Abstract
RNA polymerase II transcription is associated with cyclic phosphorylation of the C-terminal domain (CTD) of the large subunit of RNA polymerase II. To date, FCP1 is the only specific CTD phosphatase, which is required for general transcription and cell viability. To identify FCP1-associated proteins, we constructed a human cell line expressing epitope-tagged FCP1. In addition to RAP74, a previously identified FCP1 interacting factor, we determined that FCP1-affinity purified extracts contain RNAPII that has either a hyper- or a hypo-phosphorylated CTD. In addition, by mass spectrometry of affinity purified FCP1-associated factors, we identified a novel FCP1-interacting protein, named MEP50, a recently described component of the methylosome complex that binds to the snRNP’s Sm proteins. We found that FCP1 specifically interacts with components of the spliceosomal U small nuclear ribonucleoproteins. These results suggest a putative role of FCP1 CTD-phosphatase in linking the transcription elongation with the splicing process.
INTRODUCTION
RNA polymerase II is highly conserved among eukaryotes and it is subjected to reversible phosphorylation during the transcription cycle. Two forms of RNA PolII exist in vivo, namely RNA PolIIa and IIo. These two forms differ in the phosphorylation status of the C-terminal domain (CTD) of the largest subunit of RNA polymerase. The CTD consists of 27 heptad repeats in yeast and 52 in humans, and dynamic site-specific phosphorylation–dephosphorylation events during the transcription cycle occur (1). Enzymes that modify the CTD’s phosphorylation state have been identified. Several conserved cyclin-dependent kinases are capable of phosphorylating CTD. Srb10/Cdk8 appears to repress transcription by phosphorylating free RNAPIIo (2–4). Kin28/Cdk7 is a subunit of TFIIH and it plays an essential role shortly after initiation (5). Finally, Cdk9, the catalytic subunit of the P-TEFb complex plays an important role in transcription elongation both in vivo and in vitro (6).
While several kinases affect the CTD phosphorylation status during transcription, the FCP1 protein is the only CTD phosphatase identified thus far (7,8). FCP1 is a conserved CTD phosphatase that is required for transcription of most yeast genes and for cell viability (9,10). In addition, it plays a crucial role in re-cycling RNAPII in vitro (11). FCP1 protein was originally identified as an interacting protein of RAP74, a component of the general transcription initiation factor TFIIF, and it has been found as a component of the PolII holoenzyme. The FCP1 activity is stimulated by TFIIF and repressed by TFIIB. Moreover, it has been recently shown that a transcription-independent CTD dephosphorylation by FCP1 occurs in Xenopus laevis indicating that free RNAPII is a natural substrate for FCP1 activity (12).
A large number of recent observations strongly suggested that the CTD phosphorylation plays a major role in orchestrating interaction of the CTD with mRNA processing factors involved in capping, splicing and polyadenylation (13). Biochemical and genetic studies strongly suggested that different modified CTD forms predominate at different stages of transcription. CTD phosphorylated at Ser5 is localized to promoters, whereas Ser2 phosphorylation occurs primarily during elongation (14). In yeast the transition from Ser5 to Ser2 phosphorylation is regulated by Ctk1 kinase and FCP1 phosphatase, and it has been suggested that FCP1 is implicated in transcription elongation by modulating the levels of Ser2 phosphorylation (15).
As part of ongoing effort to elucidate the role of FCP1, in this study we report a biochemical characterization of FCP1 associating factors using an epitope-tagged FCP1 stably expressing cell line. We found that FCP1-affinity purified extracts contain RNAPII and we identified a novel FCP1-interacting protein, named MEP50, identified as one of three known components of the methylosome complex involved in the methylation and assembly of spliceosomal snRNPs Sm proteins (16). Furthermore, we found that FCP1 specifically interacts with components of the pre-mRNA spliceosomal snRNPs suggesting a putative role of FCP1 in the recruitment of pre-mRNA splicing factors during transcription.
MATERIALS AND METHODS
Plasmids
The p3XFLAG-FCP1 construct was obtained by inserting the R1 fragment from GFP-FCP1 (17) into the p3XFLAG-CMV-10 (Sigma). To construct the MEP50 expression vector, the DNA sequences corresponding to the full-length protein (16) were amplified by PCR from Marathon-Ready HeLa cDNA (Clontech), and subcloned into the pCMV-MYC vector (Clontech). The p3XFLAG-PIN1 vector was constructed by inserting the PIN1 coding sequences into the p3XFLAG-CMV-10.
Cell culture and transfections
The H1299 cells were cultured in DMEM supplemented with 10% fetal bovine serum. The p3XFLAG-FCP1 was transfected into H1299 cells using the standard calcium phosphate method, and the cells were cultured for 2 weeks in the presence of Geneticin (1 mg/ml). Drug-resistant clones were isolated, and the cell lines expressing FLAG-FCP1 (HFFCP1) were selected by immunoblotting with anti-FLAG M2 (Sigma). The stably derived cell line was cultured in the presence of 0.5 mg/ml G418. For transient transfections, H1299 and HFFCP1 cells (150 cm2 dishes) were transfected with the same quantities (10 µg) of expression vectors p3XFLAG-FCP1 and/or pMyc-MEP50 following the standard calcium phosphate method. Cells were harvested 48 h after transfections, and nuclear extracts were prepared as described below. The cell extracts were immunoprecipitated with FLAG monoclonal antibody (M2) and analyzed by western blotting using the anti c-Myc as described in the text.
Affinity purification of FLAG-FCP1
To obtain a large quantity of highly purified FCP1-associated polypeptides, FCP1 complexes were affinity purified from nuclear extracts from 4 × 108 HHFCP1 cells. Cells were washed in PBS and lysed in buffer A (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF). NP40 to final concentration 0.5% was added after 15 min on ice. Nuclei were collected by centrifugation and lysed in buffer C (20 mM HEPES, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF and proteinase inhibitors). Nuclear extracts were collected after centrifugation of the lysates for 2 h to 25 000 r.p.m. in a Beckman SW41 rotor at 4°C. Extracts were incubated with M2 anti-FLAG agarose-conjugated antibody (Sigma) overnight, followed by extensive washes with BC100 buffer (100 mM NaCl, 10% glycerol, 20 mM Tris–HCl pH 7.9, 0.1 NP40) and FLAG peptide elution in BC100. The eluted extracts were TCA precipitated before loading on 5–20% SDS–PAGE. The gels were treated with colloidal blue staining kit (Invitrogen). Protein bands were excised from the gel and destained by repetitive washings with 0.1 M NH4HCO3 pH 7.5 and acetonitrile. Samples were reduced and carboxyamidomethylated with 10 mM DTT and 55 mM iodoacetamide in 0.1 M NH4HCO3 buffer pH 7.5. Tryptic digestion of the alkylated samples was performed at 37°C for 18 h using 50 µl of trypsin 12.5 µg/ml. The resulting peptide mixtures were directly analyzed on a reflectron Voyager DE PRO MALDI-TOF mass spectrometer (Applied Biosystems). The mass range was calibrated using the [M+H]+ ion from the standard peptide mixture provided by the manufacturer. About 1.0 µl of sample was applied to a sample slide and mixed with 1.0 µl of a 10 mg/ml α-cyano-4-hydroxycinnamic acid solution in acetonitrile/0.2% TFA (70:30, v/v) before air-drying. Peptide mass values recorded in the MALDI spectra were used for database search using both the Mascot and the MS-Fit softwares.
Cell fractionation and glycerol gradients centrifugation
Cell fractionation into cytoplasmic and nuclear fractions was performed as described previously (18). Equal cell volume from nuclear and cytoplasmic fractions was loaded on the gel. Nuclear and total extracts were separated on 5–45% glycerol gradients at 40 000 r.p.m. in an SW41 rotor at 4°C for 15 h as described (19). Fractions (0.5 ml) were collected and 20 µl of each fraction was separated by SDS–PAGE and analyzed by western blotting.
Antibodies
The following antibodies were used for carrying out the immunological techniques. RAP74 protein was detected using a mixture of two antisera (C-18 and N-16, Santa Cruz Biotechnologies). For RNAPII detection the rabbit N-20 (Santa Cruz Biotechnologies) against the N-terminus of the RNAPII, the mouse monoclonal antibody against the RNAPII CTD (clone 8WG16; BAbCO Covance), and the specific mouse monoclonal anti-phosphorylated RNAPII CTD-Ser5 and Ser2, H14 and H5 (BAbCO Covance), respectively. The c-Myc antibody is from Clontech. For spliceosomal subunits detections the SmB (N-18) and U1 SnRNP 70 (N-20) and α-tubulin antibody are from Santa Cruz Biotechnologies. Anti-FLAG M2 affinity gel is from Sigma. The anti FCP1 antibody was a gift of Dr Reinberg (11). The anti MEP50 was a gift of Dr Dreyfuss (16). Western blots were processed for chemiluminescence as described previously (17). For immunofluorescence analysis normal human BJ1 cells (Clontech) were permeabilized in 0.1% Triton X-100 in PBS, pre-blocked in 2% BSA-3% NGS in PBS and then incubated overnight at 4°C with anti-MEP50 diluted 1:200, followed by incubation with a FITC-coniugated anti-mouse (Jackson Immunoresearch Lab) secondary antibody (1:400 dilution). Cells were washed, mounted and analyzed by fluorescence microscopy. All images were digitally processed using Adobe Photoshop Software.
Northern blots
Samples were processed and electrophoresed in 8% SDS–PAGE. RNAs were electro-transferred on Hybond N+ (Amersham). The membranes were cross-linked by UV, hybridized overnight in hybridization buffer (5 × SSPE, 5% Denhardt-s reagent, 0.5% SDS, 50 µg salmon sperm DNA) at 65°C, and washed first for 30 min at 65°C in 1 × SSC buffer and twice in 0.2 × SSC buffer supplemented with 0.1% SDS. 7SK and U1 cDNAs were labeled by nick-translation system from Invitrogen.
RESULTS
In vivo interaction between FCP1 and RNAPII
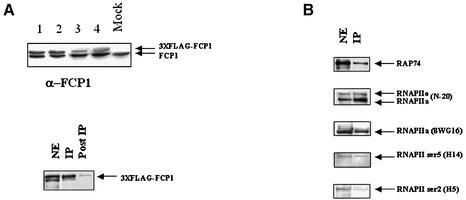
To obtain purified FCP1 complexes we carried out the isolation of FCP1 complexes from cultured human cells using the FLAG-tagged method. For this purpose, we generated a human lung carcinoma cell line H1299 that stably expressed the FLAG-epitope-tagged FCP1 protein. Several FLAG-FCP1 stably transfected H1299 clones were obtained and nuclear extracts were analyzed by western blotting using FCP1 antibody. Figure 1A shows that different clones exhibit variable expression of the FLAG-FCP1 protein and the clone 4, expressing lower amount of the exogenous versus the endogenous FCP1 protein, was chosen for further analysis. We will refer to this clone as the HFFCP1 cell line. First, we sought to verify the interaction between the FLAG-tagged FCP1 and the known partner RAP74. Nuclear cell extracts were incubated overnight at 4°C with anti-FLAG M2-agarose beads and, after extensive washing in BC100 buffer, bound proteins were eluted with the FLAG peptide. In our experimental conditions we found that almost all the FLAG-tagged FCP1 protein was purified by immunoaffinity with anti-FLAG M2-agarose beads (Fig. 1). The eluted FLAG-selected material was then analyzed by western blotting with anti-RAP74 antibodies. Figure 1B shows the presence of RAP74, indicating that the FLAG-FCP1 interacts in vivo with RAP74.
Figure 1.
(A) Western blotting analysis of FCP1-stably expressing cell clones. Different H1299 cell clones were assayed for FCP1 expression by using an FCP1-specific antibody. Clone 4, expressing lower amount of exogenous versus endogenous FCP1, was chosen for further analysis. The efficiency of the immunoaffinity of nuclear extracts from cell clone 4 with M2 anti-FLAG beads is shown below (NE, nuclear extract; IP, bound material; PostIP, unbound material). (B) Nuclear extracts from HFFCP1 cells (NE, nuclear extracts; IP, FLAG-affinity material) were assayed in western blots using the indicated antibodies.
We had previously demonstrated that artificial recruitment of FCP1 to promoter templates, through fusion to a DNA binding domain (GAL4-DBD), stimulates transcription (17). Because it was shown that FCP1 is a stoichiometric component of the human RNAPII holoenzyme complex, a plausible rationale for the GAL4-FCP1 mediated activation might involve recruitment of the RNAPII itself. Although FCP1 phosphatase has a docking site on RNAPII that is distinct from the CTD (20) a direct binding between FCP1 and RNAPII has not been clearly proved in cultured mammalian cells. The association between RNAPII and FCP1 has been proved recently in Schizosaccaromyces pombe (21).
In order to investigate if human FCP1 interacts in vivo with RNAPII, FLAG-immunoaffinity materials from HFFCP1 cell nuclear extracts were analyzed for the presence of RNAPII by western blotting using highly specific RNAPII antibodies.
As shown in Figure 1B, RNAPII was readily detected using an antibody (sc-899; N-20) that recognized the N-terminus of RNAPII. To confirm and extend these results we used different RNAPII antibodies that specifically recognize the CTD: the mouse monoclonal 8WG16 recognizes mostly the hypophosphorylated form, while the H5 and H14 the RNAPII phosphorylated in Ser2 or Ser5, respectively. We found (Fig. 1B) that all three antibodies revealed the different unphosphorylated or phosphorylated forms in FLAG-immunoaffinity extracts, suggesting that FCP1 can interact in vivo with the RNAPII independently of the state (hypo/hyperphosphorylated) and of the site (Ser2/Ser5) of CTD phosphorylation. The presence of RNAPII in the FLAG-immunoaffinity materials was specific for FCP1, since FLAG-affinity materials derived from a cell line containing an irrelevant FLAG-fusion protein (H1299 cells expressing a FLAG-GFP protein) did not show any interaction with RNAPII (data not shown).
Identification of novel FCP1-associating proteins
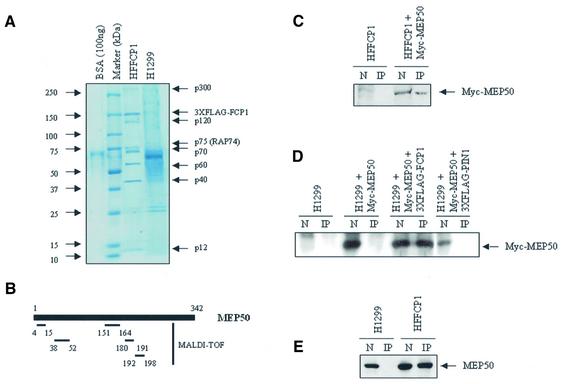
To identify new FCP1 cellular partners large amounts of nuclear extracts from the HFFCP1 cell line were prepared. Nuclear extracts were separated by affinity-immunoprecipitation by using an M2 anti-FLAG agarose antibody and bound proteins were eluted with a FLAG peptide. The affinity-eluted extracts were analyzed by SDS–PAGE and coomassie staining. The results are shown in Figure 2A. By comparison of the protein pattern with the parental cell line H1299, the FCP1 stable expressing cell line revealed some specific bands that are indicated in Figure 2 relative to their molecular weight. Western blot analysis by using a RAP74 antibody confirmed that the p75 protein corresponds to the RAP74 (data not shown).
Figure 2.
(A) Identification of FCP1-binding proteins. Coomassie staining of an SDS–PAGE gel containing the FLAG-eluates from the indicated cell nuclear extracts (see Materials and Methods). (B) MALDI-TOF analysis of affinity purified MEP50. Monoisotopic peptides corresponding to MEP50 are indicated. (C) HFFCP1 cells were transfected with the pMyc-MEP50 vector and nuclear extract (NE) and anti-FLAG materials (IP) from untransfected or transfected HFFCP1 cells were assayed with a c-Myc-specific antibody. (D) H1299 cells were co-transfected with the indicated tagged expression vectors and cell extracts were IP using a FLAG antibody and assayed with the c-Myc antibody. (E) Nuclear extracts from H1299 and HFFCP1 cells (N, nuclear extracts; IP, FLAG-affinity material) were assayed in western blot using the MEP50 antibody.
To determine the identity of the other specific proteins the bands were excised from the gel, reduced, alkylated and digested with trypsin. The resulting peptide mixtures were analyzed by mass spectrometry and the recorded mass signals used for database search. Mass spectrometry data confirmed that the p150 band corresponds to the FLAG-FCP1 protein (data not shown). Of the other specific FCP1 coimmunoprecipitated proteins here we report the identity of the p40 protein. This protein was found to be a WD repeat protein termed methylosome protein 50, MEP50. This protein, together with PRMT5 and pICln, constitutes the 20S methylosome complex involved in the arginine dimethylation of the Sm proteins, a crucial step required for in vivo assembly of snRNP (16).
To confirm the specificity of the interaction between FCP1 and MEP50 we cloned by PCR from the Marathon Ready HeLa cDNA (Clontech) the MEP50 cDNA and constructed a Myc-tagged MEP50 fusion vector. The H1299 parental cell line and the HFFCP1 derivative cells were transfected with the pMyc-MEP50 vector. Nuclear extracts were immunoprecipitated with FLAG antibody and tested for MEP50 co-immunoprecipitation by western blotting using a c-Myc antibody (Fig. 2C). As reported in Figure 2 the exogenously expressed MEP50 co-immunoprecipitate with the FCP1 protein. To further validate the interaction between MEP50 and FCP1, the H1299 cells were co-transfected with p3xFLAG-FCP1 and pMyc-MEP50 vectors, and the interaction of the exogenously expressed proteins was shown by the specific co-immunoprecipitation with a FLAG antibody followed by western blotting with anti-c-Myc. As control, H1299 cells were co-transfected with pMycMEP50 and an unrelated FLAG-expressing vector (p3xFLAG-PIN1) (see Figure 2D). Finally, HFFC1 cell extracts were immunoprecipitated with FLAG antibody and tested for specific interaction with the endogenous MEP50 protein using a recently described monoclonal anti-MEP50 antibody (16). As shown in Figure 2E specific interaction was observed only in the HFFCP1 derivative cell line with respect to the H1299 parental cell line. Collectively, these results strongly indicate that FCP1 interacts in vivo with MEP50.
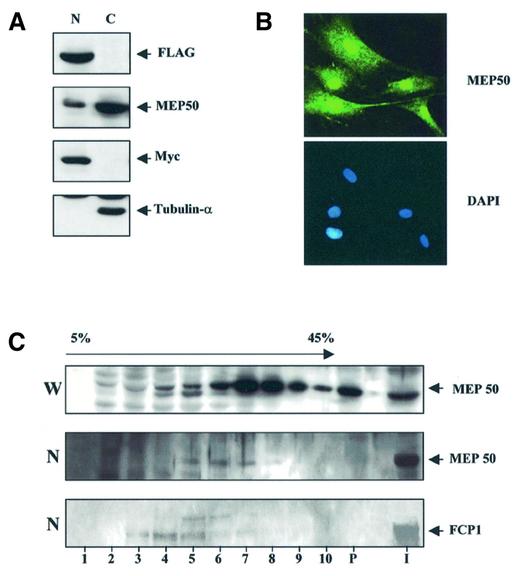
The MEP50 protein has been reported as a component of a cytoplasmic methylosome complex (16), while the FCP1 protein is found exclusively in the nucleus (17). Moreover, specific interaction between the two proteins was detected using nuclear extracts, thus suggesting that MEP50 might be present in both nucleus and cytoplasm fractions. To evaluate the presence of the MEP50 protein in the sub-cellular compartments, nuclear and cytoplasm extracts of HFFCP1 cells were analyzed by western blot by using the MEP50 antibody (Fig. 3A). As control, the fractionated extracts were analyzed for the presence of the nuclear FLAG-FCP1 and c-MYC proteins, and for the exclusively cytoplasmic located α-tubulin. Although the majority of MEP50 was indeed found in the cytoplasm, the MEP50 protein was also detected in the nucleus. The sub-cellular distribution of MEP50 was further investigated by immunofluorescence. As shown in Figure 3B MEP50 was detected in both nuclear and cytoplasmic sub-cellular compartments. Thus, biochemical cellular fractionation and immunofluorescence data concordantly indicated that MEP50 is located in both nucleus and cytoplasm.
Figure 3.
Subcellular localization of MEP50. (A) HFFCP1 cell fractionation (C, cytoplasmic fraction; N, nuclear fraction) were resolved by SDS–PAGE. Extracts were analyzed by western blotting with anti MEP50 and FLAG and subsequently the same extracts were probed with anti-Myc and anti-tubulin α antibodies as control of the purity and the integrity of nuclear and cytoplasmic protein fractions respectively. (B) MEP50 localize in both nuclear and cytoplasm sub-cellular compartments. An example of immunofluorescence microscopy of human BJ1 cells using MEP50 antibody is reported. DAPI nuclear staining of the same cells is shown. (C) Fractionation of an H1299 cell extract (W, whole cell extract, N, nuclear extract) by ultracentrifugation on a glycerol gradient. Distribution of MEP50 and FCP1 in the gradient fractions and pellet (P) was monitored by western blot using specific antibodies.
Next, we sought to determine whether FCP1 might be part of the methylosome complex, or alternatively FCP1 and MEP50 may belong to a distinct complex. To this end we carried out glycerol gradient fractionation of H1299 nuclear or whole-cell extracts and tested the fractions by western blotting with MEP50 and FCP1 antibodies, respectively. The sedimentation profile of whole-cell extract indicated that MEP50 was present in two peaks of ∼400 K and >800 K. In sharp contrast, fractionation of nuclear extract resulted in the presence of nuclear MEP50 only in the light peak (fractions 5–7). Thus, the sedimentation profile of MEP50 is clearly different from that observed with whole-cell extract. Interestingly, western analysis of the fractionated nuclear and whole-cell extracts with anti-FCP1 (Fig. 3C, and data not shown) indicated that the bulk of phosphatase is present in fractions 3–5. These results strongly suggest that FCP1 does not associate with the high molecular complex containing MEP50. Rather, it appears that FCP1 and MEP50 co-sediment in complexes of the same size (fractions 5–6).
Finally, using commercially available anti-PMRT5, a component of the 20S methylosome complex, we did not detect any specific association between FCP1 and PMRT5 (data not shown). Collectively, our data suggest that MEP50 and FCP1 associate into the nucleus and analysis of nuclear protein distribution indicate that they co-sediment in a complex of the same size distinct from the 20S methylosome complex.
FCP1 interacts with spliceosomal snRNPs
The association of the FCP1 protein with MEP50, a component of the methylosome complex, which binds to the snRNP Sm proteins, raised the possibility that FCP1 might interact with pre-mRNA spliceosomal snRNPs. It has been suggested that, because MEP50 contains six WD repeats, it can provide a platform for multiple protein interactions (16). To determine whether FCP1 might interact in vivo with pre-mRNA snRNPs complexes, FLAG-tagged FCP1 immunoaffinity materials from HFFCP1 nuclear extracts were assayed for the presence of specific components of the snRNPs by western blotting using antibodies against the snRNP proteins U1 70 K and the common Sm protein B and B′. The results (Fig. 4A) show that the FCP1 protein co-immunoprecipitates endogenous SmB and U1 SnRNP 70 proteins. The results support the finding that FCP1 is able to interact with components of the pre-mRNA machinery.
Figure 4.
(A) Nuclear extracts of HFFCP1 cells (NE, nuclear extracts; IP, FLAG-affinity materials) were assayed by western blots using the indicated antibodies. (B) Northern blots of nuclear extracts (NE) and the relative FLAG-affinity materials (IP) from HFFCP1 cells were hybridized with the indicated probes.
Given the association between FCP1 and components of the snRNP we sought to investigate if FCP1 co-immunoprecipitates the U1 snRNA, a common core element of snRNPs. Nuclear extracts from the HFFCP1 cells and from the parental cell line H1299 were prepared in the presence of RNase inhibitor and immunoprecipitated with anti-FLAG M2 beads. The immunoprecipitated extracts were run on SDS–PAGE and transferred on Hybond N+ to perform northern blot with the U1 snRNA probe. In parallel, the same extracts were analyzed by western blot for the presence of U1 70 K and SmB (data not shown). The results (Fig. 4B) show the presence of the spliceosomal U1 RNA in the FLAG-immunoprecipitated extracts. The presence of U1 RNA in the extracts was not due to an unspecific binding to small RNAs as shown by the lack of signal in northern-blotting with the 7SK RNA probe. Collectively, these findings suggest that FCP1 specifically interacts in vivo with the pre-mRNA snRNP.
DISCUSSION
In this study we used an affinity purification assay to isolate human FCP1 interacting partners. We demonstrated that, in addition to RAP74, the FCP1-affinity materials contained the RNAPII, suggesting the presence of the FCP1/TFIIF/RNAPII complex in vivo. Our data are in agreement with recent purification of a similar complex FCP1/TFIIF/PolII from S.pombe (21). Our data suggest that FCP1 interacts with both RNAPIIo and RNAPIIa forms. The association of FCP1 with the hypophosphorylated RNAPII is consistent with the previous findings showing that FCP1 is a component of the mammalian RNAPII holoenzyme (8). Likely, the association of FCP1 with RNAPII that is not engaged in transcription may prevent phosphorylation of the CTD by CTD-kinases. On the other hand, it has been recently shown that a transcription-independent CTD dephosphorylation occurs in X.laevis, suggesting that free hyperphosphorylated RNAPII is a bona fide substrate for FCP1 activity (12). Finally, it has recently shown that FCP1 is associated with polymerase during transcription elongation in vivo (15). Thus, it appears that FCP1 has the inherent ability to interact with RNAPII irrespectively from the CTD-phosphorylation status.
By mass spectrometry of affinity purified FCP1-associated factors, we identified a novel FCP1-interacting protein, named MEP50. MEP50, together with PMRT5 and pICln, constitutes the 20S methylosome complex, which is responsible for diarginine methylation of the Sm protein and it is required for snRNPs assembly. FCP1 co-immunoprecipitation analysis supported the specific interaction between FCP1 and MEP50. We demonstrate that FCP1 and MEP50 complex is present into the nucleus, thus it represents a distinct entity from the 20S methylosome. Moreover, we did not detect any specific association between FCP1 and the dimethylarginine PMRT5 component of the 20S methylosome. Conversely, we found evidence that FCP1 interacts with Sm proteins, common components of the snRNPs. Because MEP50 binds the Sm proteins, we hypothesize that association between FCP1 and Sm protein might be mediated by MEP50. It has been suggested (16,22) that the WD repeat protein MEP50 may provide a platform for multiple protein interactions. However, because FCP1 associates with RNAPII and RNAPII has been recently reported to associate the spliceosome complex (23), it is conceivable if not likely, that interaction between FCP1 and common components of the spliceosome complex might be mediated by RNAPII. Moreover, we found no evidence of specific association between MEP50 and RNAPII by immunoprecipitation experiments (data not shown).
Other mammalian protein phosphatases have been described to have a role in pre-mRNA splicing (24 and references therein). Even if there is currently only limited information on the spliceosomal substrates of these protein phosphatases it is known that several splicing factors as the SF2/ASF and SAP155 undergo phosphorylation/dephosphorylation events during the process of splicing (25–27). In contrast with other phosphatase, FCP1 is the first example of a phosphatase that is involved in transcription elongation.
Our findings add further support to the concept that there is functional intercommunication between the transcription and splicing machineries, and the RNAPII-CTD appears to play a pivotal role in coordinating transcription and pre-RNA processing (13 and references therein). Splicing factors have been detected in association with a transcriptionally active ‘holoenzyme’ containing polymerase. Furthermore, the evidence for reciprocal stimulation between splicing and transcription machineries has been reported (28). The TAT-SF1 protein, able to interact with the P-TEFb CTD kinase, was shown to bind specifically splicing factors and this association positively influences transcription (28). Interestingly, another snRNA, the 7SK RNA, was shown to associate with the P-TEFb complex and negatively modulates transcription (19,29). Using the in vivo cross-linking/chromatin immunoprecipitation assays it has been shown that FCP1 phosphatase cross-links to promoter and coding regions, suggesting that the FCP1 is associated with the elongating polymerase. Moreover, mutations in the FCP1 lead to increase levels of CTD-Ser2 phosphorylation (15). Thus, it appears that FCP1 may regulate transcription elongation by modulating the levels of CTD-Ser2 phosphorylation. The ability of FCP1 to associate and to dephosphorylate the RNAPII CTD at specific stage of transcription elongation, and to interact in vivo with MEP50, a protein associated with the common Sm factors, suggests the possibility that FCP1 might play a regulatory role in the coordination of transcription elongation and pre-mRNA splicing process. Alternatively, association between FCP1 and MEP50 might lead to de-phosphorylation of MEP50 or other components associated to MEP50. Clearly, additional studies are required to elucidate the functional significance of this association.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Dreyfuss for gift of MEP50 antibody. This work was supported by grants from the Italian Association for Cancer Research (AIRC), Istituto Superiore di Sanità, Programma Nazionale di Ricerca AIDS and MURST (COFIN).
REFERENCES
- 1.Conaway J.W., Shilatifard,A., Dvir,A. and Conaway,R.C. (2000) Control of elongation by RNA polymerase II. Trends Biochem. Sci., 25, 375–380. [DOI] [PubMed] [Google Scholar]
- 2.Hengartner C.J., Myer,V.E., Liao,S.M., Wilson,C.J., Koh,S.S. and Young,R.A. (1998) Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol. Cell, 2, 43–53. [DOI] [PubMed] [Google Scholar]
- 3.Sun X., Zhang,Y., Cho,H., Rickert,P., Lees,E., Lane,W. and Reinberg,D. (1998) NAT, a human complex containing Srb polypeptides that functions as a negative regulator of activated transcription. Mol. Cell, 2, 213–222. [DOI] [PubMed] [Google Scholar]
- 4.Gu W., Malik,S., Ito,M., Yuan,C.X., Fondell,J.D., Zhang,X., Martinez,E., Qin,J. and Roeder,R.G. (1999) A novel human SRB/MED-containing cofactor complex, SMCC, involved in transcription regulation. Mol. Cell, 3, 97–108. [DOI] [PubMed] [Google Scholar]
- 5.Lee T.I. and Young,R.A. (2000) Transcription of eukaryotic protein-coding genes. Annu. Rev. Genet., 34, 77–137. [DOI] [PubMed] [Google Scholar]
- 6.Price D.H. (2000) P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol., 20, 2629–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archambault J., Chambers,R.S., Kobor,M.S., Ho,Y., Cartier,M., Bolotin,D. Andrews,B., Kane,C.M. and Greenblatt,J. (1997) An essential component of a C-terminal domain phosphatase that interacts with transcription factor TFIIF in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 94, 14300–14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archambault J., Pan,G., Dahmus,G.K., Cartier,M., Marshall,N., Zhang,S., Dahmus,M.E. and Greenblatt,J. (1998)FCP1,the RAP74-interacting subunit of a human protein phosphatase that dephosphorylates the carboxyl-terminal domain of RNA polymerase IIO. J. Biol. Chem., 273, 27593–27601. [DOI] [PubMed] [Google Scholar]
- 9.Kobor M.S., Archambault,J., Lester,W., Holstege,F.C., Gileadi,O., Jansma,D.B., Jennings,E.G., Kouyoumdjian,F., Davidson,A.R., Young,R.A. and Greenblatt,J. (1999) An unusual eukaryotic protein phosphatase required for transcription by RNA polymerase II and CTD dephosphorylation in S.cerevisiae. Mol. Cell, 4, 55–62. [DOI] [PubMed] [Google Scholar]
- 10.Kobor M.S., Simon,L.D., Omichinski,J., Zhong,G., Archambault,J. and Greenblatt,J. (2000) A motif shared by TFIIF and TFIIB mediates their interaction with RNA Polymerase II carboxy-terminal domain phosphatase FCP1 in Saccharomyces cerevisiae. Mol. Cell. Biol., 20, 7438–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho H., Kim,T.K., Mancebo,H., Lane,W.S., Flores,O. and Reinberg,D. (1999) A protein phosphatase functions to recycle RNA polymerase II. Genes Dev., 13, 1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palancade B., Dubois,M.F., Dahmus,M.E. and Bensaude,O. (2001) Transcription-independent RNA polymerase II dephosphorylation by the FCP1 carboxy-terminal domain phosphatase in Xenopus laevis early embryos. Mol. Cell. Biol., 21, 6359–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maniatis T. and Reed,R. (2002) An extensive network of coupling among gene expression machines. Nature, 416, 499–506. [DOI] [PubMed] [Google Scholar]
- 14.Komarnitsky P., Cho,E.J. and Buratowski,S. (2000) Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev., 14, 2452–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho E.J., Kobor,M.S., Kim,M., Greenblatt,J. and Buratowski,S. (2001) Opposing effects of Ctk1 kinase and Fcp1 phosphatase at Ser2 of the RNA polymerase IIC-terminal domain. Genes Dev., 15, 3319–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friesen W.J., Wyce,A., Paushkin,S., Abel,L., Rappsilber,J., Mann,M. and Dreyfuss,G. (2002) A novel WD repeat protein component of the methylosome binds Sm proteins. J. Biol. Chem., 277, 8243–8247. [DOI] [PubMed] [Google Scholar]
- 17.Licciardo P., Ruggiero,L., Lania,L. and Majello,B. (2001) Transcription activation by targeted recruitment of the RNA polymerase II CTD phosphatase FCP1. Nucleic Acids Res., 29, 3539–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napolitano G., Licciardo,P., Carbone,R., Majello,B. and Lania,L. (2002) CDK9 has the intrinsic property to shuttle between nucleus and cytoplasm and enhanced expression of cyclin T1promotes its nuclear localization. J. Cell Physiol., 192, 209–215. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen V.T., Kiss,T., Michels,A.A. and Bensaude,O. (2001) 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature, 414, 322–325. [DOI] [PubMed] [Google Scholar]
- 20.Chambers R.S., Wang,B.Q., Burton,Z.F. and Dahmus,M.E. (1995) The activity of COOH-terminal domain phosphatase is regulated by a docking site on RNA polymerase II and by the general transcription factors IIF and IIB. J. Biol. Chem., 270, 14962–14969. [DOI] [PubMed] [Google Scholar]
- 21.Kimura M., Suzuki,H. and Ishihama,A. (2002) Formation of a carboxy-terminal domain phosphatase (Fcp1)/TFIIF/RNA polymerase II (polII) complex in Schizosaccharomyces pombe involves direct interaction between Fcp1 and the Rpb4 subunit of polII. Mol. Cell. Biol., 22, 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paushkin S., Gubitz,A.K., Massenet,S. and Dreyfuss,G. (2002) The SMN complex, an assemblyosome of ribonucleoproteins. Curr. Opin. Cell Biol., 14, 305–312. [DOI] [PubMed] [Google Scholar]
- 23.Robert F., Blanchette,M., Maes,O., Chabot,B. and Coulombe,F. (2002) A human RNA polymerase II-containing complex associated with factors necessary for spliceosome assembly. J. Biol. Chem., 277, 9302–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bollen M. and Beullens,M. (2002) Signaling by protein phosphatases in the nucleus. Trends Cell Biol., 12, 138–145. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Bruderer,S., Rafi,Z., Xue,J., Milburn,P.J., Kramer,A. and Robinson,P.J. (1999) Phosphorylation of splicing factor SF1 on Ser20 by cGMP-dependent protein kinase regulates spliceosome assembly. EMBO J., 18, 4549–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao S.-H. and Manley,J.L. (1998) Phosphorylation-dephosphorylation differentially affects activities of splicing factor ASF/SF2. EMBO J., 17, 6359–6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C., Chua,K., Seghezzi,W., Lee,E., Gozani,O. and Reed,R. (1998) Phosphorylation of spliceosomal protein SAP 155 coupled with splicing catalysis. Genes Dev., 12, 1409–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fong Y.W. and Zhou,Q. (2001) Stimulatory effect of splicing factors on transcriptional elongation. Nature, 414, 929–933. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z., Zhu,Q., Luo,K. and Zhou,Q. (2001) The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature, 414, 317–322. [DOI] [PubMed] [Google Scholar]