Abstract
BACKGROUND
Mind-body practices such as yoga are widely popular, but little is known about how such exercises impact health-related quality of life.
OBJECTIVE
To measure changes in health-related quality of life associated with 3 months of mind-body training as practiced in community-based settings.
DESIGN
Prospective cohort study.
SETTING
Eight centers for practice of mind-body training.
PARTICIPANTS
One hundred ninety-four English-speaking adults who had taken no more than 10 classes at the centers prior to enrollment in the study. One hundred seventy-one (88%) returned the 3-month follow-up questionnaire.
INTERVENTION
Administration of the SF-36 questionnaire at the start of training and after 3 months.
MEASUREMENTS AND MAIN RESULTS
At baseline, new participants in mind-body training reported lower scores than U.S. norms for 7 of 8 domains of the SF-36: mental health, role emotional, social, vitality, general health, body pain, and role physical (P < .002 for all comparisons). After 3 months of training, within-patient change scores improved in all domains (P < .0001), including a change of +15.5 (standard deviation ±21) in the mental health domain. In hierarchical regression analysis, younger age (P= .0003), baseline level of depressive symptoms (P= .01), and reporting a history of hypertension (P= .0054) were independent predictors of greater improvement in the SF-36 mental health score. Five participants (2.9%) reported a musculoskeletal injury.
CONCLUSIONS
New participants in a community-based mind-body training program reported poor health-related quality of life at baseline and moderate improvements after 3 months of practice. Randomized trials are needed to determine whether benefits may be generalizable to physician-referred populations.
Keywords: alternative medicine, behavorial, health promotion disease prevention, patient involvement, quality of life
Popular interest in yoga and other mind-body practices is strong, but there is still little data on the health effects of such training. Randomized trial designs have been used to investigate the value of mind-body training for clinical conditions including hypertension,1 osteoarthritis,2 risk of falling,3 carpal tunnel syndrome,4 and depression.5 However, no studies from the United States have specifically evaluated mind-body training programs as they are typically provided in community-based settings. Nor has any study from the United States measured the impact of mind-body training on general health-related quality of life, using validated assessment instruments.
We proposed to study health effects associated with new participation in a community-based mind-body training program. The primary objective was to measure changes in health-related quality of life after 3 months of training in dahn-hak (this practice is also offered under the trademarked name “Brain Respiration”), a modernized mind-body training program with origins in South Korea. This practice shares elements of hatha yoga (stretching, postures) and qigong (energy cultivation). The population included new participants in the program as practiced in community-based centers. Study subjects underwent training as would any other new participant at a center, without interference from the study investigators.
The primary outcome measure was the mental health domain of the Medical Outcomes Study SF-36 general health questionnaire. This domain was chosen because several prospective studies have suggested a benefit of yoga in depression and anxiety.5–9 Furthermore, the positive effects of physical exercise on mood have been well documented.10 We hypothesized that new participants in mind-body training would report a 5-point improvement (on a scale of 0 to 100) in the mental health domain of the SF-36 after 3 months of practice, a magnitude comparable to changes observed in four prior prospective studies of the effects of physical exercise on health-related quality of life.11–14 Data on demographic and clinical characteristics and frequency of participation were collected to identify whether any population subsets were more likely to experience a benefit from mind-body training, or whether training was associated with dose-response effects. A secondary objective was to identify any physical injuries or other adverse experiences associated with practice and quantify their incidence.
METHODS
Study Population
New participants in mind-body training were recruited from eight community-based centers in the metropolitan New York City area, including two in Manhattan, two in Queens, three on Long Island, and one in northern New Jersey. Participants were eligible if they were age 18 or greater, and had taken no more than 10 classes at their centers at the time of enrollment (in order for baseline data to be maximally reflective of health status prior to any impact of training). They were English speaking and signed informed consent to participate. Past history of participation in other forms of mind-body exercise was not an exclusion, as we felt that the plethora and variety of mind-body methods now available to the public (in studios, community centers, by videotape, etc.) precluded creation of legitimate exclusion criteria based on past practice. Participants were recruited during beginner orientation sessions, which generally occurred 2 to 4 times monthly at each center. Instructors at all centers were aware of the study and notified the principal investigator if a new member enrolled who met inclusion criteria and had not attended an orientation session (less than 10% of enrollees in the study). Study recruitment took place between January 8 and June 22, 2002. The study was approved by the Institutional Review Board of New York Presbyterian Hospital, Weill Cornell Medical Center.
Baseline Data Collection
Upon enrollment, demographic data were collected including age, gender, educational level, employment status, health insurance, access to a physician, marital status, and ethnic background. Clinical data were collected including medications used (prescription and over the counter), medical comorbidity as measured by the Charlson Comorbidity Index,15 and history of hypertension or arthritis (which are not included in the Charlson Index). Participants’ baseline level of physical activity in kcal per week was measured using the Paffenbarger Physical Activity and Exercise Index,16 given the known effects of physical activity on health-related quality of life.17
The questionnaire included the Medical Outcomes Study Short Form-36, a validated health assessment instrument that measures 8 domains of health-related quality of life (mental, role emotional, physical function, role physical, vitality, social, body pain, and general health; range 0 to 100 for each domain).18 The primary outcome measure was the mental health domain of the SF-36. Summary scores for the SF-36 were not used, given the known limitations of their algorithms when evaluating interventions that affect both physical and mental well-being.19
In order to discriminate between depressive and anxious symptoms, the questionnaire also included the Centers for Epidemiologic Studies Depressive Symptoms Inventory20 (range 0 to 60) and the Spielberger Trait Anxiety Inventory (range 20 to 80).21 Because yogic training is commonly described as a means of gaining greater “self-control,” we were also interested in measuring whether training would be associated with changes in self-efficacy. Self-efficacy was measured using the Generalized Self-efficacy Scale22 (range 10 to 40). Participants were contacted by telephone within 7 days of enrollment to encourage completion of the survey.
Study participants trained at their respective centers as would any other new enrollee in the practice. Training in this practice typically consists of a 1-hour class 2 to 3 times per week, which consists of 3 major segments. The class begins with stretching exercises, which increase flexibility in the large muscle groups and shoulders, neck, hips, back, and knees. In the second phase, postures are held for “energy accumulation,” followed by a brief (5- to 10-minute) period of meditation intended to facilitate “energy awareness.” Class concludes with a repetition of the large muscle group stretches.
Follow-up Data Collection
Three months after enrollment, a follow-up questionnaire was mailed to those volunteers who had returned the initial survey. This questionnaire included the SF-36, the Centers for Epidemiologic Studies Depression Scale for depressive symptoms, the Spielberger Trait Anxiety Inventory for trait anxiety, and the Generalized Self-efficacy Scale. It included questions related to level of participation in the practice (estimated number of classes attended in the preceding 3 months, number of classes attended per week, frequency of home exercise completion per week), whether any other new physical exercises were undertaken, and whether any medications had changed since enrollment (new medications, changed dosage, or discontinued). The questionnaire also asked whether participants had had any “negative experiences or injuries” associated with their practice. Volunteers were again contacted, either by telephone or in person, if questionnaires were not returned within 2 weeks of mailing. If contact was established, the questionnaire was administered directly (i.e., by telephone or in person).
Statistical Analysis
Sample size was calculated based on effect sizes observed in previous clinical trials of physical exercise to improve health-related quality of life.11–14 The weighted mean magnitude of improvements in the SF-36 mental health domain in those studies was 6 (standard deviation [SD]± 22). Based on a hypothesized change of 5 (SD ± 20) in the SF-36 mental health domain for the present study, with a two-tailed α= 0.05 and 90% power, 168 persons were required for the study.
Descriptive statistics were generated using all baseline variables. Paired t tests were used to compare baseline and 3-month follow-up scores for each participant, for all outcome variables. Linear regression was performed using within-subject change in the SF-36 mental health domain score as the dependent variable, to identify independent predictors of change in the mental health score. Demographic predictors entered into the model included the following: age, gender, educational level, ethnicity, and marital, employment, and health insurance status. Charlson comorbidity score, history of hypertension, history of osteoarthritis, baseline physical activity in kcal per week, use of psychiatric, cardiovascular, or thyroid medication, and baseline depressive symptoms were all included in the model. These variables were entered in order to determine whether mental health changes related to yogic training might be greater in particular clinical populations. Participation variables including number of classes attended and completion of home exercises were entered, to test for dose-response effects. In order to rule out possible effects related to co-interventions, history of new antidepressant medication and/or other new physical exercise since enrollment in the study was also entered into the model. For modeling of changes in the mental health domain of the SF-36 (primary outcome variable), age and predictors significant at P < .10 in bivariate analysis were entered into a backward stepwise multiple regression. Only variables significant at P < .05 were retained in the final model.
Regression analysis was performed using a hierarchical model to account for potential correlations related to individual volunteers, center of practice, or geographical location (Manhattan, Queens, Long Island, or northern New Jersey), using participant, center, and location as random factors. All calculations were performed using SAS (version 8, SAS Institute, Cary, NC).
RESULTS
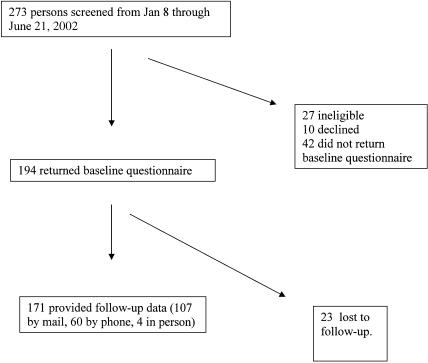
The flow of participant recruitment and data collection is shown in Figure 1. The 23 participants lost to follow-up were significantly younger (mean age 34; P = .029) and had lower self-efficacy (mean generalized self-efficacy score 28.1; P = .039) than those who provided follow-up data.
FIGURE 1.
Flow of participant recruitment and data collection.
Baseline characteristics of study participants are shown in Table 1. Most of the participants were women and were relatively well educated. A majority had completed college or graduate-level education. Over half identified their ethnic background as white. Most had health insurance. Participants were relatively free of comorbid medical conditions, with only 12% having a Charlson comorbidity score of 1 or higher, and 12% reporting a history of hypertension. Participants were relatively sedentary, reporting physical activity less than the 1,000 kcal per week recommended by the U.S. Surgeon General.23
Table 1.
Characteristics of Study Participants at Baseline (N = 194)
| Value | |
|---|---|
| Mean age, y (± SD, range) | 40 (12, 18 to 67) |
| Women, % | 77 |
| Location of practice, % | |
| Manhattan | 14 |
| Queens | 29 |
| Long Island | 49 |
| Northern New Jersey | 9 |
| Ethnicity, % | |
| African American | 7 |
| Asian or Asian American | 17 |
| White | 52 |
| Hispanic | 15 |
| Other | 10 |
| Physical activity in kcal/week (± SD) | 723 (970) |
| Educational level, % | |
| High school or less | 23 |
| Some college | 20 |
| Completed college | 31 |
| Graduate or professional school | 26 |
| Married, % | 40 |
| Has health insurance, % | 83 |
| Charlson comorbidity score ≥1, % | 12 |
| History of hypertension, % | 12 |
| Current prescription medication use, % | |
| Cardiovascular | 10 |
| Antidepressant* | 8 |
| Sedative-hypnotic† | 6 |
| Thyroid | 6 |
Includes 1 person taking St. John's wort.
Includes 2 persons taking kava.
SD, standard deviation.
Baseline scores for health-related quality of life, depressive symptoms, trait anxiety, and generalized self-efficacy are shown in Table 2, along with scores on these measures from other studies of community-based populations in the United States.18,20,21,24 Although they did not have significant medical comorbidity, new participants in mind-body training reported worse baseline scores for 7 of 8 domains of health-related quality of life, more depressive symptoms, and more trait anxiety than community-based populations. However, the mean score for self-efficacy was slightly higher than community-based norms.
Table 2.
Baseline Scores for Health-related Quality of Life, Depressive Symptoms, Trait Anxiety, and Self-efficacy, Compared to U.S. Community Samples
| Participants, n (± SD) | U.S. Samples, n (± SD) | P Value | |
|---|---|---|---|
| Health-related quality of life* | |||
| Mental health | 57.3 (21) | 74.7 (18) | <.0001 |
| Role emotional | 56.5 (44) | 81.3 (33) | <.0001 |
| Physical function | 81.8 (19) | 84.2 (23) | NS |
| Role physical | 72.7 (36) | 81.0 (34) | .0015 |
| Social | 66.9 (28) | 83.3 (23) | <.0001 |
| Vitality | 47.8 (21) | 60.9 (21) | <.0001 |
| Body pain | 62.2 (24) | 75.2 (24) | <.0001 |
| General health | 64.9 (20) | 72.0 (20) | <.0001 |
| Depressive symptoms (CESD)† | 17.4 (12) | 9.3 (9) | <.0001 |
| Trait anxiety (STAI)‡ | 44.8 (12) | 35.0 (9) | <.0001 |
| Self-efficacy (GSES)§ | 30.3 (5) | 29.5 (5) | .0270 |
Measured by the SF-36 (8 domains, range 0 to 100 for each domain; higher scores indicate better status).
Centers for Epidemiologic Studies Depression Scale (CESD; range 0 to 60; higher scores indicate more depressive symptoms).
Spielberger Trait Anxiety Inventory (STAI; range 20 to 80; higher scores indicate more anxiety).
Generalized Self-efficacy Scale (GSES; range 10 to 40; higher scores indicate greater self-efficacy).
SD, standard deviation.
After 3 months, participants reported taking a mean of 24 (SD 13; range 0 to 100) classes at their respective centers. Twenty-six percent reported beginning an additional form of physical exercise since enrolling in the study, such as using a gym, going for walks, beginning other kinds of yoga, or playing recreational sports.
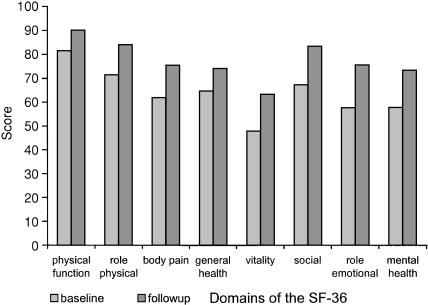
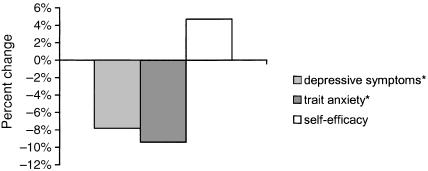
Mean baseline SF-36 scores, compared with 3-month follow-up scores, are shown in Figure 2. All domains improved (P < .0001), and the mean improvement in the mental health domain was +16. One hundred fourteen participants provided data on secondary outcome measures. On average, they reported fewer depressive symptoms, less trait anxiety, and greater self-efficacy than they did at baseline (Fig. 3
FIGURE 2.
Mean baseline and follow-up scores for domains of the SF-36 general health questionnaire (n = 171). All changes significant at P < .0001.
FIGURE 3.
Mean within-subject improvements in depressive symptoms, trait anxiety, and self-efficacy after 3 months of mind-body training (n = 114). Changes are expressed as a percentage change of scale range. All changes significant at P < .0001. *Lower scores indicate better status.
Hierarchical regression analysis was performed to identify predictors of within-subject improvement in the mental health domain score of the SF-36, the primary outcome variable. In the final multivariate model, younger age, history of hypertension, and more depressive symptoms at baseline were independent predictors of greater improvement in mental health score (Table 3). History of hypertension remained significant after excluding those persons who did not report antihypertensive medication use. Number of classes attended did not predict greater improvement in the mental health score (β= 0.14; P = .27).
Table 3.
Independent Predictors of Greater Improvement in SF-36 Mental Health Score, in Multivariate Hierarchical Regression Model
| Coefficient | P Value | |
|---|---|---|
| Age | −0.5 | .0003 |
| History of hypertension | 12.8 | .0099 |
| Baseline depressive symptoms* | 0.4 | .0054 |
As measured by Centers for Epidemiologic Studies Depression Scale (range 0 to 60; higher score indicates more depressive symptoms).
Five (3%) of the volunteers reported musculoskeletal injuries related to their exercises (back strains, calf strain, bruise on arm due to a fall). Of these, one person reported indefinitely discontinuing the practice due to the injury (back strain). Two (1%) persons reported discontinuing the practice due to the “cultish” environment of the training. One reported an inability of instructors to engage in conversation not related to the practice. The other reported a discomfort with phone calls made to her home to encourage attendance and with social affection shown by instructors and practitioners of the exercise.
DISCUSSION
To our knowledge, this is the first prospective study measuring changes in health-related quality of life associated with participation in a community-based mind-body training program. We found that at baseline, new participants in mind-body training in the New York City area reported worse health-related quality of life and mental health than other U.S. community-based populations. After 3 months, they reported improvements in all domains of health-related quality of life, fewer depressive symptoms, less trait anxiety, and greater self-efficacy. These findings suggest that participation in mind-body training was a vehicle through which some persons attained moderate, clinically significant improvements in health-related quality of life.
Prior studies have found that users of complementary and alternative health care report more depressive symptoms and anxiety than nonusers,25–31 and our findings were consistent with those data. In contrast to their general health and mood scores, study participants reported a slightly higher score for self-efficacy than community-based populations. This finding corroborates research showing that patients who choose some complementary therapies are more likely to believe in potential self-control over health.32,33
With one exception,31 the previous studies on the relationship between depressive symptoms and complementary health care use have been cross-sectional. Many questions about the effectiveness of such practices have thus remained unanswered. Using a longitudinal design, the present report documents improvements in health-related quality of life and self-reported mental health associated with new participation in mind-body training.
It is not clear what mechanisms may be responsible for health improvements related to mind-body training. Studies have suggested that yoga positively impacts endocrine and biochemical parameters associated with chronic stress,34–39 or what has more recently been termed “allostatic load.”40 Stress has been implicated in the pathogenesis of depression41 and an extensive literature has documented the association of depression with elevated cortisol levels.42 Furthermore, participants may have benefited from an emotionally supportive atmosphere at their training centers, and it is known that caring relationships have a positive impact on physiological parameters.43 To the extent that depressive disorders may be stress related, it is plausible that benefits of yogic training could be mediated through attenuated neurohormonal reactions of the stress response.
While the data did not show a dose-response effect between number of classes attended and improvement in the SF-36 mental health score, it is possible that the analysis was limited by ceiling effects. For example, only 6.7% of study participants reported a mental health score of 90 or greater at baseline (including 1.6% who scored 100). However, at 3-month follow-up, 22.8% of participants reported scores of 90 or greater (including 6.4% who scored 100). Qualitative analysis corroborated a range of emotional and behavioral improvements, such as improved skills at managing stress or anger,44 which are not directly addressed by the SF-36. The presence of ceiling effects on mental health questionnaires further evidences the need for more research on positive health and well-being.45,46
Study participants reported a relatively low rate of adverse outcomes from their training, and all of the physical injuries involved the musculoskeletal system. These injuries were of a milder nature than case reports of adverse outcomes from yogic training,47–52 but their incidence serves as a reminder that physicians may wish to advise patients who join physically oriented mind-body training programs to proceed with their training slowly and without excessive straining. The discomfort expressed by two participants with the training atmosphere suggests that some may experience psychological or cultural discordances in mind-body training centers.53,54
This study had several limitations related to its observational cohort design. We were interested in the self-reported health effects of a complementary health care practice as it is normally offered in community centers. Given this naturalistic study setting, comparison to a valid control group was not deemed practical. Thus it is impossible to know what portion of the measured improvements are attributable to statistical regression to the mean—a problem common to all studies that do not include a nonintervention control group. Although 12% of study participants were lost to follow-up, we do not believe that inclusion of their outcomes would contradict the overall findings of self-reported improvement, especially given the ceiling effects we observed at 3 months. Finally, future studies will need to follow participants over longer intervals in order to determine whether benefits are transient or enduring, and to quantify the degree of training intensity necessary to sustain improvements.
In summary, new participants in a community-based mind-body training program reported moderate improvements in health-related quality of life, fewer depressive symptoms, less trait anxiety, and greater self-efficacy after 3 months of practice. Further studies using randomized designs are warranted to assess whether improvements may be applicable to physician-referred populations.
Acknowledgments
This research was supported by a T32 training grant from the Agency for Healthcare Research and Quality.
REFERENCES
- 1.Young DR, Appel LJ, Jee SH, Miller ER. Effects of aerobic exercise and tai chi on blood pressure in older people: results of a randomized trial. J Am Geriatr Soc. 1999;47:277–84. doi: 10.1111/j.1532-5415.1999.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 2.Hartman CA, Manos TM, Winter C, et al. Effects of tai chi training on function and quality of life indicators in older adults with osteoarthritis. J Am Geriatr Soc. 2000;48:1553–9. doi: 10.1111/j.1532-5415.2000.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 3.Wolf SL, Barnhart HX, Kutner N, et al. Reducing frailty and falls in older persons: an investigation of tai chi and computerized balance training. J Am Geriatr Soc. 1996;44:489–97. doi: 10.1111/j.1532-5415.1996.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 4.Garfinkel MS, Schumacher HR, Husain A, Levy M, Reshetar RA. Evaluation of a yoga based regimen for treatment of osteoarthritis of the hands. J Rheumatol. 1994;21:2341–3. [PubMed] [Google Scholar]
- 5.Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, Harish MG, Subbakrishna DK, Vedamurthachar A. Antidepressant efficacy of Sudarshan Kriya Yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57:255–9. doi: 10.1016/s0165-0327(99)00079-8. [DOI] [PubMed] [Google Scholar]
- 6.Naga Venkatesha Murthy PJ, Janakiramaiah N, Gangadhar BN, Subbakrishna DK. P300 amplitude and antidepressant response to Sudarshan Kriya Yoga (SKY) J Affect Disord. 1998;50:45–8. doi: 10.1016/s0165-0327(98)00029-9. [DOI] [PubMed] [Google Scholar]
- 7.Khumar SS, Kaur P, Kaur S. Effectiveness of shavasana on depression among university students. Indian J Clin Psychol. 1993;20:82–7. [Google Scholar]
- 8.Malathi A, Damodaran A, Shah N, et al. Psychophysiological changes at the time of examination in medical students before and after the practice of yoga and relaxation. Indian J Psychiatry. 1998;40:35–40. [PMC free article] [PubMed] [Google Scholar]
- 9.Malathi A, Damadoran A, Shah N, et al. Effect of yogic practices on subjective well-being. Indian J Physiol Pharmacol. 2000;44:202–6. [PubMed] [Google Scholar]
- 10.Craft LL, Landers DM. Effect of exercise on clinical depression and depression resulting from mental illness: a meta-analysis. J Sport Exer Psychol. 1998;20:339–57. [Google Scholar]
- 11.Mannerkorpi K, Nyberg B, Ahlmen M, Ekdah C. Pool exercise combined with an education program for patients with fibromyalgia syndrome: a prospective, randomized study. J Rheumatol. 2000;27:2473–81. [PubMed] [Google Scholar]
- 12.Painter P, Carlson L, Carey S, Paul SM, Myll J. Physical functioning and health-related quality-of-life changes with exercise training in hemodialysis patients. Am J Kidney Dis. 2000;35:482–92. doi: 10.1016/s0272-6386(00)70202-2. [DOI] [PubMed] [Google Scholar]
- 13.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci. 1997;52A:M27–35. doi: 10.1093/gerona/52a.1.m27. [DOI] [PubMed] [Google Scholar]
- 14.Wallace JI, Buchner DM, Grothaus L, et al. Implementation and effectiveness of a community-based health promotion program for older adults. J Gerontol A Biol Sci Med Sci. 1998;53A:M301–6. doi: 10.1093/gerona/53a.4.m301. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Paffenbarger RS, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 17.Stewart AL, Hays RD, Wells KB. Long-term functioning and well-being outcomes associated with physical activity and exercise in patients with chronic conditions in the medical outcomes study. J Clin Epidemiol. 1994;47:719–30. doi: 10.1016/0895-4356(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston, Mass: Health Institute; 1993. [Google Scholar]
- 19.Simon GE, Revicki DA, Grothaus L, Vonkorff M. SF-36 summary scores: are physical and mental health truly distinct? Med Care. 1998;36:567–72. doi: 10.1097/00005650-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 21.Spielberger CD. Manual for the State-trait Anxiety Inventory. Redwood City, Calif: Mindgarden; 1983. [Google Scholar]
- 22.Schwarzer R, Jerusalem M. Generalized self-efficacy scale. In: Weinman J, Wright S, Johnston M, editors. Measures in Health Psychology: A User's Portfolio. Windsor, UK: NFER-NELSON; 1995. [Google Scholar]
- 23.U.S. Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, Ga: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, International Medical Publishing; 1996. [Google Scholar]
- 24.Scholz U, Gutiérrez-Doña B, Sud S, Schwarzer R. Is perceived self-efficacy a universal construct? Psychometric findings from 25 countries. Eur J Psychol Assess. 2002;18:242–51. [Google Scholar]
- 25.Al-Windi A, Dag E, Kurt S. The influence of perceived well-being and reported symptoms on health care utilization: a population-based study. J Clin Epidemiol. 2002;55:60–6. doi: 10.1016/s0895-4356(01)00423-1. [DOI] [PubMed] [Google Scholar]
- 26.Astin JA. Why patients use alternative medicine: results of a national study. JAMA. 1998;279:1548–53. doi: 10.1001/jama.279.19.1548. [DOI] [PubMed] [Google Scholar]
- 27.Knaudt PR, Connor KM, Weisler RH, Churchill LE, Davidson JR. Alternative therapy use by psychiatric outpatients. J Nerv Ment Dis. 1999;187:692–5. doi: 10.1097/00005053-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Druss BG, Rosenheck RA. Use of practitioner-based complementary therapies by persons reporting mental conditions in the United States. Arch Gen Psychiatry. 2000;57:708–14. doi: 10.1001/archpsyc.57.7.708. [DOI] [PubMed] [Google Scholar]
- 29.Unutzer J, Klap R, Sturm R, et al. Mental disorders and the use of alternative medicine: results from a national survey. Am J Psychiatry. 2000;157:1851–7. doi: 10.1176/appi.ajp.157.11.1851. [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158:289–94. doi: 10.1176/appi.ajp.158.2.289. [DOI] [PubMed] [Google Scholar]
- 31.Burstein HJ, Gelber S, Guadagnoli E, Weeks JC. Use of alternative medicine by women with early-stage breast cancer. N Engl J Med. 1999;340:1733–9. doi: 10.1056/NEJM199906033402206. [DOI] [PubMed] [Google Scholar]
- 32.Furnham A, Bhagrath R. A comparison of health beliefs and behaviors of clients of orthodox and complementary medicine. Br J Clin Psychol. 1993;32:237–46. doi: 10.1111/j.2044-8260.1993.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 33.Furnham A, Forey J. The attitudes, behaviors, and beliefs of patients of conventional vs. complementary (alternative) medicine. J Clin Psychol. 1994;50:458–69. doi: 10.1002/1097-4679(199405)50:3<458::aid-jclp2270500318>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.MacLean CRK, Walton KG, Wenneberg SR, et al. Effects of the Transcendental Meditation program on adaptive mechanisms: changes in hormone levels and responses to stress after 4 months of practice. Psychoneuroendocrinology. 1997;22:277–95. doi: 10.1016/s0306-4530(97)00003-6. [DOI] [PubMed] [Google Scholar]
- 35.McGrady A, Woerner M, Argueta Bernal GA, Higgins JT. Effect of biofeedback-assisted relaxation on blood pressure and cortisol levels in normotensives and hypertensives. J Behav Med. 1987;10:301–10. doi: 10.1007/BF00846543. [DOI] [PubMed] [Google Scholar]
- 36.Sudsuang R, Chentanez V, Veluvan K. Effect of buddhist meditation on serum cortisol and total protein levels, blood pressure, pulse rate, lung volume, and reaction time. Physiol Behav. 1991;50:543–8. doi: 10.1016/0031-9384(91)90543-w. [DOI] [PubMed] [Google Scholar]
- 37.McKinney CH, Antoni MH, Kumar M, Tims FC, McCabe PM. Effects of guided imagery and music therapy on mood and cortisol in healthy adults. Health Psychol. 1997;16:390–400. doi: 10.1037//0278-6133.16.4.390. [DOI] [PubMed] [Google Scholar]
- 38.Udupa KN, Singh RH. The scientific basis of yoga. JAMA. 1972;220:1365. doi: 10.1001/jama.1972.03200100075029. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt T, Wijga A, Von Zur Muhlen A, Brabant G, Wagner TO. Changes in cardiovascular risk factors and hormones during a comprehensive residential three month kriya yoga training and vegetarian nutrition. Acta Physiol Scand Suppl. 1997;640:158–62. [PubMed] [Google Scholar]
- 40.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Ann NY Acad Med. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 41.Meyer SE, Chrousos GP, Gold PW. Major depression and the stress system: a life span perspective. Dev Psychopath. 2001;13:565–80. doi: 10.1017/s095457940100308x. [DOI] [PubMed] [Google Scholar]
- 42.Stokes PE. The potential role of excessive cortisol induced by HPA hyperfunction in the pathogenesis of depression. Eur Neuropsychopharmacol. 1995;5(suppl):77–82. doi: 10.1016/0924-977x(95)00039-r. [DOI] [PubMed] [Google Scholar]
- 43.Adler HM. The sociophysiology of caring in the doctor-patient relationship. J Gen Intern Med. 2002;17:874–81. doi: 10.1046/j.1525-1497.2002.10640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SW, Mancuso CA, Charlson ME. Expectations and outcomes associated with mind-body training. Under review [Google Scholar]
- 45.Seligman ME, Csikszentmihalyi M. Positive psychology: an introduction. Am Psychol. 2000;55:5–14. doi: 10.1037//0003-066x.55.1.5. [DOI] [PubMed] [Google Scholar]
- 46.Ryff CD, Singer B. The contours of positive health. Psychol Inq. 1998;9:1–28. [Google Scholar]
- 47.Mattio TG, Nishida T, Minieka MM. Lotus neuropathy: report of a case. Neurology. 1992;42:1636. doi: 10.1212/wnl.42.8.1636. [DOI] [PubMed] [Google Scholar]
- 48.Vogel CM, Albin R, Alberts JW. Lotus footdrop: sciatic neuropathy in the thigh. Neurology. 1991;41:605–6. doi: 10.1212/wnl.41.4.605. [DOI] [PubMed] [Google Scholar]
- 49.Chusid J. Yoga foot drop. JAMA. 1971;217:827–8. [PubMed] [Google Scholar]
- 50.Fong KY, Cheung RT, Yu YL, Lai CW, Chang CM. Basilar artery occlusion following yoga exercise: a case report. Clin Exp Neurol. 1993;30:104–9. [PubMed] [Google Scholar]
- 51.Hanus SH, Homer TD, Harter DH. Vertebral artery occlusion complicating yoga exercises. Arch Neurol. 1977;34:574–5. doi: 10.1001/archneur.1977.00500210076015. [DOI] [PubMed] [Google Scholar]
- 52.Fahmy JA, Fledelius H. Yoga-induced attacks of acute glaucoma: a case report. Acta Ophthalmol. 1973;51:80–4. doi: 10.1111/j.1755-3768.1973.tb08249.x. [DOI] [PubMed] [Google Scholar]
- 53.Jung CJ. Yoga and the West. In: Read H, Forham M, Adler G, McGuire W, editors. Psychology and the East, Hull RFC, trans. Princeton, NJ: Princeton University Press; 1978. pp. 77–85. [Google Scholar]
- 54.Armstrong H. Yoga: one physician's experience. CMAJ. 1978;118:992–9. 1004. [PMC free article] [PubMed] [Google Scholar]