Abstract
BACKGROUND
U.S. professional medical societies and the national health systems of all other industrialized nations recommend that most women need not undergo Papanicolaou (Pap) smear screening annually. There are no data, however, regarding the frequency at which women actually undergo screening.
OBJECTIVE
To describe the frequency of cervical cancer screening in the United States.
DESIGN
National Health Interview Survey, a cross-sectional population-based telephone survey conducted by the National Center for Health Statistics.
PARTICIPANTS
Representative sample of U.S. women age 21 and older who denied a history of cancer (N = 16,467).
MEASUREMENTS
Pap smear screening frequency, categorized as no regular screening or screening at 1 of 3 discrete screening intervals (every year, every 2 years, or every 3 years) based on each woman's reported number of Pap smears in the previous 6 years.
RESULTS
The vast majority (93%) of American women report having had at least one Pap smear in their lifetime. Among women with no history of abnormal smears, 55% undergo Pap smear screening annually, 17% report a 2-year screening interval, 16% report being screened every 3 years, and 11% are not being screened regularly. Even the very elderly report frequent screening—38% of women age 75 to 84 and 20% of women age 85 and older reported annual Pap smears. Overall, 20% of women reported having had at least one abnormal Pap smear. Among these women, rates of frequent Pap smear screening are considerably higher—80% undergo annual screening, with only a modest decline in screening frequency with increasing age.
CONCLUSIONS
The majority of American women report being screened for cervical cancer more frequently than recommended. Lengthening the screening interval would not only reduce the volume of specimens that cytotechnologists are required to read, but would also reduce the follow-up testing after abnormal smears.
Keywords: cervical cancer screening, Pap smear, test frequency, overutilization
Although it has never been tested in a randomized trial, the effectiveness of Papanicolaou (Pap) smear screening in reducing cervical cancer mortality is almost universally accepted. Marked declines in cervical cancer incidence and mortality have accompanied the implementation of national screening programs in most industrialized countries, and the dissemination of such programs to developing nations has become a top global health care priority. There have been questions, however, about how frequently women should be screened. While Pap smear screening in the United States was initially implemented as an annual test, analysis of data from large screening programs in 8 countries suggests that annual screening confers at best a minimal advantage over triennial screening.1 Other analyses have arrived at the same conclusion.2–6
In light of this evidence, since 1988 most U.S. professional medical societies have accepted that average women need not undergo Pap smear screening annually.7 The American College of Physicians recommends screening every 3 years for average risk women,5 and the U.S. Preventive Services Task Force recommends a screening interval of up to 3 years8 depending upon a woman's risk factors. Even organizations that might be expected to argue for more aggressive screening practices, such as the American Cancer Society9 and the American College of Obstetricians and Gynecologists,10 have accepted less frequent Pap smear screening in women who have had 3 consecutive normal smears. And almost all other industrialized nations have screening programs in which women are screened every 2 to 5 years.11–15
Given this consensus, one might reasonably suppose that annual screening would be largely a historical phenomenon. In fact, the authors of a recent cost-benefit analysis examining the inclusion of human papillomavirus testing in routine cervical cancer screening did not even include annual Pap smear screening in any of the 18 screening strategies evaluated, noting that biennial and triennial screening “most closely reflect current professional guidelines and clinical practice.”16 Nonetheless, we were surprised to find that there are few data on how frequently women are screened in current practice. In this paper, we describe the reported Pap smear screening practices of American women using a nationally representative sample of women interviewed about their screening behaviors.
METHODS
Data Source
The National Health Interview Survey (NHIS) is an annual population-based face-to-face interview survey conducted since 1957 by the National Center for Health Statistics. The NHIS collects data on health status, behaviors, health care access, and utilization from a representative (multistage) sample of the civilian, noninstitutionalized, household population of the United States. In this study, we use the 2000 survey17 of 32,374 adults (response rate 72.1%18) and focus on the female respondents age 21 and older who denied a history of cancer (N = 16,467).
Study Sample
All women were asked whether they had ever had a Pap smear (described as “a routine test for women in which the doctor examines the cervix, takes a cell sample from the cervix with a small stick or brush, and sends it to the lab”). After determining the proportion of women who had ever had a Pap smear, we restricted all subsequent analyses to 11,739 women who had at least one Pap smear, had not had a hysterectomy, and did not have a history of cancer (excluding non-melanoma skin cancer). Because abnormal smears influence the timing of repeat testing, we distinguished between women with and without a history of abnormal Pap smears in our analysis of Pap smear screening intervals.
Screening Patterns
We estimated each woman's screening frequency based on how many Pap smears she reported having had in the past 6 years, and the timing of her most recent Pap smear. We assumed that a woman was not undergoing regular screening if she reported that her most recent Pap smear was more than 5 years ago, if she reported having had no Pap smears in the past 6 years, or if she did not report how many Pap smears she had had (item nonresponse 3%). For additional detail, we distinguished between women who had stopped screening (those whose last Pap smear was more than 5 years earlier), and those undergoing sporadic screening (those whose last Pap smear was within 5 years).
All other women were considered to be undergoing regular screening. We used the number of Pap smears reported in the previous 6 years to categorize these women into 1 of 3 discrete screening intervals (every year, every 2 years, or every 3 years). We inferred that a woman who averaged 0.75 Pap smears or more per year—in this case, those who reported 5 or more Pap smears in the previous 6 years—was being screened annually. Similarly, women with 3 or 4 smears during the previous 6 years (0.5 to 0.74 Pap smears per year) were being tested every 2 years, and women with 1 or 2 Pap smears during the same period (fewer than 0.5 per year) were being screened every 3 years.
Because these screening intervals were estimated based solely on women's self-reports of Pap smear screening practices, they are referred to as “reported screening intervals” for the remainder of the paper.
Analysis
Each year's NHIS sample is weighted to the respondent's probability of selection and to the gender-, age-, and race/ethnicity-specific population from the most current census data or intercensal estimates, with further adjustments for nonresponse. We report weighted proportions only. Because the poststratification weights reflect the size of the underlying stratum-specific population, we were able to estimate the number of U.S. women represented by different responses. Screening intervals were calculated as described, using a modification for women aged 21 to 23 (see Appendix A for explanation). We analyzed responses according to age, using 8 age categories (see Appendix A). All analyses were performed using STATA (version 7.0, Stata Corporation, College Station, Tex).
RESULTS
The vast majority (93%) of American women age 18 and older reported having had at least one Pap smear in their lifetime.
Screening Among Women with No Previous Abnormal Pap Smears
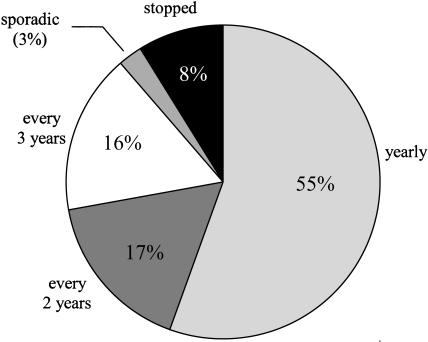
Figure 1 shows the distribution of estimated Pap smear screening intervals among women who have been screened at least once, have not had a hysterectomy, and have never had an abnormal Pap smear. More than half (55%) undergo Pap smear screening annually. Seventeen percent report being screened every 2 years, and 16% are screened at a 3-year screening interval. Eleven percent of women are not being regularly screened. (See Appendix A for women's responses to the question, “How many Pap smears have you had in the past 6 years?” upon which these estimates are based.)
FIGURE 1.
Pap smear screening intervals among women with no history of abnormal Pap smears. Includes only women who have had at least one Pap smear, and have not had a hysterectomy.
Reported Screening Intervals and Age
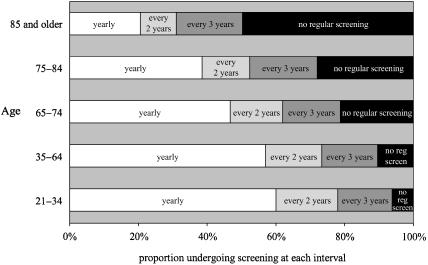
Figure 2 shows the distribution of reported screening intervals among women of different ages. Except for women age 85 and older, annual screening is the most common interval reported. Annual screening is most prevalent among younger women—60% of women age 21 to 34 and 57% of women age 35 to 64 reported getting annual Pap smears.
FIGURE 2.
Pap smear screening intervals of American women of different ages. Includes women who have had at least one Pap smear, no abnormal smears, and have not had a hysterectomy.
Figure 2 also underscores that many of the elderly continue frequent screening. Almost half of women age 65 to 74 reported being screened for cervical cancer annually. And frequent screening is common even among the very old—39% of women age 75 to 84 and 21% of women age 85 and older reported getting a Pap smear every year. Relatively few appear to have stopped screening. Nearly three-quarters of women age 75 to 84 and over half of women age 85 and older are still being regularly screened.
Screening in Women with a History of Abnormal Pap Smears
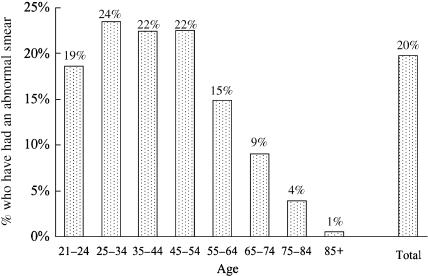
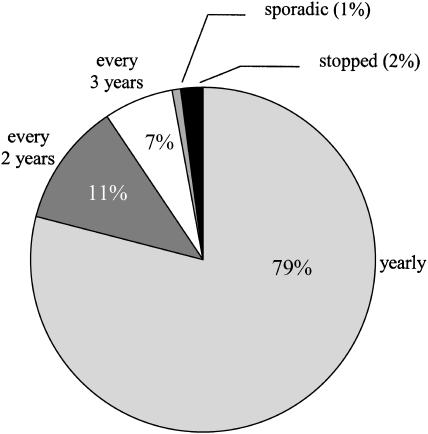
Twenty percent of American women report a history of at least one previous abnormal Pap smear. Figure 3 shows that the proportion of women who have had an abnormal Pap smear was 20% or greater for women in all age groups from 25 to 54. Among women with a history of abnormal Pap smears, the large majority (79%) report undergoing Pap smear screening at least annually (see Fig. 4), with rates falling only modestly with increasing age (from 84% among women 21 to 34, to 73% among those 65 and older). Of note, a number of women appear to undergo even more frequent screening. For example, 11% of women age 21 to 34 averaged 2 or more Pap smears per year (12 Pap smears during the 6-year period). (See Appendix B for women's responses to “How many Pap smears have you had in the past 6 years?”)
FIGURE 3.
Proportion of U.S. women with at least one abnormal Pap smear. Includes only women without a hysterectomy.
FIGURE 4.
Pap smear screening intervals among women with a history of abnormal Pap smears. Includes only women who have not had a hysterectomy.
Follow-up After Abnormal Pap Smears
Ninety percent of women with a history of at least one abnormal Pap smear had undergone further testing because of an abnormal smear; almost half (42%) had had surgery or other treatment because of the abnormal Pap smear.
DISCUSSION
There is a tremendous amount of effort devoted to cervical cancer screening in the United States. We estimate that 61 million women had at least one Pap smear in 2000. We found that Pap smear screening is common even among elderly women. Most importantly, while it has been more than a decade since most professional societies revised their recommendations for Pap smear screening intervals in favor of less frequent screening, we found that the majority of American women report being screened for cervical cancer annually.
For a number of reasons, it is difficult to quantify how many women are being screened more often than is recommended. Most agree that only women at high risk should be screened annually. Unfortunately, until very recently guidelines have been either remarkably imprecise or remarkably expansive about what constitutes high risk (see Table 1).5,8–10 Some recommend that a woman's decision about annual screening should be “determined by her risk factors.”8 Others recommend annual screening if any one of a number of “high-risk factors” is present—including (among others) herpes infection, any sexually transmitted disease, smoking, and having had a partner who has had multiple partners.10 Because it would be nonsensical to argue that the majority of American women is at higher than average risk, however, there seems little doubt that excessive screening is occurring. Were one to assume that one-fifth of the population was at high risk, our data would suggest that 25 million women currently undergoing annual screening are being screened more often than necessary. Assuming 10% of women were at high risk, the figure would rise to 33 million undergoing unnecessarily frequent screening.
Table 1.
Professional Medical Society Guidelines for Pap Smear Screening Intervals
| American Cancer Society, 20029 | After initiation of screening, cervical screening should be performed annually with conventional cervical cytology OR every 2 years using liquid-based cytology; at or after age 30, women who have had 3 consecutive, technically satisfactory normal/negative cytology results may be screened every 2 to 3 years (unless they have a history of in utero DES exposure, are HIV positive, or are immunocompromised by organ transplantation, chemotherapy, or chronic corticosteroid treatment). |
| American College of Obstetrics and Gynecology, 199510 | All women who are or who have been sexually active or who have reached age 18 should undergo an annual Pap test and pelvic examination. After a woman has had 3 or more consecutive, satisfactory annual examinations with normal findings, the Pap test may be performed less frequently in a low-risk woman at the discretion of her physician. |
| Certain high-risk factors have been associated with the development of cervical intraepithelial neoplasia and cervical carcinoma. The College recommends that when 1 or more of these risk factors is present, more frequent Pap tests may be required. High-risk factors include: | |
| Women who have had multiple sexual partners OR whose male partners have had multiple partners | |
| Women who began sexual intercourse at an early age | |
| Women whose male sexual partners have had other sexual partners with cervical cancer | |
| Women with current or prior human papillomavirus infection or condylomata or both | |
| Women with current or prior herpes simplex virus infection | |
| Women who are infected with the human immunodeficiency virus (HIV) | |
| Women with a history of other sexually transmitted diseases | |
| Women who are immunosuppressed (such as those who have received renal transplants) | |
| Smokers and abusers of other substances | |
| Women who have a history of cervical dysplasia or cervical cancer or endometrial, vaginal, or vulvar cancer | |
| Women of lower socioeconomic status | |
| American College of Physicians, 19915 | Women should be screened at least every 3 years starting in their early 20s, and continuing into their 60s. For most women, a 3-year frequency is appropriate. Some women, however, might prefer more intensive screening (for example every 2 years or even annually). If there are questions, women should be given information on the expected benefits, risks, and costs, and allowed to choose. |
| U.S. Preventive Services Task Force, 19968 | There is little evidence that annual screening achieves better outcomes than screening every 3 years. Pap tests should be performed at least every 3 years (“B” recommendation). The interval for each patient should be recommended by the physician based on risk factors (e.g., early onset of sexual intercourse, a history of multiple sex partners, low socioeconomic status). (Women infected with human immunodeficiency virus require more frequent screening according to established guidelines.) |
While observational data suggest that Pap smear screening has substantially reduced cervical cancer incidence and mortality, the same data also suggest that annual screening provides little, if any, benefit over triennial screening. In 1986, the World Health Organization's International Agency for Research on Cancer concluded, after analyzing evidence from large-scale screening programs in 8 countries, that annual screening confers a trivial, if any, advantage compared with triennial screening in reducing the incidence of cervical cancer.1 Similarly, a recent U.S. study found that rates of high-grade cytologic abnormalities did not differ among women who waited 1, 2, or 3 years after a normal Pap smear to get their next test.19 In another study, investigators found extremely low rates of histologic abnormalities in the next 2 annual Pap smears after a normal smear.20 Based upon these studies, the CDC recently concluded that annual screening shows no clear advantage over less frequent screening, and may even lead to worse health outcomes due to a greater number of questionable abnormalities requiring intervention.21
In fact, it is not difficult to recognize the potential disadvantages of annual compared with triennial screening. First, more frequent Pap smear testing means more Pap smears must be interpreted. Millions of additional Pap smears place enormous demands on the limited supply of the human resource required for smear interpretation—cytotechnologists.22 Concerns that overworked cytotechnologists were responsible for unacceptably high rates of inaccurate Pap smear readings prompted the federal government in 1988 to enact legislation mandating strict laboratory standards (Clinical Laboratory Improvement Amendments of 1988) that included a requirement that technologists examine no more than 100 smears each day.23,24 Considering that each smear contains between 50,000 and 300,000 cells, even this is an ambitious task.25 Reducing the amount of annual screening among average-risk women (and stopping screening among women who have had a hysterectomy) would decrease the annual workload for cytotechnologists by millions of Pap smears per year, allowing more time for smear review, mandatory slide rescreening, and improved quality assurance.
Second, more Pap smears interpreted means more abnormal Pap results. Each year an estimated 3.1 million Pap smears in the United States (5% of smears) are read as abnormal. Our data show that 20% of American women have had at least one abnormal smear. Investigators have found elevated levels of anxiety, distress, and negative self-image in women with abnormal smear results,26 with high levels of anxiety persisting during surveillance for mild smear abnormalities.27 Furthermore, most abnormal smear results are either atypical squamous cells of undetermined significance (comprising 60% of abnormal smears) or low-grade squamous intraepithelial lesions (33% of abnormal smears),28 the majority of which are transient lesions that would resolve spontaneously without treatment.29–31 This raises obvious questions about the benefit of promptly identifying such lesions.
Third, more abnormal results mean more follow-up procedures, including a substantial amount of testing and treatment of transient lesions. The volume of follow-up Pap smears is difficult to estimate, but our data suggest that the large majority (90%) of women with an abnormal Pap smear have one or more follow-up smears or additional tests. Many women will also undergo more invasive testing and treatment. In Australia, where national screening policy consists of routine biennial smears, 2.5% of women undergo colposcopy each year, and the lifetime risk of colposcopy for a 15-year-old woman is estimated to be 77%.32
Our study has several limitations. First, there may be legitimate concerns about our process for estimating screening intervals, which involves several assumptions. Our assumption of a single discrete screening interval for each woman is somewhat artificial, but unlikely to lead to an overestimate of screening frequency. We also implicitly assumed that each woman's Pap smears over the previous 6 years had been regularly spaced, allowing us to generalize the number of smears during that period into a periodic screening frequency. However, because we were able to stratify our analysis according to history of abnormal Pap smears, we were able to effectively eliminate the possibility that additional follow-up Pap smears artificially inflated our estimate of screening frequency among the 80% of women with no prior abnormal smears.
Second, our estimates of screening frequency are based on self-reports, which are likely to differ from actual screening history. Several studies have documented that women's self-reports of Pap smear testing consistently tend to overestimate screening.33–38 Overreporting of screening has only been demonstrated with respect to questions about timing of the most recent Pap smear. There are no data about the accuracy of self-report of the number of tests in a given time period. To the extent that women did overestimate the number of Pap smears in the past 6 years, we may have overestimated the amount of annual screening. As is the case with all survey-based research, the generalizability of our estimates is also limited by survey nonresponse. If nonresponders undergo screening less often than responders, we may have overestimated the screening frequency of American women. Our response rate of 72%, however, compares favorably with rates from other national surveys. Certainly, claims-based analysis of Pap smear screening patterns and intervals would have yielded more accurate estimates of actual practice. However, a claims-based analysis including a sample representative of civilian noninstitutionalized American women, as is included in NHIS, is not possible. In fact, ours are the first national estimates of Pap smear screening frequency.
Annual screening may be a difficult habit to break. Studies of physicians’ preferences have found that, despite professional society guidelines endorsing less frequent screening, nearly all obstetricians/gynecologists and the large majority of primary care physicians recommend annual Pap smear screening for their female patients of all ages.39 It is therefore not surprising that, of women surveyed after a revision of their HMO's Pap smear screening guideline from annual to triennial screening, the majority were skeptical or negative regarding the change, particularly those with a personal history of frequent (annual) Pap smears.40
It may be appropriate to continue annual Pap smear screening in particularly risk-averse women, as well as women at high risk for cervical cancer. However, many women may be comfortable with, indeed prefer, less frequent screening. In fact, 30% of women in the HMO survey were neutral or viewed the decrease in screening frequency favorably.40 If physicians were to recognize and discuss with patients the comparable benefits of annual and triennial screening, giving average-risk women “permission” to be screened every 2 or 3 years, many women might gladly forego annual screening, allowing time for discussion of other important preventive health issues during annual visits.
There is increasing awareness that health care priorities must be redirected away from unnecessarily frequent cervical cancer screening. The National Breast and Cervical Cancer Early Detection Program (a federal program promoting breast and cervical cancer screening among low-income and uninsured women) recently reexamined their own policy. They now require participating sites to direct more resources to women with no prior (or remote) cervical cancer screening, and to discontinue the practice of annual screening in women who have had 3 normal annual Pap smears.41 Even as screening technology and test accuracy evolve in the coming years42 with improvements in liquid-based slide preparation, automated microscopy, and human papillomavirus testing, the question of how often to test for cervical cancer and its precursors will only increase in importance. Based on our findings, we recommend that health care providers discuss screening recommendations and rationale with their female patients, and offer Pap smear screening every 2 or 3 years for women who are not at high risk of cervical cancer.
Acknowledgments
Dr. Sirovich was supported by a Veterans Affairs Ambulatory Care Fellowship at the time of this research. The views expressed herein do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
APPENDIX A
Distribution of Responses to the Question “How Many Pap Smears Have You Had in the Past 6 Years?” Among Women with No Previous Abnormal Pap Smears
| Number of Reported Pap Smears in the Past 6 Years, %* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Category | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7–11† | 12† |
| 21 to 24‡ | 1 | 14 | 13 | 13 | 14 | 8 | 28|| | 4 | 1 |
| 25 to 34 | 2 | 9 | 7 | 10 | 9 | 7 | 48|| | 3 | 2 |
| 35 to 44 | 5 | 9 | 9 | 11 | 8 | 7 | 47|| | 1 | 2 |
| 45 to 54 | 7 | 9 | 9 | 9 | 6 | 7 | 49|| | 1 | 1 |
| 55 to 64 | 6 | 11 | 9 | 9 | 7 | 5 | 49|| | 0 | 1 |
| 65 to 74 | 14 | 11 | 9 | 9 | 7 | 5 | 40|| | 0 | 1 |
| 75 to 84 | 19 | 11 | 11 | 9 | 4 | 5 | 34|| | 0 | 0 |
| ≥85 | 35|| | 11 | 9 | 7 | 3 | 1 | 20|| | 0 | 0 |
| Total | 6 | 10 | 9 | 10 | 8 | 7 | 45|| | 1 | 1 |
Percentages do not sum to 100% due to rounding and to nonresponse (item nonresponse 2% to 6% for women under 85, 15% for women 85 and older).
Response categories are combined due to small numbers of respondents. Response categories included every number from 0 through 12, and 13 or more.
We adjusted our methodology for young women as follows. Because the median age for starting screening was 18, women under age 24 would not be expected to have had Pap smears during the entire 6-year period preceding the interview. Therefore, for women age 21, 22, and 23, we assumed that all Pap smears reported in the previous 6 years had taken place since age 18 (i.e., in the last 3, 4, and 5 years, respectively). Using the same cutoffs as for women 24 and older, we inferred that a woman with 0.75 or more Pap smears per year was screened annually, 0.5 to 0.74 per year biennially, and less than 0.5 per year were being screened triennially. We did not attempt to estimate intervals for women age 18, 19, and 20.
Includes only women who have had at least one Pap smear and have not had a hysterectomy.
APPENDIX B
Distribution of Responses to the Question “How Many Pap Smears Have You Had in the Past 6 Years?” Among Women Who Have Had an Abnormal Pap Smear
| Number of Reported Pap Smears in the Past 6 Years, %* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Category | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7–11† | 12† |
| 21 to 34 | 0 | 3 | 3 | 5 | 5 | 5 | 48‡ | 19 | 11 |
| 35 to 64 | 2 | 4 | 5 | 7 | 5 | 6 | 55‡ | 10 | 5 |
| ≥65 | 3 | 5 | 6 | 6 | 7 | 5 | 61‡ | 7 | 0 |
| Total | 1 | 4 | 4 | 6 | 5 | 6 | 53‡ | 14 | 7 |
Percentages do not sum to 100% due to rounding and to nonresponders (item nonresponse < 1%).
Response categories combined. Response categories included every number from 0 through 12, and 13 or more.
Includes only women who have not had a hysterectomy.
REFERENCES
- 1.IARC Working Group on Evaluation of Cervical Cancer Screening Programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J. 1986;293:659–64. doi: 10.1136/bmj.293.6548.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frame PS, Frame JS. Determinants of cancer screening frequency: the example of screening for cervical cancer. J Am Board Fam Pract. 1998;11:87–95. doi: 10.3122/15572625-11-2-87. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Hoaglin DC, Berkey CS. Cancer incidence and mortality: the priority of screening frequency and population coverage. Milbank Q. 1997;75:147–73. doi: 10.1111/1468-0009.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahs MC, Mandelblatt J, Schecter C, Muller C. Cost effectiveness of cervical cancer screening for the elderly. Ann Intern Med. 1992;117:520–7. doi: 10.7326/0003-4819-117-6-520. [DOI] [PubMed] [Google Scholar]
- 5.American College of Physicians. Guidelines: screening for cervical cancer. In: Eddy DM, editor. Common Screening Tests. Philadelphia, Pa: American College of Physicians; 1991. pp. 413–4. [Google Scholar]
- 6.Koopmanschap MA, Lubbe KTN, van Oortmarssen GJ, van Agt HMA, van Ballegooijen M, Habbema JDF. Economic aspects of cervical cancer screening. Soc Sci Med. 1990;30:1081–7. doi: 10.1016/0277-9536(90)90294-3. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society. Summary of Current Guidelines for the Cancer-Related Checkup: Recommendations. Atlanta, Ga: American Cancer Society; 1988. [Google Scholar]
- 8.U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd edn. Baltimore, Md: Williams & Wilkins; 1996. Screening for cervical cancer; pp. 105–18. [Google Scholar]
- 9.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society Guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342–62. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous. Intl J Gynaecol Obstet. Vol. 49. 1995. American College of Obstetricians and Gynecologists; pp. 210–1. ACOG committee opinions. Recommendations on frequency of Pap test screening. Number 152, March 1995 Committee on Gynecologic Practice. [PubMed] [Google Scholar]
- 11.Parboosingh J. Screening for cervical cancer: Canadian programmatic guidelines. Can Fam Phys. 1999;45:383–93. [PMC free article] [PubMed] [Google Scholar]
- 12.Hermens RP, Hak E, Hulscher ME, Mulder J, Braspenning JCC, Grol RPTM. Do general practices adhere to organizational guidelines for effective cervical cancer screening? Fam Pract. 1998;15:112–8. doi: 10.1093/fampra/15.2.112. [DOI] [PubMed] [Google Scholar]
- 13.Bergstrom R, Sparen P, Adami H-O. Trends in cancer of the cervix uteri in Sweden following cytological screening. Br J Cancer. 1999;81:159–66. doi: 10.1038/sj.bjc.6690666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn M, Babb P, Jones J, Allen E on behalf of the United Kingdom Association of Cancer Registries. Effect of screening on incidence of and mortality from cancer of the cervix in England: evaluation based on routinely collected statistics. Br Med J. 1999;318:904–8. doi: 10.1136/bmj.318.7188.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wai D, Ferrier A, Collings S, Laverty C. Have the most recent Pap smear guidelines affected GP practices? Austral Fam Phys. 1996;(suppl 1):44–8. [PubMed] [Google Scholar]
- 16.Mandelblatt JS, Lawrence WF, Womack SM, et al. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA. 2002;287:2372–81. doi: 10.1001/jama.287.18.2372. [DOI] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. Data File Documentation, National Health Interview Survey, 2000 (machine readable data file and documentation) Hyattsville, Md: National Center for Health Statistics; 2002. Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Datasets/NHIS//. Accessed October 30, 2002. [Google Scholar]
- 18.National Center for Health Statistics. Survey Description, National Health Interview Survey, 2000. Hyattsville, Md: National Center for Health Statistics; 2002. Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2000/srvydesc.pdf. Accessed January 21, 2004. [Google Scholar]
- 19.Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol. 2000;96:219–23. doi: 10.1016/s0029-7844(00)00882-6. [DOI] [PubMed] [Google Scholar]
- 20.Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS) Ann Intern Med. 2000;133:942–50. doi: 10.7326/0003-4819-133-12-200012190-00009. [DOI] [PubMed] [Google Scholar]
- 21.Incidence of Pap test abnormalities within 3 years of a normal Pap test—United States 1991–1998 (Editorial Note) MMWR Morb Mortal Wkly Rep. 2000;49:1001–3. [PubMed] [Google Scholar]
- 22.Castleberry BM, Wargelin LL. 1998 Wage and vacancy survey of medical laboratories. Lab Med. 1999;30:174–8. [Google Scholar]
- 23.Pear R. 1988 Standards for medical labs go unenforced by administration. New York Times; March 20, 1991:1. [Google Scholar]
- 24.Centers for Disease Control. Regulations for implementing Clinical Laboratory Improvement Amendments of 1988: a summary. JAMA. 1992;267:1725–34. [PubMed] [Google Scholar]
- 25.Koss LG. The Papanicolaou test for cervical cancer detection: a triumph and a tragedy. JAMA. 1989;261:737–43. [PubMed] [Google Scholar]
- 26.Bell S, Porter M, Kitchener H, Fraser C, Fisher P, Mann E. Psychological response to cervical screening. Prev Med. 1995;24:610–6. doi: 10.1006/pmed.1995.1096. [DOI] [PubMed] [Google Scholar]
- 27.Peters T, Somerset M, Baxter K, Wilkinson C. Anxiety among women with mild dyskaryosis: a randomized trial of an educational intervention. Br J General Pract. 1999;49:348–52. [PMC free article] [PubMed] [Google Scholar]
- 28.Davey DD, Naryshkin S, Nielsen ML, Kline TS. Atypical squamous cells of undetermined significance: interlaboratory comparison and quality assurance monitors. Diagn Cytopathol. 1994;11:390–6. doi: 10.1002/dc.2840110416. [DOI] [PubMed] [Google Scholar]
- 29.Holowaty P, Miller AB, Rohan T, To T. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst. 1999;91:252–8. doi: 10.1093/jnci/91.3.252. [DOI] [PubMed] [Google Scholar]
- 30.Melnikow J, Nuovo J, Willan AR, Chan BKS, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727–35. doi: 10.1016/s0029-7844(98)00245-2. [DOI] [PubMed] [Google Scholar]
- 31.Ostör AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–92. [PubMed] [Google Scholar]
- 32.Kavanagh AM, Santow G, Mitchell H. Consequences of current patterns of Pap smear and colposcopy use. J Med Screen. 1996;3:29–34. doi: 10.1177/096914139600300108. [DOI] [PubMed] [Google Scholar]
- 33.McGovern PG, Lurie N, Margolis KL, Slater JS. Accuracy of self-report of mammography and Pap smear in a low-income urban population. Am J Prev Med. 1998;14:201–8. doi: 10.1016/s0749-3797(97)00076-7. [DOI] [PubMed] [Google Scholar]
- 34.Bowman JA, Sanson-Fisher R, Redman S. The accuracy of self-reported Pap smear utilisation. Soc Sci Med. 1997;44:969–76. doi: 10.1016/s0277-9536(96)00222-5. [DOI] [PubMed] [Google Scholar]
- 35.Suarez L, Goldman DA, Weiss NS. Validity of Pap smear and mammogram self-reports in a low-income Hispanic population. Am J Prev Med. 1995;11:94–8. [PubMed] [Google Scholar]
- 36.Whitman S, Lacey L, Ansell D, Chen EH, Dell J, Phillips CW. Do chart reviews and interviews provide the same information about breast and cervical cancer screening? Int J Epidemiol. 1993;22:393–7. doi: 10.1093/ije/22.3.393. [DOI] [PubMed] [Google Scholar]
- 37.McKenna MT, Speers M, Malin K, Warnecke R. Agreement between patient self-reports and medical records for Pap smear histories. Am J Prev Med. 1992;8:287–91. [PubMed] [Google Scholar]
- 38.Bowman JA, Redman S, Dickinson JA, Gibberd R, Sanson-Fisher RW. The accuracy of Pap smear utilization self-report: a methodological consideration in cervical screening research. Health Serv Res. 1991;26:97–107. [PMC free article] [PubMed] [Google Scholar]
- 39.Herman CJ, Lengerich EJ, Stoodt G. Variation in recommendation for breast and cervical cancer screening among primary care physicians in North Carolina, 1991. South Med J. 1996;89:583–90. doi: 10.1097/00007611-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Rolnick SJ, LaFerla JJ, Jackson J, Akkerman D, Compo R. Impact of a new cervical Pap smear screening guideline on member perceptions and comfort levels. Prev Med. 1999;28:530–4. doi: 10.1006/pmed.1998.0473. [DOI] [PubMed] [Google Scholar]
- 41.Lawson HW, Henson R, Bobo JK, Kaeser MK. Implementing recommendations for the early detection of breast and cervical cancer among low-income women. MMWR Morb Mortal Wkly Rep. 2000;49:36–55. [PubMed] [Google Scholar]
- 42.Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810–9. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]