Abstract
We report the first experimental probing of electrostatic interactions on the pyrimidine side of a bent A tract. Although the curvature of short A tracts (A4–A6) has long been studied, its physical origins remain under debate. Current hypotheses include the influence of major-groove hydrogen bonds between propeller-twisted base pairs, electrostatic effects of closely associated minor-groove cations, effects of minor-groove solvation, and stacking effects at the junctions adjacent to the A tract. We investigated this problem through the substitution of thymidines in A5 tracts by difluorotoluene deoxynucleoside (F), a non-polar molecule of the same size and shape which lacks hydrogen bonding and metal-ion complexing capabilities. Ligation experiments with phased A tracts demonstrated that F substitution has asymmetric effects on the bend angle. The strongest effects occurred at the second and third thymines where curvature was reduced from 19.8° to 5.3° and 9.6°, respectively. Moderate effects were observed with substitutions at positions 1 and 4, while substitution at position 5 had no effect on bend angle. The results support the hypothesis that highly localized electrostatic interactions are a principal cause of A-tract curvature. Furthermore, they are most consistent with the notion that local metal-ion complexation at O2 of thymine is a strong component of these interactions.
INTRODUCTION
Tracts of four to six adenines (A tracts), repeated in phase with the helical repeat of B-DNA, are known to cause curvature of the DNA helix toward the minor groove (1). A tracts exhibit other distinct features such as narrowing of the minor groove toward the 3′ end, propeller twisting of the A–T base pairs and bifurcated hydrogen bonds between adjacent base pairs (2–4). The molecular basis for the curvature exhibited by A tracts is still under debate, two decades after the first two models attempting to explain A-tract bending were proposed.
The first of these models, the wedge model proposed by Trifonov and Sussman, suggests that roll angles in ApA steps cause these adjacent base pairs to be non-parallel (5). Each non-parallel ApA step effectively forms a wedge that can curve the helix axis if they occur in DNA at regular intervals (i.e. spaced with the helical repeat) such that the wedges add constructively. The second model, the junction model put forward by Crothers and co-workers, proposes that curvature occurs as a result of an optimization of stacking where non-A-tract DNA meets A-tract DNA (6). Base pairs in an A tract are highly inclined; thus, parallel stacking of all the bases drives the non-A-tract DNA helix axis to form an angle with the A-tract DNA helix axis.
Both of these early models rely on sequence-dependent base–base interactions as the cause of A-tract curvature (7). Williams and co-workers recently proposed an alternative hybrid-solvent model which focuses on interactions between DNA and its environment. In this hypothesis, electrostatic interactions between DNA and the solution cause bending (8,9). More specifically, the model suggests that cations can partition into the minor groove spine of hydration and disperse around DNA in an asymmetric fashion depending on the DNA sequence. Cation organization is dependent on DNA sequence because localization depends on electrostatic interactions with the functional groups of the DNA bases and backbone. Localization in or around the DNA can cause phosphate neutralization. If this localization is biased toward one face of the DNA, phosphate neutralization can result in an asymmetric force on the DNA which in turn can cause narrowing of the minor groove and bending of the helical axis.
Recently, crystallographic, NMR and theoretical evidence have begun to accumulate in favor of the hybrid-solvent model. Williams and co-workers demonstrated, through separate x-ray crystal structures, localization of Na+, K+, Cs+, Mg2+ and Tl+ in the A-tract minor groove of the Dickerson dodecamer as well as narrowing of the minor groove width in response to cation binding (8–12). Rb+ was also observed in the A-tract minor groove of the Dickerson dodecamer in a separate x-ray crystal structure by Egli and co-workers (13). Using NMR and A2–5 tracts, Hud and Feigon demonstrated localization of monovalent and divalent cations in the minor groove, which they propose as the cause of axial bending (14–16). Molecular dynamics simulations by Beveridge and co-workers and by Wilson and co-workers supported these findings with a demonstration of A-tract minor groove narrowing and bending as a result of cation partitioning into the A-tract minor groove (17–20). Maher and co-workers further supported the hybrid-solvent model’s phosphate neutralization mechanism with a demonstration of DNA bending as a result of the incorporation of neutral phosphate analogs and cationic analogs (21–24).
A number of previous studies into the origins of A-tract curvature have made molecular substitutions to test the influence of specific atoms and groups on A-tract structure. Diekmann and McLaughlin substituted inosine–cytosine (I–C) for A–T pairs in order to disrupt bifurcated hydrogen bonds. They found that the effects were generally small, consistent with other localized minor groove effects being responsible for bending and not bifurcated hydrogen bonds (25). Seela and Grein presented a study of substitutions on the purine side of A5 and A6 tracts, replacing adenine with analogs lacking minor groove (N3) or major groove (N7) nitrogens (26). Interestingly, both were found to be important in curvature. In the minor groove, removal of N3 at positions 4–6 in an A6 tract was found to abolish most of the bend, whereas removal at positions 1–3 had little effect. Significantly, such previous molecular replacement studies have probed interactions on the purine side of the A tract but have largely ignored the pyrimidine half. If localized interactions in the minor groove including solvation and cation binding are an important causative factor of curved DNA, then it seems quite possible that thymine O2 might play a central role. Thymine O2 has greater negative charge density than adenine N3, and as such it forms stronger hydrogen bonds to water and is likely to have greater affinity for most cations as well. Moreover, the recent experiments of Williams, Feigon and Beveridge have all pointed to the central role of thymine O2 in cation localization in the minor groove.
Here we report on the effects of substitution of thymines in an A5 tract by 2,4-difluorotoluene deoxynucleoside (F). This analog is a nearly perfect shape mimic (isostere) of thymidine but has fluorine in place of the carbonyls at positions 2 and 4 (Fig. 1). Thus, it lacks the hydrogen bonding and metal-ion-complexation ability of thymidine. To measure the effects on duplex DNA curvature, we applied the gel mobility methods of Maher and co-workers (27). This refined method takes advantage of the well known observation that curved DNAs exhibit unusually slow migration in native gel electrophoresis. Using this approach, we found that substitution of certain thymines in an A tract causes a significant decrease in curvature. The results are consistent with localized electrostatic effects at thymine, such as minor groove solvation and cation localization, being primary causes of A-tract curvature.
Figure 1.

Structures and space-filling models of thymine and the non-polar shape mimic difluorotoluene. Calculated electrostatic potentials are mapped on the surfaces; red indicates negative potential and blue indicates positive.
MATERIALS AND METHODS
Background
The method of Maher and co-workers was applied to quantify DNA curvature (27). In brief, a duplex context representing two helical turns and containing two A tracts is used. One A tract serves as an internal reference with a known bend angle and direction while the other serves as a test A tract. In this test A tract, modifications can be made to evaluate their effects on bend angle and direction relative to the reference tract. It is in this test A tract that F was substituted for T at each possible position. In addition to A tracts, all duplexes have 3′ extensions that permit ligation to occur only at the appropriate end of another duplex. Upon ligation, the number of A tracts repeated in phase with the helical repeat of the duplex increases. The longer duplexes have a greater bend and larger mobility anomaly. It is this relationship that is exploited to calculate the bend angle and apparent orientation.
For every F substitution, three duplexes (cis, ortho and trans) were made to determine the direction of the bend. The three differ by the relative phasing of their A tracts. In the cis case, the A tracts are separated by one complete turn of the helix such that they align on the same face of the helix and result in the greatest net curvature and largest gel mobility anomaly. In contrast, in the trans duplex, the A tracts are separated by a half turn of the helix. This produces two bends in opposing directions and a duplex with little to no net curvature or mobility anomaly. Finally, in the ortho case, the A tracts are separated by about a three-quarter turn; thus, the bend sites are offset and not completely additive. These different phasings are necessary to determine the direction of the net bend angle since net bend angle depends on both the location and the direction of the individual bends (27).
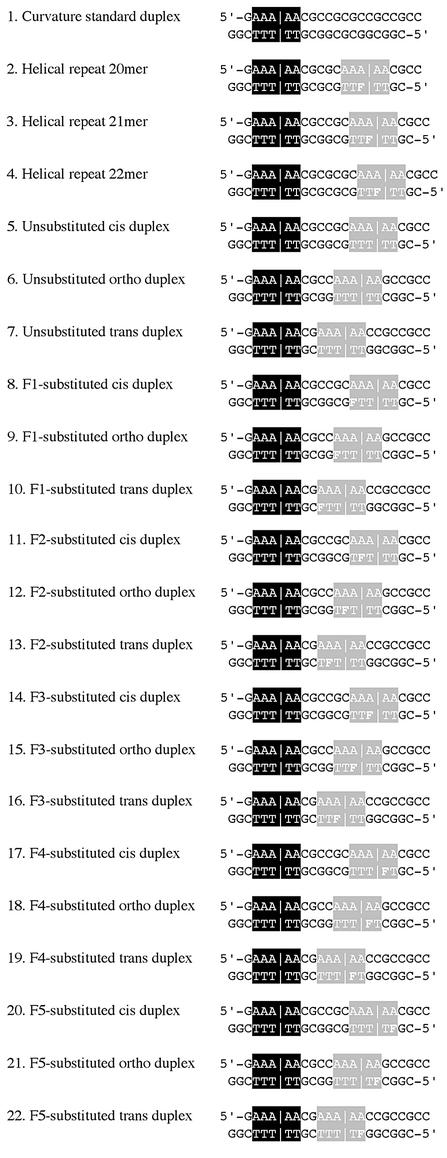
In addition to the F-substituted A-tract duplexes, a series of unsubstituted A-tract sequences, helical repeat duplexes and an external reference (or curvature standard) containing one A tract were also made. The unsubstituted A-tract duplexes were made to ensure that the published methods were reproducible in our hands. The helical repeat DNAs consisted of three duplexes of 20, 21 and 22 bp monosubstituted with F. Substitution was made at the middle thymine position of the test A tract. These DNAs were used to determine the helical repeat of the DNA when monosubstituted with F. The curvature standard was simply a 21-bp duplex with one A tract, which acted as an external control for the effect of bending on gel mobility.
Preparation of oligonucleotides
Oligodeoxyribonucleotides were synthesized on an ABI 392 synthesizer using standard β-cyanoethylphosphoramidite chemistry. F (Fig. 1; Glen Research) was coupled under these same conditions. F substitutions were made individually into each of the thymine positions of the test A tract shown in gray (Fig. 2). Oligonucleotides were deprotected in ammonium hydroxide at 55°C for 16 h. The deprotected oligonucleotides were purified by denaturing 20% polyacrylamide gel electrophoresis (PAGE) and excised from the gel. DNA was extracted from the polyacrylamide by shaking overnight with 0.2 M NaCl. The extracts were filtered then dialyzed against distilled water. Oligonucleotide concentrations were determined using their absorbance at 260 nm and their calculated molar extinction coefficients.
Figure 2.
DNA duplexes used in this study. The sequence in the black box represents the internal reference A tract, and the lines indicate the A tract center of curvature. The sequence in gray represents the test A tract, and the lines indicate the assigned center of curvature.
Ligation ladders
Purified oligonucleotide (400 pmol) was labeled at the 5′ end using 10 U of T4 polynucleotide kinase (New England Biolabs) and 10 µCi [γ-32P]ATP (5000 Ci/mmol, Amersham Biosciences) in a total volume of 10 µl. The reactions were incubated at 37°C for 45 min at which point 1 µl of 100 mM dATP (Invitrogen) was added to each reaction and incubated for an additional 20 min. Labeled top strand (400 pmol) was combined with labeled complementary bottom strand (400 pmol). The oligonucleotides were annealed by first denaturing at 80°C for 10 min then cooling to 4°C over 1.5 h. Annealed duplex (200 pmol) was ligated using 800 U of T4 DNA ligase (New England Biolabs) in a total volume of 20 µl. The reactions were incubated at 16°C for 1 h then stopped by the addition of 1 µl of 100 mM EDTA.
Exonuclease digestion
To distinguish between linear and circular products, samples of the ligation reactions were digested with Bal31. This exonuclease degrades linear but not circular products. The digestions were carried out with 8 µl of ligation reaction, 1 U of Bal31 (New England Biolabs), and the buffer supplied with the enzyme in a total volume of 18 µl. The reactions were incubated at 30°C for 30 min and terminated by the addition of 0.7 µl of 0.5 M ethylene glycol bis(2-aminoethyl ether) tetraacetic acid (EGTA; to chelate the enzymatic cofactor Ca2+).
Gel electrophoresis
Ligation ladder analysis was carried out by separating the DNA duplexes on 5% native polyacrylamide gels (29:1 acrylamide:bisacrylamide) using 1× Tris–boric acid–EDTA (TBE) buffer, pH 8. The gels were run at 500 V for 3 h. One series of ligation ladders, the corresponding Bal31 digests, curvature standard and a molecular weight marker were analyzed on each gel. Samples of the appropriate digests were analyzed with their corresponding ligation ladders in order to identify and measure only the linear bands in the ligation ladder lanes. The molecular weight marker is critical to establish the relationship between duplex length and gel mobility for normal (i.e. unbent) DNA. This marker was prepared by 5′-end labeling a 100-bp ladder (Invitrogen) using 10 U of T4 polynucleotide kinase (New England Biolabs) and 30 µCi [γ-32P]ATP (5000 Ci/mmol, Amersham Biosciences) in exchange reaction buffer (Invitrogen).
Gel analysis
The gels were visualized by autoradiography. Data analysis, curve fitting and calculations were carried out as done previously by Maher and co-workers using KaleidaGraph™ software (27). The method will be briefly outlined here; further details are given in Ross et al. (27). Using the autoradiograms, the distances migrated by the DNA duplexes were measured manually. Using the bands from DNA marker lane, a calibration curve was created by plotting migration distance (x) as a function of DNA length (L). The data were fit to the following exponential equation by a least-squares method:
L = αe(βx)1
From the curve fit results, values for α and β were determined. These parameters were substituted back into the equation and used to calculate the apparent lengths (Lapp) of the bands from 42 to 189 bp in all the ligation ladders (helical repeat, unsubstituted and F-substituted duplexes) using their migration distances. With the apparent and actual DNA lengths (Lact), the relative mobilities of the duplexes were calculated as follows:
RL = Lapp / Lact2
Using the relative mobilities of the helical repeat DNAs, the data for the band corresponding to seven ligated duplexes (i.e. 147-bp band) for each of the cis, ortho and trans duplexes were plotted as a function of Lduplex / 2. This latter value is 10, 10.5 and 11 for the 20, 21 and 22-bp duplexes, respectively. The data for the bands corresponding to eight and nine ligated duplexes were plotted in the same fashion to generate a total of three parabolic curves. These curves were fit to the following parabolic equation:
RL = i(Lduplex / 2)2 + j(Lduplex / 2) + k3
The curve fit data provide values for i, j and k. The helical repeat was determined using this data and the following equation:
h = –j / 2i4
One value of h was obtained from each curve and used to calculate an average value. The helical repeat is expected to be about 10.5, meaning that a 21-bp duplex (as employed here) corresponds to two turns of the helix. Using the F3-substituted helical repeat DNAs, the helical repeat was found to be 10.6. Thus, 21-bp duplexes were used for the rest of the experiments.
With the relative mobilities for the curvature standard, and the normal and F-substituted A-tract duplexes, the data were transformed by subtracting 1 from the relative mobility and squaring the value of the actual length. (RL – 1) was plotted as a function of Lact2. The data from the curvature standard from every gel (Fig. 2, duplex 1) were each fit by a least-squares method to the following equation:
RL – 1 = (pLact2 – q)(r2)5
where r is equal to 0.5 and the initial values for p and q were set to 9.6 × 10–5 and 0.47 respectively after Maher’s method. The value of r represents the number of A5 tracts per helical turn. The curvature standard has one A5 tract and represents two helical turns thus r = 0.5. Actual values for p and q were obtained from the curve fit. The plotted data for the bands from 105 to 189 bp for the ligation ladders run on the same gel as a given curvature standard were then fit by a least-squares method to equation 5 with the actual values of p and q from the curvature standard curve-fit data. The initial estimate for the value of r was set to 0.5. Actual values of r are obtained from these latter curve fits and used to calculate the curvature of each of the cis, ortho and trans duplexes in degrees (r′) using the following equation:
r′ = 36r6
The rotary angle (θ) is the angle between the reference A tract and the test A tract centers. For the cis duplex, the rotary angle was set to zero. For the ortho and trans duplexes, the rotary angle was determined by counting the number of base pairs between the assigned center of the test A tract (0.5 bp 3′ of the center A) and the center of the reference A tract (0.5 bp 3′ of the center A) and multiplying this number by 360° / h where h is the helical repeat. Curvature (r′) was plotted as a function of rotary angle and fit by a least-squares method to the cosine function:
r′ = a + bcos(θ – c)7
where initial guesses for a, b and c were set to 25.88, –11.16 and 202.63 as taken from Ross et al. (27). If the value of b from the curve fit data was less than zero, 180° was subtracted from the initial guess for c to make b positive. The curvature of the test A tract in degrees (t°) was finally calculated using the data from the cosine plot curve fit and the following equation:
t° = a + b – 18°8
The parameter c was used in subsequent calculations of the test A tract bend direction. It was first converted into base pair units through the calculation of s using the following equation:
s = c · h / 3609
where h is the helical repeat. The value s represents the distance (in bp) closer to the reference A tract that the test A tract would have to be (relative to the cis duplex) in order to have true cis phasing. If s is >5 bp, h – s gives the distance (in bp) further from the reference A tract that the test A tract would have to be to obtain true cis phasing. Using s, a measure of the difference between the assigned and actual bend centers in the test A tract was obtained by calculating the spacing coefficient (D) as follows:
D = h – x + s10
where h is the helical repeat and x is the number of base pairs between the center of curvature in the reference A tract and the assigned center of the test A tract for the cis duplex (i.e. x = 11). If D is greater than zero, then the assigned center of the test A tract should be moved closer to the reference A tract by D base pairs in order for the test A tract curvature to be toward the minor groove. Similarly, if D is less than zero, the assigned center of the test A tract should be moved further from the reference A tract by D base pairs.
RESULTS AND DISCUSSION
We chose the difluorotoluene nucleoside as the only available non-hydrogen-bonding analog of thymidine that does not alter size and shape. A different analog of thymidine has been used to probe minor groove interactions in non-A-tract contexts and reported previously (28,29). However, that analog lacked the 2-keto group. A functional group deletion such as this may perturb minor groove width or other steric effects possibly related to bending, and thus cannot serve as an optimal probe of the role of the 2-keto group alone. Therefore, the F analog presents a unique opportunity to probe the pyrimidine half of curved A tracts. This analog was chosen over one with only the minor groove O2 replaced by fluorine because of the expected low chemical stability of that structure.
To measure bending parameters, we adopted Maher’s refinement of the Crothers ligation ladder method, which makes use of the fact that progressively larger ligated multimers containing A tracts phased with the helical repeat exhibit increasing gel mobility anomalies (27,30). The method shows high precision and accuracy in the measurement of bend angle and position.
The curvature analysis relies on an accurate helical repeat parameter for the sequences under study. The helical repeat for the unsubstituted A5 tract was reported as 10.4 bp/helical turn by Maher and co-workers, but this value could not be assumed to be accurate for an F-substituted A tract. A previous structural study of a non-A-tract duplex containing F opposite A concluded that F causes no measurable local or global changes relative to T (31). However, we felt it prudent to evaluate the helical repeat explicitly for an F-containing sequence in the present context. We selected a central F substitution for our helical repeat DNAs, and put F in position 3 of the T5 tract (one of the more perturbing cases for DNA bending). (Note that our numbering takes on that of the A5 strand; for example, T4 is opposite A4.) The helical repeat measurement data are shown in Supplementary Material. We found a helical repeat of 10.6 bp/helical turn for this particular sequence. This confirms the notion that F is not inherently structurally perturbing relative to T and adds confidence in the bending data derived from the mobilities of phased ligation ladders containing F.
We carried out analyses of the five monosubstituted A tracts as well as the unsubstituted A-tract control. In all, 18 duplexes were studied and included in the analysis. Each ligation ladder analysis was repeated at least three times to allow for estimates of error. The experiments showed clearly that there were differences among the different F-substituted A tracts. The F5-substituted duplex showed no effect, displaying a migration anomaly similar to the unsubstituted A tract. In contrast, F substitution increased the mobility of the duplexes when put into positions 1 through 4, and substitution at position 2 resulted in the least retardation among the F-substituted duplexes (Table 1 and Fig. 3).
Table 1. DNA bend angles and bend positions for A tracts containing single F-for-T substitutions.
| Substitution | Bend angle (°)a | Bend position |
|---|---|---|
| None | 19.8 ± 1.3 | 0.20 ± 0.033 |
| F1 | 12.7 ± 0.2 | 1.7 ± 0.021 |
| F2 | 5.3 ± 0.2 | –1.1 ± 0.21 |
| F3 | 9.6 ± 0.5 | –1.7 ± 0.094 |
| F4 | 13.8 ± 1.1 | –0.91 ± 0.16 |
| F5 | 17.9 ± 1.8 | –0.21 ± 0.028 |
aAverage value ± standard deviation based on three separate experiments.
Figure 3.

Autoradiograms of representative ligation ladders for (A) F5- and (B) F2-substituted A tracts. M indicates the 100-bp DNA marker. Duplex number refers to the numbers given to the duplexes in Figure 2. Lanes 2–6 represent ligation ladders without Bal31 digestion while lanes 7–10 represent the same ligation ladders subjected to Bal31 digestion. The arrows point to the ligated nonamers as a point of reference to illustrate the relative migration anomalies.
The migration anomaly was quantified by the ratio of the apparent length of the band in base pairs to the actual length, a value termed the relative mobility (RL). The differences in gel mobility became readily apparent when the RL values for the cis duplexes were plotted against the actual lengths of the DNA (Fig. 4). F substitution clearly has effects on gel mobility and thus bending. The effects depend on the location of F in the T tract and vary in magnitude with substitution position.
Figure 4.
Relative mobilities of the cis duplexes in the range of 42–189 bp. Unsubstituted A tract (triangles) and F substitution at T1 (diamonds), T2 (open circles), T3 (crosses), T4 (squares) and T5 (filled circles).
Parameters calculated for the five F-substituted sequences are summarized in Table 1. We determined a bend angle of 19.8 ± 1.3° for the unsubstituted A tract. This is consistent with Maher’s value of 18.9° (27) and Crothers’ value of 18 ± 3° (30). The effect of F substitution on the A-tract bend angle was significant at some positions while negligible in others (Table 1). Substitution at thymines 1 and 4 in the test A tract moderately reduced curvature but had no significant effect at thymine 5. Substitution effects at thymine 2 and 3, however, were much more pronounced with substitution at position 2 having the strongest influence. At that position, nearly all of the observed curvature was lost. Overall, the effect of substitution was asymmetric and most pronounced around bases 2 and 3.
From the reference point 0.5 bp 3′ of the center of the reference A tract, one can compare the position and orientation of the bend in the test A tract. Parameter D provides a measure of the difference (in bp) between the test A tract’s assigned center of bending and its actual center. We found that the unsubstituted A-tract duplex had a negligible D value of 0.20, consistent with the known minor groove direction of A-tract curvature and confirming the validity of the method in our hands. The data for the F-substituted A-tract duplexes show that they all have bend centers within 1.7 bp of the assigned center indicating that they are clearly curved toward the minor groove as well. The positions where F substitution had the smallest influence on bend angle (F4 and F5) have bend centers similar to the unsubstituted tract (within 1 bp). The other three cases (F1, F2 and F3) show centers of curvature that are between 1 and 2 bp away from the assigned bend center. This indicates that either the center of curvature is indeed shifted in these cases or that the orientation of the bend is different by up to ±58° from perfect cis orientation (relative to the reference tract). The fact that D was positive (i.e. an apparent shift toward the reference tract) for the F1 case is somewhat surprising because this substitution leaves a full A4 tract that has a center shifted away from the reference. This suggests that the orientation (rather than bend center) may have been shifted by F substitution. The other two cases (F2 and F3) by contrast have moderate negative shifts in bend position.
The consequence of thymine replacement with F is the exchange of the major and minor groove carbonyls (C4 and C2 respectively) for fluorine atoms—the exchange of a highly polar group for a non-polar group of the same size. These carbonyls may be involved in several properties of A tracts: major-groove bifurcated hydrogen bonds, minor-groove spine of hydration (32) and minor-groove cation coordination (8,9). A brief analysis (below) suggests that the consequences of F substitution may be perturbation of more than one of these properties.
If DNA bending at A tracts were caused completely by localized effects (such as a single metal ion binding or an unusual stacking phenomenon at a junction), then A tracts would not become successively more curved as they increase in length from 3 to 6 bp. Moreover, substitution with analogs like F would not have substantial effects over nearly the whole length of the tract. Thus, it would appear that part of the physical cause of A-tract curvature is a cumulative, cooperative or delocalized effect.
If, however, the cause of the bending were entirely a delocalized, cumulative phenomenon requiring only a certain number of consecutive A–T base pairs, then substitution with analog F would have predictable effects. F at positions 1 and 5 would leave an A4 tract with much of the bend left intact. F at positions 2 or 4 would leave A3 tracts with only a moderate bend. Finally, F at position 3 would leave only two A2 tracts with most of the bend lost. Clearly, some aspects of the data from F substitution are not consistent with this. For example, F5 had no effect whereas F1 had a quite significant effect. Similarly, F2 substitution had a much stronger effect than F4. Finally, F2 had a larger effect than F3. Thus, much of the effect of F substitution is to disrupt a localized interaction with especially strong relative disruptions at positions 2 and 3 on the pyrimidine side of the tract. Overall, we conclude that both a cumulative (delocalized) effect and a localized electrostatic effect appear to be disrupted by F substitution.
The presence of bifurcated hydrogen bonds in the major groove between successive A–T base pairs has been suggested as a possible causative factor in A-tract bending, and these bonds have been observed experimentally in solution using NMR (4,33). Bifurcated hydrogen bonds were associated with the large propeller twist of base pairs in A tracts and located between adenine N6 in the n position and O4 of the thymine at n + 1. Thus, T2 would form an inter-base-pair hydrogen bond with A1 according to our numbering scheme. Because of the directionality of this interaction, the thymine at the 5′ end of the A tract (T1) is not predicted to be involved in a bifurcated hydrogen bond whereas T2–T5 are. If bifurcated hydrogen bonds were a chief cause of DNA curvature, we would then expect to see an effect on curvature from F-for-T replacement at position 5, but this was not observed. Similarly, one would expect no effect at T1, but instead we observed a relatively strong effect. Therefore, our results are not consistent with bifurcated hydrogen bond disruption as the cause of decreased curvature. Bifurcated hydrogen bonds may simply be a result of the large propeller twist inherent to A tracts. Previous inosine substitution experiments have also concluded that bifurcated hydrogen bonds are not essential for DNA curvature (25,34).
Chuprina has suggested that the minor groove spine of hydration is a stabilizing factor in curved A tracts (35). Within the spine of hydration, the interaction with water is maintained by thymine O2 and adenine N3. A study by Lan and McLaughlin in another sequence context suggested that the chain of waters is energetically cooperative in stabilizing duplexes (28,29). Replacement of this oxygen with fluorine would disrupt this hydrogen bond interaction. We hypothesize that part of the general effect of F substitution may be due to this disruption. However, the strongly localized effects we observed toward the 5′ end of the A tract (positions 2 and 3) would be difficult to explain in terms of a spine of solvation alone. Similarly, Seela and Grein also observed such localized effects on the purine side of an A tract using adenine analogs lacking N3 (26). Thus, we believe that F substitution does have a general effect on solvation and thus on bending, but disruption of solvation cannot account for all of our results.
Next, we consider the effect of F substitution on the possible coordination of cations in the minor groove. Experiments by Maher and co-workers have shown convincingly that cations associated with the minor groove of DNA can result in bending toward that groove (22,24). It is possible then that if cations were to associate closely with the minor groove of A tracts in particular, they would induce a bend that is consistent with that observed with A tracts. Recently, investigators have obtained experimental evidence of cation coordination in the A-tract minor groove and minor groove narrowing in response to cation binding (8–19). In solution-phase experiments, Hud and Feigon demonstrated localization of cations at specific locations in the A-tract minor groove and suggested that thymine O2 has a role in cation coordination. The data showed Mn2+ localized at the first two 5′ adenines of an A5 tract while in separate experiments, ammonium was localized to the third and fourth adenines in an A5 tract. There were no Mn2+ or ammonium cations in our experiments, but our samples did contain Na+ (11 mM) and Mg2+ (7.5 mM) from the reaction buffers used prior to gel analysis. Perhaps more importantly, the gel running buffer contained 3.14 mM Na+ and 89 mM Tris (of which roughly half was in cationic form). The highly localized effects on bending seen in our experiments are consistent with the specific localization of cations seen by Hud and Feigon. Cation binding centered around T2 would explain why bending was greatly reduced at this position, moderately at the adjacent bases, and not at all at the base most removed (T5) when F was substituted into the tract. Finally, cation localization might be expected to be associated with narrowing of the groove, an important structural feature of A tracts (2,8–12,17–20). However, without more detailed structural studies, it is not yet clear what effects (direct or indirect) F substitutions might have on groove width.
If minor groove cation localization causes A-tract curvature, we conclude that T1, T2 and T3 play a role in cation binding under the conditions used in this study. Thus, our results implicate possible cation sites in the first half of the tract. Interestingly, in earlier studies of N3-deaza adenine substitutions in A6 tracts, Seela and Grein noted strong effects in the last half of the tract (A4–A6). Since monosubstitutions of the deaza-A analogs were not carried out in that study, it is not yet possible to comment on the site-specific effects of nitrogen loss. At first glance, it would appear that our results are at odds with those of Seela and Grein since the effects appear at opposite ends of the sequence. However, further discussion may help explain this. First, the right-handed twist of the helix brings 5′ pyrimidines closer in space to purines located at the 3′ end of such a tract. Second, bending toward the minor groove may bring these groups even closer to one another. Thus, the first thymine O2 may be closer to the N3 of the last adenine in an A5 tract than in normal unbent DNA. It then seems possible that effects at opposing ends of an A tract may not yet be ruled out. Further studies using single N3-deaza adenine substitutions in an A5 tract would be useful to clarify this. It may also be of interest to test multiple substitutions of such analogs to probe for cooperative interactions.
The idea that cations can cause DNA curvature is not new and has even been observed by electron microscopy (36). Curvature of DNA containing A tracts in solution has also been shown to increase with higher concentrations of Zn2+, Co2+, Ba2+ and Mn2+ (36). More recently, cation-induced DNA bending has been discussed extensively by Williams and Feigon and co-workers (7,9,15,37). Very high-resolution crystal structures of the Dickerson dodecamer by Williams and co-workers have identified sites in the minor groove spine of hydration where metal ions have replaced water molecules, and thymine O2 groups are involved in the interaction with these cations. However, no such high-resolution x-ray data are available for longer curved A tracts.
Lastly, Crother’s junction model proposes that the strong stacking ability of adenine at the junction between A-tract and normal DNA may contribute to A-tract curvature (25,34). Although our data have no direct bearing on the purine side of the duplex, we can deduce from our results that stacking on the T side of the tract may not be an important contributor, at least at the 3′ end of the tract. The difluorotoluene analog stacks much more strongly than thymine (38), yet we observe that it has no effect on curvature at the 3′ junction.
SUPPLEMENTARY MATERIAL
Images of PAGE analyses for all unsubstituted and F-substituted ligation ladders, plots for helical repeat and curvature analyses are available as Supplementary Material at NAR Online.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr L. J. Maher for helpful discussions. This work was supported by the National Institutes of Health (GM52956). A.M. acknowledges support from a Boehringer Ingelheim Pharmaceuticals graduate fellowship.
REFERENCES
- 1.Koo H.-S., Wu,H.-M. and Crothers,D.M. (1986) DNA bending at adenine-thymine tracts. Nature, 320, 501–506. [DOI] [PubMed] [Google Scholar]
- 2.Burkhoff A.M. and Tullius,T.D. (1987) The unusual conformation adopted by the adenine tracts in kinetoplast DNA. Cell, 48, 935–943. [DOI] [PubMed] [Google Scholar]
- 3.Nelson H.C.M., Finch,J.T., Bonaventura,F.L. and Klug,A. (1987) The structure of an oligo(dA)-oligo(dT) tract and its biological implications. Nature, 330, 221–226. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald D., Herbert,K., Zhang,X., Polgruto,T. and Lu,P. (2001) Solution structure of an A-tract bend. J. Mol. Biol., 306, 1081–1098. [DOI] [PubMed] [Google Scholar]
- 5.Trifonov E.N. and Sussman,J.L. (1980) The pitch of chromatin DNA is reflected in its nucleotide sequence. Proc. Natl Acad. Sci. USA, 77, 3816–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H.-M. and Crothers,D.M. (1984) The locus of sequence-directed and protein-induced DNA bending. Nature, 308, 509–513. [DOI] [PubMed] [Google Scholar]
- 7.Williams L.D. and Maher,L.J. (2000) Electrostatic mechanisms of DNA deformation. Annu. Rev. Biophys Biomol. Struct., 29, 497–521. [DOI] [PubMed] [Google Scholar]
- 8.Shui X., McFail-Isom,L., Hu,G.G. and Williams,L.D. (1998) The B-DNA dodecamer at high resolution reveals a spine of water on sodium. Biochemistry, 37, 8341–8355. [DOI] [PubMed] [Google Scholar]
- 9.Shui X., Sines,C.C., McFail-Isom,L., VanDerveer,D. and Williams,L.D. (1998) Structure of the potassium form of CGCGAATTCGCG: DNA deformation by electrostatic collapse around inorganic cations. Biochemistry, 37, 16877–16887. [DOI] [PubMed] [Google Scholar]
- 10.Kruger Woods K., McFail-Isom,L., Sines,C.C., Howerton,S.B., Stephens,R.K. and Williams,L.D. (2000) Monovalent cations sequester within the A-tract minor groove of [d(CGCGAATTCGCG)]2. J. Am. Chem. Soc., 122, 1546–1547. [Google Scholar]
- 11.Sines C.C., McFail-Isom,L., Howerton,S.B., VanDerveer,D. and Williams,L.D. (2000) Cations mediate B-DNA conformational heterogeneity. J. Am. Chem. Soc., 122, 11048–11056. [Google Scholar]
- 12.Howerton S.B., Sines,C.C., VanDerveer,D. and Williams,L.D. (2001) Locating monovalent cations in the grooves of B-DNA. Biochemistry, 40, 10023–10031. [DOI] [PubMed] [Google Scholar]
- 13.Tereshko V., Minasov,G. and Egli,M. (1999) A ‘hydrat-ion’ spine in a B-DNA minor groove. J. Am. Chem. Soc., 121, 3590–3595. [Google Scholar]
- 14.Hud N.V. and Feigon,J. (1997) Localization of divalent metal ions in the minor groove of DNA A-tracts. J. Am. Chem. Soc., 119, 5756–5757. [Google Scholar]
- 15.Hud N.V., Sklenar,V. and Feigon,J. (1999) Localization of ammonium ions in the minor groove of DNA duplexes in solution and the origin of DNA A-tract bending. J. Mol. Biol., 286, 651–660. [DOI] [PubMed] [Google Scholar]
- 16.Hud N.V. and Feigon,J. (2002) Characterization of divalent cation localization in the minor groove of the AnTn and TnAn DNA sequence elements by 1H NMR spectroscopy and manganese(II). Biochemistry, 41, 9900–9910. [DOI] [PubMed] [Google Scholar]
- 17.Young M.A., Jayaram,B. and Beveridge,D.L. (1997) Intrusion of counterions into the spine of hydration in the minor groove of B-DNA: fractional occupancy of electronegative pockets. J. Am. Chem. Soc., 119, 59–69. [Google Scholar]
- 18.Young M.A. and Beveridge,D.L. (1998) Molecular dynamics simulations of an oligonucleotide duplex with adenine tracts phased by a full helix turn. J. Mol. Biol., 281, 675–687. [DOI] [PubMed] [Google Scholar]
- 19.McConnell K.J. and Beveridge,D.L. (2000) DNA stucture: what’s in charge? J. Mol. Biol., 304, 803–820. [DOI] [PubMed] [Google Scholar]
- 20.Hamelberg D., McFail-Isom,L., Williams,L.D. and Wilson,W.D. (2000) Flexible structure of DNA: ion dependence of minor-groove structure and dynamics. J. Am. Chem. Soc., 122, 10513–10520. [Google Scholar]
- 21.Strauss J.K. and Maher,L.J. (1994) DNA bending by asymmetric phosphate neutralization. Science, 266, 1829–1834. [DOI] [PubMed] [Google Scholar]
- 22.Strauss J.K., Roberts,C., Nelson,M.G., Switzer,C. and Maher,L.J. (1996) DNA bending by hexamethylene-tethered ammonium ions. Proc. Natl Acad. Sci. USA, 93, 9515–9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strauss-Soukoup J.K., Vaghefi,M.M., Hogrefe,R.I. and Maher,L.J. (1997) Effects of neutralization pattern and stereochemistry on DNA bending by methylphosphonate substitutions. Biochemistry, 36, 8692–8698. [DOI] [PubMed] [Google Scholar]
- 24.Hardwidge P.R., Lee,D.-K., Prakash,T.P., Iglesias,B., Den,R.B., Switzer,C. and Maher,L.J. (2001) DNA bending by asymmetrically tethered cations: influence of tether flexibility. Chem. Biol., 8, 967–980. [DOI] [PubMed] [Google Scholar]
- 25.Diekmann S., Mazzarelli,J.M., McLaughlin,L.W., von Kitzing,E. and Travers,A.A. (1992) DNA curvature does not require bifurcated hydrogen bonds or pyrimidine methyl groups. J. Mol. Biol., 225, 729–738. [DOI] [PubMed] [Google Scholar]
- 26.Seela F. and Grein,T. (1992) 7-Deaza-2′-deoxyadenosine and 3-deaza-2′-deoxyadenosine replacing dA within d(A6)-tracts: differential bending at 3′- and 5′-junctions of d(A6)-d(T6) and B-DNA. Nucleic Acids Res., 20, 2297–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross E.D., Den,R.B., Hardwidge,P.R. and Maher,L.J. (1999) Improved quantitation of DNA curvature using ligation ladders. Nucleic Acids Res., 27, 4135–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lan T. and McLaughlin,L.W. (2000) Minor groove hydration is critical to the stability of DNA duplexes. J. Am. Chem. Soc., 122, 6512–6513. [Google Scholar]
- 29.Lan T. and McLaughlin,L.W. (2001) Minor groove functional groups are critical for the B-form conformation of duplex DNA. Biochemistry, 40, 968–976. [DOI] [PubMed] [Google Scholar]
- 30.Crothers D.M. and Drak,J. (1992) Global features of DNA structure by comparative gel electrophoresis. Methods Enzymol., 212, 46–71. [DOI] [PubMed] [Google Scholar]
- 31.Guckian K.M., Krugh,T.R. and Kool,E.T. (1998) Solution structure of a DNA duplex containing a replicable difluorotoluene-adenine pair. Nature Struct. Biol., 5, 954–959. [DOI] [PubMed] [Google Scholar]
- 32.Kopka M.L., Fratini,A.V., Drew,H.R. and Dickerson,R.E. (1983) Ordered water structure around a B-DNA dodecamer, a quantitative study. J. Mol. Biol., 163, 129–146. [DOI] [PubMed] [Google Scholar]
- 33.Coll M., Frederick,C.A., Wang,A.H.-J. and Rich,A. (1987) A bifurcated hydrogen-bonded conformation in the d(A-T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc. Natl Acad. Sci. USA, 84, 8385–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koo H.-S. and Crothers,D.M. (1987) Chemical determinants of DNA bending at adenine-thymine tracts. Biochemistry, 26, 3745–3748. [DOI] [PubMed] [Google Scholar]
- 35.Chuprina V.P. (1985) Regularities in formation of the spine of hydration in the DNA minor groove and its influence on the DNA structure. FEBS Lett., 186, 98–102. [DOI] [PubMed] [Google Scholar]
- 36.Laundon C.H. and Griffith,J.D. (1987) Cationic metals promote sequence DNA bending. Biochemistry, 26, 3759–3762. [DOI] [PubMed] [Google Scholar]
- 37.McFail-Isom L., Sines,C.C. and Williams,L.D. (1999) DNA structure: cations in charge? Curr. Opin. Struct. Biol., 9, 298–304. [DOI] [PubMed] [Google Scholar]
- 38.Guckian K.M., Schweitzer,B.A., Ren,R.X.-F., Sheils,C.J., Paris,P.L., Tahmassebi,D.C. and Kool,E.T. (1996) Experimental measurement of aromatic stacking affinities in the context of duplex DNA. J. Am. Chem. Soc., 118, 8182–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.