Abstract
BACKGROUND
This study evaluated the efficacy of bupropion for relapse prevention in smokers with and without a past history of major depressive disorder. Changes in depressive symptoms were also examined.
DESIGN
Data were gathered prospectively from a randomized, double-blind relapse prevention trial of bupropion conducted at five study sites. A total of 784 smokers (54% female, 97% white) were enrolled. Using the Structured Clinical Interview for Depression, 17% of the subjects reported a past history of major depressive disorder at baseline. All subjects received open-label bupropion SR (300 mg/d) for 7 weeks. Subjects abstinent from smoking at the end of 7 weeks (N= 429) were randomized to bupropion SR (300 mg/d) or placebo for the remainder of the year and followed for 1 year off medication. The primary outcome measures were median time to relapse to smoking and the 7-day point-prevalence smoking abstinence rate. Self-reported abstinence from smoking was verified by expired air carbon monoxide. The Beck Depression Inventory was used to assess depressive symptoms at baseline and at weeks 8 and 12.
RESULTS
Median time to relapse did not differ by past history of major depressive disorder. Bupropion was associated with higher point-prevalence smoking abstinence at the end of medication compared to placebo (P= .007), independent of a past history of major depressive disorder. Moreover, change in depressive symptoms during the double-blind phase did not differ for those with and without a past history of major depressive disorder.
CONCLUSIONS
Extended use of bupropion for relapse prevention is effective for smokers with and without a history of major depression.
Keywords: smoking, relapse prevention, major depression, bupropion therapy
Many smokers in the population and those seen in general clinical practice present with a recent or past history of major depressive disorder (MDD) or other psychiatric diagnoses.1,2 A past history of MDD has been associated with poorer treatment outcomes in some trials using pharmacotherapy for smoking cessation.3,4 While smokers with past MDD are not currently depressed, they do report elevated levels of depressive symptoms compared to those without a past diagnosis of MDD.5,6 Moreover, some research indicates that individuals with a past history of MDD are at increased risk for developing a major depressive episode during smoking abstinence.7,8 These are important reasons to focus on smokers with a past MDD diagnosis who are the focus of the current investigation. Nonetheless, it is important to note that smokers with current MDD are a group where differences in treatment outcomes may be more pronounced than, for example, among smokers whose depression remitted several years ago. Many smokers with current MDD are excluded from clinical trials of smoking cessation given contraindications to pharmacotherapy and other considerations. Our prior research also indicates difficulty in recruiting nonmedicated smokers with current MDD for cessation trials.9
In a previous study,5 we examined the efficacy of 7 weeks of sustained-release bupropion use for smoking cessation in 615 smokers with a past history of MDD, alcoholism, both disorders, or neither disorder. At the end of 7 weeks of treatment and at 1-year follow-up, bupropion was found to be effective for improving the point-prevalence smoking abstinence rates independent of a past history of MDD or alcoholism. Thus, bupropion appears to be equally effective in smokers with and without past psychiatric comorbidity. In addition, mean changes in depressive symptoms during treatment did not differ across these diagnostic groups. That study was a short-term efficacy trial examining bupropion use for only 7 weeks. In a subsequent investigation,10 subjects achieving initial smoking abstinence after 7 weeks of open-label sustained-release bupropion therapy were randomly assigned to active bupropion or placebo for 1 year. Long-term bupropion use improved the abstinence rates during the first 6 months of treatment and increased time to relapse relative to placebo. No previous work has evaluated bupropion use for smoking relapse prevention in association with psychiatric comorbidity. Using data from the Hays et al.10 trial, the current prospective investigation adds new information on differences in treatment response (i.e., relapse prevention) among smokers with and without a history of MDD receiving bupropion for longer-term use.
METHODS
Subjects
Subjects were participants in a prospective randomized, double-blind, placebo-controlled relapse prevention trial that was performed at five study sites. Details of the study are described elsewhere.10 Subjects gave their informed consent after the procedure and side effects of the medication were fully explained. Study entry criteria required that the subjects had to be 18 years of age or older, had smoked an average of ≥15 cigarettes per day for the past year, were motivated to stop smoking, and were generally in good health as determined by a physician. Exclusion criteria included a history of a seizure disorder, severe head trauma, predisposition to seizures, current or past history of anorexia nervosa or bulimia, presence of an unstable medical or psychiatric condition, pregnancy or lactation, current use of psychotropic medications, prior use of bupropion, current use of tobacco products other than cigarettes, current use of any nicotine replacement therapy or other smoking cessation treatment, major depressive episode within the past month, and a history within the past year of dependence on alcohol or other nonnicotine substances. Based on data obtained at the Mayo Clinic Rochester site, of 362 volunteers screened by telephone, 1 reported history of an eating disorder, 12 reported alcoholism within the past year, and 2 reported another current psychiatric condition. At the information session, 2 of 92 interviewed using the Structured Clinical Interview for Depression (SCID) reported a current psychiatric disorder.
Procedure
At baseline, 784 subjects were assigned to open-label, sustained-release bupropion at a dose of 300 mg per day for 7 weeks (150 mg per day for the first 3 days followed by 150 mg twice a day). They were instructed to set a target quit date after 1 week of initiating medication (usually the eighth day of therapy). Each subject received a brief personalized message to stop smoking from the examining physician and self-help materials based on the National Cancer Institute program.11 Each subject then returned weekly during the 7-week open-label phase. Subjects who reported no smoking (not even a puff) during the seventh week of the open-label phase and had an expired air carbon monoxide (CO) level of ≤10 ppm were eligible for randomization to the double-blind phase of the study. A total of 429 were randomly assigned to sustained-release bupropion, 150 mg twice daily, or an identical placebo for the remainder of the year.
Randomized subjects returned for 14 visits during the double-blind medication phase (weeks 8, 9, 10, 12, and every 4 weeks thereafter through week 52) and 5 visits during the postmedication phase (weeks 53, 56, 64, 76, and 104). A study assistant provided brief individual counseling (approximately 10 to 15 minutes) at each visit during both the open-label and double-blind phases of the study.
Measures
At baseline, subjects were interviewed using the SCID12 to determine presence or absence of a lifetime history of MDD according to the Diagnostic and Statistical Manual, fourth edition (DSM-IV) criteria.13 Only the depressive disorders section of the SCID was administered (i.e., assessing mania, MDD, and dysthymic disorder). The Self-administered Alcohol Screening Test (SAAST)14 was completed by subjects to determine current or past history of alcohol problems. The SAAST is a 37-item, self-administered, validated screening questionnaire assessing excessive alcohol use and alcohol-related problems and used primarily in general medical settings.15 Scores are used to classify a patient's drinking habits into alcoholism risk categories. A score of 6 or less is indicative of nonalcoholic consumption or possible alcoholism, and a score of 7 or more is suggestive of probable alcoholism. Because those with a history of alcoholism in the past year were excluded, elevated scores (≥7) likely represent those in remission from an alcohol problem.
The Fagerström Tolerance Questionnaire (FTQ)16 was used to measure severity of nicotine dependence. Total scores can range from 0 to 11; a score of ≥6 indicates nicotine dependence. The Beck Depression Inventory (BDI)17 is a 21-item, self-administered questionnaire that was used to assess severity of depressive symptoms at baseline, and at weeks 8 and 12. Total scores on the BDI can range from 0 to 63. BDI scores of 9 or below are considered within normal range, scores of 10 to 18 are consistent with mild-to-moderate depression, and scores of 19 to 29 are consistent with moderate-to-severe depression. At each visit during the double-blind phase through week 53, a study assistant recorded the subject's self-reported use of study medication since the previous visit.
Smoking status was assessed by self-report at each visit and biochemically confirmed by expired air carbon monoxide (CO). The weekly point-prevalence smoking abstinence was defined as self-report of no smoking during the previous 7 days and having an expired air CO level of ≤10 ppm. Smoking relapse was defined as any self-report of smoking or an expired air CO level >10 ppm. Subjects with missing visits were not considered relapsed to smoking unless they missed two or more consecutive visits. Date of smoking relapse was determined based on self-report for the subjects that self-reported smoking. For subjects not self-reporting smoking but classified as smoking because of an elevated CO level or because of consecutive missed visits, the date of relapse was defined as the day following the most recent previous study visit attended at which they were biochemically confirmed not smoking.
Statistical Analyses
Differences in baseline subject characteristics between those with and without a history of MDD were assessed using one-way analysis of variance models for continuous variables and the χ2 test for categorical variables. A logistic regression analysis, adjusting for study site, was employed to assess whether MDD history was associated with the 7-day point-prevalence smoking abstinence at the end of the open-label bupropion phase. Time to first smoking relapse during the double-blind medication phase was analyzed using Kaplan-Meier survival estimates and a proportional hazard regression model. For this analysis, time to first relapse was defined as the number of days between date of first relapse and date of randomization. For those who did not relapse, time to first relapse was censored using the date of their final (week 104) study visit. For the proportional hazard regression analysis of time to first smoking relapse the independent variables were MDD history group and medication assignment. To assess whether the effect of medication assignment was dependent on MDD history, and vice versa, an initial analysis was performed that included the treatment by MDD history interaction. After verifying the effect of treatment was not dependent on MDD history, a proportional hazard regression analysis was used to assess differences between those with and without MDD history, adjusting for treatment and including study site as a stratification factor.
The point-prevalence smoking abstinence rates were examined at week 12 (which was 5 weeks following randomization), week 52 (end of the medication phase at 45 weeks following randomization), and week 104 (1 year after completion of the double-blind medication phase). Point-prevalence smoking abstinence rates were compared for those with and without a history of MDD using a logistic regression analysis with point-prevalence smoking status as the dependent variable, MDD history group and medication assignment as independent variables, and study site as a covariate. Again, the MDD history group by medication assignment interaction term was included to assess whether the effect of MDD history was dependent on medication assignment. After verifying that the effects of MDD history and medication assignment were not dependent on each other, a logistic regression analysis was performed to assess main effect differences in rates of smoking between subjects with and without a history of MDD, including medication assignment and study site as covariates. In addition, a repeated measures analysis was performed using data from each of the 3 time points (week 12, week 52, and week 104). This analysis was performed using Generalized Estimating Equations (GEE)18,19 with point-prevalence smoking status as the dependent variable, MDD history group, medication assignment, time, and each of the two-way interactions as independent variables, and study site as a covariate. The GEE model was fit using a logit link function and an autoregressive (AR1) working correlation matrix. After verifying that the MDD history by medication assignment and MDD history by time interactions were not significant, a repeated measures GEE analysis was performed to assess the effects of history of MDD, medication assignment, time, and the time by medication interaction on smoking status. Study site was again included as a covariate in the final model.
A history of alcoholism has been shown to be associated with poorer smoking treatment response in some studies.20 Thus, we repeated the point-prevalence abstinence and smoking relapse analyses adjusting for baseline SAAST score treated as a dichotomous variable (≤6 or ≥7).
Change in BDI depressive symptoms during the double-blind medication phase was assessed among all randomized subjects with assessments, regardless of smoking status, at weeks 8 and 12. For each time point, two-way analysis of variance models were used to compare mean change in BDI score from baseline (time minus baseline), with change in BDI as the dependent variable, MDD history group and medication assignment as crossclassification factors, and study site as a covariate. In all analyses, two-sided P values ≤.05 were considered as evidence of findings not attributable to chance.
RESULTS
Open-label Phase
Baseline Subject Characteristics
Among the 784 subjects enrolled in the study, 137 (17%) had a history of MDD. One study subject could not be classified on MDD history due to missing SCID data and was omitted from the analyses. Table 1 presents baseline characteristics according to MDD history. Those with a history of MDD were significantly more likely to be female, and to have higher BDI scores and elevated SAAST scores. For the overall sample, the BDI mean (standard deviation) score was 3.8 (4.3) with a range of 0 to 35. For those with a history of MDD, the mean BDI score was 5.3 (5.8) with a range of 0 to 35; and among those without a history of MDD, the average BDI score was 3.5 (3.8), range 0 to 26. No other baseline characteristics were significantly different between the two groups. Among those with a history of MDD, the mean number of prior depressive episodes was 1.3 (0.97), range 1 to 10. The average age at the onset of the first episode was 32.1 (10.6), range 17 to 60.
Table 1.
Baseline Characteristics of Subjects Enrolled in Open-label Phase According to History of Major Depressive Disorder (N = 783)*
| History of Major Depressive Disorder? | |||
|---|---|---|---|
| Characteristic | No (N = 646) | Yes (N = 137) | P Value† |
| Mean age, y (SD) | 45.5 (9.9) | 45.2 (9.5) | NS |
| Range | 19 to 75 | 20 to 72 | |
| Gender, % female | 51 | 66 | <.001 |
| Marital status, % married/ live w. partner | 62 | 58 | NS |
| Level of education % with post-high school education | 26 | 23 | NS |
| Beck Depression Inventory score, %‡ | <.001 | ||
| ≤9 | 92 | 82 | |
| 10 to 18 | 7 | 15 | |
| 19 to 29 | 1 | 2 | |
| ≥30 | 0 | 1 | |
| Mean cigarettes per day (SD) | 27.2 (9.8) | 28.1 (11.0) | NS |
| Fagerström Tolerance Questionnaire§ | NS | ||
| Mean (SD) | 7.3 (1.6) | 7.4 (1.6) | |
| No. of previous stop attempts, %|| | NS | ||
| 0 to 1 | 26 | 21 | |
| ≥2 | 74 | 79 | |
| SAAST score, %¶ | .008 | ||
| ≤6 (nonalcoholic or possible alcoholism) | 90 | 83 | |
| ≥7 (probable alcoholism) | 10 | 18 | |
Because of rounding, not all percentages total 100.
Two-tailed P value from a one-way ANOVA F-test or χ2 test of no difference across the two groups.
Data were missing for 1 subject in the history of MDD group and 6 subjects in the no history of MDD group.
Data were missing for 1 subject in the history of MDD group and 2 subjects in the no history of MDD group.
Data were missing for 1 subject in the history of MDD group.
Data were missing for 3 subjects in the no history of MDD group.
MDD, major depressive disorder; SAAST, Self-administered Alcoholism Screening Test; NS, not significant.
Point-prevalence Smoking Abstinence Rates
Overall, 461 subjects were abstinent from smoking at the end of the open-label phase. A history of MDD was not found to be significantly associated with smoking abstinence (P = .555) at the end of the open-label bupropion phase. The results remained unchanged after adjusting for baseline SAAST score. The 7-day point-prevalence smoking abstinence rates at the end of the open-label phase were 62% for those with a history of MDD compared with 58% for subjects without this history.
In addition to a history of MDD, baseline characteristics listed in Table 1 were compared for treatment responders versus nonresponders. Subjects abstinent from smoking at the end of the open-label phase were significantly more likely to be male (P = .041), older (P = .044), married/living with partner (P < .001), to have made two or more prior stop attempts (P = .031), and to have lower FTQ scores (P = .007) and lower (≤6) SAAST scores (P = .026).
Of the 461 subjects who were abstinent at the end of the open-label phase, 429 were randomized to the double-blind medication phase. Of the 32 who were not randomized, reasons for not participating were scheduling difficulties (n = 20), an adverse event (n = 10), or protocol deviation (n = 2). Of the 32 dropouts, 5 (16%) had a history of MDD. There was no difference (P = .671) in the proportion of dropouts among those with MDD (6%; 5 of 85) or without a history of MDD (7%; 27 of 376).
Double-blind Medication Phase
Baseline Subject Characteristics
The baseline characteristics and medication assignment for the 429 subjects with MDD history information and randomized to the double-blind medication phase are presented in Table 2 according to MDD history group. Differences between those with and without a history of MDD were again detected for gender, and BDI and SAAST scores. For the overall sample, the mean BDI score was 3.8 (4.2), with a range of 0 to 26. For those with a history of MDD, the mean BDI score was 5.3 (5.5), with a range of 0 to 25; and among those without a history of MDD, the average BDI score was 3.4 (3.7), range 0 to 26. In addition, differences between groups were detected for level of education and study site. Among those with a history of MDD, the mean number of prior depressive episodes was 1.2 ± 0.6, range 1 to 4. The average age at the onset of the first episode was 32.5 ± 11.4, range 17 to 60.
Table 2.
Baseline Characteristics of Randomized Subjects According to History of Major Depressive Disorder (N = 429)*
| History of Major Depressive Disorder? | |||
|---|---|---|---|
| Characteristic | No (N = 349) | Yes (N = 80) | P Value† |
| Mean age, y (SD) | 46.1 (9.7) | 46.8 (8.7) | NS |
| Range | 20 to 71 | 28 to 72 | |
| Gender, % female | 48 | 66 | .003 |
| Marital status, % married/ live w. partner | 68 | 63 | NS |
| Level of education % with post-high school education | 27 | 15 | .023 |
| Beck Depression Inventory score, %‡ | .008 | ||
| ≤9 | 93 | 84 | |
| 10 to 18 | 6 | 13 | |
| 19 to 29 | 1 | 4 | |
| ≥30 | 0 | 0 | |
| Mean cigarettes per day (SD) | 26.7 (9.9) | 27.3 (10.9) | NS |
| Fagerström Tolerance Questionnaire§ mean (SD) | 7.2 (1.6) | 7.4 (1.5) | NS |
| No. of previous stop attempts, % | NS | ||
| 0 to 1 | 22 | 23 | |
| ≥2 | 78 | 78 | |
| SAAST score, %|| | .034 | ||
| ≤6 (nonalcoholic or possible alcoholism) | 93 | 85 | |
| ≥7 (probable alcoholism) | 8 | 15 | |
| Medication, % | NS | ||
| Placebo | 51 | 45 | |
| Bupropion 300 mg/day | 49 | 55 | |
| Study site, % | .018 | ||
| Portland, OR | 20 | 10 | |
| Rochester, MN | 32 | 48 | |
| Providence, RI | 15 | 9 | |
| Boston, MA | 15 | 11 | |
| Palo Alto, CA | 18 | 23 | |
Because of rounding, not all percentages total 100.
Two-tailed P value from a one-way ANOVA F-test or χ2 test of no difference across the two groups.
Data were missing for 1 subject in the history of MDD group and 4 subjects in the no history of MDD group.
Data were missing for 1 subject in the no history of MDD group.
Data were missing for 2 subjects in the no history of MDD group.
MDD, major depressive disorder; SAAST, Self-administered Alcoholism Screening Test; NS, not significant.
Medication Compliance
Among the 429 randomized participants, the percentage of self-reported prescribed medication taken among subjects with a history of MDD (mean, 65%; median, 80%; range, 0% to 100%) did not differ significantly (P = .140) from those without a history of MDD (mean, 71%; median, 92%; range, 0% to 100%). A total of 347 subjects (80.9%; 175 in the placebo group and 172 in the bupropion group) remained in the study through the 45-week double-blind medication phase. Of these, 90 subjects (49 placebo recipients and 41 bupropion recipients) prematurely discontinued medication use. The 257 subjects who continued use of medication (placebo or bupropion) throughout the double-blind phase reported taking 95% of the prescribed dosages (median, 97%; range, 61% to 100%). Among these 257, the percentage of self-reported prescribed medication taken was not significantly different (P = .938) between subjects with a history of MDD (n = 41; mean, 95%; median, 97%; range, 64% to 100%) and those without such a history (n = 216; mean, 95%; median, 97%; range, 61% to 100%).
Smoking Relapse Rates
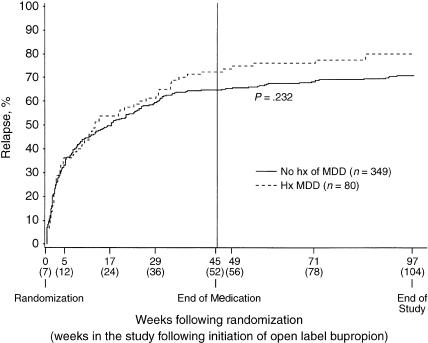
Figure 1 shows the Kaplan-Meier estimates of smoking relapse by MDD history group, unadjusted for medication assignment, over the 2 years following randomization. The median time to smoking relapse from randomization was 89.5 days for subjects with a history of MDD compared to 119 days for those without such a history (P = .232 log-rank test). After adjusting for medication assignment, there was no significant difference in relapse curves by MDD history group (P = .136). The results remained unchanged after adjusting for baseline SAAST score.
FIGURE 1.
Observed cumulative smoking relapse according to history of major depressive disorder (MDD). There was no significant difference in relapse curves across time between subjects with and without a history of MDD (P = .110, proportional hazard regression). The median time to smoking relapse from randomization was 89.5 days for subjects with a history of MDD and 119 days for those with no such history (P = .232, log-rank test).
Overall, the percent relapsed following randomization by week 104 was higher for those with a history of MDD (80%; 95% confidence interval [CI], 70% to 88%) compared to those without this history (71%; 95% CI, 66% to 75%). However, from logistic regression analysis adjusting for medication assignment, the percent of subjects who relapsed by week 104 was not significantly different for those with and without MDD (P =.063). These results remained unchanged after adjusting for baseline SAAST score.
There was no statistically significant difference (P > .10) in the extent of missing outcome information for those with and without a history of MDD. For the 64 subjects with a history of MDD who relapsed, smoking status was imputed 33 times (52%). For the 247 without a history of MDD who relapsed, smoking status was imputed 121 times (49%).
Point-prevalence Smoking Abstinence Rates
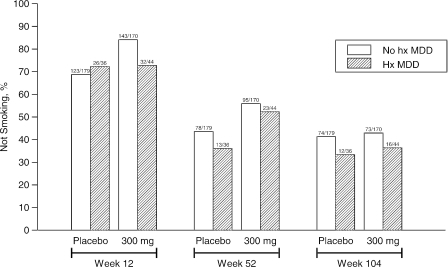
Figure 2 displays the 7-day point-prevalence smoking abstinence rates according to MDD history group and medication assignment at weeks 12, 52, and 104. Using logistic regression, there was no evidence of a significant interaction between MDD history group and medication assignment at any of the time points, suggesting that the effect of medication assignment was not dependent on MDD history (Table 3). A significant main effect for medication was evident at week 12 (P = .002) and week 52 (P = .007), consistent with higher smoking abstinence associated with the random assignment to active bupropion. At week 104, however, there was no longer a significant medication effect (P = .676). In addition, there was no evidence that a history of MDD was associated with reduced likelihood of smoking abstinence at any of these time points. These results remained unchanged after adjusting for baseline SAAST score.
FIGURE 2.
Seven-day point-prevalence smoking abstinence rates according to history of major depressive disorder (MDD) and medication treatment at weeks 12, 52, and 104. Bupropion medication was significantly associated with higher abstinence rates at week 12 (P = .002) and week 52 (P = .007) compared to placebo. A past history of MDD was not significantly associated with abstinence at any of these time points.
Table 3.
Association of History of Major Depressive Disorder and Treatment Medication with Point-prevalence Smoking Abstinence*
| Week 12 | End of Treatment Week 52 | 2 Year Follow-up Week 104 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value |
| Medication | .002 | .007 | .676 | ||||||
| Placebo | 1.00 | 1.00 | 1.00 | ||||||
| Bupropion 300 mg/day | 2.03 | 1.28 to 3.20 | 1.71 | 1.16 to 2.52 | 1.09 | 0.74 to 1.61 | |||
| MDD history group | .277 | .337 | .219 | ||||||
| No MDD history | 1.00 | 1.00 | 1.00 | ||||||
| MDD history | 0.73 | 0.41 to 1.29 | 0.78 | 0.47 to 1.29 | 0.72 | 0.43 to 1.21 | |||
Multiple logistic regression was used to determine the relationship between the MDD history group and medication assignment with 7-day point-prevalence smoking abstinence at weeks 12, 52, and 104 (5, 45, and 97 weeks following randomization, respectively). For these analyses, the dependent variable was point-prevalent smoking status and the independent variables were MDD history group and medication assignment. Each analysis was adjusted for study site. From initial analyses, there was no evidence of a significant medication-by-MDD history group interaction at any of the time points (P =.107 at week 12, P = .738 at week 52, and P = .872 at 2 years). The results presented are from logistic regression analyses that adjust for study site and include only the main effects of medication and MDD history. An odds ratio of 1.00 indicates the reference group. The results remained unchanged after adjusting for baseline SAAST score.
MDD, major depressive disorder; SAAST, Self-administered Alcoholism Screening Test; OR, odds ration; CI, confidence interval.
In an analysis restricted to those without a history of MDD, a significant bupropion effect was detected at week 52 (odds ratio [OR], 1.65; 95% CI, 1.08 to 2.53; P = .022). An analysis restricted to those with a history of MDD was not statistically significant (OR, 1.90; 95% CI, 0.74 to 4.88; P = .180).
A repeated measures GEE analysis was performed showing a beneficial bupropion effect (parameter estimate, 0.696; standard error, 0.193; P < .001), a significant time effect with abstinence rates decreasing over time (parameter estimate, −0.009; standard error, 0.001; P < .001), and a significant time by bupropion interaction (parameter estimate, −0.005; standard error, 0.002; P = .006), indicating that the effect of bupropion diminished over time. MDD history did not affect the outcome (parameter estimate, −0.283; standard error, 0.208; P = .174). These findings remained unchanged after adjusting for baseline SAAST score.
Changes in Depressive Symptoms
There was no evidence of a significant main effect of either MDD history group or medication assignment on change from baseline in BDI depressive symptom scores at either week 8 or week 12.
DISCUSSION
The findings of this prospective study suggest that sustained-release bupropion therapy is effective in delaying relapse to smoking independent of a past history of major depressive disorder. This is the first study to examine bupropion use for smoking relapse prevention in association with major depression. Our results indicate no significant difference between MDD history groups in median time to smoking relapse or the overall percent of subjects who relapsed. Thus, when used for longer-term treatment, bupropion appears equally effective across the range of smokers with and without past major depression comorbidity. This contrasts with other research findings of an association between a history of MDD and higher rates of smoking relapse following use of clonidine.1 However, that study was an efficacy trial utilizing the pharmacotherapy for only a few weeks. While there was little change in percent abstinent from smoking between weeks 52 and 104 for placebo subjects, we found there was a sizable decrease for those who had received active medication. A history of MDD did not affect this outcome. However, this does suggest that while bupropion is helpful, it may need to be maintained on a chronic basis.
Our results should be interpreted cautiously, due to the small sample size in the MDD group that limits the statistical power to detect differences in treatment outcomes. Given the sample size of 80 for those with a history of MDD randomly assigned to the double-blind phase and the week 52 abstinence rates, this study provided statistical power of 27%, 54%, 73%, and 84% to detect a medication assignment-by-MDD history group interaction with a 2-, 3-, 4-, and 5-fold increase in treatment efficacy, respectively, for those with versus without a history of MDD (i.e., x% power to detect an odds ratio for the interaction effect). Among subjects with a history of MDD, 52% of those receiving bupropion were abstinent at 1 year compared to only 36% of those using placebo. Although not statistically significant, this difference is of meaningful clinical interest, and underscores the need for replication of our results in a larger sample of smokers with a past history of major depressive disorder.
The finding of a lack of effect of MDD history on treatment outcomes should also be interpreted in light of the potential for selective attrition prior to randomization to the double-blind phase. At the end of the open-label phase, nonresponders differed from bupropion responders on several baseline characteristics, including gender and severity of nicotine dependence. It is therefore possible that a history of major depressive disorder may have affected outcome or interacted with medication assignment if the study design did not require exclusion of bupropion nonresponders.
Some subject characteristics limit generalizability of the findings. They were primarily white, well-educated, and motivated to stop smoking. The stringent screening criteria for this trial (e.g., psychiatric disorder) also limit the representativeness of the smokers with MDD history to other smokers in the population with a history of MDD. However, these study criteria were very similar to other trials of pharmacotherapy for smoking cessation.1,5,21 A population-based study indicates that about 40% of current smokers report a current or past psychiatric disorder.2 Nonetheless, we found that only 5% of those screened by telephone (based on data obtained at the Mayo site) were excluded based on a psychiatric disorder. Moreover, given the selection criteria, the dispersion of baseline BDI scores were restricted to the lower end, which likely limited our ability to detect an association between changes in depressive symptoms and MDD history and/or medication assignment. The effectiveness of bupropion for smoking abstinence and relapse prevention among patients presenting with current depression is a clinical issue that warrants empirical attention.
Acknowledgments
This study was supported by a grant from Glaxo Wellcome, Inc. Findings of this study were presented in part at the Society for Research on Nicotine and Tobacco, 7th annual meeting, Seattle, Wash, March 2001.
REFERENCES
- 1.Glassman AH, Stetner F, Walsh BT, et al. Heavy smokers, smoking cessation, and clonidine. Results of a double-blind, randomized trial. JAMA. 1988;259:2863–6. [PubMed] [Google Scholar]
- 2.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 3.Covey LS, Glassman AH, Stetner F, Becker JT. Effect of history of alcoholism or major depression on smoking cessation. Am J Psychiatry. 1993;150:1546–7. doi: 10.1176/ajp.150.10.1546. [DOI] [PubMed] [Google Scholar]
- 4.Glassman AH, Helzer JE, Covey LS, et al. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–9. [PubMed] [Google Scholar]
- 5.Hayford KE, Patten CA, Rummans TA, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. Br J Psychiatry. 1999;174:173–8. doi: 10.1192/bjp.174.2.173. [DOI] [PubMed] [Google Scholar]
- 6.Niaura R, Britt DM, Borrelli B, Shadel WG, Abrams DB, Goldstein MG. History and symptoms of depression among smokers during a self-initiated quit attempt. Nicotine Tob Res. 1999;1:251–7. doi: 10.1080/14622299050011371. [DOI] [PubMed] [Google Scholar]
- 7.Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet. 2001;357:1929–32. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- 8.Tsoh JY, Humfleet GL, Munoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000;157:368–74. doi: 10.1176/appi.ajp.157.3.368. [DOI] [PubMed] [Google Scholar]
- 9.Thorsteinsson HS, Gillin JC, Patten CA, et al. The effects of transdermal nicotine therapy for smoking cessation on depressive symptoms in patients with major depression. Neuropsychopharmacology. 2001;24:350–8. doi: 10.1016/S0893-133X(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 10.Hays JT, Hurt RD, Rigotti N, et al. A randomized controlled trial of sustained-release bupropion for pharmacologic relapse prevention following smoking cessation. Ann Intern Med. 2001;135:423–33. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- 11.Glynn TJ, Manley MW. How to Help Your Patients Stop Smoking: A National Cancer Institute Manual for Physicians. Bethesda, Md: National Cancer Institute, NIH Publication; 1990. No. 90-3064. [Google Scholar]
- 12.Spitzer RL, Williams JBW, Gibbon M, et al. User's Guide for the Structured Clinical Interview for DSM-III-R: SCID. Washington, DC: American Psychiatric Press, Inc; 1990. [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual—Revised. 4th ed. Washington, DC: American Psychiatric Association; 1994. (DSM-IV). [Google Scholar]
- 14.Swensen WM, Morse RM. The use of a self-administered alcoholism screening test (SAAST) in a medical center. Mayo Clin Proc. 1975;50:204–8. [PubMed] [Google Scholar]
- 15.Davis LJ. Self-administered Alcoholism Screening Test (SAAST) In: Maruish ME, editor. Handbook of Psychological Assessment in Primary Care Settings. Mahweh, NJ: Lawrence Erlbaum; 2000. pp. 537–54. [Google Scholar]
- 16.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–41. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Steer RA. Beck Depression Inventory. Philadelphia, Pa: Center for Cognitive Therapy; 1987. [Google Scholar]
- 18.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford: Clarendon Press; 1994. [Google Scholar]
- 19.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 20.Hays JT, Schroeder DR, Offord KP, et al. Response to nicotine dependence treatment in smokers with current and past alcohol problems. Ann Behav Med. 1999;21:244–50. doi: 10.1007/BF02884841. [DOI] [PubMed] [Google Scholar]
- 21.Hall SM, Reus VI, Munoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55:683–90. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]