Abstract
OBJECTIVES
To compare statin nonadherence and discontinuation rates of primary and secondary prevention populations and to identify factors that may affect those suboptimal medication-taking behaviors.
DESIGN
Retrospective cohort utilizing pharmacy claims and administrative databases.
SETTING
A midwestern U.S. university-affiliated hospital and managed care organization (MCO).
PATIENTS
Non-Medicaid MCO enrollees, 18 years old and older, who filled 2 or more statin prescriptions from January 1998 to November 2001; 2,258 secondary and 2,544 primary prevention patients were identified.
MEASUREMENTS
Nonadherence was assessed by the percent of days without medication (gap) over days of active statin use, a measurement known as cumulative multiple refill-interval gap (CMG). Discontinuation was identified by cessation of statin refills prior to the end of available pharmacy claims data.
RESULTS
On average, the primary and secondary groups went without medication 20.4% and 21.5% of the time, respectively (P = .149). Primary prevention patients were more likely to discontinue statin therapy relative to the secondary prevention cohort (relative risk [RR], 1.24; 95% confidence interval [CI], 1.08 to 1.43). Several factors influenced nonadherence and discontinuation. Fifty percent of patients whose average monthly statin copayment was <$10 discontinued by the end of follow-up (3.9 years), whereas 50% of those who paid >$10 but. $20 and >$20 discontinued by 2.2 and 1.0 years, respectively (RR, 1.39 and 4.30 relative to <$10 copay, respectively).
Keywords: adherence, copayment, discontinuation, HMGCoA reductase inhibitors, coroncary heart disease
The efficacy of HMG-CoA reductase inhibitors (statins) in reducing coronary heart disease (CHD)-associated morbidity and mortality is widely accepted 1–6. What may be less well known is that the absolute magnitude of the benefit attributable to statins is closely linked to the level of CHD risk in the patient population for whom they are prescribed. Among individuals with documented CHD, for example, the 4S study demonstrated that statin use prevents 1 CHD event for every 63 patients treated over the course of a year.7 Alternatively, for individuals with lower levels of CHD risk (i.e., with mildly elevated cholesterol levels), the AFCAPS/TexCAPS trial results in a number needed to treat of 429 patients to prevent 1 CHD event, excluding unstable angina, over 1 year.7 Due to these differences, the cost-effectiveness of statin therapy is highly dependent on the CHD risk of the patient population taking these agents. Thus, the incremental clinical and economic benefits of statins are enhanced when prescribed for those at higher levels of CHD risk.
Although the benefits of statin therapy are modified to a large extent by CHD risk, data are limited on whether adherence rates differ between primary and secondary prevention populations. The relationship between high adherence levels and improved CHD outcomes is documented in several populations with varying levels of CHD risk.8–10 However, the published data on statin adherence are largely derived from clinical trials and retrospective studies that enrolled either primary or secondary, but not both, prevention populations.1–6,11–13 While recent studies have reported differences in statin adherence rates among elderly patients at varying levels of CHD risk,14,15 they lack generalizability given their focus on older, publicly insured patients.
Because current evidence suggests that individuals treated for secondary prevention have relatively more to gain from statin therapy than those treated for primary prevention, our primary objective was to compare nonadherence and discontinuation rates in these 2 prevention populations. An additional aim was to identify modifiable factors that may affect those behaviors. In response to the mounting evidence suggesting that out-of-pocket expenditures are a barrier to adherence with prescription drugs,16–18 we assessed the role of patient copayment on statin nonadherence and discontinuation rates.
METHODS
Patient Population
We retrospectively reviewed the electronic medical and claims databases of a managed care organization (MCO) in the midwestern United States with approximately 200,000 enrollees. Individuals were considered eligible for the study if they were 18 years of age or older, a non-Medicaid MCO enrollee, and had filled at least 2 prescriptions for an HMG-CoA reductase inhibitor (statin) during the period of January 1, 1998 through November 30, 2001. Subjects were followed from the date of first statin prescription fill (index date) until: 1) disenrollment from the MCO; 2) switch to a non-statin antihyperlipidemic; 3) death; or 4) end of available pharmacy claims data.
Prevention Category
Through health system administrative databases, subjects were categorized as secondary prevention if they had either 1) a condition that the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)19 designated as “CHD or CHD risk equivalents” or 2) a cardiovascular event as defined by Health Plan Employer Data and Information Set (HEDIS®) 2002 “Criteria for Cholesterol Management After Acute Cardiovascular Event”20(Table 1)All other patients were classified as primary prevention.
Table 1.
Secondary Prevention Criteria
| Diagnosis or Procedure | ICD-9-CM, DRG, or CPT Codes |
|---|---|
| HEDIS® Criteria | |
| AMI | ICD-9-CM: 410.x |
| DRG: 121, 122 | |
| PTCA | ICD-9-CM: 36.01, 36.02, 36.05, 36.09 |
| DRG: 112 | |
| CPT: 92980-92982, 92984, 92995, 92996 | |
| CABG | ICD-9-CM: 36.1, 36.2 |
| DRG: 106, 107, 109 | |
| CPT: 33510-33514, 33516-33519, 33521-33523, 33533-33539 | |
| NCEP ATP III Criteria | |
| Chronic IHD | ICD-9-CM: 414.1x, 414.8, 414.9 |
| Coronary atheroschlerosis | ICD-9-CM: 414.0, 414.01, 414.02, 414.03, 414.04, 414.05 |
| Diabetes mellitus (Type I or II)* | ICD-9-CM: 250.x |
Use of insulin or oral antidiabetic agents also verified by pharmacy claims.
HEDIS®, Health Plan Employer Data and Information Set; NCEP ATP III, National Cholesterol Education Program; ICD-9-CM, International Classification of Diseases, Ninth Revision, DRG, Diagnosis Related Group; CPT, Current Procedural Terminology; AMI, Acute Myocardial Infarction; PTCA, Percutaneous Transluminal Coronary Angioplasty; CABC, Coronary Artery Bypass Graft; IHD, Ischemic Heart Disease.
A quality assurance protocol was developed a priori and conducted on the initially identified primary prevention cohort. A 10% random sample was drawn from the primary prevention group and medical records were thoroughly reviewed to assess the presence of the aforementioned secondary prevention criteria. The investigators agreed a priori that a misidentification threshold of 5% would be utilized to determine reliability of the secondary prevention identification protocol.
Outcomes
Two specific outcomes were measured: 1) nonadherence and 2) discontinuation of therapy.
Nonadherence.
Nonadherence was defined as a dichotomous variable based on a patient's cumulative multiple-refill interval gap (CMG). CMG was defined as the number of days without medication (gap) divided by days of active statin use, expressed as a percentage. CMG may range from 0% (indicating no gap days and total adherence) to 100% (indicating complete nonadherence) and has been shown to be a reliable estimate of patient adherence in previous studies utilizing pharmacy records.21,22 For this study, the time period of active statin use is between the date of first statin prescription fill and the date of last statin prescription fill. Patients were considered “nonadherent” if their CMG was greater than 10% (indicating more than 1 day without therapy out of every 10 days) and “adherent” if their CMG was less than 10%. Any oversupply obtained by the patient was allocated to subsequent treatment gaps unless a change in statin brand or dose occurred. Once a change in brand or dose was evident, all previously obtained oversupply was deemed unusable.
Discontinuation.
All patients who began statin therapy were assumed to require treatment for the remainder of their lives, regardless of their CHD risk level. Therefore, any gap in statin therapy, from the date of last prescription filled to the end of available pharmacy claims data, that could not be accounted for by the final prescription's days supply, any usable oversupply obtained up until that point, and/or a 7-day “grace period” was considered an inappropriate discontinuation of therapy. The following exceptions were allowed: 1) the patient was switched to a non-statin antihyperlipidemic agent (e.g., gemfibrozil, bile acid sequestrants); 2) the patient terminated enrollment in the MCO; or 3) the patient died. Patients who satisfied one of these exceptions were considered to have remained on their prescribed statin regimen. The grace period of 7 days is similar to that used in a previously published analysis of adherence that utilized pharmacy claims data.15
As with prior analyses of time to discontinuation of statin therapy,15 patients were required to have been enrolled in the MCO for at least 1 year prior to the date on which they had filled their first statin prescription. Patients who fulfilled these criteria were termed “statin-naive.”
Statistical Analysis
Demographic and clinical data were compared among the primary and secondary prevention populations with the χ2statistic or Fisher's exact test for categorical variables and with the Wilcoxon rank sum test for ordinal variables. A P value of less than .05 was considered significant for these comparative analyses.
A logistic regression model was used to determine the predictive ability of potential explanatory variables on the odds of a patient exhibiting nonadherent behavior (CMG > 10%). Potential predictive variables, identified through univariate analysis of each variable and CMG, included gender, race, marital status (a proxy for social support), whether the patient tried multiple brands or dosages of statin therapy, whether the patient was prescribed a multiple daily dose regimen, whether or not the patient was statin-naive, average number of cardiologist visits per year, average number of low-density lipoprotein (LDL) tests per year, average days supply, average monthly copayment, and prevention category. We performed forward stepwise selection, maintaining variables significant at a P value of .05 and requiring age to be included in the model regardless of significance. As the definition nonadherent is arbitrary, sensitivity analyses were performed with 2 additional logistic regression models allowing for nonadherent behavior to be more liberally redefined as CMG >20% and CMG >30%.
The probability of statin discontinuation over time for patients identified as statin-naive was determined using a Cox proportional hazards model adjusting for the aforementioned potential confounders. For this analysis, the dependent variable was time to discontinuation of statin, and patients who did not fulfill the criteria for inappropriate discontinuation of statin therapy (as defined earlier) were censored. The time to discontinuation was defined as the number of days from the index date until the date on which either the supply obtained with the last filled prescription ran out, all oversupply ran out, or the 7-day grace period expired, whichever came first. All statistical analyses were performed with SAS for Windows, release 8.02 (1999–2001, SAS Institute Inc., Cary, NC).
RESULTS
Study Population Characteristics
There were 4,802 patients who met the inclusion criteria (Table 2) Of these, 2,258 (47%) were identified as secondary prevention and the remaining 2,544 patients (53%) were categorized as primary prevention. The quality assurance protocol identified 5 patients (2%) out of 255 randomly selected primary prevention patients as being truly secondary prevention patients. This 2% misidentification rate fell well below the a priori defined threshold and therefore the composition of the cohorts was determined to be acceptable.
Table 2.
Population Characteristics
| Prevention Cohort | |||
|---|---|---|---|
| Primary(N= 2,544) | Secondary (N= 2,258) | P Value | |
| Demographics | |||
| Mean age, y (±SD) | 56.7 (12.9) | 63.2 (12.1) | <.0001 |
| Male, % | 52.9 | 59.5 | <.0001 |
| Race, % | |||
| White | 81.0 | 82.7 | <.0001 |
| African-American | 5.8 | 8.8 | |
| Other | 13.2 | 8.5 | |
| Married, % | 72.4 | 70.2 | NS |
| Statin naive, % | 54.0 | 54.4 | NS |
| Health Care Utilization | |||
| Cardiologist visits per year, mean (±SD) | 0.1 (0.7) | 1.5 (3.1) | <.0001 |
| Number of LDL tests per year, mean (±SD) | 2.4 (4.0) | 3.8 (5.5) | <.0001 |
| Prescription copayment, mean (±SD) | $9.92 (7.24) | $11.23 (8.07) | <.0001 |
| Medication Regimen | |||
| Single brand/strength regimens only, % | 64.3 | 53.2 | <.0001 |
| Once daily regimens only, % | 90.1 | 83.6 | <.0001 |
| Days supply count per Rx, mean (±SD) | 39.0 (18.4) | 39.3 (17.5) | NS |
SD, standard deviation; LDL, low-density lipoprotein; NS, not significant.
The population was predominately white (82%) and male (56%) with a mean age of 59.7 years. Patients in the secondary prevention cohort were approximately 6.5 years older than their primary prevention counterparts and more likely to be male. In terms of health care utilization, secondary prevention patients had more visits to a cardiologist and a greater number of LDL tests per year. Out-of-pocket statin prescription expenses were significantly higher for secondary prevention patients as compared to primary prevention patients ($11.23 vs $9.93; P < .0001). There was no difference in average days supply per prescription fill; however, the primary prevention group was more likely to be prescribed once daily dosing regimens and to remain on one brand and dose of statin throughout the observation period.
Nonadherence
There was no significant difference between the primary and secondary prevention cohorts in their mean CMG nonadherence measurement (20.4% vs 21.5%, respectively; P = .149). These CMG levels correspond to a patient not taking the prescribed statin approximately 1 out of every 5 days. Likewise, there was no significant difference between the prevention categories in the percentage of patients for whom a CMG greater than 10% was calculated (56.4% vs 56.0%; P= .793). This lack of difference holds true regardless of the definition of nonadherent behavior (Table 3)
Table 3.
Percent Nonadherence Observed over Various Definitions of Nonadherence
| Definition of Non-Adherence | |||
|---|---|---|---|
| CMG <10% | CMG <20% | CMG <30% | |
| Percent Meeting Nonadherent Definition | |||
| Prevention category | |||
| Secondary | 56.0 | 38.8 | 26.7 |
| Primary | 56.4 | 37.8 | 28.0 |
| Range of mean prescription copay * | |||
| <$10 | 49.3 | 28.6 | 22.2 |
| $10 to <$20 | 60.0 | 39.7 | 28.4 |
| $20+ | 76.2 | 59.4 | 45.1 |
| Overall | 56.2 | 38.3 | 27.3 |
χ2P < .001 within each definition of nonadherence. CMG, cumulative multiple refill-interval gap.
The factors associated with nonadherent behavior (CMG > 10%) are displayed in Table 4. Women, patients less than 65 years of age, and African Americans were more likely to be nonadherent. Patients receiving multiple doses per day, those treated with multiple brands or doses of a particular medication, and those receiving less than 2 months (i.e., 65 days) supply of a medication per prescription fill were also more likely to be nonadherent. Adherent behavior was positively associated with the average number of LDL tests a patient had per year.
Table 4.
Adjusted Odds Ratios for Nonadherence to Statin Therapy
| Definition of Non-Adherence | |||
|---|---|---|---|
| CMG <10% | CMG <20% | CMG <30% | |
| Variable | Odds Ratio (95% Confidence Interval) | ||
| Age < 65 years | 1.25 (1.08 to 1.45) | 1.31 (1.13 to 1.52) | 1.26 (1.07 to 1.49) |
| Gender, female | 1.24 (1.10 to 1.40) | 1.13 (1.01 to 1.29) | — |
| Race, African-American * | 1.73 (1.35 to 2.21) | 1.97 (1.57 to 2.48) | 2.13 (1.69 to 2.69) |
| LDL tests per year † | 0.96 (0.95 to 0.98) | 0.97 (0.96 to 0.99) | 0.97 (0.96 to 0.99) |
| Copay, $10 to <$20‡ | 1.45 (1.25 to 1.69) | 1.30 (1.11 to 1.51) | 1.25 (1.06 to 1.48) |
| Copay, ≥$20‡ | 3.23 (2.55 to 4.10) | 3.11 (2.48 to 3.89) | 2.73 (2.16 to 3.45) |
| Multiple brands or strengths tried | 1.41 (1.24 to 1.61) | 1.40 (1.22 to 1.59) | 1.48 (1.28 to 1.70) |
| Multiple doses per day | 1.88 (1.55 to 2.27) | 1.71 (1.43 to 2.05) | 1.61 (1.33 to 1.94) |
| Average days supply, 0 to <35§ | 2.17 (1.77 to 2.65) | 2.43 (1.93 to 3.06) | 3.58 (2.66 to 4.82) |
| Average days supply, 35 to <65§ | 1.74 (1.43 to 2.12) | 1.91 (1.51 to 2.40) | 2.59 (1.92 to 3.49) |
CMG thresholds indicate the number of days without therapy over the number of days the patient was actively taking statin, expressed as a percentage. Model developed through forward stepwise selection with all predictor variables required to maintain a minimal significance of P< .05.
Reference group = patients who self-identified as “white.”
For each additional unit.
Reference group = patients whose average monthly prescription copayment was less than $10.
Reference group = patients whose average days supply per prescription was 65 or greater.
CMG, cumulative multiple refill-interval gap.
The increasing magnitude of patient cost-sharing had a large, negative effect on adherent behavior as illustrated by the 76.2% of patients with a $20 or greater copay meeting the definition of nonadherent behavior (CMG > 10%) as compared to 49.4% of those who paid less than $10. This significant difference across the mean prescription copay category was observed under more liberal definitions of nonadherence as well (Table 3). As compared to patients who had a copayment of less than $10, patients who paid at least $10 but less than $20 and those who paid $20 or greater were 1.45 and 3.23 times more likely to be nonadherent with statin therapy, respectively. Other variables that are notable for their lack of statistical significance include prevention category, marital status, average yearly cardiologist visits, and statin naiveté. Sensitivity analyses with modified definitions of nonadherent behavior revealed the same variables to be predictive of noncompliant behavior as found in the original model (Table 4). The lone exception was the insignificance of gender in the model that defined nonadherent behavior as CMG >30%.
Discontinuation
In order to study discontinuation of statin therapy and identify associated factors, the analysis was restricted to statin-naive patients (n= 2,601). As shown in Figure 1, primary prevention patients were significantly more likely to discontinue statin therapy relative to the secondary prevention cohort (relative risk [RR], 1.24; 95% confidence interval [CI], 1.08 to 1.43) over the mean observation period. The time at which 50% of patients discontinued statin therapy was 3.4 years for the secondary prevention cohort and 3.7 years for the primary prevention cohort.
FIGURE 1.
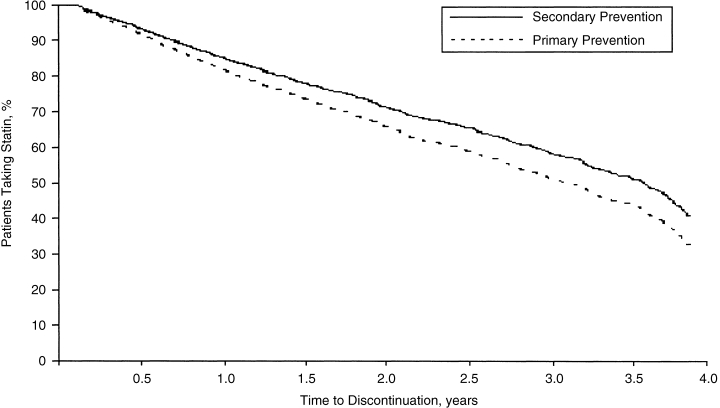
Survival curves for discontinuation of statin therapy by prevention category. Adjusted for all available covariates. The median time to discontinuation was 3.7 years for secondary prevention and 3.4 years for primary prevention.
Greater patient cost-sharing was associated with a higher likelihood of discontinuing a statin (see Fig. 2). Patients whose average monthly statin prescription copayment equaled or exceeded $20 were more than 4 times as likely to discontinue statin therapy than those patients who paid less than $10 (RR, 4.30; 95% CI, 3.39 to 5.44). Patients whose average monthly statin prescription copayment was greater than $10 but less than or equal to $20 were also more likely to discontinue statin therapy (RR, 1.39; 95% CI, 1.19 to 1.63) compared to those who paid less than $10. The time at which 50% of patients whose copay equaled or exceeded $20 discontinued statin therapy was 1.0 years and 2.2 years for those whose copayment exceeded $10 but was less than or equal to $20. The median time to discontinuation for patients who paid less than $10 was near the maximum possible observational period of 3.9 years. The effects of other factors on statin discontinuation are shown in Table 5
FIGURE 2.
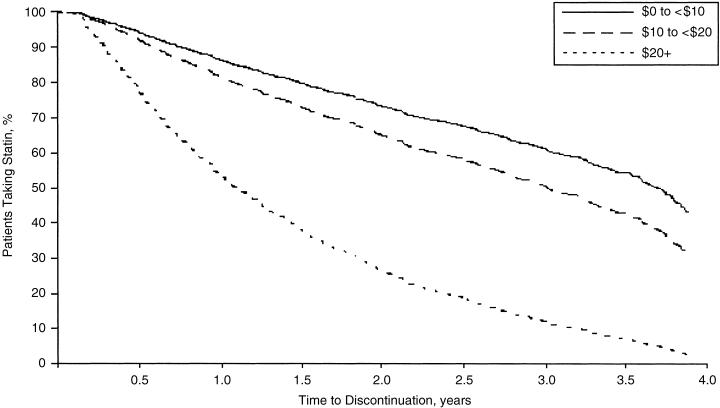
Survival curves for discontinuation of statin therapy by range of mean prescription copayment. Adjusted for all available covariates. The median time to discontinuation was 3.9+ years for $0 to <$10, 2.2 years for $10 to <$20, and 1.0 year for $20+.
Table 5.
Factors Associated with Discontinuation
| Variable | Hazard Ratio (95% Confidence Interval) |
|---|---|
| Primary prevention group | 1.24 (1.08 to 1.43) |
| Age, years * | 0.99 (0.98 to 0.99) |
| Gender, female | 1.18 (1.04 to 1.35) |
| Race, African-American † | 1.43 (1.17 to 1.74) |
| Cardiologist visits per year * | 1.07 (1.03 to 1.10) |
| LDL tests per year * | 1.03 (1.01 to 1.05) |
| Multiple brands or strengths tried | 0.48 (0.42 to 0.55) |
| Copay, $10 to <$20‡ | 1.39 (1.19 to 1.63) |
| Copay, $20 or greater ‡ | 4.30 (3.39 to 5.44) |
| Average days supply, 35 to <65§ | 1.60 (1.24 to 2.06) |
| Average days supply, 65 or greater § | 2.79 (2.46 to 3.09) |
For each additional unit.
Compared with patients who self-identified as “white”.
Compared with an average monthly prescription copayment of less than $10.
Compared with an average days supply per prescription of less than 35.
DISCUSSION
We report that adherence with statin therapy was less than optimal in cohorts treated for primary and secondary prevention and that adherence rates were not meaningfully different between groups. The observed level of nonadherence is undesirable and especially disappointing for individuals with documented CHD given the greater likelihood of negative outcomes in this high-risk group. There is no debate that clinicians, patients, and health plans should strive for optimal adherence rates in all treated patients. However, in an era of increasingly scarce resources devoted to quality-improving interventions, initiatives to enhance adherence to statin therapy, or any medical intervention for that matter, should be directed toward those individuals most likely to benefit from their use.
In an analysis of statin-naive patients, we detected a significantly higher discontinuation rate among primary prevention patients when compared to those treated for secondary prevention. This finding suggests that some degree of augmented retention occurs in those most likely to benefit from statin therapy, independent of the “day-to-day” adherence to active statin therapy. However, the likelihood of discontinuation observed was unacceptably high in both populations. The 50% discontinuation rate after 3.4 years found among secondary prevention patients in our study—higher than reported previously—should be of great concern to practitioners in lieu of recent findings documenting the risks of statin discontinuation in acute coronary syndrome or stable CHD patients.23,24 Moreover, the discontinuation rate detected in our primary prevention patients (50% at 3.7 years) was higher than those observed in WOSCOPS,1 EXCEL,2 and AFCAPS/TexCAPS,3 where the discontinuation rates were 26% at 5 years, 16% at 1 year, and 29% at 5.2 years, respectively.
Using methods that define adherence by the percentage of time a patient has drug available (or the percentage of time in which refill gaps are not present), several retrospective studies reported similar rates of statin adherence ranging from 64% to 84%.12,13,25 A study of elderly Canadian and U.S. populations observed higher adherence rates for individuals with risk factors for future cardiac event.12 A recently published study involving this same elderly Canadian population confirmed these observations.14 Similar findings were reported in a different elderly Canadian population with and without acute coronary syndrome.15 The generalizability of these studies may be limited because only publicly insured patients older than 65 were enrolled. Recent surveys have demonstrated that elderly patients and those enrolled in Medicaid or other prescription assistance programs report difficulty in the procurement of prescription medications beyond that of the general population.16–18 These poor levels of adherence to statin therapy as reported in the literature, coupled with our population where 38.3% of all patients had a CMG greater than 20%, are alarming given the recent evidence that patients with CMG levels greater than 20% have recurrent myocardial infarction and all-cause mortality outcomes not significantly different than patients who were not taking statin.26
One significant finding from our analysis was that the level of patient copayment was an independent factor for statin discontinuation. Compared to those who had less than a $10 copayment, patients who paid greater than or equal to $20 were more than 4 times more likely to discontinue their statin; patients who paid between $10 and $20 per month per statin prescription were also more likely to cease therapy. Coincidence alone cannot explain the lower rates of discontinuation in clinical trials, where study medication is almost always provided free of charge to study subjects, as compared to statin discontinuation described within our study population where mean monthly prescription copayment had such a profound effect. Previously published studies report that an increase in prescription copayment can lead to a decrease in drug utilization.27,28 Making matters worse, it is possible that this undesirable effect of copayment on statin adherence may be even greater in the secondary prevention cohort for whom the mean out-of-pocket expense per prescription was significantly higher than those taking statins for primary prevention.
Consumer cost-sharing has been a longstanding component of pharmaceutical cost containment. MCOs implement various cost-sharing arrangements to balance the demands for increased access to pharmaceuticals with pressures to constrain pharmaceutical cost growth. Conceptually, MCOs want to allow consumers to express their preferences for selected products by their willingness to pay, while ensuring that no prescription goes unfilled due to a patient's inability to pay the copay. There is a growing body of evidence demonstrating that effective therapies are not being used due to the requirement of a patient out-of-pocket expenditure. The results presented herein would support the argument that modern cost-containment strategies are failing to constrain drug cost growth while optimizing essential drug utilization.
A copay structure based on potential clinical benefit, rather than drug acquisition cost, would alleviate some financial burden and allow patients to prioritize their out-of-pocket expenditures.29 What distinguishes this “Benefit Based Copay” from existing systems is its determination of patient copays based on medical need and not drug acquisition costs, as best determined from the available medical and economic evidence. If implemented, a benefit-based copayment structure would decrease the economic burden for the secondary prevention population and thereby potentially improve adherence in those high-risk patients.
In our study, several factors in addition to cost-sharing and prevention category were predictive of nonadherence and discontinuation to statin therapy. Being female, younger, and of a minority race corresponded to both non-compliant behavior and discontinuation. These findings support previous research describing medication adherence and procurement in minority groups 30 and female patients.11,13,31 The number of cardiologist visits and LDL tests performed also were associated with improved statin adherence. The observed influence of these factors may argue that more intensive follow-up can improve patient adherence or, alternatively, these factors may simply serve as a proxy for patient adherence to a broad range of health care utilities. Finally, an increase in the complexity of the statin regimen influenced nonadherent behavior supporting previously published research examining the effects of dosing frequency on adherence.32 Patients who were prescribed their medications more frequently than once daily or experienced multiple brand and/or dosage switches were more likely to refill their medication in a nonadherent fashion. Thus, physicians and pharmacists can potentially enhance patient adherence by prescribing and filling simplified drug regimens that optimize patients’ insurance limits.
Several limitations of our study are worth noting. First, we cannot account for tablet splitting. Such behavior would artificially increase the number of apparent gaps in therapy overall, but should have an equal effect on both prevention cohorts. Second, we lack information regarding the incidence of drug-related adverse events and the exact reason for discontinuation of statin therapy. Statin discontinuation may be clinically appropriate, such as for lack of efficacy or side effects. We completed our analyses under the assumption that patients who had been initiated on statin therapy would require it for life. Operating under this assumption, any stoppage in statin therapy should have been followed by initiation of a non-statin antihyperlipidemic agent. Third, as with any study that utilizes pharmacy claims databases, we could only determine that a prescription was filled and not if the patients actually took the medications at the time of day and at the frequency prescribed by their clinicians. Fourth, our use of medical claims and administrative databases for identification of our population lends to certain inherent weakness as recent claims and services may not reflect previous illness. Our use of a misidentification quality assurance check minimized the effect of inappropriately allotting a truly secondary prevention patient to the primary prevention cohort. Last, because our analysis was performed in a single site and we did not include Medicaid enrollees, the generalizability of our findings may be limited. Nevertheless, our study population is representative of managed care enrollees nationwide, a large population for whom these outcomes have yet to be published.
Conclusions
In this study, we found that nonadherence and discontinuation with statins was suboptimal and similar in primary and secondary prevention populations. A profound predictive effect of higher prescription copayment levels on nonadherence and discontinuation of statin therapy was observed. This expected yet undesirable impact of copayment, coupled with the fact that out-of-pocket expenditures were higher for the secondary prevention population, indicates that mechanisms implemented to constrain pharmaceutical expenditures may be detrimental to patient outcomes. While universal adherence for all patients is a desirable goal, incremental efforts should focus on improving adherence and discontinuation rates in those high-risk populations who are the most likely to benefit from their use.
Acknowledgments
The authors acknowledge Katherine Young, BA, for her assistance with data acquisition and analysis.
REFERENCES
- 1.Shepherd J, Cobe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia: West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 2.Bradford RH, Shear CL, Chremos AN, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study result. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Arch Intern Med. 1991;151:43–9. doi: 10.1001/archinte.151.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of ASCAPS/TexCAPS. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 4.Scandinavian Simvastatin Survival Study Group Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 5.The LIPID Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 7.Kumana CR, Cheung B, Lauder IJ, et al. Gauging the impact of statins using number needed to treat. JAMA. 1999;282:1899–901. doi: 10.1001/jama.282.20.1899. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Schmitt B, Wallner E. Impact of medication nonadherence on coronary heart outcomes: a critical review. Arch Intern Med. 1997;157:1921–9. [PubMed] [Google Scholar]
- 9.Mäenpää H, Heinonen OP, Manninen V. Medication compliance and serum lipid changes in the Helsinki Heart Study. Br J Clin Pharmacol. 1991;32:409–15. doi: 10.1111/j.1365-2125.1991.tb03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The West of Scotland Coronary Prevention Study Group Compliance and adverse event withdrawal: their impact on the West of Scotland Coronary Prevention Study. Eur Heart J. 1997;18:1718–24. doi: 10.1093/oxfordjournals.eurheartj.a015165. [DOI] [PubMed] [Google Scholar]
- 11.Andrade SE, Walker AM, Gottleib LK, et al. Discontinuation of antihyperlipidemic drugs—do rates reported in clinical trials reflect rates in primary care settings? N Engl J Med. 1995;332:1125–31. doi: 10.1056/NEJM199504273321703. [DOI] [PubMed] [Google Scholar]
- 12.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279:1458–62. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 13.Sung JCY, Nichol MB, Venturini F, Bailey KL, McCombs JS, Cody M. Factors affecting patient compliance with antihyperlipidemic medications in an HMO population. Am J Manag Care. 1998;4:1421–30. [PubMed] [Google Scholar]
- 14.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 15.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–7. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 16.Taylor H, Leitman R. Out-of-pocket costs are a substantial barrier to prescription drug compliance. Health Care News. 2001 November 20. [Google Scholar]
- 17. Reed-Haldy-McIntosh for AARP, Prescription Drug Use Among Persons Age 45+, 2002, Survey; April.
- 18.Cunningham PJ. Center for Studying Health System Change. Washington, DC: Research Report No. 5; April 2002. Affording Prescription Drugs: Not Just a Problem for the Elderly. [Google Scholar]
- 19.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.Health Plan Employer Data and Information Set (HEDIS) Cholesterol Management After Acute Cardiovascular Events. Washington, DC: National Committee for Quality Assurance; 2002. [Google Scholar]
- 21.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records: description and validation. Med Care. 1988;26:814–23. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 23.Heeschen C, Hamm CW, Laufs U, et al. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105:1446–52. doi: 10.1161/01.cir.0000012530.68333.c8. [DOI] [PubMed] [Google Scholar]
- 24.Thomas M, Mann J. Increased thrombotic vascular events after change of statin. Lancet. 1998;352:1830–1. doi: 10.1016/S0140-6736(05)79893-7. [DOI] [PubMed] [Google Scholar]
- 25.White TJ, Chang E, Leslie S, et al. Patient adherence with HMG reductase inhibitor therapy among users of two types of prescription services. J Manag Care Pharm. 2002;8:186–91. [Google Scholar]
- 26.Wei L, Wang J, Thompson P, Wong S, Struthers AD, MacDonald TM. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow-up study. Heart. 2002;88:229–33. doi: 10.1136/heart.88.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joyce GF, Escarce JJ, Solomon MD, Goldman DP. Employer drug benefit plans and spending on prescription drugs. JAMA. 2002;288:1733–9. doi: 10.1001/jama.288.14.1733. [DOI] [PubMed] [Google Scholar]
- 28.Motheral BR, Henderson R. The effect of a copay increase on pharmaceutical utilization, expenditures, and treatment continuation. Am J Manag Care. 1999;5:1383–94. [PubMed] [Google Scholar]
- 29.Fendrick AM, Smith DG, Chernew ME, Shaw SN. A benefit-based copay for prescription drugs: patient contribution based on total benefits, not drug acquisition cost. Am J Manag Care. 2001;7:861–7. [PubMed] [Google Scholar]
- 30.Nelson K, Norris K, Mangione CM. Disparities in the diagnosis and pharmacologic treatment of high serum cholesterol by race and ethnicity. Arch Intern Med. 2002;162:929–35. doi: 10.1001/archinte.162.8.929. [DOI] [PubMed] [Google Scholar]
- 31.Salganicoff A, Beckman JZ, Wyn R, Ojeda VD. Report by The Henry J. Kaiser Foundation; 2002. Women's Health in the United States: Health Coverage and Access to Care. May. [Google Scholar]
- 32.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–10. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]


