Abstract
BACKGROUND
There is controversy surrounding the issue of whether, and how, to screen adults for type 2 diabetes. Our objective was to measure the incidence of new diabetes among outpatients enrolled in a health care system, and to determine whether hemoglobin A1c (HbA1c) values would allow risk stratification for Patients' likelihood of developing diabetes over 3 years.
METHODS
We conducted a prospective cohort study with 3-year follow-up at a single large, tertiary care, Department of Veterans Affairs Medical Center (VAMC). A convenience sample of 1,253 outpatients without diabetes, age 45 to 64, with a scheduled visit at the VAMC, were screened for diabetes using an initial HbA1c measurement. All subjects with HbA1c ≥ 6.0% (normal, 4.0% to 6.0%) were invited for follow-up fasting plasma glucose (FPG). We then surveyed patients annually for 3 years to ascertain interval diagnosis of diabetes by a physician. The baseline screening process was repeated 3 years after initial screening. After the baseline screening, new cases of diabetes were defined as either the self-report of a physician's diagnosis of diabetes, or by HbA1c ≥ 7.0% or FPG ≥ 7.0 mmol/L at 3-year follow-up. The incidence of diabetes was calculated as the number of new cases per person-year of follow-up.
RESULTS
One thousand two hundred fifty-three patients were screened initially, and 56 (4.5%) were found to have prevalent unrecognized diabetes at baseline. The 1,197 patients without diabetes at baseline accrued 3,257 person-years of follow-up. There were 73 new cases of diabetes over 3 years of follow-up, with an annual incidence of 2.2% (95% confidence interval [CI], 1.7% to 2.7%). In a multivariable logistic regression model, baseline HbA1c and baseline body mass index (BMI) were the only significant predictors of new onset diabetes, with HbA1c having a greater effect than BMI. The annual incidence of diabetes for patients with baseline HbA1c ≤ 5.5 was 0.8% (CI, 0.4% to 1.2%); for HbA1c 5.6 to 6.0, 2.5% (CI, 1.6% to 3.5%); and for HbA1c 6.1 to 6.9, 7.8% (CI, 5.2% to 10.4%). Obese patients with HbA1c 5.6 to 6.0 had an annual incidence of diabetes of 4.1% (CI, 2.2% to 6.0%).
CONCLUSIONS
HbA1c testing helps predict the likelihood that patients will develop diabetes in the future. Patients with normal HbA1c have a low incidence of diabetes and may not require rescreening in 3 years. However, patients with elevated HbA1c who do not have diabetes may need more careful follow-up and possibly aggressive treatment to reduce the risk of diabetes. Patients with high-normal HbA1c may require follow-up sooner than 3 years, especially if they are significantly overweight or obese. This predictive value suggests that HbA1c may be a useful test for periodic diabetes screening.
Keywords: diabetes, screening, hemoglobin A1c
Systematic screening for diabetes is a potentially useful intervention, because diabetes is a common,1,2 costly,3 and highly morbid illness, and because there is a long asymptomatic phase to the illness.4 Recommendations from professional organizations regarding diabetes screening are conflicting, with some organizations failing to support diabetes screening,5 and others recommending screening all patients 45 and over every 3 years.6 The U.S. Preventive Services Task Force (USPSTF) now recommends screening for all patients with hypertension every 3 years, in part because of the increased prevalence of disease in these patients.7
The utility of diabetes screening ultimately depends on the evidence that early treatment will have added benefit over treatment at the time of symptomatic diagnosis, most likely in the form of added years of complication-free life. There are no studies that provide direct evidence for this.7,8 However, computer models informed by observational data obtained from cohorts diagnosed by screening suggest borderline cost-effectiveness for diabetes screening.9 Assuming potential effectiveness and cost-effectiveness for screening, these will improve by targeting patients both at high risk of diabetes and at high risk of complications once they acquire the disease.10 This logic applies both to initial and interval screening; patients at high risk for developing diabetes can be targeted for more frequent screening than those at low risk if high-risk patients can be identified.
The role of hemoglobin A1c (HbA1c) as a diabetes-screening test is not conclusively established. Those organizations that recommend diabetes screening do not recommend use of HbA1c as a screening test due, mostly, to lack of standardization.5 However, recent reports have shown that HbA1c, measured in the standard way by high-performance liquid chromatography (HPLC), may have better sensitivity than fasting plasma glucose (FPG) in diagnosing diabetes.11,12 Additionally, HbA1c is attractive as a screening test because it is used to define treatment targets in diabetes,13 and it predicts complications of diabetes.14–19 In particular, HbA1c ≥ 6.0% is associated with increased risk of prevalent microvascular complications in multiple populations.20 If HbA1c were both able to predict future diabetes and to predict future complications, it would be an attractive test for interval screening, as it would allow targeting of the screening interval to both risk of incident diabetes and complication risk once diabetes is developed. The ability of HbA1c to predict the future onset of diabetes is unknown.
Our objective was to determine the 3-year incidence of diabetes in an outpatient population. We further sought to determine whether the HbA1c level at a baseline examination was an independent predictor of new onset diabetes, and, by extension, to identify target populations that might need more or less frequent diabetes screening.
METHODS
Patients
Patient enrollment has been described in detail elsewhere.21 Briefly, we identified all patients age 45 to 64 who had kept an outpatient visit at the Durham Veterans Affairs Medical Center (DVAMC) between October 1996 and March 1998. We sent all these patients a 1-page questionnaire that asked whether the patient had diabetes and whether we could contact them by telephone for a research study. Respondents denying knowledge of diabetes or “high blood sugar” and agreeing to be telephoned were contacted for enrollment into the study. Prior to enrollment we obtained written informed consent from all subjects. The study and enrollment strategy were approved by the Institutional Review Board of the DVAMC. At the initial visit we excluded patients who said they had diabetes, had had a prescription filled at the DVAMC pharmacy for a hypoglycemic medication, had a short life expectancy (incurable cancer, heart or lung disease requiring oxygen), or had no easy access to a telephone.
Diabetes Screening Protocol
We measured HbA1c by HPLC on all subjects; the first 476 subjects were measured on one machine (Varian, BioRad, Hercules, CA) and the remaining 777 and all subjects at follow-up were measured on another chromatograph (2.2, Tosoh, Tokyo, Japan). Both machines were calibrated to a normal range of 4.0% to 6.0%. Comparison of the two machines showed a correlation of 0.99 with a slope of 0.96. All subjects with HbA1c ≥ 6.0% were invited back for follow-up FPG to complete the baseline diabetes assessment. We then interviewed subjects annually for 2 years by telephone to determine whether they had been diagnosed with diabetes. All available subjects, regardless of baseline test results or diabetes status, were rescreened 3 years later using a protocol that was identical to the baseline assessment, including HbA1c and, for those with HbA1c ≥ 6.0%, follow-up FPG.
Ascertainment of Diabetes
At the baseline screening, we defined a case of diabetes as HbA1c ≥ 7.0% or FPG ≥ 7.0 mmol/L (126 mg/dl). These patients were then excluded from analysis of diabetes incidence. For the remaining patients, an incident case of diabetes was defined as self-report by patient at any of the 3 annual interviews, or by HbA1c ≥ 7.0% or FPG ≥ 7 mmol/L at 3-year rescreening. The use of self-reported diabetes as part of the definition is necessary because patients with diabetes may receive treatment that lowers their laboratory test values below the threshold for diagnosis; indeed, this is the goal of optimal diabetes treatment.13
Additional Measures
At enrollment, we obtained multiple demographic measures and patient-reported family history of diabetes and hypertension. We also obtained height and weight on all subjects, and calculated body mass index (BMI) by the formula BMI = wt(kg)/ht(m)2.
Analysis
We chose a target sample size of 1,253 in order to provide narrow confidence intervals (CI) around the prevalence of unrecognized diabetes at enrollment. Descriptive statistics were computed for all variables of interest. Incidence was calculated by excluding cases of diabetes diagnosed at baseline, and calculating the number of new cases diagnosed and dividing by the total number of follow-up years until diagnosis of diabetes or close-out.
We performed multivariable modeling comparing patients who did and did not develop new onset diabetes during the 3-year follow-up of the study using logistic regression. Candidate variables for the model were chosen by identifying known predictors of diabetes including age, race, family history of diabetes, BMI, and hypertension. These variables were entered into the model in a stepwise fashion along with baseline HbA1c, the primary variable of interest. As a sensitivity analysis, Cox proportional hazards modeling was performed using the same variables, with annual diagnosis of diabetes as the outcome variable. The statistical significance was the same for all variables between the two models, and the logistic regression results are presented here. All analyses were performed using the SAS analysis system (SAS Institute, Cary, NC).
RESULTS
Subjects
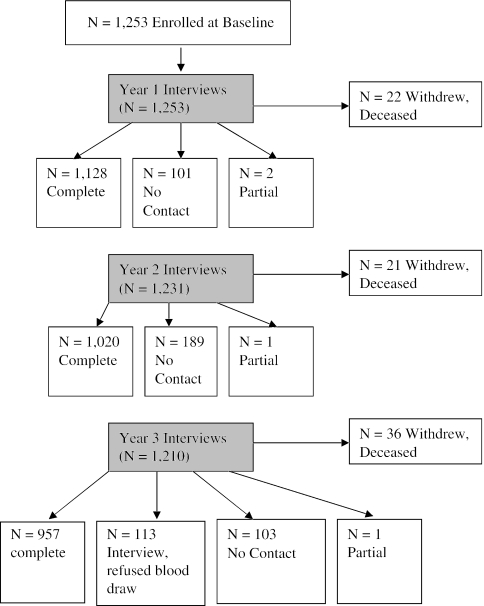
Patient flow through the study is shown in Figure 1. Of the 1,253 patients initially screened for diabetes, 56 had prevalent unrecognized diabetes. We achieved 3,257 person-years of follow-up on the remaining 1,197 patients (91%) and fully rescreened 957 subjects (80%).
FIGURE 1.
Flow of patients through the protocol.
Patient baseline characteristics are shown in Table 1. Approximately 29% of subjects described their race as African American, 43% were overweight (BMI, 25 to 30), and 35% were obese (BMI > 30). Hypertension was present in 53%. The mean HbA1c in the sample at baseline was 5.6%.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Patients (N = 1,253)
| Mean (SD) or % | |
|---|---|
| Demographic characteristics | |
| Age, y | 55 (6) |
| Gender, % male | 94 |
| Enrolled in primary care at Durham VAMC | 72 |
| Diabetes risk factors | |
| Race | |
| White | 69 |
| African American | 29 |
| Other | 2 |
| Family history of diabetes | 38 |
| BMI | |
| Normal (<25) | 22 |
| Overweight (25 to 30) | 43 |
| Obese (>30) | 35 |
| Hypertension | 53 |
| Quality of life | |
| SF-36 physical component score | 36 (12) |
| SF-36 mental component score | 50 (14) |
| Baseline HbA1c, % | 5.6 (0.7) |
VAMC, Veterans Administration medical center; BMI, body mass index; SD, standard deviation.
Incidence of Diabetes
Among the 1,197 patients who did not have diabetes diagnosed at baseline screening, we identified 73 incident cases of diabetes during 3 years of annual follow-up. The incidence of diabetes in the sample was 2.2 per 100 patient-years (73/3,257). Of the 73 incident cases, 34 (47%) were ascertained by our rescreening protocol, and 39 (53%) were ascertained by self-report during the annual telephone interview.
Relationship Between HbA1c and Incident Diabetes
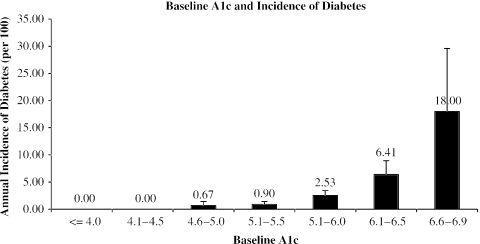
We next explored the relationship between baseline HbA1c and incidence of diabetes over the 3 years of follow-up after that HbA1c test (Fig. 2). There were no new cases of diabetes among patients with HbA1c ≤ 4.5% at baseline. There was a steady increase in incidence with increasing baseline HbA1c, to a peak incidence of 18% per year at HbA1c of 6.5% to 6.9%.
FIGURE 2.
Annual incidence of diabetes based on baseline HbA1c. Error bar is +2 S.E.M.
We then divided baseline HbA1c into 3 categories based on the standard deviation of HbA1c and traditional clinical interpretation: normal (HbA1c ≤ 5.5%, 1 standard deviation above mean); high-normal (HbA1c 5.6% to 6.0%, 1 to 2 standard deviations above mean); and elevated (HbA1c 6.1% to 6.9%, >2 standard deviations above mean). Over the 3 years of follow-up, new onset of diabetes was rare for patients with normal HbA1c, with an annual incidence of only 0.8%. Incidence after an initial high-normal HbA1c was 2.5% per year, and after an elevated HbA1c the incidence of diabetes was 7.8% per year (Table 2).
Table 2.
Incidence of Diabetes in Patients Based on Baseline Screening HbA1c
| HbA1c Category | Incidence per 100 Person-years (95% CI) |
|---|---|
| Normal (≤ 5.5%) | 0.8 (0.4 to 1.2) |
| High-normal (5.6% to 6.0%) | 2.5 (1.6 to 3.5) |
| Elevated (6.1% to 6.9%) | 7.8 (5.2 to 10.4) |
P < .0001 for difference between groups.
CI, confidence interval.
Relationship of HbA1c and Other Diabetes Risk Factors on Incidence of Diabetes
We performed logistic regression to assess the added role of known diabetes risk factors (age, race, family history of diabetes, BMI, hypertension) on incidence of diabetes after adjusting for baseline HbA1c. Only baseline BMI was associated with elevated risk of developing diabetes after adjustment for baseline HbA1c. Each additional 5 units of BMI contributed an adjusted odds ratio of 1.7 (1.4 to 2.1) for developing diabetes during follow-up. The interaction between BMI and HbA1c was not statistically significant.
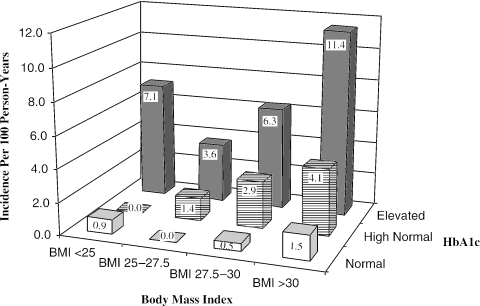
To further explore the relationship between obesity and HbA1c in predicting diabetes, we divided our subjects into categories of BMI and measured incidence of diabetes for each category of HbA1c for each category of BMI (Fig. 3). Regardless of BMI category, patients with elevated HbA1c had at least a 3.6% annual incidence of diabetes, and patients with normal HbA1c had no more than 1.5% annual incidence of diabetes. Patients with high-normal HbA1c and BMI 27.5 to 30 had a 2.9% annual incidence of diabetes, and this increased to 4.1% for obese subjects with high-normal BMI.
FIGURE 3.
Relationship of body mass index and baseline HbA1c to annual incidence of diabetes.
As sensitivity analyses, we explored other multivariable models of incident diabetes. First, to eliminate the effect our study might have had on physicians' seeking the diagnosis of diabetes during follow-up, we removed from the analysis the 39 patients diagnosed with diabetes by self-report during follow-up. In this model, both HbA1c and BMI remained significantly associated with incident diabetes, with slightly lower adjusted odds ratios than in the model using all patients. Then, in a model using all patients, we removed HbA1c from the model and used only characteristics that can be obtained from a traditional clinical history, as well as height and weight. In this model, only baseline BMI statistically significantly predicted incident diabetes, with an adjusted odds ratio of 1.8 (1.5 to 2.2) for each 5 units of BMI.
DISCUSSION
This study shows that in an outpatient population undergoing screening in a health care setting, HbA1c strongly predicts the development of diabetes. Obese patients were more likely to develop diabetes; however, obesity was not as strongly predictive of diabetes as HbA1c. We documented a very high incidence, 11% per year, of diabetes in obese patients with elevated HbA1c.
Whether adults should be screened for type 2 diabetes remains a controversial topic. The best decision analysis available suggests that diabetes screening is of borderline cost-effectiveness.9 In that analysis, the cost-effectiveness was very sensitive to the yield of screening. USPSTF now recommends screening patients with known hypertension, and agrees that a strategy targeted to high-risk patients will improve the success of the screening intervention.7 For periodic screening, the yield will depend on the incidence of diabetes in the target population; therefore, identification of people with a high risk of developing diabetes will maximize the cost-effectiveness of periodic diabetes screening. In our population of outpatients, the incidence of diabetes (2.2 per 100 person-years) was approximately 50% higher than that found in a community population of a similar age.22 This reinforces the notion that both episodic and periodic screening are more likely to be effective in health care settings (“opportunistic screening”) rather than in the community.8,21 Furthermore, our data demonstrate that HbA1c at baseline screening is strongly associated with developing diabetes, and that the yield of periodic screening can thus be further enhanced by more frequent screening of patients with higher HbA1c at first screening.
From these recent studies, we propose an opportunistic screening strategy for type 2 diabetes. First, a target population for baseline screening can be selected from patients in a medical setting based on risk factors such as hypertension, obesity, and family history of diabetes.21–23 Then, HbA1c results from the baseline screen can be used to suggest an interval until the next screening test, with follow-up testing more frequent for those patients with baseline HbA1c more than 2 standard deviations above mean and less frequent for those with HbA1c less than 1 standard deviation above mean.
Obesity also predicted new onset diabetes in our population. Patients with BMI over 27.5 and high-normal HbA1c had a modestly increased incidence of diabetes, and may also merit closer attention and more frequent periodic screening than patients of normal weight. Additionally, informing patients of the role of obesity in increasing their risk for developing diabetes, even if they are nondiabetic at their current weight, may help reinforce the message that weight loss is important.
Our data suggest that patients being tested for diabetes with HbA1c, regardless of whether testing is due to screening or as part of other medical evaluation (e.g., evaluation for cause of peripheral neuropathy), merit close follow-up for results that are outside the normal range even in the absence of diabetes. Given recent studies documenting successful strategies for diabetes prevention in patients at high risk for diabetes,24–26 patients with HbA1c in the 6.1% to 6.9% range may be candidates for lifestyle or even pharmacotherapy to prevent or delay the development of diabetes.
Our data do not directly address the contribution of HbA1c over and above traditional epidemiological risk factors for diabetes (e.g., obesity, hypertension). Most of our patients with high-normal HbA1c may have these risk factors and as such already be considered to be at high risk for diabetes. However, HbA1c measurement, as a clinically relevant, stable measure of insulin resistance, may allow refinement of diabetic risk judgments and may provide more precise identification of candidates for lifestyle intervention. It is also true that some patients develop diabetes in the absence of these risk factors, and HbA1c measurement provides risk information for those patients without traditional risk factors for diabetes. Additionally, knowledge of an elevated HbA1c might activate patients to follow lifestyle recommendations, or might persuade physicians to devote more effort to lifestyle recommendations for patients with traditional risk factors. Finally, patients with elevated HbA1c outside the diabetic range are at increased risk for cardiovascular morbidity and mortality,27 and so more aggressive treatment of traditional cardiovascular risk factors (e.g., blood pressure, lipids) may be considered in these patients.
Our data also point out the risk of developing diabetes associated with HbA1c that is above mean, but within the normal range. Other studies have pointed out the epidemiological risk associated with normal plasma glucose levels, either fasting or after an oral sugar load28–30; our study confirms that this is also true for HbA1c testing. Especially in obese patients, HbA1c between 1 and 2 standard deviations above the mean (5.6% to 6.0% in our study) requires consideration of periodic follow-up testing and potentially more urgent attention to diabetes risk reduction, given the relatively high likelihood of progression to diabetes.
Our study has limitations. First, we chose a pragmatic definition of diabetes. The study was designed to emulate a possible real-world screening strategy, and oral glucose tolerance testing, although still a standard, is rarely used in clinical settings.31 Our strategy, beginning with HbA1c and providing follow-up testing for patients with HbA1c ≥ 6.0%, has very high specificity, but a few people with diabetes will have HbA1c < 7.0% and FPG < 7.0 mmol/L. This definition causes further limitation because follow-up plasma glucose testing was only performed in those patients with HbA1c ≥ 6.0%, thereby increasing the possibility of finding diabetes (with FPG ≥ 7.0 mmol/L) in those patients as compared to those with HbA1c < 6.0%. The magnitude of this bias is small; a convenience sample of 160 patients with HbA1c <6.0% received FPG testing, and only one had FPG ≥ 7.0 mmol/L.21
We have additional limitations not related to the testing protocol. Annual screening was not performed, as the recommendation at that time was for screening every 3 years, and our pragmatic study attempted to emulate the recommended practice. Nevertheless, by adding self-report of diabetes to the definition during the study, we may have overstated the impact of screening, as 34 of the 73 cases were diagnosed not at screening but by self-report. Analysis excluding those patients diagnosed by their physicians yielded lower absolute numbers but similar and statistically significant associations for HbA1c and BMI in predicting diabetes. A further limitation is the generalizability of the study, particularly related to the demographics of the population at a single VA site (our study population was 94% male). This limitation is partially mitigated by the fact that these data are from a patient rather than a community sample, making them more generalizable in that way to the patient populations chosen for opportunistic screening.
Our study was not designed to confirm or refute the effectiveness or cost-effectiveness of diabetes screening. The answer to that question is likely to remain unknown; a randomized controlled trial of diabetes screening would be expensive and require very large numbers of patients and very long follow-up.8 In the absence of such trials, observational studies such as these will provide the best information from which to draw inference about the role of diabetes screening in health care systems, and to inform future decision analyses estimating cost-effectiveness. Identifying high-risk target populations, both at initial evaluation and in follow-up, will maximize both the effectiveness and cost-effectiveness of screening for this costly and morbid disease.
Acknowledgments
This study was funded by the Department of Veterans’ Affairs Cooperative Studies Program (study #705-D). Dr. Edelman was supported by a VA Health Services Research Career Award.
REFERENCES
- 1.Harris MI. Noninsulin-dependent diabetes mellitus in black and white Americans. Diabetes Metab Rev. 1990;6:71–90. doi: 10.1002/dmr.5610060202. [DOI] [PubMed] [Google Scholar]
- 2.Harris MI. Impaired glucose tolerance in the US population. Diabetes Care. 1989;12:464–74. doi: 10.2337/diacare.12.7.464. [DOI] [PubMed] [Google Scholar]
- 3.Huse DM, Oster G, Killen AR, Lacey MJ, Colditz GA. The economic costs of non-insulin-dependent diabetes mellitus. JAMA. 1989;262:2708–13. doi: 10.1001/jama.262.19.2708. [DOI] [PubMed] [Google Scholar]
- 4.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 years before clinical diagnosis. Diabetes Care. 1992;15:815–9. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. VA Diabetes Clinical Practice Guidelines. Available at: http://209.42.214.199/cpg/DM/DM_base.htm. Accessed October 11, 2004.
- 6.Anonymous. Screening for type 2 diabetes. Diabetes Care. 2003;26(S1):21–4. [Google Scholar]
- 7.Harris R, Donahue K, Rathore SS, Frame P, Woolf SH, Lohr KN. Screening adults for type 2 diabetes: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138:215–29. doi: 10.7326/0003-4819-138-3-200302040-00015. [DOI] [PubMed] [Google Scholar]
- 8.Engelgau MM, Narayan KMV, Herman WH. Screening for type 2 diabetes. Diabetes Care. 2000;23:1563–80. doi: 10.2337/diacare.23.10.1563. [DOI] [PubMed] [Google Scholar]
- 9.CDC Cost-Effectiveness Study Group. The cost-effectiveness of screening for type 2 diabetes. JAMA. 1998;280:1757–63. [PubMed] [Google Scholar]
- 10.Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical Epidemiology: A Basic Science for Clinical Medicine. 2nd ed. Boston, Mass: Little, Brown and Company; 1991. [Google Scholar]
- 11.Rohlfing CL, Little RR, Wiedmeyer HM, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23:187–91. doi: 10.2337/diacare.23.2.187. [DOI] [PubMed] [Google Scholar]
- 12.Gerken KL, Van Lente F. Effectiveness of screening for diabetes. Arch Pathol Lab Med. 1990;114:201–3. [PubMed] [Google Scholar]
- 13.Anonymous. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26(S1):33–50. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 15.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 16.UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 17.DCCT/EDIC Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson ML, Gerstein HC, Tsui E, Zinman B. Effect of intensive therapy on early macrovascular disease in young individuals with type 1 diabetes. Diabetes Care. 1999;22(S1):B35–B39. [PubMed] [Google Scholar]
- 19.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr RG, Nathan DM, Meigs JB, Singer DE. Tests of glycemia for the diagnosis of type 2 diabetes mellitus. Ann Intern Med. 2002;137:263–72. doi: 10.7326/0003-4819-137-4-200208200-00011. [DOI] [PubMed] [Google Scholar]
- 21.Edelman D, Edwards LJ, Olsen MK, et al. Screening for diabetes in an outpatient clinic population. J Gen Intern Med. 2002;17:23–8. doi: 10.1046/j.1525-1497.2002.10420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brancati FL, Kao WHL, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the atherosclerosis risk in communities study. JAMA. 2000;283:2253–60. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 23.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 24.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 25.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 26.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Research Group. Reduction in the incidence of type 2 diabetes with life-style intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and Nutrition (EPIC-Norfolk) BMJ. 2001;322:1–6. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dzien A, Dzien-Bischinger C, Hoppichler F, Lechleitner M. Fasting glucose concentrations and cardiovascular disease; are the new diagnostic criteria not strict enough? Diabetes Res Clin Pract. 2001;54:213–4. doi: 10.1016/s0168-8227(00)00243-6. [DOI] [PubMed] [Google Scholar]
- 29.de Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: the Hoorn Study. JAMA. 2001;285:2109–13. doi: 10.1001/jama.285.16.2109. [DOI] [PubMed] [Google Scholar]
- 30.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46:701–10. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med. 2002;136:575–81. doi: 10.7326/0003-4819-136-8-200204160-00006. [DOI] [PubMed] [Google Scholar]