Abstract
OBJECTIVE
To assess mortality associated with hormone replacement in younger and older postmenopausal women.
DESIGN
A comprehensive search of medline, cinahl, and embase databases was performed to identify randomized controlled trials of hormone replacement therapy from 1966 to September 2002. The search was augmented by scanning selected journals through April 2003 and references of identified articles. Randomized trials of greater than 6 months’ duration were included if they compared hormone replacement with placebo or no treatment, and reported at least 1 death.
MEASUREMENTS
Outcomes measured were total deaths and deaths due to cardiovascular disease, cancer, or other causes. Odds ratios (OR) for total and cause-specific mortality were reported separately for trials with mean age of participants less than and greater than 60 years at baseline.
MAIN RESULTS
Pooled data from 30 trials with 26,708 participants showed that the OR for total mortality associated with hormone replacement was 0.98 (95% confidence interval [CI], 0.87 to 1.12). Hormone replacement reduced mortality in the younger age group (OR, 0.61; CI, 0.39 to 0.95), but not in the older age group (OR, 1.03; CI, 0.90 to 1.18). For all ages combined, treatment did not significantly affect the risk for cardiovascular or cancer mortality, but reduced mortality from other causes (OR, 0.67; CI, 0.51 to 0.88).
CONCLUSIONS
Hormone replacement therapy reduced total mortality in trials with mean age of participants under 60 years. No change in mortality was seen in trials with mean age over 60 years.
Keywords: hormone replacement therapy, postmenopause, mortality, age factors, meta-analysis
In the assessment of risks and benefits of hormone replacement therapy (HRT), observational studies and clinical trials have yielded apparently conflicting results. Large prospective cohort studies have shown that women who used HRT, most of whom started treatment shortly after menopause, had significant reductions in total and cardiovascular mortality compared to nonusers.1–8 The results remained significant after adjusting for cardiovascular risk factors such as age, smoking, and blood pressure. The largest randomized trial of HRT, the Women's Health Initiative (WHI), evaluated women with mean age 63 years and found that HRT increased the risk of cardiovascular events, without changing total or cardiovascular mortality.9
It is possible that the mortality reduction seen in observational studies was due to a confounding variable that was not adequately adjusted for, such as general health status or access to health care.10–12 Another explanation is that when HRT is started in younger women, a true mortality benefit is seen. Mortality is a relatively rare outcome, even in large trials. For example, the WHI had approximately 5 deaths per 1,000 patient-years.9 A more precise estimate of the impact of HRT on mortality can be made by pooling the results of many trials. The objective of this study was to assess the effect of age on total and cause-specific mortality associated with HRT by performing a meta-analysis of randomized controlled trials.
METHODS
Search Strategy
The medline,embase,cinahl, and Cochrane databases were searched comprehensively to identify randomized controlled trials published between 1966 and September 2002 that evaluated hormone replacement in postmenopausal women. Terms used in the search were climacter,* menopause,* perimenopaus* or peri-menopaus*,postmenopaus* or post-menopaus*, andestrogen*, estrogen replacement therapy, hormone replacement therapy, hormone substitution, progesterone, progestogen, progestin, or gestagen. Trials were not excluded on the basis of language. The search was augmented by scanning selected journals through April 2003, and references of identified articles.
Trial Selection
Two investigators independently evaluated studies for inclusion, and the observed interrater agreement was measured using the κ statistic.13 Trials were included if they 1) were randomized controlled trials of postmenopausal women that compared HRT to placebo or no hormone therapy, 2) were of longer than 6 months’ duration, and 3) reported at least 1 death. Attempts were made to contact the investigators of all trials longer than 6 months, to obtain information concerning deaths during the trial.
For studies with multiple publications from the same group of participants, one publication containing the most information was chosen for inclusion. Interventions in the trials included transdermal or oral estrogens alone or in combination with a progestin. Control groups received placebo, no treatment, or calcium supplementation.
Data Extraction
Two independent reviewers extracted data from the selected articles, reconciling differences by consensus. The outcomes measured were total deaths and deaths due to cardiovascular events, cancer, and all other causes. For patients who withdrew from the study because of adverse events, deaths that were reported after withdrawal from the trial were included in the analysis.
For crossover trials, only data from the end of the first phase were used because of the potential carryover effect of HRT. For randomized trials with nonrandomized open-label extensions, only data from the randomized trial were included. For trials that provided data on other interventions, such as raloxifine, only data from the hormone and control groups were included.
Assessment of Validity
The methodological quality of each trial was assessed according to the following factors: 1) was the randomization procedure and allocation concealment adequate? 2) Were the patients and providers blinded to the intervention? 3) Were withdrawals and dropouts described, and the analysis performed as intention-to-treat? Trials received an A score when all quality criteria were met, a B score when one or more criteria were partially met, and a C score when one or more criteria were not met.14,15 Two reviewers independently assessed quality scores and the interrater agreement was calculated using the κ statistic. The quality assessment was used for a sensitivity analysis.
Data Synthesis
For each trial, the ratio of deaths to surviving patients was calculated for both the treatment and control groups, to obtain the odds of death. The net result for mortality was expressed as an odds ratio (OR), by dividing the treatment odds by the control odds. The results were pooled to obtain a summary odds ratio using the random-effects model for dichotomous outcomes, with confidence intervals (CI) set at 95% significance.16 The random-effects method was chosen as it accounts for the potential of interstudy heterogeneity. The analysis was performed using Meta View 4.1 (Update Software, Oxford, UK). Only trials that reported at least 1 death could be used in the estimation of odds ratio. To test for interstudy heterogeneity, the χ2 value and the Q-value were calculated for the assumption of homogeneity.
In order to evaluate the effect of age on mortality, trials were divided into those with mean age of participants at baseline less than or greater than 60 years. The cutoff of 60 years was arbitrarily chosen, a priori, to divide the participants into a younger and older age group. The cutoff was thought to be appropriate because the rate of cardiovascular events in women accelerates after this age, suggesting that a primary protective effect of HRT might be lost or diminished after this time.17,18 The results of the analysis were reported separately for all ages and for the younger and older age groups. The results of the two age groups were compared to each other using the logarithm of the odds ratio (log OR). A sensitivity analysis was performed to evaluate the effect of using different age cutoffs. Linear regression analysis was performed to evaluate mortality OR as a linear function of age.
A subgroup analysis compared the use of unopposed estrogen and combined treatment with estrogen and progestin. For trials that provided information on both unopposed and combined treatment, the data for each type of treatment were analyzed separately.
RESULTS
Search Results
The electronic database search identified 4,993 articles, of which 358 were potentially relevant trials on HRT in postmenopausal women. After scanning journals and references from selected articles, an additional 12 trials were identified. Of these 370 studies, 30 met inclusion criteria (Table 1).9,19–47 The κ score for interrater agreement in trial selection was 0.91 (95% CI, 0.82 to 1.00). Studies were excluded for the following reasons: 98 did not report any deaths, 110 provided data on patients already included in the analysis, 79 were of 6 months’ duration or less, 26 did not provide a control group, and 27 were not randomized.
Table 1.
Characteristics of Included Studies
| Study (Reference #) | Design, Duration | Inclusion and Exclusion Criteria | Participants (n) in HRT Placebo | Dropout Rate in HRT Placebo, % | Mean Age in HRT Placebo | Active Comparison Intervention | Outcomes | Comments |
|---|---|---|---|---|---|---|---|---|
| Angerer, 200020 | Double blind, 1 year | Inclusion: Postmenopausal women aged 40 to 70 years with more than 1 mm intima media thickness in at least one segment of the carotid arteries | 215 | 28 | 59.0 | Estradiol 1 mg/day plus gestodone 0.25 mg/day vs placebo | Carotid artery dispensability | |
| Exclusion: Myocardial infarction within 6 months, angina or any contraindication to hormone use | 66 | 29 | 59.5 | |||||
| Arrenbrecht, 200221 | Double blind, 2 years | Inclusion: Healthy postmenopausal women with hysterectomy | 108 | 24 | 50.5s | Transdermal estradiol 50 or 100 mcg/day vs placebo | Bone mineral density, bone turnover | |
| Exclusion: Medication that affects bone metabolism, smoking, or osteoporosis | 53 | combined | combined | |||||
| Binder, 200122 | Double blind, 9 months | Inclusion: Postmenopausal women over the age of 75 years | 41 | 22 | 82.0 | CEE 0.625 mg/day plus MPA 5 mg/day vs placebo | Lipid profiles | |
| Exclusion: Recent hormone use, breast, or gynecological malignancy, current treatment for thromboembolism, cardiovascular disease, or unstable thyroid disease | 22 | 9.1 | 83.0 | |||||
| Cherry, 200223 | Double blind, 2 years | Inclusion: Women aged 50 to 69 with recent myocardial infarction | 513 | 57.3 | 62.3 | Estradiol valerate, 2 mg/day vs placebo | Reinfarction, cardiac death, all-cause mortality | |
| Exclusion: Recent use of hormones or vaginal bleeding, gynecological malignancy, liver or renal disease, history of venous thromboembolism | 504 | 36.5 | 62.9 | |||||
| Gallagher, 200124 | Double blind, 3 years | Inclusion: Elderly postmenopausal women | 121 | 16.5 | 72.0 | CEE 0.625 mg/day plus MPA 2.5 mg/day vs placebo | Bone mineral density | Calcitriol with and without hormones were also studied |
| Exclusion: Severe chronic illness or medications that affect bone metabolism | 123 | 8.9 | 71.0 | |||||
| Giske, 200225 | Dαouble blind, 2 years | Inclusion: Healthy postmenopausal women with hysterectomy | 123 | 11.4 | 49.1 | Estradiol 0.5 mg, 1 mg or 2 mg/day vs placebo | Bone mineral density | |
| Exclusion: Medications that affect bone metabolism | 43 | 30.2 | 49.6 | |||||
| Guidozzi, 199926 | Open label, 4 years | Inclusion: Postmenopausal women with ovarian cancer | 62 | 4 | 51 | CEE 0.625 mg/day plus MPA 2.5 mg/day vs placebo | Disease-free survival and overall survival | |
| Exclusion: Previous hormone replacement or ovarian malignancy of low malignant potential | 68 | combined | combined | |||||
| Hall, 199427 | Open label, 2 years | Inclusion: Postmenopausal women with rheumatoid arthritis | 100 | 37 | 55.8 | Transdermal estradiol 50 mcg/day vs placebo | Bone mineral density | |
| Exclusion: Contraindications to hormone replacement, breast or gynecological malignancy, or thromboembolism | 100 | 16 | 56.1 | |||||
| Hall, 199828 | Single blind, 1 year | Inclusion: Postmenopausal women with coronary heart disease | 40 | 20 | 58.6 | Transdermal estradiol 50 mcg/day and MPA 5 mg/day vs placebo | Angina | |
| Exclusion: None listed | 20 | 30 | 61.3 | |||||
| Herrington, 200029 | Double blind, 3.2 years | Inclusion: Postmenopausal women with coronary disease | 204 | 20 | 65.9 | CEE 0.625 mg/ day plus MPA 2.5 mg/day vs placebo | Angiographic changes and lipid profiles | |
| Exclusion: Breast or gynecological malignancy, planned coronary artery surgery, thromboembolism, symptomatic gallstones, renal or liver dysfunction, uncontrolled diabetes, hypertension, or hypertryiglyceridemia | 105 | combined | 65.6 | |||||
| Hodis, 200130 | Double blind, 2 years | Inclusion: Postmenopausal women at least 45 years old with elevated cholesterol | 111 | 25.2 | 60.9 | Estradiol 1 mg/day vs placebo | Change in intimal thickness | |
| Exclusion: Breast or gynecological malignancy, recent or long- term hormone use, severe hot flushes, severe hypertension, or severe illness | 111 | 25.2 | 62.1 | |||||
| Hulley, 200231 | Double blind, 4.1 years | Inclusion: Postmenopausal women under the age of 80 years with coronary heart disease | 1380 | 11.5 | 67 | CEE 0.625 mg/day plus MPA 2.5 mg/day vs placebo | Coronary heart disease events or deaths | |
| Exclusion: Thromboembolism, breast or gynecological malignancy, recent hormone use and serious disease | 1383 | 11.2 | 67 | |||||
| Komulainen, 199932 | Open label, 5 years | Inclusion: Early postmenopausal women | 115 | 5.2 | 52.9 | Estradiol 2 mg/day plus cyproterone acetate 1 mg/day vs placebo | Bone mineral density | Vitamin D with or without hormones was also studied |
| Exclusion: Breast or gynecological malignancy, thromboembolism, or medication-resistant hypertension | 115 | 5.2 | 52.6 | |||||
| Kyllonen, 199833 | Double blind, 2 years | Inclusion: Healthy early postmenopausal women age 49 to 55 years | 52 | 22 | 52.6 | Estradiol 2 mg/day plus MPA 10 mg/day for 10 days vs placebo | Lumbar spine mobility and low-back symptoms | |
| Exclusion: None listed | 26 | combined | combined | |||||
| Lindsay, 197634 | Double blind, 5 years | Inclusion: Postmenopausal women with oophorectomy | 63 | 6.3 | 44 to 50 | Mestranol 28.4 mg/day vs placebo | Bone mineral content | |
| Exclusion: Prior estrogen use | 57 | 5.3 | ||||||
| MacDonald, 199435 | Double blind, 1 year | Inclusion: Postmenopausal women with rheumatoid arthritis | 40 | 22.5 | 53 | Transdermal estradiol 50 mcg/day with or without norethisterone 1 mg/day for 10 days vs placebo | Bone mineral density and rheumatoid arthritis disease activity | |
| Exclusion: Significant menopausal symptoms | 22 | 40.9 | 55 | |||||
| Mijatovic, 199836 | Double blind, 2 years | Inclusion: Healthy postmenopausal women with hysterectomy | 13 | 5 | 55.7 | CEE 0.625 mg/day vs placebo | Homocysteine levels | Raloxifine also studied |
| Exclusion: Hepatic, renal, endocrinologic, gastrointestinal, or cardiovascular disease or breast or gynecological malignancies | 13 | 6.7 | 54.9 | |||||
| Mosekilde, 200237 | Open label, 5 years | Inclusion: Postmenopausal women | 502 | 9.6 | 49.5 | Estradiol 2 mg/day plus norethisterone 1 mg/day for 10 days vs placebo | Bone mineral density | |
| Exclusion: Metabolic bone disease, recent estrogen or corticosteroid use, steroids, malignancy, chronic disease, or alcohol abuse | 504 | 9.1 | 50.0 | |||||
| Mulnard, 200038 | Double blind, 1 year | Inclusion: Postmenopausal women with hysterectomies and mild to moderate Alzheimer's disease | 81 | 19.8 | 75.6 | CEE 0.615 or 1.25 mg/day vs placebo | Global measures of cognition, mood, motor function, and activities of daily living | |
| Exclusion: Recent myocardial infarction, thromboembolism, hypercoagulable state, hyperlipidemia, or use of antipsychotics, anticonvulsants, anticoagulants, beta-blockers, narcotics, methyldopa, clonidine, or cognitive-enhancing or anti-Parkinson medications | 39 | 17.9 | 74.1 | |||||
| Nachtigall, 197939 | Double blind, 10 years | Inclusion: Postmenopausal women hospitalized for chronic diseases | 84 | 3.6 | 55.3 | CEE 2.5 mg/day plus MPA 2.5 mg/day vs placebo | Clinical outcomes such as medical illness or death | |
| Exclusion: Hypertension, hysterectomy, coronary heart disease, or previous hormone use | 84 | 8.3 | 54.9 | |||||
| Os, 200040 | Open label, 1 year | Inclusion: Postmenopausal women with documented coronary artery disease | 60 | 10.2 | 63 | Transdermal estradiol 50 mcg/day plus MPA 5 mg/day for 14 days vs placebo | Lipid profiles | |
| Exclusion: Previous hormone use, previous myocardial infarction or coronary artery bypass surgery, thromboembolism, breast or gynecological malignancy, alcoholism, or psychiatric disorder | 58 | 15.3 | 66 | |||||
| PEPI trial Writing Group, 199541 | Double blind, 3 years | Inclusion: Healthy postmenopausal women | 701 | 16 | 56.1 | CEE 0.625 mg/day plus MPA 2.5 mg/ day or MPA 10 mg/day for 10 days or micronized progesterone 200 mg/day for 12 days vs placebo | Lipid profiles, fibrinogen, blood pressure, and insulin | |
| Exclusion: Severe menopausal symptoms, unstable thyroid disease, serious illness, or contraindications to hormone use | 174 | 32.8 | combined | |||||
| Perez-Jaraiz, 199642 | Open-label, 1 year | Inclusion: Early postmenopausal with rapid bone loss | 26 | 5.8 | 48 | Transdermal estradiol transdermal 50 mcg/day plus MPA 10 mg for 12 days vs calcium supplementation | Bone mineral density | Calcitonin also studied |
| Exclusion: None listed | 52 | 5.8 | 50 | |||||
| Ravn, 199943 | Open label, 4 years | Inclusion: Healthy early postmenopausal women age 49 to 55 years | 110 | 25.2 | 55 | CEE 0.625 mg/day plus MPA 5 mg/day or estradiol 1 to 2 mg/day plus noresisterone 1 mg/day on days 13 to 22 vs placebo | Bone mineral density and bone turnover | Alendronate also studied |
| Exclusion: Hormone use | 109 | 26.7 | 55 | |||||
| Raz, 199344 | Double blind, 8 months | Inclusion: Postmenopausal women with 3 or more confirmed urinary tract infections during previous year | 50 | 28 | 64.7 | Intravaginal estradiol cream 0.5 mg/day vs placebo vaginal cream | Incidence of urinary tract infections, and vaginal pH and cultures | |
| Exclusion: Thromboembolism disorders, liver disease, estrogen-dependent tumors, anatomical lesions in urogenital area, indwelling urinary catheter, long-term use of antimicrobial agents, or oral estrogen therapy | 43 | 20.9 | 65.4 | |||||
| Recker, 199945 | Double blind, 3.5 years | Inclusion: Healthy women over the age of 65 years with low bone mass | 64 | 28.1 | 73.2 | CEE 0.3 mg/day with MPA 2.5 mg/day vs placebo | Bone mineral density, serum total alkaline phosphatase and osteocalcin levels, and urinary creatinine and hydoxyproline | |
| Exclusion: Previous hip fracture, use of estrogen, calcitonin, or corticosteroids in the past 6 months, any use of bisphosphonates or fluoride, endometrial thickness more than 6 mm, breast cancer, and smoking | 64 | 18.8 | 74.0 | |||||
| Viscoli, 200146 | Double Blind, 2.8 years | Inclusion: Postmenopausal women with recent stroke, cerebrovascular disease | 337 | 34.4 | 72 | Estradiol 1 mg/day vs placebo | Death and stroke incidence | |
| Exclusion: Coexisting condition that limits life expectancy, breast or gynecological malignancy, thromboembolism while on estrogen therapy, or neurological or psychiatric disease | 327 | 24.2 | 71 | |||||
| Waters, 200247 | Double blind, 2.8 years | Inclusion: Postmenopausal women with coronary stenosis documented on angiogram | 108 | 21.3 | 65 | CEE 0.625 mg/day with medroxyproge sterone acetate 2.5 mg/day vs placebo | Annualized mean change in minimum lumen diameter on angiogram, myocardial infarction, death | Antioxidant vitamins also studied |
| Exclusion: Hormone use in previous 3 months, concurrent use of vitamin C or E, gynecological or breast cancer, uncontrolled diabetes or hypertension, myocardial infarction with 1 month, renal insufficiency, gallstones, congestive heart failure, hemorrhagic stroke, thromboembolism, or untreated osteoporosis | 103 | 36.2 | 66 | |||||
| Watts, 200048 | Double Blind, 2 years | Inclusion: Early postmenopausal women | 303 | 10 | 51.8 | CEE 0.3, 0.625 or 1.25 mg/day vs placebo | Bone mineral density | |
| Exclusion: Recent hormone use, osteoporosis, smoking, or medications that affect mineral metabolism | 103 | combined | 51.3 | |||||
| Women's Health Initiative Writing Group, 200249 | Double Blind, 5.2 years | Inclusion: Postmenopausal women aged 50 to 79 years | 8506 | 6.3 | 63.2 | CEE 0.625 mg/day plus MPA 2.5 mg/day vs placebo | Coronary heart disease events, breast cancer, and a global index for risks and benefits | |
| Exclusion: Serious illness, cancer, anemia, alcohol abuse, or dementia | 8102 | 6.1 | 63.3 |
HRT, hormone replacement therapy.
Trial Characteristics
The analysis included 30 trials, with a total of 26,708 participants followed for 119,118 patient-years. The mean trial duration was 4.46 years (range 0.7 to 10 years), with a mean study size of 890 participants (range 52 to 16,608). The mean age of participants at baseline was 62.2 ± 8.9 years in the treatment group and 63.4 ± 9.1 years in the placebo group, with an age range of 36 to 87 years. The mean dropout rate was estimated to be 11.5% in the treatment group and 10.6% in the placebo group. The κ score for interrater agreement on methodological quality scores was 0.95 (95% CI, 0.6 to 1.0). Of the trials studied, 13 received a score of A, 10 received a score of B, and 7 received a score of C.
Total and Cause-specific Mortality
For all ages combined, there were 518 deaths reported in 14,147 participants in the treatment group and 501 deaths in 12,561 participants in the control group (Table 2). The summary OR for total mortality associated with HRT was 0.98 (95% CI, 0.87 to 1.18). The OR for cardiovascular mortality was 1.10 (95% CI, 0.90 to 1.34), and for cancer deaths was 1.03 (95% CI, 0.82 to 1.29). Of note, there was no increase in breast cancer deaths in those trials that reported cancer-specific mortality (OR, 1.03; 95% CI, 0.29 to 3.67). HRT was associated with a 33% reduction in deaths from causes other than cardiovascular disease or cancer (OR, 0.67; 95% CI, 0.51 to 0.88; Table 2). The specific causes of death in this category included infectious diseases, sepsis, accidents, renal failure, respiratory failure, pulmonary embolism, liver failure, gastrointestinal bleeds, and rheumatologic diseases.
Table 2.
Odds Ratio for Total, Cardiovascular, Cancer, and Other Mortality in Younger and Older Women Associated with Hormone Replacement Therapy
| HRT Deaths | N | Control Deaths | N | OR (95% CI) | ||
|---|---|---|---|---|---|---|
| All ages | Total death | 518 | 14,147 | 501 | 12,561 | 0.98 (0.87 to 1.18) |
| CV death | 215 | 187 | 1.10 (0.90 to 1.34) | |||
| Cancer death | 184 | 171 | 1.03 (0.23 to 1.29) | |||
| Other death | 91 | 126 | 0.67 (0.51 to 0.88)* | |||
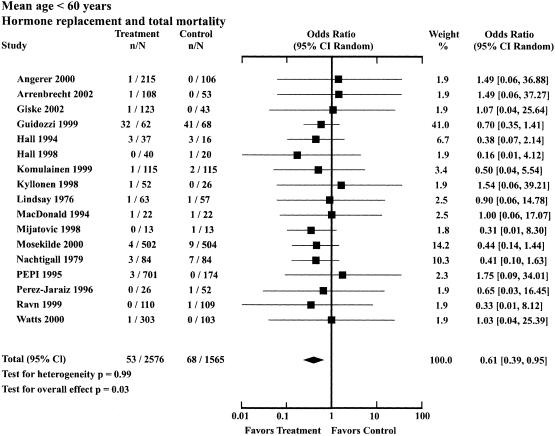
| Mean age, y < 60 | Total death | 53 | 2,576 | 68 | 1,565 | 0.61 (0.39 to 0.95)* |
| CV death | 3 | 3 | 0.68 (0.22 to 2.15) | |||
| Cancer death | 45 | 54 | 0.69 (0.59 to 1.08) | |||
| Other death | 5 | 12 | 0.44 (0.17 to 1.13) | |||
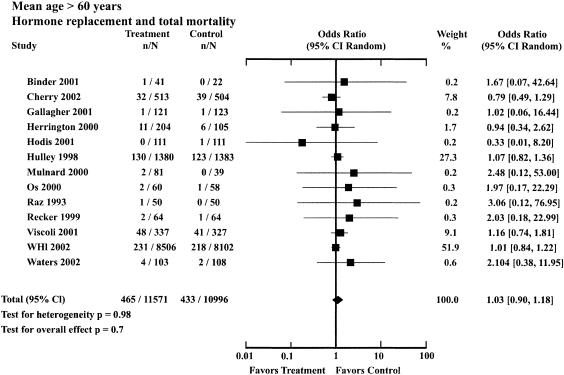
| Mean age, y > 60 | Total death | 465 | 11,571 | 433 | 10,996 | 1.03 (0.90 to 1.18) |
| CV death | 212 | 184 | 1.11 (0.91 to 1.36) | |||
| Cancer death | 137 | 123 | 1.07 (0.84 to 1.37) | |||
| Other death | 86 | 116 | 0.68 (0.56 to 0.91)* |
Statistical significance.
OR, odds ratio; CV, cardiovascular; HRT, hormone replacement therapy.
There were 17 trials in the younger age group, with 4,141 participants followed for a mean trial duration of 3.66 years. The mean age in the treatment group was 53.9 ± 3.5 years and in the control group was 53.7 ± 3.4 years. The OR for total mortality in the younger group was 0.61 (95% CI, 0.39 to 0.95), indicating a 39% reduction in mortality for those receiving HRT (Table 2; Fig. 1). The OR for cardiovascular mortality was 0.68 (95% CI, 0.22 to 2.15), for cancer mortality was 0.69 (95% CI, 0.59 to 1.08), and for other mortality was 0.44 (95% CI, 0.17 to 1.13).
FIGURE 1.
Odds ratios for total mortality associated with hormone replacement therapy: trials with mean age of participants less than 60 years.
There were 13 trials in the older age group, with 22,567 participants followed for a mean trial duration of 4.66 years. The mean age in the treatment group was 64.6 ± 7.2 and in the control group was 66.8 ± 7.0. The OR for total mortality associated with HRT in the older group was 1.03 (95% CI, 0.9 to 1.18; Table 2; Fig. 2). For this group, HRT did not significantly affect cardiovascular mortality (1.11; 95% CI, 0.91 to 1.36) or cancer mortality (1.07; 95% CI, 0.84 to 1.37), but was associated with a reduction in mortality from other causes (0.68; 95% CI, 0.56 to 0.91).
FIGURE 2.
Odds ratios for total mortality associated with hormone replacement therapy: trials with mean age of participants greater than 60 years.
When the results of the two age groups were compared to each other, HRT was associated with significantly lower mortality in the younger group as compared to the older group (P = .03). A sensitivity analysis showed that HRT was still associated with significant reductions in mortality in the younger group using an age cutoff in the range from 56 to 63 years. In a linear regression analysis the results for OR were assessed for 3 age groups, less than 56 years (0.62; 95% CI, 0.39 to 1.00), 56 to 63 years (0.74; 95% CI, 0.47 to 1.16), and greater than 63 years (1.05; 95% CI, 0.92 to 1.21). A significant trend was found for mortality OR as a function of age, with the OR increasing by 0.024 (95% CI, 0.003 to 0.038) per year (correlation coefficient 0.98).
In a subgroup analysis, there was no significant difference in total mortality for all ages combined when unopposed estrogen (OR, 0.96; 95% CI, 0.73 to 1.27) was compared to combined treatment (OR, 1.00; 95% CI, 0.86 to 1.15). There was no significant difference in results for the two types of intervention in the younger age group or in the older age group (P > .5).
A sensitivity analysis was performed to evaluate the effect of including those trials with the lowest scores for methodological quality. When trials with a C score were excluded, the OR for total mortality changed by less than 0.1 points (P > .9) for all ages and for the two age groups. No evidence for significant interstudy variance was found in any of the analyses (P > .7). The Q value was calculated and found to be compatible with homogeneity between studies.
Discussion
Pooled data from 30 trials, with 26,708 postmenopausal women followed for a mean duration of 4.5 years, indicate that hormone replacement does not increase total mortality. In the younger group, with mean age 54 years at baseline, HRT was associated with a reduction in total mortality of 39%. In the older group, with mean age 66 years, HRT was not associated with a change in total mortality. A cutoff of 60 years for mean age was chosen, although the results were still robust for cutoffs in the range from 56 to 63 years. Given that there is not one single cutoff but rather a linear relationship between young and old, linear regression analysis was performed that demonstrated a significant trend between increasing mortality OR and increasing age, with a correlation coefficient of 0.98.
These results may help to explain the discrepancies that have been seen between observational studies and randomized trials. The Nurses’ Health Study was a 20-year prospective cohort study of 120,000 women under the age of 55 years, and the WHI was a 5-year trial of 16,000 women with mean age 63 years.2,9 In both studies, HRT was associated with similar increases in breast cancer, stroke, and pulmonary embolism, and similar reductions in colorectal cancer and hip fracture.48 However, the WHI found an increase in cardiac events without a change in cardiovascular or total mortality, while the Nurses’ Health Study found significant reductions in cardiac events as well as cardiovascular and total mortality.
The Nurses’ Health Study, after adjusting for potential confounding variables, found that current hormone users, 80% of whom had started treatment within 2 years of menopause, had a total mortality risk of 0.63 (95% CI, 0.56 to 0.70) that of nonusers.2 This meta-analysis found similar results for total mortality in the younger group (OR, 0.61; 95% CI, 0.39 to 0.95), providing evidence that the mortality benefit seen in the observational studies may be a true effect of HRT when treatment is started shortly after menopause. Beneficial effects of HRT include increases in high-density lipoproteins and reductions in low-density lipoproteins, Lp(a) lipoproteins, homocysteine, fibrinogen, plasminogen activator inhibitor antigen, intrinsic coagulation factors, glucose, weight, insulin levels, and the incidence of new-onset diabetes mellitus, compared to placebo.29,40,49–58 In women with diabetes mellitus, HRT reduces central adiposity and improves glycemic control and physical functioning.59,60 HRT also causes sustained increases in nitric oxide levels and reductions in plasma norepinephrine, plasma renin activity, and endothelin.61–64 These endothelial changes have been associated with vasodilation, reduced blood pressure, increased blood flow, and improved cardiac performance.65–69 It is thought that estrogen has protective properties against cardiovascular disease in premenopausal women, and that the risk for atherosclerosis begins to rise after menopause.70,71 It is possible that if HRT is started in women in the early postmenopausal period, well before the development of atherosclerosis, primary prevention could be achieved through these improvements in metabolism, hemostasis, and endothelial function. In fact, there is some evidence from clinical trials and animal studies that HRT can halt the progression of atherosclerosis if treatment is started early in the course of the disease.29,72–76
No mortality benefit from HRT was seen for women in the WHI trial, most of whom had not taken hormones since the start of menopause at least 10 years earlier.9 The WHI included women who were under the age of 60 years, but the investigators declined to provide mortality data for those women separately. Of note, a subgroup analysis of cardiac events in the trial found a hazard ratio of 0.89 for those women within 10 years of menopause, 1.22 for those 10 to 15 years from menopause, and 1.71 for those greater than 20 years from menopause.49 The results demonstrate a nonsignificant, but suggestive, trend toward decreased events in those who initiated treatment shortly after menopause and increased events for those who started treatment many years after menopause. This meta-analysis pooled the results of 13 trials in older women and found no change in total mortality (OR, 1.03; 95% CI, 0.9 to 1.18). Of note, a post-hoc subgroup analysis showed that trials of women with known cardiovascular disease had the same OR for cardiovascular mortality (1.10; 95% CI, 0.86 to 1.41) as those trials of older women without known cardiovascular disease (1.12; 95% CI, 0.79 to 1.58). This indicates that there may be significant progression of atherosclerosis in healthy older women who have been without hormone replacement for many years, so that primary prevention of cardiac disease may not be possible at this stage. The accumulated evidence indicates that once atherosclerosis has already developed, HRT has no effect at reversing the process.28,46,76,77
When HRT is started in older women a significant time trend is seen, with increased cardiovascular events in the first year and then decreasing events over the next few years.9,78 This is thought to be due to a prothrombotic effect of HRT that is greatest within the first year of treatment.12,79 Estrogen treatment has been shown to increase levels of C-reactive protein and von Willebrand factor antigen, and may promote arterial thrombosis or plaque destabilization in women with established atherosclerosis.80–82 Few trials have followed patients beyond 5 years, so it is not possible say whether long-term treatment is associated with a net cardiovascular benefit or harm in older women.
Hormone replacement has also been shown to affect cancer risk, but its effect on cancer deaths is less clear. In the WHI trial, HRT increased breast cancer incidence by 26% and reduced colon cancers by 27%, without changing the risk for death from each disease.9,83 This meta-analysis found no change in breast cancer deaths or total cancer deaths. Observational studies have found HRT to be associated with increased mortality for breast cancer and reduced mortality for colon cancer.84,85
Hormone replacement has beneficial effects in conditions other than cancer and cardiovascular disease, such as a 35% reduction in the incidence of hip fractures and new-onset diabetes mellitus, and a 60% reduction in recurrent urinary tract infections.9,30,50,86 In this meta-analysis, hormone replacement decreased the risk of deaths from causes other than cardiovascular disease or cancer. It is possible that this is due, in part, to a reduction in the complications from hip fracture, diabetes mellitus, and sepsis.
This analysis has several limitations, some that are common to most meta-analyses.87 Standard meta-analytic results are uncertain when the numbers of events per study are small, as is the case with mortality. There was marked heterogeneity in the trials, although no heterogeneity was seen in the results. There was a wide range in study size, medication used, and method of administration. The results for the older group were mainly from a few large high-quality trials, with a majority of the data coming from one trial.9 The results for the younger group were from many smaller trials, and approximately one half of the deaths were from one trial in ovarian cancer survivors.25 However, when this trial was excluded from the analysis, there still was a significant reduction in mortality (OR, 0.56; 95% CI, 0.31 to 0.99).
Another limitation of the study is that the age groups were defined according to the mean age of participants in each trial and not based on individual patient's characteristics, allowing for some overlap of ages in the two groups. Most trials did not include mortality as a primary outcome, so it is not clear how ascertainment of deaths was made in each trial. However, all trials reported adverse outcomes that included deaths, and investigators were contacted to get more information about mortality. It is unlikely that the reporting of deaths would be different for the two age groups. It was not possible to assess the absolute mortality rates in this study, as only those trials with at least 1 death could be included in the analysis. The search revealed another 98 trials that were excluded because no deaths were reported. This analysis was based only on published literature and therefore is subject to publication bias. However, funnel plots of effect size versus standard error for the trials in this analysis showed no evidence of bias. Few trials provided data on treatment for longer than 5 years, so it is not possible to assess mortality risk with long-term treatment. Despite these limitations, we believe that this meta-analysis provides valuable evidence concerning the mortality risk associated with HRT in younger and older women.
Treatment decisions concerning hormone replacement must be made on an individual basis, taking into account the age of the woman, the degree of bothersome postmenopausal symptoms, and any associated disease risk factors. The results of this analysis indicate that the benefits of HRT may outweigh the risks if treatment is given to younger women, but the risks may outweigh benefits if treatment is started at a later age. This study could not assess the optimal age at initiation of treatment or the duration of treatment needed in order to maximize benefits while minimizing risks. More large randomized trials of long duration, studying younger women near the start of menopause, would be needed to adequately address these issues.
Acknowledgments
Financial support was provided by the Santa Clara Valley Medical Center and the University of California, San Francisco.
The authors thank Hai Emily Huang and Donald Miller for technical assistance and Christopher Stave for coordinating the trials search.
REFERENCES
- 1.Wolf PH, Madans JH, Finucane FF, Higgins M, Kleinman JC. Reduction of cardiovascular disease-related mortality among postmenopausal women who use hormones: evidence from a national cohort. Am J Obstet Gynecol. 1991;164:489–94. doi: 10.1016/s0002-9378(11)80006-2. [DOI] [PubMed] [Google Scholar]
- 2.Grodstein R, Stampfer JJ, Colditz GA, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769–75. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 3.Bush TL, Barrett-Conner E, Cowan LD, et al. Cardiovascular mortality and contraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75:1102–9. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- 4.Sourander L, Rajala T, Raiha I, Makinen J, Ekkola R, Helenius H. Cardiovascular and cancer morbidity and mortality and sudden cardiac death in postmenopausal women on oestrogen replacement therapy (ERT) Lancet. 1998;352:1965–9. doi: 10.1016/S0140-6736(98)05066-1. [DOI] [PubMed] [Google Scholar]
- 5.Petitti DB, Perlman JA, Sidney S. Noncontraceptive estrogens and mortality: long-term follow-up of women in the Walnut Creek Study. Obstet Gynecol. 1987;70:289–93. [PubMed] [Google Scholar]
- 6.Ettinger B, Friedman GM, Bush T, Quesenberry CP. Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstet Gynecol. 1996;87:6–12. doi: 10.1016/0029-7844(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 7.Criqui MH, Suarez L, Barrett-Connor E, McPhillips J, Wingard DL, Garland C. Postmenopausal estrogen use and mortality. Results from a prospective study in a defined, homogeneous community. Am J Epidemiol. 1988;128:606–14. doi: 10.1093/oxfordjournals.aje.a115008. [DOI] [PubMed] [Google Scholar]
- 8.Cauley JA, Seeley DG, Browner WS, et al. Estrogen replacement therapy and mortality among older women. The study of osteoporotic fractures. Arch Intern Med. 1997;157:2181–7. [PubMed] [Google Scholar]
- 9.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Barrett-Conner E. Post-menopausal estrogen and prevention bias. Ann Intern Med. 1991;115:455–6. doi: 10.7326/0003-4819-115-6-455. [DOI] [PubMed] [Google Scholar]
- 11.Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga PA. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol. 1996;143:971–8. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- 12.Humphrey LL, Chan BKS, Sox HC. Postmenopausal hormone replacement therapy and the primary prevention of cardiovascular disease. Ann Intern Med. 2002;137:273–84. doi: 10.7326/0003-4819-137-4-200208200-00012. [DOI] [PubMed] [Google Scholar]
- 13.Fleiss JL. Statistical Methods for Rates and Proportions. New York: Wiley; 1981. pp. 217–34. [Google Scholar]
- 14.Jadad AR, Moore A, Carroll D, et al. Assessing the quality or reports of randomized clinical trials. Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 15.Schultz KF, Chalmers I, Hayes RG, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Conteras I, Parra D. Estrogen replacement therapy and the prevention of coronary heart disease in postmenopausal women. Am J Health Syst Pharm. 2000;57:1963–71. [PubMed] [Google Scholar]
- 18.Gorodeski GI. Impact of the menopause on the epidemiology and risk factors of coronary artery heart disease in women. Exp Gerontol. 1994;29:357–75. doi: 10.1016/0531-5565(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 19.Angerer P, Kothny W, Stork S, von Schacky C. Hormone replacement therapy and distensibility of carotid arteries in postmenopausal women: a randomized, controlled trial. J Am Coll Cardiol. 2000;36:1789–96. doi: 10.1016/s0735-1097(00)00969-4. [DOI] [PubMed] [Google Scholar]
- 20.Arrenbrecht S, Boermans AJ. Effects of transdermal estradiol delivered by a matrix patch on bone density in hysterectomized, postmenopausal women: a 2-year placebo-controlled trial. Osteoporos Int. 2002;13:176–83. doi: 10.1007/s001980200010. [DOI] [PubMed] [Google Scholar]
- 21.Binder EF, Williams DB, Schechtman KB, Jeffe DB, Kohrt WM. Effects of hormone replacement therapy on serum lipids in elderly women. A randomized, placebo-controlled trial. Ann Intern Med. 2001;134:754–60. doi: 10.7326/0003-4819-134-9_part_1-200105010-00012. [DOI] [PubMed] [Google Scholar]
- 22.Cherry N, Gilmour K, Hannaford P, et al. Oestrogen therapy for prevention of reinfarction in postmenopausal women: a randomised placebo controlled trial. Lancet. 2002;360:2001–8. doi: 10.1016/s0140-6736(02)12001-0. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab. 2001;86:3618–28. doi: 10.1210/jcem.86.8.7703. [DOI] [PubMed] [Google Scholar]
- 24.Giske LE, Hall G, Rud T, Landgren BM. The effect of 17beta-estradiol at doses of 0.5, 1 and 2 mg compared with placebo on early postmenopausal bone loss in hysterectomized women. Osteoporos Int. 2002;13:309–16. doi: 10.1007/s001980200031. [DOI] [PubMed] [Google Scholar]
- 25.Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors: a randomized controlled trial. Cancer. 1999;86:1013–8. doi: 10.1002/(sici)1097-0142(19990915)86:6<1013::aid-cncr17>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Hall GM, Daniels M, Doyle DV, Spector TD. Effect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroids. Arthritis Rheum. 1994;37:1499–505. doi: 10.1002/art.1780371014. [DOI] [PubMed] [Google Scholar]
- 27.Hall G, Pripp U, Schenck-Gustafsson K, Landgren BM. Long-term effects of hormone replacement therapy on symptoms of angina pectoris, quality of life and compliance in women with coronary artery disease. Maturitas. 1998;28:235–42. doi: 10.1016/s0378-5122(97)00080-7. [DOI] [PubMed] [Google Scholar]
- 28.Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522–9. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 29.Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–53. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 30.Hulley S, Furberg C, Barrett-Connor E, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 31.Komulainen M, Kroger H, Tuppurainen MT, et al. Prevention of femoral and lumbar bone loss with hormone replacement therapy and vitamin D3 in early postmenopausal women: a population-based 5-year randomized trial. J Clin Endocrinol Metab. 1999;84:546–52. doi: 10.1210/jcem.84.2.5496. [DOI] [PubMed] [Google Scholar]
- 32.Kyllonen ES, Heikkinen JE, Vaananen HK, et al. Influence of estrogen-progestin replacement therapy and exercise on lumbar spine mobility and low back symptoms in a healthy early postmenopausal female population: a 2-year randomized controlled trial. Eur Spine J. 1998;7:381–6. doi: 10.1007/s005860050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindsay R, Hart DM, Aitken JM, MacDonald EB, Anderson JB, Clarke AC. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet. 1976;1:1038–41. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald AG, Murphy EA, Capell HA, Bankowska UZ, Ralston SH. Effects of hormone replacement therapy in rheumatoid arthritis: a double blind placebo-controlled study. Ann Rheum Dis. 1994;53:54–7. doi: 10.1136/ard.53.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mijatovic V, Netelenbos C, van der Mooren MJ, de Valk-de Roo GW, Jakobs C, Kenemans P. Randomized, double-blind, placebo-controlled study of the effects of raloxifene and conjugated equine estrogen on plasma homocysteine levels in healthy postmenopausal women. Fertil Steril. 1998;70:1085–9. doi: 10.1016/s0015-0282(98)00381-1. [DOI] [PubMed] [Google Scholar]
- 36.Mosekilde L, Beck-Nielsen H, Sorensen OH, et al. Hormonal replacement therapy reduces forearm fracture incidence in recent postmenopausal women—results of the Danish Osteoporosis Prevention Study. Maturitas. 2000;36:181–93. doi: 10.1016/s0378-5122(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 37.Mulnard RA, Cotman RA, Kawas C, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease. JAMA. 2000;283:1007–15. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 38.Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy II: a prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstet Gynecol. 1979;54:74–9. doi: 10.1097/00006250-197907000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Os I, Hofstad AE, Brekke M, et al. The EWA (estrogen in women with atherosclerosis) study: a randomized study of the use of hormone replacement therapy in women with angiographically verified coronary artery disease. Characteristics of the study population. Effects on lipids and lipoproteins. J Intern Med. 2000;247:433–41. doi: 10.1046/j.1365-2796.2000.00675.x. [DOI] [PubMed] [Google Scholar]
- 40.PEPI Trial Writing Group. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 41.Perez-Jaraiz MD, Revilla M, Alvarez de los Heros JI, Villa LF, Rico H. Prophylaxis of osteoporosis with calcium, estrogens and/or eelcatonin: comparative longitudinal study of bone mass. Maturitas. 1996;23:327–32. doi: 10.1016/0378-5122(96)00999-1. [DOI] [PubMed] [Google Scholar]
- 42.Ravn P, Bidstrup M, Wasnich RD, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med. 1999;131:935–42. doi: 10.7326/0003-4819-131-12-199912210-00005. [DOI] [PubMed] [Google Scholar]
- 43.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753–6. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 44.Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Ann Intern Med. 1999;130:897–904. doi: 10.7326/0003-4819-130-11-199906010-00005. [DOI] [PubMed] [Google Scholar]
- 45.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–9. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 46.Waters D, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women. JAMA. 2002;288:2432–40. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 47.Watts NB, Nolan JC, Brennan JJ, Yang HM. Esterified estrogen therapy in postmenopausal women. Relationships of bone marker changes and plasma estradiol to BMD changes: a two-year study. Menopause. 2000;7:375–82. doi: 10.1097/00042192-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348:645–50. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 49.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 50.Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/Progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 51.Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch Intern Med. 2000;160:3315–25. doi: 10.1001/archinte.160.21.3315. [DOI] [PubMed] [Google Scholar]
- 52.Vehkavaara S, Silveira A, Hakala-Ala-Pietila T, et al. Effects of oral and transdermal estrogen replacement therapy on markers of coagulation, fibrinolysis, inflammation and serum lipids and lipoproteins in postmenopausal women. Thromb Haemost. 2001;85:619–25. [PubMed] [Google Scholar]
- 53.Conard J, Gompel A, Pelissier C, Mirabel C, Basdevant A. Fibrinogen and plasminogen modifications during oral estradiol replacement therapy. Fertil Steril. 1997;68:449–53. doi: 10.1016/s0015-0282(97)00220-3. [DOI] [PubMed] [Google Scholar]
- 54.Demirol A, Baykal C, Kirazli S, Ayhan A. Effects of hormone replacement on hemostasis in spontaneous menopause. Menopause. 2001;8:135–40. doi: 10.1097/00042192-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Manning PJ, Allum A, Jones S, Sutherland WH, Williams SM. The effect of hormone replacement therapy on cardiovascular risk factors in type 2 diabetes: a randomized controlled trial. Arch Intern Med. 2001;161:1772–6. doi: 10.1001/archinte.161.14.1772. [DOI] [PubMed] [Google Scholar]
- 56.Haines C, Chung T, Chang A, Masarei J, Tomlinson B, Wong E. Effect of oral estradiol on Lp(a) and other lipoproteins in postmenopausal women. A randomized, double-blind, placebo-controlled, crossover study. Arch Intern Med. 1996;156:866–72. [PubMed] [Google Scholar]
- 57.Walsh BW, Kuller LH, Wild RA, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445–51. doi: 10.1001/jama.279.18.1445. [DOI] [PubMed] [Google Scholar]
- 58.Ventura P, Cagnacci A, Malmusi S, et al. Continuous combined hormone replacement therapy with oral 17beta-estradiol and norethisterone acetate improves homocysteine metabolism in postmenopausal women. Menopause. 2001;8:252–8. doi: 10.1097/00042192-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Samaras K, Hayward CS, Sullivan D, Kelly RP, Campbell LV. Effects of postmenopausal hormone replacement therapy on central abdominal fat, glycemic control, lipid metabolism, and vascular factors in type 2 diabetes: a prospective study. Diabetes Care. 1999;22:1401–7. doi: 10.2337/diacare.22.9.1401. [DOI] [PubMed] [Google Scholar]
- 60.Friday KE, Dong C, Fontenot RU. Conjugated equine estrogen improves glycemic control and blood lipoproteins in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2001;86:48–52. doi: 10.1210/jcem.86.1.7094. [DOI] [PubMed] [Google Scholar]
- 61.Cicinelli E, Ignarro LJ, Matteo MG, Galantino P, Schonauer LM, Falco N. Effects of estrogen replacement therapy on plasma levels of nitric oxide in postmenopausal women. Am J Obstet Gynecol. 1999;180:334–9. doi: 10.1016/s0002-9378(99)70209-7. [DOI] [PubMed] [Google Scholar]
- 62.Saitta A, Altavilla D, Cucinotta D, et al. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma NO concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol. 2001;21:1512–9. doi: 10.1161/hq0901.095565. [DOI] [PubMed] [Google Scholar]
- 63.Guzic-Salobir B, Keber I, Seljeflot I, Arnesen H, Vrabic L. Combined hormone replacement therapy improves endothelial function in healthy postmenopausal women. J Intern Med. 2001;250:508–15. doi: 10.1046/j.1365-2796.2001.00910.x. [DOI] [PubMed] [Google Scholar]
- 64.Manwaring P, Phoon S, Diamond T, Howes LG. Effects of hormone replacement therapy on cardiovascular responses in postmenopausal women with and without type 2 diabetes. Maturitas. 2002;43:157–64. doi: 10.1016/s0378-5122(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 65.Penotti M, Sironi L, Castiglioni E, et al. Blood flow in the internal carotid and middle cerebral arteries: effects of continuous oral conjugated equine estrogens administration with monthly progestogen supplementation on postmenopausal women. Menopause. 1999;6:225–9. doi: 10.1097/00042192-199906030-00008. [DOI] [PubMed] [Google Scholar]
- 66.Affinito P, Palomba S, Bonifacio M, et al. Effects of hormonal replacement therapy in postmenopausal hypertensive patients. Maturitas. 2001;40:75–83. doi: 10.1016/s0378-5122(01)00196-7. [DOI] [PubMed] [Google Scholar]
- 67.Lau TK, Wan D, Yim SF, Sanderson JE, Haines CJ. Prospective, randomized, controlled study of the effect of hormone replacement therapy on peripheral blood flow velocity in postmenopausal women. Fertil Steril. 1998;70:284–8. doi: 10.1016/s0015-0282(98)00148-4. [DOI] [PubMed] [Google Scholar]
- 68.Light KC, Hinderliter AL, West SG, et al. Hormone replacement improves hemodynamic profile and left ventricular geometry in hypertensive and normotensive postmenopausal women. J Hypertens. 2001;19:269–78. doi: 10.1097/00004872-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Gallinelli A, Angioni S, Matteo ML, Montaldo PL, Fenu MA, Volpe A. Variations of cardiac performance and inotropism in healthy postmenopausal women treated with estroprogestin replacement therapy. Menopause. 1999;6:49–55. [PubMed] [Google Scholar]
- 70.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–52. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 71.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–10. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 72.Hough JL, Zilversmit DB. Effect of 17 beta estradiol on aortic cholesterol content and metabolism in cholesterol-fed rabbits. Arteriosclerosis. 1986;6:57–63. doi: 10.1161/01.atv.6.1.57. [DOI] [PubMed] [Google Scholar]
- 73.Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81:1680–7. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
- 74.Wagner JD, Clarkson TB, St Clair RW, Schwenke DC, Shively CA, Adams MR. Estrogen and progesterone replacement therapy reduces low density lipoprotein accumulation in the coronary arteries of surgically postmenopausal cynomolgus monkeys. J Clin Invest. 1991;88:1995–2002. doi: 10.1172/JCI115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kushwaha RS, Lewis DS, Carey KD, McGill HC., Jr Effects of estrogen and progesterone on plasma lipoproteins and experimental atherosclerosis in the baboon (Papio sp.) Arterioscler Thromb. 1991;11:23–31. doi: 10.1161/01.atv.11.1.23. [DOI] [PubMed] [Google Scholar]
- 76.Rosenfeld ME, Kauser K, Martin-McNulty B, Polinsky P, Schwartz SM, Rubanyi GM. Estrogen inhibits the initiation of fatty streaks throughout the vasculature but does not inhibit intra-plaque hemorrhage and the progression of established lesions in apolipoprotein E deficient mice. Atherosclerosis. 2002;164:251–9. doi: 10.1016/s0021-9150(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 77.Hodis HN, Mack WJ, Azen SP, et al. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349:535–45. doi: 10.1056/NEJMoa030830. [DOI] [PubMed] [Google Scholar]
- 78.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 79.Miller J, Chan BK, Nelson HD. Postmenopausal estrogen replacement and risk for venous thromboembolism: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:680–90. doi: 10.7326/0003-4819-136-9-200205070-00011. [DOI] [PubMed] [Google Scholar]
- 80.Pradhan AD, Manson JE, Rossouw JE, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women's Health Initiative observational study. JAMA. 2002;288:980–7. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 81.Rabbani LE, Seminario NA, Sciacca RR, Chen HJ, Giardina EG. Oral conjugated equine estrogen increases plasma von Willebrand factor in postmenopausal women. J Am Coll Cardiol. 2002;40:1991–9. doi: 10.1016/s0735-1097(02)02565-2. [DOI] [PubMed] [Google Scholar]
- 82.Brussaard HE, Leuven JA, Krans HM, Kluft C. The effect of 17 beta-oestradiol on variables of coagulation and fibrinolysis in postmenopausal women with type 2 diabetes mellitus. Vascul Pharmacol. 2002;39:141–7. doi: 10.1016/s1537-1891(02)00303-8. [DOI] [PubMed] [Google Scholar]
- 83.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 84.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 85.Nanda K, Bastian LA, Hasselblad V, Simel DL. Hormone replacement therapy and the risk of colorectal cancer: a meta-analysis. Obstet Gynecol. 1999;93:880–8. doi: 10.1016/s0029-7844(98)00424-4. [DOI] [PubMed] [Google Scholar]
- 86.Cardozo L, Lose G, McClish D, Versi E, de Konning Glans H. A systematic review of estrogens for recurrent urinary tract infections: third report of the Hormones and Urogenital therapy (HUT) committee. Int Urogynecol J. 2001;12:15–20. doi: 10.1007/s001920170088. [DOI] [PubMed] [Google Scholar]
- 87.Ionnidis JP, Lau J. Pooling research results: benefits and limitations of meta-analysis. Jt Comm J Qual Improv. 1999;25:462–9. doi: 10.1016/s1070-3241(16)30460-6. [DOI] [PubMed] [Google Scholar]