Abstract
OBJECTIVE
To investigate how physicians tailor their recommendations for breast cancer prevention and risk reduction.
DESIGN
Cross-sectional, mail survey.
PARTICIPANTS
Random sample of primary care physicians in California (N = 822).
MEASUREMENTS AND MAIN RESULTS
Six standardized patient scenarios were used to assess how women's breast cancer risk factors influence physicians’ recommendations for screening mammography, counseling about lifestyle behaviors, genetic testing, the use of tamoxifen, prophylactic surgery, and referral to a breast specialist. Over 90% of physicians endorsed mammography for all of the scenarios. Similarly, approximately 80% of physicians endorsed counseling about lifestyle factors for all of the scenarios. Five-year risk of developing breast cancer and family history were both strongly associated with each of the 6 recommendations. Importantly, however, physicians were more likely to endorse the discussion of genetic testing, the use of tamoxifen, and prophylactic surgery for women with a family history of breast cancer compared with women at a higher risk of developing breast cancer but without a family history. Obstetrician-gynecologists were more likely to endorse most of these practices compared with internists.
CONCLUSIONS
Mammography and counseling about lifestyle behaviors are widely endorsed by physicians for breast cancer prevention and risk reduction. Whereas physicians are generally able to tailor their recommendations for prevention and risk reduction based on risk, they may perhaps underutilize genetic evaluation and newer therapeutic options for primary prevention for women who are at high risk of developing breast cancer but do not have a family history.
Keywords: breast cancer, prevention, risk reduction, physician survey
Breast cancer is a leading women's health concern in the United States, where women have a lifetime risk of approximately 11%.1 Several recent advances may promote the primary prevention of breast cancer, including genetic testing for BRCA1 and BRCA2,2 evidence to support the use of tamoxifen for the primary prevention of breast cancer,3 and the publication of data about the efficacy of prophylactic mastectomy for some women at high risk of developing breast cancer.4,5 Despite these advances, consensus about the indications for these therapies is lacking.3,6,7 Even for more established preventative options, there is not complete consensus. While mammography is generally recommended for women over the age of 50, there is controversy about its use for younger women.8–12 The literature has also shown conflicting results regarding the role of lifestyle modification.13–16 In the setting of this evolving evidence, physicians may not be completely informed about new developments, or may be influenced by their own personal beliefs and experiences rather than available scientific evidence. For these reasons, it is important to understand how physicians integrate these new findings into their prevention and risk reduction practices, and how they counsel their patients.
How a physician assesses a woman's risk of developing breast cancer and uses that assessment to counsel and treat is central to the practice of primary care medicine. With the increasing availability of genetic markers, medicine will increasingly move toward more individualized recommendations based on specific patient characteristics.17 Breast cancer risk reduction is an important example of how this type of “tailoring” could be implemented in clinical practice, because there is an array of risk reduction options available to physicians that could be geared to the risk and preferences of each woman. Despite the availability of well-established tools for breast cancer risk assessment18 and new tools for breast cancer risk reduction, it is possible that physicians’ personal and clinical practice characteristics may influence their recommendations. Female physicians may be more likely to recommend cancer screening tests, such as Pap smears,19,20 while physicians residing in urban settings may be more likely to adopt new medical developments, such as the use of breast-conserving therapy for the treatment of breast cancer.21,22
In this era of expanding options for breast cancer prevention and risk reduction, it is particularly timely to investigate how physicians tailor their recommendations for breast cancer prevention and risk reduction to the characteristics of individual women. To address this issue, we performed a mail survey of a random sample of primary care physicians in California, using standardized patient scenarios, to assess how women's breast cancer risk factors and physicians’ own characteristics influence physicians’ recommendations for certain breast cancer prevention therapies. These therapies included screening mammography, counseling about lifestyle behaviors, genetic testing, the use of tamoxifen, prophylactic surgery, and referral to a breast specialist.
METHODS
Study Sample
A random sample of 2,002 California physicians was identified from the 2001 American Medical Association (AMA) Masterfile stratified by 3 selected specialties: family medicine, internal medicine, and obstetrics/gynecology. Physicians who practiced in a metropolitan statistical area with a population ≥250,000, and who graduated from medical school between 1960 and 1997 were selected. Eligibility was further restricted to include only those physicians who spent at least 10% of their work time doing direct patient care, but less than 90% of their work time doing emergency or urgent care, and whose patient panel included at least 10% women.
This research was conducted with a grant from the California Breast Cancer Research Program. The funding organization did not have any role in the design, collection, analysis, or interpretation of the data. It did not review this manuscript. This study was reviewed and approved by the institutional review board of the University of California, San Francisco.
Survey
Eligible physicians were asked to complete a self-administered mail survey. The survey assessed demographic characteristics (age, gender, race/ethnicity, and country of birth), professional characteristics (country of medical training, year of medical school graduation, board certification, specialty training, and affiliations with university medical centers), practice characteristics (practice setting, hours per week spent in patient care, and a description of patient characteristics such as the proportion of patients with limited English ability and the proportion uninsured), and exposure to breast cancer (breast cancer diagnoses per year, and personal or family history of breast cancer). The survey was sent to eligible physicians between January and April 2002. Physicians who did not respond to the initial survey were sent a reminder postcard, up to two additional mailings of the survey, and received a reminder phone call or fax.
Scenarios
Physicians were asked to evaluate a series of 6 clinical scenarios (Table 1). These scenarios were chosen to reflect different levels of risk of developing breast cancer over the next 5 years. The calculated 5-year Gail risk scores of these hypothetical patients ranged from 0.7% to 5.2%.18 Although the current Gail model does not calculate a risk score for women with ductal carcinoma in situ (DCIS), we included a scenario with DCIS because of the lack of consensus for how to best manage these women.23,24 The 5-year risk of developing breast cancer among women with DCIS is estimated to be 8.2%.25 The scenarios are listed in the table in order of increasing risk. They were presented in the survey in alphabetical order (i.e., patient A was presented first). The numerical risk scores were not given to physician survey respondents.
Table 1.
Description of the Scenarios and the Calculated Risk of Developing Breast Cancer
| Scenario | Estimated Risk of Developing Breast Cancer over 5 years18,25 |
|---|---|
| Patient D is 40 years old. She was 10 at menarche and has had no children. She has no personal history of breast cancer, breast biopsy, DCIS, or LCIS. She has no first-degree relatives with breast cancer. | 0.7 |
| Patient C is 67 years old. She was 11 at menarche and 29 at the birth of her first child. She has no personal history of breast cancer, breast biopsy, DCIS, or LCIS. Having been adopted, it is unknown whether she has any first-degree relatives with breast cancer. | 2.1 |
| Patient A is 45 years old. She was 12 at menarche and 22 at the birth of her first child. She has no personal history of breast cancer, breast biopsy, DCIS, or LCIS. She has two first-degree relatives with breast cancer. | 3.4 |
| Patient F is 55 years old. She was 12 at menarche and 22 at the birth of her first child. She has no personal history of breast cancer, breast biopsy, DCIS, or LCIS. She has two first-degree relatives with breast cancer. | 4.8 |
| Patient B is 65 years old. She was 12 at menarche and 31 at the birth of her first child. She has no personal history of breast cancer, DCIS, or LCIS, but has a history of one breast biopsy showing atypical hyperplasia. She has no first-degree relatives with breast cancer. | 5.2 |
| Patient E is 40 years old. She was 13 at menarche and 20 at the birth of her first child. She has no personal history of breast cancer but was previously diagnosed with DCIS. She has no first-degree relatives with breast cancer. | 8.2 |
DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
Outcome Variables
Physicians were asked to evaluate the clinical scenarios in terms of their personal practice patterns, not their knowledge of the literature or expert opinion. For each of the 6 scenarios, physicians were asked if they would: 1) order a screening mammogram, 2) talk with the woman about her lifestyle behaviors (e.g., exercise, diet, alcohol) to reduce her risk of breast cancer, 3) discuss with her the pros and cons of getting genetically evaluated for breast cancer risk, 4) discuss with her the pros and cons of taking tamoxifen for breast cancer risk reduction, 5) discuss with her the pros and cons of getting a prophylactic mastectomy or oophorectomy, and 6) refer her to a breast clinic or specialist.
Independent Variables
The scenarios described several risk factors for developing breast cancer, including current age, age at menarche and first birth, personal history of breast cancer, prior breast biopsy, history of DCIS, and any family history of breast cancer. Because there were more risk factors than scenarios, it was not possible to model the effects of all patient risk factors simultaneously. We opted instead to model the overall effect of the 5-year risk of developing breast cancer and whether the patient had described a family history of breast cancer. Although family history is incorporated in the calculation of breast cancer risk, we hypothesized that it is independently utilized by physicians to guide their risk reduction practices. Additional independent variables included physician demographic characteristics, professional characteristics, practice characteristics, and exposure to breast cancer.
Statistical Analysis
Six logistic regression models were fit, one for each outcome variable. Because responses across scenarios were clustered within physicians, unstructured correlation of response was modeled using generalized estimating equations.26 Explanatory variables were selected on the basis of significant bivariate relationships or a priori hypotheses. For each model, explanatory variables included physician age, gender, race/ethnicity, whether they graduated from a U.S. medical school, specialty (internal medicine, family medicine, or obstetrics and gynecology), practice characteristics (percentage of patients with limited English ability, percentage of patients without health insurance), whether in the last 2 years they had consulted with a physician at an academic medical center about breast cancer issues or a patient's breast care, the average number of women they diagnosed with breast cancer each year, whether they had a personal experience with breast cancer (themselves or a family member), the estimated 5-year risk of developing breast cancer for the woman described in the scenario, and a variable to indicate whether the scenario indicated a family history of breast cancer.
RESULTS
Response Rate
Of the initial 2,002 physicians selected, 355 were found to be ineligible (e.g., not in the targeted specialties, no longer in practice in California, or not enough time spent in patient care). Completed questionnaires were returned by 822 of the remaining 1,647 eligible physicians, yielding a 49.9% response rate. Information about nonrespondents was obtained from the AMA Masterfile. Respondents and nonrespondents differed significantly by gender, with 47.2% of females responding compared with 37.6% of males (P < .001). A significant overall difference was also observed with respect to specialty, with 48.0% of obstetrician-gynecologists responding, 43.6% of internists, and 40.6% of family physicians (P < .001). There were no significant differences between respondents and nonrespondents with regard to physician age, country of birth, country of medical school, or year of graduation from medical school.
Characteristics of the Sample
The average age of the physicians who participated in the survey was 46.8 years (Table 2). The majority of the participants described themselves as white, 30.5% as Asian/Pacific Islander, and 12.4% as Latino, African-American, or “other” race/ethnicity. Sixty-one percent were born in the United States. Only 4.7% of the physicians were less than 6 years beyond medical school graduation. The majority graduated from a U.S. medical school. Almost all were board certified. The participants worked in a variety of practice settings and provided an average of 37.5 hours per week of ambulatory care. The participating physicians estimated that an average of 20.4% of their patients had limited English ability and that 6.2% were uninsured. Only 29.9% of physicians had consulted with a university-based physician in the last 2 years about a breast care issue. The majority had attended conferences or read journals often or very often to gather new medical knowledge. Fewer had obtained new information from a variety of other sources. On average, these physicians diagnosed 4.3 cases of breast cancer during the prior year. Approximately one-third of the physicians had either a personal history of breast cancer or a family member who had received this diagnosis.
Table 2.
Description of the Study Sample (N = 822)
| Mean age, y (range) | 46.8 (31 to 65) |
| Female, % | 42.2 |
| Race/ethnicity, % | |
| White | 57.1 |
| Asian/Pacific Islander | 30.5 |
| Latino/African-American/Other | 12.4 |
| Born in the U.S., % | 60.9 |
| Year graduated from medical school, % | |
| 1975 or earlier | 24.6 |
| 1976–1985 | 32.7 |
| 1986–1995 | 38.0 |
| 1996 or later | 4.7 |
| U.S. medical school, % | 76.1 |
| Specialty | |
| Obstetrician/gynecologist | 39.1 |
| Family medicine | 33.5 |
| Internal medicine | 27.5 |
| Board certified, % | 92.3 |
| Practice setting, % | |
| Solo practice | 26.9 |
| Single-specialty group practice | 22.4 |
| Multi-specialty group practice | 16.7 |
| Staff model HMO | 20.5 |
| Community health center or public hopital | 4.9 |
| Academic health center-based practice | 6.0 |
| Other | 2.7 |
| Mean hours/week doing ambulatory care (range) | 37.5 (4 to 80) |
| Average percentage of patients with limited English ability | 20.4 (0 to 98) |
| Average percentage of patients with no health insurance | 6.2 (0 to 100) |
| Consulted with a university physician about breast care during the past 2 years, % | 29.9 |
| During the past year, used the following resources to gather new medical knowledge often or very often, % | |
| Medical meetings or conferences | 77.2 |
| Medical journals | 71.9 |
| The internet | 24.0 |
| Pharmaceutical industry representatives or materials | 14.7 |
| Newspapers or magazines | 12.4 |
| Sources brought in by patients | 3.4 |
| Average number of breast cancer diagnoses within the past year | 4.3 (0 to 50) |
| Personal or family history of breast cancer, % | 32.4 |
Note: missing data for U.S. medical school (n = 1), country of birth (n = 2), gender (n = 1), race/ethnicity (n = 1), hours/week doing ambulatory care (n = 16), percentage of patients with limited English ability (n = 10), percentage of patients with no health insurance (n = 35), practice setting (n = 1), consulted with a university physician (n = 9), and personal or family history of breast cancer (n = 11). May not add to 100% due to rounding.
Physician Recommendations for Breast Care
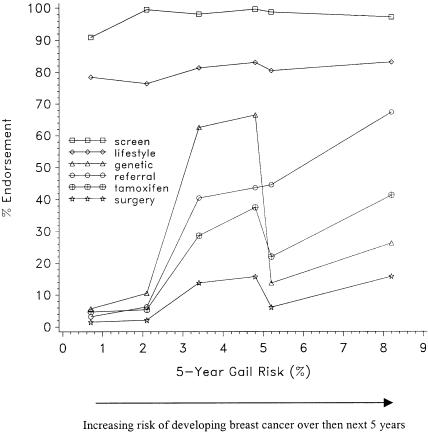
Figure 1 displays the percentage endorsement for each of the outcome measures. The cases are arranged in order of increasing risk of developing breast cancer, ranging from a calculated risk of 0.7% for scenario D to a risk of 8.2% for scenario E. Over 90% of physicians endorsed mammography screening for all of the scenarios, including a 40-year-old woman with no additional risk factors for breast cancer (scenario D). Similarly, approximately 80% of physicians endorsed talking with all of these women about lifestyle factors to reduce their risk of developing breast cancer. The remaining outcome measures demonstrated more variation in physician recommendations. Discussion of genetic testing was endorsed most frequently for women with a family history of breast cancer (scenarios A and F), followed more distantly by the scenario with DCIS (scenario E). The probability of a physician endorsing a referral to a specialist increased monotonically with the risk of developing breast cancer. Similar to referral for genetic testing, discussion of tamoxifen occurred most frequently for the scenarios that described women with a family history of breast cancer, but was also endorsed for the woman described with DCIS. Thirty-seven percent of physicians endorsed discussion of tamoxifen with the woman in scenario F, a postmenopausal woman with a family history of breast cancer, and 41% of physicians endorsed this discussion for the woman with DCIS described in scenario E. Finally, physicians were most likely to endorse discussion of prophylactic surgery with the women who had a family history of breast cancer or a personal history of DCIS. Interestingly, physicians were less likely to endorse the discussion of tamoxifen, prophylactic surgery, or genetic testing for the woman with a high calculated risk of developing breast cancer who did not have a family history or a personal history of DCIS (the patient with a personal history of a biopsy demonstrating atypical hyperplasia, described in scenario B), compared with the women whose scenarios indicated a family history of breast cancer. Risk of developing breast cancer and family history were both strongly associated with each of these 6 recommendations (Table 3).
FIGURE 1.
Percentage endorsement of breast cancer prevention recommendations for clinical scenarios.
Mammography: all scenarios significantly different from scenario D. Scenarios E and F also differed significantly (all P < .001). Lifestyle: scenario A significantly different from scenarios C and D; scenario B significantly different from scenarios C, E, and F; scenario C significantly different from scenarios E and F; scenario D significantly different from scenarios A, E, and F (all P < .001).
Discuss genetic evaluation: all scenarios significantly different from each other except scenarios A and F, and B and C (all P < .001).
Discuss tamoxifen: all scenarios significantly different from each other except scenarios C and D, and E and F (all P < .001).
Discuss surgery: all scenarios significantly different from each other except scenarios A and E, A and F, C and D, and E and F (all P < .001).
Refer to a specialist: all scenarios significantly different from each other except scenarios A and B, A and F, and B and F (all P < .001).
Table 3.
Factors Associated with Breast Cancer Prevention Recommendations
| Mammogram | Talk About Lifestyle | Discuss Genetic Evaluation | Discuss Tamoxifen | Discuss Surgery | Referral to a Specialist | |
|---|---|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | ||||||
| Patient Characteristics | ||||||
| Five-year risk of developing breast cancer | 1.28 (1.14 to 1.44) | 1.06 (1.04 to 1.07) | 1.24 (1.20 to 1.28) | 1.45 (1.39 to 1.50) | 1.40 (1.32 to 1.49) | 1.68 (1.62 to 1.74) |
| Family history | ||||||
| Yes | 2.42 (1.45 to 4.04) | 1.19 (1.11 to 1.27) | 13.92 (11.52 to 16.82) | 3.10 (2.67 to 3.59) | 3.87 (3.04 to 4.94) | 2.37 (2.06 to 2.72) |
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Physician and Practice Characteristics | ||||||
| Physician gender | ||||||
| Female | 1.49 (0.84 to 2.67) | 1.73 (1.20 to 2.49) | 0.94 (0.73 to 1.21) | 0.95 (0.73 to 1.23) | 1.02 (0.73 to 1.43) | 1.20 (0.94 to 1.54) |
| Male | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Specialty | ||||||
| OB* | 3.23 (1.59 to 6.56) | 1.00 (0.68 to 1.49) | 2.05 (1.52 to 2.77) | 1.37 (1.01 to 1.87) | 1.84 (1.25 to 2.72) | 1.54 (1.17 to 2.05) |
| FP | 1.06 (0.61 to 1.85) | 1.59 (1.03 to 2.46) | 1.10 (0.80 to 1.52) | 0.82 (0.59 to 1.13) | 0.93 (0.61 to 1.42) | 0.74 (0.55 to 1.00) |
| IM | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medical school | ||||||
| U.S. | 0.61 (0.34 to 1.09) | 0.55 (0.34 to 0.89) | 0.59 (0.43 to 0.81) | 0.97 (0.71 to 1.31) | 0.59 (0.40 to 0.86) | 1.02 (0.76 to 1.37) |
| Foreign | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Percentage of patients with limited English† | 0.91 (0.83 to 1.01) | 1.02 (0.93 to 1.12) | 1.10 (1.03 to 1.17) | 1.01 (0.95 to 1.07) | 1.04 (0.95 to 1.13) | 1.04 (0.98 to 1.09) |
| Consulted with a university– affiliated physician | ||||||
| Yes | 0.81 (0.50 to 1.32) | 1.43 (0.97 to 2.10) | 1.41 (1.09 to 1.83) | 1.18 (0.90 to 1.55) | 1.06 (0.76 to 1.49) | 1.19 (0.93 to 1.45) |
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Number of breast cancer cases/year | 1.08 (1.00 to 1.17) | 1.03 (0.98 to 1.08) | 1.02 (0.99 to 1.04) | 1.05 (1.02 to 1.08) | 1.01 (0.97 to 1.04) | 0.98 (0.96 to 1.01) |
Note: models are adjusted for physician age, gender, race/ethnicity, whether or not a U.S. medical school graduate, specialty, practice characteristics, whether or not in the last 2 years they had consulted with a physician at an academic medical center about breast cancer issues or a patient's breast care, the average number of women diagnosed with breast cancer each year, whether or not the physician had a personal experience with breast cancer, and the Gail score (5-year estimate of the risk of developing breast cancer) and family history associated with the clinical scenario.
OB, obstetrics-gynecology; FP, family practice; IM, internal medicine.
For each change of 10%.
Association of Breast Cancer Risk Recommendations with Physician Characteristics
Beyond the associations described above, we also examined the association of breast cancer prevention recommendations with physicians’ demographic, professional, and practice characteristics, and their personal exposure to breast cancer (Table 3). Whereas female physicians were more likely to talk about lifestyle behaviors for breast cancer risk reduction than males, there were no other observed differences in prevention recommendations by gender. Obstetrician-gynecologists were more likely than internists to endorse all of the breast cancer prevention recommendations except the discussion of lifestyle factors. The recommendations of family physicians and internists were more similar. Graduates of U.S. medical schools were less likely than foreign graduates to discuss lifestyle modification, genetic evaluation, or prophylactic surgery. Consultation with a university-affiliated physician about breast care was associated with an increased likelihood of discussion of genetic evaluation, but not with other practices. Finally, physicians who had diagnosed more women with breast cancer in the prior year were more likely to discuss tamoxifen, but there was no association demonstrated with other prevention practices. There were no differences in reported utilization of these breast cancer prevention recommendations by physician age, race, year of medical school graduation, or personal experience with breast cancer (not displayed in Table 3).
DISCUSSION
Mammography screening and counseling about lifestyle behaviors were endorsed by the majority of these California physicians for all of the clinical scenarios presented in this survey. These results are not unexpected. Mammography is widely endorsed irrespective of risk, and while the role of lifestyle modification in the prevention of breast cancer is less certain, it is not associated with risks and may in fact have other health benefits. In contrast, discussion of the risks and benefits of genetic testing, the use of tamoxifen for primary prevention, prophylactic surgery, or referral to a breast specialist demonstrated overall lower rates of endorsement that varied substantially with the calculated risk of developing breast cancer and family history. Of all of these preventative practices, only mammography and the use of tamoxifen for women at high risk of developing breast cancer have been reviewed and endorsed by the U.S. Preventive Services Task Force (USPSTF).27 Although the scenario describing the woman with the history of a prior breast biopsy showing atypical hyperplasia had a higher calculated risk of developing breast cancer over the subsequent 5 years, physicians were less likely to endorse discussion of genetic evaluation, tamoxifen, or prophylactic surgery for her compared with the women who were at lower risk but had family histories of breast cancer. The majority of physicians endorsed referral to a specialist for the woman with DCIS. Our findings also demonstrated fairly consistent differences in prevention recommendations by physician specialty. Obstetrician-gynecologists were more likely to endorse most of these prevention practices. In contrast to other studies,19 female physicians were not more likely to endorse mammography screening, although they were more likely to endorse the discussion of lifestyle risk factors.
These findings suggest that the vast majority of physicians have accepted the role of mammography in the early detection of breast cancer. While there is some uncertainty in the literature about the role of mammography for women between the ages of 40 and 49 years, the role of mammography screening for women 50 and above is well established.28 In this survey, physicians demonstrated similar rates of endorsement of mammography for both of these age groups. These physicians also uniformly endorsed the discussion of lifestyle risk factors for reducing the risk of breast cancer. Whereas there is less consensus on the benefits of lifestyle modification in terms of reducing breast cancer risk, there are few negative effects associated with this intervention.13,16,29–32
DCIS is associated with an increased risk of subsequent invasive breast cancer.25,33 Although the diagnosis of DCIS has increased markedly in the United States as the utilization of mammography has increased,34 the prognosis and optimal management of women with DCIS is controversial.24,35 Because of the uncertainty surrounding the most appropriate management of women with this diagnosis, and the rapid evolution of new findings, referral to a specialist is appropriate for many women with this diagnosis. Our findings suggest that almost 70% of physicians in our sample endorse referral of women with DCIS for specialty care.
Our results suggest that genetic testing may be underutilized by primary care physicians. Only 60% of physicians endorsed the discussion of genetic testing for the women with a strong family history of breast cancer. Enhanced discussion of the role of genetic testing may require an improved understanding of cancer genetics by primary care physicians or referral guidelines.36 Given the likelihood that genetic risk evaluation will become an increasingly important part of the practice of medicine for other chronic diseases, future work should consider factors that facilitate or impede the discussion of genetic risk by physicians. A minority of physicians endorsed discussion of the risks and benefits of tamoxifen or prophylactic surgery for women with a high risk of developing breast cancer. Tamoxifen, used for many years in the treatment and secondary prevention of breast cancer, was approved by the Food and Drug Administration (FDA) for the primary prevention of breast cancer for women over the age of 35 at increased risk of developing the disease, based on the results of a large clinical trial in the United States.3 This study suggests that women with ≥1.67% risk of developing the disease within 5 years, estimated by Gail's algorithm, had a 50% reduction in the rate of primary breast cancer when taking tamoxifen. However, two smaller European studies with different enrollment criteria and confounded by simultaneous estrogen use failed to show beneficial effects of tamoxifen on breast cancer incidence, contributing to clinical uncertainty.6,7 Early findings from an ongoing European trial suggests that tamoxifen reduces the risk of developing breast cancer.37 Beyond the concern resulting from these conflicting findings, physicians and their currently healthy patients may be concerned about the potential adverse effects of tamoxifen. One estimate suggests that 16% of U.S. women could consider using tamoxifen for the primary prevention of breast cancer based on the FDA's approval.38 Among white women, who are more likely to have a positive benefit/risk index than African-American or Hispanic women, over 2 million women would have a positive benefit/risk index, and approximately 28,000 cases of breast cancer would be prevented.38 The USPSTF currently recommends that clinicians discuss chemoprevention with women at high risk for breast cancer and at low risk for adverse effects.27 Prophylactic mastectomy and/or oophorectomy may also be an option for some women at high risk for developing breast cancer. Women with a strong family history of breast cancer or who are BRCA1 or BRCA2 mutation carriers and undergo surgery may have a substantial reduction in the risk of subsequent breast cancer and improved survival compared to women who do not have surgery.4,5,39 Whereas further evidence is needed to decide how best to target these therapeutic options, women should be informed of these options by their physicians. The discussion of prevention options by primary care physicians may influence the breast cancer prevention practices of women, including their participation in mammography, genetic testing, and clinical trials for breast cancer chemoprevention.40–42 These results suggest that interventions should be considered to facilitate the discussion of these options by primary care physicians for women at high risk of developing breast cancer. These interventions should recognize the time constraints typically experienced by primary care physicians.43
The availability of tools to help women and their physicians assess a woman's risk of developing breast cancer,18 along with the new options for prevention, make physician recommendations for the screening and prevention of breast cancer a particularly important model for examining factors that influence physician decision making about prevention. Unfortunately, there is very limited research in the area of physicians’ breast cancer prevention and risk reduction practices. One survey of general surgeons, plastic surgeons, and gynecologists suggests that a greater proportion of plastic surgeons recommend bilateral prophylactic mastectomies, compared to general surgeons and gynecologists.44
These results suggest that obstetrician-gynecologists are more likely to endorse the discussion of breast cancer prevention options for these clinical scenarios. Prior work suggests that obstetrician-gynecologists may do more counseling and screening than family and general practitioners and internists with respect to breast cancer.45
This paper has several limitations. Although we surveyed a large number of California physicians, our results may not be generalizable to physicians practicing in other areas of the country or in more rural areas. We did not examine how patient characteristics, such as insurance status or race, may independently influence physician practice patterns involving the primary prevention of breast cancer. Although risk of breast cancer varies by race/ethnicity, the purpose of this study was to examine whether physicians’ practices vary by established clinical characteristics. Further work should examine how a patient's race/ethnicity influences practice patterns. We did not ask physicians about actual utilization of therapies, but rather whether or not they would discuss them with the patient. Similarly, written patient scenarios cannot approach the complexity of a real patient. The initiation of a discussion by a physician about therapeutic options is, nonetheless, an initial step toward a decision about the options that may or may not be appropriate for her. Physician-reported behavior may differ from care documented in medical records or the reports of patients about their care.46 Whereas each of these perspectives may offer different, and valuable, perspectives on the quality of care, this study only examined physician-reported behavior. Finally, in an era where primary care physicians face many competing demands for limited time,43 these data cannot determine how often primary care physicians should discuss these prevention strategies with women in their practice.
This paper extends what is known about the breast cancer screening and risk reduction recommendations of physicians. These results suggest that the vast majority of physicians endorse mammography screening and the discussion of lifestyle risk factors for the primary prevention of breast cancer. Whereas physicians are generally able to tailor their recommendations for prevention based on risk, they may underrecognize the risk associated with a history of atypical hyperplasia, and perhaps underutilize genetic evaluation, referral to a specialist, and newer therapeutic options for primary prevention for some women who are at high risk of developing breast cancer. In this era of expanding options for breast cancer prevention and risk reduction, physicians need to consider strategies beyond the endorsement of mammography and lifestyle counseling to include the discussion of targeted, risk-based interventions.
REFERENCES
- 1.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001;93:1633–7. doi: 10.1093/jnci/93.21.1633. [DOI] [PubMed] [Google Scholar]
- 6.Powles T, Eeles R, Ashley S, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;352:98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomized trial among hysterectomized women. Italian Tamoxifen Prevention Study. Lancet. 1998;352:93–7. doi: 10.1016/s0140-6736(98)85011-3. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro S. Periodic screening for breast cancer: the HIP Randomized Controlled Trial. Health Insurance Plan. J Natl Cancer Inst. 1997;22:27–30. doi: 10.1093/jncimono/1997.22.27. [DOI] [PubMed] [Google Scholar]
- 9.Tabar L, Chen HH, Fagerberg G, Duffy SW, Smith TC. Recent results from the Swedish Two-County Trial. The effects of age, histologic type, and mode of detection on the efficacy of breast cancer screening. J Natl Cancer Inst. 1997;22:43–7. doi: 10.1093/jncimono/1997.22.43. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher SW. Breast cancer screening among women in their forties: an overview of the issues. J Natl Cancer Inst. 1997;22:5–9. doi: 10.1093/jncimono/1997.22.5. [DOI] [PubMed] [Google Scholar]
- 11.Kerlikowske K. Efficacy of screening mammography among women aged 40–49 years and 50–69 years. Comparison of relative and absolute benefit. J Natl Cancer Inst. 1997;22:79–86. doi: 10.1093/jncimono/1997.22.79. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher SW, Elmore JG. Clinical practice. Mammographic screening for breast cancer. N Engl J Med. 2003;348:1672–80. doi: 10.1056/NEJMcp021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mezetti M, La Vecchia C, Decarli A, Boyle P, Talamini R, Franceschi S. Population attributable risk for breast cancer: diet, nutrition and physical exercise. J Natl Cancer Inst. 1998;90:389–93. doi: 10.1093/jnci/90.5.389. [DOI] [PubMed] [Google Scholar]
- 14.Gammon MD, Schoenberg JB, Britton JA, et al. Recreational physical activity and breast cancer risk among women under age 45 years. Am J Epidemiol. 1998;147:273–80. doi: 10.1093/oxfordjournals.aje.a009447. [DOI] [PubMed] [Google Scholar]
- 15.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–11. [PubMed] [Google Scholar]
- 16.Smith-Warner S, Spiegelman D, Yaun S-S, et al. Alcohol and breast cancer in women. A pooled analysis of cohort studies. JAMA. 1998;279:535–9. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- 17.Kallioniemi A. Molecular signatures of breast cancer—predicting the future. N Engl J Med. 2002;347:2067–8. doi: 10.1056/NEJMe020152. [DOI] [PubMed] [Google Scholar]
- 18.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 19.Lurie N, Margolis KL, Mink PJ, Slater JS. Why do patients of female physicians have higher rate of breast and cervical cancer screening? J Gen Intern Med. 1997;12:34–43. doi: 10.1046/j.1525-1497.1997.12102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman CJ, Lengerich EJ, Stood G. Variation in recommendations for breast and cervical cancer screening among primary care physicians in North Carolina. South Med J. 1996;89:583–90. doi: 10.1097/00007611-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Howe HL, Katterhagen JG. Effects of physician outreach programs on rural-urban differences in breast cancer management. J Rural Health. 1997;13:109–17. doi: 10.1111/j.1748-0361.1997.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 22.Nattinger AB, Gottlieb MS, Veum J, Yahnke D, Goodwin JS. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326:1102–7. doi: 10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 23.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275:913–8. [PubMed] [Google Scholar]
- 24.Morrow M, Strom EA, Bassett LW, et al. Standard for the management of ductal carcinoma in situ of the breast (DCIS) CA Cancer J Clin. 2002;52:256–76. doi: 10.3322/canjclin.52.5.256. [DOI] [PubMed] [Google Scholar]
- 25.Kerlikowske K, Molinaro A, Cha I, et al. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95:1692–702. doi: 10.1093/jnci/djg097. [DOI] [PubMed] [Google Scholar]
- 26.Lipsitz SR, Fitzmaurice GM, Orav EJ, Laird NM. Performance of generalized estimating equations in practical situations. Biometrics. 1994;50:270–8. [PubMed] [Google Scholar]
- 27.U.S. Preventive Services Task Force. Available at: http://www.ahcpr.gov/clinic/uspstfix.htm. Accessed February 27, 2004.
- 28.Kerlikowske K, Grady D, Rubin SM, Sandrock C, Ernster VL. Efficacy of screening mammography. A meta-analysis. JAMA. 1995;273:149–54. [PubMed] [Google Scholar]
- 29.Willet WC, Browne ML, Bain C. Relative weight and risk of breast cancer among premenopausal women. Am J Epidemiol. 1985;122:731–9. doi: 10.1093/oxfordjournals.aje.a114156. [DOI] [PubMed] [Google Scholar]
- 30.Hunter DJ, Willett WC. Nutrition and breast cancer. Cancer Causes Control. 1996;7:56–68. doi: 10.1007/BF00115638. [DOI] [PubMed] [Google Scholar]
- 31.Pathak DR, Whittemore AS. Combined effects of body size, parity and menstrual events on breast cancer incidence in seven countries. Am J Epidemiol. 1992;135:153–67. doi: 10.1093/oxfordjournals.aje.a116268. [DOI] [PubMed] [Google Scholar]
- 32.Hankinson SE, Willet WC, Manson JE. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 33.Sakorafas GH. The management of women at high risk for the development of breast cancer: risk estimation and preventative strategies. Cancer Treat Rev. 2003;29:79–89. doi: 10.1016/s0305-7372(02)00107-x. [DOI] [PubMed] [Google Scholar]
- 34.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546–54. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 35.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med. 2000;160:953–8. doi: 10.1001/archinte.160.7.953. [DOI] [PubMed] [Google Scholar]
- 36.Wideroff L, Freedman AN, Olson L, et al. Physician use of genetic testing for cancer susceptibility: results of a national survey. Cancer Epidemiol Biomarkers Prev. 2003;12:295–303. [PubMed] [Google Scholar]
- 37.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomized prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 38.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526–32. doi: 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- 39.Schrag D, Kuntz KM, Garber JE, Weeks JC. Decision analysis—effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336:1465–71. doi: 10.1056/NEJM199705153362022. [DOI] [PubMed] [Google Scholar]
- 40.Kinney AY, Richards C, Vernon SW, Vogel VG. The effect of physician recommendation on enrollment in the breast cancer chemoprevention trial. Prev Med. 1998;27(5 Part 1):713–9. doi: 10.1006/pmed.1998.0349. [DOI] [PubMed] [Google Scholar]
- 41.Fox SA, Siu AL, Stein JA. The importance of physician communication on breast cancer screening of older women. Arch Intern Med. 1994;154:2058–68. [PubMed] [Google Scholar]
- 42.Armstrong K, Stopfer J, Calzone K, Fitzgerald G, Coyne J, Weber B. What does my doctor think? Preferences for knowing the doctor's opinion among women considering clinical testing for BRCA1/2 mutations. Genet Test. 2002;6:115–8. doi: 10.1089/10906570260199366. [DOI] [PubMed] [Google Scholar]
- 43.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–41. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houn F, Helzlsouer KJ, Friedmna NB, Stefanek ME. The practice of prophylactic mastectomy. A survey of Maryland surgeons. Am J Public Health. 1995;85:801–5. doi: 10.2105/ajph.85.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank E, Rock J, Sara D. Characteristics of female obstetrician-gynecologists in the United States. Obstet Gynecol. 1999;94(5 Part 1):659–65. doi: 10.1016/s0029-7844(99)00425-1. [DOI] [PubMed] [Google Scholar]
- 46.Gandhi TK, Francis EC, Puopolo AL, Burstin HR, Haas JS, Brennan TA. Inconsistent report cards: assessing the comparability of various measures of the quality of ambulatory care. Med Care. 2002;40:155–65. doi: 10.1097/00005650-200202000-00010. [DOI] [PubMed] [Google Scholar]