Abstract
Activation of transcription factors by receptor mediated signaling is an essential step for T lymphocyte effector function. Following antigenic stimulation of T cells the two central cytokines IL-2 and TNFα are co-expressed and co-regulated. Two important transcription factors, i.e., early growth response (EGR) protein EGR-1 and nuclear factors of activated T cells (NFAT) protein NFATc, regulate transcription of the human IL-2 cytokine and the same combination of EGR and NFAT proteins seems relevant for coordinated cytokine expression. Here we demonstrate that the zinc finger protein EGR-1 and two members of the NFAT protein family bind simultaneously to adjacent elements position –168 to –150 within the TNFα promoter. Both promoter sites are important for TNFα gene transcription as shown by transfection assays having the IL-2 and TNFα promoters linked to a luciferase reporter. The use of promoter deletion constructs with the zinc finger protein (ZIP), the NFAT binding element or a combination of both deleted show a functional cooperation of these elements and of their binding factors. These experiments demonstrate that EGR-1 as well as EGR-4 functionally cooperate with NFAT proteins and induce expression of both cytokine genes. Using tagged NFATc and NFATp in glutathione S-transferase pull down assays showed interaction and physical complex formation of each NFAT protein with recombinant, as well as native, EGR-1 and EGR-4 proteins. Thus EGR-NFAT interaction and complex formation seems essential for human cytokine expression as adjacent ZIP and NFAT elements are conserved in the IL-2 and TNFα gene promoters. Binding of regulatory EGR and NFAT factors to these sites and the functional interaction and formation of stable heterodimeric complexes indicate an important role of these factors for gene transcription.
INTRODUCTION
The four ‘early growth response’ proteins EGR-1 to EGR-4 represent a family of DNA-binding zinc finger proteins that act as nuclear effectors of extracellular signals. EGR genes are transiently and coordinately induced upon activation of peripheral blood T lymphocytes (1,2). In addition EGR-genes are expressed in a wide range of cell types, including lymphoid and myeloid cells such as thymocytes, B cells and monocytes, as well as non lymphoid cells such as fibroblasts, kidney cells and neurons (3,4). The individual cDNAs have been isolated from several sources and consequently different names are used for EGR-1 (zif268, Krox24, tis8, NGFI-A and pAT 225) (5–7), EGR-2 (Krox 20, pAT591) (8,9), EGR-3 (PILOT) (10,11) and EGR-4 (NGFI-C, pAT133) (1,12). EGR transcripts are induced by a wide range of stimuli, such as activation and differentiation signals, tissue injury and apoptotic signals (3,4).
The four EGR proteins have highly related DNA-binding zinc finger domains, but their divergent flanking regions indicate specific functions of the individual proteins. Binding of all four recombinant EGR proteins to the same consensus sequence GCG G/TGG GCG is in agreement with the high conservation of their zinc finger domains (8,13). EGR-binding elements, indicative of EGR target genes, have been identified in a large panel of gene promoters, including immune effector genes, such as the cytokine IL-2 (14), the pro-inflammatory immune mediator TNFα (15), and cell surface molecules such as the IL-2 receptor (16), Fas/CD95 (17), ICAM-1 (18) and FasL (19). In addition EGR-1 binding elements are reported within the promoter domains of genes coding for transcription factors such as EGR-1, EGR-4, junD and nur77 (13,20–22), growth factors, such as insulin like growth factor-II, basic fibroblast growth factor, epidermal growth factor receptor, platelet-derived growth factor (PDGF), tissue growth factor (TGF) (3,23–28), cell cycle regulators, including the retinoblastoma susceptibility gene Rb (29), cyclin D1 (30) and in hormones, like the luteinizing hormone β (LH-β) (31).
The DNA binding role of the four EGR proteins is well established, but their contribution to gene transcription, tissue specific gene expression and precise mode of action are poorly defined so far. Over-expression of individual EGR factors in reporter gene assays shows rather low transcriptional activities, while in contrast deletion of the EGR-binding sites in the promoter reveals significant effects of these elements in gene transcription (14,15,31). A selective and specific role of individual EGR proteins in gene transcription is confirmed by the distinct phenotype of EGR knock-out animals: EGR-1 knock-out mice show infertility of female animals, due to the lack of luteinizing hormone β (LH-β) gene expression (32), EGR-2 knock-out mice have defective nerve development (33), EGR-3 knock-out mice lack formation of muscle spindles (34) and EGR-4 knock-out mice show infertility of male animals due to arrest in spermatogenesis (35).
Despite the ubiquitous synthesis of EGR proteins, EGR-regulated genes are expressed in a tissue-restricted manner. Thus specificity of EGR function may be due to interaction with other proteins, most likely transcription factors that bind to adjacent sites in a given promoter context, or by factors of the basic transcription network. Synergistic function of EGR-1 with other nuclear factors has been reported, e.g., with the homeobox protein Ptx1 and the steroidogenic factor 1 (Sf-1) for regulation of the pituitary luteinizing hormone β (LH-β) (31,32), with RelA in the regulation of NFkB1 (p50) transcription (36), with the cAMP response element-binding protein (CBP)/p300 in transcription of the lipoxygenase gene (37) and with NFATc in IL-2 gene expression (14). In addition functional interaction of EGR-1 with Sp1 is reported for macrophage colony-stimulating factor (M-CSF) gene expression (38) and interaction of EGR-1 with the viral proteins Tat (39), Tax (40), HBx (41) and IE2 (42) results in upregulation of genes like FasL, PDGF-B or TGF-β. In contrast complexes of EGR-1 with NAB1 or NAB2 are described as transcriptional inhibitors (43,44).
Given the unique role of EGR-1 in the transcriptional regulation of T-cell growth factor IL-2, the mechanism by which this ubiquitously expressed nuclear protein mediates T cell-specific gene transcription is of special interest. Cytokine gene expression is a hallmark for T cell effector function and consequently transcriptional regulation of the IL-2 and the pro-inflammatory TNFα gene are studied in great detail. In addition TNFα expression is deregulated in a number of human inflammatory diseases (45). Previously we described a functional, synergistic interaction of EGR-1 with the nuclear factor of activated T cells (NFATc) in regulation of IL-2 expression (14,46). NFAT proteins are considered key regulators of cytokine expression such as IL-2, IL-3, IL-4, IL-5, granulocyte-macrophage colony stimulated factor and TNFα genes (47,48). Five members of this protein family have been identified: NFATp (NFAT1, c2), NFATc (NFAT2, c1), NFAT3, NFATx (NFAT4, c3) and NFAT5. NFATp and NFATc are predominantly expressed in lymphoid cells, while NFATx is specifically detected in the thymus, NFAT3 is preferentially synthesized in non-lymphoid tissues and NFAT5 is a constitutive nuclear protein (47,49).
Here we identify a regulatory 35 bp promoter region which is conserved in sequence, position and orientation in the human IL-2 and TNFα gene promoters. These regions include binding sites for zinc finger proteins EGR-1 and Sp1 and for NFAT proteins and seem responsible for the coordinated expression of the two cytokine genes. Mobility shift assays show that the individual elements serve as binding sites for unique zinc finger—and for NFAT proteins. The ubiquitously expressed Sp1 and the inducible EGR-1 zinc finger protein bind to the ZIP elements of both promoters, while EGR-4 binds to the TNFα, but not the IL-2 site. Similarly NFATp binds to both promoters, while NFATc binds specifically to the IL-2 promoter. On a functional level interaction of EGR and NFAT proteins, but not of Sp1 contributes to transcription of both cytokine genes. This interaction is mediated by stable physical heterodimeric protein complexes. In summary these results explain how ubiquitously expressed transcription factors like EGR-proteins contribute to tissue or cell specific gene expression, like that of T cell specific effector genes.
MATERIALS AND METHODS
Plasmid construction
The TNFα reporter deletion constructs p-191T-Luc, p-163T-Luc, p-142T-Luc, p-41T-Luc and p-191Tm-Luc contain various domains of the human TNFα promoter. A vector with a 286 bp promoter region of the human TNF gene (kindly provided by Martin Krönke, University of Köln) was used as template in PCRs using primers TTTAGATCTGTCCC CAACTTTCCAAATCC (∼191T), TTTAGATCTGCGATG GAGAAGAAACCG (∼162T), TTTAGATCTGACAGAAGG TGCAGGGC (∼143T), TTTAGATCTGTCCCCAACTTTCC AAATCCCCGCCCCCGCGATGGATAATAAATCGAGAC (∼191Tm) and TTTAAGCTTCTGCTGTCCTTGCTGAGG (+34). The generated fragments were ligated into firefly luciferase vector pGL2-Basic (Promega). The reporter plasmid pTZN3-Luc has three copies of the ZIP/NFAT region (–172/–145) of the human TNFα promoter ligated to a minimal IL-2 promoter fragment (–63/+51) and inserted 5′ to the coding region of the luciferase gene in the pGL2-Basic vector. The reporter plasmid pZNA3-Luc contains three copies of the ZIP/NFAT/AP1 binding site of the IL-2 promoter (–302/–285) linked to a minimal IL-2 promoter fragment (–63/+51) ligated into the pGL2-Basic vector as previously described (46).
Expression plasmids pSG5-EGR-1 and pSG5-EGR4 representing the full-length human cDNAs under control of a SV40 promoter have been reported (22). Plasmid pPacSp1 that has 2.1 kb of the human Sp1 cDNA (encoding the C-terminal 696 amino acids of Sp1) inserted, was kindly provided by Robert Tjian, University of California, Berkeley, CA. Plasmid pSH107c that contains the full-length human NFATc cDNA and plasmid pSH210 that codes for the C-terminal 755 amino acids of NFATp were a generous gift of Gerald R. Crabtree, Stanford University, Stanford, CA. Expression plasmids pGEX-NFATc and pGEX-NFATp coding for NFAT-GST fusion proteins were provided by Edgar Serfling, University of Würzburg, Würzburg, Germany. All plasmids were sequenced using a standard dideoxy method.
Cell culture and transfection
The human helper T cell line Jurkat and the kidney cell line 293T were maintained in RPMI 1640 (Bio Whittaker), supplemented with 10% heat-inactivated fetal calf serum (FCS), penicillin, streptomycin and fungizone at a density between 0.5 × 105 and 8 × 105 cells per ml. For transient transfections, 1 × 107 cells were washed with PBS and resuspended in 0.8 ml transfection buffer [10 mM glucose, 0.1 mM dithiothreitol (DTT) in RPMI 1640]. Jurkat cells were transfected by electroporation at 300 V and 960 µF using 5 µg reporter construct and 7 µg of the indicated expression plasmid and 0.1 µg of pRL-SV40 vector (Promega) to control for transfection efficiency. In all experiments the total amount of transfected DNA was kept constant by addition of pSG5 plasmid DNA. After transfection, cells were incubated for 48 h in culture medium. When indicated, cells were stimulated 24 h after transfection with 1 µg/ml Phytohemagglutinin (PHA; Murex Diagnostics) and 20 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma Chemical Co., St. Louis, MO). Cells were harvested by washing two times with PBS, lysed by incubation in 250 µl lysis buffer (Promega) for 8 min at room temperature and centrifuged for 10 s at maximum speed in an Eppendorf microfuge. Cell supernatant (50 µl) was mixed with 50 µl Luciferase assay reagent (Promega) and the light emission was measured immediately at 25°C using a luminometer (Berthold Biolumat LB 9500C). Transfection of 293T cells was performed by calcium phosphate precipitation as described (50). About 4 × 105 cells were transfected with 2 µg of reporter plasmid and 3 µg of the various expression plasmids, together with 0.02 µg of pRL-SV40 vector. After 24 h, cells were washed twice with PBS and harvested. Cell lysis and measurement of luciferase activities were performed using the dual luciferase reporter assay according the manufacturer’s recommendation (Promega). The initial 10 s integral of light emission was recorded. All assays were performed in triplicate.
Expression of recombinant proteins and preparation of cell extract
Recombinant EGR-1 and EGR-4 were expressed in the baculovirus system as described (22). Briefly, Spodoptera frugiperda (Sf9) cells were grown in Grace’s medium (Bio Whittaker), supplemented with 10% heat-inactivated FCS (Gibco BRL), streptomycin (100 µg/ml, Bio Whittaker), penicillin (100 U/ml, Bio Whittaker) and fungizone (250 ng/ml, Gibco BRL) at 27°C in monolayer cultures. Sf9 cells were infected with recombinant virus in Insect-Xpress medium (Bio Whittaker), using a multiplicity of infection of 5. After three days cells were harvested, resuspended in lysis buffer (20 mM HEPES pH 7.8, 100 mM NaCl, 0.4 mM EDTA, 0.5 mM DTT, 10 mM ZnCl2 and 25% Glycerol), containing the proteinase inhibitors phenylmethylsulfonyl fluoride (PMSF) (0.5 mM; Sigma), aprotinin (2 mg/ml; Boehringer Mannheim) and leupeptin (5 mg/ml; Boehringer Mannheim) and lysed by three freeze/thaw cycles. The extracts were cleared by centrifugation at 12 000 g for 20 min at 4°C and stored in aliquots at –70°C.
Recombinant NFATc and NFATp protein were expressed as glutathione S-transferase (GST)-fusion proteins in Escherichia coli. An overnight culture of pGEX-NFATc and NFATp transformed cells (DH5α/BL21) was added to 100 ml LB medium containing 50 µg/ml ampicillin, 0.1% glucose and grown for 2 h at 37°C. After induction of protein expression by IPTG cells were grown at room temperature for an additional hour. Cells were harvested, resuspended in 2 ml PBS containing 1% Triton X-100 (Sigma), 1 mM DTT, 1 mM PMSF lysed on ice by sonication and cellular debris were pelleted by centrifugation. Upon addition of 1 ml glutathione– agarose beads (Sigma) (50% v/v) the mixture was incubated for 20 min at 4°C on a rotating platform. Beads were collected by brief centrifugation (1 min at 800 g) and washed five times with PBS (containing DTT and PMSF). GST-NFATc and NFATp fusion proteins were eluted with 50 mM Tris–HCl, pH 8.0, containing 10 mM glutathione (Boehringer Mannheim). Eluted proteins were aliquoted and stored at –7°C. Recombinant Sp1 protein was obtained from Promega.
Jurkat cells (5 × 107) were stimulated for 2.5 h with 1 µg/ml PHA and 20 ng/ml PMA, washed twice in PBS and harvested. Cells were allowed to swell for 10 min at 4°C in RSB (10 mM Tris–HCL pH 7.4, 10 mM NaCl, 1.5 mM MgCl2, 10 mM NaF, 0.5 mM DTT and 0.5 mM PMSF) and were lysed by incubation for 45 min in buffer C (20 mM HEPES pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerin, 10 mM NaF, 0.5 mM DTT and 0.5 mM PMSF). Lysates were cleared by centrifugation for 10 min (14 000 r.p.m.; Eppendorf centrifuge) and frozen in aliquots at –70°C.
Protein binding assay
GST fusion proteins were dialyzed against PBS buffer, using a Biomax-5K-concentrator (Millipore). About 400–600 µg of recombinant proteins were incubated for 30 min with 200 µl glutathione–agarose beads (50% v/v) in a total volume of 1.1 ml PBS (containing 0.5 mM DTT and 0.5 mM PMSF). After centrifugation Sf9 cell extract, containing 300–500 µg of recombinant EGR proteins or Jurkat cell extract (∼400– 600 µg) were added to the pellets in a total volume of 1.1 ml NENT buffer (20mM Tris–HCl pH 8, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40 and 0.15 µg/ml BSA). This mixture was incubated for 40–60 min, centrifuged and the pelleted beads were washed four times using 1 ml NENT buffer. Proteins were eluted with 100 µl glutathione in 50 mM Tris. All fractions were tested by gel electophoresis followed by silver staining and western blot analysis.
SDS–PAGE and western blot analysis
SDS–PAGE was performed as described (50) using 10% separating gels and prestained high and low molecular weight marker proteins (Bio-Rad). Proteins were transferred onto nitrocellulose membrane by semidry blotting. Membranes were blocked with 5% (w/v) dried milk in PBS for 30 min and incubated overnight at 4°C using polyclonal rabbit anti-human EGR-1 (Santa Cruz Biotechnologies), EGR-4 (44), Sp1 (PEP2, Santa Cruz Biotechnologies) antisera, or monoclonal mouse anti-human NFATc (ABR, Inc., CO) or NFATp (UBI/Biomol) antibodies. For detection of the expressed control protein FH 15-20 a specific goat anti Factor H antiserum (Calbiochem) was used.
Electrophoretic mobility shift assay (EMSA)
For EMSAs, 1–5 pM of double-stranded oligonucleotides were end-labelled with [γ-33P]dATP (specific activity: 3000 Ci/mM; Amersham Buchler). 10–30 µM of the labelled oligonucleotides were incubated at 4°C with recombinant proteins in 20 µl buffer containing 20 mM HEPES, pH 7.9, 50 mM KCl, 0.5 mM DTT, 1 mM MgCl2 and 4% (v/v) Ficoll for 30 min in the presence of 0.5 µg poly(dI-dC) (Pharmacia). The resulting DNA–protein complexes were separated by electrophoresis on a 5% non-denaturing polyacrylamide gel at 4°C in 0.25 × TBE at 150 V and 20 mA. Double-stranded oligonucleotides representing the ZIP/NFAT site of the human TNFα promoter (T; position –174 to –142) as well as the complete IL-2 ZIP/NFAT region, (ZN; position –302 to –258) were used.
RESULTS
Conservation of ZIP and NFAT elements within the human IL-2 and TNFα gene promoters
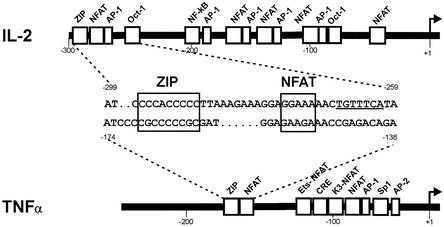
In T lymphocytes, EGR-1 and NFATc function as central regulators of IL-2 gene expression as these factors bind to adjacent regulatory ZIP and NFAT promoter elements (14,46). As the pro-inflammatory TNFα and the IL-2 gene are co-expressed in T cells, it is of interest to identify regulatory elements that mediate co-expression. A sequence alignment reveals a stretch of highly related sequences, which include the regulatory ZIP and NFAT elements in a similar position, distance and orientation (Fig. 1). This conservation suggested a conserved role of the ZIP and the NFAT sites for TNFα gene regulation.
Figure 1.
Comparison of the human IL-2 and TNFα promoter segments. The individual regulatory sites together with the corresponding binding proteins are indicated in the human IL-2 (top) and TNFα gene promoters (bottom). Direct sequence alignment shows conservation of ZIP/NFAT binding regions (boxed) at positions –296 to –288 (IL-2) and –169 to –150 (TNFα). The AP-1 binding site within the human IL-2 gene promoter is underlined. Numbers indicate positions relative to the start site of transcription (+1).
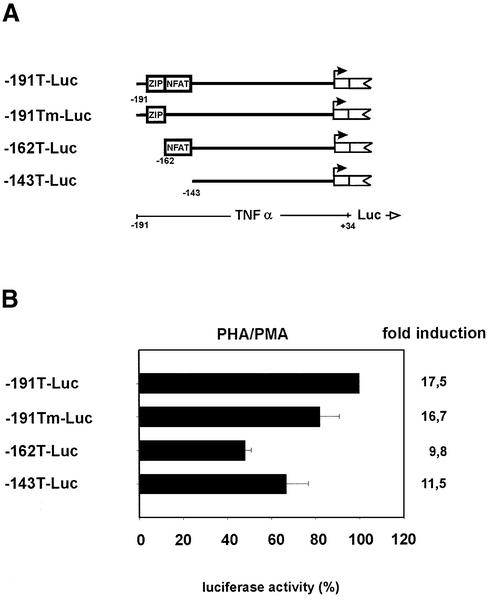
The conserved promoter elements play a role in TNFα gene transcription
Reporter gene constructs were created in order to functionally characterize the ZIP and the NFAT sites of the human TNFα gene promoter. The four constructs represent either the wild type configuration (p-191T-Luc), have the individual ZIP or NFAT binding elements deleted (p-191Tm-Luc and p162T-Luc) or lack both elements (p-143T-Luc) (Fig. 2A). This set of constructs was tested for expression and regulation in Jurkat T cells. Upon transfection all four constructs showed only background activity in unstimulated cells (1% of that of activated cells) and responded differently to PHA/PMA activation (Fig. 2B). The wild type construct (p-191T-Luc), containing both the EGR and NFAT sites, and the construct with the deleted NFAT site (p-191Tm-Luc), were induced 17.5- and 16.7-fold, respectively. In contrast, the constructs which had either the EGR site (p-162T-Luc) or both the EGR and NFAT sites deleted (p-143T-Luc) showed a reduced activity (9.8- and 11.5-fold induction). These activities represent ∼50 and 67% of those obtained with the activated wild type construct (100%), thus showing that the EGR site together with the NFAT element contribute to TNFα promoter activity.
Figure 2.
Regulatory elements within the human TNFα gene promoter. (A) Schematic structure of the reported constructs representing the wild type TNFα promoter (-191T-Luc) and the deletion mutants having the NFAT binding site (-191Tm-Luc), the ZIP site (-162T-Luc) or both the ZIP and NFAT sites deleted (-143T-Luc). (B) The indicated reporter constructs were transfected into Jurkat T cells left untreated for 18–24 h or stimulated for the same time with PHA/PMA. The luciferase activity obtained in PHA/PMA stimulated cells is shown in comparison to the wild type construct, p-191-Luc which was set at 100%. Luciferase activities shown as fold inductions were compared between transfected uninduced cells and transfected but PHA/PMA induced cells. (±SEM).
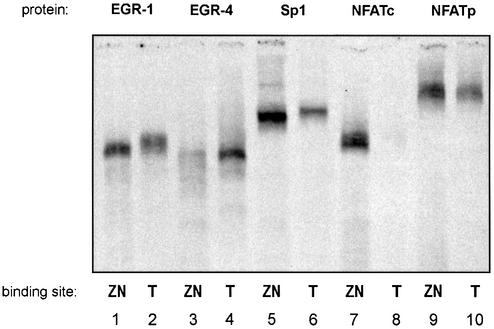
Proteins binding to the conserved promoter regions
Having demonstrated a regulatory effect of both the ZIP and the NFAT consensus sites within the human TNFα gene promoter we were interested to identify which proteins bind to these sites. Recombinant EGR-1, EGR-4, Sp1 and NFAT proteins were used in EMSA with oligonucleotides representing the ZIP-NFAT region either of the human IL-2 (ZN) or the TNFα gene promoter (T). EGR-1 and Sp1 bound to the IL-2, as well as to the TNFα sites although with different affinities. A stronger binding is observed to the IL-2 element (Fig. 3, lanes 1, 2, 5 and 6). In contrast recombinant EGR-4 bound rather weakly to the IL-2 site, but strongly to the TNFα element (Fig. 3, lanes 3 and 4). Similarly NFATc and NFATp proteins showed different binding. NFATc bound to the IL-2, but not the TNFα site (Fig. 3, lanes 7 and 8), while NFATp bound to both promoter elements (Fig. 3, lanes 9 and 10). These data demonstrate different binding of the inducible EGR and NFAT proteins to the human IL-2 and TNFα gene promoters.
Figure 3.
Binding of zinc finger and NFAT proteins to the ZIP/NFAT binding regions of the IL-2 and TNFα gene promoters. Binding of recombinant proteins to 33P-labelled oligonucleotides representing the ZIP/NFAT sites of the IL-2 (ZN) or of the TNFα gene (T) was assessed by electophoretic mobility shift assays. EGR-1, Sp1 and NFATp bind to both the ZIP/NFAT binding elements (lane 1, 2, 5, 6, 9 and 10). EGR-4 binds with low affinity to the ZIP/NFAT region of the IL-2 gene (lane 3), and with high affinity to the TNFα element (lane 4). Similarly recombinant NFATc protein binds to the ZIP/NFAT region of the IL-2 promoter (lane 7), but not to the TNFα region (lane 8), while NFATp binds to both sites (lanes 9 and 10).
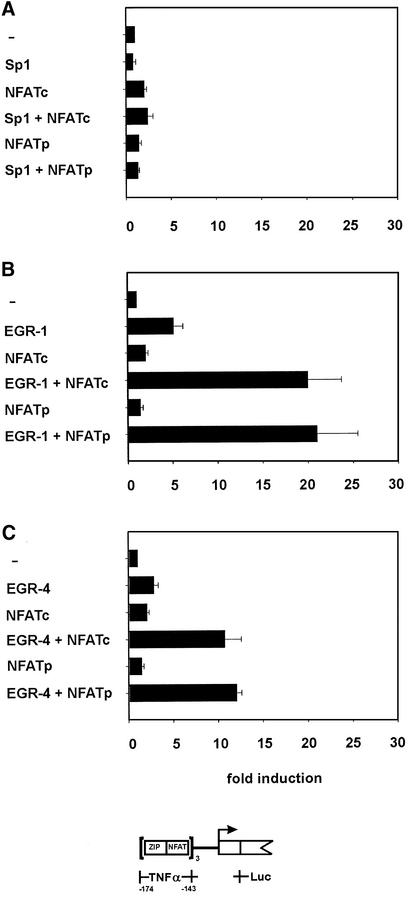
EGR-1 and EGR-4 regulate the TNFα reporter gene transcription in combination with NFATc and NFATp
Having demonstrated binding of three different zinc finger proteins to the ZIP site and specific binding of NFATp to the NFAT site of the TNFα gene promoter, we wanted to identify the factor or the combination of factors which mediate transcription of the TNFα gene. To this end, cotransfection experiments were performed in non-TNFα expressing 293T cells using several expression vectors together with reporter construct pTZN3-Luc. This reporter construct has three copies of the TNFα ZIP-NFAT region and a minimal promoter element linked to the firefly luciferase gene. Expression of the zinc finger protein Sp1 alone or in combination with NFATc or NFATp showed minimal effects on reporter gene expression (2–3-fold, Fig. 4A). Thus neither Sp1 nor NFATc or NFATp proteins alone, or a combination of these factors, contribute to TNFα gene expression.
Figure 4.
Effect of the ZIP-binding zinc finger proteins Sp1, EGR-1 and EGR-4 on regulation of NFAT transcriptional activity. Human 293 kidney cells were transfected with reporter construct pTZN3-Luc together with expression vectors coding for EGR-1 or EGR-4, or Sp1 or NFATc or NFATp or combinations thereof. (A) Cells were transfected with an expression vector coding for Sp1 alone or in combination with NFATc or NFATp. (B) Cells were transfected with an expression construct coding for EGR-1 alone or in combination with NFATc or NFATp. (C) A reporter construct encoding EGR-4 was used alone or in combination with NFATc or NFATp. The transcriptional activity is shown as fold induction of the activity of the reporter construct alone, which was set to 1. Each column represents the mean of at least three independent experiments and standard deviations are indicated.
Expression of EGR-1 resulted in a 5-fold increase in reporter gene activity (Fig. 4B), which is in agreement with previous results (15). Co-expression of EGR-1 with either NFATc or NFATp resulted in a 20-fold induction of reporter gene expression, demonstrating synergistic interaction of EGR-1 with both NFAT proteins. Expression of EGR-4 as a single protein showed ∼3-fold induction of the reporter construct (Fig. 4C). This zinc finger protein showed a similar cooperative effect with the analysed NFAT proteins, resulting in a 10-fold increase in reporter gene activity. Although three zinc finger proteins Sp1, EGR-1 and EGR-4 bind to the ZIP element of the human TNFα gene promoter (Fig. 3), the two inducible EGR proteins, but not the constitutively expressed Sp1 protein cooperate with NFATc and NFATp in TNFα gene expression. Interaction of EGR-1 with both NFAT proteins results in a 20-fold induction of the reporter construct.
EGR-1 and EGR-4 cooperate with NFAT proteins in context of the wild type TNFα promoter
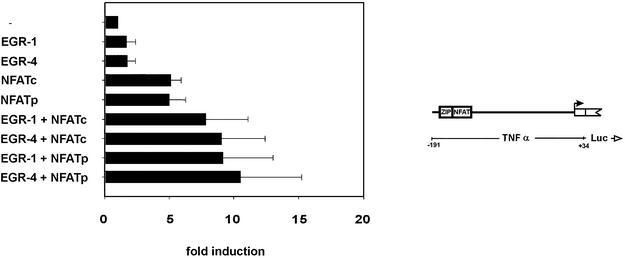
Having demonstrated synergistic cooperation of EGR and NFAT proteins in the regulation of a synthetic construct, we also tested their effect on a wild type construct, that includes the complete 191 bp of the TNFα promoter. Used as single proteins both EGR proteins induced transcription ∼1.7-fold and both NFAT proteins induced transcription ∼5-fold. In combination EGR and NFAT proteins showed higher activities, ranging from 7.5- to 11-fold induction (Fig. 5). The highest levels, ∼11-fold activation was observed with EGR-4 in combination with NFATp. This value corresponds to 65% of the induction of the same reporter construct by PHA and PMA (17.5-fold induction; data not shown).
Figure 5.
Individual combinations of EGR and NFAT family members affect transcription of the wild type TNFα gene promoter. Human 293 kidney cells were transfected with a reporter construct containing the wild type (–191 bp promoter region) of human TNFα gene together with expression vectors coding for the indicated EGR and NFAT proteins. Mean values and standard deviations of at least three independent experiments are indicated.
EGR-1 and EGR-4 interact with NFATc and NFATp proteins in activation of a IL-2 reporter gene
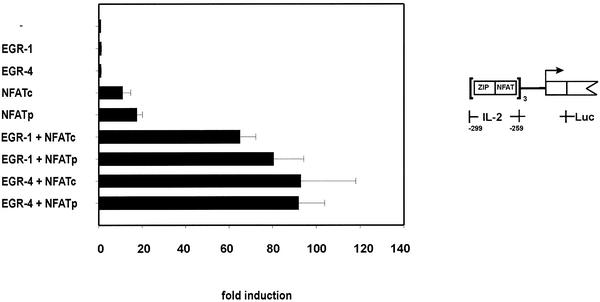
The functional interaction of EGR-1 and EGR-4 with the two NFAT proteins was also tested for the IL-2 gene promoter. To this end cotransfection assays were performed in 293T cells using a synthetic reporter construct which includes three copies of the IL-2 ZIP:NFAT binding elements and a minimal IL-2 promoter linked the luciferase gene (Fig. 6). As single proteins EGR-1 and EGR-4 showed little activity on reporter gene transcription (1.25-fold) and NFATc and NFATp induced luciferase expression 11- and 17.6-fold. Synergistic effects were observed upon coexpression of EGR and NFAT proteins. In combination with NFATc EGR-1 enhanced transcription 65-fold and together with NFATp 80.5-fold. EGR-4 coexpressed with NFATc or NFATp showed similar effects, reaching ∼90-fold induction of the reporter gene. These results demonstrate a strong functional interaction of both EGR proteins with the two analysed NFAT proteins.
Figure 6.
Functional interaction of EGR and NFAT proteins. Human 293 kidney cells were transfected with a reporter construct pZNA3-Luc and expression vectors coding for the indicated transcription factors used alone or in combination. The values represent fold induction and are compared to the luciferase activity obtained with the reporter construct in the absence of expressed proteins (1-fold). Each column represents the mean value of four independent experiments and standard deviations are indicated.
Recombinant EGR and NFAT proteins form stable complexes
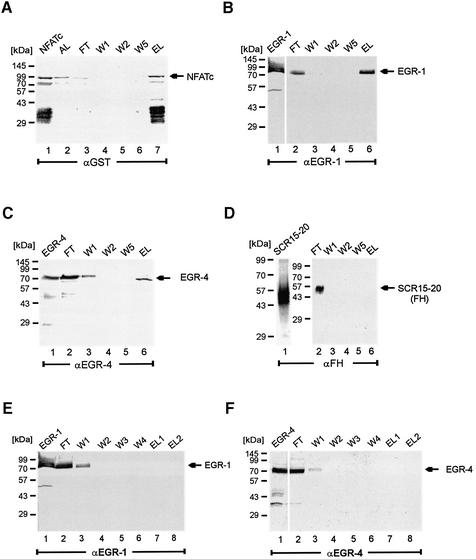
Given the functional synergy between the analysed EGR and NFAT proteins in the regulation of TNFα and IL-2 gene transcription (Figs 4 and 6), we next asked whether these proteins form physical complexes. EGR-1 and NFATc bind simultaneously to adjacent sites within the human IL-2 promoter, thus in their promoter bound context the two factors are in close contact (46). By mobility shift assays we were unable to demonstrate a direct physical interaction of these proteins (12, and data not shown). Therefore an affinity based system was used to analyse heterodimer formation. NFATp and NFATc expressed as GST tagged fusion proteins were attached to a gluthatione matrix and binding of recombinant and native EGR proteins was assayed. Cell extract, prepared from insect cells expressing either recombinant EGR-1 or EGR-4 was applied to the NFAT matrix and after extensive washing bound proteins were eluted, separated by SDS–PAGE and analysed by western blotting. Specificity of coupling, binding and elution of NFATc is shown in Figure 7A. Even in the presence of protease inhibitors proteolytic degradation occurred, as detected by the appearance of additional bands of lower molecular mass (Fig. 7A, lanes 1 and 7). Recombinant EGR-1 and EGR-4 proteins were detected by western blotting as 80 and 79 kDa proteins (Figs 7B, lane 1 and 7C, lane 1). Both EGR proteins did not bind completely to the NFATc matrix, and were still detectable in the flow through fractions (Fig. 7B, lane 2 and Fig. 7C, lane 2). Specific attachment is observed as both proteins were present in the elute, but not in the wash fractions (compare Fig. 7B, lanes 5 and 6; with Fig. 7C, lanes 5 and 6). These experiments demonstrate a direct physical interaction between EGR-1 and EGR-4 with NFATc. Specificity of the interaction was further demonstrated by using cell extract from cells expressing an unrelated protein [a deletion mutant of complement factor H (SCRs 15–20)] (Fig. 7D). In this control assay no bound proteins were detected (Fig. 7D, lane 6). To exclude non-specific binding of EGR-1 and EGR-4 to the GST matrix, recombinant proteins were incubated with the GST matrix and wash and elute fractions were tested for the presence of EGR proteins. Neither EGR-1 (Fig. 7E) nor EGR-4 (Fig. 7F) bound to the matrix.
Figure 7.
Interaction of recombinant EGR-1 and EGR-4 proteins with NFATc. (A) Recombinant GST-NFATc protein expressed in E.coli was coupled to GST. Various wash and elute fractions were obtained, separated by SDS–PAGE, transferred to nitrocellulose and assayed by western blotting using the indicated antibodies. The individual lanes represent the recombinant protein (lane 1), the material obtained after loading the column (lane 2), the flow through, (lane 3), several wash (lanes 4, 5 and 6) and the elute fraction (lane 7). (B) Interaction of EGR-1 and NFATc. EGR-1 expressed in insect cells (lane 1) was applied to an NFATc matrix and wash and elute fractions were assayed using an EGR-1 specific antiserum. (C) Interaction of EGR-4 and NFATc. EGR-4 expressed in insect cells (lane 1) was added to the NFATc matrix and wash and elute fractions were assayed with an EGR-4 specific antiserum. (D) As control an unrelated recombinant protein (SCRs 15–20 of complement factor H) (lane 1) was assayed for binding and the individual fractions were assayed using an anti factor H antiserum. (E) To exclude non-specific binding of EGR-1 and (F) of EGR-4, the GST matrixes were incubated with the recombinant EGR proteins and wash and elute fractions were assayed for presence of EGR proteins.
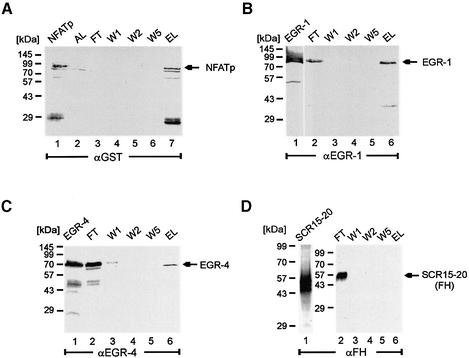
The same approach was used to analyse interaction of recombinant EGR-1 and EGR-4 with NFATp. NFATp was expressed as GST-tagged fusion protein, coupled to the matrix and analysed for binding. Both recombinant EGR-1 and EGR-4 were detected in the elute, but not in the wash fractions (Fig. 8B lanes 5 and 6; Fig. 8C lanes 5 and 6). These experiments demonstrate a direct physical interaction between recombinant EGR-1 and EGR-4 proteins with NFATp.
Figure 8.
Interaction of recombinant EGR-1 and EGR-4 proteins with NFATp. (A) Recombinant GST-NFATp protein expressed in E.coli was coupled to GST affinity matrix. Various wash and elute fractions were obtained, separated by SDS–PAGE electrophoresis, transferred to nitrocellulose and assayed by western blotting using the indicated antibodies. The individual lanes represent the recombinant protein (lane 1), the material obtained after loading the column (lane 2), the flow through, (lane 3), various wash (lanes 4, 5 and 6) and the elute fraction (lane 7). (B) Interaction of EGR-1 and NFATp. EGR-1 expressed in insect cells (lane 1) was added to an NFATp matrix and wash and elute fractions were assayed with an EGR-1 specific antiserum. (C) Interaction of EGR-4 and NFATp. EGR-4 expressed in insect cells (lane 1) was applied to the NFATp matrix and wash and elute fractions were assayed with an EGR-4 specific antiserum. (D) As control an unrelated recombinant protein (FH SCR 15–20) (lane 1) was assayed for binding and an anti factor H antiserum was used for detection of the individual fractions.
Native EGR-1 and EGR-4 expressed in Jurkat cells interact with NFATc and NFATp
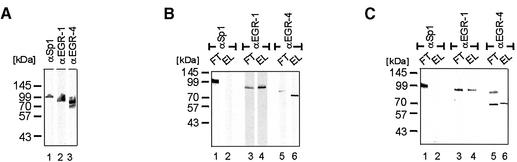
The relevance of the interaction observed with recombinant proteins was confirmed using native EGR proteins expressed in Jurkat T cells. Jurkat T cells were stimulated by PHA/PMA and the three zinc finger proteins Sp1, EGR-1 and EGR-4 were detected by western blotting as 99, 80 and 79 kDa bands (Fig. 9A, lanes 1, 2 and 3). This extract was applied to NFATc or NFATp matrixes and interaction was tested by analysing the proteins in the elute fractions. Sp1 was detected in the flow through, but not in the elute fractions (Fig. 9B, lanes 1 and 2; Fig. 9C lanes 1 and 2), showing that Sp1 did not bind to NFATc or to NFATp. In contrast both Jurkat derived EGR proteins were detected in the elute fractions (Fig. 9B, lanes 4 and 6, Fig. 9C, lanes 4 and 6). These experiments demonstrate physical interaction between native, Jurkat expressed EGR-1 or EGR-4 with both NFATc and NFATp and show formation of stable EGR–NFAT protein complexes.
Figure 9.
Native EGR proteins interact with recombinant NFAT proteins. (A) Detection of Sp1, EGR-1 and EGR-4 proteins in cell extract prepared from stimulated Jurkat T cells by SDS–PAGE and western blotting, using specific antisera for Sp1 (lane 1), EGR-1 (lane 2) and EGR-4 (lane 3). (B) Following application of Jurkat cell extract to a NFATc matrix, the flow through (FT) and elute (EL) fractions were assayed by SDS–PAGE and western blotting with antisera for Sp1 (lanes 1 and 2), EGR-1 (lanes 3 and 4) and EGR-4 (lanes 5 and 6). (C) Following application of cell extract derived from stimulated Jurkat T cells and added to a NFATp matrix, the flow through (FT) and elute (EL) fractions were assayed by SDS–PAGE and western blotting using the indicated antisera for Sp1 (lanes 1 and 2), EGR-1 (lanes 3 and 4) and EGR-4 (lanes 5 and 6). The mobility of the marker proteins is indicated on the left.
DISCUSSION
Binding of extracellular ligands to the T-cell receptor complex generates cytoplasmic signals that are transferred to the nucleus and consequently a large panel of transcription factors are induced. These nuclear factors include EGR, NFAT, AP-1 and NF-κB proteins which initiate transcription of immune effector genes including the T-cell specific growth factor IL-2 and the proinflammatory mediator TNFα. The activated transcription factors bind to adjacent promoter elements where they may interact with each other. Here we show that members of the EGR and NFAT protein families form stable heterodimeric complexes and cooperate in tissue specific expression of the immune effector genes IL-2 and TNFα. These stable EGR/NFAT complexes may help or even initiate assembly of the higher ordered protein complex, which controls gene transcription. Consequently a higher ordered protein complex can form, which combines inducible transcription factors and proteins of the basal transcriptional machinery like transcription associated factors (TAFs). Such a network of proteins is responsible for positioning RNA-polymerase II and initiation of mRNA transcription.
The human cytokine gene IL-2 and the pro-inflammatory mediator TNFα are co-expressed and co-regulated in human T-lymphocytes and based on their central immune regulatory roles both genes represent paradigms for cytokine gene expression. A 300 bp promoter element of the human IL-2 gene is essential for T cell specific expression and mediates the effects of the immune suppressive drug CSA (51). Similarly a 250 bp region of the human TNFα gene is sufficient for T cell specific expression. The essential pro-inflammatory role of TNFα is highlighted by the deregulated expression in a number of diseases e.g. consequently low TNFα levels contribute to the pathophysiology rheumatoid arthritis and septic shock (45).
In the human IL-2 and TNFα cytokine gene promoters, the sequence, position, distance and orientation of the EGR/Sp1 (ZIP) and NFAT binding sites are highly conserved. In the human IL-2 gene promoter the 37 bp promoter region, which includes the zinc finger protein (ZIP), the NFAT and the AP-1 binding elements (position –297 to –261) is important for transcription (14). The ZIP-element serves as a binding site for two distinct zinc finger proteins Sp1 and EGR-1. In non IL-2 producing cells the ubiquitously expressed Sp1 protein binds to this site and in IL-2 secreting cells Sp1 is replaced by the transiently induced EGR-1 protein (14,46). Individual members of the NFAT protein and AP-1 protein families bind to the NFAT and the adjacent AP-1 site (51,52). The human TNFα gene promoter contains a similar region located at positions –169 to –150, which represents binding sites for the zinc finger proteins Sp1, EGR-1, EGR-4 and NFATp (Figs 1 and 3) (15,53).
The conservation of these regulatory sites indicates a general role for cytokine expression. The importance of the ZIP and NFATp sites for TNFα gene transcription is demonstrated by transfection experiments, as the intact wild type promoter conferred transcriptional activity in T cells (Fig. 2). Deletion of the ZIP site reduces transcriptional activity by ∼50% and deletion of both the ZIP and NFAT sites by ∼40% (Fig. 2). Thus the ZIP promoter element contributes to TNFα promoter activity and gene expression. These experiments also reveal a regulatory role for the additional downstream elements, which include a cAMP response element (CRE) that binds STF-2/Jun and a k3 and a proximal NFAT binding site (position –121 to –67) (53,54).
Although highly related in sequence, distance and orientation the ZIP and NFAT elements display sequence differences which affect protein binding. The ZIP sites of both the IL-2 and TNFα promoter serve as binding sites for Sp1 and EGR-1, but EGR-4 binds specifically to the TNFα and not, or rather weakly to the IL-2 element (Fig. 3). Similarly NFATp, but not NFATc binds to the TNFα promoter (Fig. 3), and both NFATc and NFATp bind to the IL-2 promoter (Fig. 3). The difference in sequences affects the affinity for each protein and thus selects binding of specific proteins. Formation of unique heterodimeric protein complexes at the particular promoter site seems of functional relevance as combinations of distinct EGR and NFAT proteins affect transcription differently (Figs 4 and 6).
Upon binding to adjacent promoter sites EGR and NFAT proteins are in close contact to each other and as demonstrated by GST pull down assays, form heterodimers (Figs 7, 8 and 9). Clearly EGR-1, as well as EGR-4 form stable physical complexes with both NFATc and NFATp (Figs 7, 8 and 9). These complexes are specific; as (i) the components are detected in the elute but not in the wash fractions (Figs 7, 8 and 9); (ii) are observed with both recombinant and the native proteins expressed in insect cells or Jurkat T cells, respectively (Figs 7, 8 and 9); (iii) are not detected with the transcription factor Sp1, which also binds to the ZIP site (Fig. 9); and (iv) are independent of the surface, as they are not detected with a matrix lacking NFAT (Fig. 7).
Physical complex formation and the functional interaction highlights the cooperative effects of these DNA binding EGR and NFAT proteins on human cytokine gene transcription. As heterodimer formation is observed in the absence of DNA, promoter binding of one single component seems sufficient. The functional cooperativity is also observed for combinations, where only one partner binds to the promoter [e.g. NFATc in combination with EGR-1 and EGR-4 in TNFα context (Fig. 5) and for EGR-4 in IL-2 gene transcription (Fig. 6)].
Due to their sequence specific DNA-binding activities, EGR-proteins are considered transcription factors which regulate in positive and negative manner transcription of specific target genes. However in reporter gene assays, the single proteins display only marginal effects on transcription. This lack of activity indicates the requirement for cooperating factors. EGR-1 interacting proteins, with highly tissue specific expression have been identified for specific promoter contexts such as: (i) the homeobox protein Ptx1 and the steroidogenic factor 1 (Sf-1) in regulation of luteinizing hormone β (LH-β) expression (31,32); (ii) the p65 protein in NFkB1 (p50) gene induction (36); (iii) the CBP/p300 upon upregulation of the lipoxygenase gene transcription (37); (iv) the tumour suppressor p53 in programmed cell death (26); (v) the NFATc in FasL promoter activity (19); and (vi) the repressor proteins termed NAB1 and NAB2 (55,56). In summary this functional cooperation explains how inducible and ubiquitously expressed nuclear factors, like the EGR-proteins, contribute to tissue specific gene transcription.
Acknowledgments
ACKNOWLEDGEMENTS
The work of the authors is funded by the Thüringer Ministerium für Wissenschaft, Forschung und Technologie (TMWFK). We thank M. Kroenke at the University of Cologne, B. Tjian and E. Serfling, for their kind gift of plasmids.
REFERENCES
- 1.Müller H.-J., Skerka,C., Bialonski,A. and Zipfel,P.F. (1991) Clone pAT 133 identifies a gene that encodes another human member of a class of growth factor induced genes with almost identical zinc-finger domains. Proc. Natl Acad. Sci. USA, 88, 10079–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Castillo A., Pipaon,C., Garcia,I. and Alemany,S. (1993) NGFI-A gene expression is necessary for T lymphocyte proliferation. J. Biol. Chem., 268, 19445–19450. [PubMed] [Google Scholar]
- 3.Gashler A. and Sukhatme,V.P. (1995) Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog. Nucleic Acid Res. Mol. Biol., 50, 191–224. [DOI] [PubMed] [Google Scholar]
- 4.Beckmann M.A. and Wilce,P.A. (1997) Egr transcription factors in the nervous system. Neurochem. Int., 31, 477–510. [DOI] [PubMed] [Google Scholar]
- 5.Milbrandt J. (1987) A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science, 238, 797–799. [DOI] [PubMed] [Google Scholar]
- 6.Suggs S.V., Katzowitz,J.L., Tsai-Morris,C. and Sukhaktme,V.P. (1990) cDNA sequence of the human cellular early growth response gene Egr-1. Nucleic Acids Res., 18, 4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright J.J., Gunter,K.C., Mitsuya,H., Irving,S.G., Kelly,K. and Siebenlist,U. (1990) Expression of a zinc finger gene in HTLV-I and HTLV-II transformed cells. Science, 248, 588–591. [DOI] [PubMed] [Google Scholar]
- 8.Chavrier P., Zerial,M., Lemaire,P., Almendral,J., Bravo,R. and Charney,P. (1988) A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO J., 7, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph L.J., Le Beau,M.M., Jamieson,G.A.,Jr, Acharya,S., Shows,T.B., Rowley,J.D. and Sukhatme,V.P. (1988) Molecular colning, sequencing and mapping of EGR2, a human early growth response gene encoding a protein with ‘zinc-binding finger’ structure. Proc. Natl Acad. Sci. USA, 85, 7164–7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mages H.W., Stamminger,T., Rilke,O., Bravo,R. and Kroczek,R.A. (1993) Expression of Pilot, a putative transcription factor, requires two signals and is cyclosporin A sensitive in T cells. Int. Immunol., 5, 63–70. [DOI] [PubMed] [Google Scholar]
- 11.Patwardhan S., Gashler,A., Siegel,M.G., Chang,L.C., Joseph,L., Shows,T.B., Le Beau,M.M. and Sukhatme,V.P. (1991) EGR-3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene, 6, 917–928. [PubMed] [Google Scholar]
- 12.Crosby S.D., Puetz,J.J., Simburger,K.S., Fahrner,T.J. and Milbrandt,J. (1991) The early response gene NGFI-C encodes a zinc finger transcriptional activator and is a member of the GCGGGGGCG (GSG) element-binding protein family. Mol. Cell. Biol., 11, 3835–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao X., Guy,G.R., Sukhatme,V.P. and Tan,Y.H. (1993) Regulation of the EGR-1 gene by tumor necrosis factor and interferons in primary human fibroblasts. J. Biol. Chem., 267, 1345–1349. [PubMed] [Google Scholar]
- 14.Skerka C., Decker,E.L. and Zipfel,P.F. (1995) A regulatory element in the human interleukin 2 gene promoter is a binding site for zinc finger proteins Sp1 and EGR-1. J. Biol. Chem., 270, 22500–22506. [DOI] [PubMed] [Google Scholar]
- 15.Krämer B., Meichle,A., Hensel,G., Charnay,P. and Krönke,M. (1994) Characterization of an Krox-24/Egr-1 responsive element in the human tumor necrosis factor promoter. Biochim. Biophys. Acta, 1219, 413–421. [DOI] [PubMed] [Google Scholar]
- 16.Lin J.-X. and Leonard,W.J. (1997) The immediate-early gene product Egr-1 regulates the human interleukin-2 receptor b-chain promoter through noncanonical Egr and Sp1 binding sites. Mol. Cell. Biol., 17, 3714–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinkel A., Aicher,W.K., Haas,C., Zipfel,P.F., Peter,H.H. and Eibel,H. (1997) Transcription factor Egr-1 activity down-regulates Fas and CD23 expression in B cells. J. Immunol., 159, 2678–2684. [PubMed] [Google Scholar]
- 18.Maltzman J.S., Carmen,J.A. and Monroe,J.G. (1996) Transcriptional regulation of the Icam-1 gene in antigen receptor- and phorbol ester-stimulated B lymphocytes: role for transcription factor EGR-1. J. Exp. Med., 183, 1747–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li-Weber M., Laur,O. and Krammer,P.H. (1999) Novel Egr/NFAT composite sites mediate activation of the CD95 (APO/Fas) ligand promoter in response to T cell stimulation. Eur. J. Immunol., 29, 3017–3027. [DOI] [PubMed] [Google Scholar]
- 20.Christy B. and Nathans,D. (1989) DNA binding site of the growth factor-inducible protein Zif268. Proc. Natl Acad. Sci. USA, 86, 8737–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z.Y. and Duel,T.F. (1992) An S1 nuclease-sensitive homopurine/homopyrimidine domain in the PDGF A-chain promoter contains a novel binding site for the growth factor-inducible protein EGR-1. Biochem. Biophys. Res. Commun., 188, 433–439. [DOI] [PubMed] [Google Scholar]
- 22.Zipfel P.F., Decker,E.L., Holst,C. and Skerka,C. (1997) The human zinc finger protein EGR-4 acts as autoregulatory transcriptional repressor. Biochim. Biophys. Acta, 1354, 134–144. [DOI] [PubMed] [Google Scholar]
- 23.Biesiada E., Razandi,M. and Levin,E.R. (1996) Egr-1 activates basic fibroblast growth factor transcription. J. Biol. Chem., 271, 18576–18581. [DOI] [PubMed] [Google Scholar]
- 24.Hu R.M. and Levin,E.R. (1994) Astrocyte growth is regulated by neuropeptides through Tis 8 and basic fibroblast growth factor. J. Clin. Invest., 193, 1820–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khachigian L.M., Lindner,V., Williams,A.J. and Collins,T. (1996) Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science, 271, 1427–1430. [DOI] [PubMed] [Google Scholar]
- 26.Liu J., Grogan,L., Nau,M.M., Allegra,C.J., Chu,E. and Wright,J.J. (2001) Physical interaction between p53 and primary response gene Egr-1. Int. J. Oncol., 18, 863–870. [DOI] [PubMed] [Google Scholar]
- 27.Cui M.-Z., Party,G.C.N., Oeth,P., Larson,H., Smith,M., Huang,R.-P., Adamson,E.D. and Mackman,N. (1996) Transcriptional regulation of the tissue factor gene in human epithelial cells is mediated by Sp1 and EGR-1. J. Biol. Chem., 271, 2731–2739. [DOI] [PubMed] [Google Scholar]
- 28.Lui C., Adamson,E. and Mercola,D. (1996) Transcription factor EGR-1 suppresses the growth and transformation of human HAT-1080 fibrosarcoma cells by induction of transforming growth factor b1. Proc. Natl Acad. Sci. USA, 93, 11831–11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day M.L., Wu,S. and Basler,J.W. (1993) Prostatic nerve growth factor inducible A gene binds a novel element in the retinoblastoma gene promoter. Cancer Res., 53, 5597–5599. [PubMed] [Google Scholar]
- 30.Philipp A., Schneider,A., Vasrick,I., Finke,K., Xiong,Y., Besch,D., Alitalo,K. and Eilers,E. (1994) Repression of cyclin D1: a novel function of MYC. Mol. Cell. Biol., 14, 4032–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay J.J. and Drouin,J. (1999) Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone β gene transcription. Mol. Cell. Biol., 19, 2567–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S.L., Sadovsky,Y., Swirnoff,H., Polish,J.A., Goda,P., Gavrilina,G. and Milbrandt,J. (1996) Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science, 273, 1219–1221. [DOI] [PubMed] [Google Scholar]
- 33.Schneider-Maunoury S., Topilka,P., Seitandou,T., Levi,G., Cohen-Tannoudji,M., Pournin,S., Babinet,C. and Charnay,P. (1993) Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell, 75, 1199–1214. [DOI] [PubMed] [Google Scholar]
- 34.Tourtellotte W.G. and Milbrandt,J. (1998) Sensory ataxis and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nature Genet., 20, 87–91. [DOI] [PubMed] [Google Scholar]
- 35.Tourtellotte W.G., Nagarajan,R., Auyeung,A., Mueller,C. and Milbrandt,J. (1999) Infertility associated with incomplete spermatogenic arrest and oligospermia in Egr-4 deficient mice. Development, 126, 5061–5071. [DOI] [PubMed] [Google Scholar]
- 36.Cogswell P.C., Mayo,M.W. and Baldwin,A.S.,Jr (1997) Involvement of Egr-1/RelA synergy in distinguishing T cell activation from tumor necrosis factor-α-induced NF-kB1 transcription. J. Exp. Med., 185, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman E.S., Du,J., Williams,A.J., Wadgaonkar,R., Drazen,J.M. and Collins T. (1998) cAMP-response-element-binding-protein-binding-protein (CBP) and p300 are transcriptional co-activators of early growth response factor-1 (Egr-1). Biochem. J., 336, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava S., Weitzmann,M.N., Kimble,R.B., Rizzo,M., Zahner,M., Milbrandt,J., Ross,F.P. and Pacifici,R. (1998) Estrogen blocks M-CSF gene expression and osteoclast formation by regulating phosphorylation of Egr-1 and its interaction with Sp1. J. Clin. Invest., 102, 1850–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y., Dong,B., Mittelstadt,P.R., Xiao,H. and Ashwell,J.D. (2002) HIV Tat binds Egr proteins and enhances Egr-dependent transactivation of the Fas ligand promotor. J. Biol. Chem., 277, 19482–19487. [DOI] [PubMed] [Google Scholar]
- 40.Trejo S.R., Fahl,W.E. and Ratner,L. (1997) The Tax protein of human T-cell leukemia virus type 1 mediates the transactivation of the c-sis/platelet-derived growth factor-B promoter through interactions with the zinc finger transcription factors Sp1 and NGFI-A/Egr-1. J. Biol. Chem., 272, 27411–27421. [DOI] [PubMed] [Google Scholar]
- 41.Yoo Y.D., Ueda,H., Park,K., Flanders,K.C., Lee,Y.I., Jay,G. and Kim,S.-J. (1996) Regulation of transforming growth factor-β1 expression by Hepatitis B virus (HBV) X transactivator. J. Clin. Invest., 97, 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoo Y.D., Chiou,C.-J., Choi,K.S., Yi,Y., Michelson,S., Kim,S., Hayward,G.S. and Kim,S.-J. (1996) The IE2 regulatory protein of human cytomegalovirus induces expression of the human transforming growth factor β1 gene through an Egr-1 binding site. J. Virol., 70, 7062–7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo M., Sevetson,B.R. and Milbrandt,J. (1995) Identification of Nab1, a repressor of NGFI-A- and Krox20-mediated transcription. Proc. Natl Acad. Sci. USA, 92, 6873–6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Svaren J., Sevetson,B.R., Apel,E.D., Zimonjic,D.B., Popescu,N.C. and Milbrandt,J. (1996) NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and diferentiative stimuli. Mol. Cell. Biol., 16, 3545–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locksley R.M., Killeen,N. and Lenardo,M.J. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell, 104, 487–501. [DOI] [PubMed] [Google Scholar]
- 46.Decker E.L., Skerka,C. and Zipfel,P.F. (1998) The early growth response protein (EGR-1) regulates interleukine-2 transcription by synergistic interaction with the nuclear factor of activated T cells. J. Biol. Chem., 273, 26923–26930. [DOI] [PubMed] [Google Scholar]
- 47.Rao A., Luo,C. and Hogan,P.G. (1997) Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol., 15, 707–747. [DOI] [PubMed] [Google Scholar]
- 48.Kuklina E.M. and Shirshev,S.V. (2001) Role of transcription factor NFAT in the immune response. Biochemistry, 66, 467–475. [DOI] [PubMed] [Google Scholar]
- 49.Lopez-Rodriguez C., Aramburu,J., Rakeman,A.S. and Rao,A. (1999) NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc. Natl Acad. Sci. USA, 96, 7214–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skerka C., Decker,E.L. and Zipfel,P.F. (1997) Coordinate expression and distinct DNA-binding characteristics of the four EGR-zinc finger proteins in Jurkat T lymphocytes. Immunobiology, 198, 179–191. [DOI] [PubMed] [Google Scholar]
- 51.Durand D.B., Busch,M.R., Morgan,J.G., Wess,A. and Crabtree,G.R. (1988) Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol. Cell. Biol., 8, 1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain J., McCaffrey,P.G., Valge-Archer,V.E. and Rao,A. (1992) Analysis of the AP-1 sites in the IL-2 promoter. Nature, 356, 801–804. [PubMed] [Google Scholar]
- 53.Tsai E.Y., Jain,J., Pesavento,P.A., Rao,A. and Goldfeld,A.E. (1996) Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol. Cell. Biol., 16, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldfield A.E., McCaffrey,P.G., Stromonger,J.K. and Rao,A. (1993) Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor α gene promoter. J. Exp. Med., 178, 1365–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russo M.W., Sevetson,B.R. and Milbrandt,J. (1995) Identification of NAB1, a repressor of NGFI-A- and Krox20-mediated transcription. Proc. Natl Acad. Sci. USA, 92, 6873–6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svaren J., Sevetson,B.R., Apel,E.D., Zimonjic,D.B., Popescu,N.C. and Milbrandt,J. (1996) Nab2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol. Cell. Biol., 16, 3545–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]