Abstract
OBJECTIVE
To explore the preferences of male primary care patients and their spouses for the outcomes of prostate cancer screening and treatment, and quality of life with metastatic prostate cancer.
DESIGN
Cross-sectional design.
SETTING
Primary care clinics in Galveston County, Texas.
PATIENTS
One hundred sixty-eight couples in which the husband was a primary care patient and a candidate for prostate cancer screening.
MEASUREMENTS AND MAIN RESULTS
Preferences were measured as utilities for treatment outcomes and quality of life with metastatic disease by the time trade-off method for the husband and the wife individually and then conjointly for the couple. For each health state considered, husbands associated lower utilities for the health states than did their wives. Couples’ utilities fell between those of husbands and wives (all comparisons were significant at P < .01). For partial and complete impotence and mild-to-moderate incontinence, the median utility value for the wives was 1.0, indicating that most wives did not associate disutility with their husbands having to experience these treatment complications.
CONCLUSIONS
Male primary care patients who are candidates for prostate cancer screening evaluate the outcomes of prostate cancer treatment and life with advanced prostate cancer as being far worse than do their wives. Because the choice between quantity and quality of life is a highly individualistic one, both the patient and his partner should be involved in making decisions about prostate cancer screening.
Keywords: prostate cancer, medical decision making, primary health care, patient preferences
The American Cancer Society estimated that 189,000 new cases of prostate cancer and 30,200 prostate cancer-related deaths would be reported in 2002.1 Many professional organizations endorse early detection as the best, most effective way to decrease the substantial health burden of prostate cancer.2,3 Nevertheless, the benefits of detecting prostate cancer early in asymptomatic men remain uncertain,4,5 and this uncertainty has led many professional organizations to advocate an individualized approach to decision making about prostate cancer screening.6,7
Kassirer8 used the term “utility sensitive” to describe those medical decisions in which a patient's preferences for the various potential outcomes of treatment are central to choosing a treatment strategy. Several decision analyses have shown that the optimal decision for prostate cancer screening is sensitive to a patient's preferences for the complications of treatment.9,10 Screening can lead to a cascade of events, from biopsy to treatment to treatment-related complications.11 Complications resulting from surgical and radiotherapeutic treatment of prostate cancer are common and include impotence, urinary incontinence, and bowel problems.12–16 These treatment complications may affect quality of life and functional status, and can directly affect a man's sense of self and challenge the most intimate aspects of a couple's relationship.17–19
Herein, we explore the preferences of male primary care patients who were candidates for prostate cancer screening with regard to the outcomes of such screening (e.g., treatment complications, quality of life with metastatic prostate cancer). We consider the preferences for the screening outcomes from the perspectives of men who are making the screening decision. Because close family members can play a significant role in how patients adjust to cancer treatment and recovery,20 the perspectives of spouses are also examined.
METHODS
Subjects and Setting
Subjects for this study were recruited from among patients presenting for general medical care at the Family Practice Center at The University of Texas Medical Branch in Galveston, Texas. They were recruited by responding to posted flyers at the study clinical site, by direct solicitation in clinic waiting rooms, and by telephone using rosters of patients with scheduled appointments. To be eligible, a man must have been 45 to 70 years of age, must not have had a history of prostate cancer, must not have had any chronic disabling physical illness that would preclude screening (e.g., advanced heart disease), and must have a partner or a spouse.
Research assistants reviewed the study procedures and completed the informed consent process with each couple. The husband and wife were then escorted to separate interview rooms where they completed a questionnaire that included sociodemographic characteristics and other project-related measures. Once their questionnaires were completed, each husband and wife separately completed the utility-assessment procedure (described below). The husband and wife were then reunited, and the utility assessment procedure was repeated with them responding as a couple. The entire procedure required from 90 minutes to 3 hours to complete, and most couples were finished within 2 hours. Short breaks were taken during the interviews, and refreshments were provided.
Utility-assessment Procedures
A utility is a quantitative measure referring to the preference for or desirability of a particular health state.21–23 For the purpose of this study, a utility represented the subject's evaluation of the quality of life associated with a complication of treatment for prostate cancer or life with metastatic disease. The values of the utilities we used ranged from 0.0 (death) to 1.0 (perfect or full health). The lower the utility, or the greater the disutility, the more undesirable the health state was perceived to be by the subject. The utility assessments proceeded in 3 phases. The first phase involved a detailed education period, during which the subjects were shown laminated cards with text and graphics that described the function of the prostate gland, cancer of the prostate, approaches to cancer screening and diagnosis, available treatment options (i.e., radical prostatectomy and radiation therapy), and potential complications of treatment. For each treatment complication, the health state and the possible options for treating the specific complication were described. Two health states corresponding to metastatic prostate cancer—hormonally responsive prostate cancer and hormonally refractory prostate cancer—were also described. The interviewers reviewed and summarized each card with each subject, and all of the cards were made available to all of the subjects for later reference. The treatment complications and the metastatic prostate cancer health states addressed in the study are described in Table 1.
Table 1.
Description of Treatment Complications and Metastatic (Advanced) Prostate Cancer States*
| State | Description | Possible Treatments for Complications |
|---|---|---|
| Treatment complications | ||
| Partial impotence | Inability to always have erections when wanted, or erections not always firm enough for sexual activity. | Patient adaptability; medications. |
| Complete impotence | Entire loss of ability to have an erection. | Medications; injections/insertions; vacuum-assisted devices; penile implants. |
| Mild-to-moderate incontinence | The inability to control the urine stream, resulting in leakage or dribbling of urine. | Absorbent pads; behavioral modification; Kegel exercises. |
| Severe incontinence | The complete and constant loss of urine. | Medications; penis clamp; condom catheter; artificial sphincter. |
| Urethral stricture | Blockage or narrowing of the urethra, which makes it difficult for urine to easily pass. | Dilating the urethra; surgery. |
| Rectal injury | Cut or damage to rectal wall, which may allow feces to pass through rectal wall. | Surgical repair; colostomy. |
| Metastatic (advanced) prostate cancer | ||
| Hormonally responsive prostate cancer | Cancer that has spread to other parts of the body. The purpose of treatment is to slow the growth of prostate cancer cells by stopping the production of testosterone. | |
| Hormonally refractory prostate cancer | Cancer that has spread throughout the body. Hormone treatment is no longer effective. The purpose of treatment is to slow its spread and to control symptoms, in particular pain. |
The health state descriptions and the possible treatments for complications are abbreviated versions excerpted from the utility-assessment procedures.
During the next 2 phases, the utilities of the subjects with regard to the various health states were elicited. The utilities were determined from 3 perspectives: that of the husband, that of the wife, and that of the couple. The referent for each health state was always the husband (i.e., husbands were asked to assume they would experience the health state, and wives were asked to assume that their husbands would experience the health state). The outcome states of urinary incontinence and impotence were demarcated by severity, because previous studies have shown that patients experience a range of functioning after treatment for prostate cancer.12–16,24,25 For assessments with the couple, the interviewers asked the couple to decide on a single response.
The second phase involved the use of a category-scaling technique, wherein subjects were asked to rank the health states on a continuum (or “feeling thermometer”) from 0, representing the health state of “death,” to 100, representing the health state of “perfect health.” A training exercise, in which the subjects were asked to evaluate blindness in one eye and blindness in two eyes on a continuum, was completed first. After the subjects demonstrated an understanding of the procedure, they were asked to rank each of the health states described in Table 1. A holistic approach, in which the outcome health states are described within the context in which they would be experienced,26 was used rather than a decomposed one. For example, the partial impotence state was presented to the wife as follows:
Your husband has a P[rostate]-S[pecific] A[ntigen] test and a D[igital] R[ectal] E[xamination] which are abnormal, and a biopsy shows he has prostate cancer. He is treated for his cancer, and as a result of treatment experiences partial impotence.
A similar strategy was used for each health state. The interviewers presented each outcome state sequentially, asked the subjects to clarify their rankings during the process, and gave subjects the opportunity to modify their rankings.
In the third phase, the time trade-off method22,23 was used to elicit each subject's utilities. A training exercise was conducted, again using the example of blindness in one or both eyes. The time trade-off assessment determined the point of indifference between a period of time in an outcome state and a shorter period of time in perfect health. The maximum period of time in the health state was based on the husband's life expectancy, as determined by U.S. life tables.27 The order by which health states were assessed was based on their ranking from the category-scaling assessment. The complication of partial impotence is used here to describe these assessments. The husbands’ assessment took the following form, wherein (n) represents the life expectancy of the man:
Assume you were to live (n) years with partial impotence, or 1 year without partial impotence and in otherwise perfect health. Which would you choose?
Assuming the patient preferred the longer period of time with partial impotence, the trade-off was reoffered, each time increasing the number of years in perfect health until the subject became indifferent to choosing between the options. The same procedure was followed for the wives, wherein the assessment took the following form:
Assume your husband were to live (n) years with partial impotence, or 1 year without partial impotence and in otherwise perfect health. Which would you choose?
When the couple completed the assessment together, the assessment took this form:
We want you [the couple] to decide on a single answer. Assume you [the husband] were to live (n) years with partial impotence, or 1 year without partial impotence and in otherwise perfect health. Which would you [the couple] choose?
The subjects were encouraged to explain their responses and were given the opportunity to review and modify them. The interviewers recorded any comments made by the subjects.
Statistical Analysis
The validity of the subjects’ responses was evaluated by examining the relationship between the 2 impotence states and the 2 incontinence states using the category-scaling technique. Responses were considered to be valid 1) if the ranking for partial impotence was equal to or higher than the ranking for complete impotence, and 2) if the ranking for mild-to-moderate incontinence was equal to or higher than the ranking for severe incontinence.28 Of the 168 couples, 10 had an invalid ranking for either impotence or incontinence from at least one perspective. Dropping these couples from the analyses did not change the findings in a meaningful way, so we included their responses in our findings.
Responses to the time trade-off assessments were reexpressed as utilities by dividing the indifference points by the husband's life expectancy. The values ranged from 0.00 (greatest disutility or least desirable state) to 1.00 (least disutility or most desirable health state). For example, if a subject was indifferent to a choice between 7 years of perfect health and 10 years of partial impotence (the life expectancy of the husband being 10 years), then the utility value for partial impotence was determined to be 7/10, or 0.70. Boxplots were used to graphically display the distribution of the utility values (see the Appendix for the descriptive statistics for the utility values). The Friedman test, a nonparametric test for related samples, was used to test for differences in utility values between the perspectives of husbands, wives, and couples. Racial/ethnic differences in utilities were also explored by using the Kruskal–Wallis H test, a nonparametric version of ANOVA.29 To more fully describe intracouple differences, we determined the percentage of couples in which the husband's utility was less than, the same as, or greater than the wife's utility. Finally, to examine the degree of association between perspectives, Spearman rank-order correlation coefficients (rho coefficients) were calculated. All of the analyses were carried out with SPSS for Windows, version 11 (SSPS Inc., Chicago, Ill).30
RESULTS
Characteristics of the Sample
The sociodemographic characteristics of the 168 couples are given in Table 2. Husbands were on average 4 years older than their wives, and 17.9% of the husbands were 65 years of age or older. For 149 couples (88.7%), the husband and the wife reported the same race/ethnicity. Educational attainment and annual income was varied among the participants; more than 50% of them were currently employed. The couples had been married an average of 24 years, with a range of 1 to 51 years. Nearly half of the husbands (48.8%) reported having been tested for prostate cancer with PSA in the past, although only 25% indicated being tested every year. A family history of prostate cancer was reported by 17.3% of the husbands.
Table 2.
Characteristics of the Sample
| Variable | Husbands (N = 168) | Wives (N = 168) |
|---|---|---|
| Sociodemographic Characteristics | ||
| Age, y | ||
| Mean | 56.4 | 52.2 |
| Median | 56.0 | 52.0 |
| Minimum | 45.0 | 29.0 |
| Maximum | 70.0 | 72.0 |
| Race/ethnicity, n (%) | ||
| African American | 30 (17.9) | 26 (15.5) |
| White | 110 (65.5) | 111 (66.1) |
| Mexican American | 24 (14.3) | 26 (15.5) |
| Other | 4 (2.4) | 5 (3.0) |
| Education, n (%) | ||
| Not a high school graduate | 32 (19.0) | 30 (17.9) |
| High school graduate | 32 (19.0) | 31 (18.5) |
| Some college or vocational training | 58 (34.5) | 64 (38.1) |
| College graduate | 22 (13.1) | 26 (15.5) |
| Postgraduate degree | 24 (14.3) | 17 (10.1) |
| Employment status, n (%) | ||
| Full time | 78 (46.4) | 75 (44.9) |
| Part time | 12 (7.1) | 20 (11.9) |
| Retired | 42 (25.0) | 19 (11.3) |
| Homemaker | 1 (0.6) | 33 (19.6) |
| Disabled | 26 (15.5) | 13 (7.7) |
| Unemployed | 9 (5.4) | 7 (4.2) |
| Variable | Couples (N = 168) |
|---|---|
| Annual family income, n (%) | |
| <$10,000 | 25 (14.9) |
| $10,000–$19,999 | 27 (16.1) |
| $20,000–$39,999 | 46 (27.4) |
| $40,000–$69,999 | 42 (25.0) |
| >$70,000 | 22 (13.1) |
| Not reported | 6 (3.6) |
| Married, y | |
| Mean | 24.1 |
| Median | 24.0 |
| Minimum | 1.0 |
| Maximum | 51.0 |
| Husband's past PSA testing (lifetime), n (%) | |
| Yes | 82 (48.8) |
| No | 62 (35.9) |
| Unsure | 24 (14.3) |
| Husband's PSA testing every year, n (%) | |
| Yes | 42 (25.0) |
| No | 103 (61.3) |
| Unsure | 23 (13.7) |
| Family history of prostate cancer, n (%) | |
| Yes | 29 (17.3) |
| No | 121 (72.0) |
| Unsure | 18 (10.7) |
Utilities for Treatment Complications and Metastatic Cancer
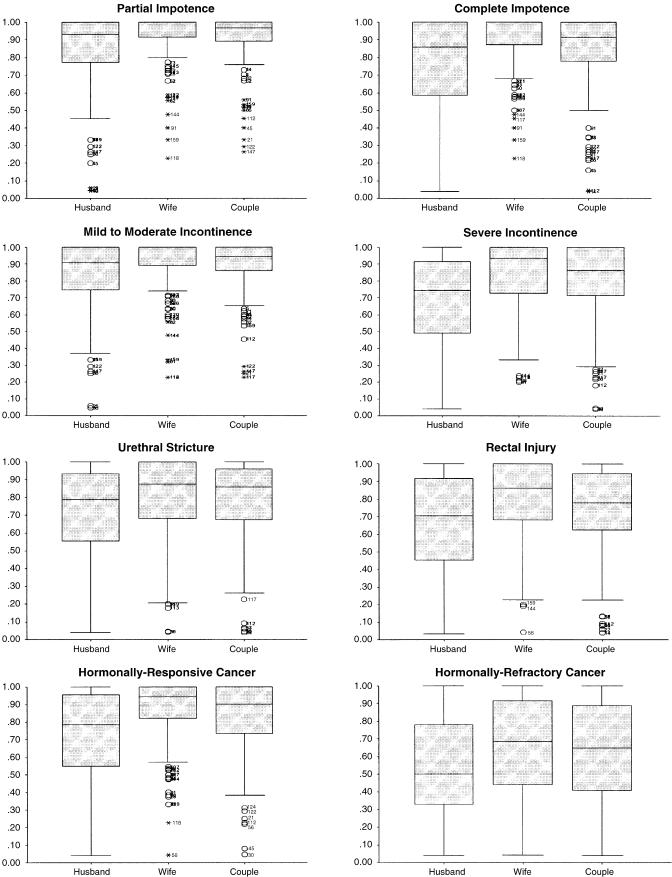
Figure 1 gives boxplots of the time trade-off utilities for each health state by perspective (see the Appendix for the descriptive statistics on the health-state distributions). Each boxplot depicts the median value (the horizontal line within the box), the 25th and 75th percentiles (the lower and upper borders of the box, respectively), and the range of scores (the lines extending above and below the box).
FIGURE 1.
Boxplots of utilities for health states elicited from three perspectives (husband, wife, couple).
Several patterns are apparent from the aggregate data depicted in Figure 1. First, for each health state considered, husbands reported lower utilities than did their wives, while the couples’ utilities fell at a point in-between (all comparisons were significant at P < .01 using the Friedman test). Second, for partial and complete impotence and for mild-to-moderate incontinence, the median utility value for the wives was 1.00, indicating that most wives were not willing to trade away any time (of their husbands’ life expectancy) to avoid these treatment complications. Utility values of 1.00 were observed among 61.9% of wives for partial impotence, among 53.6% for complete impotence, and among 57.7% for mild-to-moderate incontinence. In general, the variability in the utilities was far less for the wives than for the husbands.
The largest absolute differences in median utilities between husbands and wives were observed for severe incontinence (0.19), hormonally refractory prostate cancer (0.18), rectal injury (0.16), hormonally responsive prostate cancer (0.15), and complete impotence (0.14). For the 2 impotence states and mild-to-moderate incontinence, the couples’ utilities were more similar to those of the husbands. In contrast, for the more severe health states, including urethral stricture and the two metastatic cancer states, the couples’ utilities were more similar to those of the wives.
Overall, there was a pattern in which the utilities were lower for the more severe treatment complications and for hormonally refractory prostate cancer, for which pain is a concern; the range of values for these states was far greater as well. The median utilities for hormonally refractory prostate cancer were 0.50 for husbands and 0.68 for wives, representing an absolute difference of 0.20 and 0.18, respectively, when compared to the closest values for rectal injury.
Table 3 summarizes the utility data by showing the percentage of husband-and-wife pairs whose utilities were the same or different. For most couples (55.6%), the husband's utilities were lower than the wife’s. In fact, partial impotence (48.2%) and urethral stricture (48.5%) were the only states for which the husband's utilities were lower than the wife's in fewer than half of the couples. The health state with the largest percentage of couples in which the wife's utility was lower than the husband's was hormonally refractory prostate cancer (31.1%). Finally, couples agreed most about partial impotence (33.3%), complete impotence (25.6%), and mild-to-moderate incontinence (29.2%).
Table 3.
Comparison of Time Trade-off Utilities for Husband and Wife Pairs*
| Percentage of Husband-Wife Pairs* | |||
|---|---|---|---|
| Health States | Wife's Utility Less Than Husband's (%) | Husband's Utility Equals Wife's (%) | Husband's Utility Less Than Wife's (%) |
| Partial impotence | 18.5 | 33.3 | 48.2 |
| Complete impotence | 19.0 | 25.6 | 55.4 |
| Mild-to-moderate incontinence | 20.8 | 29.2 | 50.0 |
| Severe incontinence | 20.4 | 13.2 | 66.5 |
| Urethral stricture | 31.1 | 20.4 | 48.5 |
| Rectal injury | 25.1 | 15.0 | 59.9 |
| Hormonally responsive prostate cancer | 24.6 | 15.0 | 60.5 |
| Hormonally refractory prostate cancer | 31.1 | 13.2 | 55.7 |
| All states combined | 23.8 | 20.6 | 55.6 |
For “Wife's less than Husband’s,” the numbers indicate the percentage of wives who associated lesser utility, or greater disutility, with a health state than did their husbands; for “Husband's less than Wife’s,” the numbers indicate the percentage of husbands who associated lesser utility, or greater disutility, with a health state than did their wives; and for “Husband's equal to Wife’s,” the numbers indicate the percentage of pairs for which the utility values were the same.
The only difference across racial/ethnic groups was in the utility for hormonally refractory prostate cancer from the couple's perspective; African-American couples had higher utilities (median = 0.90) than did white (median = 0.63) or Mexican-American (median = 0.50) couples (χ2(2) from Kruskal–Wallis H = 7.47; P < .05).
Concordance Between Perspectives
Concordance of the utility values across the various perspectives was examined by using Spearman rank-order correlation coefficients. Table 4 shows a low correlation between husbands’ and wives’ time trade-off utilities, with coefficients ranging from 0.04 to 0.19. Similarly, there were modest to strong correlations between the perspectives of the wives and of the couples (range, 0.34 to 0.43). In contrast, there were consistently larger correlations between the husbands’ utilities and those of the couples (range, 0.58 to 0.73). For each health state, the association with the couples’ utilities was slightly greater for the husbands’ than for the wives’ perspectives. No racial/ethnic group differences were observed.
Table 4.
Concordance of Time Trade-off Utilities Between Perspectives*
| Correlation Coefficients Between Perspectives | |||
|---|---|---|---|
| Health States | Husband & Wife | Husband & Couple | Wife & Couple |
| Partial impotence | 0.19† | 0.58† | 0.41† |
| Complete impotence | 0.16† | 0.67† | 0.34† |
| Mild-to-moderate incontinence | 0.14 | 0.59† | 0.36† |
| Severe incontinence | 0.16† | 0.73† | 0.39† |
| Urethral stricture | 0.16† | 0.67† | 0.38† |
| Rectal injury | 0.14 | 0.71† | 0.43† |
| Hormonally responsive prostate cancer | 0.04 | 0.69† | 0.35† |
| Hormonally refractory prostate cancer | 0.16† | 0.60† | 0.35† |
Spearman rank-order correlation coefficients are reported.
Coefficients significant at P < .05.
DISCUSSION
Our study demonstrates that male primary care patients who are candidates for prostate cancer screening have preferences for the outcomes of prostate cancer treatment and quality of life with advanced prostate cancer that differ from the preferences of their wives. Generally, the husbands evaluated these health states as being far worse than did the wives. Many of the wives would not trade any (of their husband’s) quantity of life for quality of life when impotence and mild-to-moderate incontinence were considered. Most husbands indicated that they would be willing to trade some longevity to avoid these complications. The outcome rated most negatively by both husbands and wives was life with advanced prostate cancer that is refractory to hormonal treatment. When preferences were evaluated individually for each husband and wife pair, there was little agreement between the perspectives. When preferences were determined jointly, both partners influenced the final preference.
The utilities for impotence and incontinence that were obtained from the couples in our study were remarkably similar to those obtained in studies of male prostate cancer patients after treatment. The mean utilities from the perspective of couples in our study were 0.91 for partial impotence and 0.84 for complete impotence (Appendix). In a study of 209 radical prostatectomy patients, the utility for men reporting that sexual function had been at least a small problem for them in the past month was 0.87.31 A second cohort study of 50 prostate cancer survivors yielded a utility for men reporting current sexual dysfunction of 0.90.32 Both of these studies used the same time trade-off method we used for our utility assessment. Similarly, Krahn et al.33 used the UCLA Prostate Cancer Index34 to stratify prostate cancer patients’ preferences by degree of sexual dysfunction. Utilities assessed by the standard gamble method for men in the lower (poorer function) quartile and upper (better function) quartile of sexual function scores were identical to those observed from the couples’ perspective for complete impotence and partial impotence, respectively.
In our study, the mean utilities from the perspective of couples were 0.89 for mild-to-moderate incontinence and 0.79 for severe incontinence. A utility of 0.89 has been observed among prostate cancer patients reporting current urinary problems.32 Utilities from the Krahn et al. study33 were 0.79 for men scoring in the lower (poorer function) quartile of urinary function and 0.87 for men scoring in the upper (better function) quartile. Altogether, these findings suggest that the couples’ preferences may more closely approximate the experiences of prostate cancer patients who have treatment-related complications.
It is noteworthy that in our study the utilities of husbands and wives for impotence and incontinence varied by severity of the condition. Previous research on the quality of life of prostate cancer patients has shown that generic measures tend to miss some key determinants of functioning, including sexual, urinary, and bowel dysfunction.24 In a decision analysis by Krahn et al.10 the model included impotence and incontinence stratified by severity of dysfunction. Therefore, there is good evidence supporting the importance of considering not only patients’ preferences for these health states but also of considering the degree of dysfunction. Many studies have shown that sexual functioning after treatment for prostate cancer is very important to men, and our study supports that finding from the perspective of screening candidates. Erectile dysfunction is related to poorer general health perceptions and greater role limitations in prostate cancer patients.35 In a Swedish study of prostate cancer patients and similarly aged men from the general population, most men reported being distressed by their waning sexual capacity.36 Yet, about 1 in 5 men were not willing to risk their current sexual functioning for a treatment that could improve life expectancy, while 2 in 5 would accept treatment unconditionally. For the remainder, treatment acceptance was related to the potential length of prolonged life.
The importance of sexual functioning for many men cannot be overstated. In a classic study of the trade-offs between quality and quantity of life, Singer et al.37 demonstrated that some men will choose treatment options with a shorter-term survival benefit if the chances of maintaining sexual potency are greater. The findings from our study are consistent with those of other studies that used qualitative methods to investigate couples’ treatment decision-making processes for prostate cancer38 and their adjustment to hormonal treatment.18,19 In these studies, sexual dysfunction was considered by husbands to be a highly significant complication of treatment.
In our study, husbands consistently associated greater disutility with each health state than did wives. Contradictorily, studies of spouses of cancer patients have shown that they experience greater distress than do the patients themselves. One to two years after diagnosis and treatment, about half of husbands and three quarters of wives experience some degree of general distress about the cancer.20 Spouses of prostate cancer patients in particular experience greater psychological problems, such as worry and tension, and more frequently report problems with insomnia and fatigue than do their patient-husbands.39 Similar patterns have been found for spousal adjustment to colon cancer.40 In studies of breast cancer and prostate cancer, partners have been found to overestimate the distress experienced by the patient.41,42 This paradoxical finding therefore lends support to the need to collect preferences from spouses whose partners are prostate cancer patients experiencing treatment complications or living with advanced disease. This finding may also underscore the core issue of spouses’ willingness to trade quantity of life with their partner for quality of life.
The couple's relationship can have a wide-ranging impact on prostate cancer survival. Although one provocative study of a large Norwegian birth cohort showed an excess incidence of prostate cancer in married men, their survival was favorably affected by their marital status.43 The higher incidence of prostate cancer among married men may be explained by the role of the spouse in encouraging early detection. Interestingly, unmarried men regain sexual and urinary functioning at higher rates than do married men following radical prostatectomy.25
There is consistent evidence that treatment for cancer can have a significant impact on a couple's relationship. Studies of the long-term impact of treatment for testicular cancer on the marital relationship have shown that most couples adjust well and report that their relationship is strengthened as a result of experiencing the disease. In a study of adjustment to prostate cancer treatment in men treated with radiation therapy, high levels of marital satisfaction were reported.41 On the other hand, studies of men treated hormonally for prostate cancer have shown that, in many couples, both husbands and wives avoid discussing treatment complications and quality-of-life issues.18,19
During our assessments of preferences with regard to hormonally refractory prostate cancer, several wives indicated that they did not want their husbands to suffer. Their concern was reflected in the overall lower utility values for this health state. Husbands also indicated that hormonally refractory prostate cancer had the greatest disutility among the states considered in this study. Concern about pain20 and the chances of a prolonged battle with progressive disease are particularly distressing for patients with cancer. Bennett et al.44 compared time trade-off utilities for metastatic prostate cancer from 3 perspectives: those of urologists, those of patients with localized disease, and those of patients with metastatic disease. The 2 groups of patients associated the greatest disutility with the state characterized by the severe pain and fatigue associated with late progression of the disease. Interestingly, physicians overall had utility values that were higher than those of patients.
Our study has several important limitations. The time trade-off method can be problematic for patients who are unwilling to trade time based on personal values, thus indicating no disutility for a health state.45 This bias would likely result in higher utility values and less variability than might otherwise be observed. Given the variability in the utility values we observed, this bias only reinforces our conclusions. It could be argued that preferences for different health states are best considered when elicited from patients and spouses who are actually experiencing a particular health state.21 The scenarios we evaluated were largely hypothetical. This issue cannot be fully addressed empirically, but it would be important to examine the preferences of couples experiencing a health state and compare them to those of a screening population. Finally, the mean age of the men in this study was 56 years, and they represent a younger cohort than men typically diagnosed with prostate cancer. Utilities from this cohort may be different than utilities expected from an older group of men with prostate cancer.
Deber et al.46 have noted that involving patients in treatment decisions is more common than involving patients in screening decisions. The involvement of spouses in treatment decisions is also common and is encouraged when men are to be treated for prostate cancer.38 Nevertheless, involving spouses in screening decisions is not a widespread practice and will require a shift in how preventive health care services are delivered for conditions in which the best strategy is uncertain. Screening decisions, including those relevant to prostate cancer, need not be made immediately, and there is an opportunity to involve others who might be invested in the outcome. Decision aids that clarify the values for the patient and his spouse can be used to facilitate this process.47 Further studies should consider whether a couple's communication during and after treatment would be improved if the wife was more actively involved and informed about the screening decision.
Acknowledgments
The authors wish to acknowledge the assistance of Carol A. Carlson, BS, and Kristi J. O’Dell, PhD, in collecting the data, of Pamela Paradis Tice, ELS(D), in editing the manuscript, and of Michael J. Barry, MD, in giving critical feedback on the utility assessment procedures. This study was supported by a grant (R01 HS08992) from the Agency for Healthcare Research and Quality.
APPENDIX
Descriptive Statistics for Time Trade-off Utilities by Subjects’ Perspectives
| Prostate Cancer Treatment Complications | ||||||
|---|---|---|---|---|---|---|
| Partial impotence*,† | Complete impotence*,†,‡ | |||||
| Husbands | Wives | Couples | Husbands | Wives | Couples | |
| 0.84 | 0.93 | 0.91 | Mean | 0.76 | 0.90 | 0.84 |
| 0.93 | 1.00 | 0.97 | Median | 0.86 | 1.00 | 0.91 |
| 0.77 | 0.91 | 0.89 | 25th Percentile | 0.59 | 0.87 | 0.78 |
| 1.00 | 1.00 | 1.00 | 75th Percentile | 1.00 | 1.00 | 1.00 |
| Mild-to-moderate incontinence*,† | Severe incontinence*,†,‡ | |||||
|---|---|---|---|---|---|---|
| Husbands | Wives | Couples | Husbands | Wives | Couples | |
| 0.83 | 0.91 | 0.89 | Mean | 0.69 | 0.86 | 0.79 |
| 0.91 | 1.00 | 0.94 | Median | 0.74 | 0.93 | 0.86 |
| 0.75 | 0.89 | 0.86 | 25th Percentile | 0.49 | 0.71 | 0.71 |
| 1.00 | 1.00 | 1.00 | 75th Percentile | 0.91 | 1.00 | 0.96 |
| Urethral stricture*,† | Rectal injury*,†,‡ | |||||
|---|---|---|---|---|---|---|
| Husbands | Wives | Couples | Husbands | Wives | Couples | |
| 0.72 | 0.80 | 0.78 | Mean | 0.66 | 0.79 | 0.73 |
| 0.79 | 0.88 | 0.86 | Median | 0.70 | 0.86 | 0.78 |
| 0.56 | 0.68 | 0.67 | 25th Percentile | 0.45 | 0.68 | 0.63 |
| 0.93 | 1.00 | 0.96 | 75th Percentile | 0.92 | 1.00 | 0.95 |
| Metastatic Prostate Cancer Health States | ||||||
|---|---|---|---|---|---|---|
| Hormonally responsive prostate cancer*,† | Hormonally refractory prostate cancer*,†,‡ | |||||
| Husbands | Wives | Couples | Husbands | Wives | Couples | |
| 0.72 | 0.86 | 0.83 | Mean | 0.55 | 0.66 | 0.62 |
| 0.79 | 0.94 | 0.90 | Median | 0.50 | 0.68 | 0.65 |
| 0.55 | 0.82 | 0.73 | 25th Percentile | 0.33 | 0.43 | 0.41 |
| 0.96 | 1.00 | 1.00 | 75th Percentile | 0.78 | 0.92 | 0.89 |
Comparisons based on Wilcoxon Signed Ranks Test.
Utility significantly lower for Husbands compared to Wives.
Utility significantly lower for Husbands compared to Couples.
Utility significantly lower for Couples compared to Wives (all P values < .01).
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2002. Atlanta, Ga: 2002. [Google Scholar]
- 2.von Eschenbach A, Ho R, Murphy GP, Cunningham M, Lins N. American Cancer Society Guideline for early detection of prostate cancer: update 1997. CA Cancer J Clin. 1997;47:261–4. doi: 10.3322/canjclin.47.5.261. [DOI] [PubMed] [Google Scholar]
- 3.American Urological Association. Prostate-specific antigen (PSA) best practice policy. Oncology (Huntingt) 2000;14:267–72. 77–8, 280 passim. [PubMed] [Google Scholar]
- 4.Barry MJ, Fleming C, Coley CM, Wasson JH, Fahs MC, Oesterling JE. Should Medicare provide reimbursement for prostate-specific antigen testing for early detection of prostate cancer? Part I: framing the debate. Urology. 1995;46:2–13. doi: 10.1016/s0090-4295(99)80151-4. [DOI] [PubMed] [Google Scholar]
- 5.Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ. 2002;325:740. doi: 10.1136/bmj.325.7367.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Preventive Services Task Force. Screening for prostate cancer: recommendation and rationale. Ann Intern Med. 2002;137:915–6. doi: 10.7326/0003-4819-137-11-200212030-00013. [DOI] [PubMed] [Google Scholar]
- 7.Ferrini R, Woolf SH. American College of Preventive Medicine Practice Policy: screening for prostate cancer in American men. Am J Prev Med. 1998;15:81–5. doi: 10.1016/s0749-3797(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 8.Kassirer JP. Incorporating patients’ preferences into medical decisions. N Engl J Med. 1994;330:1895–6. doi: 10.1056/NEJM199406303302611. [DOI] [PubMed] [Google Scholar]
- 9.Cantor SB, Spann SJ, Volk RJ, Cardenas MP, Warren MM. Prostate cancer screening: a decision analysis. J Fam Pract. 1995;41:33–41. [PubMed] [Google Scholar]
- 10.Krahn MD, Mahoney JE, Eckman MH, Trachtenberg J, Pauker SG, Detsky AS. Screening for prostate cancer. A decision analytic view. JAMA. 1994;272:773–80. [PubMed] [Google Scholar]
- 11.Mold JW, Stein HF. The cascade effect in the clinical care of patients. N Engl J Med. 1986;314:512–4. doi: 10.1056/NEJM198602203140809. [DOI] [PubMed] [Google Scholar]
- 12.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92:1582–92. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 13.Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol. 2001;19:3750–7. doi: 10.1200/JCO.2001.19.17.3750. [DOI] [PubMed] [Google Scholar]
- 14.Potosky AL, Reeve BB, Clegg LX, et al. Quality of life following localized prostate cancer treated initially with androgen deprivation therapy or no therapy. J Natl Cancer Inst. 2002;94:430–7. doi: 10.1093/jnci/94.6.430. [DOI] [PubMed] [Google Scholar]
- 15.Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354–60. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 16.Smith DS, Carvalhal GF, Schneider K, Krygiel J, Yan Y, Catalona WJ. Quality-of-life outcomes for men with prostate carcinoma detected by screening. Cancer. 2000;88:1454–63. [PubMed] [Google Scholar]
- 17.Volk RJ, Cantor SB, Spann SJ, Cass AR, Cardenas MP, Warren MM. Preferences of husbands and wives for prostate cancer screening. Arch Fam Med. 1997;6:72–6. doi: 10.1001/archfami.6.1.72. [DOI] [PubMed] [Google Scholar]
- 18.Boehmer U, Clark JA. Married couples’ perspectives on prostate cancer diagnosis and treatment decision-making. Psychooncology. 2001;10:147–55. doi: 10.1002/pon.504. [DOI] [PubMed] [Google Scholar]
- 19.Boehmer U, Clark JA. Communication about prostate cancer between men and their wives. J Fam Pract. 2001;50:226–31. [PubMed] [Google Scholar]
- 20.Cliff AM, MacDonagh RP. Psychosocial morbidity in prostate cancer. II. A comparison of patients and partners. BJU Int. 2000;86:834–9. doi: 10.1046/j.1464-410x.2000.00914.x. [DOI] [PubMed] [Google Scholar]
- 21.Gold MR, Patrick DL. Identifying and valuing outcomes. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press;; 1996. pp. 82–134. [Google Scholar]
- 22.Sox HC, Jr, Blatt MA, Higgins MC, Marton KI. Medical Decision Making. Boston, Mass: Butterworths;; 1988. [Google Scholar]
- 23.Torrance GW. Utility approach to measuring health related quality of life. J Chronic Dis. 1987;40:593–600. doi: 10.1016/0021-9681(87)90019-1. [DOI] [PubMed] [Google Scholar]
- 24.Krahn M, Ritvo P, Irvine J, et al. Construction of the Patient-Oriented Prostate Utility Scale (PORPUS): a multiattribute health state classification system for prostate cancer. J Clin Epidemiol. 2000;53:920–30. doi: 10.1016/s0895-4356(00)00211-0. [DOI] [PubMed] [Google Scholar]
- 25.Litwin MS, Melmed GY, Nakazon T. Life after radical prostatectomy: a longitudinal study. J Urol. 2001;166:587–92. [PubMed] [Google Scholar]
- 26.Naglie G, Krahn MD, Naimark D, Redelmeier DA, Detsky AS. Primer on medical decision analysis: part 3—estimating probabilities and utilities. Med Decis Making. 1997;17:136–41. doi: 10.1177/0272989X9701700203. [DOI] [PubMed] [Google Scholar]
- 27.National Center for Health Statistics. Vital Statistics of the United States, 1988. Part A. Vol. 2. Washington, DC: U.S. Department of Health and Human Services, Public Health Service;; Publication PHS 91 1101. [Google Scholar]
- 28.Nease RF, Jr, Kneeland T, O'Connor GT, et al. the Ischemic Heart Disease Patient Outcomes Research Team. Variation in patient utilities for outcomes of the management of chronic stable angina. Implications for clinical practice guidelines. JAMA. 1995;273:1185–90. [PubMed] [Google Scholar]
- 29.Siegel S, Castellan NJ., Jr . Nonparametric Statistics for the Behavioral Sciences. New York, NY: McGraw-Hill, Inc.; 1988. [Google Scholar]
- 30.Statistical Package for the Social Sciences. 11.0. 1 ed. Chicago, Ill: SPSS, Inc.; 2001. [Google Scholar]
- 31.Smith DS, Krygiel J, Nease RF, Jr, Sumner WII, Catalona WJ. Patient preferences for outcomes associated with surgical management of prostate cancer. J Urol. 2002;167:2117–22. [PubMed] [Google Scholar]
- 32.Albertsen PC, Nease RF, Jr, Potosky AL. Assessment of patient preferences among men with prostate cancer. J Urol. 1998;159:158–63. doi: 10.1016/s0022-5347(01)64043-6. [DOI] [PubMed] [Google Scholar]
- 33.Krahn M, Ritvo P, Irvine J, et al. Patient and community preferences for outcomes in prostate cancer: implications for clinical policy. Med Care. 2003;41:153–64. doi: 10.1097/00005650-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–12. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13:159–66. doi: 10.1046/j.1525-1497.1998.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helgason AR, Adolfsson J, Dickman P, Fredrikson M, Arver S, Steineck G. Waning sexual function—the most important disease-specific distress for patients with prostate cancer. Br J Cancer. 1996;73:1417–21. doi: 10.1038/bjc.1996.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer PA, Tasch ES, Stocking C, Rubin S, Siegler M, Weichselbaum R. Sex or survival: trade-offs between quality and quantity of life. J Clin Oncol. 1991;9:328–34. doi: 10.1200/JCO.1991.9.2.328. [DOI] [PubMed] [Google Scholar]
- 38.O'Rourke ME. Narrowing the options: the process of deciding on prostate cancer treatment. Cancer Invest. 1999;17:349–59. doi: 10.3109/07357909909032877. [DOI] [PubMed] [Google Scholar]
- 39.Kornblith AB, Herr HW, Ofman US, Scher HI, Holland JC. Quality of life of patients with prostate cancer and their spouses. The value of a data base in clinical care. Cancer. 1994;73:2791–802. doi: 10.1002/1097-0142(19940601)73:11<2791::aid-cncr2820731123>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Northouse LL, Mood D, Templin T, Mellon S, George T. Couples’ patterns of adjustment to colon cancer. Soc Sci Med. 2000;50:271–84. doi: 10.1016/s0277-9536(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 41.Ptacek JT, Pierce GR, Ptacek JJ, Nogel C. Stress and coping processes in men with prostate cancer: the divergent views of husbands and wives. J Soc Clin Psychol. 1999;18:299–324. [Google Scholar]
- 42.Carlson LE, Ottenbreit N, St Pierre M, Bultz BD. Partner understanding of the breast and prostate cancer experience. Cancer Nurs. 2001;24:231–9. [PubMed] [Google Scholar]
- 43.Harvei S, Kravdal O. The importance of marital and socioeconomic status in incidence and survival of prostate cancer. An analysis of complete Norwegian birth cohorts. Prev Med. 1997;26(5 Part 1):623–32. doi: 10.1006/pmed.1997.0153. [DOI] [PubMed] [Google Scholar]
- 44.Bennett CL, Chapman G, Elstein AS, et al. A comparison of perspectives on prostate cancer: analysis of utility assessments of patients and physicians. Eur Urol. 1997;32(suppl 3):86–8. [PubMed] [Google Scholar]
- 45.Read JL, Quinn RJ, Berwick DM, Fineberg HV, Weinstein MC. Preferences for health outcomes: comparison of assessment methods. Med Decis Making. 1984;4:315–29. doi: 10.1177/0272989X8400400307. [DOI] [PubMed] [Google Scholar]
- 46.Deber RB, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Arch Intern Med. 1996;156:1414–20. [PubMed] [Google Scholar]
- 47.O'Connor AM, Fiset V, DeGrasse C, et al. Decision aids for patients considering options affecting cancer outcomes: evidence of efficacy and policy implications. J Natl Cancer Inst Monogr. 1999;25:67–80. doi: 10.1093/oxfordjournals.jncimonographs.a024212. [DOI] [PubMed] [Google Scholar]