Abstract
The p41 splice variant of major histocompatibility complex (MHC) class II-associated invariant chain (Ii) contains a 65 aa segment that binds to the active site of cathepsin L (CatL), a lysosomal cysteine protease involved in MHC class II-restricted antigen presentation. This segment is absent from the predominant form of Ii, p31. Here we document the in vivo significance of the p41–CatL interaction. By biochemical means and electron microscopy, we demonstrate that the levels of active CatL are strongly reduced in bone marrow-derived antigen-presenting cells that lack p41. This defect mainly concerns the mature two-chain forms of CatL, which depend on p41 to be expressed at wild-type levels. Indeed, pulse–chase analysis suggests that these mature forms of CatL are degraded by endocytic proteases when p41 is absent. We conclude that p41 is required for activity of CatL by stabilizing the mature forms of the enzyme. This suggests that p41 is not merely an inhibitor of CatL enzymatic activity, but serves as a chaperone to help maintain a pool of mature enzyme in late-endocytic compartments of antigen-presenting cells.
Keywords: antigen-presenting cells/cathepsin L/chaperone/invariant chain/p41
Introduction
Antigen presentation via major histocompatibility complex (MHC) class II molecules relies on the intersection of their biosynthetic route with the endocytic pathway (Wolf and Ploegh, 1995). There, resident proteases attack macromolecules, ensuring that even complex particulate antigens will be converted into presentable peptides (Villadangos et al., 1999; Villadangos and Ploegh, 2000). In the endoplasmic reticulum (ER), three class II αβ dimers assemble onto a scaffold of a trimeric type II membrane protein, the invariant chain (Ii) to yield an (αβ–Ii)3 complex (Roche et al., 1991). Ii not only serves as a chaperone to facilitate the folding and assembly of class II αβ dimers, but also contains the address code for their delivery to the endocytic pathway (Bakke and Dobberstein, 1990; Lotteau et al., 1990). During export of (αβ–Ii)3 complexes, non-cysteine and cysteine proteases sequentially degrade Ii (Roche and Cresswell, 1991; Villadangos et al., 1997). CLIP, the Ii fragment that remains associated with MHC class II molecules, is ultimately dislodged and replaced with antigenic peptides, which are likewise generated by lysosomal proteases (Roche and Cresswell, 1990, 1991; Wolf and Ploegh, 1995). Antigen presentation is therefore dependent on the collection of proteases to which αβ–Ii complexes and newly internalized antigens are exposed (Villadangos and Ploegh, 2000). Analysis of mice deficient in specific cysteine proteases identified cathepsins S (CatS) and L (CatL) as two key enzymes required for Ii degradation (Riese et al., 1996; Nakagawa et al., 1998, 1999; Shi et al., 1999; Villadangos and Ploegh, 2000). The final step of Ii proteolysis into αβ-CLIP is mediated by CatS in B cells and dendritic cells (DCs), while CatL performs this cleavage in cortical thymic epithelial cells (cTECs) (Nakagawa et al., 1998, 1999; Shi et al., 1999).
Most lysosomal hydrolases are synthesized in the ER as proenzymes (Erickson, 1989; McGrath, 1999). The CatL propiece consists of a 96 amino acid (aa) polypeptide that occupies its active site cleft, maintaining the enzyme in an inactive state (Coulombe et al., 1996). In addition, the CatL pro-region is necessary for folding, stability (at neutral pH) and intracellular trafficking of the enzyme (McIntyre and Erickson, 1991; Coulombe et al., 1996; Jerala et al., 1998; McGrath, 1999). During export along the endocytic pathway, proCatL undergoes several proteolytic cleavages to generate the CatL single-chain (30 kDa) and two-chain mature forms, composed of a heavy-chain (25 kDa) linked to a light-chain (6 kDa) by disulfide bonds (Erickson, 1989; Ishidoh et al., 1998; McGrath, 1999). Most of the CatL single-chain form is detected in late endosomes and lysosomes, while the two-chain form is found mainly in the latter (Ishidoh et al., 1998).
Antigen-presenting cells (APCs) express two Ii isoforms, p31 and p41, which result from alternative RNA splicing (Strubin et al., 1986; Koch et al., 1987; O’Sullivan et al., 1987) and are expressed in different ratios in the different types of APCs. Whereas p41 represents no more than 10% of the total pool of Ii in splenocytes, its expression levels are considerably higher in macrophages, DCs and Langerhans cells (Koch and Harris, 1984; Pierre and Mellman, 1998). In addition to their indistinguishable function as chaperones for class II folding and intracellular trafficking (Peterson and Miller, 1992; Takaesu et al., 1995, 1997), both p31 and p41 can be converted into CLIP (Takaesu et al., 1995, 1997), presumably by CatS.
The p41-specific 65 aa segment resembles a thyroglobulin type 1 domain, rich in cysteine residues (O’Sullivan et al., 1987). This p41 segment was found non-covalently bound to the mature form of CatL purified from human kidney (Ogrinc et al., 1993). The crystal structure of CatL in a complex with the p41 segment shows that this fragment occupies the CatL active site (Guncar et al., 1999). In agreement with this observation, in vitro studies show that the 65 aa segment of p41 inhibits CatL enzymatic activity (Bevec et al., 1996; Fineschi et al., 1996). However, neither the origin of CatL–p41 association nor its functional relevance in vivo is known. In addition, a recent report shows that H2-DM molecules are degraded by cysteine proteases in various APCs from Ii–/– mice (Pierre et al., 2000). Whether the expression of p31 and p41 directly affects the protease activity of primary APCs in vivo has not been addressed.
In the present study, we used several approaches to analyze the expression and activity of CatL in bone marrow-derived APCs (BM APCs) from mutant mice that selectively express the p31 or p41 isoform of Ii. Contrary to expectations, we show that CatL expression and activity are considerably diminished in cells lacking Ii. Furthermore, we demonstrate that it is the p41 Ii isoform that is required for full CatL activity. Indeed, in cells that lack p41, the mature forms of CatL are partially degraded. We conclude that p41 enhances the intracellular levels of active CatL by preventing its premature destruction by the surrounding endocytic proteases. We propose that the p41 isoform of Ii serves as a chaperone for CatL, to help maintain a pool of mature enzyme in late-endocytic compartments of APCs.
Results
Decreased levels of mature CatL in the absence of Ii
The isolation of a complex composed of CatL and the 65 aa segment specific to the p41 isoform of Ii (Ogrinc et al., 1993) suggested that Ii expression might influence CatL activity. Experiments carried out in vitro demonstrated that the 65 aa p41 fragment inhibited CatL’s enzymatic activity (Bevec et al., 1996), as would be expected of any compound that blocks the enzyme’s active site. What then is the relationship of Ii expression to CatL activity in vivo? To answer this question, we first compared CatL expression in APCs isolated from Ii-positive and Ii-negative (Ii–/–) mice by immunoblotting. CatL–/– mice served as a control for specificity of the CatL antiserum, which recognizes both the mature single-chain and two-chain forms of the enzyme, in addition to the zymogen (McIntyre et al., 1994). CatL expression was examined in cell lysates from BM cells exposed to granulocyte/macrophage colony-stimulating factor (GM-CSF) for 6 days (enriched for macrophages, DCs and granulocytes) and from splenocytes (largely comprised of B cells). CatL was not detected in splenocytes under these experimental conditions (Figure 1A, lanes 1–3). Cultured BM cells, however, expressed the proform and the different mature forms of the enzyme (Figure 1A, lanes 4–6). The minor 30 kDa polypeptide corresponds to the single-chain form of CatL, and the 24–25 kDa doublet presumably represents distinct heavy-chain variants of the two-chain mature form of the enzyme. For convenience, these two heavy-chain isoforms will be designated as the ‘CatL-24 kDa and -25 kDa two-chain forms’. These results are in agreement with several studies that demonstrate the prevalence of the two-chain form of CatL in macrophages (Erickson, 1989; Claus et al., 1998), with only low levels of the CatL single-chain form (30 kDa). Interestingly, the levels of mature CatL were significantly decreased in BM cells from Ii–/– mice (Figure 1A, lanes 4–6), an unexpected finding in light of reports that demonstrated in vitro inhibition of CatL by the 65 aa segment of the p41 Ii isoform. The lysates prepared from the different APCs were analyzed with an anti-proCatD serum to ensure that equal amounts of protein from each sample were loaded on the gel (Figure 1B). Even though we detected less proCatD in cell lysates from splenocytes (Figure 1B, lanes 1–3) compared with BM-derived APCs (Figure 1B, lanes 4–6), its level was not affected by the absence of Ii.
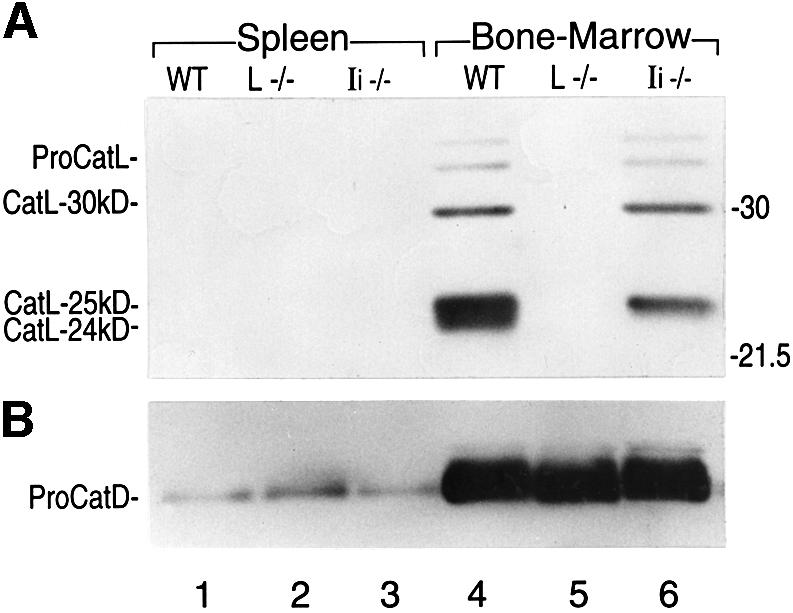
Fig. 1. Expression of CatL in splenocytes and BM APCs from WT and Ii–/– mice. Immunoblot analysis performed on 15 µg of cell lysate obtained from splenocytes or BM cells cultured during 6 days in GM-CSF, and analyzed by SDS–PAGE on a 15% gel under reducing conditions. An antiserum recognizing the proform and the mature forms of CatL (A), or an antiserum raised against CatD (B) was used.
To identify the BM cell types that were the source of the detected CatL, we separated BM macrophages, DCs and granulocytes by exploiting their different adherence properties and by FACS sorting (see Materials and methods). Lysates prepared from these three cell populations were analyzed for CatL expression as described above. We detected mature CatL in BM-derived APCs, that is, in macrophages and, to a lesser extent, in DCs (data not shown). Mature CatL was not found in the non-APC granulocytes. Thus, we conclude that in BM APCs, the expression of mature CatL two-chain forms relies on the presence of Ii. Because BM macrophages display higher levels of CatL and are more readily obtained in adequate numbers as compared with DCs, all subsequent experiments were performed using this cell population.
The p41 isoform of Ii controls the intracellular levels of mature active CatL
Are the decreased amounts of CatL in BM APCs from Ii–/– mice due to the absence of p41? To address this question we used mutant mice that express either the p31 or p41 isoform of Ii, referred to hereafter as p31 and p41 mice (Takaesu et al., 1995, 1997). These animals express p31 or p41 at levels similar to WT mice, since transcription is driven by the cis-acting regulatory elements of the Ii gene (Takaesu et al., 1995, 1997; see below; Figure 6B). CatL expression level was examined by immunoblotting in BM macrophages isolated from WT, CatL–/–, Ii–/–, p31 and p41 mice. Ii–/– and p31 BM macrophages lacked CatL-24 kDa and displayed slightly decreased CatL-25 kDa levels (Figure 2A, lanes 3 and 4). In marked contrast, both forms were present in cells that expressed p41 (Figure 2A, lanes 1 and 5). Thus, the level of expression of mature CatL in BM-derived APCs depends on the presence of p41.
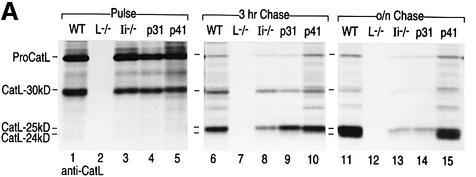
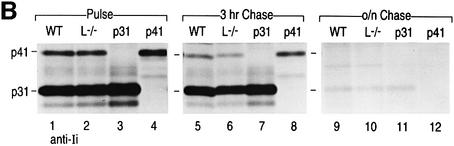
Fig. 6. CatL and Ii biogenesis in BM macrophages from WT, Ii–/–, p31 and p41 mice. Immunoprecipitation was performed on cell lysates prepared from BM macrophages treated with 100 U/ml of IFNγ for 48 h, pulsed with [35S]cysteine and [35S]methionine for 40 min, and chased for 3 h or overnight (16 h). Antisera directed against CatL (A) or Ii (B) were used. The samples were analyzed by SDS–PAGE on a 12.5% gel under reducing conditions.
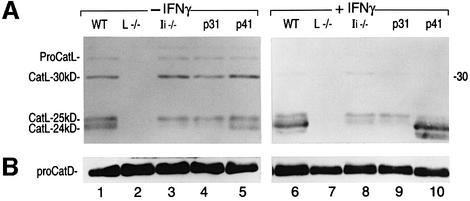
Fig. 2. Expression of CatL in resting and IFNγ-stimulated BM macrophages from WT, Ii–/–, p31 and p41 mice. Immunoblot analysis performed on 15 µg of cell lysate obtained from day 6 BM macrophages cultured for 48 h in the absence (–) or presence (+) of IFNγ, and analyzed by SDS–PAGE on a 15% gel under reducing conditions. An antiserum recognizing the proform and the mature forms of CatL (A), or an antiserum raised against CatD (B) was used. p31 and p41 mice selectively express one Ii isoform.
The levels of the 24 kDa form of CatL, whose expression was completely dependent on p41, increased when macrophages were treated with interferon-γ (IFNγ) (Figure 2A, lanes 6 and 10). Therefore, the difference in the total amount of active CatL between cells that do or do not express p41 was even more drastic after treatment with IFNγ (Figure 2A, lanes 6, 8, 9 and 10). Interestingly, IFNγ did not enhance the levels of proCatL (which was not readily detectable in IFNγ-treated cells) but only its conversion into the CatL-24 kDa mature form. This suggests that the regulatory effect of IFNγ is not due entirely to increased CatL gene transcription as reported previously (Lah et al., 1995).
Are the two-chain forms of CatL enzymatically active? To address this question we used an affinity-labeled active site-directed probe, LHVS-biotin (Figure 3A), which covalently modifies the cysteine active site when added to cell lysates. Covalent modification by LHVS-biotin is mechanism based, and therefore reflects the enzymatic activity of the protease targeted (Palmer et al., 1995). LHVS-biotin is a derivative of the previously described LHVS (Palmer et al., 1995) and targets mainly the active sites of CatS and CatB. However, when used at high concentrations, LHVS-biotin also targets active CatL (Figure 3B). LHVS-biotin labeled both the 24 and 25 kDa two-chain forms of CatL in IFNγ-treated cells expressing p41 (Figure 3B, lanes 1 and 5). Thus, both of these forms of CatL are active. As expected from our immunoblot experiments, the predominant 24 kDa form of CatL was not detected in Ii–/– or p31 cell lysates (Figure 3B, lanes 3 and 4). In addition, this LHVS-biotin labeling experiment showed that the effect of p41 on the activity of cysteine proteases is CatL specific, since labeling of CatB and CatS is unaffected by p41 status (Figure 3B). We conclude that the total levels of mature CatL depend on the presence of p41, and that p41 is therefore required for full CatL activity in BM-derived APCs.
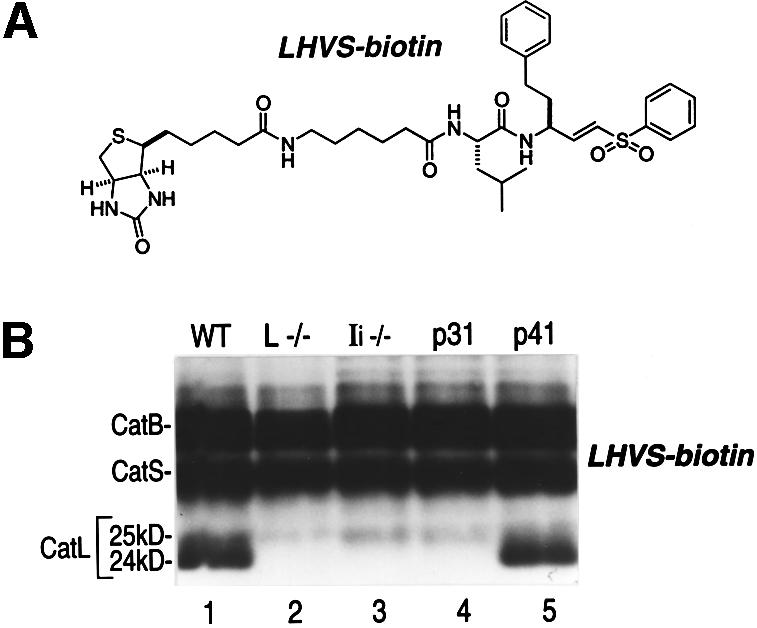
Fig. 3. Activity of the two-chain forms of CatL in WT, Ii–/–, p31 and p41 macrophages. (A) LHVS-biotin. (B) Twenty-five micrograms of cell lysates (pH 5) obtained from BM macrophages treated with IFNγ for 48 h were labeled with 1 mM LHVS-biotin and analyzed by SDS–PAGE on a 15% gel under reducing conditions. CatB and CatS were identified by comparison with cell lysates from CatB and CatS knock-out mice. CatL-30 kDa was not detected in this assay, probably because it co-migrates with CatB.
p41 regulates the activity of CatL in late endocytic compartments
Because p41 selectively affects the two-chain forms of CatL, which reside mainly in lysosomes (Erickson, 1989; Claus et al., 1998), p41 may enhance maturation and/or stability of CatL in late endocytic compartments. Alternatively, p41 could provide the signal motif(s) necessary for delivery of a fraction of proCatL to the endocytic pathway, similar to its role in escorting class II molecules. Indeed, trafficking of proCatL to endosomes can occur independently of mannose-6-phosphate receptors, implicating alternative modes of CatL sorting to endosomes (McIntyre and Erickson, 1991). To examine intracellular trafficking of CatL, we used a subcellular fractionation technique consisting of two successive Percoll gradients (Castellino and Germain, 1995; Driessen et al., 1999). As assessed by the β-hexosaminidase activity in each fraction (Figure 4A), this method allows the separation of lysosomes (peak A/27% Percoll gradient) from other intracellular compartments, including endosomes, which were further fractionated on a 10% Percoll gradient. This second fractionation step generates two peaks of β-hexosaminidase activity; peak B corresponds to late-endosomes, while peak C comprises early-endosomes, Golgi, ER and plasma membrane (Driessen et al., 1999) (Figure 4A).
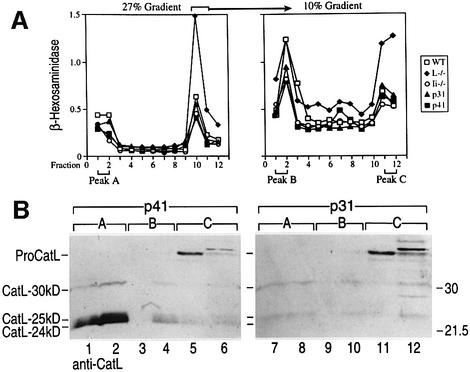
Fig. 4. Maturation of CatL along the endocytic pathway of BM macrophages from p31 and p41 mice. (A) Subcellular fractionation of BM cells was performed by successive Percoll gradients (27 and 10%) and the β-hexosaminidase activity (absorbance at 448 nm) of each fraction was quantified as described in Materials and methods (Driessen et al., 1999). Characterization of the fractions allowed the identification of peaks A, B and C, as corresponding to lysosomes (peak A), late-endosomes (peak B), and ER, Golgi, early endosomes and plasma membrane (peak C), respectively. (B) Immunoblot analysis of CatL expression in the two subcellular fractions corresponding to peaks A, B and C (see Figure 3A). The fractions were lysed in 1% NP-40 buffer, centrifuged at 100 000 g to remove the Percoll, and analyzed by SDS–PAGE on a 12.5% gel under reducing conditions (90 µl of each fraction/well). An antiserum recognizing the proform and the mature forms of CatL was used. ProCatL appears as two polypeptides of different size, which may result from differential glycosylation.
CatL content in the different fractions was assessed by immunoblotting on peaks A, B and C from p31 and p41 BM macrophages (Figure 4B). As expected, proCatL was recovered primarily in peak C, reflecting its prevalence in the ER and Golgi compartments (Figure 4B, lanes 5, 6, 11 and 12). As proCatL arrived in late-endosomal compartments, it was converted into the 30 kDa single-chain form and subsequently processed into the two-chain forms (Figure 4B, lanes 3, 4, 9 and 10). CatL-24 kDa and -25 kDa two-chain forms were detected predominantly in lysosomes (Figure 4B, lanes 1, 2, 7 and 8), with a small proportion of these CatL species present in late-endosomal compartments (lanes 3, 4, 9 and 10). This distribution pattern of the different forms of CatL corroborates the validity of the fractionation protocol.
No significant difference in the amounts of proCatL in peak C and of the CatL-30 kDa form in peaks A and B was observed for p31 and p41 cells (Figure 4B, lanes 3–6 and 9–12). In striking contrast, the fully active 24 and 25 kDa two-chain forms of CatL, abundantly expressed in the lysosomal fractions from p41 APCs, were barely detectable in p31 cells (Figure 4B, lanes 1, 2, 7 and 8). We conclude that trafficking of proCatL to endocytic compartments occurs independently of p41. In contrast, the presence in late-endosomes and lysosomes of the 24 and 25 kDa mature forms of CatL strictly requires p41. Identical conclusions were reached analyzing distribution of CatL in WT and Ii–/– BM macrophages (data not shown).
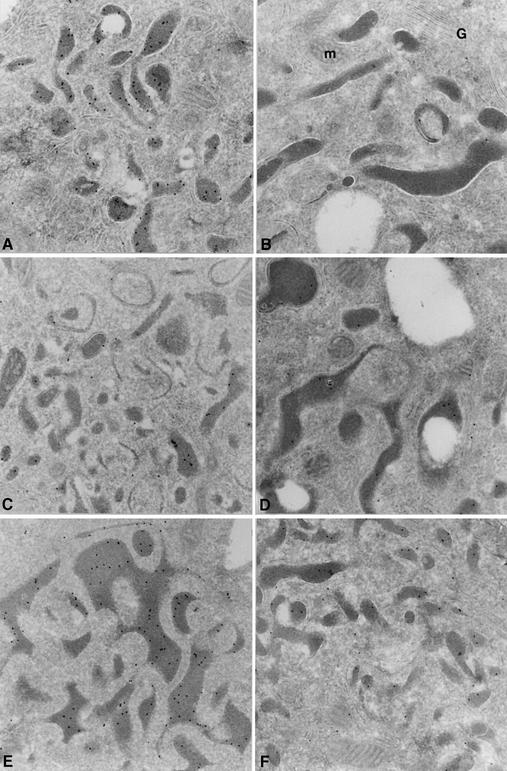
BM macrophages were further analyzed for their CatL content by cryoimmunogold electron microscopy, using the CatL polyclonal antiserum described above. While lysosomes from WT and p41 cells show strong labeling for CatL, lysosomes from p31 and Ii cells display little staining (Figure 5A–E). Furthermore, when labeling the cells with an antiserum directed against the thyroglobulin domain of p41, we observed its presence in lysosomal compartments (Figure 5F), evidence of colocalization of this p41 fragment with active CatL. In summary, the data presented thus far show that p41 is required for maintenance of wild-type (WT) levels of mature CatL in late-endocytic compartments of BM APCs.
Fig. 5. Analysis of CatL and p41 expression by cryoimmunogold electron microscopy in BM macrophages derived from WT, Ii–/–, p31 and p41 mice. Cryosections from WT (A), CatL–/– (B), Ii–/– (C), p31 (D) and p41 (E and F) BM macrophages, treated with IFNγ during 48 h and fixed with 2% paraformaldehyde plus 0.2% glutaraldehyde for 2 h were used for staining. Sections were incubated with antisera recognizing the proform and the mature forms of CatL (anti-CatL, A–E) or the p41 65 aa-specific fragment (anti-p41, F), followed by incubation with protein A coupled to gold particles.
The biogenesis of CatL is altered in cells lacking p41
The role of p41 in CatL biogenesis was examined in pulse–chase experiments performed on BM macrophages obtained from WT, CatL–/–, Ii–/–, p31 and p41 mice and metabolically labeled with [35S]methionine and [35S]cysteine (Figure 6A). To prevent post-lysis artifacts, cells were directly lysed in 1% SDS and lysates immediately boiled after sampling each time-point. Cell lysates were immunoprecipitated with both anti-CatL (Figure 6A) and anti-Ii (Figure 6B) sera. Immunoprecipitation of Ii showed no change in p31 and p41 biosynthesis and turnover in cells that lack CatL (Figure 6B, lanes 2, 6 and 10).
After a 40 min pulse, proCatL was the predominant species retrieved by immunoprecipitation with the CatL antiserum, even though a small portion of proCatL was already converted into the 30 kDa mature form (Figure 6A, lanes 1–5). In agreement with the fractionation results, which show that the CatL two-chain forms are generated in late-endocytic compartments, the 24 and 25 kDa mature forms of CatL were not detected at this early time-point (Figure 6A, lanes 1–5). As expected, there was no difference between Ii+/+ and Ii–/– cells. The rate of synthesis of proCatL was thus independent of the presence of p41. After 3 h of chase, the bulk of CatL had reached the two-chain state, but only the 25 kDa form of CatL was detected (Figure 6A, lanes 6–10). Its levels were slightly decreased in p31 and Ii–/– cells (Figure 6A, lanes 8 and 9). After an overnight chase, both CatL-25 kDa and -24 kDa were readily detected in WT and p41 cells (Figure 6A, lanes 11 and 15) whereas only small amounts of CatL-25 kDa and no CatL-24 kDa was seen in p31 and Ii–/– cells (Figure 6A, lanes 13 and 14). In addition, the levels of proCatL and CatL-30 kDa were also affected by the absence of p41 at these late chase-points (Figure 6A, lanes 6–15). These results are in agreement with the data presented above, confirming the strong dependency of the CatL two-chain forms on p41. Furthermore, these data suggest that the stability of the proform and all mature forms of CatL is compromised when p41 is absent.
p41 protects mature CatL from degradation by cysteine proteases
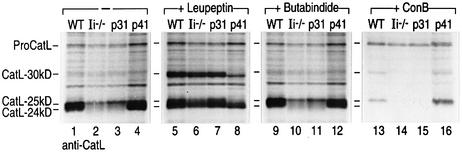
The decrease in stability of all CatL forms observed in pulse–chase experiments in cells that lack p41 (Figure 6A) may reflect enhanced degradation of mature CatL. To investigate whether, in the absence of p41, CatL was vulnerable to destruction by lysosomal proteases, including perhaps CatL itself, a pulse–chase experiment was performed in the presence of different protease inhibitors. Cells were pulsed for 40 min and after a 3 h chase (to allow the generation of both CatL-30 kDa and -25 kDa; Figure 6A), either leupeptin (a cysteine protease inhibitor), butabindide (a serine protease inhibitor) (Rose et al., 1996) or Concamycin B (ConB) (the vacuolar H+-ATPase inhibitor) (Yilla et al., 1993) was added to the chase medium (Figure 7). Butabindide had no effect on CatL levels or proteolytic conversion of the proenzyme, yielding results essentially similar to untreated cells (Figure 7, lanes 9–12). As expected, ConB inhibited CatL maturation in all cell types. However, this inhibition was more pronounced in cells lacking p41 (Figure 7, lanes 13–16). Leupeptin markedly increased the overall levels of CatL and resulted in accumulation of the 30 kDa form of CatL. More importantly, leupeptin rescued the defect in CatL expression observed in cells that lack p41 (Figure 7, lanes 5–8). In the presence of leupeptin, the 24 kDa CatL two-chain form was detected at equivalent levels in WT, Ii–/–, p31 and p41 cells, showing that leupeptin mimics the effect of p41. Collectively, our findings suggest that the presence of p41 stabilizes the mature forms of CatL and protects them from degradation by cysteine proteases.
Fig. 7. CatL biogenesis in BM macrophages from WT, Ii–/–, p31 and p41 mice treated with different protease inhibitors. Immuno precipitations were performed on cell lysates prepared from BM macrophages treated with 100 U/ml of IFNγ for 48 h using an antiserum recognizing the proform and mature forms of CatL. Cells were pulsed with [35S]cysteine and [35S]methionine for 40 min and chased overnight (16 h). Leupeptin (1 mM), butabindide (0.5 mM) or ConB (20 nm) was added to the cells after 3 h of chase. The samples were analyzed by SDS–PAGE on a 12.5% gel under reducing conditions.
Discussion
The data presented here demonstrate that Ii is required in vivo for full activity of CatL, a thiol protease implicated in Ii degradation and T-cell development (Nakagawa et al., 1998; Villadangos and Ploegh, 2000). We show that, in BM APCs that lack the p41 isoform of Ii, CatL activity is impaired due to decreased levels of the mature forms of CatL. This defect concerns mainly the two-chain forms of CatL, which are generated late during maturation of the enzyme in acidic subcellular compartments. In particular, active CatL-24 kDa was not detected in the absence of p41, while the levels of CatL-25 kDa were slightly decreased. In IFNγ-treated APCs that lacked p41, the reduction of the total amount of CatL two-chain forms (24 and 25 kDa combined) was ∼80% when assessed by immunoblotting. In general, the effect of p41’s absence on the levels of Cat-30 kDa and -25 kDa was always more pronounced when examined after long chase-points in pulse–chase experiments, compared with levels of CatL examined at steady state by immunoblotting. Nonetheless, CatL-24 kDa was undetectable in both types of experiment in cells that lack p41. Hence, our data show that p41 is essential in vivo for full CatL expression and activity (summarized in Figure 8).
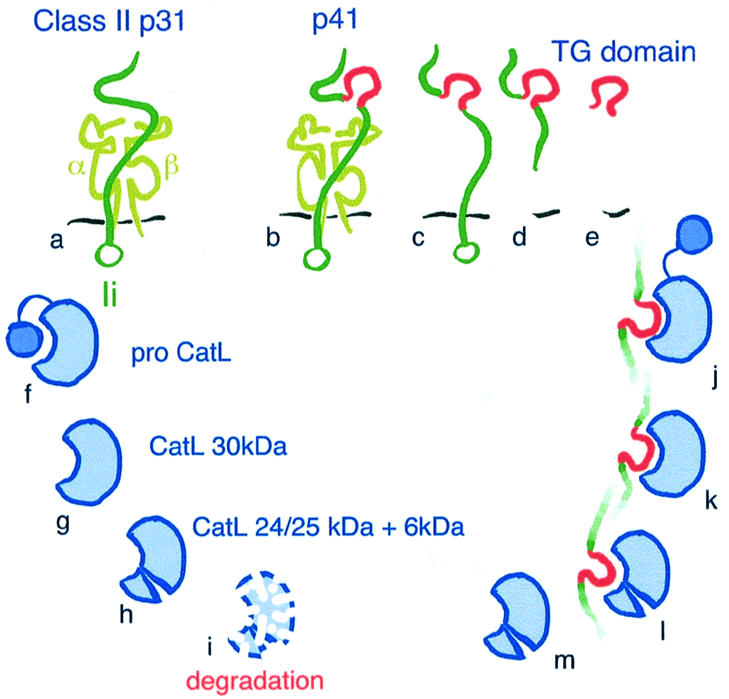
Fig. 8. Model for the protective effect of P41 on CatL. (A and B) MHC class II molecule (α and β chain) associated with the p31 (A) or p41 (B) isoform of Ii. (B–E) Different forms of p41 potentially capable of interacting with CatL. The thyroglobulin-specific domain of p41 is colored in red. The species designated (D) and (E) are hypothetical and represent putative p41 breakdown intermediates. (F–I) CatL maturation and degradation/turnover (I). The maturation of CatL involves removal of the propeptide and cleavage of the 30 kDa single-chain form to generate the 24/25 kDa + 6 kDa two-chain forms (F–H). The structural difference(s) between both two-chain forms is not known. In the absence of p41, CatL-24 kDa is degraded (I). (J–M) Interaction of p41, via its thyroglobulin domain, with the active site of CatL. This association has been documented for mature CatL (K and L), but could also involve proCatL (J) followed by proteolytic processing of the propiece. Interaction of p41 with CatL protects the mature two-chain form from degradation (M versus I).
ProCatL has a molecular weight of ∼38 kDa, the processed single-chain form of CatL is 30 kDa, and cleavage at Asn169 generates the two-chain form of the enzyme (heavy-chain: 24/25 kDa plus light-chain: 6 kDa) (Figure 8F–H). The difference between the CatL-24 kDa and -25 kDa two-chain forms is presently not understood. Cathepsins undergo trimming at the N- and/or C-terminal ends of the single-chain and two-chain forms (Erickson, 1989). These proteolytic cleavage events occur late in cathepsin biosynthesis, and thus could explain the difference between the CatL-25 kDa and -24 kDa forms, the latter being detected only after an overnight chase. Both two-chain forms of CatL are enzymatically active. Interestingly, it has been suggested that such trimming could play a role in the turnover of lysosomal enzymes (Erickson, 1989).
What is the composition of CatL–p41 complexes in vivo? Ii is synthesized in molar excess over class II molecules in many cell types. Indeed, we observed sizeable levels of Ii in untreated BM macrophages, while class II molecules themselves were difficult to detect (data not shown). In addition, expression of Ii in the absence of class II molecules has also been reported for various tissues (Koch and Harris, 1984). The pool of Ii/p41 available for interactions with proteins other than class II is the most likely source of CatL-modulating activity. Given the tight interaction of Ii/p41 with itself (Ii trimer) and with class II molecules (via CLIP), a simultaneous interaction of CatL with a class II–p41 complex (via the 65 aa p41 segment) would be highly constrained (Figure 8B). This observation is supported by the crystallography data obtained for CatL complexed to the p41 fragment (Guncar et al., 1999). In addition, it is not known whether CatL–p41 interaction in vivo involves the full-length p41 molecule (Figure 8C), its 65 aa thyroglobulin domain (Figure 8E) or some p41 proteolytic intermediate (Figure 8D). We have not been able to demonstrate a direct CatL–p41 interaction by co-immunoprecipitation of a CatL–Ii complex, or by precipitating active-site-labeled CatL from cell lysates using anti-Ii antibodies (data not shown). Covalent modification of the active site of CatL may not be compatible with its interaction with p41.
Let us consider briefly the possibilities for the different form(s) of CatL that could interact with p41. In agreement with the in vivo role of p41 in stabilizing predominantly the mature two-chain form of CatL, the p41-specific fragment was previously found to interact with both the single-chain and the two-chain forms of the enzyme (Ogrinc et al., 1993; Guncar et al., 1999) (Figure 8K and L). However, it is possible that the p41 fragment interacts with proCatL by displacing the propeptide (Figure 8J). Indeed, our pulse–chase experiments show decreased stability of proCatL in cells that lack p41 (Figure 6A). This association may occur transiently, just before the propeptide is detached from the active site of the enzyme in an endocytic compartment.
How does p41 protect the active forms of CatL from degradation? Protection might be linked to the ability of p41 to interact with mature CatL by binding to its active site (Guncar et al., 1999). The interaction of the p41 fragment with CatL is reminiscent of the interaction of CatL with its propeptide. Of course the latter occurs early during CatL biosynthesis in neutral pH compartments such as the ER and the Golgi (Erickson, 1989; McGrath, 1999). There, the CatL propeptide not only maintains the enzyme in an inactive state but also assists its folding and stabilizes its conformation (Coulombe et al., 1996; Jerala et al., 1998; McGrath, 1999). As proCatL traverses endocytic compartments, the attendant drop in pH induces conformational changes in the propeptide. The CatL propeptide then detaches from the CatL active site and is cleaved to generate the 30 kDa single-chain form of the enzyme (Jerala et al., 1998). Even though removal of the CatL propeptide is necessary for enzymatic activation, it may destabilize the tertiary structure of the enzyme sufficiently to allow partial unfolding and subsequent degradation. In late-endocytic compartments p41 may act as a CatL chaperone, not unlike the propeptide in neutral compartments. This would allow the cell to maintain a pool of (latent) mature CatL in late-endocytic compartments that is protected from destruction by other hydrolases. We observed by both pulse–chase and immunoblotting that maturation of CatL is more sensitive to ConB when p41 is lacking (Figure 7 and data not shown). By raising the pH of the late-endocytic compartments, ConB might destabilize CatL and render it susceptible to attack by those cysteine proteases that retain some activity at pH 7. In other words, p41 would attenuate the effect of ConB by stabilizing CatL, which would then be less sensitive to the increase in pH induced by the drug. This agrees well with the observations of Ogrinc et al. (1993), who found that, unlike free CatL, CatL complexed to the p41 fragment is stable when exposed to neutral pH.
Our proposal for the significance of the p41–CatL interaction is conceptually similar to the situation encountered in Fabry’s disease, a disorder of glycosphingolipid metabolism that results from deficient maturation of lysosomal α-galactosidase. Indeed, the addition of a competitive inhibitor of α-galactosidase restored the maturation and activity of the enzyme, indicating that the inhibitor can act as a chaperone when administrated at sub-inhibitory concentrations (Fan et al., 1999).
We demonstrate that the level of active CatL-24 kDa relies not only on p41 but is also strongly increased by IFNγ, an agent known to potentiate many components of the class I- and class II-restricted antigen presentation pathways. This suggests that the pool of CatL complexed to p41 might play a specific role in this process. This would require further investigation, for example, in an infectious disease model. One attractive possibility would be to propose that the pool of CatL complexed to p41 is packaged in lysosomal secretory vesicles and is released into extracellular space, since—unlike free mature CatL—the enzyme complexed to p41 can survive in a neutral pH environment (Ogrinc et al., 1993). We were unable to detect any mature CatL in the supernatant of our macrophage cultures (data not shown). However, secretion of CatL–p41 complexes may need to be triggered by a specific event, such as a microbial infection or an inflammatory stimulus. Secreted active CatL could play a role in the degradation of the extracellular matrix to promote cellular migration during inflammation (Reddy et al., 1995), or in the generation of antigenic peptides, to be loaded on the empty class II molecules reported to be present at the surface of some APCs (Santambrogio et al., 1999). Indeed, secretion of active proteases by activated macrophages has been reported (Punturieri et al., 2000). In particular, mature CatK has been found to be secreted in association with its high affinity competitive inhibitor, cystatin C (Punturieri et al., 2000). Similar to the role we propose for p41, cystatin C may stabilize the conformation of mature CatK at neutral pH, favoring survival of the enzyme in the extracellular environment. This could be equally true for the interaction between cystatin C and CatS, which has likewise been invoked as a mechanism for control of CatS activity in the course of DC maturation (Pierre and Mellman, 1998). Together, these observations suggest that in vivo p41 and cystatins may serve not only as inhibitors to regulate protease activity, but also as chaperones that preserve a pool of (latent) active enzymes.
The need for p41 to achieve WT levels of active CatL was abolished in leupeptin-treated cells. One interpretation of these data is that p41 protects mature CatL from degradation by leupeptin-sensitive (cysteine) proteases. This chaperone function of p41 is presumably due to its ability to interact directly with CatL, as was shown in vitro for the 65 aa segment particular to this Ii isoform. Just as the p41-specific fragment occupies the active site of CatL (Ogrinc et al., 1993), so would leupeptin. Therefore, an alternative interpretation of leupeptin’s protective effect is that the mere occupation of mature CatL’s active site by leupeptin (and/or p41) may stabilize it. Our data cannot distinguish between inhibition of other cysteine protease(s) by leupeptin, versus occupation of CatL’s active site by leupeptin, or both, as the mechanism(s) by which the mature forms of CatL are protected. However, we have experimental evidence showing that the levels of CatL-24 kDa are increased in BM macrophages from CatS and CatB knock-out mice (A.-M.Lennon-Duménil, unpublished data). These results suggest that leupeptin-sensitive cysteine proteases contribute to the turnover of CatL in late-endocytic compartments by partially degrading its mature forms. In addition to CatS and CatB, CatL could regulate its own levels of activity by self-degradation. In this context, p41 would exert a protective effect by preventing self-destruction of mature CatL.
Our observation that Ii, and more specifically p41, controls the expression of mature CatL in vivo indicates yet another function for Ii. The requirement for p41 in maintaining the levels of active CatL was altogether unexpected, and the impact of this finding on antigen presentation in vivo, for example, in an infectious disease model, must now be assessed. The essential role of proteolysis in class II-restricted antigen presentation asserts itself at multiple levels: proteolysis of Ii and generation of antigenic determinants. The proteolytic activation of cathepsin zymogens necessitates autocatalytic removal of the propieces, or the action of other lysosomal proteases that can perform such cleavages. Thus, the activities of cathepsins are likely to be controlled by a complex network of interactions. We now add to this network p41, as an example of a protein that is not enzymatically active itself, yet serves as a chaperone to maintain a pool of active CatL in acidic compartments of APCs. Interestingly, this chaperone function of p41 relies on its ability to occupy the active site of CatL via its thyroglobulin domain in the manner of a competitive inhibitor, suggesting that the physiological role of these compounds may be more complex than initially thought.
Materials and methods
Mice
C57BL/6 and Ii–/– mice were purchased from the Jackson laboratory (Bar Harbor, ME). CatL–/–, p31 and p41 mice were described elsewhere (Takaesu et al., 1995, 1997; Nakagawa et al., 1998; Shi et al., 1999). Similar data were obtained when using Ii–/–, p31 and p41 mice in an h-2b or h-2k background.
Preparation of BM-derived APCs
BM was obtained from 2- to 4-month-old mice, and BM-derived APCs were prepared as described (Inaba et al., 1992), using RPMI + 10% fetal calf serum (FCS) + 1% of a culture supernatant containing recombinant mouse GM-CSF (Pierre and Mellman, 1998), obtained from J558L cells transfected with the mouse GM-CSF cDNA (a gift from Dr I.Mellman, Yale University, New Haven, CT). Removal of non-adherent and semi-adherent cells on day 6 combined with extensive washes of the adherent population enabled the adherent macrophages to be separated from DCs and granulocytes. Non-adherent and semi-adherent cells were double-labeled with anti-Gr1-FITC and anti-Ab-PE Abs (Pharmingen). Ab+Gr1– cells (corresponding to mature DCs) and Ab–Gr1+ (corresponding to granulocytes) were separated on a cell-sorter (Coulter). For macrophage preparation, if necessary, 100 U/ml of recombinant mouse IFNγ were added, which were then cultured for two additional days. Phosphate-buffered saline (PBS) + 10 mM EDTA was used to detach macrophages from the plates.
Immunoblotting
Cells were lysed in NP-40 pH 5 lysis buffer (50 mM sodium acetate pH 5, 5 mM MgCl2, 0.5% NP-40) and lysates loaded on a polyacrylamide gel. After electrophoresis, proteins were transferred to a PVDF membrane, which was blocked in 10% non-fat milk. Membranes were probed with a 1/20 000 dilution of the proCatL antiserum (McIntyre and Erickson, 1991) or a 1/1000 dilution of the purified anti-CatD rabbit polyclonal antibody (a gift from Dr E.Weber, Martin-Lutter-University Halle-Wittenberg, Halle, Germany), followed by a 1/5000 dilution of a secondary anti-rabbit IgG antibody coupled to peroxidase. ECL was used for visualization.
Active site labeling
LHVS-biotin was prepared as follows: tert-butyloxycarbonyl-leucinyl-d-homophenylalanyl-vinyl-phenyl-sulfone (Boc-LHVS) was synthesized (Palmer et al., 1995) and made to react with trifluoroacetic acid/water/triisopropylsilane (95/5/5). After evaporation of solvent, the residue was reacted with EZ-Link™ NHS-LC-biotin (Pierce). After concentration in vacuo, the residue was dissolved in ethyl acetate, resulting in the precipitation of crude LHVS-biotin. Purification was performed by reverse-phase HPLC.
BM cells were lysed in NP-40 pH 5 lysis buffer (see above) and 25 µg of lysates were incubated with 1 mM LHVS-biotin for 1 h at 37°C. The labeling reaction was terminated by addition of SDS reducing sample buffer and boiling. Samples were analyzed by SDS–PAGE and transferred onto a PVDF membrane. Labeled polypeptides were visualized after blocking the membrane in PBS + 5% non-fat milk and incubating it at room temperature with a Streptavidin-HRP solution diluted in PBS + 0.2% Tween-20.
Cryoimmunogold electron microscopy
Cryoimmunogold labeling was performed as described (Peters and Hunziker, 2001). Material was fixed with 2% paraformaldehyde and 0.2% glutaraldehyde for 2 h. The p41 antiserum was obtained by immunizing a rabbit with a 33mer peptide (aa 192–225) specific for mouse p41. The specificity of this antibody was verified by both immunoblotting and immunoprecipitation.
Pulse–chase analysis
IFNγ-treated BM macrophages were starved for 45 min at 37°C in 1 ml of cysteine/methionine-free Dulbecco’s modified Eagle’s medium (DMEM) plus 10% FCS. Cells were pulsed with 0.5 mCi/ml of [35S]methionine/cysteine (Dupont New-England Nuclear) for 40 min and either lysed or chased in complete DMEM for 1, 3, 6 h or overnight. ConB (20 nM; Ajinimoto Co., Kanagawa, Japan) (Yilla et al., 1993), leupeptin (1 mM; Boerhinger) or butabindide (500 nM; a gift from Dr Schwartz, Paris, France) (Rose et al., 1996) was added to the medium after 3 h of chase. Butabindide activity was assayed in vitro for inhibition of tri-peptidyl peptidase II. After each chase-point, cells were lysed in NP-40 lysis buffer pH 7.4 (50 mM Tris, 150 mM NaCl, 0.5% NP-40) supplemented with 1% SDS, boiled for 5 min and reconstituted to 1.2 ml with SDS-free NP-40 solution. Immunoprecipitations were performed as described (Bryant et al., 1999), using 5 µl of CatL antiserum or of a 1:1 mix of the JV5 and JV11 antisera raised against the N- and C-terminal portions of Ii, respectively (Driessen et al., 1999).
Subcellular fractionation
108 BM macrophages were subjected to subcellular fractionation as described (Driessen et al., 1999). Briefly, postnuclear supernatants obtained from cells labeled for 5 h with [35S]methionine/cysteine and homogenized in a sucrose-containing buffer were fractionated by centrifugation on a 27% Percoll gradient. The fractions obtained were characterized for β-hexosaminidase activity as previously described (Driessen et al., 1999). The high-density peak obtained from this first gradient (fractions 1 + 2) is referred to as peak A and represents lysosomes. Fractions containing the low-density peak of β-hexosaminidase activity (fractions 9 and 10) were pooled, applied to a 10% Percoll gradient and fractionated by centrifugation. This yielded a predominant intermediate density peak of β-hexosaminidase activity at the bottom of the gradient, peak B (fractions 1 + 2 of the 10% gradient), which represents late endosomal compartments. Peak C (fractions 11 + 12 of the 10% gradient) was defined based on distribution of radioactivity, although it contains a small amount of β-hexosaminidase. This peak contains ER, Golgi, early endosomes and plasma membrane. As explained above, the distribution of both the β-hexosaminidase activity and various endosomal markers along the gradient (Driessen et al., 1999) suggested that the different subcellular compartments were predominantly contained in two fractions. Both fractions were therefore analyzed by immunoblotting.
Acknowledgments
Acknowledgements
The authors thank J.A.Villadangos, M.Maurice, E.Fiebiger, C.Lagaudrière and A.Bakker for their comments on the manuscript, and Dr Weber for providing the anti-CatD antibody. The authors are recipients of post-doctoral fellowships from the Juvenile Diabetes Foundation (A.-M.L.-D.), the Cancer Research Institute (R.A.R.), the Deutsche Forschungsgemeinschaft (DR378/1-1; C.D.) and the Netherlands Organization for Scientific Research (H.S.O.). This work was supported by grants to H.L.P. from the Juvenile Diabetes Foundation International, through the JDF Center for Islet Transplantation at Harvard Medical School, from the National Institutes of Health (AI34893 and CA14051) and from Boehringer Ingelheim.
References
- Bakke O. and Dobberstein,B. (1990) MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell, 63, 707–716. [DOI] [PubMed] [Google Scholar]
- Bevec T., Stoka,V., Pungercic,G., Dolenc,I. and Turk,V. (1996) Major histocompatibility complex class II-associated p41 invariant chain fragment is a strong inhibitor of lysosomal cathepsin L. J. Exp. Med., 183, 1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant P.W., Roos,P., Ploegh,H.L. and Sant,A.J. (1999) Deviant trafficking of I-Ad mutant molecules is reflected in their peptide binding properties. Eur. J. Immunol., 29, 2729–2739. [DOI] [PubMed] [Google Scholar]
- Castellino F. and Germain,R.N. (1995) Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity, 2, 73–88. [DOI] [PubMed] [Google Scholar]
- Claus V., Jahraus,A., Tjelle,T., Berg,T., Kirschke,H., Faulstich,H. and Griffiths,G. (1998) Lysosomal enzyme trafficking between phagosomes, endosomes, and lysosomes in J774 macrophages. Enrichment of cathepsin H in early endosomes. J. Biol. Chem., 273, 9842–9851. [DOI] [PubMed] [Google Scholar]
- Coulombe R., Grochulski,P., Sivaraman,J., Menard,R., Mort,J.S. and Cygler,M. (1996) Structure of human procathepsin L reveals the molecular basis of inhibition by the prosegment. EMBO J., 15, 5492–5503. [PMC free article] [PubMed] [Google Scholar]
- Driessen C., Bryant,R., Lennon-Duménil,A.M., Villadangos,J., Bryant,P., Shi,G.P., Chapman,H.A. and Ploegh,H.L. (1999) Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J. Cell Biol., 147, 775–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson A.H. (1989) Biosynthesis of lysosomal endopeptidases. J. Cell. Biochem., 40, 31–41. [DOI] [PubMed] [Google Scholar]
- Fan J.Q., Ishii,S., Asano,N. and Suzuki,Y. (1999) Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nature Med., 5, 112–115. [DOI] [PubMed] [Google Scholar]
- Fineschi B., Sakaguchi,K., Appella,E. and Miller,J. (1996) The proteolytic environment involved in MHC class II-restricted antigen presentation can be modulated by the p41 form of invariant chain. J. Immunol., 157, 3211–3215. [PubMed] [Google Scholar]
- Guncar G., Pungercic,G., Klemencic,I., Turk,V. and Turk,D. (1999) Crystal structure of MHC class II-associated p41 Ii fragment bound to cathepsin L reveals the structural basis for differentiation between cathepsins L and S. EMBO J., 18, 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba,M., Romani,N., Aya,H., Deguchi,M., Ikehara,S., Muramatsu,S. and Steinman,R.M. (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med., 176, 1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishidoh K., Saido,T.C., Kawashima,S., Hirose,M., Watanabe,S., Sato,N. and Kominami,E. (1998) Multiple processing of procathepsin L to cathepsin L in vivo. Biochem. Biophys. Res. Commun., 252, 202–207. [DOI] [PubMed] [Google Scholar]
- Jerala R., Zerovnik,E., Kidric,J. and Turk,V. (1998) pH-induced conformational transitions of the propeptide of human cathepsin L. A role for a molten globule state in zymogen activation. J. Biol. Chem., 273, 11498–11504. [DOI] [PubMed] [Google Scholar]
- Koch N. and Harris,A.W. (1984) Differential expression of the invariant chain in mouse tumor cells: relationship to B lymphoid development. J. Immunol., 132, 12–15. [PubMed] [Google Scholar]
- Koch N., Lauer,W., Habicht,J. and Dobberstein,B. (1987) Primary structure of the gene for the murine Ia antigen-associated invariant chains (Ii). An alternatively spliced exon encodes a cysteine-rich domain highly homologous to a repetitive sequence of thyroglobulin. EMBO J., 6, 1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lah T.T., Hawley,M., Rock,K.L. and Goldberg,A.L. (1995) γ-interferon causes a selective induction of the lysosomal proteases, cathepsins B and L, in macrophages. FEBS Lett., 363, 85–89. [DOI] [PubMed] [Google Scholar]
- Lotteau V., Teyton,L., Peleraux,A., Nilsson,T., Karlsson,L., Schmid,S.L., Quaranta,V. and Peterson,P.A. (1990) Intracellular transport of class II MHC molecules directed by invariant chain. Nature, 348, 600–605. [DOI] [PubMed] [Google Scholar]
- McGrath M.E. (1999) The lysosomal cysteine proteases. Annu. Rev. Biophys. Biomol. Struct., 28, 181–204. [DOI] [PubMed] [Google Scholar]
- McIntyre G.F. and Erickson,A.H. (1991) Procathepsins L and D are membrane-bound in acidic microsomal vesicles. J. Biol. Chem., 266, 15438–15445. [PubMed] [Google Scholar]
- McIntyre G.F., Godbold,G.D. and Erickson,A.H. (1994) The pH-dependent membrane association of procathepsin L is mediated by a 9-residue sequence within the propeptide. J. Biol. Chem., 269, 567–572. [PubMed] [Google Scholar]
- Nakagawa T. et al. (1998) Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science, 280, 450–453. [DOI] [PubMed] [Google Scholar]
- Nakagawa T.Y. et al. (1999) Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity, 10, 207–217. [DOI] [PubMed] [Google Scholar]
- Ogrinc T., Dolenc,I., Ritonja,A. and Turk,V. (1993) Purification of the complex of cathepsin L and the MHC class II-associated invariant chain fragment from human kidney. FEBS Lett., 336, 555–559. [DOI] [PubMed] [Google Scholar]
- O’Sullivan D.M., Noonan,D. and Quaranta,V. (1987) Four Ia invariant chain forms derive from a single gene by alternate splicing and alternate initiation of transcription/translation. J. Exp. Med., 166, 444–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J.T., Rasnick,D., Klaus,J.L. and Bromme,D. (1995) Vinyl sulfones as mechanism-based cysteine protease inhibitors. J. Med. Chem., 38, 3193–3196. [DOI] [PubMed] [Google Scholar]
- Peters P.J. and Hunziker,W. (2001) Subcellular localization of Rab17 by cryo-immunogold electron microscopy in epithelial cells grown on polycarbonate filters. Methods Enzymol., 329, 210–225. [DOI] [PubMed] [Google Scholar]
- Peterson M. and Miller,J. (1992) Antigen presentation enhanced by the alternatively spliced invariant chain gene product p41. Nature, 357, 596–598. [DOI] [PubMed] [Google Scholar]
- Pierre P. and Mellman,I. (1998) Developmental regulation of invariant chain proteolysis controls MHC class II trafficking in mouse dendritic cells. Cell, 93, 1135–1145. [DOI] [PubMed] [Google Scholar]
- Pierre P., Shachar,I., Matza,D., Gatti,E., Flavell,R.A. and Mellman,I. (2000) Invariant chain controls H2-M proteolysis in mouse splenocytes and dendritic cells. J. Exp. Med., 191, 1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punturieri A., Filippov,S., Allen,E., Caras,I., Murray,R., Reddy,V. and Weiss,S.J. (2000) Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J. Exp. Med., 192, 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V.Y., Zhang,Q.Y. and Weiss,S.J. (1995) Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc. Natl Acad. Sci. USA, 92, 3849–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese R.J., Wolf,P.R., Bromme,D., Natkin,L.R., Villadangos,J.A., Ploegh,H.L. and Chapman,H.A. (1996) Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity, 4, 357–366. [DOI] [PubMed] [Google Scholar]
- Roche P.A. and Cresswell,P. (1990) Invariant chain association with HCA-DR molecules inhibits immunogenic peptide binding. Nature, 345, 615–618. [DOI] [PubMed] [Google Scholar]
- Roche P.A. and Cresswell,P. (1991) Proteolysis of the class II-associated invariant chain generates a peptide binding site in intracellular HLA-DR molecules. Proc. Natl Acad. Sci. USA, 88, 3150–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche P.A., Marks,M.S. and Cresswell,P. (1991) Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature, 354, 392–394. [DOI] [PubMed] [Google Scholar]
- Rose C. et al. (1996) Characterization and inhibition of a cholecystokinin-inactivating serine peptidase. Nature, 380, 403–409. [DOI] [PubMed] [Google Scholar]
- Santambrogio L., Sato,A.K., Fischer,F.R., Dorf,M.E. and Stern,L.J. (1999) Abundant empty class II MHC molecules on the surface of immature dendritic cells. Proc. Natl Acad. Sci. USA, 96, 15050–15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G.P., Villadangos,J.A., Dranoff,G., Small,C., Gu,L., Haley,K.J., Riese,R., Ploegh,H.L. and Chapman,H.A. (1999) Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity, 10, 197–206. [DOI] [PubMed] [Google Scholar]
- Strubin M., Berte,C. and Mach,B. (1986) Alternative splicing and alternative initiation of translation explain the four forms of the Ia antigen-associated invariant chain. EMBO J., 5, 3483–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaesu N.T., Lower,J.A., Robertson,E.J. and Bikoff,E.K. (1995) Major histocompatibility class II peptide occupancy, antigen presentation, and CD4+ T cell function in mice lacking the p41 isoform of invariant chain. Immunity, 3, 385–396. [DOI] [PubMed] [Google Scholar]
- Takaesu N.T., Lower,J.A., Yelon,D., Robertson,E.J. and Bikoff,E.K. (1997) In vivo functions mediated by the p41 isoform of the MHC class II-associated invariant chain. J. Immunol., 158, 187–199. [PubMed] [Google Scholar]
- Villadangos J.A. and Ploegh,H.L. (2000) Proteolysis in MHC class II antigen presentation: who’s in charge? Immunity, 12, 233–239. [DOI] [PubMed] [Google Scholar]
- Villadangos J.A., Riese,R.J., Peters,C., Chapman,H.A. and Ploegh,H.L. (1997) Degradation of mouse invariant chain: roles of cathepsins S and D and the influence of major histocompatibility complex polymorphism. J. Exp. Med., 186, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadangos J.A. et al. (1999) Proteases involved in MHC class II antigen presentation. Immunol. Rev., 172, 109–120. [DOI] [PubMed] [Google Scholar]
- Wolf P.R. and Ploegh,H.L. (1995) How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu. Rev. Cell Dev. Biol., 11, 267–306. [DOI] [PubMed] [Google Scholar]
- Yilla M., Tan,A., Ito,K., Miwa,K. and Ploegh,H.L. (1993) Involvement of the vacuolar H+-ATPases in the secretory pathway of HepG2 cells. J. Biol. Chem., 268, 19092–19100. [PubMed] [Google Scholar]