Abstract
BACKGROUND
Diabetes causes 45% of incident end-stage renal disease (ESRD). Risk of progression is higher in those with clinical risk factors (albuminuria and hypertension), and in ethnic minorities (including blacks, Asians, and Latinos). Angiotensin-converting enzyme inhibitors (ACE) and angiotensin receptor blockers (ARB) slow the progression of diabetic nephropathy, yet little is known about their use among patients at high risk for progression to ESRD.
OBJECTIVES
To examine the prevalence of ACE or ARB (ACE/ARB) use overall and within patients with high-risk clinical indications, and to assess for ethnic disparities in ACE/ARB use.
DESIGN
Observational cohort study.
SETTING
Kaiser Permanente Northern California (KPNC) Diabetes Registry, a longitudinal registry that monitors quality and outcomes of care for all KPNC patients with diabetes.
PATIENTS
Individuals (N= 38,887) with diabetes who were continuously enrolled with pharmacy benefits during the year 2000, and had self-reported ethnicity data on survey.
INTERVENTIONS AND MEASUREMENTS
Pharmacy dispensing of ACE/ARB.
RESULTS
Forty-one percent of the cohort had both hypertension and albuminuria, 30% had hypertension alone, and 12% had albuminuria alone. Fourteen percent were black, 11% Latino, 13% Asian, and 63% non-Latino white. Overall, 61% of the cohort received an ACE/ARB. ACE/ARB was dispensed to 74% of patients with both hypertension and albuminuria, 64% of those with hypertension alone, and 54% of those with albuminuria alone. ACE/ARB was dispensed to 61% of whites, 63% of blacks, 59% of Latinos, and 60% of Asians. Among those with albuminuria alone, blacks were significantly (P = .0002) less likely than whites to receive ACE/ARB (47% vs 56%, respectively). No other ethnic disparities were found.
CONCLUSIONS
In this cohort, the majority of eligible patients received indicated ACE/ARB therapy in 2000. However, up to 45% to 55% of high-risk clinical groups (most notably individuals with isolated albuminuria) were not receiving indicated therapy. Additional targeted efforts to increase use of ACE/ARB could improve quality of care and reduce ESRD incidence, both overall and in high-risk ethnic groups. Policymakers might consider use of ACE/ARB for inclusion in diabetes performance measurement sets.
Keywords: diabetes, renal disease, ACE inhibitors, angiotensin receptor blockers, secondary prevention
In 1999, over 38,000 individuals, approximately 45% of all incident end-stage renal disease (ESRD) cases, initiated dialysis for diabetes-related renal failure.1 The doubling of ESRD incidence over the past decade 1 is due almost entirely to diabetic nephropathy and parallels the worsening “epidemic” of diabetes in the United States, where an estimated 7% of the adult population has diagnosed diabetes.2 At current rates of growth, over 175,000 individuals will receive a new diagnosis of ESRD in 2010 with the majority of cases attributable to diabetes.1,3 Once renal replacement therapy is initiated, those with diabetes are more likely to develop complications, be hospitalized, or die than those without diabetes.1,3,4
Known clinical risk factors for progression of nephropathy include hypertension and albuminuria. In addition, several ethnic minority groups are known to be at higher risk to develop ESRD due to diabetes.1,5–9 Nationwide, the incidence of diabetes-related ESRD is 4 times higher among blacks, 1.5 times higher among Asians, and 2 times higher among Latinos, when compared to the incidence among whites.1,3,10 Previous research from the population on which the present study is based 9 suggests that even in a population with uniform access to care, statistically and clinically significant excess ESRD incidence among minorities remains even after adjustment for differences in clinical, socioeconomic, behavioral, and other access to care confounders.
While ESRD can be a devastating complication of diabetes, effective prevention exists. Treatment of high-risk diabetes patients with an ACE inhibitor (ACE) or angiotensin receptor blocker (ARB) can reduce the risk of progression to ESRD by 23% to 50%.11–15 Since 1997, professional society clinical guidelines and Kaiser Permanente Clinical Practice Guidelines (April 1998) have recommended the use of ACE or ARB for 2 high-risk groups of patients with diabetes: those with hypertension and those with albuminuria (microalbuminuria or overt nephropathy).16,17 Although guidelines do not make special recommendations for ACE or ARB use in high-risk ethnic minorities, prior literature has shown important ethnic disparities in the use of other effective treatments.18–27
Despite evidence of efficacy and the existence of guideline recommendations, little is known about rates of ACE or ARB (ACE/ARB) use among patients at high risk for progression of nephropathy. In this study, we assessed the prevalence of ACE/ARB use, and ethnicity-based differences in use, among patients with diabetes and among those subgroups at higher risk of progression to ESRD based on additional clinical risk factors.
METHODS
Setting
Kaiser Permanente Northern California (KPNC) is a fully integrated, nonprofit, group practice, prepaid health plan providing comprehensive medical services to over 3 million members throughout Northern California (25% to 30% of the region's population). Plan members are mainly employed individuals and approximate the general population ethnically and socioeconomically except at the extremes of the income distribution.28–30
The Kaiser Permanente Northern California Diabetes Registry was established in 1993 to monitor the quality of care and health outcomes for plan members with diabetes. Details regarding the methodology used for this diabetes registry have been published previously.9,31–40 The registry is updated annually by identifying all plan members with diabetes from automated databases of pharmacy data, laboratory data, hospitalization records, and outpatient diagnoses. A chart review study in 1996 estimated a 2% false positive rate and a validation study in 2000 found the registry was 99% sensitive for detecting members with diabetes. Between 1994 and 1996, the ~98,000 noninstitutionalized diabetic members over 18 years of age were surveyed (in English or Spanish) to assess key patient-specific history and risk factors. The survey had a response rate of 83% and provided information on ethnicity, information needed to classify diabetes type, education, lifestyle and behavioral risk factors, and diabetes family history.
Study Cohort
Our study cohort included active KPNC members with diabetes who had continuous plan enrollment with pharmacy benefits for at least 10 months during the year 2000 and had responded previously to the patient survey (sole source of ethnicity data). From these 50,989, we excluded those missing data on diabetes type (n= 2,225) and those missing laboratory data on albuminuria status (n= 3,706). Members whose prescription copayment exceeded 80% of medication cost (n= 700) were excluded to minimize bias related to underascertaining medication use among individuals with high out-of-pocket costs who may obtain their medications at non-Kaiser pharmacies. We also excluded those who reported “other” ethnic origin or various mixed heritages (n= 6,087) because it is difficult to make meaningful inferences about this heterogenous group, and difficult to report on the individual subgroups because of small sample sizes.
Data Sources and Definitions
Patient characteristics were ascertained on or prior to December 31, 1999, from clinical, laboratory, administrative, and survey records. The outcome of interest was at least one dispensing of an ACE/ARB at any time during the calendar year 2000. We assumed that contraindications to treatment would be rare because most individuals not tolerating an ACE can receive an ARB with a low likelihood of side effects.41,42
Individuals with diabetes were separated into 4 clinical risk strata: those with hypertension only, those with albuminuria only, those with both hypertension and albuminuria, and those with neither risk factor. We hypothesized that individuals with 2 clinical indications would have higher rates of ACE/ARB use than those with a single clinical indication, and those with neither indication would have the lowest rates of use. Among those with a single clinical indication, we hypothesized that those with albuminuria would have higher rates of ACE/ARB use than those with hypertension as their indication, reflecting the strength of guideline support for ACE use in these groups as well as multiple alternative therapy options for hypertension, but not albuminuria.16,43 A patient was classified as having hypertension based on either self-report on survey or a diagnosis coded on an outpatient encounter form. A patient was classified as having albuminuria based on laboratory data demonstrating albumin excretion >30 mg/24 hours or >20 µg/minute on timed urine specimen, or an albumin to creatinine ratio >30 mg/g on a spot urine specimen. Throughout this report, we use the term albuminuria to describe any abnormal urinary albumin excretion including microalbuminuria or macroalbuminuria. When albuminuria was more stringently defined as those with 2 or more positive urine tests, rates of ACE/ARB use were 3% to 6% higher, but because ACE/ARB can reverse albuminuria on subsequent testing, we report rates of use using the less stringent definition.
Ethnicity was determined by self-report on survey and categorized as black, Latino, Asian, and non-Latino white. Patients were also classified according to age, gender, and educational attainment (high school or less, some college, or college graduate). Diabetes type was determined based upon a previously described algorithm 38 incorporating age and BMI (obesity) at diagnosis, use of insulin, and length of time between diagnosis and insulin initiation. Other disease-related factors examined included duration since diabetes diagnosis (<10 years, ≥10 years), and level of glycemic control as measured by hemoglobin A1c (<7.0, 7.0 to 7.9, 8.0 to 9.5, or ≥9.5%).
Analyses
We tabulated patient characteristics and prevalence of ACE/ARB use for the entire cohort, and for each clinical risk stratum (hypertension alone, albuminuria alone, hypertension and albuminuria, and neither risk factor). For the overall cohort and within each clinical risk stratum, we also calculated the prevalence of ACE/ARB use for each racial/ethnic group. We assessed the statistical significance of all comparisons using analysis of variance for continuous variables and Wald χ2tests for categorical variables using whites as the reference group for ethnic contrasts. We did not risk-adjust ethnic comparisons because we were evaluating ACE/ARB use as a clinical performance standard (i.e., process measure), rather than making inferences about the causes of differences. All analyses were performed using SAS, version 8.0 (SAS Institute, Cary, NC), with two-tailed P values less than or equal to .05 considered statistically significant.
RESULTS
There were 38,887 eligible individuals with diabetes in the study cohort. Approximately half were over the age of 65, and half were female (Table 1). The vast majority had type 2 diabetes. Eighty-three percent of the cohort had at least one clinical indication, identified by guidelines, for ACE/ARB: 41% of the sample had both hypertension and albuminuria, 30% had hypertension alone, and 12% had albuminuria alone. Thirty-seven percent of the cohort comprised high-risk ethnic minority groups: 14% of the sample (n= 5,310) was black, 11% (n= 4,063) Latino, 13% (n= 5,028) Asian, and 63% (n= 24,486) were non-Latino white.
Table 1.
Baseline Characteristics Overall and by Clinical Risk Group
| Clinical Risk Group | |||||
|---|---|---|---|---|---|
| Characteristic | Neither | HTN | Alb | HTN and Alb | |
| Sample size | 38,887 | 6,557 | 11,585 | 4,741 | 16,004 |
| % | |||||
| Age, y | |||||
| 18–40 | 3.9 | 9.9 | 1.4 | 8.2 | 2.0 |
| 41–65 | 47.0 | 56.8 | 45.0 | 53.9 | 42.4 |
| 65–79 | 41.9 | 29.1 | 46.2 | 32.6 | 46.8 |
| ≥80 | 7.1 | 4.2 | 7.4 | 5.4 | 8.7 |
| Female | 47.8 | 43.3 | 51.5 | 44.9 | 47.8 |
| Ethnicity | |||||
| White | 63.0 | 65.0 | 65.1 | 60.9 | 61.2 |
| Black | 13.7 | 9.2 | 14.8 | 10.4 | 15.6 |
| Latino | 10.5 | 12.0 | 8.7 | 13.5 | 10.2 |
| Asian | 12.9 | 13.9 | 11.4 | 15.2 | 13.0 |
| Education | |||||
| ≤ High school | 38.3 | 31.6 | 39.3 | 35.6 | 41.2 |
| Some college | 28.6 | 29.2 | 28.6 | 27.5 | 28.7 |
| ≥ College | 24.0 | 28.4 | 24.8 | 23.4 | 21.9 |
| Missing | 9.0 | 10.8 | 7.4 | 13.5 | 8.2 |
| Diabetes type, % | |||||
| Type 1 | 5.2 | 11.7 | 2.3 | 9.4 | 3.3 |
| Type 2 | 92.3 | 84.1 | 96.0 | 87.2 | 94.6 |
| Unclear | 2.5 | 1.8 | 1.8 | 3.4 | 2.1 |
| Diabetes duration ≥10 years | 50.0 | 44.5 | 44.1 | 51.5 | 56.1 |
HTN, hypertension; Alb, albuminuria.
Several characteristics differed notably between ethnic groups (Table 2). Whites were significantly older and had significantly better glycemic control than any of the other ethnic groups. The prevalence of hypertension was significantly higher among blacks than among the other ethnicities. Compared to the other ethnic groups, Latinos were less likely and Asians were more likely to have had education past high school.
Table 2.
Baseline Characteristics by Ethnicity
| Characteristic | White | Black | Latino | Asian |
|---|---|---|---|---|
| Sample size | 24,486 | 5,310 | 4,063 | 5,028 |
| % | ||||
| Age, y | ||||
| 18–40 | 4.0 | 3.5 | 4.5 | 3.6 |
| 41–65 | 42.7 | 54.5 | 52.4 | 55.9 |
| 65–79 | 44.4 | 36.7 | 39.6 | 37.0 |
| ≥80 | 8.9 | 5.3 | 3.5 | 3.6 |
| Female | 46.3 | 53.6 | 48.6 | 48.3 |
| Education | ||||
| ≤ High school | 38.2 | 37.5 | 54.3 | 27.1 |
| Some college | 30.0 | 34.6 | 21.0 | 21.6 |
| ≥ College | 24.7 | 17.6 | 10.2 | 38.7 |
| Missing | 7.1 | 10.3 | 14.5 | 12.7 |
| Hypertension | 70.8 | 79.4 | 65.0 | 67.6 |
| Albuminuria | 51.8 | 56.4 | 55.9 | 55.7 |
| Diabetes type, % | ||||
| Type 1 | 6.9 | 2.5 | 2.2 | 1.7 |
| Type 2 | 90.4 | 94.3 | 96.1 | 96.6 |
| Unclear | 2.7 | 3.2 | 1.7 | 1.7 |
| Glycemic control (HbA1c %) | ||||
| <7.0 | 31.1 | 26.3 | 23.5 | 22.2 |
| 7.0–7.9 | 28.3 | 23.7 | 23.7 | 27.9 |
| 8.0–9.5 | 24.3 | 23.9 | 25.2 | 27.4 |
| ≥9.5 | 15.0 | 25.6 | 26.2 | 21.3 |
| Missing | 1.4 | 1.5 | 1.3 | 1.2 |
| Diabetes duration ≥10 years | 50.5 | 51.4 | 50.7 | 45.1 |
Overall, 61% of the study cohort received an ACE or ARB. An ACE/ARB was dispensed to 74% of patients with both hypertension and albuminuria, 64% of those with hypertension alone, 54% of those with albuminuria alone, and 23% of those with neither hypertension nor albuminuria (Fig. 1). In general, decreasing age and longer duration since diabetes diagnosis were associated with higher rates of ACE/ARB use in all of the clinical risk strata (data not shown).
FIGURE 1.
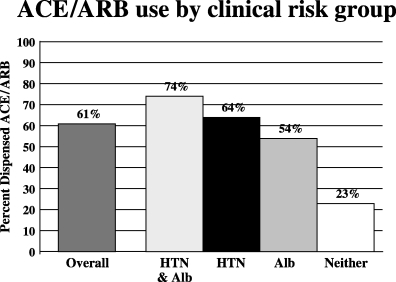
ACE/ARB use by clinical risk group. HTN, hypertension; Alb, presence of albuminuria.
There were no significant differences in the rates of ACE/ARB use among the different ethnic groups overall. An ACE or ARB was dispensed to 61% of whites, 63% of blacks, 59% of Latinos, and 60% of Asians (Fig. 2). Among the clinical risk strata, the only ethnic disparity was noted for those who had albuminuria alone as their indication for ACE/ARB treatment (Fig. 3); ACE/ARB was dispensed less frequently to blacks (46.6%) than to whites (55.8%) and this difference was statistically significant (odds ratio 0.69; 95% confidence interval, 0.57 to 0.84). No other racial/ethnic disparities in ACE/ARB use were noted.
FIGURE 2.
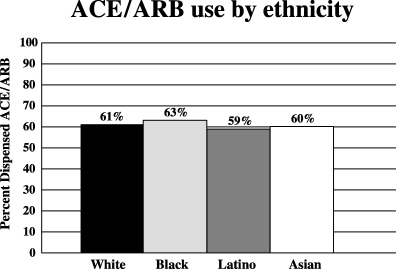
ACE/ARB use among ethnic groups (all patients).
FIGURE 3.
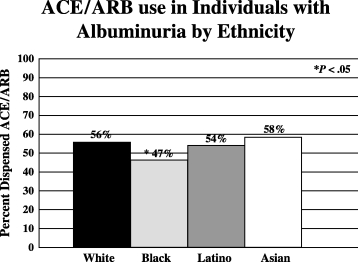
ACE/ARB use among ethnic groups with albuminuria. The asterisk indicates a significant (P < .05) difference in rates of ACE/ARB use compared to rates of use in whites with albuminuria in the absence of hypertension.
DISCUSSION
In a large cohort of health plan enrollees with diabetes, we found that between 55% and 75% of patients with important clinical risk factors were receiving ACE/ARB therapy to prevent progression of nephropathy. Among the high-risk clinical groups, ACE/ARB use was significantly lower in those with albuminuria alone than among those with hypertension (regardless of whether albuminuria was concomitantly present) as a risk factor. Additionally, within this high-risk group with the lowest rates of use (those with albuminuria alone), we found that blacks were less likely than whites (47% vs 56%) to receive ACE/ARB. No other racial/ethnic disparities were apparent.
To our knowledge, ours is the first large, population-based study to examine ACE/ARB use with data collected after guidelines started recommending ACE/ARB 16,17 for high-risk clinical subgroups with diabetes. Studies predating the wide dissemination of guidelines found rates of use ranging from 40% to 45% depending on the setting and clinical risk of the population.44–46 One study found that only 40% of high-risk patients with diabetes and no contraindication to therapy had ever been prescribed an ACE inhibitor.47 While rates of use in the current study are higher than reported in prior studies, between 25% and 45% of those with clear clinical indications for ACE/ARB were still not receiving therapy. Additionally, our results suggest that, despite clear guidelines for ACE/ARB use for patients with diabetes and albuminuria, physicians may not be as likely to recognize or treat isolated albuminuria with ACE/ARB as strongly as they are to treat hypertension with ACE/ARB.
Ours is the first study to examine the association between race/ethnicity and ACE/ARB use among individuals with diabetes. In contrast to prior studies,18–27 we found few racial/ethnic disparities in ACE/ARB use among patients with diabetes. There were no racial/ethnic disparities in ACE/ARB use among patients with isolated hypertension or combined hypertension and albuminuria. However, blacks with isolated albuminuria received ACE/ARB less frequently than whites. Some past ACE inhibitor trials suggested that blacks may not achieve the same degree of blood pressure reduction as whites,48,49 but reductions in cardiovascular and renovascular event rates occur independent of blood pressure lowering effects in individuals with diabetes.48,50 Because of this and hypertension guidelines that recommend ACE or ARB as the preferred first-line agents for blacks with diabetes or renal disease,51 we would not expect rates of use to be lower in the black population.
Our findings may have important implications for improving the quality of diabetes care. Our data suggest that more could be done to encourage ACE/ARB use particularly among normotensive diabetic patients with microalbuminuria. Efforts to increase ACE/ARB use in this group should improve overall quality of care and may also help to narrow the one racial gap we found in this clinical risk group.
Our results also highlight an important opportunity for quality measurement efforts such as the National Committee for Quality Assurance's (NCQA) Health Plan Employer Data and Information Set (HEDIS), which, in recent years, has focused on the quality of diabetes care. In 1998, the NCQA adopted 6 diabetes performance measures, including a measure for annual microalbuminuria screening. In 2000, only 41% of individuals with diabetes enrolled in HEDIS-reporting health plans were screened for albuminuria.52 Prior literature suggests that, of those screened and found to have microalbuminuria, only 40% are placed on ACE/ARB in response to a positive screening test.53 It appears, therefore, that a large number of patients who would potentially benefit from ACE/ARB use are not receiving indicated screening or indicated treatment. In addition, the indications in diabetes for renin-angiotensin blockade continue to expand with evidence of cardiac benefit for ACE 54–57 and for ARB,11,58 and likely renal benefits of ACE for normotensive, normoalbuminuric individuals.59–61 Given the expanding eligible population, low screening rates, and low treatment rates in response to positive screens, in the future it may be desirable to replace the current HEDIS measure of microalbuminuria screening with an alternative performance measure assessing ACE/ARB treatment for all patients with diabetes.
Our study had some limitations. Our sample excluded patients who failed to report their race/ethnicity on a survey administered during 1994–1996. However, past research suggests that ethnicity data are more reliable when obtained from self-report than from administrative data.62 The survey response rate was high (83%) but nonresponse bias cannot be excluded. Because rates of ACE/ARB use were comparable in all ethnic minorities except in blacks with albuminuria, it is possible that this represents a chance finding. While the registry had excellent completeness of laboratory follow-up (albuminuria screening done in 91% of the population compared to an average of 41% nationally 52), we did not estimate rates of ACE/ARB use among the 9% of patients who did not undergo screening for microalbuminuria. Because of incomplete ascertainment of key clinical factors, such as history of adverse reaction to ACE, we could not exclude all individuals with contraindications to treatment. However, because our end point was a composite of ACE or ARB use and contraindications to ARBs in those not tolerating ACE are rare,41,42 we would expect any bias to be small. Only 0.1% to 0.2% of patients develop ACE-related angioedema. Although this contraindication may be 3 times more frequent in blacks than in whites,63 the low incidence makes it an unlikely explanation of the racial disparity we observed. Our estimates may not generalize directly to other health care settings because KPNC enrolls an insured, predominantly employed population, and has quality improvement programs in place (such as guidelines and the use of physician opinion leaders) to improve ACE/ARB use in at-risk populations. By defining our outcome as a single ACE- or ARB-dispensing event during the year 2000, we were unable to estimate rates of sustained ACE/ARB use. We expect that sustained use would be lower because of nonadherence. Finally, we were unable to determine whether patients with clinical indications (i.e., albuminuria or hypertension) not using ACE/ARB were never prescribed these therapies by their physician, or that they were prescribed ACE/ARB but did not fill the prescription. It is unlikely, however, that the prevalence of failure to fill prescriptions would differ across the clinical risk groups we studied.
Conclusion
In summary, we found that between 55% and 75% of high-risk diabetic patients with known clinical indications for preventive therapy were treated with an ACE or ARB during 2000. Those with albuminuria as their sole clinical indication of increased risk were significantly less likely than those with hypertension to receive an ACE/ARB; and among those with albuminuria alone, blacks were significantly less likely than whites to receive ACE/ARB. No other significant racial/ethnic differences in ACE/ARB use were found. To further increase rates of ACE/ARB use and narrow the racial gap we observed, educational efforts to patients and physicians should highlight microalbuminuria as an important clinical indication for ACE/ARB. In addition, policymakers may want to consider designing a measure of ACE/ARB use for inclusion in future diabetes performance measurement sets.
Acknowledgments
Dr. Rosen was supported by an AHRQ Health Services Research Fellowship at the Harvard School of Public Health, grant number 5 T32 HS00020-16. Drs. Karter and Selby and Ms. Liu's work was supported by grants from the American Diabetes Association and the Kaiser Foundation Research Institute.
REFERENCES
- 1.U.S. Renal Data System. USRDS 2001 annual data report: atlas of end-stage renal disease in the United States. Bethesda, Md: National Institute of Diabetes and Digestive and Kidney Diseases; 2001. [Google Scholar]
- 2.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 3.Hostetter TH. Prevention of end-stage renal disease due to type 2 diabetes. N Engl J Med. 2001;345:910–2. doi: 10.1056/NEJM200109203451209. [DOI] [PubMed] [Google Scholar]
- 4.Marcelli D, Spotti D, Conte F, Limido A, Malberti F, Locatelli F. Prognosis of diabetic patients on dialysis: analysis of Lombardy Registry data. Nephrol Dial Transplant. 1995;10:1985–901. [PubMed] [Google Scholar]
- 5.Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med. 1989;321:1074–9. doi: 10.1056/NEJM198910193211603. [DOI] [PubMed] [Google Scholar]
- 6.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ. The excess incidence of diabetic end-stage renal disease among blacks: a population-based study of potential explanatory factors. JAMA. 1992;268:3079–84. [PubMed] [Google Scholar]
- 7.Krop JS, Coresh J, Chambless LE. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes. Arch Intern Med. 1999;159:1777–83. doi: 10.1001/archinte.159.15.1777. [DOI] [PubMed] [Google Scholar]
- 8.Powers DR, Wallin JD. End-stage renal disease in specific ethnic and racial groups: risk factors and benefits of antihypertensive therapy. Arch Intern Med. 1998;158:793–800. doi: 10.1001/archinte.158.7.793. [DOI] [PubMed] [Google Scholar]
- 9.Karter AJ, Ferrarra A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–27. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 10. NIDDK NIH, National Kidney Disease Education Program Available at: http://www.ncqa.org/somc2001/diabetes/cdc6_datx.html. Accessed September 12, 2002.
- 11.Brenner BM, Cooper ME, deZeeuw D, et al. Effect of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 12.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 13.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with neuropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 14.Ravid M, Lang R, Rachmani R, Lishner M. Long-term renoprotective effect of angiotensin-converting-enzyme inhibition in non-insulin-dependent diabetes mellitus. A 7-year follow-up study. Arch Intern Med. 1996;156:286–9. [PubMed] [Google Scholar]
- 15.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Amer P. Irbesartan in patients with type 2 diabetes and microalbuminuria study group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 16.Bennett PH, Haffner S, Kasiske BL, et al. Screening and management of microalbuminuria in patients with diabetes mellitus: recommendations to the Scientific Advisory Board of the National Kidney Foundation from an ad hoc committee of the Council on Diabetes Mellitus of the National Kidney Foundation. Am J Kidney Dis. 1995;25:107–12. doi: 10.1016/0272-6386(95)90636-3. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 1997;20:S5–S13. [Google Scholar]
- 18.Ayanian JZ, Udvarhelyi IS, Gatsonis CA, Pashos CL, Epstein AM. Racial differences in the use of revascularization procedures after coronary angiography. JAMA. 1993;269:2642–6. [PubMed] [Google Scholar]
- 19.Fiscella K, Franks P, Gold MR, Clancy C. Inequality in quality: addressing socioeconomic, racial and ethnic disparities in health care. JAMA. 2000;283:2579–84. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- 20.Escarce JJ, Epstein KR, Colby DC, Schwartz JS. Racial differences in the elderly's use of medical procedures and diagnostic tests. Am J Public Health. 1993;83:948–54. doi: 10.2105/ajph.83.7.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gornick ME, Eggers PW, Reilly TW, et al. Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med. 1996;335:791–9. doi: 10.1056/NEJM199609123351106. [DOI] [PubMed] [Google Scholar]
- 22.Hegarty V, Burchett BM, Gold DT, Cohen HJ. Racial differences in use of cancer prevention services among older Americans. J Am Geriatr Soc. 2000;48:735–40. doi: 10.1111/j.1532-5415.2000.tb04746.x. [DOI] [PubMed] [Google Scholar]
- 23.Peterson E, Shaw L, DeLong E, Pryor D, Califf R, Mark D. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? N Engl J Med. 1997;336:480–6. doi: 10.1056/NEJM199702133360706. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academy Press; 2002. [Google Scholar]
- 25.Schneider EC, Cleary PD, Zaslavsky AM, Epstein AM. Racial disparities in influenza vaccination: does managed care narrow the gap between African Americans and whites? JAMA. 2001;286:1455–60. doi: 10.1001/jama.286.12.1455. [DOI] [PubMed] [Google Scholar]
- 26.Schneider EC, Zaslavsky AM, Epstein AM. Racial disparities in the quality of care for enrollees in Medicare managed care. JAMA. 2002;287:1288–94. doi: 10.1001/jama.287.10.1288. [DOI] [PubMed] [Google Scholar]
- 27.Zaslavsky AM, Hochheimer JN, Schneider EC, et al. Impact of sociodemographic case mix on the HEDIS measures of health plan quality. Med Care. 2000;38:981–92. doi: 10.1097/00005650-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Gordon NP, Kaplan GA. Some evidence refuting the HMO “favorable selection” hypothesis: the case of Kaiser Permanente. Adv Health Econ Health Serv Res. 1991;12:19–29. [PubMed] [Google Scholar]
- 29.Hiatt RA, Friedman GD. Characteristics of patients referred for treatment of end-stage renal disease in a defined population. Am J Public Health. 1982;72:829–33. doi: 10.2105/ajph.72.8.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selby JV, Karter AJ, Ackerson LM, Ferrara A, Liu J. Developing a prediction rule from automated clinical databases to identify high-risk patients in a large population with diabetes. Diabetes Care. 2001;24:1547–55. doi: 10.2337/diacare.24.9.1547. [DOI] [PubMed] [Google Scholar]
- 32.Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20:1396–402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 33.Selby JV, Ettinger B, Swain B, Brown JB. First 20 months’ experience with use of Metformin for type 2 diabetes in a large health maintenance organization. Diabetes Care. 1999;22:38–44. doi: 10.2337/diacare.22.1.38. [DOI] [PubMed] [Google Scholar]
- 34.Selby JV. Linking automated databases for research in managed care settings. Ann Intern Med. 1997;127:719–24. doi: 10.7326/0003-4819-127-8_part_2-199710151-00056. [DOI] [PubMed] [Google Scholar]
- 35.Karter AJ, Rowell SE, Ackerson LM, et al. Excess maternal transmission of type 2 diabetes. The Northern California Kaiser Permanente Diabetes Registry. Diabetes Care. 1999;22:938–43. doi: 10.2337/diacare.22.6.938. [DOI] [PubMed] [Google Scholar]
- 36.Karter AJ, Newman B, Rowell S, et al. Large-scale collection of family history data and recruitment of informative families for genetic analysis. J Regist Manag. 1998;25:7–12. [Google Scholar]
- 37.Karter AJ, Ferrara A, Darbinian J, Ackerson LM, Selby JV. Self-monitoring of blood glucose: language and financial barriers in a managed care population with diabetes. Diabetes Care. 2000;23:477–83. doi: 10.2337/diacare.23.4.477. [DOI] [PubMed] [Google Scholar]
- 38.Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111:1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 39.Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–73. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 40.Ferrara A, Karter AJ, Ackerson LM, Liu JY, Selby JV. Hormone replacement therapy is associated with better glycemic control in women with type 2 diabetes: the Northern California Kaiser Permanente Diabetes registry. Diabetes Care. 2001;24:1144–50. doi: 10.2337/diacare.24.7.1144. [DOI] [PubMed] [Google Scholar]
- 41.Paster RZ, Snavely DB, Sweet AR, et al. Use of losartan in the treatment of hypertensive patients with a history of cough induced by angiotensin-converting enzyme inhibitors. Clin Ther. 1998;20:978–89. doi: 10.1016/s0149-2918(98)80079-9. [DOI] [PubMed] [Google Scholar]
- 42.Tanser PH, Campbell LM, Carranza JK, Toutouzas P, Watts R. for the Multicentre Cough Study Group. Candesartan cilexetil is not associated with cough in hypertensive patients with enalapril-induced cough. Am J Hypertens. 2000;13:214–8. doi: 10.1016/s0895-7061(99)00165-x. [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 1999;22:S32–S41. [PubMed] [Google Scholar]
- 44.Dunn EJ, Burton CJ, Feest TG. The care of patients with diabetic nephropathy: audit, feedback, and improvement. Q J Med. 1999;92:443–9. doi: 10.1093/qjmed/92.8.443. [DOI] [PubMed] [Google Scholar]
- 45.Gold JA. A word from WIPRO: improving use of ACE inhibitors in diabetic nephropathy. Wis Med J. 1996;95:588–9. [PubMed] [Google Scholar]
- 46.Scarsi KK, Bjornson DC. The use of ACE inhibitors as renoprotective agents in Medicaid patients with diabetes. Ann Pharmacother. 2000;34:1002–6. doi: 10.1345/aph.19346. [DOI] [PubMed] [Google Scholar]
- 47.Gordian ME, Kelly J. Why patients with diabetes, hypertension and/or proteinuria are NOT on angiotensin converting enzyme inhibitors. Alaska Med. 1998;40:51–4. [PubMed] [Google Scholar]
- 48.Flack JM, Mensah GA, Ferrario CM. Using angiotensin converting enzyme inhibitors in African-American hypertensives: a new approach to treating hypertension and preventing target-organ damage. Curr Med Res Opin. 2000;16:66–79. [PubMed] [Google Scholar]
- 49.Weir MR, Chrysant SG, McCarron DA, et al. Influence of race and dietary salt on the antihypertensive efficacy of an angiotensin-converting enzyme inhibitor or a calcium channel antagonist in salt-sensitive hypertensives. Hypertension. 1998;31:1088–96. doi: 10.1161/01.hyp.31.5.1088. [DOI] [PubMed] [Google Scholar]
- 50.Fenves A, Ram CV, et al. Are angiotensin converting enzyme inhibitors and angiotensin receptor blockers becoming the treatment of choice in African-Americans? Curr Hypertens Rep. 2002;4:286–9. doi: 10.1007/s11906-996-0006-y. [DOI] [PubMed] [Google Scholar]
- 51.Douglas JG, Bakris GL, Epstein M. for the Hypertension in African Americans Working Group. Management of high blood pressure in African Americans. Consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163:525–41. doi: 10.1001/archinte.163.5.525. [DOI] [PubMed] [Google Scholar]
- 52.National Committee for Quality Assurance (NCQA) The state of managed care quality. 2001 2002 Available at: http://www.ncqa.org/somc2001/diabetes/cdc6_datx.html. Accessed September 12.
- 53.Hueston WJ, Scibelli S, Mainous AG. Use of microalbuminuria testing in persons with type 2 diabetes: are the right patients being tested? J Fam Pract. 2001;50:669–73. [PubMed] [Google Scholar]
- 54.Gerstein HC, Mann JFE, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–6. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 55.Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 56.Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 57.Niskanen L, Hedner T, Hansson L, Lanke J, Niklason A. for the CAPPP Study Group. Reduced cardiovascular morbidity and mortality in hypertensive diabetic patients on first-line therapy with an ACE inhibitor compared with a diuretic/beta-blocker-based treatment regimen: a subanalysis of the Captopril Prevention Project. Diabetes Care. 2001;24:2091–6. doi: 10.2337/diacare.24.12.2091. [DOI] [PubMed] [Google Scholar]
- 58.Lindholm LH, Ibsen H, Dahlof B, et al. for the LIFE Study Group. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–10. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 59.Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. Ann Intern Med. 1998;128:982–8. doi: 10.7326/0003-4819-128-12_part_1-199806150-00004. [DOI] [PubMed] [Google Scholar]
- 60.Kvetny J, Gregersen G, Pedersen RS. Randomized placebo-controlled trial of perindopril in normotensive, normoalbuminuric patients with type 1 diabetes mellitus. Q J Med. 2001;94:89–94. doi: 10.1093/qjmed/94.2.89. [DOI] [PubMed] [Google Scholar]
- 61.The EUCLID study group. Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. Lancet. 1997;349:1787–92. [PubMed] [Google Scholar]
- 62.Boehmer U, Kressin NR, Berlowitz DR, et al. Self-reported vs administrative race/ethnicity data and study results. Am J Public Health. 2002;92:1471–2. doi: 10.2105/ajph.92.9.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vleeming W, van Amsterdam JGC, Stricker BHC, de Wildt DJ. ACE inhibitor-induced angioedema: incidence, prevention and management. Drug Saf. 1998;18:171–88. doi: 10.2165/00002018-199818030-00003. [DOI] [PubMed] [Google Scholar]


