Abstract
The RAD54 gene of Saccharomyces cerevisiae encodes a conserved dsDNA-dependent ATPase of the Swi2/Snf2 family with a specialized function during recombinational DNA repair. Here we analyzed the consequences of the loss of Rad54 function in vegetative (mitotic) cells. Mutants in RAD54 exhibited drastically reduced rates of spontaneous intragenic recombination but were proficient for spontaneous intergenic recombinant formation. The intergenic recombinants likely arose by a RAD54-independent pathway of break-induced replication. Significantly increased rates of spontaneous chromosome loss for diploid rad54/rad54 cells were identified in several independent assays. Inter estingly, the increase in chromosome loss appeared to depend on the presence of a homolog. In addition, the rate of complex genetic events involving chromosome loss were drastically increased in diploid rad54/rad54 cells. Together, these data suggest a role for Rad54 protein in the repair of spontaneous damage, where in the absence of Rad54 protein, homologous recombination is initiated but not properly terminated, leading to misrepair and chromosome loss.
INTRODUCTION
In Saccharomyces cerevisiae double-stranded DNA breaks (DSBs) are primarily repaired by homologous recombination (HR). In vegetative (mitotic) wild-type cells, the primary HR pathway appears to be gene conversion without associated crossing-over, whereas other mechanisms such as break-induced replication (BIR) and single-strand annealing (SSA) may become more prominent with special DNA substrates (e.g. direct and inverted repeats) or in certain mutants (1–4). DSB repair by a conversion-type mechanism initiates with processing of the DSB to produce 3′ ssDNA tails that are presumably covered with the eukaryotic ssDNA-binding protein RPA. The mediator proteins, Rad52 and the Rad55/Rad57 complex, catalyze the exchange of the RPA filament for the Rad51:ssDNA filament, which is active in homology search and DNA strand exchange (5). Strand invasion and heteroduplex DNA (hDNA) formation by the Rad51:ssDNA filament are enhanced by its interaction with the Rad54 protein (6–9). Rad54 protein also augments hDNA extension in Rad51 protein-mediated DNA strand exchange (10). Thus, biochemical experiments using purified proteins suggest a role of Rad54 protein in HR after the initial DSB processing and assembly of the Rad51 nucleoprotein filament. This notion is supported by genetic and cytological data that place RAD54 downstream of RAD51, RAD52, RAD55 and RAD57 in the recombination pathway (4,11–14).
Rad54 protein is a member of the Swi2/Snf2 family of DNA-dependent ATPases that modulate protein:dsDNA interactions in various molecular processes (15). Inactivation of RAD54, as well as of RAD51 and RAD52, leads to extreme sensitivity to DSBs. Genetically, RAD52 is required for almost all forms of HR, whereas the requirement for RAD51 and RAD54 is not as strict. Most studies with rad54 cells have been performed using repeat recombination substrates. An increase or decrease of direct repeat recombination, depending on the particular assay used (16–20), and a consistent decrease in two inverted repeat systems (3,11) were described. The limited data with natural chromosomes suggested a decrease in spontaneous intragenic recombination in two studies using the identical assay system (19,21) but an increase in intergenic recombination and chromosome loss (22). During meiotic recombination, the effect of a rad54 mutation is far less pronounced due to the function of the TID1 gene which encodes a protein highly related to Rad54 (19,21,23–25). The effects of a tid1 mutation during vegetative growth are somewhat strain-dependent and less severe compared with the DNA damage sensitivity of a rad54 mutant (19,23). It has been proposed that Rad54 protein functions primarily in recombination between sister chromatids, whereas Tid1 protein mediates primarily recombination between homologs (19,21,23).
Given the critical function of Rad54 protein during DSB repair by HR, we were interested to study the effect of rad54 null mutations on recombination and genome stability involving natural chromosomes during vegetative growth of S.cerevisiae cells in the absence of external genotoxic stress. DSBs and other types of DNA damage (e.g. ssDNA gaps) that are repaired by HR may occur during growth even in the absence of external genotoxic stress (4). We found that Rad54 protein is important for proper recombination between homologous chromosomes. Chromosome loss and complex genetic events that included chromosome loss were greatly elevated in diploid rad54/rad54 cells. Strikingly, chromosome loss was only found elevated in diploid cells containing two copies of the test chromosome. This suggests that genomic instability in rad54 cells is caused not by the absence of HR but by misrepair leading to elevated mutation and chromosome loss rates.
MATERIALS AND METHODS
Strains, plasmids and media
The S.cerevisiae strains used in this study are listed in Table 1. Deletion/substitution alleles were constructed by the one-step gene disruption method (26). To disrupt the RAD54 gene, plasmids pSM31 (rad54-Δ::LEU2) (kindly supplied by Dr D. Schild) and pWDH252 (rad54-Δ::URA3) were digested with StuI/BglII and XbaI/EcoRI, respectively, to transform strains selecting for the respective marker gene. The marker genes replaced a 1.9 kb internal BamHI fragment, eliminating approximately 2/3 of the RAD54 coding sequence. The rad54-Δ::KanR mutation substituting the RAD54 coding region between the first 10 and last 27 amino acids with the kanamycin-resistance gene was created by PCR product-mediated transformation (27). In the his1-Δ::KanR allele, the entire HIS1 open-reading frame was replaced by the kanamycin-resistance gene (27). All deletion/disruption alleles were verified by appropriate Southern blot analysis. Plasmid YCp50-RAD54 (pWDH205) was constructed by inserting the PstI–SalI RAD54 fragment of YEp13-RAD54-216A (28) into SalI–EcoRI cleaved YCp50 (29) after converting all ends with Klenow DNA polymerase to blunt ends. Standard media and growth conditions were used (30). l-Canavanine sulfate (60 mg/l) was added to synthetic media lacking arginine cooled to 60°C.
Table 1. Saccharomyces cerevisiae strains used in this study.
| Strain | Genotype |
|---|---|
| FF18728 | MATa lys9 met2 leu2-3,112 |
| FF18733 | MATa leu2-3,112 trp1-289 ura3-52 his7-2 lys1-1 |
| FF18734 | MATα leu2-3,112 trp1-289 ura3-52 his7-2 lys1-1 |
| FF18973 | MATa leu2-3,112 trp1-289 ura3-52 his7-2 lys1-1 rad54-Δ::LEU2 |
| FF18974 | MATα leu2-3,112 trp1-289 ura3-52 his7-2 lys1-1 rad54-Δ::LEU2 |
| FF18984 | MATa leu2-3,112 ura3-52 his7-1 lys2-1 |
| JH320 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ1 cyh2R +CF[CEN6 URA3 SUP11 CYH2S] |
| REE209 | MATα ura3-13 ade2 ade5 can1 leu1-12 trp5-d his7-2 tyr1-1 lys2-1 met13-d |
| REE218 | MATa ura3-1 ade2 cyh2 leu1-c trp5-c his7-1 tyr1-2 lys2-2 met13-c |
| WDHY543 | MATa leu2-3,112 ura3-52 his7-1 lys2-1 rad54-Δ::LEU2 |
| WDHY707 | MATa lys9 met2 leu2,3-112 rad54-Δ::LEU2 |
| WDHY768 | MATα ura3-13 ade2 ade5 can1 leu1-12 trp5-d his7-2 tyr1-1 lys2-1 met13-d rad54-Δ::URA3 |
| WDHY769 | MATa ura3-1 ade2 cyh2 leu1-c trp5-c his7-1 tyr1-2 lys2-2 met13-c rad54-Δ::URA3 |
| WDHY909 | MATα ura3-52 lys2-801amb ade2-101och trp1-·63 lys3-·200 leu2-·1 cyh2R [CF (CEN6) URA3 SUP11 CYH2S] |
| WDHY910 | MATα ura3-52 lys2-801amb ade2-101och trp1-·63 lys3-·200 leu2-·1 cyh2R [CF (CEN6) URA3+ SUP11+ CYH2S] rad54-Δ::KanR |
| WDHY980 | MATa leu2-3,112 trp1-289 ura3-52 his7-2 lys1-1 trt1-Δ::KanR |
| WDHY982 | MATa leu2-3,112 trp1-289 ura3-52 his7-2 lys1-1 trt1-Δ::KanR rad54-Δ::LEU2 |
| WDHY984 | MATα leu2-3,112 ura3-52 his7-1 lys2-1 trt1-Δ::KanR |
| WDHY985 | MATα leu2-3,112 ura3-52 his7-1 lys2-1 trt1-Δ::KanR rad54-Δ::LEU2 |
| WDHY1074 | MATα can1R ura3-52 his1-Δ::KanR iso1 ade5 lys1 leu2-3,112 trp1-289 |
| WDHY1138 | MATα can1R ura3-52 his1-Δ::KanR iso1 ade5 lys1 leu2-3,112 trp1-289 rad54-Δ::LEU2 |
| WDHY1216 | MATa ura3-52 lys2-801 ade2-101 trp1-·1 cyh2R +CF[CEN6 URA3 SUP11 CYHS] rad54-Δ::KanR |
| WDHY1246 | MATa/MATα CAN1/CAN1 ura3/URA3 his1-Δ::KanR/HIS1 ilv1/ILV1 leu2/leu2 lys1/LYS1 lys9/LYS9 trp1/TRP1 met2/MET2 ade5/ADE5 |
| WDHY1251 | MATa/MATα CAN1/CAN1 ura3/URA3 his1-Δ::KanR/HIS1 ilv1/ILV1 leu2/leu2 lys1/LYS1 lys9/LYS9 trp1/TRP1 met2/MET2 ade5/ADE5 rad54-Δ::LEU2/rad54-Δ::LEU2 |
| WDHY1657 | MATa/MATα leu2/leu2 ade2/ade2 lys2/lys2 trp1/trp1 his4/HIS4 ura3/ura3 can1/CAN1[YAC 18ED5::URA3::ADE2, YAC WXD4932::LEU2::TRP1] |
| WDHY1700 | MATa/MATα leu2/leu2 ade2/ade2 lys2/lys2 trp1/trp1 his4/HIS4 ura3/ura3 can1/CAN1 [YAC 18ED5::URA3::ADE2, YAC 18ED5::LEU2::TRP1] |
| WDHY1792 | MATa/MATα leu2/leu2 ade2/ade2 lys2/lys2 trp1/trp1 his4/HIS4 ura3/ura3 can1/CAN1 rad54-Δ::KanR/rad54-Δ::KanR [YAC 18ED5::URA3::ADE2, YAC 18ED5::LEU2::TRP1] |
| WDHY1805 | MATa/MATα Leu2/leu2 ade2/ade2 lys2/lys2 trp1/trp1 his4/HIS4 ura3/ura3 can1/CAN1 rad54-Δ::KanR/rad54-Δ::KanR [YAC 18ED5::URA3::ADE2, YAC WXD4932::LEU2::TRP1] |
All FF strains were kindly supplied by Dr F. Fabre. The REE strains were kindly provided by Dr R. Easton Esposito. JH320 was a kind gift of Dr H. Hegemann. All WDHY strains were constructed during this study.
Mitotic recombination, chromosome loss and mutation rates
Rates of spontaneous mitotic recombination were determined in fluctuation tests by the median method (31). Nine independent single colonies were analyzed in each experiment. All diploid strains were freshly constructed before each experiment. To determine mitotic intragenic recombination rates, single colonies were picked and inoculated in 10 ml of YPD medium and incubated at 30°C for 20 h. Cells (5 × 108) were collected by centrifugation, resuspended in 1.6 ml of H2O, and appropriate dilutions were plated on selective plates to determine the number of recombinants and on full medium to determine the total live cell count. Plates were scored after incubation for 4 days at 30°C. Mitotic intergenic recombination and chromosome loss rates for chromosome V markers were determined by measuring the rates of canavanine-resistant colonies in a fluctuation test after incubation for 4 days at 30°C. Genotypes of colonies were inferred from phenotypic analysis on the appropriate media to follow all chromosome V markers. Due to the extremely low rate, the p(0) method (31) was used to determine the wild type rate in Figure 5.
Figure 5.
Diploid S.cerevisiae rad54/rad54 cells exhibit increased genomic instability. (A) Schematic representation of the chromosome V marker system used in this experiment. The HIS1 locus was replaced with the kanamycin-resistance gene on the upper chromosome. Note that the CAN1 is homozygous and that two genetic events are needed to generate a canavanine-resistant cell. (B) Genetic analysis of events leading to canavanine resistance. Rates, numbers and percent of total for all phenotypic classes (–) in isogenic wild-type (WDHY1246) and rad54/rad54 (WDHY1251) diploid strains.
Rates of chromosome fragment loss
The rates were determined by fluctuation tests using the method of the median (31). Single colonies were grown on SD-uracil to select for the presence of the chromosome fragment (CF) and inoculated in 1 ml of YPD. After growth for 20 h at 30°C, ∼200 cells were spread per YPD plate. Plates were scored after 4 days of incubation at 30°C and 1 day at 4°C. Nine independent colonies were used per data point. To determine the rate for the loss of a single CF in a diploid cell, white colonies grown on YPD plates were taken, as they contain two copies of the CF (32).
Rates of YAC loss
The rates of yeast artificial chromosome (YAC) instability were determined by the method of the median (31). Briefly, single yeast colonies harboring the YACs were resuspended in water and appropriate dilutions were plated onto YPD. After 16–24 h of growth at 30°C, five to nine colonies were resuspended in water, and an appropriate dilution was plated on YPD for total cell counts, while the remaining suspension or dilution was plated on selective complete media containing 1 mg of 5-fluoro-orotic acid per milliliter. To ensure reproducibility, a minimum of three repetitions of the fluctuation assay were performed per strain, and at least three independently isolated clones were tested.
Targeted integration assay
PCR product-mediated transformation was performed as described (27) using the transformation protocol of Schiestl et al. (33). Transformants were analyzed by Southern blotting using the kanamycin-resistance gene as a probe and several restriction enzymes that cleave outside the fragment used for transformation. PCR primer P1 (5′-biotin-gATTCgATA CTAACgCCgCCA-3′) was biotinylated to allow direct sequencing of the genomic amplification products with primer P2 (5′-CTACCgTCAgAAACTgCTgTAC-3′) as described (34).
UV survival
UV survival curves were performed at fluencies of 0–150 J/m2 with cells spread on YPD plates. Plates were incubated in the dark at 30°C and scored after 4 days.
Statistical analysis
The ranking method described by Wierdl et al. (35) was used to determine the statistical significance of differences in the rate data.
RESULTS
RAD54 is important for spontaneous intragenic recombination between homologs
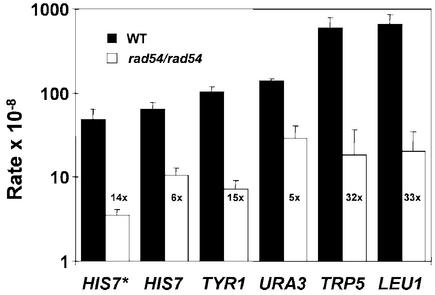
RAD54 is an important member of the recombinational DNA repair pathway in S.cerevisiae. Mutants in this gene show an almost complete deficiency in DSB repair. We wanted to ascertain the function of RAD54 in spontaneous intragenic mitotic recombination between homologs using heteroallelic mutations in five different genes. Rate determinations demonstrated a significant decrease in prototrophic recombinant formation in diploid rad54/rad54 strains compared with their isogenic wild-type strains (Fig. 1). Depending on the strain background, the rate decrease was 14-fold or 6-fold for the his7-1/his7-2 heteroalleles. The rate decreases for the TYR1, URA3, TRP5 and LEU1 genes ranged from 5- to 33-fold (Fig. 1). This is consistent with previous reports of a 10- to 12-fold reduction in prototroph formation between HIS4 heteroalleles (19,21). From these data, we conclude that RAD54 plays an important role in mitotic intragenic recombination between homologs. Since intragenic recombination is largely achieved by gene conversion (4), we conclude that rad54 mutants are severely defective in gene conversion between homologs.
Figure 1.
Diploid S.cerevisiae rad54 cells exhibit a defect in spontaneous, intragenic mitotic recombination. Intragenic mitotic recombination rates at the indicated gene loci were determined in two different isogenic wild-type and rad54/rad54 strain pairs by fluctuation tests as described in Materials and Methods. The black and white bars labeled HIS7* show the data for one set of strains: wild-type diploid (FF18734xFF18984, WT) and rad54/rad54 diploid (FF18974xWDHY543, rad54), respectively. Shown are means from five independent determinations. The black and white bars labeled HIS7, TYR1, URA3, TRP5 and LEU1 show the data for a second set of strains: wild-type diploid (REE209xREE218, WT) and rad54/rad diploid (WDHY768xWDHY769, rad54), respectively. Shown are means of three independent determinations. Error bars represent one standard deviation. The extent of the rate differences between the mutant and the wild type is given as fold reduction.
Spontaneous intergenic mitotic recombination and chromosome loss in rad54/rad54 cells
Intergenic recombinants in vegetative cells can arise by various mechanisms, including BIR, long tract gene conversion, and crossing-over (4). Using a marker system on chromosome V [see Fig. 2A; derived from (36)], we determined the rates for mitotic intergenic recombination and chromosome loss in diploid rad54/rad54 and isogenic wild-type strains (Fig. 2B). The upper chromosome was marked by the recessive canavanine-resistance marker. CAN1 codes for the arginine permease of S.cerevisiae, and CAN1 mutants cannot take up arginine or its toxic analog canavanine, leading to canavanine resistance. In addition, this chromosome carried three additional recessive markers (ura3, his1, ilv1) on both sides of the centromere and a dominant kanamycin-resistance marker (KanR) replacing the HIS1 gene on the right arm of chromosome V. The lower chromosome was wild-type for all yeast markers but lacked the kanamycin-resistance gene. After selecting for canavanine-resistant colonies, we determined the rates of all phenotypic classes for the chromosome V markers. The overall rate of canavanine-resistant colonies was elevated in rad54/rad54 cells 13-fold. Class 1 (CanR Ura+ His+ KanR Ilv+; Fig. 2B) can arise from conversion of the CAN1S marker to can1R in G1 or G2, from cross-over between the CAN1 and ura3 genes in G2, or from BIR of CAN1S to can1R in G1 or G2. It was the majority class in wild-type cells, representing 62.2% of all events. This class experienced a slight rate reduction (1.5-fold) in rad54/rad54 cells. This may represent the net result of several independent changes depending on the effect of RAD54 on conversion, BIR, and cross-over. Classes 2, 4 and 5 cannot be explained by a single event and due to the low rates of occurrence are not discussed here. Class 3 (CanR Ura– His+ KanR Ilv+), which accounts for 29.4% of the wild-type events, can arise from conversion of the CAN1S and URA3 markers to can1R and ura3 in G1 or G2, by cross-over between ura3 and the centromere in G2, or by BIR of the CAN1S and URA3 markers in G1 or G2. This class experienced a 2.4-fold rate increase in the rad54 strain. Class 6 (CanR Ura– His– KanR Ilv–), representing 7.7% of the wild-type events, is presumably caused by loss of the lower chromosome, exposing all recessive markers and retaining the dominant kanamycin-resistance marker. Importantly, in rad54/rad54 cells the rate of chromosome loss is increased 134-fold, representing 90.5% of the events. These data were independently confirmed in a second set of isogenic diploid strains identical to those shown in Figure 2A, except having a his1 missense mutation, therefore lacking the dominant KanR marker on the upper chromosome. The rate data for these wild-type and mutant strains as well as the class distributions were nearly identical to those reported in Figure 2 (data not shown). Loss of chromosome V in rad54/rad54 cells was recently reported to be increased only 29-fold in a different strain background using a different protocol (22).
Figure 2.
Saccharomyces cerevisiae rad54/rad54 cells exhibit increased chromosome loss. (A) Schematic representation of the chromosome V marker system used in this experiment. The HIS1 locus was replaced with the kanamycin-resistance gene on the upper chromosome. (B) Genetic analysis of events leading to canavanine resistance. Rates, numbers and percent of total for all phenotypic classes (1–6) in isogenic wild-type (FF18728xWDHY1074, WT) and rad54/rad54 (WDHY707xWDHY1138, rad54) diploids are given for 1024 colonies analyzed. The data are means of five independent determinations; standard deviations are given in brackets.
To test if the elevated rate of chromosome V loss in rad54/rad54 cells was specific for that chromosome, we measured loss of chromosome III using the co-dominant MATa and MATα markers. A statistically significant (P < 0.01) 8-fold increase in the frequency of chromosome III loss was found (data not shown). The reasons for the quantitative difference in effect of the rad54 mutation on chromosome III loss compared to chromosome V are unclear but may be related to the size difference between the two chromosomes (III, 320 kb; V, 580 kb) or may be due to chromosome-specific characteristics.
Taken together, we conclude from these data that genome-wide chromosome loss is significantly increased in rad54 mutants of S.cerevisiae.
Analysis of intergenic recombinants
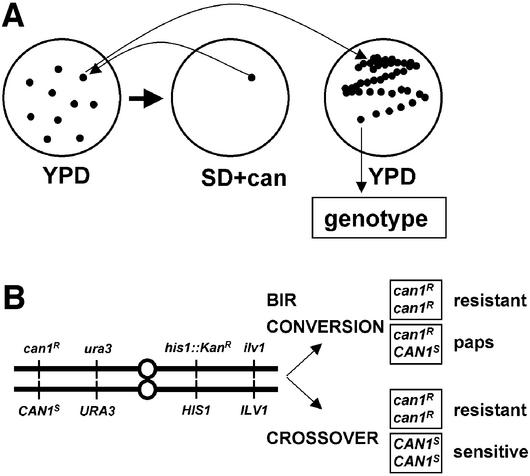
While intragenic recombination is largely accomplished by a conversion-type mechanism, the intergenic recombinants analyzed in the chromosome V system (Fig. 2B) could arise from either conversion, BIR, or crossing-over. As rad54/rad54 mutants are highly defective in intragenic recombination, we concluded that they drastically reduce conversion. To distinguish whether the intergenic recombinants found in classes 1 and 3 of Figure 2B were generated by BIR or crossing-over in G2, we devised the scheme shown in Figure 3A. Using non-selectively grown cells, we identified colonies containing canavanine-resistant cells by replica plating. The original colony was identified, re-streaked on fresh medium, and single colonies were phenotyped. The rationale to distinguish BIR/conversion from cross-over is shown in Figure 3B. A BIR/conversion event leads to two daughter cells, of which one is resistant (homozygous can1R/can1R) and the other is heterozygous (can1R/CAN1S). The heterozygous daughter cells will give rise to a colony that will form canavanine-resistant papillae (paps), which are easily scored. In contrast, a cross-over event leads to two products, of which one is resistant (homozygous can1R/can1R) and the other is sensitive (CAN1S/CAN1S). In analyzing 94 events, we did not detect canavanine-sensitive cells, the evidence for the reciprocal cross-over product, in wild-type (0/94). Only four in 77 rad54/rad54 events showed evidence for the reciprocal cross-over product. Thus, we conclude that either cross-over is not the major mechanism to generate mitotic intergenic recombinants or that the cross-over process in mitotic cells is not reciprocal, leading to loss of the unselected chromosome.
Figure 3.
Analysis of apparent intergenic recombinants in chromosome V system. (A) Outline of the strategy to distinguish between intergenic recombinants resulting from crossing-over or BIR/conversion. Canavanine-resistant colonies were identified by replica plating from YPD plates on SD plates containing canavanine. Colonies found to contain canavanine-resistant cells were re-streaked from the non-selective YPD plate, and 12 of the resultant single colonies for each original can1R cells containing colony were genetically characterized. (B) Diagram to illustrate the expected outcomes of reciprocal recombination events versus BIR and conversion events in the G2 phase of the cell cycle. Cross-over events are signaled by the occurrence of canavanine-sensitive colonies in addition to canavanine-resistant colonies, whereas BIR/conversion events are signaled by the occurrence of papillating canavanine-sensitive colonies (paps) in addition to canavanine-resistant colonies.
Chromosome loss in rad54 cells depends on the presence of a homolog
Mutants that increase chromosome loss identify genes which function in different cellular processes, including recombination, DNA replication, and chromosome segregation. To learn more about the role of RAD54 in chromosome stability, we analyzed chromosome loss in haploid cells. Since all S.cerevisiae chromosomes carry essential genes, the loss of a non-essential CF was determined (32). The left arm of the CF was 125 kb in size and is derived from the left arm of chromosome III of S.cerevisiae. The right arm of the CF carried URA3 and the SUP11, encoding an ochre-suppressor tRNA, separated by CEN6 from the left arm. Chromosome loss rates were measured by a visual assay that utilizes the red pigment phenotype of S.cerevisiae colonies carrying the ade2-1 ochre mutation. The haploid indicator strain with the CF containing the SUP11 suppressor formed white colonies because the ade2-1 mutation was suppressed by the suppressor tRNA, whereas CF loss leads to red colonies. Rate determinations in isogenic haploid wild-type and rad54 strains containing the CF did not reveal a statistically significant difference in the loss of the CF (Table 2). Thus, we conclude that loss of a single chromosome in haploid cells is not increased in rad54 cells.
Table 2. Loss of one non-essential CF is increased in diploid but not in haploid rad54 cells.
| Strainsa | Rate/1000 cell divisionsb | |
|---|---|---|
| 1n + 1 CF | Wild type | 5.55c |
| rad54 | 5.15c | |
| 2n + 2 CF | Wild type | 13.2d |
| rad54/rad54 | 33.1d |
aHaploid strains were WDHY909 (wild type) and WDHY910 (rad54), diploid strains were WDHY909xJH320 (wild type) and WDHY910x WDHY1216 (rad54/rad54).
bGiven are means from two (haploid strains) or three (diploid strain) independent rate determinations.
cDifference between wild type and rad54 is statistically not significant (P > 0.05).
dDifference between wild type and rad54 is statistically significant (P < 0.01).
Since we identified greatly enhanced chromosome loss in diploid rad54/rad54 cells using a test system with native homologous chromosomes (Fig. 2) but no increase in haploid cells using the CF system, we determined the rate of CF loss in diploid cells. Diploid strains carrying two copies of the CF were isolated as white colonies in which the ade2-1 mutations were fully suppressed. Loss of a single CF is signaled by the appearance of pink colonies, loss of both CFs leads to red colonies (32). The rates for the loss of one CF in isogenic diploid wild-type and rad54/rad54 cells containing two CFs were determined to be 13.2 × 10–3 and 33.1 × 10–3, respectively (Table 2). This represents a 2.5-fold increase in rad54/rad54 cells, which was statistically significant. It is interesting to note that the CF was 2.4-fold less stable in diploid compared to haploid cells even in wild-type cells. We conclude that in diploid rad54/rad54 cells containing two CFs, the CF loss rates increased. Since there was no increase in haploid cells containing one CF, this result suggests that the presence of the homolog contributed to the elevated CF loss rate. In wild-type diploid cells loss of both CFs was never observed (rate estimated to be <2 × 10–4), whereas in rad54/rad54 cells the rate of losing both CFs was ∼1 × 10–3.
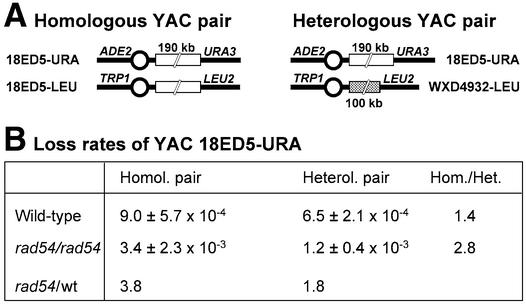
The magnitude of the increase in CF loss in diploid cells was less than expected from the data with the native chromosomes III and V (Fig. 2). Several reasons may have contributed to this. For both haploid and diploid wild-type cells, the CF loss rate is already significantly higher than that of natural chromosomes. For comparison, chromosome V is lost at a rate of 3 × 10–6 in wild-type cells (Fig. 2), an over 4000-fold difference relative to the diploid CF loss rate. This may be due to the size difference or to the lack of stabilizing DNA sequences found in natural chromosomes but lacking in the CF. Another contributing factor to the lower stability of the CF might be its significant sequence homology with chromosome III from which it was derived. To overcome this limitation and to test the idea that the presence of a homolog destabilizes chromosomes in rad54/rad54 cells, we devised the system shown in Figure 4A. We made use of YACs that contained inserts of homologous or non-homologous human DNA. The homologous YAC pair 18ED5-URA and 18ED5-LEU differs only in their content of marker genes, whereas 18ED5-URA and WXD4932-LEU represent a heterologous YAC pair. The loss rates of the heterologous and homologous YAC pairs were determined in isogenic wild-type and rad54/rad54 diploid strains by establishing the rate of Ura– cells and determining the proportion of the events where also the second marker gene, ADE2, was lost. In wild-type diploid cells, the loss rates of the homologous and heterologous YAC pairs were very similar, and the difference was not statistically significant (Fig. 4B). In contrast, in rad54/rad54 cells the homologous pair exhibited a significantly higher loss rate of YAC 18ED5-URA than the heterologous pair (P < 0.025). The inherent instability of the WXD4932-URA YAC precluded a meaningful analysis of the reciprocal heterologous and homologous YAC pairs (data not shown). From the CF and YAC loss data we conclude that chromosome loss in rad54 cells is specifically increased by the presence of two homologous chromosomes.
Figure 4.
Homolog-dependent increase of chromosome loss in rad54/rad54 cells. (A) Diagram of the two pairs of YACs used in this experiment. 18ED5-URA and 18ED5-LEU contained the same 190 kb human DNA insert in vector backbones differing in their marker genes as indicated. WXD4932 contains a 100 kb human DNA insert that is different from the 18ED5 YACs. (B) Chromosome loss rates of YAC 18 ED5-URA in wild-type cells containing the homologous (WDHY1700) or heterologous (WDHY1657) YAC pair and rad54/rad54 strains containing the homologous (WDHY1792) or heterologous YAC pair (WDHY1805). The rates for chromosome loss were determined as described in Materials and Methods. Means and one standard deviation of at least three independent experiments are shown.
Complex genetic events are highly elevated in rad54/rad54 cells
The analysis of spontaneous chromosome loss using selected (Fig. 2) and unselected events (data not shown) suggested the possibility that complex genetic events may be more frequent in rad54/rad54 cells. While the previous experiments were not designed to test this question directly, we modified our chromosome V marker system to determine rates of more complex genetic events. As a baseline reference, we first determined the mutation rate in the CAN1 gene (Table 3). rad54 cells showed a statistically significant 2.3-fold increase over the isogenic wild type. This increase was complemented to wild-type rates, when the wild-type RAD54 gene was re-introduced on a centromeric plasmid (Table 3). This is consistent with published data of a 6-fold increase in the CAN1 mutation rate in a different strain background (18).
Table 3. Increased mutation rate in haploid rad54 cells.
| Straina | canR rate × 10–7 | Fold increase |
|---|---|---|
| Wild type | 5.9 ± 0.9 | 1× |
| rad54 | 13.3 ± 2.9 | 2.3 |
| rad54 + YCp50-RAD54 | 3.7 ± 0.9 | 0.6 |
Data shown are means from five independent fluctuation tests with one standard deviation.
aStrains were FF18733 (wild type) and FF18973 (rad54).
To measure the rate of complex genetic events, we determined the rate of canavanine-resistant cells in isogenic diploid wild-type and rad54/rad54 strains homozygous for the CAN1S gene (Fig. 5A). Generation of canavanine-resistant derivatives necessitates at least two genetic events which were inferred from genetic analysis. The rate of canavanine-resistant derivatives was extremely low in wild-type cells (3.1 × 10–10), as expected (Fig. 5B). In rad54/rad54 cells this rate was elevated 84-fold. Six genotypic classes could be distinguished in the ensuing genetic analysis (Fig. 5B). Class 1 of Figure 5, which represented the great majority (95.8%) of the events in wild-type cells, has likely arisen by a mutation in one CAN1S gene followed by conversion or BIR of the remaining CAN1S gene, or by cross-over between CAN1 and ura3. The rate for this class is elevated 3.7-fold in rad54/rad54 cells, but this class represents only 10.9% of all mutant events. The only two other classes in wild-type show one homolog with a mutation at CAN1S and loss of the upper (class 5 of Fig. 5) or lower (class 6 of Fig. 5) chromosome. Both were found at a very low rate (7 × 10–12) represented by single cases constituting together only 4.2% of the wild-type events. Both class 5 and 6 events (Fig. 5B) were found to exhibit a tremendous rate increase in rad54/rad54 cells of 2206- and 1229-fold, respectively. In addition, three additional classes were identified (classes 2–4 of Fig. 5). They represent together 3.6% of the mutant events and were not found in wild-type cells. These classes can arise by a variety of different pathways that were not further explored due to their low frequency. From this data we conclude that complex genetic events are highly elevated in rad54/rad54 diploid cells.
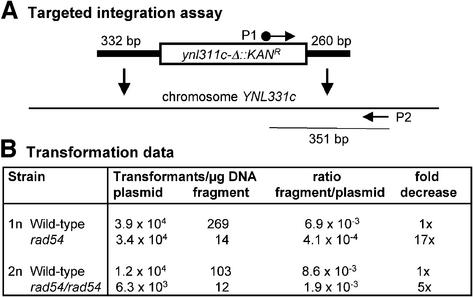
Gene targeting is reduced, but not absent, in rad54 cells
Gene targeting of linear fragments into the chromosome is mediated by HR. Since Rad54 protein plays an important role in HR, we determined the ability of rad54 cells to integrate a linear fragment that had 332 and 260 bp of homology at its ends (Fig. 6A). We chose the YNLc311 gene, whose deletion in a systematic analysis of S.cerevisiae open-reading frames did not demonstrate any appreciable phenotype (M. Sigrist and W.-D. Heyer, unpublished results). As a control for transformation efficiency, the competent cells were also transformed with the centromeric plasmid YCp50. The transformation efficiency of wild-type and rad54 cells was found to be very similar within a 2-fold or smaller difference (Fig. 6B). To determine the efficiency of homologous integration of the linear fragment independent of the transformation efficiency, we calculated the ratio of the number of kanamycin-resistant transformants obtained with the linear fragment per microgram DNA over the number of plasmid transformants per microgram DNA. Haploid rad54 cells showed a highly significant 17-fold decrease in the gene-targeting efficiency in comparison with an isogenic wild-type strain. In diploid rad54/rad54 cells the reduction was 5-fold (Fig. 6B).
Figure 6.
Gene-targeting defect in rad54 cells. (A) Diagram of the targeted integration assay using PCR product-mediated transformation with a ynl311c-Δ::KANR fragment containing 332 and 260 bp of homology to the chromosome on either end, respectively. The positions of the PCR primers (biotinylated P1 and P2) to amplify the right-hand integration junction as a 351 bp fragment are indicated. (B) Transformation data for haploid wild type (FF18984) and rad54 (WDHY543) as well as diploid wild type (FF18984xFF18734) and rad54/rad54 (WDHY543xFF18974) are given for the plasmid YCp50 and the PCR fragment shown in (A).
To determine the accuracy of the integration events, Southern blot and PCR analysis of fragment transformants were performed. In 43 wild-type and 30 rad54 transformants, all integration events were found to be at the chromosomal YNL311c locus and appeared to be correct (data not shown). To provide higher resolution, the right junction of the integration (Fig. 6A) was amplified by PCR and a 351 bp fragment covering the junction was entirely sequenced in 12 diploid wild-type and 13 rad54/rad54 integrants. No changes in the DNA sequence were identified (data not shown). From these data we conclude that gene targeting is reduced, but not eliminated, in rad54 cells and that the targeting events are not detectably mutagenic.
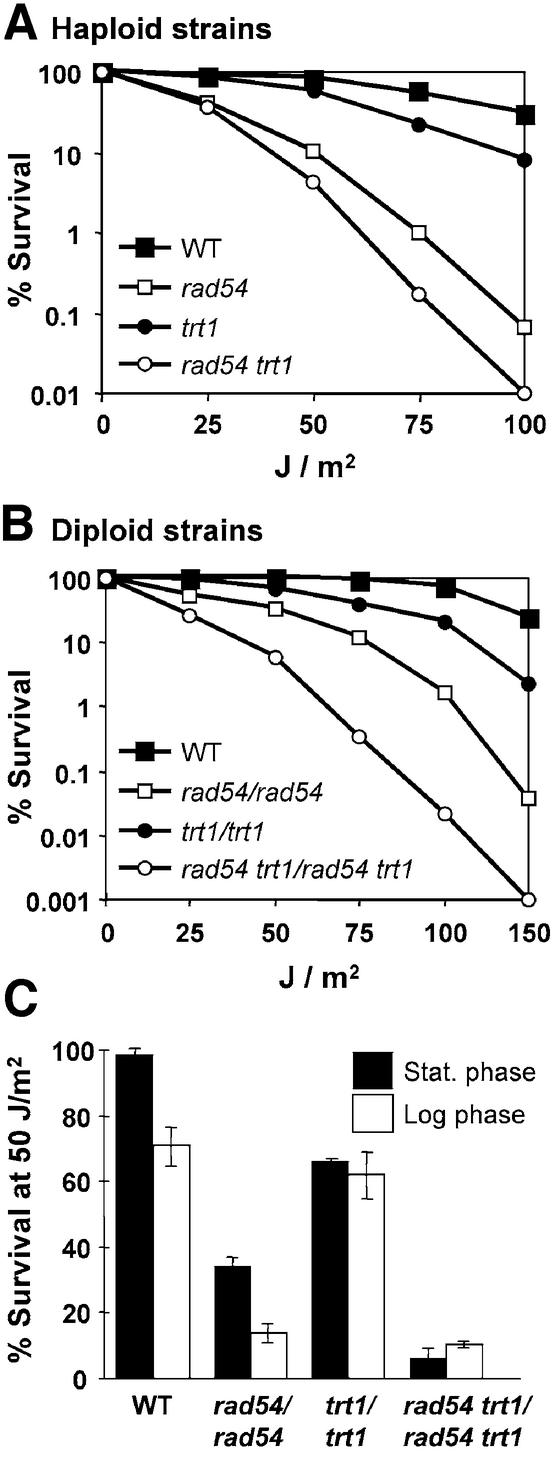
The UV sensitivity of a histone H2A/B mutant is not epistatic with rad54 and does not suppress the UV sensitivity, mitotic intragenic recombination defect, and enhanced mutation rate of rad54
Rad54 protein is a member of the Swi2/Snf2 family of ATPases, of which many members are active in chromatin remodeling. It has been speculated that Rad54 might function to remodel chromatin during recombinational repair (37). The transcriptional activation defect of snf2 cells can be suppressed by a deletion of TRT1 locus, one of two loci encoding histone H2A and histone H2B (38). trt1 mutant cells are viable but exhibit gross, genome-wide changes in chromatin structure (39), which apparently render transcriptional activation independent of chromatin remodeling by Snf2/Swi2. Thus, we hypothesized that a deletion of TRT1 might also suppress the phenotypes of a rad54 mutation. The deletion of TRT1 caused a mild UV-sensitive phenotype, which was less pronounced than that caused by rad54 (Fig. 7). These findings permitted epistasis analysis. In isogenic haploid and diploid strains, trt1 did not suppress the UV sensitivity caused by rad54 (Fig. 7A and B). On the contrary, trt1 rad54 double mutants were more sensitive than either single mutant to an extent that suggests that both mutations caused additive sensitivity to UV. These data were confirmed by single-dose experiments comparing stationary phase cells that have a G1 DNA content and logarithmically grown cells that represent a mixture of G1, S and G2 cells (Fig. 7C). In addition to UV sensitivity, MMS sensitivity, spontaneous mutation rates, and spontaneous mitotic intragenic recombination rates were measured in isogenic wild-type, rad54, trt1 and rad54 trt1 strains. Deletion of TRT1 did not cause a phenotype in any of these assays and did not suppress the defects caused by the rad54 mutation (data not shown). From these data we conclude that TRT1 contributes to resistance to UV radiation, acting in a pathway that is independent of the recombinational repair pathway. Furthermore, we conclude that unlike with the transcriptional activation defect in snf2 cells caused by defective chromatin remodeling, the deletion of the TRT1 locus encoding histone H2A/histoneH2B cannot suppress the DNA repair defects, the mutation rate increase, or recombination defect of rad54 cells.
Figure 7.
The UV sensitivity of rad54 cells is not suppressed by a deletion of the histone H2A/H2B TRT1 locus. (A) UV survival curves of haploid wild-type (FF18984), rad54 (WDHY543), trt1 (WDHY980) and rad54 trt1 (WDHY982) strains. Shown are the means of three independent determinations. (B) UV survival curves of diploid wild-type (FF18984xFF18734), rad54/rad54 (WDHY543xFF18974), trt1/trt1 (WDHY980xWDHY984) and rad54 trt1/rad54 trt1 (WDHY982xWDHY985) strains. Shown are the means of three independent determinations. Error bars were omitted for clarity. (C) Comparison of UV survival of stationary phase and logarithmically growing cultures of the diploid strains analyzed in (B) at a fluency of 50 J/m2. Shown are the means of two independent determinations. Error bars represent one standard deviation.
DISCUSSION
Rad54 is essential for accurate mitotic gene conversion between homologous chromosomes
Using marker systems in five different genes and two strain backgrounds, we showed that rad54/rad54 mutants are highly deficient in generating prototrophs during spontaneous mitotic intragenic recombination (Fig. 1). The multiple heteroallele system used for most of the intragenic recombination analysis with rad54/rad54 strains had been previously used with rad52-1/rad52-1 diploids (40). rad52-1 is a missense mutation changing amino acid 90 from alanine to valine (41) and displays a null phenotype in most assays, including mitotic recombination. RAD52 is essential for all forms of HR in S.cerevisiae, reducing it to ∼1% of wild-type levels when this gene is mutated (4). The rad52 analysis reported frequency data which allows comparison with our frequency data underlying the rate data of Figure 1. Although frequency data are subject to experimental variation, we observed that for three heteroallele pairs the reported wild-type data (40) and our wild-type data are near identical, allowing direct comparison. In these heteroallele pairs, rad52-1 caused a 6-fold reduction in prototroph formation between his7-1 and his7-2, a 32-fold reduction between tyr1-1 and tyr1-2, and a 9-fold reduction between ura3-1 and ura3-13. For the same markers, rad54/rad54 diploids showed a reduction of 8-, 26- and 21-fold, respectively. It appears that within likely experimental error, the reduction of the frequency of spontaneous intragenic mitotic recombination in rad52-1/rad52-1 diploids and rad54/rad54 diploid is equivalent. These data demonstrate the importance of Rad54 protein for the interaction between homologous chromosomes. It appears from studies of meiotic recombination (19,21,23) that during meiosis the function of the related Tid1 protein overlaps with that of Rad54. It is likely that this reflects the distinct and overlapping meiotic roles of Rad51 and Dmc1, the respective interaction partners of Rad54 and Tid1 (13,42–44). While Tid1 may not play a role in sister chromatid recombination (21,23), Rad54 is important for recombination between sister chromatids and homologs. This conclusion is consistent with the genetic requirement for RAD54 in the repair of an HO-induced DSB by conversion (45). Incidentally, DSB-induced gene conversion, a recombination event involving a broken and an unbroken homologous chromosome, is independent of TID1 (45).
The defect in prototroph formation during intragenic recombination in rad54 cells could result from the absence of recombination or from mis-recombination, producing recombinant chromosomes that contain mutations in the marker gene and hence cannot be selected for. We tried to distinguish between both possibilities by analyzing a gene-targeting experiment where the accuracy of the recombination event is not strictly selected for. Gene targeting was significantly reduced, but not absent, in rad54 cells, a result that is consistent with observations made in chicken and mouse RAD54-deficient cells (46,47). Physical analysis of integration junctions failed to detect any recombination-associated mutations, suggesting that at least the selectable targeted integration events are not mutagenic in rad54 cells. However, the reduced frequency of targeted integration events could mean that the mutagenic events have been lost, possibly by associated chromosome loss.
rad54/rad54 cells are proficient in BIR to generate intergenic recombinants
The analysis of a marker system on chromosome V showed that rad54/rad54 cells were proficient in generating intergenic recombinants. The most abundant recombinant classes (classes 1 and 3 in Fig. 2) could result from gene conversion, BIR, or crossing-over. Since rad54/rad54 cells are highly deficient in intragenic recombination which is primarily the result of conversion events, we consider it unlikely that gene conversion is contributing much to these classes in wild-type or rad54/rad54 cells. Hence, we tried to distinguish between BIR and crossing-over as the mechanism to generate the observed recombinants. From further genetic analysis it appeared that these recombinants were almost exclusively the result of BIR. This is consistent with previous observations that crossing-over is quite rare in vegetatively growing cells (4). An HO-induced DSB can be repaired by BIR in a RAD54-independent fashion (45), and it appears from our data that also spontaneous DNA damage can result in RAD54-independent BIR. It is unlikely that this spontaneous damage, which triggered the recombination events scored here, is a DSB, because rad54 mutants are extremely sensitive to DSB damage with most of the cells not surviving a single DSB (48). It was recently realized that direct repeat recombination can be the result of a combination of BIR and SSA (2). The proficiency of rad54/rad54 cells in BIR and SSA is consistent with the observed disparate effects of RAD54 mutants in direct repeat recombination assays (16–20).
Genomic instability in rad54/rad54 cells
Cells lacking Rad54 exhibit significantly increased levels of genome-wide chromosome loss (22; this study). Importantly, we showed that the increased chromosome loss phenotype is unlikely to be caused by a defect in chromosome segregation. Defects in chromosome segregation (e.g. mutations of the centromere or the spindle apparatus) lead to increased chromosome loss of non-essential test chromosomes in haploid cells (32). rad54 mutants, however, did not lead to increased chromosome loss in haploid cells. Instead, it appeared that chromosome loss was specifically increased in diploid cells carrying two homologous, but not two heterologous, chromosomes (Table 2 and Fig. 4). This suggests that attempted recombination between homologous chromosomes results in chromosome loss. Biochemical, genetic and cytological data support the notion that Rad54 protein acts after the initial formation of the Rad51-ssDNA filament (see Introduction), which could lead to recombination intermediates that cannot be properly processed in the absence of Rad54.
Mutation rates to canavanine-resistant cells in haploid rad54 cells are only 2–6-fold elevated over wild type (18) (Table 3). In determining the rate of events leading to canavanine-resistance in homozygous CAN1S/CAN1S diploid cells, we found an over 80-fold increase in rad54/rad54 cells (Fig. 5). The generation of canavanine-resistant offspring required at least two events. By far the majority of these events in rad54/rad54 mutants, but not in wild-type cells, were a mutation in one CAN1S gene and loss of the other chromosome. The rates of these two classes (classes 5 and 6 in Fig. 5) were elevated over 1700-fold compared with wild type. Since the rates of the component events (mutation of CAN1S, Table 3; chromosome V loss, Fig. 2) have been determined independently, it is possible to evaluate the independence of both mechanisms. The rates of CAN1 mutation + chromosome loss was 1.4 × 10–11 in wild type and ∼2400 × 10–11 in rad54/rad54 cells (classes 5 and 6 in Fig. 5). In wild type, the component rates were 5.9 × 10–7 (haploid mutation rate, Table 3) and 3 × 10–6 (loss rate of one chromosome in CAN1w/can1R diploid, Fig. 2) resulting in an expected rate of 1.8 × 10–12, if both events were independent. The observed rate of 1.4 × 10–11 was ∼8-fold higher, indicating that the two events might not be entirely independent in wild-type cells. In rad54/rad54 cells, the component rates (13.3 × 10–7 × 401 × 10–6) predicted a rate of 53 × 10–11, but the observed rate was 2400 × 10–11, an over 45-fold difference, suggesting that both mutation and chromosome loss are highly coincident.
This calculation makes no assumption about the possible mechanisms and order of events leading to canavanine-resistant clones with loss of one chromosome V. If one were to assume that the mutation happens first and the chromosome loss second, one might argue to multiply the haploid mutation rate by a factor of two, since there are two CAN1S genes. In this sequence of events, mutation would result in a CAN1S/can1R diploid, which after loss of the CAN1S chromosome, leads to the observed class (can1R with loss of the other chromosome). Thus, only one half of the possible chromosome V loss events leads to the selected class, leading to consider another factor of two in the calculation. However, one could equally assume that the chromosome loss events happens first and the mutation second, since chromosome loss is considerably more frequent than the haploid mutation event in both wild-type and rad54 cells. In this case, the loss would produce an aneuploid cell with a single chromosome V where the CAN1S gene would then mutate to can1R. Here one could argue to introduce a factor of two because either of the two chromosomes could be lost, whereas in the determination of the component rate one particular chromosome must be lost to produce a selectable event. For the mutation rate, the haploid rate would apply under the assumption that the mutation rate is not influenced by overall ploidy and mating-type status. In the absence of specific insight into the mechanisms leading to the can1R + chromosome loss events, we favor not introducing any factors. This operation may lead to an overestimate of the absolute extent by how much the two events are coincident, but the relative difference between wild-type and rad54/rad54 cells remains constant.
Keeping the above mentioned caveat in mind, the calculation suggests that in wild-type cells both events, mutation and chromosome loss, may not be entirely independent showing an 8-fold coincidence, whereas in rad54/rad54 cells both events are highly coincident (45-fold). We suggest that attempted recombinational repair of spontaneous DNA damage in rad54/rad54 cells leads to associated chromosome loss and mutations.
Previous studies in diploid S.cerevisiae cells have identified an association between spontaneous mutation and homozygosis, leading to diploid cells with homozygous mutations (49,50). These authors suggested that the mutations arose from recombinogenic lesions causing the coincidence between spontaneous mutagenesis and homozygosis. This observation fits our data in wild type, where many more canavanine-resistant cells retaining both chromosomes (rate 2.97 × 10–10, Fig. 5, class 1) were found than predicted from the haploid mutation rate (5.9 × 10–7 × 5.9 × 10–7 = 3.5 × 10–14, Table 3). We surmise that the associated recombination in rad54/rad54 cells leads to the observed chromosome loss, explaining the high coincidence between both events in the mutant. In RAD52 mutants, the rare intragenic recombination events were found to be highly associated with loss of one chromosome, an association seen in wild-type cells only infrequently (51).
Together, these data suggest that HR is an important factor in genomic stability, particularly in diploid cells. It will be interesting to expand the present studies on genomic instability, which are being performed in haploid cells (52), to diploid cells in order to understand the interplay between DNA replication, HR, DNA checkpoints and the chromosome segregation apparatus to maintain genomic stability.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Drs R. Easton Esposito, F. Fabre, H. Hegemann, D. Schild and G. Simchen for kindly supplying strains, plasmids and YACs. We thank S. Bärtsch, V. Bashkirov, K. Ehmsen, E. Haghnazari and J. Solinger for critical comments on the manuscript. This work was supported by ACS postdoctoral fellowship PF-01-238-01-GMC to M.R. and NIH grant ROI-GM58015 to W.-D.H.
REFERENCES
- 1.Bärtsch S., Kang,L.E. and Symington,L.S. (2000) RAD51 is required for the repair of plasmid double-stranded DNA gaps from either plasmid or chromosomal templates. Mol. Cell. Biol., 20, 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang L.E. and Symington,L.S. (2000) Aberrant double-strand break repair in rad51 mutants of Saccharomyces cerevisiae. Mol. Cell. Biol., 20, 9162–9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malagon F. and Aguilera,A. (2001) Yeast spt6-140 mutation, affecting chromatin and transcription, preferentially increases recombination in which Rad51p-mediated strand exchange is dispensable. Genetics, 158, 597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paques F. and Haber,J.E. (1999) Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev., 63, 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung P., Trujillo,K.M. and Van Komen,S. (2000) Recombination factors of Saccharomyces cerevisiae. Mutat. Res., 451, 257–275. [DOI] [PubMed] [Google Scholar]
- 6.Petukhova G., Stratton,S. and Sung,P. (1998) Catalysis of homologous pairing by yeast Rad51 and Rad54 proteins. Nature, 393, 91–94. [DOI] [PubMed] [Google Scholar]
- 7.Van Komen S., Petukhova,G., Sigurdsson,S., Stratton,S. and Sung,P. (2000) Superhelicity-driven homologous DNA pairing by yeast recombination factors Rad51 and Rad54. Mol. Cell, 6, 563–572. [DOI] [PubMed] [Google Scholar]
- 8.Mazin A.V., Bornarth,C.J., Solinger,J.A., Heyer,W.-D. and Kowalczykowski,S.C. (2000) Rad54 protein is targeted to pairing loci by the Rad51 nucleoprotein filament. Mol. Cell, 6, 583–592. [DOI] [PubMed] [Google Scholar]
- 9.Solinger J.A., Lutz,G., Sugiyama,T., Kowalczykowski,S.C. and Heyer,W.-D. (2001) Rad54 protein stimulates heteroduplex DNA formation in the synaptic phase of DNA strand exchange via specific interactions with the presynaptic Rad51 nucleoprotein filament. J. Mol. Biol., 307, 1207–1221. [DOI] [PubMed] [Google Scholar]
- 10.Solinger J.A. and Heyer,W.-D. (2001) Rad54 protein stimulates the postsynaptic phase of Rad51 protein-mediated DNA strand exchange. Proc. Natl Acad. Sci. USA, 98, 8447–8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rattray A.J. and Symington,L.S. (1995) Multiple pathways for homologous recombination in Saccharomyces cerevisiae. Genetics, 139, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schild D. (1995) Suppression of a new allele of the yeast RAD52 gene by overexpression of RAD51, mutations in srs2 and ccr4, or mating-type heterozygosity. Genetics, 140, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara M., Gasior,S.L., Bishop,D.K. and Shinohara,A. (2000) Tid1/Rdh54 promotes colocalization of Rad51 and Dmc1 during meiotic recombination. Proc. Natl Acad. Sci. USA, 97, 10814–10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim P.M., Paffett,K.S., Solinger,J.A., Heyer,W.D. and Nickoloff,J.A. (2002) Spontaneous and double-strand break-induced recombination and gene conversion tract lengths, are differentially affected by overexpression of wild-type or ATPase-defective yeast Rad54. Nucleic Acids Res., 30, 2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pazin M.J. and Kadonaga,J.T. (1997) SWI2/SNF2 and related proteins: ATP-driven motors that disrupt protein–DNA interactions? Cell, 88, 737–740. [DOI] [PubMed] [Google Scholar]
- 16.McDonald J.P. and Rothstein,R. (1994) Unrepaired heteroduplex DNA in Saccharomyces cerevisiae is decreased in RAD1 RAD52-independent recombination. Genetics, 137, 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan H.Y. and Klein,H.L. (1994) Characterization of mutations that suppress the temperature-sensitive growth of the hpr1 mutant of Saccharomyces cerevisiae. Genetics, 137, 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liefshitz B., Parket,A., Maya,R. and Kupiec,M. (1995) The role of DNA repair genes in recombination between repeated sequences in yeast. Genetics, 140, 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinohara M., ShitaYamaguchi,E., Buerstedde,J.M., Shinagawa,H., Ogawa,H. and Shinohara,A. (1997) Characterization of the roles of the Saccharomyces cerevisiae RAD54 gene and a homologue of RAD54, RDH54/TID1, in mitosis and meiosis. Genetics, 147, 1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petukhova G., Van Komen,S., Vergano,S., Klein,H. and Sung,P. (1999) Yeast Rad54 promotes Rad51-dependent homologous DNA pairing via ATP hydrolysis-driven change in DNA double helix conformation. J. Biol. Chem., 274, 29453–29462. [DOI] [PubMed] [Google Scholar]
- 21.Arbel A., Zenvirth,D. and Simchen,G. (1999) Sister chromatid-based DNA repair is mediated by RAD54, not by DMC1 or TID1. EMBO J., 18, 2648–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein H.L. (2001) Spontaneous chromosome loss in Saccharomyces cerevisiae is suppressed by DNA damage checkpoint functions. Genetics, 159, 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein H.L. (1997) RDH54, a RAD54 homologue in Saccharomyces cerevisiae, is required for mitotic diploid-specific recombination and repair and for meiosis. Genetics, 147, 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petukhova G., Sung,P. and Klein,H. (2000) Promotion of Rad51-dependent D-loop formation by yeast recombination factor Rdh54/Tid1. Genes Dev., 14, 2206–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmuckli-Maurer J. and Heyer,W.D. (2000) Meiotic recombination in RAD54 mutants of Saccharomyces cerevisiae. Chromosoma, 109, 86–93. [DOI] [PubMed] [Google Scholar]
- 26.Rothstein R.J. (1983) One-step gene disruption in yeast. Methods Enzymol., 101, 202–211. [DOI] [PubMed] [Google Scholar]
- 27.Wach A., Brachat,A., Pohlmann,R. and Philippsen,P. (1994) New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast, 10, 1793–1808. [DOI] [PubMed] [Google Scholar]
- 28.Schild D., Calderon,I.L., Contopolou,C.R. and Mortimer,R.K. (1983) Cellular Responses to DNA Damage. Alan R. Liss, Inc., New York, pp. 417–427.
- 29.Rose M.D., Novick,P., Thomas,J.H., Botstein,D. and Fink,G.R. (1987) A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene, 60, 237–243. [DOI] [PubMed] [Google Scholar]
- 30.Sherman F., Fink.,G.R. and Hicks,J.B. (1982) Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Lea D.E. and Coulson,C.A. (1949) The distribution of the numbers of mutants in bacterial populations. J. Genet., 49, 399–406. [DOI] [PubMed] [Google Scholar]
- 32.Jehn B., Niedenthal,R. and Hegemann,J. (1991) In vivo analysis of the Saccharomyces cerevisiae centromere CDEIII sequence: requirements for mitotic chromosome segregation. Mol. Cell. Biol., 11, 5212–5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiestl R.H., Manivasakam,P., Woods,R.A. and Gietz,R.D. (1993) Introducing DNA into yeast by transformation. Methods Enzymol., 5, 79–85. [Google Scholar]
- 34.Clever B., Schmuckli-Maurer,J., Sigrist,M., Glassner,B. and Heyer,W.-D. (1999) Specific negative effects resulting from elevated levels of the recombinational repair protein Rad54p in Saccharomyces cerevisiae. Yeast, 15, 721–740. [DOI] [PubMed] [Google Scholar]
- 35.Wierdl M., Greene,C.N., Datta,A., Jinks-Robertson,S. and Petes,T.D. (1996) Destabilization of simple repetitive DNA sequences by transcription in yeast. Genetics, 143, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivanov E.L., Korolev,V.G. and Fabre,F. (1992) XRS2, a DNA repair gene of Saccharomyces cerevisiae, is needed for meiotic recombination. Genetics, 132, 651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugawara N., Ivanov,E.L., Fishman Lobell,J., Ray,B.L., Wu,X. and Haber,J.E. (1995) DNA structure-dependent requirements for yeast RAD genes in gene conversion. Nature, 373, 84–86. [DOI] [PubMed] [Google Scholar]
- 38.Hirschhorn J.N., Brown,S.A., Clark,C.D. and Winston,F. (1992) Evidence that SNF2/SWI2 and SNF5 activate transcription in yeast by altering chromatin structure. Genes Dev., 6, 2288–2298. [DOI] [PubMed] [Google Scholar]
- 39.Norris D., Dunn,B. and Osley,M.A. (1988) The effect of histone gene deletions on chromatin structure in Saccharomyces cerevisiae. Science, 242, 759–761. [DOI] [PubMed] [Google Scholar]
- 40.Malone R.E. and Esposito,R.E. (1980) The RAD52 gene is required for homothallic interconversion of mating types and spontaneous mitotic recombination in yeast. Proc. Natl Acad. Sci. USA, 77, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adzuma K., Ogawa,T. and Ogawa,H. (1984) Primary structure of the RAD52 gene in Saccharomyces cerevisiae. Mol. Cell. Biol., 4, 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinohara A., Gasior,S., Ogawa,T., Kleckner,N. and Bishop,D.K. (1997) Saccharomyces cerevisiae recA homologues RAD51 and DMC1 have both distinct and overlapping roles in meiotic recombination. Genes Cells, 2, 615–629. [DOI] [PubMed] [Google Scholar]
- 43.Dresser M.E., Ewing,D.J., Conrad,M.N., Dominguez,A.M., Barstead,R., Jiang,H. and Kodadek,T. (1997) DMC1 functions in a Saccharomyces cerevisiae meiotic pathway that is largely independent of the RAD51 pathway. Genetics, 147, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clever B., Interthal,H., Schmuckli-Maurer,J., King,J., Sigrist,M. and Heyer,W.D. (1997) Recombinational repair in yeast: functional interactions between Rad51 and Rad54 proteins. EMBO J., 16, 2535–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Signon L., Malkova,A., Naylor,M.L., Klein,H. and Haber,J.E. (2001) Genetic requirements for RAD51- and RAD54-independent break-induced replication repair of a chromosomal double-strand break. Mol. Cell. Biol., 21, 2048–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bezzubova O., Silbergleit,A., YamaguchiIwai,Y., Takeda,S. and Buerstedde,J.M. (1997) Reduced X-ray resistance and homologous recombination frequencies in a RAD54(–/–) mutant of the chicken DT40 cell line. Cell, 89, 185–193. [DOI] [PubMed] [Google Scholar]
- 47.Essers J., Hendriks,R.W., Swagemakers,S.M.A., Troelstra,C., deWit,J., Bootsma,D., Hoeijmakers,J.H.J. and Kanaar,R. (1997) Disruption of mouse RAD54 reduces ionizing radiation resistance. Cell, 89, 195–204. [DOI] [PubMed] [Google Scholar]
- 48.Schmuckli-Maurer J. and Heyer,W.-D. (1999) The Saccharomyces cerevisiae RAD54 gene is important but not essential for natural homothallic mating-type switching. Mol. Gen. Genet., 260, 551–558. [DOI] [PubMed] [Google Scholar]
- 49.Esposito M.S., Ramirez,R.M. and Bruschi,C.V. (1994) Nonrandomly-associated forward mutation and mitotic recombination yield yeast diploids homozygous for recessive mutations. Curr. Genet., 26, 302–307. [DOI] [PubMed] [Google Scholar]
- 50.Esposito M.S. and Bruschi,C.V. (1993) Diploid yeast cells yield homozygous spontaneous mutations. Curr. Genet., 23, 430–434. [DOI] [PubMed] [Google Scholar]
- 51.Haber J.E. and Hearn,M. (1985) RAD52-independent mitotic gene conversion in Saccharomyces cerevisiae frequently results in chromosomal loss. Genetics, 111, 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolodner R.D., Putnam,C.D. and Myung,K. (2002) Maintenance of genome stability in Saccharomyces cerevisiae. Science, 297, 552–557. [DOI] [PubMed] [Google Scholar]