Abstract
OBJECTIVE
To evaluate and synthesize the evidence on the effect of supplements of vitamin E on the prevention and treatment of cardiovascular disease.
DESIGN
Systematic review of placebo-controlled randomized controlled trials; meta-analysis where justified.
MEASUREMENTS AND MAIN RESULTS
Eighty-four eligible trials were identified. For the outcomes of all-cause mortality, cardiovascular mortality, fatal or nonfatal myocardial infarction, and blood lipids, neither supplements of vitamin E alone nor vitamin E given with other agents yielded a statistically significant beneficial or adverse pooled relative risk (for example, pooled relative risk of vitamin E alone = 0.96 [95% confidence interval (CI), 0.84 to 1.10]; 0.97 [95% CI, 0.80 to 1.90]; and 0.72 [95% CI, 0.51 to 1.02] for all-cause mortality, cardiovascular mortality, and nonfatal myocardial infarction, respectively.
CONCLUSIONS
There is good evidence that vitamin E supplementation does not beneficially or adversely affect cardiovascular outcomes.
Keywords: vitamin E, antioxidants, meta-analysis, systematic review, cardiovascular disease
Cardiovascular disease, defined as coronary artery disease, hypertensive heart disease, congestive heart failure, peripheral vascular disease, and atherosclerosis including cerebral artery disease and strokes, is the leading cause of death in the United States. In 1999, 1 in 5 Americans had cardiovascular disease, and 958,775 died from it that year. This figure represented 40% of all deaths in the United States that year, equal to the next 7 leading causes of death.1 Globally, cardiovascular disease accounts for an estimated 31% of worldwide mortality and burden of disease from all noncommunicable diseases.2
The epidemiologic and observational literature have suggested a beneficial effect of antioxidant-rich foods, as well as specific antioxidants, on the risk of cardiovascular disease.3–9 The purpose of this study was to conduct a systematic review and meta-analysis of randomized trials to assess the evidence for the efficacy of supplements of the antioxidant vitamin E for the prevention and treatment of cardiovascular disease.
METHODS
Identification of Literature
This report is part of a larger review of the literature regarding the antioxidants vitamin C, vitamin E, and coenzyme Q10.10 This report deals only with vitamin E. Potential evidence for the report came from 3 sources: published reports listed in online databases and in the reference lists of relevant articles, and other sources such as experts in the field and the personal libraries of project staff and their associates. “Gray” literature was included (abstracts, etc.) but we did not specifically search for unpublished data.11 We conducted 4 searches on the specific interventions of interest with no language restriction. The full search strategies and antioxidant terms are displayed in the Appendix (available at http://www.jgim.org). In brief, we searched Medline, Embase, MANTIS, Allied & Complementary Medicine, Biosis Previews, CAB Health, Cancerlit, the Cochrane Library, Social SciSearch, SciSearch Cited Ref Sci, and TGG Health & Wellness Database using supersensitive search terms for clinical trials and vitamin E, alpha-tocopherol, d-alpha-tocopherol, rrr-alpha-tocopherol, and all rac-alpha-tocopherol.
Two trained reviewers (a physician and a PhD) independently evaluated lists of titles and abstracts (from which duplicates were removed) generated by the online database searches, as well as the additional titles from other sources to identify clinical trials in humans that assessed the effect of supplements of vitamin C, vitamin E, or coenzyme Q10 and reported clinical outcomes or intermediate (surrogate) outcomes that are closely associated with clinical outcomes. Fifty-two abstracts or articles in 7 languages other than English were reviewed by physicians in the language, with the assistance of a member of the study team. Non-English language articles did not undergo dual review.
Data Extraction
Detailed information from each study was collected on a specialized data collection instrument. The reviewers, working independently, extracted data in duplicate and resolved disagreements by consensus. A senior physician resolved any disagreements not resolved by consensus.
To evaluate the quality of the design and execution of trials, we collected information on the study design, appropriateness of randomization, blinding, description of withdrawals and dropouts, and concealment of allocation.12,13 A quality score was calculated for each trial using a system developed by Jadad.12 Empirical evidence has shown that studies scoring 2 or less on the Jadad scale report exaggerated results compared with studies scoring 3 or more.14 Whereas other elements of the design and execution of controlled trials have been proposed as quality measures, empiric evidence supporting their use as generic quality measures is lacking.
Data Synthesis
For groups of studies that assessed interventions, populations, and outcomes that were sufficiently similar, we performed meta-analysis. We estimated the Dersimonian and Laird random effects15 pooled log risk ratio if the outcome was binary, or effect size if the outcome was continuous, and associated 95% confidence interval. We applied a random effects model as it incorporates both within and between study variation. We transformed the pooled log risk ratio to the risk ratio scale for interpretability. For each subgroup of comparable studies, we calculated the χ2 test for heterogeneity based on Cochran's Q,16 and the I2 statistic and its 95% uncertainty interval.17 This latter statistic represents the percentage of study variability due to heterogeneity rather than chance, and is independent of the number of studies and the effect size metric. We present a forest plot for each meta-analysis in which each individual trial intervention effect is shown with its confidence interval as a box whose area is inversely proportional to the estimated trial variance. The pooled estimate and its confidence interval are shown as a diamond at the bottom of the plot with a dotted vertical line indicating its location. A vertical solid line either at 1 (for risk ratio analyses) or at 0 (for effect size analyses) indicates no treatment effect. We performed some posthoc sensitivity analyses motivated by the observed heterogeneity among the trials. These posthoc sensitivity analyses included removing any trials that appeared to have extreme estimates.
For each subgroup of trials for which we conducted a meta-analysis, we assessed the possibility of publication bias graphically by evaluating a funnel plot of the log risk ratios or effect sizes for asymmetry, which would result from the nonpublication of small trials with negative outcomes. Because graphical evaluation can be subjective, we also conducted an adjusted rank-correlation test18 and a regression asymmetry test19 as formal statistical tests for publication bias.
RESULTS
Description of the Evidence
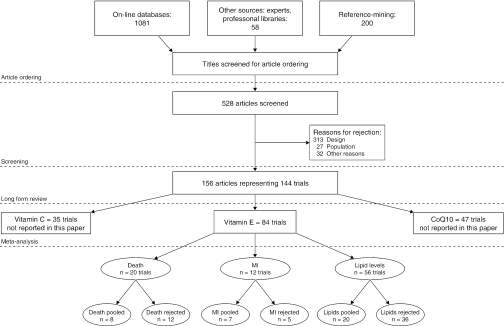
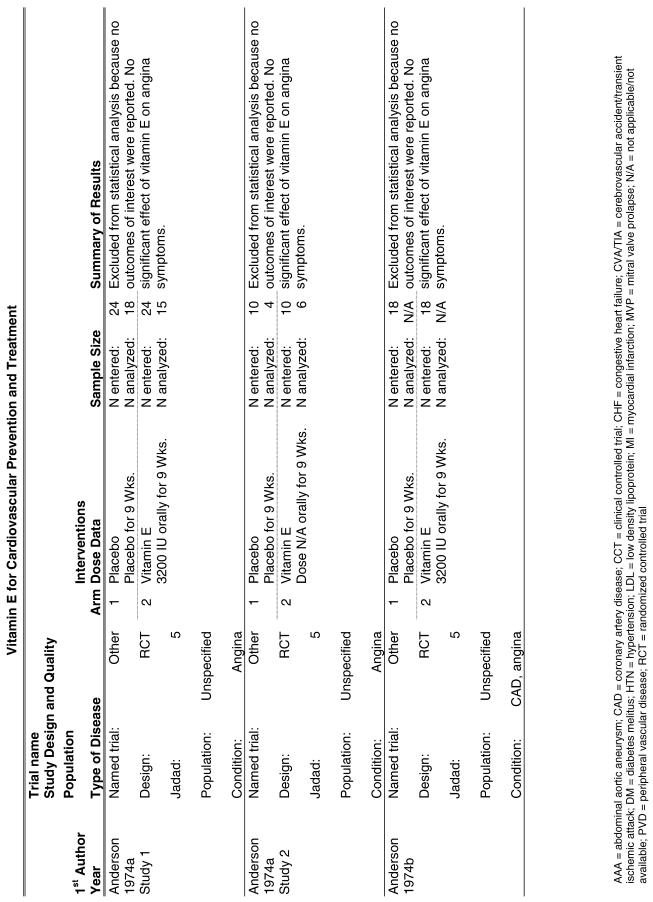
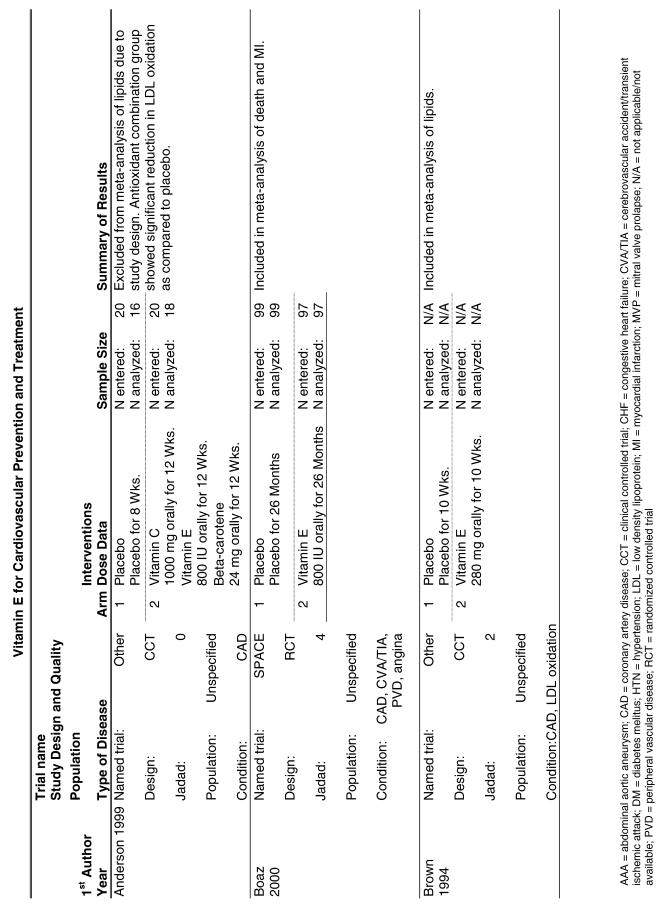
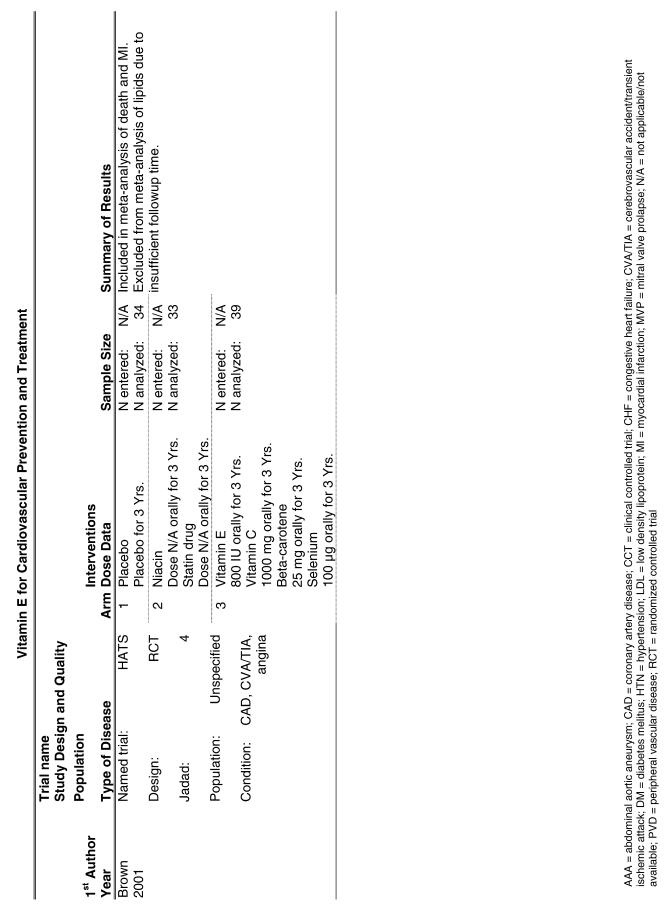
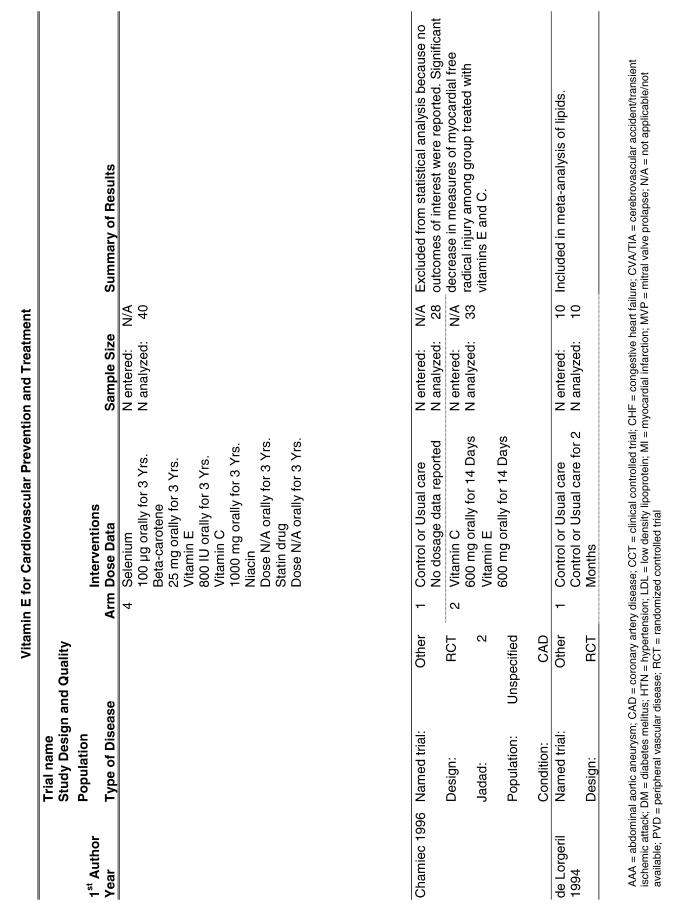
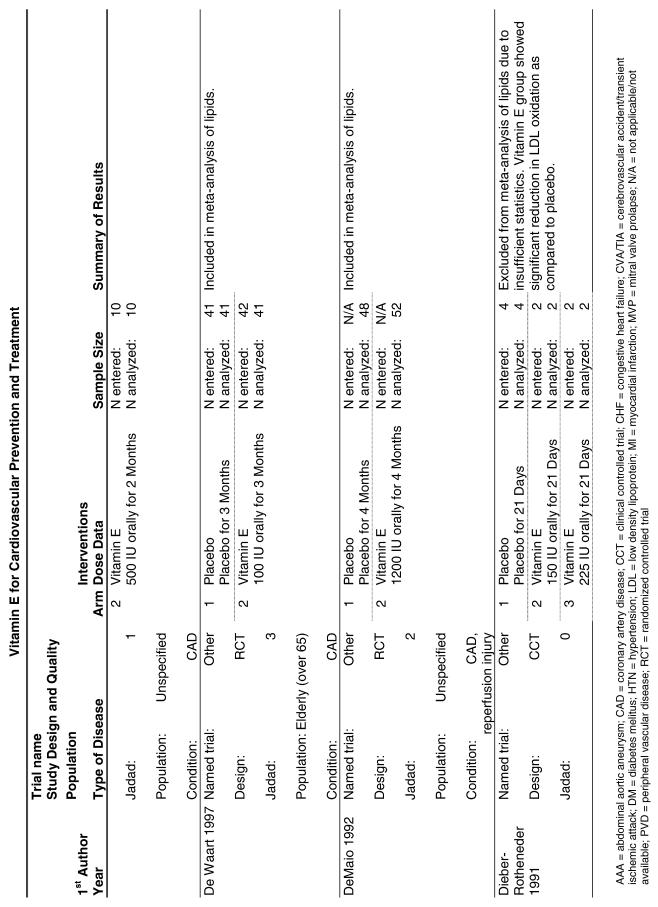
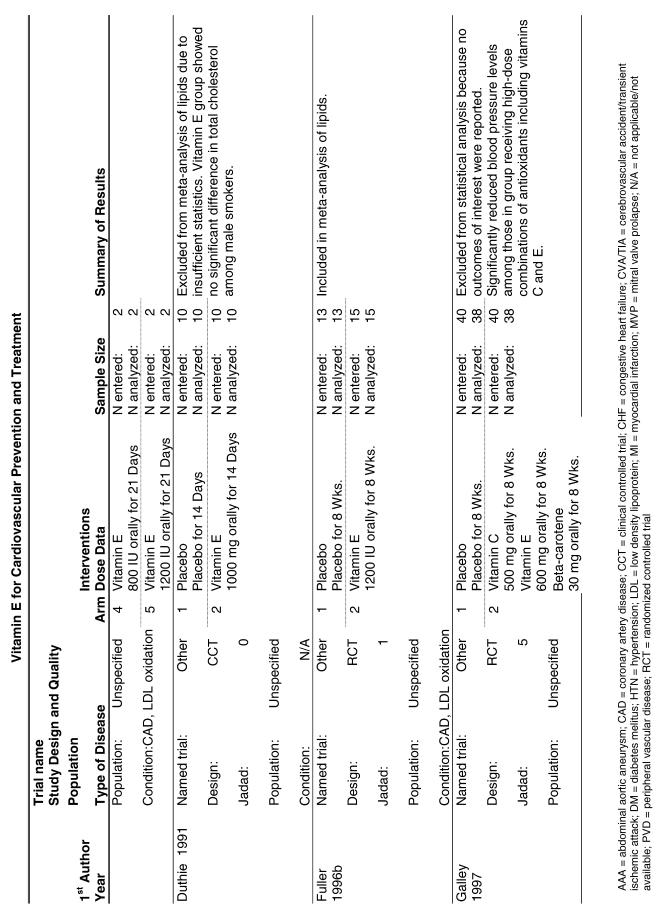
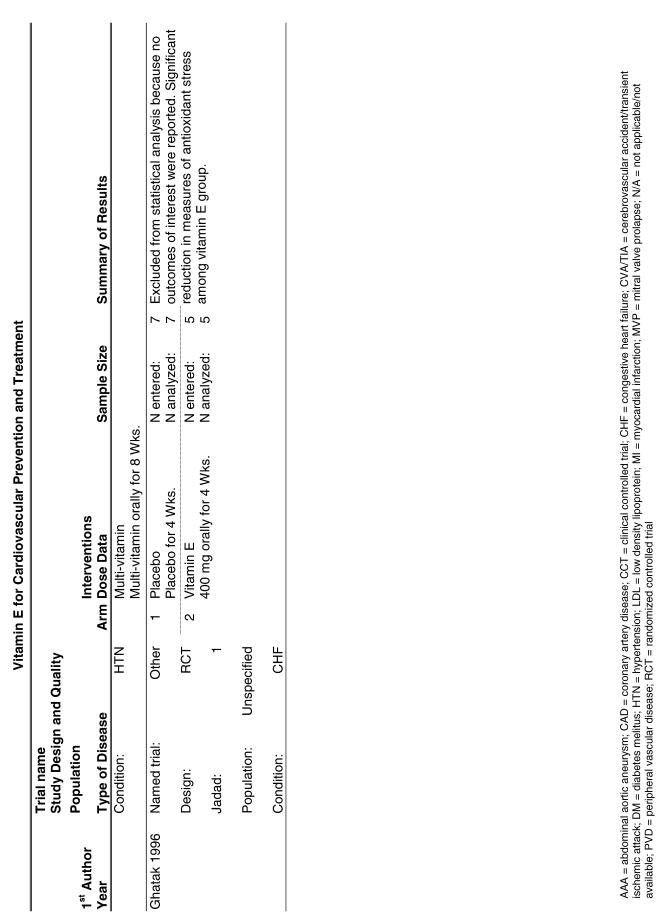
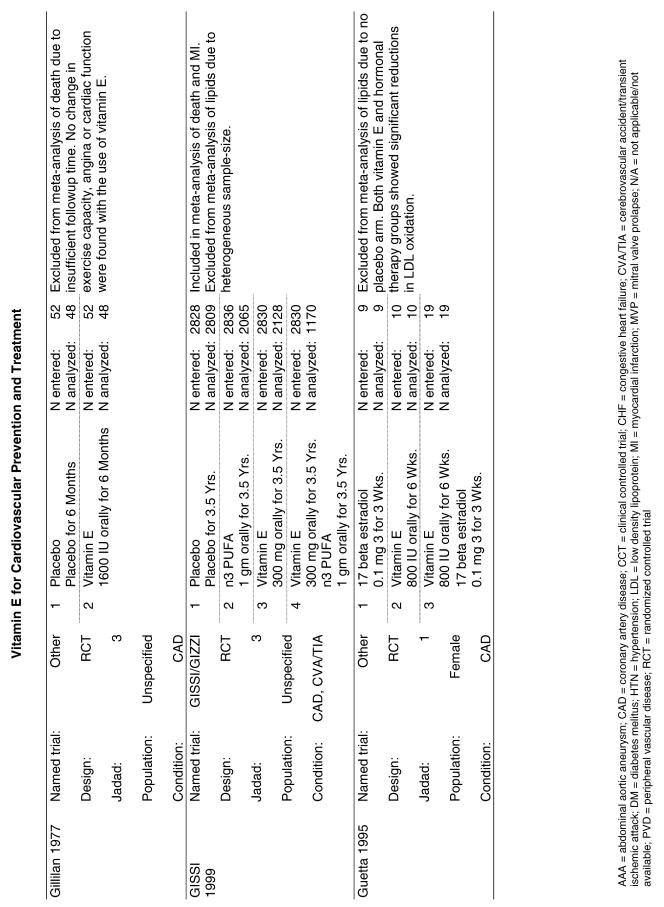
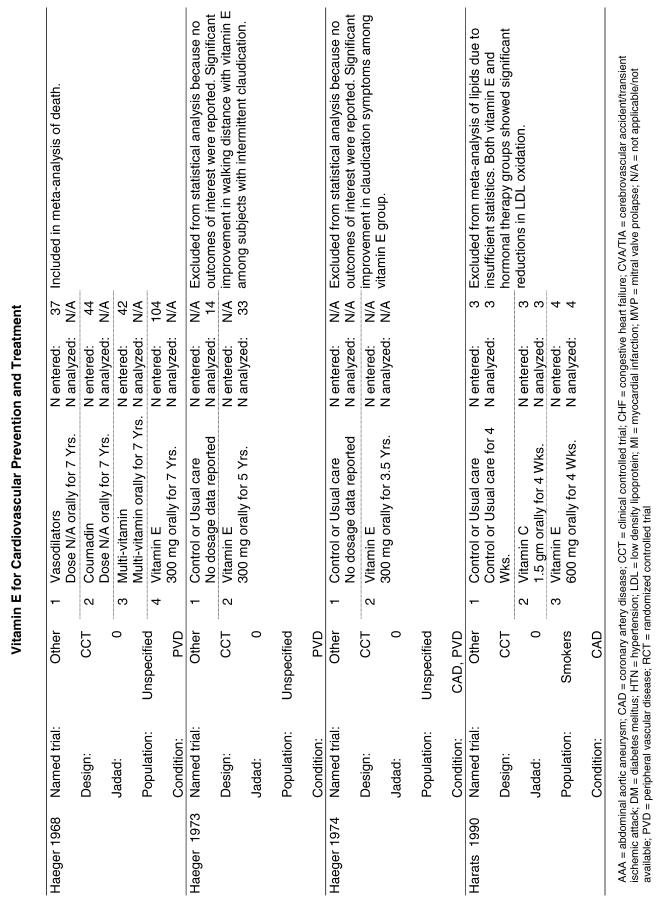
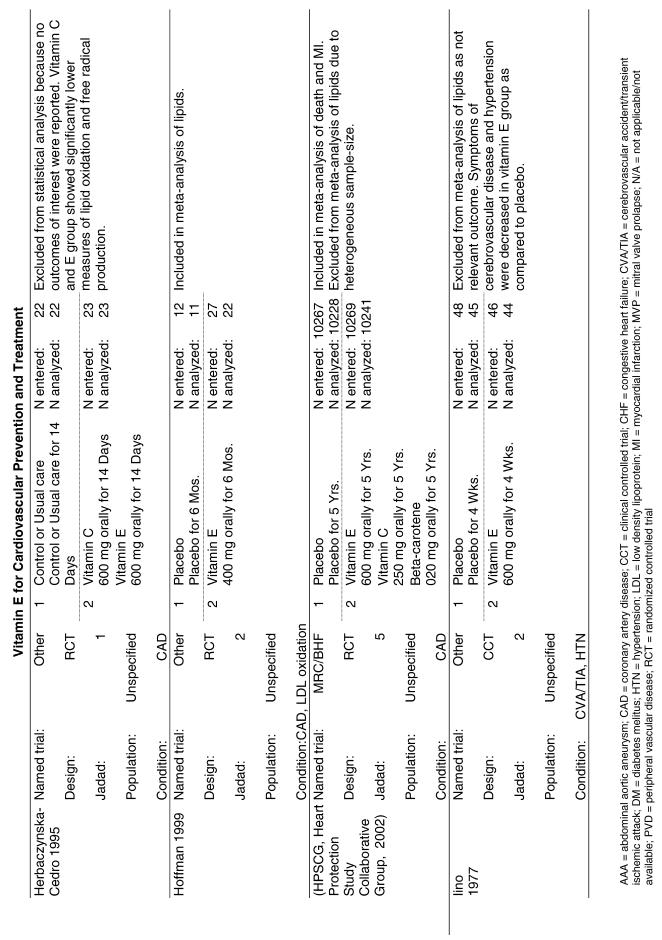
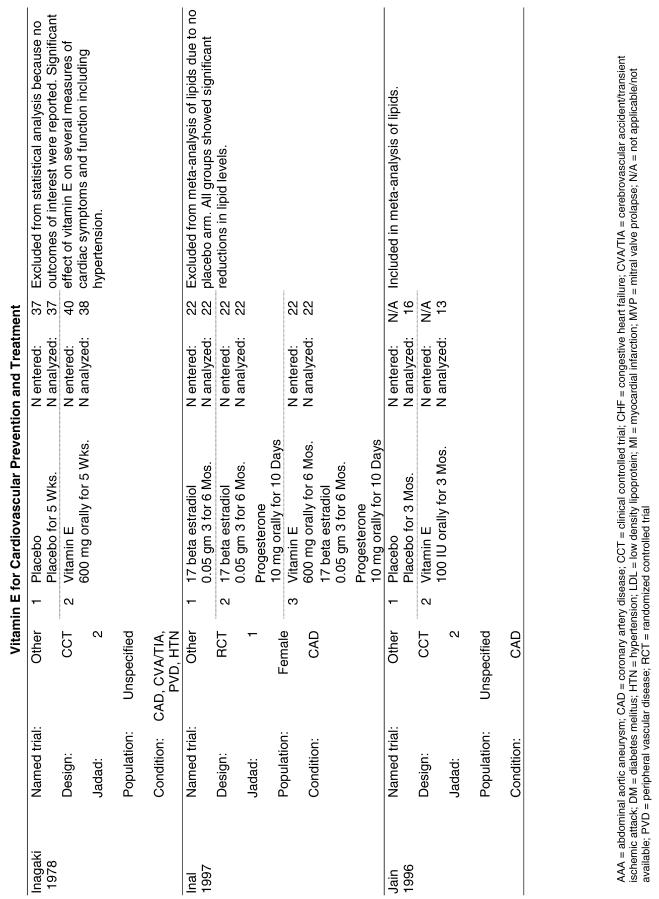
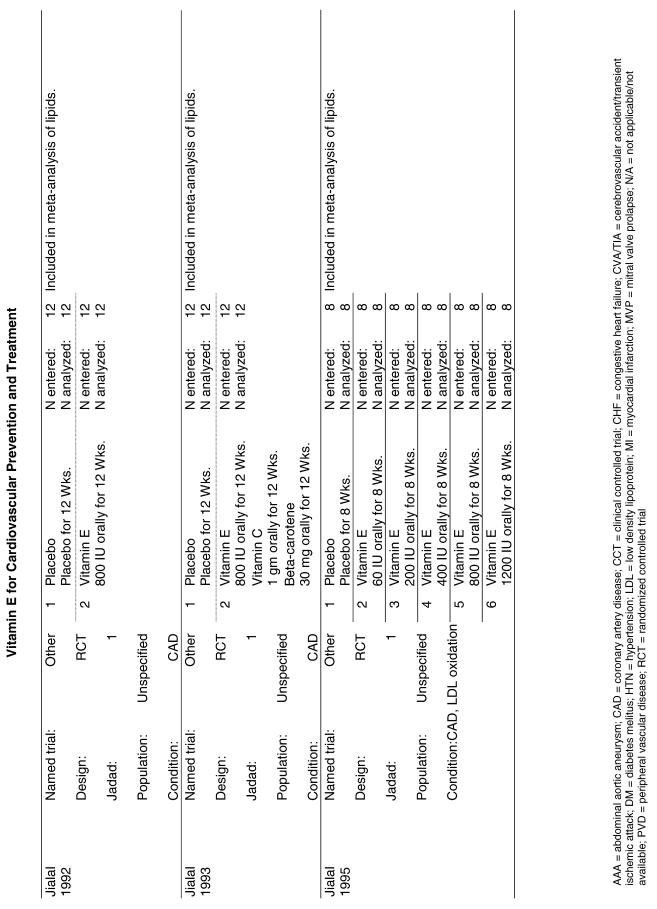
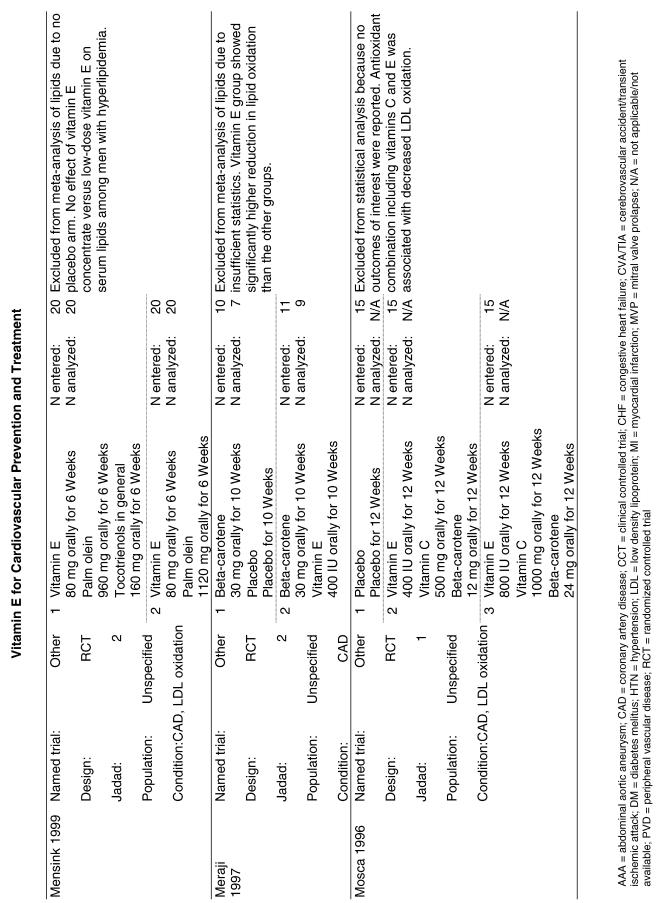
Our literature search identified 8,173 titles, which yielded 156 articles that represented results from 144 unique trials (see Fig. 1). Of these, 84 trials concerned vitamin E. A number of articles reported on different aspects of several large clinical trials. These large trials are des-cribed in Table 1 and are categorized as primary or secondary prevention trials based on the population enrolled. All studies reviewed in depth are summarized in the evidence table in the Appendix.
FIGURE 1.
Flowchart of trials.
Table 1.
Large Clinical Trials Included in Various Pooled Analyses*
| Primary Prevention Trials | |
|---|---|
| ASAP | The Antioxidant Supplementation in Atherosclerosis Prevention Study (ASAP) tested in a randomized placebo-controlled trial the effect of vitamin C (250 mg) and vitamin E (91 mg d-alpha-tocopherol) in progression of carotid atherosclerosis.20 The subjects (N = 520) all had elevated lipid levels and included both smokers and nonsmokers. Serum lipids were measured as secondary outcomes. |
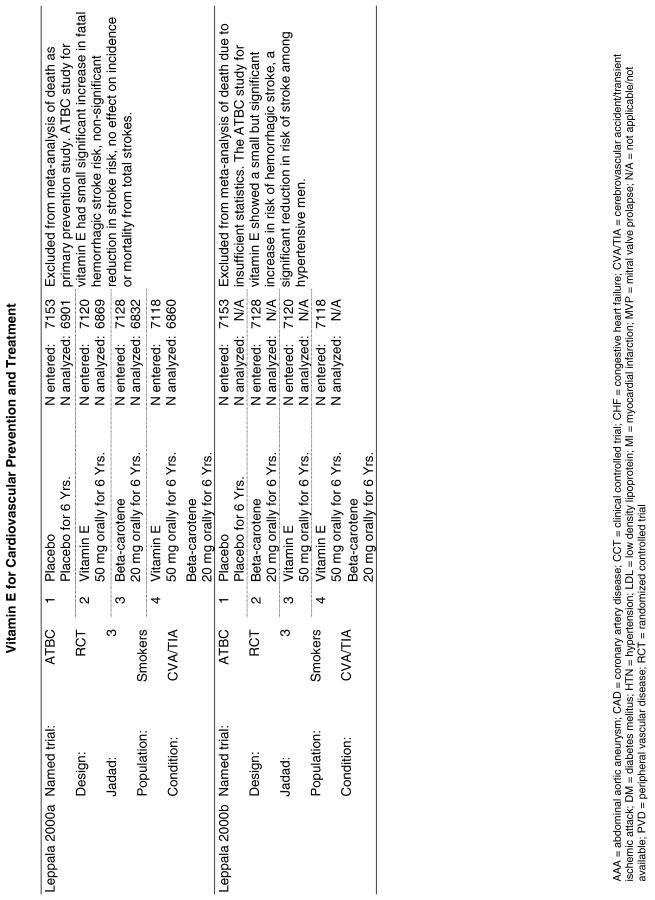
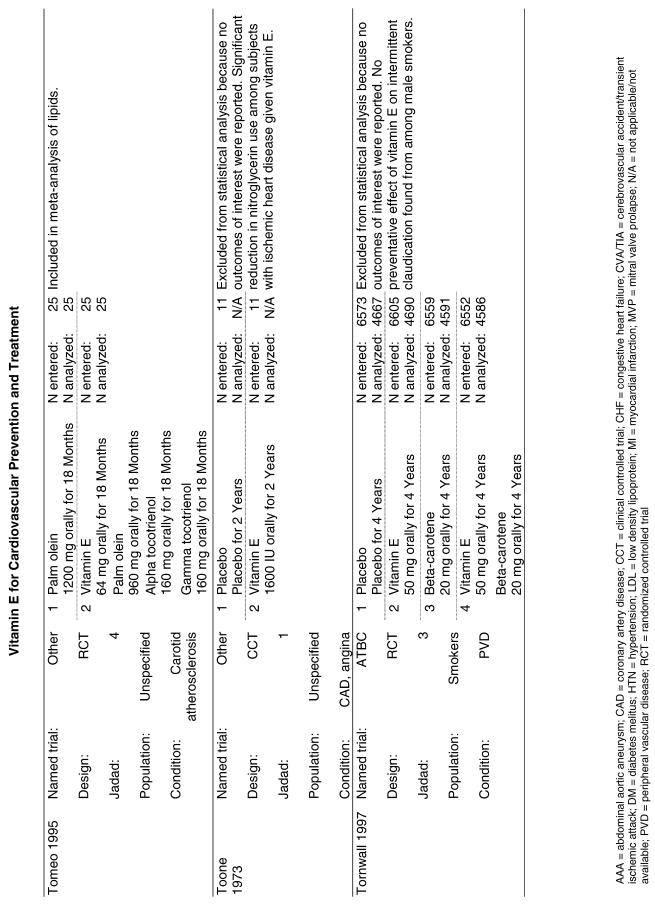
| ATBC | A primary prevention trial designed to assess cancer prevention, the Alpha Tocopherol Beta Carotene (ATBC) trial, randomized 29,133 male smokers from Finland to receive 1 of 4 possible regimens: placebo, d-, l-alpha-tocopherol acetate (AT) alone (50 mg/day), beta-carotene (BC) alone (20 mg/day), or both vitamins. CVD endpoints were analyzed as secondary endpoints for this trial. Patients were followed for a minimum of 5 years and a maximum of 8 years.32 |
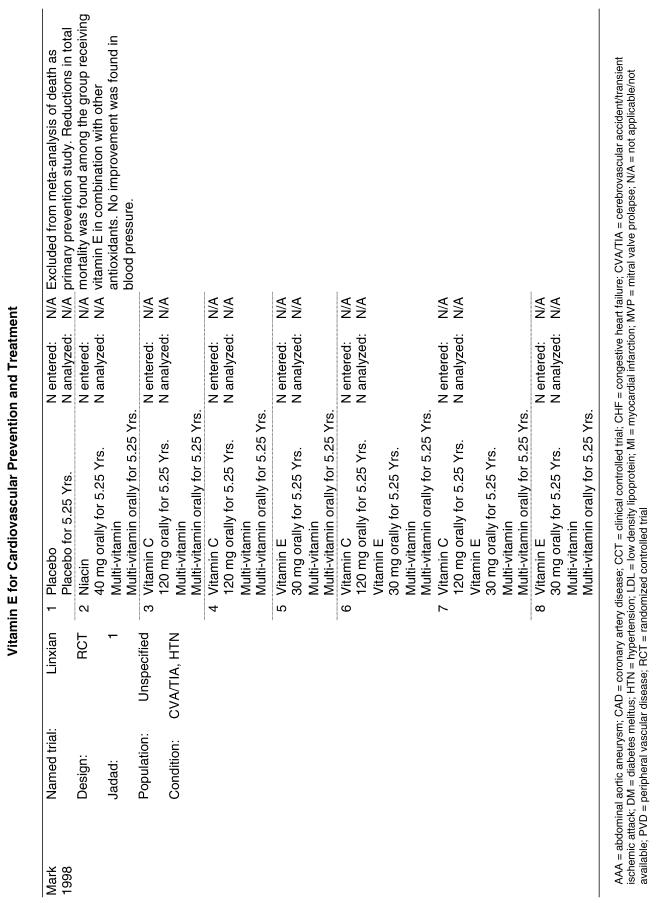
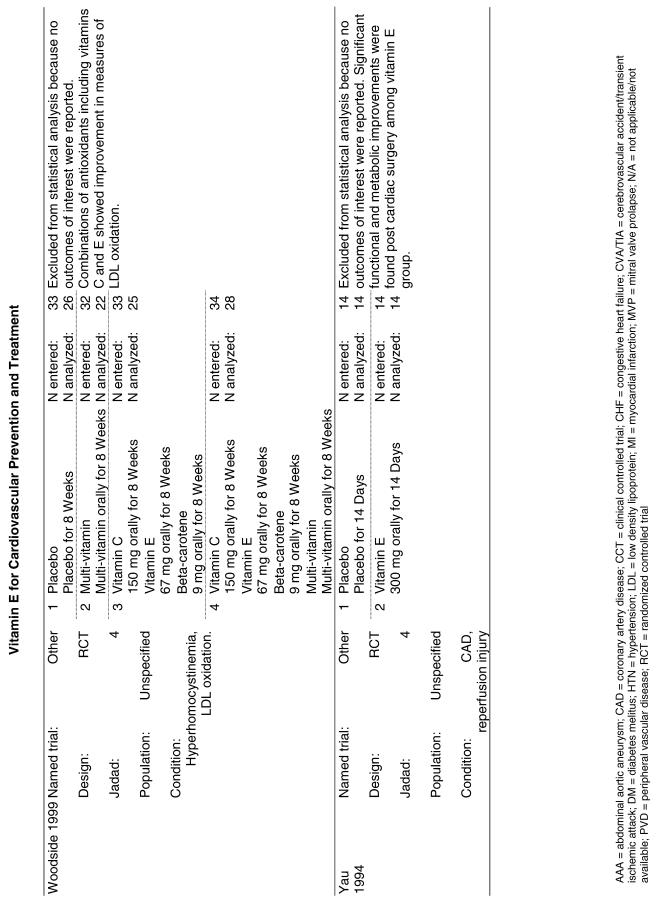
| Linxian | The Linxian Nutrition Intervention trial (Linxian), also a primary prevention trial, enrolled approximately 30,000 apparently healthy but vitamin-deficient members of the general population in an area of southwestern China that had a very high incidence of carcinoma of the esophagus and stomach. This trial was designed to assess risk of developing esophageal and gastric cancer, so the analysis of CVD endpoints represented a secondary outcome analysis. In addition, the baseline clinical examination of CVD and the measurement of outcomes for these parameters were not as rigorous for these secondary outcomes. These patients (the general population group) were randomized to receive 1 of 5 treatments singly and in combination for 5.2 years. They were given either placebo or formula A (retinol [5,000 IU] and zinc oxide [22.5 mg]), formula B (riboflavin [3.2 mg] and niacin [40 mg]), formula C (ascorbic acid [120 mg] and molybdenum [30 µg]), or formula D (selenium [50 µg] and beta-carotene [15 mg] and alpha-tocopherol [30 mg]). Each of these formulas was given alone and in combination with the other formulas. All 4 formulas were given together and a placebo group was included.21 |
| MASI | The MASI trial enrolled 60 healthy male smokers in a single blind placebo controlled trial to evaluate the effect of vitamin E on lipid oxidation. Volunteers were given either a placebo, 200 mg of RRR-alpha-tocopherol acetate daily, or 200 mg RRR-alpha-tocopherol acetate plus 500 mg ascorbic acid daily for 2 months. Lipid oxidation, lipid levels, and vitamin serum concentration were measured.42 |
| PPP | The Primary Prevention trial (PPP) involved 4,495 subjects in a 2 × 2 factorial design testing the effects of low-dose aspirin (110 mg/day) and vitamin E (synthetic alpha-tocopherol, 500 mg/day) in patients with risk factors for cardiovascular disease. Follow-up in this study was stopped after 3.6 years because of the proven benefit of aspirin supplementation in atherosclerosis (ASA) for cardiac patients.19 |
| Secondary Prevention Trials (testing the effects of antioxidants in preventing further disease in patients with preexisting cardiovasculardisease)or persons judged to be at high risk for developing coronary artery disease. | |
|---|---|
| ATBC (Subgroup) | The ATBC investigators also analyzed their trial for the subgroup of enrolled subjects with cardiovascular disease at the baseline examination.27,33 The median time for follow-up was 510 days, this is the value used in this analysis. |
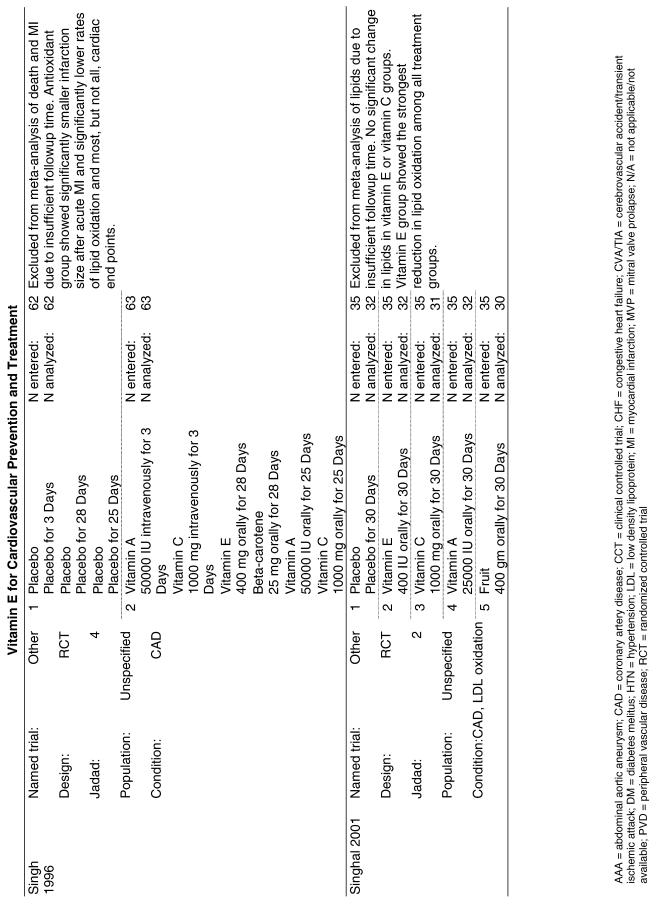
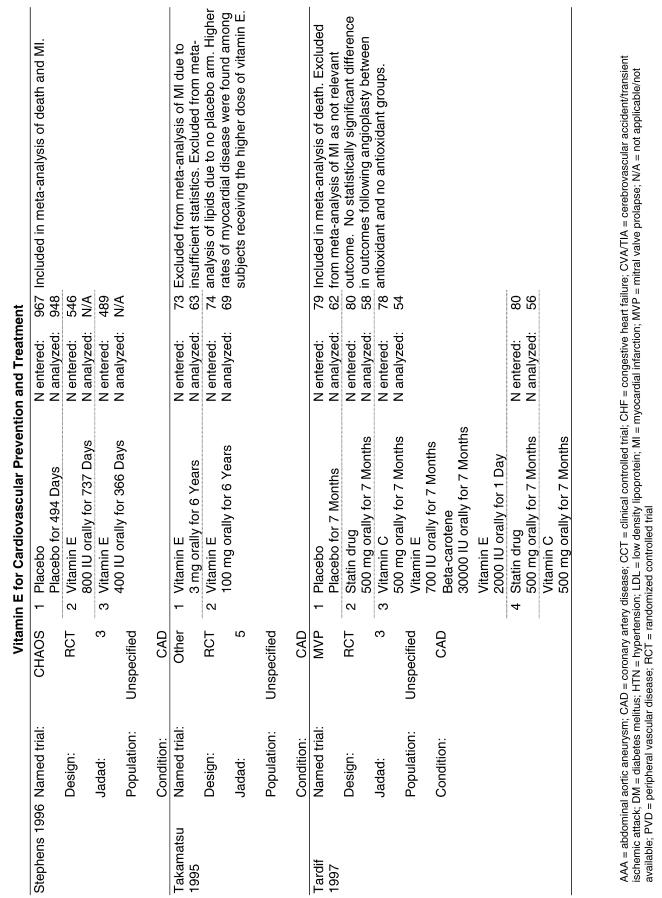
| CHAOS | The Cambridge Heart Antioxidant Study (CHAOS) assessed 2,002 subjects with angiographically proven coronary artery disease who were randomized to receive either vitamin E (400 or 800 IU/day of alpha-tocopherol) or placebo and were followed for a median of 510 days.23 |
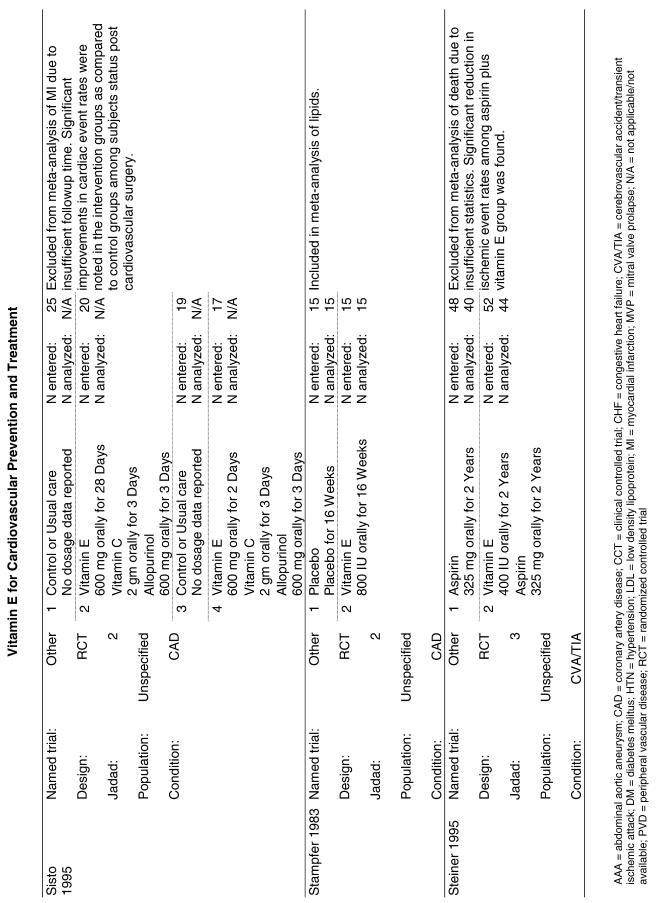
| GISSI | In the GISSI-Prevenzione trial, investigators enrolled 11,324 subjects surviving recent MI into four groups: vitamin E (300 mg/day as synthetic alpha-tocopherol), n-3 polyunsaturated fatty acids (PUFA) (1 g/day), both, or placebo for 3.5 years—and evaluated the risk of developing death, nonfatal MI, or nonfatal stroke as primary outcomes.26 |
| HATS | The HDL-Atherosclerosis Treatment Study (HATS) enrolled 160 subjects with preexisting cardiovascular disease and tested them with the following combinations: simvastatin (10 to 20 mg/day) plus niacin (500–1000 mg/day slow release); antioxidants including vitamin E alone (800 IU of d-alpha-tocopherol); simvastatin, niacin, and vitamin or placebo.34 The primary endpoint for this study was the change in angiogram over the course of the trial, but secondary endpoints included death and nonfatal MI. Treatment was continued for 3 years. |
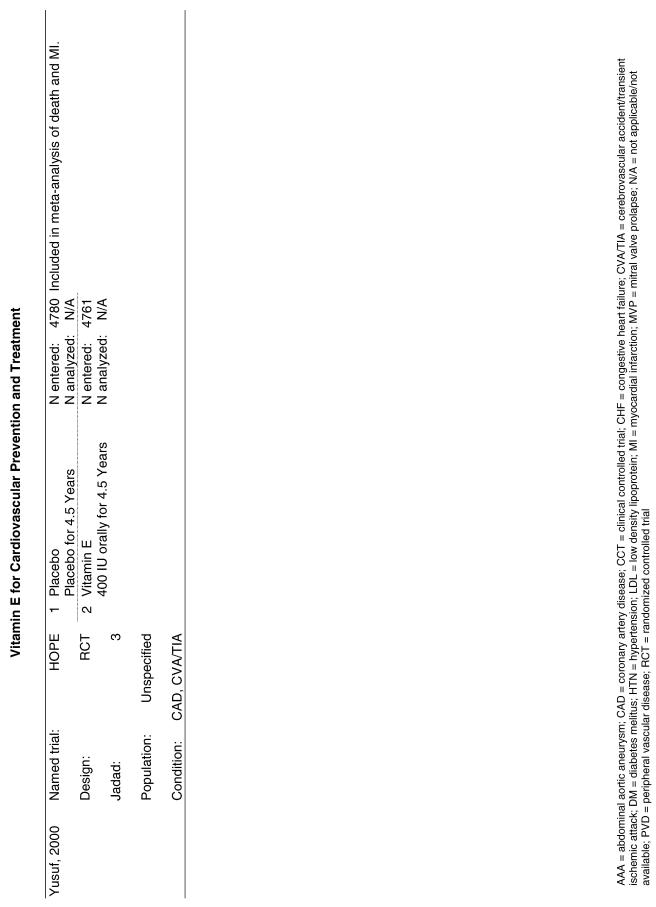
| HOPE | The Heart Outcomes Prevention Evaluation Study (HOPE)25 enrolled 2,545 men and 6,996 women more than 55 years old who were judged at increased risk for CVD due to the presence of certain risk factors in a 2 × 2 factorial trial for 4.5 years. The interventions tested were vitamin E (400 IU) from natural sources, ramipril (an angiotensin converting enzyme inhibitor), both, or neither. |
| MRC/BHF | The MRC/BHF trial enrolled 20,536 British adults with preexisting coronary artery disease, peripheral vascular disease, or diabetes in a 5-year trial evaluating the effects of a combination of vitamin E (600 mg of synthetic vitamin E), beta carotene (20 mg), and vitamin C (250 mg) versus placebo on the primary outcomes of MI, stroke, and death from cardiovascular causes.28 |
| MVP | The Multi-vitamins and Probucol Study (MVP) enrolled 317 patients scheduled for percutaneous angioplasty and having preexisting coronary artery disease in a 6-month study of a combination of vitamin E (700 IU as d-, l-alpha-tocopherol), vitamin C (500 mg), and beta-carotene (30,000 IU), with and without probucol versus placebo.31 |
| SPACE | The Secondary Prevention with Antioxidants of Cardiovascular Disease in End-stage Renal Disease (SPACE) trial22 enrolled 196 subjects receiving hemodialysis and with known cardiovascular disease who were randomized to receive vitamin E (800 IU/day as natural alpha-tocopherol) or placebo. They were followed for a median of 519 days and the CVD outcomes were the primary outcomes in this trial. |
Data on vitamin E dosages, which were often in the form of alpha-tocopherol, are sometimes reported in milligrams and sometimes in international units (IU). One milligram of alpha-tocopherol is approximately equal to 1.5 IU of vitamin E.
Vitamin E Trials that Report Death as an Outcome
Twenty trials reported on death as an outcome and were therefore considered for pooled analysis. We decided not to pool the primary prevention trials with the secondary prevention trials, as the death rates in the primary prevention trials are expected to be lower because the patients did not have known preexisting disease. We considered pooling primary prevention trials (ATBC, PPP, ASAP, Linxian)20–23 but judged these 4 trials to be too heterogeneous in terms of interventions to support statistical pooling; thus the findings for these studies are reported narratively.
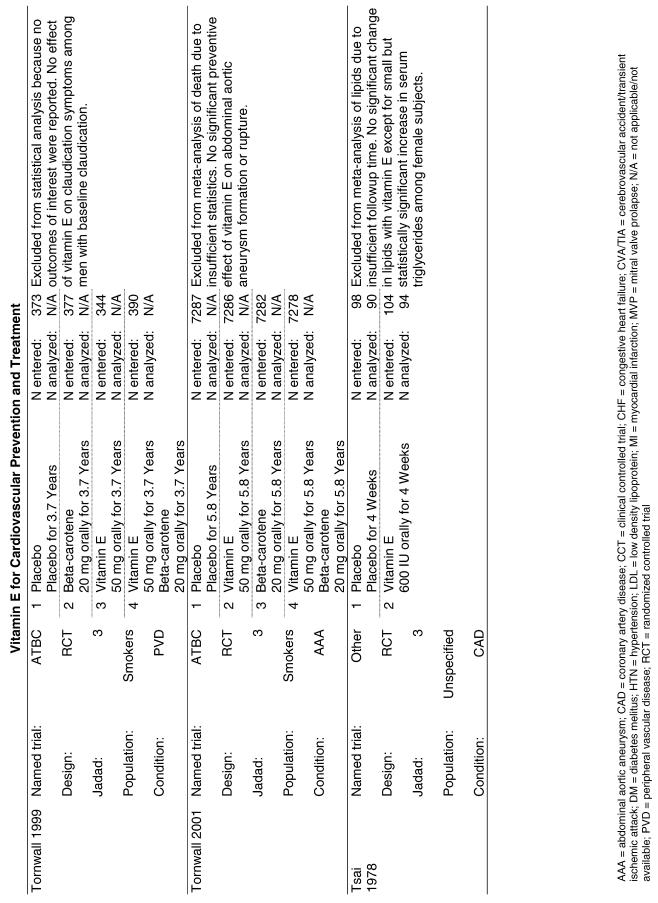
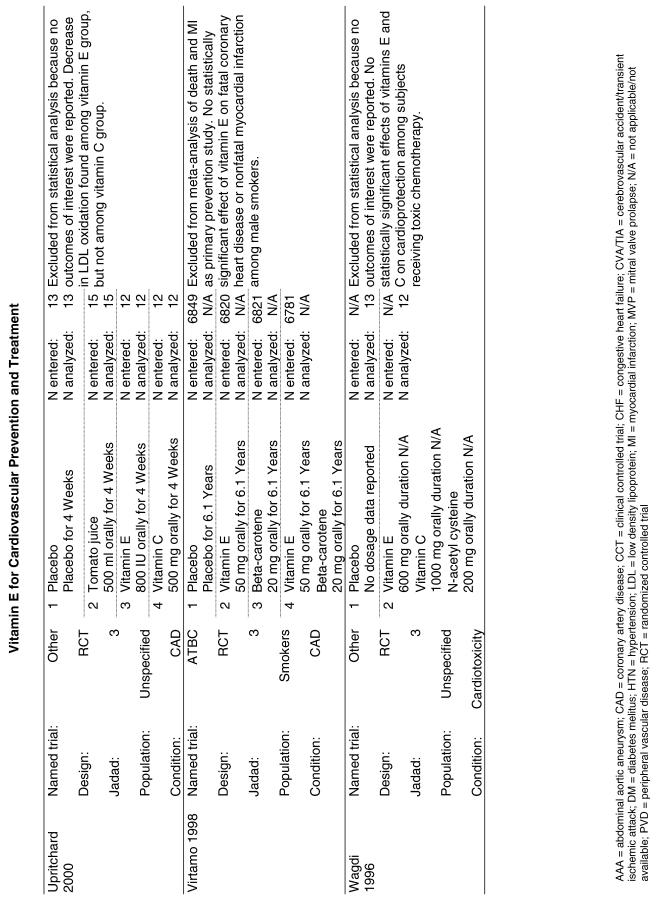
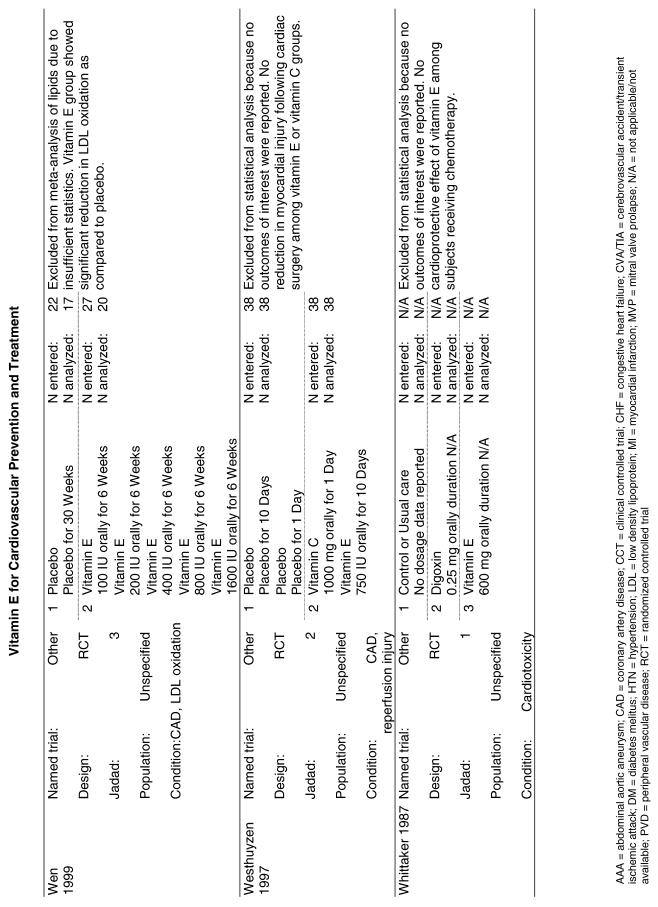
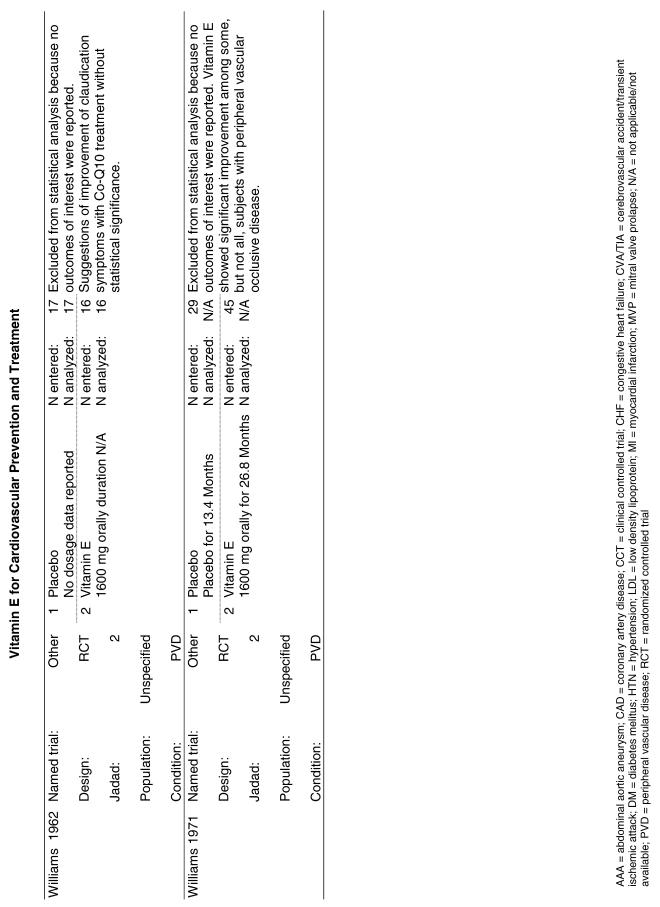
The follow-up of the included trials ranged from 224,25 to 7 years.26 The interventions consisted of vitamin E alone or in combination with other antioxidants, typically vitamin C or beta-carotene. Four of the trials tested a relatively low-dose vitamin E supplement (i.e., less than or equal to 400 IU),26–29 and the remaining 4 trials tested a higher dose of vitamin E (greater than 400 IU).24,25,30,31 For details of these trials, please see the evidence table in the Appendix.
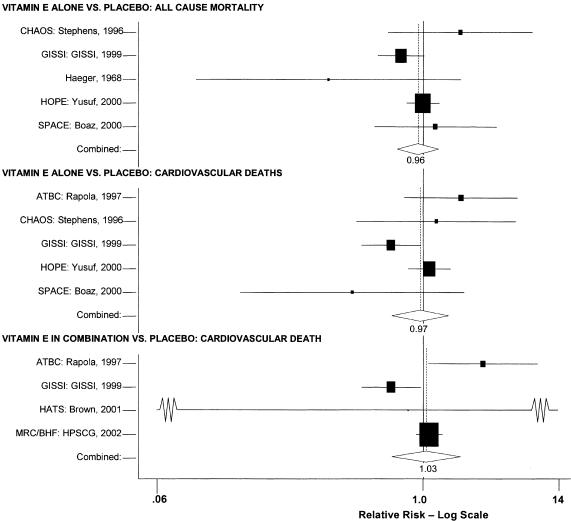
Meta-analysis of Vitamin E Alone Versus Placebo: All-cause Mortality
Four large secondary prevention trials reported on all-cause mortality using vitamin E alone as an intervention: the SPACE trial,24 the HOPE trial,27 the GISSI trial,28 and the CHAOS trial.25 A fifth, smaller, trial by Haeger and colleagues is also included in this meta-analysis.26 The random effects pooled estimate was a relative risk ratio of 0.96 (95% confidence interval [CI], 0.84 to 1.10) as shown in Fig. 2. The χ2 test did not demonstrate significant heterogeneity (P = .22). The I2 statistic was 31% (with 95% uncertainty interval 0% to 74%). Sensitivity analyses did not alter these results. Neither formal test demonstrated evidence of publication bias (Appendix Table). The results of these tests are presented in the Appendix.
FIGURE 2.
Pooled relative risk ratios for all-cause mortality.
A small secondary prevention study by Gillian32 that was excluded from the pooled analysis because of insufficient follow-up time (6 months) reported a relative risk ratio for all-cause mortality of 0.85 (95% CI, 0.13 to 5.52). The remaining 2 studies were primary prevention trials, and were therefore not included in the pooled analysis of the secondary prevention trials. The ASAP trial22 reported a relative risk ratio of 3.00 (95% CI, 0.32 to 28.47) and the PPP trial21 reported a relative risk ratio of 1.07 (95% CI, 0.78 to 1.49). Thus, the results of the 3 trials that were not included in the pooled analysis support the findings of the pooled analysis that there is no evidence of a significant effect of vitamin E alone on all-cause mortality, either in primary or secondary prevention trials.
Meta-analysis of Vitamin E in Combination Versus Placebo: All-cause Mortality
Five trials were considered for this pooled analysis. Two of the trials were primary prevention trials, so they were not considered for pooling with the secondary prevention trials for the reasons previously listed.22,23 Of the secondary prevention trials, one33 had a follow-up time of 6 months, too short for pooling with the other studies. Eliminating those 3 trials left only 2 clinically similar trials, the GISSI trial28 and the MRC/BHF Heart Protection Study,30 an insufficient number for meta-analytic pooling. Therefore we describe the results for these 5 studies narratively.
The Linxian study23 and the GISSI study28 alone reported statistically significant benefits. The effect on all-cause mortality in the GISSI trial was almost certainly a result of the omega-3 polyunsaturated fatty acids that were given with vitamin E, with the former providing all of the benefits. In an analysis of the effect of the individual components in this 2 × 2 factorial trial, omega-3 polyunsaturated fatty acid supplementation was beneficial as measured by all-cause mortality (risk ratio [RR], 0.80; 95% CI, 0.68 to 0.95), whereas vitamin E supplementation was not (RR, 0.86; 95% CI, 0.73 to 1.01). Therefore, the beneficial effect reported for the combination of these 2 agents is most probably due to the omega-3 polyunsaturated fatty acids alone. The Linxian trial reported a statistically significant 9% reduction in all-cause mortality for subjects who received beta-carotene, selenium, and vitamin E (RR, 0.91; 95% CI, 0.84 to 0.99).23 The other 3 studies all reported no statistically significant beneficial or adverse effect on all-cause mortality.22,30,33
Meta-analysis of Vitamin E Alone Versus Placebo: Cardiovascular Deaths
Five trials were pooled.24,25,27–29 The ATBC trial reported on the results at 2 different time intervals.29,34,35 To avoid double-counting the data, only the results with the longer follow-up period29 were pooled. The random effects pooled estimate for all studies was a relative risk ratio of 0.97 (95% CI, 0.80 to 1.19) as shown in Fig. 2. The χ2 test did not demonstrate significant heterogeneity with a P value of .09. The I2 statistic was 50% (with 95% uncertainty interval 0% to 82%). Sensitivity analyses did not alter these results. The GISSI study reported a significant benefit on mortality (RR, 0.80), whereas 3 of the other 4 studies actually reported nonsignificant increases in mortality in the treated groups. Neither formal test demonstrated evidence of publication bias (Appendix Table).
Meta-analysis of Vitamin E in Combination Versus Placebo: Cardiovascular Death
Four trials were included in this analysis. A small secondary prevention trial, the HATS trial, was pooled36 with 3 large secondary prevention trials: the ATBC trial29 (cardiovascular disease subpopulation); the GISSI trial;28 and the MRC/BHF trial.30 The random effects pooled estimate for the 4 studies was a relative risk ratio of 1.03 (95% CI, 0.81 to 1.32) as shown in Fig. 2. The χ2 test did demonstrate significant heterogeneity (P = .02). The I2 statistic was 70% (with 95% uncertainty interval 12% to 89%). Similar to the findings for vitamin E alone, the GISSI trial reported a statistically significant benefit, while 2 of the other 3 trials reported increases in the numbers of negative outcomes in the vitamin E-treated group. There was no evidence of publication bias (Appendix Table).
The results of 2 analyses of the ATBC trial34,35 that were not included in the pooled analysis demonstrated no evidence of a significant association of vitamin E in the combinations tested on the risk of mortality due to cardiovascular disease.
Summary of the Results of Vitamin E Alone and in Combination on Risk of Death
For the 4 preceding syntheses, the results did not generally support any positive benefit associated with the use of vitamin E either alone or in the combinations tested for the prevention of all-cause death or cardiovascular death. Neither was there any evidence of significant harm from the same interventions. The effects on all-cause mortality and on cardiovascular mortality reported in the GISSI trial were observed only in the “four-way” analysis (that is, comparing each arm of the 2 × 2 factorial study separately), but not in the “two-way” analysis (comparing all subjects who received vitamin E to all those who did not). The GISSI investigators suggested that the results in the four-way analysis were likely due to chance and concluded that vitamin E supplementation conferred no benefit. The reduction in all-cause mortality reported in the Linxian study was primarily due to a decrease in cancer deaths, not cardiovascular deaths, although the relative risk reported for stroke deaths also achieved statistical significance (RR, 0.91; 95% CI, 0.84 to 0.99). Therefore, there is little evidence that vitamin E supplementation results in a reduction in cardiovascular mortality.
After we completed our analyses, results were reported for a new randomized controlled trial that assessed the effect of vitamin E, vitamin C, and estrogen in 423 postmenopausal women with preexisting cardiovascular disease. No benefit was reported for patients treated with vitamins E and C. A potential for increased mortality was reported in the antioxidant-treated group.37
Vitamin E Trials that Report on Myocardial Infarction as an Outcome
Fifteen trials were considered for inclusion in this analysis. We judged the 2 primary prevention trials not clinically appropriate to pool with secondary prevention studies because of the differences in the populations studied.21,34 We determined that 2 years of follow-up was the minimum appropriate for adequate assessment. Therefore, 4 studies were eliminated for insufficient follow-up time.38–41 Seven trials were judged suitable for inclusion in the pooled analysis.24,25,27–30,36 All of the included trials were secondary prevention trials; therefore, all the populations tested had a previous history of or significant risk factors for cardiovascular disease.
Three of the trials tested a low dose of vitamin E (i.e., less than or equal to 400 IU),27–29 and the remaining 4 trials tested a high dose of vitamin E (greater than 400 IU). 24,25,30,31 The outcome of myocardial infarction (MI) was reported in 2 ways in these trials: as fatal MI or as nonfatal MI. We pooled these 2 outcomes separately. For details of these trials, please see the evidence table in the Appendix.
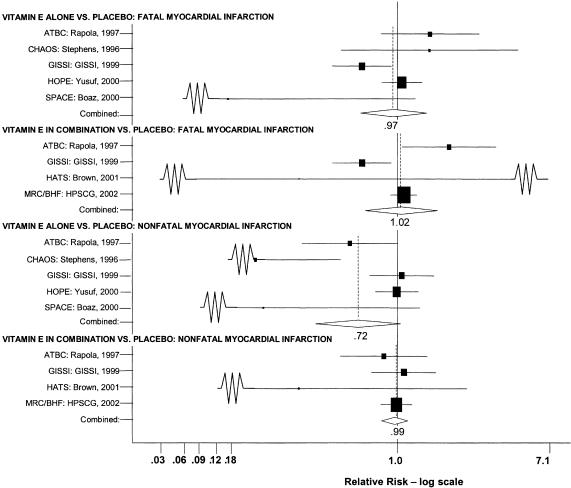
Meta-analysis of Vitamin E Alone Versus Placebo: Fatal Myocardial Infarction
Five trials were included in the pooled analysis: the SPACE trial,24 the HOPE trial,27 the report of the ATBC subpopulation with preexisting cardiovascular disease,29 the GISSI trial,28 and the CHAOS trial.25
The random effects pooled estimate of the relative risk ratio was 0.97 (95% CI, 0.74 to 1.27) as shown in Fig. 3. The χ2 test did demonstrate significant heterogeneity (P = .03). The I2 statistic was 63% (with 95% uncertainty interval, 2% to 86%). Sensitivity analyses did not alter these results. No evidence of publication bias was demonstrated. As with the analyses of vitamin E and mortality, the GISSI study differed from the others in that it alone reported a statistically significant result (RR, 0.75; 95% CI, 0.59 to 0.96). However, this effect was seen only in the four-way analysis; in the two-way analysis the effect was not significant. Three of the remaining 4 trials reported nonsignificant results with the point estimates actually reflecting increased fatal MI in the vitamin E treated group.
FIGURE 3.
Pooled relative risk ratios for myocardial infarction.
Risk ratios were calculated for the primary prevention ATBC and PPP trials that were not included in the pooled analysis.21,29,34,35 Neither of these primary prevention studies reported a statistically significant benefit for vitamin E on fatal MI.
Meta-analysis of Vitamin E in Combination Versus Placebo: Fatal Myocardial Infarction
Four trials were included in this pooled analysis: a secondary prevention trial, HATS;36 the longer version of the ATBC trial, which focused on patients with prior cardiovascular disease;29 the GISSI trial;28 and the MRC/BHF trial.30 The random effects pooled estimate of the 4 studies was 1.02 (95% CI, 0.77 to 1.37) as shown in Fig. 3. This result was not significant, but the χ2 test did demonstrate significant heterogeneity (P = .01). The I2 statistic was 73% (with 95% uncertainty interval, 25% to 90%). Sensitivity analyses did not alter these results. No evidence of publication bias was demonstrated (Appendix Table).
As in previous analyses, the GISSI study was the only individual study to report a benefit of vitamin E supplementation (RR, 0.75; 95% CI, 0.59 to 0.96). Also similar to the previous case, in the two-way analysis of the GISSI data the effect on fatal MI was not statistically significant. In contrast to the results of previous analyses, 1 trial, the ATBC trial, which enrolled subjects with prior cardiovascular disease, reported a statistically significant adverse effect of vitamin E supplementation (RR, 1.51; 95% CI, 1.04 to 2.20). Although the GISSI trial used a higher dose of vitamin E than did the ATBC, it would be rare for 2 different doses of a supplement to have completely opposite effects and for each of those effects to be real. It is possible that the adverse effect reported for the ATBC and the beneficial effect reported for the GISSI result was due to chance.
An additional trial,39 not included in the pooled analysis due to insufficient follow-up time, demonstrated no significant effect of vitamin E supplementation in the risk of fatal MI. The primary prevention sample of the ATBC trial34 reported no effect on fatal MI.
Meta-analysis Vitamin E Alone Versus Placebo: Nonfatal Myocardial Infarction
The same 5 trials included in a prior pooled analysis of fatal MI also reported on the outcome of nonfatal MI.24,25,27–29 The random effects pooled estimate was 0.72 (95% CI, 0.51 to 1.02) as shown in Fig. 3. The χ2 test did demonstrate significant heterogeneity (P = .001). The I2 statistic was 78% (with 95% uncertainty interval, 47% to 91%). Sensitivity analyses did not alter these results. There was no evidence of publication bias (Appendix Table).
In contrast to prior analyses, in this analysis the GISSI trial did not report a statistically significant effect of vitamin E. In fact, the point estimate of effect for nonfatal MI was in the opposite direction (RR, 1.04; 95% CI, 0.80 to 1.34). Surprisingly, in this analysis the ATBC trial, which reported a statistically significant adverse effect of vitamin E on fatal MI, reports a beneficial effect for nonfatal MI that just fails to reach conventional levels of statistical significance (RR, 0.68; 95% CI, 0.46 to 1.01).
The results of 2 primary prevention studies, the ATBC study and the PPP trial,21,34 reported no significant effect of vitamin E alone for reducing the risk of nonfatal MI.
Meta-analysis of Vitamin E in Combination Versus Placebo: Nonfatal Myocardial Infarction
Four trials were included in this pooled analysis: the HATS trial,36 the longer version of the ATBC trial of subjects with prior cardiovascular disease,29 the GISSI trial,28 and the MRC/BHF trial.30 The random effects pooled estimate was a relative risk ratio of 0.99 (95% CI, 0.89 to 1.10) as shown in Fig. 3. The χ2 test did not demonstrate significant heterogeneity (P = .60). The I2 statistic was 0% (with 95% uncertainty interval, 0% to 89%). There was no evidence of publication bias.
Summary of the Results of Vitamin E Alone and in Combination on Risk of Myocardial Infarction
The effects of treatment with vitamin E alone or in combination on the risk of MI, both fatal and nonfatal, are mixed. No pooled analysis yielded a beneficial or adverse effect for vitamin E supplementation, either alone or in combination. However, individual studies did report significant effects. The GISSI study reported a benefit on fatal MI but a nonsignificant adverse effect on nonfatal MI. Furthermore, the beneficial effects in GISSI were seen only in the four-way analysis and not in the larger two-way analysis. The ATBC trials reported just the opposite results of the GISSI four-way analysis: a significant adverse effect of vitamin E on fatal myocardial infarction but a nearly significant beneficial effect of vitamin E on nonfatal MI. These trials had distinct differences (ATBC assessed 50 mg of vitamin E whereas the GISSI assessed 300 mg; but the baseline risk of both fatal and nonfatal MI was approximately equivalent in the 2 studies), but such disparities in results cast doubt on the observed effects being due to a causal relationship, because consistency of effect and a dose–response relationship are 2 important criteria of causality.
Vitamin E Trials that Reported on Lipids as an Outcome
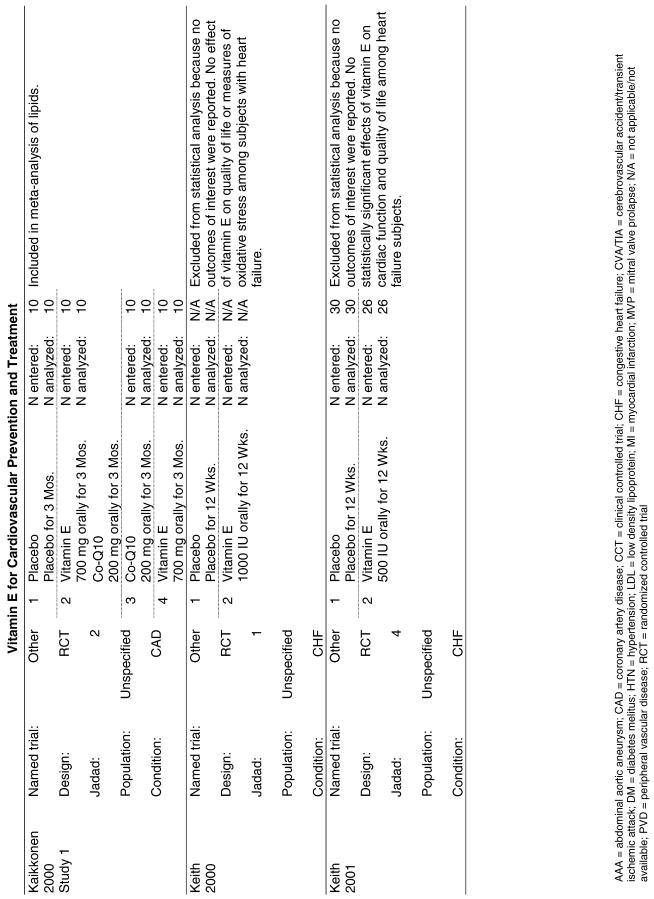
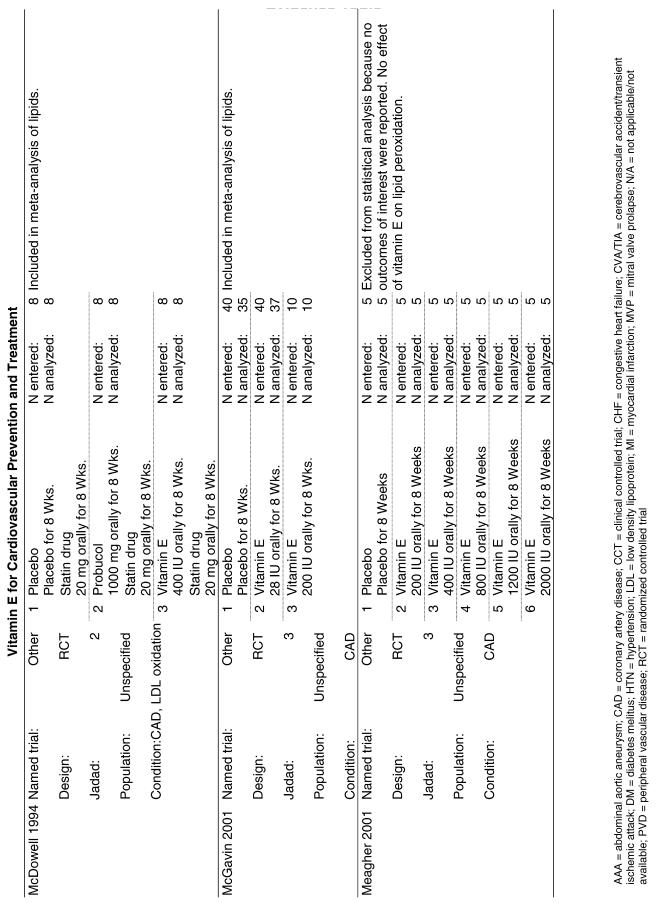
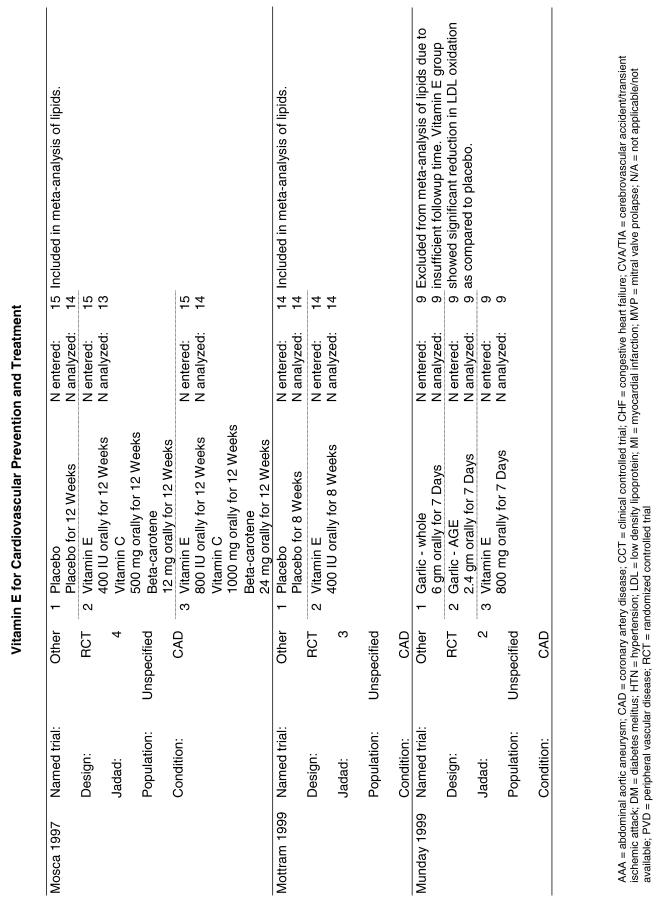
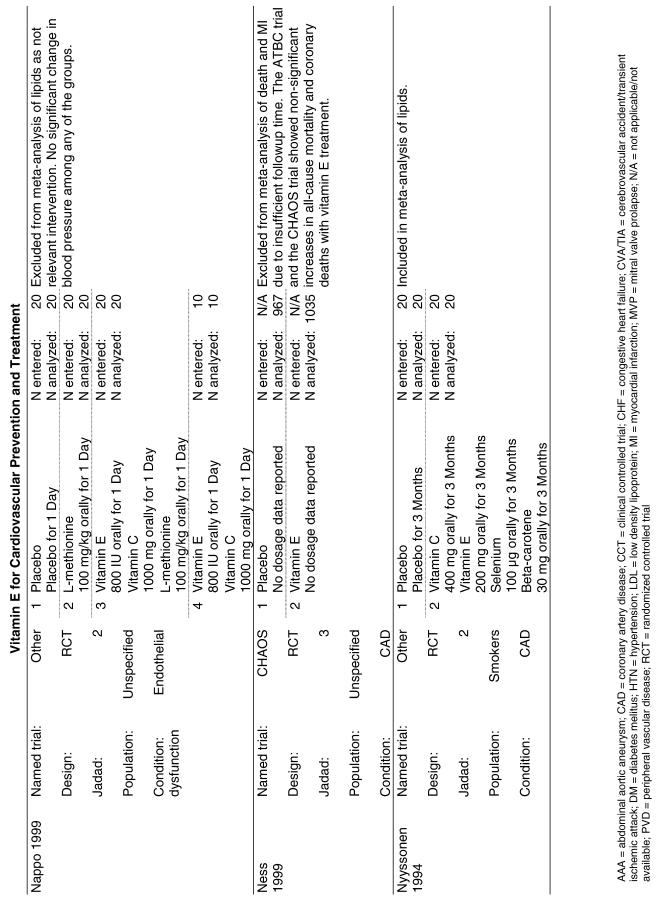
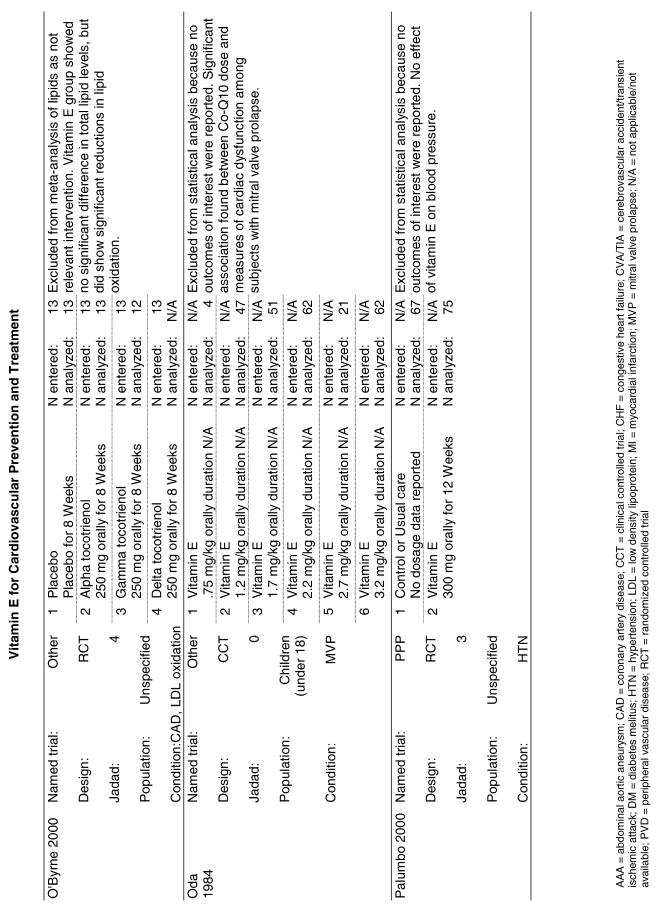
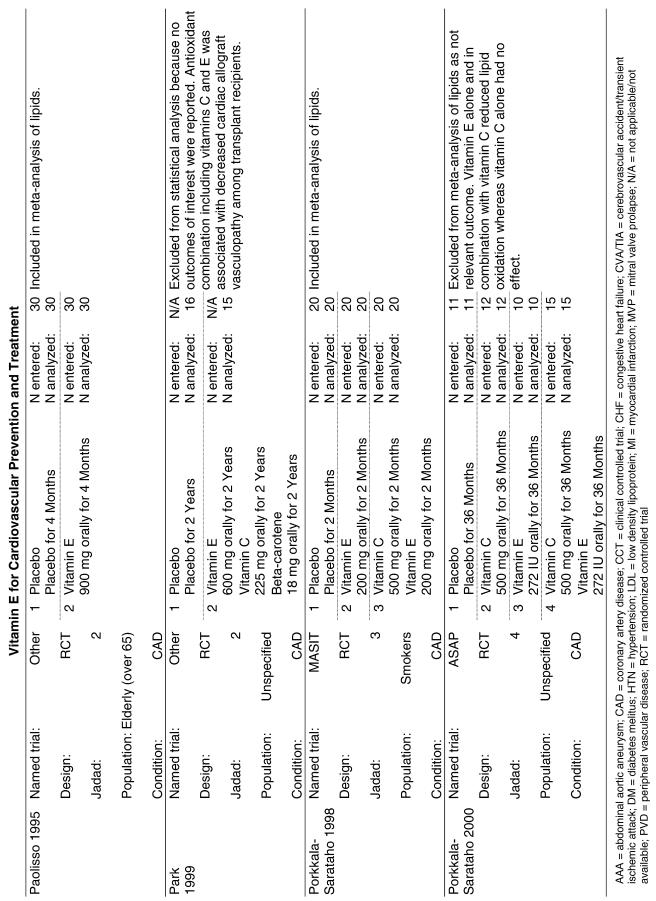
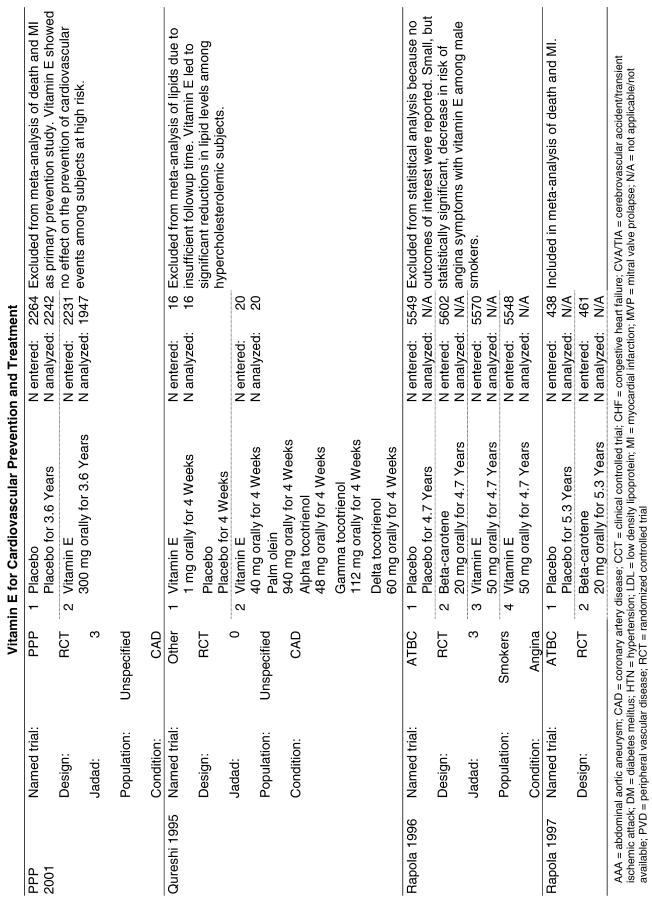
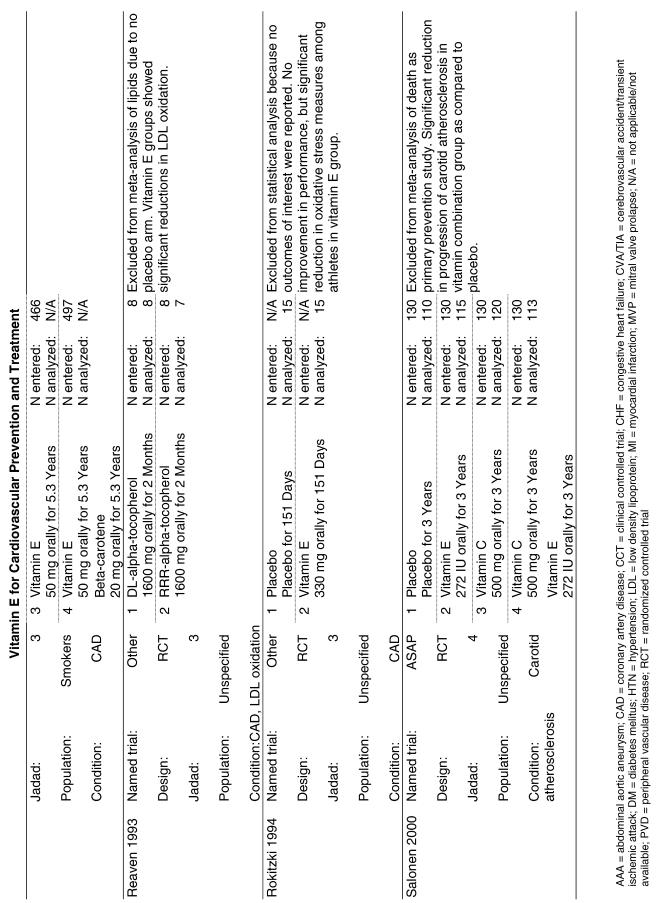
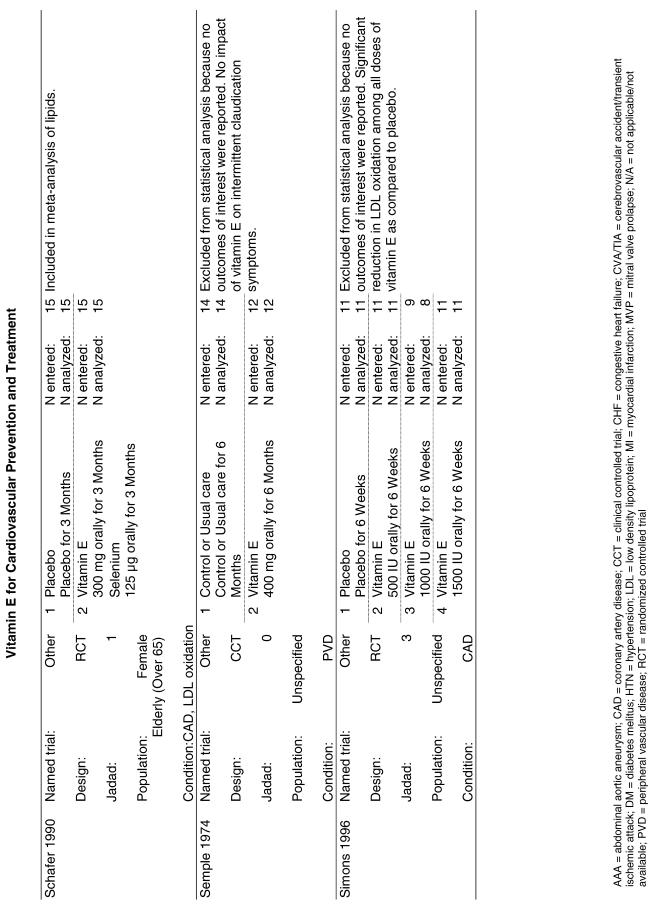
Fifty-six trials were identified that examined the effects of the antioxidants on the intermediate outcome of blood lipids. Intermediate outcomes that have a demonstrated relationship to cardiovascular disease clinical outcomes, namely total cholesterol, LDL cholesterol, and HDL cholesterol, were chosen for continued analysis. Other intermediate outcomes, such as lipid or LDL oxidation, were not chosen for analysis because no direct evidence links them to clinical cardiovascular disease outcomes such as mortality. Therefore, 4 trials that reported only on the indirect outcome of lipid oxidation were excluded from pooling.42–45 A fifth trial46 was excluded because none of the chosen lipid outcomes was identified. A sixth trial47 was excluded because it was a pharmacokinetics study of coenzyme Q10.
Two trials, the GISSI28 and the MRC/BHF trials,30 were excluded from pooled analysis because their sample sizes were more than an order of magnitude larger than those of the rest of the trials and would have rendered the results of any smaller trials statistically meaningless in pooled analysis. Instead, we compared the results of these large trials with the pooled results of the smaller trials.
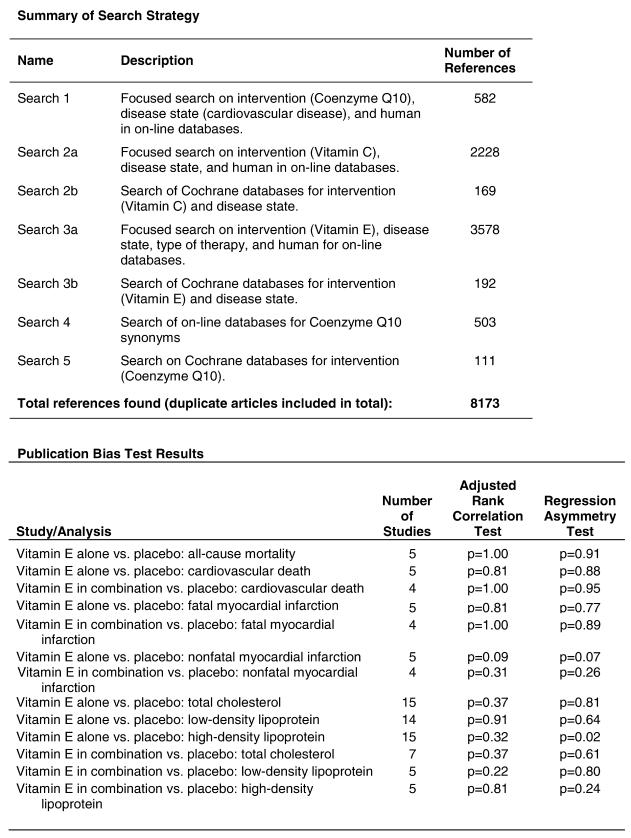
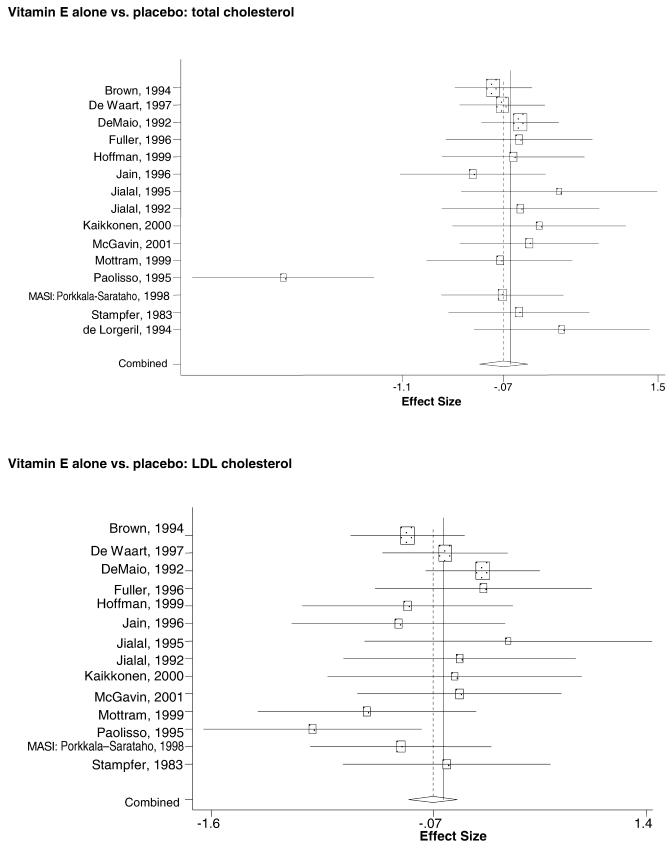
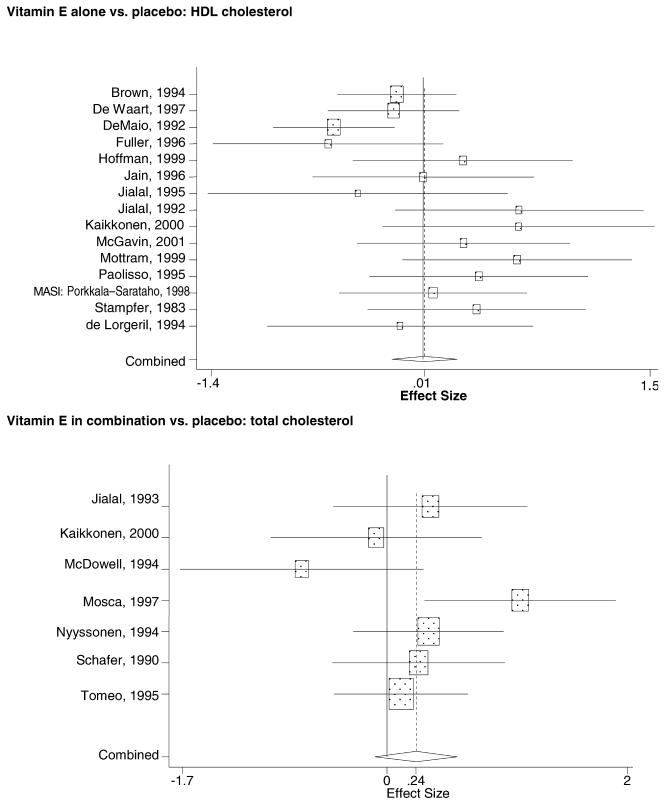
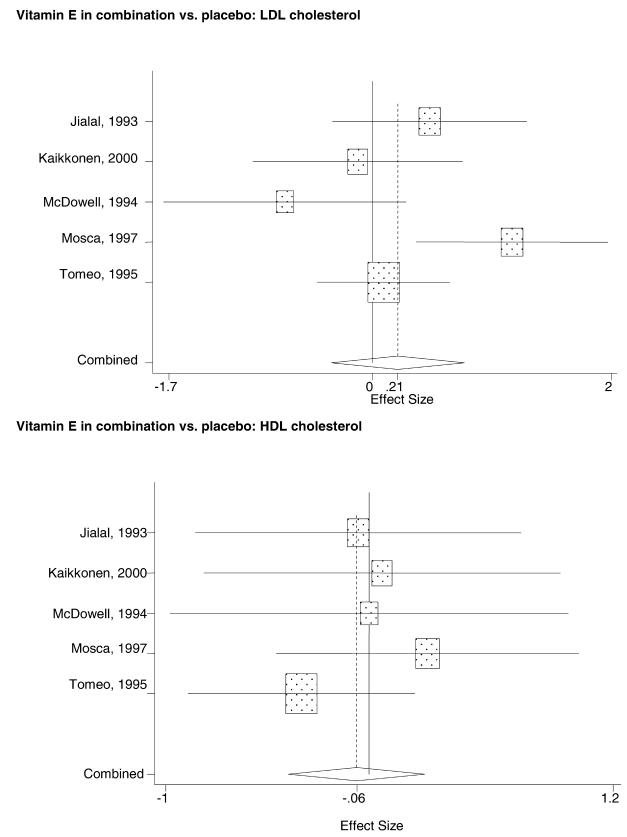
In the populations studied, interventions with vitamin E (alone and in combination with other antioxidants) in doses ranging from 100 IU to 1200 IU and treatment durations of 8 to 24 weeks did not demonstrate a statistically significant effect on serum lipids (effect sizes for vitamin E alone were −0.07, −0.07, and 0.01 for total cholesterol, LDL cholesterol, and HDL cholesterol, respectively; full results are in the Appendix.) The 2 large primary prevention trials reported clinically insignificant (but statistically significant) changes in these outcomes. Thus, there is no evidence that vitamin E alone or in combination has a clinically and statistically significant favorable or unfavorable effect on lipids.
DISCUSSION
The available scientific studies offer little evidence that supplementation with vitamin E has any benefit on cardiovascular disease prevention or treatment. Indeed, supplementation with vitamin E at the doses tested appears to provide no benefit: large placebo-controlled, randomized trials have reported no benefit in terms of all-cause mortality, cardiovascular mortality, myocardial infarction, or blood lipids (e.g., the MRC/BHF trial, GISSI, HOPE, PPP, ATBC). Isolated examples of possible benefits for vitamin E supplementation reported for specific outcomes in particular trials failed to be confirmed by other outcomes in the same trials or in other trials. Either these disparate results within and across trials are due to chance, or the mechanism of action of vitamin E with respect to MI is very complicated. This lack of consistency in the evidence casts doubt on any of the reported associations having a cause-and-effect relationship. There is good evidence that vitamin E supplementation has no clinically important effect on lipid levels.
After finishing our report, 2 new reviews have been published assessing the effect of vitamin E on cardiovascular disease. The first was a meta-analysis of vitamin E and beta-carotene.48 This meta-analysis restricted its selection criteria to only very large studies, and therefore included fewer studies than ours and used somewhat different methods for assessing outcomes. However, despite these differences, this meta-analysis also concluded that vitamin E supplementation has no appreciable effect on mortality or cardiovascular outcomes. The second review was performed for the U.S. Prevention Services Task Force.49 This review searched fewer databases, included cohort studies, and synthesized their evidence qualitatively rather than quantitatively. They included fewer RCTs assessing mortality and myocardial infarction outcomes than our review, but included studies assessing angina outcomes that we did not assess. This review concluded that the randomized trial evidence showed “no effect” on cardiovascular events or cardiovascular or all-cause mortality. Taken together, these 3 reviews, conducted independently, using somewhat different inclusion criteria and methods for synthesis, provide strong convergent validity that supplementary use of vitamin E has no effect on cardiovascular outcomes.
An explanation that has been proposed for the lack of effect reported in many of the reviewed trials is that the vitamin E was not administered in a sufficient dose or combined with other agents essential for its success, or given for a long enough period of time, or given to a population sufficiently likely to benefit. Both the GISSI study and the HOPE study were prematurely terminated due to evidence of benefits from other intervention arms. It has been suggested that if these studies had been allowed to continue for longer, a benefit of antioxidants would have become more apparent. Some experts have called for new ways to identify populations most likely to benefit, such as selecting participants based on some measure of oxidative stress or low levels of antioxidants. Whether higher doses or different formulations or longer treatment durations will prove more effective is unknown. The findings we report here make it less likely, in our view, that a particular antioxidant intervention will be found that proves to be markedly beneficial.
Our review and meta-analysis have several limitations. The first, common to many systematic reviews, is the quality of the original studies. Only a third of our trials achieved a Jadad score of 3 or more. Other elements of the design and execution of the studies may also be important. For example, the Linxian trial was not designed to assess cardiovascular disease outcomes as its primary purpose, hence the baseline data on cardiovascular disease were not as complete as those from some of the other studies. However, recent attempts to define elements of study design and execution that are related to bias have shown that in many cases, proposed criteria and scales are not reproducible and do not distinguish studies based on their results.50,51
Heterogeneity existed in the trial design, populations, size, interventions, and outcomes and affected our ability to pool studies. We describe explicitly the clinical judgments we made about pooling studies. We tested other combinations of studies in sensitivity analyses; no differences in results were seen. Furthermore, almost without exception, individual studies also failed to demonstrate a benefit of antioxidant supplementation. Therefore, while there was heterogeneity among studies, we do not think our choices for pooling studies introduced significant bias in either direction.
In summary, there is good evidence that supplements of the antioxidant vitamin E do not substantially affect cardiovascular disease either positively or adversely.
Acknowledgments
This research was performed by the Southern California Evidence-Based Practice Center based at RAND, Santa Monica, Calif under contract to the Agency for Healthcare Research and Quality (contract no. 290-97-0001). The research was requested and funded by The National Center for Complementary and Alternative Medicine, National Institutes of Health. The authors of this article are responsible for its contents, including any clinical or treatment recommendations. No statement in this article should be construed as an endorsement by an official position of the Agency for Healthcare Research and Quality or of The National Center for Complementary and Alternative Medicine, National Institutes of Health, or of the U.S. Department of Health and Human Services.
Dr. Shekelle is a Senior Research Associate of the Veterans Affairs Health Services Research and Development Service.
Appendix
REFERENCES
- 1.American Heart Association. 2001 Heart and Stroke Statistical Update. Dallas, Tex: American Heart Association;; 2002. [Google Scholar]
- 2.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I. General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 3.Jha P, Flather M, Lonn E, et al. The antioxidant vitamins and cardiovascular disease: a critical review of epidemiologic and clinical trial data. Ann Intern Med. 1995;123:860–72. doi: 10.7326/0003-4819-123-11-199512010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Diplock AT. Antioxidant nutrients and disease prevention. Am J Clin Nutr. 1991;53:S189–S93. doi: 10.1093/ajcn/53.1.189Sb. [DOI] [PubMed] [Google Scholar]
- 5.Hennekens CH, Gaziano JM. Antioxidants and heart disease: epidemiology and clinical evidence. Clin Cardiol. 1993;16(4 suppl):I10–I15. doi: 10.1002/clc.4960161305. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell S. Antioxidant vitamin supplements. Update of their potential benefits and possible risks. Drug Safety. 1999;21:253–66. doi: 10.2165/00002018-199921040-00002. [DOI] [PubMed] [Google Scholar]
- 7.Clifton PM. Antioxidant vitamins and coronary heart disease risk. Curr Opin Lipidol. 1995;6:20–4. doi: 10.1097/00041433-199502000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Asplund K. Antioxidant vitamins in the prevention of cardiovascular disease: a systematic review. J Intern Med. 2002;251:372–92. doi: 10.1046/j.1365-2796.2002.00973.x. [DOI] [PubMed] [Google Scholar]
- 9.Tribble DL. Antioxidant consumption and risk of coronary heart disease. Emphasis on vitamin C, vitamin E, and beta-carotene: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;99:591–5. doi: 10.1161/01.cir.99.4.591. [DOI] [PubMed] [Google Scholar]
- 10.Shekelle P, Morton S, Hardy M. Evidence Report/Technology Assessment no83 (Prepared by Southern California RAND Evidence-Based Practice Center, under contract no. 290-97-0001) AHRQ Publication no. 03-E043. Rockville, Md: Agency for Health care Research and Quality; 2003. Effect of Supplemental Antioxidants Vitamin C, Vitamin E, and Coenzyme Q10 for the Prevention and Treatment of Cardiovascular Disease. [PMC free article] [PubMed] [Google Scholar]
- 11.McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of the grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356:1228–31. doi: 10.1016/S0140-6736(00)02786-0. [DOI] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 13.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego, Calif: Academic Press; 1985. [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leppala JM, Virtamo J, Fogelholm R, et al. Controlled trial of alpha-tocopherol and beta-carotene supplements on stroke incidence and mortality in male smokers. Arterioscler Thromb Vasc Biol. 2000;20:230–5. doi: 10.1161/01.atv.20.1.230. [DOI] [PubMed] [Google Scholar]
- 21.de Gaetano G. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group Primary Prevention Project. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 22.Salonen JT, Nyyssonen K, Salonen R, et al. Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3-year progression of carotid atherosclerosis. J Int Med. 2000;248:377–86. doi: 10.1046/j.1365-2796.2000.00752.x. [DOI] [PubMed] [Google Scholar]
- 23.Mark SD, Wang W, Fraumeni JF, et al. Do nutritional supplements lower the risk of stroke or hypertension? Epidemiology. 1998;9:9–15. [PubMed] [Google Scholar]
- 24.Boaz M, Smetana S, Weinstein T, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356:1213–8. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 25.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease. Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–6. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 26.Haeger K. The treatment of peripheral occlusive arterial disease with alpha-tocopherol as compared with vasodilator agents and antiprothrombin (dicumarol) Vasc Dis. 1968;5:199–213. [PubMed] [Google Scholar]
- 27.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 28.GISSI-Prevenzione Investigators. (Gruppo Italiano per lo Studio della Sopravvianza nell’Infarto Miocardico). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 29.Rapola JM, Virtamo J, Ripatti S, et al. Randomised trial of alpha-tocopherol and beta-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet. 1997;349:1715–20. doi: 10.1016/S0140-6736(97)01234-8. [DOI] [PubMed] [Google Scholar]
- 30.MRC/BHF Heart Protection Study Collaborative group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 31.Haeger K. Letter: vitamin E in intermittent claudication. Lancet. 1974;1:1352. doi: 10.1016/s0140-6736(74)90735-1. [DOI] [PubMed] [Google Scholar]
- 32.Gillilan RE, Mondell B, Warbasse JR. Quantitative evaluation of vitamin E in the treatment of angina pectoris. Am Heart J. 1977;93:444–9. doi: 10.1016/s0002-8703(77)80406-7. [DOI] [PubMed] [Google Scholar]
- 33.Tardif JC, Cote G, Lesperance J, et al. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins Probucol Study Group. N Engl J Med. 1997;337:365–72. doi: 10.1056/NEJM199708073370601. [DOI] [PubMed] [Google Scholar]
- 34.Virtamo J, Rapola JM, Ripatti S, et al. Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch Intern Med. 1998;158:668–75. doi: 10.1001/archinte.158.6.668. [DOI] [PubMed] [Google Scholar]
- 35.Rapola JM, Virtamo J, Ripatti S, et al. Effects of alpha tocopherol and beta carotene supplements on symptoms, progression, and prognosis of angina pectoris. Heart. 1998;79:454–8. doi: 10.1136/hrt.79.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown BG, Xue-Qiao Z, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–92. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 37.Waters DD, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288:2432–40. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 38.Ness A, Smith GD. Mortality in the CHAOS trial. Cambridge Heart Antioxidant Study. Lancet. 1999;353:1017–8. doi: 10.1016/s0140-6736(05)70733-9. [DOI] [PubMed] [Google Scholar]
- 39.Singh RB, Niaz MA, Rastogi SS, Rastogi S. Usefulness of antioxidant vitamins in suspected acute myocardial infarction (the Indian experiment of infarct survival-3) Am J Cardiol. 1996;77:232–6. doi: 10.1016/s0002-9149(97)89384-8. [DOI] [PubMed] [Google Scholar]
- 40.Sisto T, Paajanen H, Metsa-Ketela T, Harmoinen A, Nordback I, Tarkka M. Pretreatment with antioxidants and allopurinol diminishes cardiac onset events in coronary artery bypass grafting. Ann Thorac Surg. 1995;59:1519–23. doi: 10.1016/0003-4975(95)00197-s. [DOI] [PubMed] [Google Scholar]
- 41.Singh RB, Wander GS, Rastogi A, et al. Randomized, double-blind placebo-controlled trial of coenzyme Q10 in patients with acute myocardial infarction. Cardiovasc Drugs Ther. 1998;12:347–53. doi: 10.1023/a:1007764616025. [DOI] [PubMed] [Google Scholar]
- 42.Kaikkonen J, Kosonen L, Nyyssonen K, et al. Effect of combined coenzyme Q10 and d-alpha-tocopheryl acetate supplementation on exercised-induced lipid peroxidation and muscular damage: a placebo-controlled double-blind study in marathon runners. Free Radic Res. 1998;13:1528–33. doi: 10.1080/10715769800300101. [DOI] [PubMed] [Google Scholar]
- 43.Wen Y, Cooke T, Feely J. The effect of pharmacological supplementation with vitamin C on low-density lipoprotein oxidation. Br J Clin Pharmacol. 1997;44:94–7. doi: 10.1046/j.1365-2125.1997.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porkkala-Sarataho E, Salonen JT, Nyyssonen K, et al. Long-term effects of vitamin E, vitamin C, and combined supplementation on urinary 7-hydro-8-oxo-2′-deoxyguanosine, serum cholesterol oxidation products, and oxidation resistance of lipids in nondepleted men. Arterioscler Thromb Vasc Biol. 2000;20:2087–93. doi: 10.1161/01.atv.20.9.2087. [DOI] [PubMed] [Google Scholar]
- 45.Raitakari OT, McCredie RJ, Witting P, et al. Coenzyme Q improves LDL resistance to ex vivo oxidation but does not enhance endothelial function in hypercholesterolemic young adults. Free Radic Biol Med. 2000;28:1100–5. doi: 10.1016/s0891-5849(00)00201-x. [DOI] [PubMed] [Google Scholar]
- 46.Iino K, Abe K, Kariya S, Kimura H, Kusaba T. A controlled, double-blind study of dl-alpha-tocopheryl nicotinate (Juvela-Nicotinate) for treatment of symptoms in hypertension and cerebral arteriosclerosis. Jpn Heart J. 1977;18:277–83. doi: 10.1536/ihj.18.277. [DOI] [PubMed] [Google Scholar]
- 47.Kaikkonen J, Nyyssonen K, Tomasi A, et al. Antioxidative efficacy of parallel and combined supplementation with coenzyme Q10 and d-alpha-tocopherol in mildly hypercholesterolemic subjects: a randomized placebo-controlled clinical study. Free Radic Res. 2000;33:329–40. doi: 10.1080/10715760000301501. [DOI] [PubMed] [Google Scholar]
- 48.Vivekananthan DP. Use of antioxidant vitamins for the prevention of cardiovascular disease. Lancet. 2003;361:2017–23. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 49.Morris CD, Carson S. Routine vitamin supplementation to prevent cardiovascular disease. A summary of the evidence for the U.S. Preventive Services Task Force. Ann Int Med. 2003;139:56–70. doi: 10.7326/0003-4819-139-1-200307010-00014. [DOI] [PubMed] [Google Scholar]
- 50.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–60. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 51.Balk EM, Bonis PA, Moskowitz H, et al. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. JAMA. 2002;287:2973–82. doi: 10.1001/jama.287.22.2973. [DOI] [PubMed] [Google Scholar]