Abstract
BACKGROUND
Angiotensin-converting enzyme inhibitors are effective for many cardiovascular diseases and are widely prescribed, but cough sometimes necessitates their withdrawal.
OBJECTIVE
To develop and validate a model that predicts, by using information available at first prescription, whether a patient will develop cough within 6 months.
DESIGN
Retrospective cohort study with derivation and validation sets.
SETTING
Outpatient clinics affiliated with an urban tertiary care hospital.
PATIENTS
Clinical data were collected from electronic charts. The derivation set included 1,125 patients and the validation set included 567 patients.
INTERVENTIONS
None.
MEASUREMENTS
Angiotensin-converting enzyme inhibitorinduced cough assessed by predetermined criteria.
RESULTS
In the total cohort, 12% of patients developed angiotensin-converting enzyme inhibitor-induced cough. Independent multivariate predictors of cough were older age, female gender, non-African American (with East Asian having highest risk), no history of previous angiotensin-converting enzyme inhibitor use, and history of cough due to another angiotensin-converting enzyme inhibitor. Patients with a history of angiotensin-converting enzyme inhibitor-induced cough were 29 times more likely to develop a cough than those without this history. These factors were used to develop a model stratifying patients into 4 risk groups. In the derivation set, low-risk, average-risk, intermediate-risk, and highrisk groups had a 6%, 9%, 22%, and 55% probability of cough, respectively. In the validation set, 4%, 14%, 20%, and 60% of patients in these 4 groups developed cough, respectively.
CONCLUSIONS
This model may help clinicians predict the likelihood of a particular patient developing cough from an angiotensin-converting enzyme inhibitor at the time of prescribing, and may also assist with subsequent clinical decisions.
Keywords: adverse drug events, angiotensin-converting enzyme inhibitors, cough, clinical prediction rule
Angiotensin-converting enzyme (ACE) inhibitors have been shown to improve the prognosis of patients with variety of cardiovascular diseases, such as hypertension, myocardial infarction, and congestive heart failure, as well as diabetic nephropathy and other renal diseases. 1,2 As a result, the number of patients on ACE inhibitors has dramatically increased. In the United States alone, approximately 15 million prescriptions of lisinopril (the 11th most frequently prescribed medication) were issued for ambulatory patients in 2000.3
Although generally not severe, adverse events due to ACE inhibitors lead to drug discontinuation in an important fraction of patients. The most frequent adverse event is a dry cough, with an incidence of 5% to 25%,4–6 accounting for 2.5% of chronic cough.7 Cough due to ACE inhibitors causes patient discomfort, necessity for medication change, and occasionally unnecessary diagnostic evaluation. In one study, adverse drug events in outpatients were associated with increased worry or discomfort in 49% of patients, and were also associated with lower patient satisfaction.8
As a response to ACE inhibitor-induced cough, angiotensin II receptor blockers (ARBs) are often substituted for ACE inhibitors.9 However, pooled evidence suggests that ARBs are not as effective as ACE inhibitors and are 1.5 to 3 times more costly in the United States.9 Thus, ACE inhibitors will likely be used as the first-line therapy in a variety of cardiovascular diseases for years to come. However, switching from an ACE inhibitor to an ARB can often require additional clinic visits and medication costs. For these reasons, it would be useful to be able to differentiate patients with a high risk of developing ACE inhibitor-induced cough and avoid prescribing ACE inhibitors in those patients.
Therefore, to identify the independent correlates of ACE inhibitor-induced cough, we retrospectively collected consecutive data on 1,692 outpatient prescriptions for ACE inhibitors. These data were used to develop and validate a decision rule that may help physicians judge, when initially prescribing a specific ACE inhibitor, whether a patient is likely to develop cough or, if a cough develops, whether the cough is caused by ACE inhibitors.
METHODS
Patient Population
Data collection took place at Brigham and Women's Hospital in Boston, Mass. The outpatient clinic of the hospital has an electronic longitudinal medical record, which includes problem lists, medication lists, allergies, patient demographics, visit notes, and laboratory data. In addition, physicians write prescriptions electronically and the information (including drug start and discontinuation date) is also recorded. Patients enrolled in the study included all outpatients prescribed a specific ACE inhibitor for the first time between January 2000 and December 2001. We extracted all new prescriptions of ACE inhibitors from an electronic medical record and then manually reviewed each prescription to ensure it met inclusion criteria. Patients who had been prescribed other kinds of ACE inhibitors in the past, but not at the time of inclusion in the study, were included. Those who had received some kind of ACE inhibitors in the past were excluded. We also excluded patients for whom physicians had refilled ACE inhibitors previously prescribed at other clinics, or for whom reviewers could not identify the start date of the drug. Patients not followed after the prescription of ACE inhibitors were also excluded. The Human Research Committee at Brigham and Women's Hospital approved this study.
Risk Factor Variables
To be eligible for inclusion in the model, potential risk factors (Table 1) ) had to be documented when the ACE inhibitor was prescribed. Factors associated with cough due to ACE inhibitors identified by previous studies included older age,10 female gender,10–14 ethnicity,13,15–17 smoking status,12 diabetes mellitus,18,19 congestive heart failure,6 respiratory diseases (chronic obstructive pulmonary disease, asthma, or others),6 elevated creatinine,19,20 and concurrent use of nonsteroidal anti-inflammatory drugs,21,22 aspirin,23 cyclo-oxygenase-2 inhibitors,24 and nifedipine.22 Because a high incidence of ACE inhibitor-induced cough had previously been reported in patients of Chinese and Japanese ethnicity,15,16 we divided Asian ethnicity into East Asian (Chinese, Korean, and Japanese) and non-East Asian (other Asian). We also included the concurrent use of other medications (diuretics, beta-blockers), history of using other types of ACE inhibitors, history of cough caused by other types of ACE inhibitors, history of angioedema caused by other types of ACE inhibitors, hypertension, coronary heart disease, depression and other psychiatric disorders, and hemodialysis.
Table 1.
Definitions of Potential Risk Factor Variables
| Variable | Definition |
|---|---|
| Current smoker | Tobacco smoking at the point of enrollment |
| Past smoker | Tobacco smoking in the past but in cessation at the point of enrollment |
| Never smoked | No tobacco smoking in the past |
| History of other ACE inhibitors | Taking other types of ACE inhibitors in the past |
| History of ACE inhibitor-induced cough | Experience of ACE inhibitor-induced cough in the past |
| History of ACE inhibitor-induced angioedema | Experience of ACE inhibitor-induced angioedema in the past |
| Hypertension | Clinical diagnosis with systolic blood pressure ≥140, diastolic blood pressure ≥90, or receiving anti-hypertensive therapy |
| Diabetes mellitus | Clinical diagnosis with educational or pharmacological therapy |
| Coronary artery disease | Typical angina or myocardial infarction diagnosed by electrocardiogram or coronary angiography |
| Congestive heart failure | Clinical diagnosis or left ventricular ejection fraction ≤40% |
| Chronic obstructive pulmonary disease | Clinical diagnosis, typical chest radiograph findings, or FEV1.0 ≤70% |
| Asthma | Clinical diagnosis with history of use of inhalers, aminophyline, or steroids |
| Other respiratory diseases | Any kind of lung parenchymal disease documented by physician |
| Depression/anxiety | Clinical diagnosis or use of antidepressant |
| Other psychiatric diseases | Any kind of psychiatric disease documented by physician |
| Hemodialysis | On hemodialysis or peritoneal dialysis at the point of enrollment |
| Creatinine | Creatinine value (mg/dL) just before enrollment. If none available, closest value before the end of study |
| Concurrent medications | Prescription of the subsequent drugs at the point of enrollment |
| Diuretics | Any kind of loop diuretics, thiazide, or derivatives |
| Beta-blockers | Any kind of beta-blocking agents including alpha/beta-blocking agents |
| Calcium antagonists | Any kind of calcium antagonists |
| Low dose (≤325 mg/day) aspirin | Aspirin prescribed ≤325 mg/day |
| High dose (≥325 mg/day) aspirin | Aspirin prescribed >325 mg/day |
| Nonsteroidal anti-inflammatory drugs | Any kind of nonsteroidal anti-inflammatory drugs except for aspirin and cyclo-oxygenase-2 inhibitors |
| Cyclo-oxygenase-2 inhibitors | Any kind of cyclo-oxygenase-2 inhibitors |
ACE, angiotensin-converting enzyme; FEV, forced expiratory volume.
ACE inhibitor-associated cough was defined as cough with all of the following characteristics: dry cough that developed during the 6-month period after the prescription of ACE inhibitors; lack of constitutional or infectious signs such as fever, rhinorrhea, or myalgia; cough recognized as ACE inhibitor induced and the medication discontinued; and the cough disappeared or was alleviated after withdrawal.
Data Collection
All data were collected from the electronic record. One investigator reviewed all electronic records. For binary variables, missing information for a patient was coded as absent. For categorical or continuous variables, missing data were found in ethnicity and creatinine values, 155/1,692 for ethnicity and 26/1,692 for creatinine. We developed dummy variables for ethnicity and a binary variable for creatinine for making the prediction rule, and treated the missing data as absent.
Statistical Analysis
Before beginning the analysis, all observations were randomly assigned to the derivation set and the validation set at a ratio of 2 to 1. The univariate relationships between ACE inhibitor-induced cough and categorical and continuous variables in the derivation set were evaluated using the χ2test with appropriate degrees of freedom and t test. Variables that showed substantial correlation (P < .10) with ACE inhibitor-induced cough were then entered into a stepwise logistic regression analysis in addition to history of other ACE inhibitors to evaluate whether it would provide a safer profile. To make the prediction rule clear and easy for physicians to use, we categorized age into 4 groups (<50, 50 to 59, 60 to 69, and ≥70). We also categorized ethnicity into 3 groups (African-American, East Asian, and neither African-American nor East Asian). In the logistic regression model, factors with P values ≤.05 were retained. We looked for interactions between these variables, and did not find any to be significant at the .05 level.
The results of the multivariate analysis were then used to develop a clinical prediction model.25 Each beta coefficient was divided by 0.26 (one fifth the added beta coefficient for 2 of the correlates: aged 60 to 69 and aged 70 or older) and rounded to the nearest integer. The risk score for an individual patient was determined by assigning points for each factor present and summing. The resulting continuous distribution of total risk scores across all patients in the derivation set was then stratified into 4 categories of points that grouped patients according to the level of risk (lower, average, higher, and extremely higher risks). Although this stratification method resulted in relatively few episodes in the highest risk group, it allowed for discrimination of this small subset of patients at extremely high risk. The χ2test was used to compare the derivation and validation sets.
The discriminatory performance of the model was validated by comparing the receiver-operating characteristic (ROC) curve analysis in the derivation set with that in the validation set.26 All statistical analyses were carried out using SAS software (version 8.02, SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
During the study period, 2,250 patients were prescribed ACE inhibitors for the first time. Among them, 1,692 patients were followed for at least 180 days after an initial prescription without ACE inhibitor-induced cough or who developed cough within 180 days. The mean age of the excluded cohort was 59, which was the same as the included cohort. The proportion of men was 41% in both the excluded and included cohort. The proportion of African Americans in the excluded cohort was 26%, versus 27% in the included cohort. In addition, the proportion of patients with a history of other ACE inhibitor exposure in the excluded cohort was 21%, versus 22% in the included cohort. Thus, the excluded cohort was similar to the included cohort. Thereafter, we allocated 1,125 patients at random to the derivation set and the remaining 567 to the validation set (Table 2) Differences in patient characteristics were not significant across the groups. ACE inhibitor-induced cough developed in 12% (202/1,692) of patients in the derivation and validation sets combined.
Table 2.
Patient Characteristics in the Derivation and Validation Sets for Patients Prescribed ACE inhibitors
| Characteristic | Derivation Set (N= 1,125) | Validation Set (N= 567) |
|---|---|---|
| Mean age, y ± SD | 58.9 ± 13.9 | 57.9 ± 14.1 |
| Male, n(%) | 476 (42) | 210 (37) |
| Ethnicity | ||
| Caucasian, n(%) | 498 (44) | 232 (41) |
| African American, n(%) | 303 (27) | 160 (28) |
| Latino, n(%) | 175 (16) | 103 (18) |
| East Asian (Chinese, Korean, Japanese), n(%) | 17 (1.5) | 4 (0.7) |
| Smoking Status | ||
| Current smoker, n(%) | 125 (11) | 72 (13) |
| Past smoker, n(%) | 284 (25) | 154 (27) |
| Never smoked, n(%) | 716 (64) | 341 (60) |
| History of other ACE inhibitors, n(%) | 271 (24) | 108 (19) |
| History of ACE inhibitor-induced cough, n(%) | 24 (2) | 8 (1) |
| History of ACE inhibitor-induced angioedema, n(%) | 1 (0.1) | 1 (0.2) |
| Medical Conditions | ||
| Hypertension, n(%) | 960 (85) | 482 (85) |
| Diabetes mellitus, n(%) | 407 (36) | 197 (35) |
| Coronary artery disease, n(%) | 224 (20) | 106 (19) |
| Congestive heart failure, n(%) | 86 (8) | 48 (8) |
| Chronic obstructive pulmonary disease, n(%) | 39 (3) | 24 (4) |
| Asthma, n(%) | 119 (11) | 50 (9) |
| Other respiratory diseases, n(%) | 15 (1) | 9 (2) |
| Depression/anxiety, n(%) | 314 (28) | 163 (29) |
| Other psychiatric diseases, n(%) | 42 (4) | 27 (5) |
| Hemodialysis, n(%) | 14 (1) | 6 (1) |
| Creatinine ≥1.6 mg/dL, n(%) | 66 (6) | 29 (5) |
| Concurrent Medications | ||
| Diuretics, n(%) | 436 (39) | 208 (37) |
| Beta-blockers, n(%) | 408 (36) | 208 (37) |
| Calcium antagonists, n(%) | 174 (15) | 81 (14) |
| Low dose (≤325 mg/day) aspirin, n(%) | 373 (33) | 177 (31) |
| High dose (>325 mg/day) aspirin, n(%) | 1 (0.1) | 2 (0.4) |
| Nonsteroidal anti-inflammatory drugs, n(%) | 178 (16) | 98 (17) |
| Cyclo-oxygenase-2 inhibitors, n(%) | 64 (6) | 28 (5) |
| ACE inhibitor-induced cough, n(%) | 130 (12) | 72 (13) |
ACE, angiotensin-converting enzyme; SD, standard deviation.
Univariate and Multivariate Analysis
Univariate correlates of ACE inhibitor-induced cough in the derivation set included age, gender, ethnicity, history of ACE inhibitor-induced cough, hypertension, diabetes mellitus, depression or anxiety, and creatinine equal to or greater than 1.6 mg/dL (Table 3) History of other ACE inhibitors was not significantly correlated with ACE inhibitor-induced cough (P>.1), but we included this variable in the multivariate analysis because of our predetermined hypothesis. In the stepwise logistic regression model for ACE inhibitor-induced cough, the variables listed in Table 4 were retained as independent predictors (P < .05) of ACE inhibitor-induced cough.
Table 3.
Univariate Correlates of ACE Inhibitor-Induced Cough in the Derivation Set
| Variable | Cough (N= 130) | No Cough (N= 995) | P Value * |
|---|---|---|---|
| Age | <.001 | ||
| 49 or less, n(%) | 20 (15) | 282 (28) | |
| 50 to 59, n(%) | 31 (24) | 288 (29) | |
| 60 to 69, n(%) | 43 (33) | 197 (20) | |
| 70 or greater, n(%) | 36 (28) | 228 (23) | |
| Male, n(%) | 33 (25) | 443 (45) | <.001 |
| Ethnicity | .06 | ||
| White, n(%) | 61 (47) | 437 (44) | |
| African-American, n(%) | 25 (19) | 278 (28) | |
| Latino, n(%) | 18 (14) | 157 (16) | |
| East Asian (Chinese, Korean, Japanese), n(%) | 4 (3) | 13 (1) | |
| Smoking Status | .4 | ||
| Current smoker, n(%) | 13 (10) | 112 (11) | |
| Past smoker, n(%) | 39 (30) | 245 (25) | |
| History of other ACE inhibitors, n(%) | 28 (22) | 243 (24) | .5 |
| History of ACE inhibitor-induced cough, n(%) | 15 (12) | 9 (0.9) | <.001 |
| History of ACE inhibitor-induced angioedema, n(%) | 0 (0) | 1 (0.1) | .7 |
| Medical Conditions | |||
| Hypertension, n(%) | 120 (92) | 840 (84) | .02 |
| Diabetes mellitus, n(%) | 39 (30) | 368 (37) | .1 |
| Coronary artery disease, n(%) | 30 (23) | 194 (20) | .3 |
| Congestive heart failure, n(%) | 10 (8) | 76 (8) | 1.0 |
| Chronic obstructive pulmonary disease, n(%) | 6 (5) | 33 (3) | .4 |
| Asthma, n(%) | 16 (12) | 103 (10) | .5 |
| Other respiratory diseases, n(%) | 1 (0.8) | 14 (1.4) | .6 |
| Depression/anxiety, n(%) | 44 (34) | 270 (27) | .1 |
| Other psychiatric diseases, n(%) | 4 (3) | 38 (4) | .7 |
| Hemodialysis, n(%) | 1 (0.8) | 13 (1.3) | .6 |
| Creatinine ≥1.6 mg/dL, n(%) | 3 (2) | 63 (6) | .07 |
| Concurrent Medications | |||
| Diuretics, n(%) | 44 (34) | 392 (39) | .2 |
| Beta-blockers, n(%) | 53 (41) | 355 (36) | .3 |
| Calcium antagonists, n(%) | 19 (15) | 155 (16) | .8 |
| Low dose (≤325 mg/day) aspirin, n(%) | 46 (35) | 327 (33) | .6 |
| High dose (>325 mg/day) aspirin, n(%) | 0 (0) | 1 (0.1) | .7 |
| Nonsteroidal anti-inflammatory drugs, n(%) | 22 (17) | 156 (16) | .7 |
| Cyclo-oxygenase-2 inhibitors, n(%) | 7 (5) | 57 (6) | .9 |
t tests were used for age; χ2tests were used for other variables.
ACE, angiotensin-converting enzyme.
Table 4.
Independent Predictors Identified by Multivariate Analysis
| Crude | Adjusted | |||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | Beta | Odds Ratio (95% CI) | Points * |
| Intercept | 4. | |||
| Age, 60 to 69 | 2.0 (1.3 to 3.0) | 0.75 | 2.1 (1.3 to 3.4) | 3 |
| Age, 70 or more | 1.3 (0.9 to 1.9) | 0.56 | 1.7 (1.1 to 2.8) | 2 |
| Female | 2.4 (1.6 to 3.6) | 0.83 | 2.3 (1.5 to 3.5) | 3 |
| Neither African-American nor East Asian | 1.4 (0.9 to 2.2) | 0.56 | 1.7 (1.1 to 2.9) | 2 |
| East Asian (Chinese, Korean, Japanese) | 2.4 (0.8 to 7.5) | 1.46 | 4.3 (1.2 to 14.8) | 6 |
| No history of other ACE inhibitors | 1.2 (0.8 to 1.8) | 0.88 | 2.4 (1.3 to 4.4) | 3 |
| History of ACE inhibitor-induced cough | 14 (6.1 to 33.4) | 3.38 | 29 (10.4 to 82.5) | 13 |
The risk score for an individual patient was determined by assigning points for each factor present and summing. The resulting risk score was then used in Table 5 to estimate the probability of ACE inhibitor-induced cough. The reference group for variable “Age, 60 to 69” is the group “Age less than 60 or equal to or greater than 70.” That for “Age, 70 or more” is the group “Age less than 70.” That for “Neither African-American nor East Asian” is the group “African-American or East Asian.” That for “East Asian” is the group “Ethnicity other than African-American or East Asian.” Model R2was 0.14.
Calculated by dividing the beta coefficient by 0.26 and rounding to the nearest integer.
ACE, angiotensin-converting enzyme; CI, confidence interval.
Development of the Clinical Prediction Rule
To develop the clinical prediction rule, we assigned each of the 7 identified risk factors an integer score proportional to the beta coefficient (Table 4). For each patient, all applicable risk score values were summed to attain a total risk score for that patient. This rule was then used to categorize the patients in the derivation set into 4 risk groups with varying likelihood of ACE inhibitor-induced cough (Table 5) ): 1) those patients with approximately half the risk of the entire cohort (low-risk group); 2) those with similar risk to the entire cohort (average-risk group); 3) those with approximately twice the risk (intermediate-risk group); 4) those with extremely high risk (high-risk group). As a result, the low-risk group was defined by a risk score of 5 or less, the average-risk group by a score of 6 to 8, the intermediate-risk group by a score of 9 to 11, and the high-risk group by a score of 12 or more. For example, a patient who was 65 years old (3 points), female (3 points), white (2 points), and with no history of other ACE inhibitors (3 points) or history of ACE inhibitor-induced cough (0 points) would have a risk score of 11, and would have roughly twice the baseline risk for ACE inhibitor-induced cough.
Table 5.
Performance of the Prediction Rule
| Risk Score | ||||
|---|---|---|---|---|
| Variable | 0 to 5 | 6 to 8 | 9 to 11 | ≥12 |
| Derivation set (N= 1,125), n(%) | ||||
| ACE inhibitor-induced cough developed | 24 (6) | 43 (9) | 45 (22) | 18 (55) |
| Did not develop | 392 (94) | 430 (91) | 158 (78) | 15 (45) |
| Validation set (N= 567), n(%) | ||||
| ACE inhibitor-induced cough developed | 7 (4) | 37 (14) | 22 (20) | 6 (60) |
| Did not develop | 172 (96) | 233 (86) | 86 (80) | 4 (40) |
When the threshold is score 6: positive predictive value (PPV) and negative predictive value (NPV) in the derivation set were 15% and 94%, respectively; PPV and NPV in the validation set was 17% and 96%, respectively. When the threshold is score 9: PPV and NPV in the derivation set were 27% and 93%, respectively; PPV and NPV in the validation set were 24% and 90%, respectively. When the threshold is score 12: PPV and NPV in the derivation set were 55% and 90%, respectively; PPV and NPV in the validation set were 60% and 88%, respectively.
ACE, angiotensin-converting enzyme.
Of 1,125 patients in the derivation set, 416 (37%) fell into the low-risk group (risk score ≤5). This low-risk group had a 6% probability (24/416) of ACE inhibitor-induced cough, whereas the high-risk group (risk score ≥12) had a 55% probability of ACE inhibitor-induced cough. Of the two intermediate-risk groups, the average-risk group (risk score, 6 to 8) had ACE inhibitor-induced cough rates of 9% and the intermediate-risk group (risk score, 9 to 11) had 22% (Table 5).
The rule performed well on ROC curve analysis for predicting ACE inhibitor-induced cough (area under the curve 0.71 ± 0.03;Fig. 1). Calibration of the model was tested on the entire cohort and proved satisfactory (Fig. 2Fig. 3).
FIGURE 1.

Receiver-operating characteristic (ROC) curves for ACE inhibitor-induced cough. The area under the curve was 0.71 ± 0.03 for the derivation set, and 0.68 ± 0.03 for the vali-dation set. The difference was not statistically significant (P= .4). The straight dashed diagonal line represents a test of no discriminative ability.
FIGURE 2.
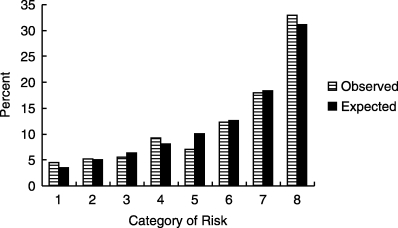
Observed versus predicted probability of ACE inhibitor-induced cough. Results were obtained by stratifying patients into 8 categories by level of risk. Derivation and validation sets were combined.
FIGURE 3.

Observed versus predicted incidence of ACE inhibitor-induced cough. Scatterplot allowing a visual assessment of the linearity of increasing event rates across risk groups. The straight dashed diagonal line represents perfect calibration and deviations from this line represent over- and underprediction of actual risk.
Validation
The validation set contained 567 patients who received ACE inhibitors, 72 of whom (13%) had ACE inhibitor-induced cough. In the validation set, the clinical prediction rule again stratified patients into different degrees of risk of developing ACE inhibitor-induced cough (Table 5). The low-risk group (risk score, 0 to 5) of 179 patients, accounting for 32% of the validation set, included only 7 (4%) patients who developed cough within 6 months. The average-risk group (risk score, 6 to 8) had ACE inhibitor-induced cough rates of 14%, the intermediate-risk group (risk score, 9 to 11) had 20%, and the high-risk group (risk score ≥12) had 60%. The area under the ROC curve for the derivation set was 0.71 ± 0.03 and that for the validation set was 0.68 ± 0.03 (Fig. 1), only a slight degradation in performance. This difference was not statistically significant (P= .4).
DISCUSSION
We developed and internally validated a clinical prediction rule for ACE inhibitor-induced cough. The independent predictors of ACE inhibitor-induced cough, all of which were available at the time of prescription, included being elderly, being female, being non-African American (with East Asian having highest risk), having no history of exposure to another ACE inhibitor, and having a history of cough due to another ACE inhibitor. A model based on these 6 factors allowed rapid classification of patients into groups with varying risks of cough due to ACE inhibitors.
Our findings are consistent with prior reports regarding individual risk factors associated with ACE inhibitor-induced cough. However, those studies differ from ours in definition of outcome, study design, and patient population, and did not consider multiple factors simultaneously or develop a prediction rule. In particular, most of the studies were byproducts of randomized controlled trials and, therefore, the risk factors were observed in patients of the treatment arm without adjustment for other potential risk factors. Several reports show that cough is more likely to develop among women on ACE inhibitors.10–14 From the data of Studies of Left Ventricular Dysfunction, the risk of cough due to enalapril was 2.4 times higher in women than men.14 Although the relative risk was calculated only from the treatment arm and without adjustment in Studies of Left Ventricular Dysfunction, it is similar to the odds ratio of 2.3 in our study. In addition, a report from postmarketing surveillance data showed that patients aged 65 to 79 had a higher incidence of adverse events associated with perindopril compared with younger patients,10 which also supports our results.
Chinese, Japanese, and African-American patients have also been reported to be at increased risk of ACE inhibitor-induced cough, compared with other groups.13,15–17 After adjustment for other covariates in our study, East Asian ethnicity remained a risk factor while African-American ethnicity was protective (negative risk). Several other risk factors for ACE inhibitor-induced cough have been reported, including renal insufficiency,19,20 diabetes mellitus,18,19 and nonsmoking status.12 In our study, however, these risk factors were not independent predictors of ACE inhibitor-induced cough.
Previous studies have suggested that concurrent use of nonsteroidal anti-inflammatory drugs,21,22 intermediate dose of aspirin,23 cyclo-oxygenase-2 inhibitors,24 and nifedipine 22 decreased the risk of ACE inhibitor-induced cough. In this study, we took into account the concurrent use of these drugs, but none of these drugs was independently associated with the outcome. Because our study was not a clinical trial, the dose and frequency of these drugs were not consistent.
Our prediction rule is based on demographics and whether the patient has previously taken or had a reaction to ACE inhibitors. Thus, using the rule in clinical practice to estimate the risk of ACE inhibitor-induced cough when prescribing these medications should be straightforward. For example, patients with a previous history of ACE inhibitor-induced cough would have a risk score of 13 points or more and probably not be given ACE inhibitors because they would have an approximately 60% probability of developing cough. In patients who develop dry cough while on ACE inhibitors for the first time, physicians can quantify the likelihood of that cough being caused by the ACE inhibitor. If the patient's risk score is low and the probability of ACE inhibitors being the cause is low, prompt work-up for other causes may be justified.
A major issue with clinical prediction rules is that physicians have found them difficult to use.27 However, this is likely to change soon, especially with computerization of prescribing.28 Computerization of medication ordering associated with decision support has been shown to reduce medication error rates,29,30 and it would be straightforward to make available rules such as this one using such applications. All the correlates in our study are simple, descriptive variables and a calculation could be performed in the background, with risk then being presented to clinicians at the time of ordering. For example, Bates and colleagues developed a clinical prediction rule for true positive blood cultures and implemented it in a computerized order entry system.28,31 They found that 28% of physicians changed their action according to the computerized guidance based on the prediction rule. Although the clinical prediction rule developed here is simple enough for physicians to calculate the risk manually, the increasing use of computerized prescribing makes it likely that this will be how such decision support is delivered in the future.32–34
Our results must be interpreted within the inherent limitations of the study design, which was a retrospective chart review. First, not all data may have been recorded. To be conservative, we strictly defined the criteria for presence of the primary outcome—ACE inhibitor-induced cough, that is, cough being too severe to continue the medication. However, we may have missed cases, either because they were mild, or because patients went to another physician outside the network. If anything, this suggests that the incidence we identified represents a lower bound. Also, the risk factor variables could be biased because records were incomplete, or because of subjective physician judgment. Random measurement error and misclassification can lead to dilution bias and underestimation of the effects of the tested risk factors. However, the factors in our prediction rule included age, gender, ethnicity, history of other ACE inhibitors, and history of ACE inhibitor-induced cough, all of which are recorded regularly and are relatively unlikely to be influenced by physician judgment. Second, one of the important factors in the final prediction rule was East Asian ethnicity. Although this group included only 21 patients, this factor has previously been found to be associated with ACE inhibitor-induced cough.15,16 Finally, because this study was conducted at an urban tertiary care hospital and the applicability of clinical prediction rule to other settings is always challenging,27,35 prospective validation of the rule in other settings should be performed, and its performance may not be as good in other settings.
In conclusion, we identified independent predictors of ACE inhibitor-induced cough and developed a decision rule that allows rapid estimation of risk of cough due to an ACE inhibitor within 6 months. This information may be valuable in clinical decision making regarding starting ACE inhibitors and considering withdrawal of an ACE inhibitor or the need for work-up for cough. Some patients are probably at sufficiently high risk that ACE inhibitors should be avoided altogether.
Acknowledgments
The authors would like to acknowledge Erin Hartman, MS, for her suggestions regarding the manuscript.
This work was supported in part by grants from the Pfizer Health Research Foundation and the Health Care Science Institute and grant R01-HS11169 from the Agency for Healthcare Research and Quality.
Dr. Morimoto received fellowship grant support from the St. Luke's Life Science Institute, Tokyo.
REFERENCES
- 1.Khalil ME, Basher AW, Brown EJ, Jr., Alhaddad IA. A remarkable medical story: benefits of angiotensin-converting enzyme inhibitors in cardiac patients. J Am Coll Cardiol. 2001;37:1757–64. doi: 10.1016/s0735-1097(01)01229-3. [DOI] [PubMed] [Google Scholar]
- 2.Halkin A, Keren G. Potential indications for angiotensin-converting enzyme inhibitors in atherosclerotic vascular disease. Am J Med. 2002;112:126–34. doi: 10.1016/s0002-9343(01)01001-4. [DOI] [PubMed] [Google Scholar]
- 3.Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 Summary. Advance Data from Vital and Health Statistics; No. 328. Hyattsville, Md: National Center for Health Statistics; 2002. [PubMed] [Google Scholar]
- 4.Sebastian JL, McKinney WP, Kaufman J, Young MJ. Angiotensin-converting enzyme inhibitors and cough. Prevalence in an outpatient medical clinic population. Chest. 1991;99:36–9. doi: 10.1378/chest.99.1.36. [DOI] [PubMed] [Google Scholar]
- 5.Simon SR, Black HR, Moser M, Berland WE. Cough and ACE inhibitors. Arch Intern Med. 1992;152:1698–700. [PubMed] [Google Scholar]
- 6.Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117:234–42. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- 7.Smyrnios NA, Irwin RS, Curley FJ, French CL. From a prospective study of chronic cough: diagnostic and therapeutic aspects in older adults. Arch Intern Med. 1998;158:1222–8. doi: 10.1001/archinte.158.11.1222. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi TK, Burstin HR, Cook EF, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15:149–54. doi: 10.1046/j.1525-1497.2000.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman E, Messerli FH, Neutel JM. Angiotensin II receptor blockers: equal or preferred substitutes for ACE inhibitors? Arch Intern Med. 2000;160:1905–11. doi: 10.1001/archinte.160.13.1905. [DOI] [PubMed] [Google Scholar]
- 10.Speirs C, Wagniart F, Poggi L. Perindopril postmarketing surveillance: a 12 month study in 47,351 hypertensive patients. Br J Clin Pharmacol. 1998;46:63–70. doi: 10.1046/j.1365-2125.1998.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore N, Noblet C, Joannides R, Ollagnier M, Imbs JL, Lagier G. Cough and ACE inhibitors. Lancet. 1993;341:61. doi: 10.1016/0140-6736(93)92543-3. [DOI] [PubMed] [Google Scholar]
- 12.Os I, Bratland B, Dahlof B, Gisholt K, Syvertsen JO, Tretli S. Female preponderance for lisinopril-induced cough in hypertension. Am J Hypertens. 1994;7:1012–5. doi: 10.1093/ajh/7.11.1012. [DOI] [PubMed] [Google Scholar]
- 13.Elliott WJ. Higher incidence of discontinuation of angiotensin converting enzyme inhibitors due to cough in black subjects. Clin Pharmacol Ther. 1996;60:582–8. doi: 10.1016/S0009-9236(96)90155-1. [DOI] [PubMed] [Google Scholar]
- 14.Kostis JB, Shelton B, Gosselin G, et al. Adverse effects of enalapril in the Studies of Left Ventricular Dysfunction (SOLVD). SOLVD Investigators. Am Heart J. 1996;131:350–5. doi: 10.1016/s0002-8703(96)90365-8. [DOI] [PubMed] [Google Scholar]
- 15.Woo KS, Norris RM, Nicholls G. Racial difference in incidence of cough with angiotensin-converting enzyme inhibitors (a tale of two cities) Am J Cardiol. 1995;75:967–8. doi: 10.1016/s0002-9149(99)80703-6. [DOI] [PubMed] [Google Scholar]
- 16.Ishimitsu T, Yagi S, Ebihara A, et al. Long-term evaluation of combined antihypertensive therapy with lisinopril and a thiazide diuretic in patients with essential hypertension. Jpn Heart J. 1997;38:831–40. doi: 10.1536/ihj.38.831. [DOI] [PubMed] [Google Scholar]
- 17.Ajayi AA, Adigun AQ. Angioedema and cough in Nigerian patients receiving ACE inhibitors. Br J Clin Pharmacol. 2000;50:81–2. doi: 10.1046/j.1365-2125.2000.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malini PL, Strocchi E, Fiumi N, Ambrosioni E, Ciavarella A. ACE inhibitor-induced cough in hypertensive type 2 diabetic patients. Diabetes Care. 1999;22:1586–7. doi: 10.2337/diacare.22.9.1586. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Chiang YF, Tsai JC. Severe nonproductive cough and cough-induced stress urinary incontinence in diabetic postmenopausal women treated with ACE inhibitor. Diabetes Care. 2000;23:427–8. doi: 10.2337/diacare.23.3.427. [DOI] [PubMed] [Google Scholar]
- 20.Keane WF, Polis A, Wolf D, Faison E, Shahinfar S. The long-term tolerability of enalapril in hypertensive patients with renal impairment. Nephrol Dial Transplant. 1997;12(suppl 2):75–81. [PubMed] [Google Scholar]
- 21.Gilchrist NL, Richards AM, March R, Nicholls MG. Effect of sulindac on angiotensin converting enzyme inhibitor-induced cough: randomised placebo-controlled double-blind cross-over study. J Hum Hypertens. 1989;3:451–5. [PubMed] [Google Scholar]
- 22.Fogari R, Zoppi A, Tettamanti F, Malamani GD, Tinelli C, Salvetti A. Effects of nifedipine and indomethacin on cough induced by angiotensin-converting enzyme inhibitors: a double-blind, randomized, cross-over study. J Cardiovasc Pharmacol. 1992;19:670–3. [PubMed] [Google Scholar]
- 23.Tenenbaum A, Grossman E, Shemesh J, Fisman EZ, Nosrati I, Motro M. Intermediate but not low doses of aspirin can suppress angiotensin-converting enzyme inhibitor-induced cough. Am J Hypertens. 2000;13:776–82. doi: 10.1016/s0895-7061(00)00268-5. [DOI] [PubMed] [Google Scholar]
- 24.Knox AJ, Pang L. Tackling ACE inhibitor cough. Lancet. 1997;350:814. doi: 10.1016/S0140-6736(05)62610-4. [DOI] [PubMed] [Google Scholar]
- 25.Tu JV, Naylor CD. Clinical prediction rules. J Clin Epidemiol. 1997;50:743–4. doi: 10.1016/s0895-4356(97)89028-2. [DOI] [PubMed] [Google Scholar]
- 26.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–98. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 27.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA. 1997;277:488–94. [PubMed] [Google Scholar]
- 28.Bates DW, Lee TH. Rapid classification of positive blood cultures. Prospective validation of a multivariate algorithm. JAMA. 1992;267:1962–6. [PubMed] [Google Scholar]
- 29.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–6. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 30.Raschke RA, Gollihare B, Wunderlich TA, et al. A computer alert system to prevent injury from adverse drug events: development and evaluation in a community teaching hospital. JAMA. 1998;280:1317–20. doi: 10.1001/jama.280.15.1317. [DOI] [PubMed] [Google Scholar]
- 31.Wang SJ, Kuperman GJ, Ohno-Machado L, Onderdonk A, Sandige H, Bates DW. Using electronic data to predict the probability of true bacteremia from positive blood cultures. Proc AMIA Symp. 2000:893–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman L, Cook EF, Brand DA, et al. A computer protocol to predict myocardial infarction in emergency department patients with chest pain. N Engl J Med. 1988;318:797–803. doi: 10.1056/NEJM198803313181301. [DOI] [PubMed] [Google Scholar]
- 33.Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to the emergency departments with acute chest pain. N Engl J Med. 1996;334:1498–504. doi: 10.1056/NEJM199606063342303. [DOI] [PubMed] [Google Scholar]
- 34.Bates DW, Ebell M, Gotlieb E, Zapp J, Mullins HC. A proposal for electronic medical records in U.S. primary care. J Am Med Inform Assoc. 2003;10:1–10. doi: 10.1197/jamia.M1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randolph AG, Guyatt GH, Calvin JE, Doig G, Richardson WS. Understanding articles describing clinical prediction tools. Evidence Based Medicine in Critical Care Group. Crit Care Med. 1998;26:1603–12. doi: 10.1097/00003246-199809000-00036. [DOI] [PubMed] [Google Scholar]


