Abstract
Locked nucleic acids (LNA) are novel high-affinity DNA analogs that can be used as genotype-specific drugs. The LNA oligonucleotides (LNA PO ODNs) are very stable in vitro and in vivo without the need for a phosphorothiolated backbone. In this study we tested the biological fate and the efficacy in tumor growth inhibition of antisense oligonucleotides directed against the gene of the large subunit of RNA polymerase II (POLR2A) that are completely synthesized as LNA containing diester backbones. These full LNA oligonucleotides strongly reduce POLR2A protein levels. Full LNA PO ODNs appeared to be very stable compounds when injected into the circulation of mice. Full LNA PO ODNs were continuously administered for 14 days to tumor-bearing nude mice. Tumor growth was inhibited sequence specifically at dosages from 1 mg/kg/day. LNA PO ODNs appeared to be non-toxic at dosages <5 mg/kg/day. Biodistribution studies showed the kidneys to have the highest uptake of LNA PO ODNs and urinary secretion as the major route of clearance. This report shows that LNA PO ODNs are potent genotype-specific drugs that can inhibit tumor growth in vivo.
INTRODUCTION
Over the last 20 years, antisense oligonucleotides (ODNs) have been shown to be potent inhibitors of gene expression (1). DNA antisense oligonucleotides with phosphorothioate chemistry (DNA PS) have been widely used both in vitro and in vivo. DNA PS antisense ODNs have been used in many experiments to down-regulate mRNA levels of their target. RNase H is thought to be important for antisense PS ODN effects. Antisense PS ODNs bind to mRNA and the resulting RNA–DNA heteroduplex is cleaved by RNase H (2). The DNA PS ODNs are the most widely used class of ODNs because they are relatively stable and cheap to synthesize. However, the inclusion of a PS backbone mediates non-sequence-specific effects due to their polyanionic nature causing interactions with proteins. This was already observed in the early nineties when antisense ODNs were used as inhibitors of HIV-1 (3,4). Despite these non-sequence-specific effects, several of the PS ODNs are already in Phase II and III clinical trials. These include inhibitors of Ha-ras (5), c-raf kinase (6), PKC-α (7,8) and ICAM (9).Vitravene, an antisense ODN against human cytomegalovirus (HCMV), is the first antisense drug that was registered. This shows that, despite the scepticism that sometimes is associated with ODNs, there is room for optimism. Novel chemical modifications of antisense ODNs have been shown to be of great value for the biological stability and efficacy of antisense ODNs. At this moment antisense ODNs with different types of chemistry like peptide nucleic acids, N3′,P5′-phosphoroamidates, morpholino phosphoroamidates and 2′-O-methoxyethyl modifications, are under investigation by multiple groups of researchers around the world (for reviews see 10,11)
Locked nucleic acids (LNA) are a novel class of DNA analogs that provide major improvements in a number of key properties. LNA combine by far the highest affinity ever reported for a DNA analog towards complementary DNA and RNA with a good ability to discriminate between matched and mismatched target sequences (12). LNA contain a methylene bridge that connects the 2′-oxygen of ribose with the 4′-carbon. By virtue of this bicyclic structure, the furanose ring of the LNA monomers is locked in a 3′-endo conformation, thus structurally mimicking the standard RNA monomers. LNA induce large increase in thermal stability (melting temperature, Tm) when bound to a matching RNA sequence (for reviews see 13,14).
In vivo studies by Wahlestedt et al. (15) have shown that DNA/LNA co-polymers can be used as stable, non-toxic and potent antisense ODNs that are able to recruit RNase H provided a ‘stretch’ of DNA is present in the ODN. Importantly, this study very clearly showed that the LNA-containing ODNs were in vivo more potent in knocking down the rat δ opioid receptor as compared to the classic full DNA ODNs. Kurreck et al. (16) performed a systematic study where they compared the stability and the ability to activate RNase H and the Tm of LNA/DNA chimeras as compared with classic DNA phosphorothioates and 2′-O-methyl gapmers. These studies showed that introduction of LNA into a DNA ODN could greatly enhance the Tm and resulted in a greater stability in human serum as compared with isosequential phosphorothioates. Full LNA PO ODNs, which have a very high affinity for their complementary mRNA but cannot recruit RNase H, might inhibit protein translation directly without the aid of RNase H recruitment. Such a steric block mechanism of gene expression was shown for several other ‘next generation’ ODN chemistries, like 2′-O-(2-methoxyethyl) (MOE) ODNs (17) and peptide nucleic acid (PNA) ODNs (18). Recently, LNA were also shown to be able to act as steric block ODNs (19).
In previous studies we have shown that antisense ODNs against the gene encoding the large subunit of RNA polymerase II (POLR2A) can be used as genotype-specific drugs that inhibit tumor growth in vivo (20,21). However, when DNA PS ODNs were used as genotype-specific drugs against POLR2A, our studies showed limited toxicity issues (21). LNA as a new class of antisense ODNs that do not need PS backbone modifications for their stability in vivo might have superior properties when compared to the classic DNA PS ODNs. Therefore, we explored the use of LNA PO ODNs as anticancer drugs in our established model system. In this study we examine the possibility of using the new class of LNA antisense ODNs as genotype-specific inhibitors of tumor growth in vivo. Full LNA PO ODNs (16mers) with diester linkages directed against POLR2A were tested in vitro and in vivo. The stability of the full LNA PO ODNs in vivo and their biodistributions were studied. In addition we studied the efficacy of tumor growth inhibition and the toxicity during 14 days of continuous treatment with full LNA PO ODNs.
MATERIALS AND METHODS
LNA oligonucleotide synthesis
All ODNs were synthesized by Cureon AS (Copenhagen, Denmark). In all ODNs methyl-C was used to prevent immune stimulation.
All syntheses were carried out on the 1 µmol scale in a MOSS Expedite instrument platform. The synthesis procedures are essentially carried out as described in the instrument manual.
Preparation of the LNA succinyl hemiester
5′-O-dimethoxytrityl (Dmt)-3′-hydroxy-LNA monomer (500 mg), succinic anhidride (1.2 equiv.) and DMAP (1.2 equiv.) were dissolved in DCM (35 ml). The reaction was stirred at room temperature overnight. After extractions with 0.1 M NaH2PO4 pH 5.5, (2×) and brine (1×), the organic layer was further dried with anhydrous Na2SO4, filtered and evaporated. The hemiester derivative was obtained in 95% yield and was used without any further purification.
Preparation of the LNA–CPG resin
The above prepared hemiester derivative (90 µmol) was dissolved in a minimum amount of dimethylformamide (DMF). N,N-diisopropylethylamine and benzotriazole-1- yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate (90 µmol) were added and mixed together for 1 min. This pre-activated mixture was combined with long chain alkyl amino (LCAA)-controlled pore glass (CPG) (500 Å, 80–120 mesh size, 300 mg) in a manual synthesizer and stirred. After 1.5 h at room temperature, the support was filtered off and washed with DMF, dichloromethane and methanol. After drying the loading was determined, and found to be 57 µmol/g.
Phosphorothioate cycles
5′-O-Dmt (benzoyl-A, benzoyl-C, isobutyryl-G and T) linked to CPG were deprotected using a solution of 3% trichloroacetic acid (v/v) in dichloromethane. The resin is washed with acetonitrile. Coupling of phosphoramidites (benzoyl-A, isobutyryl-G, 5-methylbenzoyl-C or T-β-cyanoethylphosphoramidite) is performed by using a solution of 0.08 M 5′-O-Dmt-protected amidite in acetonitrile and activation is done by using 4,5-dicyanoimidazole in acetonitrile (0.25 M). Coupling is carried out in 2 min. Thiolation is carried out by using Beaucage reagent (0.05 M in acetonitrile) and is allowed to react for 3 min. The support is thoroughly washed with acetonitrile and the subsequent capping of unreacted 5′-hydroxyl groups is carried out by using a solution of acetic anhydride in tetrahydrofuran (THF) (Cap A) and N-methylimidazole/pyridine/THF (1:1:8) (Cap B). The capping step is then repeated and the cycle is concluded by acetonitrile washing.
LNA cycles
5′-O-Dmt LNA (benzoyl-A, benzoyl-C, isobutyryl-G or T) monomer linked to CPG is deprotected by using the same procedure as above. Coupling is performed by using 5′-O-Dmt LNA (benzoyl-A, benzoyl-C, isobutyryl-G or T-β-cyanoethylphosphoramidite) monomer (0.1 M in acetonitrile) and activation is done with 4,5-dicyanoimidazole (0.25 M in acetonitrile). Coupling is prolonged to 7 min. Capping is done with Cap A and Cap B for 30 s. The phosphite triester is oxidized to the more stable phosphate triester by using a solution of I2 and pyridine in THF for 30 s. The support is washed with acetonitrile and the capping step is repeated. The cycle is concluded by thorough acetonitrile wash.
Cleavage and deprotection
The oligomers are cleaved from the support and the β-cyanoethyl protecting group removed by treating the support with 35% NH4OH for 1 h at room temperature. The support is filtered off and the base protecting groups are removed by raising the temperature to 65°C for 4 h. The oligomer solution is then evaporated to dryness.
Purification
The oligomers were purified by reversed phase (RP)-HPLC or anion exchange (AIE). RP-HPLC purification was on a VYDAC™ column (catalog no. 218TP1010; Vydac). A buffer gradient, composed of different percentages of acetonitrile in 0.1 M ammonium acetate pH 7.6, was used with a flow rate of 3 ml/min: 10 min at 5%, 18 min at 30%, 45 min at 100%, 28 min at 0%. AIE purification was on a Resource™ 15Q column (Amersham Pharmacia). A buffer gradient, composed of different percentages of 0.1 M NaOH, 2.0 M NaCl in 0.1 M NaOH, was used with a flow rate of 1.2 ml/min: 1 min at 25%, 27 min at 55%, 60 min at 100%, 33 min at 0%.
Sequences
The following sequences were used: Cur616, 5′-TCGCCGTAGCGCAGCT-3′; Cur222, 5′-TCGCCATAGCGCAGCT-3′; Cur942, which is a four base mismatch, 5′-TCGCCTCAGGCCAGCT-3′. All three ODNs were HPLC purified (see above) and checked using MALDI-TOF analysis (see below). Melting temperatures were calculated using the LNA Tm calculation program at www.lna-tm.com. Tritium labeling of ODNs was performed using the heat exchange method described by Graham et al. (22).
Cell lines
The prostate cancer cell line 15PC3 were maintained by serial passage in Dulbecco’s modified Eagle’s medium (DMEM). Cells were grown at 37°C and 5% CO2. Media were supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin.
Transfections
ODN transfections were performed in 6-well culture plates as described previously (20) with DAC-30 (Eurogentec) as liposomal transfection agent. Fluorescently labeled LNA PO ODNs were used to determine the transfection efficiency. The cells were lysed in lysis buffer (150 mM NaCl, 50 mM Tris–HCl pH 8, with 1% Nonidet P40) 48 h post-transfection. Cellular proteins were separated by denaturing SDS–PAGE in the presence of β-mercaptoethanol and analyzed on western blots according to standard protocols. For detection of POLR2A on western blots and immunofluorescence the anti-POLR2A monoclonal antibody 8WG16 (RDI, Flanders, NJ) was used.
In vivo model
Eight to ten week old NMRI nu/nu mice (Charles River, Maastricht, The Netherlands) were injected subcutaneously in the flank with 106 15PC3 cells in 300 µl Matrigel (Collaborative Biomedical Products, Bedford, MA). The cells were injected within 1 h after harvesting by trypsin treatment. Before injection the cells were washed with cold phosphate-buffered saline (PBS), counted with a hemocytometer and subsequently mixed with the Matrigel on ice. One week after tumor cell injection, when tumor take was positive, an osmotic minipump (model 1002; Alzet Corp., Palo Alto, CA) was implanted dorsally according to the instructions of the manufacturer. The osmotic minipumps were incubated in PBS for 20 h at 37°C prior to implantation to start up the pump, in order to quickly reach a steady delivery rate after implantation. In vitro testing showed that the Alzet 1002 minipumps reached a steady pumping rate within 24 h. The osmotic minipumps were filled with oligonucleotides (using the dosages as indicated in the figure legends) or 0.9% saline. For each treatment, five mice per group were used. Tumor growth was monitored daily following the implantation of the osmotic minipump. Tumor volume was measured and calculated as described previously (23). Tissue distribution studies of tritiated ODNs were performed according to Bijsterbosch et al. (24). The radioactivity in the different organs was corrected for serum present at the time of sampling as determined by the distribution of 125I-labeled BSA (K. Kruijt, University of Leiden, The Netherlands, personal communication). Liver samples were fixed in 4% formaldehyde in PBS and subsequently embedded in paraffin according to standard procedures. Hematoxylin and eosin (HE) stains were used to visualize the effects of ODN treatment on the liver, kidneys and tumor xenografts. Aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) levels in serum were determined using standard diagnostic procedures with the H747 (Hitachi/Roche) with the appropriate kits (Roche Diagnostics).
MALDI-TOF and HPLC analysis
After coagulation blood cells were spun down and the serum fraction was used for analysis of LNA PO ODN stability. One volume of 1% Nonidet P40 was added to the serum followed by 2 vol of ice-cold acetonitrile to precipitate proteins. Urine samples were not processed prior to purification. The LNA was purified by ion exchange on the purification plates from the Nucleave genotyping kit (Waters, Milford, MA) and analyzed using Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) on a Biflex III MALDI spectrometer (Brucker Instruments, Leipzig, Germany).
RESULTS
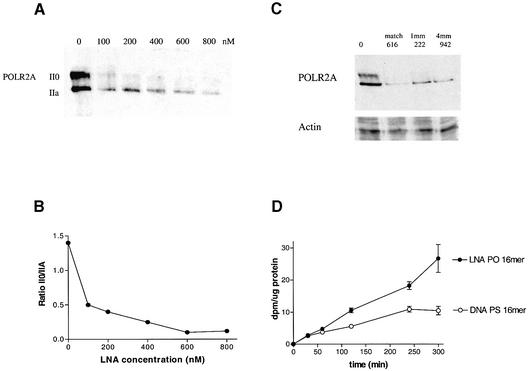
Phosphorothioate DNA antisense ODNs directed against POLR2A can inhibit tumor growth in vitro and in vivo (20,21). In these previous publications we described a set of antisense ODNs that are specific for a single nucleotide polymorphism of POLR2A. For the current study, full LNA non-phosphorothiolated versions of the previously described ODNs directed against POLR2A were tested for their ability to inhibit POLR2A expression in 15PC3 cells (prostate cancer cell line). These full LNA PO ODNs are unable to recruit RNase H activity (data not shown). Therefore, we studied their ability to reduce POLR2A protein expression directly by western blot (Fig. 1). The antibody (8WG16) against POLR2A that is used in this study recognizes POLR2A as two distinct bands on western blots, the hyperphosphorylated (II0) form migrating as a 240 kDa protein and the hypophosphorylated (IIA) form which migrates as a 214 kDa protein. Transfection with the full LNA PO ODN Cur616 that is matched to the genotype of POLR2A in 15PC3 cells results in a dose-dependent decrease in both the II0 and IIA forms of POLR2A. However, the hyperphosphorylated II0 form of POLR2A is affected more strongly, resulting in a decrease of the II0/IIA ratio (Fig. 1A and B). Since LNA confers a strong increase in Tm it is important to determine the sequence specificity of a full LNA PO ODN. The calculated Tm values in a 115 mM salt concentration of Cur616, Cur222 and Cur942 are 87, 84 and 88°C, respectively. The sequence specificity of the full match Cur616 was compared to two other full LNA PO ODNs, Cur222 and Cur942, which have one and four mismatches, respectively. In our previous studies we demonstrated that the anti-POLR2A DNA PS ODNs are very sequence specific (20,21). But all three LNA versions of these ODNs showed similar efficacy in reduction of POLR2A protein levels in vitro, suggesting that the sequence specificity was much reduced because of the LNA-mediated increase in the Tm (Fig. 1C). However, the expression of a totally different gene (RPA70), encoding the 70 kDa subunit of replication protein A, was not affected as detected on western blots. The LNA PO ODNs showed a very high efficacy in inhibiting POLR2A protein levels. 15PC3 cells already showed an ∼40% reduction in POLR2A protein levels at an ODN concentration of 50 nM. This effective concentration is much lower than what we observed for the DNA PS ODNs as published previously (20). However, the amount of LNA that was taken up by the 15PC3 cells during transfection was larger as compared to the DNA PS ODN (Fig. 1D). So the concentration of the LNA inside the 15PC3 cells is much higher. The increased uptake of LNA PO ODNs in vitro may be an important factor for the specificity of the ODN (see below).
Figure 1.
(A) Western blot of POLR2A protein isolated from 15PC3 cells 48 h post-transfection with LNA PO ODNs. Protein was isolated from 15PC3 cells transfected with increasing doses of from 0 to 800 nM Cur616 LNA ODN. (B) Ratio of II0/IIA subunits of POLR2A in response to increasing concentrations of Cur616 as quantified from western blots. (C) Western blot of POLR2A protein isolated from 15PC3 cells 48 h post-transfection with LNA PO ODNs. Protein was isolated from 15PC3 cells transfected with 800 nM LNA. 616, matched ODN; 222, single base mismatch; 942, four base mismatched ODN. (D) Comparison of the uptake of tritiated 16mer LNA PO ODN and tritiated 16mer DNA PS ODN by 15PC3 cells over time during transfection with 800 nM ODN with DAC-30 as transfection agent.
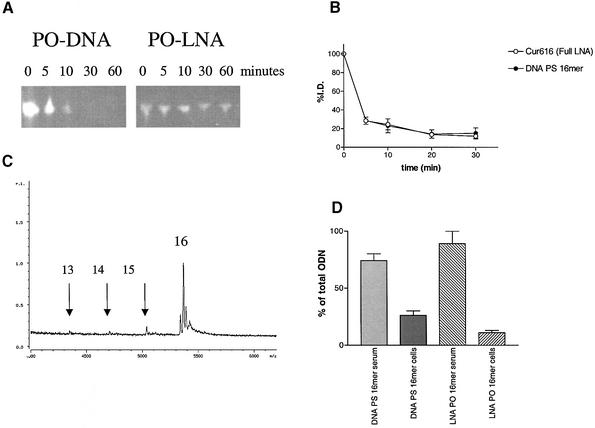
LNA PO ODNs are very stable molecules in serum (15,16). Chimeric DNA/LNA oligonucleotides are more stable than isosequential DNA PS ODNs, which have half-lives of >10 h (16). In line with these earlier findings we found that full LNA PO ODNs with a normal diester backbone remained stable in fresh mouse serum for >2 h while a non-phosphorothiolated DNA ODN is degraded rapidly within 1 h (Fig. 2A). To study and compare the serum decay and biodistribution of the LNA PO ODNs with a DNA PS ODN in vivo, two 16mer ODNs, a LNA 16mer and a DNA PS 16mer, were radiolabeled with 3H using the heat exchange method (22). The radiolabeled ODNs were injected i.v. into nude mice (NMRI nu/nu). The serum decay of a radiolabeled full LNA 16mer ODN was very rapid and similarly biphasic to the serum decay of a radiolabeled DNA PS ODN 16mer (Fig. 2B). After 30 min only 20% of the injected dose remained in the serum. The LNA PO ODNs present in the serum samples were analyzed using MALDI-TOF. After 30 min of circulation most of the injected LNA PO ODN was still intact as a 16mer. Only minor peaks of 15mers, 14mers and 13 mers were found, indicating that LNA PO ODNs with a diester backbone are very stable compounds in vivo (Fig. 2C). When radiolabeled ODNs were injected it was found that the radioactivity of both the LNA PO ODNs and the DNA PS ODNs were almost completely recovered in the serum; only 10–20% was found associated with the blood cells (Fig. 2D).
Figure 2.
(A) Stability of 16mer DNA PO ODN and 16mer LNA PO ODN in fresh mouse serum at 37°C. FAM-labeled ODNs were mixed with fresh mouse serum and incubated at 37°C. At the indicated time intervals samples were taken and analyzed on 16% acrylamide gels. (B) Clearance of two radiolabeled 16mer ODNs: LNA PO ODN Cur616 and DNA PS ODN PS Tas16 from mouse serum after i.v. injection. At the indicated time points blood samples were taken and the amount of radioactivity in serum was counted. The data is represented as percentage of the injected dose ± SEM. (C) MALDI-TOF analysis of a 16mer LNA PO ODN in mouse serum 30 min after injection. The 15mer, 14mer and 13mer possibly resulting from 3′ exonuclease activity are indicated with arrows. (D) Amount of radioactive 16mer LNA PO ODN or DNA PS ODN recovered in serum or associated with coagulated blood cells. Thirty minutes after injection (i.v.) blood samples were taken from mice and after coagulation of the blood, the coagulate was spun down and the amount of radioactivity was counted in serum and coagulated cells.
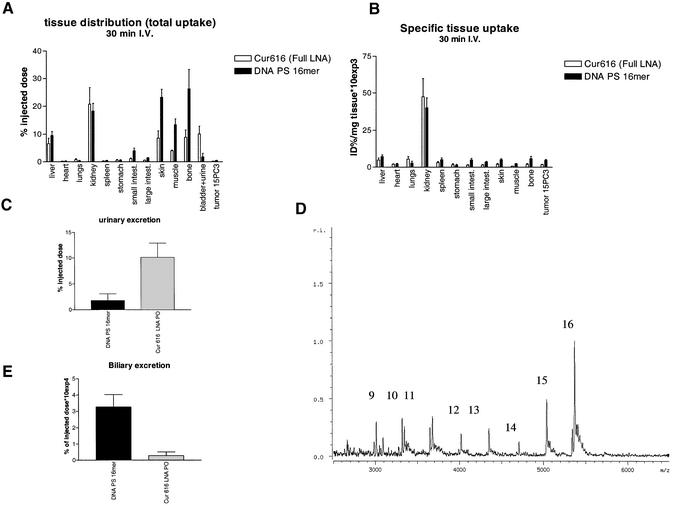
The biodistribution of the LNA PO ODN versus the PS DNA ODN was overall very similar. Except for ‘large’ tissues such as skin, muscle and bone (Fig. 3A), the kidney was the main site of uptake of both ODNs when corrected for tissue weight (Fig. 3B). The urinary excretion of the LNA PO ODN was extremely fast and over 4-fold higher than the excretion of the DNA PS ODN (Fig. 3C). This difference in urinary excretion between the DNA PS ODN and the LNA PO ODN could be due to the thiolation. When a 16mer LNA PS ODN was injected into the mouse, similar low amounts were secreted in urine as compared to the DNA PS ODN (data not shown). MALDI-TOF analysis of the urine revealed that the LNA PO ODN appeared to be partly degraded (Fig. 3D). Possibly, as a consequence of the rapid urinary secretion of the LNA PO ODNs, the total uptake by ‘large’ tissues such as the skin, muscle and bone was much lower as compared to the DNA PS ODN. The biliary secretion of the DNA PS ODN was several fold higher as compared to the LNA PO ODN, indicating that the liver might be handling the two types of ODN differently (Fig. 3E). It must be noted that the biliary secretion is only a very minor route of clearance as compared to the urinary secretion.
Figure 3.
Tissue distribution studies of radiolabeled 16 mer LNA PO ODN or DNA PS 16mers in NMRI nude mice. Tritiated ODNs were injected i.v. and after 30 min of circulation the distribution of radioactivity was determined. The radioactivity in the different tissues was corrected for serum present at the time of sampling as determined by the distribution of 125I-labeled BSA. The results are presented as (A) total uptake as a percentage of the injected dose by the tissues and (B) as specific uptake (percentage of total administered radioactivity divided by tissue weight) ± SEM (n = 3 per treatment). (C) Amount of radioactivity expressed as a percentage of injected dose ± SEM recovered in urine 30 min post-injection. (D) MALDI-TOF analysis of the LNA PO ODN recovered in urine 30 min after injection. The numbers at each peak indicate the size of the ODN fragment, e.g. 16 = 16mer, 15 = 15mer, etc. (E) Amount of radioactivity expressed as a percentage of injected dose ± SEM recovered in bile 30 min post-injection.
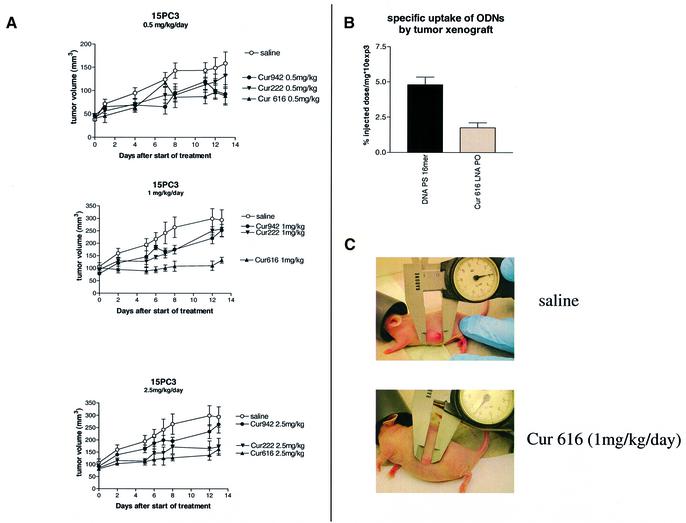
To test the efficacy of the LNA PO ODNs against POLR2A in vivo, a nude mouse model was used. We have previously demonstrated tumor growth inhibition with the DNA PS ODNs against POLR2A (21). In the previous study we showed that the DNA PS ODNs could inhibit tumor growth in vivo at a dosage of 5 mg/kg/day specifically for a single nucleotide polymorphism of POLR2A. In this present study we tested the full LNA PO ODNs in the same in vivo model as was used in the earlier studies. The nude mice (NMRI strain) were xenografted with 15PC3 tumors and subsequently treated with the LNA PO ODNs for 14 days using Alzet osmotic minipumps. Three LNA PO ODNs were compared in their efficacy to inhibit tumor growth in vivo: Cur616 that is matched to the genotype of POLR2A in 15PC3 cells and Cur222 and Cur942 that have one and four mismatches, respectively. Four dosages were used: 0.5, 1, 2.5 and 5 mg/kg/day. Unlike the uptake of the LNA PO ODNs by the 15PC3 cells in culture, the uptake of radiolabeled LNA PO ODN by the tumor xenografts was half the uptake of the DNA PS ODN (Fig. 4B). Tumor growth inhibition was minimal at the 0.5 mg/kg dose (Fig. 4A). However, increasing the dosage to 1 mg/kg resulted in a clear inhibition of 15PC3 xenograft growth by the completely matched ODN Cur616. We observed a sequence-specific effect. Both the one and four nucleotide mismatch ODNs were less effective. Increasing the dosage up to 2.5 mg/kg resulted in a loss of sequence specificity. Tumor growth was now also inhibited by the one base mismatched LNA PO ODN Cur222, while the four base mismatched LNA PO ODN (Cur942) was still less effective (Fig. 4A). Further increasing the dosage up to 5 mg/kg/day, the dosage that was found to be the most effective for the DNA PS ODNs in the previous study (21) fully negated any difference in the inhibition of tumor growth by either LNA PO ODN (data not shown). This indicates that the observed sequence specificity of the LNA PO ODNs is concentration dependent.
Figure 4.
Tumor growth inhibition of tumor xenografts in nude NMRI mice treated with LNA PO ODNs. (A) NMRI nu/nu mice were injected s.c. in the flank with 106 15PC3 cells from culture in 300 µl Matrigel. After 1 week of tumor growth the mice received an osmotic minipump (Alzet model 1002) which was implanted dorsally. The osmotic minipumps were filled with LNA PO ODNs against POLR2A: Cur616 (full match), Cur222 (single mismatch), Cur942 (four mismatch) or saline control at dosages of 0.5, 1 and 2.5 mg/kg/day. The tumor growth was monitored after implantation of the osmotic pump. The results are expressed as mean tumor volume ± SEM (n = 5 per group). (B) Amount of radioactivity expressed as specific uptake (percentage of injected dose divided by tissue weight) ± SEM recovered in 15PC3 tumor xenografts 30 min post-injection. (C) Photographs of representative tumors of a mouse treated with saline (top) and a mouse treated with a full matching LNA PO ODN against POLR2A at 1 mg/kg/day dose (bottom).
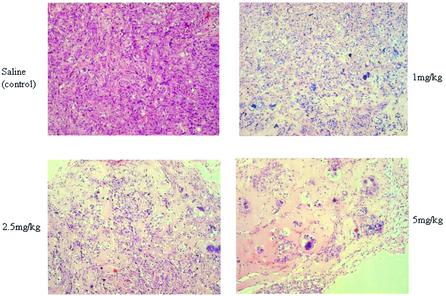
Histological studies of the tumor xenografts revealed that not only overall tumor size, but also the number of viable tumor cells within the tumor was severely reduced in a dose-dependent way (Fig. 5). To test the toxicity of the LNA PO ODNs on the liver, ASAT and ALAT levels in serum were measured after the 14 day period of treatment. Only after a 14 day long treatment at the 5 mg/kg dose was an increase in both ASAT and ALAT levels in serum found, indicating some liver damage at this dose (Table 1). At the 1 and 2.5 mg/kg dosages no significant increases in serum values of ASAT and ALAT were found. During treatment with the LNA PO ODNs the body temperature of the mice was monitored using IPTT-200 temperature transponders. The body temperature did not change significantly during treatment with the LNA PO ODNs, even at the high 5 mg/kg dose. Histological analysis of liver and kidneys showed no damage as a result of the 14 day period of treatment at any concentration. No minor and diffuse mixed mononuclear cell infiltrates that are characteristic after treatment with DNA PS ODNs were discovered within the livers of mice treated with LNA PO ODNs. Together these data indicate that the continuous treatment with LNA PO ODNs only gave minor toxicity issues in a concentration range well above the optimal dosage.
Figure 5.
Hematoxylin and eosin (HE) staining of tumor xenografts treated for 14 days with LNA PO ODN Cur616 at the indicated dosage or saline control (100× magnification).
Table 1. ASAT and ALAT levels in serum of NMRI nude mice treated for 14 days with LNA PO ODNS (Cur616 and Cur942) at the indicated dosages or mice treated with 0.9% saline.
| Dose (mg/kg) | Enzyme | Saline (U/l ± SEM) | Cur616 (U/l ± SEM) | Cur942 (U/l ± SEM) |
|---|---|---|---|---|
| 1 | ASAT | 150 ± 8.5 | 165 ± 32 | 139 ± 26 |
| ALAT | 49 ± 5 | 43 ± 2 | 73 ± 25 | |
| 2.5 | ASAT | 150 ± 8 | 139 ± 9 | 160 ± 19 |
| ALAT | 49 ± 5 | 38 ± 1 | 70 ± 17 | |
| 5 | ASAT | 165 ± 10 | 705 ± 204 | 348 ± 109 |
| ALAT | 45 ± 2 | 127 ± 29 | 72 ± 19 |
The enzymatic activities of ASAT and ALAT were measured in fresh mouse serum. The enzymatic activities are expressed as U/l at 37°C ± SEM.
DISCUSSION
LNA are a novel class of DNA analogs that have the highest reported affinity for binding of matching nucleotides of all ‘new generation’ DNA analogs (12). In addition, LNA PO ODNs appear to be very stable and resistant to nucleases in vivo. The need of a phosphorothiolated backbone can be negated and a ‘normal’ phosphorodiester backbone is sufficiently stable when LNA PO ODNs are used. This is in accordance with previous studies where mixed DNA/LNA PO ODNs showed a great stability in fresh serum (15,16). Normally, antisense ODNs are phosphorothiolated because this greatly enhances the resistance of the ODN against nucleases. However, the PS ODNs may cause toxic side effects by stimulation of the immune system. Minor and diffuse mixed mononuclear cell infiltrates are present in the livers of mice treated with phosphorothioate ODNs. These effects are non-sequence-specific and probably caused by the phosphorothioate chemistry (25). The stability of LNA ODNs with diester linkages in vivo makes it possible to synthesize potent non-toxic antisense oligonucleotides with a very high affinity for the target mRNA. However, like other DNA analogs which are modified at the 2′ position of the ribose, LNA cannot recruit RNase H activity. For LNA PO ODNs to recruit RNase H activity a stretch of at least six normal or phosphorothiolated DNA residues has to be incorporated into the ODN (16). The activity of RNase H, which degrades the RNA in a DNA/RNA heteroduplex, can result in an increased efficacy of an antisense ODN (14). However, the loss of RNase H recruitment capability of an ODN with a very high Tm might be advantageous since it will reduce the possibility of cleavage of non-target mRNA when an ODN is only partially complementary to another stretch of mRNA.
In this report we show that full LNA PO ODNs directed against POLR2A can directly inhibit the POLR2A protein levels without mRNA degradation.
In untreated 15PC3 cells the hyperphosphorylated II0 and the hypophosporylated IIA forms are present in similar proportions. The II0 form is thought to be associated with the elongation complex that synthesizes the mRNA and the hypophosporylated IIA form is preferentially associated with the transcription pre-initiation complex and is not yet actively transcribing mRNA transcripts (26). In this study we see that the levels of both the hyperphosphorylated II0 and hypophosporylated IIA forms of the targeted POLR2A are reduced. However, the II0 form is degraded more rapidly as compared to the IIA form after treatment with the full LNA against POLR2A. It may be that the II0 form is simply less stable. But it is worth noticing that POLR2A degradation that is triggered by addition of α-amanitin or UV irradiation of cells results in a more rapid degradation of the IIA form as compared to the II0 form (27,28). The degradation triggered by α-amanitin is directly caused by binding of the toxin to the POLR2A protein, inducing a specific degradation of POLR2A protein (27). Damage of DNA by UV irradiation triggers a complex reaction resulting in a ubiquitination-mediated proteolysis of POLR2A (28). In both cases the IIA form is more rapidly degraded while the II0 form is allowed to finish ongoing transcription. Since, the inhibition caused by LNA does not resemble the degradation pattern of both forms of POLR2A as seen with α-amanitin or UV irradiation it is not likely to be caused by either direct binding of LNA to POLR2A or ‘DNA damage’ by binding of the LNA ODN to genomic DNA.
The inhibitory effects of the full LNA ODNs might be caused by a steric block mechanism. The full LNA ODN has such a high affinity for the matching mRNA, the calculated Tm of Cur616 is 87°C, that it can be envisaged that the translation complex cannot dislodge such an LNA ODN that blocks its path. The full LNA PO ODNs are more potent as compared to the DNA PS versions. The effective concentration of the LNA PO ODN in vivo is five times lower than was previously published for the DNA PS version of this ODN (20). This may be caused by the increased affinity of the LNA PO ODN. Recently, work by Martinez et al. showed that single-stranded RNA can mediate RNA interference-like gene silencing (29). Since LNA structurally mimics the sugar backbone conformation of RNA when bound to mRNA (30) it might be hypothesized that full LNA ODNs can function in a likewise manner. However, activation of a RNA interference-like gene silencing effect implies that mRNA would be degraded and in these experiments we did not observe degradation of the mRNA of POLR2A. Therefore, the most likely mechanism through which our full LNA ODNs inhibit POLR2A expression is a steric block mechanism.
Since LNA PO ODNs have a very high affinity for their targeted mRNA it may be difficult to create very sequence-specific ODNs. In a previous study we explored the possibility of creating genotype-specific antisense ODNs which could distinguish single base mismatches. We developed highly specific PS ODNs against POLR2A that were able to inhibit tumor growth depending on which particular single nucleotide polymorphism the tumor possessed (21). In this current report we used the same sequences as in the previous studies, but the ODNs were synthesized with LNA instead of phosphorothiolated DNA. We show that in vitro the new LNA PO ODNs are very potent in inhibiting POLR2A protein levels, but at the expense of the high sequence specificity in vitro. ODNs with a single mismatched nucleotide or a four nucleotides mismatch were as effective in inhibiting the POLR2A protein levels in vitro. When these LNA PO ODNs were administrated in vivo we observed sequence-specific and dose-dependent inhibition of tumor growth. At a dose of 1 mg/kg/day we observed that 15PC3 tumor xenografts were inhibited in their growth with the matching LNA PO ODN. The other two LNA PO ODNs with the single or four base mismatch showed less efficacy in tumor growth inhibition, indicating that the observed effects are sequence-dependent and ‘true antisense’ effects. Increasing the dosage increased the tumor growth inhibitory effects but at the expense of sequence specificity. The reduced sequence specificity in vitro might be caused by a relatively high uptake of LNA PO ODN by 15PC3 cells during transfection as compared to the DNA PS ODNs. In the xenografts the uptake of LNA PO ODN is significantly lower as compared with the DNA PS ODN. We observed a dosage-dependent and sequence-specific inhibition of tumor growth at lower doses than we previously observed for DNA PS ODNs. The low dosages required for tumor growth inhibition and the lower uptake in vivo of the LNA ODN versus the DNA PO ODN indicate that the LNA PO ODNs are much more potent. Only at the highest dose of 5 mg/kg/day were increased ASAT and ALAT levels observed, indicating some toxic effects on the liver. Since the LNA ODNs are not phosphorothiolated, the LNA ODNs themselves might be the cause. The polr2a sequence of the mouse differs only slightly from the targeted human sequence (21). Thus the LNA PO ODNs may inhibit the mouse polr2a protein levels at high dosages in the tissues that take up most LNA PO ODNs, resulting in some toxicity. Therefore, careful consideration must be taken in the design and use of LNA PO ODNs as genotype-specific drugs, since problems might arise due to their extremely high Tm values.
The biodistribution of the LNA PO ODNs in nude mice was quite similar to the biodistribution of a DNA PS ODN, with the kidney as the major site of uptake. The major difference was the high urinary excretion of intact LNA PO ODNs as compared to the PS ODNs. It might be that the PS ODNs get stuck in the kidney due to binding to large proteins in the serum while LNA PO ODNs are easily filtrated out of the kidney. The LNA PO ODNs clearly show increased efficacy as compared with classic DNA PS ODNs, while their biodistribution profile is seemingly not as attractive. The low dosages required for tumor growth inhibition in comparison with DNA PS ODNs, despite the higher renal excretion rate and lower uptake by the tumors, indicates that the LNA PO ODNs are much more potent.
To date, no oligonucleotide has been approved as a drug to treat cancer, though several are in clinical trials. Although ODNs have a great potential to supply a new class of chemotherapeutic agents, due to their inherent specificity, some problems were encountered in the last 20 years mainly due to aspecific side effects. The gap between promise and achievement has resulted in the continued development of novel types of ODNs to improve potency, specificity and efficacy. LNA is one of these new classes of ODNs with great promise. To our knowledge this is the first time that LNA oligonucleotides have been demonstrated to inhibit tumor growth in vivo. LNA PO ODNs might be developed as chemotherapeutic agents, but careful considerations must be made regarding their extremely high affinity. Subsequent studies must show whether shortening of the LNA PO ODNs or strategic placement of LNAs within chimeric ODNs may result in the desired improvements in potency, specificity and efficacy.
Acknowledgments
ACKNOWLEDGEMENTS
This research was supported by the Stichting Kindergeneeskundig Kankeronderzoek and Stichting Vanderes. K.F. is funded by a VENI grant of the Innovational Research Incentives Scheme by ZonMw.
REFERENCES
- 1.Dove A. (2002) Antisense and sensibility. Nat. Biotechnol., 20, 121–124. [DOI] [PubMed] [Google Scholar]
- 2.Hausen P. and Stein,H (1970) Ribonuclease H, an enzyme degrading the RNA moiety of DNA-RNA hybrids. Eur. J. Biochem., 14, 278–283. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal S. (1999) Importance of nucleotide sequence and chemical modifications of antisense oligonucleotides Biochim. Biophys. Acta, 1489, 53–68. [DOI] [PubMed] [Google Scholar]
- 4.Stein C.A. (1999) Two problems in antisense biotechnology: in vitro delivery and the design of antisense experiments. Biochim. Biophys. Acta, 1489, 45–52. [DOI] [PubMed] [Google Scholar]
- 5.Yu R., Geary,R., Leeds,J., Watanabe,T., Moore,M., Fitchett,J., Matson,J., Burckin,T., Templin,M. and Levin,A. (2001) Comparison of pharmacokinetics and tissue disposition of an antisense phosphorothioate oligonucleotide targeting human Ha-ras mRNA in mouse and monkey. J. Pharm. Sci., 90, 182–193. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson J.P., Yao,K.S., Gallagher,M., Friedland,D., Mitchell,E.P., Cassella,A., Monia,B., Kwoh,T.J., Yu,R., Holmlund,J. et al. (1999) Phase I clinical/pharmacokinetic and pharmacodynamic trial of the c-raf-1 antisense oligonucleotide ISIS 5132 (CGP 69846A). J. Clin. Oncol., 17, 2227–2236. [DOI] [PubMed] [Google Scholar]
- 7.Dennis J.U., Dean,N.M., Bennett,C.F., Griffith,J.W., Lang,C.M. and Welch,D.R. (1998) Human melanoma metastasis is inhibited following ex vivo treatment with an antisense oligonucleotide to protein kinase C-alpha. Cancer Lett., 128, 65–70. [DOI] [PubMed] [Google Scholar]
- 8.Yuen A.R., Halsey,J., Fisher,G.A., Holmlund,J.T., Geary,R.S., Kwoh,T.J., Dorr,A. and Sikic,B.I. (1999) Phase I study of an antisense oligonucleotide to protein kinase C-alpha (ISIS 3521/CGP 64128A) in patients with cancer. Clin. Cancer Res., 5, 3357–3363. [PubMed] [Google Scholar]
- 9.Yacyshyn B.R., Bowen-Yacyshyn,M.B., Jewell,L., Tami,J.A., Bennett,C.F., Kisner,D.L. and Shanahan,W.R.,Jr (1998) A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn’s desease. Gastroenterology, 114, 1133–1142. [DOI] [PubMed] [Google Scholar]
- 10.Braasch D.A. and Corey,D.R. (2002) Novel antisense startegies for controlling gene expression. Biochemistry, 41, 4503–4510. [DOI] [PubMed] [Google Scholar]
- 11.Dias N. and Stein,C.A.(2002) Antisense oligonucleotides: basic concepts and mechanisms. Mol. Cancer Ther., 1, 347–355. [PubMed] [Google Scholar]
- 12.Koshkin A., Singh,S.K., Nielsen,P., Rajwanshi,V.K., Kumar,R., Melgaard,M., Olsen,C.E. and Wengel,J. (1998) LNA: synthesis of the adenine, cytosine, guanine 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation and unprecedented nucleic acid recognition. Tetrahedron, 54, 3607–3630. [Google Scholar]
- 13.Braasch D.A. and Corey,D.R. (2001) Locked nucleic acid (LNA): fine-tuning the recognition of DNA and RNA. Chem. Biol., 8, 1–7. [DOI] [PubMed] [Google Scholar]
- 14.Elayadi A.N. and Cory,D.R. (2001) Application of PNA and LNA oligomers to chemotherapy. Curr. Opin. Invest. Drugs, 2, 558–561. [PubMed] [Google Scholar]
- 15.Wahlestedt C., Salmi,P., Good,L., Kela,J., Johnsson,T., Hokfelt,T., Broberger,C., Porreca,F., Lai,J., Ren,K. et al. (2000) Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc. Natl Acad. Sci. USA, 97, 5633–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurreck J., Wyszko,E., Gillen,C. and Erdmann,V.A. (2002) Design of antisense oligonucleotides stabilized by locked nucleic acids. Nucleic Acids Res., 30, 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker B.F., Lot,S.S., Condon,T.P., Cheng-Flournoy,S., Lesnik,E.A., Sasmor,H.M. and Bennett,C.F. (1997) 2′-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J. Biol. Chem., 272, 11994–12000. [DOI] [PubMed] [Google Scholar]
- 18.Dias N., Dheur,S., Nielsen,P.E., Gryaznov,S., Van Aerschot,A., Herdewijn,P., Helene,C. and Saison-Behmoaras,T.E. (1991) Antisense PNA tridecamers targeted to the coding region of Ha-ras mRNA arrest polypeptide chain elongation. J. Mol. Biol., 294, 403–416. [DOI] [PubMed] [Google Scholar]
- 19.Arzumanov A., Walsh,A.P., Rajwanshi,V.K., Kumar,R., Wengel,J. and Gait,M.J. (2001) Inhibition of HIV-1 Tat-dependent trans activation by steric block chimeric 2′-O-methyl/LNA oligoribonucleotides. Biochemistry, 40, 14645–14654. [DOI] [PubMed] [Google Scholar]
- 20.ten Asbroek A.L., Fluiter,K., van Groenigen,M., Nooij,M. and Baas,F. (2000) Polymorphisms in the large subunit of human RNA polymerase II as target for allele-specific inhibition. Nucleic Acids Res., 28, 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fluiter K., ten Asbroek,A.L.M.A., van Groeningen,M., Nooij,M., Aalders,M.C.G. and Baas,F. (2002) Tumor genotype specific growth inhibition in vivo by antisense oligonucleotides against a polymorphic site of the large subunit of human RNA Polymerase II. Cancer Res., 62, 2024–2028. [PubMed] [Google Scholar]
- 22.Graham M.J., Freier,S.M., Crooke,R.M., Ecker,D.J., Maslova,R.N. and Lesnik,E.A. (1993) Tritium labeling of antisense oligonucleotides by exchange with tritiated water. Nucleic Acids Res., 21, 3737–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer T., Regenass,U., Fabbro,D., Alteri,E., Rosel,J., Muller,M., Caravatti,G. and Matter,A. (1989) A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int. J. Cancer, 43, 851–856. [DOI] [PubMed] [Google Scholar]
- 24.Bijsterbosch M.K., Manoharan,M., Rump,E.T., De Vrueh,R.L., van Veghel,R., Tivel,K.L., Biessen,E.A., Bennett,C.F., Cook,P.D. and van Berkel,T.J. (1997) In vivo fate of phosphorothioate antisense oligodeoxynucleotides: predominant uptake by scavenger receptors on endothelial liver cells. Nucleic Acids Res., 25, 3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin A. (1999) A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim. Biophys. Acta, 1489, 69–84. [DOI] [PubMed] [Google Scholar]
- 26.Hirose Y. and Manley,J.L.(2000) RNA polymerase II and the integration of nuclear events. Genes Dev., 14, 1415–1429. [PubMed] [Google Scholar]
- 27.Nguyen V.T., Giannoni,F., Dubois,M.F., Seo,S.J., Vigneron,M., Dedinger,C. and Bensaude,O. (1996) In vivo degradation of RNA polymerase II largest subunit triggered by alpha-amanitin. Nucleic Acids Res., 24, 2924–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKay B.C., Chen,F., Clarke,S.T., Wiggin,H.E., Harley,L.M. and Ljungman,M. (2001) UV light-induced degradation of RNA polymerase II is dependent on the Cockayne’s syndrome A and B proteins but not p53 or MLH1. Mutat. Res., 485, 93–105. [DOI] [PubMed] [Google Scholar]
- 29.Martinez J., Patkaniowska,A., Urlaub,H., Luhrman,R. and Tuschl,T. (2002) Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell, 110, 563–574. [DOI] [PubMed] [Google Scholar]
- 30.Petersen M., Bondensgaard,K., Wengel,J. and Jacobsen,J.P. (2002) Locked nucleic acid (LNA) recognition of RNA: NMR solution structures of LNA:RNA hybrids. J. Am. Chem. Soc., 124, 5974–5982. [DOI] [PubMed] [Google Scholar]