Abstract
Modified single-stranded DNA oligonucleotides can direct the repair of genetic mutations in yeast, plant and mammalian cells. The mechanism by which these molecules exert their effect is being elucidated, but the first phase is likely to involve the homologous alignment of the single strand with its complementary sequence in the target gene. In this study, we establish the importance of such DNA pairing in facilitating the gene repair event. Oligonucleotide-directed repair occurs at a low frequency in an Escherichia coli strain (DH10B) lacking the RECA DNA pairing function. Repair activity can be rescued by using purified RecA protein to catalyze the assimilation of oligonucleotide vectors into a plasmid containing a mutant kanamycin resistance gene in vitro. Electroporation of the preformed complex into DH10B cells results in high levels of gene repair activity, evidenced by the appearance of kanamycin-resistant colonies. Gene repair is dependent on the formation of a double-displacement loop (double-D-loop), a recombination intermediate containing two single-stranded oligonucleotides hybridized to opposite strands of the plasmid at the site of the point mutation. The heightened level of stability of the double-D-loop enables it to serve as an active template for the DNA repair events. The data establish DNA pairing and the formation of the double-D-loop as important first steps in the process of gene repair.
INTRODUCTION
DNA pairing plays a critical role in the cellular response to DNA damage. In the most traditional repair pathway, single strands of DNA arising from nicks, gaps or breaks in the chromosome invade a sister homolog and hybridize to a region of DNA that has a complementary sequence, usually initializing its repair. This invasion forms a DNA structure known as a heteroduplex joint containing paired strands donated by aligned parental duplexes. Biochemical work, focused on defining the activity of the bacterial recombinase RecA protein, modeled this reaction using a single strand of DNA and a superhelical duplex molecule, the conjoining of which forms a structure known as a displacement loop (D-loop) (1–6). This triple-stranded configuration consists of two strands paired by complementarity and a displaced strand that can be removed by endogenous nuclease activity. If the donor strand remains hybridized to its complement in the recipient, it can initialize a DNA repair reaction or a recombination event.
The D-loop structure has served as the primary experimental model for many studies aimed at elucidating the molecular and enzymatic activities that regulate the initiation of DNA pairing. As such, molecular requirements, including the energy found in a superhelix in the recipient duplex, for promoting efficient uptake of the single strand have been identified. In addition, the stereochemistry of the triple-helix structure created by D-loop formation has been informative for studies focusing on the biochemical function of RecA protein (1,7). Energy requirements also entail ATP hydrolysis, which produces a regulated reaction known as the D-loop cycle (8,9).
We have been studying targeted nucleotide exchange (TNE), a process in which a single-stranded oligonucleotide assimilates into an episomal or chromosomal target region, perhaps using the D-loop as a reaction intermediate. After formation, the oligonucleotide directs the repair (or exchange) of a targeted nucleotide in the parent duplex (10). The specificity of nucleotide exchange is dictated by the creation of a mismatched base pair between the oligonucleotide vector and a nucleotide in the coding region of the targeted gene. But the reaction is entirely dependent on the successful pairing of the single-stranded vector with its complement in the duplex target. Previous data using cell-free extracts established a role for mismatch repair genes in the repair phase of the reaction (11,12), and genetic studies suggested that proteins with DNA pairing activity that assimilate the single-stranded oligonucleotides into recipient plasmid targets are essential for the TNE reaction (13–15). It seems probable, therefore, that a D-loop or D-loop-like structure serves as the central intermediate in the overall TNE pathway. Such a hypothesis gains support from the pioneering work of Holloman et al. (1,7). In these studies, D-loops were formed by hybridization and the joint molecule was transformed into Escherichia coli. The preformation of the D-loop in phage ØX174 DNA alleviated the need for a DNA pairing function in E.coli. It is generally believed that these experiments helped define ‘… one of the most obscure aspects of genetic recombination’ (7)—its initiation.
The importance of elucidating the structural intermediate of TNE emerges from the belief that the success of TNE is likely to depend (at least in part) on the stability of the joint molecule serving as the reaction intermediate. And factors contributing to increased stability of the intermediate would in turn lead to higher rates of repair. Parekh-Olmedo et al. (16) achieved higher frequencies of repair by simultaneously targeting a chromosomal gene with two complementary oligonucleotides. The use of dual vectors in the TNE reaction is based upon previously reported biochemical evidence that RecA protein can catalyze the formation of ‘double-D-loops’, structures in which the two oligomers are paired, side-by-side, to parallel, complementary sequences at the same site in a recipient plasmid (17). These double-D-loops, also known as complement-stabilized D-loops, increase stability by providing a second oligonucleotide ‘trap’ to hybridize with the displaced complementary strand of the duplex.
Since elevated frequencies of TNE were found in experiments using two oligomers by Parekh-Olmedo et al. (16), we wondered whether the double-D-loop structure was serving as an efficient, primed template for the TNE reaction. Thus, in the present work, we set out to establish reaction conditions for the facile formation of stable double-D-loops bearing a single mismatched base in vitro, and asked whether a gene repair reaction could occur within these preformed templates. A genetic readout system in E.coli was chosen because of its successful use in previous studies (11,18) and the fact that correction results in a phenotypic change. Because we were working in a bacterial system, we utilized the DNA pairing function of the RecA protein to preform double-D-loops in vitro. The complexes were then electroporated into recA-deficient cells, and the frequency of the TNE reaction was measured by the appearance of antibiotic-resistant bacterial colonies. Our results strongly suggest that DNA pairing is an important step in TNE, and that the double-D-loop can serve as an active template upon which the nucleotide exchange event takes place.
MATERIALS AND METHODS
RecA and oligonucleotides
RecA protein was obtained from USB (Cleveland, OH). Single-stranded oligonucleotides were obtained from Integrated DNA Technologies, Inc. (Coralville, IA). Oligo nucleotides were quantified on the basis of spectrophotometric Abs260 values and using the conversion factor of 33 µg/ml OD. Oligonucleotides were subsequently labeled by standard procedures using [γ-32P]ATP and T4 polynucleotide kinase.
D-loop preparation
Formation of D-loops was carried out in two steps. A 15-min pre-synaptic step occurred at 37°C using 40 nM of the appropriate ‘incoming’ oligonucleotide and 1.5 µM RecA (ratio of two nucleotide bases per one RecA monomer) in a solution containing 1 mM ATPγS, 25 mM Tris–OAc (pH 7.5), 1 mM (CH3CO2)2Mg·4H2O and 1 mM DTT. The synaptic phase began with the addition of 10 nM supercoiled plasmid and an additional 9 mM (CH3CO2)2Mg·4H2O for 5 min. Double-D-loops were formed with the addition of the required 320 nM 32P-labeled-‘annealing oligonucleotide’ having a length of between 30 and 50 bp. Additional incubation at 37°C occurred for 10 min, and joint molecules were deproteinated with the addition of 1% SDS at 4°C. Buffer exchange into 10 mM Tris, 1 mM EDTA was conducted using either CentriSpin-20 (Princeton Separations, Adelphia, NJ) or Chromaspin-400 (Clontech Laboratories, Inc., Palo Alto, CA) columns. Excess unbound oligonucleotide was removed with the Chromaspin-400 columns. The presence of joint molecules was confirmed by 1% agarose gel electrophoresis prior to use in the in vivo targeting experiments. Quantification of D-loop and double-D-loop complexes was carried out using ImageQuant 5.2 software after visualization using a Typhoon 8600 Variable Mode Imager (Molecular Dynamics Inc., Sunnyvale, CA) to scan for either phosphorescence or fluorescence.
Electroporation, plating and selection
Five microliters of DNA sample (20 µl total) was used to transform 20 µl aliquots of electrocompetent DH10B (Invitrogen, Carlsbad, CA) or BL21(DE3) (Clontech Laboratories, Inc., Palo Alto, CA) bacteria using a Gibco cell-porator apparatus (Life Technologies, Cleveland, OH) under the following conditions: 330 µF, 4 ΩK, 400 V/sample. Each mixture was transferred to a 1 ml SOC culture [2.0% (w/v) tryptone, 0.5% (w/v) yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2 and 20 mM Glc] and incubated at room temperature for 3 h. In cases where joint complexes were not used for transforming bacteria, 50 ng plasmid DNA, 1 µg incoming oligonucleotide and 2 µg annealing oligonucleotide were added to the electrocompetent cells immediately prior to electroporation. Plasmid DNA was amplified by adding ampicillin to 100 µg/ml and an equal volume of SOC media. These cultures underwent an additional incubation of 24 h at room temperature with shaking at 300 r.p.m. One hundred microliter aliquots of 10–1 and 10–2 diluted cultures were plated onto Luria–Bertani (LB) agar plates containing 50 µg/ml kanamycin, and 10–5 and 10–6 diluted cultures were plated onto LB agar plates containing 100 µg/ml ampicillin. Plating was performed in duplicate using sterile Pyrex beads. Both sets of plates were incubated for 24 h at 37°C and colonies were counted. Targeted conversion of the kanr gene was determined by normalizing the number of kanamycin-resistant colonies and dividing by the number of ampicillin-resistant colonies, with correction efficiencies calculated per 105 ampicillin colonies. Resistant colonies were confirmed by selecting isolated clones for mini preparation of plasmid DNA followed by sequencing using an automated ABI Prism 3100 Genetic Analyzer.
RESULTS
Assay system for targeted nucleotide exchange
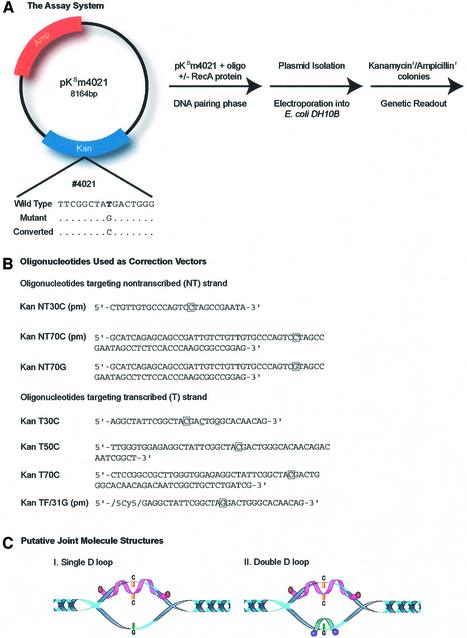
A genetic readout was used as an assay system to measure the correction efficiency of TNE. Plasmid pKSm4021 (Fig. 1A), bearing a point mutation in the gene that confers kanamycin resistance onto E.coli, served as the target. A stop codon (TAG) was created at the indicated site replacing the wild-type TAT codon. The nucleotide exchange reaction, directed by specific oligonucleotides (see below), results in the creation of a TAC triplet and reversal of phenotype; kanamycin sensitivity to resistance. The creation of the TAC codon can be confirmed by DNA sequence analyses of kanr colonies. If the TAC codon was present, kanamycin resistance arose from a repair event, rather than having arisen from contamination of a wild-type plasmid, which contains a TAT codon at the targeted position. Plasmid pKSm4021 also contains the gene for ampicillin resistance, which is used as an internal control and a measure of electroporation efficiency.
Figure 1.
The assay system and sequences of modified oligonucleotides. (A) Plasmid pKsm4021 has an expression cassette bearing a kanamycin and an ampicillin gene. The kanamycin gene contains a single-base transversion at nucleotide 4021, a replacement mutation of TAT (tyrosine) to TAG (stop codon). Correction of pKsm4021 requires the replacement of the mutant G residue with a C residue. The plasmid and an (incoming) oligonucleotide are incubated in the presence or absence of the RecA protein, followed by the addition of a second (annealing) oligonucleotide. After joint complexes are formed and isolated, the complex is electroporated into E.coli DH10B cells, which are deficient in RecA protein. A genetic readout of the correction efficiency is then analyzed by normalizing the number of kanamycin-resistant colonies to the number of ampicillin-resistant colonies. (B) Synthetic oligonucleotides were used to direct reversion of kanr genes to restore resistance to kanamycin. KanNT30C, KanNT70C and KanNT70G are all single-stranded oligonucleotides that target the non-transcribed strand. KanT30C, KanT50C, KanT70C and KanTF/31G are oligonucleotides that target the transcribed strand of the plasmid target. KanTF/31G contains a Cy5 fluorescent dye at its 5′ end. pm refers to an oligonucleotide being perfectly matched to its complementary target sequence. (C) Structure of D-loop (I) and double-D-loop (II) joint molecules as putative reaction intermediates.
The oligonucleotides used in this study are listed in Figure 1B. KanNT30C(pm) and KanNT70C(pm) are complementary to the non-transcribed (NT) strand of plasmid pKSm4021 and have lengths of 30 and 70 nt, respectively. KanNT70G is also complementary to the non-transcribed strand, except at position 4021 where a G·G mismatch is formed with the target sequence. The second group of oligonucleotide vectors hybridizes to the transcribed (T) strand of pKSm4021 at the same site. These molecules differ only in their length (30, 50 or 70 bases), but all create a C·C mismatch with the target strand of pKSm4021. KanTF/31G (pm) contains a fluorescent-Cy5 tag on the 5′ end, is 31 bases in length, and hybridizes with perfect complementarity (pm) to the target site.
The experimental protocol involves the pairing of two oligonucleotides with pKSm4021 in a reaction catalyzed by the RecA protein. RecA protein has the capacity to transfer oligonucleotides into the plasmid at the homologous site, creating a structure known as a D-loop or, in our case, a double-D-loop. Some of the oligonucleotides used in these experiments align in perfect register with either the ‘transcribed or non-transcribed’ strand of pKSm4021, while others form a single mismatched base pair located in the center of the conjoined molecules. The structure of a D-loop or a double-D-loop is represented in Figure 1C. We use the terminology ‘transcribed’ (T) or ‘non-transcribed’ (NT) for convenience simply to distinguish the strands of plasmid pKSm4021.
Once the complexes are assembled, they can be separated from excess single-stranded molecules by using a chromaspin column (see Materials and Methods). The isolated complexes can be electroporated directly into DH1OB (recA–) E.coli cells to measure their competency for nucleotide repair. Since this strain has a disrupted RECA gene, which inactivates RecA function, the majority of DNA pairing events must, therefore, occur outside the cell. After electroporation of the preformed joint molecule, the repair function is provided by the bacterial cell. The conversion events can be monitored by growth on agar plates containing either kanamycin or ampicillin. The correction efficiency of each reaction is calculated by dividing the number of kanr colonies by the number of ampr colonies, the latter value normalizing for differences in electroporation efficiency. Thus, we use an experimental protocol in which the DNA pairing phase of this reaction occurs outside the cell, while the repair of the point mutation in pKSm4021 takes place in the cell. Such a scenario addresses the need for DNA pairing in TNE because, in effect, electroporation of preformed, ‘pre-paired’ substrates should rescue a recA– strain for the TNE reaction.
The importance of RecA protein in TNE was demonstrated directly in the following experiment. Two bacterial strains—DH10B, which lacks functional RecA protein, and BL21(DE3), which has normal RecA protein activity—were electroporated with plasmid pKSm4021, which was maintained under ampicillin selection. Subsequently, oligonucleotides KanT70C and KanNT30C were co-electroporated into each strain, respectively, and the correction of pKSm4021 monitored by the appearance of Kanr colonies. As shown in Table 1, both E.coli strains support the repair of pKSm4021, but at significantly different levels. In DH10B cells, TNE occurs at a low level, while in contrast, correction frequency in BL21 is 0.89. The reduction in activity observed in DH10B cells is attributable, in all likelihood, to the absence of active RecA protein. This observation is consistent with the data of Holloman and Radding (1) and forms the basis for the implication that DNA pairing function is an important requirement for high levels of TNE. A low level of correction did occur in the DH10B cells, indicating that other recombination activity(s) may promote TNE in the absence of RecA function. Such activities could include, but would not be limited to, strand annealing or DNA condensation, which could juxtapose DNA sequences that could serve as repair templates.
Table 1. TNE in DH10B and BL21 cells.
| Strain | DNA substrates | Kanr colonies | Ampr colonies(/105) | Correction efficiency [Kanr/Ampr (105)] |
|---|---|---|---|---|
| DH10B | – | 0 | 642 | 0.00 |
| DH10B | KanT70C/KanNT30C | 42.5 | 512 | 0.08 |
| BL21 | – | 0 | 512 | 0.00 |
| BL21 | KanT70C/KanNT30C | 600 | 709 | 0.89 |
Escherichia coli strains DH10B [F– mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 endA1 araΔ139 Δ(ara, leu)7697 galU galK λ– rpsL nupG tonA] and BL21(DE3) [F– ompT hsdSB(rB–mB–) gal dcm (DE3)] were electroporated with either 50 ng pKSm4021 plasmid alone or 50 ng plasmid, 1 µg T70C, and 2 µg NT30C (see Materials and Methods). Average kanamycin-resistant and ampicillin-resistant colony numbers and correction efficiencies per 105 cells are presented.
To test this hypothesis directly, however, we established an experimental protocol in which double-D-loops were constructed with the same plasmid and oligonucleotides prior to electroporation using purified RecA protein to supply the DNA pairing function ex vivo.
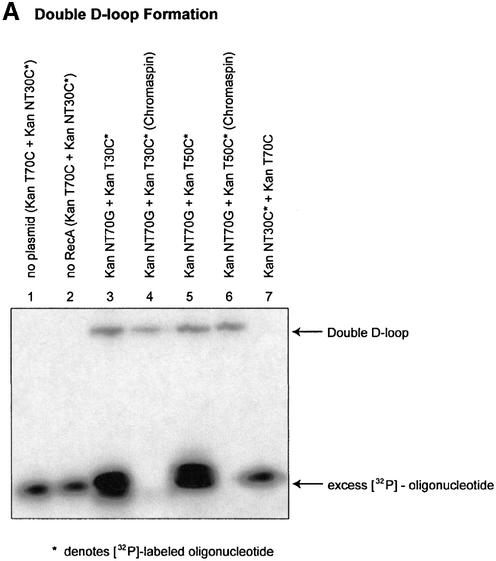
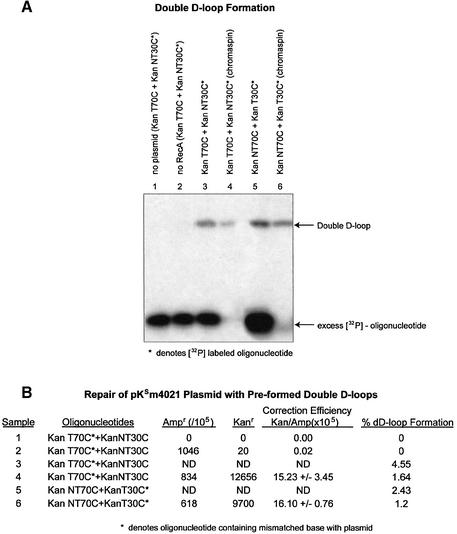
In the first iteration, double-D-loops were formed using an unlabeled incoming oligonucleotide and a 32P-labeled annealing oligonucleotide. As shown in Figure 2A, double-D-loops are visualized by the change in position of the 32P-labeled annealing oligonucleotide. The position of the 32P-labeled annealing oligonucleotide is altered from a fast moving species to one that co-migrates with the superhelical DNA once incorporated into the plasmid. In our reaction protocol, the annealing oligonucleotide will hybridize to the complement strand of the D-loop only if RecA protein remains associated with the initial joint molecule consisting of the plasmid DNA and the incoming oligonucleotide (this fact is confirmed by lane 7 below). In the experiment presented in Figure 2A, KanNT70G was used as the incoming oligonucleotide and either 32P-labeled KanT30C or 32P-labeled KanT50C was used as the annealing oligonucleotide. Under these reaction conditions, double-D-loop formation is dependent on the presence of RecA protein and both DNA substrates (Fig. 2A, lanes 1 and 2). In this case, double-D-loops bear two mismatched base pairs, one created by the non-transcribed strand with KanNT70G and one created by the transcribed strand with KanT30C or KanT50C. Figure 2A, lanes 3, 4 (30mer), 5 and 6 (50mer), respectively, demonstrates the efficiency of separating the double-D-loop complexes from excess, unbound, 32P-labeled oligonucleotides by using the chromaspin column technique (see Materials and Methods). Of particular interest is the observation that a significant percentage of stable double-D-loops were recovered due, in all likelihood, to the enhanced level of complex stability engendered by the 50mer. We observed (Fig. 2A) that a higher level of double-D-loops are isolated when the annealing oligonucleotide has a length of 50 bases, rather than 30 bases. Lane 7 illustrates the results of a reaction in which the 30mer (KanNT30C) was used as the initial or incoming oligonucleotide. No double-D-loops are formed, suggesting that the 30mer cannot enter the complex or does not remain in the complex as the incoming oligomer, but it can assimilate if the 70mer and the plasmid DNA have combined to form the initial D-loop structure.
Figure 2.
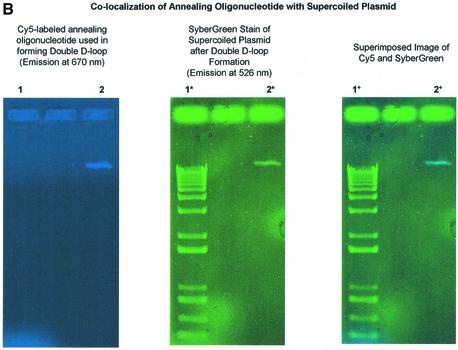
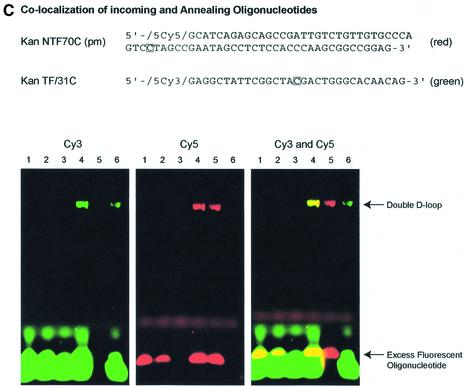
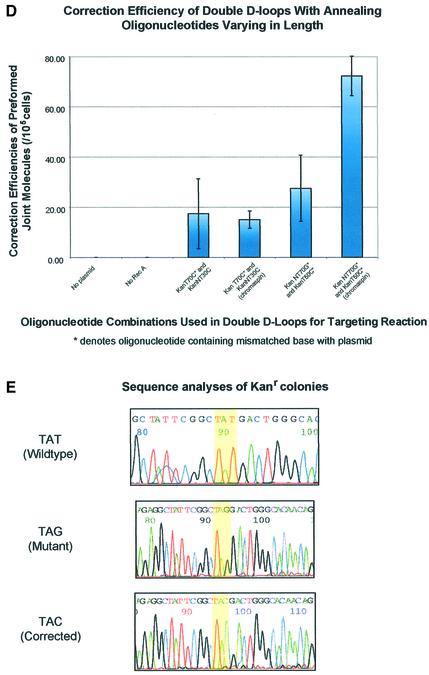
Length effect of annealing oligonucleotide in TNE. (A) Double-D-loops were formed by adding either a 30mer (KanT30C) (lanes 3 and 4) or 50mer (KanT50C) (lanes 5 and 6) as the annealing oligonucleotide after the initial synaptic step in which the incoming oligonucleotide (KanNT70G) is paired to plasmid pKanSm4021. The presence of double-D-loops are visualized by the migration of the 32P-labeled oligonucleotide on an agarose gel. An amount of 5 µl joint complex sample is loaded on a 1% agarose gel with the addition of 1× loading dye (0.25% bromophenol blue, 0.25% xylene cyanol, 25% ficoll), and electrophoresed at 97 V for 2 h at room temperature. No double-D-loops are formed when a 30mer is used as an incoming oligonucleotide and a longer 70mer as the annealing oligonucleotide (lane 7). (B) Double-D-loops were formed using KanTF/31G, a Cy5-labeled annealing oligonucleotide perfectly matched to the target strand (lanes 2, 2* and 2+). To ensure formation of a double-D-loop complex, the gel was stained with SyberGreen to monitor the presence of the double-stranded plasmid target. Lanes 1, 1* and 1+ represent a DNA ladder with no base pair periodicity. (C) Double-D-loops were formed using both Cy5-labeled and Cy3-labeled oligonucleotides. Additional oligonucleotides were used in conjunction with those listed in Figure 1B. Kan NTF70C contains a Cy5 tag at its 5′ end and is perfectly matched to the non-transcribed strand of the plasmid. KanTF/31C contains a Cy3 tag on its 5′ end and a C·C mismatch with the plasmid. The following combinations were tested in the following reactions: no RecA protein (lane 1), no pKanSm4021 (lane 2), no incoming oligonucleotide, NTF70C (lane 3), NTF70C (Cy5) + TF31C (Cy3) (lane 4), NTF70C (Cy5) + T30C (lane 5) and NT70C + TF31C (Cy3) (lane 6). The presence of Cy3-labeled oligonucleotides in double-D-loops are shown in green when visualized at 580 nm, whereas Cy5-labeled oligonucleotides are red when visualized at 670 nm. When both oligonucleotides are incorporated into double-D-loops, yellow bands appear in the gel. (D) The bar graph indicates the correction efficiency using double-D-loops containing either 30 or 50mer as the annealing oligonucleotide. After monitoring double-D-loop complex formation on an agarose gel, an additional 5 µl sample was transfected into DH10B competent cells. The designation ‘chromaspin’ indicates that these double-D-loops were isolated by passage through a chromaspin column, removing excess oligonucleotide. The correction efficiencies were determined by normalizing the number of kanamycin-resistant colonies to the number of ampicillin-resistant colonies. (E) Kanamycin- resistant colonies were picked for colony PCR, and the PCR products were sequenced as described in Materials and Methods. Wild-type pKSm4021 has a sequence of TAT, while the mutant pKSm4021 plasmid contains a sequence of TAG at the target position. All kanamycin-resistant colonies tested were found to have a TAC sequence, exhibiting a perfect replacement of a G base at position 4021. The standard deviation for these experiments and others that follow was calculated using data from four independent experiments.
To show that the annealing oligonucleotide co-migrates with supercoiled plasmid DNA after agarose gel electrophoresis, we carried out a double-D-loop reaction using a fluorescently tagged, single-stranded oligonucleotide. KanTF/31G, which contains a Cy5 fluorescent moiety attached to the 5′ end, was used as the annealing oligonucleotide. This molecule was synthesized with this fluorescent tag so that its location in an agarose gel could be detected at a wavelength of 670 nm. Lane 1 contains a 1 kb ladder used as a double-stranded molecule. By forming double-D-loops and removing excess oligonucleotide, the presence of oligonucleotide can be identified because of Cy5 emission at 670 nm (Fig. 2B, lane 2). When the same gel is stained with SyberGreen, which demarcates the position of double-stranded DNA (pKSm4021) with emission at 526 nm, a green fluorescent band is visualized not only on the 1 kb DNA ladder (lane 1*), but also at the position of superhelical plasmid DNA (lane 2*). Using ImageQuant software, the two scans of the gel can be overlaid, identifying the co-migration of oligonucleotide vector and plasmid pKSm4021 (lane 2+).
To more accurately define the oligonucleotide components of the double-D-loop, we used two new oligonucleotides modified at the 5′ end. KanNTF70C(pm) is analogous to KanNT70C (pm), except it contains a Cy5 fluorescent tag on its 5′ end (see Fig. 1B). KanTF/31C is the same as KanTF/31G, except that it has a Cy3 fluorescent tag on its 5′ end instead of a Cy5 tag. It also creates a C·C mismatch instead of being perfectly matched to the transcribed strand (Fig. 2C). We used KanNTF70C and KanTF/31C in double-D-loop reactions, following each oligonucleotide by its unique fluorescent label. As shown in Figure 2C, double-D-loop formation is dependent on the presence of RecA protein (lane 1), plasmid DNA (lane 2) and incoming oligonucleotide (lane 3). In these three lanes, no detectable fluorescence is observed. Lane 4 represents a reaction in which KanNTF70C and KanTF/31C are both present and, as seen in the far left panel, green fluorescence indicates the presence of Cy3-labeled KanTF/31C oligonucleotides. The center panel (lane 4) confirms the presence of the Cy5-labeled KanNTF70C (red). Data in the far right panel confirm that both oligonucleotides co-localize (lane 4, yellow band). Lanes 5 and 6 represent reaction mixtures in which either KanTF/31C or KanNTF70C is used in conjunction with a partner oligonucleotide that does not contain a fluorescent tag (see Fig. 1B). Taken together, the data establish the presence of both oligonucleotides in the double-D-loop complex.
Complexes from Figure 2A were electroporated into E.coli and the correction efficiency was determined by selection on plates containing either ampicillin or kanamycin. As shown in Figure 2D, kanamycin-resistant colonies were observed when the initial mixtures contained all the appropriate reaction components. The double-D-loop reactions containing KanT70C and KanNT30C were tested for correction activity in the isolated (chromaspin) and non-isolated forms. Both exhibited gene repair activity at approximately the same level indicating that, in all likelihood, the corrected plasmids conferring antibiotic resistance are likely to be those that contained preformed double-D-loops. The combination of KanNT70G/KanT50C exhibited a higher level of nucleotide exchange than the combination of KanT70C/KanNT30C, perhaps reflecting the increased percentage (40% higher) of isolated double-D-loops from the combination of KanNT70G and KanT50C with passage through a chromaspin column. This increase may indicate that the complex bearing KanNT70G and KanT50C is more amenable to the repair reaction because the half-life of the whole complex within the cell is increased. Another possibility for the higher level of correction observed with the KanNT70G/KanT50C combination is that two mismatched base pairs are created when these oligonucleotides hybridize to the target. Finally, the purified complexes from lane 6 (Fig. 2A) are more active than the unpurified complexes, represented in lane 5 (Fig. 2A), and thus again, the data suggest that the corrected kanamycin genes are likely to have arisen from the preformed double-D-loop complexes.
Colonies exhibiting kanamycin resistance as a result of the action of KanNT70G/KanNT50C were processed for DNA sequence analyses (Fig. 2E). Isolated plasmid molecules from these colonies were found to contain the converted TAC codon rather than the TAG codon. In addition, no TAT codons were found, validating the assay and suggesting that kanamycin resistance arose from a DNA repair process and not from wild-type plasmid contamination. Resistant colonies generated by the electroporation of the 70/30mer oligonucleotide combination showed the same DNA sequence results (data not shown). These results suggest that purified double-D-loops can serve as templates for the gene repair reaction and that the specific nucleotide base targeted for repair is, in fact, altered.
In the next series of experiments, we compared the efficiency of repair generated from double-D-loop complexes in which the 70mer or the 30mer served as the oligonucleotide that created the single mismatch with the transcribed (T) strand. Preformed double-D-loops created through the combinations of KanT70C/KanNT30C or KanNT70C/KanT30C were isolated and electroporated into DH10B cells for genetic readout. When KanT70C and KanNT30C are used, a single mismatch (C·C) is made between the incoming 70mer and the ‘transcribed’ strand of pKSm4021 (Fig. 3A). While the overall percentage of 32P-labeled oligonucleotide transferred into the complex by the action of RecA protein is estimated to be 4.5%, relative to the amount of labeled excess oligo (Fig. 3B, lane 3), half of the preformed complexes were successfully isolated by the chromaspin technique (Fig. 3B, lane 4). When electroporated into E.coli, this combination of oligonucleotides produces a correction frequency of 15.23. Under these reaction conditions, plasmid DNA is limiting and it is likely that most of it will be assembled into joint complexes (see Fig. 4). When KanNT70C and KanT30C are used to preform the double-D-loop, the incoming oligonucleotide, KanNT70C, hybridizes completely to the non-transcribed strand of pKSm4021 and KanT30C forms a C·C mismatch to the transcribed strand (Fig. 3A, lanes 5 and 6). After electroporation into DH10B cells, the isolated molecules give rise to a correction efficiency of 16.10. Since the two values (15.23 and 16.10) are similar, we conclude that the length of the oligonucleotide creating the mismatched base pair with the transcribed strand of pKSm4021 has little impact on the overall correction frequency.
Figure 3.
Alternate mismatches in double-D-loop intermediates. (A) Joint molecules were formed under standard conditions so that either the incoming or annealing oligonucleotide created the mismatch with the target base; in either case, the mismatch targeted the transcribed strand of plasmid pKSm4021. (B) Correction efficiency was measured in samples lacking plasmid or RecA protein (lanes 1 and 2). The formation of joint double-D-loop complexes were quantified and compared with the correction efficiencies for samples 4 and 6. The amount of correction is similar, despite the mismatch location change from the long incoming oligonucleotide to the short annealing oligonucleotide. KanT70C* and KaNT30C complexes observed a correction efficiency of 15.23 ± 3.45, whereas KanNT70C and KanT30C* observed a correction efficiency of 16.10 ± 0.76 after excess oligonucleotide removal. The amount of complex in these samples transfected into the competent cells was also similar—1.64 and 1.2%, respectively.
Figure 4.
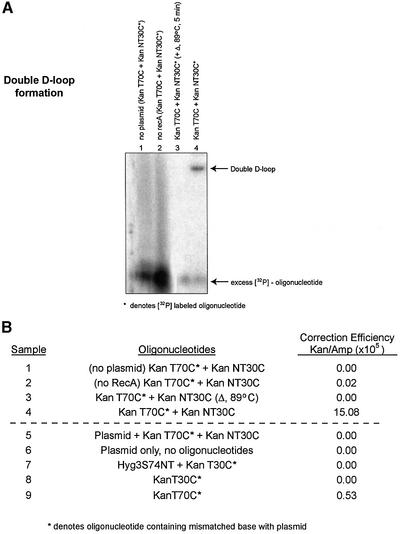
Parameters of the reaction. (A) The two oligonucleotides, KanT70C and KanNT30C, were used to study joint complex formation in the absence of either plasmid (lane 1) or RecA protein (lane 2). A stable double-D-loop formed only under standard reaction conditions (lane 4). In one case, the sample was heated to 89°C for 5 min before loading on the gel (lane 3). (B) The same samples from the agarose gel in (A) were transfected into DH10B cells. Additional samples were also transfected into DH10B cells (samples 5–9). Plasmid and oligonucleotides were added without RecA, ATPγS or Mg2+ (sample 5). Sample 6 contained only plasmid, and sample 7 contained a non-specific oligonucleotide with no complementarity to the kanamycin sequence. This oligonucleotide was a 74mer (17,18) and was used in place of a 70mer oligonucleotide. D-loops were formed in samples 8 and 9 using KanT30C and KanT70C, respectively, prior to electroporation in DH10B cells.
A series of controls in which several reaction conditions were altered either before or after the formation of the double-D-loop were carried out. For most experiments, KanT70C and KanNT30C were used as incoming and annealing oligonucleotides, respectively. As shown in Figure 4A, the formation of double-D-loops does not occur when either plasmid DNA or RecA protein is excluded from the reaction (lanes 1 and 2), whereas a complete reaction mixture results in double-D-loop formation (lane 4). In one modified reaction, isolated double-D-loop complexes were heated to 89°C for 5 min and, as shown in Figure 4A (lane 3), the population of joint molecules was no longer observed. Figure 4B illustrates the correction efficiencies of samples 1–4 and, consistent with earlier data, only sample 4 produces a significant correction frequency. The heat-treated sample does not give rise to kanr colonies after electroporation into DH10B cells. Furthermore, in a reaction in which pKSm4021, KanT70C and KanNT30C were electroporated simultaneously without the in vitro pairing step (sample 5), no detectable correction events took place, as evidenced by the correction efficiency value of 0.0. The same results were observed when only the plasmid was electroporated into E.coli (sample 6), and align with the results presented in Figure 2. When a non-homologous 74mer (Hyg3S/74NT) (13) replaced KanT70C as the incoming oligonucleotide, no gene repair of pKSm4021 was found (lane 7). The formation of D-loops, as opposed to double-D-loops, only produced a low correction efficiency of 0.53 (lane 9). No correction efficiencies were observed when using a D-loop formed with a 30mer oligonucleotide. The results of all of these control experiments suggest that a DNA pairing event creating a double-D-loop reaction intermediate is a critical step in the pathway of TNE.
DISCUSSION
We have used synthetic oligonucleotides to correct a mutation in the kanamycin gene contained in plasmid pKSm4021. Using the DNA repair pathways in E.coli, the gene is corrected and its expression enables the bacteria to grow on plates laden with kanamycin. A simple assay system (11) in which the plasmid and the oligonucleotides are introduced into E.coli at various times and in various combinations facilitates an evaluation of reaction parameters. In this study, we examine how the preformation of joint molecules comprised of a plasmid and oligonucleotides influences the repair of the kanamycin mutation. More specifically, we focus on the creation of double-D-loops—a conjoined molecule in which oligonucleotides hybridize to opposite strands of the target helix forming a complement-stabilized structure—and its capacity to serve as a template for gene repair. Our results indicate the following: first, the creation of double-D-loops through a DNA pairing reaction greatly enhances the number of kanamycin-resistant bacterial cells arising from the repair activity in E.coli; secondly, the frequency of repair is elevated when the length of the oligonucleotides bound within the double-D-loop complex is increased; and thirdly, either oligonucleotide can serve as the molecule forming the mismatch with the target, but the highest level of correction is attained when both oligonucleotides form mismatches with both strands at the specific site.
The success of the gene repair reaction depends largely on the DNA pairing step, which, in our experimental protocol, is catalyzed by the action of a RecA protein prior to electroporation into E.coli. The reaction is also dependent on each oligonucleotide bearing sequence homology to the target sequence, and the dissociation of the preformed complex eliminates gene repair activity as measured by the appearance of kanr colonies. Preformation of this complex requires a strict order of addition in which the longer incoming oligonucleotide is first assembled with RecA protein in the presence of ATP-γS, followed by the addition of the shorter (annealing) oligonucleotide. These reaction conditions insure that RecA will not catalyze the reverse reaction and, as a side reaction, anneal the two juxtaposed oligonucleotides, leading to the disablement of the double-D-loop structure. We are aware, however, that once the complex enters the cell, there is still a possibility that the structure of the complex is modified in some fashion. But all of the controls presented argue against this notion and in favor of the hypothesis that the pre-pairing, preformation of double-D-loop complexes greatly enhances the frequency of gene repair. This conclusion is also supported by the fact that isolated/purified double-D-loops are as effective in producing corrected plasmids as double-D-loops that have not been purified.
The critical characteristic of the double-D-loop structure in promoting high levels of gene repair is most likely its inherent stability. This stability is enabled because both oligonucleotides are hybridized to their complementary strand at the target site. Previous data have shown that D-loops are kinetically stable in superhelical plasmid DNA, even after the removal of RecA proteins (17,19–21), and that this stability is due to the slow dissociation step once the joint is formed. In these studies, however, the incoming oligonucleotide is pre- assembled into a RecA filament, but our work establishes a more simple strategy for double-D-loop formation in which only the incoming molecule is pre-bound by RecA. Recent data from Parekh-Olmedo et al. (22) indicate that these stable double-D-loops, serving as intermediates in the gene repair reaction, produce a higher level of correction compared with correction levels attained using a single oligonucleotide.
Initialization of the gene repair reaction depends on DNA pairing. In this scenario, the oligonucleotides are positioned into homologous register with the target sequence with subsequent cellular activities leading to the exchange of a single nucleotide. Here we have emphasized the importance of the pairing step in achieving substantial levels of gene repair. And DNA pairing events that lead to the creation of a more stable joint molecule (here, the double-D-loop) can increase correction efficiency, as compared with TNE efficiencies obtained without a pre-pairing phase. Similar observations were made for single D-loop structures in the pioneering studies of Holloman and Radding (1) and Holloman et al. (7). This work was important not only for showing that genetic information can be transferred from single-stranded DNA to progeny, but also for establishing a transformation system in E.coli that simulated the early steps of recombination (7). We have used this system and extended their observations to implicate the double-D-loop as a putative intermediate in the gene repair reaction. The correction of the mismatched base pairs in E.coli was originally reported by Razin et al. (23), a reaction used extensively to biochemically define the components of the mismatch repair system (reviewed in 24).
The DNA mismatch repair pathway corrects aberrant base pairs created through the hybridization of the oligo(s) with the plasmid sequence (11,12,25). While our objective in the current study is not to elucidate more fully this pathway in the gene repair reaction, we are aware that the majority of correction events herein arise from a C·C base mismatch formed in the double-D-loop. And it is widely accepted that the C·C mismatch is corrected in the least efficient fashion, due perhaps to the structural configuration adopted by this specific base mispair (26–30). Currently, we are investigating the repair of other mismatched base pairs in order to establish a ‘base repair hierarchy’. But it is also important to note that the template upon which such repairs take place is dissimilar to the one used to establish the current hierarchy. In our case, the mismatch repair enzymes would encounter a double-D-loop configuration rather than a double helix containing a simple mismatch. The efficiency with which mismatches are corrected is believed to be determined by structural properties of individual mispairs, with those corrected most efficiently forming a structure that induces a rigid deformation of the helix. In contrast, those corrected more poorly deform the helix into a more dynamic state based on cooperative hydrogen binding and reduced interhelical stacking. The latter conformation enables a higher intrinsic flexibility that allows C/C mispairs, for example, to escape recognition by the mismatch repair enzymes (26,31). Evidence has been put forward indicating that double-D-loops containing heterologous inserts, perhaps a single mismatched base pair, adopt an ‘anti-rotational lock’ conformation (20). As such, there is a strong potential that a quadruplex evolves primarily through guanine base pairing, a structure will clearly produce a more rigid conformation, which in turn would obviate the accepted hierarchy. Future studies focused on the DNA conformation of the double-D-loop will hopefully shed more light on this possibility, but, regardless of its structural characteristics, we now establish the double-D-loop as an active template upon which gene repair can take place. Furthermore, the pathway taken to form this intermediate depends on DNA pairing activities.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to members of the Kmiec laboratory for comments during the course of the work. We acknowledge the continuing efforts of Ms Elizabeth Feather in manuscript preparation and Mr Eric Roberts in graphics. This work was supported by NIH grant RO1 DK56134 and NIH training grant T32 GM-08550.
REFERENCES
- 1.Holloman W.K. and Radding,C.M. (1976) Recombination promoted by superhelical DNA and the recA gene of Escherichia coli. Proc. Natl Acad. Sci. USA, 73, 3910–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotchkiss R.D. (1971) Toward a general theory of genetic recombination in DNA. Adv. Genet., 16, 325–348. [PubMed] [Google Scholar]
- 3.Hotchkiss R.D. (1974) Models of genetic recombination. Annu. Rev. Microbiol., 28, 445–468. [DOI] [PubMed] [Google Scholar]
- 4.Miller R.C. Jr (1975) Replication and molecular recombination of T-phage. Annu. Rev. Microbiol., 29, 355–376. [DOI] [PubMed] [Google Scholar]
- 5.Radding C.M. (1973) Molecular mechanisms in genetic recombination. Annu. Rev. Genet., 7, 87–111. [DOI] [PubMed] [Google Scholar]
- 6.Lacks S. (1966) Integration efficiency and genetic recombination in pneumococcal transformation. Genetics, 53, 207–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holloman W.K., Wiegand,R., Hoessli,C. and Radding,C.M. (1975) Uptake of homologous single-stranded fragments by superhelical DNA: a possible mechanism for initiation of genetic recombination. Proc. Natl Acad. Sci. USA, 72, 2394–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata T., Ohtani,T., Iwabuchi,M. and Ando,T. (1982) D-loop cycle. A circular reaction sequence which comprises formation and dissociation of D-loops and inactivation and reactivation of superhelical closed circular DNA promoted by recA protein of Escherichia coli. J. Biol. Chem., 257, 13981–13986. [PubMed] [Google Scholar]
- 9.Shibata T., Ohtani,T., Chang,P.K. and Ando,T. (1982) Role of superhelicity in homologous pairing of DNA molecules promoted by Escherichia coli recA protein. J. Biol. Chem., 257, 370–376. [PubMed] [Google Scholar]
- 10.Brachman E.E. and Kmiec,E.B. (2002) The ‘biased’ evolution of targeted gene repair. Curr. Opin. Mol. Ther., 4, 171–176. [PubMed] [Google Scholar]
- 11.Cole-Strauss A., Gamper,H., Holloman,W.K., Munoz,M., Cheng,N. and Kmiec,E.B. (1999) Targeted gene repair directed by the chimeric RNA/DNA oligonucleotide in a mammalian cell-free extract. Nucleic Acids Res., 27, 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice M.C., Bruner,M., Czymmek,K. and Kmiec,E.B. (2001) In vitro and in vivo nucleotide exchange directed by chimeric RNA/DNA oligonucleotides in Saccharomyces cerevisae. Mol. Microbiol., 40, 857–868. [DOI] [PubMed] [Google Scholar]
- 13.Liu L., Rice,M.C. and Kmiec,E.B. (2001) In vivo gene repair of point and frameshift mutations directed by chimeric RNA/DNA oligonucleotides and modified single-stranded oligonucleotides. Nucleic Acids Res., 29, 4238–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L., Cheng,S., van Brabant,A.J. and Kmiec,E.B. (2002) Rad51p and Rad54p, but not Rad52p, elevate gene repair in Saccharomyces cerevisiae directed by modified single-stranded oligonucleotide vectors. Nucleic Acids Res., 31, 2742–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L., Rice,M.C., Drury,M., Cheng,S., Gamper,H. and Kmiec,E.B. (2002) Strand bias in targeted gene repair is influenced by transcriptional activity. Mol. Cell. Biol., 22, 3852–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parekh-Olmedo H., Krainc,D. and Kmiec,E.B. (2002) Targeted gene repair and its application to neurodegenerative disorders. Neuron, 33, 495–498. [DOI] [PubMed] [Google Scholar]
- 17.Sena E.P. and Zarling,D.A. (1993) Targeting in linear DNA duplexes with two complementary probe strands for hybrid stability. Nature Genet., 3, 365–372. [DOI] [PubMed] [Google Scholar]
- 18.Gamper H.B., Parekh,H., Rice,M.C., Bruner,M., Youkey,H. and Kmiec,E.B. (2000) The DNA strand of chimeric RNA/DNA oligonucleotides can direct gene repair/conversion activity in mammalian and plant cell-free extracts. Nucleic Acids Res., 28, 4332–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayasena V.K. and Johnston,B.H. (1993) Complement-stabilized D-loop. RecA-catalyzed stable pairing of linear DNA molecules at internal sites. J. Mol. Biol., 230, 1015–1024. [DOI] [PubMed] [Google Scholar]
- 20.Belotserkovskii B.P., Reddy,G. and Zarling,D.A. (1999) DNA hybrids stabilized by heterologies. Biochemistry, 38, 10785–10792. [DOI] [PubMed] [Google Scholar]
- 21.Belotserkovskii B.P. and Zarling,D.A. (2002) Peptide nucleic acid (PNA) facilitates multistranded hybrid formation between linear double-stranded DNA targets and RecA protein-coated complementary single-stranded DNA probes. Biochemistry, 41, 3686–3692. [DOI] [PubMed] [Google Scholar]
- 22.Parekh-Olmedo H., Drury,M. and Kmiec,E.B. (2002) Targeted nucleotide exchange in Saccharomyces cerevisiae directed by short oligonucleotides containing locked nucleic acids. Chem. Biol., 9, 1073–1084. [DOI] [PubMed] [Google Scholar]
- 23.Razin A., Hirose,T., Itakura,K. and Riggs,A.D. (1978) Efficient correction of a mutation by use of chemically synthesized DNA. Proc. Natl Acad. Sci. USA, 75, 4268–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marra G. and Schar,P. (1999) Recognition of DNA alterations by the mismatch repair system. Biochem. J., 338 (Pt 1), 1–13. [PMC free article] [PubMed] [Google Scholar]
- 25.Rice M.C., May,G.D., Kipp,P.B., Parekh,H. and Kmiec,E.B. (2000) Genetic repair of mutations in plant cell-free extracts directed by specific chimeric oligonucleotides. Plant Physiol., 123, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter W.N., Brown,T., Anand,N.N. and Kennard,O. (1986) Structure of an adenine-cytosine base pair in DNA and its implications for mismatch repair. Nature, 320, 552–555. [DOI] [PubMed] [Google Scholar]
- 27.Cornelis A.G., Haasnoot,J.H., den Hartog,J.F., de Rooij,M., van Boom,J.H. and Cornelis,A. (1979) Local destabilisation of a DNA double helix by a T–T wobble pair. Nature, 281, 235–236. [DOI] [PubMed] [Google Scholar]
- 28.Ho P.S., Frederick,C.A., Quigley,G.J., van der Marel,G.A., van Boom,J.H., Wang,A.H. and Rich,A. (1985) G.T wobble base-pairing in Z-DNA at 1.0 Å atomic resolution: the crystal structure of d(CGCGTG). EMBO J., 4, 3617–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown T., Hunter,W.N., Kneale,G. and Kennard,O. (1986) Molecular structure of the G.A base pair in DNA and its implications for the mechanism of transversion mutations. Proc. Natl Acad. Sci. USA, 83, 2402–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hare D., Shapiro,L. and Patel,D.J. (1986) Extrahelical adenosine stacks into right-handed DNA: solution conformation of the d(C-G- C-A-G-A-G-C-T-C-G-C-G) duplex deduced from distance geometry analysis of nuclear Overhauser effect spectra. Biochemistry, 25, 7456–7464. [DOI] [PubMed] [Google Scholar]
- 31.Werntges H., Steger,G., Riesner,D. and Fritz,H.J. (1986) Mismatches in DNA double strands: thermodynamic parameters and their correlation to repair efficiencies. Nucleic Acids Res., 14, 3773–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]