Abstract
Previously, progesterone was found to regulate the initiation and biosynthetic rate of myelin synthesis in Schwann cell/neuronal cocultures. The mRNA for cytochrome P450scc (converts cholesterol to pregnenolone), 3β-hydroxysteroid dehydrogenase (3β-HSD, converts pregnenolone to progesterone), and the progesterone receptor were found to be markedly induced during active myelin synthesis. However, the cells in the cocultures responsible for these changes were not identified. In this study, in situ hybridization was used to determine the localization of the enzymes responsible for steroid biosynthesis. The mRNA for cytochrome P450scc and 3β-HSD were detected only in actively myelinating cocultures and were localized exclusively in the Schwann cells. Using immunocytochemistry, with minimal staining of the Schwann cells, we found the progesterone receptor in the dorsal root ganglia (DRG) neurons. The progesterone receptor in the neurons translocated into the nuclei of these cells when progesterone was added to neuronal cultures or during myelin synthesis in the cocultures. Additionally, a marked induction of the progesterone receptor was found in neuronal cultures after the addition of progesterone. The induction of various genes in the neurons was also investigated using mRNA differential display PCR in an attempt to elucidate the mechanism of steroid action on myelin synthesis. Two novel genes were induced in neuronal cultures by progesterone. These genes, along with the progesterone receptor, were also induced in cocultures during myelin synthesis, and their induction was blocked by RU-486 (a progesterone receptor antagonist). These genes were not induced in Schwann cells cultured alone after the addition of progesterone. These results suggest that progesterone is synthesized in Schwann cells and that it can indirectly regulate myelin formation by activating transcription via the classical steroid receptor in the DRG neurons.
INTRODUCTION
Myelin is a unique component of the nervous system that allows for efficient saltatory conduction of action potentials transmitted along axons. While many factors have been identified as affecting the overall myelination process, the molecules responsible for signaling and regulating specific steps in myelin synthesis remain to be determined. Recently, steroid biosynthesis and the influence of various hormones on the central and peripheral nervous systems have received widespread attention. Specifically, hormones such as thyroid hormones and corticosteroids have been implicated in regulating the differentiation of glial cells, suggesting a role for various hormones in the myelination process (Walters and Morell, 1981; Almazan et al., 1985; Koper et al., 1986; Warringa et al., 1987; Kumar et al., 1989; Tosic et al., 1992; Barres et al., 1994). Estradiol and progesterone have also been implicated in increasing the expression of myelin proteins (Jung-Testas et al., 1994; Robel and Baulieu, 1994; Koenig et al., 1995; Jung-Testas et al., 1996b; Notterpek et al., 1999), while progesterone and dexamethasone have been found to activate the promoters of peripheral myelin protein-22 and P0 (Desarnaud et al., 1998; Melcangi et al., 1999). The enzymes responsible for progesterone biosynthesis, cytochrome P450scc and 3β-HSD, have been identified in the brain and spinal cord of the rat. These enzymes also have been localized and identified in various cell types [e.g., the neurons of the vestibular and hypoglossal nuclei, cerebellar granule cells, Purkinje cells, primary glial cells, and purified oligodendrocytes (Hu et al., 1987; Jung-Testas et al., 1989; Robel and Baulieu, 1994; Dupont et al., 1994; Sanne and Krueger, 1995; Furukawa et al., 1998)].
Myelin synthesis can be induced in cocultures of Schwann cells and DRG neurons. Recently, we demonstrated that progesterone added to Schwann cell/neuronal cocultures decreased the time required for initiation and increased the biosynthetic rate of myelin synthesis (Chan et al., 1998). RU-486 (a progesterone receptor antagonist) was found to inhibit myelin formation, demonstrating that progesterone is an essential factor involved in myelin synthesis and that its major mechanism of action is through the classical progesterone receptor. The expression of the mRNA for cytochrome P450scc, 3β-HSD, and the progesterone receptor were dramatically induced in cocultures at the time of myelin synthesis. However, the cells in these cocultures responsible for these changes were not identified.
During development or after nerve injury, complex interactions between glial cells and neurons are responsible for the reciprocal regulation and dramatic modulation of gene expression in both types of cells (for reviews see Doyle and Colman, 1993; Reynolds and Woolf, 1993). Previously, the expression of differentially regulated genes has been investigated during peripheral nerve injury, and during the differentiation of oligodendrocytes in the CNS (Stahl et al., 1990; Gillen et al., 1995; Schaeren-Wiemers et al., 1995). Similarly, axonal/Schwann cell contacts in the peripheral nervous system are essential for the differentiation of Schwann cells and for the morphogenesis of the axon (Salzer et al., 1980; Joe and Angelides, 1992; De Waegh et al., 1992; Bolin and Shooter, 1993; Einheber et al., 1993; Dugandzija-Novakovic et al., 1995; Yin et al., 1998). The presence of inducible proteins responsible for steroid biosynthesis and the dramatic effects of progestins and glucocorticoids on myelin synthesis suggest that steroids and their metabolites may act as signaling molecules between Schwann cells and neurons. Because cytochrome P450scc, 3β-HSD, and steroid hormone receptors are inducible proteins, they may be present or absent in cells under various conditions. Consequently, to determine the mechanism of the interactions that exist during myelin synthesis and to differentiate between paracrine and/or autocrine signaling, it is important to determine the localization of these enzymes and the corresponding steroid receptors. Using oligonucleotide probes specific for cytochrome P450scc and 3β-HSD, the localization and expression of the mRNA for the enzymes were investigated by in situ hybridization. Immunocytochemistry was employed for localization of the progesterone receptor. In addition, mRNA differential display technology (Liang and Pardee, 1992; Liang et al., 1994) was used on premyelinating and myelinating cocultures, as well as on neuronal cultures, with and without the addition of progesterone to identify changes in gene expression (for a review utilizing differential display and hormone-inducible gene expression, see Averboukh et al., 1997). The results suggest that progesterone is synthesized in Schwann cells and that it is an essential signaling molecule that regulates myelin synthesis through the classical steroid receptor in the DRG neurons by activating transcription.
MATERIALS AND METHODS
DRG Neuron/Schwann Cell Cocultures
Purified neuronal and Schwann cell cultures were prepared using modified methods according to Eldridge et al. (1987) (Chan et al., 1998). Briefly, neuronal cultures were established from dorsal root ganglia neurons obtained from 15-d gestation Sprague Dawley rat embryos (Harlan, Indianapolis, IN). The dorsal root ganglia neurons were dissociated with trypsin and plated onto collagen-coated coverslips. Nonneuronal cells were eliminated by cycling with a medium containing fluorodeoxyuridine. Neurons were then maintained 1 wk in a medium consisting of 10% fetal bovine serum in Eagle's Minimal Essential Medium (MEM) and 200 ng/ml nerve growth factor (M1 medium). Schwann cells were isolated from the sciatic nerve of 4-day-old rat pups (Brockes et al., 1979). The sciatic nerves were dissociated with trypsin and collagenase. The dissociated Schwann cells were maintained for 3 to 5 days in a medium consisting of 10% fetal bovine serum in DMEM (DMEM) with gentamicin and cytosine arabinoside to eliminate fibroblasts. The purified Schwann cells were then used to seed the purified neuronal cells and establish cocultures. The purified neuronal cultures of ∼ 70,000 cells were seeded with ∼ 100,000 Schwann cells. Cocultures were then maintained in MEM with the addition of 10% charcoal-filtered fetal calf serum (delipidated) and 200 ng/ml nerve growth factor. Myelination was induced with the addition of ascorbate.
Oligonucleotides
Primer pairs for PCR were designed in conserved regions from sequences available in the GenBank/EMBL database as described by Chan et al. (1998). Primers were synthesized at the Genetic Engineering Facility at the University of Illinois (Urbana, IL). Oligonucleotide probes for in situ hybridization were designed according to the PCR primers and were also synthesized at the Genetic Engineering Facility at the University of Illinois. Biotin-dT was incorporated into each oligonucleotide probe at approximately every 15 bases. Listed below are the probes used. Bases in bold represent the conjugated biotin label.
L19
5′-ATAGTGTCGGACATGGACTTCCAGTTTCCCTTACACAAGTTTTTG-3′
Myelin Basic Protein (MBP)
5′-CCCCTCCTTCTCTGTCGGCGAGACCTAGAGGGTACCGTTC-3′
3β-HSD
5′-CTTACCCCGGAGGCGGAACTAAGGTCGACCTCGGAA-GGAGACGGGGAC-3′
Cytochrome P450scc
5′-GGACTACGGACTCTTCGGATAGAAGAAGTTGAAGG-TCGGA-3′
Reverse Transcription PCR
RT-PCR was performed according to Chan et al. (1998). Briefly, RNA from cultured cells was isolated using RNAgents Total Isolation System (Promega, Madison, WI). The concentration and purity of total RNA was determined by measuring the optical density at 260 and 280 nm. The RNA was subjected to DNase treatment (DNase I, FPLCpure, Pharmacia Biotech, Piscataway, NJ) and then reverse transcription. Residual RNA was then digested with Ribonuclease H. The cDNA was subjected to 30 cycles of amplification using a Minicycler (MJ Research, Watertown, MA). The amplification reactions and conditions are described previously by Chan et al. (1998). Detection and quantitation were accomplished with a phosphoimager, the ImageQuant software (Molecular Dynamics, Sunnyvale, CA), and the IPlab Images Software (Signal Analytics, Vienna, VA). The relative levels of gene expression were measured by determining a ratio between the products generated from the target gene and the endogenous internal standard in separate reactions (Horikoshi et al., 1993). The linear range for amplifying regions of the genes of interest and the gene for the internal standard were determined by serial dilutions of the cDNA. The slopes of the lines for the intensity of the amplified cDNA versus concentration were calculated and the ratios of the gene of interest to the internal standard were compared. The fold increase observed is relative to premyelinating cultures at 3 days after seeding.
mRNA Differential Display PCR
RNA from cultured cells was isolated using the RNAgents Total Isolation System. The RNA was subjected to Dnase treatment at 37°C for 15 min to remove residual genomic DNA. Differential display was accomplished with the RNAimage mRNA Differential Display System (GenHunter Corp., Nashville, TN). Briefly, three separate reverse transcription reactions were performed with each RNA sample, using the one-base anchored oligo-dT primers. Reverse transcription reactions without the reverse transcriptase were heated to 65°C for 5 min, then to 37°C for 60 min. After 10 min at 37°C, the MMLV reverse transcriptase was added to each reaction. After the 37°C incubation, the samples were heated to 75°C for 5 min to inactivate the enzyme. PCR was performed for 40 cycles using α-[33P]dATP (2000 Ci/mmol), the anchored oligo-dT primers (HT11A, HT11C, HT11G), the eight different degenerate primers provided by the RNAimage kit (HAP-1-HAP-8), and a degenerate primer synthesized by the Genetic Engineering Facility at the University of Illinois (HAP-0). Each cycle consisted of a denaturation step (94°C, 30 s), an annealing step (40°C, 2 min), and an elongation step (72°C, 30 s), with a final 5-min elongation after the last cycle. The PCR products were electrophoresed on a 6% denaturing polyacrylamide gel and analyzed using a phosphoimager and the ImageQuant software. Autoradiograms were oriented on the dried gels, and the bands of interest were isolated and purified using 3 M sodium acetate, glycogen (10 mg/ml), and EtOH at -80°C. The products were reamplified, gel purified, subcloned using the PCR-TRAP Cloning System (GenHunter Corp., Nashville, TN), sequenced and purified using the ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Foster City, CA) and Centri-sep Spin Columns (Princeton Separations, Adelphia, NJ).
In Situ Hybridization
In situ hybridization was performed using the Life TechnologiesBRL In Situ Hybridization and Detection System (Life TechnologiesBRL, Gaithersburg, MD). This detection system employs the alkaline phosphatase enzyme conjugated to strepavidin. Biotinylated oligonucleotides were designed in conserved regions from sequences available in the GenBank/EMBL database. The 40-mer oligonucleotide probes for cytochrome P450scc, 3β-HSD, MBP, and L19 were synthesized at the Genetic Engineering Facility at the University of Illinois (Urbana, IL). Biotin-dT was incorporated into each oligonucleotide probe at approximately every 15 bases. The final probe concentration was determined by serial dilutions until an adequate signal was obtained (0.05–0.1 μg/μl). All probes were verified by Northern Blot analysis using the Life TechnologiesBRL BlueGene Nonradioactive Nucleic Acid Detection System (Life TechnologiesBRL). Dilutions were made in a hybridization buffer composed of 4X SSC [1X SSC (pH 7.0): 0.15 M sodium chloride, 0.015 M sodium citrate], 0.2 M sodium phosphate (pH 6.5), 2X Denhardt's solution, 10% dextran sulfate in formamide, and 0.1 μg/ml sodium azide. Pretreatment and hybridization were accomplished by fixing Schwann cell/neuronal cocultures with 4% paraformaldehyde in phosphate buffer saline [8.1 mM sodium phosphate, 1.5 mM potassium phosphate (pH 7.0), 137 mM sodium chloride, and 2.7 mM potassium chloride] at room temperature for 5 min. The slides were washed twice with phosphate-buffered saline before dehydration with 50, 70, 90, and 100% ethanol (2 min each). Cultures were incubated with oligonucleotide probes overnight at 45°C in a humid atmosphere. After hybridization, the slides were washed twice with 0.2X SSC at 25°C for 15 min. Slides were then treated with a blocking solution for 15 min at 25°C and incubated with a strepavidin-alkaline phosphatase conjugate (40 μg/ml) in 100 mM Tris-HCl (pH 7.8), 150 mM magnesium chloride, 10 mg/ml bovine serum albumin, and 0.2 mg/ml sodium azide for 15 min at 25°C. The slides were washed twice with Tris buffer for 15 min and once with an alkaline substrate buffer [0.1 M Tris-base (pH 9.5), 0.15 M sodium chloride, and 0.05 M magnesium chloride]. Cultures were incubated in a prewarmed (37°C) solution of 0.30 mg/ml nitroblue tetrazolium (NBT), 0.17 mg/ml 4-bromo-5-chloro-3-indolylphosphate (BCIP), dimethylformamide, and alkaline substrate buffer for 10 min to 1 h. The color development was stopped by rinsing the slides several times with deionized water. The samples were finally dehydrated through a graded ethanol series, as previously described, and visualized by light microscopy.
Immunocytochemistry
Coverslips with cultured cells were washed in phosphate-buffered saline (pH 7.4) and fixed 4% paraformaldehyde before dehydration through a graded ethanol series (50, 70, 90, and 100%). Cultures were blocked in SuperBlock (Pierce, Rockford, IL) for 20 min at 25°C and then incubated with the selected primary monoclonal antibody [antiprogesterone receptor antibody (Affinity Bioreagents, Inc., Golden, CA) and an anti-S-100 β-subunit antibody (Sigma, St. Louis, MO)] at 4°C overnight. The coverslips were washed in phosphate-buffered saline and treated with a rat adsorbed biotinylated antimouse secondary antibody (Vector Laboratories, Burlingame, CA) for 30 min at 25°C. Coverslips were washed and stained using the horseradish peroxidase conjugated Vectastain ABC staining kit (Vector Laboratories) and the ImmunoPure Metal Enhanced DAB Substrate (Pierce). The horseradish peroxidase-avidin complex was applied to the cultures for 30 min at 25°C. The cultures were washed thoroughly, and the Metal Enhanced DAB substrate solution was added and incubated for ∼ 5 min, or until adequate staining was achieved. Cells stained positively resulted in an intense brown/black signal.
Electron Microscopy
Electron microscopy was performed at the Center for Microscopy and Imaging at the University of Illinois (Urbana, IL). Samples were fixed and embedded using rapid microwave technology according to Hanker and Giammara (1993) and Login and Dvorak (1993). Briefly, samples were fixed in a glutaraldehyde/formalin solution and chilled on ice. The samples were then irradiated using microwave employing the use of water load. The samples were then chilled and rinsed with Cacodylate buffer. OsO4 was then used as a secondary fixative combined with microwave. After incubation, the samples were dehydrated using an ethanol gradient (25, 50, 75, 100%) and immersed in propylene oxide. Infiltration of the samples was accomplished by vigorous vortexing and rapid microwave. Finally, the samples were embedded in pure epoxy and allowed to polymerize for 8–15 h at 90°C.
RESULTS
Induction of Cytochrome P450scc, 3β-HSD, and the Progesterone Receptor
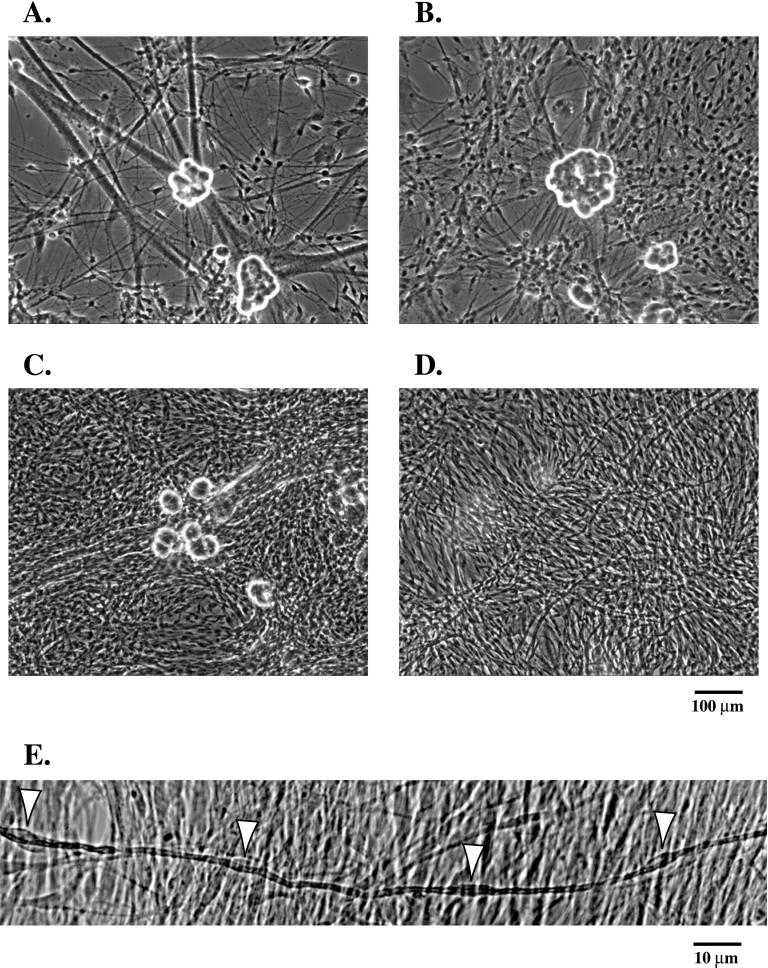
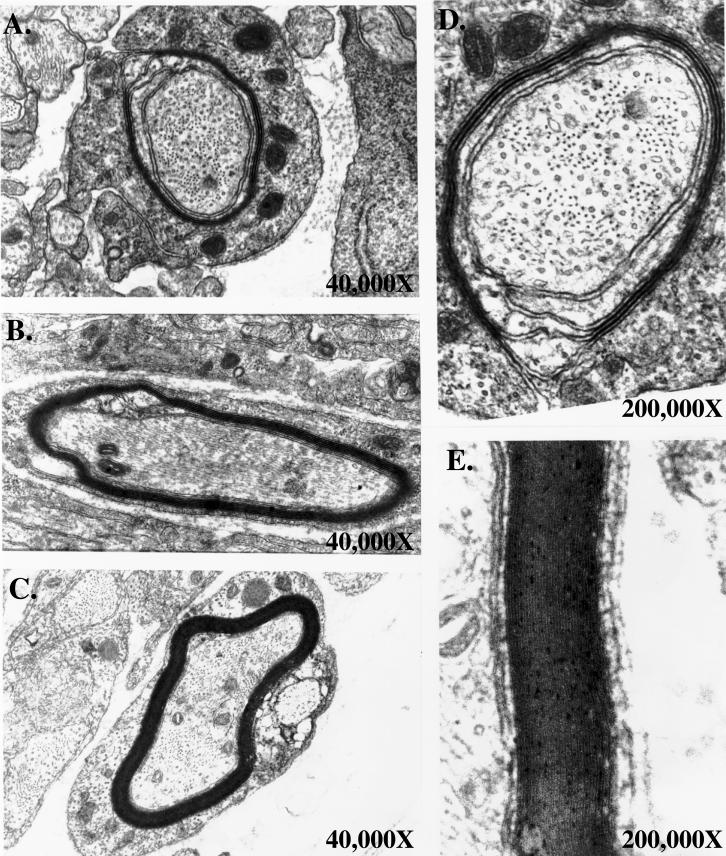
Previously, we developed a method to quantitate the biosynthetic rate of myelin synthesis in Schwann cell/neuronal cocultures and to determine the period of active myelin synthesis (Bilderback et al., 1997). Schwann cell/neuronal cocultures were established as described in the MATERIALS AND METHODS. The cells were grown to maturity separately, and contaminating cells were removed by cycling with an antimitotic in the neurons and Schwann cells. After 1 wk off of the antimitotic, neuronal cultures were seeded with the Schwann cells (Figure 1A). Upon contact with the axons, Schwann cells proliferated rapidly. Three days after seeding, the axons were populated with Schwann cells; however, bare axons were still detected (Figure 1B). Seven days after seeding, the axons were fully populated and proliferation had ceased (Figure 1C). The Schwann cells then began to elongate and ensheath the axons. At this time the cocultures were induced to myelinate with the addition of ascorbic acid (50 μg/ml). Four days after induction, myelin internodes could be detected (Figure 1D). Figure 1E illustrates a magnified view of four adjacent Schwann cells actively forming myelin. Arrows point to the Schwann cell bodies located in the middle of the internodes. Active myelin synthesis occurred between 4–7 days after induction, while the premyelinating period included the first 3 days after induction. This allows for a distinction to be made between the premyelinating period and the time of active myelin synthesis (Bilderback et al., 1997). In addition, electron microscopy was performed on the Schwann cell/neuronal cocultures at various days after induction (Figure 2). The bulk of myelin synthesis also occurred between 4–7 days after induction. At 3 days after induction, Schwann cells ensheathed axons, while very few myelinated fibers were detected (0–2 wraps of myelin). At 5 days after induction, ∼ 8 wraps of myelin were deposited by each Schwann cell. At 7 days after induction, 10–12 wraps of myelin were observed, while after 7 days, only a few additional wraps of myelin were deposited (maximum of 15 wraps) (Figure 2).
Figure 1.
Establishment of Schwann cell/neuronal cocultures. (A) Schwann cells were seeded onto neuronal cultures. (B) Day 3 after seeding. (C) Day 7 after seeding. (D) Three days after induction of myelin synthesis with ascorbate. (E) Magnified view of four Schwann cells that are actively forming myelin (arrows indicate Schwann cell bodies). Images were obtained with phase contrast microscopy. Bars,100 μm (A-D) and 10 μm (E).
Figure 2.
Electron micrographs at different time points during myelin synthesis. Schwann cell/neuronal cocultures at 3 days after induction (A, D), 5 days after induction (B), and 7 days after induction (C, E). Electron micrographs were at 40,000X magnification (A, B, C). D and E are identical micrographs at 200,000X magnification of A and C, respectively.
RT-PCR was employed to measure the relative gene expression of several genes throughout the myelination process. Basic FGF is associated with cell proliferation and was used as a control to aid in the characterization of the cocultures. The mRNA for basic FGF was found to increase by 4-fold during the proliferation phase in the cocultures (our unpublished results). Figures 1B and C illustrate the proliferation phase that corresponds to the induction of basic FGF. As the Schwann cells began to differentiate and then myelinate, the expression of the basic FGF mRNA decreased (our unpublished results). In previous work, we showed that the mRNA for MBP increased by ∼ 9-fold during active myelin synthesis (5 days after induction) and that cytochrome P450scc, 3β-HSD, and the progesterone receptor were induced 17-fold, 30-fold and 10-fold, respectively, during active myelin synthesis in the cocultures (Chan et al., 1998). When RT-PCR was performed on Schwann cells and dorsal root ganglia neurons cultured separately, the mRNA for cytochrome P450scc, 3β-HSD, and the progesterone receptor were undetectable at 30 cycles of PCR (our unpublished results). Thus, substantial expression of the mRNA for cytochrome P450scc, 3β-HSD and the progesterone receptor occurred only in cocultures during myelin synthesis.
In Situ Hybridization of Cytochrome P450scc and 3β-HSD
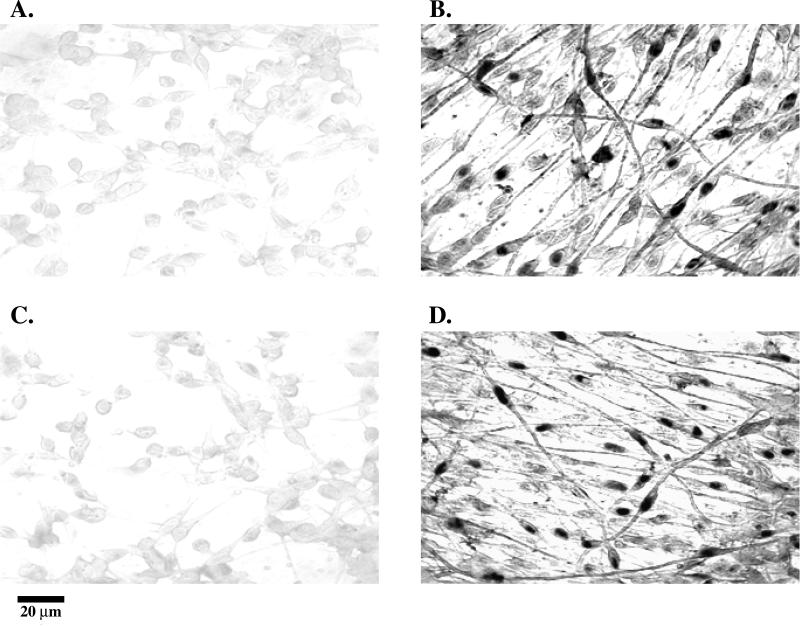
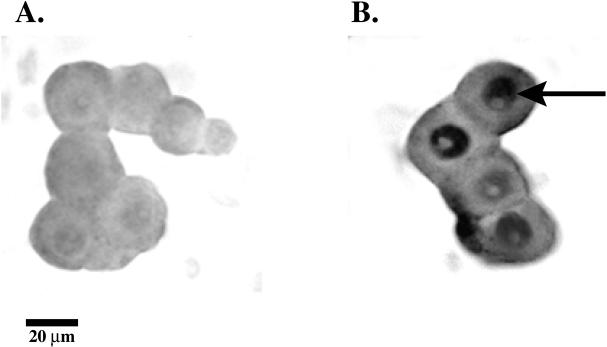
The presence of inducible enzymes responsible for steroid biosynthesis and the dramatic effects of progestins and glucocorticoids on myelin synthesis suggest that these steroids and their metabolites may act as signaling molecules between Schwann cells and neurons. Using biotinylated oligonucleotide probes specific for cytochrome P450scc and 3β-HSD, the localization and expression of the mRNA for the enzymes were investigated by in situ hybridization. In primary Schwann cell/neuronal cocultures, Schwann cells, neurons, and fibroblasts were easily identifiable by their morphology and immunocytochemical staining using antibodies against galactocerebroside (for Schwann cells), S-100 (for Schwann cells), and neurofilament proteins (for neurons). Oligonucleotide probes for L19, an internal standard ribosomal protein, and MBP were used as controls to examine the specificity of the labeling procedure. L19 was detected in both cell types, while MBP was exclusively localized to the myelin forming cells (our unpublished results). The expression of cytochrome P450scc and 3β-HSD was also examined throughout the myelination process. The mRNA for cytochrome P450scc and 3β-HSD were undetectable in premyelinating Schwann cell/neuronal cocultures (Figure 3A, C). Cytochrome P450scc and 3β-HSD mRNA were only detected in the Schwann cells of actively myelinating cocultures (Figure 3B, D). The negative results (Figure 3A, C) were comparable to in situ hybridization staining using sense oligonucleotide probes (our unpublished results), and illustrate the background level of staining under these conditions. These results demonstrate that the localization of the induced mRNA for the enzymes is in Schwann cells and that this occurs during the period of myelin formation. The Schwann cells that stained more intensely were elongated and actively myelinating, as expected for a close association between steroid biosynthesis and myelin formation.
Figure 3.
In situ hybridization of Schwann cell/neuronal cocultures. Cells were probed with biotin-labeled oligonucleotides and visualized under light microscopy with a strepavidin-alkaline phosphatase conjugate and NBT/BCIP (Life Technologies In Situ Hybridization and Detection System). The final probe concentrations were determined by serial dilutions until an adequate signal was obtained. All probes were verified by Northern blot analysis using the Life TechnologiesBRL (Gaithersburg, MD) BlueGene Nonradioactive Nucleic Acid Detection System. An oligonucleotide probe for 3β-HSD was applied to premyelinating (2 days after induction) and myelinating (5 days after induction) Schwann cell/neuronal cocultures (A, B). An oligonucleotide probe for cytochrome P450scc was also applied to premyelinating (C) and myelinating (D) cocultures.
Immunocytochemistry and Induction of the Progesterone Receptor
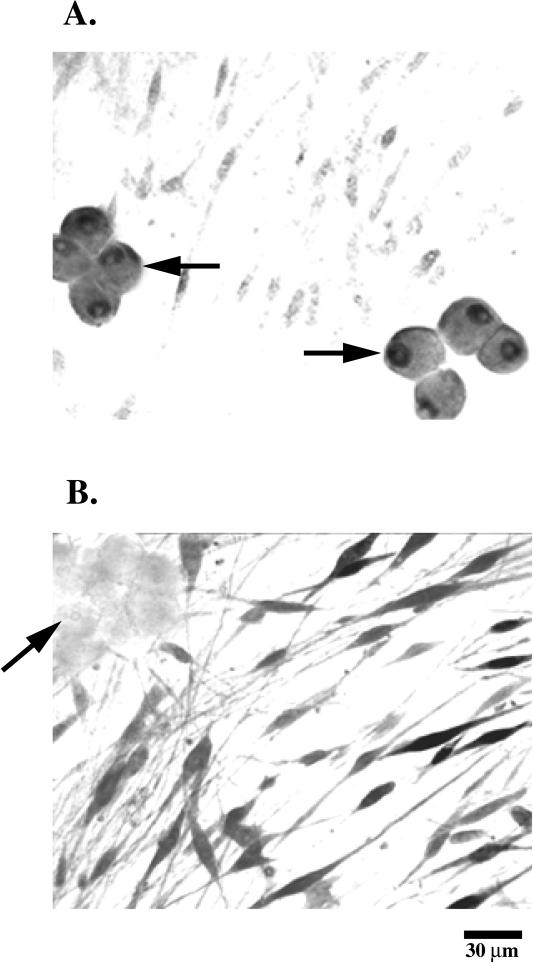
The mRNA for the progesterone receptor was not detectable by in situ hybridization. The induced message for the progesterone receptor during the myelinating stage was ∼ 100-fold less than the message for 3β-HSD at the premyelinating stage, which was already below the sensitivity of the in situ hybridization technique. Consequently, a monoclonal antibody to the progesterone receptor was employed to localize the receptor. The receptor was found in the dorsal root ganglia neurons and localized in the cell nucleus, while minimal staining was observed in Schwann cells (Figure 4A). The staining of Schwann cells cultured either alone or in cocultures throughout the myelination process was comparable to background staining observed when the primary or secondary antibodies were omitted (our unpublished results). A monoclonal antibody to S-100, a cytosolic Schwann cell specific protein, was also employed as a positive control to examine the specificity of the staining. Schwann cells were stained for S-100, while minimal staining (comparable to background) was observed in the neurons (Figure 4B).
Figure 4.
Immunocytochemistry of the progesterone receptor in Schwann cell/neuronal cocultures. Monoclonal antibodies were applied to Schwann cell/neuronal cocultures and were visualized under light microscopy using the horseradish peroxidase conjugated Vectastain ABC staining kit (Vector Laboratories, Burlingame, CA) and the ImmunoPure Metal Enhanced DAB Substrate (Pierce, Rockford, IL) The coverslips were then treated with a rat-adsorbed biotinylated antimouse secondary antibody. Antibodies to the progesterone receptor (A) and S-100 (B) were applied to myelinating cocultures (4 days after induction). The arrows indicate the neuronal cell bodies.
Neurons cultured in the absence of Schwann cells and in medium lacking steroid hormones gave a diffuse positive staining of the progesterone receptor in the neuronal cell bodies (Figure 5A), while the addition of progesterone (100 nM) resulted in an intense localized staining of the nuclei in the neurons (Figure 5B). The localization of the receptor in the nucleus suggested a nuclear translocation of the receptor in the presence of the steroid hormone. The translocation event in the neurons was also observed in actively myelinating Schwann cell/neuronal cocultures consistent with steroid hormone production by Schwann cells. The neuronal axons were not stained and are not visible in Figure 5. It is possible that the receptors were present in the axons, but at a level too low to be detected. Addition of hormones to Schwann cells cultured separately resulted in background levels of staining (our unpublished results).
Figure 5.
Immunocytochemistry of the progesterone receptor in neuronal cell cultures. A monoclonal antibody to the progesterone receptor was applied to dorsal root ganglia neurons in media either lacking steroid hormones (A) or cultured in the presence of 100 nM progesterone for 24 h (B). The arrow indicates the nucleus of a neuron.
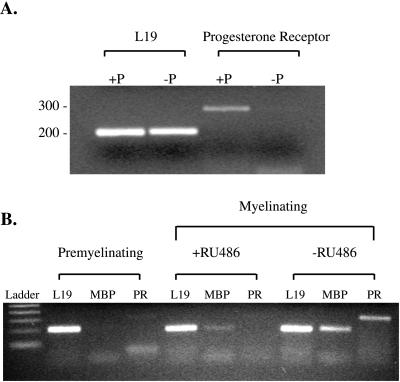
Besides the translocation of the receptor in neurons, the immunocytochemical staining suggested an overall induction of the receptor in the presence of progesterone. Using RT-PCR as described previously, a dramatic auto-induction of the progesterone receptor was observed in neuronal cultures (Figure 6A). Furthermore, to investigate the mechanism of the progesterone receptor in myelin synthesis, the mRNA from premyelinating and myelinating cocultures were extracted, as well as corresponding cocultures after the addition of RU-486 (100 nM, a progesterone receptor antagonist). Previously, the progesterone receptor mRNA was found to be induced by 10-fold during active myelin synthesis in cocultures (Chan et al., 1998). Upon addition of RU-486, the induction was completely abolished in the cocultures (Figure 6B). RU-486 was also found to inhibit the induction of MBP in the cocultures, consistent with its inhibition of myelin synthesis (Koenig et al., 1995; Chan et al., 1998). The addition of progesterone or RU-486 to Schwann cell cultures did not result in detectable quantities of the progesterone receptor mRNA.
Figure 6.
(A) RT-PCR on cDNA generated from neuronal cells cultured with and without progesterone. (B) RT-PCR on cDNA from premyelinating (3 days after induction) and myelinating (5 days after induction) cocultures with and without the addition of RU-486 (RU-486 was added on the day of induction). L19 and the progesterone receptor (PR) were amplified from neuronal cDNA (A), while L19, MBP, and PR were amplified from the coculture cDNA (B). The linear range of amplification for each gene was determined by serial dilutions of the cDNA samples. The cDNA were subjected to 30 cycles of PCR and electrophoresed in ethidium bromide-containing 2% agarose gels.
mRNA Differential Display
If progesterone acts on neurons via the progesterone receptor, it should alter gene expression specifically in neurons. mRNA differential display PCR (Liang and Pardee, 1992; Liang et al., 1994) was employed to identify novel genes involved in the regulation of myelin synthesis and the specific genes in neuronal cells that are directly induced by the action of progesterone. The mRNA from premyelinating (3 days after induction) and myelinating (5 days after induction) cocultures was isolated and screened for novel genes that were induced during myelin synthesis (Figure 7). One gene, identified by differential display PCR in myelinating cocultures was 3β-HSD. This finding represents a confirmation of the previously reported induction of 3β-HSD during active myelin synthesis and illustrates that differential display technology has the potential to identify essential genes throughout the myelination process in the cocultures. In addition, calcineurin A (a calcium dependent phosphatase) and a rat placenta cDNA clone were also identified in myelinating cocultures (Table 1). While the rat cDNA clone has not yet been identified, calcineurin A was slightly induced in myelinating cocultures. Because both 3β-HSD and calcineurin A were unaffected by the RU-486 treatment, the induction of these genes was not a result of the action of progesterone through the progesterone receptor. In addition, both 3β-HSD and calcineurin A were examined in either Schwann cells cultured alone or DRG neurons cultured alone, with and without the addition of progesterone (Table 1). While 3β-HSD was undetectable in both Schwann cells and neurons cultured separately (30 cycles of PCR), calcineurin A was detected, but was not modulated by progesterone. Although a total of five different genes were isolated using differential display on myelinating cocultures, only three out of the five genes were identified in the GenBank/EMBL database. Only a limited number of degenerate primer sets were used in the differential display experiments, and additional degenerate primers should reveal more inducible genes.
Figure 7.
Representative mRNA differential display gel between premyelinating and myelinating Schwann cell/neuronal cocultures. Using the HAP-0 and HT11C primers, the cDNA from premyelinating (A, B) (3 days after induction) and myelinating (C, D) (5 days after induction) cocultures were amplified in duplicate. The arrows indicate the location of potentially differentially expressed genes. The arrow in the left column illustrates a gene that is down-regulated.
Table 1.
Genes induced during myelin synthesis using mRNA differential display PCR from premyelinating (3 days after induction) and myelinating (5 days after induction) Schwann cell/neuronal co-cultures
| Identified Genes | Primersa | Cocultures (fold change)b | Cocultures + RU-486 (fold change)b | Schwann cells + P (fold change)c | DRG Neurons + P (fold change)c |
|---|---|---|---|---|---|
| 3-β-hydroxysteroid dehydrogenase | HT11C/HAP-0 | 30 ± 2.5 | 25 ± 3.0 | Not detectedd | Not detectedd |
| Calcineurin A | HT11C/HAP-0 | 3.3 ± 0.3 | 2.5 ± 0.7 | 0.8 ± 0.3 | 1.4 ± 0.2 |
| Rat placenta cDNA clone no. RPLCT57 | HT11A/HAP-4 | Undetermined | Undetermined | Undetermined | Undetermined |
Designated primers were provided by the RNAimage Differential Display System (GenHunter Corp.).
The fold induction of genes were relative to cocultures at 3 days before induction. All genes were verified using RT-PCR of cDNA from premyelinating and myelinating cocultures ± RU-486. (See MATERIALS AND METHODS section).
The fold induction of genes was relative to Schwann cells or DRG neurons without the addition of progesterone.
3β-HSD was undetectable using RT-PCR for Schwann cells or DRG neurons with and without the addition of progesterone.
To elucidate the specific genes induced by progesterone in neuronal cells, differential display was applied to the mRNA from neuronal cultures (no Schwann cells) with and without the addition of progesterone (100 nM). The progesterone receptor was auto-induced by progesterone, and differential display identified four additional genes, two of which were induced by progesterone and inhibited by RU-486 in cocultures (Table 2). These two genes are Rap 1b (a small Ras-like GTP-binding protein) and PRPP (phosphoribosyl diphosphate) synthase-associated protein. The third gene, nonmuscle myosin light chain, was not induced in the neurons with progesterone, in cocultures during myelin synthesis, nor was it effected by RU-486 in cocultures. Therefore, the myosin light chain most likely represents a false positive from the differential display PCR and is not directly related to myelin synthesis. The fourth gene was in the database (rat ovary cDNA clone #ROVAA30), but its identity is not known. A total of ten different genes were isolated using differential display on neuronal cultures with and without progesterone, but only four of the ten genes were identified in the GenBank/EMBL database.
Table 2.
Genes induced by progesterone using mRNA differential display PCR from DRG neuronal cultures with and without the addition of progesterone (100 nM)
| Identified genes | Primersa | Cocultures (Fold Change)b | Cocultures + RU-486 (Fold Change)b | Schwann cells + P (Fold Change)c | DRG Neurons + P (Fold Change) |
|---|---|---|---|---|---|
| Rap 1 b | HT11C/HAP-5 | 5.0 ± 1.0 | 1.3 ± 0.1 | 0.7 ± 0.2 | Inducedd |
| PRPP synthase-associated protein-39 kDa | HT11C/HAP-3 | 9.5 ± 1.5 | 1.1 ± 0.5 | 1.3 ± 0.4 | Inducedd |
| Nonmuscle myosin light chain | HT11C/HAP-2 | 0.72 ± 0.2 | 1.3 ± 0.2 | 0.9 ± 0.3 | 0.7 ± 0.4 |
| Rat ovary cDNA clone no. ROVAA30 | HT11G/HAP-4 | Undetermined | Undetermined | Undetermined | Undetermined |
Designated primers were provided by the RNAimage Differential Display System (GenHunter Corp.).
The fold induction of genes was relative to cocultures at 3 days before induction. All genes were verified using RT-PCR of cDNA from premyelinating and myelinating co-cultures ± RU-486. (See MATERIALS AND METHODS section).
The fold induction of genes was relative to Schwann cells or DRG neurons without the addition of progesterone.
The genes were undetectable using RT-PCR in DRG neurons without the addition of progesterone.
As a control, differential display was also conducted on Schwann cell cultures with and without the addition of progesterone. In contrast to the results with the neurons, no bands were identified as being induced with progesterone, and the results suggest that progesterone has a little effect on Schwann cell transcription. Using RT-PCR, Rap 1b and PRPP synthase-associated protein were detected in Schwann cell cultures, but they were not induced by progesterone. This also means that the induction (fold-change) of the genes in the neurons observed in the cocultures (Table 2) was underestimated due to the constitutive levels of Rap 1b and PRPP synthase-associated protein in Schwann cells. In addition, progesterone added to Schwann cell cultures also did not show an induction in the mRNA for MBP, the progesterone receptor, or the myosin light chain.
DISCUSSION
Steroid hormones have dramatic and widespread effects on the central and peripheral nervous systems. The biosynthesis and interactions of steroids in the rat brain and spinal cord independent of steroidogenic gland secretion have been well-documented (Le Goascogne et al., 1987; Robel and Baulieu, 1994; Sanne and Krueger, 1995). We have shown that endogenously synthesized steroids in Schwann cell/neuronal cocultures affect the initiation and regulate the biosynthetic rate of myelin synthesis (Chan et al., 1998). Progesterone may be synthesized in either Schwann cells or neurons and may act as signaling molecules in the formation of myelin. Therefore, the localization of cytochrome P450scc and 3β-HSD expression are essential in elucidating the mechanism of steroid hormone signaling during myelin synthesis. Using premyelinating and myelinating cocultures, the induced expression of the mRNA for cytochrome P450scc and 3β-HSD was localized exclusively to the Schwann cells. Furthermore, this expression was limited to the period of myelin synthesis.
The progesterone receptor was found in the dorsal root ganglia neurons of cocultures during the myelination process. The dorsal root ganglia neurons of premyelinating and myelinating cocultures were positively stained with an antibody to the progesterone receptor with minimal staining of the Schwann cells. The presence of the progesterone receptor has been reported in Schwann cells by Jung-Testas et al. (1996a) but under different conditions than the present experiments. While in the present study the Schwann cells did not exhibit the typical brown/black staining, these results do not exclude the possibility that the progesterone receptor is present in lower amounts in the Schwann cells. Our results clearly demonstrate, however, that the induction of the enzymes responsible for progesterone synthesis occurred in the Schwann cells, and the induction of the progesterone receptor occurred in the DRG neurons. A prediction stemming from these results is that progesterone should alter the expression of genes specifically in neurons. The addition of progesterone was found to auto-induce the progesterone receptor and to cause the translocation of the receptor into the nuclei of the DRG neurons cultured alone. The addition of progesterone to Schwann cells cultured alone did not induce any nuclear staining of the progesterone receptor, and displayed background staining. In addition, both MBP and progesterone receptor mRNA were not induced by progesterone in Schwann cell cultures.
Using mRNA differential display PCR, Rap 1b and PRPP synthase-associated protein have been identified for the first time to be induced by progesterone in neurons. These genes were also induced in cocultures during myelin synthesis and their induction was inhibited by RU-486. The mRNA for these genes were detected in Schwann cell cultures but were not modulated by the addition of progesterone. Recently, using differential display, a Ras-like protein similar to Rap 1b was found to be regulated by dexamethasone in AtT-20 cells (Kemppainen and Behrend, 1998). The induction of these novel genes by progesterone suggests an important function, but this remains to be determined.
Other studies have been carried out to determine the mechanism of how progesterone enhances myelin gene expression. Progesterone causes an approximate 2-fold stimulation of a luciferase reporter gene under the control of the promoter regions of PMP22 and P0 in Schwann cell cultures (Desarnaud et al., 1998). The stimulation was not inhibited by RU-486, but rather RU-486 acted as an agonist under the conditions of the experiments. Progesterone did not stimulate the expression of the PMP22 or P0 constructs in a human carcinoma cell line. The authors suggest that progesterone may indirectly activate myelin gene promoters through Schwann cell-specific transcription factors. In another study, progesterone did not significantly stimulate PMP22 expression in Schwann cells, but rather its derivatives, dihydroprogesterone and tetrahydroprogesterone, induced PMP22 expression. The progesterone derivatives were thought to be acting through the GABA receptor rather than the classical progesterone receptor (Melcangi et al., 1999). Recently, it was shown that progesterone alone, as opposed to forskolin pretreated Schwann cells used by Desarnaud et al. (1998), was not sufficient to promote PMP22 expression in a MSC80 Schwann cell line (Sabéran-Djopneidi et al., 2000). The response of steroid hormones acting through their classical receptors is modulated by complex interactions with coactivators and corepressors, as well as chromatin remodeling mechanisms (for a review see Di Croce et al., 1999). Consequently, a response to a hormone depends on the cell type and the cellular conditions. The present study was directed at understanding what happens during myelin synthesis. Schwann cells and neurons under other culture and developmental conditions may behave differently. In Schwann cell/neuronal cocultures, RU-486 dramatically inhibits myelin synthesis (Koenig et al., 1995; Chan et al., 1998). While Schwann cells were still found to ensheath axons in the presence of RU-486, compact myelin was not observed in electron micrographs, even after 15 days of induction. The present results demonstrate that RU-486 acts as an antagonist by blocking the action of progesterone on the classical progesterone receptor and gene expression in neurons. The results do not exclude the possibility that progesterone has some direct effect on Schwann cells, but they suggest that the predominant effect of progesterone in myelinating cocultures is on neurons. Neuronal signals are necessary for Schwann cells to form myelin and progesterone is part of the cross talk that prepares neurons for myelination. By further elucidating the genes activated in the neurons by progesterone, additional factors that regulate neuronal-glial interactions and myelin synthesis may be identified.
ACKNOWLEDGMENTS
We are grateful to Dr. Edward J. Roy for technical advice on immunocytochemistry; Dr. Eric V. Shusta and Adrian O. Rodriguez for their technical assistance and helpful discussions, and special thanks to Dr. H. Edward Conrad for novel insight and for reviewing the paper. This work was sponsored by a grant from the Multiple Sclerosis Society; Grant number: RG 2660.
REFERENCES
- Almazan G, Honegger P, Matthieu JM. Triiodothyronine stimulation of oligodendrocyte differentiation and myelination. Dev Neurosci. 1985;7:45–54. doi: 10.1159/000112275. [DOI] [PubMed] [Google Scholar]
- Averboukh L, Liang P, Douglas SA, Pardee AB. Hormone-inducible genes in prostate cells. In: Liang P, Pardee A B, editors. Methods in Molecular Biology, Differential Display Methods and Protocols. Vol. 85. Humana Press Inc.; 1997. pp. 163–172. [DOI] [PubMed] [Google Scholar]
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- Bilderback T, Chan JR, Harvey J, Glaser M. Measurement of the rate of myelination using a fluorescent analogue of ceramide. J Neurosci Res. 1997;49:497–507. [PubMed] [Google Scholar]
- Bolin LM, Shooter EM. Neurons regulate Schwann cell genes by diffusible molecules. J Cell Biol. 1993;123:237–243. doi: 10.1083/jcb.123.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Chan JR, Phillips LJ, Glaser M. Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc Natl Acad Sci USA. 1998;95:10459–10464. doi: 10.1073/pnas.95.18.10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desarnaud F, Do Thi AN, Brown AM, Lemke G, Suter U, Baulieu EE, Schumacher M. Progesterone stimulates the activity of the promoter of peripheral myelin protein-22 and protein zero genes in Schwann cells. J Neurochem. 1998;71:1765–1768. doi: 10.1046/j.1471-4159.1998.71041765.x. [DOI] [PubMed] [Google Scholar]
- De Waegh SM, Lee VM-Y, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- Di Croce L, Okret S, Kersten S, Gustafsson J-A, Parker M, Wahli W, Beato M. Steroid and nuclear receptors, Villefranche-sur-Mer, France, May 25–27. EMBO J. 1999;18:6201–6210. doi: 10.1093/emboj/18.22.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JP, Colman DR. Glial-neuron interactions and the regulation of myelin formation. Curr Opin Cell Biol. 1993;5:779–785. doi: 10.1016/0955-0674(93)90025-l. [DOI] [PubMed] [Google Scholar]
- Dugandzija-Novakovic S, Koszowski AG, Levinson SR, Shrager P. Clustering of Na+ channels and node of ranvier formation in remyelinating axons. J Neurosci. 1995;15:492–503. doi: 10.1523/JNEUROSCI.15-01-00492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont E, Simard J, Luu-The V, Labrie F, Pelletier G. Localization of 3β-hydroxysteroid dehydrogenase in rat brain as studied by in situ hybridization. Mol Cell Neurosci. 1994;5:119–123. doi: 10.1006/mcne.1994.1014. [DOI] [PubMed] [Google Scholar]
- Einheber S, Milner TA, Giancotti F, Salzer JL. Axonal regulation of Schwann cell integrin expression suggests a role for α6β4 in myelination. J Cell Biol. 1993;123:1223–1236. doi: 10.1083/jcb.123.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge CF, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina formation and myelin formation. J Cell Biol. 1987;105:1023–1034. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y. Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: Colocalization of StAR, cytochrome P450scc (CYP XIA1), and 3β-hydroxysteroid dehydrogenase in the rat brain. J Neurochem. 1998;71:2231–2238. doi: 10.1046/j.1471-4159.1998.71062231.x. [DOI] [PubMed] [Google Scholar]
- Gillen C, Gleichmann M, Spreyer P, Muller HW. Differentially expressed genes after peripheral nerve injury. J Neurosci Res. 1995;42:159–171. doi: 10.1002/jnr.490420203. [DOI] [PubMed] [Google Scholar]
- Hanker J, Giammara B. Microwave-accelerated cytochemical stains for the image analysis and the electron microscope examination of light microscopy diagnostic slides. Scanning. 1993;15:67–80. doi: 10.1002/sca.4950150203. [DOI] [PubMed] [Google Scholar]
- Horikoshi T, Danenberg K, Volkenandt M, Stadlbauer T, Danenberg PV. Quantitative measurement of relative gene expression in human tumors. In: White B, editor. PCR protocols: Current methods and applications. Humana Press Inc.; 1993. pp. 177–188. [DOI] [PubMed] [Google Scholar]
- Hu ZY, Bourreau E, Jung-Testas I, Robel P, Baulieu EE. Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc Natl Acad Sci USA. 1987;84:8215–8219. doi: 10.1073/pnas.84.23.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe E-H, Angelides K. Clustering of voltage-dependent sodium channels on axons depends on Schwann cell contact. Nature. 1992;356:333–335. doi: 10.1038/356333a0. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Schumacher M, Robel P, Baulieu EE. Demonstration of progesterone receptors in rat Schwann cells. J Steroid Biochem Molec Biol. 1996a;58:77–82. doi: 10.1016/0960-0760(96)00009-x. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Schumacher M, Robel P, Baulieu EE. The neurosteroid progesterone increases the expression of myelin proteins (MBP and CNPase) in rat oligodendrocytes in primary culture. Cell Mol Neurobiol. 1996b;16:439–443. doi: 10.1007/BF02088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Testas I, Schumacher M, Robel P, Baulieu EE. Actions of steroid hormones and growth factors on glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1994;48:145–154. doi: 10.1016/0960-0760(94)90261-5. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Hu ZY, Baulieu EE, Robel P. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology. 1989;125:2083–2091. doi: 10.1210/endo-125-4-2083. [DOI] [PubMed] [Google Scholar]
- Kemppainen RJ, Behrend EN. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J Biol Chem. 1998;273:3129–3131. doi: 10.1074/jbc.273.6.3129. [DOI] [PubMed] [Google Scholar]
- Koenig HL, Schumacher M, Ferzaz B, Do Thi AN, Ressouches A, Guennoun R, Jung-Testas I, Robel P, Akwa Y, Baulieu EE. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995;268:1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- Koper JW, Hoeben RC, Hochstenbach F, van Golde L, Lopes-Cardozo M. Effects of triiodothyronine on the synthesis of sulfolipids by oligodendrocyte-enriched glial cultures. Biochem Biophys Acta. 1986;887:327–334. doi: 10.1016/0167-4889(86)90162-x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Cole R, Chiappelli F, De Vellis J. Differential regulation of oligodendrocyte markers by glucocorticoids: Post-transcriptional regulation of both proteolipid protein and myelin basic protein and transcriptional regulation of glycerol phosphate dehydrogenase. Proc Natl Acad Sci USA. 1989;86:6807–6811. doi: 10.1073/pnas.86.17.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goascogne C, Robel P, Gouezou P, Sananes N, Baulieu EE, Watermann M. Neurosteroids. cytochrome P-450scc in rat brain. Science. 1987;237:1004–1007. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Liang P, Zhu W, Zhang X, Guo Z, O'Connell RP, Averboukh L, Wang F, Pardee AB. Differential display using one-base anchored oligo dT primers. Nucleic Acids Res. 1994;22:5763–5764. doi: 10.1093/nar/22.25.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Login GR, Dvorak AM. A review of rapid microwave fixation technology: its expanding niche in morphologic studies. Scanning. 1993;15:58–66. doi: 10.1002/sca.4950150202. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Magnaghi V, Cavarretta I, Zucchi I, Bovolin P, D'Urso D, Martini L. Progesterone derivatives are able to influence peripheral myelin protein 22 and Po gene expression: possible mechanisms of action. J Neurosci Res. 1999;56:349–357. doi: 10.1002/(SICI)1097-4547(19990515)56:4<349::AID-JNR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Notterpek L, Snipes GJ, Shooter EM. Temporal expression pattern of peripheral myelin protein 22 during in vivo and in vitro myelination. Glia. 1999;25:358–369. [PubMed] [Google Scholar]
- Reynolds ML, Woolf CJ. Reciprocal. Schwann cell-axon interactions. Curr Opin Neurobiol. 1993;3:683–693. doi: 10.1016/0959-4388(93)90139-p. [DOI] [PubMed] [Google Scholar]
- Robel P, Baulieu EE. Neurosteroids. Biosynthesis and function. TEM. 1994;5:1–8. doi: 10.1016/1043-2760(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Sabéran-Djoneidi D, Sanguedolce V, Assouline Z, Lévy N, Passage E, Fontés M. Molecular dissection of the Schwann cell specific promoter of the PMP22 gene. Gene. 2000;248:223–231. doi: 10.1016/s0378-1119(00)00116-5. [DOI] [PubMed] [Google Scholar]
- Salzer JL, Bunge RP, Glaser L. Studies of Schwann cell proliferation III. Evidence for the surface localization of the neurite mitogen. J Cell Biol. 1980;84:767–778. doi: 10.1083/jcb.84.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanne JL, Krueger KE. Expression of cytochrome P450 side-chain cleavage enzyme and 3β-hydroxysteroid dehydrogenase in the rat central nervous system: a study by polymerase chain reaction and in situ hybridization. J Neurochem. 1995;65:528–536. doi: 10.1046/j.1471-4159.1995.65020528.x. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Schaefer C, Valenzuela DM, Yancopoulos GD, Schwab ME. Identification of new oligodendrocyte- and myelin-specific genes by a differential screening approach. J Neurochem. 1995;65:10–22. doi: 10.1046/j.1471-4159.1995.65010010.x. [DOI] [PubMed] [Google Scholar]
- Stahl N, Harry J, Popko B. Quantitative analysis of myelin protein gene expression during development in the rat sciatic nerve. Mol Brain Res. 1990;8:209–212. doi: 10.1016/0169-328x(90)90018-9. [DOI] [PubMed] [Google Scholar]
- Tosic M, Torch S, Comte V, Dolivo M, Honegger P, Matthieu JM. Triiodothyronine has diverse and multiple stimulating effects on expression of the major myelin protein genes. J Neurochem. 1992;59:1770–1777. doi: 10.1111/j.1471-4159.1992.tb11009.x. [DOI] [PubMed] [Google Scholar]
- Walters SN, Morell P. Effects of altered thyroid states on myelinogenesis. J Neurochem. 1981;36:1792–1801. doi: 10.1111/j.1471-4159.1981.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Warringa RA, Hoeben RC, Koper JW, Sykes JE, vanGolde LM, Lopes-Cardozo M. Hydrocortisone stimulates the development of oligodendrocytes in primary glial cultures and affects glucose metabolism and lipid synthesis in these cultures. Brain Res. 1987;431:79–86. doi: 10.1016/0165-3806(87)90197-0. [DOI] [PubMed] [Google Scholar]
- Yin X, Crawford TO, Griffin JW, Tu P-H, Lee VM-Y, Li C, Roder J, Trapp BD. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci. 1998;18:1953–1962. doi: 10.1523/JNEUROSCI.18-06-01953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]