln at position 22 and Trp to Ser at position 23 (Gln22, Ser23) in the p53 transactivation domain impaired the ability of the wild-type p53 protein to activate transcription (Lin et al., 1994). Furthermore, the p53 281 (Asp to Gly) mutant, which was shown to transactivate MDR-1 and c-myc, lost this ability upon insertion of these two N-terminal mutations (Lin et al., 1995; Frazier et al., 1998). Thus, it appears that the transcription domain of mutant p53 is similar to that identified in the wild-type p53 protein. Therefore, we generated M1/2 stable clones, which express a p53 molecule harboring the 22 Gln and 23 Ser mutations in conjunction with the 143 Ala core mutation (referred to as the 22,23,143 triple mutant). M1/2 clones expressing wild-type p53 harboring the same N-terminal mutations [hereafter termed p53 (22-23)] were generated in parallel.
Effect of the 22-23 mutations on the apoptotic activity of wild-type p53
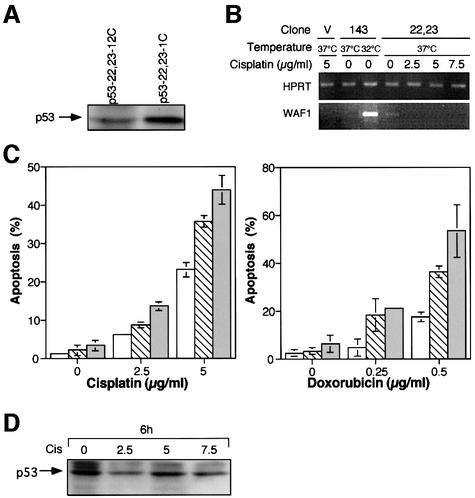
We next examined the p53 (22-23) expressing clones. Our infections yielded a series of M1/2-derived clones expressing various levels of p53 (22-23). Western blot analysis of the clones that were selected for further study is presented in Figure 5A. We found that the p53 (22-23)-expressing clones exhibit a normal cell cycle over a prolonged incubation. The percentage of apoptotic cells in these clones is very low and is comparable to that in the parental cells (2–5%). The fact that we have generated viable clones harboring a plasmid coding for a transcription-deficient p53 but otherwise wild type in structure, further confirms the notion that apoptosis mediated by wild-type p53 is transcription dependent (Sabbatini et al., 1995; Attardi et al., 1996; Yonish-Rouach et al., 1995; Chao et al., 2000; Jimenez et al., 2000). To confirm that the p53 (22-23) protein expressed in our M1/2 clones is transcriptionally defective, we analyzed the expression of p21/WAF1, a p53 downstream gene (El-Deiry et al., 1993) involved in growth arrest, before and following DNA damage with cisplatin. p53 (22-23) expressing cells were treated with various concentrations of cisplatin, and RNA was extracted following 4 h of treatment. RT–PCR analysis revealed that without treatment WAF1 is expressed at very low levels (Figure 5B). Furthermore, after treatment with cisplatin, WAF1 expression could not be detected. As a positive control, we demonstrated WAF1 induction by p53 143 (Val to Ala) expressed as its wild-type p53 conformation (see Figure 5B). We therefore concluded that the p53 (22-23) in our experimental system is indeed transcriptionally inactive.
Fig. 5. Characterization of M1/2 clones expressing the p53 (22-23) molecule. (A) Western blot analysis of representative M1/2 clones expressing the p53 (22-23) transactivation-deficient mutant. (B) p53 (22-23) expressed in M1/2-derived clones is transactivation-defective. RT–PCR analysis to examine WAF1 gene expression in p53-22,23-1C clone, before and after treatment with cisplatin. cDNAs from clone p53ts-143-106, expressing the p53 143 (Val to Ala) ts mutant, at 37 and 32°C, were used as negative and positive controls, respectively. HPRT gene expression was used for all cDNAs as a quantity control. V, vector expressing cells. (C) Enhancement of DNA damage-induced apoptosis in p53 transactivation-deficient expressing clones. FACS analysis of cisplatin and doxorubicin treatments of p53 (22-23) expressing clones. Open bars represent a clone generated by infection with empty vector, hatched bars represent clone p53-22,23-12C and gray bars represent clone p53-22,23-1C. (D) Western blot for p53 expression in p53-22,23-1C cells treated with cisplatin (Cis).
We next examined whether the p53 transcription-deficient mutant, p53 (22-23), can synergize with drug-induced apoptosis, as we observed for intact wild-type p53 (Li et al., 1998). As can be seen in Figure 5C, treatment with cisplatin or doxorubicin induced significant enhancement in apoptosis in the p53 (22-23) expressing clones, compared with the control clone. Western blot analysis confirmed that p53 (22-23) is expressed during cisplatin treatment (Figure 5D). These observations suggest that synergism of wild-type p53 with drug-induced apoptosis involves a transcription-independent mechanism, supporting the notion that wild-type p53-mediated apoptosis may be transcription independent (Caelles et al., 1994; Wagner et al., 1994; Haupt et al., 1995; Roemer and Mueller-Lantzsch, 1996; Ding et al., 2000; Kokontis et al., 2001). Based on these observations, we propose that the capacity of p53 to initiate apoptosis is dependent on the activation of p53 downstream genes. However, in drug-induced apoptosis, p53 may facilitate this process in a manner that does not require p53 to be transcriptionally active.
Effect of the 22-23 mutations on mutant p53-mediated interference with apoptosis
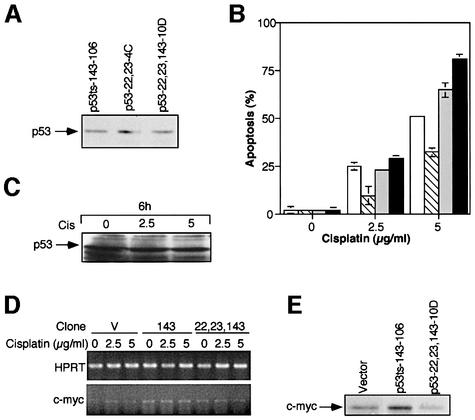
We then analyzed the triple mutant clones expressing the p53 143 (Val to Ala) core mutation in conjunction with the N-terminal 22Gln and 23Ser mutations. We sought to determine the response of these clones to treatment with cisplatin, for which we found the single mutant p53 143 (Val to Ala) protective. To that end, we chose a set of clones expressing comparable levels of either the p53 143 (Val to Ala) mutant, the p53 (22-23) double mutant or the p53 (22-23),143 triple mutant. Protein levels in the various clones were determined by western blot analysis and immunoprecipitations. A typical western blot analysis for the clones selected, p53ts-143-106, p53-22,23-4C and p53-22,23,143-10D, is represented in Figure 6A. We subjected the set of chosen clones to treatment with cisplatin at 37°C, the permissive temperature for the mutant conformation. As can be seen in Figure 6B, addition of the 22Gln and 23Ser mutations to the p53 143 (Val to Ala) mutant abolished the protective effect against cisplatin-induced apoptosis. It should be mentioned that the percentage of apoptosis in the p53-22,23,143-10D clone, expressing the triple mutant, was even higher than in the p53 (22-23) expressing clone. Western blot analysis of p53-22,23,143-10D cells revealed that the cisplatin treatment did not affect the levels of the p53 triple mutant protein (Figure 6C). Thus, the loss of protection from drug-induced apoptosis in these cells cannot be attributed to a loss of expression of the mutant p53 protein, and is most likely directly related to the lack of mutant p53 transcription activity. Protection of mutant p53 against cisplatin-induced apoptosis requires a transcriptionally active mutant p53.
Fig. 6. Protection of mutant p53 from cisplatin-induced apoptosis requires a transcriptionally active mutant p53. (A) A representative western blot analysis of selected clones expressing similar levels of p53 143 (Val to Ala), p53 (22-23) or p53-22,23,143. (B) M1/2 stable clones expressing comparable levels of mutated p53, 143Ala or 22Gln and 23Ser, or these three mutations all together, were treated with cisplatin at 37°C and assayed by AO for percentage of apoptosis. Open bars represent a clone generated by infection with empty vector, hatched bars represent clone p53ts-143-106, light gray bars represent clone p53-22,23-4C and dark gray bars represent clone p53-22,23,143-10D. (C) Cell lysates from p53-22,23,143-10D cells, before and after treatment with cisplatin, were analyzed by immunoblotting for p53 triple mutant expression. Cis, cisplatin. (D) p53 (22-23),143 expressed in M1/2-derived clones is transactivation defective. RT–PCR analysis to examine c-myc gene expression in clones expressing the empty vector (V), the p53 single mutant or the p53 triple mutant, before and after treatment with cisplatin. HPRT gene expression was used for all cDNAs as a quantity control. (E) Western blot analysis of c-myc protein expression in the various clones. Vector, vector expressing cells.
Modulations in c-myc expression pattern in the various mutant p53-expressing clones
To further resolve the pathway that controls the activity of mutant p53 in drug resistance, it was important to characterize specific changes in the expression of downstream genes. At present, several candidates have been suggested (reviewed in Sigal and Rotter, 2000). We have focused our experiments on c-myc, which has been suggested as a specific target gene of mutant p53 (Frazier et al., 1998). In our experiments we examined whether activation of c-myc correlated with mutant p53 activity in drug resistance. As it has been shown that c-myc induction by mutant p53 is dependent on the integrity of the transcription domain (Frazier et al., 1998), it was important to examine whether the p53 triple mutant protein, which lost the ability to block cisplatin-induced apoptosis, is also deficient in the ability to activate the c-myc promoter. We therefore used RT–PCR analysis to compare the RNA levels of c-myc in the empty vector expressing clone, the p53ts-143-106 clone and the p53-22,23,143-10D clone, before and after a 4 h treatment with cisplatin. We noticed that while c-myc is upregulated in the single mutant expressing clone, this upregulation is absent in the triple mutant-expressing clone (Figure 6D). Analysis of c-myc protein levels in these clones indicated that, like the RNA, the elevation in c-myc expression observed in the p53 core domain mutant was abolished in the p53 triple mutant expressing clone (Figure 6E). We therefore conclude that the p53 (22-23),143 expressed in M1/2 is transcriptionally inactive in this pathway.
Discussion
p53, ‘the guardian of the genome’, has been shown to exert tumor suppressor activity by the activation of cell cycle checkpoints (Schwartz and Rotter, 1998; Sionov and Haupt, 1999), involvement in DNA repair (Tlsty, 1997; Wahl et al., 1997; Janus et al., 1999), induction of apoptosis (Levine, 1997; Gottlieb and Oren, 1998) and cell differentiation (Almog and Rotter, 1997). In cancerous cells, however, it appears that not only the loss of the wild-type p53 form, but also the accumulation of mutant p53 forms, plays a critical role in the process of establishing the malignant phenotype (Sigal and Rotter, 2000).
In our previous studies using a murine model we observed that while the wild-type p53 form synergizes with p53-independent apoptosis induced by DNA- damaging agents, mutant p53 interferes with these apoptotic pathways (Peled et al., 1996; Li et al., 1998).
The mechanism by which wild-type p53 mediates apoptosis is still unclear. In particular, the question of whether p53-induced apoptosis requires transcriptional activity is debatable. Several studies suggest that the human p53 (22-23) transcription-deficient mutant is capable of inducing apoptosis (Haupt et al., 1995; Ding et al., 2000; Kokontis et al., 2001). Others have shown that the same p53 mutant was incompetent for the induction of apoptosis (Sabbatini et al., 1995; Attardi et al., 1996; Yonish-Rouach et al., 1995). Findings that support the latter possibility were published recently for the analogous murine p53 mutant (Chao et al., 2000; Jimenez et al., 2000).
Here we show that p53 may mediate apoptosis by both transcription-dependent and -independent pathways. Our success in establishing M1/2 stable clones expressing the transcriptionally deficient p53, p53 (22-23), with a very limited apoptotic population, argues for a transcription-dependent mechanism for initiating p53-mediated apoptosis. On the other hand, the ability of the p53 (22-23) to synergize with drug-induced apoptosis supports the notion that p53-dependent apoptosis may occur in the absence of transcription.
It appears that in these clones the transcription-independent pathway for apoptosis by p53 is silent in normal growing conditions and is turned on only following exposure to DNA damage. Further support for the idea that a transcription-independent pathway is induced by p53 following stress is provided by the observations presented in this report, and previously shown by others (Caelles et al., 1994), that treatment with the transcription inhibitors actinomycin D or α-amanitin does not interfere with wild-type p53-induced apoptosis. It is conceivable that during the normal cell cycle transcription may take place to execute programmed cell death. However, the presence of damaged DNA requires an immediate response. Thus, a transcription-coupled pathway, which is time consuming, might be less appropriate. It may well be that the mechanism chosen for apoptosis by p53 is also dependent on the cell type, the genetic background and the type of damage. Taken together, this may explain the controversial findings in this field. We suggest that the net result of this complex equation determines the choice of either a transcription-dependent or -independent pathway for wild-type p53-mediated apoptosis.
According to the current state of knowledge, some wild-type p53 activities are dependent on the transcriptional activity of the protein, whereas others are independent of this activity. While growth arrest has been shown to be dependent on the transactivation of specific downstream genes (El-Deiry et al., 1993), p53-dependent apoptosis may involve either transcription-dependent or -independent mechanisms. Interestingly, we have observed that in DNA repair, particularly in the base excision repair pathway, p53 acts through a transcription-independent mechanism (Offer et al., 2001).
Mutant p53 has been shown to act either by a transdominant mechanism involving inactivation of the wild-type p53 form or a by gain of function, independent of wild-type p53 (Sigal and Rotter, 2000). Protection from chemotherapy can be the result of wild-type p53 loss, but we and others have previously demonstrated that it can also result from mutant p53 gain of function (reviewed in Hickman and Dive, 1999). We have observed that p53 135 (Ala to Val), expressed in M1/2 p53 non-producer cells, provides protection from p53-independent apoptosis mediated by γ-irradiation and several chemotherapeutic agents (Li et al., 1998). Likewise, drug resistance following mutant p53 expression has been reported in the H1299 cell line (Blandino et al., 1999).
In this report we demonstrate that various human p53 mutant proteins can interfere with several drug-induced apoptotic pathways. Expression of the p53 mutants 143 (Val to Ala), 248 (Arg to Trp) or 273 (Arg to His) resulted in resistance to cisplatin and etoposide, drugs commonly used in chemotherapy, and also to α-amanitin, which may serve as a potential agent for cancer treatment (Koumenis and Giaccia, 1997). These effects of mutant p53 were observed in the absence of wild-type p53 and therefore represent a gain of function activity of the mutant p53 protein. Interestingly, mutant p53 expression did not interfere with doxorubicin-, DRB- or actinomycin D-induced apoptosis, suggesting that mutant p53 protein forms may affect differently the outcome of various drugs. The fact that mutant p53 proteins did not confer resistance to all drugs may have important implications for the choice of drugs in patients with p53 mutations.
Our present study shows that, as in the case of wild-type p53, core mutant p53 acts as a transcription factor. However, it is expected that the two will engage different downstream pathways. We observed that alterations in the N-terminal domain of core mutant p53 abrogated the apoptosis-blocking activity. This conclusion is further substantiated by studies showing that mutant p53 can transactivate genes involved in malignancy. Among the specific target genes of mutant p53 that were identified, several are candidates for mediating the anti-apoptotic effect. p53 mutants can transactivate the MDR-1 gene promoter (Dittmer et al., 1993; Lin et al., 1995), and insulin-like growth factor II (Lee et al., 2000), as well as genes that are involved in proliferation (Deb et al., 1992; Margulies and Sehgal, 1993; Ludes-Meyers et al., 1996).
The c-myc oncoprotein has also been identified as a specific target gene of mutant p53 (Frazier et al., 1998). In agreement with these observations, we found that in M1/2 cells c-myc RNA and protein expression are elevated by mutant p53 protein expression, and that this upregulation is dependent on an intact mutant p53 transactivation domain. The elevated expression of c-myc in the mutant p53 expressing clone may be directly linked to the resistant phenotype of these clones. This idea is supported by studies showing that activation of c-myc contributes to chemoresistance to cisplatin and other drugs (Niimi et al., 1991; Sklar and Prochownik, 1991; Kinashi et al., 1998). Furthermore, decreasing the expression of c-myc, by using antisense oligonucleotides, has been shown to enhance the efficacy of cisplatin treatment both in vitro and in vivo (Mizutani et al., 1994; Van Waardenburg et al., 1996; 1997; Citro et al., 1998; Leonetti et al., 1999). However, it should be borne in mind that others have reported that overexpression of c-myc increases the sensitivity to drugs by activating apoptosis (Lotem and Sachs, 1993, 1995; Dong et al., 1997). If the latter is the case, mutant p53 expressing cells may tolerate higher levels of c-myc than the empty vector clone, and therefore do not undergo c-myc-induced apoptosis upon c-myc expression. Such tolerance to c-myc overexpression has been shown (Zindy et al., 1998) for p53-null murine embryonic fibroblasts, compared with their normal counterparts. In this scenario, c-myc elevation in mutant p53 expressing cells may play a role in gain of function activities of mutant p53 other than chemoresistance, such as immortalization and tumorigenicity.
Since the p53 (22-23) was also shown to be defective in the repression of promoters (Roemer and Mueller-Lantzsch, 1996), it may well be that mutant p53-mediated chemoresistance is acquired by the repression of apoptotic genes.
Our present results show that integrity of the N-terminus is critical for mutant p53 anti-apoptotic function. Furthermore, we recently found that modification of the extreme C-terminus of the murine mutant p53 135 (Ala to Val) by alternative splicing resulted in the loss of this gain of function activity (Sigal and Rotter, 2000). These results are consistent with a transactivation-based mechanism for mutant p53 gain of function, since truncation of the extreme C-terminus of the p53 281 (Asp to Gly) mutant rendered it transcriptionally inactive (Frazier et al., 1998).
It should be borne in mind that the N-terminus of p53 is also the site that interacts with mdm2. Therefore, it could not be entirely ruled out that the abolishment of the apoptosis-blocking activity of mutant p53, by insertion of the 22Gln and 23Ser mutations, could at least be partly due to the disruption of this pathway. Indeed, it has recently been demonstrated that while various p53 mutants, including p53 281 (Asp to Gly), may stabilize the mdm2 oncoprotein, p53 22,23,281 is defective in this activity (Peng et al., 2001). In addition, it has been reported recently (Buschmann et al., 2000) that mutant p53 may interact with mdm2 or JNK, known components in the control of wild-type p53 stability (Woods and Vousden, 2001). Interestingly, we observed that treatment of cells with cisplatin, doxorubicin and actinomycin D induced the accumulation of mutant p53. These observations further support the idea that, in addition to the existence of the mdm2–wild-type p53 feedback loop, mdm2 may also affect the mutant p53 protein.
Taken together, we propose that the protective activity of mutant p53 relies upon an intact N-terminus (as shown in this report) as well as C-terminus (Sigal et al., 2001). This hypothesis is supported by the molecular epidemiology of p53, in which mutations in primary human tumors were found to be clustered at the core domain of p53, leaving both the N- and the C-terminus unaffected (Hussain and Harris, 2000). This suggests that, like the wild-type p53, activity of mutant p53 requires the extreme parts of the molecule, which contain various functional domains. Our present findings support the notion that mutant p53 protein is transcriptionally active and that in malignant transformation it acts via a gain of function mechanism. This conclusion provokes the need for a specific search of target genes that are activated in this mutant p53 gain of function pathway.
Materials and methods
Plasmids and cell lines
The cDNAs encoding the various p53 mutants were cloned into the pBabe-puro retroviral vector (Morgenstern and Land, 1990). Retroviral stocks were generated by using the highly efficient 293GP packaging cell line (Yee et al., 1994). The 293GP cells were transfected with the cDNA constructs by the calcium phosphate method. Stable clones expressing the p53 mutations were generated by infection of the M1/2 parental p53 non-producer cell line as described previously (Peled et al., 1996). For each p53 mutant, several clones expressing different levels of the mutated protein were selected. Clones were grown continuously in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum and CM. Each set of experiments was performed with the same batch of CM.
Drugs
Cisplatin (Abic) and α-amanitin (Sigma) were prepared as 1 mg/ml stock solutions in water. Actinomycin D and doxorubicin (Sigma) were prepared, respectively, as 500 µg/ml and 2 mg/ml stock solutions in water. DRB (Sigma) was prepared as a 0.5 M stock solution in DMSO. Etoposide (Sigma) was dissolved in DMSO to a concentration of 50 mM.
Western blot analysis
Cell extracts for western blot analysis were lysed in TLB buffer (50 mM Tris pH 7.5, 100 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS). The samples were normalized according to quantitation by Bradford assay (Bio-Rad). Protein sample buffer (140 mM Tris pH 6.8, 22.4% glycerol, 6% SDS, 10% β-mercaptoethanol, 0.02% bromophenol blue) was added, and the samples were boiled for 10 min and loaded onto 10% SDS–polyacrylamide gel. The proteins were transferred to nitrocellulose membranes using a semi-dry transfer cell (Bio-Rad). The p53-specific monoclonal antibody Pab-1801 was used to detect p53. c-myc protein was detected by an antibody kindly provided by H.Kahana. The protein–antibody complexes were detected by using a horseradish peroxidase-conjugated secondary antibody by the super-signal enhanced chemiluminescence system (Pierce).
FACS analysis of apoptosis
The AO DNA denaturability assay was performed as described previously (Li et al., 1998). Briefly, cells were fixed in 80% ethanol–20% Hanks balanced salt solution (HBSS) and stored at –20°C. Cells were washed once with HBSS, resuspended in HBSS containing 0.25 mg/ml RNase, and incubated at 37°C for 1 h and 15 min.
Cells were then harvested and resuspended in HBSS. Cell suspensions (200 µl) were added to 0.5 ml of 0.1 M HCl for a 40 s incubation. Acid denaturation was quenched by the addition of 2 ml of AO (Molecular Probes) staining solution pH 2.6, containing 90% v/v 0.1 M sodium citrate, 10% v/v 0.2 M Na2HPO4 and 6 µg/ml AO (Darzynkiewicz et al., 1994). The PI (Sigma) exclusion assay was performed as for the AO assay, except that following the incubation with RNase, 20 µg/ml PI was added to the cell suspensions (500 µl). The cells were analyzed by the FACScan flow cytometer (Beckton-Dickinson), using the CellQuest (Beckton-Dickinson) software.
RT–PCR analysis
Total RNA was extracted from 2 × 106 cells using the Tri-Reagent kit (Sigma) according to the manufacturer’s instructions. For the generation of cDNA, 3 µg of RNA were incubated with 0.1 µg of oligo dT at 70°C for 10 min. Reverse transcription was performed using Superscript II following the manufacturer’s instructions (Gibco-BRL). The following primers were used for PCR amplification under the following conditions: HPRT, 23 cycles at 50°C (5′ primer, CAGAGGACTAGAACACCT; 3′ primer, GCTGGTGAAAAGGACCTCT); waf1, 30 cycles at 58°C (5′ primer, GATTGCGATGCGCTCATGG; 3′ primer, CCTCCTGACCCACAGCAGAAG); and c-myc, 22 cycles at 58°C (5′ primer, GGAAACTTTGCCCATTGCAG; 3′ primer, ACGTAGCGACCGCAA CATAG).
Acknowledgments
Acknowledgements
We would like to thank Professor H.Kahana for the generous gift of the anti-c-myc antibody. This work was supported in part by grants from the Israel–USA Binational Science Foundation (BSF) the DIP (Deutsch–Israelische Projektkooperation) and the Kadoori Foundation. V.R. is the incumbent of the Norman and Helen Asher Professorial Chair in Cancer Research at the Weizmann Institute.
References
- Almog N. and Rotter,V. (1997) Involvement of p53 in cell differentiation and development. Biochim. Biophys. Acta, 1333, F1–F27. [DOI] [PubMed] [Google Scholar]
- Andera L. and Wasylyk,B. (1997) Transcription abnormalities potentiate apoptosis of normal human fibroblasts. Mol. Med., 3, 852–863. [PMC free article] [PubMed] [Google Scholar]
- Attardi L.D., Lowe,S.W., Brugarolas,J. and Jacks,T. (1996) Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J., 15, 3702–3712. [PMC free article] [PubMed] [Google Scholar]
- Blandino G., Levine,A.J. and Oren,M. (1999) Mutant p53 gain of function: differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. Oncogene, 18, 477–485. [DOI] [PubMed] [Google Scholar]
- Buschmann T., Minamoto,T., Wagle,N., Fuchs,S.Y., Adler,V., Mai,M. and Ronai,Z. (2000) Analysis of JNK, Mdm2 and p14(ARF) contribution to the regulation of mutant p53 stability. J. Mol. Biol., 295, 1009–1021. [DOI] [PubMed] [Google Scholar]
- Caelles C., Helmberg,A. and Karin,M. (1994) p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature, 370, 220–223. [DOI] [PubMed] [Google Scholar]
- Chao C., Saito,S., Kang,J., Anderson,C.W., Appella,E. and Xu,Y. (2000) p53 transcriptional activity is essential for p53-dependent apoptosis following DNA damage. EMBO J., 19, 4967–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chene P. (1998) In vitro analysis of the dominant negative effect of p53 mutants. J. Mol. Biol., 281, 205–209. [DOI] [PubMed] [Google Scholar]
- Citro G., D’Agnano,I., Leonetti,C., Perini,R., Bucci,B., Zon,G., Calabretta,B. and Zupi,G. (1998) c-myc antisense oligodeoxy nucleotides enhance the efficacy of cisplatin in melanoma chemotherapy in vitro and in nude mice. Cancer Res., 58, 283–289. [PubMed] [Google Scholar]
- Cochet-Meilhac M. and Chambon,P. (1974) Animal DNA-dependent RNA polymerases. 11. Mechanism of the inhibition of RNA polymerases B by amatoxins. Biochim. Biophys. Acta, 353, 160–184. [DOI] [PubMed] [Google Scholar]
- Crook T. and Vousden,K.H. (1992) Properties of p53 mutations detected in primary and secondary cervical cancers suggest mechanisms of metastasis and involvement of environmental carcinogens. EMBO J., 11, 3935–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Li,X. and Gong,J. (1994) Assays of cell viability: discrimination of cells dying by apoptosis. Methods Cell Biol., 41, 15–38. [DOI] [PubMed] [Google Scholar]
- Deb S., Jackson,C.T., Subler,M.A. and Martin,D.W. (1992) Modulation of cellular and viral promoters by mutant human p53 proteins found in tumor cells. J. Virol., 66, 6164–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mercoyrol L., Job,C. and Job,D. (1989) Studies on the inhibition by α-amanitin of single-step addition reactions and productive RNA synthesis catalysed by wheat-germ RNA polymerase II. Biochem. J., 258, 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H.F., Lin,Y.L., McGill,G., Juo,P., Zhu,H., Blenis,J., Yuan,J. and Fisher,D.E. (2000) Essential role for caspase-8 in transcription-independent apoptosis triggered by p53. J. Biol. Chem., 275, 38905–38911. [DOI] [PubMed] [Google Scholar]
- Dittmer D., Pati,S., Zambetti,G., Chu,S., Teresky,A., Moore,M., Finlay,C. and Levine,A. (1993) Gain of function mutations in p53. Nature Genet., 4, 42–46. [DOI] [PubMed] [Google Scholar]
- Dong J., Naito,M. and Tsuruo,T. (1997) c-myc plays a role in cellular susceptibility to death receptor-mediated and chemotherapy-induced apoptosis in human monocytic leukemia U937 cells. Oncogene, 15, 639–647. [DOI] [PubMed] [Google Scholar]
- Dubois M.F., Nguyen,V.T., Bellier,S. and Bensaude,O. (1994) Inhibitors of transcription such as 5,6-dichloro-1-β-d-ribofuranosylbenz imidazole and isoquinoline sulfonamide derivatives (H-8 and H-7) promote dephosphorylation of the carboxyl-terminal domain of RNA polymerase II largest subunit. J. Biol. Chem., 269, 13331–13336. [PubMed] [Google Scholar]
- El-Deiry W.S. (1998) Regulation of p53 downstream genes. Semin. Cancer Biol., 8, 345–357. [DOI] [PubMed] [Google Scholar]
- El-Deiry W.S. et al. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell, 75, 817–825. [DOI] [PubMed] [Google Scholar]
- Frazier M.W., He,X., Wang,J., Gu,Z., Cleveland,J.L. and Zambetti,G.P. (1998) Activation of c-myc gene expression by tumor-derived p53 mutants requires a discrete C-terminal domain. Mol. Cell. Biol., 18, 3735–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz D.A. (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol., 57, 727–741. [DOI] [PubMed] [Google Scholar]
- Gottlieb T.M. and Oren,M. (1998) p53 and apoptosis. Semin. Cancer Biol., 8, 359–368. [DOI] [PubMed] [Google Scholar]
- Graeber T., Osmanian,C., Jacks,T., Housman,D., Koch,C., Lowe,S.W. and Giaccia,A.J. (1996) Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature, 379, 88–91. [DOI] [PubMed] [Google Scholar]
- Hande K.R. (1998) Etoposide: four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer, 34, 1514–1521. [DOI] [PubMed] [Google Scholar]
- Haupt Y., Rowan,S., Shaulian,E., Vousden,K.H. and Oren,M. (1995) Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev., 9, 2170–2183. [DOI] [PubMed] [Google Scholar]
- Hickman J.A. and Dive,C. (1999) Apoptosis and Cancer Chemotherapy. Humana Press, Totowa, NJ.
- Hortobagyi G.N. (1997) Anthracyclines in the treatment of cancer. An overview. Drugs, 54, 1–7. [DOI] [PubMed] [Google Scholar]
- Hsiao M., Low,J., Dorn,E., Ku,D., Pattengale,P., Yeargin,J. and Haas,M. (1994) Gain-of-function mutations of the p53 gene induce lymphohematopoietic metastatic potential and tissue invasiveness. Am. J. Pathol., 145, 702–714. [PMC free article] [PubMed] [Google Scholar]
- Hussain S.P. and Harris,C.C. (1998) Molecular epidemiology of human cancer: contribution of mutation spectra studies of tumor suppressor genes. Cancer Res., 58, 4023–4037. [PubMed] [Google Scholar]
- Hussain S.P. and Harris,C.C. (2000) Molecular epidemiology and carcinogenesis: endogenous and exogenous carcinogens. Mutat. Res., 462, 311–322. [DOI] [PubMed] [Google Scholar]
- Iwamoto K.S., Mizuno,T., Ito,T., Tsuyama,N., Kyoizumi,S. and Seyama,T. (1996) Gain-of-function p53 mutations enhance alteration of the T-cell receptor following X-irradiation, independently of the cell cycle and cell survival. Cancer Res., 56, 3862–3865. [PubMed] [Google Scholar]
- Janus F., Albrechtsen,N., Dornreiter,I., Wiesmuller,L., Grosse,F. and Deppert,W. (1999) The dual role model for p53 in maintaining genomic integrity. Cell. Mol. Life Sci., 55, 12–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez G.S., Nister,M., Stommel,J.M., Beeche,M., Barcarse,E.A., Zhang,X.Q., O’Gorman,S. and Wahl,G.M. (2000) A transactivation-deficient mouse model provides insights into trp53 regulation and function. Nature Genet., 26, 37–43. [DOI] [PubMed] [Google Scholar]
- Kastan M.B., Onyekwere,O., Sidransky,D., Vogelstein,B. and Craig,R.W. (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res., 51, 6304–6311. [PubMed] [Google Scholar]
- Kern S.E., Pietenpol,J.A., Thiagalingam,S., Seymor,A., Kinzler,K.W. and Vogelstein,B. (1992) Oncogenic forms of p53 inhibit p53-regulated gene expression. Science, 256, 827–830. [DOI] [PubMed] [Google Scholar]
- Kinashi Y., Akaboshi,M., Masunaga,S., Ono,K. and Watanabe,M. (1998) Resistance to 195mPt-radiolabeled cis-diaminedichloroplatinum (II) of SHOK cells transfected with various oncogenes. Radiat. Med., 16, 233–237. [PubMed] [Google Scholar]
- Ko J.L. and Prives,C. (1996) p53: puzzle and paradigm. Genes Dev., 10, 1054–1072. [DOI] [PubMed] [Google Scholar]
- Kokontis J.M., Wagner,A.J., O’Leary,M., Liao,S. and Hay,N. (2001) A transcriptional activation function of p53 is dispensable for and inhibitory of its apoptotic function. Oncogene, 20, 659–668. [DOI] [PubMed] [Google Scholar]
- Koumenis C. and Giaccia,A. (1997) Transformed cells require continuous activity of RNA polymerase II to resist oncogene-induced apoptosis. Mol. Cell. Biol., 17, 7306–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi A., Deb,D., Seymour,R.C., Ludes-Meyers,J.H., Subler,M.A. and Deb,S. (1998) ‘Gain of function’ phenotype of tumor-derived mutant p53 requires the oligomerization/nonsequence-specific nucleic acid-binding domain. Oncogene, 16, 3169–3176. [DOI] [PubMed] [Google Scholar]
- Lee Y.I., Lee,S., Das,G.C., Park,U.S., Park,S.M. and Lee,Y.I. (2000) Activation of the insulin-like growth factor II transcription by aflatoxin B1 induced p53 mutant 249 is caused by activation of transcription complexes; implications for a gain-of-function during the formation of hepatocellular carcinoma. Oncogene, 19, 3717–3726. [DOI] [PubMed] [Google Scholar]
- Leist M., Gantner,F., Bohlinger,I., Germann,P.G., Tiegs,G. and Wendel,A. (1994) Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-α requires transcriptional arrest. J. Immunol., 153, 1778–1788. [PubMed] [Google Scholar]
- Leonetti C., Biroccio,A., Candiloro,A., Citro,G., Fornari,C., Mottolese,M., Del Bufalo,D. and Zupi,G. (1999) Increase of cisplatin sensitivity by c-myc antisense oligodeoxynucleotides in a human metastatic melanoma inherently resistant to cisplatin. Clin. Cancer Res., 5, 2588–2595. [PubMed] [Google Scholar]
- Levine J.A. (1997) p53, the cellular gatekeeper for growth and division. Cell, 88, 323–331. [DOI] [PubMed] [Google Scholar]
- Li R. et al. (1998) Mutant p53 protein expression interferes with p53-independent apoptotic pathways. Oncogene, 16, 3269–3277. [DOI] [PubMed] [Google Scholar]
- Lin J., Chen,J., Elenbaas,B. and Levine,A.J. (1994) Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev., 8, 1235–1246. [DOI] [PubMed] [Google Scholar]
- Lin J., Teresky,A.K. and Levine,A.J. (1995) Two critical hydrophobic amino acids in the N-terminal domain of the p53 protein are required for the gain of function phenotypes of human p53 mutants. Oncogene, 10, 2387–2390. [PubMed] [Google Scholar]
- Ljungman M., Zhang,F., Chen,F., Rainbow,A.J. and McKay,B.C. (1999) Inhibition of RNA polymerase II as a trigger for the p53 response. Oncogene, 18, 583–592. [DOI] [PubMed] [Google Scholar]
- Lotem J. and Sachs,L. (1993) Regulation by bcl-2, c-myc and p53 of susceptibility to induction of apoptosis by heat shock and cancer chemotherapy compounds in differentiation-competent and -defective myeloid leukemic cells. Cell Growth Differ., 4, 41–47. [PubMed] [Google Scholar]
- Lotem J. and Sachs,L. (1995) A mutant p53 antagonizes the deregulated c-myc-mediated enhancement of apoptosis and decrease in leukemogenicity. Proc. Natl Acad. Sci. USA, 92, 9672–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S.W., Ruley,H.E., Jacks,T. and Housman,D.E. (1993) p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell, 74, 957–967. [DOI] [PubMed] [Google Scholar]
- Lowe S.W., Bodis,S., McClatchey,A., Remington,L., Ruley,H.E., Fisher,D.E., Housman,D.E. and Jacks,T. (1994) p53 status and the efficacy of cancer therapy in vivo. Science, 266, 807–810. [DOI] [PubMed] [Google Scholar]
- Ludes-Meyers J.H., Subler,M.A., Shivakumar,V., Munoz,R.M., Jiang,P., Bigger,J.E., Brown,D.R., Deb,S.P. and Deb,S. (1996) Transcriptional activation of the human epidermal growth factor receptor promotor by human p53. Mol. Cell. Biol., 16, 6009–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman W. and Czyzyk,L. (1984) UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol. Cell. Biol., 4, 1689–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies L. and Sehgal,P.B. (1993) Modulation of the human interleukin-6 promoter (IL-6) and transcription factor C/EBPb (NF-IL6) activity by p53 species. J. Biol. Chem., 268, 15096–15100. [PubMed] [Google Scholar]
- Mizutani Y., Fukumoto,M., Bonavida,B. and Yoshida,O. (1994) Enhancement of sensitivity of urinary bladder tumor cells to cisplatin by c-myc antisense oligonucleotide. Cancer, 74, 2546–2554. [DOI] [PubMed] [Google Scholar]
- Morgenstern P.J. and Land,H. (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res., 18, 3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi S., Nakagawa,K., Yokota,J., Tsunokawa,Y., Nishio,K., Terashima,Y., Shibuya,M., Terada,M. and Saijo,N. (1991) Resistance to anticancer drugs in NIH3T3 cells transfected with c-myc and/or c-H-ras genes. Br. J. Cancer, 63, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offer H., Milyavsky,M., Erez,N., Matas,D., Zurer,I., Harris,C. and Rotter,V. (2001) Structural and functional involvement of p53 in BER in vitro and in vivo. Oncogene, 20, 581–589. [DOI] [PubMed] [Google Scholar]
- Peled A., Zipori,D. and Rotter,V. (1996) Cooperation between p53-dependent and p53-independent apoptotic pathway of myeloid cells. Cancer Res., 56, 2148–2156. [PubMed] [Google Scholar]
- Peng Y., Chen,L., Li,C., Lu,W., Agrawal,S. and Chen,J. (2001) Stabilization of the MDM2 oncoprotein by mutant p53. J. Biol. Chem., 276, 6874–6878 [DOI] [PubMed] [Google Scholar]
- Pratt W.B. and Ruddon,R.W. (1979) The Anticancer Drugs. Oxford University Press, New York, NY.
- Roemer K. and Mueller-Lantzsch,N. (1996) p53 transactivation domain mutant Q22, S23 is impaired for repression of promoters and mediation of apoptosis. Oncogene, 12, 2069–2079. [PubMed] [Google Scholar]
- Rotter V. (1983) p53, a transformation-related cellular encoded protein, can be used as a biochemical marker for the detection of primary mouse tumor cells. Proc. Natl Acad. Sci. USA, 80, 2613–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini P., Lin,J.Y., Levine,A.J. and White,E. (1995) Essential role for p53-mediated transcription in E1A-induced apoptosis. Genes Dev., 9, 2184–2192. [DOI] [PubMed] [Google Scholar]
- Schwartz D. and Rotter,V. (1998) p53-dependent cell cycle control: response to genotoxic stress. Semin. Cancer Biol., 8, 325–336. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lin,A.W., McCurrach,M.E., Beach,D. and Lowe,S.W. (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell, 88, 593–602. [DOI] [PubMed] [Google Scholar]
- Shaulian E., Zauberman,A., Ginsberg,D. and Oren,M. (1992) Identification of minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol. Cell. Biol., 12, 5581–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal A. and Rotter,V. (2000) Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res., 60, 6788–6793. [PubMed] [Google Scholar]
- Sigal A., Matas,D., Almog,N., Goldfinger,N. and Rotter,V. (2001) The C-terminus of mutant p53 is necessary for its ability to interfere with growth arrest or apoptosis. Oncogene, in press. [DOI] [PubMed] [Google Scholar]
- Sionov R.V. and Haupt,Y. (1999) The cellular response to p53: the decision between life and death. Oncogene, 18, 6145–6157. [DOI] [PubMed] [Google Scholar]
- Sklar M.D. and Prochownik,E.V. (1991) Modulation of cis-platinum resistance in Friend erythroleukemia cells by c-myc. Cancer Res., 51, 2118–2123. [PubMed] [Google Scholar]
- Srivastava S., Wang,S., Tong,Y.O., Hao,Z.M. and Chang,E. (1993) Dominant negative effect of a germ-line mutant p53: a step fostering tumorigenesis. Cancer Res., 53, 4452–4455. [PubMed] [Google Scholar]
- Subler M.A., Martin,D.W. and Deb,S. (1994) Overlapping domains on the p53 protein regulate its transcriptional activation and repression functions. Oncogene, 9, 1351–1359. [PubMed] [Google Scholar]
- Tlsty T.D. (1997) Genomic instability and its role in neoplasia. Curr. Top. Microbiol. Immunol., 221, 37–46. [DOI] [PubMed] [Google Scholar]
- Unger T., Nau,M.M., Segal,S. and Minna,J.D. (1992) p53: a transdominant regulator of transcription whose function is ablated by mutations occurring in human cancer. EMBO J., 11, 1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T., Mietz,J.A., Scheffner,M., Yee,C.L. and Howley,P.M. (1993) Functional domains of wild-type and mutant p53 proteins involved in transcriptional regulation, transdominant inhibition and transformation suppression. Mol. Cell. Biol., 13, 5186–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waardenburg R.C., Prins,J., Meijer,C., Uges,D.R., De Vries,E.G. and Mulder,N.H. (1996) Effects of c-myc oncogene modulation on drug resistance in human small cell lung carcinoma cell lines. Anticancer Res., 16, 1963–1970. [PubMed] [Google Scholar]
- Van Waardenburg R.C., Meijer,C., Burger,H., Nooter,K., De Vries,E.G., Mulder,N.H. and De Jong,S. (1997) Effects of an inducible anti-sense c-myc gene transfer in a drug-resistant human small-cell-lung-carcinoma cell line. Int. J. Cancer, 73, 544–550. [DOI] [PubMed] [Google Scholar]
- Wagner A.J., Kokontis,J.M. and Hay,N. (1994) Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev., 8, 2817–2830. [DOI] [PubMed] [Google Scholar]
- Wahl G.M., Linke,S.P., Paulson,T.G. and Huang,L.C. (1997) Maintaining genetic stability through TP53 mediated checkpoint control. Cancer Surv., 29, 183–219. [PubMed] [Google Scholar]
- Wolf D., Harris,N. and Rotter,V. (1984) Reconstitution of p53 expression in a nonproducer Ab-MuLV-transformed cell line by transfection of a functional p53 gene. Cell, 38, 119–126. [DOI] [PubMed] [Google Scholar]
- Woods D.B. and Vousden,K.H. (2001) Regulation of p53 function. Exp. Cell Res., 264, 56–66. [DOI] [PubMed] [Google Scholar]
- Yankulov K., Yamashita,K., Roy,R., Egly,J.M. and Bentley,D.L. (1995) The transcriptional elongation inhibitor 5,6-dichloro-1- β-d-ribofuranosylbenzimidazole inhibits transcription factor IIH-associated protein kinase. J. Biol. Chem., 270, 23922–23925. [DOI] [PubMed] [Google Scholar]
- Yee J.K., Friedmann,T. and Burns,J.C. (1994) Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol., 43, 99–112. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Deguin,V., Zaitchouk,T., Breugnot,C., Mishal,Z., Jenkins,J.R. and May,E. (1995) Transcriptional activation plays a role in the induction of apoptosis by transiently transfected wild-type p53. Oncogene, 11, 2197–2205. [PubMed] [Google Scholar]
- Zamble D.B. and Lippard,S.J. (1995) Cisplatin and DNA repair in cancer chemotherapy. Trends Biochem. Sci., 20, 435–439. [DOI] [PubMed] [Google Scholar]
- Zhang W., Guo,X.Y., Hu,G.Y., Liu,W.B., Shay,J.W. and Deisseroth,A.B. (1994) A temperature-sensitive mutant of human p53. EMBO J., 13, 2535–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F., Eischen,C.M., Randle,D.H., Kamijo,T., Cleveland,J.L., Sherr,C.J. and Roussel,M.F. (1998) Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev., 12, 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]