Abstract
OBJECTIVE
To assess whether newer antipsychotic medications are associated with weight gain and development of diabetes.
DESIGN
Retrospective cohort study.
SETTING
Data from a comprehensive electronic medical record serving an urban public hospital and a citywide network of mental health clinics.
PATIENTS/PARTICIPANTS
Three thousand one hundred fifteen patients at least 18 years old who were prescribed a single antipsychotic drug for at least 1 year.
METHODS
We identified independent predictors of significant weight gain (≥7%) and new onset of diabetes mellitus in the first year of antipsychotic drug treatment, using logistic regression adjusted for demographic characteristics, obesity, preexisting psychiatric diagnoses, alcohol and drug abuse, number of primary care, psychiatric clinic, and emergency department visits, and pretreatment weight.
MEASUREMENTS AND MAIN RESULTS
Twenty-five percent of patients taking older phenothiazines developed significant weight gain in the first year of treatment compared to 40% of the patients taking olanzapine (adjusted odds ratio [OR], 2.8; 95% confidence interval [CI], 1.7 to 4.6; P < .0001) and 37% of patients taking risperidone (adjusted OR, 2.3; 95% CI, 1.5 to 3.4; P < .0001). New diabetes developed in 3% of patients taking older phenothiazines was new onset diabetes compared to 8.0% of patients taking olanzapine (adjusted OR, 1.9; 95% CI, 1.1 to 3.3; P= .03) and 3.5% of patients taking risperidone (adjusted OR, 0.7; 95% CI, 0.4 to 1.4; P= .3). No association was found between significant weight gain and developing diabetes (adjusted OR, 0.7; 95% CI, 0.4 to 1.4; P= .4).
CONCLUSIONS
Olanzapine and risperidone use was associated with gaining weight in the first year, but only olanzapine was associated with developing diabetes mellitus.
Keywords: olanzapine, risperidone, diabetes, weight gain, schizophrenia
In the last half-century, several new medications have been developed to help treat schizophrenia. Initially, phenothiazines were the only medication available to control psychotic Patients' agitation and aggression. However, these medications were found to have many severe and potentially irreversible side effects including acute dystonia, akathisia, Parkinsonism, tardive dyskinesia, and weight gain.1,2 In 1990, the first atypical antipsychotic, clozapine, was introduced with marked reduction in the risk of extrapyramidal symptoms and tardive dyskinesia. In the past 10 years, several other atypical antipsychotic medications have been approved for clinical use. While there is some disagreement over whether the atypical antipsychotic drugs are more effective at alleviating the symptoms of schizophrenia, the atypical drugs have a clear advantage in lowering adverse drug effects.3,4
However, the new atypical antipsychotic medications, including olanzapine and risperidone, have been associated with significant weight gain5–7 and new onset diabetes mellitus.8–10 Yet, recent epidemiological evidence casts doubt on the association with diabetes and suggests that there may be differences in the risk associated with use of typical versus atypical antipsychotic drugs.11 Moreover, prior studies have not adjusted for comorbid conditions that could affect the rate at which patients with schizophrenia gain weight or develop clinical diabetes mellitus.9 Therefore, we performed a retrospective case-control study using data from a large electronic medical record system serving an inner-city public hospital to determine whether risperidone and olanzapine were independently associated with weight gain or the development of diabetes mellitus among newly treated psychotic patients.
METHODS
Study Site and Subjects
After approval by the Indiana University (IU) Institutional Review Board, data for this study were obtained from the Regenstrief Medical Record System (RMRS),12 a comprehensive electronic medical record system that serves as the main clinical data repository for Wishard Memorial Hospital (Indianapolis's urban public teaching hospital), Wishard's primary and specialty care outpatient practices, and Midtown Mental Health (a comprehensive network of 26 tax-supported mental health centers in Indianapolis). The RMRS contains all inpatient, outpatient, and emergency department data including diagnoses, diagnostic test results, drug therapy, procedures, vital signs, and charges from Wishard Hospital and Midtown Mental Health. These two systems are the main and often the only sources of allopathic and mental health care for indigent persons in Indianapolis. Wishard's primary care practices (general internal medicine, pediatrics, and obstetrics and gynecology) are located on the campus of IU Medical Center and in five community health centers spread across Indianapolis.
We selected patients who had ever been prescribed olanzapine, risperidone, or one of the following typical antipsychotic drugs: chlorpromazine, flufenazine, mesoridazine, piperacetazine, thioridazine, triavil, or trifluoperazine. If a patient was prescribed more than one of these drugs, the first recorded prescription was used in the analysis. The date of this first recorded prescription of a target antipsychotic drug was the inception date for the patient in the study cohort. We excluded patients less than 18 years of age at the time of their first antipsychotic drug prescription and those who were treated with their initial antipsychotic drug for less than 1 year. Further, we excluded patients prescribed haloperidol (which is most often used in this practice for reasons other than schizophrenia, mainly among older adults) or selected phenothiazines (e.g., promethazine) that are mainly prescribed as anti-emetics. For the analysis of weight gain, we only included patients with weights recorded both before and during their first year of antipsychotic drug treatment. Weight is routinely recorded at all primary care, specialty care, and mental health visits. For the analysis of new onset diabetes, we excluded patients with no prior weight measurement and those who, prior to first antipsychotic drug prescription, had any of the following evidence of diabetes: any diagnosis of type 1 or type 2 diabetes, treatment with any antidiabetic medication, more than one recorded blood glucose value greater than 200 mg/dl, or a single glycated hemoglobin greater than 9%.
Data Extraction
From each patient's RMRS medical record, we extracted the following information from all data available on or before the inception date (i.e., first recorded antipsychotic drug treatment): demographic data (age, gender, and race), the last recorded weight, the diagnosis of obesity, use of benztropine, evidence of diabetes mellitus (as described above), selected measures of prior outpatient health care utilization, and previously recorded mental health diagnoses (i.e., schizophrenia, depression, anxiety, bipolar disorder, personality disorder, dementia, alcohol dependence, or elicit drug abuse). Then, for the year following each patient's inception date, we extracted any evidence of new onset diabetes mellitus and the highest recorded weight. Weight gain of 7% or more was considered clinically significant and is consistent with the Food and Drug Administration's definition of significant weight gain for studies of psychotropic drugs.13 Each dichotomous variable was coded 1 if evidence was found in the patient's electronic medical record prior to first antipsychotic drug treatment, or coded 0 if no such evidence was found.
Statistical Analysis
To assess the effects of olanzapine and risperidone treatment on the incidence of new onset diabetes and weight gain of 7% or more, we used univariable and multivariable conditional logistic regression.14 The multivariable analyses adjusted for demographic data, comorbid psychiatric diagnoses, the preexisting diagnosis of obesity, benztropine use, and prior outpatient health care utilization (as described above). These adjusting variables were selected to account for intensity of observation and additional potential risk factors for weight gain and the development of diabetes. To maximize the power of the analysis, we performed multivariate statistical analyses separately for olanzapine versus typical phenothiazines, and risperidone versus typical phenothiazines. A two-sided P value ≤ .05 was considered significant in all analyses.
The study was performed under a contract with Bristol-Myers Squibb that established the outcomes to be assessed (weight gain and the development of diabetes). However, the sponsor had no role in how these dependent variables were defined, the selection of other independent variables or how they were defined, or in the analyses.
RESULTS
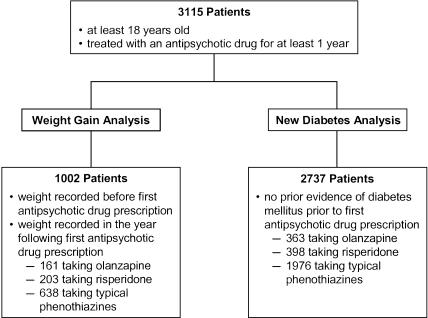
Between 1972 and 2000, we identified 3,115 adults at least 18 years of age who were treated for at least 1 year with one of the target antipsychotic drugs: 438 (14%) were first prescribed olanzapine, 482 (15%) risperidone, and 2,195 (70%) a typical phenothiazine (Fig. 1). Risperidone was first prescribed in 1994, while olanzapine was first prescribed in 1996. Table 1 contains descriptive statistics for all 3,115 study patients. Of those taking a typical phenothiazine, 870 (40%) were prescribed thioridazine, 503 (23%) chlorpromazine, 409 (19%) fluphenazine, 267 (12%) trifluperazine, 140 (6%) perphenazine, 5 (0.2%) mesoridazine, and 1 (0.04%) piperacetazine. Approximately half of the study patients were men and half African American. In the first year of antipsychotic drug therapy, 25% of patients initially prescribed a typical phenothiazine had significant weight gain compared with 40% for patients taking olanzapine and 37% of those on risperidone. Also, 3.2% of patients on a typical phenothiazine developed evidence of new onset diabetes compared with 8.0% of those taking olanzapine and 3.5% of patients taking risperidone.
FIGURE 1.
Numbers of eligible subjects, antipsychotic drug treatment, cases of significant weight gain (≥7% body weight), and new onset diabetes during the first year of antipsychotic drug therapy.
Table 1.
Characteristics of Adult Patients at First Treatment with an Antipsychotic Drug
| First Antipsychotic Drug Prescribed | |||
|---|---|---|---|
| Characteristic | Olanzapine (n = 438) | Risperidone (n = 482) | Typical Phenothiazine (n = 2,195) |
| Female gender, % | 49.3 | 51.9 | 57.8 |
| Black race, % | 45.1 | 47.9 | 47.9 |
| Mean age, y (SD) | 42.9 (12.0) | 42.4 (14.5) | 42.6 (14.9) |
| Mean number of years on drug (SD) | 2.3 (1.2) | 2.3 (1.5) | 6.0 (4.8) |
| Schizophrenia, %* | 35.2 | 22.6 | 0.2 |
| Depression, % | 17.1 | 18.5 | 5.0 |
| Anxiety, % | 10.5 | 11.0 | 5.6 |
| Personality disorder, % | 14.4 | 11.4 | 3.1 |
| Dementia, % | 6.2 | 9.3 | 1.6 |
| Alcohol dependence, % | 29.0 | 28.0 | 6.8 |
| Drug abuse, % | 15.3 | 12.7 | 1.8 |
| Obesity, % | 10.7 | 13.1 | 4.7 |
| Benztropine, % | 46.6 | 41.3 | 20.4 |
| Primary care visits (mean, SD) | 5.6, 11.7 | 5.3, 10.4 | 0.4, 2.1 |
| Mental health visits (mean, SD) | 66.2, 132.4 | 36.6, 100.9 | 0.5, 6.1 |
| Emergency room visits (mean, SD) | 9.8, 14.9 | 9.8, 16.2 | 2.0, 5.0 |
| Mean weight, lb (SD) | 185.4 (46.6) | 187.6 (50.6) | 168.6 (45.2) |
| ≥7% weight gain during the first year of antipsychotic drug treatment, % | 39.8 | 37.0 | 24.9 |
| New onset diabetes in the first year of antipsychotic drug treatment, % | 8.0 | 3.5 | 3.2 |
All diagnoses were those available in Patients' electronic records prior to the visit in which the first antipsychotic drug was prescribed.
SD, standard deviation.
We identified predictors of weight gain among the 1,002 patients who had a weight recorded both before their first antipsychotic drug treatment and during the following year. Of these 1,002 patients, 161 were treated with olanzapine, 40% of whom had significant weight gain; 203 were treated with risperidone (37% with significant weight gain); and 638 were treated with a typical phenothiazine (25% with significant weight gain) (see Fig. 1Table 1). As shown in Table 2, adjusting for baseline weight, psychiatric comorbid conditions, and number of primary care, psychiatric clinic, and emergency department visits, olanzapine was significantly associated with significant weight gain compared with those on typical phenothiazines, the reference group (adjusted odds ratio [OR], 2.8; 95% confidence interval [CI], 1.7 to 4.6; P < .0001). Likewise, risperidone was associated with significant weight gain (adjusted OR, 2.3; 95% CI, 1.5 to 3.4; P < .0001).
Table 2.
Independent Multivariable Predictors of ≥ 7% Weight Gain in the First Year of Antipsychotic Drug Therapy
| Independent Variables | OR | 95% CI | χ2 | P Value |
|---|---|---|---|---|
| Olanzapine use | 2.8 | 1.7 to 4.6 | 17.8 | <.0001 |
| Risperidone use | 2.3 | 1.5 to 3.4 | 15.4 | <.0001 |
| Schizophrenia | 0.7 | 0.4 to 1.2 | 1.8 | .2 |
| Depression | 1.2 | 0.8 to 1.7 | 0.7 | .4 |
| Anxiety | 1.2 | 0.8 to 1.9 | 1.1 | .3 |
| Personality disorder | 0.8 | 0.5 to 1.5 | 0.5 | .5 |
| Dementia | 0.8 | 0.4 to 1.4 | 0.8 | .4 |
| Alcohol abuse | 1.1 | 0.7 to 1.6 | 0.1 | .8 |
| Drug abuse | 1.1 | 0.6 to 2.0 | 0.02 | .9 |
| Obesity | 1.3 | 0.8 to 2.0 | 1.2 | .3 |
| Cognitive dysfunction | 1.1 | 0.8 to 1.6 | 0.2 | .6 |
| Primary care visits | 0.98 | 0.97 to 1.0 | 0.28 | .1 |
| Mental health visits* | 1.0 | 1.0 to 1.01 | 0.6 | .4 |
| Emergency visits | 1.0 | 1.00 to 1.02 | 2.5 | .1 |
| Baseline weight, lb | 0.99 | 0.96 to 0.99 | 38.2 | <.0001 |
Odds ratio for 10 visits.
OR, adjusted odds ratio; CI, confidence interval.
We identified predictors of new onset diabetes mellitus among the 2,737 patients who had a previously recorded weight and no prior evidence of diabetes at the time of first antipsychotic drug treatment. Of these, 363 were taking olanzapine, of whom 8.0% developed diabetes mellitus in their first year on antipsychotic drug therapy; 398 were treated with risperidone (3.5% developed diabetes); and 1,976 were treated with a typical phenothiazine (3.2% developed diabetes). As shown in Table 3, olanzapine use was a significant predictor of developing new onset diabetes (adjusted OR, 1.9; 95% CI, 1.1 to 3.3; P = .03). But risperidone use was not associated with an increased risk of developing diabetes (adjusted OR, 0.7; 95% CI, 0.4 to 1.4; P = .3).
Table 3.
Independent Multivariable Predictors of New Onset Diabetes in the First Year of Antipsychotic Drug Therapy
| Independent Variables | OR | 95% CI | χ2 | P Value |
|---|---|---|---|---|
| Olanzapine use | 1.9 | 1.1 to 3.3 | 5.0 | .03 |
| Risperidone use | 0.7 | 0.4 to 1.4 | 1.1 | .3 |
| Schizophrenia | 1.1 | 0.5 to 2.3 | 0.02 | .9 |
| Depression | 1.3 | 0.7 to 2.5 | 0.7 | .4 |
| Anxiety | 1.1 | 0.5 to 2.2 | 0.06 | .8 |
| Personality disorder | 1.1 | 0.5 to 2.3 | 0.04 | .8 |
| Dementia | 1.8 | 0.8 to 4.4 | 1.8 | .2 |
| Alcohol abuse | 1.7 | 0.9 to 2.9 | 3.1 | .08 |
| Drug abuse | 2.0 | 1.0 to 4.3 | 3.3 | .07 |
| Obesity | 2.6 | 1.4 to 4.8 | 8.7 | .003 |
| Cognitive dysfunction | 0.7 | 0.4 to 1.1 | 2.0 | .2 |
| Primary care visits | 1.02 | 0.99 to 1.05 | 2.0 | .2 |
| Mental health visits* | 1.0 | 0.96 to 1.02 | 0.7 | .4 |
| Emergency visits | 1.0 | 0.98 to 1.0 | 0.0 | .9 |
Odds ratio for 10 visits.
OR, adjusted odds ratio; CI, confidence interval.
To assess whether the increased risk of new onset diabetes associated with olanzapine use was associated with weight gain, we repeated the multivariable logistic regression analyses of new onset diabetes in the subset of 744 (112 taking olanzapine, 150 taking risperidone, and 482 taking typical phenothiazines) who had no prior evidence of diabetes and had weights recorded both before first treatment with antipsychotic drugs and during the first year of treatment. We then included in the multivariable analysis an indicator of significant weight gain (≥7% body weight) during the first year of antipsychotic drug therapy. Significant weight gain was not significantly associated with the development of evidence of new onset diabetes in this subgroup (OR, 0.7; 95% CI, 0.4 to 1.4; P = .4), while olanzapine remained a significant independent risk factor for the development of diabetes (adjusted OR, 2.2; 95% CI, 1.0 to 5.0; P = .05).
The use of atypical antipsychotic drugs was confounded by time: once atypical antipsychotic drugs became available in this practice in 1994, few patients were prescribed typical antipsychotic drugs as initial therapy.15 However, we identified 104 patients begun on typical phenothiazines after 1994. We repeated our multivariable analyses including only these 104 patients and those receiving olanzapine or risperidone. The resulting odds ratios for olanzapine and risperidone were slightly lower for weight gain (1.8 and 1.6, respectively) but similar for developing new onset diabetes (1.7 and 0.7) to those in the primary analysis. However, all confidence intervals included one because of the smaller number of patients taking typical phenothiazines.
DISCUSSION
In accord with previous research,16 we found that patients taking either olanzapine or risperidone had significant weight gain as compared to patients taking typical phenothiazines. Several explanations have been proposed for gaining weight while taking atypical antipsychotic drugs.17–19 Atypical antipsychotic medications may have an affinity for dopamine, serotonin, and histamine receptors that could lead to increased eating and weight gain.6 Unfortunately, efforts to ameliorate this effect using behavior treatments or additional medications to modify serotonin and histamine receptor actions have had limited success.20,21 Genetic studies performed to date have failed to identify which patients taking atypical antipsychotics are at increased risk for weight gain.22,23
Prior case reports and studies of the association of atypical antipsychotics with weight gain and diabetes have been criticized for not controlling for the weight gain and increased incidence of diabetes that may be associated with schizophrenia itself or the use of typical antipsychotic drugs.9,24–26 We therefore compared patients taking olanzapine or risperidone with patients taking typical phenothiazines and confirmed the increased relative risk of developing diabetes mellitus among patients taking olanzapine.27 However, we did not find that patients taking risperidone have an increased risk of developing diabetes in relation to patients taking typical phenothiazines.10,26 In fact, the multivariable odds ratio was in the other direction, though not significantly different from 1.0.
We also found no evidence that the development of diabetes among patients taking olanzapine was associated with weight gain. These results support previous reports that also failed to find a significant link between weight gain, treatment with an atypical antipsychotic drug, and development of frank diabetes mellitus.9,24–26 Our data therefore support the hypothesis that weight gain is not the cause of diabetes mellitus among patients taking atypical antipsychotics but suggest that the development of diabetes is due to an independent effect of atypical antipsychotic drugs on metabolism.8,9,26,28
There were dramatically fewer preexisting psychiatric diagnoses in the electronic medical records of patients taking typical antipsychotic drugs. This could have been due to a number of factors: there may actually be an increasing incidence of psychiatric disease in the inner city or, more likely, an enhanced recognition of comorbid psychiatric conditions by treating physicians. There could also have been changes in billing systems that may have stimulated increased recording of comorbid diagnoses. However, we included all of these diagnoses in the multivariable analysis, and none was a significant predictor of weight gain or diabetes in the first year of antipsychotic drug therapy.
We also found that a lower weight at the time of antipsychotic drug therapy was a significant multivariable predictor of weight gain (Table 2). This may represent regression to the mean: some of these psychiatric patients may have had eating disorders—both over- and undereating—and in both conditions baseline weight would be inversely associated with change in weight in response to antipsychotic drug therapy.
This study has several limitations. It was performed using data from inner-city patients receiving care at publicly financed mental health clinics with a high prevalence of African Americans who are at greater risk of both weight gain and diabetes.29–31 This may limit the generalizability of our results to other populations. Because only the outpatient medicine and mental health clinics routinely measured patient weight, the selective availability of weights may have introduced biases for which our case-control design may not have adequately adjusted. The analyses are also confounded by time: once atypical antipsychotic medications became available, few typical phenothiazines were prescribed as first-choice antipsychotic drugs.15 Thus, we could adequately adjust the multivariable analyses for the year of first antipsychotic drug treatment. Temporal changes in the environment or health care delivery may have affected the risk of gaining weight or developing diabetes.32,33 Reliance on medical records rather than uniform independent, prospective assessments of diabetes may have introduced measurement bias, especially if providers monitored for weight gain or diabetes more intensively among patients treated with atypical antipsychotic drugs.34 However, the lack of an affect of risperidone on the risk of diabetes makes this bias less likely. Finally, Patients' heights were not routinely available, which prevented us from calculating body mass index and determining the extent to which the observed weight gain is related to clinical obesity.
Our results contribute to the growing evidence that atypical antipsychotic medications, especially olanzapine, have clinically significant metabolic side effects including weight gain and the development of diabetes mellitus. However, the development of diabetes mellitus among patients taking olanzapine did not appear to be associated with weight gain. Additional studies are needed to further delineate the metabolic causes of increased risk of diabetes among patients treated with olanzapine or other atypical antipsychotic drugs. A greater understanding of these metabolic disturbances is likely to improve the clinical management of patients prescribed antipsychotic medications.
Acknowledgments
This research was supported by a contract to Dr. Tierney from the Bristol-Myers Squibb Research Institute.
This study was performed under a contract with Bristol-Myers Squibb that contained no restraints on publication of the study's results. The authors thank Mark Olfson, MD, MPH for his editorial assistance with the article. The information and opinions in this report are solely those of the authors and do not necessarily reflect the opinions and policies of the authors’ institutions.
REFERENCES
- 1.Klett CJ, Caffey EM., Jr Weight changes during treatment with phenothiazine derivatives. J Neuropsychiatry. 1960;2:102–8. [PubMed] [Google Scholar]
- 2.Holden JM, Holden UP. Weight changes with schizophrenic psychosis and psychotropic drug therapy. Psychosomatics. 1970;11:551–61. doi: 10.1016/S0033-3182(70)71576-4. [DOI] [PubMed] [Google Scholar]
- 3.Kane JM. Pharmacologic treatment of schizophrenia. Biol Psychiatry. 1999;46:1396–408. doi: 10.1016/s0006-3223(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 4.Rosenheck R, Perlick D, Bingham S, et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia—a randomized controlled trial. JAMA. 2003;290:2693–702. doi: 10.1001/jama.290.20.2693. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DM, McAskill R. Atypical antipsychotics and weight gain—a systematic review. Acta Psychiatr Scand. 2000;101:416–32. doi: 10.1034/j.1600-0447.2000.101006416.x. [DOI] [PubMed] [Google Scholar]
- 6.Wetterling T. Bodyweight gain with atypical antipsychotics: a comparative review. Drug Saf. 2001;24:59–73. doi: 10.2165/00002018-200124010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Sussman N. Review of atypical antipsychotics and weight gain. J Clin Psychiatry. 2001;62(S23):5–12. [PubMed] [Google Scholar]
- 8.Lindenmayer JP, Nathan AM, Smith RC. Hyperglycemia associated with the use of atypical antipsychotics. J Clin Psychiatry. 2001;62(S23):30–8. [PubMed] [Google Scholar]
- 9.Meyer JM. A retrospective comparison of weight, lipid, and glucose changes between risperidone- and olanzapine-treated inpatients: metabolic outcomes after 1 year. J Clin Psychiatry. 2002;63:425–33. doi: 10.4088/jcp.v63n0509. [DOI] [PubMed] [Google Scholar]
- 10.Sernyak M, Leslie D, Alarcon R, Losonczy M, Rosenheck R. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry. 2002;159:561–6. doi: 10.1176/appi.ajp.159.4.561. [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Cavazzoni P, Hornbuckle K, Hutchins D, Breier A, Jovanovic L. A retrospective cohort study of diabetes mellitus and antipsychotic treatment in the United States. J Clin Epidemiol. 2003;56:164–70. doi: 10.1016/s0895-4356(02)00588-7. [DOI] [PubMed] [Google Scholar]
- 12.McDonald CJ, Overhage JM, Tierney WM, et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54:225–53. doi: 10.1016/s1386-5056(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 13.Sachs G, Guille C. Weight gain associated with use of psychotropic medications. J Clin Psychiatry. 1999;60(S2):16–19. [PubMed] [Google Scholar]
- 14.Stokes ES, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System. Cary, NC: SAS Institute, Inc; 1995. [Google Scholar]
- 15.Williams CL, Johnstone BM, Kesterson JG, Javor KA, Schmetzer AD. Evaluation of antipsychotic and concomitant medication use patterns in patients with schizophrenia. Med Care. 1999;37(4 suppl Lilly):AS81–AS86. doi: 10.1097/00005650-199904001-00011. [DOI] [PubMed] [Google Scholar]
- 16.Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry. 2001;62(S7):22–31. [PubMed] [Google Scholar]
- 17.Kraus T, Haack M, Schuld A, et al. Body weight and leptin plasma levels during treatment with antipsychotic drugs. Am J Psychiatry. 1999;156:312–4. doi: 10.1176/ajp.156.2.312. [DOI] [PubMed] [Google Scholar]
- 18.Casey DE, Zorn SH. The pharmacology of weight gain with antipsychotics. J Clin Psychiatry. 2001;62(S7):4–10. [PubMed] [Google Scholar]
- 19.Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58(S10):45–9. [PubMed] [Google Scholar]
- 20.Poyurovsky M, Pashinian A, Gil-Ad I, et al. Olanzapine-induced weight gain in patients with first-episode schizophrenia: a double-blind, placebo-controlled study of fluoxetine addition. Am J Psychiatry. 2002;159:1058–60. doi: 10.1176/appi.ajp.159.6.1058. [DOI] [PubMed] [Google Scholar]
- 21.Sacchetti E, Guarneri L, Bravi D. H2 antagonist nizatidine may control olanzapine-associated weight gain in schizophrenic patients. Biol Psychiatry. 2000;48:167–8. doi: 10.1016/s0006-3223(00)00872-6. [DOI] [PubMed] [Google Scholar]
- 22.Rietschel M, Naber D, Fimmers R, Moller HJ, Propping P, Nothen MM. Efficacy and side-effects of clozapine not associated with variation in the 5-HT2C receptor. Neuroreport. 1997;8:1999–2003. doi: 10.1097/00001756-199705260-00040. [DOI] [PubMed] [Google Scholar]
- 23.Basile VS, Masellis M, McIntyre RS, Meltzer HY, Lieberman JA, Kennedy JL. Genetic dissection of atypical antipsychotic-induced weight gain: novel preliminary data on the pharmacogenetic puzzle. J Clin Psychiatry. 2001;62(S23):45–66. [PubMed] [Google Scholar]
- 24.Goldstein LE, Sporn J, Brown S, et al. New-onset diabetes mellitus and diabetic ketoacidosis associated with olanzapine treatment. Psychosomatics. 1999;40:438–43. doi: 10.1016/S0033-3182(99)71210-7. [DOI] [PubMed] [Google Scholar]
- 25.Rigalleau V, Gatta B, Bonnaud S, et al. Diabetes as a result of atypical anti-psychotic drugs—a report of three cases. Diabet Med. 2000;17:484–6. doi: 10.1046/j.1464-5491.2000.00296.x. [DOI] [PubMed] [Google Scholar]
- 26.Wirshing DA, Pierre JM, Eyeler J, Weinbach J, Wirshing WC. Risperidone-associated new-onset diabetes. Biol Psychiatry. 2001;50:148–9. doi: 10.1016/s0006-3223(01)01087-3. [DOI] [PubMed] [Google Scholar]
- 27.Koro CE, Fedder DO, L’Italien GJ, et al. Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ. 2002;325:243–7. doi: 10.1136/bmj.325.7358.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newcomer JW, Haupt DW, Fucetola R, et al. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry. 2002;59:337–45. doi: 10.1001/archpsyc.59.4.337. [DOI] [PubMed] [Google Scholar]
- 29.Burke GL, Bild DE, Hilner JE, Folsom AR, Wagenknecht LE, Sidney S. Differences in weight gain in relation to race, gender, age and education in young adults: the CARDIA Study. Coronary Artery Risk Development in Young Adults. Ethn Health. 1996;1:327–35. doi: 10.1080/13557858.1996.9961802. [DOI] [PubMed] [Google Scholar]
- 30.Lewis CE, Smith DE, Wallace DD, Williams OD, Bild DE, Jacobs DR. Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA Study. Am J Public Health. 1997;87:635–42. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyle JP, Honeycutt AA, Narayan KMV, et al. Projection of diabetes burden through 2050. Diabetes Care. 2001;24:1936–40. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 32.Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987–1996. Schizophr Res. 2002;55:277–84. doi: 10.1016/s0920-9964(01)00256-0. [DOI] [PubMed] [Google Scholar]
- 33.Freedman DS, Khan LK, Serdula MK, et al. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA. 2002;288:1758–61. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- 34.Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical Epidemiology: A Basic Science for Clinical Medicine. Boston, Mass: Little, Brown, and Company; 1991. p. 182. [Google Scholar]