Abstract
OBJECTIVE
The prevalence of major depression is approximately 2-fold higher in patients with diabetes mellitus compared to medical controls. We explored the association of major depression with 8 cardiac risk factors in diabetic patients with and without evidence of cardiovascular disease (CVD).
DESIGN
A mail survey questionnaire was administered to a population-based sample of 4,225 patients with diabetes to obtain data on depression status, diabetes self-care (diet, exercise, and smoking), diabetes history, and demographics. On the basis of automated data we measured diabetes complications, glycosylated hemoglobin, medical comorbidity, low-density lipid levels, triglyceride levels, diagnosis of hypertension, and evidence of microalbuminuria. Separate analyses were conducted for subgroups according to the presence or absence of CVD.
SETTING
Nine primary care clinics of a nonprofit health maintenance organization.
MAIN RESULTS
Patients with major depression and diabetes were 1.5- to 2-fold more likely to have 3 or more cardiovascular risk factors as patients with diabetes without depression (62.5% vs 38.4% in those without CVD, and 61.3% vs 45% in those with CVD). Patients with diabetes without CVD who met criteria for major depression were significantly more likely to be smokers, to have a body mass index (BMI) ≥ 30 kg/m2, to lead a more sedentary lifestyle, and to have HbA1c levels of >8.0% compared to nondepressed patients with diabetes without heart disease. Patients with major depression, diabetes, and evidence of heart disease were significantly more likely to have a BMI ≥ 30 kg/m2, a more sedentary lifestyle, and triglyceride levels > 400 mg/dl than nondepressed diabetic patients with evidence of heart disease.
CONCLUSIONS
Patients with major depression and diabetes with or without evidence of heart disease have a higher number of CVD risk factors. Interventions aimed at decreasing these risk factors may need to address treatment for major depression in order to be effective.
Keywords: depression, risk factors, coronary, diabetes, epidemiology
Behavioral, genetic, and biomedical disorders have long been recognized as associated with an increased risk of heart disease. These include: smoking, sedentary lifestyle, obesity, high levels of low-density lipids (LDL) and triglycerides, hypertension, diabetes, microalbuminuria, and family history of coronary vascular disease.1 There has been an increased interest in both the association of major depression as a risk factor for the development of coronary heart disease2 as well as a risk factor for mortality after onset of coronary vascular disease.3 A recent meta-analysis of 11 studies found that major depression was a significant predictor for development of coronary artery disease. Depressed subjects had an overall relative risk of 1.64 (95% confidence interval [CI], 1.29 to 2.08) for developing heart disease compared to nondepressed subjects.4 Major depression has also been shown to increase the risk of mortality in patients after myocardial infarction by as much as 4-fold.5
Major depression has also been found in several longitudinal studies of patients with diabetes mellitus to be associated with a higher incidence of coronary heart disease.6–8 This is important because the mortality from cardiovascular disease (CVD) is increased by a factor of 2 to 3 in patients with diabetes as compared to the general population.6,7 Clouse et al. found that major depression increased the risk of development of CVD over a 10-year period in a sample of 76 women with either type 1 or type 2 diabetes.6 A study of Mexican Americans 65 years of age and older showed that patients with comorbid diabetes and major depression had increased incidence of morbidity and mortality from CVD over a 7-year period compared to patients with diabetes alone.7 The Pittsburgh Epidemiology of Diabetes Complication Study8 also reported that a high degree of depressive symptoms on the Beck Depression Inventory9 increased the risk of CVD over a 10-year period in 658 men and women with type 1 diabetes.
Several possible mechanisms have been proposed to explain why depression increases the longitudinal risk of development of CVD as well as mortality from CVD. These include the association of depression with behavioral mediators of CVD such as smoking,10 obesity,11,12 sedentary lifestyle,13,14 and lack of adherence to medication.15 Other research has focused on biologic explanations such as possible connections between depression and altered autonomic regulation marked by decreased heart rate variability16; increased platelet dysfunction characterized by heightened platelet aggregation or adhesiveness17,18; an association with inflammatory processes that may be important in developing CVD such as increased C-reactive protein or cytokine levels2,19; and abnormalities in endothelial function.2
Although prior studies have focused on the association with individual cardiac risk factors and depression in patients with type 2 diabetes, we are not aware of research that has examined whether depression was associated with an increased number of a comprehensive set of cardiac risk factors. In a population-based sample of primary care patients with predominantly type 2 diabetes, we assessed whether major depression was associated with a higher number of 8 known risk factors for CVD (smoking, obesity, lack of exercise, high triglycerides, LDL, glycemic control, hypertension, and microalbuminuria/macroalbuminuria).1 The study will also determine which cardiac risk factors are significantly associated with major depression.
METHODS
The Pathways Study was developed by a multidisciplinary team in the Department of Psychiatry at the University of Washington and the Center for Health Studies at Group Health Cooperative.20 Group Health is a nonprofit health maintenance organization with 30 primary care clinics in western Washington State. The study protocol was reviewed and approved by Institutional Review Boards (IRB) at the University of Washington and Group Health Cooperative.
Study Setting
Nine Group Health Cooperative (GHC) primary care clinics in western Washington were selected for the study. We selected clinics based on 3 criteria: 1) clinics with the largest number of diabetic patients; 2) clinics within a 40-mile geographic radius of Seattle; and 3) clinics with the highest racial and ethnic diversity. These 9 primary care clinics range from 20 miles north to 40 miles south of Seattle, and the population is representative of the western Washington State region, with the exception of having fewer patients in the highest income range.
Sample Recruitment
Case identification was facilitated by GHC's prior development of a population-based diabetes registry that supports patient care.21 Patients are added to the diabetes registry based on: 1) whether they are currently taking any diabetic agent; or 2) a fasting glucose ≥ 126 mg/dl confirmed by a second out-of-range test within 1 year; or 3) a random plasma glucose ≥ 200 mg/dl also confirmed by a second test within 1 year; or 4) a hospital discharge diagnosis of diabetes at any time during GHC enrollment or 2 outpatient diagnoses of diabetes.21 The goal of the epidemiologic survey was to successfully screen at least 4,500 primary care patients with diabetes for major depression.20
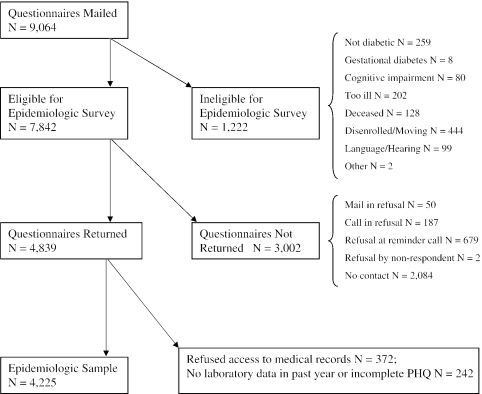
Patients were surveyed by mail in sequential waves with approximately 700 questionnaires sent per month. A $3 gift certificate for a local store was included with the mailing to encourage response. If the patient did not return a mailed packet by 4 weeks, a second packet was sent. If this second packet was not returned by 2 weeks, the patient received a telephone reminder call. The final response rate for this epidemiologic study was 61.7%. Figure 1 describes the recruitment and the reasons for ineligibility or refusal at each phase of the study.
FIGURE 1.
Mail survey description.
The Patient Health Questionnaire (PHQ-9) was used to screen for depression. This questionnaire provides both a dichotomous diagnosis of major depression and a continuous severity score.22,23 The PHQ-9 diagnosis of major depression has been found to have high agreement with the diagnosis of major depression based on structured interview.22 The criteria for major depression required the patient to have, for at least 2 weeks, 5 or more depressive symptoms present for more than half of the days, with at least 1 of these symptoms being either depressed mood or anhedonia.22,23
Eight cardiac risk factors were selected for study based on the United Kingdom Prospective Diabetes Study (UKPDS)24 and the Atherosclerosis Risk in Community Study (ARIC),25 including smoking, hypertension, HbA1c levels, LDL, triglyceride levels, sedentary lifestyle, body mass index (BMI), and micro- or macroalbuminuria. The UKPDS showed that traditional risk factors for coronary heart disease in patients with type 2 diabetes (smoking, hypertension, HbA1c levels, LDL and triglyceride levels) were all significant independent predictors of incident coronary heart disease.24,25 The ARIC study also showed that the addition of multiple nontraditional risk factors such as BMI, sedentary lifestyle, and evidence of kidney disease significantly increased the predictivity of the model.25
The survey also asked about demographics including age, gender, years of education, employment status, race, and marital status. Questions about clinical status included age of onset of diabetes, duration of diabetes, current diabetic treatments (insulin, oral hypoglycemic medication, diet), diabetes treatment at onset of disease, smoking status,26 recent level of exercise,26 height, and weight. Patients were diagnosed as having type 1 diabetes if their age of onset was less than 30 and insulin was the first treatment prescribed.
We utilized GHC's automated utilization, diagnostic, and laboratory data to identify whether patients were hypertensive or had microalbuminuria or macroalbuminuria as well as assessing LDL and triglyceride levels. We selected the LDL and triglyceride level closest to the date in which the epidemiologic survey was returned to reflect these lipid values. Patients were classified as having high LDL based on a level > 130 mg/dl and high triglycerides based on a level > 400 mg/dl.27 Microalbuminuria was based on automated laboratory data demonstrating presence of macroalbuminuria (>300 µg/mg creatinine) or abnormal microalbuminuria (>30 µg/mg creatinine) on spot urine collection.28 We used a physician outpatient or inpatient diagnosis of hypertension in the preceding 18 months prior to the date the survey was returned to define whether patients had hypertension. The International Classification of Diseases, Ninth Revision (ICD-9) codes for hypertension included any code starting with 401, 402, 403, 404, or 405.
Computerized pharmacy records were used to compute a revised chronic disease score known as Rx Risk, which measured medical comorbidity based on prescription drug use over the 12 previous months.29 The Rx Risk has been found to be comparable to using Ambulatory Care Groups30 in predicting total future health costs.29
The presence of CVD was identified by automated diagnostic data from the previous 18 months. ICD-9 codes for myocardial infarction, ventricular fibrillation, cardiac arrest, other acute/subacute ischemic heart disease, old myocardial infarction, angina pectoris, other chronic ischemic heart disease, or heart failure indicated the presence of CVD.31
Statistical Analyses
Assessing nonresponse bias: after obtaining IRB approval, we examined differences in de-identified data between survey respondents and nonrespondents using automated health care data. We excluded 369 survey participants who did not give permission to use automated medical records data. We estimated response propensity scores (probability of being a respondent) as a function of the following variables (all of these within the prior year): age; gender; most recent HbA1c value; treatment with insulin; use of oral hypoglycemic medicines; received specialty mental health care; depression diagnosis in primary or specialty care; any prescriptions filled for antidepressant medication; hospitalization; Rx Risk score omitting diabetes and psychiatric medications; number of primary and specialty care visits; patient inclusion on the GHC heart disease registry; and patient primary care clinic. We predicted response/nonresponse status as a function of these variables using PROC LOGISTIC of SAS (SAS Institute, Cary, NC).32 Using these predictors, we estimated a response probability for each survey respondent (response propensity score). We used a weighted analysis (weights inversely proportional to estimated probability of response) rescaled to sum to the observed sample size (i.e., the number of survey respondents). In weighted analysis, persons with a low probability of responding would be given a higher weight in the analysis to represent the larger number of nonrespondents with similar characteristics. We then compared weighted and unweighted analyses to see whether postsurvey adjustment for factors related to nonresponse resulted in meaningful differences in survey estimates. Differences in weighted and unweighted data were negligible; therefore, we report analyses based on observed data.
Due to the importance of CVD status to our risk factor analyses, the sample was stratified by the presence or absence of cardiovascular disease (CVD+, CVD−). Clinical and demographic variables were compared for the groups with and without major depression within CVD strata using χ2 tests for the dichotomous variables and independent group, two-tailed t tests for the continuous variables. To determine whether patients with major depression had a higher number of risk factors, unadjusted χ2 analyses were performed to compare the number of risk factors in patients by major depression status (yes vs no), for both CVD groups separately. Two analyses of covariance (ANCOVAs) were also performed (one for each CVD stratum) using the continuous number of risk factors (0 to 8) as the dependent variable and the major depression group as the independent variable. These analyses adjusted for gender, age, marital status, education, race, clinic, type 1 diabetes, and duration of diabetes in years. In the event of a significant association between total number of risk factors and major depression, each of the 8 risk factors was examined individually to assess its relationship with major depression. This was completed for each CVD stratum, adjusted and unadjusted for covariates. χ2 tests relating major depression status to the presence or absence of each risk factor were used in unadjusted analyses, and logistic regressions were used in adjusted analyses. In the logistic analyses, the outcome was the presence or absence of a given risk factor, and the independent variable of interest was the presence of major depression. The covariates in the analyses were gender, age, marital status, education, race, clinic, type 1 diabetes, and duration of diabetes in years. Adjusted odds ratios for major depression and their 95% confidence intervals (CI) are presented.
RESULTS
Of the 4,225 patients, 3,010 had no CVD (71%, CVD−) and 1,215 (29%, CVD+) had CVD. Within the CVD− group, 10.6% had major depression, while 14.2% of the CVD+ group had major depression. Within both CVD strata, there were significantly more women than men with major depression, and depressed patients were significantly younger (Table 1). In the CVD− group, depressed subjects were significantly less likely to be married than the nondepressed group. In the CVD+ group, depressed subjects had greater medical comorbidity than the nondepressed group.
Table 1.
Demographics and Clinical Characteristics (N = 4,225)
| Patients with No CVD (N = 3,010) | Patients with CVD (N = 1,215) | |||||
|---|---|---|---|---|---|---|
| Variables | Major Depression (n = 320) % (n ) | No Depression (n = 2,690) % (n) | χ2 Test Df = 1 | Major Depression (n = 173) % (n) | No Depression (n = 1,042) % (n) | χ2 Test Df = 1 |
| Female, % | 65.9 (211) | 50.7 (1,365) | 26.47† | 47.4 (82) | 38.5 (401) | 4.92* |
| Some college, % | 77.0 (241) | 77.0 (2,050) | 0.01 | 64.5 (111) | 71.1 (729) | 3.05 |
| White, % | 77.6 (239) | 78.2 (2,058) | 0.06 | 82.2 (139) | 84.5 (863) | 0.56 |
| Married, % | 56.8 (179) | 66.8 (1,788) | 12.49† | 64.0 (110) | 69.0 (710) | 1.73 |
| Type 1 diabetics, % | 4.1 (13) | 5.2 (140) | 0.76 | 2.9 (5) | 2.1 (22) | 0.14 |
| Mean (SD) | Mean (SD) | t Test (df = 3,008) | Mean (SD) | Mean (SD) | t Test (df = ) | |
| Age, y | 54.8 (12.2) | 61.5 (13.1) | 9.26† | 67.0 (11.9) | 70.7 (10.3) | 3.85† |
| Duration of diabetes, y | 8.3 (7.0) | 8.8 (9.1) | 1.27 | 12.0 (9.7) | 11.1 (10.1) | −1.02 |
| Chronic disease score | 2,787.0 (2,475.2) | 2,709.2 (2,423.7) | −0.54 | 6,389.6 (5,207.7) | 5,466.7 (3,419.1) | −2.25* |
P < .05.
P < .001.
CVD, cardiovascular disease; SD, standard deviation; df, degrees of freedom.
Table 2 shows the unadjusted relationships between number of risk factors and major depression for the CVD strata. Patients with major depression were significantly more likely to have higher numbers of risk factors. This relationship is highly significant for patients with diabetes with or without CVD. Patients with major depression were 2-fold more likely to have 4 or more risk factors in comparison to patients without depression (32% vs 15% for CVD− groups and 36% vs 19% for CVD+ groups) and had almost twice the likelihood of having 3 or more risk factors (62.5% vs 38.4% in CVD− groups and 61.3% vs 45.0% in CVD+ groups). The adjusted analyses for the continuous number of risk factors (covariates of age, gender, marital status, education, race, type 1 diabetes, duration of diabetes, and clinic) had the same pattern of results. For diabetic patients with no CVD, the ANCOVA showed a significant major depression effect: F(1,2906) = 49.97, P < .001, with adjusted means of 2.75 (standard deviation [SD]= 1.30) and 2.20 (SD = 1.30) risk factors for the depressed and nondepressed groups, respectively. For diabetic patients with CVD, the ANCOVA adjusted for all the above-listed covariates showed a significant major depression effect: F(1,1163) = 15.58, P < .001, with adjusted means of 2.82 (SD = 1.43) and 2.39 (SD = 1.33) risk factors for the depressed and nondepressed groups, respectively.
Table 2.
Number of Risk Factors for the Depression Groups
| Patients with No CVD (N = 3,010) | Patients with CVD (N = 1,215) | |||
|---|---|---|---|---|
| Number of Risk Factors | Major Depression (n = 320) % (n) | No Depression (n = 2,690) % (n) | Major Depression (n = 173) % (n ) | No Depression (n = 1,042) % (n) |
| 0 | 1.6 (5) | 8.9 (240) | 2.9 (5) | 6.5 (68) |
| 1 | 13.4 (43) | 23.2 (623) | 13.3 (23) | 22.2 (231) |
| 2 | 22.5 (72) | 29.5 (793) | 22.5 (39) | 26.3 (274) |
| 3 | 30.9 (99) | 23.0 (620) | 24.9 (43) | 25.5 (266) |
| 4+ | 31.6 (101) | 15.4 (414) | 36.4 (63) | 19.5 (203) |
| χ2 unadjusted test | χ2= 87.26, df = 4, P < .001 | χ2= 29.12, df = 4, P < .001 | ||
CVD, cardiovascular disease; df, degrees of freedom.
Table 3 shows the unadjusted and adjusted analyses for subjects with and without CVD. Patients without CVD and major depression had statistically significantly higher rates using unadjusted χ2 analyses of smoking (P < .001), BMI ≥ 30 kg/m2 (P < .001), lower rates of exercise (P < .001), higher rates of HbA1c > 8.0% (P < .001), and higher rates of microalbuminuria (P < .004). For subjects without CVD, 29% of the depressed group in comparison to 17% of the nondepressed group reported no days of exercise.
Table 3.
Relationship of Major Depression to Risk Factors for Patients Without CVD (N = 3,010)
| Risk Factor | N* | Major Depression % (N)† | No Major Depression % (N)† | χ2 Df = 1 | Adjusted‡ Odds Ratio for Major Depression | Wald's Adjusted χ2 Df = 1 | 95% CI for Odds Ratios |
|---|---|---|---|---|---|---|---|
| Currently smoking | 2,967 | 20.1 (63) | 8.2 (218) | 45.96 (P < .001) | 2.22 | 21.15 (P < .001) | 1.58 to 3.11 |
| BMI ≥ 30.0 | 2,969 | 69.0 (216) | 48.4 (1,285) | 47.67 (P < .001) | 1.69 | 13.48 (P < .001) | 1.28 to 2.23 |
| Lower exercise level <3 days per week average | 3,010 | 63.8 (204) | 48.2 (1,296) | 27.74 (P < .001) | 1.72 | 17.56 (P < .001) | 1.34 to 2.22 |
| Hypertension | 3,010 | 34.1 (109) | 35.8 (963) | 0.38 (P = .54) | 1.10 | 0.48 (P = .49) | 0.84 to 1.43 |
| High LDL level 130+ | 2,017 | 38.8 (87) | 33.0 (592) | 3.02 (P = .08) | 1.19 | 1.27 (P = .26) | 0.88 to 1.62 |
| High triglyceride level 400+ | 2,022 | 11.5 (26) | 8.4 (150) | 2.51 (P = .11) | 1.08 | 0.09 (P = .76) | 0.67 to 1.74 |
| Microalbuminuria | 3,010 | 24.4 (78) | 17.8 (479) | 8.18 (P = .004) | 1.29 | 2.79 (P = .09) | 0.96 to 1.73 |
| HbA1c > 8.0 | 3,101 | 48.4 (155) | 33.0 (888) | 30.05 (P < .001) | 1.71 | 17.27 (P < .001) | 1.33 to 2.20 |
Number of subjects with either written questionnaire or automated data for each risk factor.
Number of depressed and not depressed for each risk factor.
Adjusted for gender, age, marital status, education, racial ethnicity, clinic, type 1 diabetes, and duration of diabetes in years.
CVD, cardiovascular disease; df, degrees of freedom; CI, confidence interval; BMI, body mass index; LDL, low-density lipids.
Depressed patients without CVD also had higher rates of high LDL levels (P = .08) and higher levels of triglycerides (P = .11) in the unadjusted analyses, but these were only significant at a trend level. Patients with and without major depression were fairly equal in the proportion with hypertension. Adjusting for the clinical and demographic factors did not appreciably decrease the relationship between major depression and the risk factors. The only differences were that microalbuminuria was only significant at a trend level (P = .12), and triglyceride and LDL levels did not approach statistical significance.
Among patients with CVD (Table 4), the unadjusted analyses show that the depressed group had greater proportions on all risk factors; however, only BMI ≥ 30 kg/m2, lower exercise levels, and high triglyceride levels reach statistical significance (P < .001). There is trend-level significance for hypertension (P = .13) and HbA1c > 8.0% (P > .06). Logistic regressions adjusting for the covariates show that patients with depression are almost two times more likely than patients without depression to have a BMI ≥ 30 kg/m2, higher triglyceride levels, and lower exercise levels compared to patients without depression. For subjects with CVD, 37% of the depressed patients had no exercise days compared to 22% of those without depression.
Table 4.
Relationship of Major Depression to Risk Factors for Patients with CVD (N = 1,215)
| Risk Factor | N* | Major Depression % (N)† | No Major Depression % (N)† | χ2 Df = 1 | Adjusted ‡ Odds Ratio for Major Depression | Wald's Adjusted χ2 Df = 1 | 95% CI for Odds Ratios |
|---|---|---|---|---|---|---|---|
| Currently smoking | 1,190 | 8.8 (15) | 6.1 (62) | 1.39 (P = .24) | 0.90 | 0.10 (P = .75) | 0.46 to 1.75 |
| BMI ≥ 30.0 | 1,194 | 60.6 (103) | 41.8 (428) | 20.09 (P < .001) | 1.91 | 11.07 (P = .001) | 1.30 to 2.80 |
| Lower exercise level <3 days per week average | 1,215 | 69.9 (121) | 52.5 (547) | 17.55 (P < .001) | 2.02 | 14.27 (P < .001) | 1.40 to 2.90 |
| Hypertension | 1,215 | 69.4 (120) | 63.1 (657) | 2.30 (P = .13) | 1.22 | 1.20 (P = .27) | 0.85 to 1.76 |
| High LDL level 130+ | 991 | 19.3 (26) | 17.0 (145) | 0.29 (P = .59) | 0.94 | 0.07 (P = .79) | 0.57 to 1.54 |
| High triglyceride level 400+ | 990 | 16.3 (22) | 6.9 (59) | 12.52 (P < .001) | 2.02 | 5.77 (P = .02) | 1.14 to 3.58 |
| Microalbuminuria | 1,215 | 20.8 (36) | 19.7 (205) | 0.06 (P = .81) | 1.07 | 0.09 (P = .76) | 0.70 to 1.63 |
| HbA1c > 8.0 | 1,215 | 42.2 (73) | 34.7 (362) | 3.59 (P = .06) | 1.22 | 1.17 (P = .28) | 0.85 to 1.73 |
Number of subjects with either written questionnaire or automated data for each risk factor.
Number of depressed and not depressed for each risk factor.
Adjusted for gender, age, marital status, education, racial ethnicity, clinic, type 1 diabetes, and duration of diabetes in years.
CVD, cardiovascular disease; df, degrees of freedom; CI, confidence interval; BMI, body mass index; LDL, low-density lipids.
DISCUSSION
In a population-based primary care sample of patients with diabetes, major depression was associated with a significantly higher number of cardiovascular risk factors in the presence or absence of heart disease. In both groups, diabetic patients with major depression were significantly overrepresented among patients who had 3 or more cardiovascular risk factors. These patients with 3 or more risk factors presumably have the highest risk for development of either a new cardiovascular event or progression of CVD severity. Among diabetic patients without heart disease, depression was significantly associated with being a smoker, being obese (BMI ≥ 30 kg/m2), having an HbA1c level > 8.0%, and being less physically active. In patients with diabetes and evidence of heart disease, depression was significantly associated with obesity (BMI ≥ 30 kg/m2), lower exercise levels, and high triglyceride levels. Among diabetic patients with heart disease, the percentage of patients who smoked was lower, perhaps because a cardiovascular event motivated these patients to quit smoking.
This is the first study we are aware of that describes the association of major depression with a large number of cardiac risk factors in patients with predominantly type 2 diabetes. The results are similar to findings from a recent community-based study that reported the association of depression with multiple cardiac risk factors and cardiac mortality in a study of 93,676 women (age 50 to 79).33 This large community study reported that depressive symptoms were associated with a higher risk of smoking, having a BMI > 27.3, being sedentary, and having hypertension, diabetes, and high cholesterol.
In the Pittsburgh Epidemiology of Diabetes Complications Study, researchers found a longitudinal relationship between depression and later development of CVD among patients with type 1 diabetes.8 These researchers also found that this relationship appeared to be, in part, explained by the association of depression with potential mediators such as insulin resistance, autonomic dysregulation (based on increased heart rate and decreased heart rate variability), markers of inflammation, smoking, and complications associated with diabetes such as microalbuminuria.8 Our finding of a greater rate of smoking in patients with major depression and diabetes without CVD supports these findings. Whereas in bivariate analyses, increased microalbuminuria levels were significantly associated with major depression in the diabetic group without CVD, this association disappeared after controlling for covariates.
Our data also suggest that in patients with depression and diabetes, behavioral mediators such as smoking, obesity, sedentary lifestyle, and glycemic control may contribute to the increased risk of heart disease that has been found in longitudinal studies in depressed versus nondepressed patients with diabetes. Several of these behavioral mediators, including sedentary lifestyle and obesity (as well as both diabetes and depression), have also been found to be related to higher C-reactive protein levels, an inflammatory risk factor for coronary artery disease.34 Research has consistently demonstrated that major depression is more common in women than in men.35 Patients with diabetes are no exception.36 These findings may partially explain why improved biomedical prevention and treatment of heart disease has decreased mortality in men, but not in women.37
An important research question is whether interventions that improve accuracy of diagnosis and quality of treatment of depression along with behavioral treatment of risk factors would improve cardiac morbidity and mortality in patients with diabetes, especially women. The higher rate of obesity, smoking, and triglyceride levels in patients with depression, diabetes, and heart disease found in this study may also contribute to the increased risk of mortality in patients with diabetes and a history of heart disease that has been found in prior studies.38,39 Although our data is cross-sectional, limiting our ability to ascertain whether depression or the cardiac risk factors came first, longitudinal studies have shown that depression earlier in life raises the risk of both obesity11,12 and smoking.40 Several studies have also found that depression is associated with an increased risk of sedentary lifestyle among adults.13,14 Moreover, recent data suggest that depression is associated with less success in quitting smoking over a decade of adult life,41 higher dropout rates in exercise rehabilitation programs of patients with heart disease,42 as well as higher dropout from weight loss programs in patients with diabetes.43 Major depression has also been found to be associated with decreased adherence to oral hypoglycemic medications in patients with type 2 diabetes44 and to aspirin in patients with CVD.45 These data suggest that intervention programs aimed at decreasing the rates of obesity, smoking, and sedentary lifestyle and improving adherence to medication among patients with both diabetes and heart disease may need to address the high rate of depression in these patients, especially patients with multiple risk factors.
Biological studies have also found that both major depression and diabetes have been shown to be independently associated with increased platelet adhesiveness and exaggerated aggregation,17,18,46,47 increased markers of inflammatory response such as C-reactive protein,19,48 and endothelial dysfunction.49,50 When major depression is comorbid with diabetes, there may be additive risk from these the behavioral mediators (i.e., smoking, obesity, lack of exercise, and poor glycemic control) and biologic factors that are associated with heart disease.
Strengths of the study include the large population-based sample of patients with type 2 diabetes observed in naturalistic care in a large primary care system. However, the use of naturalistic laboratory data limited obtaining timely measurement of lipids and markers of microalbuminuria. Using automated data to ascertain the presence or absence of heart disease may also contain misclassifications. The cross-sectional design of the study also precludes explanations of causality. For instance, we cannot be certain whether depression preceded lack of exercise and the development of heart disease or whether having heart disease led to depression and associated inactivity. Other limitations include lack of physiologic measures such as markers of inflammation and autonomic nervous system regulation. A final limitation is that our analyses did not control for medical comorbidity (there were no differences in medical comorbidity in the depressed vs nondepressed patients without evidence of heart disease) because our comorbidity measure overlapped with outcome variables such as hypertension, hyperlipidemia, and kidney disease.
Conclusion
Major depression is associated with an increased number of known cardiac risk factors in patients with diabetes mellitus. Systems of care that integrate diagnosis and treatment of major depression into medical management of diabetes may be needed in order to lower cardiac risks and complications.
Acknowledgments
This work was supported by grants MH41739 and MH016473 from the National Institute of Mental Health Services Division, Bethesda, Md (Dr. Katon).
REFERENCES
- 1.Kannel W, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 2.Strike P, Steptoe A. Depression, stress and the heart. Heart. 2002;88:441–3. doi: 10.1136/heart.88.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary artery disease. BMJ. 1999;318:1460–7. doi: 10.1136/bmj.318.7196.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 5.Frasure-Smith, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 6.Clouse R, Freedland K, Carney R, Griffith L, Lustman P. Depression accelerates the presentation of coronary heart disease (CHD) in women with diabetes mellitus. Psychosom Med. 2001;63:103. [Google Scholar]
- 7.Black S, Markides K, Ray L. Depression predicts increased incidence of adverse death outcomes in older Mexican-Americans with type 2 diabetes. Diabetes Care. 2003;26:2822–8. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 8.Kinder L, Kamarck T, Baum A, Orchard T. Depressive symptomatology and coronary heart disease in type 1 diabetes mellitus. A study of possible mechanisms. Health Psychol. 2002;21:542–52. doi: 10.1037//0278-6133.21.6.542. [DOI] [PubMed] [Google Scholar]
- 9.Beck A, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1998;8:77–100. [Google Scholar]
- 10.Glassman A, Helzer J, Covey L, et al. Smoking, smoking cessation and major depression. JAMA. 1990;264:1583–4. [PubMed] [Google Scholar]
- 11.Goodman E, Whitaker R. A prospective study of the role of depression in the development and persistence of adult obesity. Pediatrics. 2002;110:497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- 12.Pine D, Goldstein R, Wolk S, Weissman M. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107:1049–55. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- 13.Steptoe A, Wardle J, Fuller R, et al. Leisure-time physical exercise: prevalence, attitudinal correlate and behavior correlates among young Europeans from 21 countries. Prev Med. 1997;26:845–54. doi: 10.1006/pmed.1997.0224. [DOI] [PubMed] [Google Scholar]
- 14.Rajala U, Uusimaki A, Keinanen-Kiukaanniemi S, Kivela SL. Prevalence of depression in a 55-year-old Finnish population. Soc Psychiatry Psychiatr Epidemiol. 1994;29:126–30. doi: 10.1007/BF00796492. [DOI] [PubMed] [Google Scholar]
- 15.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 16.Gorman J, Sloan R. Heart rate variability in depressive and anxiety disorders. Am Heart J. 2000;140(suppl 4):S77–S83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- 17.Langhrissi-Thode F, Wagner W, Pollock B, Johnson P, Finkel M. Elevated platelet factor 4 and beta-thromoglobin plasma levels in depressed patients with ischemia heart disease. Biol Psychiatry. 1997;42:290–5. doi: 10.1016/S0006-3223(96)00345-9. [DOI] [PubMed] [Google Scholar]
- 18.Musselman D, Tomer A, Manatunga A, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry. 1996;153:1313–7. doi: 10.1176/ajp.153.10.1313. [DOI] [PubMed] [Google Scholar]
- 19.Konsman J, Parret J, Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Trends Neurosci. 2002;25:154–9. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 20.Katon W, Von Korff M, Lin E, et al. Improving primary care treatment of depression among patients with diabetes mellitus: the design of the Pathways Study. Gen Hosp Psychiatry. 2003;25:158–68. doi: 10.1016/s0163-8343(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 21.McCulloch D, Price M, Hindmarsh M, Wagner E. A population-based approach to diabetes management in a primary care setting: early results and lessons learned. Eff Clin Pract. 1998;1:12–22. [PubMed] [Google Scholar]
- 22.Spitzer R, Kroenke K, Williams J. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders: Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens R, Kothari V, Adler A, Stratton I, Holman R. The UKPDS Risk Engine: a model for the risk of coronary artery disease in type 2 diabetes (UKPDS 56) Clin Sci. 2001;101:671–9. [PubMed] [Google Scholar]
- 25.Folsom A, Chambless L, Duncan B, Gilbert A, Pankow J. Prediction of coronary heart disease in middle-aged adults with diabetes. Diabetes. 2003;26:2777–84. doi: 10.2337/diacare.26.10.2777. [DOI] [PubMed] [Google Scholar]
- 26.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–50. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2003;26(suppl 1):S83–S86. doi: 10.2337/diacare.26.2007.s83. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2002;25(suppl 1):S33–S42. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- 29.Fishman P, Goodman M, Hornbrook M, Meenan RT, Bachman DJ, O'Keefe Rosetti MC. Risk adjustment using automated pharmacy data: the Rx Risk model. Med Care. 2003;41:84–99. doi: 10.1097/00005650-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Starfield B, Weiner J, Mumford L, Steinwachs D. Ambulatory care groups: a categorization of diagnoses for research and management. Health Serv Res. 1991;26:53–74. [PMC free article] [PubMed] [Google Scholar]
- 31.Selby J, Karter A, Ackerson L, Ferrara A, Lia J. Developing a prediction rule from automated clinical databases to identify high-risk patients in a large population with diabetes. Diabetes Care. 2001;24:1547–55. doi: 10.2337/diacare.24.9.1547. [DOI] [PubMed] [Google Scholar]
- 32.SAS/Statistics Software. Changes and EnhancementsSAS Technical Release. Cary, NC: SAS Institute, Inc.; 1994. [Google Scholar]
- 33.Wassertheil-Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in post-menopausal women: The Women's Health Initiative. Arch Intern Med. 2004;164:289–98. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- 34.Pitsavos C, Chrysohoou C, Panagiotakos D, et al. Association of leisure-time physical activity on inflammatory markers (C-reactive protein, white blood cell count, amyloid A, fibrinogen) in health subjects (from the Attica study) Am J Cardiol. 2003;91:368–70. doi: 10.1016/s0002-9149(02)03175-2. [DOI] [PubMed] [Google Scholar]
- 35.Kessler R, McGonagle K, Zhao S, et al. Lifetime and 12-month prevalence of DSM III-R psychiatric disorders in the United States. Results from the National Comorbidity Study. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 36.Anderson R, Freedland K, Clouse R, Lustman P. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 37.Gu K, Cowil C, Harris M. Diabetes and decline in heart disease mortality in U.S. adults. JAMA. 1999;281:1291–7. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- 38.Raza A, Movahed A. Current concepts of cardiovascular diseases in diabetes mellitus. Int J Cardiol. 2003;89:123–34. doi: 10.1016/s0167-5273(02)00510-7. [DOI] [PubMed] [Google Scholar]
- 39.Blendea M, McFarlane I, Isernovic E, Gick G, Sowers J. Heart disease in diabetic patients. Curr Diab Rep. 2003;3:233–9. doi: 10.1007/s11892-003-0068-z. [DOI] [PubMed] [Google Scholar]
- 40.Patton G, Carlin J, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: a prospective study over 3 years. Am J Public Health. 1998;88:1518–22. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anda R, Williamson D, Escobedo L, Most E, Gioviro G, Remington P. Depression and dynamics of smoking. JAMA. 1990;264:1541–3. [PubMed] [Google Scholar]
- 42.Blumenthal J, Williams R, Wallace A, Williams R, Needles T. Physiological and psychological variables predict compliance to prescribed exercise in patients recovering from myocardial infarction. Psychosom Med. 1982;44:519–27. doi: 10.1097/00006842-198212000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Marcus M, Wing R, Guare J, Blaire E, Jawad A. Lifetime prevalence of major depression and its effect on treatment outcome in obese type 2 diabetic patients. Diabetes Care. 1992;15:253–5. doi: 10.2337/diacare.15.2.253. [DOI] [PubMed] [Google Scholar]
- 44.Ciechanowski P, Katon W, Russo J. Depression and diabetes: impact of depression symptoms on adherence, function and costs. Arch Intern Med. 2000;160:3278–85. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 45.Carney R, Freedland K, Eisen S, Rich M, Skala J, Jaffe A. Adherence to a prophylactic medication regimen in patients with symptomatic versus asymptomatic ischemic heart disease. Behav Med. 1998;24:35–9. doi: 10.1080/08964289809596379. [DOI] [PubMed] [Google Scholar]
- 46.Sobol AB, Watala C. The role of platelets in diabetes-related vascular complications. Diabetes Res Clin Pract. 2000;50:1–16. doi: 10.1016/s0168-8227(00)00160-1. [DOI] [PubMed] [Google Scholar]
- 47.Collier A, Tymkewycz P, Armstrong R, Young RJ, Jones RL, Clarke BF. Increased platelet thromboxane receptor sensitivity in diabetic patients with proliferative retinopathy. Diabetologia. 1986;29:471–4. doi: 10.1007/BF00453495. [DOI] [PubMed] [Google Scholar]
- 48.Frohlich M, Imhof A, Berg G, et al. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–9. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 49.Broadley A, Korzun A, Jones C, Freeneaux M. Arterial dysfunction is impaired in treated depression. Heart. 2002;88:521–3. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen RA. Dysfunction of the vascular endothelium in diabetes mellitus. Circulation. 1993;87(suppl):V67–V76. [Google Scholar]