Clinical Vignettes
A CASE OF ACYCLOVIR-INDUCED RESPIRATORY DEPRESSION IN PATIENT WITH END-STAGE RENAL DISEASE
W. Hester1; V.T. Martin1; S. Bansil1; C.J. Fichtenbaum1. 1University of Cincinnati, Cincinnati, OH. (Tracking ID #115632)
LEARNING OBJECTIVES
1. Recognize the clinical setting of acyclovir-induced neurotoxicity. 2. Diagnose and manage acyclovir-induced neuroxicity.
CASE
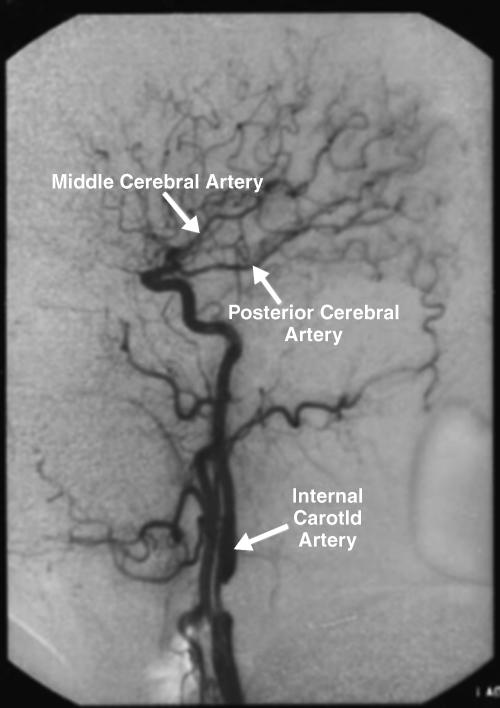
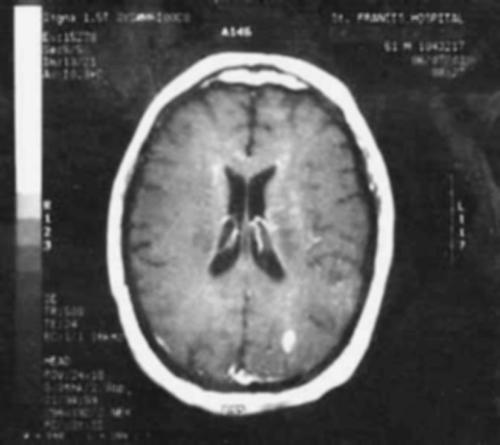
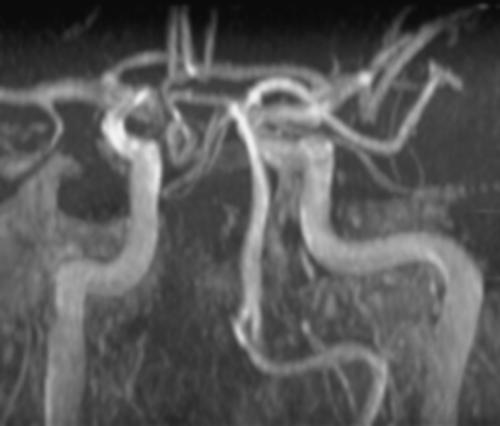
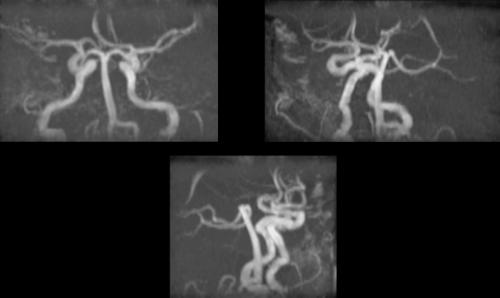
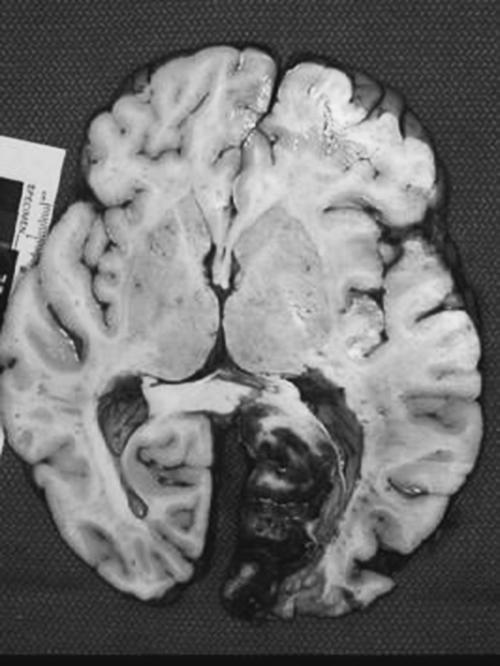
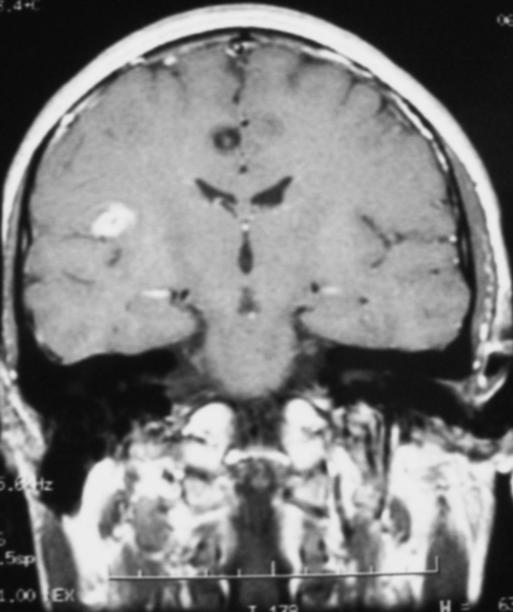
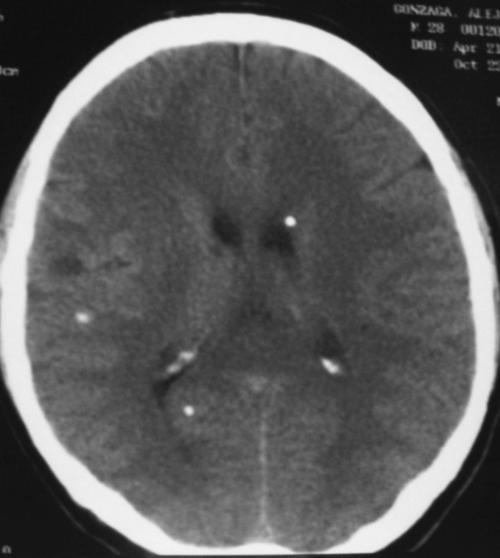
A 46 year-old woman with AIDS (recent CD 4 + lymphocyte count 145 per mL & plasma HIV RNA level <400 copies per mL) and ESRD on continuous ambulatory peritoneal dialysis (CAPD) presented with pain in her right thigh for three months. Doppler ultrasounds were negative for deep venous thrombosis on two different tests. She had been in stable health without recent opportunistic infections. Her past medical history was significant for a history of shingles, orolabial herpes simplex disease, pancreatitis secondary to nucleosides, Candida esophagitis, and asthma. Her physical exam revealed an edematous right thigh and a tender 3 × 5 cm irregular ecchymotic area present on her lateral thigh near a small shallow ulcer. A small vesicle has preceded the ulcer. Laboratory data included a white blood cell count of 8.1K with 74% neutrophils; blood urea nitrogen of 74 mg/dL; creatinine of 15.7 mg/dL; and creatinine kinase 1,303 U/L. The Alveolar-arterial gradient was 71053.25 mmHg. Computerized Tomography of the right lower extremity with contrast and multiplanar 3D reconstructions revealed no abscess. Empiric therapy with intravenous acyclovir 5 mg/kg/day was empirically started on hospital day 2. The next day the patient developed delirium and hypoxemia. The arterial blood gas revealed a pH 7.21, PaCO2 67 mmHg, PaO2 163, HCO3 27 mmol/L, O2 saturation 97.8% on 100% oxygen via a non-rebreathable mask. Chest radiography demonstrated pulmonary edema. Cultures of the peritoneal fluid, bronchoalveolar lavage, blood, and spinal fluid, were all negative. The skin biopsy demonstrated findings consistent with calciphylaxis and pressure necrosis and the absence of viral inclusions. Acyclovir was discontinued on the sixth hospital day and a serum acyclovir level 12 hours after stopping the acyclovir was 5.5 mcg/mL (reported therapeutic peak range of 0.40–2.0 mcg/mL). Twenty-four hours after stopping the acyclovir the patient became alert and was extubated within 48 hours. Given all of these findings, the patient was diagnosed with acyclovir-induced respiratory depression.
DISCUSSION
Neurotoxicities such as lethargy, confusion, and delirium have been reported with acyclovir and seem to be more prevalent in the setting of kidney dysfunction, but have been identified in otherwise healthy individuals. To our knowledge, this is the first case report of acyclovir leading to respiratory failure in a patient with chronic renal disease. As demonstrated in this case, acyclovir should be used cautiously in those with renal failure to prevent neurotoxicities.
A CASE OF AMIODARONE-INDUCED THYROTOXICOSIS
J.E. Adams1. 1University of California, San Francisco, San Francisco, CA. (Tracking ID #115759)
LEARNING OBJECTIVES
1. Review Amiodarone's effects on thyroid function. 2. Diagnose and treat thyrotoxic effects of Amiodarone.
CASE
62 y/o male presented to his primary medical doctor complaining of a several month history of weakness, fatigue, hand tremor, and a ten pound weight loss. The patient was started on Amiodarone 2 years ago for paroxysmal atrial fibrillation and had remained in sinus rhythm without further complications. Upon initial work-up patient was found to have an undetectable TSH, and an elevated free T4.
DISCUSSION
Up to 20% of patients on long-term therapy will develop hypothyroidism as a result of toxic effects of Amiodarone, and 3% will develop hyperthyroidism. Hypothyroidism occurs by several mechanisms, the most common being a destructive thyroiditis which is often preceded by a hyperthyroid phase. Additionally, Amiodarone decreases the peripheral conversion of T4 to T3 and acts to directly block the T3 receptor. Lastly, synthesis of thyroid hormone is inhibited by high levels of iodine in Amiodarone (Wolff-Chaikoff effect). Treatment of hypothyroidism is with replacement therapy and is rarely an indication to discontinue therapy. Hyperthyroidism secondary to Amiodarone toxicity also occurs by a variety of mechanisms. In Type 1, synthesis of T4 is increased due to iodine load in a patient with underlying autonomy secondary to a nodule or goiter. In Type 2, patients develop a destructive thyroiditis often followed by hypothyroidism. Clinically, determining the mechanism of hyperthyroidism can be challenging but can direct therapy. Detectable uptake on thyroid scan or nodules on exam suggest Type 1. Patients with Type 2 sometimes have elevated IL-6 levels. Doppler sonography to assess vascularity and diagnose small nodules is successful in classifying 80% of cases. Type 1 disease is treated with anti-thyroid drugs such as Methimazole and response may be slow. Patients with Type 2 are treated with steroids and often respond quickly. In clinical practice, patients are often treated with both, with the rapidity of response guiding further treatment. In considering stopping therapy, it is important to weigh the risks of chronic hyperthyroidism against the risk of arrhythmia. Amiodarone has a very long half-life which prevents any immediate benefit in stopping the drug, and symptoms may actually be exacerbated when the beta-blocking effects of Amiodarone are lost. In general, thyrotoxicity is not an absolute contraindication for continuation of Amiodarone and risks and benefits must be weighed carefully. In monitoring patients on long-term therapy, TSH and FT4 should be followed every six months.
A CASE OF AMNESIA RESPONSIVE TO PHLEBOTOMY
H.A. Younes1; R. Parker1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #116839)
LEARNING OBJECTIVES
1) To diagnose and recognize the different kinds of erythrocytosis and polycythemia, 2) To learn about the complications of erythrocytosis, 3) To learn about the treatment options of different kinds of erythrocytosis.
CASE
A 63 y.o. gentleman, with a past medical history significant for CAD, hypertension, and recurrent DVT's, presented to his PCP office complaining of frequent forgetfulness for 2 weeks. He reported the problem starting while he was on a plane trip from Denver to Pittsburgh where he felt some shortness of breath with mild headache. When he reached Pittsburgh, he couldn't tell where he came from, or the time of the day. He was even somewhat confused about his personal belongings. This episode was followed with several incidences of forgetfulness in his daily tasks, such as forgetting the water tap was open and forgetting his daily schedule of activities. Physical exam revealed no localized neurological deficit. An MRI of brain showed no evidence of a new ischemic or hemorrhagic stroke, although it showed an old right MCA occipital lobe infarct. A hypercoagulable workup was negative. A CBC showed a Hgb of 18 g/dL, a Hct of 54.3, and an RBC mass of 5.83 × 10 to power 12 per L. WBC count was 6.6, and platelet count 147,000. A repeat CBC confirmed above values. Serum viscosity and erythropoitin level proved normal. A blood volume study showed normal RBC volume, with a low plasma volume, and a low normal total blood volume; all findings consistent with a relative polycythemia. In view of the persistent neurological findings, therapeutic phlebotomy of the patient was done, with 500 ml removed each time. After a few sessions, the patient reported his symptoms improving significantly, with a decrease in his hemoglobin and hematocrit levels.
DISCUSSION
As opposed to absolute polycythemia (polycythemia vera or PV) where there is an absolute increase in red cell mass, ‘relative’, ‘stress’, or ‘apparent’ polycythemia is defined as an increase in hematocrit with normal red cell mass. Approximately 25% of cases have reduced plasma volume. Possible causes of this relative polycythemia include dehydration, alcohol, smoking, obesity, hypoxia, acute MI, and hypertension. PV is known to cause both microvascular disturbances, such as peripheral ischemia and atypical cerebral ischemic attacks, as well as major arterial and venous thromboembolism. However, the risk of vascular occlusive episodes in relative polycythemia is not well known. A study by Schwartz et al. comparing relative polycythemia with PV revealed significantly more thromboembolic events (DVT/PE) in PV, but equal risk of cardiac and cerebrovascular events. The fact that our patient's symptoms improved after phlebotomy as his hematocrit decreased, suggests that his symptoms were atypical cerebral attacks. Although phlebotomy is not considered a typical treatment modality of relative polycythemia, it worked well with our patient.
A CASE OF CAMPYLOBACTER FETUS MENINGITIS IN A FORTY-YEAR OLD MAN
M.S. Divakaruni1; A. Hwang2. 1Stanford University, Palo Alto, CA; 2Santa Clara Valley Medical Center, San Jose, CA. (Tracking ID #116215)
LEARNING OBJECTIVES
1. Recognize Camplyobacter species as a potential etiology of bacterial meningitis in patients with predisposing illness, including recent or distant neurosurgery, or alcohol abuse. 2. Treat CNS infections with C. fetus with carbapenems or a third-generation cephalosporin and an aminoglycoside.
CASE
Campylobacter is an uncommon cause of bacterial meningitis in adults. We report the case of a 40-year old Vietnamese gentleman with a prior history of partial craniotomy and alcohol abuse who was admitted with headache, fever, neck pain, and weight loss over the preceding two weeks. The patient had reported a history of a flu-like illness preceded by one day of non-bloody diarrhea, but these symptoms had resolved several days prior to admission. On admission, he was febrile to 39.4 degrees centigrade and had prominent nuchal rigidity and positive Kernig's and Brudzinski's signs, but an otherwise normal neurological exam except for marked confusion. Laboratory data showed serum WBC 15,000/mm3 with a left shift. Cerebrospinal fluid analysis showed 543 WBC/mm3 with 85% neutrophils and 15% lymphocytes, glucose of 24 mg/dl, and protein of 117 mg/dl. The fluid was India ink negative, cryptococcal antigen negative, and acid-fast bacilli negative. The patient was initially treated with intravenous vancomycin and cetriaxione. On the third hospital day, one out of four blood cultures began to grow out gram-negative rods. The subsequent day, the patient's cerebrospinal fluid grew out comma-shaped gram-negative rods suspicious for Campylobacter species. Vancomycin was discontinued, and the patient was started on gentamicin in addition to ceftriaxone. The patient responded rapidly to antibiotic therapy. Both the patient's blood and cerebrospinal fluid cultures eventually returned with a final result of Campylobacter fetus species. The patient was treated with a total of five days of parenteral gentamicin, fifteen days of parenteral ceftriaxone, and an additional seven days of oral ciprofloxacin for a total antibiotic course of twenty-one days. At the time of discharge the patient was doing well, and had no further gastointestinal or neurologic symptoms.
DISCUSSION
In this case of Campylobacter fetus meningitis, the patient had predominately extra-intestinal manifestations as is normally seen with C. fetus species, though with a one-day history of diarrheal illness not usually reported with the organism. The patient had a predisposing immunosuppressed state secondary to his alcohol abuse, as well as a prior history of neurosurgery, consistent with previously reported cases. Given the incidence of mortality reported in the case literature, and this patient's rapid response to the selected antibiotic regimen, the early and appropriate treatment of C. fetus meningitis appears to be clinically important.
A CASE OF HERPES ZOSTER ENCEPAHLITIS
S. Ramamurthy1; M. Graham2. 1Medical College of Wisconsin, Germantown, WI; 2Medical College of Wisconsin, Milwaukee, WI. (Tracking ID #115575)
LEARNING OBJECTIVES
Viral pathogens can cause a variety of syndromes when affecting the central nervous system including aseptic meningitis and encephalitis.Varicella zoster virus (VZV) is a rare cause of central nervous system syndromes. We discuss a patient who initially presented with dermatomal zoster whose clinical course was complicated by the development of VZV encephalitis with complications both from the primary disease process and the appropriate therapy.
CASE
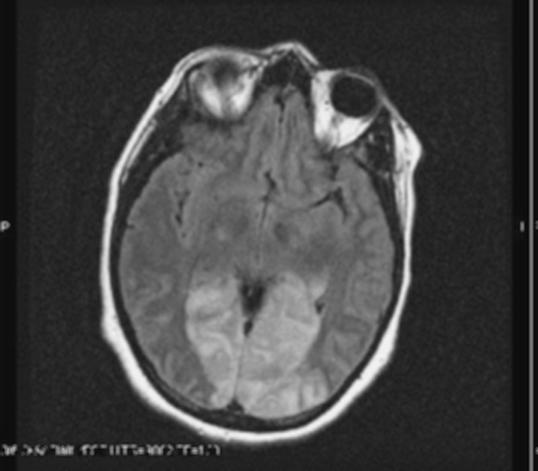
66 yr old Caucasian female with history of rheumatoid arthritis treated with methotrexate who presented with mental status changes. Seven days prior to admission she developed an erythematous rash on back and chest confined to right side of thorax. She was treated with valacyclovir for two days and complained of pain at the site for which she was prescribed vicodin and amitryptyline. The next day she was noted to be disoriented by family and brought to ER. Physical exam revealed an erythematous vesicular rash on her chest and back confined to the T2–T3 dermatome on right side. Neurological exam was within normal limits except that she had difficulty finding words. CT scan of the head on admission was normal and her labs were significant for hyponatremia (123 mmol/l). Urine osmolality was 708 mosm/kg and serum osmolality was 267 mosm/kg consistent with SIADH. She was placed on intravenous (IV) acyclovir and fluid restriction for SIADH. Cerebrospinal fluid (CSF) analysis revealed an elevated white blood cell count (321/cmm) with lymphocytic predominanace (90%) and elevated protein level (115 mg/dL). CSFanalysis for VZV by PCR was positive. On day 3 her creatinine level increased and urinalysis revealed numerous crystals consistent with acyclovir induced nephropathy. The acyclovir dose was adjusted based on renal function and she was given IV fluids. On day 4 she complained of hallucinations and double vision. MRI of the head was normal. She continued to improve with IV acyclovir and hyponatremia resolved. A repeat CSF analysis was negative for VZV by PCR. Patient completed a two week course of IV acyclovir and was discharged on oral valacyclovir for an additional week and neurontin for pain.
DISCUSSION
Herpes zoster encephalitis is rare and very few cases have been reported.We postulate that in this case her immunosuppresed state on methotrexate was the main predisposing factor. This case also highlights the complications of disease process, specifically hyponatremia (SIADH) and adverse effect of treatment (acyclovir induced nephropathy) and how to manage them astutely.
A CASE OF INTERNAL MAMMARY ARTERY STEAL SYNDROME
H.L. Korlakunta1; D. Lakkireddy1; N. Mehta1; T. Lanspa1; I. Khan1. 1Creighton University, Omaha, NE. (Tracking ID #115718)
LEARNING OBJECTIVES
To report a case of a patient with IMA steal syndrome after a LIMA bypass grafting to the LAD who was successfully treated with percutaneous transcatheter endovascular coiling of the anomalous lateral internal thoracic artery.
CASE
A 53-year male presented for evaluation of recurrent exertional angina more so with upper body exercise. He had known diabetes, hypertension, hyperlipidemia, paroxysmal atrial fibrillation and coronary artery disease with a 3-vessel coronary artery bypass surgery done 4 years prior to presentation. A transradial coronary angiogram revealed total occlusion of the RCA graft with 99% stenosis of mid RCA, which was successfully treated with angioplasty and stent placement. The LCX graft was patent, LIMA was patent but appeared to be a small vessel. There was 80% stenosis of mid LAD just proximal to the LIMA insertion. During LIMA injection a parallel branch running lateral to the LIMA graft was seen giving rise to anterior intercostals and perforating branches. This fits the anatomic description of an anomalous IMA with a lateral internal mammary artery. Patient then underwent an adenosine cardiolyte stress perfusion imaging which showed mild to moderate reversible ischemia in the antero-septal and anterior walls. Patient was started on a long acting nitrate in addition to his regular dose of beta blockers, diuretic, angiotensin converting enzyme inhibitor and was advised to abstain from upper body exertional activities. He was brought back a month later and a selective catheterization followed by a coil embolization of the lateral internal thoracic artery was performed with successful closure. There was a dramatic improvement to the flow through the LIMA graft after closing the lateral branch. An exercise stress was performed with no ischemic symptoms or EKG manifestations.
DISCUSSION
The internal mammary artery (IMA) is a conduit of choice for myocardial revascularization, especially when the target vessel is the left anterior descending artery (LAD). Occasionally IMA hypo perfusion occurs when there is inadequate flow through the IMA graft to the LAD artery. The graft hypo perfusion can occur both acutely and chronically resulting in Malperfusion Syndrome and Dysfunctional Graft with persistent ischemia in the region of supply. This is a case of symptomatic LAD ischemia from a hypoperfusing IMA graft which was experiencing vaso-steal phenomenon from a persistent anomalous lateral internal thoracic artery. It was subsequently embolized with coils with improved perfusion in LAD and symptomatic improvement.
A CASE OF MONDOR's DISEASE: SUPERFICIAL THROMBOPHLEBITIS OF THE BREAST
D. Cywinski1; E. Caiola1. 1University of Rochester, Rochester, NY. (Tracking ID #117108)
LEARNING OBJECTIVES
1. Recognize that thrombophlebitis of superficial veins of the breast is an uncommon condition that is usually self-limited. 2. Review the potential etiologies of Mondor's disease: most commonly idiopathic, post breast surgery and uncommonly due to underlying breast cancer. 3. Review that Mondor's disease can be diagnosed with color flow Doppler examination of the breast and if no other abnormalities are detected can be followed and treated symptomatically.
CASE
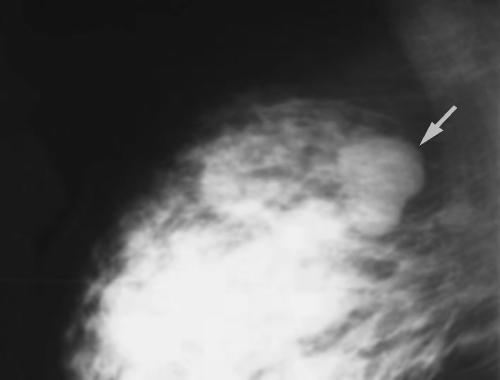
A previously healthy 26-year-old female presented with a two-day history of a painful left breast with a palpable cord. She denied a history of breast-feeding, trauma, fever or chills. She was G2P1011 with a 2-year-old child. Depo-Provera was her only medication. She denied alcohol, tobacco or drug use. She had no previous history of thrombophlebitis or deep venous thrombosis. Family history was negative for thromboembolism or breast cancer. Physical examination was notable for an approximate 10-cm palpable, tender venous cord over the upper outer quadrant of the left breast. There was minimal surrounding erythema and induration. There were no palpable breast masses or axillary adenopathy. There was no extension to the axillary veins and there was no arm edema or asymmetry. The remainder of the exam was non-focal. A Doppler examination demonstrated a hypoechoic tubular structure without vascular flow consistent with Mondor's thrombophlebitis. No other abnormalities were detected. The patient was treated symptomatically with warm compresses and NSAIDs and had complete resolution of her symptoms 4 weeks post presentation.
DISCUSSION
Mondor's disease is a rare condition of superficial thrombophlebitis of the breast veins. It is usually a self-limited condition that can be followed and treated supportively with NSAIDs. In most cases a cause is not found but Doppler examination and possible mammography are indicated. Mondor's disease may be a complication of breast surgery and uncommonly associated with underlying breast cancer.
A CASE OF NON-MENSTRUAL STAPHYLOCOCAL TOXIC SHOCK SYNDROME
S. Arora1. 1University of Connecticut, Farmington, CT. (Tracking ID #117432)
LEARNING OBJECTIVES
To recognize and manage toxic shock syndrome (TSS).
CASE
A 24-year old previously healthy male deli worker presented with a painful, marble sized swelling posterior to right greater trochanter, high fever and vomiting for 2 days with generalized red skin rash involving the entire body for a day. He had not passed urine for 12 hours. There was no preceding history of trauma or any outdoor activity. Examination revealed tachycardia with HR of 140 bpm, fever with temperature of 104oF and hypotension with BP of 80/58, pierced lower lip with lip ring, intensely red, blanchable erythema involving the entire skin and oro-pharyngeal mucous membranes. There was 2 × 2 cm tender, fluctuant swelling, mobile over underlying muscle located 5 cm posterior to right greater trochanter over the posterolateral aspect of right hip. Incision and drainage of the swelling yielded 5 ml of yellow pus which grew staphylococcus aurues sensitive to oxacillin. Investigations revealed WBC of 28,000/cmm with 18% bands and 81% neutrophils, platelets: 90,000/cmm, BUN/Cr: 44/4.8 and FeNa of 0.4%, the patient was diagnosed with staphylococcal toxic shock syndrome and was treated with IV fluids, IV Vancomycin and supportive care of acute renal failure. The patient's renal function started improving by D2, he became afebrile on D4 with gradual resolution of rash subsequently. He was discharged on D4 on Dicloxacillin and recovered with no sequelae.
DISCUSSION
Staphylococcal TSS is an acute life-threatening toxin-mediated intoxication caused by TSS toxin 1 or staphylococcal enterotoxin B. Although menstruation remains the most well-known setting for TSS, 50% of TSS is non-menstrual and can complicate the use of barrier contraceptives, child birth, superinfection of various skin lesions including burns, insect bites, varicella, surgical wounds and post-influenza pneumonia. The primary site of colonization often appears entirely benign. CDC criteria for diagnosis includes presence of all of the following: hypotension or orthostatic drop in BP, temperature >102oF, diffuse macular erythroderma, desquamation of palms and soles 1–2 weeks after onset, negative results of blood, throat or CSF cultures which may suggest an alternative diagnosis and involvement of at least three of the following organ systems: gastrointestinal (nausea and vomiting), muscular (severe myalgias or elevated CPKs, mucous membranes, renal, hepatic, hematological (thrombocytopenia <100,000, central nervous system (disorientation but no focal neurological signs). Treatment includes site drainage, aggressive fluid resuscitation, anti-staphylococcal antibiotics for 14 days, pressors for hypotension and correction of dyselectrolytemia. Critically ill or unstable patients benefit from intravenous immunoglobulin.
A CASE OF POLYMICROBIAL ENDOCARDITIS IN AN INTRAVENOUS DRUG ABUSER DUE TO ANAEROBES
S. Oh1; N. Hussain1; P.R. Havlen1. 1University of Texas Medical Branch at Galveston, Galveston, TX. (Tracking ID #115818)
LEARNING OBJECTIVES
1. Gain awareness of Infective Endocarditis (IE) due to anaerobic organisms 2. Compare IE in intravenous drug abusers (IVDA) from other cases 3. Recognize that peculiar habits of IVDA can result into unusual polymicrobial IE.
CASE
A 33-year-old white male presented to our hospital with a two-week history of subjective fevers, chills, and rigors. He had history of intravenous drug abuse and a habit of licking the needle to the dorsum of the tongue before injection into his arm. Blood cultures grew Actinomyces odontolytica, Veillonella species, and Prevotella melaninogenica. CT of the thorax showed multiple cavitary lesions in both lungs and echocardiogram showed vegetations on the tricuspid valve. The patient was treated with a six-week course of penicillin G and metronidazole. He responded well with complete resolution of symptoms.
DISCUSSION
Endocarditis in intravenous drug users are usually right sided and of the tricuspid valve. Right-sided endocarditis presents with a syndrome of persistent fever and pulmonary symptoms due to septic emboli including cough, dyspnea, and hemoptysis. The peripheral stigmata of endocarditis are not classically found in right-sided endocarditis. Although the most common organism isolated is Staphlococcus aureus, it is important to consider other more fastidious causes of infection in this population including those of endogenous origin. Anaerobes are predominant components of normal human skin and mucous membranes and are an uncommon cause of endocarditis. Most cases are caused by anaerobic cocci, Propionibacterium acnes and Bacteroides fragilis group. Actinomyces odontolytica, Veillonella species, and Prevotella melaninogenica reside predominantly in saliva and the dorsum of the tongue as compared to other organisms. We believe that his peculiar habit of licking the needle to the dorsum of the tongue to gauge the strength of the injection, subjected our patient to infection by these particular anaerobes. Polymicrobial endocarditis is a rare entity that is found almost exclusively in intravenous drug abusers. Although uncommon, it is important to consider since it carries a mortality rate exceeding 30%. There are documented cases in which cultures from the vegetations grew more organisms than the blood cultures, further exemplifying the fastidious nature of the organisms causing endocarditis in intravenous drug users. Therefore, some authors recommend empiric coverage of both skin and oral flora when endocarditis is suspected in this population. Penicillin G or other bactericidal agents appear to be the treatment of choice for these three organisms. Metronidazole is often added due to the growing resistance of anaerobes towards penicillins.
A CASE OF POST-OBSTRUCTIVE PNEUMONIA SECONDARY TO BRONCHOLITHIASIS
S.E. Luckhaupt1; L. Coberly1. 1University of Cincinnati, Cincinnati, OH. (Tracking ID #115743)
LEARNING OBJECTIVES
1) Distinguish post-obstructive pneumonia from uncomplicated community acquired pneumonia 2) Recognize broncholithiasis as a cause of bronchial obstruction 3) Manage bronchial obstruction to prevent recurrent pneumonia.
CASE
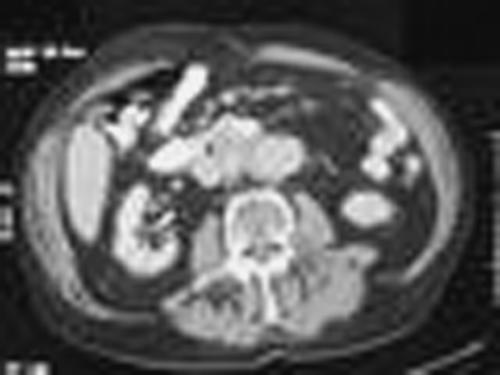
A 54-year-old male smoker with an unremarkable past medical history presented with a 2-week history of shortness of breath, cough, purulent sputum, pleuritic chest pain, and orthopnea. On exam, he had a temperature of 101.4, respirations of 28 and a pulse ox of 89% on room air. Chest exam revealed bibasilar rhonchi and intermittent wheezing over the left lung base. Initial laboratory data: WBC 20.5 with 12% bands, Hb 15.6. ABG on room air: pH 7.44, pCO2 36, pO2 64. A chest x-ray suggested left lower lobe consolidation with pleural effusion. Despite treatment with iv antibiotics, his oxygen requirement increased and serial x-rays showed increasing infiltrate and effusion. A CT on hospital day #3 revealed extensive loculated left pleural effusion with a compressed lower lobe, possibly caused by calcified left hilar lymph nodes. An ultrasound was negative for free-flowing fluid, so chest tubes were placed, and t-PA was used to assist in drainage. A repeat CT showed improvement in the effusion, but compression of the left lower lobe persisted. Bronchoscopy ultimately revealed obstructing broncholiths. The broncholiths could not safely be removed, so left lower lobectomy was performed. Pathology showed four hard tan-gray stones measuring 0.4 cm to 1.5 cm in diameter and lymph nodes with necrotizing granulomas, negative for neoplasia. No fungi, acid fast bacilli, or other organisms were identified in the pathology specimens or in the pleural fluid.
DISCUSSION
This patient's presentation provided several clues that he did not have a typical case of community acquired pneumonia. Despite having an unremarkable medical history, he was very ill on presentation with hypoxemia, which progressed even after treatment with antibiotics. Localized wheezing raised suspicion for bronchial obstruction and concern about the possibility of carcinoma. Broncholithiasis is a less common cause of bronchial obstruction, which usually presents with hemoptysis (from erosion of pulmonary vessels), wheezing, shortness of breath, or chronic cough. It is often associated with fungal infection, such as histoplasmosis, or tuberculosis. The cause of broncholithiasis in this case was unclear. The diagnosis can usually be confirmed by bronchoscopy, but bronchoscopic removal carries a high risk of bleeding, so surgical resection is often required to relieve obstruction.
A CASE OF RAPIDLY FATAL ASPERGILLOSIS IN AN IMMUNOCOMPETANT PATIENT
F.K. Salahuddin1; S. Chitavellue2; K. Karamchandanni3. 1University of Illinois at Peoria,SFMC., Peoria, IL; 2University of Illinois College of Medicine,@Peoria,SFMC, Peoria, IL; 3University of Illinois College Of Medicine,@Peoria, Peoria, IL. (Tracking ID #117272)
LEARNING OBJECTIVES
1. Diagnosis of massive hemoptysis. 2. Aspergilloma as a cause of hemoptysis. 3. Management of life threatening hemoptysis using various means.
CASE
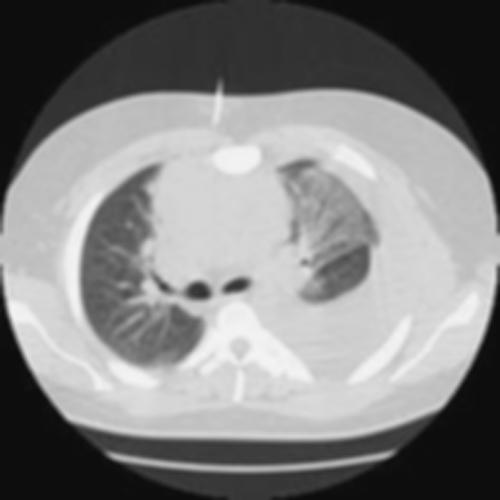
A 60 year old male was admitted into the hospital because of pleuritic chest pain, hemoptysis, fever, lethargy and significant weight loss. He was a retired janitor in a school. At the time of presentation, he was in respiratory distress and examination revealed bilateral crackles and wheezes. Chest X-ray and CT scan showed consolidation and cavitation of right upper lobe (Figure below). He underwent diagnostic flexible bronchoscopy which confirmed the bleeding from right upper lobe without any intra-bronchial pathology. Bronchoalveolar lavage grew aspergillious. Patient was treated with Amphotericin B because of massive hemoptysis. He continued to have massive hemoptysis which required mechanical ventilatory support with double lumen endotracheal intubation. Patient was sent for an emergent bronchial arteriogram and had control of bleeding with coiling. After 24 hours patient developed another episode of maasive hemoptysis which lead to his demise. Autopsy confimed the angioinvasive aspergillosis.
DISCUSSION
Angioinvasive pulmonary aspergillosis is commonly seen as a serious complication in immunosupressed individuals such as patients with AIDS and leukemia. It is rare to encounter angioinvasive aspergillosis in immunocompetant individuals. Aspergillosis can develop as a fungal ball in preexisting pulmonary cavities causing lifethreatening massive hemoptysis. Routine surgical recection of aspergillious is not recommended but should be reserved for patients with recurrent severe refractory hemoptysis. Pleuro-pneumonectomy should be avoided. Lung necrosis can result from invasion of fungus into the vasculature, leading to vascular thrombosis and hemorrhage. Massive hemoptysis can be managed with mechanical ventilation using double lumen endotracheal tube, bronchial artery embolization and or surgery. Prognosis in immunocompetant patients is usually good with above therapies.
A CASE OF RHODOCOCCUS EQUI PNEUMONIA IN A RENAL TRANSPLANT PATIENT
T.S. Bischof1; J. Hariharan1; M. Graham1. 1Medical College of Wisconsin, Milwaukee, WI. (Tracking ID #116015)
LEARNING OBJECTIVES
(1) To recognize the clinical presentation of atypical pneumonia in transplant patients. (2) To educate the clinician on the presentation, radiography, pathology, and treatment of Rhodococcus equi pneumonia.
CASE
A 48 y/o male with IgA nephropathy and 4 renal transplants presented with a one week history of nausea, vomiting, and diarrhea. He related dehydration, weakness, low-grade fevers, night sweats, and weight loss. He denied chest pain, shortness of breath or cough. The patient was taking immunosuppressive and antihypertensive medicines. Physical exam revealed an afebrile, normotensive, cachectic male in no acute distress. Exam was within normal limits, and lungs were clear. BMP was normal except for Bun/Cr of 37 mg/dL and 2.0 mg/dL. WBC was 9.0, Hgb 11.9 g/dL, and urinalysis revealed no proteinuria or white cells. Blood, urine and stool cultures were negative. Patient was hydrated, and CXR revealed a new opacity in the left lung. A chest CT revealed a 4.5 × 2.3-cm consolidation in the left lower lobe, but was negative for bony lesions and lymphadenopathy. A bronchoscopy and CT guided biopsy were done, and cultures from both subsequently grew Rhodococcus equi. On directed questioning, it was found the patient lives near a farm with routine exposures to horses and had a new dog. Therapy with moxifloxacin and azithromycin was planned until the lesion cleared on repeat CT scan.
DISCUSSION
Rhodococcus equi is a gram-positive coccobacillus that usually causes infections in grazing animals. Infection in humans is rare, but over 100 cases have been reported. Rhodococcus is often overlooked in cultures as a non-pathogenic organism and its insidious onset often leads to delays in diagnosis. Pulmonary infection is the most common, and symptoms include fever, cough, and weight loss. On radiography, the superior lobes are mainly involved, and cavitation is frequent, as well as effusion and empyema. Diagnosis is based on positive culture. Most isolates are susceptible to erythromycin, ciprofloxacin, and aminoglycosides. Oral and parenteral combinations of the above are used for treatment for at least two months. This patient was treated for 5 months and repeat CT 3 months later showed decreased consolidation. It is well known that immunocompromised patients are more prone to atypical infections. This case represents a rare cause of a treatable bacterial infection in a transplant patient and the value of social and personal history in medical management. It is important to recognize that when patients present with vague complaints and lack of physical signs, a good history and continually pursuing identification of treatable causes is important. Rhodococcus equi pneumonia is rare but understanding the nature of its presentation is highlighted in this case.
A CASE OF UNSTABLE ANGINA IN A YOUNG MAN
B. Barmar1; G. Tabas1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #103913)
LEARNING OBJECTIVES
Learning Objectives: (1) Recognize angina in young patients. (2) Consider cardiac and noncardiac causes of chest pain in young patients.
CASE
A 30-year-old Korean man with a history of gastritis and gastroesophageal reflux disease presented to his primary care physician's office with exertional chest pain relieved by rest. Because of his underlying gastrointestinal problems, young age, and lack of cardiac risk factors, his physician prescribed pantoprazole, obtained a complete blood count (CBC), and scheduled him for a treadmill stress test later that week. The next day, when the CBC revealed a platelet count of 2,800,000/uL (normal range 150–450,000/uL) the patient was instructed to go to the emergency department. He reported chest pain at rest, but this resolved after 2 sessions of emergent platelet pheresis and treatment with aspirin and nitroglycerin. Cardiac enzyme levels and electrocardiographic findings were normal. Peripheral smear showed numerous platelets, and bone marrow biopsy confirmed the diagnosis of essential thrombocytosis. After the initiation of anagrelide, a platelet-reducing agent, the patient's platelet count dropped to 1,700,000/uL by the second hospital day. To avoid a positive stress test result attributable only to platelet sludging in the coronary arteries, the physician waited until the platelet count was below 600,000/uL to perform a stress test. When the test was performed, it yielded negative results for ischemia. At 8 months after diagnosis, the patient is asymptomatic and has a platelet count of about 300,000/uL.
DISCUSSION
In young patients, cardiac causes of chest pain can be found in about 16% of cases, noncardiac causes in 68%, and unknown causes in 16%. The common noncardiac causes include musculoskeletal problems (in 36% of cases), gastrointestinal problems (in 19%), psychological problems (in 8%), and pulmonary problems (in 5%). The noncardiac causes that are most serious and require immediate treatment are pulmonary embolus, pneumothorax, and aortic dissection. A thorough history and physical examination and focused laboratory studies usually exclude life-threatening causes of cardiac chest pain. Although the patient in this case was young, his chest pain was typical for angina, so further investigation was initiated. Investigation uncovered essential thrombocytosis, an unusual cause of angina.
A CASE OF WIDE ANION GAP NON-ACIDOSIS
E. Cichowski1; H. Sakowski1; H. Hashish1; R. Baltaro1. 1Creighton University, Omaha, NE. (Tracking ID #117266)
LEARNING OBJECTIVES
1) Recognize laboratory error in the measurement of serum bicarbonate. 2) Utilize the Henderson-Hasselbach equation to indentify blood gas analysis errors. 3) Identify a previously unrecognized interfering substances as potential causes of laboratory errors.
CASE
A 72 year-old male was admitted for respiratory distress and confusion, and found to have a right upper lobe lung mass and hypercalcemia. He was intubated on the second hospital day due to worsening of his respiratory status. Propofol was initiated for sedation and methylprednisolone and levofloxacin were given for a presumed post-obstructive pneumonia. His initial arterial blood gas after intubation showed a pH 7.38 pCO2 38 pO2 143 on an Fio2 of .60. His measured HCO3 was 26 meq/l. Over the next 4 days, his measured bicarbonate progressively dropped to 8 meq/l despite no change in his arterial blood gas (pH 7.38 pCO2 36 pO2 103 on an FiO2 of .45). His anion gap was calculated at 19. Serum lactate was normal, and serum ketones were absent. Consultation with the pathology department revealed the patient's serum to be grossly lipemic. A review of the chart revealed the patient did receive lipid infusions with TPN 36 and 18 hours prior to this discovery. A lipid panel was obtained and revealed marked hypertriglyceridemia at 4,426 mg/dl. The lipid infusions were discontinued, and the propofol was weaned off. The bicarbonate level dropped to a low of 3 meq/l approximately 7 hours after the medication was discontinued. Four hours later, the bicarbonate had corrected to 21 meq/l. The serum, however, remained grossly lipemic. The patient's condition continued to decline with the development of septic shock, multi-organ failure and ventricular arrythmias. Results of a previous bronchoscopy demonstrated small cell carcinoma. The patient's family requested no further aggressive treatment and he expired later that day.
DISCUSSION
This patient developed marked derangement in his measured bicarbonate levels that did not correspond to his arterial blood gas analysis (according to the Henderson-Hasselbach equation). A laboratory error was hypothesized as the cause. Due to the finding of lipemic serum, the hypertriglyceridemia was initially suspected as the interfering substance. Upon discontinuing the propofol, the serum bicarbonate level normalized, the serum, however remained lipemic. In a review of the literature, neither propofol nor hypertriglyceridemia have been reported as potential causes of this lab error. Further testing is needed to determine the role of propafol as an interfering substance in bicarbonate laboratory analysis.
A CASE REPORT OF OXYGEN EMBOLISM FOLLOWING HYDROGEN PEROXIDE INGESTION
D. Misra1; B. Legere1. 1New Hanover Regional Medical Center, Wilmington, NC. (Tracking ID #116225)
LEARNING OBJECTIVES
Recognize that ingestion of concentrated solution of hydrogen peroxide can result in significant morbidity and mortality owing to venous or arterial oxygen embolization. We intend to share our experience through a case report to emphasize this fact .
CASE
We present the case of an 82 yr old caucasian female with prior history of emphysema who had inadvertently ingested a large quantity of concentrated hydrogen peroxide solution. Following this, she vomited and developed resiratory distress which required intubation and mechanical ventilation. On examination, she was sedated, tachycardic and had hemoccult positive stool. Blood work revealed elevated white cell count and a low hematocrit. Her basic metabolic panel, urine drug screen, liver function tests were within normal limits. Chest xray showed emphysema. CT scan of abdomen/pelvis was significant for portal venous gas and pneumatosis involving duodenal and jejunal wall. Upper endoscopy revealed hemorrhagic gastritis and distal esophagitis. She was started on empiric antibiotics and followed with serial abdominal radiographs. CT scan of the abdomen obtained five days later revealed no free air or pneumatosis. On the sixth day of hospitalization, patient was extubated and at that point of time was noted to have right sided hemiparesis. MRI scan of the brain revealed multiple areas of acute/subacute non-hemorrhagic infarction. Patient was evaluated by a neurologist and it was felt that her neurologic deficits were a result of oxygen embolization. Patient gradually improved with physical and occupational therapy and currently awaits discharge to a rehabilitation facility.
DISCUSSION
Hydrogen peroxide is widely used as an oxidant/disinfectant. It is sold in health food stores also as means of “improving oxygenation” in people with coronary artery disease. Literature search revealed several cases of accidental hydrogen peroxide ingestion. A retrospective review of all exposures reported to a poison control center revealed that 0.34% were hydrogen peroxide related. Although exposure to diluted (3%) hydogen peroxide is benign, ingestion of the concentrated form can be dangerous. Following ingestion, hydrogen peroxide breaks up into water and oxygen in the presence of catalase. When the amount of oxygen produced exceeds the maximum blood solubility, embolization occurs. We emphasize that physicians should be alert to the possibilty of multiorgan embolization in patients presenting with accidental ingestion of concentated hydrogen peroxide.
A CASE REPORT OF RECURRENT COCCIDIOIDES MENINGITIS (CM)
S.M. Maiorano1; P. Radhakrishnan2. 1St. Joseph's Medical Center, Phoenix, Phoenix, AZ; 2Catholic Healthcare West, Phoenix, AZ. (Tracking ID #117418)
LEARNING OBJECTIVES
1. Recognize that CM recurrence can occur despite prolonged antifungal treatment. 2. Recognize that indwelling CFS shunt can mask the hallmark symptoms of hydrocephalus associated with CM. 3. Recognize that diagnosis of CM can be made on serum serologies without positive CSF cultures.
CASE
A 62 year old male, presented with a 2 month history of worsening diplopia, ataxia and headache. Past History-CM with obstructive hydrocephalus and VP shunt. He was treated with Amphotericin B (intrathecal and systemic) for 2 years followed by Fluconazole for 8 years. He had been off Fluconazole for the last 5 years. Physical exam—He was somnolent, but arousable. Eyes-limited upward movement with downbeating nystagmus, disconjugate gaze with mild right lateral ocular deviation. Lab. data-CT head—mild right encephalomalcia, enlargement of 3rd and 4th ventricles, catheter in the right lateral ventricle. CSF-(from the shunt and a lumber puncture)-including Gram stain-negative. Positive CSF Coccidioides IgG and serum IGG,IGM antibodies. Complement fixation (CF) titer 1:64. Catheter tip-Coagulase negative Staphylococcus. MRI of the head—ventriculomegaly, increased periventricular and meningeal enhancement. He was diagnosed with recurrent CM, shunt failure due to presumed Staphyloccal infection. He was started on Voriconazole and Vancomycin. The shunt was replaced. He improved with resolution of his neurological symptoms and signs. He was discharged with the plan to continue the Voriconazole indefinitely.
DISCUSSION
CM is a grave form of disseminated Coccidiodes infection. Of the nearly 100,000 cases per year, only 0.1 percent present as meningitis. This case has several interesting aspects, the first being the recurrence of the CM after several years. Recurrences usually occur shortly after discontinuing therapy, as despite adequate antifungal penetration the fungus is not easily cleared. In this case, the patient remained symptom free for 5 years after stopping therapy. Second, the temporal association of shunt blockage and recurrence of symptoms of CM made us postulate that the patient remained symptom free due to the drainage of CSF and clearance of the fungus. Little data is available as to the incidence or common etiologies of shunt failure, but many case reports have found bacterial shunt obstruction through colonization as well as fungal biofilm occlusion. Third, the diagnosis of CM recurrence was made based on the CSFand serum studies. As CSF cultures are positive in only one third of cases, positive CSF IgG or IgM and CF antibodies are very helpful in diagnosing CM in patients with a high pre-test probability and negative cultures. While there are definitive guidelines for the duration of treatment of CM, patients who experience a relapse should be continued lifelong therapy.
A DIAGNOSIS AT BOTH ENDS: A CASE OF CELIAC DISEASE AND MICROSCOPIC COLITIS
D. Nataraj1; R. Granieri1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115730)
LEARNING OBJECTIVES
(1) To recognize the clinical history and histopathology of celiac disease and microscopic colitis (2) To recognize an association of celiac disease with microscopic colitis (3) To manage celiac disease and microscopic colitis.
CASE
A 31 year-old female presents with 6 months of nausea, bilious emesis, abdominal cramps, watery diarrhea, and 30 lb weight loss. Diarrhea occurs 5 to 15 times daily. Physical examination reveals cachexia, tachycardia, dry mucous membranes, normal bowel sounds, and abdominal distention with mild but diffuse tenderness to palpation. Initial laboratory values demonstrate hypokalemia, contraction alkalosis, normal amylase and lipase, hypoalbuminemia, normal liver function tests, hemoglobin of 13, leukocytosis of 22,000, and urinalysis suggestive of an infection. Additional studies show low iron level, increased RDW and decreased % saturation. EGD and colonoscopy are grossly normal. Colonic biopsy reveals increased intraepithelial lymphocytes. Duodenal and jejunal biopsies show villous atrophy with cryptitis, and mucosal lymphocytes and plasma cells. She is found to have antigliadin and antiendomysial antibodies. The diagnoses of both celiac sprue and microscopic colitis are made. With initiation of a gluten/lactose-free diet, prednisone, and octreotide, her symptoms improve considerably.
DISCUSSION
Iron deficiency anemia is the most common presentation of celiac disease. Abdominal discomfort and bloating, also common features, often incorrectly lead to the diagnosis of irritable bowel syndrome. Significant diarrhea is present in 50% of patients. Serologic studies including antiendomysial antibody (sensitivity 85%–98%; specificity 97%–100%) and tissue transglutaminase antibody (sensitivity 90%–98%; specificity 95%–97%) have facilitated diagnosis of this disease; however, the gold standard remains small bowel biopsy. Standard therapy is dietary gluten restriction, which results in symptomatic improvement in 70% of patients within 2 weeks and a decrease in antibody titers within 6 months. Conditions associated with celiac disease include type 1 diabetes and microscopic colitis (either lymphocytic or collagenous). Lymphocytic colitis typically presents in the sixth decade as watery diarrhea. The diagnosis is made by colonic biopsy revealing increased intraepithelial lymphocytes. Medications such as lansoprazole and NSAIDS have been associated with this condition. Large randomized controlled trials regarding treatment have not been conducted. Therapy is based on case reports involving small numbers of patients and includes removal of the offending drug and addition of antidiarrheals, aminosalicylates, octreotide, prednisone, or budesonide. A prospective study of 81 patients treated with a variety of the above medications demonstrated a 70% resolution of diarrhea. Bismuth subsalicylate has been promising in small trials.
A DIFFERENT TWIST TO AN ABDOMINAL PAIN
C.E. Landaverde1; S. Dea1. 1UCLA-San Fernando Valley Program, Sylmar, CA. (Tracking ID #115130)
LEARNING OBJECTIVES
1) Recognize that a volvulus can have an atypical age of presentation and occur in someone with no predisposing risk factors. 2) Recognize clinical and radiological features of a volvulus.
CASE
A 39 y/o Hispanic nulliparous female presented to the emergency room with a one day history of abdominal pain, distension, nausea and vomiting. The abdominal pain was described as sudden onset, continuous, severe, crampy, lower abdominal pain worse with eating. The patient reported having explosive, watery, brown diarrhea soon after the onset of the abdominal pain for a couple of hours but since had not had a bowel movement nor passed flatus. Patient denied prior history of constipation or use of psychotropic medications or a diet high in fiber. The patient was afebrile with a blood pressure of 168/56. The abdominal exam revealed moderate distension, no bowel sounds, tympanic with tenderness to palpation in the lower abdominal quadrants, left more than the right. There was no rebound tenderness or guarding. The rest of the physical exam was unremarkable. Laboratory results were unremarkable except for a bicarbonate level of 31. An abdominal plain film revealed a dilated, ahaustral loop of large bowel extending from the pelvis to the right upper quadrant in an “inverted U” appearance. A CT scan of the abdomen confirmed the diagnosis of a sigmoid volvulus with findings of a dilated sigmoid colon. A gastrograffin enema revealed a partial sigmoid volvulus, which had reduced upon the post-evacuation examination. Subsequently, the patient had a sigmoid resection with primary anatamosis performed.
DISCUSSION
Sigmoid volvulus is produced when a long redundant sigmoid twists about its mesenteric axis in either direction and forms a partial or complete loop obstruction. It occurs more commonly in the elderly, individuals with neurologic conditions, and in patients in nursing homes or mental health facilities. The common factor is chronic constipation. Other predisposing risk factors include megacolon, an excessively mobile colon, high-roughage diet, and lead poisoning. Furthermore, volvulus has been observed to occur most commonly in young patients in settings such as Crohn's disease, pregnancy, Chagas and in individuals with prior history of roundworm infestation. Patients present with abdominal pain, distension, nausea and absolute constipation with vomiting as a late sign. Findings on abdominal plain films include a markedly distended sigmoid loop, inverted U-shaped appearance, loss of colonic haustra and elevation of the sigmoid loop under one of the diaphragms. The involved bowel walls are edematous, resulting in a coffee bean–shaped structure (the “coffee bean” sign). CT findings of ischemia in a sigmoid volvulus include the “whirl sign”, which represents tension on the tightly twisted mesocolon by the afferent and efferent limbs of the dilated colon.
A FATAL CASE OF VARICELLA-ZOSTER PANENCEPHALO-MENINGO-RADICULO-MYELITIS IN A PATIENT WITH AIDS.
D.B. Van Schyndel1. 1Hennepin County Medical Center Internal Medicine Dept., Minneapolis, MN. (Tracking ID #117292)
LEARNING OBJECTIVES
1. Recognize that varicella-zoster virus infection of the central nervous system is a sign of probable immunocompromise. 2. Recognize that the characteristic rash often seen in varicella-zoster infections may not appear in immunocompromised patients. 3. Diagnose varicella-zoster infection of the central nervous system using PCR amplification.
CASE
A 38 year old previously healthy Canadian woman presented to the emergency department with a four-day history of frontal headache and one day of lower extremity weakness and numbness. The initial exam revealed 4/5 lower extremity strength. The initial head CT was normal and a lumbar puncture was performed. CSF studies revealed increased protein and white blood cell counts. Empiric acyclovir was started. Six hours after the patient was admitted, she complained of worsening leg weakness and numbness extending to her chest. On exam she was areflexic in her lower extremities, paraplegic, and had a sensory level at T4. She became hypoxic and was intubated. Several hours later the patient was reexamined and no brain stem reflexes were present. A repeat head CT revealed brain stem swelling and leptomeningeal enhancement of the brain stem and cerebellum. The next day, the varicella-zoster PCR performed on cerebrospinal fluid was found to be positive. The patient's family reported that she had been exposed to a child with chicken pox two weeks before her admission. They did not remember the patient complaining of a rash. An HIV test was also positive. A MRI showed changes consistent with acute disseminated encephalomyelitis. Her neurologic exam was unchanged. The patient's family decided to withdraw support and the patient died approximately 72 hours after admission. An autopsy revealed lymphocytic encephalomeningoradiculomyelitis.
DISCUSSION
Zoster is not viewed as an AIDS-defining illness, but it can indicate immunodeficiency and tends to occur more often in patients with HIV. Varicella-zoster virus is likely to be associated with HIV in central and east Africa, where the positive predictive value of a history of VZV can be up to 90%. Among opportunistic CNS infections in AIDS patients, VZV accounts for 2–4% of neurological disease. In one series of 11 AIDS patients with VZV encephalitis, four did not report a rash. Health care providers should therefore keep VZV on their differential in patients with HIV risk factors who present with neurologic symptoms but do not report a rash. Examination of the CSF usually reveals mild mononuclear pleocytosis, a normal or elevated level of protein, and a normal glucose level. Varicella-zoster virus cannot be cultured from cerebrospinal fluid, but the virus can be detected with PCR. The varicella-zoster PCR has a specificity of 98.6% and a sensitivity of 100%.
A FIRM HANDSHAKE. PRESENTATION OF AN ECTOPIC GROWTH HORMONE SECRETING TUMOR
M. Chan1; M. Ziebert1. 1Medical College of Wisconsin, Milwaukee, WI. (Tracking ID #116152)
LEARNING OBJECTIVES
1. Recognize the importance and early diagnosis and treatment of acromegaly. 2. Recognize the importance of a good history and physical exam. 3. To develop a basic understanding of pathophysiology and current treatment modalities.
CASE
A 58-year old Caucasian woman who presented to establish primary care was noted to have very large hands on initial introduction. Her only complaints were chronic bilateral hip and knee pain. Past medical history included hypertension, hypercholesterolemia, and scoliosis. Review of systems revealed difficulty sleeping with excessive daytime sleepiness, back pain, headaches, and polyuria. Physical exam revealed a woman with significant mandibular overgrowth and prognathism, a deep resonant voice, a large fleshy nose and very large hands. The patient was asked to bring an old ring and pictures for comparison. Laboratory studies included a basic metabolic panel, complete blood count, TSH, prolactin, FSH, LH, cortisol, ACTH, growth hormone (GH), and somatomedin C or insulin-like growth factor-1 (IGF-1). Both GH and IGF-1 were extremely elevated, up to five times the upper limit of normal. An MRI of her pituitary revealed a large 1.7 cm ectopic tumor in the sphenoid sinus. The patient was referred to endocrinology, neuroophthalmology, and neurosurgery for evaluation. The patient subsequently underwent sublabial, transphenoidal resection of her tumor and pathology confirmed isolated GH producing cells. The patient currently feels like a “new person”. Her arthralgias, headaches, and probable obstructive sleep apnea have significantly diminished. She is currently on cabergoline or Dostinex and finishing her adjuvant radiation.
DISCUSSION
Acromegaly is a rare, chronic syndrome that is often diagnosed by the general internist. Most commonly, it is caused by excessive secretion of GH by the somatotroph adenoma of the anterior pituitary. However, very rarely, ectopic tumors may secrete GH and present in an indolent fashion. Diagnosis is usually delayed for many years resulting in significant morbidity and mortality. In the era of healthcare reform, the emphasis is on a problem focused clinical encounter. This case illustrates that a rare, debilitating disease can be diagnosed by simply shifting the focus back to the patient. A firm handshake or first impression can still be a valuable clinical tool.
A GIFT FROM THE TOOTH FAIRY
D. Blackmon1; M. Panda1. 1University of Tennessee, Chattanooga, chattanooga, TN. (Tracking ID #106696)
LEARNING OBJECTIVES
To recognize the similarities in clinical and radiographical presentation of pulmonary actinomycosis and neoplasms.
CASE
53 year old male with a heavy tobacco history, presented with malaise, non-productive cough and weight loss for 3 months. On exam he had normal vitals, appeared non-toxic but cachetic, with dental caries and diminished breath sounds on the left. Labs were only significant for an elevated wbc count with microcytic anemia. CXR showed opacity in left hemithorax. CT chest revealed 10 × 10 × 7 cm necrotic mass abutting the pericardium and pleural suspicious for carcinoma. Biopsy revealed no neoplastic cells. Aspirate cytology revealed filamentous sulfur granules consistent with Actinomycosis confirmed by culture. Patient was treated sucessfully with penicillin and dental extractions.
DISCUSSION
Actinomycosis is a gram-positive anaerobic filamentous bacteria. Humans are the only host. It resides in the oropharynx, GI and female genital tract and commonly causes cervicofacial infections. Aspiration of oropharyngeal secretions commonly cause pulmonary actinomycosis—50% of which is associated with dental caries. These bacteria invade bony structures and cross-anatomic borders, making its appearance similar to neoplasms. Diagnosis is by identification of “sulfur granules” on cytology or isolation of organism on culture. Bronchoscopy, CT guided biopsy/aspiration or thoracotomy is often required for diagnosis due similarity in presentation to neoplasm. Treatment requires PCN for 12 months and extraction of dental caries when indicated. Diagnosis of Actinomycosis requires a high clinical index of suspicion and must be considered in individuals with lung masses and poor dental hygiene in order to spare the patient from unnecessary tests and invasive procedures.
A HIGHLY FUNCTIONING CASE OF DEMENTIA
G. Prakash1; P. Koneru1; R.D. Hobbs1. 1Oakwood Healthcare System, Dearborn, MI. (Tracking ID #117199)
LEARNING OBJECTIVES
To recognize a common error in making the diagnosis of dementia.
CASE
A 74-year-old woman with Alzheimer's disease presented for a physical exam. She had been institutionalized in another city and had recently moved to be near her sister. Her history was significant for resection of a pituitary tumor with resultant hypopituatrism, hypogonadism, hypothyroidism and later, diabetes mellitus. She was a nurse by profession. Her husband had died two years before. Physical examination revealed a dysconjugate gaze, a dilated fixed right pupil and a visual field defect. During the exam she remarked “Oh, you're checking my visual fields by direct confrontation.” She then explained how these findings were “chronic since 1955.” She was alert and oriented, performed serial sevens accurately, interpreted proverbs abstractly, and had only minor difficulty remembering a name and address. When asked to spell “world” backwards she did so and then asked the examiner if he would like to hear the alphabet spelled backwards. Without an error or pause, she then accurately spelled the alphabet backwards. The examiner later remarked jokingly, “This was the most highly functioning case of Alzheimer's disease” that he had ever seen. Her miraculous improvement had occurred after moving closer to her sister.
DISCUSSION
Studies done during the 1970's showed that between 10–20% of nursing home patients diagnosed with dementia were actually suffering from untreated depression. Unfortunately, since most dementia is incurable, such a diagnosis frequently labels an individual as medically untreatable and condemns them to their continued existence with scant hope of improvement. With more modern care the contribution of depression to dementia has been recognized and is now frequently treated. Our patient did not have Alzheimer's disease but was suffering from severe bereavement and isolation that improved when she moved nearer her sister. This case should serve as a cautionary tale to clinicians and underscore the point that in 2004 there are still individuals whose severe depression can mimic dementia to the point of institutionalization.
A HIP FRACTURE ALREADY?
C. Christopher1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117403)
LEARNING OBJECTIVES
1. Recognize risk factors for osteoporosis in a young woman 2. Distinguish causes of secondary osteoporosis.
CASE
A 44 year-old woman was admitted following a displaced left femur fracture. She also noted four months of irregular menses and depression with poor appetite. Her body mass index was 21, and the early fracture prompted an evaluation for osteopenia. She smoked but did not consume alcohol. She noted a past history of a stomach ulcer that required surgical intervention. She had no pallor, thyromegaly, or dental caries. Her breast exam was normal. Her calcium level was 7.6 mg/dL, albumin 1.7 g/dL, phosphorous 3.0 mg/dL, alkaline phosphatase 279; her renal function and CBC were normal. An intact PTH level was 53 pg/mL (normal 8–97). Her TSH was 1.65 uIU/mL with a free T4 of 0.50 ng/dL (normal 0.8–1.9). FSH, LH, and estradiol levels were consistent with premenopause. Her 1,25-dihydroxycalciferol was 12.5 (15.9–55.6); the 25-hydroxycalciferol was 8.4 (8.9–46.7). Bone densitometry showed T-scores of −2.8 (hip) and −3.3 (spine). Alendronate therapy was initiated with supplemental calcium and vitamin D. An extensive past medical history revealed that the surgery for the duodenal ulcer required a Bilroth I anastomosis, later revised to a Roux-en-Y re-anastomosis.
DISCUSSION
Risk factors for osteoporosis include gender, race, tobacco use, alcohol consumption, low body weight, and nulliparity. Our patient's young age for a hip fracture prompted an evaluation of secondary etiologies of osteoporosis. These include renal or liver disease, malignancy, primary hyperparathyroidism, vitamin D deficiency, malabsorption, malnutrition, myeloma, and hyperthyroidism. In our patient, the Roux-en-Y anastamosis had led to malabsorbtion of fat-soluable vitamin D. She was started on parenteral vitamin D in addition to alendronate and calcium supplements. Diagnosis of osteoporosis is based on T-scores from bone densitometry studies that compare the patient to sex and race matched young controls. T-scores below −1.5 is the recommended level for therapy in patients with risk factors, and therapy should begin at T-scores below −2.0 in the absence of risk factors.
A LARGE SPLENIC CYST: “INCIDENTALOCYST”
A. Sequeira1; N.K. Atray1; T.J. Vachharajani1. 1Louisiana State University Medical Center at Shreveport, Shreveport, LA. (Tracking ID #116793)
LEARNING OBJECTIVES
To discuss the differential diagnosis of an incidental splenic cyst.
CASE
A 31-year old type 1 diabetic male presented with a 5 day history of nausea, vomiting, diarrhea and upper abdominal pain, 2 months after a motor vehicle collision. He denied any prior symptoms of gastroparesis. Examination was remarkable for an afebrile patient with epigastric tenderness. Laboratory data: Hb 13.3 g/dL, WBC 15 k/cmm, serum amylase 75 U/L, serum lipase 227 U/L, T.bil 0.5 mg/dL, albumin 3.9 mg/dL, Alk phos 126 U/L, SGOT 12 U/L, SGPT 56 U/L, BUN 28 mg/dL, Creat 1.9 mg/dL, anion gap 18, urine ketones 4 + and an ABG with pH 7.31, PaCO2 27, PaO2 120, HCO3 12, SaO2 99% on 1.5 liters oxygen. His symptoms of nausea, vomiting and abdominal pain persisted despite correcting his ketoacidosis. A CT abdomen showed a calcified multiseptate splenic cyst measuring 12 × 8 cm, which was compressing the stomach. The possibility of a splenic abscess precipitating ketoacidosis was entertained. His blood cultures were negative for bacteria, fungi and acid-fast bacilli. The splenic aspirate was sterile for any organisms. Subsequently, he underwent splenectomy for multiseptated cystic spleen with pressure symptoms. Pathology revealed a 490 gm spleen measuring 16 × 13 × 8 cm. Histopathology revealed a cyst without lining cells with organized fibrin and old hemorrhages, suggestive of a posttraumatic pseudocyst.
DISCUSSION
Splenic cysts are rare, many of which are asymptomatic and incidental findings. They are classified as true or false based on the presence or absence of an epithelial lining. In the absence of an Echinococcal infection, cysts are commonly congenital or post traumatic. The above case highlights the need to suspect posttraumatic splenic cyst as a possible differential in a patient with a LUQ mass following an abdominal trauma. As in the above case, large splenic cysts may mimic the symptoms of gastroparesis in a diabetic.
“A LAZY HOUSEWIFE”: CASE OF LUPUS PNEUMONITIS
J.E. Cho1; D. Yick1. 1University of California, Los Angeles—San Fernando Valley Program, Sylmar, CA. (Tracking ID #115612)
LEARNING OBJECTIVES
1) Recognize lupus pneumonitis as an etiology of pulmonary effusion 2) Describe the typical presentation of lupus pneumonitis 3) Recognize the treatment options and prognosis of lupus pneumonitis.
CASE
A previously healthy 22 year old female presented to the emergency department with acute shortness of breath. Her shortness of breath was worse with exertion and associated with pleuritic chest pain for two days. She also noted fever, non-productive cough, nausea, vomiting, and arthralgia. On presentation, she was febrile with temperature 38.6, blood pressure 88/54, pulse of 104 beats per minute, respiratory rate of 28 and oxygen saturation of 88% on room air improved to 92% with 2 liters of supplemental oxygen. She was in moderate respiratory distress; however, she was speaking in full sentences. Physical examination revealed absent breath sounds throughout right thorax with decreased breath sounds half way up on the left thorax. There was associated egophony, decreased fremitus, and dullness to percussion on the right thorax. There was no jugular venous distension, lower extremity edema, or skin rash. Chest radiograph revealed small pleural effusion on the left side one-fourth way up in addition to the right-sided pleural effusion three-fourths way up with mediastinal shift to the left. She was admitted to the intensive care unit for acute respiratory distress. Chest CT with contrast confirmed findings of right-sided pleural effusion and small left sided effusion. Thoracentesis was performed and it showed negative culture, gram stain, and cytology with increased LDH and protein consistent with Light's criteria for exudative process. The pleural fluid was positive for ANA at 1:10,000, lupus anticoagulant, anti double stranded DNA 1:40, and anticardiolipin antibody. The diagnosis of lupus pneumonitis was made, and she improved on intravenous solumedrol, and discharged home on oral prednisone several days later.
DISCUSSION
Systemic lupus erythematosus (SLE) is a multifactorial autoimmune disorder that may affect one or multiple organ systems. Acute lupus pneumonitis is an uncommon presentation that appears in 1% to 10% of the cases, and the symptoms include severe dyspnea, tachypnea, fever, pleurisy, cough, basilar rales, hypoxia, and no apparent infection with radiographic findings of bilateral lower lobe infiltrate and atelectasis. In addition, pleural effusion is observed in up to 30% of cases. Lupus pneumonitis responds with glucocorticoids. However, intravenous pulse steroid therapy or immunosuppressive drugs may be considered if no improvement in 3 days. Lupus pneumonitis may progress to pulmonary fibrosis and eventually develop into pulmonary hypertension. The prognosis of lupus pneumonitis is poor with short-term mortality approaching as high as 50% with persistent pulmonary function abnormalities, including severe restrictive pulmonary defect.
A METABOLIC MESS: A CASE OF ETHYLENE GLYCOL POISONING
S. Khan1; B. Taqui1. 1Temple University, Philadelphia, PA. (Tracking ID #116218)
LEARNING OBJECTIVES
1. Recognize causes of potentially fatal alcohol intoxication. 2. Recognize clinical features of ethylene glycol poisoning. 3. Review management of ethylene glycol posioning.
CASE
A 55 year old African American female with depression, hypothyroidism, and breast cancer presented with altered mental status. She lives with her mother, but history taking was limited by the mother's Alzheimer's disease. The mother reported that the patient had been vomiting earlier in the day. In the emergency room, patient became unresponsive and was intubated. Her vitals were T 96.9F, HR 96, BP 125/90. Her exam revealed left, fixed pinpoint pupil, flaccid extremities, absent reflexes. Her labs revealed: Na 161, K 3.6, Cl 120, HCO3 7, BUN 14, Cr 1.7, glucose 114, anion gap 34. Her calculated osmolarity was 320, measured osmolarity 551 and osmolar gap 231. Her lactate was 9.5 and ammonia 98. Her WBC was 15.7 (no shift), Hgb 14.4, platelets 151. Her liver function tests and TSH were normal. Her urine showd calcium oxalate crystals. ABG prior to intubation revealed pH 6.93 pCO2 21 HCO3 4. Head CT and LP were negative. Her ethanol level was 31 mg/dl and ethylene glycol level was 900 mg/dl. She received two doses of fomepizole and D5W with 3 amps of bicarbonate. She was then placed on an ethanol drip. She subsequently improved, was extubated and transferred to inpatient psychiatry after she admitted to drinking antifreeze.
DISCUSSION
Three alcohols can produce fatal intoxication: methanol, isopropanolol, and ethylene glycol. All can increase the osmolal gap, but only methanol and ethylene glycol cause an anion gap metabolic acidosis. Ethylene gylcol is a component of antifreeze and solvents. The lethal dose is 100ml. Clinical presentation ranges from from drunkenness to coma. Complications involve the heart, lungs and kidneys. Two types of urinary calcium oxalate crystals can be seen: needle shaped and envelope shaped. The absence of crystalluria does not preclude the diagnosis. Urine examination by Wood's light may reveal fluorescence if the patient has ingested antifreeze which commonly contains fluorescin dye. Ethylene glycol is metabolized to toxic metabolites: glycolic acid and oxalic acid. Glycolic acid falsely elevates lactate. Management consists of supportive care, prevention of drug absorption, bicarbonate, and antidotes. Fomepizole, which rapidly inhibits alcohol dehydrogenase, is the drug of choice for ethylene glycol and methanol intoxication. Ethanol can also be used, but is not as potent. Both treatments need to be initiated quickly, prior to alcohol metabolism. In severe cases, hemodialysis may be required.
A MIDDLE AGE WOMAN WITH WORSENING SHORTNESS OF BREATH
N. Latif1; G.H. Tabas1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115826)
LEARNING OBJECTIVES
Recognize a non-embolic cause of pulmonary occlusive disease(PVOD) in hypoxemic patients.
CASE
A 55 year old white female with a history of coronary artery disease, insulin dependent diabetes mellitus and hypertension presented with a one week history of worsening shortness of breath, dry cough, wheezing and chest discomfort. She was treated with azithromycin as an outpatient but because her symptoms did not improve she presented herself to the emergency department. There, her temperature was 39 degrees C and her physical examination revealed some neck stiffness. Lumbar puncture was performed and cerebrospinal fluid examination was unremarkable. Her chest X-ray was interpreted as normal. The patient was empirically treated with intravenous levofloxacin. In the hospital her oxygen saturation decreased to 86% and a repeat chest X-ray showed bilateral pleural effusions and pulmonary edema. Computerized tomography of the chest revealed no pulmonary embolism. Because of the onset of atrial flutter, echocardiography was performed and showed an increased pulmonary artery pressure of 55 mmHg. To diagnose the cause of her pleural effusions and hypoxemia she underwent video assisted thoracoscopy and lung biopsy that revealed pulmonary veno-occlusive disease (PVOD). She was treated with prednisone and coumedin with symptomatic improvement.
DISCUSSION
PVOD is rare but important cause of hypoxemia and pulmonary hypertension. PVOD has no known etiology, can present at any age group with equal male and female distribution. The pathologic hallmark of PVOD is occlusion of small pulmonary veins by fibrous tissue; large veins are rarely affected. Pulmonary arteries may exhibit moderate to severe medial hypertrophy and alveolar capillaries may become engorged and tortous. Interstitial fibrosis may develop in the pulmonary parenchyma. There is no curative therapy and immunosuppressive agents including prednisone are of unproved benefit. Anticoagulation may improve survival but long-term prognosis is poor.
A MULTIPLE SCLEROSIS-LIKE ILLNESS ASSOCIATED WITH LEBER's HEREDITARY OPTIC NEUROPATHY
A.J. Huang1. 1University of California, San Francisco, San Francisco, CA. (Tracking ID #116277)
LEARNING OBJECTIVES
1. Review the clinical features of Leber's hereditary optic neuropathy (LHON). 2. Recognize LHON as a risk factor for developing multiple sclerosis. 3. Distinguish between LHON and the optic neuritis of multiple sclerosis.
CASE
A 27 year-old man with a family history of Leber's hereditary optic neuropathy (LHON) presented for physical examination before enrolling in a rehabilitation program for the visually impaired. The patient reported 2 years of progressive, bilateral central vision loss, similar to a female cousin who had been diagnosed with LHON after genetic testing confirmed the presence of a characteristic mitochondrial DNA mutation, G11778A. Unlike his cousin, the patient also suffered from patchy numbness and clumsiness in both hands and lower extremities, which he attributed to drinking too much alcohol (over 8 pints of beer per day). Physical exam revealed severe bilateral optic atrophy with an otherwise normal cranial nerve exam, decreased vibration and joint position sense in both feet, and a slow and wide-based gait. Routine laboratory studies, including TSH, B12, RPR, and HIV, were normal. The patient was counseled to stop drinking, but continued to have problems with coordination and ambulation after discontinuing alcohol, and returned to clinic 3 months later after a mechanical fall. Follow-up exam revealed interval development of moderate spasticity in both lower extremities, a mildly positive Romberg sign, and worsened vibration and joint position sensory defects. Electromyography was negative for lower motor neuron abnormalities. An MRI showed multiple focal areas of T2 prolongation in the periventricular white matter of the corpus collosum, brainstem, and cervical spinal cord, consistent with a demyelinating disease such as multiple sclerosis. The patient was referred to neurology for management of multiple sclerosis associated with LHON.
DISCUSSION
Leber's hereditary optic neuropathy (LHON) is a mitochondrially transmitted disease affecting young adults, with a male to female ratio of approximately 4 to 1. It is characterized by subacute, bilateral, central vision loss resulting in permanent optic atrophy, with relative sparing of peripheral vision. While a tentative diagnosis of LHON can often be made based solely on patients' clinical history, fluorescein angiography and electrophysiology studies may be helpful in confirming the diagnosis. Over 95% of patients with LHON have one of three mitochondrial DNA point mutations, G3460A, G11778A, or T14484C, but only 50% of men and 10% of women who harbor one of these mutations develop the optic neuropathy. A multiple sclerosis (MS)-like illness has been described in patients with LHON, especially those with mutation G11778A, in which MRI and CSF findings are identical to those of the MS population in general. The vision loss associated with LHON differs from the optic neuritis more commonly seen in multiple sclerosis in that it is bilateral rather than unilateral, is not accompanied by eye pain, is not associated with pupillary reflex defects, and rarely responds to corticosteroids. Screening LHON patients for MS, particularly if they have neurologic symptoms other than visual loss, may be appropriate if one accepts that immunomodulatory treatment should be started early in MS.
A NECROTIC PENIS
M. Glass1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117485)
LEARNING OBJECTIVES
1. Recognize the clinical presentation of calciphylaxis. 2. Recognize the risk factors for calciphylaxis.
CASE
A 52-year-old man with was admitted for necrosis at the tip of his penis. He had a history of diabetic renal failure, and was scheduled for dialysis following a permacath placement. He was afebrile and a systolic murmur was noted at the base and apex of the heart. His labs were notable for a phosphate of 8.1, and a calcium of 8.3. An echocardiogram revealed calcified mitral and aortic valves. The necrotic area was treated with surgical debridement. The surgical pathology report described acute and chronic inflammation with extensive coagulative necrosis consistent with calciphylaxis-induced ischemia.
DISCUSSION
Calciphylaxis is the deposition of calcium-phosphate crystals in the setting of either hypercalcemia or hyperphosphatemia. Deposition in peripheral arteries can result in ischemia with subsequent peripheral necrosis. A calcium-phosphate product of greater than fifty should prompt suspicion of this complication. The diagnosis is suggested by ischemic skin lesions and is confirmed by biopsy showing arterial occlusion and calcification without vascultic changes. In this case, a diagnosis of calciphylaxis was suggested by the clinical presentation and his history of renal failure; the elevated calcium-phosphate product of 67 sufficiently increased the pre-test probability to prompt a skin biopsy. In the setting of a calcium-phophate product greater than 50, physicians should consider calciphalaxis as a potential cause of vascular insufficiency and valvular calcification.
A NOT-SO-BENIGN CASE OF PROSTATIC HYPERPLASIA
A.N. Githaiga1; P.K. Han1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115075)
LEARNING OBJECTIVES
1) To recognize bladder diverticuli and spontaneous bladder perforation as possible complications of benign prostatic hyperplasia (BPH). 2) To identify clinical findings suggestive of bladder perforation.
CASE
A 69-year-old man with a history of mild BPH, colonic diverticulosis and hyperlipidemia presented to his doctor's office with a two-day history of lower abdominal pain, dysuria, urinary urgency and frequency. He was treated empirically with ciprofloxacin and tamsulosin, but his symptoms progressed to include generalized abdominal pain and distension, constipation, nausea and vomiting. He presented to the emergency room two days later, in distress from pain. Vital signs were normal.On physical examination his abdomen was distended and tympanitic with absent bowel sounds. There was severe generalized tenderness but no peritoneal signs and no masses. Rectal examination revealed an empty rectal vault and a firm, moderately enlarged and non-tender prostate gland. Four attempts were made at bladder catheterization but the catheter failed to pass through the urethra. Bladder ultrasound showed an empty bladder. Laboratory tests included WBC 12.2 K, BUN 38, creatinine 5.2 (baseline 0.9), and normal serum electrolytes. Urinalysis showed 0–3 WBC, 5–10 RBC, and few bacteria. Plain abdominal X-ray demonstrated a dilated transverse colon with absence of gas in the distal colon. A non-contrast CT scan showed inflammatory changes around the mesentery and bladder; gastrograffin enema and renal ultrasound were normal. CT scan of the abdomen and pelvis was repeated with IV contrast, and demonstrated communication in the superior aspect of the bladder with a contrast-filled collection in the extraperitoneal space, consistent with a bladder leak. A cystogram was obtained, which demonstrated bladder perforation along with trabeculation and diverticuli, consistent with chronic bladder outlet obstruction. These findings were confirmed by cystoscopy. The patient was managed conservatively with bladder catheterization and had rapid resolution of his symptoms and renal insufficiency.
DISCUSSION
BPH is a common condition, although serious complications are unusual. Chronic bladder outlet obstruction, however, may occasionally cause urinary retention, bladder diverticuli, and, rarely, spontaneous bladder perforation, which presents with progressive abdominal pain. Intraperitoneal rupture may cause peritonitis; in this particular patient, however, the perforation was extraperitoneal, and thus there were no signs of peritoneal inflammation. The case illustrates that although bladder perforation presents dramatically, affected patients may have minimal prior symptoms of bladder outlet obstruction; therefore, clinicians should be aware of this potential complication.
A PAIN IN THE BUTT
H. Segrest1; J. Brice1. 1Tulane Health Sciences Center, New Orleans, LA. (Tracking ID #117509)
LEARNING OBJECTIVES
1. Recognize the criteria for diagnosing endocarditis. 2. Recognize the surgical indications in endocarditis
CASE
A 29-year-old man presented with one week of a right buttock lesion and fevers. He denied any past medical history or IV drug use. His temperature was 37°C, pulse 113 bt/min, respirations 32 breaths/min, blood pressure of 83/59 mmHg. With the exception of the lesion, his exam was normal. His white count was 33,000 with 40% bands. He was taken to the operating room for incision and drainage of the abscess and started on vancomycin and piperacillin. The wound culture grew methacillin-resistant staph aureus. On the third hospital day, he was noted to have Janeway lesions, a new murmur, and chest X-ray with fluffy infiltrates. Gentamicin was added; an echocardiogram revealed a small vegetation on the mitral valve. Blood cultures were positive for methacillin-resistant staph aureus and remained positive despite antibiotics. CT surgery was consulted, but declined to operate given his positive cultures. On the eighth hospital night he complained of headache and blurred vision. A CT of the head was performed. By the end of the CT scan, his Glasgow Coma scale had decreased to 4. The CT scan revealed a large occipital intra-cranial hemorrhage. He was pronounced brain dead the following morning.
DISCUSSION
While seemingly commonplace in urban hospitals, acute endocarditis carries a high morbidity and mortality. The indications for sugery in endocarditis include acute aortic or mitral regurgitation with heart failure, fungal infection, acute aortic regurgitation with tachycardia and early closure of the mitral valve on echocardiogram, and evidence of annular or aortic abscess. In these situations, surgery should not be delayed due to persistently positive blood cultures. One of the roles of the general internist is to advocate for the patients who cannot advocate for themselves. Internists should be fully aware of the indications for surgery in the setting of endocarditis to permit this advocacy where indicated.
A PAIN IN THE NECK
J.A. Kasher1; P.P. Balingit1; A. El-Bialy1; J. Wheat1. 1UCLA San Fernando Valley Program, Sylmar, CA. (Tracking ID #115816)
LEARNING OBJECTIVES
1. Appreciate the multiple possible clinical manifestations of aortic dissection.
CASE
A 60-year-old male with history of hypertension presented with complaint of neck discomfort and increasing shortness of breath for 2 weeks. Initially, he felt a sudden “severe, pressure sensation” in his jaw which radiated to his chest, neck, and back of the head. In the next few days, the pain localized to the neck and he also developed progressive dyspnea and orthopnea. On examination heart rate was 96, blood pressure was 171/65, and breating rate was 30. Lungs had bibasilar rales, and heart had a diastolic murmur best heard over the aortic area and S3 gallop. Distal extremities revealed normal, equal peripheral pulses and moderate edema. Serum electrolytes, CBC, and EKG were not significant. Troponin level was 1.1, but normalized later. Chest radiograph demonstrated cardiomegaly, an unfolded aorta, bilateral pleural effusions, and pulmonary vascular congestion. Subsequent trans-thoracic and trans-esophageal echocardiograms revealed a type A aortic dissection involving both the ascending and descending aorta and causing significant aortic regurgitation. The patient underwent emergent repair of aortic dissection and was discharged shortly thereafter in good condition.
DISCUSSION
Aortic dissection is a relatively uncommon but catastrophic illness classically thought to present with sudden-onset, unrelenting, tearing pain localized in the thorax and radiating posteriorly. However, clinical presentations are variable, and dependent on which areas of the aorta are involved. For instance, involvement of the coronaries could lead to acute MI. Patient could also present with severe aortic insufficiency, heart failure, cardiac tamponade. Involvement of the carotid or renal arteries may lead to cerebrovascular accident or acute renal failure, respectively. Peripheral vascular involvement may result in pulse and neurologic deficits, whereas abdominal pain may develop with involvement of the mesenteric arteries. Type A dissection can present as severe chest pain (79%), back pain (46%), abdominal pain (22%), syncope (13%), CHF (9%), or cerebrovascular accident (6%). In one case series, only 64% of patients described their pain as being sharp. Another series reports that the treating clinician fails to initially entertain the diagnosis of aortic dissection in up to 35% of cases. Many patients later found to have aortic dissection are initially suspected to have other conditions, such as acute coronary syndrome, non-dissecting aneurysms, pericarditis, pulmonary embolism, aortic stenosis, or even cholecystitis. The high mortality associated with aortic dissection makes its early diagnosis critical. This case serves as a cautionary tale for the clinician to recognize the often “unusual” presentations of this deadly disease, and to consider the diagnosis of aortic dissection in any patient presenting acutely with chest or abdominal pain.
A PUZZLING CASE OF HYPOGYLECEMIA: THE CLUE IN THE MEDICATION HISTORY
S. Estes1; M. Panda1. 1University of Tennessee, Chattanooga, Chattanooga, TN. (Tracking ID #115171)
LEARNING OBJECTIVES
1. Recognize the importance of taking a detailed history. 2. Recognize the interactions of herbal medications with prescription drugs.
CASE
A 30 year old white female with well controlled insulin dependent diabetes mellitus for thirteen years presented with 2 months of numerous hypoglycemic episodes. A decrease in her insulin regimen did not resolve the hypoglycemia. On further review of the patient's medications, the only new addition was ginseng, which she began taking 2 months ago for “increased energy”. Complete work-up including renal function was normal. The ginseng was discontinued and her hypoglycemia resolved. She was able to resume her previous insulin regimen.
DISCUSSION
Herbal therapy is an ancient practice that appears to be experiencing resurgence in the U.S. In numerous previous studies, the ginseng glycopeptides (GGP) from the roots of Panax ginseng had hypoglycemic activity on both normal and hyperglycemic animals. Studies in diabetic humans have also suggested that ginseng lowers blood glucose. The hypoglycemia is due to the enhancement of aerobic glycolysis. The administration of GGP decreases both the level of plasma lactic acid and the activities of plasma and liver LDH while enhancing the rate limiting enzymes in aerobic glycolysis (tricarboxylic acid cycle). The hypoglycemic action of GGP could last up to 16 hours. This case reflects the increasing frequency of herbal and alternative medication use and supports the fact that patients often neglect to tell their physicians. Direct inquiry about herbal medication use should be a routine part of history taking.
A RARE CASE OF INTRATHORACIC ECTOPIC GOITER
Q. Saleheen1; H.J. Freidman1; O. Marzouki1; S. Nizar1. 1Saint Francis Hospital, Evanston, IL. (Tracking ID #116561)
LEARNING OBJECTIVES
1. To emphasize the need to consider ectopic goiter in the differential of a mediastinal mass. 2. To understand that a mediastinal mass with hemorrhagic changes can cause acute stridor. 3. To think about mediastinal mass as a cause of cough especially when cough is positional.
CASE
A 38-year-old African American woman presented with a history of dry cough for 3 weeks and shortness of breath with a loud noisy breathing for 1 day. Patient also complained of generalized fatigue but no fever, no phlegm or weight loss. Patient is a non-smoker and works as a construction worker. On examination, patient vital signs were stable with 98% O2 saturation on room air with audible stridor. There was fullness in the lower anterior neck but no well-demarcated mass, no lymphadenopathy or thyroid enlargement, and no audible bruit in the neck was appreciated. The rest of examination was unremarkable. Laboratory workup showed microcytic hypochromic anemia with Hemoglobin of 7.4. TSH, FT4 and T3 were normal. Chest x-ray revealed a superior mediastinal mass with deviation of trachea towards the right side. A subsequent CT scan showed a 7 cm mediastinal mass with inhomogeneous enhancement extending from anterior to middle mediastinum with no lymphadenopathy. Patient underwent surgical resection with removal of a cystic mass arising from the chest beneath, but separate from the left inferior lobe of thyroid. Both lobes of the thyroid gland appeared normal. A preliminary post-operative diagnosis of bronchogenic cyst with tracheal compression was made. The final diagnosis was made on biopsy, which showed benign nodular thyroid tissue with involution and hemorrhagic changes. Patient subsequently discharged home without complications.
DISCUSSION
We present here a rare case of an ectopic intrathoracic goiter (a goiter with no attachment to the cervical thyroid gland). Most of the ectopic goiters are reported in the neck but rarely in the mediastinum. On review of the literature, there were sporadic cases reported as ectopic intrathoracic goiters. A study in Germany of 61 surgically treated intra-thoracic goiters from 1980 to 1999 showed that only 2 cases were ectopic. When present as a mediastinal mass the ectopic goiter can cause compression symptoms in about 40%–50% of the cases. A mediastinal goiter can cause stridor that can be gradual in onset or sudden if there are hemorrhagic changes in goiter (as in our patient). Also a mediastinal goiter can cause cough that can be positional in character. Thus it is important to consider ectopic goiter in the differential of a mediastinal mass with the evidence of airway obstruction.
A RARE CAUSE OF CIRRHOSIS IN AN EPILEPTIC
M.A. Kalpakian1; S. Dea2. 1UCLA San Fernando Valley Program, Sylmar, CA; 2University of California, Los Angeles, Sylmar, CA. (Tracking ID #115614)
LEARNING OBJECTIVES
1. Diagnose the etiology of cirrhosis when the cause is not obvious. 2. Review monitoring tests for patients on antiepileptic medications.
CASE
A 31-year-old male with a history of epilepsy for over ten years presented with complaints of nausea, vomiting, and dull left upper quadrant pain developing over the past two months. His generalized tonic-clonic seizures and absence seizures have been controlled with carbamazepine for ten years and valproic acid for three years. The patient did not have diabetes or hyperlipidemia. On review of symptoms, he complained of decreased appetite with 20 lb weight loss over the past year. The patient denied tobacco, alcohol, or drug abuse. Physical exam was significant for a slender afebrile male with tenderness to palpation in the left upper quadrant and hepatosplenomegaly. There was no rebound or guarding. The patient was not jaundiced and did not have stigmata of chronic liver disease. Labs were significant for an ALT of 104, AST of 79, alkaline phosphatase 229, INR 1.04, WBC of 3.2, Hb 12.5, Hct 37.4 and platelet count of 87. Pancytopenia was thought to be a result of massive splenomegaly. An abdominal ultrasound showed a liver span of 18.2 cm and the spleen was 15.2 cm × 15.3 cm with a patent portal vein with appropriate flow. Liver biopsy revealed cirrhosis. Hepatitis B and C serologies were all negative. Antinuclear antibody, anti-smooth muscle antibody, and antimitochondrial antibody were negative. Iron, ferritin, iron saturation and ceruloplasmin levels were normal. A comprehensive literature search showed no reported cases of carbamazepine-induced cirrhosis and a few case reports of valproic acid associated with cirrhosis. Carbamazepine and valproic acid were stopped and the patient was started on levetiracetam.
DISCUSSION
Many drugs are hepatically metabolized but drugs that induce cirrhosis are relatively rare. Valproic acid is one of these drugs and is being used by internists for many indications ranging from migraine headache prophylaxis to seizure disorders. Routine monitoring of transaminases in patients on anti-epileptics is still controversial. Some clinicians argue that since hepatic failure caused by valproic acid is an acute idiosyncratic reaction, checking transaminases in patients who have been on valproic acid for years may not prevent liver failure. However, as this case illustrates, chronic liver damage and cirrhosis can result from chronic anti-epileptic drug use. A toxic metabolite of valproic acid may be responsible for inducing non-alcoholic fatty liver disease that may progress to cirrhosis. Perhaps monitoring transaminases every 6 months in these patients could have diagnosed liver toxicity prior to the onset of cirrhosis. Monitoring may have allowed this patient to be switched to another anti-epileptic drug earlier, preventing him from developing end-stage liver disease.
A RATHER SIGNIFICANT EOSINOPHILIA
S.Y. Chien1; A.M. Fogelman2. 1University of California, Los Angeles, Sylmar, CA; 2University of California, Los Angeles, Los Angeles, CA. (Tracking ID #115722)
LEARNING OBJECTIVES
1. Recognize the differential diagnosis and clinical aspects of eosinophilia. 2. Distinguish between different vasculitides.
CASE
A 43 year-old female with complicated medical history was transferred to our hospital for six months of bilateral neck swelling and recent right-sided weakness. She reported several other conditions that had begun in the previous 2 years including alopecia, allergy, chronic otitis media, and whole body pruritis. She was now complaining of a new cough, dyspnea, and exertional chest pain. Previous diagnostic work-ups found pulmonary infiltrates, bilateral internal carotid artery aneurysms, and confirmed a recent stroke. Her initial CBC was particularly signifcant for a WBC of 11,000 with 50% eosinophils on the differential. ESR was negative. A work up of this appreciable eosinophilia ensued, with normal infectious cultures and rheumatologic tests (ANA and ANCA). Because of her history of angina, a cardiac nuclear stress perfusion scan was done, showing multiple defects with a depressed ejection fraction. Subsequent cardiac catheterization demonstrated no significant atherosclerosis, but found aneurysmal dilatation of all three main coronary arteries. Based on her carotid and coronary aneurysms and significant eosinophilia, our patient was diagnosed with a vasculitis, most likely Churg-Strauss syndrome or Takayasu's.
DISCUSSION
Eosinophilia is defined as >500 per microliter in the blood or tissue. Patients may have multiple end-organ dysfunction, leading to thrombosis and fibrosis. Besides parasitic or helminthic infections, other common causes are allergies, collagen vascular diseases, and malignancies. However, the etiology of this patient's eosinophilia was due to vasculitides, likely ANCA-associated small to medium-vessel disease (Churg-Strauss syndrome, microscopic polyangitis, or Wegener's granulomatosis) versus large-vessel disease (Takayasu's). It is important to realize that approximately 10% of patients with ANCA-associated vasculitis have negative assays for ANCA. Often, there is a substantial overlap among different vasculitides, such as in this patient. Churg-Strauss syndrome has a characteristic triad: allergic rhinitis and asthma, systemic granulomatous inflammation of small vessel, and virtually all patients have eosinophilia. Ofteh, it has less renal involvement. However, coronary arteritis and myocarditis are very frequent, accounting for major morbidity and mortality. On the other hand, Takayasu's arteritis (aortic arch syndrome) has a strong predilection for the aortic arch and its branches. Pulses are commonly absent, particularly if subclavian artery is involved. Although less common, inflammation can also be found in other major arteries, including carotid and coronary. The mainstay of treatment for most vasculitides includes corticosteroids with or without cytotoxic drugs such as cyclophosphamide. Combined therapy induces improvement in 90% and complete remission in 75% of patients.
A RED EYE AND VISION LOSS: NOT YOUR USUAL CONJUNCTIVITIS
T. Pestana1; M. Landry1. 1Tulane Health Sciences Center, New Orleans, LA. (Tracking ID #117498)
LEARNING OBJECTIVES
1) Identify ocular manifestations of fungal infections. 2) Recognize risk factors for fungal ocular infections. 3) Establish available treatments for fungal ocular infections.
CASE
A 25 year-old woman presented with left eye pain and vision loss. She noted eye injection and pain with movement. She also complained of lower back pain. She had fever, chills, night sweats, and a recent fifty-pound weight loss. Her past medical history included hepatitis C and intravenous drug abuse. Her needle-sharing companions had developed similar ocular symptoms several weeks prior. She was afebrile, and had conjunctival, scleral, and limbal injection with a hazy cornea, and clear ocular discharge. The left pupil was fixed at four millimeters; visual acuity was 20/200. Direct fundoscopic exam revealed fluffy white vitreous opacities obscuring the optic disc, and retinal detachment. The L1–L2 right paraspinal region was tender to palpation. MRI of the spine showed discitis, osteomyelitis, and a psoas abscess in the paraspinal region. The patient was diagnosed with retinal detachment and fungal endophthalmitis. Intravitreal and systemic amphotericin B were initiated. Cultures obtained from the psoas abscess yielded Candida albicans, confirming disseminated fungal infection.
DISCUSSION
Fungal endophthalmitis is a serious infection that can lead to visual deficits. Risk factors include intravenous drug abuse, immunosuppression, parenteral nutrition, and ophthalmologic surgery. There is no consensus on standard antifungal treatment, but amphotericin B, flucytosine, and fluconazole may be used. Vitrectomy may also be considered for vision salvage. Early diagnosis, ophthalmologic evaluation, and treatment are essential for preventing vision loss.
A RESTAURANT, AN ONION, A LIVER: A CASE OF FULMINANT HEPATIC FAILURE FROM HEPATITIS A
P.K. Nair1; B.S. Berk1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115944)
LEARNING OBJECTIVES
1) Recognize the epidemiology of Hepatitis A (HA), 2) Diagnose Fulminant Hepatic Failure (FHF), 3) Manage FHF in HA.
CASE
A 57-year-old male who is on prednisone for ulcerative colitis presented with elevated liver enzymes and change in mental status. He is a non-smoker who drinks about two beers per week. On October 11th, he and his wife ate at a local restaurant. On October 29th, they both developed anorexia, myalgia, and diaphoresis. The patient's wife improved, but he continued to deteriorate. On November 5th, he went to his community hospital. His labs showed the following: ALT 6438, AST 8689, INR 3.0, and Total Bili 2.5. The next day he became poorly responsive and was transferred to our MICU. He was deeply jaundiced, had brisk reflexes, and right ankle clonus. Intracranial pressure was elevated. No stigmata of chronic liver failure was noted. Labs revealed the following: glucose 50, lactate 5, ammonia 101, ferritin >5,000, ALT 3624, AST 1108, Total Bili 11.3, PTT >100, and non-calculable PT/INR. He was promptly transferred to the Liver Transplant ICU. Hepatitis serologies were negative for HepB, HepC, CMV, EBV, HSV, VCZ, Wilson's, and autoimmune markers. HepA IgM was positive. The next morning he underwent orthotopic liver transplantation. He was discharged one month later in stable condition.
DISCUSSION
There were 650 confirmed cases of HA from a recent outbreak in western Pennsylvania. It was linked to a restaurant that served tainted green onions from Mexico. Four people developed FHF, and only this patient survived. In the United States, there has been a gradual decline in the number of confirmed HA cases from 1980 (29,087 reported cases) to 2001 (10,616 reported cases). On average only 0.2% to 0.4% of cases of HA progress to FHF. The risk of developing FHF from HA infection increases with advancing age and in patients with chronic liver disease, especially from hepatitis C. In FHF from HA, there is a rapid progression of severe acute liver injury with impaired synthetic function as evidenced by decreasing AST/ALT and increasing INR. Stigmata of chronic liver disease are absent. To be classified as having FHF one must have encephalopathy develop within eight weeks of onset of symptoms if they had a previously healthy liver, or within two weeks of onset of jaundice regardless of any underlying liver disease. Management of FHF from HA should involve early referral to a liver transplant center of high-risk patients. Transplantation improves survival rates in FHF by as much as 70% to 80%. Based on the King's College Criteria, referral for transplant of FHF from HA should take place if the following criteria are met: 1. PT >100, irrespective of the grade of encephalopathy, or 2. Any three of the following variables: age <10 or >40, duration of jaundice before onset of encephalopathy >7 days, PT >50, or Total Bili >18.
A SEVERE CASE OF ALCOHOLIC HEPATITIS
J.E. Guy1. 1University of California, San Francisco, San Francisco, CA. (Tracking ID #117466)
LEARNING OBJECTIVES
1. Review the differential diagnosis of right upper quadrant abdominal pain. 2. Recognize laboratory findings seen in alcoholic hepatitis. 3. Discuss treatment options for severe cases.
CASE
A 45 year old woman with a 23 year history of significant alcohol abuse presents to the emergency room with a several week history of nausea, nonbloody emesis, constant, nonradiating right upper quadrant pain, and jaundice. She reports intermittent subjective fevers. Her last drink was one day prior to admission, and she reports drinking 9 shots of brandy a week. On physical exam she was afebrile. She was somnolent but arousable. Asterixis was present. Her abdominal exam was distended and diffusely tender to palpation, with marked right upper quadrant pain without rebound. Her liver span was enlarged to 12 cm but there was no fluid wave or frank ascites. Skin exam was markedly jaundice but without spider angiomata or palmar erythema. Pertinent laboratory data included a WBC of 41.5K, total bilirubin 17.6, AST 132, ALT 14, albumin 1.7, Cr 0.9, and PT 21.7. A CXR, urinalysis and blood cultures were negative. A right upper quadrant US did not reveal signs of cholecystitis, obstruction or ascites. An abdominal/pelvic CT was without evidence of cholangitis or masses. Of note, the patient was admitted to the surgical service three weeks prior to admission with similar complaints, a WBC of 14.8K, AST 130, ALT 20, and total bilirubin 4.6. At that time a RUQ ultrasound, HIDA scan, abdominal CT, white blood cell scan, urine and blood cultures were within normal limits. The patient was diagnosed with alcoholic hepatitis and told to abstain from drinking.
DISCUSSION
This patient's clinical picture was consistent with severe alcoholic hepatitis. On two occasions an in-depth evaluation was undertaken to evaluate for cholecystitis, cholangitis, obstructing lesion, systemic infection or other explanations of right upper quadrant pain, abnormal liver function tests and leukocytosis. As this case underscores, alcoholic hepatitis manifests as an inflammatory state of the liver and can be confused for infection or obstruction. Patients often present with right upper quadrant pain, hepatomegaly, jaundice and fever. Hepatic encephalopathy is a poor prognostic indicator. The mortality of the disease is high, and can be stratified by calculating the discriminant function [formula 4.6 (PT-control) + tbili]. In this patient with a discriminant function (DF) of greater than 32, her mortality at one month is 50%. A small mortality benefit at two and six months has been demonstrated with corticosteroids and at four weeks with pentoxifylline in patients with DF >32 and no evidence of infection or bleeding. This patient received pentoxifylline without significant change in her laboratory or clinical parameters after 4 weeks of treatment. She did survive the acute period, and in follow-up six months later, the patient had abstained from drinking with improvement in her physical exam and laboratory values.
A SURPRISING CAUSE OF ACUTE-ONSET ALTERED MENTAL STATUS AND HYPOXIA IN A PREVIOUSLY STABLE PATIENT
M.R. Heller1. 1University of California, San Francisco, San Francisco, CA. (Tracking ID #115003)
LEARNING OBJECTIVES
1) Recognize air embolus as a possible cause of respiratory distress and altered mental status in hospitalized patients. 2) Become familiar with preventative measures and treatment options.
CASE
A 58 year old man with AML was admitted to the hospital for consolidation chemotherapy. Three weeks into his hospital stay, he was found on the ground in the doorway to his room, confused and unable to get up. The patient was oriented only to person, but did not complain of any localized pain or difficulty breathing. On physical exam, his vitals were stable with the exception of his O2 saturation, which was 84% on room air, but increased to 97% on 10L face mask. He had no obvious signs of trauma, except that the cap to one of the lumens of his central line was off. The remainder of his cardiopulmonary and neurological exam was within normal limits, to the extent that he could cooperate. Labs were unremarkable. A head CT was negative, and a chest x-ray and CT did not show signs of new infection, pneumothorax, or pulmonary embolus. By the following morning, the patient's altered mental status and hypoxia had resolved. His symptoms were ascribed to an air embolus, which likely occurred during a normal tidal volume inhalation after the cap to his central line became disconnected.
DISCUSSION
Air embolus (AE) is most commonly associated with surgical procedures, penetrating chest injuries, barotrauma, and central venous catheterization. Slow infusions of small amounts of air are tolerated better than large rapid boluses —it is thought that 300–500 cc of air infused over a few seconds can be fatal in humans. Dyspnea is found in almost all cases of AE, and may be accompanied by tachypnea, respiratory failure, chest pain, hypotension, and tachycardia. Neurological findings are present in up to 40% of cases, and range from dizziness and a subjective “sense of doom” to altered mental status or focal neurological deficits. Diagnosis of AE can be difficult, as labs, chest x-rays, VQ scans, and chest CTs are most often normal. Therefore, a high index of suspicion is required in a patient undergoing a surgical procedure or with central venous access. Treatment focuses on identifying the source of air entry and preventing further air flow. Hyperbaric oxygen may be helpful in extreme cases to reduce the intravascular air bubble size. Furthermore, positioning the patient in the left lateral decubitus position may help to prevent an air bubble in the right ventricle from obstructing the pulmonary outflow tract. Finally, aspiration through the central venous catheter may be attempted if the introduction of the AE is witnessed. Emphasis should be placed on prevention —patients should be in Trendelenburg position and they should be instructed to valsalva or exhale during the placement or removal of a central venous catheter.
A THORNY PROBLEM: MYCOBACTERIUM KANSASII INFECTION OF THE SKIN
K. Pachipala1; S. Naidu1; L. Adhikesavan1; R. Gotoff1; D.R. Gutknecht1. 1Geisinger Medical Center, Danville, PA. (Tracking ID #103207)
LEARNING OBJECTIVES
Appreciate that M.kansasii can cause water-borne skin infections.
CASE
A 43-year-old woman developed erythema and swelling over a PIP joint of her left hand three months after cutting that finger on a thorn. Pus was aspirated but no bacteria were found on gram stain or culture. Subsequent biopsy showed a granulomatous reaction and cultures grew pigmented mycobacteria. The patient had a history of exposure to both an inground swimming pool and a hot tub and was empirically treated with Bactrim for presumed infection with M.marinum, since that agent is the commonest pigmented mycobacterium causing skin infections. The organism was later identified as M.kansasii and the patient was given INH, rifampin and ethambutol. INH and rifampin could not be continued because of hepatotoxicity, and after consultation with a national expert, an alternative treatment regimen of clarithromycin, gatifloxacin and ethambutol was instituted and the patient improved.
DISCUSSION
M.kansasii is a slow growing, photochromogenic mycobacterium found in potable water supplies, swimming pools and sewage. Cutaneous M.kansasii infections are rare, with 44 cases so far reported in the literature. These infections are sporadic and usually due to inoculation following minimal cutaneous trauma. Most occur in patients with immunological problems, and patients may present with papules, nodules, pustules, crusted ulcers, cellulitis or sporotrichial lesions. The American Thoracic Society recommends treatment with INH, rifampin and ethambutol. In our patient the history suggested both thorn injury and water exposure as possible vectors of infection. Thorn prick is usually associated with infections with gram-negative bacteria, clostridial species or sporotrichosis. Bacteria associated with water exposure include Aeromonas sps, Edwardsiella tarda, Erysipelothrix rhusiopathiae, Vibrio vulnificus and Mycobacterium marinum. The surprise finding of M.kansasii confirmed this was a water-borne infection, and an unusual one at that.
A VERY RARE PRESENTATION OF A COMMON ILLNESS, INFLUENZA A INFECTION WITH A MASSIVE PERICARDIAL EFFUSION
S.G. Khurshid1; U. Ahmed2; P. Sherchan3. 1Saint Francis Hospital at Evanston, Evanston, IL; 2Saint Francis Hospital, Evanston, IL; 3Saint Fancis Hospital at Evanton, evanston, IL. (Tracking ID #115934)
LEARNING OBJECTIVES
To recognize an unusual complication of Influenza A virus.
CASE
21-year-old previously healthy male presented with seven days history of pleuritic chest pain and fever. Chest pain was persistent and worsened with deep breathing and lying down. Patient also described a recent upper respiratory illness associated with fever, chills and myalgias. Physical examination revealed a temperature of 100.5 F, pulse rate of 111, BP of 105/65 and respiratory rate of 18. The heart sounds were distant with a prominent pericardial rub. Decreased breath sounds and dullness were present at the left lung base. No rales were audible, liver was not enlarged and there was no peripheral edema. No jugular venous pulsation was visible and pulses paradoxus could not be detected. Lab workup revealed WBC count of 11.4 with 40 percent lymphocytes. Serological screening for viruses including CMV, Coxsackie, EBV, VZV, HIV and Echovirus was negative. Rapid influenza antigen detection from nasophyrengeal swab was positive for influenza A virus. EKG revealed sinus tachycardia with no evidence of electrical alternans. CXR showed cardiomegaly with bilateral pleural effusions. CT scan of the chest showed a fluid collection in the pericardium with abnormal enhancement of the pericardial lining indicating pericardititis. Echocardiogram revealed massive pericardial effusion with no evidence of cardiac tamponade. Patient improved with NSAIDs, oseltamivir and fluid resuscitation.
DISCUSSION
Influenza A and B infections are recognized causes of pericarditis. Viral pericarditis may be associated with myocarditis and in this case, the pericardial inflammation was sufficient to cause a massive effusion. Myocarditis and pericarditis were reported in association with influenza viral infection during the 1918–1919 pandemic; these reports were largely based on histopathologic findings, and these complications have been reported only infrequently since then. To our knowledge only a few cases of a massive pericardial effusion causing tamponade secondary to influenza have been reported but all those patients had underlying cardiac disease. This case appears to be unique in that the presence of massive pericardial effusion did not cause clinical or echocardiographic evidence of tamponade. We attribute this to the young age and normal underlying myocardium.
ACQUIRED FACTOR VIII INHIBITOR PRESENTING AS REFRACTORY GI BLEEDING
R.R. Yeldandi1; M. Peek1. 1Rush University/Rush- Presbyterian-St. Luke's Medical Center, Chicago, IL. (Tracking ID #116937)
LEARNING OBJECTIVES
(1) Recognize underlying coagulopathies as etiologies of refractory GI bleeding. (2) Understand the treatment options for acquired factor VIII inhibitors.
CASE
The patient is a 49 year old woman with a history of hypertension, coronary artery disease, and CHF who presented after two episodes of hematemesis; she denied hematochezia or melena. She had no history of peptic ulcer disease, dyspepsia or NSAIDs use. Nasogastric lavage yielded 500 ccs of coffee grounds and her stool was heme positive. An emergent EGD revealed a gastric ulcer whose actively bleeding vessel was coagulated with electrocautery. She was started on Protonix. Three days later, the patient had hematochezia and hematemesis. Repeat EGDs showed an adherent clot but no active bleeding. H. pylori titers and a colonoscopy were negative. With no other source of active bleeding identified, the gastric ulcer was surgically resected, but the patient had persistent post-operative bleeding. On admission, the patient had a prolonged aPTT (>240 sec) which was previously normal; aPTT mixing studies were positive for a coagulation factor inhibitor. Factor VIII inhibitor levels were elevated, thus diagnosing the patient with an acquired factor VIII inhibitor coagulopathy. The patient was treated with Factor Eight Inhibitor Bypass Activator (FEIBA), recombinant activated factor VIIa, steroids and Imuran. The patient was discharged in stable condition.
DISCUSSION
Factor VIII inhibitor is an autoantibody directed against coagulation factor VIII. Patients can present with hematomas, hematuria, GI bleeding, retropharyngeal or retroperitoneal bleeding, cerebral hemorrhages and/or post-operative bleeding. Recombinant factor VIIa infusions can activate the coagulation cascade by bypassing factor VIII and control acute bleeding. Maintenance therapy with immunosupressants can lower inhibitor titers. Our patient's acute bleeding resolved with infusions of FEIBA and recombinant factor VIIa. Although she presented with a common clinical manifestation of factor VIII inhibitor, the etiology of her uncontrolled bleeding was uncommon. Our patient had a clear source of GI bleeding, but she did not respond to standard treatment. Without her underlying coagulopathy, her bleeding may have ceased with cautery. We present this case in order to bring attention to possible uncommon etiologies for common scenarios. Failure of conventional therapy for GI bleeding should prompt further evaluation for an underlying coagulopathy. Early identification of acquired coagulopathies and treatment with effective agents can reduce excessive blood loss and reduce mortality.
ACTINOMYCOSIS PRESENTING AS A SKIN ABSCESS
J. Blank1; M. Traina1. 1UCLA— San Fernando Valley Program, Sylmar, CA. (Tracking ID #116705)
LEARNING OBJECTIVES
1) Recognize Actinomyces as a cause of skin abscess. 2) Discuss the diagnosis and management of disseminated Actinomycosis.
CASE
A 46 year old male presented to the clinic with a three month history of left shoulder pain and progressive loss of range of motion. The patient noticed a lump on his shoulder 6 weeks ago that has been increasing in size. He admits to a productive cough, dyspnea on exertion, and fatigue. The patient smokes 2 packs per day, drinks 6 beers per day, and works as a repairman, often underneath houses. Physical examination revealed a fluctuant 13 × 13 cm mass over the left scapula, poor dentition, and rhonchi over the left lung base without evidence of lymphadenopathy. Significant laboratory results included a WBC 12.9, Hemoglobin 7.8, and Folate 2.7. Chest x-ray showed a nodule in the left lower lobe. CT scan of the chest revealed a 3 cm irregular soft tissue mass in the left lower lobe with apparent sinus tracts extending to the back. Fine needle aspiration of the shoulder mass was completed and Gram stain demonstrated “sulfur granules” consistent with Actinomyces infection. After surgical drainage of the abscess, intravenous ampicillin-sulbactam was initiated for 4 weeks followed by oral penicillin. Repeat CT scan after 4 weeks of treatment revealed a decreased lung mass size.
DISCUSSION
Actinomycosis in humans is commonly caused by Actinomyces israelii. It frequently occurs in adult males with dental infections but also can occur in patients with diabetes, immunosuppression, malnutrition and local tissue damage with neoplastic disease or irradiation. Actinomycosis generally arises from a dental source but in rare cases it can originate from the thyroid gland, thyroidectomy incision sites or lung as seen with this patient. Typically, infection spreads by direct invasion without respect to anatomical barriers including fascial planes, forming multiple abscesses. Pulmonary infections usually arise after aspiration of oropharyngeal or gastrointestinal secretions. The most common pulmonary clinical presentation is an indolent, progressing pneumonia with or without pleural involvement. Patients present with a productive cough, fever, chest pain, and weight loss. Actinomyces is difficult to culture and should be suspected when “sulfur granules” are seen on visual or microscopic examination. Sulfur granules which are rarely seen with Nocardiosis, are common with Actinomycosis and have a characteristic cauliflower-like appearance upon microscopic examination. Abscesses require surgical drainage and the infection is highly responsive to antibiotic treatment with long term penicillin.
ACUTE ONSET GENERALIZED LYMPHADENOPATHY IN A 58 YO MALE
B.E. Gewurz1; J. Beach2. 1Beth Israel Deaconess Medical Center, Boston, MA; 2Beth Israel Deaconess Medical Center, Brookline, MA. (Tracking ID #115887)
LEARNING OBJECTIVES
Distinguish between local and generalized adenopathy. Diagnose etiology of generalized lymphadenopathy. Recognize when a lymph node biopsy is indicated.
CASE
Mr. JW is a 58 yo male with no significant PMHx who presented to an urgent care visit with a chief complaint of “bumps” on his neck of several days duration. He denied associated symptoms, including fevers, chills, night sweats, cough, myalgia or arthralgia. He denied any recent travel, sick contacts, or unusual exposures at the supermarket that he manages. He takes no medications. He has been married for 30 years, has a remote smoking history, and denied IV drug use. He has a pet cat. Physical examination was notable for numerous 1–3 cm nontender, firm, mobile lymph nodes in the preauricular, postauricular, occipital, anterior and posterior cervical, axillary and inguinal chains. There was no hepatosplenomegaly, rashes, or mucosal lesions. An extensive work-up did not reveal likely infectious causes of adenopathy: PPD was negative and serologic tests failed to show acute infection by HIV-1, CMV, EBV, toxoplasma, syphilis, borrelia, or bartonella. No HIV RNA was detected. ESR was 16 mm/hr. No acute cardiopulmonary process was evident on chest film. CBC revealed WBC of 5,000 cells/uL, HCT of 40.8%, and PLT of 282,000 cells/uL. Regressing adenopathy was noted at a subsequent visit, with interval onset of night sweats, low grade fever, 5-lb weight loss over two weeks, fatigue, persistent dry cough and generalized pruritis. A chest CT scan revealed striking systemic adenopathy of all major nodal groups. Submandibular biopsy revealed features consistent with angioimmunoblastic T-cell lymphoma (AILD). Bone marrow biopsy revealed AILD involvement.
DISCUSSION
Peripheral lymphadenopathy may be the only sign of an underlying systemic process. We review the importance of recognizing regional versus generalized adenopathy, their differential diagnoses, and studies that should be considered. Generalized adenopathy signifies a serious associated condition and that requires further evaluation. When should a lymph node biopsy be obtained? Although many patients with adenopathy fear a diagnosis of cancer, adenopathy often results from an infectious illness and only rarely represents malignancy in the primary care setting. The need for definitive diagnosis should be weighed against the morbidity of an invasive procedure. We discuss circumstances where an observation period is a safe alternative and review algorithms that predict the appropriateness of lymph node biopsy.
ACUTE RENAL FAILURE CAUSED BY A RARE BLEEDING COMPLICATION OF ENOXAPARIN
M. Bandara1; B.P. Sankarapandian1; S.K. Thambidorai1; S. Dhanireddy2; D. Schuller1. 1Creighton University, Omaha, NE; 2Creighton University Medical School, Omaha, NE. (Tracking ID #115704)
LEARNING OBJECTIVES
1. Recognize the potential for enoxaparin induced intra-abdominal bleed 2. Identify acute renal failure as a potential secondary complication of intra-abdominal bleed 3. Assess causes for post obstructive uropathy.
CASE
A 43-year-old white female with recent history of multiple upper respiratory tract infections presents to the emergency room with dyspnea. She was found to be in atrial fibrillation with rapid ventricular response. The patient was admitted and treated with enoxaparin and diltiazem infusion. She was subsequently cardioverted to normal sinus rhythm using direct current cardioversion. During the hospitalization she developed left lower quadrant abdominal pain with associated oliguria. She rapidly progressed to anuria prior to the obtaining of urine studies. Renal ultrasound showed bi-lateral hydronephrosis. Urology performed a cystoscopy, which showed a compression of the bladder from an external source. Bi-lateral ureteral stents were placed extending from the renal pelvis into the urethra. Post operatively the patient became severely hypotensive requiring multiple transfusions and vasopressor support. Computerized tomography (CT) of the abdomen showed a massive hematoma in the pelvis surrounding and compressing the bladder. The patient remained anuric for several days requiring continuous venous-venous hemodialysis. Her renal function eventually improved and hemodialysis was discontinued.
DISCUSSION
Enoxaparin is a rare cause for intra-abdominal bleed. This patient presented with obstructive uropathy resulting in acute renal failure. The initial cause for the obstruction was not apparent. Evaluation of the renal failure led to the discovery that the patient had extrinsic bladder compression from a massive intra-pelvic hematoma. The severity of the bleeding led to a prolonged state of renal hypoperfusion, which resulted in acute tubular necrosis. In patients where the cause of obstruction is not readily apparent, one must consider other potential factors. Post-obstructive renal failure is commonly due to tumors, prostatic hypertrophy, calculi, and strictures. This case demonstrates that pelvic bleeding should also be included in the differential diagnoses of post-obstructive renal failure in patients receiving anti-coagulation therapy.
ADRENAL INCIDENTALOMA, CUSHING's SYNDROME, AND INSULIN RESISTANCE SYNDROME
M.N. Phan1; N. Mikhail1; M. Rotblatt1. 1UCLA SFVP-Olive View Medical Center Department of Internal Medicine, Sylmar, CA. (Tracking ID #117285)
LEARNING OBJECTIVES
1. Recognize an approach to diagnosing adrenal incidentaloma. 2. Be aware of the possibility of Cushing's syndrome in adult patients with an insulin resistance syndrome.
CASE
A 51 year old male was referred for further evaluation and management of an adrenal incidentaloma found on abdominal CT, which was originally performed to rule out nephrolithiasis. There were no kidney stones, but instead, a 3 cm left adrenal mass was reported. His PMH included hypertension for 6 years and diabetes mellitus for 3 years. The patient did not report easing bruising, headache, sweating, or palpitations. Review of systems was significant for weight gain of 50 lbs over 5 years. Family history was also positive for diabetes and hypertension. Vitals signs were T37, BP 181/90, P115, RR16, and WT 296 lbs. Physical exam was significant for obesity, moon facies, and supraclavicular fat pads, but no buffalo hump or abdominal striae. Cushing's syndrome was suspected, and laboratory investigation revealed an elevated 24-hour urine cortisol of 134.2 ug/24 hrs (4–50), and suppressed ACTH of <5 PG/ML (15–70). Serum renin and aldosterone were normal, as were urine catecholamines. The patient was diagnosed with Cushing's syndrome secondary to an adrenal mass and was subsequently referred for adrenalectomy.
DISCUSSION
The incidental discovery of an adrenal mass is not an uncommon event due to the routine use of common imaging techniques. The prevalence of adrenal incidentaloma is about 2.3 % at autopsy and 0.5–2% by abdominal CT scan. Hyperfunctioning develops in 1.7% of cases, and the risk is higher in patients with lesions larger than 3 cm, of which cortisol hypersecretion is the most likely disorder. Evaluation of an adrenal incidentaloma includes hormone studies to determine whether the patient has pheochromocytoma, glucocorticoid excess, primary aldosteronism, or virilizing or feminizing tumors. This is especially important if signs and symptoms of hormonal excess are present. Cortisol secreting adrenal incidentaloma has been implicated in causing obesity, glucose intolerance/type 2 diabetes, hypertension, and dyslipidemia or the insulin resistance syndrome. Screening for Cushing's syndrome in this patient population, i.e., those with larger masses and/or potential signs or symptoms of hormonal hypersecretion, can be critical. Adrenalectomy should ameliorate insulin resistance as well as the vascular risk profile of these patients.
ADULT PRESENTING WITH A PEDIATRIC DISEASE
M. Bandara1; B.P. Sankarapandian1; S.K. Thambidorai1; S. Dhanireddy2; J. Derby1. 1Creighton University, Omaha, NE; 2Creighton University Medical School, Omaha, NE. (Tracking ID #115703)
LEARNING OBJECTIVES
1. Identify the clinical manifestations of Henoch-Schonlein purpura. 2. Recognize the organ systems affected by the disease. 3. Demonstrate that pediatric diseases can present in adulthood.
CASE
A 21 year old white male with a 4 month history of bloody diarrhea and poly-arthritis presents to an outpatient clinic with pain, stiffness, swelling and purplish discoloration of the right ankle. These skin changes progressed to multiple purpuric lesions over the bilateral lower extremities. Erythrocyte sedimentation rate, urine analysis, rheumatoid factor, human leukocyte antigen B-27 and radiologic studies of the ankles were all normal. Skin puncture biopsy of these lesions showed evidence of leukocytoclastic vasculitis. IgA antigen was positive on the vascular wall confirming the diagnosis of Henoch-Schonlein purpura (HSP). The patient was then started on oral prednisone therapy. He responded well to the steroids, and they were subsequently tapered.
DISCUSSION
HSP is a subtype of Leukocytoclastic vasculitis which affects multiple organ systems. Skin involvement is seen in virtually all patients, manifesting as a palpable purpura in the lower extremities and the buttocks. Polyarthralgias are also a common clinical manifestation. Gastrointestinal symptoms are characterized by colicky abdominal pain, vomiting, diarrhea, and the passage of blood. Renal involvement is seen in almost 80% of the patients and has a nephritic urine sediment and moderate proteinuria. The presence of mesangial IgA on immunofluorescence microscopy is diagnostic. Less than 10% of the patients will progress to chronic renal failure and persistent hypertension. HSP has the highest incidence in children with a median age group of 5 years. Classic skin manifestations occurring early in the disease help diagnose HSP in the pediatric age group. Our case presented with bloody diarrhea demonstrating the need for physicians to be cognizant that HSP may initially manifest in atypical organ systems in adult populations. Steroids are the first line of treatment for this disease.
ALCOHOLIC HEPATITIS—A CAUSE OF FEVER OF UNKNOWN ORIGIN
M. Gaddamanugu1; F. Salahauddin1; M. Aiyer2. 1University of Illinois at Peoria, Peoria, IL; 2University of Illinois at Chicago, Peoria, IL. (Tracking ID #117117)
LEARNING OBJECTIVES
Identify alcoholic hepatitis as one of the causes of fever of unknown origin. Recognize the classic presentation of alcoholic hepatitis Discuss the diagnostic work up of fever in a patient presenting with alcoholic hepatitis.
CASE
A 52-year-old female with significant history of alcohol and IV drug abuse presented with 3-week history of jaundice, fever, and malaise. Past medical history was significant for hypertension and asthma. Review of systems was remarkable only for a 30 lb weight loss. Initial evaluation revealed markedly elevated liver enzymes, and anemia. Blood cultures, urine cultures and chest radiographs were normal. The diagnosis of alcoholic hepatitis was entertained. However patient was persistently febrile even 10 days after hospital admission. Exam revealed a cachetic female with marked scleral icterus. Vital signs showed temp 102.3 F, B.P 98/52, RR 20. Abdominal exam revealed hepatosplenomegaly with minimal ascites. Patient underwent an extensive workup including CT scan of abdomen, pelvis and chest, gallium scan, bone marrow studies and temporal artery biopsy, all of which were normal. Her ANA, HIV and hepatitis panel were negative. Liver biopsy revealed alcoholic steatohepatitis. Over the course of 6 weeks of hospital stay, patient gradually deferveresed with supportive treatment and was discharged in stable condition
DISCUSSION
Malaise, fever, jaundice and tender hepatomegaly represent the classic syndrome of alcoholic hepatitis. However, the full syndrome occurs only in a minority of patients with alcoholic liver disease. In addition, the fever in alcoholic hepatitis is very modest usually less than 101 degree Fahrenheit. High temperatures warrant a work up for alternative diagnosis. This case represents a patient who presented with the classic syndrome of alcoholic hepatitis and persistent high fever all attributed to alcoholic hepatitis. Alcoholic hepatitis can be considered as a cause for fever of unknown origin.
ALTERED MENTAL STATUS AND AN ABDOMINAL MASS
L.B. Chun1; R.V. Liddicoat1. 1Massachusetts General Hospital, Boston, MA. (Tracking ID #115932)
LEARNING OBJECTIVES
1) To recognize the clinical manifestations of unmanaged renal failure 2) To understand the complications of decompressing a massively distended bladder
CASE
A 59 year old male presented with three months of nausea, altered mental status, difficulty urinating, and a 30lb weight loss. Physical exam revealed a pale, cachectic man with slowed speech, fetor uremicus, and a midline 20 × 20 cm, hard, nontender abdominal mass. Labs revealed a hematocrit of 15, potassium 5.0, HCO3 8.6, BUN 243, creatinine 17, phosphorus 8.2 and iCa 0.81, and a reticulocyte count of 2.1. His abdominal CT revealed a markedly distended bladder, severe hydronephrosis, and an enlarged prostate. A foley catheter was placed and 1.7L of blood tinged urine was drained. A renal ultrasound after bladder drainage showed a 10 × 10 cm bladder mass and moderate hydronephrosis with no significant cortical thinning. Cystoscopy revealed over 4 units of malodorous blood clot in the bladder, a normal sized prostate, and markedly friable bladder walls. The clots were removed and bilateral ureteral stents placed. The patient continued to bleed from the bladder despite the use of desmopressin acetate and required 14 units of packed red blood cells over 9 days to increase his hematocrit from 15 to 32. Despite expedient removal of the patient's obstruction, the patient did not regain full renal function and required dialysis.
DISCUSSION
This case illustrates an unusual cause of renal failure as well as many of its complications. It also demonstrates the problems that can arise from decompression of a massively distended bladder. The electrolyte abnormalities commonly seen in untreated renal failure include uremia, hyperkalemia, metabolic acidosis, and hyperphosphatemia. Nausea, altered mental status, anorexia, and fetor uremicus are classic signs of uremia. High levels of blood urea nitrogen can also inhibit platelet action and lead to excessive bleeding. In addition, hypocalcemia and anemia can result from decreased 1,25- OH vitamin D and erythropoetin production. Acute decompression of the bladder can result in bleeding through re-expansion of previously compressed bladder wall veins. This is difficult to ameliorate through intermittent clamping. Reflex vasodilation occurs with bladder decompression leading to hypotension, particularly in hypovolemic patients. Although it has been suggested that more gradual bladder decompression can reduce hypotensive episodes, this is of unproven efficacy. Post-renal causes of acute renal failure account for 5–15% of cases of renal failure cases, the vast majority due to BPH. Early recognition of renal failure secondary to obstruction and prompt relief of the obstruction are important in preventing permanent renal damage. Normal urine output does not rule out obstructive causes of renal failure. Most recovery of renal function occurs within 7–10 days after relief of the obstruction.
AN ESSENTIAL CASE OF NON-ESSENTIAL HYPERTENSION
M. Guidry1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117468)
LEARNING OBJECTIVES
1. Recognize the symptoms and signs that suggest secondary hypertension 2. Diagnose adrenal adenoma using clinical and radiographic criteria
CASE
A 31-year-old man presented with five days of shortness of breath. His baseline dyspnea on exertion increased from shortness of breath at five blocks, to walking 30 feet. His one-pillow orthopnea increased to sleeping for minutes at a time sitting straight up in bed; he developed new onset paroxysmal nocturnal dyspnea and pedal edema. The shortness of breath was associated with a cough productive of clear sputum and fatigue. He has a past history of hypertension and systolic dysfunction. His out-patient medications included clonidine, metoprolol, and K-dur 40 meq. His blood pressure was 195/126 mmHg, pulse 80 beats/min, respiratory rate 26 breaths/min, and temperature 38.3°C. He had eight cm of JVD, an S3 gallop, and bibasilar crackles. Despite his supplementation, his potassium was 2.7 mg/L. He had LVH on EKG and bilateral cephalization on chest X-ray. He was admitted with the diagnosis of a CHF exacerbation and treated with diuresis and blood pressure control. The inability to control his blood pressure despite several medications and the continuing potassium supplementation requirements in the absence of a diuretic prompted an evaluation for the diagnosis of primary hyperaldosteronism. This was confirmed with a serum aldosterone of 21.3 (normal <16) and a rennin level of <0.8. An abdominal/pelvic CT showed an 18 × 14 mm round adrenal mass with central attenuation.
DISCUSSION
Primary hypertension is so common as to mask the red flags of secondary hypertension. A search for secondary causes of hypertension should be initiated when faced with any of the following: hypertension in youth, hypertension requiring multiple medications, episodes of flash pulmonary edema or unexplained congestive heart failure, hypertension with unexplained electrolyte abnormalities, and any of the above coupled with an incidentaloma found on CT. The patient in this case presented was diagnosed with hypertension at the age of 26, and his blood pressure was poorly controlled while being treated with multiple medications. He required potassium supplementation despite not being on potassium-wasting medications. He was hospitalized twice for unexplained congestive heart failure and had a benign adenoma was discovered on abdominal CT. Following the removal of his adrenal adenoma, his hypertension resolved.
AN INTERESTING CASE OF HYPERTHYROIDISM IN PREGNANCY
N. Mehta1; R.O. Powrie2; L. Larson2; K. Rosene-Montella2. 1Women and Infants' Hospital, Providence, RI; 2Brown University, Providence, RI. (Tracking ID #116673)
LEARNING OBJECTIVES
LEARNING OBJECTIVES: 1) Correctly interpret thyroid function tests (TFTs) in pregnancy 2) Review the relevant differential diagnosis and management of hyperthyroidism in the pregnant patient.
CASE
We were asked to consult on a 22-year-old woman at 18 weeks gestation for elevated blood pressure and abnormal TSH. She had been healthy until 3 months prior to presentation when she first noted increasing lower extremity edema. She had also noted blurry vision and headache in the preceding week. In the emergency room, she was noted to be tachycardic (102/min) and hypertensive (190/110 mmhg). Her physical exam was unremarkable except for a fine tremor of the hands and 3+ pitting pedal edema. A urine dipstick revealed 3+ proteinuria. Lab abnormalities were consistent with preeclampsia. A TSH level was found to be <.01 U/ml. Her full thyroid function panel was consistent with hyperthyroidism. She was admitted with a diagnosis of thyrotoxicosis and severe preeclampsia at early gestational age. A fetal ultrasound revealed multiple congenital anomalies consistent with triploidy. The patient chose to terminate the pregnancy. Placental pathology was consis-tent with a partial hydatiform mole. Postpartum, the patient's tachycardia, hypertension and tremor resolved within a day. A -hCG level on the third postpartum day was still elevated at 42,700 u/ml. At her 6-week postpartum check, the patient was well, with normal thyroid function tests and -hCG level <5 u/ml.
DISCUSSION
Hyperthyroidism is the second most common endocrine problem encountered in pregnant women. Recent evidence has emphasized the importance of a euthyroid state in pregnancy for favorable maternal and fetal outcomes. Internists should be prepared to diagnose and manage thyroid disease in pregnancy. Changes in serum concentrations of thyroid hormones and thyroxine-binding globulin during pregnancy make the interpretation of TFTs in pregnancy difficult. The expected changes in TFTs with each trimester and their relationship to hCG levels will be dis-cussed. The pathophysiology, clinical presentation and management of hyperthyroidism in pregnancy will be discussed. Typical causes of hyperthyroidism in pregnancy, including Grave's disease and Hashimoto's thyroiditis will be reviewed and differentiated from hyperthyroidism associated with gestational trophoblastic disease and hyperemesis gravidarum.
AN OVERLOOKED DIAGNOSIS IN THE ELDERLY
C.L. Cullinane1. 1Boston Medical Center, Boston, MA. (Tracking ID #116564)
LEARNING OBJECTIVES
1. To enhance clinician awareness of the need for HIV testing in the elderly. 2. To recognize the importance of a complete history. 3. To recognize the need for further research on HIV prevention and treatment among the elderly.
CASE
A 77 year old Cape Verdean-male was admitted with trigeminal zoster. Past medical history was significant for hypertension, pneumonia with sepsis, recurrent urinary tract infections, anemia of chronic disease, malnutrition, ischemic cardiomyopathy, and renal insufficiency. He was a nonsmoker and denied IVDU or prior transfusions. His wife had recently died of unknown causes. Three years prior to admission, a persistently elevated total protein prompted an evaluation for multiple myeloma. Bone marrow biopsy revealed a polyclonal gammopathy and a skeletal survey was negative. Subsequently a leukemia and lymphoma panel was unremarkable. Prior to admission he was being followed by a hematologist for Monoclonal Gammopathy of Undetermined Significance. As an inpatient with trigeminal zoster he tested positive for HIV with a CD4 count of 70 and a viral load of 38,386, suggesting advanced disease. HAART therapy was instituted after discharge with suppression of his viral load. He died of an arrhythmia almost 2 years later in the setting of decompensated heart failure.
DISCUSSION
Individuals over 50 years of age account for up to 10% of AIDS cases reported to the CDC, a number that is expected to rise as a result of improved survival of patients with treated disease. Older adults are less likely to use a condom during sexual intercourse or to participate in HIV testing. Older adults with HIV infection are more likely to be diagnosed late in disease due to delayed recognition, they experience progression more quickly, and they survive for shorter periods of time than their younger counterparts. Co-morbidities often complicate management and controlled data on tolerability and responses to HAART are lacking. The possibility of HIV infection must be considered among elderly patients with clinical features of immunodeficiency in order to avoid delay in counseling and treatment. This case emphasizes the importance of conducting the sexual history, regardless of age, and it underscores the need for age-appropriate prevention and treatment strategies.
AN UNCOMMON CAUSE OF CIRRHOSIS, OR IS IT?
S. Evans1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117456)
LEARNING OBJECTIVES
1. Recognize the complications of jejeunoileal bypass prior to the onset of liver failure 2. Recognize the presentation of vitamin deficiency.
CASE
A 62 year-old white woman presented with a two-year history of intermittent jaundice and a three-week history of increasing abdominal girth. She had a history of jejeunoileal bypass in 1978 for morbid obesity. She had been admitted fourteen months prior with a hemoglobin of 4 g/dl, an elevated bilirubin, and a prolonged prothrombin time. She was diagnosed with B12 deficiency and malabsorption of fat soluble vitamins. A CT at that time showed diffuse fatty infiltration of the liver. On exam she had peripheral wasting, spider angiomas, shifting dullness, jaundice, and peripheral edema. On admission, she had a prolonged prothrombin time, a normal hemoglobin, an elevated bilirubin, and low cholesterol levels. An abdominal CT revealed large volume ascites and a cirrhotic liver. Paracentesis was consistent with portal hypertension as an etiology for the ascitic fluid. A liver biopsy showed severe steatosis with cirrhosis.
DISCUSSION
Jejeunoileal bypass has been a common treatment for morbid obesity, but has rapidly lost favor due to its severe long term consequences, including arthritis, B12 deficiency, cirrhosis, and chronic diarrhea. Patients who received this procedure are now coming to the attention of physicians because of cirrhosis. Unfortunately, this patient was regularly followed in the medicine clinic but did not undergo hepatic evaluation because her symptoms were attributed to the altered physiology of bypass. The intermittent episodes of jaundice following the correction of the B12 deficiency suggested another underlying pathology. Similarly, the prolongation in the prothrombin time following replacement of vitamin K should have been a clue that there was impaired hepatic synthetic function. The CT scan of the abdomen was also potentially confusing: because of the morbid obesity, hepatic steatosis was attributed to NASH. Prompt recognition of this complication of jejeunoileal bypass is important to refer patients to a hepatologist to prevent the expected fifty percent mortality after the development of ascites. At present our patient is awaiting liver transplant.
AN UNEXPECTED CAUSE OF TREMOR AND MYOCLONUS
K. Nashar1; E. Anish1; N. Busis1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #116914)
LEARNING OBJECTIVES
1. To recognize Creutzfeldt-Jakob Disease (CJD) as a cause of tremor and myoclonus. 2. To appreciate the value of performing a brain biopsy to confirm a diagnosis of CJD.
CASE
A 70 year-old female presented with a 6-week history of abnormal motor movements. Initially, she developed an action tremor in the right arm. After 4 weeks, the left arm also became affected and she began to experience myoclonus in both arms. On physical exam, the patient appeared well-nourished and was afebrile. Her Mini-Mental Status Exam score was 30/30. CNs II-XII were intact. Action-induced myoclonic jerks were noted in both arms (R > L). Strength was 5/5 in all extremities. Sensory testing was normal. DTR's were +2 and symmetric. Romberg test was negative. Cerebellar testing was normal. A brain MRI was performed revealing “gyriform” signal changes in the parietal-occipital region bilaterally. This raised suspicion for a diagnosis of encephalitis. As a result, a lumbar puncture was performed that showed no evidence of pleocytosis or increased protein. Over the next 4 weeks, the patient developed worsening neurological changes, including: dysarthria, ataxia, and increased myoclonus. A repeat brain MRI was unchanged. An EEG was normal. Serological studies looking for evidence of an autoimmune disease were negative. Anti-Hu and anti-Yo antibodies were negative. Since a diagnosis remained elusive and the patient's clinical condition continued to deteriorate, a brain biopsy was performed. The pathology revealed spongiform changes consistent with CJD. The patient's neurological impairment progressed, including the development of dementia, and she died 2 weeks later.
DISCUSSION
CJD is a degenerative disease of the central nervous system that is caused by infectious proteins called prions. Various forms of CJD have been recognized including: sporadic, familial, iatrogenic, and new-variant CJD. Most patients with CJD have the sporadic form and present with some degree of cognitive impairment and tend to progress rapidly to a state of dementia. A myriad of other neurological signs and symptoms may develop throughout the course of the illness with >90% of patients developing myoclonus. A typical clinical course may suggest CJD, but confirming this diagnosis can be challenging. Blood and CSF analyses can help exclude other conditions that may mimic CJD. Most patients demonstrate characteristic periodic complexes on EEG and/or abnormal signal patterns on MRI, but these findings are nonspecific. The gold standard for diagnosis remains brain biopsy. This case is unique in that cognitive impairment did not develop until extremely late in the course of the illness. It also emphasizes how an atypical clinical presentation, the absence of the more common EEG and/or MRI abnormalities, and a lack of risk factors for prion disease, can result in the delay of a diagnosis of CJD being made.
AN UNUSUAL CASE OF ACUTE SICKLE HEPATIC CRISIS
D. Zell1; E. Choe1; D. Spruill1. 1Tulane Health Sciences Center, New Orleans, LA. (Tracking ID #117441)
LEARNING OBJECTIVES
1. Recognize the common and uncommon abnormal laboratory findings associated with sickle cell disease. 2. Recognize hepatitis as a complication of vaso-occlusive crisis.
CASE
A 36 year-old woman presented with one week of worsening pain in her back, lower extremities, and nauseaand vomiting. She had a history of sickle cell anemia and a recent pulmonary embolism Her vital signs were normal; she had hepatomegaly and tenderness in the right upper quadrant. There was also pain in her back and lower extremities. Her alkaline phosphatase was 71, total bilirubin 1.3, AST 1968, and ALT 2088. Her viral hepatitis panel was negative; she denied alcohol use. Her acetaminophen and salicylate were normal. She was treated for five days with intravenous fluids and pain medication, and her liver enzymes decreased with resolution of her abdominal pain. She returned ten days later; her has AST (53) and ALT (67) has both decreased.
DISCUSSION
Sickle cell disease is characterized by arterial occlusions due to micro-thrombi from the sickled cells. Peripheral vaso-occlusion is the most common since systemic vascular resistance is higher in these vessels and they are of smaller caliber. The result is the typical bone and muscle pain of a sickle cell crisis. Solid organs can also be involved, however, resulting in myocardial infarction, stroke, renal impairment and, in this case, ischemic hepatitis. Although ischemic hepatitis is seen in only ten percent of all sickle patients, physicians should be vigilant for the complication, especially in the setting of right upper quadrant pain, hepatomegaly, jaundice, and a low grade fever. The usual laboratory findings are elevated AST and ALT levels. Treatment is supportive care with IVF and pain control; hepatitis that does not resolve warrants exchange transfusions.
AN UNUSUAL CAUSE OF FEVER IN AN HIV+ PATIENT
R. Gardner1. 1University of California, San Francisco, San Francisco, CA. (Tracking ID #117316)
LEARNING OBJECTIVES
1. Diagnose Multicentric Castleman's Disease. 2. Recognize the association between Castleman's Disease, HHV-8, and HIV.
CASE
The patient is a 39 year-old HIV+ man (CD4 23) who presents with worsening fevers and increasing abdominal pain for 1 week. He reports onset of intermittent fevers 18 months ago; 1 week ago, the fevers began to occur daily, accompanied by abdominal pain, headaches, back pain, and nausea. The patient recalled a similar symptom constellation 7 months before that resolved spontaneously. He has Karposi's Sarcoma (KS) and takes abacavir/3TC/AZT, lopinavir, tenofovir, azithromycin, and TMP/SMX. On admission the patient was afebrile with normal vital signs. His exam was remarkable for axillary and inguinal lymphadenopathy (LAD); a soft abdomen, mildly tender to palpation diffusely; KS lesions on the left lower extremity. Laboratory studies showed anemia but were otherwise normal. While hospitalized the patient spiked daily fevers, unrelated to antiretroviral administration. Blood, urine, CSF and MAC cultures all were negative, as were an influenza panel, monospot test, and cryptococcus serology. Chest radiography and head CT were normal. Abdominal CT revealed splenomegaly and diffuse LAD. Biopsy of an axillary lymph node showed Castleman's disease (CD) and stained positive for human herpes virus 8 (HHV-8).
DISCUSSION
The differential diagnosis for fever and LAD, already extensive, is even broader in patients with advanced HIV. In this case, the leading diagnoses included disseminated TB, MAC, and non-Hodgkin's lymphoma. CD, which also manifests with fever and LAD, is a rare lymphoproliferative disorder which has received renewed interest as increasing case reports link it to HIV and HHV-8. The unicentric form of CD is isolated, usually asymptomatic, and often discovered incidentally. Not typically associated with HHV-8, it can be cured with surgical resection. Multicentric CD (MCD), as seen in this patient, has systemic symptoms. Effective therapeutic options are limited, contributing to a poor prognosis. The disease manifests differently in patients with and without HIV and HHV-8. HIV+ patients with MCD are universally positive for HHV-8, and are more likely to have a rapidly progressive course with a shorter survival. New studies suggest that HHV-8 may contribute more to this pattern than HIV. Patients typically die of fulminant infection or associated malignancies. Optimal treatment is unclear given the rarity of the disease, the variety of clinical presentation, and the paucity of literature. Most therapies offer a temporary response with relapse after discontinuation, but combination chemotherapy and rituximab show promise for more durable responses. Castleman's disease is a rare disorder with a clinical course shaped by the presence of HIV and HHV-8; it should considered in any HIV+ patient with KS, fevers, and lymphadenopathy.
AN UNUSUAL CAUSE OF RECURRENT RHABDOMYOLYSIS
D.L. Stern1; R. Warrier2; J. Fixley2; E. Adickes2; J. Derby3. 1Creighton University Medical Center, Omaha, NE; 2CUMC, Omaha, NE; 3Creighton University, Omaha, NE. (Tracking ID #115653)
LEARNING OBJECTIVES
1. Recognize that viral infections are a common cause of rhabdomyolysis. 2. Recognize that a muscle biopsy is the gold standard for diagnosis of rhabdomyolysis. 3. Realize that congenital diseases can have initial presentation in adulthood.
CASE
Case Presentation: A 23 yo AA girl with PMH of asthma was admitted to the hospital with a 3 day history of generalized muscle pain. She reported upper respiratory tract symptoms approximately one week prior to admission. She denied muscle weakness, changes in urine color or urine output. She had no history of trauma, no new meds, seizures, or extraordinary physical exertion. She had been admitted two other times with similar symptoms in the past four years. These episodes were treated as rhabdomyolysis believed to be precipitated by viral illnesses. Physical exam was unremarkable except she displayed generalized muscle tenderness without any objective muscle weakness. Her CPK was 35,136 with a serum myoglobin 3207. Other labs of significance included potassium of 3.9, BUN/Cr of 13.0/1.0. Phosphorus was 4.1, AST was 133, ALT was 64, while the remainder of her liver function tests were normal. Complete blood count was normal. A urinalysis showed a myoglobin of 32,300. Free carnitine, acyl carnitine, total carnitine, pyruvate and lactate were all within normal limits. A diagnostic muscle biopsy was performed which revealed Nemaline myopathy. Patient was treated for rhabdomyolysis and was asymptomatic with low levels of CPK at the time of discharge.
DISCUSSION
Discussion: Nemaline myopathy is a congenital muscle disease with a wide spectrum of phenotypes, ranging from forms with neonatal onset and fatal outcome to asymptomatic forms. Muscle biopsy reveals atrophy, variation in muscle fiber size and a lattice like appearance typical of nemaline rod bodies emanated from the Z-discs of affected muscle fibers. Adult-onset cases usually manifest with symptoms as a child. Our patient denied any problems as a child and was actually was very active in athletics. Most case reports of adult-onset cases are of patients with progressive proximal weakness or generalized weakness. These patients had either a normal or slightly elevated CPK. None of these case reports displayed such a markedly elevated CPK or recurrent rhabdomyolysis as manifested in our patient. We believe that this is the first case report of nemaline myopathy presenting as recurrent rhabdomyolysis.
AN UNUSUAL ETIOLOGY OF LEFT INGUINAL LYMPHADENOPATHY IN A 53 YEAR OLD MAN
D. Takahashi1; M.M. Schapira1; S.R. Pandit1. 1Medical College of Wisconsin, Milwaukee, WI. (Tracking ID #116307)
LEARNING OBJECTIVES
1. Recognize unusual etiologies of localized lymphadenopathy. 2. Recognize clinical features of Castleman's disease. 3. Determine when there is a need for biopsy in patients presenting with lymphadenopathy.
CASE
A 53 year old Caucasian male presents with left groin lump and 10 kg weight loss over a period of one month. The lump is painful to touch and has been progressively enlarging in size over the past month. The patient also complains of generalized malaise and night sweats. His past medical history is significant for essential hypertension. The physical exam reveals an ill-appearing, 72 inch, 81 kg male with stable vital signs. The general physical exam is unremarkable except for a left sided groin mass measuring 2 –3 cm in size. The mass is discrete, firm and mobile with moderate tenderness to touch and not associated with erythema or induration. Laboratory exam initially reveals a normal CBC and differential and normal electrolytes. An ultrasound of the groin mass reveals clusters of enlarged inguinal lymph nodes, the largest of which measures 3.6 cm. A CT scan of the chest, abdomen, and pelvis reveals no additional lymphadenopathy. An inguinal lymph node biopsy was done, with initial pathology impression being a low grade lymphoma, but a second opinion from a reference laboratory was reported as follicular hyperplasia with expanded mantle zones, atretic germinal centers and monotypic lambda expression by plasma cells (Castleman's disease like changes).
DISCUSSION
Lymphadenopathy is often a diagnostic challenge for general internists. There is a rather large list of possible etiologies, some of which require immediate attention and management. Definitive diagnosis is often obtained by biopsy, which is invasive and not necessary in some cases. The role of general internists is crucial to identifying patients who require lymph node biopsy through a detailed history and physical exam. Age, location, duration, and associated signs and symptoms aid in deciding when a biopsy is necessary. The presence of enlarging lymphadenopathy and systemic symptoms in this patient indicate the need for a lymph node biopsy. Castleman's disease or angiofollicular lymph node hyperplasia is an uncommon etiology of lymphadenopathy that was first described by Benjamin Castleman. Castleman's disease can present as localized lymphadenopathy (unicentric Castleman's disease) or generalized lymphadenopathy (multicentric Castleman's disease). These two forms of disease carry different prognoses. Unicentric Castleman's disease, as was found in this case, is potentially curable with surgical excision of lymph node. Multicentric Castleman's disease, in contrast, has a median survival of only 8 to 14 months. Clinically, it is difficult to differentiate Castleman's disease from other more malignant lymphoproliferative disorders. Most cases of unicentric Castleman's disease are asymptomatic, the median age of the patient is approximately 35, it occurs equally in males and females, and the median size of the lesion is 5 to 9 cm. Unicentric Castleman's disease is of two subtypes: hyaline-vascular (90%) and the plasma cell type. The hyaline-vascular type is considered a reactive chronic lymphoid hyperplasia. The plasma cell type is considered to have an inflammatory pathogenesis, either through chronic antigenic stimulation (i.e. infection) or via an autoimmune mechanism. A plasma cell dyscrasia which includes polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin changes (POEMS syndrome) is sometimes associated with Castleman's disease. Interleukin-6 has been defined to have a role in the pathophysiology of this disease, and the systemic manifestations of Castleman's disease. In contrast, multicentric Castleman's disease usually occurs in patients with a median age between 52 and 65, and presents with systemic symptoms such as fever, malaise, weight loss, and peripheral lymphadenopathy. Hepatomegaly and splenomegaly are also common findings at presentation. Mediastinal and abdominal lymphadenopathy is less common at presentation although approximately 50% will progress to involve mediastinal or abdominal lymph nodes.
ANAPHYLACTIC REACTION TO TOPICAL LIDOCAINE
A. Pleister1; J.L. Sebastian1; M. Glisczinski2. 1Medical College of Wisconsin, Milwaukee, WI; 2Clement J. Zablocki VA Hospital, Milwaukee, WI. (Tracking ID #116735)
LEARNING OBJECTIVES
To recognize the potential for topical anesthetic agents to cause severe allergic reactions, including anaphylaxis, in susceptible individuals.
CASE
On the day of admission, a 73-year old man underwent a surveillance cystoscopy for transitional cell carcinoma of the bladder and dilation of a recurrent urethral stricture. During an uneventful 30-minute outpatient procedure, the patient received no medications other than 20 cc of a 2% lidocaine gel that was used as a topical anesthetic. Within 30 minutes of leaving the cystocopy suite, the patient developed intense pruritis and a feeling that he was about to pass out. The patient's wife noticed that he was markedly weak and pale and she immediately brought him to the Emergency Room. Initial physical exam revealed a diaphoretic man with a blood pressure (BP) of 61/34 and a weak pulse at 76 beats/minute. The chest and heart exams were normal but a diffuse urticarial skin rash was noted on the trunk and extremities. The patient's BP responded to the administration of two liters of IV normal saline and 50 mg of IV diphenhydramine. Additional medical history revealed that the patient had a previous allergic reaction to Bactrim that caused a similar urticarial skin rash. His active prescription list included propylthiouracil, lisinopril, felodipine and simvastatin for treatment of hyperthyroidism, hypertension and hyperlipidemia. None of these medications were new and the patient specifically denied use of any over-the-counter medications, supplements or nutraceuticals. A literature search determined that the formulation of topical lidocaine used in this case contained sodium metabisulfite, a sulfite responsible for causing allergic type reactions, including anaphylaxis, in susceptible individuals. The patient's chart was subsequently marked ALLERGIC TO TOPICAL LIDOCAINE and an adverse drug reaction form was filed with the hospital pharmacy. After a brief period of hospital observation, the patient was discharged home in stable condition and an outpatient allergy appointment was scheduled for further evaluation and sensitivity testing.
DISCUSSION
Although usually considered a benign agent, some commercially available formulations of topical lidocaine contain additives that have the potential to cause serious allergic reactions in susceptible individuals. This vignette reinforces the importance of obtaining a thorough allergic history before beginning any new medication or using a new formulation of a previously administered medication. Systemic absorption of topically applied medications can cause serious, and potentially life-threatening, complications.
ANEMIA DUE TO LAMOTRIGINE
N. Milojkovic1; M. Elnicki1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #116715)
LEARNING OBJECTIVES
Learning Objectives: 1. Recognize possible causes of anemia in a young woman. 2. Recognize the hematologic side effects of Lamotrigine.
CASE
A 28 yo African American female with a history of seizures was admitted for worsening shortness of breath and weakness. Review of systems was otherwise negative. Her seizures were partial complex seizures controlled with Lamotrigine and Levetiracetam. She had been seizure free for over 2 years. Her family history was positive for thyroid disease and iron deficiency anemia. She does not use alcohol, tobacco or street drugs. The patient's vital signs were normal except for a regular heart rate of 108, and her physical exam was otherwise completely normal. Laboratory evaluation revealed Hemoglobin of 4 mg/dL, Hematocrit 8.8%, MCV 93 fL, MCH 32 pg, MCHC 34 g/dL, Platelets 117.000/mcL, WBC 3.000/mcL with 45% neutrophils, 2% bands, 42% lymphocytes, 3% monocytes, 8% nucleated RBCs, reticulocyte count 5%. Serum iron was 150 µg/dL, transferrin saturation 60% and ferritin 600 ng/mL. LDH, haptoglobin and indirect bilirubin were normal as well as her TSH, B12, folate, LFTs and sickle cell prep. Viral antibody titers were negative. Bone marrow biopsy showed trilineage hematopoiesis, marked erythroid predominance and marked megaloblastic changes.
DISCUSSION
In this patient with anemia and seizure disorder, the differential diagnosis of severe anemia includes: 1. Hypoproliferative bone marrow (e.g. medication induced, viral infections, leukemic infiltration) 2. Maturation disorders including thalassemia, sideroblastic anemia; B12 or folate deficiency. 3. Hemorrhage or hemolysis. We can narrow our differential diagnosis based on the initial negative evaluation. The patient's bone marrow has a poor reticulocyte response to this degree of anemia suggesting inefficient erythropoiesis. Viral infections affecting the bone marrow, particularly Parvo B19, can cause an aplastic crisis. However, her parvovirus antibodies were negative. The bone marrow biopsy with evidence of megaloblastic picture in the setting of normal B12, folate can be attributed to medications, particularly to Lamotrigine. Erythroblastopenic crisis secondary to Lamotrigine has been described. Inhibition of dihydrofolate reductase is probably the mechanism of erythroblastopenia and it occurs more often in patients with underlying abnormal hematopoiesis, such as heterozygous [beta]-thalassemia. That brings the question of possible underlying abnormality of hematopoiesis in our patient and need for further work up such as hemoglobin electrophoresis. Treatment with folinic acid results in complete resolution of the erythroblastopenic crisis. Using this therapeutic approach, long-term treatment with Lamotrigine can be administered without any further complication. However, because of the severity of anemia in our patient Lamotrigine was discontinued and her hemoglobin recovered.
AORTODUODENAL FISTULA – A DIAGNOSTIC CHALLENGE
N. Nathan1; K. Muniyappa1; S. Parikh1; H. Friedman1. 1St. Francis Hospital, Evanston, IL. (Tracking ID #116043)
LEARNING OBJECTIVES
1. A high index of suspicion for ADF based on history and physical examination should be maintained in the presence of equivocal or even negative diagnostic tests. 2. The finding of a primary aortoduodenal fistula in a patient with previous aortic aneurysm repair is extremely rare.
CASE
An 84-year-old male with past medical history of hypertension, congestive heart failure, aortic valve replacement (on warfarin) and AAA repair, presented with one episode of bright red blood per rectum. On physical examination, he was afebrile and vitals were stable. Orthostatic hypotension was absent. Hemoglobin was 12 gm/dL, BUN was 55 mg/dL, serum creatinine was 1.8 mg/dL and INR was 2.99. An emergent EGD revealed no active bleeding. An abdominal CT scan with IV contrast revealed a saccular aneurysm arising from the infrarenal abdominal aorta with contrast entering the distal duodenum. This was highly suspicious for an aortoenteric fistula. Vascular surgery was immediately consulted. The patient suffered another episode of bright red blood per rectum with massive exsanguination and died despite aggressive resuscitation efforts. An autopsy evidenced a fistulous communication between the atherosclerotic aneurysm of orta and the third part of the duodenum, proximal to the suture line of the previous vascular graft. This was consistent with a primary aortoduodenal fistula, as defined below, and was quite unexpected.the a
DISCUSSION
A primary aortoduodenal fistula (PADF) is defined as a communication between the native aorta and the duodenum. In contrast, secondary aortoduodenal fistulas (SADF) arise between the suture line of a vascular graft and the duodenum and are far more common than PADFs. The most frequent cause of PADF is atherosclerosis. The triad of pain, GI bleeding and an abdominal mass is seen in only 40% of patients. The initial bleeding, commonly known as a `herald bleed', is often transient and self limiting owing to thrombus formation. EGD is useful to refute other causes of GI bleeding, but does not rule out ADF, which is often in the distal duodenum. Abdominal CT is specific but has low sensitivity for ADF. Treatment consists of emergent exploratory laparotomy with graft repair of the aorta and closure of the fistula tract. A high index of clinical suspicion, based on history and physical examination, is the key to correct diagnosis. Although the suspicion for SADF was high, the presence of a PADF in our patient makes this case unique.
APLASTIC ANEMIA IN PREGNANCY
V.R. Patel1; N. Le1. 1Baylor College of Medicine, Houston, TX. (Tracking ID #116919)
LEARNING OBJECTIVES
1) Review the presentation of aplastic anemia in pregnancy 2) Review the possible pathogenesis and current treatment options of aplastic anemia in pregnancy.
CASE
A 26 year old G2P1 16 week pregnant African American woman with a history of sickle cell trait and iron deficiency anemia was referred by her doctor for an abnormal CBC: WBC 4.1 (62N, 2B, 31L, 5M), Hgb. 9.1 (MCV 98.5) and platelets of 11K. The patient reported fatigue for several months and easy bruising for 2 years. The patient had a prior uncomplicated pregnancy with a vaginal delivery on 10/01 except for iron deficiency anemia and thrombocytopenia (70K). The patient's vital signs included a temperature of 98.1F, BP 109/64, and HR 88. On exam there was a 2 cm. ecchymosis on her left thigh and numerous petechiae on her chest. Pertinent laboratory results included normal PT, PTT, folate, B12, TSH. Her reticulocyte index was .6, Ferritin 14, and Iron Sat. 31%. Bone marrow biopsy revealed markedly hypocellular marrow (20%) with severely decreased megakaryocyte and myeloid cell content and no tumors or other abnormalities. She was treated successfully with multiple platelet and blood transfusions, along with cyclosporine, antithymocyte globulin (ATG), and corticosteroids.
DISCUSSION
Aplastic anemia (AA) in pregnancy is rare, but has long been recognized. Currently, there is no proven association between pregnancy and AA. Fleming postulated a hormonal mechanism due to an imbalance between erythropoietin and placental lactogen which both increase erythropoiesis and estrogens which inhibit hematopoiesis. This idea could be supported by the spontaneous resolution of aplasia in 1/3 of cases after delivery. Historically, AA results in a high maternal and fetal morbidity and mortality. However, in recent years with modern supportive therapy, maternal mortality has been 15% and more than 90% of patients survive in remission. Treatment options depend on the timing of AA during pregnancy and its severity. AA occurring early in pregnancy may be treated by termination of pregnancy and if necessary a bone marrow transplant. AA occurring in the 2nd half of pregnancy should be treated with ATG +/– cyclosporine and supportive care. ATG is effective for those with non-severe AA who are transfusion dependent and for severe AA in the absence of an HLA compatible sibling. ATG used in combination with cyclosporine results in a 50% survival rate. Supportive care remains the main treatment and includes the minimization of transfusions to decrease the likelihood of sensitization. Transfusion with HLA-matched or single-donor platelets is recommended.
ARTERIAL EMBOLUS PRESENTING AS ACUTE ABDOMINAL PAIN
M. Roschewski1. 1Eisenhower Army Medical Center, Augusta, GA. (Tracking ID #116713)
LEARNING OBJECTIVES
1. Recognize challenges of making diagnosis of arterial embolus 2. Identify risk factors for arterial emboli 3. Recognize management difficulty in patients with unidentifiable cause of arterial emboli.
CASE
30 y.o. African-American female without significant medical history presented to the emergency department after experiencing the acute onset of sharp, right-sided abdominal pain. Contrasted abdominal CT revealed the presence of a wedge-shaped infarct in the right renal cortex consistent with infarcted tissue. Magnetic resonance imaging of the kidney and its associated arteries confirmed the diagnosis of arterial embolism. Transesophageal echocardiogram and renal arteriogram was unable to identify a source of embolus. Extensive laboratory evaluation to identify an inherited or acquired thrombophilia was negative. Despite the uncertain etiology of the renal infarction, our patient was placed on warfarin anticoagulation indefinitely. Six months later our patient presented again with acute onset of chest pain and EKG changes of her inferior leads. Her cardiac troponin enzymes were elevated, revealing ongoing myocardial infarction. Coronary arteriography revealed the presence of a thrombus in her right coronary artery without associated atherosclerotic disease.
DISCUSSION
Systemic arterial emboli have variable presentations that range from asymptomatic to the acute onset of abdominal pain mimicking surgical emergencies. Arterial embolic phenomena, in general, most often affect older persons with mural thrombus from atrial fibrillation or erosive atheromatous disease following aortic manipulation. Such phenomena have, however, also been described as the presenting feature of inherited and acquired thrombophilias such as antiphospholipid antibody syndrome, hyperhomocysteinemia, and paroxysmal nocturnal hemoglobinuria. This case illustrates the challenge in making diagnoses as well as long-term management in patients who present with unusual embolic phenomena of arterial origin. Despite advances in our understanding of thrombophilias, up to 20% of patients will remain undiagnosed and be subjected to years of anticoagulation without well-studied outcomes.
AUTOIMMUNE HEPATITIS MASQUERADING AS HEMOCHROMATOSIS
B. Konicek1; J. Franco1; J.L. Sebastian1; D. Torre1. 1Medical College of Wisconsin, Milwaukee, WI. (Tracking ID #116751)
LEARNING OBJECTIVES
1) Recognize the clinical presentation and differential diagnosis of autoimmune hepatitis (AIH) and 2) recognize iron overload as a condition that can accompany many types of acute liver injury.
CASE
A 57-year-old Caucasian man with a history of type 2 diabetes and alcohol use of 40 g/day presented with a one-week history of anorexia, fatigue and dark-colored urine. He denied any history of fever, chills, abdominal pain, pruritis, hematuria, myalgias or change in bowel habits. There was no past history of HIV disease, blood transfusions, IV drug use, exposure to sick contacts or excessive use of acetaminophen. Because the patient was adopted, no family history was available. Physical exam was remarkable for normal vital signs, tanned skin, scleral icterus and hepatomegaly. Admitting lab tests revealed a total bilirubin of 19.2 mg/dl, AST = 3093 U/L, ALT = 2092 U/L, alkaline phosphatase = 171 IU/L, protime = 14.1 seconds, ferritin = 7497 ng/ml and ceruloplasmin = 36 mg/dl. Viral hepatitis titers were all negative as were the titers for ANA and anti-mitochondrial antibodies. Anti-smooth muscle antibody titers were mildly elevated at 1 : 80. An abdominal CT scan demonstrated diffuse hepatosplenomegaly without dense changes in the liver parenchyma or mineralized lymph nodes typical of hemochromatosis. Liver biopsy revealed a minimal amount of stainable iron within hepatocytes and thin, bridging fibrocollagenous strands between hepatocytes. After two weeks, aminotransferase levels declined to 2–3 times normal, but the patient continued to complain of anorexia and fatigue. Follow-up blood work three months later revealed a serum gamma globulin level of 1.62 g/dl, elevated IgG = 1800 mg/dl and IgM = 501 mg/dl. Taken together, all of these findings suggested a probable diagnosis of AIH.
DISCUSSION
Autoimmune hepatitis is a rare disorder of unknown etiology characterized by persistent inflammation of the liver. Clinical manifestations of AIH display marked variability, ranging from asymptomatic periods to fulminant hepatic failure. The diagnosis of AIH is based on a scoring system that includes gender, drug/alcohol history, immunoglobulin levels, antibody titers, viral hepatitis markers, liver histology, HLA serotypes and the presence of additional autoimmune diseases. Although our patient was a middle-aged male, AIH is usually more common in women (3.6 : 1) and younger patients (peak incidence ages 16–30). This clinical vignette reminds clinicians that iron overload can accompany any form of acute hepatitis and reinforces the need to include AIH in the differential diagnosis of suspected hemochromatosis.
AUTOIMMUNE THROMBOCYTOPENIC PURPURA AND GRAVES' DISEASE: A CASE OF WORSENING THROMBOCYTOPENIA DUE TO THYROTOXICOSIS
J. Baez-Escudero1; F. Grzywacz1; B. Taqui1. 1Temple University, Philadelphia, PA. (Tracking ID #116186)
LEARNING OBJECTIVES
1. Recognize the rare association between Autoimmune Thrombocytopenic Purpura and Graves' disease. 2. Recognize that treatment of Graves' disease with return to an euthyroid state can improve thrombocytopenia.
CASE
A 23 year old nulliparous Vietnamese woman with Idiopathic Thrombocytopenic Purpura (ITP) presented with palpitations, hair loss, tremor, anxiety, weight loss, heat intolerance and fatigue. She had been diagnosed with steroid responsive ITP two years prior. She denied history of bleeding complications. She had stable platelet counts of 20,000 on 20 mg of oral prednisone. On exam, she was tachycardic. She had a tremor, exophthalmos, mild epistaxis, gingival bleeding, diffuse nontender non-nodular goiter, hyperreflexia, and a severe petechial rash. Platelet count was 8,000. Her thyroid studies were: TSH 0.00, Total T3,714.9 (80–220 ng/dl), Total T4 24.5 (4.5–12.5 mcg/dl), Free T4 5.6 (1.0–2.3 ng/dl). Thyrotropin receptor autoantibodies were positive. Thyroid radioactive iodine uptake scan showed diffusely increased uptake. Serum beta-hcg and HIV were negative. Graves' disease was diagnosed and propranolol was started for symptomatic treatment. She was given a higher dose of prednisone for her worsening thrombocytopenia, but she did not respond. Due to the risk of bleeding, the patient was not offered a subtotal thyroidectomy. She was treated with a one time dose of radioactive iodine (131 I) after which she became euthyroid. Four weeks after treatment she was tapered off prednisone and maintained platelet counts above 50,000 for the next few months without further need for steroid therapy.
DISCUSSION
Autoimmune Thrombocytopenic Purpura is a rare but previously reported hematologic manisfestation of Graves' disease. The association between hyperthyroidism and thrombocytopenia is a known although infrequent occurrence. Distinct mechanisms are probably active in each condition, but the thyrotoxic state has been implicated as having a key effect on the fall in the number of platelets. Theories for this event include a common immunologic cause or a thyrotoxic-induced decrease in platelet survival. We describe a patient with coexisting Graves' disease and “idiopathic” thrombocytopenic purpura who showed minimal response to treatment of thrombocytopenia in the thyrotoxic state, but who promptly recovered and was able to sustain higher platelet counts while she was euthyroid. Evaluation of the thyroid condition in patients with refractory thrombocytopenia is advised. Specific therapy for the hyperthyroid state might lead to a moderate increase of the platelet count.
B12 DEFICIENCY AND DRUG ABUSE: N0 LAUGHING MATTER
M.T. Reyes1; M. Rotblatt2. 1UCLA-SFVP Olive View Medical Center Department of Internal Medicine, Sylmar, CA; 2UCLA SFVP-Olive View Medical Center Department of Internal Medicine, Sylmar, CA. (Tracking ID #115053)
LEARNING OBJECTIVES
1. Recognize nitrous oxide (N2O) exposure as a cause of subacute combined degenerative (SCD) myelopathy. 2. Recognize the neurologic deficits associated with a dorsal column myelopathy.
CASE
A 24 year old Latino male presented with 2 weeks of progressively worsening balance problems and numbness in his distal extremities. His symptoms started as a tingling sensation in the fingers of both hands and both feet, later progressing to clumsiness with fine motor movements of his hands. Mild balance problems progressed to being chair-bound at the time of admission. He denied taking any medications, but admitted to binge drinking alcohol roughly twice a month for 2 years. He reported having a conventional Western diet. Physical examination was significant for diminished vibration sense and proprioception in both hands and feet, but preserved sensation to light touch and pinprick. His cranial nerves and DTR's were normal. The only weakness noted was in the intrinsic muscles of his hands. Marked ataxia, with a wide-based and unsteady gait, and mild finger-to-nose and heel-to-shin dysmetria, were present. After the initial assessment, the patient's wife disclosed that he had been abusing inhaled N2O for 5 years, increasing his use in the previous 4 weeks. Basic labs were normal. MRI of the C-spine showed increased T2 signal of the posterior columns bilaterally, suggestive of B12 deficiency. Serum B12 level was 110 pcg/ml (normal 250–1000), and methylmalonic acid level was 7236 (90–279). He was started on B12 injections and was transferred to a rehabilitation facility. After 2 weeks of B12 injections and PT, the patient showed mild improvement in his ataxia and sensory complaints. He was subsequently lost to follow up.
DISCUSSION
Layzer et al. first described neuropathy after recreational nitrous oxide (laughing gas) abuse in 1978. Subsequently there have been about 10 published cases of a similar presentation either after exposure to N2O anesthesia or chronic recreational use. The symptoms are consistent with findings of B12 deficiency, that is, the classic picture of subacute combined degeneration of the dorsal and lateral spinal columns. N2O oxidizes cobalamin into its inactive form, thereby causing B12 deficiency. The methionine synthase enzyme and myelin production are inhibited, thus producing the classic neurologic manifestations. Clinically, patients present with parasthesias and ataxia, with diminished vibration sense and proprioception. Patients may or may not have macrocytic anemia. MRI findings typically reflect the pathophysiology of dorsal column demyelination of the cervical spinal cord. Symptoms and MRI findings may be reversible if B12 replacement is initiated early.
BACILLARY PELIOSIS HEPATITIS: IF SCRATCH DEEP ENOUGH, YOU'LL GET THE DIAGNOSIS
S. Irani1; B. Taqui1. 1Temple University, Philadelphia, PA. (Tracking ID #116209)
LEARNING OBJECTIVES
1. Review the diseases caused by Bartonella species. 2. Recognize bacillary peliosis as an important differential in HIV patients with fever and abdominal pain. 3. Review diagnosis and treatment strategies for peliosis hepatitis and splenitis.
CASE
A 34 year old African American female with AIDS (last CD4 207) presented with two week history of fever, malaise, nausea, vomiting, diarrhea, diffuse abdominal pain. On exam, she had T = 102.7, small 1cm sub-mandibular nodule, soft diffusely tender abdomen. Lab data revealed WBC 12.7 without shift, Hgb 7.5. Blood chemistries, liver function tests, blood cultures, stool studies, CXR were all negative. CT abdomen showed multiple 2–7 mm lesions in liver and spleen. During admission, her diarrhea resolved, but fever and malaise persisted. Upon further questioning, patient recalled being scratched one month prior by stray cats that she fed to a ward of rats. Liver biopsy revealed scattered granulomatoid inflammation with rod shaped bacilli on Warthin Starry stain. Electron micrograph showed multiple trilaminar cell-walled bacillary organisms leading to a diagnosis of bacillary peliosis hepatitis.
DISCUSSION
The genus Bartonella contains four species demonstrated to be pathogenic in humans (bacilliformis, henselae, quintana, elizabethae). Disease syndromes are of variable severity, ranging from cat-scratch disease (CSD) to systemic diseases such as trench fever and bartonellosis. In general, immunocompetent patients who are otherwise healthy tend to present with classic CSD. Patients who are immunocompromised tend to have systemic manifestations such as bacillary angiomatosis, extracutaneous lesions, bacteremia, and bacillary peliosis hepatitis and splenitis. Bacillary peliosis was first reported in HIV patients in 1990. It is the occurrence of multiple blood-filled cysts in the liver and/or spleen. Biopsy remains the gold standard for diagnosis, open having higher yield than percutaneous or transvenous. First line treatment is with erythromycin and doxycycline. Optimal duration of treatment is unknown, but most sources recommend 3 months. Relapses are not uncommon and usually occur with shorter courses of treatment. Our case highlights the importance of including bacillary peliosis in the differential of fever and abdominal pain in HIV patients.
BACTEREMIA IN A NURSING HOME RESIDENT: LOOK BEYOND THE FOLEY
S. Kumar1; E. Anish1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115905)
LEARNING OBJECTIVES
1. To recognize diverticulitis as a potential source of Providencia stuartii bacteremia. 2. To appreciate an atypical presentation of diverticultis.
CASE
A 77 year-old female, nursing home resident presented with a one-week history of malaise and chills. Her PMH was significant for CAD, HTN, and urinary incontinence that resulted in the placement of a chronic, indwelling Foley catheter. Physical exam revealed an elderly appearing female in no acute distress. She was afebrile with a BP-126/78 mmHg and HR-78 beats/minute.The remainder of her exam was unremarkable with no localizing signs of infection. Initial diagnostic tests revealed a UA significant for 5–10 WBCs. CBC and serum chemistries were WNL. A CXR was unremarkable. After obtaining blood and urine cultures, empiric antibiotic therapy with levofloxacin was initiated for a presumptive diagnosis of a urinary tract infection. The next day, 2 sets of blood cultures returned positive for Providencia stuartii, resistant to levofloxacin. The urine culture revealed no growth. The patient's antibiotics were changed to piperacillin/tazobactam and gentamicin and a work-up was initiated to determine the source of the bacteremia. Additional testing included an ultrasound of the abdomen/pelvis and a transthoracic ECHO that were both unremarkable. A CT scan of the abdomen/pelvis revealed mild sigmoid diverticulosis, but no other abnormalities. An indium-labeled WBC scan was then performed that showed increased activity in the right lower quadrant of the abdomen. Based on this result, her prior abdominal CT was re-evaluated and, in fact, changes consistent with cecal diverticulitis were found. A 14-day course of IV antibiotics was completed and the patient's symptoms resolved. Of note, throughout the entire illness, the patient remained asymptomatic from a GI standpoint and she never demonstrated any abdominal tenderness on exam.
DISCUSSION
Most cases of Providencia stuartii bacteremia occur in patients who develop catheter-related urinary tract infections. Although Providencia stuartii can be found as a component of the normal bowel flora, it is not a common organism associated with bacteremia in the setting of gastrointestinal disease. In fact, a MEDLINE search revealed no published cases of Providencia stuartii bacteremia related to diverticulitis. Although our patient had a Foley catheter, the absence of bacteriuria compelled a search for an alternative source of her bacteremia. This patient lacked the more common manifestations of diverticulitis (i.e., fever, abdominal pain and tenderness, leukocytosis), but a diagnosis was able to be made based on the results of diagnostic imaging studies. This case serves to illustrate how diverticulitis can present in an unusual manner in an older adult and it emphasizes the importance of re-evaluating prior diagnostic tests when necessary, even if the initial reports are unremarkable.
BE SENSITIVE TO SENSITIVITY: ASPIRIN-INDUCED ASTHMA
C.L. Spagnoletti1; M.A. McNeil1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115007)
LEARNING OBJECTIVES
1) Recognize the clinical presentation of aspirin-induced asthma (AIA) 2) Understand the pathophysiology of AIA 3) Discuss the treatment of AIA.
CASE
Mr. A is a 68 year old male who was hospitalized with back pain secondary to a compression fracture. His past medical history was significant for asthma, nasal polyps, and osteoporosis. His medications included fluticasone plus salmeterol inhaler and alendronate. He reported an allergy to aspirin, but was unable to provide further details. He denied alcohol or tobacco use. On exam, he was afebrile with stable vital signs. His oxygen saturation was 98% on room air. His pulmonary exam revealed no wheezes or rhonchi and good air movement. He was given intravenous etodolac for pain relief. One hour later, he developed severe shortness of breath and nasal congestion, with a drop in his oxygen saturation to 80% on room air. His repeat pulmonary exam revealed decreased air movement and diffuse inspiratory and expiratory wheezes. The CXR was clear. He was felt to have developed bronchospasm secondary to etodolac and was treated with albuterol/atrovent nebulizers, oxygen, and intravenous steroids. His symptoms resolved within 12 hours.
DISCUSSION
Five to fifteen percent of asthmatics are intolerant to aspirin, and aspirin intolerance (AI) is a risk factor for the development of asthma. AI is more common in asthmatics with nasal polyps and sinusitis. Most asthmatics who are sensitive to aspirin are also sensitive to non-steroidal anti-inflammatory medications (NSAIDs). AIA usually presents in the third or fouth decade in individuals who were not previously sensitive to aspirin or NSAIDs. Patients with AI tend to have more severe asthma than those without. Wheezing, congestion, rhinorrhea, tearing, facial flushing, or angioedema occur one-half to two hours after ingestion. The pathogenesis relates to the metabolism of arachnidonic acid. Patients with AIA react to compounds that inhibit cyclooxygenase (COX) I, which catalyzes the formation of prostaglandins and thromboxanes from cell membrane arachnidonic acid. These inhibitors include aspirin and most NSAIDs. When COX I is inhibited, arachidonic acid metabolism is shunted to the 5-lipoxygenase pathway, the byproducts of which are leukotrienes. They are potent inflammatory mediators which can induce bronchconstriction, mucus secretion, nasal mucosal swelling, airway edema, and attract eosinophils into the airways in predisposed people. AIA patients have increased amounts of 5-lipoxygenase pathway enzymes. Non-AIA asthmatics do not overproduce leukotrienes in response to COX I blockade. Treatment of AIA is according to usual asthma guidelines. Leukotriene modifying drugs are pivotal in long term management. Aspirin and NSAIDs should be avoided. Desensitization can be done if either is absolutely necessary. COX 2 inhibitors are safe for use.
BILATERAL CORTICAL BLINDNESS AS PRESENTING SYMPTOM OF INFECTIVE ENDOCARDITIS (IE)
P. Cunningham1; P. Radhakrishnan2. 1St. Joseph Hospital, Phoenix, AZ; 2St Joseph Hospital, Phoenix, AZ. (Tracking ID #116483)
LEARNING OBJECTIVES
LEARNING OBJECTIVES: 1. Diagnose cortical blindness. 2. Recognize the rare association of IE and occipital infarction.
CASE
A 41 year old female with a history of untreated hypertension and IV drug use presented with history of sudden onset of blurring of vision, stating that she was unable to distinguish any objects. She also complained of weakness on her left side and mild shortness of breath. PHYSICAL EXAM: The patient was obese . There was mild confusion. Vital signs were normal except for a temperature of 39.0 C. Eye exam revealed normal fundi, PERL, no light perception. There were track marks on the arms, bilateral splinter hemorrhages, and Janeway lesions. There were mild sensory and motor deficits in the upper extremities. LABS: MRI brain: bilateral occipital and subcortical infarcts c/w septic emboli. CT Chest: multiple airspace densities bilaterally c/w septic emboli. WBC 11.6 with normal differential, ESR 39, Drug screen +cocaine. HOSPITAL COURSE: Patient was treated with antibiotics for presumptive endocarditis with significant sequelae of CVA w/residual left-sided partial paralysis, blindness and pulmonary emboli. Transesophageal echocardiogram (TEE) showed a large tricuspid vegetation, moderate mitral vegetation, and a patent foramen ovale (PFO) by bubble study. Blood and sputum cultures: Oxacillin Sensitive Staphylococcus aureus. There was no evidence of aneurysm per CT angiogram. The patient experienced a slight improvement in vision prior to hospital discharge with return of light perception.
DISCUSSION
IV drug use accounts for half of IE cases in developed countries. The tricuspid valve is involved in 30–70% of IVDU-related IE, Staphylococcus aureus accounting for 50% of IVDU IE. PFOs are estimated to be present in 30% of the normal population. While embolization is a common complication of IE, the incidence of neurologic complications being 20–40%, cortical blindness is exceedingly rare. Cortical blindness is defined as bilateral visual defects with normal ocular and pupillary exam, most often caused by lesions of the vertebral-basilar arteries, often due to atherosclerosis, malformations. Review of literature has revealed a handful of case reports of infective endocarditis causing cortical blindness. The causes have ranged from rupture of a mycotic aneurysm, infarction to TIA due to septic emboli. Our case has several interesting aspects. Cortical blindness due to embolic stroke caused by infective endocarditis. The presence of Patent Foramen Ovale in this patient was possibly responsible for the widespread dissemination of the emboli. To summarize, consider infective endocarditis in the differential diagnosis of cortical blindness, in a young patient.
BREAST MASS AS AN ATYPICAL PRESENTATION OF BLASTOMYCOSIS
B.A. Kisiel1; A. Marhatta1; K. Rajkotia1; H. Friedman1. 1St. Francis Hospital, Evanston, IL. (Tracking ID #116244)
LEARNING OBJECTIVES
Distinguish breast involvement of Blastomycosis from breast malignancy.
CASE
A 48-year-old woman presented with dry cough, generalized weakness and a right breast mass for one month. She denied fever, chest pain or hemoptysis. She was not a smoker and denied alcohol use. Physical exam: afebrile; BP: 135/80 mmHg; pulse: 70/min; RR: 14/min. Breast exam: 3 by 2 cm nontender, erythematous nodule within the outer quadrant of the right breast. Lungs: Diminished breath sounds at the right base. Remaining exam was unremarkable. Laboratory data: WBC 28.000 (83% neutrophils). AFB sputum and HIV test were negative. CT chest revealed right middle and lower lobes consolidation with pretracheal lymphadenopathy and the right breast mass was identified. Mammogram confirmed the mass in the right breast and showed a smaller mass within the left breast. The patient underwent bronchoscopy and breast biopsy. Histopathological analysis of transbronchial biopsy and breast tissue revealed broad-based budding yeast accompanied by chronic granulomatous inflammation. A diagnosis of blastomycosis was made which was further confirmed by positive fungal cultures. The patient was started on Itraconazole with significant clinical improvement.
DISCUSSION
Blastomycosis is a systemic pyogranulomatous infection caused by the dimorphic fungus Blastomyces Dermatitidis. Initial infection results from inhalation of the conidia into the lungs. The clinical spectrum is varied, including asymptomatic infection, acute or chronic pnemonia, and disseminated disease involving the skin, bones and CNS. Alveolar infiltrates, mass lesions, and fibronodular infiltrates are the most common radiographic findings. Disseminated disease in immunocompetent hosts is uncommon. Blastomycosis of the breast is reported infrequently and clinically mimics carcinoma. Treatment of choice for mild to moderate disease is Itraconazole for 6–12 months. Amphotericin B is used for severe disease and CNS involvement. In patients presenting with breast and pulmonary masses blastomycosis in addition to cancer should be considered. (attached illustrations are the mamogram and the silver methenamine staining of the breast tissue)
BRONCHIECTASIS IN A PREGNANT PATIENT
B. Springgate1; M. Landry1. 1Tulane Health Sciences Center, New Orleans, LA. (Tracking ID #117516)
LEARNING OBJECTIVES
1. Recognize major diagnoses associated with bronchiectasis. 2. Identify occult cystic fibrosis in the adult patient. 3. Demonstrate the need for optimization of health status and genetic counseling for cystic fibrosis patients considering pregnancy.
CASE
A 32 year-old woman was transferred to the intensive care unit (ICU) with purulent respiratory secretions following delivery of twins at 32 weeks gestational age. Her follicle-stimulation-assisted pregnancy was complicated by mild cough, transient pruritus, and moderate elevations of liver transaminases, alkaline phosphatase, and amylase at 27 weeks gestation. In the ICU, the chest x-ray demonstrated diffuse patchy infiltrates. Sputum culture revealed pan-sensitive Staphylococcus aureus. The patient rapidly improved on intravenous antibiotics. An outpatient CT of the chest revealed diffuse bronchiectasis, and a CT of the abdomen failed to demonstrate a gall bladder. Sweat chloride levels were 102.5 and 91.0 meq/L, and pulmonary function testing revealed FEV1of 31% of the predicted value.
DISCUSSION
Bronchiectasis is associated with allergic bronchopulmonary aspergillosis, hypogammaglobulinemia, immotile cilia syndrome, and cystic fibrosis (CF). Cystic fibrosis is uncommonly diagnosed during pregnancy. Clues to diagnosis may include evidence of infertility, mild sinus or pulmonary disease, cholestasis, pancreatitis, and absent gallbladder. Genetic counseling and family planning assume paramount importance in patients with CF, as prognosis for mother and infant can be adversely affected in the setting of advanced lung or pancreatic disease.
CAN CHEMOTHERAPY INDUCE RADIATION-LIKE EFFECT? RADIATION RECALL MYOSITIS INDUCED BY GEMCITABINE
A.P. Amin1. 1John H. Stroger Jr. Hospital of Cook County, Chicago, IL. (Tracking ID #116671)
LEARNING OBJECTIVES
1. To recognize a rare cause of leg swelling in a cancer patient. 2. Beware of the radiation recall phenomenon. 3. To emphasize the association of 'radiation recall phenomenon' with gemcitabine.
CASE
A 47-year-old male with pancreatic cancer, metastasized to the right hip and femur bone, received a ten-day course of radiation (300 cGy daily) to the right hip and thigh. On completion of radiotherapy, there were no skin changes or swelling of the right thigh. One month later the patient was started on weekly gemcitabine infusions (1000 mg/m2) as palliative chemotherapy — which he received for six weeks. On presentation for the 7th week of chemotherapy, he complained of right thigh pain, swelling and inability to walk. On examination, he was afebrile. The right and left thighs measured 32 cm and 18 cm respectively. The swelling was strictly confined to the area of previous irradiation. A CT scan revealed diffuse, massive enlargement of all muscles in the right thigh. There was no localized abscess or pus collection. The creatinine kinase (CK) was elevated (624 U/L) and the total leukocyte count was normal (3,400/mm3). A duplex scan for deep vein thrombosis was negative. A diagnosis of radiation recall myositis was made on clinical grounds, and chemotherapy was stopped.
DISCUSSION
Radiation recall phenomenon is an acute inflammatory reaction that develops exclusively at a previously irradiated site, in response to an anti-neoplastic agent. The anti-neoplastic agent triggers the `recall' of the effect of radiation given earlier, and the quiescent tissue becomes inflamed as if freshly irradiated. Recall reactions have been described by various agents (doxorubicin, paclitaxel, etoposide, gemcitabine, bleomycin, vinblastine) and at various sites (skin, muscle, lung, oral mucosa, vagina). Of all recall reactions, dermatitis is the most common while myositis is quite rare. Only three cases of radiation recall myositis have been previously reported, all induced by gemcitabine. The exact mechanism of radiation recall reactions remains unclear. Possible hypotheses include radio-sensitization by the precipitating agent, a drug hypersensitivity-like mechanism, a Koebner phenomenon like reaction and radiation-induced up-regulation of thymidine phosphorylase. No treatment guidelines currently exist for this condition. Generally the triggering agent is discontinued, and there are anecdotal beneficial results with steroids. Clinicians should be aware of this unusual complication of cancer treatment.
CARDIAC TAMPONADE: A RARE COMPLICATION OF ADULT ONSET STILL's DISEASE
L. Crawford1; M. Panda1; R. Enzenauer1. 1University of Tennessee, Chattanooga, chattanooga, TN. (Tracking ID #115170)
LEARNING OBJECTIVES
To recognize the clinical features of Adult Onset Still's Disease (AOSD) and the potentially fatal complication of cardiac tamponade.
CASE
A 19 year old white female with a past history of Hodgkin's lymphoma currently in remission and recently diagnosed AOSD presented with the complaints of chest pain and shortness of breath. The chest pain was sharp, 8/10 in severity, exacerbated by deep inspiration and coughing and it radiated posteriorly. This was associated with severe dyspnea on exertion, paroxysmal nocturnal dyspnea and orthopnea. Leaning forward relieved both symptoms. She described recent subjective fevers, diaphoresis, diffuse arthralgias specifically in her right shoulder and knees, and a sore throat. On physical exam she was hypotensive (systolic BP 90 mmHg) tachycardic (pulse 130/min) and tachypneic (respiratory rate 40/min). She additionally had jugular venous distension of approximately 13 cm and a pulsus paradoxsus of 12 mmHg. Cardiopulmonary examination revealed clear breath sounds and a very prominent pericardial friction rub. Posterior pharynx was erthyematous with out exudates. Musculoskeletal exam revealed decreased range of motion in her right shoulder and tenderness to palpation without evidence of effusion. Laboratory values: white blood cell count of 51,800, neutrophil count of 97%, sedimentation rate of 114 mm/hr, c-reactive protein >9, and a ferritin level greater than 100,000. Emergent echocardiogram was obtained which revealed a large circumferential pericardial effusion with early diastolic collapse of the ventricles. The patient was taken to the operating room for an emergent pericardial window placement. A pericardial catheter was placed and drained 330 ml of serosanguinous fluid which was exudative in nature. Immediately after the procedure the patients tachycardia resolved, her blood pressure increased and her dyspnea dramatically improved. All cultures obtained returned without growth and the patient's antibiotics were discontinued. The patient was placed on high dose steroids and methotrexate. She reports doing well to date with no further complications.
DISCUSSION
Still's Disease is an infrequent rheumatological disease that until recently was poorly described in medical literature. It is a systemic inflammatory disorder of unknown etiology that typically affects young adults. The vast majority of cases present with a constellation of symptoms reminiscent of a viral syndrome including quotidian fevers, salmon colored macular rash, sore throat, myalgias, arthralgias, lymphadenopathy, and serositis. Pleuritis and Pericarditis occur in approximately 25% of patients. Pleural effusions are usually bilateral and asymptomatic, and thoracentesis often reveals bloody exudative effusions. Pericarditis is the most alarming, as it may herald of impending acute cardiac tamponade. Though pericarditis is common, there have been very few actual reports of cardiac tamponade reported in relation to Still's disease. This case report allows us the opportunity to discuss the salient features of AOSD and it's diagnostic criteria. This report also allows us to describe the rare and potentially fatal complication of cardiac tamponade. This report should enable clinicians to improve their diagnostic awareness of a complicating illness that presents itself in many ways.
CAROTID ARTERY STENOSIS AND OCCIPITAL. INFARCTION
T. Thenappan1; S. Parikh1; P. Kapoor1; D. O'Brien1. 1St. Francis Hospital, Evanston, IL. (Tracking ID #117447)
LEARNING OBJECTIVES
1. Recognize the anomalous origin of the posterior cerebral artery from the internal carotid artery. 2. Magnetic resonance imaging (MRI) is superior to computerized tomography (CT scan) in evaluating cerebrovascular events. 3. Investigate cerebrovascular disease with both carotid duplex ultrasound and magnetic resonance angiography (MRA) irrespective of intracranial localization.
CASE
We report a case of a 64-year-old male, with a history of hypertension and diabetes, who noted sudden onset of a vaguely characterized visual disturbance. The patient denied similar episodes in the past. The neurological examination was notable only for a right homonymous hemianopia. Duplex ultrasound of the carotid arteries was suggestive of high grade occlusion of the left internal carotid artery. The non-enhanced CT scan of the brain was normal, but MRI revealed an acute infarct of the left occipital lobe, consistent with examination. The magnetic resonance angiogram showed an anomalous left posterior cerebral artery originating directly from the left internal carotid artery (instead of the basilar artery), a hypoplastic A1 segment of the left anterior cerebral artery, with significant eccentric narrowing of the left internal carotid artery suggestive of plaque formation. These findings were confirmed by conventional angiography. The patient subsequently underwent a left carotid endarterectomy.
DISCUSSION
The Circle of Willis, as classically described, is present in only 40% of the population. The emerging vascular imaging technologies have enhanced our ability to recognize anatomical variants in the acute clinical setting, and the rational application of these modalities helps direct appropriate intervention.This case highlights three major points: First, although an infarct in the posterior fossa due to ipsilateral carotid artery disease has rarely been reported, the possibility of an anomalous posterior cerebral artery origin from the carotid artery should be kept in mind. This underscores the necessity of a complete cerebrovascular evaluation, always including both carotid studies and magnetic resonance angiography, irrespective of specific intracranial localization. Second, this case emphasizes the superiority of MRI over CT scan in the evaluation of cerebrovascular disease. Finally, this report reinforces the importance of a thorough vascular evaluation in the management of cerebrovascular disease, so that these anomalies are recognized and, if necessary, factored into medical and surgical interventional planning.
CAROTID SINUS HYPERSENSITIVITY
M.M. Vasudevan1; J.T. Bates1; A.A. Taylor1. 1Baylor College of Medicine, Houston, TX. (Tracking ID #116888)
LEARNING OBJECTIVES
1. Review the clinical presentation and diagnosis of carotid sinus hypersensitivity. 2. Recognize the usefulness of carotid sinus massage (CSM). 3. Understand how to differentiate the cardio-inhibitory, vasodepressor, and mixed subtypes of carotid sinus hypersensitivity (CSH).
CASE
A 72 year-old African American man with a history of hypertension and bipolar disorder was admitted for his second syncopal episode, which occurred while he was seated, and it was not associated with a prodrome or seizure activity. Pertinent physical findings included a supine blood pressure of 197/90 and heart rate of 56, without orthostasis. Carotid exam was normal. Electrocardiogram and telemetry demonstrated sinus bradycardia and first-degree heart block without arrhythmias. Bedside carotid sinus massage produced asystole that lasted at least ten seconds. Formal cardiovascular testing on a tilt table demonstrated a drop in blood pressure from 187/76 (supine) to 120/70 (with an 80-degree tilt), and there was no associated change in heart rate. Right-sided CSM produced the same 70 mmHg drop in systolic BP with a concurrent eight second period of asystole. Left-sided CSM was unremarkable. Exam findings were therefore suggestive of the mixed subtype of carotid sinus hypersensitivity (CSH). Treatment was initiated with the placement of a dual chamber cardiac pacemaker.
DISCUSSION
The geriatric population has a high rate of syncope, accounting for up to 3% of emergency room visits. The differential diagnosis of syncope should include CSH, which is often overlooked or misdiagnosed. CSM is essential for appropriate identification and differentiation of the three subtypes of CSH, and it can be performed safely with minimal cardiac or neurological complications. Appropriate therapy depends on the specific subtype of CSH. In patients with the cardio-inhibitory subtype, CSM produces at least three seconds of asystole; pacemaker implantation should abolish this cardio-inhibitory type. In patients with the vasodepressor subtype, CSM produces a fall in systolic blood pressure of at least 50 mmHg. The third subtype is a combination of both cardio-inhibitory and vasodepressor components. CSM during cardiac pacing can differentiate between pure vasodepressor and mixed types. Therapeutic options for patients with either the vasodepressor or mixed subtypes include surgical interruption of the afferent or efferent components of the baroreflex or stripping the vascular adventitia in the area of the carotid sinus. In summary, a thorough investigation of syncope includes consideration of CSH, which can safely and effectively be diagnosed with the CSM.
CARPE DIEM
S. Dravid1; M. Guidry1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117417)
LEARNING OBJECTIVES
1. To stress the importance of a complete differential diagnosis and systematically to rule out potential causes of mental status changes. 2. Recognize non-convulsive status epilepticus as a possible cause for mental status changes.
CASE
A 29 year-old man recently started on HAART for HIV presented with four weeks of fever and confusion. He described one month of night sweats and weight loss. Within three days of admission, he became completely unresponsive to verbal and physical stimuli. His vital signs were normal and he had small, but reactive pupils. His reflexes were normal, and he had no posturing. His electrolytes, liver function tests, CBC, d-dimer, fibrinogen, coagulation studies, and serum and urine toxicology screens were normal. A CT of the head showed mild cerebral atrophy with no masses, lesions, or midline shift. An LP was negative for meningitis. Blood and urine cultures were negative. He was empirically started on ceftriaxone and vancomycin; his HAART therapy was held. After no significant improvement, an EEG was obtained that revealed alpha waves consistent with non-convulsive status epilepticus. A loading dose of phenytoin was administered; his mental status returned to normal within 24 hours.
DISCUSSION
Altered mental status is a frequently encountered admitting problem. Easily diagnosed problems such as infection, electrolyte abnormalities, or metabolic encephalopathy are usually the etiology and should be excluded first. When these are excluded, less common diagnoses should be investigated. Non-convulsive status epilipticus is an important cause of altered mental status that is frequently missed because of its lack of symptoms. Unlike other varieties of seizure disorders, it is frequently not preceeded by an antecedent seizure history. Once the diagnosis is established by EEG, subsequent studies should be performed to discover the underlying cause.
CAVITATING LUNG LESION: IT's NOT ALL TUBERCULOSIS
J.S. Nguyen1; J. Cofrancesco1. 1Johns Hopkins University, Baltimore, MD. (Tracking ID #115854)
LEARNING OBJECTIVES
(1) To recognize the causes of cavitating lung lesion in HIV negative patients, and (2) to recognize HTLV-1 as a cause of immunosupression.
CASE
A 43 year old gentleman from St Croix without significant past medical history presents with 4 months of progressively increasing fatigue, weakness, lower extremity swelling, and shortness of breath; as well as a 30 pound weight loss, low grade fevers and night sweats. On physical exam, patient was afebrile, had normal vital signs with an oxygen saturation of 96% on room air, and had a pulmonary exam notable for decreased bibasilar lung sounds with crackles appreciated in the right mid and upper lung fields. Preliminary laboratory results were remarkable for WBC = 30,000 with a lymphocyte predominance, platelets = 114,000, serum calcium = 13.9, LDH = 1375, blood cultures which were sterile and an HIV antibody which was negative by ELISA. A chest radiograph revealed an ill defined right upper and middle lobe alveolar infiltrate as well as bilateral pleural effusions. A chest CT scan revealed a 3.1 × 3.1 cm cavitary mass in the right upper lobe as well as infiltrates in the right upper lobe, right middle lobe and left upper lobe. PPD was negative. Bronchoscopy was performed and the bronchoalveolar lavage was positive for Pneumocystis jiroveci by direct immunofluorescence. The transbronchial biopsy revealed a focal atypical lymphocytic infiltrate as well as Cytomegalovirus infection by immunostaining. Upon further testing, the patient was found to be Human T-cell Lymphotrophic Virus, type 1 (HTLV-1) antibody positive and peripheral blood flow cytometry revealed a predominantly abnormal T cell population. The combination of these findings was diagnostic for Adult T-Cell Lymphoma Leukemia and secondary opportunistic infection with PCP and CMV.
DISCUSSION
Tuberculosis is often considered in a patient with a cavitary lung lesion. However, a broad differential diagnosis exists and will be discussed including: (1) pulmonary infections (tuberculosis, atypical mycobacterium, aspergillosis, pneumocystis jeroveci, lung abscesses, septic emboli), (2) pulmonary infarction, (3) malignancies (lymphoma, metastatic disease, or primary lung cancer) or (4) vasculitis (Wegener's granulomatosis, micropathic polyangitis), rheumatoid nodules or sarcoidosis. Given the high calcium and unusual findings of both pneumocystis jeroveci and CMV pneumonia in a previously healthy gentleman on no prior medications, immunocompromise was considered. Adult T-Cell Lymphoma Leukemia (ATLL) will be discussed. ATLL is a peripheral T-cell neoplasm associated with Human T-cell Lymphotrophic Virus, type 1 (HTLV-1), infection that is endemic to the Caribbean islands, Japan and Africa. Typical presentation includes lymphocytosis with an abnormal T-cell population, hypercalcemia, hepatosplenomegaly, and interstitial pulmonary infiltrates. Patients with ATLL have a functional T-cell immunodeficiency which predisposes them to opportunistic infections, as this case has illustrated. Overall prognosis for patients with ATLL is poor with a median survival of 4 months.
CELLULITIS GONE WILD
B. Weinberg1; R. Cader2. 1University of California, Los Angeles, Sylmar, CA; 2Sepulveda VA Ambulatory Care Center, North Hills, CA. (Tracking ID #116311)
LEARNING OBJECTIVES
1. Recognize an unusual presentation of necrotizing fascititis. 2. Recognize the importance of an early surgical consult when you suspect necrotizing fasciitis.
CASE
A 42 yo white female with a history of intravenous drug use, Hepatitis C, and skin popping presented to an outside hospital with complaints of abdominal wall cellulitis and fevers. During her hospitalization there she was afebrile with normal vital signs, and was described as having a cellulitis on her right abdominal wall. The patient's cellulitis worsened despite ampicillin/sulbactam and the addition of levofloxacin. On hospital day 3 the patient was transferred to our facility with a “refractory cellulitis.” Upon arrival in the late evening the patient was afebrile with stable vital signs and appeared to be in significant pain related to the local site of infection. On exam she had an extensive area of erythema, firmness, and induration encompassing her entire right flank, extending to her hip and into her groin. This area was exquisitely tender but without creptitance or areas of fluctuance. Antibiotics were continued, a CT scan was ordered to evaluate for an underlying abscess, and an ID consult was to be obtained in the AM. The following morning the patient became hypothermic and hypotensive. Her wound was more erythematous and had spread further down her flank and groin. She was transferred to the ICU, evaluated by surgery and taken to the operating room for debridement of necrotizing fasciitis. A 40 × 30 cm area was debrided to muscle over her right flank. After surgery the patient was continued on broad spectrum antibiotics and discharged on day 10 with follow-up by Plastic Surgery for a future skin graft.
DISCUSSION
Necrotizing infections of the skin include necrotizing forms of cellulitis and fasciitis. Necrotizing fasciitis is a deep infection of the subcutaneous tissue that leads to progressive destruction of fascia and fat. Causative organisms include Group A Streptococcus, mixed anaerobic/aerobic bacteria and C. Perfrigens. Early recognition of necrotizing fasciitis is paramount since it can rapidly progress over hours, causing extensive destruction, toxicity, loss of limb or death. Unexplained pain which increases rapidly may be the first sign of this process. If untreated this progresses to localized swelling, edema and tenderness. Later the skin typically becomes dark red with bluish bullae. These patients may exhibit signs of sepsis and hemodynamic instability seemingly out of proportion to the lesion. Creptitus on exam and subcutaneous air demonstrated radiographically are extremely suggestive of necrotizing fasciitis or gas gangrene. Any suspicion of necrotizing fasciitis requires immediate surgical consultation with potential surgical exploration and debridement, as well as the initiation of broad spectrum antibiotics.
CEREBROVASCULAR ACCIDENT IN AN ELDERLY PATIENT—IMPORTANCE OF ECHOCARDIOGRAM
S. Dodla1; T. Townley1; H. Hashish1; Z. Gatalica1. 1Creighton University, Omaha, NE. (Tracking ID #115697)
LEARNING OBJECTIVES
1) To report a case of Papillary fibroelastoma, a rare cardiac tumor. 2) Recognize the importance of surgical resection of tumor in symptomatic patients. 3) Emphasize the importance of echocardiogram in patients with symptoms of CVA.
CASE
Patient is a 73-year-old female who presented with symptoms of numbness of the tongue, decreased sensation of the mouth and the lips along with difficulty swallowing, chewing and talking. Her other significant past medical history included Coronary artery disease, Hypertension and Hyperlipidemia. She was found to have Paroxysmal atrial fibrillation during the stay in the hospital. MRI and MRA of head showed a focal acute infarct in the right posterior frontal lobe with an old ischemic infarct in the left lenticular nucleus. Trans Thoracic Echocardiography showed no evidence of mass or thrombus. Trans Esophageal Echocardiography (TEE) revealed a 1.3 × 1.1 cm mobile, broad based echo density on the ventricular surface of the right coronary cusp of the aortic valve with trace central aortic regurgitation. The mass was surgically excised and the diagnosis of PF was histologically confirmed. Follow up TEE revealed no residual tumor and no aortic regurgitation. The patient did not have any embolic episodes for a follow up period of twelve months.
DISCUSSION
PF is a rare, benign, slowly growing cardiac tumor. These tumors have been reported sporadically in live patients since the introduction of echocardiography especially TEE, during evaluation of patients with transient ischemic attack, cerebrovascular accident and myocardial infarction. Although this patient had paroxysmal atrial fibrillation it was deemed necessary to surgically remove the aortic valve mass to prevent recurrent strokes, as the possibility of the latter being the source of embolus was high. Although PF may possess some characteristic echocardiographic features, histopathologic evaluation is essential to differentiate them from other intra-cardiac benign and malignant tumors.
CHEST PAIN IN A YOUNG MAN: SPONTANEOUS PNEUMOMEDIASTINUM
T. Sai1. 1John H. Stroger Hospital of Cook County/Rush University Medical Center, Chicago, IL. (Tracking ID #115900)
LEARNING OBJECTIVES
1. Recognize spontaneous pneumomediastinum as a cause of chest pain in young individuals. 2. Review the clinical features, diagnosis and treatment of spontaneous pneumomediastinum.
CASE
A 17 year-old man presented to the emergency department with the acute onset of substernal chest pain . The patient was well until the afternoon of admission when he developed the sudden onset of sore throat one hour after eating lunch. He then developed sharp substernal chest pain followed by anterior neck stiffness. There was no history of trauma, dyspnea, cough, dysphagia or ingestion of sharp objects. On examination, his vital signs were normal. There was subcutaneous crepitation of the neck, supraclavicular fossae and anterior chest wall. Cardiac examination was normal as well as the remainder of the physical examination. Chest and neck x-ray revealed subcutaneous linear lucencies without evidence of free mediastinal air. CT scans of the neck and chest showed free mediastinal air with dissection into the posterior pharynx. All mediastinal structures were normal. Fiberoptic laryngoscopic evaluation and contrast esophagram were normal. A diagnosis of spontaneous pneumomediastinum was made. The patient was treated with ibuprofen for analgesia and bed rest. The patient's symptoms resolved after 48 hours. A repeat chest CT scan showed reduction in mediastinal air. To date, the patient has had no recurrence of symptoms.
DISCUSSION
Spontaneous pneumomediastinum is the non-traumatic presence of free air in the mediastinum. Although it is second only to spontaneous pneumothorax as the admitting diagnosis for healthy young people with the sudden onset of chest pain or dyspnea, it occurs in only 1 in 13,000 young persons presenting with chest pain. It results when alveoli rupture in the absence of trauma, causing escaped air to dissect along the interstitial bronchovascular sheath into the mediastinum. The Valsalva maneuver and illicit drug use are predisposing factors. Patients most commonly experience chest pain; other complaints include dyspnea, dysphagia and throat discomfort. Findings on physical examination include subcutaneous emphysema and Hamman's sign, a cardiac systolic crunch best heard at the apex. Chest x-ray may reveal the presence of free air in the mediastinum as well as subcutaneous emphysema. CT imaging confirms the diagnosis and helps evaluate mediastinal structures. Esophageal imaging to rule out rupture is sometimes warranted. Management includes symptom control and observation. Most patients have resolution of symptoms within 2 to 7 days and seldom require further intervention. Although uncommon, recurrences have been reported. Awareness of this rare, benign entity may help avoid unnecessary invasive tests and procedures.
CHRONIC ALCOHOLISM PRESENTING AS SEVERE ACUTE AXONAL NEUROPATHY
G.G. Tapia1; L.D. Stagner1; L.M. Tapia1. 1Henry Ford Hospital Detroit, Detroit, MI. (Tracking ID #115809)
LEARNING OBJECTIVES
Recognize acute cases of alcohol-related polyneuropathy and distinguish clinical features between guillain-barre syndrome and alcohol-related acute axonal polyneuropathy
CASE
A 33 year-old female with a four-year history of alcohol abuse was admitted to the hospital with generalized ascending weakness and numbness for three weeks. The patient denied flu-like or gastrointestinal symptoms. A physical exam revealed diminished power 3/5 of the extremities, decreased sensation to pinprick, pain, and temperature distally, areflexia, flaccid muscle tone, dysphagia and a vital capacity (VC) of 900cc. RBC folate, thiamine, and urine methylmalonic acid levels were normal. The CSF revealed normal protein without pleocytosis, a negative VDRL, and the absence of CMV DNA, Lyme antibodies, cryptococcal antigen, and oligoclonal bands. TSH, ANA, anti SS-A, anti SS-B and RF were all normal. HIV was non-reactive. Additional studies including anti-Hu antibodies, VZV IgM, EBV capsid IgM, HTLV-1and 2, and a viral hepatitis screen were all unremarkable. Urine and stool porphyrins were unremarkable. A 24-hour urine showed normal lead, thallium, aluminum, and mercury levels. A serum ganglioside antibody panel was negative and ceruloplasmin level normal. An EMG revealed an axonal sensorimotor polyneuropathy with signs of acute denervation and reinnervation. The patient did not require 20. Following abstinence from alcohol, her motor strength, deep tendon reflexes, and VC improved.
DISCUSSION
Approximately 9% of patients with chronic alcohol abuse suffer from insidious peripheral neuropathy. The few reported cases of acute alcoholic neuropathy (AAN) describe onset of symptoms to be from few days to several weeks. This entity must be distinguished from acute polyneuropathies such as Guillain-Barre syndrome (GBS). Severe sensory loss is uncommon in classic GBS but frequent in axonal GBS. Unlike any GBS patient with severe tetraparesis, cranial nerve abnormalities and respiratory insufficiency were less severe in our patient. Less than 5% of GBS cases in North america are primary axonal forms. Although autoimmunity in GBS is debatible, antibodies to gangliosides (GM1, GM1b, GD1a) correlate with the presence of axonal GBS. All patients with AAN may regain their ability to walk within months of alcohol abstinence as opposed to 15% of GBS patients who are unable to walk after one year of diagnosis. The pathophysiology of AAN is still uncertain. Vitamin levels and nutritional state poorly correlate with polyneuropathy; however, preliminary data describe the direct toxic effect of alcohol in peripheral nerves. Alcohol abstinence is the treatment of AAN while immunotheraphy is probably unnecessary compared to cases with GBS.
COLA-COLORED URINE IN VIRAL SYNDROME: RHABDOMYOLYSIS WITH A CPK OF OVER ONE MILLION UNITS WITHOUT ACUTE RENAL FAILURE
G.T. Arbour1; L. Mitchell1; R. Palmer1; D. Strain1; J. Huang1. 1Louisiana State University Medical Center at Shreveport, Shreveport, LA. (Tracking ID #115650)
LEARNING OBJECTIVES
1. Recognize that viral illness and flu-like syndromes can cause severe rhabdomyolysis. 2. Recognize that renal failure can be prevented in rhabdomyolysis if diagnosed and intervened early.
CASE
A 20-year old previously healthy black male presented with the complaint of “cola-colored urine” for one day. 7 days prior to presentation, he developed fever, headache, dry cough, rhinorrhea, sore throat and general malaise. 5 days later, he developed severe myalgias and muscle weakness, especially in his thighs, shoulders and back, followed by reddish-brown colored urine. The patient denied alcohol or cocaine use, recent strenuous activity, or trauma. Physical exam was unremarkable except for diffuse tenderness of most muscle groups. Motor strength of all extremities was also decreased at 4/5. UA on admission demonstrated large blood on dipstick with 0–2 RBCs on microscopy. Serum creatine phosphokinase (CPK) revealed a value of 395,800 U/L which peaked 3 days later to a maximum of 1,213,650 U/L. Elevated AST and ALT paralleled CPK in kinetics; however, alkaline phosphatase remained normal. Urine myoglobin peaked at 162 ng/mL. A UDS was negative for cocaine. Electrolytes were normal and a viral hepatitis panel and ANA were negative. Nasal aspirate was negative for influenza by ELISA. Upon diagnosis, IV fluid with bicarbonate was promptly initiated in the ED. After 3 days, the CPK, transaminases, and myoglobin began to trend down until they normalized. The serum creatinine remained normal. The patient's symptoms resolved and he was discharged home a week later.
DISCUSSION
Rhabdomyolysis is commonly caused by drugs, alcohol, seizures, and trauma. It has also been reported in viral and bacterial infections. Influenza is the most common viral etiology. The pathophysiology underlying virus-induced muscle damage is unclear. Two mechanisms have been postulated: direct viral invasion and myotoxins mediated by cytokines. Viral syndrome seems to be responsible for this case based on the patient's symptoms and exclusion of other etiologies. Influenza is likely, although not confirmed, given the epidemic season and several confirmed local cases. The highest CPK in influenza was reported at over 500,000 U/L and nearly half of the patients developed acute renal failure (ARF). Although CPK remains the most reliable indicator of muscle damage, it does not always correlate with the development of ARF. This case demonstrates that even a CPK value of over 1.2 million U/L does not necessarily lead to ARF. With a high index of suspicion during flu season, diagnosis of rhabdomyolysis can be made by history, physical exam, and simple lab tests. Early intervention with aggressive hydration is the key to the prevention of ARF.
COLCHICINE OVERDOSE — NOT JUST DIARRHEA
B.P. Sankarapandian1; S.K. Thambidorai1; M. Bandara1; S. Dodla1; L. Morrow1. 1Creighton University, Omaha, NE. (Tracking ID #115553)
LEARNING OBJECTIVES
1. Demonstrate the classic pattern of the clinical course after colchicine overdose. 2. Recognize the current modalities in managing colchicine overdose. 3. Recognize the fatal potential of a common drug.
CASE
23-year-old male with history of depression was admitted to the intensive care unit after ingesting 60 mg of colchicine. He initially presented to a rural hospital where he underwent gastric lavage followed by activated charcoal administration. The patient's initial symptoms upon presentation to our hospital included weakness, lethargy and nausea. During the course of the hospitalization the patient developed an upper gastrointestinal bleed (GI) with subsequent hypotension and anuria. The patient's condition worsened when he became progressively more dyspneic eventually leading to acute respiratory distress syndrome (ARDS). Hepatic failure ensued with the following enzymes values: alk phos 929 IU/L, AST 1466 IU/L, ALT 366 IU/L, and T bili 2.6 mg/dL. He later developed rhabdomyolysis with a serum myoglobin of 480 ng/mL, urine myoglobin 5100 mg/L, CPK 16260 IU/L, BUN 38 mg/dL, and serum creatinine of 3.6 mg/dL. His white blood cell count was initially high, however he later developed signs of agranulocytosis. The serum colchicine level was noted to be 12 ng/mL on admission. The patient underwent continuous renal replacement therapy to try and correct his metabolic abnormalities, but despite the supportive management the patient died 5 days after the suspected time of ingestion. The patient's death was secondary to multi-organ failure.
DISCUSSION
Colchicine overdose is a rare occurrence. Fatalities have been reported after ingestion of just 7 to 12 mg. The morbidity and mortality are directly related to the dose ingested. Toxic manifestations appear after a delay of 2 to 12 hours following ingestion. Symptoms progress in 3 stages. Stage I (Days 1–3) includes a GI phase (Nausea, vomiting, abdominal cramps, diarrhea) and a circulatory phase (hypovolemia, hypotension and cardiogenic shock). This is followed by hypoventilation and acute respiratory distress syndrome. Stage II (Days 3–10) includes bone marrow aplasia, coagulation disorders, polyneuritis, myopathy, and acute renal failure. Stage III: (10 + days) is the recovery phase. There is no specific antidote for colchicine and the only treatment available is supportive management. There are case reports of patients treated with an antibody made from goat serum. This antidote is not readily available and is no longer produced.
CONJUNCTIVITIS, COUGH AND FEVER IN A NEPALI MOUNTAIN PORTER
H.K. Liss1. 1University of Washington, Seattle, WA. (Tracking ID #116042)
LEARNING OBJECTIVES
1) Recognize the clinical features of measles, particularly prior to the development of exanthem. 2) Assess and manage the public health issues posed by a measles outbreak in a population with low levels of passive and active immunity. 3) Identify and treat measles and the associated complications.
CASE
An 18 year old male Nepali mountain porter presented to a new healthcare post in the Everest region of Nepal with a chief complaint of red eyes, malaise, coryza, anorexia and cough of 3 days duration. Physical exam revealed fever of 39.8°C, tachycardia of 110 bpm, significantly injected conjunctiva bilaterally, and several 1–3 mm white spots on the posterior buccal mucosa bilaterally. The rest of the exam was significant only for bibasilar crackles and the absence of rash. Concern for prodrome of measles prompted immediate isolation of the patient in a room in the trekking lodge where the clinic was situated. Initial management also included oral hydration, anti-pyretics, and antibiotics for possible measles pneumonia with bacterial super-infection. By the following morning, a blanching erythematous macular-papular rash erupted over the patient's entire body, sparing his palms and soles. Generally such a patient would be evacuated on foot, by porter or yak to the permanent hospital 5–7 hours away for further management. However, a scheduled immunization day at the hospital precluded such an evacuation, which would possibly expose many unimmunized children and adults to measles. Thus, a porter walked to the hospital, notified the staff of the patient's illness, and requested doses of measles vaccine for contacts. When the porter returned with 10 doses of measles immunization, those Nepalis assessed to be at highest risk of contracting measles were administered vaccine. On the following day, the patient was escorted to the hospital and kept in respiratory isolation for a total of 5 days after the initial appearance of the rash. The patient recovered, returning to work as a porter the next week. In the following month, approximately 10 additional suspected cases of measles were seen at the hospital; however, none of these cases had known contact with this patient.
DISCUSSION
This case underscores the importance of early diagnosis of highly communicable diseases in populations with low levels of immunity. In the case of measles, making the diagnosis and isolating the patient prior to development of rash may help stem epidemics. In addition, the case highlights geographically specific issues to the Himalayas, as conjunctivitis and cough are very common complaints, posing diagnostic confusion with the presentation of prodromal measles. The challenge of managing both patient and population during an outbreak of measles in remote regions which are resource-poor, difficult to access, and fraught with crowded living conditions emphasizes the need for improved primary prevention through immunization.
CONSERVATIVE MANAGEMENT OF CHRONIC MESENTERIC ISCHEMIA
D.N. Goldson-Prophete1. 1University of Medicine and Dentistry of New Jersey, Newark, NJ. (Tracking ID #102070)
LEARNING OBJECTIVES
1. Recognize the signs/symptoms of chronic mesenteric ischemia. 2. Recognize treatment options for mesenteric ischemia.
CASE
Mr. S is a 61-year-old man who complained of sharp “stabbing” mid-epigastric abdominal pain radiating towards his umbilicus for approximately one year. He further complained of decreased appetite with avoidance of meals and a 30lb. weight loss. He denied any correlation between the onset of pain and his position or activity. His past medical history is significant for diabetes, hypertension, hyperlipidemia, atrial fibrillation (AF), coronary artery disease, and congestive heart failure (CHF) with an ejection fraction of ~15%. His current medications include Esomeprazole, Glyburide/Metformin, Metoprolol , Pravastatin, Losartan, Furosemide, Warfarin and enteric-coated Aspirin. No significant family history. He denies tobacco, alcohol. On physical examination, Mr. S was an obese male. Bowel sounds were normoactive and abdomen was soft with mid-epigastric tenderness. No rebound or guarding. No abdominal bruit. Rectal examination was normal and he was guiaic negative. CT scan of the abdomen and pelvis was notable for focal narrowing of the superior mesenteric artery and mural calcification. Subsequent angiography revealed mild to moderate stenosis of 50–60% in the proximal portion of main superior mesenteric artery (SMA). However, it was not flow limiting and there was no pressure gradient across the stenosis. The patient subsequently underwent dilatation and stenting of his SMA with reduction of the stenosis to 10–20%, after which he reported complete relief of his symptoms.
DISCUSSION
Mesenteric ischemia due to chronic arteriosclerotic occlusive disease usually results in the gradual development of postprandial pain associated with avoidance of meals and resultant weight loss. Diagnosis is based on clinical symptoms and arteriographic demonstration of an occlusive process of the splanchnic vessels. Endarterectomy or bypass procedures of the celiac and/or SMA have shown the greatest success in treating this condition. However, percutaneous ballon angioplasty with/without stenting, becomes the procedure of choice for high-risk patients in whom a more conservative approach is warranted, such as Mr. S. The literature has generally concentrated on the management of those patients with a “severe” stenosis of one or more of the splanchnic vessels. This case demonstrates that even a “moderate” stenosis of the SMA can be clinically significant and may warrant an attempt at revascularization. A prospective trial of endarterectomy vs. percutaneous angioplasty in these patients (as opposed to those with severe stenoses) has not been done.
CRACKING THE NECK CAN CAUSE A STROKE
F.K. Salahuddin1; M. Gaddamanugu2; S.G. Nace3. 1University of Illinois College of Medicine at Peoria, Peoria, IL; 2University of Illinois College of Medicine Peoria,SFMC, Peoria, IL; 3University of Illinois College of Medicine, Peoria,SFMC, IL, IL. (Tracking ID #117189)
LEARNING OBJECTIVES
1. Sudden repeated neck movements can cause a vertebrobasilar artery dissection. 2. Thorough history taking is the key towards an accurate diagnosis. 3. Isolated episodes of vertigo may be the only manifestation of vertebrobasilar ischemia.
CASE
This was a 51 year old white male who presented to the ED with sudden onset of vertigo,lightheadedness and severe right sided headache following sudden neck movement. He closed his eyes in an attempt to stop the vertigo with out much relief, he then described his gait as preferential tilting to his right side. Patient required assistance in getting out of the car because of his leg weakness. Symptoms occurred as the patient “popped” his neck as he was in the habit of doing it daily, and especially that day he repeatedly “popped” his neck many times in an attempt to “pop” into place. Past medical history was significant for neck pain for which patient had consulted chiropractor; his last visit being was a few months ago. Upon arrival his neurological exam was unremarkable. Initial non contrast CT of the head and lumbar puncture were normal. MRI/MRA demonstrated right vertebral artery dissection at C1 vertebral level with infarction in the distribution of the PICA. These finding were consistent with dissection and frank occlusion of right vertebral artery. Patient was successfully treated with clopidogrel due to his allergy to aspirin. He had complete recovery. He was however advised to avoid rapid neck movements as well as manipulations.
DISCUSSION
Concerns about vertebrobasilar artery dissection after chiropractic as well as self manipulation are common. Anatomical variations of the vertebral arteries and their branches are not infrequent and may constitute a predisposing factor to complications after neck manipulation. Vertebrobasilar artery dissection is a reported complication of sudden neck movements. There are reported cases in the literature as a result of chiropractic manipulation, blunt trauma to the neck, and in sports requiring sudden and rapid movements. Neurological symptoms may improve depending upon the severity of the injury. Long-term anticoagulation is the recommended. A careful history in the proper setting is essential to a diagnosis and can prompt the clinicians to do appropriate diagnostic study.
CULTURAL ISSUES IN PRIMARY CARE MANAGEMENT OF RUSSIAN IMMIGRANTS
B.H. Warren1; I. Pines2. 1Denver Health and Hospital Authority, Denver, CO; 2University of Colorado Health Sciences Center, Denver, CO. (Tracking ID #116362)
LEARNING OBJECTIVES
1. Become familiar with the cultural conditions of Russian immigrants that may interfere with succesful management of chronic disease for these individuals. 2. Apply knowledge of cultural barriess to solving clinical problems among Russians with chronic disease.
CASE
68 YO Russian speaking engineer who immigrated to the US in 1999. He has CAD, diabetes and uncontrolled hypertension. His BP is 195/100, HgA1C is 11.8, LDL cholesteral is 155 and he is chronicall depressed. He has no concept of the physiology and pathogenesis of hyperlipidemia and takes previously prescribed blood pressure medicines “PRN” for not feeling well. Dietery instructions given him earlier in English were not understood. (On or two other vignettes will be offered to illustrate the issues addressed)
DISCUSSION
BACKGROUND Federal mandates and National Quality Guidelines and Policies require that practitioners address cultural competence for special populations in the care of our patients. Healthy People 2010 sets goals for reducing cultural disparities in preventive management of diseases. Cultural issues have a significant impact on the successful management of chronic diseases according to guidelines and expectations when measuring the clinical performance of general internists. Addressing these goals requires explicit knowledge of the cultural issues, barriers and opportunities that are relevant for each of the diverse old and new populations seeking health care services in the United States. Newer immigrants are more vulnerable as they bring foreign experience and expectations of medical health care with which American providers may be unfamiliar. One such population about whom little has been published is that of recent Russian immigrants. DISCUSSION Examples of Russian cultural barriers and opportunities addressed in this presentation include attitudes towards American medicine, skepticism about preventive health care, reluctance to use medications regularly, and fear of chronic disease and hospitalization. High expectations for absolute cure of chronic illness, religious interventions (in some populations), and free access to medical care add to complicating variables. Underlying high prevalence of chronic depression, previous adverse experiences with chronic disease and poor availability of medications also require special consideration.
CUSHING's SYNDROME DUE TO A PULMONARY CARCINOID
E.H. Orth1; T. Dorsch1; L. Cation1. 1University of Illinois at Peoria, Peoria, IL. (Tracking ID #116346)
LEARNING OBJECTIVES
Recognize the clinical features of Cushing's Syndrome. Recognize the classic features of a pulmonary carcinoid tumor.
CASE
A 40 year-old man with a 3-year history of congestive heart failure due to a presumed viral cardiomyopathy presented with hypokalemia despite vigorous oral potassium replacement. He also noted increasing proximal muscle weakness. On physical exam, plethora, striae, bruising, muscle wasting, and central obesity were present. Analysis revealed the potassium on admission was 2.0 mmol/L. A 24-hour urine free cortisol was 350 mcg/24 hours (normal <50). There was no suppression of urinary free cortisol with dexamethasone 2 mg po every 6 hours for 48 hours. CRF testing showed baseline serum cortisol was 31 mcg/dl and peak cortisol was 34 mcg/dl; baseline plasma ACTH was 52 pg/ml and peak was 64 pg/ml. A chest film showed healing rib fractures. Further imaging with CT scan of the chest showed a small infiltrate in the left upper lobe thought to represent inflammatory changes. Petrosal sinus sampling for ACTH did not demonstrate a gradient. An octreotide scan showed uptake in the left lung, corresponding to the infiltrate on CT scan. A carcinoid tumor was resected from the left lung. Immunoperoxidase staining of the tumor was markedly positive for ACTH.
DISCUSSION
Bronchial carcinoid tumors comprise approximately 2% of all ectopic ACTH secreting neoplams, and 3.5% of all carcinoid tumors. Patients with bronchial carcinoid are less likely to have flushing or diarrhea than carcinoid tumors from the embryonic midgut or hindgut. Ectopic ACTH secrection and right-sided heart disease can be features of carcinoid syndrome. Typically these tumors pursue an indolent course, with onset of symptoms preceding diagnosis by a mean of 4.5 years. In summary, our case represents a patient with a history of heart disease and Cushing's Syndrome due to a pulmonary carcinoid tumor.
CYCLIC FEVER IN A PREGNANT WOMAN
T.T. Tran1; L.B. Lu2. 1Baylor College of Medicine, Houston, TX; 2Baylor College of Medicine, Friendswood, TX. (Tracking ID #117249)
LEARNING OBJECTIVES
1) Construct a differential diagnosis for cyclic fever in pregnant women. 2) Recognize malaria as the cause of cyclic fever in a patient from an endemic area. 3) Review relapse, perinatal outcome, and treatment of Plasmodium vivax infection during pregnancy.
CASE
A 19-year-old G2P1 woman, 21 weeks pregnant, presented to the emergency room with 10 day history of cyclic fever and chills. She was in her usual state of health until 10 days prior to presentation when she developed intermittent shaking chills and fever up to 104 F. Each episode would last an hour, usually in early morning and late afternoon. The patient denied cough, abdominal pain, diarrhea, dysuria, and weight loss. She was from El Salvador and immigrated to the United States 6 months ago. On arrival, her vital signs were with temperature of 101.4, blood pressure of 90/40 with no orthostatic hypotension, heart rate of 105, and respiration rate of 18. She had pale conjunctiva, supple neck, clear lungs, normal heart sounds, gravid abdomen with no tenderness, no costovertebral angle tenderness, and no palpable lymphadenopathy. Blood work revealed WBC 7.7 with 68% neutrophils and 21% lymphocytes, Hgb 11.1, Hct 31.3, platelets 146, normal chemistries, and normal liver function tests. LDH was 329. Urine and blood cultures were negative. A peripheral blood smear revealed multiple red blood cells with rings consistent with malaria. Pathology confirmed that the morphology was consistent with Plasmodium vivax in different stages of development. The patient was treated with oral chloroquine with resolution of her fever. After her delivery, primaquine was started to eradicate the liver stages of Plasmodium vivax.
DISCUSSION
In a pregnant woman, cyclic fever has a limited differential diagnosis which includes cyclic neutropenia, lymphoma, and malaria. Malaria should be considered in a patient with cyclic fever from an endemic area. The initial infection with P. vivax can present with mild or no symptoms. However, P. vivax produces dormant liver stages that may cause late relapse 6 to 11 months after the initial infection. Pregnancy predisposes to relapse due to depressed cell-mediated immunity and sequestration of parasites in the placenta. P. vivax infection during pregnancy is associated with maternal anemia and decreased fetal birthweight. The preferred drug during pregnancy is chloroquine. For chloroquine-resistant malaria, quinine combined with clindamycin is the treatment of choice. Primaquine is used after delivery to eradicate latent liver stages of P. vivax. In conclusion, malaria should always be in the differential in all synchronized cyclic fever.
DEEP VENOUS THROMBOSIS SECONDARY TO DEEP VENOUS THROMBOSIS PROPHYLAXIS
K. Paranada1; M. Lim1. 1University of Connecticut, Farmington, CT. (Tracking ID #116572)
LEARNING OBJECTIVES
Diagnose and manage deep venous thrombosis (DVT) secondary to heparin-induced thrombocytopenia as a complication of heparin for DVT prophylaxis.
CASE
A 72 year old female with a history of hypertension, type 2 diabetes mellitus and obesity, underwent bilateral knee replacement for osteoarthritis. She was given enoxaparin for DVT prophylaxis and was discharged stable on her fourth post-operative day. She returned 6 days later complaining of left leg pain localized below the knee. Examination showed the leg to be markedly swollen, cold, pale, with weak pulses. The patient was immediately taken for fasciotomy of the involved leg where there was note of muscle edema but no hematoma or myonecrosis. Pedal pulses improved. Laboratory data showed: platelet of 13,000/ml3 (normal on discharge), PT of 17.7, INR of 2.42 and PTT of 30. However, a few hours post-fasciotomy, the left leg was noted to be progressively more mottled, cyanotic and edematous. The patient was started on IV lepirudin. Venous angiogram showed massive DVT involving the left common femoral vein down to the popliteal vein. Thrombolytic therapy using urokinase for limb salvage was started. An IVC filter was also placed. INR was kept between 2 to 3 with lepirudin.
DISCUSSION
Heparin has long been considered the standard in DVT prophylaxis. However, complications arising from heparin therapy, such as that seen in this patient, can occasionally be life or limb threatening. Type II heparin-induced thrombocytopenia occurs in 3% of patients receiving heparin for 5 or more days. The major target antigen is a multimolecular complex of platelet factor 4 and heparin. Immune complexes interact with the platelet Fc gamma II receptor, which leads to platelet activation, formation of prothrombotic microparticles, and generation of thrombin. The key in diagnosis of HIT is a high index of suspicion as assays with high sensitivity and specificity may not be readily available. The appearance of otherwise unexplained thrombocytopenia, thrombosis associated with thrombocytopenia, or even a normal platelet count which has fallen 50 percent or more from a prior value should raise the possibility of HIT in any patient begun on heparin therapy within the preceding five to ten days. Once HIT is clinically suspected, therapeutic management consists of removal of the immune stimulus, by discontinuing heparin therapy and inhibition of thrombin, either directly (lepirudin and argatroban) or by blocking the generation of new thrombin (danaparoid). In the face of thrombosis, thrombolytic therapy may also be considered. Thus, the decision to put patients on heparin comes with the responsibility and vigilance of screening for this important complication.
DEMENTIA WITHOUT MEMORY LOSS?
P. Koneru1; G. Prakash1; R.D. Hobbs1. 1Oakwood Healthcare System, Dearborn, MI. (Tracking ID #117109)
LEARNING OBJECTIVES
To recognize the existence of atypical dementias that are language based and without significant memory loss.
CASE
A 71-year-old man accompanied by his wife presented for the evaluation of dementia. His history was significant for hypertension and hyperlipidemia for which he took benazepril and lovastatin. He was healthy until five years previously when he noted word-finding difficulties. He could now only express himself in monosyllables. There were no focal neurological deficits. His behavior, mood, ability to recall recent events and recognize people were intact. He had mild difficulty dressing. Social graces, facial expressions, coordination and gait were normal. He followed commands. He accurately drew a clock and wrote a sentence without spelling errors. His MMSE was measured at 10/30. CBC, metabolic panel, Vitamin B-12, Folate, TSH, Ceruloplasmin levels, and HIV and Syphilis serologies were normal. CT and MRI only showed generalized cerebral atrophy. Neuropsychological testing revealed Primary Progressive Aphasia (PPA).
DISCUSSION
Unlike most common dementias where memory problems are common and an integral part of the diagnosis, PPA is an atypical dementia characterized by a relentless dissolution of language with relative preservation of memory. Patients usually present with word-finding difficulties, spelling errors or abnormal speech patterns. Unlike Alzheimer's disease, these patients can recall and evaluate recent events despite an inability to express their knowledge verbally. Folstein's MMSE because of its reliance on verbal expression overestimates the degree of the patient's cognitive dysfunction in PPA and may lead to inappropriate labeling and ineffective treatment. Neuropsychological testing is the diagnostic modality of choice. There is no effective pharmacological treatment for PPA although speech therapy is useful in exploring alternative communication strategies. Explaining the nature of the condition is one of the greatest benefits in terms of coping with the impairment and recognizing the primary problem to be expressive rather than cognitive.
DEPRESSION AS A RISK FACTOR FOR HYPOGLYCEMIA IN A WELL-CONTROLLED DIABETIC
M.A. Mendiola1; E. Coffey1. 1Hennepin County Medical Center, Minneapolis, MN. (Tracking ID #117183)
LEARNING OBJECTIVES
1) Recognize that diabetics have a higher incidence of depression than non-diabetics. 2) Recognize that depression is associated with poor glycemic control. 3) Recognize that anorexia associated with depression may put a diabetic a risk for hypoglycemia.
CASE
A 71 year old female with a history of type 2 diabetes presented to her primary physician for routine follow up. She had been maintained on a regimen of NPH and regular insulin with a recent glycohemoglobin of 6.3%. She had previously experienced few hypoglycemic reactions, but the episodes had become more frequent over the previous two months. At this visit, she also described symptoms consistent with depression, including anorexia and poor motivation for meal preparation. Venlafaxine was initiated, and her insulin regimen was switched to Glargine and Lispro, to allow more flexibility. She was specifically instructed not to take her short acting insulin if she was not eating. However, she continued to have frequent serious hypoglycemic events requiring emergent medical care. Her insulin regimen was significantly liberalized until her depression could be brought under better control.
DISCUSSION
It has been shown in a meta-analysis by Anderson et al. in Diabetes Care, 2001, that diabetics are twice as likely as non-diabetics to suffer from depression (14–26% in diabetics vs. 5–9% in non-diabetics, with an overall odds ratio of 1.9). Depression is also associated with poor glycemic control, increased microvascular and macrovascular complications, as well as increased healthcare expenditures. Total healthcare expenditures for people with depression and diabetes were 4.5 times as high as for those with diabetes without depression, in a 2002 study by Egede et al. in Diabetes Care. This case demonstrates the need for close follow up in diabetics diagnosed with depression. Moreover, it may be necessary to tolerate hyperglycemia in the short run to reduce the risk of life-threatening hypoglycemia until the depression can be controlled. Further research is necessary to investigate the risk of hypoglycemia in diabetics with depression.
DIABETES INSIPIDUS COMPLICATING GASTRIC BYPASS SURGERY
D.L. Mercado1; P. Liew2. 1Baystate Medical Center, Wilbraham, MA; 2Baystate Medical Center, springfield, MA. (Tracking ID #116467)
LEARNING OBJECTIVES
To appropriately screen preoperative gastric bypass surgery patients for Diabetes Insipidus
CASE
A 26 year old woman with morbid obesity was admitted for gastric bypass surgery. Her past history was notable only for polycystic ovarian syndrome and depression. On postoperative day 1, she had a high urine output, but had clinical signs of volume depletion. She complained of thirst but was unable to take adequate oral fluids due to intake limits from the postoperative gastric bypass diet. Her initial serum sodium was 151, but her urine specific gravity was 1.006; renal function was normal. IV fluid resuscitation was initiated resulting in urine output of 7 liters in 24 hours. She was suspected of having Diabetes Insipidus. History from the patient's mother revealed chronic polydipsia and polyuria, which the patient had failed to mention. Further labs showed serum Na 149, urine sodium 140, and serum osms 302. A trial of vasopressin decreased her urine output dramatically from 300 cc/hr to 50 cc/hr with a > 100% increase in urine osms. Work up revealed no specific cause and the condition was deemed idiopathic central DI. With daily vasopressin nasal spray she was able to maintain adequate hydration while adhering to her post-surgical fluid limits, and her polyuria and polydipsia resolved.
DISCUSSION
Central DI is a rare neurohypophyseal disease defined as polyuria of 2–10 L/day with dilute urine (SpGr 1.000–1.005) in conjunction with high serum osmolarity and high serum sodium. The most common causes are trauma or neurosurgery, but 30–50% are idiopathic. Our patient had daily symptoms consistent with DI which she did not think to mention preoperatively because of their chronicity. Postoperatively undiagnosed DI can result in severe volume depletion because of limited access to free water. This is particularly true of gastric stapling and gastric bypass patients, who have limited oral intake allowances, especially during their first few days postoperatively. Preoperative screening for DI is critical in these patients since they would not be able to maintain hydration without IV fluids due to their limited oral intake postoperatively. In fact, the presence of nephrogenic DI, which is poorly responsive to vasopressin, is an absolute contraindication to this type of surgery. Preoperative patients should be questioned about polyuria and polydipsia. If symptoms are present and not due to hyperglycemia, urine and serum osms, and urine and serum sodium should be checked. If the clinical findings and history are suspicious, water deprivation testing can be considered to confirm the diagnosis.
DIAGNOSIS AND MANAGEMENT OF A PREGNANT FEMALE WITH BRUISES AND SPONTANEOUS ABORTIONS: A CATCH 22
J. Cunningham1; M. Panda1; W.P. Caine1. 1University of Tennessee, Chattanooga, Chattanooga, TN. (Tracking ID #115585)
LEARNING OBJECTIVES
1. Recognize the effect of pregnancy on platelet aggregation disorders 2. Discuss the diagnosis, categorization, and treatment of thrombophilic disorders 3. Recognize the dilemma in the evaluation and treatment of combined hypercoagulable and hypocoaguable disease.
CASE
A 19 year old black female G4P0030, at 9 weeks of gestation presented with spontaneous bruising. She was diagnosed with von Willebrand factor deficiency 3 months ago. She has a history of menorrhagia but denies history of bleeding from other sites. No family history of bleeding disorders. She has had two spontaneous first trimester abortions. No history of alcohol, tobacco, or illicit drug use. Meds include ASA (started during this pregnancy by PCP) and prenatal vitamins. Physical exam revealed new bilateral pretibial bruises and multiple bruises in different stages of healing on legs and arms. Labs revealed a normal CMP, CBC, PT 13 (INR 0.96), aPTT 34, negative ASO &nANA titer, monospot, RPR, HIV, hepatitis panel. Fibrinogen was elevated at 509, D-dimer 0.6. Ristocetin cofactor, Factor VIII, beta-2-glycoprotein and hexagonal antibodies were normal. All vWF multimers were present and normal. Mixing study and dilute Russell Venom Viper Test (dRVVT) were abnormal confirming the presence of a lupus anticoagulant.
DISCUSSION
Thrombophilic disorders can be hereditary, acquired, or both. The antiphospholipid antibody syndrome (APAS) is an acquired disorder associated with arterial and venous thrombosis, recurrent miscarriages and thrombocytopenia due to the presence of anticardiolipin antibodies, lupus anticoagulant, or subgroup of other antibodies. Types of thromboses associated with the APAS are categorized into syndromes with specific treatment. Mixing study and the dRVVT are specific tests to evaluate presence of a lupus anticoagulant. As commonly seen, our patient's platelet aggregation disorder (vWF def) corrected during pregnancy. Her history of spontaneous abortions prompted an APAS workup.The presence of lupus anticoagulant confirmed our patient had the Fetal Wastage variant of the APAS. Treatment for this is aspirin immediately before conception and heparin immediately after continued till 6 weeks postpartum. In our patient Aspirin exacerbated her vWF def causing bruising. We anticipate that the vWF def will resurface in the postpartum period which together with treatment will increase bleeding. In an attempt to prevent another abortion Aspirin was continued and heparin was added after discussion of the risk and benefits of receiving anticoagulation and antiplatelet therapy with the patient. A team approach by a high risk obstetrician, internist and hematologist for close monitoring during and after pregnancy will be utilized.
DIARRHEA GIVES ME A HEADACHE: SAGITTAL VENOUS THROMBOSIS AS A COMPLICATION OF AN EXACERBATION OF INFLAMMATORY BOWEL DISEASE
S. Homsi1; J. Hefner1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #116222)
LEARNING OBJECTIVES
1.) Recognize an uncommon extraintestinal manifestation of Inflammatory Bowel Disease (IBD); 2.) Diagnose and treat a sagittal vein thrombosis; 3.) Recognize IBD as a hypercoagulable state.
CASE
A 39-year-old woman, recently diagnosed with IBD, was admitted with severe watery, bloody diarrhea associated with crampy abdominal pain, weight loss, and night sweats. The patient was diagnosed six months prior to admission and never had a complete remission of her symptoms. She was treated on this admission with solu-medrol (methylprednisolone), pentasa (5-ASA) and rowasa (5-ASA) enema. On the fourth hospitalization day, the patient developed a frontal headache, described as pressure-like radiating to the back of her neck. The severity of the headache was initially 5/10, and increased significantly over the next 24 hours to become 10/10. The headache worsened in the supine position and improved mildly with sitting. There was associated nausea, vomiting and severe photophobia without any focal neurological symptoms. She was initially treated with NSAID's without any improvement. A non-contrasted head CT was negative for SAH or a hemorrhagic CVA. Her GI symptoms continued despite IV steroids. 6MP was added without improvement and then started on Infliximab. Physical examination revealed no nucal rigidity and no focal neurologic deficits. Fundoscopic exam revealed no papillary edema. Brain MRI revealed a thrombus involving the superior sagittal sinus extending into the left transverse sinus. A hypercoagulable work up revealed decreased levels of Protein S. The patient was started on a heparin drip and the headache improved dramatically over the next 48 hours. She was discharged on warfarin.
DISCUSSION
The incidence of extraintestinal manifestations of inflammatory bowel disease is reported to be between 25 and 35%. Neurological complications are rare and are usually the result of thromboembolic events. The pathophysiology is not fully understood but there may be an increased frequency of thrombophilic mutations such as factor V Leiden, prothrombin, or methylene tetrahydrofolate reductase. The coagulation abnormalities that have been reported to be associated with Crohn's disease include accelerated thromboplastin generation, increased concentration of factor V, factor VIII, and fibrinogen. Low antithrombin III concentrations, thrombocytosis, decreased platelet survival, and spontaneous platelet aggregation causing coagulation abnormalities have also been reported. The most common presentation of intracranial venous thrombosis is headache associated with symptoms of increased intracranial pressure. MRI is the gold standard for diagnosis and treatment consists of anticoagulation.
DIC AND TTP: TWO, THREE LETTER WORDS
M. Hamblin1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117472)
LEARNING OBJECTIVES
1. Appreciate the overlapping clinical and laboratory findings in DIC and TTP. 2. Recall the pathogenesis behind these two disorders.
CASE
A 45-year-old man with HIV presented with ten days of headaches. His examination was normal with no signs of meningismus. Cerebrospinal fluid was positive for cryptococcal antigen, and he was treated with intravenous amphotericin. On the third day he developed an acute change in his mental status thought to be due to sepsis. His altered mental status persisted over the ensuing seven days despite antibiotics. He developed a fever and his creatinine increased from 1.5 to 3.0; his platelet count decreased from 164 to 20. His fibrinogen and PTT remained in the normal range, but the D-dimer was positive. His PT was 16.7, and his LDH was 1,124. A blood smear revealed schistocytes indicating microangiopathic hemolytic anemia.
DISCUSSION
Thrombotic thrombocytopenic purpura (TTP) and disseminated intravascular coagulation (DIC) have overlapping lab features that can make a definitive diagnosis difficult. TTP is due to an acquired antibody-mediated deficiency of von Willebrand factor (vWF)-cleaving protease. It is thought that antigenic mimicry from an antecedent infection stimulates the development of this antibody. In HIV patients a link between CMV infection and the development of TTP has been established. The decreased levels of vWF-cleaving protease lead to intravascular fibrils that induce the thrombocytopenia and microangiopathic hemolytic anemia with a markedly elevated LDH. The clinical symptoms of fever, neurologic changes, and renal insufficiency result from obstructed small-vessel blood flow. The neurological findings are often the first clinical indication of disease, and may range from headache and confusion to aphasia, lethargy, and coma. In DIC the depletion of inhibitors of coagulation activate thrombin. The coagulation cascade becomes unregulated resulting in the hallmark lab findings of DIC: thrombocytopenia, hypofibrinogenemia, increased fibrin degradation products, and a prolonged prothrombin time (PT). In twenty-five percent of cases, microangiopathic hemolytic anemia may be present. Distinguishing between these two diseases is important as they warrant different treatments. In DIC, treating the underlying disease process is essential. Supportive measures, such as transfusions with platelets and cryoprecipitate maintain coagulation factors necessary to prevent bleeding until the underlying disease is eradicated. Low-dose heparin may prevent errant thrombosis. In the case of TTP, treatment is aimed at removing the antibody to von-Willebrand factor cleaving protease using large volume plasmapheresis.
DIFFUSE ALVEOLAR HEMORRHAGE, PULMONARY CAPILLARITIS AND RENAL FAILURE: A CASE OF CATASTROPHIC ANTIPHOSPHOLIPID SYNDROME
B.C. Clark1. 1University of California, San Francisco, San Francisco, CA. (Tracking ID #116421)
LEARNING OBJECTIVES
1) Recognize the major clinical manifestations of catastrophic antiphospholipid syndrome. 2) Review treatment strategies for catastrophic APLS.
CASE
A 42 year old woman with a remote history of infective endocarditis and mitral valve replacement was transferred from an outside hospital for work-up of hemoptysis and pulmonary hypertension. She reported 8 months of daily expectorated blood clots and blood streaked sputum as well as marked progressive dyspnea on exertion. Review of old records revealed long-standing mild thrombocytopenia, elevated PTT, false positive RPR and strongly positive anticardiolipin (aCL) IgG anti-body. On exam, lungs were clear. Laboratory data revealed a platelet count of 114,000, PTT of 62, and creatinine of 2.0 mg/dL. Urinalysis was bland. Anticardiolipin IgG was markedly elevated at 122 GPL, and Russell viper venom test was elevated to 112. ANA was borderline positive at 1:40 but DS DNA and anti-Smith were both negative, and complement levels were normal. ANCA and glomerular basement membrane anti-bodies were also negative. A chest radiograph showed interstitial infiltrates and a high-resolution CT scan showed diffuse ground glass opacities. Broncho-alveolar lavage revealed hemosiderin laden macrophages with no evidence of infection. A mini-thoracotomy with lung biopsy revealed evidence of hemorrhage with capillaritis. Shortly after the procedure the patient's clinical condition worsened further, with development of acute renal failure despite aggressive hydration. Creatinine rose to 3.8 mg/dL and pulmonary function deteriorated. The patient was treated with high dose solumedrol and underwent plasmapheresis three times. Her clinical condition rapidly improved, pulmonary hemorrhage cleared, creatinine fell to 2.3 mg/dL and aCL antibody declined to 35. Shortly after she underwent renal biopsy which revealed microthrombi of the arterioles consistent with renal involvement in antiphospholipid syndrome.
DISCUSSION
The antiphospholipid syndrome is defined by venous or arterial thromboses, recurrent fetal loss and the presence of elevated levels of antiphospholipid antibodies. A subset of patients with APLS develop a “catastrophic” clinical picture characterized by vaso-occlusive events, typically microthromboses, affecting multiple organs over a period of days to weeks. Diverse organs, including the heart, lungs, kidneys, liver, spleen, CNS and extremities can be involved. In the largest case series of 80 patients, renal involvement occurred in 72% and pulmonary involvement in 64% of cases. Lung manifestations can include pulmonary emboli, pulmonary hypertension, ARDS, and intra-alveolar hemorrhage and capillaritis as in this patient. Thrombotic microangiopathy is the most common renal manifestation. Laboratory findings include elevated titers of aCL (98%), lupus anticoagulant (68%), and thrombocytopenia (60%). Treatment modalities include anticoagulation, high-dose steroids, plasmapheresis, IV gamma globulin, and immunosuppression with cyclosporine or azathioprine. In a 1998 case series of 50 patients, treatment with a combination of anticoagulation, steroids and plasmapheresis or IV GG resulted in a 70% survival rate. The mainstay of therapy, anticoagulation, was delayed in this patient until she was safely past the immediate post-operative period and pulmonary hemorrhage had subsided.
DIFFUSE LYMPHADENOPATHY IN A PATIENT WITH LONG-STANDING HIV
K. Ozer1; J. Madrazo1; L. Lu1. 1Baylor College of Medicine, Houston, TX. (Tracking ID #116772)
LEARNING OBJECTIVES
1. Construct the differential diagnosis of diffuse lymphadenopathy. 2. Review the clinical presentation and histopathologic features of Castleman's Disease. 3. Recognize intra-abdominal lymphadenopathy as a cause of biliary obstruction
CASE
A 34 year-old man with an eleven-year history of HIV presented with a 3-week history of right upper quadrant pain, fever, and 10 lb weight loss. The pain was described as a constant, stabbing pain with radiation to the back and was not associated with food intake. Physical examination revealed a temperature of 98.7°, mild scleral icterus; bilateral rubbery, mobile, non-tender submandibular, cervical and axillary lymphadenopathy; tender right upper quadrant, with no rebound tenderness or guarding. Hepatosplenomegaly was not present. Labs revealed CD4 count of 105, viral load greater than 750,000, WBC: 3.4, Hb: 9.4, Hct: 27.4, MCV: 86.9, PLT: 158, Total bilirubin: 3.1, AST: 90, ALT: 122, alkaline phosphatase: 666. CT of the abdomen showed extensive retroperitoneal lymphadenopathy, including a node on the gastrohepatic ligament compressing the biliary tree. Work up for Bartonella, mycobacterial, fungal, CMV and EBV infections were negative. The patient had an excisional biopsy of a cervical lymph node, which was consistent with the plasma cell variant of Castleman's Disease (CD). The patient was treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) and responded well with rapid improvement of the biliary obstructive symptoms.
DISCUSSION
Diffuse lymphadenopathy is associated with a wide range of conditions including infections, immunologic diseases, malignancies, and other diseases of lymphoid tissues such as histiocytic necrotizing lymphadenitis, lymphomatoid granulomatosis, dermatopathic lymphadenitis and Castleman's Disease. Castleman's Disease is a rare entity and its prevalence has not been well documented. It can present in two forms, localized form (unicentric) and diffuse form (multicentric). Unicentric CD is found in non-HIV patients. Multicentric CD is often associated with HIV infection, especially with co-infection of Human Herpesvirus 8 (HHV8). CD is prevalent in young males who typically present with vague constitutional symptoms of weight loss, fever, malaise, and diffuse lymphadenopathy. Lymph node biopsy is required for definitive diagnosis. Treatment options include combination chemotherapy with several regimens, the most commonly used being CHOP. Unicentric CD is curable with surgical resection. Multicentric CD has poor prognosis with overall mortality of 70–85% and a mean median survival of 8 to 14 months. In conclusion, multicentric CD should be on the differential diagnosis in HIV patients presenting with diffuse lymphadenopathy.
DISSEMINATED COCCIDIOIDOMYCOSIS
P. Hu1; M. Pillai1; N.C. Le1. 1Baylor College of Medicine, Houston, TX. (Tracking ID #116476)
LEARNING OBJECTIVES
1) Recognize the presentation and diagnosis of disseminated coccidioidomycosis 2) Treatment for coccidioidomycosis
CASE
A 49-year-old African American man with a past medical history of hepatitis C, cirrhosis, and frequent travel to Arizona, presented with a two-week history of fever, chills, and cough. Significant findings on admission were temperature 101.3, RR 28 and decreased breath sounds on the left base. Lab results showed WBC 15K, with 85% neutrophils and 2% bands. Chest x-ray showed a left pleural effusion. Various antibiotics were started without improvement. On day #9, sputum culture grew a fungal pathogen, which was later identified as Coccidioides immitis. On day #10, patient developed several subcutaneous nodules in his forearms. Biopsy of the nodules showed C. immitis. He is currently on fluconazole 400 mg daily with resolution of symptoms.
DISCUSSION
C. immitis is a soil saprophyte endemic to California, Arizona, and Western Texas. Primary pulmonary disease usually goes unnoticed. Patients can present with a range of symptoms from cough to severe pneumonia. The disease is usually self-limited, but two important sequelae can occur: 1) hypersensitivity reactions and 2) dissemination. In hypersensitivity, the patient can develop conditions such as erythema nodosum, erythema multiforme. Dissemination occurs primarily with hematogeneous spread and is more likely in African Americans, pregnant women, and immunosuppressed patients. Diagnosing coccidioidomycosis involves recovery of C. immitis from clinical specimens or detection of anti-coccidioidal antibodies in serum or other body fluids. Recovery of C. immitis from respiratory secretions, tissue and other specimens establishes the diagnosis since it is not a normal flora. Anti-coccidioidal antibodies are highly specific (80–90% sensitivity in disseminated disease), but these antibodies may lag behind the onset of illness from weeks to even months. Thus, the absence of the antibodies does not exclude the diagnosis of coccidioidomycosis. Treatment depends on the severity of the disease. Primary pulmonary disease usually resolves without treatment. For disseminated disease, oral azole antifungal agents are the drugs of choice. Amphotericin B is used in patients with severe disease (eg. spread to the spine) and during pregnancy. Treatment with antifungal should be continued for years. Prognosis for complete cure remains uncertain.
DOC, I COUGHED UP A WORM!
M. Traina1; R.R. Cader2. 1University of California, Los Angeles—San Fernando Valley Program, Sylmar, CA; 2University of California, Los Angeles, North Hills, CA. (Tracking ID #117536)
LEARNING OBJECTIVES
1) To learn the clinical presentation of anisakis infection. 2) To learn management of anisakis infection.
CASE
A 56 year old male presented to urgent care complaining of coughing up a worm. The patient killed the worm and kept it in a wrapper. He denied fevers, chills, diarrhea or abdominal discomfort. Upon further review, he noted that he had been consuming raw beef for the past few months. One week prior to presentation, he had started eating raw fish including salmon and mackerel purchased at the Santa Monica Fish Market. Physical examination was normal with no evidence of abdominal distention or tenderness. The worm was taken for analysis and identified to be Anisakis simplex.
DISCUSSION
Anisakiasis is caused by infection with the nematode Anisakis simplex. A. Simplex is found in whales, dolphins, seals and sea lions. Eggs are ingested by tiny crustaceans which are then ingested by fish, where Anisakis matures into larvae. Humans are accidental hosts upon ingestion of raw parasitized fish and crustaceans. In the US, infection occurs most commonly with ingestion of salmon, mackerel, herring and cod. Patients usually present with an itchy sensation in the back of the throat after ingestion, followed by coughing up the worm as was seen with our patient. If the parasite is swallowed, Anisakis can be invasive causing a severe eosinophilic granulomatous response usually 1–2 weeks after ingestion. Patients can present with abdominal distention or obstruction and present with symptoms that mimic appendicits or crohn's disease. Spontaneous regurgitation or endoscopic removal of worm cures infection. Occasionally, surgical debridement may be needed for invasive disease. Diagnosis is made by removal of larvae with subsequent microscopic analysis. There is no known antiparasitic therapy for anisakiasis. Infection can be prevented by cooking to >60°C or freezing to lower than −20°C for at least 48 hours. It is important to educate patients as to the risks of eating improperly prepared foods. Our patient was advised to discontinue eating raw meats and fish.
DON'T EAT AT JOES
M. Cordone1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117407)
LEARNING OBJECTIVES
1. Recognize the presenting features of Hepatitis A. 2. Diagnose and manage patients with Hepatitis A 3. Repeat labs if there remains a strong clinical suggestion of a common etiology.
CASE
A 29 year-old woman presented with two weeks of nausea, fever and vomiting. She reported having a hamburger at a Cajan barbecue less than two weeks ago. She worked in a veterinary hospital where she incurred numerous animal scratches and bites. She was tachycardic, hypotensive, and had tenderness of the right upper quadrant. She had 23% bands, but a normal WBC. Her amylase was 152; she had a negative pregnancy test, and an AST and ALT of 158 and148. Her alkaline phosphatase and total bilirubin were normal. Her TSH was 0.24. Fearing thyroid storm, the emergency physician administered PTU and propranolol. Her transaminases rose to AST 3,281 and ALT of 3,265 over the next day. A CT scan revealed peri-portal edema secondary to hepatitis. A viral hepatitis panel was normal. Her toxicology screen, including aspirin and acetominophen were normal. ANA, CMV, and a Monospot test were negative; the ferritin was 6,018. Owing to a high clinical suspicion, a second hepatitis panel was ordered. The second hepatitis panel was reactive to IgM Hep A antigen.
DISCUSSION
The incubation period of hepatitis A is four weeks. It is transmitted via a fecal-oral route. Clinical symptoms include nausea, anorexia, vomiting, fatigue, malaise, headache, fever, pharyngitis, cough, and myalgias. Aminotransferases have a variable rise from 400 to 4,000, but do not correlate with liver damage. Neutropenia and lymphocytopenia are also present with some atypical lymphocytes, making it difficult to distinguish from infectious mononucleosis. The high clinical suspicion in this case prompted repeating a study that made sense in the first place, thereby obviating more invasive surgical and biopsy procedures. Physicians should be aware of the incubation period of Hepatitis A. This case illustrates an important second lesson: when a clinical case is confusing, it is always better to repeat tests that made sense in the first place then to embark on invasive and fantastical work-ups.
EFFUSION CONFUSION
K. Casey1; J. Newman1; J.M. Huddleston1. 1Mayo Clinic, Rochester, MN. (Tracking ID #117052)
LEARNING OBJECTIVES
To recognize pulmonary amyloid as a cause for persistent non-cardiac pleural effusions.
CASE
An 84-year-old male presented with persistent pleural effusions. The patient was evaluated three months prior for shortness of breath. His only history was hypertension and a bypass surgery six years prior. Chest x-ray revealed a left-sided pleural effusion. The patient was treated for presumptive congestive heart failure and discharged on furosemide and an ACE-I with moderate improvement. His dyspnea persisted for three months and he was readmitted with a diagnosis of pneumonia. After a course of levofloxacin, the patient was discharged from the hospital. He returned one week later with worsening dyspnea, cough and hemoptysis. Chest x-ray revealed a slightly worse pleural effusion. A 2D-echo showed normal left ventricular function. Ultrasound-guided thoracentesis yielded one liter of slightly hemorrhagic fluid. The fluid was negative for organisms, acid fast bacilli, and malignancy. CT scan and bronchoscopy were also not diagnostic. Subsequently, the patient underwent wedge resection of the left upper lobe for definitive diagnosis. A chest tube drained sero-sanguinous fluid. He was transferred to the Mayo Clinic for further treatment of the effusion. Pathology review of the lung biopsy at the Mayo Clinic revealed alveolar septal amyloidosis.
DISCUSSION
Amyloidosis refers to a group of conditions characterized by the deposition of abnormal protein material in extracellular tissue. Major sites of clinically significant amyloid deposition are in the kidneys, heart and liver. Primary amyloid (AL) is due to deposition of protein derived from immunoglobulin light chain fragments. Though pulmonary involvement with primary systemic amyloidosis has been shown in various post-mortem autopsy reports, cases presenting with pulmonary amyloid have been reported infrequently. Because cardiac involvement in systemic amyloid is common, it is often difficult to discern the origin of pleural effusions. Our patient was noted to have hemorrhagic effusions and no evidence of heart failure by echo. This suggests that the etiology of the effusions was pulmonary rather than cardiac. In our patient, fat biopsy confirmed the diagnosis of systemic amyloid and bone marrow biopsy revealed 5% monoclonal lambda plasma cells. The effusions subsequently improved and the chest tube was removed. The patient was discharged in stable condition and shortly after was started on melphalan and prednisone for the treatment of systemic amyloid. He is presently doing well.
ELEVEN ISN'T ENOUGH: FACTOR XI DEFICIENCY AND CORONARY ARTERY DISEASE
F. Yafai1; B. Taqui1. 1Temple University, Philadelphia, PA. (Tracking ID #116261)
LEARNING OBJECTIVES
1. Recognize the clinical manifestations of factor XI deficiency. 2. Recognize that factor XI deficiency does not protect against myocardial infarction (MI). 3. Recognize the importance of risk benefit analysis in starting aspirin in patients with factor XI deficiency and coronary disease (CAD).
CASE
An 85 year old Caucasian male physician was seen following 3-vessel coronary bypass after a myocardial infarction. Twenty years prior, the patient was diagnosed with factor XI deficiency after an incidental finding of an elevated PTT. He denied history of epistaxis, melena, hematochezia, but endorsed easy bruising and occasional bleeding with teeth-brushing. He denied history of spontaneous bleeding, but reported increased bleeding after an inguinal hernia repair, dermatologic procedure and an attempted steroid injection. He had an uncomplicated cholecystectomy, dental extractions and colonoscopies. During the bypass, he received fresh frozen plasma. On exam, there were several ecchymoses along his upper extremities but no signs of active bleeding. His hospital course was uneventful, without excess bleeding. Admission labs revealed PTT 66.1, (baseline 60–80). Post-operative (post fresh frozen plasma) labs revealed PTT 32.4, PT 12.2 INR 1.1, Hgb 9.6 and platelets 131. The surgeons requested consultant input on aspirin administration to prevent graft restenosis. After lengthy discussions between hematology, cardiology and cardiothoracic surgery, it was decided that the patient should be discharged on a low dose aspirin. The decision was based on a review of the literature and a review of the patient's history.
DISCUSSION
Factor XI deficiency, previously known as hemophilia C, is diagnosed by an isolated elevation in PTT. It tends to present with milder bleeding episodes than other coagulopathies. In addition, it has not been shown to be protective against myocardial infarction. Studies of patients with hemophilia A and B have demonstrated 80% reduced risk of myocardial infarction. There is 25% reduced risk among female carriers of hemophilia. In contrast, a recent study of 96 Israelis with factor XI deficiency found that the incidence of acute myocardial infarction was similar to that of the general Israeli population. Furthermore, anecdotal reports of patients with factor XI deficiency and coronary disease have demonstrated that aspirin is well tolerated. However, more research is required. Until then, risk/benefit ratios should be defined for each individual. Our patient had significant coronary disease, recent bypass and no serious bleeding episodes. Based on risk benefit analysis and physician-patient discussion, it was decided to initiate therapy with low dose aspirin.
EPHEDRINE STRIKES AGAIN? A CASE REPORT AND LITERATURE REVIEW
E. Mcdonald1; J.I. Lane1. 1Mayo Clinic, Rochester, MN. (Tracking ID #116553)
LEARNING OBJECTIVES
Recognize uncommon causes for stroke in young adults.
CASE
A 36-year-old woman presented to the Emergency Department (ED) with headache, dizziness, nausea, vomiting, and abdominal discomfort. She had already been seen at an outside ED and was given a course of ciprofloxacin and prochlorperazine, which she had completed. A migraine headache was diagnosed and she was sent out with promethazine. She returned to the ED three days later, still complaining of headache. She was referred to an outpatient resident's clinic without ED evaluation. The resident noted hypokalemia in the second ED evaluation and admitted the patient to the hospital for rehydration. On admission, her family stated that she had been extremely lethargic the past few days, only getting out of bed to go to the bathroom, with assistance. A non-contrast CT of the head showed an hemispheric ischemic infarct in two-thirds of the right cerebellum with associated mass effect. A diffusion weighted MRI and MRA of the head the next morning showed a subacute infarct in the right posterior inferior cerebellar artery (PICA) with compression of the fourth ventricle and dilation of the third and lateral ventricles. A cerebral angiogram showed occlusion of the right PICA and evidence for bilateral vertebral artery dissections with no evidence of fibromuscular dysplasia (images, including 3D, presented in poster). An extensive thrombophilia work-up did not reveal any coagulation disorder. The patient had no risk factors for or family history of stroke. She was started on anticoagulation and showed no further signs of cerebral edema. A urine drug screen was done shortly after admission, which confirmed the presence of phenylpropanolamine (PPA) and ephedrine. After questioning, the patient's husband brought in a bottle of “diet” pills that had ephedrine listed as one of the ingredients. It was unclear whether the patient had taken these pills recently and/or an over-the-counter cough and cold medicine.
DISCUSSION
PPA used to be commonly found in appetite suppressants and in some cough and cold medicines. It was slated to be taken off the market after an FDA advisory in November of 2000. It is associated with hypertension (Cantu, et al., 2003) and is an independent hemorrhagic stroke risk factor in women (Kernan, et al., 2000). Dietary supplements with ephedra alkaloids (ma huang) are also associated with cardiovascular and central nervous system adverse events (Haller and Benowitz, 2000). The FDA announced plans to ban the sale of ephedrine on December 30th, 2003. Scientific evidence for this action is reviewed in the poster. PPA and ephedrine ingestion with resulting hypertension and subsequent vertebral artery dissection may be the mechanism of massive stroke in this otherwise healthy young woman.
EXTRA ADRENAL PARAGANGLINOMA: SYMPTOMS IT MAY CAUSE
T.M. Feinstein1; T. Bui1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115446)
LEARNING OBJECTIVES
1. Recognize pheochromocytoma as a cause of glucose intolerance and ischemic bowel. 2. Diagnose pheochromocytoma using clinical, biochemical and radiological indicators.
CASE
71 year old Caucasian female with a history of COPD, bronchiectasis, myelodysplastic syndrome, recently diagnosed diabetes and worsening anxiety presented with left lower quadrant abdominal pain, headache, excessive perspiration, orthostatic hypotension and elevated WBC. Abdominal CT and ultrasound revealed a sigmoid mass (6 × 5 cm) with central necrosis, separated from the uterus and ovaries, with vascularity within the sigmoid mesocolon. Colonoscopy showed ischemic colitis. Her blood pressure became labile and fluctuated from 200/100 to 80/50 in minutes. She experienced momentary mental status changes, nausea and constipation. EKG and cardiac enzymes show no evidence of a myocardial infarction. 24 hour urine catecholamines and serum catecholamines were significantly elevated. I-131 metaiodobenzylguanidine (MIBG) scintigraphy showed increased uptake in her left pelvic mass, without other foci. Phenoxybenzamine and clonidine controlled blood pressure. Nitroprusside was used during the open, uncomplicated laporatomy. Pathology revealed a 70.7 gram extra adrenal paraganglioma. Urine catecholamines normalized. The patient's symptoms improved and her blood pressure was controlled with a low dose of metoprolol.
DISCUSSION
Pheochromocytoma is a catecholamine-secreting tumor. Common manifestations are headache, excessive perspiration and palpitations. Altered mental status, focal neurological signs, seizures, or stroke may be observed during a paroxysm. Unusual presentations include diabetes mellitus and gastrointestinal complications. Glucose intolerance is present in one-third of patients. Decreased insulin sensitivity and secretion cause carbohydrate intolerance. Pheochromocytoma has an atypical relationship with type 2 diabetes. Diabetic symptoms worsen as the patient's body mass index decreases from catecholamine-induced lipolysis. Glucose intolerance associated with normal body weight and hypertension is a clue in the diagnosis. Gastrointestinal ischemic complications are rare but are more frequent with larger tumors (>70 grams). Biochemical markers are usually elevated 3 times normal. Urine normetanephrine is 97% sensitive. Norepinephrine and epinephrine or normetanephrine and metanephrine are 100% sensitivity when combined. Hydroxymethoxymandelic acid has a sensitivity of 70% with the 95% confidence level (48 mg/g). Plasma catecholamines are more useful when urine values are equivocal. The MIBG scan reveals uptakes by the pheochromocytoma, and it can localize tumors with a sensitivity of 85% and a specificity of 99%.
FALANGA: AN UNEXPECTED CAUSE OF PERIPHERAL NEUROPATHY
E.J. Rourke1; S.S. Crosby1; M. Paasche-Orlow1; M.A. Grodin1. 1Boston University, Boston, MA. (Tracking ID #116708)
LEARNING OBJECTIVES
1. Diagnose history of falanga as a cause of peripheral neuropathy 2. Recognize the possibility of torture as an etiology for otherwise unexplained symptoms in patients from high-risk areas.
CASE
A 38-year-old male from Mauritania with a history of chronic hepatitis B, latent TB infection treated with isoniazid for nine months, depression being treated with mirtazapine, and post traumatic stress disorder presented to Primary Care complaining of longstanding bilateral foot pain. The pain was described as severe burning, associated with numbness, which radiated into the midcalf. The patient described the sensation as “stones in the bottom of his feet,” and stated that the pain was most noticeable at night, preventing him from sleeping. Physical exam revealed decreased sensation below the mid-shin to vibration, light touch, and cold, with minimal pain to palpation on the soles of the feet bilaterally, no visible deformity, and normal deep tendon reflexes. The rest of his exam was normal. CBC, chemistries, liver function tests, hemoglobin A1C, TSH, B12, folate, RPR, cryoglobulins, SPEP, UPEP, and ANA were all normal. HIV 1 and 2, hepatitis C, and hepatitis Be serologies were negative, although hepatitis B antigen was positive. EMG testing revealed moderate to severe peripheral neuropathy, primarily demyelinating in nature, and the patient was diagnosed with sensory distal polyneuropathy, possibly related to chronic hepatitis B infection. At this time, the patient revealed a history of falanga while detained in Mauritania in 1989. The patient stated that he had been suspended by his wrists and ankles from a horizontal pole, and beaten on the soles of his feet repeatedly with a baton, causing severe swelling and pain that prevented him from walking for three weeks. MRI of the feet revealed increased signal within the plantar interosseous and lumbrical muscles bilaterally, consistent with muscle injury or inflammation. The patient was treated with amitriptyline and a lidocaine patch, producing partial relief of his symptoms, and also continued therapy for depression and post traumatic stress disorder.
DISCUSSION
Amnesty International documents the practice of torture in more than 150 different countries, including 30 countries in which falanga is practiced. There may be over 500,000 survivors of torture residing in the United States. Torture survivors seek attention in primary care settings for medical and psychological consequences of torture, and may have difficulty disclosing or describing their experiences due to traumatization. Physicians should be aware of the possibility of a torture history in patients from high-risk areas. Comprehensive information about the diagnosis and treatment of torture survivors is available in the Istanbul Protocol. [http://www.phrusa.org/research/istanbul_protocol/]
FATAL MIGRAINOUS STROKE IN YOUNG MALE
S. Habis1; L. Cation1. 1University of Illinois at Peoria, Peoria, IL. (Tracking ID #115846)
LEARNING OBJECTIVES
To recognize the typical and atypical features and presentations of stroke as complication of migraine.
CASE
A 37 year old male with a history of migraine with aura (IHS criteria) was found unresponsive in bed. He was on no medication, and there was no history of smoking, Alcohol or illicit drug abuse. Upon arrival to ER, he was intubated. Vital signs were stable and neurological exam showed Glasgow coma scale 6, PERRL, positive gag and corneal reflexes, dense right hemiplegia, spontaneous left side movement, symmetric DTR's with downgoing toes. The rest of the exam was normal. The initial evaluation showed normal CBC, chemistries, coagulation profile and negative toxicology screen. A head CT scan (Figure 1) showed acute ischemic infarct in the ACA and MCA territories. Further evaluation including echocardiogram, CXR, ESR, and hypercoagulable state profile were normal. Brain MRI showed acute ischemic infarct in the MCA and distal ACA territories. MRA was normal (Figure 2). The patient was stable on the second day, but Glasgow coma scale was 3 on the third and fourth day and he died on the fifth day. The brain autopsy (Figure 3) showed cerebral ischemia (ACA and MCA) and edema with evidence of subfalcial and uncal herniation on the left —despite maximal medical management—, and new hemorrhagic infarct in the left PCA territory due to compression of the PCA by hippocampal and parahippocampal notching. There was no evidence of atherosclerosis, vasculopathy or embolic event. The final diagnosis was migrainous stroke.
DISCUSSION
Migrainous stroke reportedly causes 1.4% of all strokes in young adults with female gender predominance (statistical differences were noticed in the few studies found in the literature). The posterior circulation especially the PCA territory is the more frequently involved area in migrainous stroke and the size of the infarct is generally small. Fatality in acute stroke, regardless of etiology, range between 1.5–7.3%. Review of literature regarding migraine and stroke between 1977 and 1997 showed that only 44 of 500 patient reported presented with ischemic stroke occurred during acute migraine and that more cases occurred with migraine without aura than with aura. This case is particularly unusual because it affected a male, in anterior circulation territory, and had fatal outcome. We were unable to find a case of fatal migrainous stroke in a young male previously reported in the literature. In summary, we report a case of migraine associated fatal stroke.
FEVER AND CERVICAL LYMPHADENOPATHY
N.S. Shah1; P. Bhat1. 1Columbia University, New York, NY. (Tracking ID #115419)
LEARNING OBJECTIVES
1) Discuss the differential diagnosis of cervical lymphadenopathy and fever in a young, healthy patient, and 2) Identify Kikuchi-Fujimoto disease as a rare, benign cause of cervical lympadenopathy.
CASE
A 24 year-old healthy female presented with 1 month of fevers and unilateral cervical lymphadenopathy. Two-weeks prior to admission she completed a course of broad-spectrum antibiotics without clinical improvement. The patient immigrated from Trinidad 6 years prior and had no history of recent travel, trauma, or contact with animals. She is married, and does not smoke or drink alcohol. She was PPD-negative within the past year. Review of systems was notable for absence of weight loss, dysphagia, or rash. Exam revealed right cervical fullness with multiple soft, mobile, tender lymph nodes, and no erythema or fluctuance. Initial leukocyte count was 5800/L with 81% neutrophils; basic chemistries, liver enzymes, and rheumatologic screen were within normal limits. Sedimentation rate was markedly elevated at 131 mm/hr. Blood and urine cultures were negative. PPD was again negative during the admission. Neck CT revealed multiple, large 1.5–2.0 cm, right upper neck nodes with heterogeneous enhancement, and extensive subcutaneous edema. Fine needle aspiration (FNA) and core biopsy were non-diagnostic, so excisional biopsy was pursued. Histopathology showed near-total effacement of lymph node architecture and replacement by histiocytes, apoptotic cells and nuclear debris consistent with Kikuchi-Fujimoto disease. Special stains for fungi, bacteria, acid-fast bacilli, Epstein-Barr virus, and spirochetes were negative. No atypical cells consistent with malignancy were found. At evaluation two months after hospital discharge the patient reported complete resolution of symptoms.
DISCUSSION
The differential diagnosis of cervical lymphadenopathy with fever is extensive, and includes a variety of infectious causes such as cat scratch disease, mononucleosis, mycobacterial infections, bacterial adenitis; head and neck cancers such as Hodgkin's and non-Hodgkin's lymphoma; and other causes such as Kikuchi-Fujimoto disease. Careful history provides important diagnostic clues, so patients should be probed for exposures, travel, high-risk behaviors, and constitutional symptoms. Definitive diagnosis is made by culture and histology. FNA may be useful in cases of infection, however excisional biopsy provides greater detail about lymph node architecture for diagnosis of malignancy. Kikuchi-Fujimoto disease (histiocytic necrotizing lymphadenitis) is a rare, benign condition of unknown etiology characterized by cervical lymphadenopathy and fevers seen predominantly in young women. Initially described in Asians, the disease is now reported in persons of all races. No effective treatment has been established, but the majority of cases are self-limited within 2–3 months.
FEVER OF UNKNOWN ORIGIN ASSOCIATED WITH LABILE HYPERTENSION
Y.Y. Li1; J. Hefner1; R. Granieri1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115952)
LEARNING OBJECTIVES
(1) Recognize the differential diagnosis of fever of unknown origin (FUO) (2) Recognize the unusual presentations of pheochromocytoma (3) Manage hypertension in patients with pheochromocytoma.
CASE
An 83 year-old female with history of valvular heart disease was admitted to the hospital with a 2 month history of fever, sweats and labile hypertension resistant to therapy with levofloxacin and amoxicillin/clavulanate. She complained of headache, dizziness and dyspnea on exertion. She denied cough, chest pain, nausea, vomiting, abdominal pain, or dysuria. On admission the patient developed rapid atrial fibrillation. Physical examination was remarkable for a temperature of 38.4 degree Celsius, BP 160/100 mmHg, an irregularly irregular heart rhythm, a III/VI pansystolic murmur at the apex, and lower extremity edema. Chest x-ray did not show infiltrate. She was started on ampicillin and gentamicin for subacute bacterial endocarditis although multiple blood cultures were negative and TEE failed to demonstrate definitive vegetations. When fevers persisted, the antibiotics were changed to vancomycin and ceftazidime, again without benefit. Antibiotics were discontinued. CT scans of the chest and abdomen showed congestive failure with a left pleural effusion and a 5 × 5 cm left adrenal mass which was confirmed to be pheochromocytoma by measurement of catecholamines and histopathology. She continued to have fevers, tachyarrhythmias and labile hypertension despite therapy with alpha and beta blockers. She was not thought to be a surgical candidate. The family elected hospice care according to the patient's advance directives.
DISCUSSION
A precise diagnosis of FUO in the elderly can be made 87–95% of the time. Often FUO in the elderly is the result of an atypical presentation of common diseases. Infection is the etiology in 25–35%, connective tissue disease in 25–31%, and malignancy in 12–23% of the cases. After thorough history and physical, focusing on symptoms and signs of intra-abdominal diseases, cardiac and musculoskeletal disorders, tuberculosis and cancers, CXR and basic laboratory studies including ESR, imaging of the abdomen, blood cultures and TEE should be done. All non-essential drugs should be discontinued. Gallium-67 or indium-111 labeled leukocyte scanning may identify infection or malignancy. The presentation of fever, labile hypertension and tachyarrhythmia in this patient can be explained by the pheochromocytoma. Pheochromocytoma may present with fever by producing the internal pyogen interleukin-6. The definitive treatment is removal of the tumor after blood pressure is controlled with both alpha and beta blockers. Beta blockers alone are contraindicated due to resultant unopposed alpha stimulation.
FOR PULMONARY EMBOLISM IS D-DIMER REALLY A SNOUT?
J. Kamali1; M. Elnicki1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #116489)
LEARNING OBJECTIVES
1) To recognize the importance of pre-test probability of disease in the interpretation of diagnostic test results, 2) To recognize limitations in the use of the D-Dimer test to rule out pulmonary embolism (PE).
CASE
A 46 year-old woman with a history of hypertension, type 2 diabetes mellitus and obesity presented with a sudden onset of right-sided pleuritic chest pain of 10 hours duration. The pain began at rest and was sharp, 8/10 in severity and nonradiating. There were no alleviating factors. The patient denied any trauma, recent operations, fever or chills but had a sedentary life style. She was nonsmoker and denied alcohol or illicit drug use. No family history of coagulopathy. Vital signs were normal except respiration rate of 22/minute. Pulse oximetry was 99% on room air. Heart, lung and extremities were unremarkable, but there was moderate right chest wall tenderness on palpation. Laboratory data were notable for normal electrolytes, cardiac enzymes and electrocardiogram. D-Dimer test was negative and a V/Q Scan was read as low probability for PE. Lower extremity dopplers were negative for deep venous thrombosis (DVT). Due to the continued high clinical suspicion of a PE, a pulmonary angiogram was performed which demonstrated filling defects in 3 segmental branches of the right lower lobe pulmonary artery consistent with PE. Subsequent evaluation revealed the presence of the Factor V Leiden mutation.
DISCUSSION
Tests with high sensitivity rule out (Snout) and high specifity rule in (Spin) diseases. These, however, are strongly influenced by the prevalence of the disease in the studied population. Using published clinical prediction rules, this patient has a high pre-test probability of a PE (> 70%). The sensitivity of the D-Dimer ranges from 85%–99% and the specifity from 45%–68%. Based on these, the negative likelihood ratio (LR-) would range from 0.33–0.15 and the post test probability from 35%–1.5%. Given the potential risk of mortality from a PE (26% if untreated), applying an invasive gold standard test (pulmonary angiography) is appropriate. The case illustrates the limitations of the D-Dimer assay in this particular diagnostic strategy. D-Dimer is the primary product of the enzymatic degradation of cross-linked fibrin by plasmin and is elevated in the presence of thrombosis. However elevated D-Dimer levels are not limited to PE or DVT, and the absence of elevated D-Dimer levels does not always rule out PE. While the D-Dimer assay has a high sensitivity, its negative predictive value depends upon the pre-test probability of disease, and must be evaluated within the clinical context.
FORMULARY CONVERSION PROGRAMS POSE A SIGNIFICANT RISK TO PATIENT SAFETY
S. Singh1; R. Shrivastava1; V. Das1. 1Unity Health System, Rochester, NY. (Tracking ID #116917)
LEARNING OBJECTIVES
Recognise that potential for adverse outcomes exist when formulary conversion programs are implemented without adequate post-conversion pharmaco-vigilance; and understand the differences in the pharmocokinetic properties of statins which affect their potential for interaction with other drugs.
CASE
A 74-year-old white female was admitted for Pleural effusion. Her medical history included orthotopic cardiac transplantation for ischemic cardiomyopathy, hypertension, hypercholesterolemia, peripheral vascular disease, diabetes mellitus and renal insufficiency. She was on a stable dose of cyclosporine, azathioprine and atorvastatin.Atorvastatin 20 mg was switched to simvastatin 40 mg on admission because of formulary restrictions. On the 12th day the patient complained of generalised weakness. She gradually developed increasing weakness of her limbs requiring ICU monitoring. Her renal function deteriorated further requiring dialysis. A peak CK level of over 10,000 mg/dl was noted by Day-29. Cyclosporine levels which were normal earlier peaked at 681 ng/ml on Day-29. Statin induced rhabdomyolysis was diagnosed. She died a few days later.
DISCUSSION
CYP3A4 is responsible for more than half of clinically significant drug interactions seen in clinical practice. Lovastatin, simvastatin and atorvastatin are metabolized by CYP3A4 while pravastatin and fluvastatin are not. Cyclosporine is a potent inhibitor of CYP3A4 increasing the risk of myopathy induced by statins using CYP3A4 pathways. In this case automatic substitution from a stable dose of atorvastatin to a higher dose of simvastatin contributed to the outcome as statin related myopathy is known to be dose dependant. Additional risk factors for myopathy included age, female sex, and renal disease. Health Care organizations' use of formulary conversion programs, such as an automatic drug substitution policy to maximize resources raise several concerns regarding patient safety. This case demonstrates the need to screen for drug-drug interactions, contraindications, and appropriate dosage conversion to minimize risks of Adverse Drug Reactions while implementing a formulary conversion program. On-going provider education, provisions allowing physicians to use their judgment in using a particular drug overriding the substitution policy, and better institutional pharmaco-vigilance should be integral parts of any safe drug substitution policy.
FUSOBACTERIUM PERICARDIAL EFFUSION CAUSING TAMPONADE—A RARE ENTITY
S.A. Dharashivkar1; R. Goodman1; A. Waissbluth1; S. Nix1; S. Goldberg1. 1The Jewish Hospital, Cincinnati, OH. (Tracking ID #115812)
LEARNING OBJECTIVES
1. Recognize anaerobic pericarditis as an uncommon but potentially fatal condition. 2. Know the management of anaerobic pericardial effusion.
CASE
A 50 year old lady on chronic steroids (5 mg/day) for lupus presented with complaints of severe chest pain which started four hours prior to presentation. The pain was worse on deep inspiration, lying down and on activity and improved on sitting. Initial physical exam revealed a blood pressure of 115/87 mmHg, pulse of 85/min, oxygen saturation 99% and a normal cardiovascular exam. During the ER stay, her pain worsened, blood pressure dropped to 64/48 mmHg and oxygen saturation dropped to 85% on room air. An emergent CT scan for suspected pulmonary embolism showed the presence of a large pericardial effusion. Echocardiogram confirmed findings of pericardial tamponade, and pericardiocentesis yielded 600 cc of opaque, thick, yellowish brown fluid. Pericardial fluid results showed 158,000 WBCs (neutrophils 97%), 10,000 RBCs, Glucose <2 mg/dL, LDH 1,850. Gram stain showed gram-negative rods. The patient was empirically started on piperacillin-tazobactam and vancomycin. Anaerobic cultures showed a growth of Fusobacterium susceptible to Ertapenem. The patient was discharged home on intravenous antibiotics and was stable after two weeks.
DISCUSSION
Anaerobic pericarditis is uncommon and associated with significant morbidity and mortality. The source of origin is usually either a contiguous source of infection, endocardial spread, hematogenous seeding or direct inoculation. The high mortality associated with anaerobic organisms makes it imperative to identify the organism quickly to initiate the right antibiotics. A search for the source of infection should be done. In our patient no obvious source of infection was identified. An oral panoramic scan which was planned after discharge could not be done as the patient did not follow-up. Her immunocompromised status due to chronic steroids predisposed her to this unusual condition most likely caused by oral anaerobes. Appropriate treatment with pericardiocentesis and antimicrobials had a good outcome in this potentially fatal condition. To the best of our knowledge, of the six reported cases of Fusobacterium pericarditis including this one, this is the second case where the patient survived.
GENES! ANSWER TO AN ELUSIVE CASE OF RECURRENT ABDOMINAL PAIN
S. Daya1; F.V. Caplan1; D.T. Francois1. 1York Hospital, York, PA. (Tracking ID #116792)
LEARNING OBJECTIVES
1) Recognize familial mediterranean fever as a etiology of recurrent abdominal pain. 2) Recognize the right testing to make the diagnosis following clinical suspicion. 3) Recognize the significant reduction in morbidity and prevention of a life-threatening complication with institution of therapy.
CASE
A 36 year old male physician of Ashkenazi Jewish descent presented to the office with more than 20yr history of recurrent self-limited episodes of abdominal pain. Pain was characterized by insidious onset of incapacitating central abdominal pain along with right sided pleuritic chest pain. There was associated “sensation” of fever with no documented raise in temperature. The episodes apparently had started as a teenager. Ibuprofen was the only medication thought to relieve pain. He was diagnosed to have irritable bowel syndrome after extensive workup of his symptoms upto this stage. The patients brother was being investigated for similar symptoms at a university center. During his last episode, abdominal exam by his physician spouse was remarkable for diffuse rigidity and decreased bowel sounds. Laboratory investigation was significant for a microcytic anemia with low iron indices and a raised CRP and sedimentation rate. Endoscopic evaluation for anemia was unremarkable. After careful review of the patients history and progress, a syndrome of hereditary periodic fever was entertained. He was referred to National Institutes of Health for genetic testing of familial Mediterranean fever. A homozygous familial mediterranean fever (FMF) mutation V726A was revealed confirming the diagnosis. Following the institution of therapy with colchicine the frequency of episodes of abdominal pain diminished significantly.
DISCUSSION
Familial mediterranean fever is a genetic disorder inherited as an autosomal recessive trait, prevalent in individuals of jewish descent. The gene named MEFV(MEditerranean FeVer) is located on short arm of chromosome 16 and encodes a protein called pyrin or marenostrin. The expression of the protien is considered to be most likely restricted to mature neutrophils and appears to act as an intranuclear regulator of transcription of peptides involved in inflammation. The clinical presentation is characterized by sporadic, acute self-limited attacks of fever often accompanied by inflammation of serosal surfaces like peritoneum or pleura and erythematous skin lesions. Diagnosis is made in individuals of appropriate ethnic background, history and confirmation by genetic analysis. Treatment is effected by colchicine, which significantly reduces the number of acute attacks and prevents AA amyloidosis which dictates life expectancy and prognosis. Genetic testing is a relatively new modality used for diagnosis of FMF. Early diagnosis and institution of colchicine can prevent significant morbidity and a life threatening complication.
GUAIFENESIN AND EPHEDRINE NEPHROLITHIASIS
R.S. De Jesus1. 1Mayo Clinic, Rochester, MN. (Tracking ID #116979)
LEARNING OBJECTIVES
1. Identify features of guaifenesin and ephedrine stones. 2. Recognize data from history that give rise to this entity. 3. Alert clinicians to yet another sequelae of ephedrine/guaifenesin abuse.
CASE
A 37 year old Caucasian female presented with approximately 5–6 hour duration of right flank pain that radiated to her right groin and nausea. There was no vomiting, fever, chills, dysuria or urinary urgency. Her medical history was significant for recurrent uric acid stones, renal tubular acidosis and prior lithotripsy. She had smoked one pack per day for 20 years but had only minimal alcohol use. She had a remote history of substance abuse. Vital signs on admission were T: 36.7, PR: 85/min., RR 18/min, BP: 130/68. There was right CVA tenderness and right inguinal discomfort on palpation. Laboratory tests showed no leukocytosis, normal creatinine and uric acid levels. Urinalysis showed 51–100 rbc/hpf and 4–10 wbc/hpf. A CT of the abdomen and pelvis showed a focal area of high attenuation in the distal right ureter proximal to UV junction, which measured 5 mm; there was perinephric stranding, right hydroureteronephrosis and the presence of several bilateral renal stones. She was dismissed to outpatient follow-up with scheduled cystoscopy and right ureteroscopy. She returned to the clinic three days later bringing with her several stones, which she had passed out at home. Analysis revealed guaifenesin metabolites as well as traces of ephedrine. She subsequently admitted to taking “cold medications”.
DISCUSSION
Guaifenesin and ephedrine induced renal calculi have only been recently described in the literature as a novel form of drug-induced renal stones in patients who consume large amounts of over-the-counter preparations containing these two substances for use mainly as stimulants. The stones are light tan in color and have radiographic properties similar to those of uric acid, which may lead to diagnostic confusion. They are radiolucent on standard X-ray imaging but can be demonstrated on unenhanced CT. Stone analysis using high performance liquid chromatography would show 70% beta-2-methoxyphenoxy-lactic acid (a guaifenesin metabolite) and only 5% ephedrine. The exact mechanism of stone generation has yet to be elucidated but hypocitraturia is thought to play a role. The initial management is similar to that of individuals with other types of calculi. An opportunity is given to pass ureteral sones spontaneously before resorting to minimally invasive techniques such as ureteroscopic extraction or shock-wave lithotripsy. Substance abuse counseling is strongly recommended after stone passage or removal to prevent future recurrence.
HEMODIALYSIS FOR VALPROIC ACID OVERDOSE
T. Siddiqi, MD1. 1The University of Connecticut, Farmington, CT. (Tracking ID #116033)
LEARNING OBJECTIVES
To illustrate the effectiveness of hemodialysis (HD) in clinically severe overdose of valproic acid (VPA).
CASE
A 43 year old woman, with a history of bipolar disorder, opioid anelgesic drug addiction and alcohol abuse, was admitted with obtundation following a suicide attempt with VPA ingestion (up to 60g). On admission she was comatose (GCS 6), hypotensive, and had fixed dilated pupils. Her initial VPA level was >1500 mg/l. She was hypocalcemic with prolonged QTc in her EKG. Her CBC, chem 7, NH3 level and LFTs were within normal limits. Imaging of the head and brain was unremarkable. She was intubated for airway protection and respiratory depression. 2 doses of charcoal were administered for gastrointestinal decontamination. HD was started within a few hours of presentation. 3 hours into dialysis, her VPA level was found to be 1107 mg/l and dropped further to 591, 261, and 182 mg/l with continuing HD (T1/2=2.6 hrs). The following day, it was 148 mg/l, and finally 70 mg/l. Further HD was not required. On awakening and extubation a few days later, the patient underwent a psychiatric evaluation and was subsequently transferred to a psychiatric facility.
DISCUSSION
VPA is an anti-epileptic agent that is widely used for prophylaxis of bipolar disorders and migraines. Its toxicity is being reported to poison control centers with increasing frequency in recent years. Severe symptoms include coma, respiratory depression, hemodynamic instability, multi-organ failure, and death. VPA's low molecular weight (144 daltons) and low volume of distribution suggest a potential benefit of HD. Although there is a high degree of protein binding, in severe toxicity proteins are saturated and most of the drug is found in its free form in the body. Thus, efficacy of HD increases in this situation. However, there is limited data guiding the use of HD for extracorporeal removal of VPA in severe overdose cases. Among the handful of reported cases of HD use (with or without hemoperfusion) for VPA toxicity, there has been a >60% success rate. Elimination half-life was markedly reduced in these cases. A similar effect was noted in our patient. Controlled trials are required to assess whether removal of VPA by HD significantly improves outcome among overdose patients.
JUST STUNG BY A BEE ... WHAT WENT WRONG
T. Thenappan1; S. Parikh1; P. Kapoor1; K. Shankar1; H. Friedman1. 1St. Francis Hospital, Evanston, IL. (Tracking ID #115559)
LEARNING OBJECTIVES
1.Diagnose Henoch Schonlein Purpura (HSP) as a late complication of bee sting. 2.Recognize HSP in an adult patient presenting with purpuric rash, abdominal pain and arthralgia.
CASE
A 41-year-old Caucasian male, with no significant past medical history, presented with a non pruritic rash of both the legs for three weeks. The rash started one week after a bee sting. Subsequently, he developed diffuse, crampy abdominal pain accompanied with swelling of the knee and ankle joints. The physical examination was notable for epigastric tenderness and a palpable purpuric rash in both the lower extremities. His stool was positive for occult blood. Laboratory investigations revealed leukocytosis (20,000 cells/cumm), elevated ESR, microscopic hematuria and proteinuria. The CT scan of the abdomen showed marked thickening of the third and fourth parts of the duodenum and the proximal ileum. Subsequent upper endoscopy visualized multiple linear deep serpiginous ulcerations in the duodenum. The skin biopsy of the purpuric rash demonstrated leukocytoclastic vasculitis associated with fibrinod deposits. His serological work up for hepatitis B and C were negative as were the HIV, EBV, CMV and Mycoplasma titers. ANA, ANCA and cryoglobulins were within normal limits. The diagnosis of HSP was established based on the skin lesions and joint, renal and gastrointestinal involvement. The patient responded remarkably to intravenous corticosteroids.
DISCUSSION
HSP, also referred to as anaphylactoid purpura, is a diffuse systemic, small vessel hypersensitivity vasculitis occurring predominantly in children. It is characterized by non-thrombocytopenic purpuric rash of the lower extremities, arthralgia, abdominal pain and renal involvement. The presumptive pathogenic mechanism is IgA dominant immune complex deposition in venules, capillaries and arterioles. The common inciting antigen is an upper respiratory infection. However, various drugs, food, immunization and insect bite have also been suggested. In our patient, in the absence of other triggers, the most probable precipitating factor is the bee sting. A small percentage of patients with HSP progress to renal failure. Therefore, diagnosing HSP as a late complication of bee sting is important. Close follow up with repeated urinalysis is warranted. HSP, a disease once thought to be confined to children is increasingly being diagnosed in adults. This case underscores the need to consider classic syndromes even in patients who, because of age or other demographic factors, are at relatively low risk.
HEPATIC SARCOIDOSIS
J.A. Kasher1. 1UCLA San Fernando Valley Program, Sylmar, CA. (Tracking ID #116588)
LEARNING OBJECTIVES
1) Recognize clinical presentation of hepatic sarcoidosis. 2) Recognize hepatic sarcoidosis as an unusual cause of end-stage liver disease.
CASE
45-year-old female presented with acute upper gastrointestinal bleeding as well as complaints of progressive jaundice, fatigue, pruritis, and unintentional weight loss over a one year period. Endoscopic evaluation revealed esophageal variceal bleeding which was treated with banding. Examination revealed jaundice as well as several stigmata of chronic liver disease including hepatosplenomegaly, mild ascites, prominent superficial abdominal veins, hemorrhoids, malar telangiectasias, and palmar erythema. Also present were numerous skin excoriations and lichenification due to scratching. The remainder of the physical exam, including assessment of neurologic status, was unremarkable. Her liver tests showed total bilirubin 2.9, alkaline phosphatase 544, AST 52, and ALT 62. Other routine chemistries, blood count, and chest x-ray were normal. Liver biopsy showed cirrhosis, non-caseating granulomas, bile duct obstruction, and small bile duct loss. Fungal and acid fast bacillus cultures showed no growth. Mitochondrial antibody titers were negative, and angiotensin converting enzyme (ACE) level was elevated at 92 (normal 9–67). The diagnosis of hepatic sarcoidosis was made. Subsequently, the patient's hepatic dysfunction progressed over the next few months and she developed encephalopathy. The patient consequently underwent liver transplantation and clinically improved. Six months postoperatively, her course was complicated by CMV infection of the liver, but no recurrence of granulomatous disease was present on repeat liver biopsy.
DISCUSSION
Sarcoidosis is a disease of unclear etiology characterized pathologically by the presence of non-caseating granulomas. Pulmonary involvement is present in 95% of cases, but many other organ systems can be affected. About 11% of patients have hepatic involvement. Most cases are subclinical, and symptomatic liver involvement is reported in less than one percent of patients with sarcoidosis. Patients with symptomatic hepatic sarcoidosis usually present with fever, malaise, weight loss, jaundice, and pruritis. Chest x-ray may not necessarily show evidence of pulmonary involvement. Hepatosplenomegaly is common, and elevation of alkaline phosphatase is commonly seen. A Kveim-Siltzback skin test may be positive, and mitochondrial antibody test is negative. ACE level is elevated in about 75% of cases. These findings help differentiate sarcoidosis from other causes of granulomatous hepatitis including mycobacterial, fungal, and toxoplasma infections, drug reactions, Crohn's disease, primary biliary cirrhosis and Hodgkin's disease. Hepatic sarcoidosis may spontaneously improve or may show a relentless progression as occurred in this patient. Prednisone may be of benefit in the treatment of hepatic sarcoisosis, and liver transplantation remains an option in cases of end-stage liver disease.
HEPATIC SINUSOIDAL DILATATION WITH ISOLATED ELEVATED ALKALINE PHOSPHATASE AS PARANEOPLASTIC SYNDROME IN HODGKIN's DISEASE
S. Habis1; L. Cation1. 1University of Illinois at Peoria, Peoria, IL. (Tracking ID #116257)
LEARNING OBJECTIVES
To recognize the clinical presentation of Hodgkin's disease despite the absence of lymphadenopathy. To recognize the hepatomegaly and elevated alkaline phosphatase as paraneoplastic effect of Hodgkin's disease.
CASE
21 years old male presented with weight loss, severe fatigue, and night sweats of several months duration. No past medical history. Physical examination was remarkable for hepatomegaly and no lymphadenopathy. Laboratory evaluation revealed hemoglobin 9.5 g/dL, MCV 71.6 FL, normal WBC and differential, albumin 2.6 g/dL, ANA 1:640 speckled, ESR 131 mm/hr, and alkaline phosphatase 330 U/L. Chest X-ray was normal. CT scan of the abdomen revealed hepatomegaly without distinct mass, no lymphadenopathy. Iron saturation was 6.5%. The following subsequent tests were normal or negative: urinalysis, PT/INR, PTT, HIV Ab, TSH, CMV PCR, anti-DNA Ab, SSA, SSB, SCL-70, RNP, anti-mitochondrial Ab, anti-smooth muscle Ab, anti-gliadin Ab, anti-endomysial Ab, anti-phospholipid Ab screen, bone scan, small bowel follow-through X-ray. Upper and lower GI endoscopy revealed mild gastritis, small pre-pyloric ulcer with upper and lower GI biopsies negative for malignancy. The patient was started on Omeprazole and iron therapy. Over the next 3 months he felt progressively worse. Repeat labs were unchanged. A bone marrow biopsy revealed no malignancy. A liver biopsy revealed prominent sinusoidal dilatation and no malignancy. A repeat chest X-ray was interpreted as having mediastinal widening and a chest CT revealed a mediastinal mass. Biopsy of a lymph node revealed Hodgkin's disease. The patient was started on chemotherapy and his hepatomegaly and alkaline phosphatase elevation resolved at the completion of therapy.
DISCUSSION
Lymphoma was high on the differential diagnosis list for this patient at presentation as patients with lymphoma commonly present with fatigue, night sweats, and weight loss. The initial normal chest X-ray, hepatomegaly, and elevated alkaline phosphatase prompted an exhaustive, expensive, and time-consuming search for a hepatic or GI etiology to his complaints. An earlier chest CT scan may have revealed the diagnosis closer to his presentation. The paraneoplastic effects of Hodgkin's disease are less common but can involve many organs including the liver. The finding of an elevated alkaline phosphatase and liver sinusoidal dilatation have been described in the setting of Hodgkin's disease. An unexplained elevated alkaline phosphatase should prompt the primary care physician to consider Hodgkin's disease in the differential. Our case illustrates an uncommon presentation of a common malignancy and the pearls and pitfalls in the evaluation of a suspected lymphoma case.
HIV & GLOMERULUS—IS RENAL BIOPSY NEEDED?
S. Parikh1; P. Kapoor1; T. Thenappan1; H. Friedman1. 1St. Francis Hospital, Evanston, IL. (Tracking ID #102039)
LEARNING OBJECTIVES
1. Identify HIV associated immune complex diseases (HIVICDs) with its relatively good prognosis as distinct from the classic HIV associated nephropathy (HIVAN). 2. Type I membranoproliferative glomerulonephritis (MPGN) can occur in patients infected with HIV without hepatitis C virus (HCV) co-infection.
CASE
A 28-year-old African American male presented with progressively increasing shortness of breath and generalized swelling of one week duration. His blood pressure was 174/100 mmHg. The physical examination revealed anasarca and bilateral crackles on auscultation of the lungs. The serum creatinine was 1.6 mg/dl and serum albumin was 2.1 mg/dl. The urine dipstick revealed 4+ protein and 11 RBCs/ hpf but no casts. A 24 hour urine collection demonstrated 6.25 gm of protein and a creatinine clearance of 55 ml/min. The renal sonogram showed normal sized kidneys. C3 and C4 levels were normal. Serum cryoglobulins, HCV and Hepatitis B antibodies were undetectable. HIV status, confirmed by western blot was positive. His CD4 count was 1154/µl and HIV RNA viral load was 26,000 copies/ml. The kidney biopsy revealed subendothelial and mesangial deposits on electron microscopy that were positive for IgG and C3 on immunofluoresence staining, consistent with the diagnosis of Type I MPGN.
DISCUSSION
A wide spectrum of glomerulopathies is associated with HIV infection. HIVAN accounts for about 90% of such lesions. The classic clinical picture of HIVAN is azotemia, proteinuria and enlarged echogenic kidneys on sonogram. Hypertension and edema are conspicuously absent. Histologic features are consistent with focal segmental glomerulosclerosis. It seems to be related to a direct effect of HIV on the renal epithelium. On the other hand, HIVICDs account for only about 10% of renal lesions in HIV infected patients. These include MPGN and IgA nephropathy, which occur secondary to the deposition of circulating immune complexes against an HIV related antigen in the renal tissue. Type I MPGN usually occurs in the subset of HIV patients co-infected with HCV. However, it can occur independent of HCV co-infection, as illustrated by our case. The relatively less aggressive course of renal disease and good prognosis in HIVICDs contrasts the rapid course and poor prognosis associated with HIVAN. These features emphasize the importance of differentiating the two subsets accurately with a renal biopsy. Our patient was started on an ACE inhibitor, his symptoms gradually improved and proteinuria decreased to 300 mg/day four weeks later.
HIV INFECTION REVEALED BY RAMSAY HUNT SYNDROME
C. Rathnakumar1; K. Subramanian1; T. Vallur1; J. Patel1; R. Mills1; A. Grigoriu1. 1Jersey City Medical Center, Jersey City, NJ. (Tracking ID #117239)
LEARNING OBJECTIVES
Ramsay Hunt Syndrome, caused by Herpes Zoster of the geniculate ganglion, consists of Ipsilateral facial palsy associated with zoster oticus (herpetic eruptions of the pinna and some times of the palate & of the occipital region) frequently with deafness. Herpes Zostercan present as Ramsay Hunt Syndrome in the background of immunodeficiency.
CASE
A 48-year old male with no significant past medical problems, presented with dizziness, unsteady gait, tinnitus and decreased hearing in the left ear. Symptoms started two weeks earlier, with tingling sensation and sharp pain in his left ear, followed by serosanguinous discharge, for which he was treated with an oral antibiotic for one week, with no benefit. Thereafter, he started to have vertigo, tinnitus & deceased hearing in his left ear and then asymmetry of his face. On physical exam, the patient was found to have left peripheral facial palsy, dry dark crusts & wet excoriations with surrounding erythema of the left external auditory canal, shinny intact tympanic membrane and left sensorineural deafness. Based on the characteristic clinical presentation, the diagnosis of Ramsay Hunt Syndrome was made. Patient was treated with a course of acyclovir and prednisone, in addition to tarsorrhaphy. As a part of work up, HIV test was done (both ELISA and Western Blot) and the patient was diagnosed to have HIV positive. The absolute CD4 count (1,349) and CD4/CD8 ratio (1.34) were within the normal range. Patient was discharged and physical therapy was implemented for gait training.
DISCUSSION
Ramsay Hunt Syndrome can be the sole manifestation of HIV infection in an otherwise healthy young adult, as presented in our patient. Physicians should recognize that it is essential to test for HIV in any person presenting with Herpes Zoster infection, including Ramsay Hunt Syndrome.
HUMAN IMMUNODEFICIENCY VIRUS ASSOICATED NEPHROPATHY
S. Moparty1; J. Aliota1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117539)
LEARNING OBJECTIVES
1. HIV infection is associated with multiple types of renal disease 2. The most common type of HIV associated nephropathy is focal glomerulosclerosis (FGS)
CASE
A 38 year-old man presented with three-months of progressively worsening headaches. On admission, the cachectic patient was noted to be hypertensive and azotemic with a blood pressure of 185/98 mmHg and a serum creatinine of 33 mg/dl. The patient was emergently dialyzed. A renal biopsy was performed revealing collapsing glomerulosclerosis consistent with human immunodeficiency virus (HIV) associated nephropathy. Upon further questioning the patient revealed a remote history of intravenous drug use, and admitted to rare but unprotected sexual relations. The patient tested positive for both hepatitis C and HIV, with a HIV viral load of 62,000, and a CD4 count of 290.
DISCUSSION
HIV infection is associated with many renal diseases, the most common of which is focal segmental glomerulosclerosis (FSGS) affecting two to ten percent of all patients with HIV, is a often accompanied by severe tubulointerstitial damage. This type of injury can be seen in asymptomatic or primary HIV infection as well as in advanced disease. The mechanism of HIV-mediated renal cell injury is unknown though it is thought that the interaction between mesangial cells and HIV-infected CD4 cells may lead to mesangial hypertrophy and sclerosis. It is postulated that preexisting hypertension or diabetes accelerates HIV nephropathy, perhaps by further stimulating mesangial cell hypertropthy. Treatment of this disease centers on HAART therapy and ACE inhibition (in patients with serum creatinine <2.0 mg/dl), although both of these modalities lack a well-controlled clinical trial. General internists should be aware of the potential for aggressive renal disease in HIV-infected patients, and should be particularly vigilant in caring for HIV-infected patients that also have hypertension or diabetes.
HYPERCALCEMIA AND IMMBOLITY: NEED WE KNOW MORE?
P. Chahal1. 1Mayo Clinic, Rochester, MN. (Tracking ID #115158)
LEARNING OBJECTIVES
1. Recognize the consequences of prolonged immobilization in hospitalized population 2. Recognize immobilization as one of the etiologies of hypercalcemia
CASE
63 year old male with multiple medical problems was admitted with the history of ankylosing spondylitis, disseminated coccidiomycosis involving lumbar spine leading to multiple surgeries of his lumbar spine and prolonged hospitalization for almost three months was found to have serum calcium of 11.9 mg/dl. His physical examination was unrevealing. His past medical history was significant for colonoscopic removal of malignant polyp fourteen months ago. His medications were also noncontributory. Consequently, his workup of hypercalcemia revealed normal alkaline phosphatase, phosphorous, creatinine, 25 hydroxy vitamin D, serum protein electrophoresis, TSH, Angiotensin converting enzyme level, parathyroid hormone related peptide, mildly suppressed PTH at 0.4 pmol/L (range 1.0–5.2 pmol/L), normal bone scan. He also underwent extensive workup to exclude malignancy as etiology with CT scan of chest, abdomen and pelvis, MRI of abdomen revealed two lesions in liver, which were biopsied revealing benign reactive tissue. He also underwent repeat colonoscopy, which was also normal. He was started on scheduled physical therapy sessions and short course of Calcitonin and single dose of Pamidronate with subsequent normalization of his serum calcium level at one and half month follow-up.
DISCUSSION
Hypercalcemia is frequently encountered metabolic abnormality. The most common etiology in the hospitalized population is the malignancy. Hence, this fact leads to battery of laboratory and radiologic tests in pursuit of finding occult or overt malignancy in the hospitalized population. Evident etiologies like prolonged immobilization leading to high bone turnover escape physician's attention. It is important to consider prolonged immobility as one of the etiologies of hypercalcemia. This could avoid unnecessary, expensive and potentially harmful investigations.
Table 1.
Laboratory features of common causes of Hypercalcemia
| Laboratory | Malignancy | Hyperparathyroidism | Immobilization |
|---|---|---|---|
| PTH | low | high | low to low-normal |
| PTHrp | High | Normal | Normal |
| Vitamin D | Low | Normal | Low or normal |
| Phosphorous | Loq | Low | normal |
HYPERCALCEMIA AND NORMAL CALCIUM ON ADMISSION?
N. Gupta1; S. Wali2. 1Olive View- UCLA Medical Center/UCLA-San Fernando Valley Program, Sylmar, CA; 2Olive View-UCLA Medical Center/UCLA-San Fernando Valley Program, Sylmar, CA. (Tracking ID #117408)
LEARNING OBJECTIVES
1) Recognizing hyperparathyroidism as an unusual cause of acute severe hypercalcemia in an inpatient setting. 2) Review the main causes of hypercalcemia and their typical clinical presentations/treatment.
CASE
A 70 y.o. Hispanic female was admitted to the surgical service for elective adrenalectomy for a 6 cm mass found on CT, suspicious for tumor. Past medical history includes hypertension, Hepatitis C, and liver cirrhosis. The surgery was complicated by severe blood loss. Replacement blood products, IV fluids, and FFP were given with secondary development of iatrogenic pulmonary edema, requiring ICU transfer for intubation and diuresis. ICU course was further complicated by Pseudomonas pneumonia, and Stenotrophomonas and Enterococcus sepsis. After 2 weeks in the ICU, the patient was extubated and transferred to the general medicine team in stable condition, with subsequent development of altered mental status, nausea, vomiting, and rapidly rising calcium from baseline levels (8.8 on admission, 9.5 on transfer from ICU, peaking to 12.2 over 4 days). Significant lab values include: Na+= 151; Cr = 1.3; glucose = 154, Mg = 2.2, phosphorus = 2.3; albumin = 3.0; ionized calcium = 6.2 at peak (normal: 4.4–5.2); PT= 20; INR = 1.8; intact PTH = 132 (normal: 10–65); 24 hour urine calcium = 418 mg/24 hour (normal: 100–200). A head CT without contrast was negative for any acute CVA/intracranial hemorrhage/mass. The patient also received a CT chest/abdomen/pelvis, which showed a cirrhotic liver and a large right subcapsular renal hematoma, but no other evidence of any masses suggestive of malignancy. EKG was normal. The patient was treated with IV pamidronate with subsequent resolution of hypercalcemia over 2 days. Furosemide and IV fluid treatment for hypercalcemia were held secondary to mild hypernatremia. The patient was diagnosed with hypercalcemia secondary to hyperparathyroidism despite normal calcium levels on hospital admission.
DISCUSSION
IMPLICATION/DISCUSSION: Hypercalcemia is a relatively common clinical problem. Although the differential for hypercalcemia is extensive, hyperparathyroidism and malignancy account for approximately 90% of all cases. Hyperparathyroidism is the most common cause in an ambulatory setting, accounting for greater than 90% of cases. In hospitalized patients, cancer is the most common cause, accounting for approximately 65% of cases, with hyperparathyroidism accounting for approximately 25% of inpatient cases. In primary hyperparathyroidism, calcium levels are usually only slightly elevated, usually less than 11 mg/dL and rarely exceed 12.5 mg/dL. Calcium levels greater than the aforementioned values, are usually attributable to a malignancy. Clinical manifestations include lethargy, weakness, constipation, pancreatitis, nephrolithiasis, shortened QT interval, psychiatric disturbances. Treatment includes heavy hydration, lasix for calciuresis, calcitonin, biphosphonates, and steroids depending on the degree of hypercalcemia.
HYPERTENSION IN PATIENTS WITH UNILATERAL ATROPHIC KIDNEY: AN UNCOMMON ETIOLOGY OF A VERY COMMON MEDICAL DIAGNOSIS
Y.Y. Li1; R. Granieri1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115244)
LEARNING OBJECTIVES
1) Recognize the relationship between hypertension and unilateral atrophic kidney 2) Recognize multiple causes of unilateral atrophic kidney 3) Recognize the difference in the management of hypertension in patients with unilateral atrophic kidney or essential hypertension.
CASE
A 37 year-old male presented with a 3 week history of mild right flank pain. He denied dysuria, hematuria, fever, chills, nausea or vomiting. He was not on any medications. Physical examination was unremarkable except blood pressure was 130/85 mmHg. An abdominal ultrasound revealed an atrophic right kidney, a compensated left kidney with no hydronephrosis. The flank pain subsided without further therapy. About 2 years later, the patient developed hypertension with blood pressure of 150/100 mmHg. MRA showed minimal blood flow through the right renal artery and a widely patent left renal artery. Additional studies showed 24 hour urine protein 90 mg, creatinine clearance 93 ml/min, plasma renin activity 21U/ml (normal 5–13 U/ml; >13 suggests renovascular hypertension), normal VMA, metanephrine, catecholamines, and aldosterone. Therapy with ramipril was started, 2.5 mg daily, for 2 weeks, then 5 mg daily, which lowered blood pressure to below 120/80 mmHg.
DISCUSSION
Unilateral small kidney may result from a variety of causes, including chronic pyelonephritis, obstructive renal atrophy, renal artery stenosis with ischemia, congenital hypolastic kidney, tuberculosis, radiation therapy and partial nephrectomy. Hypertension in patients with unilateral small kidney may be essential or secondary hypertension. The small kidney in renal artery stenosis is usually associated with difficult to control hypertension. In animal models of renovascular hypertension (2K1C Goldblatt), the elevated blood pressure is associated with renin hypersecretion from the underperfused kidney and overactivation of the renin-angiotensin-aldosterone system. However, elevated plasma renin activity is found in only 50–85% of patients with renovascular hypertension, and may be seen in 16% of patients with essential hypertension. Medical therapy should be the primary management except in uncomplicated fibromuscular dysplasia, which is usually amenable to angioplasty. One should be cautious in using high dose ACE inhibitors in patients with high renin state because of tendency of causing profound hypotension, and in patients with possible bilateral renal artery stenosis because of the risk of reversible acute renal failure. The present patient developed hypertension several years after the unilateral small kidney was incidentally found. The hypertension may be caused by the small kidney because the plasma renin activity was significantly elevated, which may also account for the hypersensitivity to ACE inhibitor therapy.
HYPERTHERMIC EMERGENCY: A CASE OF POST-CARDIAC ARREST HYPERPYREXIA
J. Silberman1; B. Taqui1. 1Temple University, Philadelphia, PA. (Tracking ID #116239)
LEARNING OBJECTIVES
1. Review differential for hyperthermic emergencies. 2. Recognize post-cardiac arrest hyperthermia as a rare, but established clinical entity. 3. Review treatment options for hyperthermic emergencies.
CASE
A 60 year old Hispanic male with ischemic, dilated cardiomyopathy (EF 20%) complained of chest pain and was subsequently found unconscious. He presented in a ventricular fibrillation cardiac arrest. His initial ABG revealed pH 6.24, pCO2 73, pO2 282, HCO3 15.3, saturation of 98% on AC with an FiO2 of 1.0. After an hour long resuscitation, he converted to sinus tachycardia with 6mm ST segment depressions in the inferolateral leads. His troponin I peaked at 220. At this time, he had rectal temperature of 108F. No inhaled anesthetics had been used during resuscitation. His family denied history of malignant hyperthermia or use of psychiatric medications or illicit drugs. Urine drug screen was negative. He was packed in ice and administered intravenous dantrolene with a resultant decrease in his temperature to 103F. He remained hypotensive despite multiple inotropes and was too unstable to be transported for head CT. He subsequently developed multiorgan failure. Because of the grave prognosis, his family withdrew care and the patient expired.
DISCUSSION
The most important causes of severe hyperthermia (temperature greater than 104F) are heat stroke, neuroleptic malignant syndrome, and post-anesthetic malignant hyperthermia. Although not well recognized, there is also an established relationship between cardiac arrest and post-resuscitation hyperthermia. Central nervous system insult is believed to be the cause of this type of hyperthermia. Two recent studies cited 194 patients with post-cardiac arrest hyperthermia. In these patients, the hyperthermia was a poor prognostic indicator, often resulting in brain death. Treatment options for post-resuscitation hyperthermia are not well established. Our approach was to use methods indicated for other hyperthermia syndromes. The approach favors discontinuation of culprit medications and symptomatic relief with ice, fans, cooling blankets, and infusion of cool saline. Refractory cases may require gastric or peritoneal lavage with cold saline or cardiopulmonary bypass with external cooling of blood. Pharmacologic therapy with dantrolene may also be indicated, depending upon the cause. Our case illustrates the importance of prevention and early treatment of hyperthermic emergencies. It also defines post-cardiac arrest hyperthermia as an entity that requires further study.
HYPERTHYROIDISM WITHOUT A THYROID GLAND?
C. Burgdorf1; J. Wiese1. 1Tulane Health Sciences Center, New Orleans, LA. (Tracking ID #117388)
LEARNING OBJECTIVES
1. Recognized that retained thyroid tissue following thyroidectomy can result in recurrent hyperthyroidism. 2. Distinguish recurrent primary hyperthyroidism from factitious or exogenous hyperthyroidism.
CASE
A 42 year-old woman presented with insomnia, anxiety, and weight loss. She had been diagnosed with Graves disease one year prior. She was treated with a subtotal thyroidectomy because of a large, compressive goiter. Per the operative report, the subtotal thyroidectomy included the isthmus and the right and left lobes. She was taking no medications and had been asymptomatic until one week prior to presentation. At presentation, her pulse was 86 beats/min; the blood pressure was 158/76 mmHg. She had no palpable thyroid tissue and no tenderness to palpation. She had an evident tremor and was anxious. Despite the history of the thyroidectomy, repeat thyroid function tests were ordered. Her TSH was 0.01 and the free T4 was 2.8. The patient was determined to be clinically and biochemically hyperthyroid.
DISCUSSION
Obtaining an accurate past medical history is instrumental to refining the differential diagnosis. This patient's case is a reminder, however, that a past history of thyroidectomy does not exclude the possibility of recurrent hyperthyroidism. Recurrent hyperthyroidism occurs in two percent of patients with a history of thyroidectomy, usually from small remnants of the thyroid gland located posterior to the plane of dissection. In these patients, it is important to evaluate for ectopic thyroid tissue. This tissue can arise in locations such as the neck, mediastinum and ovary. These sources can be diagnosed by radioactive iodine total body scan. Physicians must also consider exogenous thyroid hormone intake and overtreatment of surgically-induced hypothyroidism with levothyroxine as causes for recurrent hyperthyroidism. Physicians can diagnose surreptitious thyroid hormone intake by a low thyroglobulin and normal radioactive iodine uptake scan; subclinical hyperthyroidism can be diagnosed by a low TSH and elevated T3 or free T4 levels. Aside from diabetes, thyroid disease is the most common of endocrine diseases presenting to the general internists. Knowledge of these complications is important to the proper management of the hyperthyroid patient.
HYPOTHYROID HEART FAILURE
J.A. Kasher1; J. Wheat1; P.J. De Silva1; P.P. Balingit1. 1UCLA San Fernando Valley Program, Sylmar, CA. (Tracking ID #116171)
LEARNING OBJECTIVES
1) Recognize hypothyroidism as an etiology of heart failure. 2) Identify hypothyroid-induced cardiomyopathy as an easily treatable and reversible condition.
CASE
A 56-year-old Hispanic male presented with a two-month history of progressive substernal chest pain, dyspnea, and decreased exercise tolerance. His chest pain occurred both at rest and with exertion. Past medical history was significant for hypertension, hypothyroidism, and atrial fibrillation. Patient denied palpitations. There was no previous or current use of alcohol or other illicit substances. The patient admitted to non-compliance with his medications for about six months. Vital signs were notable for heart rate 97 and blood pressure 147/81. Jugular venous pressure was normal. Chest auscultation revealed bibasilar rales, irregularly irregular heart rate, and an S3 gallop. Routine chemistries, blood count, and cardiac enzymes were normal. EKG showed atrial fibrillation without evidence of ishcemia. Thyroid stimulating hormone level was 55.3, and free T4 and T3 were within normal limits. Echocardiography demonstrated global hypokinesis and ejection fraction (EF) 15%, while cardiac catheterization revealed clean coronary arteries with EF 35%. A regimen of L-thyroxine 0.125 mg, benazepril 20 mg, and metoprolol 25 mg was started. Initial improvement of his CHF followed, and on return visit 3 months later, TSH had normalized. He remained free of any signs or symptoms of heart failure after normalization of his thyroid tests.
DISCUSSION
Thyroid hormone affects many aspects of cardiovascular function including systemic vascular resistance, heart rate, ejection fraction, cardiac output, isovolumic relaxation time and blood volume. It also acts directly on the cardiac muscle and is a regulator of cardiac gene expression. Hyperthyroidism is a well documented cause of heart failure. However, hypothyroidism is much less common and rarely mentioned in the literature as a cause for cardiac dysfunction. The exact incidence of hypothyroid-induced heart failure has not been determined since it does not typically occur in the absence of other cardiac diseases. Hypothyroid cardiomyopthy is rare, and is often associated with mxyedema coma. In this case, the patient developed heart failure only when he became hypothyroid. His blood pressure and heart rate were still relatively controlled and it is unlikely that they induced a cardiomyopathy. Hypothyroidism and idiopathic cardiomyopathy were the other possibilities. However, idiopathic cardiomyopathy is not as reversible as that due to hypothyroidism. This patient responded rapidly to thyroid replacement and appropriate CHF management. Unlike most causes of cardiac dysfunction, hypothyroidism represents a reversible etiology and therefore it is important to exclude hypothyroidism as a potential cause in patients with cardiac failure.
“I CAN'T MOVE”
C. Chen1; S. Wali1; P.P. Balingit1. 1UCLA San Fernando Valley Program, Sylmar, CA. (Tracking ID #117488)
LEARNING OBJECTIVES
1. Recognize hypokalemic periodic paralysis (HPP). 2. Recognize the association of HPP with hyperthyroidism.
CASE
23-year-old male with presented with sudden onset of weakness of his extremities. The patient was watching TV after eating pizza for dinner, and at 4am he noticed that he was unable to rise up from a sitting position. By the time he arrived at the ER, he was unable to walk. He complained of mild anxiety, and denied recent upper respiratory tract infection symptoms. Prior to the onset of symptoms, the patient denied any unusual ingestions and did not perform strenuous exercise. The patient denied any drug, alcohol, or tobacco use. Family history was noncontributory. On physical examination, the patient was afebrile, normotensive, breathing comfortably, with a heart rate of 68. There was no goiter present. Neuromuscular examination revealed good muscle mass in the extremities, normal cranial nerve function and intact sensation. However, proximal muscle strength was 3/5 though symmetric. Laboratory results included K 3.0 and TSH 1.2. EKG showed normal sinus rhythm with U waves present. 60 mEq potassium replacement was given with resolution of his deficits. Endocrinology consultation was obtained for management of newly-diagnosed hyperthyroidism.
DISCUSSION
HPP is a syndrome in which a patient experiences episodes of flaccid weakness or paralysis, which may be potentially fatal if respiratory muscles are affected. Acute attacks are often precipitated by exercise, stress, or a large carbohydrate meal and can last for several days. During acute attacks, sudden potassium shifts toward the intracellular space may lower serum levels to as low as 1.5 to 2.5. However, potassium levels are usually normal between attacks. This syndrome may be familial, with an autosomal dominant inheritance pattern with variable penetrance, but may also be associated with hyperthyroidism. Asian/Native American males seem to be particularly susceptible to this association, with an estimated incidence of about 15% to 20% of thyrotoxic patients. The syndrome can occur with few thyrotoxic symptoms. The mechanism by which hyperthyroidism causes HPP is not well understood. It is hypothesized that thyroid hormone may increase Na-K-ATPase activity (which drives potassium into cells), thus precipitating hypokalemia when increased levels of epinephrine and insulin are present (e.g. from stress, exercise, large meals). Another hypothesis suggests that susceptible patients have a mutated calcium channel, its function being diminished during thyrotoxic states. In the familial syndrome, most cases are due to a defect in the calcium channel of skeletal muscle. The treatment of thyrotoxic-associated HPP is the same as in euthyroid patients, namely replacement of potassium. Resolution of symptoms often occurs after 15–20 minutes. Maintaining a euthyroid state often prevents further attacks. Beta blockers may be helpful in reducing the severity of attacks.
I THOUGHT IT WAS ACUTE CHOLECYSTITIS! FOOLED BY A LIVER ABSCESS
M. Kanbour1; R. Granieri1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115750)
LEARNING OBJECTIVES
1.) Identify the clinical features of pyogenic liver abscesses; 2.) Recognize the treatment of pyogenic liver abscesses 3.) Identify potential underlying causes of pyogenic liver abscesses.
CASE
CASE INFORMATION: A 73-year-old male presented with 4 days of unremitting, dull right upper quadrant (RUQ) pain, fever (39.4°C), chills, and anorexia without nausea, vomiting, or change in bowel habits. He had a history of myocaridal infarction and prostate cancer. Medications were simvastatin and aspirin. His exam was unremarkable except for RUQ tenderness, positive Murphy's sign, and rebound. His Hb was 11.9, WBC 22.1, (85 PMN, 6 bands), albumin 2.5, ALT 133, AST 132, ALK PHOS 177, total billirubin 1.1, INR 1.2. His right hemidiaphragm was elevated on CXR. CT abdomen/pelvis showed gallstones without cholecystitis or biliary ductal dilatation, diverticular disease without diverticulitis, and a 10 cm right lobe liver abscess. He was started on IV ampicillin/sulbactam. An 8 French pigtail catheter was placed under CT guidance and 250 cc of foul smelling, blood tinged fluid were drained. 5 days later, the fluid culture grew Peptostreptococcus. Blood cultures remained negative. Clinically, the patient improved dramatically. A CT on day 5 showed a 2nd communicating abscess, located superiorly. A 10 French drainage catheter was advanced. The patient did well and was discharged on oral amoxicillin/clavulinic acid. After 4 weeks of therapy, CT showed persisting abscess despite a well placed catheter. He was referred for open surgical drainage and cholecystectomy.
DISCUSSION
IMPLICATION/DISCUSSION: Pyogenic liver abscesses present as RUQ pain, tenderness, fevers, anorexia, and weight loss and can mimic the presentation of acute cholecysitis. Ultrasonography is cost effective for diagnosis and can be used to guide aspiration. Empiric antibiotics should be started as soon as an abscess is diagnosed and continued for 2–4 weeks. The antibiotics should cover E. coli, K. pneumoniae, bacteroides, enterococcus, and anaerobic streptococci. Drainage is not required in small abscesses that respond to antibiotics. A percutaneous needle aspiration is required if an abscess <6 cm. If an abscess >6 cm, however, a percutaneous catheter should be placed for drainage. If drainage fails, if there are large multilocular abscesses, or if there is an associated intra-abdominal infection requiring surgery, referral for open surgery is recommended. Antibiotics alone are effective in only a few patients; most will require aspiration or drainage. In 85% of cases, an underlying etiology is identified. Most commonly, gallstones, diverticulitis, and appendicitis are implicated. Less common causes include biliary infection, stricture, cholangiocarcinoma, gallbladder empyema, Crohn's, perforated ulcer, trauma, liver biopsy, dental infection, or cryptogenic. In all cases the underlying cause should be sought and treated.
I'M DIZZY. COULD THIS BE CANCER?
A. Nadimpalli1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117491)
LEARNING OBJECTIVES
1. Recognize the clinical presentation of CML 2. Understand the indications and methods of treatment of CML
CASE
A 37 year-old man presented with one episode of dizziness minutes after urinating. He did not lose consciousness, had no vertigo symptoms, and had intact bowel and bladder function. He had a headache the prior day for which he took aspirin and acetominophen. He also recounted large melanotic stools over the past five days. He denied hematochezia or hematemesis. His blood pressure was 120/70; heart rate 120, and he was orthostatic. His abdomen was soft without hepatosplenomegaly. He had no lymphadenopathy. His rectal exam was normal; his stool was hemoccult positive. His hematocrit was 19 mg/dl; WBC 170,000; platelets 800,000. The bleeding time was significantly elevated, prompting platelet aggregation studies. The response to ristocetin was normal; his platelets did not aggregate in response to ADP. After he was transfused, an EGD revealed a non-bleeding ulcer in his duodenum. A peripheral smear and bone marrow biopsy were consistent with CML. He was started on Imatinib mesylate (Gleevec) and evaluated for a bone marrow transplant.
DISCUSSION
The initial presentation of CML is variable. Fifty percent are asymptomatic until the end stage of the disease; a small percentage present with a bleeding episode even in the face of an elevated platelet count. In these patients, the dysfunctional platelets are due to abnormal von Willibrand factor and Ristocetin co-factor levels or platelet receptors, as confirmed by a decreased response to pro-coagulant factors, including ADP, epinephrine and collagen. Both the bleeding times and the platelet aggregation qualities may be adversely affected in patients with CML Although hydroxyurea and busulfan have been used for treatment of CML, neither are curative. Interferon alpha 2A induces complete cytogenic remission (i.e. Philadelphia chromosome negative) in a minority of patients. Bone marrow transplant, while having an initially increased mortality in the first 18 months, has a better long-term prognosis than chemotherapy. Imatinab mesylate, an oral pill that inhibits the tyrosine kinase molecule produced by the aberrant Bcr/Abl translocation, has also been shown to have better hematologic and cytogenic response than chemotherapy alone. Recognizing CML is important to the general internist as it is a common malignancy that with the advent of Imatinib mesylate can be curable.
IBUPROFEN INDUCED LEUKOPENIA
R. Nica1; E. Warm1. 1University of Cincinnati, Cincinnati, OH. (Tracking ID #115633)
LEARNING OBJECTIVES
1. Recognize the neutropenia as one of the ibuprofen side effects. 2. Manage the ibuprofen induced neutropenia.
CASE
Ibuprofen is well known to cause gastrointestinal side effects but many other types of adverse reactions can occur. We report a case of ibuprofen-induced leukopenia that began shortly after introduction of the medication and resolved completely with discontinuation. A 78-year-old African American male with no prior significant past medical history presented for his annual physical exam. He noted only mild pain in his right knee, presumed to be from osteoarthritis. He was prescribed ibuprofen 600 mg twice a day for the pain. A CBC drawn at the time of the office visit as a part of a routine battery of labs was normal. Three weeks later the patient presented to the emergency department for a minor trauma. On this occasion he was noted to have an abnormal CBC. His hematocrit, hemoglobin, and platelet levels were normal, but his white blood cell count was approximately 0.3 mm3. In order to avoid any lab error the CBC was redrawn and a blood smear was analyzed by the pathologist The patient did not have any complaints, the physical exam was unremarkable, and no other lab abnormalities were noted. He was advised to stop the ibuprofen. One month later, with no interventions and no complications noted, the patient's white blood cell count was normal.
DISCUSSION
Ibuprofen is a nonsteroidal anti-inflammatory agent that is widely used for treatment of pain and fever. Although ibuprofen has a favorable therapeutic risk-benefit ratio, a number of potentially serious adverse reactions have been associated with its use. Leukopenia, especially with neutropenia or agranulocytosis, is a rare side effect occurring at a reported rate of less than 1%. The mechanism of this side effect is not known. One proposed mechanism is the presence of an antibody that inhibits the growth of myeloid progenitors in the presence of ibuprofen. As seen in our case, the leukopenia is completely reversible if the drug is stopped. It is also thought that the frequency of this side effect is underestimated because of this property. It is, however, important to recognize the leukopenia before expensive and invasive studies are undertaken to determine the cause. To our knowledge there have been no reported cases of ibuprofen specific induced neutropenic complications. As with other drug related neutropenia, we assume that the risk of complications is increased with the degree and duration of the neutropenia. Because the mechanism of ibuprofen and NSAID induced neutropenia is unknown, we decided not to prescribe any NSAIDs for our patient in the future.
INFLAMMATORY BREAST CANCER OR UNRESOLVED MASTITIS-THE SIGNIFICANCE OF “HIGH INDEX OF SUSPICION”
K. Ghosh1; A.C. Degnim1; D.L. Adamczyk1. 1Mayo Clinic, Rochester, MN. (Tracking ID #116695)
LEARNING OBJECTIVES
1) Recognize clinical features of inflammatory breast cancer. 2) Manage a patient with persistent signs/ symptoms of mastitis.
CASE
A 58 year-old lady presented with a history of redness, warmth and fullness in the left upper breast for three weeks. She had completed a 10-day course of Clindamycin few days earlier prescribed by her primary physician for suspected mastitis. Her breast symptoms improved slightly on the antibiotics but recurred soon after the medication was discontinued. She also reported that she had noticed a lump in her left axilla about 7 months earlier that had been evaluated with a fine-needle aspiration biopsy revealing benign findings. She denied fever, but reported fatigue and night sweats. Clinical examination revealed an asymmetrically enlarged left breast with an area of erythema, warmth and fullness extending for about 4 × 8cm in the upper left breast. Palpation of the breast revealed an ill-defined thickening deep to the area of inflammation measuring about 4 cm, and a 2 cm lymph node was palpable in the right axilla. Diagnostic mammogram was negative for abnormality. Ultrasound evaluation revealed skin thickening with vague areas of shadowing in the deeper tissues, but no definite mass; the axillary lymph node had benign features. Skin biopsies taken from two areas of inflammation were negative for malignancy. An ultrasound-guided biopsy of the ill-defined hypoechoic area demonstrated an infiltrating ductal carcinoma. The patient underwent sentinel lymph node biopsy that was positive for metastasis, and is currently undergoing neo-adjuvant chemotherapy.
DISCUSSION
Inflammatory breast cancer is the most aggressive form of primary breast cancer. Clinical features include breast swelling with erythema, edema, tenderness, induration, and rapid spread to axillary lymph nodes. Since the early presentation is similar to mastitis, any non-lactational mastitis should be viewed with suspicion and followed until resolution. Diagnostic imaging (mammogram, ultrasound, or even breast MRI in select cases) is indicated if the mastitis does not resolve completely with antibiotics. Since skin biopsy may be negative in inflammatory breast cancer, image-guided biopsy, or even open surgical biopsy of deeper tissue must be pursued in suspicious cases, even in the absence of a well-defined mass lesion.
INTRAPULMONARY PERCUSSIVE VENTILATION (IPV)—A NEWER MODALITY IN THE MANAGEMENT OF BRONCHIAL ASTHMA AND ATELECTASIS—A PRELIMINARY STUDY
A. Devarajan1; R. Blejeru1; N. Maddukuri1; P. Venkataswamy1; R. Dharmaji1; D. Flores2. 1Jersey City Medical Center, Jersey City, NJ; 2Jersey City Medcial Center, Jersey City, NJ. (Tracking ID #116500)
LEARNING OBJECTIVES
To determine the effectiveness of Intra Pulmonary Percussive Ventilation in cases of respiratoratory failure requiring mechanical ventilation.
CASE
Case 1. Ms. MM is a 58 year old white female with a history of severe asthma and COPD admitted in ICU for hypercapnic respiratory failure. After being treated with inhaled bronchodilators, antibiotics and IV steroids, she improved initially and has later developed severe respiratory failure with unresponsiveness and low oxygen saturation (O2 saturation—60–70% on 100% Fi02). Since the patient refused mechanical ventilation, continuous Albuterol and Ipratropium along with 100% oxygen and IPV treatment were administered. After 10 minutes of treatment, patient expectorated a large amount of thick yellow sputum, and soon thereafter her O2 saturation rose to 88–92%. The patient regained consciousness. The was discharged home in her usual condition several days after. Case 2. 59 year old AAM with history of hypertension developed intracerebral hemorrhage and was managed in ICU with intubation and control of hypertension and Intra cranial pressure. During the 2nd week of ICU stay, the patient developed marked atelectasis of right lower lobe as was evident in chest X-Ray. The atelectasis did not respond to usual chest percussions, frequent turnings of the patient, and nebulized bronchodilator treatment. Intra Pulmonary ventilation treatment was added to the regular nebulized bronchodilator treatment for 3 days. This resulted in marked improvement in symptoms and signs, including drastic improvement in X-Ray findings.
DISCUSSION
Essentially, IPV is a form of mechanical ventilation, assists the respiration of patients with diseases which limit their normal respiration by helping to clear retained secretions from the lungs and then providing deep breathing to increase oxygen delivery to the alveoli as well as flushing carbon dioxide from the pulmonary airways. This form of mechanical ventilation delivers rapid, high flow, mini-bursts (percussions) of Air or Oxygen into the lungs while simultaneously delivering therapeutic aerosols. IPVloosens and helps propel deep retained airway secretions upward from the lungs where they can be more easily expectorated. The two cases of respiratory failure are presented here to stress the effectiveness of Intra Pulmonary Percussive Ventilation in the management of bronchoconstriction from Asthma and airway blockage from atelectasis.
IS IT A CASE OF ACUTE CORONARY SYNDROME OR AORTIC DISSECTION?
B. Xie1; A. Sohnen1. 1University of Pittsburgh Medical Center, Pittsburgh, PA. (Tracking ID #115517)
LEARNING OBJECTIVES
Learning Objectives: 1) Recognize the signs and symptoms that distinguish acute coronary syndrome from acute aortic dissection 2) Diagnose acute aortic dissection
CASE
A 59-year-old female with hypertension, diabetes, hypercholesteremia, s/p colon cancer resection 3 months previously, presented with sudden, severe, dull, substernal chest pain. The pain radiated to left arm and jaw, and was associated with nausea, diaphoresis and shortness of breath. Vitals on admission: temperature 36.4 C, heart rate 62 and regular, respiration rate 20, blood pressure 157/72 right arm and 154/68 left arm. Remaining physical examination was unremarkable. Labs were significant for H&H 11.5/35.6, CPK 30, CK-MB 0.3, Troponin I 0.1. EKG showed sinus rhythm with rate 62 and no ST-T changes in comparison with a previous EKG. Chest X-ray was normal. Patients was initially diagnosed as having an acute coronary syndrome and started on intravenous nitroglycerin, morphine and heparin. Four hours after onset (and 3 hours after treatment), the pain persisted. At that time, patient mentioned that the pain radiated to lower back and left hip. Repeat EKG, CPK, CK-MB and Troponin I showed no change. Intravenous heparin was discontinued immediately, and a spiral CT scan of chest was ordered. It showed a dissection involved both ascending and descending aorta. The patient was immediately transferred to the operation room for dissection repair. The surgery was a success and the patient was discharged to home 10 days later.
DISCUSSION
Distinguishing the chest pain of aortic dissection from acute coronary syndrome is very important. Although both illnesses are catastrophic, the treatments are quite different. The onset of myocardial ischemic pain is often gradual with an increasing intensity over time, while in aortic dissection the onset is typically abrupt with greatest intensity at the beginning. The pain of ischemia commonly radiates to the neck, upper extremity or shoulder but rarely to lower back or hip. Migratory chest pain occurs in most patients with aortic dissection. Ischemic pain usually lasts more than 2 but less than 20 minutes, unless a myocardial infarction is occurring. Persistent chest pain without EKG changes should raise concern that the pain is not due to an acute myocardial infarction. A normal chest X-ray and symmetric upper extremity blood pressures do not exclude the diagnosis of aortic dissection. As in this patient, the suspicion of the diagnosis of acute aortic dissection is raised when 1) pain is severe, sudden in onset, with radiation initially to arm and jaw then to back and hip (migratory); 2) pain persists for 3-4 hours without EKG change. Definitive diagnosis of aortic dissection is made with aortography (standard but uncommonly used) or noninvasive techniques (commonly used) including CT scanning, MRI and trans-esophageal echocardiography.
IS SINUS NODE DYSFUNCTION AN ADVERSE EFFECT OF INFLIXIMAB?
S. Dodla1; T. Townley1. 1Creighton University, Omaha, NE. (Tracking ID #115699)
LEARNING OBJECTIVES
1. To identify sick sinus syndrome as a possible adverse effect of Infliximab. 2. To realize the importance of being aware of the potential side effects of Infliximab considering its wide spread use in the recent years.
CASE
The patient is a 41-year-old male with three-year history of Rheumatoid arthritis who was treated with Steroids and Methotrexate without symptomatic improval. He was later started on Infliximab infusions along with Methotrexate with good relief in symptoms. Three months after starting the infusion he developed edema of the feet with pleural effusions and was given the new diagnosis of CHF. One month after that he was admitted to hospital again because of three syncopal episodes with complains of “passing out” while asleep and while awake. He was noted to have six-second sinus pauses with no escape rhythm on electrocardiographic monitoring associated with the symptoms. Other medications that he was on included Methotrexate, Naprelan, Imitrex, Remeron, Celebrex, Neurontin, Glipizide, Bumetanide, Lipitor, Roxicet and Insulin 70/30. Infliximab was discontinued and Dual chamber pacemaker was placed. On one year follow up, he had no more syncopal episodes, symptoms of Congestive Heart Failure had resolved and pacer interrogation revealed little to no pacer dependence and no significant bradyarrythmias. Other PMH include DM and Hyperlipidemia.
DISCUSSION
Infliximab is a human murine chimeric anti TNF-a monoclonal antibody, which has been widely used for the treatment of Rheumatoid arthritis and crohn's disease. Studies are underway regarding the cardiovascular effects of this drug especially after the recent clinical trial of 7 deaths out of 101 patients with moderate to severe CHF and another case of sudden death in a patient without CHF after Infliximab infusion. Bradycardia is a rare adverse effect of Infliximab. Although Diabetes and Rheumatoid arthritis have been associated with sick sinus syndrome, patient had these before and after the episodes of sinus pauses. The presence of no syncopal episodes, no pacer dependence after discontinuing Infliximab supports that the sinus node dysfunction could be related to Infliximab. The pathogenesis behind it, the absence of escape rhythm and confirmation of the correlation between both will need to be further assessed by future clinical trials
ISOLATED PULMONARY MAC IN HIV PATIENTS: A CASE OF IMMUNE RECONSTITUTION SYNDROME
P. Huang1; B. Taqui1; M. Keith1. 1Temple University, Philadelphia, PA. (Tracking ID #116211)
LEARNING OBJECTIVES
1. Recognize immune reconstitution inflammatory syndrome as a consequence of initiation of antiretroviral therapy in HIV patients. 2. Recognize clinical features of reconstitution syndrome.
CASE
A 40 year old male presented with one week of cough productive of clear, white sputum associated with dyspnea, fevers and night sweats. Two months prior, he had been diagnosed with HIV (CD4 7). He had been started on antiretroviral therapy (lamividine, zidovidudine, and tenofovir) which subsequently improved his CD4 count to 149. On physical exam, he was afebrile. He had oral thrush, submandibular lymphadenopathy, and crackles in left upper lung field. Lab data revealed Hgb 6.6, LDH 253. Chest xray revealed diffuse alveolar and interstitial opacities in bilateral upper lobes, left greater than right. CT abdomen/pelvis revealed marked prominence of the pancreatic head and scattered nodular densities at the lung bases, but no retroperitoneal or mesenteric lymphadenopathy. Bronchoscopy revealed many acid fast bacilli by staining, but no pneumocystis carinii (PCP) or fungi. Routine cultures were negative. He was started on isoniazid, rifabutin, ethambutol, pyrazinamide and clarithromycin for mycobacterial infection. After culture confirmation of Mycobacteria avium complex (MAC), his regimen was simplified to ethambutol and clarithromycin. At this time, the patient was also diagnosed with immune reconstitution inflammatory syndrome. He continues to do well.
DISCUSSION
Immune reconstitution inflammatory syndrome is a relatively new entity described in HIV patients who have been started on highly active antiretroviral therapy (HAART). It represents a recovery in the immune response leading to an excessive response to previously latent infections. There are no well defined predisposing factors for reconstitutuion syndrome. However, it tends to occur in antiretroviral naive patients with low CD4 counts (<50–100). It occurs 4–24 weeks after initiation of HAART. The syndrome typically is associated with ophthamologic and dermatologic manifestations. It has also been linked to herpes, MAC, tuberculosis, cytomegalovirus, hepatitis B and C, cryptococcus and PCP. It causes atypical presentations of opportunistic infections. Our patient presented with isolated pulmonary MAC infection, seen more often in chronic lung disease than in HIV. There are only 24 reported cases of isolated pulmonary MAC in HIV patients. Patients with reconstitution syndrome recover nicely with treatment of the underlying infection and continued HAART therapy.
IT's NOT OVER UNTIL IT's OVER
K. Jones1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117475)
LEARNING OBJECTIVES
1. To recognize that a normal Physical exam, normal serial EKGs and normal echocardiogram do not rule out Myocardial infarction 2. To review the timing, specificities, and sensitivities of cardiac enzymes
CASE
A 38 year-old man with a history of gastritis, panic attacks, and hyperlipidemia presented with one day of intermittent retrosternal chest pain that occurred after an evening of cigarette smoking and binge-drinking. The pain was relieved with ranitidine, but returned within an hour and persisted until presentation. He had no family history of coronary disease. His physical exam and EKG were normal. He was given pantropazol that relieved the pain. A troponin I was drawn, but he was discharged prior to its return. The next morning, the patient's troponin was found to be elevated at 4.74 (normal <1.0). The patient was called and asked to return to the emergency department, where he stated that he was without pain and felt entirely well. His examination was normal. A second troponin, 36 hours post-chest pain, was 24.5. His EKG remained normal. Despite his lack of current symptoms, the patient underwent a left heart catheterization that showed a complete blockage of his left circumflex coronary artery.
DISCUSSION
By GUSTO criteria, myocardial infarction is defined as two of three criteria: enzyme elevation, EKG changes, and clinical story. This patient's presentation was suggestive of myocardial infarction, but the response to a proton-pump antagonist falsely masked the diagnosis. Pain from myocardial infarction is intermittent, and an intervention such as a PPI can coincide with the natural resolution of the anginal pain, fooling the physician into believing the diagnosis is due to gastric reflux. This patient's past medical history of alcohol abuse and anxiety attacks induced cynicism on the part of the physician that further clouded judgment. No test on its own has a sufficiently strong likelihood ratio to confirm or exclude the diagnosis of myocardial infarction. For this reason, serial EKG's should be used to detect ischemic changes, as this improves the likelihood ratios of the test (+LR 14; –LR 0.3). Troponin I levels are sensitive indicators of cardiac damage especially after 36 hours, but may remain normal for the first twelve hours after cardiac injury (+LR 14; –LR 0.05). This case illustrates that if there is sufficient pre-test probability to exclude myocardial infarction, serial EKG's and troponin assays should be obtained. Physicians should be aware of the coincident cause-effect heuristic that may prevent accurate diagnosis of ischemic coronary disease, and the effect of moral judgment (i.e., a history of alcohol and drug use) on their clinical decision-making.
ITCH WITHOUT RASH
T. Tanabe1. 1University of Pennsylvania, Philadelphia, PA. (Tracking ID #117370)
LEARNING OBJECTIVES
1) Recognize the presenting symptoms of bullous pemphigoid. 2) Assess the differential diagnosis of pruritis.
CASE
A 74-year-old man with a history of hypertension presented with intensive pruritis for two weeks. The patient complained of itches on anterior shoulder, axillae, upper chest, abdomen and lower legs. He could not recall the initial site of an itch but denied rashes. His medication included amlodipine only, which he had taken for years. He denied any changes in home environment, recent sun exposure, intake of herbal medicine and history of herpes simplex. Review of system was negative for weight loss, diarrhea, and visual changes except for insomnia due to intensive pruritis. Physical exam revealed multiple scratches on his upper arms, chest, abdomen and lower legs, especially close to ankles. No bullae, papules, vesicles, erythema or scales were seen. There was no involvement of mucous membranes. Direct microscopic exam of the skin with potassium hydroxide preparation was negative for fungus or yeast. Laboratory values revealed normal white cell counts with normal distribution, normal renal function and normal electrolytes. The patient was referred to the dermatology clinic. Bullous pemphigoid was diagnosed by skin biopsy and the patient was treated with systemic corticosteroid successfully.
DISCUSSION
This is a fairly common presentation of bullous pemphigoid. Initially patients complain of moderate to severe pruritis without skin lesion and subsequently develop an eruption. Erythematous, popular lesions may precede bullae formation by weeks to months. Patients are often misdiagnosed as generalized eczema, scabies, allergic contact dermatitis or drug reaction. Histopathology and immunology permit a differentiation from scabies, dermatitis herpetiformis and the distribution of rash allows one to differentiate from contact dermatitis or atopic dermatitis. It is crucial for internists to obtain a detailed history including medications, diet, and the presence of similar symptoms in a patient's family. It is also imperative to examine mucous membranes carefully.
JUGGLING A DIAGNOSIS WITH TWO FEET AND ONE LEFT HAND
J.S. Dubow1; M. Rotblatt2. 1UCLA Medical Center, Los Angeles, CA; 2UCLA SFVP-Olive View Medical Center Department of Internal Medicine, Sylmar, CA. (Tracking ID #116172)
LEARNING OBJECTIVES
1. Recognize how to diagnosis and treat pneumocystis carinii pneumonia (PCP) 2. Recognize the significance of the “two foot and one hand” syndrome.
CASE
A 32-year-old hispanic man was admitted to the hospital complaining of shortness of breath, fevers and dsypnea on exertion. He also reported recent night sweats, a 15-20 pound weight loss over 6 weeks and a yellowing of his toenails on both feet and fingernails on one hand. The patient had moved to the United States from Mexico ten years previously and he admitted to having sexual intercourse with a prostitue 2 years ago. On exam, the patient was tachypneic and tachycardiac; he had coarse breath sounds and onychomycosis on all ten of his toe nails and all five of the finger nails on his left hand. The rest of the exam was only significant for a large tatoo on his torso. The CXR showed diffuse hazy interstitial infiltrates. His labs were significant for pO2 of 65, a LDH of 1087 and a normal CBC. Although we did not know the patient's HIV status, based on our high clinical suspicion for PCP, the patient was started on high-dose trimethoprim-sulfamethoxazole IV and oral prednisone. An HIV test was later positive with a CD4 count of 33, and the sputum PCP DFA was positive.
DISCUSSION
Based on this patients' symptoms, chest X-ray, LDH and fungal nail infection, it was suspected that this patient had PCP and HIV. Fungal infections of all toenails and fingernails on only one hand (two foot and one hand syndrome) have been shown to be a nonspecific finding in patients with immune deficiency states and a marker for AIDS. The reason why it only affects one hand is unknown, but this clinical finding suggests an underlying immunodeficiency. It usually affects the proximal portion of the nail and extends distally. This increased our suspicion for PCP and the diagnosis of HIV in our patient, who had a fairly classic presentation of PCP. The clinical manifestations of PCP most commonly include fever, cough and progressive dyspnea. The most common findings on physical exam are fever and tachypnea, with possible crackles or rhonchi. The most common radiographic abnormalities are diffuse, bilateral interstitial or alveolar infiltrates. The two most common abnormal laboratory values associated with PCP are a CD4 count below 200 cells/mm3 and an elevated LDH level. However, specific diagnosis of PCP requires documentation of the organism in respiratory specimens by sputum induction or BAL. First line treatment for PCP is trimethoprim-sulfamethoxazole or pentamidine for 21 days. For moderate to severe cases of PCP (defined as arterial oxygen tension of 70 mmHg or less and/or alveolar-arterial oxygen gradient of 35 or greater on room air) it is recommended to use adjunctive corticosteroids. Our patient defervesced well and was discharged with follow up in the infectious disease clinic.
LANGERHANS CELL HISTIOCYTOSIS
A. Ramakrishnan1; L. Lu1. 1Baylor College of Medicine, Houston, TX. (Tracking ID #117428)
LEARNING OBJECTIVES
1) Recognize the symptoms and clinical presentation of Langerhans Cell Histiocytosis. 2) Learn the treatment options for Langerhans Cell Histiocytosis.
CASE
A 35 year-old female with no significant past medical history presented with a one week history of worsening mental status. She was well until one week prior to presentation when her family noted that the change in her mental status along with a decreased in her fluid intake. Physical examination revealed normal vital signs and a disoriented female with diffuse erythematous maculopapular rash on her scalp and multiple ulcerated lesions on her vagina. The rest of neurological exam was unremarkable. Laboratory results revealed serum sodium of 157 and a low urine osmolality, which was consistent with diabetes insipidus. She was treated with hypotonic fluid and vasopressin with improvement of her sodium and mental status. The patient subsequently underwent biopsies of her scalp and vaginal lesions. Pathology showed dendritic cells with abundant vacuolated cytoplasm and vesicular oval nuclei surrounded by eosinophils, neurtophils and lymphocytes, and these findings were diagnostic of Langerhans Cell Histiocytosis (LCH). With her new onset of central diabetes insipidus, a MRI of the brain was obtained revealing a pituitary lesion, consistent with intracranial LCH. She was treated with a combination of chemotherapy and radiation and had good clinical response.
DISCUSSION
LCH is a group of disorders that represent abnormal proliferation of the Langerhans' cell. These disorders present more commonly in children, but can occur at any age. Their clinical presentation can be very variable but these disorders can be divided into three major clinical entities: unifocal LCH, multifocal LCH, and acute disseminated LCH. Unifocal LCH is characterized by solitary or multiple osteolytic bone lesions and prognosis is good. Multifocal LCH can involve numerous organs including the skin and reticuloendothelial system. In about 20–50% of cases, there is involvement of the pituitary gland leading to diabetes insipidus. Acute disseminated LCH is a severe form of multifocal LCH, which usually presents in infancy, is characterized by a diffuse cutaneous rash, hepatosplenomegaly, lymphadenopathy, pulmonary lesions and osteolytic bony destruction. Therapy is based on the extent of disease and prognosis for limited disease is generally very good. Single lesions can be monitored or surgically excised. Local radiotherapy has also been effective for skin and bony lesions. For more aggressive disease various chemotherapeutic agents such as vinca alkaloids, etoposide, methotrexate, prednisone and 2-chlorodeoxyadenosine have been used with varying rates of success. For patients with refractory disease, allogenic bone marrow transplantation is also an option.
LEARNING TO BREATHE CORRECTLY: WHAT YOUR MOTHER NEVER TAUGHT YOU
C.J. Amin1; E.F. Yee1; B.L. Horowitz1; G.H. Murata1. 1New Mexico VA Health Care System/University of New Mexico, Albuquerque, NM. (Tracking ID #116853)
LEARNING OBJECTIVES
1. Recognize the clinical presentation and differential diagnoses of laryngospasms 2. Discuss the work-up and management of laryngospasms 3. Recognize functional disorder as a cause of laryngospasms
CASE
A 52 year-old male presented with 2 weeks of subclinical fevers, and 4 days of cough, hoarseness, stridor and dyspnea. When he talked, coughing ensued immediately, resulting in stridor and inability to breathe for a few seconds. His past medical history was remarkable for gastroesophageal reflux disease (GERD) treated with ranitidine. The physical exam was unremarkable except for stridor. A diagnosis of laryngospasms due to GERD and upper respiratory infection (URI) was made upon admission, and treatment begun with humidified air, anti-tussives, prednisone, and lansoprazole. Pertussis antibody and culture were sent, and azithromycin started empirically. In spite of significant antitussive and nebulizer treatments given for several days, his laryngospasms increased to hourly episodes. He was transferred to the MICU where bo-tox paralysis of one vocal cord, and tracheotomy/intubation were considered. Nebulized morphine, racemic epinephrine, IV ativan and IV lidocaine all failed to prevent episodes. CT and x-rays of the head and neck were unremarkable. Laryngoscopy revealed mobile vocal cords with paroxysmal motion, but no obstruction or lesions. With these findings, a learned functional voice disorder was diagnosed. Speech therapy taught the patient to manage spasms by breathing slowly, pursing his lips, and whispering words during attacks. Symptoms, frequency, severity, and distress of his attacks subsequently all improved. The patient was discharged on anti-tussive medications, lansoprazole, and paxil, with speech therapy follow up.
DISCUSSION
Episodes of laryngitis causing laryngospasms are frequently brought on by URIs or GERD (including subclinical GERD). Bordetella pertussis, anatomical defects, Zencker's diverticulum, hyperparathyroidism, and cricopharyngeal spasm can also cause symptoms. Anatomical lesions must be excluded when laryngospasms do not resolve. Work up can include laryngoscopy, radiological imaging studies, swallowing studies, and EGD with a pH probe. Treatment depends on the etiology. This patient had a functional laryngeal dysfunction from voluntary, non-conscious learned behavior (in response to initial irritation from a URI or GERD). It is important to recognize this disorder as symptoms are very distressing to the patient and caregivers, and treatment lies in exercises, behavioral therapy, and speech therapy. Intubation and bo-tox are never used for a functional disorder. Long acting anxiolytics and SSRI's may help to manage associated anxiety or panic attacks.
LEFT SIDED HEPATIC HYDROTHORAX IN THE ABSENCE OF ASCITES—3RD KNOWN CASE
M.J. Ashraf1; S. Ryzewicz1; K.T. Hinchey2. 1Baystate Medical Center/ Tufts University, Springfield, MA, Springfield, MA; 2Baystate Medical Center, Springfield, MA. (Tracking ID #117323)
LEARNING OBJECTIVES
Background: Hepatic hydrothorax is a known complication in patients with cirrhosis and ascites. However, hepatic hydrothorax in the absence of ascites (HHAA) is extremely rare. We describe a case of “left sided” hepatic hydrothorax in the absence of ascites and review the literature.
CASE
A 52 year old woman with a ten year history of Primary Biliary Cirrhosis was admitted with symptomatic, recurrent, left sided pleural effusion. Biochemistry analysis revealed transudative pleural fluid on both occasions. There were no clinical signs of ascites and ultrasound of abdomen was also negative for ascitic fluid. There was no evidence of congestive heart failure, nephrotic syndrome or marked hypoalbuminemia and the TSH was also normal. A CAT scan of lungs post thorancentesis did not reveal any primary pulmonary pathology as a cause of recurrent effusion. The patient was managed with thoracentesis for acute symptomatic relief of respiratory distress. Subsequently, the patient underwent transjugular intrahepatic portosystemic shunt (TIPS) and 4 months post procedure the patient remained asymptomatic without recurrence of hydrothorax.
DISCUSSION
The pathophysiology of HHAA remains unclear. The proposed mechanism involves transfer of ascitic fluid into pleural space through small congenital diaphragmatic defects because of cyclical negative intrathoracic pressure. The diagnosis can be established by a radioisotope scan which reveals one-way transdiaphragmatic flow of fluid from the peritoneal to pleural cavity. Liver transplant is the treatment of choice, however, TIPS is an effective temporary alternative. The literature search revealed 29 reported cases of HHAA. The majority of them are right sided. Only two other cases of “left sided” HHAA have been reported so far.
LEFT UPPER QUADRANT ABDOMINAL PAIN IN A PATIENT WITH ULCERATIVE COLITIS: THE UNUSUAL SUSPECT
D.S. Kazi1; L. Lu1. 1Baylor College of Medicine, Houston, TX. (Tracking ID #117083)
LEARNING OBJECTIVES
1. Recognize an atypical presentation of pulmonary embolism. 2. Recognize the increased incidence of thromboembolic complications in patients with inflammatory bowel disease (IBD).
CASE
A 34 year-old Latino male with a two-year history of ulcerative colitis (UC) presented with left upper quadrant pain for seven days. He was on prednisone (60 mg) and mesalamine, but continued to have 12–15 watery bowel movements a day. Seven days prior to admission, he began to experience sharp, intermittent left upper quadrant pain that was aggravated by deep inspiration. He denied chest pain, shortness of breath, cough, hemoptysis, melena and hematochezia. On examination, he was afebrile, tachycardic and breathing comfortably with an oxygen saturation of 100% on room air. The chest was clear to auscultation but mild tenderness was noted over the left lower ribs in the mid-axillary line. There was minimal, diffuse abdominal tenderness without guarding or rebound. Stool guaiac was negative. Labs were unremarkable and plain films showed a small pleural effusion at the left base and a few dilated small bowel loops. Our initial diagnosis was an ulcerative colitis exacerbation; thus his dose of prednisone was increased. Over the next few hours, the patient reported increasing left upper quadrant pain with radiation to the left lower chest and aggravated by deep inspiration. Because of the pleuritic nature of the pain and the increased risk for thromboembolic events during acute UC flare-ups, a ventilation-perfusion scan was obtained, which showed an intermediate probability for pulmonary embolism. A helical CT scan revealed multiple, bilateral pulmonary emboli with one large segmental embolus involving the left base. The workup for hypercoagulable states (Factor V Leiden mutation, anticardiolipin antibody, lupus anticoagulant, Prothrombin 20210 mutation, hyperhomocysteinemia, and deficiencies of proteins C and S and Antithrombin III) was negative. Venous dopplers of bilateral lower extremities showed no evidence of deep venous thrombosis. Anticoagulation was initiated and the patient had an uneventful recovery.
DISCUSSION
Patients with IBD have a 1.3–6.4% lifetime risk of thromboembolic events, with the risk being highest during exacerbations. The pathophysiology is not clearly understood, but one hypothesis is an increased prevalence of thrombophilic gene mutations in patients with IBD. Although an uncommon extra-intestinal manifestation of IBD, thromboembolism is a cause of significant morbidity and mortality—about 25% of IBD patients who have a thromboembolic episode will die during the acute event. Therefore, early recognition and prompt treatment are crucial. This case illustrates the importance of maintaining a high index of suspicion for thromboembolic events in patients with inflammatory bowel disease.
LEFT VENTRICULAR OUTFLOW OBSTRUCTION CONFUSED FOR
J. Aliota1; S. Martin-Schild2; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117533)
LEARNING OBJECTIVES
1. In a hypovolemic patient, left ventricular outflow obstruction and autonomic dysfunction can be clinically similar 2. Appropriate imaging is essential in making the diagnosis of left ventricular outflow obstruction
CASE
A 65 year-old man presented with three weeks of watery diarrhea, weakness, and pre-syncope. He had a history of hypertension and old lacunar infarctions and had been hospitalized in a rehabilitation facility up until the time of admission. He was orthostatic with a supine blood pressure of 129/60 mmHg and standing blood pressure of 75/49 mmHg. He had no murmurs or carotid bruits. The physical exam was unremarkable. His hypertension while at the rehabilitation facility was uncontrolled despite the use of a dihydroperidine, oral nitrates, and clonidine. All blood pressure medications were held and despite aggressive hydration the patient's symptoms and orthostasis persisted. A trans-thoracic echo was ordered, but errantly delayed. Considering the patient's old cerebral injury, neurology presumed a diagnosis of autonomic dysfunction in the absence of a cardiac etiology. The patient was started on meclizine without improvement. Two days later, a transthoracic echo showed a significant left ventricular outflow obstruction.
DISCUSSION
The clinical presentation of hypertrophic cardiomyopathy with left ventricular outflow obstruction can appear similar to autonomic dysfunction if obvious physical exam findings are absent and appropriate imaging is not acquired. Hypertrophic cardiomyopathy may be inherited, idiopathic, or a result of long standing hypertension. While a left ventricular outflow obstruction may be symptomatic at rest, syncope, dyspnea, or weakness are common when a patient's activity increases left ventricular activity or dehydration decreases left ventricular volume. In our patient, the diarrhea contributed to a decline in left ventricular preload, thereby decreasing the left ventricular stretch that was keeping the left outflow tract patent. Treatment with beta-blockade decreases myocardial contractility and heart rate allowing an increase in diastole, thereby minimizing the outflow obstruction by increasing the left ventricular diastolic volume. These patients should be counseled to avoid strenuous activity and to maintain adequate hydration. Medications that predominately reduce preload or afterload, such as nitrates, diuretics, ace inhibitors or clonidine should be avoided.
LEG PAIN AS A PRESENTING SYMPTOM OF CARCINOMA OF THE LUNG
J.L. Wall1; G. Bryant2. 1University of Cincinnati, Cincinnati, OH; 2University of Cincinnati College of Medicine, Cincinnati, OH. (Tracking ID #115860)
LEARNING OBJECTIVES
Recognize and diagnose the skeletal manifestations of bronchogenic cancer.
CASE
Introduction: Hypertrophic pulmonary osteoarthropathy (HOA) is a skeletal paraneoplastic syndrome that is strongly associated with intrathoracic malignancies, especially bronchogenic carcinoma. We report a case of HOA that was the presenting finding in large-cell bronchogenic carcinoma. Case Presentation: A 64 year old white male with a past medical history of oxygen-dependent COPD presented with intense bilateral lower leg and ankle pain. The pain had been present for approximately 2 years and had progressively worsened. He had been previously treated by another physician with narcotics without relief and wanted a second opinion. Of note, the patient smoked 2-3 packs of cigarettes per day and had done so for approximately 50 years. On exam, the patient was cachectic and in severe respiratory distress. There was impressive clubbing of all fingers. Radiologic studies revealed hypertrophic osteoarthropathy of the tibial bones bilaterally. A chest x-ray revealed left upper-lobe infiltrate. On biopsy, this was determined to be a large-cell carcinoma.
DISCUSSION
Hypertrophic pulmonary osteoarthropathy is a paraneoplastic syndrome particularly associated with large-cell bronchogenic cancer. Manifestations of HOA include periostitis, clubbing of the fingers, and synovitis. The long bones are frequently involved and bone pain is the presenting symptom in approximately 30% of patients. HOA can be primary or secondary to pulmonary infections, cystic fibrosis, lung neoplasm, cardiac infections or cardiac malformations. When secondary, treatment is directed at the underlying etiology. Prognosis varies depending on the primary etiology, but pain remission and/or recurrence reflect activity of the primary neoplasm. Physicians should be aware of the clinical presentation of HOA as knowledge of this disease process could lead to earlier diagnosis of an underlying malignancy.
LESSONS LEARNED FROM AN UNFORTUNATE CASE OF STRONGYLOIDES HYPERINFECTION
R. Pechulis1; B. Taqui1; C. Veloski1. 1Temple University, Philadelphia, PA. (Tracking ID #116207)
LEARNING OBJECTIVES
1. Recognize risk factors for Strongyloides infection and hyperinfection. 2. Recognize importance of excluding Strongyloides infection prior to initiation of steroid therapy.
CASE
A 43 year old previously healthy Cambodian male presented with acute worsening of chronic abdominal pain, painful swallowing and 20 lb weight loss/one month. Exam revealed cachexia, temporal wasting, oral thrush, periumbilical tenderness and heme positive brown stool. Labs revealed Na 126, K 4.8, WBC 8.2 (1.9 % eosinophils), albumin 2.2, and microcytic anemia. HIV test was negative. Gastrointestinal bleeding due to ulcer or malignancy was suspected. A random cortisol level was 1 mcg/dl and a cosyntropin-stimulation test confirmed adrenal insufficiency. Hydrocortisone 100 mg IV q8 was started. That evening, the patient became progressively tachycardic and hypotensive. Blood smear drawn that morning showed gram negative rods. The patient was transferred to the intensive care unit, where he developed septic shock, ARDS and intra-alveolar hemorrhage. Multiple sets of blood cultures grew Enterococcus faecalis, Klebsiella pneumoniae and Bacteroides thetaiotaomicron. Gastric aspirate and stool samples grew Strongyloides stercoralis larvae and Ivermectin was initiated. The patient developed DIC, acute renal failure and cerebral infarct. Due to his grave prognosis, the patient's family wished to withdraw care and the patient expired on hospital day 18.
DISCUSSION
Strongyloides stercoralis is an intestinal nematode endemic to Africa, West Indies, South East Asia, South America and southeastern United States. Infection should be suspected in immigrants and travelers from endemic areas, veterans, and institutionalized individuals. Chronic infection can be present up to twenty years after travel. Hyperinfection has been reported in patients with HTLV, HIV, diabetes, renal failure, alcoholism, steroid use, hematologic malignancy and organ transplantation. Disseminated Strongyloides hyperinfection presents most commonly with gastrointestinal and pulmonary symptoms. Other manifestations include nephrotic syndrome and gram-negative sepsis. Eosinophilia, usually prominent in chronic infection, may be absent in hyperinfection. Its absence is a predictor of poor prognosis. Hyperinfection mortality is as high as 50–86%. Treatment consists of ivermectin or thiabendazole and supportive therapy. In retrospect, unexplained gram negative bacteremia in a malnourished patient from an endemic area should prompt a search for Strongyloides as the cause. In endemic areas, Strongyloides is always excluded prior to steroid initiation. Our patient's hyperinfection and subsequent demise was probably due to malnutrition and steroids. There may have also been underlying immunosuppression as suggested by the adrenal insufficiency.
LEUKEMOID REACTION IN PATIENT WITH BEHCET DISEASE
D.S. Lababidi1; L. Cation1. 1University of Illinois at Peoria, Peoria, IL. (Tracking ID #116391)
LEARNING OBJECTIVES
1. Review the clinical manifestations of Behcet's Disease (BD). 2. Review the causes of and clinical criteria for leukemoid reaction (LR). 3. Report a possible case of LR associated with BD.
CASE
A 63 yo female with HTN, CAD, asthma, chronic anemia, and long history of BD presented with stomach perforation secondary to BD. The patient had multiple complications from her BD in the past including dermatological, ophthalmologic, GI (Lower GI bleeding), cardiovascular (atheroslerotic CV disease), and renal insufficiency. The patient had surgery for her stomach perforation and then developed sepsis (slight leukocytosis 13,000 18,000 with fever and hypotension). Thereafter, the patient improved, but had worsening of leukocytosis and anemia with persistent thrombocytosis. Her highest WBC value was 30.1 K with 62–95% neutrophils, 6–29% bands, 3–15% metamyelocytes, and 1–4% myelocytes. Her Hb dropped down from 11.7 g/dL to 8.0 g/dL and her platelets rose from 347 K to 730 K. All blood, urine, wound cultures were negative. The patient was on azathioprine and prednisone before hospitalization and during this hospitalization she did not receive azathioprine. A Hematology consult was obtained when the leukocyte alkaline phosphatase was found to be high 169 units (15–70). Myeloproliferative disorder was ruled out and hepatitis C antibody was negative. A diagnosis of LR was rendered and the patient was started on erythropoieten to improve her anemia. The LR in this patient was thought to be multifactorial: BD, steroid use, and surgery. The patient's WBC count subsequently returned to normal, and anemia was improved.
DISCUSSION
Neutrophilia is an absolute blood neutrophil count greater than 7,500 × 106/l in adults. The most common reason for neutrophilia is inflammatory disease due to microbial infection, ischemic, autoimmune, or traumatic (like surgery) injury, and adrenergic stimulation (demargination). When there is combination of more than one factor, very high neutrophil counts can be encountered as well as immature myeloid forms in the blood, causing what is known as LR. This is a reaction that resembles leukemia but is due to other conditions such as infection, stress, surgery, etc. As with leukemia, a person with LR has a disorganized proliferation of immature white blood cells in the blood and bone marrow. The main differential diagnosis of mature neutrophilia is CML, LR or other myeloproliferative disorder. Leukocyte alkaline phosphatase should be normal or high in a LR and low in CML. Absence of leukemic blast cells in the blood or marrow differentiates LR from AML. Behçet's syndrome is a systemic vasculitis of unknown etiology with many clinical manifestations including aphthous ulceration, arthritis, skin lesions, thrombo-phlebitis, and cardiac, neurological, and GI involvement. There is minimal data about the hematological manifestations of BD. Reported possible hematologic manifestations include myelodysplastic syndrome, Hairy Cell Leukemia, chronic neutropenia, and one case report of LR. Our patient had many of the clinical manifestations of BD including a possible related LR.
LIMB LOSS FROM HEPARIN-INDUCED THROMBOCYTOPENIA IN A CARDIAC TRANSPLANT PATIENT
Y. Zafar1; S. Wang1; E. Warm1; Y. Nikiforov1; J. Palascak1. 1University of Cincinnati, Cincinnati, OH. (Tracking ID #115680)
LEARNING OBJECTIVES
1. Diagnose heparin-induced thrombocytopenia (HIT). 2. Recognize HIT as an etiology for catastrophic thrombosis. 3. Prevent HIT-related thrombosis and limb loss.
CASE
A 58-year-old man with ischemic cardiomyopathy and a prosthetic mitral valve underwent right heart catheterization for cardiac transplant evaluation and was maintained on a heparin infusion. His platelet counts declined but spontaneously returned to normal while on heparin. One month later the patient was readmitted for cardiac transplantation and was started on a continuous heparin infusion. His platelet counts fell over six days from an initial value of 213,000 mm3 to 87,000 mm3 on the day of transplant. Post-operative day (POD) 1, the patient received 6 units of platelets for a mediastinal bleed with an incremental increase of only 24,000 mm3. Platelet counts reached a nadir of 50,000 mm3 on POD 7, and the patient received additional platelets. By POD 7 he had developed frank ischemia of three digits on his right hand and all ten toes, and heparin exposure was discontinued. Only the third of three serial heparin-induced platelet aggregation studies was consistent with HIT, but the more sensitive platelet serotonin release assay was negative. The patient was treated with Argatroban starting on POD 8 but required amputation of both feet due to extensive arterial thromboses.
DISCUSSION
HIT type I is a self-limited, non-immune mediated, mild thrombocytopenia of approximately 10% due to the direct aggregation effect of heparin on platelets. HIT Type I is not associated with thrombosis. HIT type II, seen in 1–3% of patients treated with unfractionated heparin, is a clinical diagnosis based on a 50% reduction in the platelet count within 5–10 days of heparin exposure. Thrombocytopenia may occur within 12 hours of re-exposure to heparin. The incidence of HIT is increased in patients having undergone cardiopulmonary bypass. HIT may result in severe thrombocytopenia due to antibodies formed against the heparin/platelet factor 4 complex on the platelet membrane. Activated platelets release thrombogenic microparticles that produce a severe hypercoagulable state and consequent arterial and/or venous thrombosis. Laboratory testing is supportive. Heparin-induced platelet activation assays are less sensitive than the platelet serotonin release assay, but both may give false-negative results. Treatment involves immediate discontinuation of all sources of heparin. Direct thrombin inhibitors are indicated for anticoagulation. Platelet transfusions are relatively contraindicated as they may promote thrombosis. This case underscores the need to monitor platelet levels in patients receiving heparin.
LYMPHOMA PRESENTING AS CARDIAC TAMPONADE
M.S. Patil1. 1Stoger Hospital of Cook County, Chicago, Chicago, IL. (Tracking ID #116260)
LEARNING OBJECTIVES
1) Recognize that acute pericardial effusion may be the presenting symptom of a non-pericardial neoplasm. 2) Use imaging studies of the chest early in the course of the effusion to look for underlying malignancy.
CASE
A 26-year-old man was admitted with shortness of breath of two weeks duration. He had no significant past medical or family history and did not recall a viral prodrome. He was tachycardic with a blood pressure of 124/94 mmHg. Pulsus paradoxus was present. EKG showed sinus tachycardia, diffuse ST segment elevation, and PR segment depression in inferolateral leads. A bedside echocardiogram showed massive pericardial effusion with tamponade. He underwent emergent pericardiocentesis under fluoroscopy,and 1.5 liters of hemorrhagic exudative fluid was drained. Culture for bacteria and viruses was negative. Cytology showed no malignant cells. A workup for connective tissue disorders was unrevealing. The effusion reaccumulated over the next several days despite treatment with indomethacin. MRI showed pericardial thickening and effusion, pretracheal and mediastinal lymphadenopathy, and a large heterogenous anterior mediastinal soft tissue mass invading the pericardium. CT guided needle biopsy of this mass proved it to be Hodgkin's lymphoma-nodular sclerosis type. The patient is now receiving chemotherapy for the lymphoma.
DISCUSSION
Upto 23% of new unexplained large pericardial effusions may be malignant. The most frequent malignancies presenting in this way are lung and breast cancer, and Hodgkin's lymphoma. CT and MRI of the chest are useful not only for delineation of pericardial anatomy and loculations, but also for detection of associated neoplasms in the chest. Neoplasms should be considered and looked for with early imaging in unexplained and non-resolving effusions.
MANAGEMENT OF ESOPHAGEAL ULCERS IN PATIENTS WITH AIDS
M.J. Hamilton1. 1Boston City Hospital, Boston, MA. (Tracking ID #116887)
LEARNING OBJECTIVES
Esophageal disease is a common complication in HIV, and it is the second most common GI disease after diarrhea. It is important to tease out the classic symprtoms of esophageal disease, most commonly odynophagia. Once the esophagus is implicated in the symptom complex, the decision must be made whether or not to treat the patient empirically before further invasive testing. Finally, if upper endoscopy is performed, a treatment plan must be initiated based on the findings and/or pathology results. This case highlights diagnosis and treatment strategies for esophageal ulceration in the patient with AIDS.
CASE
A 51 year old African-American male with AIDS (CD 4 count of 4, viral load of 106,000), not taking HAART medicines, presented to the hospital with odynophagia and weight loss of one month's duration. He denied abdominal pain, nausea, vomiting, or bleeding. His physical exam was noteable for a cachetic middle aged man, afebrile, with oral thrush and a normal abdominal exam. Initial treatment with fluconazole cleared the oral thrush, however the odynophagia persisted. Upper endoscopy was performed which revealed Savary-Miller Grade III esophagitis consistent with severe candidial infection as well as 3 discrete ulcers in the distal esophagus. One ulcer was biopsied in two locations and the patient was continued on fluconazole and sent home. Patholgy was negative for infection. The pateint returned to the hospital two weeks later with continued odynophagia and repeat endoscopy showed the persistence of the ulcers which were rebiopsied, in six locations. Pathology later revealed CMV, the patient was started on ganciclovir and had dramatic improvement in his symptoms.
DISCUSSION
Over the course of the last twenty years in studying patients with AIDS, the etiologies of esophageal ulceration have expanded. The infectious causes primarily HSV and CMV are well known, however, a noninfectious etiology “Idiopathic Esophageal Ulceration” or IEU also exists. Dr. C. Mel Wilcox at Emory University in the early 1990's wrote several articles designed to study the prevalence of each in a cohort of AIDS patients with esophageal symptoms. Of the 100 patients who underwent endoscopy, 41 had IEU's, and 50 had CMV. The remainder had HSV or a combination. Wilcox's study highlighted the importance of obtaining multiple biopsies from an ulcer as the physical appearance of the ulcers on endoscopy are not sufficient to make a diagnosis. In this case, a man with severe enough disease to cause a 15 pound weight loss from lack of eating, was not correctly diagnosed on first endoscopy, and IEU was not considered. Repeat endoscopy with multiple biopsies allowed for a diagnosis and treatment plan which in the end reversed his symptoms. A third follow up endoscopy revealed complete resolution of his ulcerative disease.
MANAGEMENT OF FLUCONAZOLE RESISTANT CANDIDAL ESOPHAGITIS IN AN HIV-INFECTED PATIENT
K. Luce1; M. Panda1. 1University of Tennessee, Chattanooga, Chattanooga, TN. (Tracking ID #115219)
LEARNING OBJECTIVES
1. Recognize the increasing prevalence of resistant Candida albicans infections in HIV-infected patients 2. Discuss the role of intravenous (IV) Caspofungin as an alternative to IV Amphotericin B 3. Recognize the role of highly active anti-retroviral therapy (HAART) in the treatment of candidal esophagitis.
CASE
Our patient was a 36 year old female who had been on fluconazole treatment for several months with continued severe odynophagia and dysphagia of solid foods. Her CD4 count was 23, with a viral load of >750,000. Patient was non-compliant with HAART. Exam revealed extensive whitish plaques in the oropharynx. Upper endoscopy revealed severe, disfiguring esophagitis. Pathology showed only candidal yeast forms. Culture revealed Candida albicans species only. Treatment was initiated with amphotericin B. Initially patient tolerated this well with minimal improvement in symptoms. Despite prehydration efforts patient showed evidence of nephrotoxicity which resolved after stopping amphotericin B. At this time a trial of caspofungin was initiated to avoid any renal complication and the recurrent prolonged nature of her infection. In conjunction frequent nystatin swish and swallow, amphotericin B mouthwash and HAART was intiated. Within a week, patient reported significantly less odynophagia and dysphagia. We thus elected to stop intravenous therapy and follow patient's esophagitis on nystatin swish and swallow and alone. At one month after discharge, patient reported resolution of symptoms and repeat endoscopy revealed a normal appearing esophagus with negative biopsies. Her CD4 count also increased to 132.
DISCUSSION
The current treatment of candidal esophagitis in HIV-infected persons is frequently oral fluconazole followed by amphotericin B as second line treatment. Nonetheless, recent literature reports increasing frequency of fluconazole and amphotericin B -resistant Candida species in this patient population. In such cases, there are few options available to the treating physician. We report response with IV caspofungin in an HIV-infected patient with fluconazole-resistant Candida albicans esophagitis unable to tolerate amphotericin B. At least two randomized, double-blind trials have hailed the effectiveness and tolerability of IV caspofungin. Further, recent data suggests that institution of HAART with improvement of a patient's immunity has led to improvement and even resolution of Candida infection. This case supports the use of caspofungin as an effective agent against candidal esophagitis.Moreover, the striking resolution of our patient's esophagitis on HAART and nystatin swish and swallow alone supports the premise that improvement of patient's immune response can be a successful approach to treating HIV-associated candidal esophagitis.
MAY-THURNER SYNDROME AND DEEP VENOUS THROMBOSIS: AN UNUSUAL RISK FACTOR FOR A COMMON DISORDER
M. Daly1; T. Beckman1; D. McNaughton1. 1Mayo Clinic, Rochester, MN. (Tracking ID #103847)
LEARNING OBJECTIVES
1. Recognize May-Thurner syndrome as an unusual but important risk factor for Deep Venous Thrombosis (DVT) 2. Appreciate the potential variety of risk factors for DVT in a single patient.
CASE
A 51 year-old female presented with two days of increasing left lower extremity swelling and pain. Her past medical history was remarkable for recent surgeries, recent prolonged car and airplane travel, and chronic smoking. She denied a family history of thrombosis. She denied a personal history of thrombosis, cancer, or estrogen use. Her examination was remarkable for an edematous, mildly erythematous, and tender left lower extremity. Laboratory studies revealed a mild leukocytosis with absolute neutrophilia, normal baseline coagulation studies, and D-dimer elevated above the upper detectable limit. A chest x-ray was negative. Lower extremity dopplers showed an obstructive thrombus extending from the left common iliac to the left common femoral vein. Importantly, the patient's left iliac vein was observed to course between the right iliac artery and her lumbar spine. Hence, May-Thurner syndrome was diagnosed. The patient then underwent successful mechanical thrombolysis and iliac stent placement. Finally, she was initiated on unfractionated heparin as bridging therapy to warfarin anticoagulation.
DISCUSSION
May-Thurner syndrome is a little known risk factor for DVT. This was first recognized in 1851 by Virchow, who observed compression of the left iliac vein by the overlying right iliac artery. May and Thurner subsequently described the mechanism by which this anatomic relationship leads to thrombosis. May-Thurner syndrome should be considered in all patients with DVTs involving the left common iliac vein. Diagnosing this disorder is essential, since endovascular management has proven safe and effective in decreasing morbidity. Notably, this case highlights the challenge of recognizing May-Thurner syndrome in patients with multiple risk factors for DVT. Moreover, this case underscores the importance of diagnosing May-Thurner syndrome, since it leads to an unconventional treatment for DVT.
MENTAL STATUS CHANGES IN A MAN WITH HIV: A CASE OF MYCOSIS PSYCHOSIS
T. Wassenaar1; J.M. Sosman1. 1University of Wisconsin Medical School, Madison, WI. (Tracking ID #116768)
LEARNING OBJECTIVES
1). To identify the epidemiology of histoplasmosis. 2). To recognize the common and uncommon presentation of disseminated histoplasmosis in an immunocompromised patient. 3). To identify new therapeutic approaches and prognostic outcomes.
CASE
A 32 y/o male with a history of HIV/AIDS diagnosed in 2000 (CD4#80/ul, viral load>500,000cps/ml) only one-week prior was started on antiretroviral therapy and Bactrim prophylaxis, was admitted with a fever (39.3 C) and a two-week history of mental status changes, including word finding difficulty and visual/auditory hallucinations. His initial workup included a head MRI that was unremarkable and a LP that showed 3 nucleated cells—all lymphocytes, protein 52 mg/dl, glucose 46 mg/dl, cryptococcal Ag negative, VDRL negative. He was started on broad-spectrum antibiotics pending the LP results. His admission labs revealed pancytopenia (Wbc 3.7 K, Hgb 10.3 gm/dl, Plt 31K) and hepatic insufficiency (AST 740, ALT 218, alkaline phosphatase 306, total bilirubin 4.2), all new from labs drawn two weeks prior. Despite negative cultures he continued to spike fevers, developed worsening hepatic insufficiency (AST 1070, ALT 315, alkaline phosphatase 739, and total bilirubin 41.0), and was found to be in DIC (haptoglobin <6, D-Dimer 2–4, fibrinogin 85). A bone marrow biopsy was performed for his persistent pancytopenia, which revealed granulomas with budding yeast cells and a positive culture for Histoplasma capsulatum.
DISCUSSION
Histoplasma capsulatum is a fungal spore that grows naturally in soil and is often picked up by birds and bats and passed on in their feces. Primary infections from dust inhalation have been reported. Over fifty million people in North America have reportedly been infected with this fungus, which is endemic in the Ohio and Mississippi River valleys. The vast majority of these infections go unrecognized. Our patient had many of the classic presenting symptoms of disseminated histoplasmosis such as fever, hepatic insufficiency, and anorexia as well as some rare manifestations including mental status changes, DIC, and severe pancytopenia. The mortality rate of untreated disseminated histoplasmosis is >80%; but can be decreased to <25% with treatment. The standard of care for outpatients is treatment with an azole antifungal. However, IV amphotericin B is usually preferred in patients requiring hospitalization, as is often the case with disseminated histoplasmosis. Our patient received IV then PO azole therapy and made a complete recovery.
MIDWEST REGIONAL RESIDENT AWARD WINNER: DIFFUSE ALVEOLAR HEMORRHAGE RESULTING FROM MARIJUANA
N. Cummins1; V.T. Martin1. 1University of Cincinnati, Cincinnati, OH. (Tracking ID #115625)
LEARNING OBJECTIVES
1. Recognize diffuse alveolar hemorrhage as a potential complication from inhalational illicit drug use.
CASE
A 31 year old black male presented with a six hour history of bright red hemoptysis and dyspnea, which began 30 minutes after smoking marijuana. The patient denied chest pain, fever, chills, leg swelling, trauma or other ingestions. He had no significant past medical history and consumed no medications. Physical exam revealed normal vital signs and diffuse rhonchi in the lung fields bilaterally. A chest x-ray revealed diffuse bilateral alveolar infiltrates. Initial laboratory exam revealed a Pa02 of 66 mmHg and hemoglobin of 15.4 g/dl, which subsequently dropped to 12.9 g/dL over the ensuing twelve hours. Bronchoscopy revealed a diffuse alveolar hemorrhage in all lung lobes. Autoimmune disease, vasculitis and infection were ruled out as causes for the alveolar hemorrhage. A urine toxicology screen was positive for tetrahydrocannibinol and cocaine. Upon further questioning, the patient stated the marijuana may have been laced with cocaine. The patient was monitored with supportive care only and was discharged two days later in good condition.
DISCUSSION
Diffuse Alveolar hemorrhage (DAH) can be caused by various autoimmune diseases, vasculitis, infection and certain medicines. This represents the ninth case report linking DAH with the inhalation of free base cocaine. Inhalation of cocaine may induce vasoconstriction of the pulmonary vascular bed or lead to direct alveolar damage resulting in DAH. To our knowledge there have been no case reports linking marijuana use with DAH and therefore it remains speculative as to whether this drug could have played a role in the development of DAH. In conclusion, DAH is a potentially life threatening complication that should be in the differential diagnosis of dyspnea after inhalation of illicit drugs.
MITRAL VALVE HEMANGIOMA WITH SUPERIMPOSED INFECTIVE ENDOCARDITIS
U. Ahmed1; S.G. Khurshid2. 1Saint Francis Hospital, Evanston, IL, Evanston, IL; 2Saint Francis Hospital, Evanstion IL, Evanston, IL. (Tracking ID #116481)
LEARNING OBJECTIVES
To recognize an unusual presentation of cardiac tumors.
CASE
A twenty-four old previously healthy woman presented with sudden onset of facial asymmetry and left arm weakness. She had been having fatigue, generalized weakness and fever for four weeks for which she was being treated with oral antibiotics. Physical examination revealed a cachectic woman with left lower motor neuron facial paralysis, flaccid paralysis of left upper extremity and an apical ejection systolic murmur. CT scan of brain revealed acute ischemic infarction involving right basal ganglia and internal capsule. Transthoracic echocardiogram demonstrated vegetations on atrial and ventricular sides of the mitral valve. The patient was treated empirically for suspected infective endocarditis. Blood cultures revealed no growth. She remained febrile with spiking fever up to 102. CT scan of abdomen demonstrated multiple infarcts in kidneys and spleen. Transesophageal echocardiogram showed a pedunculated mass on the anterior mitral leaflet. The patient was taken to surgery for excision of the mass. Histopathology showed aggregates of small blood vessels suggestive of mitral valve hemangioma with a dense neutrophillic infiltrate. Gram stain from the tissue did not show any organism and bacterial, viral and fungal cultures were negative. The post operative course was unremarkable.
DISCUSSION
Primary cardiac tumors are rare and their incidence ranges from 0.002% to 0.3% at autopsy. Benign tumors account for 75% of the primary tumors; among which myxoma accounts for 50% whereas hemangioma represents only 2.8%. Cardiac tumors can cause obstruction, valve dysfunction, arrhythmias, pericardial effusions or systemic embolization and constitutional symptoms. These cardiac lesions can induce endocardial trauma via high-pressure jets of blood creating a platelet-fibrin nidus that may become infected. Most cardiac hemangiomas are asymptomatic and are discovered incidentally by echocardiography, CT, MRI or autopsy. Review of literature shows only two reported cases of mitral valve hemangioma: first incidentally found on autopsy, and second presented with chest tightness and palpitations. This patient presented with signs of infective endocarditis and was incidentally found to have mitral valve hemangioma. The main reason for negative blood cultures was thought to be prior administration of antibiotics. No similar case has been reported in literature.
MORE THAN A PAIN IN THE NECK: LEMIERRE's SYNDROME
S. Jain1; S.R. Ranji1. 1University of California, San Francisco, San Francisco, CA. (Tracking ID #117395)
LEARNING OBJECTIVES
1. Recognize the clinical features of Lemierre's Syndrome. 2. Institute the appropriate diagnostic workup in Lemierre's Syndrome. 3. Understand the rationale for aggressive, lengthy antibiotic therapy.
CASE
LR is a 63 year old female who presented with neck pain. The patient had been homebound for the past 30 years due to severe agoraphobia and a schizoid personality disorder, and was only visited by Meals on Wheels and a physician making house calls. She reported left sided neck pain with movement and subjective fevers for 2–3 days, and denied chest pain or shortness of breath. On exam, she was febrile, tachycardic, and hypoxic. She had poor dentition without any obvious abscesses. She was able to move her neck with pain, but had no frank meningismus. She had shotty anterior cervical lymphadenopathy, but no cord could be palpated. Neurologic exam was grossly normal. Labs showed a white blood cell count of 16.3, and lumbar puncture was negative for meningitis. A CT scan was then performed, which revealed thrombosis of left internal jugular vein with extension into the left sigmoid sinus and soft tissue swelling in the parapharyngeal space. This confirmed the diagnosis of Lemierre's syndrome. Subsequently, blood cultures grew out Staphylococcus aureus sensitive to methicillin. Chest CT did not reveal septic pulmonary emboli. The patient was treated with intravenous vancomycin and clindamycin for four weeks and recovered completely.
DISCUSSION
Lemierre's syndrome (also known as postanginal sepsis or necrobacillosis) is septic thrombophlebitis of the internal jugular vein, most commonly caused by anaerobic organisms such as Fusobacterium necrophorum. It is rare and affects mostly young adults. The precipitant is usually a primary dental infection or pharyngitis, followed by invasion though the parapharyngeal space to the IJV causing thrombophlebitis. Metastatic infections complications include septic pulmonary emboli (present in up to 85% of cases), septic arthritis, splenic or hepatic abscesses, and glomerulonephritis. Patients can present with local neck findings (in 52% of cases), but fever (occurring in 83% of cases) and pharyngitis (83%) are more common. CT scan with contrast is the recommended diagnostic test. Treatment with synergistic antibiotics for 3–6 weeks is recommended to prevent spread of infection. Anticoagulation remains controversial but is not routinely recommended. If a patient fails antibiotics, surgical ligation or excision of the internal jugular vein may be necessary. In the antibiotic era, prevalence and mortality has markedly decreased, but mortality remains high at 6.4%. Our case is unusual in that the inciting organism was Staphylococcus aureus rather than the more common anaerobes, and in that the patient had no evidence of septic embolization.
MORE THAN SKIN DEEP: HYPERCALCEMIA ASSOCIATED WITH MALIGNANT MELANOMA
E. Lee1; H. Jasti1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115959)
LEARNING OBJECTIVES
1. To recognize the role of parathyroid-related protein (PTH-rP) in hypercalcemia of malignancy 2. To identify an uncommon association between malignant melanoma and PTH-rP induced hypercalcemia.
CASE
A 32 year old female with a PMH significant for recurring metastatic melanoma to the left thigh with resection × 2 presented with several days of nausea and vomiting. She denied fever, chills, cough, diarrhea or other complaints. Physical exam revealed a thin Caucasian female in mild distress with a 5 cm ulcerated mass on her left thigh. Initial laboratory results were significant for a calcium of 17.6 mg/dL. Whole body CT/PET scan revealed a large mass in the posterolateral left thigh and enlarged lymph nodes in the external iliac and inguinal regions, which on biopsy were negative for malignancy. Serum PTH-rP levels came back elevated at 17.8 pmol/L. The patient was treated with intravenous fluids and pamidronate with resolution of her symptoms. At time of discharge, the calcium level was within normal limits and the PTH-rP level was nearly undetectable. Several weeks later, the patient returned with similar complaints and was again treated for hypercalcemia with fluids and pamidronate. Since that time, she has undergone successful surgical excision of the left thigh mass and has had no recurrence of hypercalcemia.
DISCUSSION
PTH-rP mediated hypercalcemia, also known as humoral hypercalcemia of malignancy, is commonly associated with renal, squamous, and bladder cell carcinomas. It is rarely associated with malignant melanoma. As a structural analog to parathyroid hormone (PTH), PTH-rP binds to the same receptors. This results in an increase in calcitriol-mediated bone resorption, an increase in calcium absorption in the distal tubules, and an inhibition of phosphate transport in the proximal tubules. Diagnosis can be made in a patient with hypercalcemia and an elevated PTH-rP level. Treatment consists of intravenous hydration and bisphosphonates. Clinically, PTH-rP levels can have significance, with higher levels predictive of a shorter median survival time that is independent of calcium levels. In addition, levels above 12 pmol/L are associated with a decreased response to bisphosphonate therapy and a greater incidence of recurrence after treatment. This patient had recurrent hypercalcemia with no evidence of metastatic disease. PTH-rP induced hypercalcemia should be suspected, and considered a poor prognostic indicator, in any patient with a solid tumor in the absence of bony metastases.
MOUNTAIN WEST REGIONAL RESIDENT AWARD WINNER: ‘TENSION HEADACHE’ WITH A SURPRISING ETIOLOGY—A CASE OF NEUROCYSTICERCOSIS
M.C. Kruer1; P. Radhakrishnan2. 1University of Arizona, Tucson, AZ; 2St Joseph Hospital, Phoenix, AZ. (Tracking ID #116970)
LEARNING OBJECTIVES
1. Recognize the distinctive clinical and neuroradiologic features of neurocysticercosis (NC). 2. Recognize that eosinophilic meningitis may be caused by NC.
CASE
A 28 year old previously healthy Hispanic male presented with a history of gradual onset bitemporal headache. It was pulsating, waxing and waning in character. During initial assessment, he had a normal physical exam. He was instructed to take analgesics. The patient subsequently returned with worsening left-sided fronto-temporal headache, nausea, vomiting, fever, photophobia, back pain. His wife reported recent irritability and emotional lability. There was no history of any recent illness, sick contacts or trauma. The patient had immigrated to the U.S. five years ago. He was born in Mexico. On examination, the patient appeared uncomfortable. The rest of his examination, including fundal and neurological examinations were normal. Laboratory studies—CBC and electrolytes were normal. CSF Protein 76 mg/dl, Glucose 40 mg/dl, WBC 267 cells/mm3, predominantly lymphocytes, eosinophils and foamy macrophages. CT scan showed numerous, bilateral calcified lesions in the brain parenchyma (figure 1). A subsequent MRI showed multiple cysts in various stages of evolution, including a racemose cyst within the right Sylvian fissure, and several active and involuting cysts, notably in the frontal temperocortex. (figures 2&3). He was treated with Prednisolone and Albendazole.
DISCUSSION
This case is interesting as it follows the evolutionary stages of cysticercosis of the brain. The clinical presentation of neurocysticercosis varies with the number, location, and status of the cysticerci. Intact cysts tend to be asymptomatic, but all cysts tend to degenerate over time. Symptoms are commonly due to the associated immune response that occurs. In addition, completely degenerate, calcified cysts can serve as epileptic foci. In our patient, the fronto-temporal headache was the primary clinical manifestation, and may have resulted from involuting cysts in that region. The eosinophilia seen in the CSF strongly supports our theory that it was the immunologic response to the involuting cysts present on the surface of the brain that caused the meningoencephalitis and sudden deterioration in the patient's condition. To summarize, in patients originating from areas endemic for T. solium infection presenting with worsening headache and eosinophilic meningitis, neurocysticercosis should be strongly considered.
MUSIC TO THE EARS? NOT!
Y. Ng1; R.C. Brooks2. 1University of Pittsburgh, Pittsburgh, PA; 2Pittsburgh VA Healthcare System, Pittsburgh, PA. (Tracking ID #115803)
LEARNING OBJECTIVES
1) To identify the different categories of tinnitus and their clinical significance. 2) To determine the appropriate work up for a patient with new onset tinnitus. 3) To recognize calcium channel blockers as a cause of drug-induced tinnitus.
CASE
Mr. L is a 60 year old man with a history of hypertension, neurofibromatosis and TIA, who presented with new onset tinnitus of 4 weeks duration. It was a constant ringing in both ears, but had minimally effected his life. He did not recall any recent trauma, although he did admit to a noisy work environment. There were no other new or associated symptoms, including no change in hearing acuity. Physical exam was only notable for many cutaneous neurofibromas and axillary freckling. Otologic and neurologic exams were normal. An audiologic evaluation revealed mild, bilateral high frequency hearing loss with normal (100%) speech discrimination. MRI of the brain was normal. Nifedipine, which was the only new medication initiated prior to the onset of his symptom, was discontinued. During his follow up visit a month later, the patient reported that his tinnitus had subsided after stopping the calcium channel blocker.
DISCUSSION
Tinnitus is a common complaint in ambulatory medicine. A careful, focused history and physical examination will dictate further workup. When taking a history, tinnitus should be categorized as pulsatile or non-pulsatile. Pulsatile tinnitus points to an underlying vascular malformation, which may be potentially life-threatening. It is frequently audible to the third party during examination and hence exam should be focused on 1) auscultation for bruits over the neck, periauricular, temple and orbital region, 2) otoscopic exam to look for vascular lesions. CT scan is the first step in the investigation of pulsatile tinnitus; if negative, angiography will be the gold standard. 95% of patients however, will have non-pulsatile tinnitus. Non-pulsatile tinnitus is usually subjective and originates in the auditory system. Associated symptoms, like hearing loss and vertigo, and precipitating factors, including medications, should be sought. A thorough neurologic exam is important in this category. Audiometric testing to assess for hearing loss is the best initial study. Unilateral hearing loss coupled with poor speech discrimination is highly suggestive of a tumor; if present, MRI is the imaging of choice, with nearly 100% sensitivity and specificity. Only 5% of hearing tests will be normal in patients with acoustic neuroma, so initial audiometric testing can reduce unnecessary use of MRIs. Most patients with non-pulsatile tinnitus will have a negative work-up for a tumor. However, a large number of commonly used medications can cause or exacerbate the symptom and discontinuation often provides significant relief, even if the symptom does not completely resolve.
MYCOPLASMA PNEUMONIAE AND SEPSIS SYNDROME
N.D. Hare1; K. Kieffer1. 1Dartmouth Hitchcock Medical Center, lebanon, NH. (Tracking ID #117449)
LEARNING OBJECTIVES
Recognize the diverse presentations of Mycoplasma pneumoniae infections. Diagnose and treat Mycoplasma pneumoniae infections.
CASE
A 23 year-old white male presented with three days of malaise, fever, chills, headache, odynophagia, lymphadenopathy, non-productive cough, myalgias, watery stools, and rash. He was previously healthy, took no medications and had no allergies. Family history was non-contributory. He was single, heterosexual, smoked 1 ppd cigarettes, rarely drank alcohol, and used IV drugs 8 years previously. Vital signs on admission were: T 38.0°C, BP 80/40, HR 120, RR 20, and oxygen saturation 94% on 2 L/min. Physical exam revealed an erythematous pharynx, tender cervical adenopathy, clear lungs, and a total-body macular rash. Laboratory results included: WBC 19,200 (39% bands); platelets 108,000; creatinine 1.5; CPK 453, and troponin T 0.29 (normal <0.03). A rapid streptococcal screen and a Monospot test were negative. Chest X-ray showed a widened mediastinum. Neck and chest CT scan showed cervical and mediastinal adenopathy. EKG showed sinus tachycardia without ischemic changes. An echocardiogram revealed ejection fraction 20%, global biventricular hypokinesis, and no valvular vegetations. He was treated with clindamycin and moxifloxacin, intravenous fluids and pressors. He continued to have fevers to 40.7°C, but cultures of blood, sputum, urine, and CSF, as well as serum and CSF viral studies, failed to reveal a source. An HIV test was negative. On hospital day #14, serology for Mycoplasma pneumoniae IgM returned positive. Moxifloxacin had been stopped on hospital day 7, but was resumed for a total course of 12 days. The patient defervesced, and a repeat echocardiogram on hospital day #23 showed normalization of ventricular function, with ejection fraction of 60%. Despite several complications, he recovered fully and was discharged to home.
DISCUSSION
Mycoplasma pneumoniae infections are usually mild and self-limiting. The most common target is the respiratory tract. However, M. pneumoniae can rarely cause severe infections, affecting multiple organ systems. Besides the lungs, the skin and heart are most commonly involved. M. pneumoniae has been documented to cause exanthems, arrhythmias, myocarditis, neurologic problems (e.g. Guillain-Barré), and Stevens-Johnson syndrome. M. pneumoniae lacks a cell wall, so it is not seen on Gram staining. It is not easily cultured. Confirmation of infection is by enzyme-linked immunoassay for IgM and IgG directed against M. pneumoniae. Due to the lack of a cell wall, Mycoplasmas are resistant to penicillins, cephalosporins, and vancomycin. Treatment with a macrolide, a fluoroquinolone, or doxycycline is recommended This patient presented with sepsis syndrome, which was ultimately attributed to Mycoplasma pneumoniae and resolved with appropriate antimicrobial therapy.
MYELITIS FROM MOSQUITOS
D. King1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117480)
LEARNING OBJECTIVES
1. Recognize the association of West Nile Virus (WNV) with acute febrile progressive paralysis. 2. Consider viral encephalitis in the differential for immunocompromised patients who present with neurological dysfunction.
CASE
A 43-year-old man with HIV (CD4 130) presented with five days of back pain, diarrhea and bilateral calf cramping. He was an obese, febrile man with symmetrical 3/5 muscle strength in upper and lower extremities. His cranial nerves and mental status were normal. Within 48 hours his weakness progressed to 0/5 proximal muscle strength, 1/5 distal muscle strength, and 2/5 hand grip and plantar-flexion. He developed areflexia, bilateral hearing loss and respiratory failure. A lumbar puncture was attempted but was unsuccessful. The patient's obesity prevented an MRI. A CT scan of the head showed only sinusitis. He was empirically treated for bacterial meningitis versus Guillian-Barre syndrome (GBS). Encephalitis panels and serological screening for CNS infections were drawn. The patient was discovered to have West Nile IgM; all other serological tests were negative. EMG was performed and showed a mixed neuropathic/myopathic picture, that, when taken into account with his hearing loss, effectively excluded GBS.
DISCUSSION
West Nile virus (WNV) is a flavivirus transmitted by mosquitos. The most common presentation is a febrile illness and meningoencephalitis. Less common, though still prevalent, is a syndrome of acute progressive febrile paralysis similar to poliomyelitis. Proximal muscle weakness predominates over distal, and cranial nerve involvement is expected. Although bacterial and fungal meningitides are the most commonly considered causes of acute-onset neurological changes in immunocompromised individuals, viral encephalitides should also be considered, especially in areas where mosquitos and other insect vectors are prevalent. Physicians should recognize the poliomyelitis-like syndrome associated with the West Nile Virus, especially in the immunocomprised.
MYOCARDIAL INFARCTION IN A YOUNG ADULT WITH ELEVATED APTT
A. Kalyanasundaram1; A. Quiery1; K. Gavlick1. 1Geisinger Medical Center, Danville, PA. (Tracking ID #116911)
LEARNING OBJECTIVES
1) Recognize antiphospholipid syndrome as a rare cause of myocardial infarction in the young adult 2) Reinforce that antiphospholipid syndrome is a procoagulant disorder despite the elevated APTT 3) Management of a myocardial infarction in the setting of antiphospholipid syndrome.
CASE
42-year-old woman presented at an outside hospital with shortness of breath and stuttering chest pain for one week. She was admitted in the same hospital less than a month earlier for dysfunctional uterine bleeding when she was diagnosed with lupus anticoagulant. Her history is otherwise unremarkable. She was transferred to our hospital with the diagnosis of an acute MI based on EKG changes and enzyme elevation. She was tachycardic, hypotensive and hypoxic. Chest exam revealed rales at both bases. Her PTT was elevated at 75. Medical management was optimized and she was started on heparin after an emergent hematology consult. An echocardiogram revealed evidence of a large inferior, inferolateral and anterolateral myocardial infarction with left ventricular ejection fraction of 30–35%. Cardiac enzymes and Troponin-T were positive. Her dilute Russell Viper Venom Time (dRVV) was positive (150 seconds). Her anticardiolipin antibody IgG was strongly positive (94.7 GPL U/ml) and IgM (56.2 GPL U/ml) was medium positive. The elevated aPTT failed to correct after mixing studies. Her heparin was titrated with serial thromboelastograms. Cardiac catheterization revealed critical ostial left main coronary artery disease that lead to CABG. Coumadin therapy was initiated and she was discharged in a satisfactory condition. Repeat anticardiolipin antibodies at 6 weeks remained elevated.
DISCUSSION
Primary antiphospholipid syndrome, also known as Hughes syndrome, is a thrombotic disorder characterized by antiphospholipid antibodies—anticardiolipin (aCL) antibodies and lupus anticoagulant. This syndrome presenting as coronary artery disease has rarely been reported in the literature. Antiphospholipid syndrome resulting in a myocardial infarction with critical ostial left main artery disease necessitating CABG has never been reported to the best of our knowledge. ACL antibodies are strongly associated with venous and arterial thrombosis, both in patients with systemic lupus erythematosus and in the primary antiphospholipid syndrome. Antiphospholipid syndrome should be considered in the differential diagnosis of myocardial infarction in the young adult. It is important to be cognizant of the fact that despite the elevated aPTT, antiphospholipid syndrome is a hypercoagulable state requiring anticoagulation when appropriate. Also, thromboelastogram, a global test of coagulation, has a possible role in the management of patients on intravenous heparin.
NEISSERIA SICCA: SO CLOSE TO MY HEART
C. Burgdorf1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117400)
LEARNING OBJECTIVES
1. Recognize N. Sicca as a cause of endocarditis in HIV-positive patients 2. Recognize the clinical risk factors for developing N. Sicca endocarditis.
CASE
A 43 year-old man presented with a three-day history of fever and myalgias. He noted the absence of headache, nausea, vomiting, weight loss, cough, or diarrhea. His past medical history was notable for hepatitis C and HIV. He routinely used intravenous drugs, but did not smoke or drink. He had no history of opportunistic infections; his CD4 count was 344 cells/mm3 three months prior. His temperature was 38.4 °C; his pulse was 109 beats/min. He had a 2/6 systolic murmur at the apex that increased with hand grip. The remainder of his exam was normal. A diagnosis of endocarditis was entertained despite prior medical records that noted a 1/6 murmur, Blood cultures were positive for Neiserria sicca. Although considered a contaminant, a transesophageal echocardiogram was performed to exclude the diagnosis. This revealed vegetations on the tricuspid valve consistent with endocarditis. He was treated with ceftriaxone for six weeks.
DISCUSSION
Although Neisseria meningitidis and Neisseria gonorrhoeae are well-known pathogens, most of the genus Neisseria species are considered commensal in as much as they colonize but do not cause disease. Neisseria sicca is a commensal organism that is typically found in the throat and rarely causes disease. In the setting of immunosuppression, however, organisms that are normally commensal in nature can initiate disease. The modified Duke criteria includes a history of intravenous drug use as a minor criteria for the disease. The impurities in intravenous drug injection have been shown to disrupt the endothelial lining of normal valves, predisposing the patient to endocarditis by allowing a site of attachment for the organism. This patient's HIV and intravenous drug use history predisposed to infection, even to a normally commensal organism. It is important for physicians to remember that there is a higher incidence of commensal organisms causing pathologic disease in those that are immunocompromised.
NEUROSARCOIDOSIS INITIALLY DIAGNOSED AS MULTIPLE SCLEROSIS
M. Quate-Operacz1; E. Warm1. 1University of Cincinnati, Cincinnati, OH. (Tracking ID #115637)
LEARNING OBJECTIVES
1. Recognize the multiple presentations of neurosarcoidosis. 2. Differentiate neurosardoidosis from multiple sclerosis.
CASE
Differentiating between the neurologic manifestations of sarcoidosis and multiple sclerosis (MS) can be exceedingly difficult, especially when other signs of sarcoidosis are missing. To do so is important as the therapies and prognoses of these two diseases are vastly different. We present a case of a patient initially diagnosed with MS who developed classic sarcoidosis 2 years later. A 38 year old male with a purported history of MS complained of dyspnea on exertion, cough with yellow-white sputum production and chest pain worsening over 4 months. Three skin lesions on the upper extremities and abdomen had appeared one month prior to presentation. Two years prior to this he had complaints of paresthesias from the umbilicus to the toes, increasing weakness of both lower extremities, and occasional weakness and paresthesias of the right upper extremity. A chest x-ray was normal at this time. An MRI revealed signal abnormalities consistent with a demyelinating process at C4–C5 and T8–T9 levels. An LP demonstrated elevated protein and oligoclonal bands. The patient was diagnosed with MS and started on interferon-beta therapy with resolution of symptoms. On the current presentation the patient's chest x-ray revealed diffuse nodular infiltrates. Biopsy of the skin lesions was consistent with sarcoidosis. In retrospect, it was concluded that the initial presentation with paresthesias and weakness was most likely attributable to neurosarcoidosis.
DISCUSSION
Neurosarcoidosis is an uncommon manifestation of systemic sarcoidosis. In clinical studies, it was present in 5–16% of sarcoid cases. Neurologic complaints are the presenting symptom of neurosarcoidosis in 50% of cases. The majority will also have disease in other organ systems. The diagnostic criteria include a compatible clinical picture, typical radiologic findings, and histologic evidence of sarcoid from any tissue. MRI findings vary but white matter lesions can be present and mimic MS. Up to 80% will have CSF abnormalities that include mononuclear pleocytosis, increased CSF pressure, and some cases there has been evidence of oligoclonal bands making it very difficult to distinguish from MS. Before treatment is instituted 35–50% of cases will improve spontaneously. One third can relapse again simulating MS. This remission and relapse are likely what occurred in our patient as studies have that shown sarcoid and other autoimmune diseases can actually be exacerbated during interferon therapy.
NEUROSYPHILIS PRESENTING AS BILATERAL VOCAL CORD PARALYSIS AND GASTROINTESTINAL AUTONOMIC DYSFUNCTION
D. Blenner1; C. Woods1; R. Levy1; J.C. Byrd1. 1East Carolina University, Greenville, NC. (Tracking ID #117338)
LEARNING OBJECTIVES
To emphasize the importance of including neurosyphilis in the differential diagnosis of unusual or unexplained neurological findings in patients who are immunocompromised.
CASE
A 34 year old male presented to our service after sustaining head trauma from a MVC causing a subarachnoid hemorrhage (SAH) and subsequent short-term memory deficits and generalized weakness. On day 16 after his injury he developed stridor. Laryngoscopy revealed bilateral vocal cord paralysis (BVCP). A head CT scan showed no acute processes. A tracheostomy was performed to protect his airway. A percutaneous gastostomy tube was placed to provide adequate nutrition but he had persistent residuals with his feedings. On day 29, the patient developed a left-sided T6 dermatomal herpes zoster rash. A thorough sexual history revealed that the patient had engaged in unprotected sexual activities with men. STD serologies were ordered with the patient's consent. The RPR and HIV were positive. A lumbar puncture was performed which revealed 43 white blood cells that were predominantly lymphocytes and a positive VDRL. Treatment for neurosyphillis was initiated with 24 million units of Penicillin G per day. After four days of treatment, the patient's voice began to return and his feeding residuals resolved. He was discharged one week into therapy with normal phonation. Subsequent laryngoscopy showed near complete recovery of his vocal cords. Literature review revealed that syphilis can be rare cause of vocal cord paralysis.
DISCUSSION
With the increasing rates of syphilis in the past 2 years and its frequent association with HIV, it is important to recognize that neurosyphilis is not uncommon and may present in a cryptic fashion.
NO DRUG IS BENIGN: NEUROLEPTIC MALIGNANT SYNDROME CAUSED BY AN ATYPICAL ANTI-PSYCHOTIC
V. Chan1; B. Taqui1. 1Temple University, Philadelphia, PA. (Tracking ID #116214)
LEARNING OBJECTIVES
1. Recognize clinical features of Neuroleptic Malignant Syndrome (NMS). 2. Recognize that newer, atypical agents can still cause NMS. 3. Recognize managment options for NMS.
CASE
A 65 year old Caucasian male with schizophrenia presented with a two days of progressive change in mental status. According to his care givers, he was no longer performing usual activities of daily living including eating, dressing, and bathing. He had been found with bowel and bladder incontinence and was no longer speaking or following commands. His medications included olanzapine 10 mg po qd and temazopam 30 mg po qhs. On exam, he was alert, but with a masked facies. Orientation and cranial nerve function could not be assessed. He could move all extremities spontaneously, but had diffusely increased tone and a 3 second upper extremity resting tremor. Non-contrast head CT, EEG and blood and urine studies were negative. He was diagnosed with neuroleptic induced extrapyramidal symptoms. Fluids were started and olanzepine was discontinued. On hospital day #3, he developed a fever to 100.6 F and was acutely confused. He had pulse 128, blood pressure 120–160 systolic. He had lead pipe muscular rigidity, increased tone compared to admission, and diffusely brisk deep tendon reflexes. Lab data revealed CPK = 2,890 U/L, Na = 154. CBC, chemistries, blood/urine cultures and CXR were negative. Brain MRI showed chronic small vessel ischemic changes, but no acute pathology. The patient was diagnosed with olanzapine induced neuroleptic malignant syndrome. He responded nicely to treatment with dantrolene, bromocriptene, and intravenous fluids.
DISCUSSION
Neuroleptic Malignant Syndrome (NMS), an idiosyncratic reaction to antipsychotic agents, is assumed to be due to reduced dopaminergic activity. It is characterized by severe rigidity, tremor, altered mental status, fever, and autonomic dysfunction. Complications include rhabdomyolyis, acute renal failure, and thromboembolism. It is traditionally associated with older antipsychotics (haloperidol, risperidone), but there have been some case reports with newer agents such as olanzepine. Risk factors include dehydration, poorly controlled neuroleptic induced extrapyramidal symptoms (EPS), treatment resistant EPS, and rapid rate of neuroleptic loading. Treatment involves discontinuation of neuroleptics and supportive care with antipyretics, fluids, and electrolytes. Refractory cases require initiation of dopamine agonists and muscle relaxants, such as bromocriptine and dantrolene. Severe NMS has 20–30% mortality. Our case underscores the importance for generalists to recognize the side effect profile of medications prescribed by other specialties. It also demonstrates that newer agents can still cause NMS.
NONTUBERCULOUS MYCOBACTERIUM AS A CAUSE OF BURSITIS
H.E. Woo1; C.H. Fung2. 1University of California, Los Angeles, Los Angeles, CA; 2VA Greater Los Angeles Healthcare System, Los Angeles, CA. (Tracking ID #116278)
LEARNING OBJECTIVES
1. Recognize nontuberculous mycobacterium as a cause of bursitis. 2. Manage Mycobacterium chelonae infection with consultation from infectious diseases and surgery.
CASE
A 63-year old retired probation officer and former alcoholic presented with a chief complaint of one month of right elbow pain and swelling. He had been performing physical therapy floor exercises, which included weight-bearing stances on both elbows, for chronic back pain. His favorite location to practice the exercises was at the local beach in southern California. Physical exam revealed a well-developed, well-nourished male in no acute distress. He was afebrile and had a 5 × 5 cm fluctuant, warm, tender, erythematous swelling over the right olecranon without open wound or overlying rash. Fine needle aspiration was performed during the initial clinic visit. Culture of the aspirated bursa fluid grew Mycobacterium chelonae, sensitive only to clarithromycin and aminoglycosides. He started oral clarithromycin and had such a dramatic response that the patient refused recommended treatment with intravenous (IV) amikacin and bursectomy. He agreed to discontinue physical therapy exercises involving elbow weight-bearing stances. Seventeen months later he presented with pain and swelling involving the opposite (left) olecranon bursa; the right olecranon was normal on examination. Aspiration again showed Mycobacterium chelonae sensitive only to clarithromycin and aminoglycosides. With consultation from infectious diseases and orthopedics, he had a successful left bursectomy while receiving a six-week course of oral clarithromycin and two-week outpatient course of IV amikacin via percutaneous intravenous central catheter (PICC line).
DISCUSSION
M. chelonae is a nontuberculous mycobacterium with worldwide distribution that can be found in natural and processed water sources such as tap water and sewage. Nosocomial infections can occur as a result of instruments contaminated with colonized tap water. Presentation varies according to the site, but superficial infections typically are nonhealing, nonspreading wounds. Diagnosis requires smear for acid-fast bacilli and culture. Unlike tuberculosis, M. chelonae infections do not need to be reported to local health departments. Management with a surgeon is indicated, because without surgical debridement recurrence is likely. Consultation with infectious disease specialists is appropriate for therapeutic guidance. M. chelonae is resistant to typical antituberculous medications such as rifampin and isoniazid. However, it is usually sensitive to clarithromycin and amikacin. Resistance to single-drug therapy occurs, so dual antibiotic therapy is typically recommended. The optimal duration of antibiotic therapy is unknown, but ranges from weeks to months.
NOT JUST ANOTHER HEART FAILURE (HF) EXACERBATION: SIGNIFICANT VOLUME OVERLOAD FROM A THIAZOLIDINEDIONE (TZD)
R.M. Malone1; M.P. Pignone1; A.B. Weil1; B. Bryant1. 1University of North Carolina at Chapel Hill, Chapel Hill, NC. (Tracking ID #115896)
LEARNING OBJECTIVES
1) Recognize the potential of TZDs to potentiate or exacerbate symptoms of HF; 2) Discuss proper use of TZDs in light of the potential adverse effects; 3) Identify patient education and counseling as a way to avoid complications from volume overload when TZDs are used.
CASE
JN is a 59 year old woman with type 2 diabetes, chronic renal insufficiency, and HF from viral myocarditis in 1991. An echocardiogram from February 2000 revealed a LVEF of 32%. She presented to clinic July 2001 with increased fatigue, decreased exercise tolerance for 3 months, DOE, and edema. She denied SOB at rest, PND, or orthopnea. Functional status had worsened from NYHA class II to III. Medications included: Pioglitazone 45 mg qd (Added 4/2001), Digoxin 0.0625 mg qd, Furosemide 80 mg qam 40 mg qpm, Lisinopril 20 mg qd, Metoprolol XL 50 mg qd, Warfarin 5 mg qd, Simvastatin 20 mg qd, Spironolactone 25 mg bid, NPH insulin 20u am and 10u pm, REG insulin 10u bid. Vitals: 271 lbs (15 lb increase over the preceding 2 months), BP 90/70, P 88. Physical Exam: 15 cm of JVD, displaced PMI, distant S1 and S2, 2–3+ bilateral lower extremity peripheral edema. Recent labs: SCr 1.6, BUN 59, ALT 27, and A1c 7% (previous A1c 11.1%). Furosemide was increased to 80 mg bid and genotyping for cardiac transplantation was ordered. Over the following 3 months, JN had 6 cardiology and medicine clinic visits and continued to complain of symptoms of DOE, edema, and an eventual weight gain of 30 lbs. She was hospitalized October 2001 for intravenous diuresis. After discharge, the General Medicine Diabetes Program decreased Pioglitazone to 30 mg over the telephone. This resulted in improved symptoms and a 28 lb weight loss over the following month. The Diabetes Program discontinued the TZD December 2001, her weight returned to baseline and functional status improved to NYHA class II.
DISCUSSION
Pioglitazone and Rosiglitazone are TZD antidiabetic agents that activate PPAR-gamma thus reducing insulin resistance. When used as monotherapy, TZDs can decrease A1c approximately 1.5%. TZD package inserts list HF, dose related edema, and weight gain as potential adverse effects. Average weight gain ranges from 0.8 to 5.4 kg and edema occurs in 4.8 to 15% of patients. In a recent cohort study, the incidence of HF symptoms and hospitalization for HF was 8.8% and 2.5%, respectively. Volume related adverse events occur frequently, are often misdiagnosed, and may lead to significant patient morbidity. Providers should recognize these potential adverse effects and avoid TZDs in patients with or at risk for HF. Careful monitoring for weight gain, edema, and HF symptoms is crucial with TZD use.
NOT JUST ANOTHER RASH
A. Harzstark1; P.P. Balingit2. 1University of California, Los Angeles, Los Angeles, CA; 2UCLA San Fernando Valley Program, Sylmar, CA. (Tracking ID #117547)
LEARNING OBJECTIVES
1. Recognize epidermodysplasia verruciformis (EV) as a predisposing factor for cutaneous squamous cell malignancies. 2. Describe the etiology and presentation of EV.
CASE
A 21 year old Honduran female with no significant past medical history presented to an urgent care clinic with a painless, nonpruritic, scaly lesion with associated eschar in the right forehead area. The lesion bled intermittently from the margins and slowly increased in size during the three years prior to presentation. The patient also reported the eruption of generalized, erythematous plaques over her back, trunk, and extremities starting at the age of eight. These skin lesions were also present in three paternal cousins. Physical examination was notable for a well-defined two by two centimeter eschar on the patient's right forehead with slight bleeding from the lateral margin. Lesions were present, most notable on her neck, upper chest, and axillae, and also involved her abdomen, back, arms, and legs. The rash consisted of flattened, erythematous and hyperpigmented papules with a scaly surface and irregular borders. Areas of confluent plaques were present as well. Biopsy of the forehead lesion showed squamous cell carcinoma in-situ with adjacent actinic keratosis. Punch biopsy of a lesion on the back revealed slight hyperkeratosis and irregular mild acanthosis with scattered areas of atypical keratinocytes in the upper half of the epidermis, consistent with EV. Plastic surgery consultation was obtained for excision of the lesion located on the patient's forehead. Additionally, routine primary care follow-up was recommended for periodic examination of the patient's skin to identify possible developing malignancies.
DISCUSSION
EV is an autosomal recessive disorder of the skin associated with chronic human papillomavirus (HPV) infection. Rare cases of autosomal dominant and X-linked transmission have also occurred. EV is associated with impaired cellular immunity to HPV, making patients susceptible to widespread viral infection. Resulting skin lesions transform to skin cancers, particularly in sun-exposed areas, in one-third of patients and usually after the age of thirty. EV typically presents during childhood but can also appear during infancy and adolescence. On histologic examination, more than 90% of EV skin lesions contain evidence of infection with HPV subtypes 5, 8, and 47. Excision is required for EV lesions which have transformed into malignant skin cancers. Topical retinoid and intralesional interferon treatments for growing EV lesions have been found to be of possible benefit in slowing the progression to skin cancer. By providing periodic examination of the skin and surveillance of lesions, the primary care physician plays a vital role in the management of EV. Biopsy of enlarging lesions is necessary to exclude malignant transformation.
ONE FLU OVER THE CUCKOO's NEST
H.T. Ly1; M. Rotblatt2. 1University of California, Los Angeles, Sylmar, CA; 2UCLA SFVP-Olive View Medical Center Department of Internal Medicine, Sylmar, CA. (Tracking ID #115582)
LEARNING OBJECTIVES
1. To recognize the severity of complications and the impact of influenza illness 2. To ultimately realize the importance of the flu vaccine.
CASE
A 50 year old saxophone player presented to the ED with a week of “chest cold,” cough productive of yellowish sputum, intermittent fevers as high as 102 F, and nightsweats. He was a smoker but had no significant PMH. He had no recent travel or risk factors for tuberculosis, although he had been visiting his ill wife in the hospital in recent weeks. She was a surgical patient whose hospital course was complicated by pneumonia. His vital signs were critical: T 38.3 C, BP 71/52, P 150, R 28, Pulse Ox 84% on 2L nasal cannula. There were diffuse crackles and tachycardia on exam. His WBC was 7.0 (N 79.6, L 9.9, M 10.4), and his BUN/Cr were 36/2.2. His CXR showed large bibasilar infiltrates. He was admitted to the ICU with a diagnosis of severe community acquired pneumonia and sepsis. He was intubated and received piperacillin/tazobactam, azithromycin, and aggressive fluid hydration. Sputum and blood cultures were sent, and the patient was stabilized but remained febrile. On day 2, both sputum and blood cultures were overwhelmingly positive for methicillin resistant Staphylococcus aureus (MRSA). Vancomycin was started, and he defervesced within 2 days. His PPD, sputum PCP DFA, AFB smears, and fungal cultures were negative. However, his pulmonary status remained poor, prompting a chest CT that showed extensive bilateral bronchiectasis and cavitation that was attributed to the Staph infection. His hospital course was long, eventually requiring transfer to a ventilation weaning facility. Interestingly, he was initially tested and found positive for influenza A. He had not received the flu vaccine that season.
DISCUSSION
Our patient likely had influenza initially, which predisposed his lungs to a secondary bacterial infection. Pneumonia, especially with Staph, is a classic complication of influenza. Acquiring an MRSA infection, however, was a curiosity and eventually was attributed to his recent visits to the hospital to visit his ill wife. This case highlights a serious course that followed a seemingly commonplace flu. Given the severity of complications and dramatic rise in influenza cases this season, it becomes paramount to realize the impact of the flu and the potential protection offered by the vaccine to the general public.
PARALYSIS IN A YOUNG ADULT: AN UNUSUAL DIAGNOSIS
D. Fotino1; M. Landry1. 1Tulane Health Sciences Center, New Orleans, LA. (Tracking ID #117459)
LEARNING OBJECTIVES
1) Recognition of causes of macrocytic anemia 2) Identify diagnosis and treatment of pernicious anemia 3) Develop a diagnosis in ascending progressive neurologic deficits.
CASE
A 39 year-old woman with diabetes mellitus presented with inability to walk. Her symptoms began eight months prior with gradual progression leading to inability to ambulate. She noted decreased bilateral lower extremity proprioception with progressive ascending sensorimotor loss. She reported decreased appetite, weight loss, dysphagia, incontinence and worsening back pain. Physical examination revealed thyromegaly, sacral decubiti and lower extremity edema. The neurologic examination was remarkable for short-term memory loss. Her lower extremity examination revealed sensory deficits to light touch and pin-prick, absent reflexes and motor paralysis. Rectal tone was poor. Diagnostic studies included a hemoglobin of 8.8 g/dl with an MCV 112 Ym£, B12 level <100, iron saturation 6% and normal thyroid studies. Electromyogram revealed diffuse axonal and demyelinating loss in her lower extremities. Her esophagogastroduodenoscopy demonstrated atrophic gastritis and her anti-intrinsic factor antibody was positive. She underwent B12 replacement with normalization of her MCV, but her neurologic deficiencies worsened. A repeat EMG was consistent with Amyotrophic Lateral Sclerosis (ALS).
DISCUSSION
Common causes of macrocytic anemia include folate and B12 deficiency, and liver and thyroid disease. Initial evaluations include folate, B12, liver and thyroid studies. Pernicious anemia causes B12 deficiency with antibodies against intrinsic factor and parietal cells. Neurologic sequelae of B12 deficiency may be reversed with B12 replacement and normalization of the MCV. Further evaluation may be necessary for progressive neurologic symptoms. ALS is a severe form of progressive degenerative neurologic disease and must be considered in the differential of worsening neurologic deficits. Patients with ALS often become sufficiently malnourished to lead to concomitant vitamin deficiencies. The failure to respond to parenteral B12 was an important clue in investigating ALS as an etiology for her neurologic deficits.
PARATHYROID CARCINOMA: TWO CASES DIAGNOSED PRE OPERATIVELY
K. Pachipala1; S. Naidu1; D. Bucaloiu1; R. Pierce1; R. Monsaert1. 1Geisinger Medical Center, Danville, PA. (Tracking ID #109732)
LEARNING OBJECTIVES
Recognize that parathyroid carcinoma can be clinically suspected preoperatively.
CASE
Case 1 A 66-year-old man presented with a 5-month history of pain in his right heel. Serum calcium and iPTH were elevated at 12.6 and 1800 respectively. Skull x-ray showed a salt and pepper appearance and a large “brown tumor”. A bone scan showed increased uptake in the heel and skull. Because of significant bone involvement and a markedly elevated PTH, parathyroid carcinoma was suspected. A large left inferior parathyroid gland was found and resected. Microscopic examination confirmed parathyroid carcinoma. The patient developed the “hungry bone syndrome” following surgery, and responded to calcium and vitamin D. Case 2 A 55-year-old woman with a history of symptoms typical of hypercalcemia came to attention when a biopsy of bone from a pathologic fracture of her patella revealed a “brown tumor”. Serum calcium and iPTH were 18.4 and 1120 respectively. Because of severe hypercalcemia, bone involvement and a markedly elevated PTH, parathyroid carcinoma was suspected. A totally intrathyroidal parathyroid carcinoma was resected. She also developed the “hungry bone syndrome”.
DISCUSSION
Hyperparathyroidism-dependent hypercalcemia is commonly due to parathyroid hyperplasia or an adenoma. Parathyroid carcinoma is an uncommon cause with 390 cases reported so far in the literature. Parathyroid carcinoma is an indolent tumor and the clinical features are predominantly due to the effects of excess PTH secretion. It is important to suspect parathyroid carcinoma preoperatively, as a complete resection of the tumor at the time of initial operation is required for an optimal outcome. Often, unfortunately the diagnosis is made when hypercalcemia recurs after surgical treatment of primary hyperparathyroidism. Parathyroid carcinoma may be suspected clinically by the presence of severe and symptomatic hypercalcemia, markedly elevated PTH levels (3–10 times), palpable neck mass and a high prevalence of renal and skeletal involvement. Our high suspicion for parathyroid carcinoma alerted the surgeon to do a more aggressive and potentially curative, surgical procedure. Significant hypocalcemia from hungry bone syndrome is common postoperatively and is regarded as a sign that the surgery is successful.
PARATHYROID STORM
E.H. Orth1; S. Mangers1; L. Cation1. 1University of Illinois at Peoria, Peoria, IL. (Tracking ID #116240)
LEARNING OBJECTIVES
Recognize the clinical features of severe hypercalcemia. Recognize benign primary hyperparathyroidism can not be excluded in the differential of severe hypercalcemia.
CASE
A 48 year old female presented with a 5 day history of progressive weakness. She complained of anorexia, vomiting, abdominal pain, as well as constipation and polyuria. Further history included headache, difficulty thinking, cough, and dysphagia. On physical exam, she was alert but displayed slow and labored conversation. The exam was remarkable for dehydration, tachypnea, and fullness of the right neck without discernible lesion. The abdomen had decreased bowel sounds and was soft with moderately diffuse tenderness. There was no guarding or rebound. The remainder of the exam was unremarkable. Laboratory analysis revealed serum calcium 23.6 mg/dl, phosphate 2.2 mg/dl, intact parathyroid hormone (iPTH) 1435 pg/ml, and serum electrophoresis with polyclonal increase in gamma globulin. The chest radiograph was unremarkable. A limited parathyroid scan had increased activity throughout an elongated right thyroid lobe. The patient was rehydrated, then given furosemide and pamidronate. Serum calcium fell to 11.3 mg/dl. Parathyroid surgery with neck exploration was performed and an 18.21gram mass was removed from the right inferior parathyroid gland. Pathologic examination revealed a parathyroid adenoma with mild nuclear atypia, low mitotic activity, and no malignancy. One day post surgery, serum calcium was 9.6 mg/dl. The patient had clinical resolution of her signs and symptoms.
DISCUSSION
Most patients with primary hyperparathyroidism are asymptomatic with only mild elevation of serum calcium. Extreme calcium level elevations are more typical of parathyroid malignancy. Upon literature review, the highest reported serum calcium in benign PHPTH was 26.3 mg/dl in 1987. We report a case of a symptomatic patient with serum calcium 23.6 mg/dl and iPTH 1435 pg/ml resulting from a benign parathyroid adenoma. Benign primary hyperparathyroidism can not be excluded in the differential of severe hypercalcemia.
PARTIAL SPINAL CORD SYNDROME IN A PATIENT WITH NASOPHARYNGEAL CARCINOMA
R. Bomprezzi1; P. Radhakrishnan2. 1St. Joseph Hospital, Phoenix, AZ; 2Catholic Healthcare West, Phoenix, AZ. (Tracking ID #116023)
LEARNING OBJECTIVES
1. Recognize the rare neurological manifestations of invasive neck tumors and the treatment. 2. Diagnose partial spinal cord syndromes. 3. Recognize that autonomic dysfunction should be considered in the differential diagnosis of patients with bradycardia and hypotension.
CASE
A 77 year-old male patient presented with a history of syncope. He was found unresponsive for an unknown duration. His past medical history included nasopharyngeal carcinoma diagnosed four years before, for which he underwent extensive surgery to the right side of his neck, including laminectomy at the level of C3–C4 and subsequent radiation therapy. He also had Rheumatic Heart Disease with moderate mitral and aortic regurgitation and hypertrophic cardiomyopathy . Physical Exam—He was alert and oriented. BP 85/50 mmHg, HR 40/min. Pupils were equal and reactive bilaterally. Neck exam. revealed a left submandibular mass. There was swelling of the left base of the tongue with mild dysarthria. CVS-apical systolic murmur with no radiation. Neurological exam-decreased sensation in the left half of the face. Sensation to temperature was decreased on the left side of the body, more pronounced on the lower extremity; proprioceptive and tactile sensations were preserved. Motor examination revealed moderate impairment to fine movements of right hand and decreased strength (grade 3/5) of the right leg. The rest of the exam was normal.
DISCUSSION
This case highlights the neurologic sequele of invasive neck tumors. The Brown-Sequard syndrome is due to hemisection of the spinal cord, usually due to trauma or spinal cord tumors. There is ipsilateral motor weakness, proprioceptive and vibratory loss, and contralateral loss of pain and temperature sensation. The neurological findings described in this case are consistent with partial (incomplete) Brown-Sequard syndrome. Injury to the descending fibers of the corticospinal tract leads to the ipsilateral motor impairment. Damage to the ascending decussated fibers of the spinothalamic tract accounts for the loss thermal sensation in the contralateral body. The left facial hypoesthesia and dysarthria was due to local invasion of the recurrent tumor. The bradycardia and hypotension in this patient could be due to either autonomic dysfunction secondary to the neck mass or the patient's underlying cardiac disease. To summarize, it is important for physicians to familiarize themselves with the different clinical manifestations of neck masses and surgery.
PATIENCE IS A VIRTUE: A SEVERE LAB ABNORMALITY WITH CAUTIOUS MANAGEMENT
E. Lee1; L. Thomas1; H. Jasti1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115958)
LEARNING OBJECTIVES
1. To identify the multi-factorial etiologies of hyponatremia 2. To recognize the importance of history and clinical presentation in hyponatremia.
CASE
A 71 year old woman with a PMH significant for hypothyroidism, atrial fibrillation, and mild mental retardation presented with generalized weakness, bilateral lower extremity swelling, and “aphasia” for several weeks. On presentation, she was able follow all commands and communicate non-verbally but with an extremely blunted affect. Physical exam revealed mild jugular venous distension, an irregularly irregular heart beat, moderate rales in the lung bases, and 2+ pitting edema of the legs bilaterally. MRI/MRA of the head was normal. Chest radiograph showed pulmonary edema, a right-sided solitary pulmonary nodule, and a small pleural effusion on the same side. Laboratory results were significant for an elevated beta-natriuretic peptide (BNP) of 241 pg/ml, normal TSH and cortisol levels, and a sodium of 103 mEq/L. Diagnostic thoracentesis revealed a transudative fluid, negative for infection or malignancy. Additional history later obtained from the family revealed that the patient had a decreased appetite over the last 2–3 weeks, but had continued to ingest large quantities of water. At baseline, she had a blunted affect and recently had been started on paroxetine for depression. The drug was discontinued and the patient was placed on water restriction. The sodium levels gradually normalized over the next five days with resolution of her symptoms.
DISCUSSION
Hyponatremia is a common lab abnormality that reflects an underlying problem with free water balance in the body. This balance can be upset in states of excess free water, either through impaired excretion or excess intake. In this patient, multiple factors potentially affected her free water balance: 1) excess free water intake, also known as primary polydipsia; 2) CHF with volume overload; 3) inadequate dietary solute intake, or the “tea and toast diet;” 4) the syndrome of inappropriate anti-diuretic hormone (SIADH) associated with pulmonary processes, such as malignancy; and 5) SIADH that has been associated with paroxetine, a selective serotonin reuptake inhibitor (SSRI). This case illustrates the importance of taking a thorough history and assessing the clinical presentation. The etiology of the patient's hyponatremia was not clear, as she had numerous predisposing factors. It was more important to realize the gradual nature of her impairment. Rapid correction of her sodium levels was not necessary, and in fact may have been more harmful. A conservative approach was taken and her hyponatremia resolved slowly over time.
PATIENT STILL HAS FEVER OF UNKNOWN ORIGIN
F. Aslam1; A. Mirza2. 1Geisinger Medical Center Danville PA, Danville, PA; 2Geisinger Medical Center, Danville, PA. (Tracking ID #115629)
LEARNING OBJECTIVES
1. To identify role of adult Still's Disease (ASD) in the differential diagnosis of Fever of Unknown origin (FUO). 2. To appreciate the difficulty in diagnosing ASD due to lack of availability of specific diagnostic test. 3. Recognize importance of marked hyperferritinemia in association with other diagnostic criterion in diagnosing ASD.
CASE
A 40 year white male was transferred to our tertiary care medical center for evaluation of FUO. He was extensively investigated and evaluated by many sub-specialists during 4-week stay at another hospital. On admission to our hospital his symptoms were persistent muscle pain, lethargy and non-specific joint pain. Physical examination revealed temperature 39°C and pulse 90/minute, however no localizing signs were present. Laboratory studies revealed White Cell Count of 25.3 K/uL (normal range 4–10.8), Erythrocyte Sedimentation Rate (ESR) 98 mm/hr (0–15) and Alkaline Phosphates 162 U/L (25–125). He continued to be febrile with peak temperature of up to 40°C. An extensive workup including chest X-ray, duplex scan of lower extremities, Bone Marrow biopsy and CT scan of chest abdomen and pelvis was unrevealing. Tests were negative for HIV-1, heterophil antibodies, Hepatitis A, B, C viruses, IgM and IgG antibodies against Cytomegalovirus, Epstein Barr virus, Borrelia Burgdorferi and Brucella Abortus. Serology for dsDNA, Antinuclear antibodies and Rheumatoid factor (RF) was negative. Blood and Urine cultures were sterile. Suspicion of ASD was raised by markedly elevated serum ferretin level to 10,154 ng/mL (30–400) and diagnosis of ASD was made on the basis of Yamaguchi and Kahn's criterion. Patient was treated with systemic corticosteroids with resolution of fever and symptoms within 24 hours. At 2 weeks follow-up visit patient was symptom free, leukocytosis had resolved and serum ferritin level had decreased to 1,068 ng/mL.
DISCUSSION
FUO is due to infection in 30 to 50 percent of cases, to cancer in 25 to 30 percent, and to autoimmune disease in 15 to 25 percent. Among autoimmune diseases ASD is one of the commonest cause of FUO. In ASD fever, elevated ESR and negative RF are present in 100% patients. Other features include arthralgias (90%), Leukocytosis (100%), rash (85%) and arthritis (65%). Since no specific test is available diagnosis is based on exclusion of other diseases and applying diagnostic criterion. For this reason average delay for the diagnosis is 3 to 8 weeks. Acute phase reactants are characteristically elevated. One of the features although nonspecific is extreme elevation in serum ferritin levels. Depending on the severity of disease nonsteroidal anti-inflammatory drug, corticosteroids, and immunomodulating drugs can be used for treatment. Neutralization of serum ferritin level is reliable index of success of therapy.
PERNICIOUS ANEMIA WITH SPLENOMEGALY IN A YOUNG MAN
M. Pillai1; P. Hu1; N. Le1. 1Baylor College of Medicine, Houston, TX. (Tracking ID #116881)
LEARNING OBJECTIVES
1) Review the clinical manifestations of severe B12 deficiency. 2) Review the diagnosis and treatment of Pernicious Anemia.
CASE
A 37-year-old white man with no significant past medical history presented to our clinic complaining of progressive fatigue. One year ago he was able to run 3 miles daily. Upon presentation he had shortness of breath and fatigue with minimal activity. In the past six months, he lost 40 lbs and had symptoms of abdominal pain and early satiety. He had gone to several doctors who just prescribed him pantoprazole which minimally alleviated his symptoms. Physical exam was significant for a smooth red tongue, III/VI systolic flow murmur, and splenomegaly of 15 cm. There were no neurological deficits. Laboratory results showed WBC 2.1, Hg 6.0, Hct 17.7, MCV 102.5 and Platelets 75. A peripheral blood smear displayed macroovalocytes and multilobulated neutrophils. His B12 level was 48 pg/ml, and serum was positive for intrinsic factor antibody. The patient was diagnosed with pernicious anemia, (PA), and treated with cyanocobalamin injections with marked improvement.
DISCUSSION
PA typically presents later in life. Only about 10% of cases involve patients <40 years old. Typically, elderly patients present with macrocytosis with or without anemia and multilobulated neutrophils. However, severe cases of cobalamin deficiency can present with pancytopenia, neurological deficits involving the dorsal and lateral spinal columns, and even splenomegaly. Prior to early detection and treatment the reported incidence of splenomegaly in PA varied from 3% to 45%. With advances in medicine resulting in earlier detection, it is now rare to see splenomegaly in PA. A peripheral blood smear showing macrocytic anemia and multilobulated neutrophils is highly specific for B12 deficiency. The diagnosis is clinched with low serum B12 levels. Today, the use of the Schilling test has been supplanted by serologic testing for intrinsic factor antibodies. The presence of anti-intrinsic factor antibodies is highly specific and confirmatory for the diagnosis of PA, with a sensitivity varying from 50 to 84%, depending upon the population tested. Treatment with parenteral or oral cobalamin is highly effective. B12 supplementation normalizes hemoglobin levels within months and can reverse neurological deficits as well as splenomegaly.
PICK THE DEMENTIA
A.M. Wilson1; P. Koneru1; G. Prakash1; R.D. Hobbs1. 1Oakwood Healthcare System, Dearborn, MI. (Tracking ID #117213)
LEARNING OBJECTIVES
To recognize the clinical significance of language impairment in the diagnosis of dementia.
CASE
A 71 year-old independently living woman with a two-year history of cognitive dysfunction presented two weeks following a car accident. She was alert, socially active and regularly played tennis. The MMSE was 9/31. Her spelling ability had decreased (diner is reddy), her speech was fluid and well enunciated but complicated by word substitution errors such as describing blowing leaves as “flies.” The remainder of the exam and the laboratory tests were normal. An MRI showed nonspecific cortical atrophy with nondiagnostic white matter changes. She was diagnosed with Pick's Disease.
DISCUSSION
Arnold Pick first described Pick's disease in 1892 while reporting a series of unusual dementias. In 1911 Alois Alzheimer described the ballooning degeneration of neurons and the eosinophilic intraneuronal inclusions that are now known as “Pick bodies.” Unlike Alzheimer's disease with which it is frequently confused, Pick's disease is characterized by early expressive aphasia, personality changes and prominent problems with language such as substitution and syntactical errors. These language problems may complicate the diagnosis since most screening tests are language based and may lead to falsely lowered scores. Memory loss is not an early finding as it is in Alzheimer's disease. This distinction is significant since the problem—memory or language, occurs at different times and may affect families and caregiver expectations. It also underscores the fact that memory loss is not necessary to make a diagnosis of dementia.
POLYMICROBIAL ENDOCARDITIS IN AN INTRAVENOUS DRUG ABUSER
R.P. Warrier1; J.A. Chang1; J. Jarrett1; A.C. Maio1. 1Creighton University Medical Center, Omaha, NE. (Tracking ID #116419)
LEARNING OBJECTIVES
1. Recognize that polymicrobial endocarditis occurs in about 4–6% of all endocarditis. 2. Identify risk factors for polymicrobial endocarditis. 3. Manage infective endocarditis with surgical intervention in cases of persistent bacteremia despite maximal antimicrobial therapy.
CASE
Patient is a 39-year-old African-American male with history of current IVDA who presented with cough, fever, chills, and increasing dyspnea of 10 days duration. His past medical history included Hepatitis C, hypertension, and COPD. He had been treated for Staphylococcal bacteremia with four weeks of Nafcillin about three months ago. At that time TEE was negative for vegetations. Blood cultures were negative at the time of discharge. On physical examination, his temperature was 102.7 F. Vital signs were stable. He had no peripheral stigmata of endocarditis. A soft holosystolic murmur was heard along the left sternal border. There was no clinical evidence of heart failure. Multiple needle tracks were visible in his skin. Initial labs revealed leukocytosis (WBC 28.4), with 13% bands. Chest X-ray and EKG were normal at the time of admission. Blood cultures persistently grew Staphylococcus aureus and Bacillus species. TEE was positive for 2.6 × 1.5 cm vegetation on the tricuspid valve with severe tricuspid regurgitation. Patient was started on Vancomycin and Rifampin based on sensitivities. Chest CT scan showed evidence of septic pulmonary emboli on the 7th day of hospitalization. Blood cultures remained positive after two weeks of adequate therapy. He then underwent partial tricuspid valve replacement with mitral valve homograft and valve annuloplasty. Vegetation was positive for the same organisms. Following surgery, patient was treated with intravenous antibiotics for four weeks and was discharged in stable condition.
DISCUSSION
Polymicrobial endocarditis is rare, about 4–6% of all cases of IE. Risk factors include IVDA, cardiac abnormalities, and presence of a central venous catheter or prosthetic valves. Right side of the heart is more commonly involved than the left. 42% of polymicrobial IE affect the tricuspid valve alone. Emboli are the most common complication presenting as septic pulmonary focci. Bacillus species have been implicated as the most common contaminant in drug paraphernalia, placing patients with history of IVDA at risk of Bacillus infections. When appropriate antibiotic therapy fails to clear bacteremia, surgical intervention to remove the nidus of infection should be pursued.
POST-CRANIOTOMY RASH AND JAUNDICE
K.M. Coyle1; K.J. Smith1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115569)
LEARNING OBJECTIVES
1. To recognize the presentation of Anticonvulsant Hypersensitivity Syndrome (AHS). 2. To identify causes, complications and medical management of AHS.
CASE
AS is a 47 year old male with PMH of hypertension who was transferred to our institution on post-operative day (POD) #32 status-post meningioma resection. He was placed postoperatively on phenytoin for seizure prophylaxis. On POD #20 AS developed a confluent, whole body, non-pruritic macular erythematous rash. Phenytoin was discontinued and neurontin prescribed. Three days later his prescription was changed to phenobarbital. The rash initially resolved following phenytoin discontinuation but worsened POD #28. On POD #30 he developed oral ulcerations, generalized edema, yellow sclerae and fever. On presentation to an outside hospital emergency department he was febrile with painful cervical lymphadenopathy, generalized macular rash and icteric sclerae. Serum ALT = 1648, AST = 2091, total bili = 27, NH3 = 53, INR = 1.4 and % eosinophils = 28. He was transferred to our institution and liver biopsy revealed eosinophilic infiltration consistent with hypersensitivity reaction. AHS was diagnosed; oral prednisone 40 mg/day was initiated. AS received supportive intravenous fluid hydration, daily liver function test monitoring and lactulose to minimize ammonia levels. He was discharged 20 days following admission with normalized liver function tests, resolved rash, oral lesions and eosinophilia.
DISCUSSION
AHS is an acute, life-threatening, idiosyncratic, non-dose-related drug reaction occurring 1 to 8 weeks after anticonvulsant exposure. The incidence of AHS is 1 in 1,000–10,000 exposures. It is characterized by multisystemic involvement, fever, lymphadenopathy, mucocutaneous rash, hypertransaminasemia and peripheral eosinophilia. Internal organ involvement most often involves the liver, but the renal, pulmonary and central nervous systems may also be affected. Although reported in patients taking lamotigrine, AHS is associated with the aromatic antileptic drugs phenytoin, carbamazepine, phenobarbital and primidone. It is thought to be related to inadequate detoxification of arene oxide metabolites of these drugs by a structural or functional defect in the enzyme epoxide hydroxylase; the metabolites subsequently cause cell necrosis or a secondary immunologic response. Cross-reactivity between drugs is 70–80%; there is a familial occurrence of AHS with an autosomal pattern of inheritance. Management consists of withdrawl of the offending agent and supportive care. Systemic corticosteroids are successful in the presence of internal organ involvement. Slow steroid taper is recommended following resolution of AHS as relapses are reported. Transient hypothyroidism can occur 1–3 months after the initial reaction.
POSTERIOR REVERSIBLE ENCEPHALOPATHY SYNDROME: WHEN A STROKE IS NOT A STROKE
A.B. Benson1; S. Desai1. 1Oregon Health & Science University, Portland, OR. (Tracking ID #116016)
LEARNING OBJECTIVES
1. Clinically recognize and diagnose posterior reversible encephalopathy syndrome (PRES). 2. Recognize patients who develop acute renal failure often have rapid elevations in blood pressure increasing the risk for the development of PRES. 3. Differentiate PRES from a bilateral posterior cerebral stroke to ensure appropriate acute management.
CASE
A 19 y/o female was admitted to the ICU with fulminant hepatic and acute renal failure following a suicide attempt with alcohol and acetaminophen. She received N-acetylcysteine and had rapid improvement in her liver function. From day 2–9 her renal failure, secondary to acute tubular necrosis, necessitated hemodialysis and caused progressive blood pressure elevation with episodic mean arterial pressures between 111–117 mmHg. On day 7, the patient complained of headache, nausea, increasing confusion and blurry vision rapidly progressing to complete blindness. Her BP was noted to be 150/105. She then experienced a generalized tonic-clonic seizure and was given lorazepam and phenytoin. An initial head CT was read as “multiple bilateral cerebellar, occipital and parietal infarcts.” A subsequent head MRI demonstrated extensive bilateral gray and white matter edema in the cerebellar, occipital, posterior parietal and temporal lobes. After neurology consultation, the patient was diagnosed with PRES based on her initial symptom complex and MRI findings. By discharge, her liver and kidney function had returned to normal and she remained seizure free without visual or mental deficit.
DISCUSSION
PRES is defined by headache, nausea, vomiting, mental status changes, visual changes and seizures associated with the characteristic head MRI findings of extensive bilateral subcortical edema most commonly in the posterior cerebral hemispheres. The two most common predisposing factors to the development of PRES are acute episodic and sustained elevations in blood pressure, common in acute renal failure, and/or the use of cyclosporine and other immunosuppressants. Treatment is to rapidly lower BP to baseline levels, and if the situation warrants, withdraw or decrease the immunosuppressant dosage and initiate seizure prophylaxis. This aggressive approach can induce cerebral ischemia in the setting of an ischemic stroke, but irreversible cases of leukomalacia and progression to infarction have been described making acute recognition, differentiation and management of PRES essential. An appropriate clinical scenario coupled with an MRI read by an experienced neuroradiologist differentiates PRES from a stroke and potentiates appropriate treatment. Symptoms usually resolve without sequelae in days to weeks.
POSTPARTUM CARDIOMYOPATHY
H. Shishodia1; J. Miller1; M. Bohning1. 1Temple University, Philadelphia, PA. (Tracking ID #116444)
LEARNING OBJECTIVES
1. Recognize the clinical manifestation of congestive heart failure (CHF). 2. Recognize postpartum cardiomyopathy as an infrequent but known cause of CHF that can present up to 6 months after delivery. 3. Discuss the implications of medical management of postpartum cardiomyopathy.
CASE
A 22 year old G1P1 woman presented to outpatient clinic 6 weeks postpartum with progressive dyspnea on exertion, and now short of breath at rest. She reported a non-productive cough, orthopnea, paroxysmal nocturnal dyspnea and lower extremity edema. Her medical history is significant only for a pregnancy complicated by preeclampsia and peripartum hemorrhage requiring transfusion. On exam her was BP 112/90 P 118, RR 24, Pulse Ox 99% on room air. She had gained 5 lbs. since her last clinic visit a month prior. She was able to speak in full sentences at rest. Her lungs were clear, she had 11 cm JVP. She had a laterally displaced hyperdynamic PMI, right ventricular heave, III/VI blowing systolic murmur radiating to the axilla, an S3 and bilateral 2+ pitting edema above the ankles. EKG showed sinus tachycardia. The patient was hospitalized and a bedside echocardiograph showed 4 chamber dilatation, depressed left ventricular systolic function of 10–15%, severe mitral and tricuspid regurgitation, mild aortic regurgitation and moderate pulmonic regurgitation. Hgb 11.5, Hct 35.5, TSH 4.49. She was given diuretics, an ACE-inhibitor and digoxin and her symptoms improved dramatically.
DISCUSSION
Postpartum cardiomyopathy is a dilated cardiomyopathy which results in signs and symptoms of heart failure. It is an uncommon cardiomyopathy with an incidence of 1/3000–1/4000 births. Symptoms typically begin during the last trimester of gestation and the diagnosis is usually made in the early peripartum period, but can develop upto 6 months after delivery. Common signs and symptoms include shortness of breath, fatigue, chest pain, palpitations, peripheral edema. Physical exam usually demonstrates an enlarged heart, S3, murmurs of mitral and tricuspid regurgitation. Echocardiography usually shows enlargement of all four chambers, with severe reduction in left ventricular systolic function. Multiparous women, women with preeclampsia and twin pregnancies are at a higher risk for peripartum cardiomyopathy. Acute management involves oxygen, diuretics, digitalis and vasodilators. Other modalities such as dopamine, dobutamine, milrinone have been used in pregnant women in a few cases. There is a high risk of mortality in patients with severe heart failure who do not recover and these women are referred for cardiac transplant. Risk of mortality ranges from 0–2% when the left ventricular function has normalized, while the risk of mortality is 8–17% in women with depressed systolic function prior to subsequent pregnancy. It is for these reasons that subsequent pregnancies are deemed high risk and therefore discouraged.
POST-PARTUM MASTITIS AND CONSEQUENCE OF DELAYED INTERVENTION
K. Ghosh1; A.C. Degnim1; K.R. Brandt1. 1Mayo Clinic, Rochester, MN. (Tracking ID #116645)
LEARNING OBJECTIVES
1) Emphasize the significance of imaging tests in the assessment of postpartum mastitis. 2) Address the role of antibiotics in the treatment of postpartum mastitis. 3) Emphasize supportive care for women with evidence of postpartum mastitis.
CASE
A 24 year-old primiparous woman presented with recurrent postpartum mastitis, and persistent pain and redness of the breast. The patient first noticed a lump in the inner left breast about nine weeks after childbirth that was associated with redness and warmth. Symptoms improved spontaneously but recurred soon after and she was evaluated by her primary physician and advised to start Cephalexin with some improvement. After three weeks on Cephalexin, she noticed drainage from the midst of the area of erythema, and was switched to Amoxicillin/Clavulanate and sent to the Breast Clinic for further treatment. Examination revealed an asymmetrically enlarged, tender, engorged, left breast with a 5 cm area of redness and warmth in the infero-medial quadrant. Ultrasound evaluation revealed a fluid collection measuring over 10 cm in the left breast with features suggestive of an infected galactocele/breast abscess. She underwent incision and drainage of the abscess with removal of approximately 160 cc of pus, and debridement of the abscess wall. The cavity measured 15 × 10 cm. Post-operatively, the abscess cavity was packed frequently enabling complete wound healing in four weeks. The patient is now contemplating plastic surgery to improve cosmesis.
DISCUSSION
Postpartum mastitis can present a clinical spectrum from mild focal breast inflammation to breast abscess formation and sepsis, occurring in 2 to 33% of breast-feeding women. Management of postpartum mastitis includes supportive measures such as hot compresses, analgesics and the expression of breast milk either by breast feeding, manual expression or use of a breast pump. In mild mastitis, these measures often suffice to resolve symptoms. However, breast abscess and septicemia are known complications of post-partum mastitis, and therefore, the role of antibiotic therapy has been an area of controversy. Ultrasound evaluation of the area of mastitis is recommended to rule out an underlying abscess, or infected galactocele. The presence of an infected fluid collection requires treatment with drainage as well as antibiotic therapy to prevent the spread of infection and enlargement of the abscess cavity.
PRESCRIBING SELECTIVE SEROTONIN REUPTAKE INHIBITORS IN THE ELDERLY: WATCH OUT FOR MENTAL STATUS CHANGES
A.L. Puswella1; K. Barnard1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #116219)
LEARNING OBJECTIVES
1) To recognize the Syndrome of Inappropriate ADH Secretion (SIADH) as a common side effect of selective serotonin reuptake inhibitor (SSRI) therapy. 2) To recognize the importance of early detection of SIADH in elderly patients starting SSRI therapy. 3) To recognize the risk factors that predispose patients to developing SSRI-induced SIADH.
CASE
An 88 y/o female was brought to the emergency department by her neighbor for increasing confusion, weakness, and altered mental status for two days. Past medical history is significant for depression, hypertension, hyperlipidemia, osteoporosis and urinary incontinence. Her medications included Losartan, Propanolol, Alendronate, Tolterodine. She had been started on Escitalopram 10mg two days prior to the onset of symptoms. In the emergency department, her serum sodium was 118 mEq/L and she was judged to be euvolemic. Her baseline serum sodium, taken two days earlier was 134 mE/L. Urine electrolytes showed Na+= 96 mEq/L, Cl = 114 mEq/L and osmolarity = 447 mOsmol/kg. A diagnosis of SSRI-induced hyponatremia due to SIADH was made. The patient was admitted, placed on fluid restriction, and the Escitalopram discontinued. Her mental status gradually improved, returning to baseline over the next five days.
DISCUSSION
The development of SIADH secondary to SSRI use in the elderly appears to be a more common occurence than previously thought. Some series show an incidence as high as 28%, with severe symptomatic hyponatremia occuring in 12% of these cases. Most episodes occur within 13 days (range 3–120 days) from the onset of treatment. Our patient demonstrates that severe symptomatic hyponatremia can occur in as little as two days, and can result in expensive hospitalization. Elderly women on multiple medications are at especially high risk of developing SIADH. The reasons for this are the higher rates of depression and SSRI use, decreased kidney function, and polypharmacy, especially the concomitant use of thiazide diuretics and neuroleptics. This case demonstrates the need for close monitoring of mental status and serum sodium when initiating SSRI drugs in the elderly. Females and those taking thiazide diuretics or neuroleptic medications appear to be at higher risk.
PRIMARY CHORIOCARCINOMA OF THE STOMACH WITH LIVER METASTASES—A CASE REPORT
A. Devarajan1; K. Subramanian1; C. Rathnakumar1; A. Regaila1; E. Christina2; U. Hitendra1; R. Jeyachandran1. 1Jersey City Medical Center, Jersey City, NJ; 2JerseyCity Medical Center, Jersey City, NJ. (Tracking ID #117440)
LEARNING OBJECTIVES
Choriocarcinoma is a malignant proliferation of the Langerhans cells and of syncytial cells of trophoblastic origin that is normally situated in the female genital tract after a gestational event such as molar pregnancy, term pregnancy, abortion or ectopic pregnancy. Rarely it occurs in either sex as a midline lesion in the retroperitoneum, mediastinum and the region of the pineal gland. Less frequently it is found in the bladder, liver, stomach and colon, where it is seen in combination with adenocarcinoma. Primary choriocarcinoma of the stomach is an extremely rare and highly malignant tumor.
CASE
We report a case of choriocarcinoma of the stomach associated with adenocarcinoma in a 51 year old female, who presented with epigastric pain and vomiting for one month with manifestations of liver failure and elevated Ò-HCG (38748 MIU/ml). No evidence of gestational malignancy was found on endometrial biopsy. Upper GI endoscopy revealed a single acute friable ulcer in the antrum. CT scan of the abdomen showed multiple liver metasteses. Histopathologic examination of gastric antral and liver biopsy specimens demonstrated ulcerated, infiltrating choriocarcinoma with poorly differentiated adenocarcinoma. The diagnosis was further confirmed by immunohistochemical staining, which showed strong positivity for Ò-HCG, CEA and CAM 5.2, and focal positivity for AFP, CK and EMA. The patient was treated with one cycle of methotrexate based chemotherapy, as patient refused dactinomycin and VP-16 based chemotherapy. The level of serum Ò-HCG was reduced (7311 MIU/ml) with no parallel clinical improvement. Patient continued to deteriorate and expired within one month of diagnosis.
DISCUSSION
Primary choriocarcinoma of the stomach presents with a picture similar to adenocarcinoma with an average age of onset of 50 to 60 years with greater preponderance among males. It spreads by hematogenous route with an average survival of only a few months from the time of diagnosis. Although chemotherapy is successful in treating gestational choriocarcinoma, its effectiveness had not been shown in gastric choriocarcinoma. Standardized treatment protocols are lacking due largely to the paucity of cases presented at any one institute. This calls for an organized multicenter or multinational double blind, case-control randomized study in order to establish a treatment protocol for this life threatening malignancy.
PULMONARY CRYPTOSPORIDIOSIS
L. Subramanyam1; S. Parikh1; M. Eapen1; H. Friedman1. 1St. Francis Hospital, Evanston, IL. (Tracking ID #116549)
LEARNING OBJECTIVES
1. Recognize the importance of Cryptosporidium species not only as a cause of intractable diarrhea and malabsorption in immunocompromised patients but also as an agent that leads to respiratory failure and death. 2. Realize that early immune reconstitution with Highly Active Anti-Retroviral Therapy (HAART) and better nutrition improves the final outcome in patients with cryptosporidiosis and Acquired Immune Deficiency Syndrome (AIDS); prognosis without immune restoration is generally poor.
CASE
The patient is a 34-year-old African-American man who presented with a 4 week history of progressively worsening shortness of breath, cough associated with mucoid sputum, moderate epigastric pain, loose watery diarrhea and a weight loss of 30 lbs during this period. He appeared emaciated with obvious clinical signs of dehydration and moderate respiratory distress, saturating 88% on room air. Cardiac and lung exam was essentially benign. The abdomen was diffusely tender to palpation without any evidence of peritoneal signs. He tested positive for HIV with a viral load of 685,354 and a CD4 count of 177. Chest X-ray showed diffuse interstitial infiltrates. He was started on HAART and Trimethoprim-sulfamethoxazole empirically for Pneumocystis carinii pneumonia. Stool specimens and a subsequent duodenal biopsy demonstrated Cryptosporidial oocysts. During the course of hospital stay, his respiratory symptoms worsened. Pneumocystis was persistently negative in the induced sputum, whereas the sputum sent for AFB staining revealed Cryptosporidia in three consecutive specimens. He was treated with paramomycin, azithromycin and intravenous hyperalimentation. Despite aggressive treatment, he succumbed to the infection and died of respiratory failure.
DISCUSSION
While there is a well-documented association of Cryptosporidium with severe diarrheal disease in immunosuppressed individuals, Cryptosporidium in the respiratory tract has been rarely described. The few case reports that are available in the literature show that these patients ultimately develop fulminant respiratory failure and die. But, the causal association could not be clearly established in these cases because of the presence of other respiratory pathogens especially, Pneumocystis. In our patient, we could not isolate any other microorganism even with multiple sputum studies. So, we consider this case as one of the rare reported cases of pulmonary cryptosporidiosis with an adverse outcome. The risk of fulminant cryptosporidiosis increases in profoundly immunocompromised patients with AIDS as measured by the low CD4 count. Immune reconstitution with HAART has shown to decrease mortality and morbidity in these individuals. Early consideration of this diagnosis and prompt institution of treatment might improve the final outcome in these patients.
PULMONARY HYPERTENSION: IS IT FIRST, OR IS IT SECOND?
A. Toprani1; J. Hutchings1. 1Tulane University, New Orleans, LA. (Tracking ID #117519)
LEARNING OBJECTIVES
1. Recognize the clinical presentation of primary pulmonary hypertension. 2. Understand the criteria for diagnosis and treatment of primary pulmonary hypertension.
CASE
A 31 year-old woman presented with hemoptysis. She had no history of trauma or anticoagulation, and no recent tuberculosis exposures. She had presented to an outpatient clinic six months earlier with similar complaints and was prescribed an antibiotic. Her symptoms resolved, but the hemoptysis recurred one week prior to presentation. She had a III/VI diastolic murmur; the remaining exam including a PPD test was normal. The chest radiograph revealed cardiomegaly and clear lungs. An echocardiogram revealed a pulmonary artery pressure of 130 mmHg. Serologies for HIV, RF and ANA were negative. PFT's were normal. Bronchoscopy was negative, and a HRCT was negative for parenchymal lung disease. The V/Q scan was normal. A right heart catheterization showed a positive response to epoprostenol.
DISCUSSION
Primary pulmonary hypertension (PPH) is a pulmonary artery pressure of greater than 25 mmHg in the absence of an identifiable cause. The pathogenesis is related to an imbalance between vasodilating and vasoconstricting factors, leading to pulmonary vasoconstriction and vascular remodeling. A mutation of the bone morphogenetic protein receptor type II (BMPR2) gene has been identified in the familial form of PPH. Many of the sporadic cases of PPH are also attributable to abnormalities of BMPR2. In order to diagnose primary pulmonary hypertension secondary causes must be excluded. This is accomplished by performing pulmonary function tests, connective tissue serologies, echocardiography, cardiac catheterization and ventilation/perfusion lung scanning. Cocaine, amphetamines and the appetite suppressant fenfluramine should also be excluded. The response to epoprostenol or nitric oxide should be investigated. The 30% of patients who do respond can be treated with oral calcium channel blockers. Patients who do not qualify for oral therapy are treated with continuous intravenous infusion of epoprostenol. Chronic anticoagulation with warfarin has been shown to improve survival.
PURULENT PERICARDITIS: A RARE COMPLICATION OF STREPTOCOCCAL PNEUMONIA
H. Bhuria1; M. Karmegam1; H. Friedman1. 1Saint Francis Hospital of Evanston, Evanston, IL. (Tracking ID #116654)
LEARNING OBJECTIVES
Recognize purulent pericarditis as a complication of pneumococcal pneumonia.
CASE
A 55 year old white female with history of alcohol abuse presented with nausea, vomiting and RUQ pain for 4 days, followed by dry cough and right sided pleuritic chest pain. She denied fever or shortness of breath. On exam temperature 97 F, pulse 122, BP 135/115, RR 28, no JVD, S1S2 audible and decreased breath sounds at bases. Abdomen showed mild RUQ tenderness but no Murphy's sign. CXR showed bibasilar effusions and infiltrates with cardiomegaly. CT abdomen was unremarkable. Blood culture subsequently grew Streptococcus pneumoniae. She was started on IV antibiotics for pneumonia. The next day she developed shortness of breath and hypotension. A chest CT scan showed bilateral pleural and pericardial fluid. Cardiac echocardiogram showed tamponade. 450 cc of purulent fluid was drained from the pericardial cavity. Analysis revealed white cell count of 56,700 with 92% neutrophils and a negative bacterial culture. Both pleural cavities were drained by tube thoracostomies aided by repeated instillation of tPA. Plerual fluid showed a white cell count of 2,600 with 89% neutrophils, ph 6.8, glucose 2, LDH 12,540 and a negative bacterial culture. She required a pericardial window for reaccumulating fluid and was eventually discharged in a stable condition.
CT chest showing bilateral pleural effusions (R > L) and pericardial effusion.
DISCUSSION
Purulent pericarditis in the antibiotic era has become a rare entity. Prior to the widespread use of antibiotics, purulent pericarditis was usually a complication of pneumococcal pneumonia; in modern times, most cases are associated with dialysis, thoracic surgery, and chemotherapy. It is occasionally seen in patients with risk factors for invasive pneumococcal disease like alcoholism. A high index of suspicion should be maintained in patients with pneumonia and pericardial effusion as prompt diagnosis and treatment with drainage of pericardial cavity is essential for a good outcome.
PYODERMA GANGRENOSUM OF THE HANDS FOLLOWING VENIPUNCTURE INJURY
C. Nassaralla1; R. McCurdy1; S. Frost1. 1Cleveland Clinic Foundation, Cleveland, OH. (Tracking ID #115494)
LEARNING OBJECTIVES
1) Diagnose and treat pyoderma gangrenosum. 2) Recognize that pyoderma gangrenosum is often misdiagnosed as bacterial skin infection, potentially resulting in devastating outcomes due to delay in therapy.
CASE
A 28-year-old woman was admitted to the hospital with excruciatingly painful hand ulcers. Eighteen days prior to presentation, she had experienced multiple traumatic attempts at intravenous (IV) access on the dorsum of both hands before undergoing cholecystectomy. After surgery, small blisters appeared at the attempted IV access sites that rapidly enlarged and ulcerated. Five days prior to admission she received amoxicillin-clavulanate for the presumptive diagnosis of impetigo, yet the ulcers continued to enlarge. Physical examination revealed tender 10 cm and 6 cm circular ulcers with hemorrhagic exudate on the dorsal aspects of the left and right hands respectively. The ulcers were demarcated with an irregular, raised, and boggy border. The surrounding skin was erythematous and edematous. The ulcers did not appear infected, and blood and wound cultures were sterile. There was no fever, and laboratory evaluation was remarkable only for mild leukocytosis. Prednisone and pain medications were prescribed after pyoderma gangrenosum (PG) was diagnosed based on clinical history, and typical ulcer appearance. Extensive evaluation revealed no associated systemic illness, and the ulcers were well healed after 14 days of therapy.
DISCUSSION
PG is a rare, idiopathic inflammatory neutrophilic dermatosis akin to Sweet's syndrome. Most cases are associated with a systemic illness such as inflammatory bowel disease. However, 15% to 30% of patients have no underlying medical condition. The pathogenesis of PG is unclear, but altered immunological reactivity is likely operative. PG lesions begin as tender, red macules, papules, nodules, or bullae that evolve into pustules or vesicles surrounded by erythematous and edematous skin. Ulceration eventually occurs, which then extends in a centrifugal pattern. PG confined to the hands is rare and has been variably referred to as “neutrophilic dermatosis of the dorsal hands”, “pustular vasculitis of the hands”, and “variant erythema elevatum diutinum.” Diagnosis of PG is based on clinical findings, including a history of minor trauma as the precipitating event, which is commonly associated with PG of the hand. Biopsy specimens generally yield nonspecific results. Systemic immunosuppressive therapy with prednisone is the cornerstone of therapy. Dapsone may be useful in decreasing inflammation and edema. Surgical procedures are contraindicated, as they may provoke a pathergic response that exacerbates tissue injury. PG is frequently misdiagnosed as bacterial skin infection, and antimicrobial therapy is ineffective. Inaccurate diagnosis and delay in appropriate treatment can result in devastating outcomes such as amputation of the affected areas.
RASH, ABDOMINAL PAIN AND WEIGHT LOSS
S. Prall1; G. Babameto2. 1Geisinger Medical Center, Danville, PA; 2Geisinger Mediacal Center, Danville, PA. (Tracking ID #115169)
LEARNING OBJECTIVES
1. Recognize atypical presentation of adult onset diabetes. 2. Diagnose hypertriglyceridemia related to uncontrolled diabetes.
CASE
A 41 yo white male presented with complaints of abdominal pain. Over the past few months he had lost 40 pounds. One month ago he developed a nontender rash on his extremities and trunk. 24 hours ago he developed pain after eating supper, not relieved with over the counter analgesics. Denied nausea, vomiting, diarrhea, or fevers. PMH included reflux, hiatal hernia, carpal tunnel syndrome b/l. No tobacco or alcohol use. Currently disabled from carpal tunnel syndrome. Physical exam: BP 130/73, HR 87, RR 20, T 37.5. Obese. HEENT: sclera anicteric. Remarkable for tense, distended abdomen with epigatric tenderness, normal bowel sounds, no rebound. Yellow nontender papules varying in size on extremities and trunk (xanthomata). Initial laboratories: lipase 1,468, cholesterol 401, triglyceride 14,798, ast 31, alt 59, alkaline phosphatase 80, total bilirubin 0.5, WBC 12.8, Na 125, K 3.6, glucose 325, CO2 21, TSH 2.04. Pt was admitted for management of acute pancreatitis likely secondary to his hypertriglyceridemia resulting from uncontrolled diabetes. Initial HgA1C was 14.2. With insulin therapy HgA1C improved to 5.8, however, cholesterol, LDL, and TG remained elevated requiring lipid lowering medications.
DISCUSSION
Pancreatitis is an uncommon presentation for new adult onset diabetes. Frequently patient's present with hyperglycemia, obesity, ketonuria, weight loss, nonketotic acidosis. Hyperlipidemia is frequently seen in uncontrolled diabetes, specifically hypertriglyceridemia. Hyperinsulinemia plus insulin resistance in uncontrolled diabetes cause increased TG production coupled with reduced clearance of plasma TG result in hypertriglyceridemia. Hypertriglyceridemia is a rare cause of pancreatitis. Levels greater then 1,000 place an individual at increased risk for pancreatitis. The hypertriglyceridemia resulting from pancreatitis is mild to moderate and should not be confused with the markedly elevated levels seen causing pancreatitis. Significantly elevated TG levels in the uncontrolled diabetic should also raise the question of an underlying lipid disorder. In this case the patient presented with hypertrigylceridemia induced pancreatitis and was found to have diabetes, but we can not assume that the diabetes alone resulted in his clinical presentation. The presence of xanthomas indicates that he likely had a longstanding hyperlipidemia. The concomitant development of diabetes probably exacerbated the preexisting hyperlipidemia. Despite ideal glycemic control with a follow up HgA1C of 5.8, the patient's lipid profile failed to normalize and he continued to require lipid lowering agents. Think of hypertriglyceridemia induced pancreatitis in the uncontrolled diabetic who presents with abdominal pain.
RECOGNIZING MAC TO AVOID THE KNIFE
S. Kahlon1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117476)
LEARNING OBJECTIVES
1. Recognize a complication of a common AIDS-related opportunistic infection, disseminated mycobacterium avium-intracellulare complex (MAC).
CASE
A 32 year-old HIV-positive man (CD4 = 28) presented with four days of progressively worsening diffuse, crampy abdominal pain with fever, night sweats, vomiting, and inability to tolerate oral intake. His examination was normal with the exception of a temperature of 38.5°C. He had mild abdominal tenderness without guarding. An initial radiograph of the abdomen suggested small bowel obstruction versus adynamic ileus. As his symptoms did not resolve, he was scheduled for exploratory laparotomy. In preparation, a CT scan of the abdomen was performed that revealed diffuse retroperitoneal and mesenteric lymphadenopathy with a partial ileus. A subsequent CT-guided needle biopsy of a retroperitoneal node revealed reactive lymphadenopathy due to Mycobacterium avium. Supportive care for nausea and pain was provided, as well bowel rest and nutritional support, until he was again able to tolerate oral intake, began having bowel movements, and symptomatically improved.
DISCUSSION
Mycobacterium avium (MAC) bacteremia can occur in HIV-positive patients with CD4 counts less than 50. The syndrome includes fever, weight loss, night sweats, diarrhea, and lymphadenopathy. Organs involved include the liver, spleen, gastrointestinal tract, lymph nodes, and bone marrow. Pancytopenia, elevated lactate dehydrogenase, and elevated alkaline phosphatase are laboratory indicators for the disease. The organism has a predilection for lymph nodes and can induce a diffuse reactive lymphadenopathy that can be sufficient to cause obstruction of contiguous organs. Gastrointestinal lymphadenopathy can be symptomatic due to mass effect and inflammation of the bowel wall. Physicians should be aware of this common complication from disseminated MAC, as unlike its imitator, lymphoma, it is readily contained with ethambutol and clairthromycin.
RECURRENT ABDOMINAL PAIN: CHECK THE MEDICATION LIST
J. Kamali1; G.L. Arnold1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #116496)
LEARNING OBJECTIVES
1) To recognize angiotensin-converting enzyme (ACE) inhibitors as a cause of angioedema of the small bowel. 2) To list the typical symptoms of angioedema of the small bowel.
CASE
A 49 year-old African American woman with past medical history of essential hypertension and recurrent abdominal pain for two years presented to the emergency department (ED) with progressive abdominal pain of four hours duration. The pain was periumbilical, constant, sharp and without radiation. It started at home as 2/10 severity and progressed to 6/10 in the ED. She had two similar episodes within the past two years, one requiring exploratory laparoscopy. The patient reported associated nausea, but denied vomiting, diarrhea, constipation, bloody stools, fever or chills. On further questioning she recalled previous episodes of swelling of her lips lasting several days, beginning after two months of intermittent abdominal symptoms. Her medications included Lotrel® (amlodipine + benazepril), iron sulfate and occasionally alprazolam. Physical examination was notable for normal vital signs. Her sclera were anicteric. Heart and lung examination was unremarkable. Abdomen was soft and non-distended. There was moderate periumbilical tenderness without guarding. Her laboratory studies showed WBC of 15.5, lipase 61 and amylase 89. CT of the abdomen demonstrated edematous loops of the small bowel. C1 esterase inhibitor level as well as C1 and C2 levels were normal.
DISCUSSION
Angioedema has been reported to occur in 0.2% of patients taking ACE inhibitors. It usually involves the deep layers of the skin but may also occur at the mucosal surfaces of the upper respiratory tract or gastrointestinal (GI) tract. The clinical presentation of the angioedema involving the GI tract includes abdominal pain, nausea, vomiting, diarrhea and/or ascites. There have been nine previous case reports of ACE inhibitor induced angioedema of the small bowel. CT of the abdomen demonstrating small bowel edema can suggest the diagnosis. The time between starting the medication and the onset of GI tract symptoms has been reported to range from a few hours to four months, but in most cases is less than seven days. The patient in this case was started on benazepril two months prior to the first episode of the abdominal pain. Failure to think of this diagnosis can result in delay of diagnosis and unnecessary procedures, such as exploratory laparoscopy and even bowel resection. No prior case reports have included angiotensin inhibitor level measurements during the acute episode. Angioedema of the GI tract caused by ACE inhibitors is probably underdiagnosed and underreported. It should be considered in all patients taking ACE inhibitors who present with the typical abdominal symptoms.
RECURRENT EPISODIC CHEST PAIN IN A WOMAN WITH INFERTILITY
L.N. Dyrbye1; C. Rohren1. 1Mayo Clinic, Rochester, MN. (Tracking ID #101799)
LEARNING OBJECTIVES
Learning objectives 1. Clinical recognition of catamenial hemothorax 2. Treatment of catamenial hemothorax
CASE
40 year old woman presented with two days of dull, non-pleuritic right chest pain aggravated by recumbency on the right side, with associated dyspnea. She reported monthly chest pain since 1993. No fever, chills, or cough. Her period started five days ago. The patient's past medical history included infertility and right-sided hydropneumothorax in 2001 with biopsy proven pleural and peritoneal endometriosis and diaphragmatic fenestration on thoracotomy. Diaphragmatic repair and chemical pleurodesis were performed. On exam, her vital signs were normal. Lung sounds were diminished over the right upper lung. Chest x-ray showed a small, right-sided hydropneumothorax. After consultation with thoracic surgery, the patient elected to undergo hormonal treatment.
DISCUSSION
Catamenial thoracic syndromes (CTS) are rare. Clinically, women between 30–34 years of age present with chest pain and dyspnea that starts within the first two days of menstrual flow. Hemoptysis is rarely reported. Chest radiograph commonly shows right-sided pneumothorax, occasionally hemothorax, and rarely nodules. [1][2] Long-term therapy involves suppression or removal of existing endometrial plaques and prevention of further plaque development. Hormonal suppression with oral contraceptives, progesterone, GnRH, and danazol is unsuccessful long-term. CTS recur in up to 50%. [1] Surgical treatment includes removal of endometrial plaques and mechanical or chemical pleural abrasion. Resection of endometrial implants on the pleura and diaphragm, along with repair of diaphragmatic abnormalities have had variable short-term success. [2][3] Chemical pleurodesis is more successful than hormones in preventing recurrence. [1] Regardless of the surgical method used, cyclical chest pain, however, may remain. [1] Surgery followed by GnRH to prevent recurrence and pain maybe the best option. [4] 1. Joseph, J.M.D. and S.A.M.D. Sahn, Am J Med 1996. 100(2): p. 164–170. 2. Alifano, M., et al., Chest, 2003. 124(3): p. 1004–8. 3. Sakamoto, K., T. Ohmori, and H. Takei, Ann Thorac Surg, 2003. 76(1): p. 290–1. 4. Blanco, S., et al., J Thorac Cardiovasc Surg, 1998. 116(1): p. 179–80.
RECURRENT PARAPNEUMONIC PLEURAL EFFUSIONS AND WORSENING DYSPNEA IN A YOUNG WOMEN
N. Latif1; G.H. Tabas1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115832)
LEARNING OBJECTIVES
Consider non-pneumonic causes of dyspnea and pleural effusion in a patient with a history of pneumonia.
CASE
A 34-year-old Caucasian woman with a history asthma, reflex sympathetic dystrophy and obesity was referred from another institution because of recurrent bilateral pleural effusions one year after an episode of pneumonia. Her symptoms included worsening dyspnea, pleuritic chest pain and a 100-pound weight loss. Physical examination showed bilaterally decreased breath sounds and ankle edema. Her chest x-ray revealed bilateral pleural effusions and computerized tomography of the chest revealed a small pericardial effusion and no pulmonary embolism. Examination of the pleural fluid showed it was transudative and pulmonary function testing demonstrated restrictive lung disease. An echocardiogram showed a normal ejection fraction and a dilated inferior vena cava. Abdominal ultrasound revealed dilated intrahepatic ducts and hepatic congestion. Right heart catheterization showed equalization of the right ventricular diastolic pressure and the pulmonary diastolic pressure. The pulmonary capillary wedge pressure was 20 mmHg. The patient had symptomatic improvement after pericardiectomy and pathologic examination revealed fibrosis and hyalinization.
DISCUSSION
Post-pneumonic pericarditis is a rare complication of pneumonia but important to diagnose. The major categories of constrictive pericarditis are idiopathic, post-radiotherapy and post-cardiac surgery. Infectious causes account for only 6% of cases of constrictive pericarditis. Patients with constrictive pericarditis usually present with congestive heart failure but rarely with cardiac tamponade. Classic clinical sign are increased jugular venous pressure, peripheral edema, pulsatile liver, pulsus paradoxus, Kussmauls sign and pericardial knock. Diagnostic testing includes electrocardiogram showing low voltage and echocardiography showing pericardial effusion and pericardial thickening. Computerized tomography of the chest shows pericardial thickening. Right heart catheterization reveals equalization of right-sided pressures. The treatment is pericardiectomy.
RECURRENT SYNCOPE AS A RARE SYMPTOM OF MASTOCYTOSIS
A.T. Czajka-Giermasz1; W.N. Kapoor1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #116571)
LEARNING OBJECTIVES
1) to recognize systemic mastocytosis (SM) as a rare cause of syncope 2) to manage patients with SM.
CASE
A 44-year old white female was transferred to the MICU after her fourth episode of syncope. Three weeks prior, she experienced sudden onset of flushing followed by severe lightheadedness and palpitations. Her husband found her unresponsive and incontinent of feces. When paramedics arrived 20 minutes later she was hypotensive, tachycardiac, confused, and could not follow commands. Initial work-up revealed normal EKG, head CT, chest CT, echocardiogram, CBC and electrolytes. One week later, while eating dinner, she felt flushed and dizzy, and slumped to the floor, losing consciousness. Cardiac catheterization, tilt-table test, electrophysiologic studies, thyroid studies, urine metanephrines, 5-HIAA, and serum serotonin levels were normal. She continued to intermittently experience flushing and headache. Her third episode of syncope, preceded by flushing, nausea, and vomiting, occurred a week later. After admission she had another severe episode of prolonged and profound hypotension with syncope, requiring transfer to the MICU. At that time the history of urticaria pigmentosa in her daughter was obtained, and the patient was found to have dermatographism on physical examination. Abdominal CT scan, brain MRI, VIP, and cortisol were normal. She had elevated serum histamine, tryptase, and urine histamine levels. Bone marrow biopsy revealed increase in mast cell numbers, with aggregates of spindle-shaped mast cells, consistent with SM. Treatment with cromolyn, H1-blocker, and H2-blocker was started. She continued to experience symptoms, and required massive doses of montelukast, H1 and H2-blockers, and cromolyn for adequate symptomatic control.
DISCUSSION
Mastocytosis is a disorder of mast cell proliferation. Intense vascular collapse is caused by spontaneous, or induced mast cell degranulation, and release of vasoactive madiators like histamine, prostaglandin D2, and leukotriens. Loss of consciousness is usually accompanied by prodromal symptoms of flushing, palpitations, and lightheadedness. Patients also can experience pruritus, gastrointestinal and neurologic symptoms. In the absence of characteristic skin lesions, diagnosis is difficult, but should be suspected in the patient with unexplained hypotensive episodes and flushing. The evaluation of the patient with suspected SM includes skin inspection, measurement of markers of mast cell activation, and bone marrow biopsy. There is no current cure for SM. Successful treatment of symptoms may require massive doses of H1 and H2-blockers, steroids, montelukast, and cromolyn.
RENAL ARTERY STENOSIS IN A PATIENT WITH UNCONTROLLED HYPERTENSION
T.T. Tran1; L.B. Lu2. 1Baylor College of Medicine, Houston, TX; 2Baylor College of Medicine, Friendswood, TX. (Tracking ID #116996)
LEARNING OBJECTIVES
1) Recognize the importance of workup for secondary causes of hypertension. 2) Review the presentation, diagnosis, and treatment options of renal artery stenosis.
CASE
A 50-year-old man with a ten month history of hypertension and chronic renal insufficiency presented with sudden onset of severe shortness of breath and diffuse diaphoresis. He had been admitted 6 times for similar symptoms due to hypertensive urgencies despite being on multiple antihypertensive agents. On this admission, his vital signs revealed BP 248/108, HR 85, RR 16, and temperature 98.1 F. Physical examination was unremarkable. Specifically, there were no papilledema, abdominal bruits, or peripheral edema. Blood work was significant for Cr of 1.9, which was at his baseline. Due to his history of multiple admissions with hypertensive urgencies, the patient underwent a workup for secondary causes of hypertension that showed no evidence of hyperaldosteronism, thyroid disease, pheochromocytoma, or Cushing's syndrome. A renal ultrasound revealed a small left kidney of 8 cm and a normal-sized right kidney of 11 cm. Subsequent renal arteriogram showed total occlusion of the left renal artery and 90% stenosis of the right renal artery. After undergoing an angioplasty with stent placement in the right renal artery, his blood pressure came under better control.
DISCUSSION
Secondary hypertension accounts for 5–10% of hypertensive cases. Features suggesting of secondary hypertension include age at onset <30 or >55, abrupt onset, > stage 3 (160/100) hypertension, and resistance to effective medical therapy. Causes include renal insufficiency, renal artery stenosis, coarctation of the aorta, aldosteronism, Cushing's syndrome, pheochromocytoma, and thyroid disease. Renal artery stenosis accounts for 0.2 to 4% of all hypertension cases and can be due to atherosclerosis (70%) or fibromuscular dysplasia (30%). Renal arteriography is the diagnostic gold standard. The captopril test has a sensitivity of 79% and specificity of 89%. Renography after captopril has sensitivity and specificity of about 85%. Duplex ultrasonography achieves sensitivity and specificity of about 90%. Both spiral computed tomographic angiography and gadolinium-enhanced three-dimensional magnetic resonance angiography are excellent noninvasive diagnostic tests with sensitivity and specificity of about 95%. There is not a specific recommended screening non-invasive test for renal artery stenosis; the clinical index of suspicion should determine the degree of evaluation. Angioplasty or surgery has been shown to modestly improve blood pressure, but not renal functions in the presence of renal insufficiency. In summary, refractory hypertension should warrant the work up for secondary causes.
RENAL TUBERCULOSIS
C. Patel1; M. Taswin1; V. Bengualid1. 1St. Barnabas Hospital, Bronx, NY. (Tracking ID #117555)
LEARNING OBJECTIVES
In USA and New York State, Genitourinary Tuberculosis is the fourth most common form of extrapulmonary tuberculosis. It represents 6% of all EPTB.
It is easily overlooked as it usually presents with symptoms of a bacterial pyelonephritis. Key presentation includes recurrent urinary tract infections despite treatment or sterile pyuria. Imaging studies (plain film, CT scan, IVP, or USG) can show calcification, ureteral dilation or obstruction. Diagnosis made by: AFB stain/culture from urine or biopsy (sensitivity 43,3% and specificity 100%) or PCR of MTB DNA in urine (sensitivity 53.8% and specificity 96.5%).
Renal involvement includes: tubulointerstitial nephritis, stricture/obstruction, calcification (24–50%), cavitating disease, with progression to renal failure. Calcification of the kidney can be identifiable as renal or ureteric stone in up to 19% of cases. It can mimic a neoplasm, as it can spread to outside the renal capsule and produce a mass lesion.
CASE
A 73 year old Hispanic HIV-negative male with a history of recurrent urinary tract infections and bilateral staghorn kidney stones was admitted for evaluation of weight loss, fever and abdominal pain. He was found to have a pansensitive Proteus mirabilis UTI and was started on Levofloxacin.
A CT scan of abdomen showed bilateral staghorn calculi with obstruction and left hydronephrosis. He underwent placement of left percutaneous nephrostomy tube from which pus also grew pansensitive Proteus mirabilis. Renal scan then was performed, showing non-functioning left kidney. A decision to do a left nephrectomy was made. Pathology of the left kidney showed extensive necrotizing granulomatous nephritis with a few AFB and marked pyonephrosis. AFB culture of the pus was negative.
With these findings, PPD was placed (was negative), sputum and urine AFB smear and culture were negative. Chest X-ray showed bilateral apical pleural thickening and a left pleural effusion which was tapped. About 400 cc of exudative fluid with lymphocytosis was obtained. AFB culture and cytology were negative.
Started on tuberculosis treatment and the patient showed clinical improvement.
DISCUSSION
Although renal TB is an uncommon cause of progressive renal failure, it is potentially preventable and easily treatable. Diagnosis depends on the physician considering the possibility of TB and obtaining appropriate specimens for culture.
If TB is found early as the cause of obstruction, this may prevent unnecessary nephrectomy or late complication of chronic TB infection that includes metaplasia that may be a potential risk factor for squamous cell carcinoma.
RENAL TUBERCULOSIS AND CHRONIC RENAL INSUFFICIENCY: CHICKEN OR EGG FIRST?!
J. Schrader1; J. Sheila1; B.L. Houghton1. 1Creighton University, Omaha, NE. (Tracking ID #116526)
LEARNING OBJECTIVES
Learning objectives: 1. Recognize that after lymphnodal involvement, most common form of nonpulmonary tuberculosis is genitourinary disease. 2. Recognize that 26–75% of renal tuberculosis coexists with active pulmonary tuberculosis. 3. Identify renal tuberculosis as one of the rare causes of chronic renal insufficiency, and that chronic renal insufficiency can predispose to renal tuberculosis.
CASE
Patient is a 57 year old Native American male who presented with generalized swelling and progressive dyspnea of 4 months duration. He denied fever, chills, night sweats or urinary symptoms. He had chronic dry cough, fatigue and weakness. Past medical history included diabetes mellitus, hypertension, rheumatoid arthritis (on steroids) and he was a recovering alcoholic. Approximately a year ago he was treated for culture negative pyuria with antibiotics. He had history of positive PPD 10 years ago, untreated. On admission, he was afebrile, and vital signs were stable. He had palor and significant bilateral pitting edema. JVP was not elevated. Heart exam was unremarkable. Chest exam revealed small bilateral pleural effusions and bibasilar rales. There was no hepatosplenomegaly or free fluid in the abdomen. Initial labs showed hemoglobin 10.5 gm%, WBC 9.7, BUN 82 mg/dl and creatinine 3.3 mg/dl. Urinalysis was positive for protein >500 mg/dl, WBC 25–50, RBC >100 and hyaline/fine granular casts. Pulmonary edema was noted on Chest Xray. Ultrasound of the kidneys suggested intrinsic renal disease without hydronephrosis. He was admitted, treated initially with diuretics. His renal failure and dyspnea worsened requiring initiation of hemodialysis. Chest films showed worsening infiltrates. Further workup showed positive AFB in sputum, urine and stool confirmed as Mycobacterium tuberculosis. Rectal biopsy ruled out amyloidosis. He was started on antituberculous therapy. Despite maximal therapy his condition continued to decline. After detailed family discussion, it was decided to take patient home with hospice, but continue ATT.
DISCUSSION
Genitourinary tuberculosis is the most common form of extrapulmonary tuberculosis after lymphadenopathy. Renal involvement occurs through hematogenous spread from a primary focus (most common being lungs) and is usually bilateral. Predisposing conditions of renal tuberculosis include diabetes, chronic renal insufficiency, steroids and alcoholism (all four present in our patient). Renal tuberculosis can present as sterile pyuria, or as chronic interstitial nephritis (biopsy shows granuloma and caseation), amyloidosis and end stage renal disease. Given the patient's treatment for sterile pyuria in the past, it is uncertain if he had insidious onset of tuberculosis starting then or whether chronic renal insufficiency predisposed to tuberculosis.
RETINAL HEMORRHAGES AND PAPILLOEDEMA IN PSEUDOTUMOR CEREBRI PRESENTING WITH SUDDEN OF LOSS VISION
A. Devarajan1; P. Patel1; A. Varadarajan1; E. Floranda1; C. Castillo1; C. Rathnakumar1; R. Jayachandran1. 1Jersey City Medical Center, Jersey City, NJ. (Tracking ID #116491)
LEARNING OBJECTIVES
A benign process affecting the brain which appears to be, but is not a tumor. It is characterized by increased intracranial pressure and normal brain ventricle size. There is no evidence of tumor, infection, and blocked drainage of the fluid surrounding the brain or any other cause. The major symptoms of pseudotumor are increased pressure within the skull (increased intracranial pressure—ICP). The cause for the condition itself is unknown, and the diagnosis is made when other health conditions are ruled out.
CASE
A 24 year old African American female with no toxic habits, and no known medical problems except extreme obesity (BMI 44) presented with sudden loss of vision in both the eyes, headache, nausea, and vomiting for one day. She denied dizziness, loss of consciousness, seizure, photophobia, neck pain, tingling, numbness and weakness of extremities, Review of other systems, family and social history were normal. She denies any medication including oral contraceptive pills. Physical examinations including neurological examination were normal except fundus showing bilateral papilloedema and retinal hemorrhage in both eyes. Initial working diagnosis Pseudo tumor cerebri was made in view of the sudden loss of vision in young obese female with no significant past medical history including medications like oral contraceptive pills with benign neurological examination. Initial CBCD and serum chemistry were normal. Lumbar puncture showed an opening pressure of over 550 mm of water and a closing pressure of 210 mm of water. 15 cc of CSF was removed during lumbar puncture. The CSF was clear, Glucose 80 mg, protein 20 mg, no WBC and RBC. CSF stains and culture were negative for pathogens. Patient was started on Acetazolamide. Patient relieved of her headache, nausea and vomiting and regained partial vision after 24 hrs and complete vision in 6 days. Patient continued to have residual papilloedema and hemorrhages in left eye.
DISCUSSION
Significance of clinical suspicion of Pseudotumor cerebri to be entertained in any young obese female patients presenting with sudden loss of vision, nausea, vomiting, and headache with no co-morbid conditions and no clinical evidence of any other causes. Early institution of diagnostic and therapeutic lumbar puncture to relieve intra cranial pressure and medical management with acetazolamide helps to prevent permanent visual damage. Our case demonstrated clinical evidence of Pseudotumor cerebri with papilloedema, and retinal hemorrhages and early diagnostic and therapeutic intervention restored the vision within a few days, though the retinal changes remained unchanged for some time.
RHABDOMYOLYSIS: AN UNUSUAL PRESENTATION OF HYPOTHYROIDISM
M. Derakhshani1; J. Huang1. 1Louisiana State University Medical Center at Shreveport, Shreveport, LA. (Tracking ID #115705)
LEARNING OBJECTIVES
1) Recognize rhabdomyolysis as a rare presentation of hypothyroidism. 2) Emphasize the importance of history.
CASE
A 50 year old African-American female with no past medical history initially presented to emergency room with a complaint of bilateral leg pain associated with lower back pain progressively worsening for the past 3 months. Her symptoms did not respond to a course of NSAIDS prescribed for “arthritis” by her local primary care physician. She described the pain as dull, primarily in the lower back and both legs, constant throughout the day and not related to physical activity. She reported ambulating and performing activities of daily life without difficulty. She also denied any recent history of vigorous exercise, over-ingestion of alcohol, or use of cocaine. Upon further history, she admitted to cold intolerance, easy fatigue, and 20 lb weight gain within the past 3–4 months. Her physical exam was unremarkable except for decreased deep tendon reflexes. Musculoskeletal exam revealed normal tone, strength, and bulk in all muscle groups. Laboratory data were significant for normal electrolytes, normal renal function, elevated AST of 145 U/L (14–36) and ALT of 63 U/L (9–52) with normal alkaline phosphotase of 46 U/L (38–126), decreased hematocrit of 31.4% (34–46), and elevated serum creatine phosphokinase (CPK) of 16,000 U/L (30–135). Urine analysis showed large blood on dipstick with only 10–20 RBC/HP suggesting myoglobinuria. Serum myoglobin was elevated at 549 ng/ml (19–56). Further workup included ANA, viral hepatitis panel, and urine drug screen which were all negative. Evaluation of thyroid function revealed low T3 of 0.15 ng/ml (0.45–1.37), low T4 of 1.01 microg/dl (4.5–12.0), and elevated TSH of >100 microU/ml (0.47–5.01) which confirmed the diagnosis of hypothyroidism. After the initial intravenous fluid infusion, patient was followed in outpatient clinic with thyroxin replacement. Her symptoms resolved and her CPK gradually normalized. Her renal function remained normal throughout the course.
DISCUSSION
Muscle involvement in adults with hypothyroidism is common and includes stiffness, myalgias, and mild weakness. However, overt rhabdomyolysis is quite rare with only a few reported cases. The exact mechanism of rhabdomyolysis remains unclear. This case suggests that rhabdomyolysis is a rare, but potentially serious complication in hypothyroidism. It can be one of the initial presenting manifestations in undiagnosed hypothyroidism. High index of suspicion and a thorough history, combined with readily available thyroid function test, are essential for diagnosis. Earlier diagnosis in this case might have prevented the indiscriminate use of NSAIDS that could have resulted in renal dysfunction.
RHABDOMYOLYSIS AS A DRUG INTERACTION BETWEEN SIMVASTATIN AND NEFAZADONE: INCREASING EVIDENCE
H.L. Korlakunta1; S. Dodla1; R. Kizer1; S. Gonzalez1. 1Creighton University, Omaha, NE. (Tracking ID #115719)
LEARNING OBJECTIVES
To recognize rhabdomyolysis as an adverse reaction of concurrent use of simvastatin and nefazodone. We emphasize this by presenting, to our knowledge the fourth reported case of myopathy or rhabdomyolysis caused by concurrent use of these drugs.
CASE
A 59-year-old white male was admitted to the hospital for sudden-onset of severe back and lower extremity pain, weakness, and dark urine. He had no history of recent trauma or prolonged heat exposure. He had a history of hyperlipidemia and depression, and was taking simvastatin 80mg orally once daily and nefazodone 150 mg orally once daily. Three weeks prior to admission, his simvastatin dose was increased from 40 mg to 80 mg orally once daily. On admission, laboratory studies revealed elevated creatine kinase (CK) at 26,862 IU/L, elevated liver transaminases, elevation of serum creatinine to nearly twice baseline, and urine sediment consistent with acute tubular necrosis. Urine analysis demonstrated hematuria and positive urine myoglobin. Erythrocyte sedimentation rate (ESR), antinuclear antibody (ANA), CMV and EBV serologies and thyroid stimulating hormone were normal. White blood cell count was normal, and patient was afebrile. Simvastatin and nefazodone were discontinued, and the patient was treated with IV hydration. The patient's CK, liver transaminases, and urine sediment normalized with resolution of his symptoms over the course of one week. He remained asymptomatic at 2 and 6-month follow-up examinations, and CK, liver transaminases, and serum creatinine were normal.
DISCUSSION
Although myopathies and elevated liver transaminases are recognized as common side-effects of HMG-CoA reductase inhibitors, susceptibility to rhabdomyolysis is significantly increased by interactions with offenders such as cyclosporine, itraconazole, and some macrolide antibiotics. These drugs inhibit the CYP3A4 enzyme family pathway, as does nefazodone. This pathway metabolizes simvastatin. Inhibition of the CYP3A4 pathway leads to increased levels of active statin metabolites, which increases the risk of myopathies and rhabdomyolysis. The risk for rhabdomyolysis may be dose-dependent. The increasing number of case reports of rhabdomyolysis associated with use of nefazodone and simvastatin along with known interactions between simvastatin and other CYP3A4 inhibitors suggest that the combination should be avoided if possible. Though this drug combination may be tolerated at certain doses, an increase in the simvastatin dose may lead to rhabdomyolysis, as was demonstrated in the above case.
SARCOIDOSIS OR RIGHT HEART FAILURE? OR BOTH?
C. Leggett1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117483)
LEARNING OBJECTIVES
1. Recognize that the presenting feature of chest pain and shortness of breath in a patient with sarcoidosis may represent worsening right heart failure.
CASE
A 37 year-old woman with a past medical history of sarcoidosis presented with worsening shortness of breath and chest pain when walking only one block. She had associated nausea and dizziness without syncope or diaphoresis; all symptoms were relieved by rest. She had a right ventricular heave, but the remaining examination was normal. Her CXR showed an enlarged cardiac silhouette and patchy infiltrates. An echocardiogram revealed a pulmonary artery pressure of 70 mmHg with poor right ventricular function. Left ventricular function was preserved. A chest CT confirmed stage III sarcoidosis. She was started on prednisone. She was discharged with home oxygen and a diagnosis of cor pulmonale secondary to her pulmonary sarcoidosis.
DISCUSSION
Physicians should consider the possibility of cardiac sarcoidosis in any patient with a known history of sarcoid or in an otherwise healthy young person who develops arrhythmias, conduction disease, or heart failure. A diagnosis of cardiac sarcoidosis is difficult to diagnose without positive endomyocardial biopsy, and biopsy is only 20% sensitive as lesions are often not confluent. There are several tests that can help to define cardiac involvement. Patient's with granulomatous infiltration of the myocardium can present solely with heart failure but there is also an increased incidence of arrythmias or conduction abnormalities. An ECG or 24-hour holter monitor can detect if these abnormalities are present. In a few patients with cor pulmonale, as suspected in this patient, right ventricular hypertrophy regresses when the pulmonary disease is improved with long term corticosteroids.
SATURDAY NIGHT FEVER
E. Chuong1; S. Shaw1; N. Feldman1. 1UCLA/San Fernando Valley Program, Sylmar, CA. (Tracking ID #116965)
LEARNING OBJECTIVES
1. Identify fever of unknown origin (FUO) and common etiologies of FUO 2. Describe a systematic diagnostic approach to FUO 3. Recognize a rare case of intravascular lymphoma as a cause of FUO
CASE
A 47 year-old woman with hypertension and untreated latent tuberculosis (TB) presented with 2 weeks of myalgia, arthralgia, weakness, fever, and confusion. Her physical exam was significant for a temperature of 39.7°C, waxing and waning mental status, and a II/VI systolic murmur. Initial laboratory data was notable for WBC 4,000 cells/uL, hemoglobin 8 g/dL, platelets 29,000 cells/uL, unrevealing peripheral blood smear, LDH 6046 U/L, ESR 60 mm/hr, D-dimer <5 ug/mL, fibrinogen 378 mg/dL, and troponin-I 21.8 ng/mL. Her chest radiograph suggested pulmonary edema, and she was intubated for respiratory distress. CT and MRI scan of her head revealed ischemic changes; CT scan of her chest indicated mutifocal pneumonia; CT scan of her abdomen and pelvis demonstrated hepatomegaly with splenic infarct. Lumbar puncture, CSF studies, echocardiogram, and bronchoscopy failed to demonstrate underlying disease. Bone marrow biopsy revealed normocellular reactive tissue. The patient continued to be febrile throughout her two-week hospitalization despite a negative evaluation for malignancy, hematological disorder, infection, and collagen-vascular disease. Multiple studies were repeated, but all remained undiagnostic. Broad-spectrum antibiotics, fluconazole, and anti-TB medications were empirically started. The patient also received corticosteroids at stress and pulse doses for presumptive collagen-vascular disease. Her critical illness required multiple blood product transfusions and prohibited nuclear medicine scanning, further biopsies, and exploratory laparotomy. The patient was diagnosed postmortem with intravascular natural killer T-cell lymphoma.
DISCUSSION
Peterdorf and Beeson defined a fever of unknown origin in 1961 as a temperature greater than 38.3°C on several occasions, illness duration longer than 3 weeks, and uncertain diagnosis despite 1 week of inpatient investigation. Common causes of FUOs in the adult population are malignancy, infection, and collagen-vascular disease. The evaluation of an FUO begins with a detailed history and regular physical exams to identify probable sources of FUO. Basic diagnostic testing should include complete blood count with differential, serum chemistries and cultures, urinalysis and culture, applicable immune and serological studies, tuberculin skin testing, and chest radiographs. Additional serum testing, noninvasive imaging, radionucleotide studies, and invasive procedures including biopsies may be helpful in the appropriate clinical context. Five to fifteen percent of FUOs are undiagnosed despite exhaustive studies. Therapeutic trials of antimicrobials and corticosteroids do not substitute for a carefully directed investigation for the underlying cause of an FUO but may be warranted in cases of severe illness.
SAVING MY LIFE, BUT BREAKING MY HEART
B. Anderson1; J. Wiese2. 1Tulane, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117386)
LEARNING OBJECTIVES
1. Recognize metabolic abnormalities associated with protease inhibitors. 2. Increase awareness of the association between HIV-positivity and ischemic heart disease.
CASE
A 43 year-old man presented with one hour of left-sided chest pain that radiated to the left arm. The chest pain occurred the day of admission while walking to work. He was diagnosed with HIV fourteen years prior, and a history of untreated hyperlipidemia that developed after instituting HIV therapy. He began treatment for HIV in 1995 with a regimen including a protease inhibitor. His CD4 count and viral load two months prior to admission were 504 cells/mm3 and <50 copies/mL. He denied any family history of heart disease. His medications included efavirenz, lamivudine/zidovudine, and lopinavir/ritonavir. His vital signs and physical exam was normal. An EKG revealed ST-segment elevation in leads V2–V6. His initial serum troponin-I was 0.20 ng/ml. A percutaneous coronary intervention was immediately performed, demonstrating a 70% stenosis of the proximal left anterior descending artery. A stent was placed to the lesion with resultant TIMI 3 flow.
DISCUSSION
Metabolic abnormalities associated with protease inhibitors include hypertriglyceridemia, hypercholesterolemia, and diabetes mellitus. As patients with HIV live longer, physicians should be cognicsent of these side effects of anti-retroviral therapy that can lead to accelerated atherosclerosis and increased myocardial risk. In this way, chronic HIV infection is similar to diabetes as a comorbidity that predisposes to heart disease. Because patients with HIV are increasingly living longer, coronary disease should be aggressively pursued in this patient population.
SCLERITIS AS A PRESENTING MANIFESTATION OF WEGENER's GRANULOMATOSIS
Y. Ozawa1; J. Blank1. 1UCLA—San Fernando Valley Program, Sylmar, CA. (Tracking ID #116296)
LEARNING OBJECTIVES
(1) Recognize scleritis as a manifestation of Wegener's Granulomatosis (WG). (2) Review the various ocular and oral presenting symptoms of WG.
CASE
A 64 year old Guatemalan female presented to the ER with eye pain and decreasing visual acuity for two months. One year ago she developed red, painful eyes which were treated with antibiotics and cortisone in her country. Six months later, her eye condition recurred resulting in bilateral corneal perforations. On review of systems in the ER, she reported anorexia and a 30 pound weight loss over the previous 6 months and recurrent rhinorrhea. She denied fever, cough, dyspnea, hemoptysis, joint pains or hematuria. On exam, both sclerae were erythematous and her left eye had an opacified cornea. A 3x1 cm ulcer was present on her lower anterior buccal mucosa with pronounced erythematous gingiva and gingival hyperplasia. The rest of her exam was unremarkable. Her labs were significant for a creatinine of 4.1 and urinalysis revealed microscopic hematuria. A chest X-ray showed right upper lobe and right middle lobe cavitary lesions as well as multiple left upper lobe nodules. A sinus CT demonstrated maxillary sinus mucosal thickening. A C-ANCA (proteinase-3) was found to be elevated at 13. The presence of three out of four clinical criteria (nasal/oral inflammation, abnormal chest radiograph and abnormal urinary sediment) and the positive C-ANCA was considered diagnostic for WG. Once infectious etiologies were ruled out, cyclophophamide was initiated with gradual resolution of her symptoms.
DISCUSSION
WG is a systemic vasculitis of the small arteries and veins. It typically involves the upper and lower respiratory tracts and the kidneys. The joints, eyes, mouth, skin, heart, and nervous system may also become involved. Eye involvement (52% of patients) may range from a mild conjunctivitis to proptosis, dacryocystitis, episcleritis, scleritis, and corneal ulcerations. Over 50% of patients diagnosed with scleritis are ultimately diagnosed with a connective tissue or vasculitic disease. Oral involvement (10% of patients) in WG may include mucosal ulcerations, gingivitis and gingival hyperplasia. Ulcerations are commonly found in the buccal mucosa and biopsy usually reveals necrotizing vasculitis. Gingiva may be strikingly red with white, yellow, or blue punctate lesions, clinically resembling over-ripe strawberries. Gingival biopsies reveal pseudoepitheliomatous hyperplasia, multinucleated giant cells, and inflammatory infiltrates, a constellation that is specific for WG. Most oral and ocular findings are nonspecific. However, scleritis, which is a common manifestation of systemic disease, may alert the clinician to early diagnosis and initiation of treatment.
SCLERODERMA UNMASKED: A CASE OF HYPERTENSIVE RENAL CRISIS
M. Hadian1; B. Taqui1; N. Marchetti1. 1Temple University, Philadelphia, PA. (Tracking ID #116188)
LEARNING OBJECTIVES
1. Recognize the clinical manifestations of scleroderma and scleroderma renal crisis. 2. Recognize the importance of prompt treatment of scleroderma renal crisis. 3. Recognize treatment options for scleroderma.
CASE
A 44 year old Hispanic male with hypertension presented with one day of headache, chest pain, dyspnea and six months of progressive leg weakness, dysphagia and post-prandial vomiting. Upon specific questioning, he also endorsed cold induced pain in his fingers. Exam revealed a thin young man with minimal facial expression. His blood pressure was 200/110. He had thickened facial skin and telangiectasia around nasolabial folds. He had papilledema with AV nicking and an S4 gallop. He had purple atrophic fingertips and thickened skin in the extremities. He had edematous lower extremities with 4–/5 proximal muscle strenth. Labs revealed BUN 42, Cr 5.7 (baseline 3.0), Hgb 9.5. His cardiac enzymes were negative. His urine showed protein >300 mg/dl, 4–10 RBC. Intravenous metoprolol, hydralazine and enalapril reduced his blood pressure to 160/85. Serologies revealed a ANA 1/1280 with nuclear pattern, normal C3 and C4, negative anti-Scl 70. Barium swallow revealed decreased motility. Sural nerve/quadriceps muscle biopsy showed sclerodermal neuropathy and myopathy. Echocardiogram showed LV hypertrophy, EF 45%, and increased pulmonary artery pressure. Subsequently, a kidney biopsy demonstrated severe arteriolosclerosis suggestive of malignant hypertension, as well as focal segmental glomerulosclerosis. The patient was diagnosed with scleroderma and discharged on enalapril, prednisone and nifedipine.
DISCUSSION
Scleroderma is a disorder characterized by fibrosis of the skin, blood vessels and visceral organs. Two major subsets are identified. The diffuse subset, characterized by the rapid development of symmetric skin thickening of proximal and distal extremities, face and trunk, is associated with visceral organ (heart, lung, kidney) involvement. The limited cutaneous subset, involving distal extremities and face, is associated with CREST syndrome (calcinosis, raynauds,esophageal dysmotility, sclerodactyly, telangiectasia). Scleroderma renal crisis is defined by acute worsening of renal function and is usually associated with abrupt onset of marked hypertension with retinopathy. Urine sediment is usually normal, or with mild proteinuria. Blood pressure control is the mainstay of therapy, with ace inhibitors as the agent of choice. Early recognition and treatment of the crisis can preserve renal function. Our patient's creatinine returned to baseline upon discharge. Renal crisis may unmask a diagnosis of scleroderma. Although there is no cure, patients with scleroderma may benefit from d-penicillamine, steroids, and calcium channel blockers.
SIMPLE HEART FAILURE OR SOMETHING MORE?
S.Y. Chien1; J.H. Tillisch2. 1UCLA San Fernando Valley Program, Sylmar, CA; 2University of California, Los Angeles, Los Angeles, CA. (Tracking ID #115658)
LEARNING OBJECTIVES
1. Recognize that the differential diagnosis of peripheral embolization and CHF symptoms includes atrial myxoma. 2. Recognize imaging modalities necessary for the diagnosis of cardiac tumors.
CASE
A 54-year-old woman was admitted with one month history of typical heart failure symptoms. Echocardiogram showed diffuse global hypokinesis and EF 20%. Because the etiology of her cardiomyopathy remained unclear, she was taken to cardiac catheterization. It showed nonsignificant coronary stenosis. She responded to initial treatment with diuretics. However, on additional questioning, she reported word-finding difficulty and that her right leg had become more painful and cooler to touch for the past four months. Eight months previously, she was found to have right renal artery occlusion on ultrasound with elevated creatinine. A current work-up for possible stroke ensued, including an MRI/MRA of the brain which demonstrated a recent ischemic infarction in the right cerebellar hemisphere and posterior MCA territory. A doppler ultrasound of the neck was negative. The stroke distribution suggested an embolic phenomenon, but TTE was negative. Thus, an MRI of the heart was performed, which showed a small round defect in the left atrium, with a bright rim. A TEE again confirmed a pedunculated mass, attached near the interatrial septum with 1-cm stalk and 1-cm irregular spherical portion, consistent with atrial myxoma. Irregular strands of swirling echoes were also consistent with thrombus formation.
DISCUSSION
Primary cardiac tumors are exceedingly rare. Three quarters of them are benign, nearly half are myxomas. Myxomas can occur in all age groups with a female predominence. Sporadic cases are more frequent, but familial cases have also been reported. These tumors usually develop in the atria, left more than right. Very rarely do they develop in the ventricles. These tumors also have an array of interesting clinical manifestations including systemic embolization involving the cerebral, retinal, renal, coronary, pulmonary, and abdominal aorta circulations. Myxomas commonly give rise to obstruction of cardiac filling. Signs of dyspnea, pulmonary edema, syncope, or sudden death may occur, mimicking mitral or tricuspid-valve stenosis. Rarely does one find predominant mitral or tricuspid insufficiency. Systolic or diastolic murmurs may be heard as well as pericardial friction rubs. The first heart sound is often loud and widely split. Laboratory findings might show anemia, thrombocytopenia, leukocytosis, and elevated ESR or CRP. EKG findings and routine chest films are often nonspecific. The introduction of echocardiography, CT, and MRI has greatly facilitated the diagnosis of cardiac tumors. Treatment of choice is surgical resection. It is usually curative. In any case, the systemic signs disappear after the tumor has been removed.
STENT PLACEMENT IN THE TREATMENT OF PULMONARY ARTERY STENOSIS SECONDARY TO FIBROSING MEDIASTINITIS
R. Satpathy1; C. Satpathy2; I.A. Khan1; V. Aguila1. 1Creighton University, Omaha, NE; 2SCB Medical College, Cuttack, Orissa. (Tracking ID #115678)
LEARNING OBJECTIVES
Fibrosing mediastinitis is a rare benign disorder caused by proliferation of acellular collagen and fibrous tissue within the mediastinum. Although many case are idiopathic, many (and perhaps the most) cases in the United States are thought to be caused by an abnormal immunologic response to Histoplasma Capsulatum infection. Affected patients are typically young and present with signs and symptoms of obstruction or compression of the superior vena cava, pulmonary veins or arteries, central airways, or esophagus. Pulmonary artery (PA) stenosis is an infrequent complication of fibrosing mediastinitis.
CASE
We report a case of 41-year-old male who presented with fever. On examination he was found to have cardiomegaly on chest x-ray and high ESR. The transthoracic echo (TTE) showed pericardial effusion, which eventually resolved on its own. At that time, it was thought to be viral in etiology. However, the patient continued to be symptomatic in terms of chest pain, increasing fatigue, dizziness and progressively increasing shortness of breadth with exercise over next 6 to 8 months. He had a repeat TTE done showing supra pulmonic stenosis. A transesophagial echo was obtained and suggested a mass compressing the pulmonary artery. The thoracic CT confirmed a 7 × 5 cm anterior mediastinum mass, which on biopsy was found to be granulomatous with fibrosing mediastinitis. AFB and fungal culture were negative. However, the serological test established the diagnosis of histoplasmosis. He had a cardiac catheterization done afterwards, which showed normal coronary arteries, SVC and pulmonary veins. His right heart pressures included right ventricle (RV) 37/4, main PA 37/7, right PA 13/7 and left PA 23/7. He had a large stent placed in the right pulmonary artery and post-stenting pressures were RV 33/5, main PA 31/8 and right PA 26/8. He has been asymptomatic since then.
DISCUSSION
Despite varied forms of pharmacologic treatment and surgical interventions, most previously reported patients with PA stenosis died of right heart failure as a result of severe pulmonary hypertension. Although the placement of stents has been described as successful treatment of congenital PA stenosis, there has been less description of PA stent placement for fibrosing mediastinitis. This article describes a patient who was moderately symptomatic from PA stenosis and has remained symptom-free for approximately 2 years now after treatment.
STEROID USE IN GLOMERULONEPHRITIS ASSOCIATED WITH INFECTIOUS ENDOCARDITIS
M. Lim1; C. Graeber2. 1University of Connecticut, Farmington, CT; 2New Britain General Hospital, New Britain, CT. (Tracking ID #116110)
LEARNING OBJECTIVES
Recognize corticosteroid as an option in the management of renal dysfunction secondary to glomerulonephritis associated with infectious endocarditis (IE) that does not improve with appropriate antibiotic treatment.
CASE
A 66 year old Caucasian male with history of DM II, CHF, pacer for sick sinus syndrome and atrial fibrillation presented with a 4 week history of night chills and 1 week history of raised, tender, red lesions on all extremities. PE showed temperature of 102.6oF; 1/6 systolic murmur at the tricuspid area; and tender, palpable purpura diffusely scattered over all four extremities, sparing the palms and soles. Laboratory showed creatinine of 2.3 mg/dl (1.0 mg/dl 4 months PTA), BUN of 53 mg/dl; proteinuria (1.5 g/24 h) and hematuria. Serologies were as follows: ANA, RF, ANCA, anti-GBM, cryoglobulin and HCVab were undetected. C3 and C4 were low. Blood cultures grew Streptococcus bovis. 2-D echo showed a 2 × 2 cm mass on the anterior leaflet of triculpid valve, sparing the pacemaker. Colonoscopy disclosed diverticulosis and non-neoplastic colonic polyps. The patient was treated with Ceftriaxone (2 g IV QD). Despite defervescence and sterile blood cultures, his renal function deteriorated with creatinine reaching 5.3 mg/dl on D22. Kidney and skin biopsy revealed focal necrotizing glomerulonephritis and perivascular inflammatory changes, respectively. Prednisone (60 mg/d) was introduced (D22) and creatinine dropped to 2.9 mg/dl on D29.
DISCUSSION
Glomerulonephritis complicates ∼20% of endocarditis cases. Patients usually present with hematuria, red cell casts, variable degrees of hypertension and renal insufficiency. This condition resolves with appropriate antibiotic treatment. However, as in the case of this patient, a steady decline in renal function sometimes persists despite apparent sterility of blood cultures, leading to death or ESRD. Plasmapharesis and immunosuppressive therapy, alone and in combination have been reported in the literature as management for these cases. There have been 8 reported cases in which corticosteroids, along with antibiotics was shown to be effective in reversing azotemia, without compromising treatment of endocarditis. The rationale for steroid use is thought to be the suppression of immune reaction and immune complex formation since glomerulonephritis is believed to be secondary to the deposition of immune complexes in the glomeruli and injuries associated with this. This case report, in addition to the other reported cases in the literature, suggests potential benefit from steroid use in IE-related glomerulonephritis refractory to appropriate antibiotic treatment. The combination of rapidly progressive glomerulonephritis and palapable purpura may be particularly common in IE due to Streptococcus Bovis.
STREPTOCOCCUS AGALACTIAE PRESENTING AS A SARCOMATOUS MASS OF THE LOWER EXTREMITY
P.J. DiGiacomo1; G. Sokos2. 1Allegheny General Hospital, Sewickley, PA; 2Allegheny General Hospital, Pittsburgh, PA. (Tracking ID #117390)
LEARNING OBJECTIVES
To recognize the peripheral embolic manifestations of endocarditis. To recognize the risk factors for Group B Steptococcal infections. To recognize a rare cause of endocarditis in a healthy individual.
CASE
This is the case of a thirty-seven-year-old woman who presented with a painful mass lesion of the right lower extremity. The lesion had developed over the course of two to three weeks. On physical exam the patient had an eight centimeter by eight centimeter firm tender mass of the posterior distal right lower extremity. The remainder of the exam was normal. Computed tomography of the lesion suggested sarcoma or lymphoma as the likely diagnosis. Prior to a surgical biopsy being performed the patient presented to the emegency department with new onset seizure disorder. Evaluation of the patient at this time revealed multiple bilateral mass lesions of the brain. Given the suggestion of a more systemic process, cytology was performed on the lower extremity mass. This revealed gram positive cocci. Given the findings the patient underwent echocardiography revealing a large echogenic lesion of the aortic valve. Six sets of blood cultures confirmed Streptococcus agalactiae as the causative organism. The periphreal embolic manifestations in this patient included abscess of the lower extrmity, multiple brain abscesses with new onset seizure, and an acute occlusion of the left superficial femoral artery. Because of persistant embolic events the patient underwent an expidited aortic valve replacement without further complication.
DISCUSSION
The common manifestations of endocarditis such as petechiae, Osler's nodes, Janeway lesions, and splinter hemorrhages were not present in this patient. The more striking large peripheral emboli created the morbidity in this patient. Streptococcus agalactiae is a causative organism of adult infections in the peripartum period. Chorioamnionitis, puerperal sepsis, endometritis, and urinary tract infection are the most frequent. Our patient had an uncomplicated NSVD four months prior to presentation. This was assumed to be the origin of her endocarditis. Risk factors for infecion at that time are failure to screen for Group B streptococcal disease, failure to initiate intrapartum prophylaxis, premature or prolonged rupture of membranes, prolonged labor, and delivery at less than thirty-seven weeks of gestation. Historically these risks did not apply to our patient. Risk factors for infection outside the genitourinary tract include immunodeficiency states and common medical conditions such as diabetes. Our patient had no such history. A history of acquired heart disease was also not present further highlighting this as an unusual case of endocarditis in an otherwise healthy patient.
STREPTOCOCCUS INTERMEDIUS CAUSING RECURRENT PNEUMONIAS
B.P. Sankarapandian1; S.K. Thambidorai1; M. Bandara1; M. Ricardo-Dukelow1; S. Dhanireddy2; L. Preheim1. 1Creighton University, Omaha, NE; 2Creighton University Medical School, Omaha, NE. (Tracking ID #115701)
LEARNING OBJECTIVES
1.Recognize Streptococcus Intermedius (Strep Int.) as a potential cause for recurrent pneumonias 2.Delineate risk factors associated with Strep Int. pulmonary infections 3.Emphasize the discordance between the clinical presentation and severity of illness.
CASE
57-year-old male with a history of alcoholism and smoking has symptoms of cough and pleuritic chest pain. He had a history multiple pulmonary infections in the months prior to admission. These infections were treated with several courses of flouroquinalone and macrolide antibiotics. Despite the treatment the patient's condition did not improve and he was eventually diagnosed at another facility with loculated empyema. He refused inpatient therapy at that time and opted for continued outpatient therapy with oral antibiotics. He subsequently presented to our facility with progressively worsening dyspnea and pleuritic chest pain. Physical exam noted poor dentition with multiple dental caries, absent breath sounds and dullness to percussion over the right lower lung fields. Computerized tomography of the chest revealed right sided effusion with multiple fluid filled loculations. Thoracentesis drained 2000 cubic centimeters of exudative fluid. Pleural fluid cultures produced strains of Strep Int. The patient was then treated with intravenous clindamycin and his condition rapidly improved.
DISCUSSION
Strep Int. is a rare but well reported pathogen in producing a variety of abscesses in the body. There are several case reports identifying Strep Int. as a cause of pulmonary infections. This patient presented with recurrent pneumonias that were treated with standard antibiotics used for community acquired pneumonia. The patient subsequently developed empyema allowing us to identify the organism responsible for the recurrent infections. Appropriate treatment of pulmonary infections caused by Strep Int. will include the addition of antibiotics with anaerobic coverage. Physicians must be cognizant of the fact that patients with periodontal disease and alcoholism are susceptible to Strep Int. infection. Respiratory infections with Strep Int. are characterized by mild symptoms (without toxic features), the presence of predisposing factors (i.e., periodontal disease, alcoholism), thoracic empyemas and prolonged hospitalizations.
STRUMA OVARII PRESENTING WITH ASCITES AND AN ELEVATED CA-125 IN A PATIENT WITH KNOWN GOITER
N.M. Dookeran1. 1Boston Medical Center, Boston, MA. (Tracking ID #117524)
LEARNING OBJECTIVES
1) Recognize the variability in clinical features manifested by struma ovarii tumors. 2) Recognize that an elevated CA-125 level is not entirely specific to ovarian cancer.
CASE
This is a case of a 67-year-old woman with a history of chronic atrial fibrillation, mitral regurgitation and right heart failure who was admitted with complaints of four months of increasing abdominal girth, not responsive as in the past to her usual diuretic. She had also recently been diagnosed with hyperthyroidism secondary to a multi-nodular goiter and was being treated with methimazole. Physical exam revealed a cachectic woman with a small, palpable left thyroid nodule, no jugular venous pressure elevation, a heart exam consistent with atrial fibrillation and mitral regurgitation, and a normal lung exam. Her abdomen was distended and non-tender, with shifting dullness and a fluid wave. Her labs were significant for marked thrombocytosis, microcytic anemia and hyponatremia. TSH and liver function tests were normal except for an elevated INR due to warfarin use. Chest X-ray was normal. Abdominal CT scan revealed ascites, a 7.1 × 5.3 cm complex left ovarian mass and serosal thickening of the stomach, colon, omentum and gallbladder. The patient's CA-125 level was markedly elevated at 889 units/ml. However, ascites cytology was negative for tumor cells and the patient eventually underwent an exploratory laparotomy and left salpingo-oophorectomy. Pathology revealed a non-functional struma ovarii.
DISCUSSION
Struma Ovarii, a rare cystic ovarian teratoma, consists mainly of thyroid tissue and can vary widely in presentation—from being asymptomatic to having ascites and possibly pseudo-Meigs' syndrome. The current case, prior to surgery, was concerning for ovarian cancer. There have been few similar cases of struma ovarii presenting with an elevated CA-125 level. In addition, the co-existence of a multi-nodular goiter made this patient's case more unique. This, along with reports of struma ovarii occurring more often in countries where goiter is endemic, raises the question of whether there are genetic and/or environmental factors that predispose to both conditions.
SWEET's SYNDROME
O.F. Osi-Ogbu1; R. Granieri1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115885)
LEARNING OBJECTIVES
1 To recognize the dermatologic manifestations of Sweet's syndrome 2 To recognize the potential association of Sweet's syndrome with malignancy 3 To recognize the essential role of skin biopsies in uncommon dermatosis
CASE
A 43 year old previously healthy male presented with a diffuse, vesicular, painful rash and fever of a day's duration. One month earlier, he was treated for an upper respiratory tract infection with azithromycin. Physical examination revealed a temperature of 38.2°(C), multiple erythematous 10–40 mm papulovesicular lesions, predominantly on his neck, shoulders, trunk, face and extremities. Laboratory studies revealed WBC 11.5 (85% neutrophils). The initial impression was disseminated herpes vs Sweet's syndrome. Culture was negative for herpes. A punch biopsy revealed neutrophilic infiltrates consistent with Sweet's syndrome. Chest CT showed a left lower lobe thyroid nodule. FNA was negative for malignancy.
DISCUSSION
Originally described in 1964 by Robert Sweet, Sweet's syndrome is characterized by the abrupt onset of tender, red to purple circinate plaques. The syndrome ranges from this classic presentation, occurring in young women after mild respiratory illness, to a more aggressive neutrophilic process that may be associated with inflammatory diseases or malignancy. Massive epidermal edema may produce a deceptively vesicular appearance. Ulcers and bullae are more common in malignancy associated disease. Sweet's syndrome demonstrates pathergy. Although the skin is the primary target organ, extracutaneous manifestations occur and include pulmonary infiltrates, proteinuria, hematuria and decreased GFR. The female to male ratio is 2–3:1. Several malignant and nonmalignant disease have been associated with Sweet's syndrome, including myelodysplasia, CML, AML, lymphoma, malignancy of the genital tract and upper respiratory infections. The presence of 2 major and 2 minor clinical findings are criteria for diagnosis. Major criteria include (1) abrupt onset of tender or painful erythematous plaques or nodules, occasionally with vesicles, pustules, or bullae and (2) predominant neutrophilic dermal infiltrates without leukocytoclastic vasculitis. The minor criteria are (1) antecedent respiratory or GI tract infection, vaccination or associated inflammatory disease, hemoproliferative disorders, solid malignant tumor or pregnancy (2) malaise and fever >38 (3) ESR > 20 mm, elevated C-reactive protein, segmented neutrophils and bands >70 %, leukocytosis > 8000, (4) excellent response to systemic corticosteroids. Skin biopsy is the main stay of diagnosis. Medical management includes prednisone (40–80 mg qd). Prognosis depends on the underlying cause.
SYMPTOMATIC HYPOMAGNESEMIA AND HYPOKALEMIA: A CASE OF GITELMAN's SYNDROME COMPLICATING A THIRD TRIMESTER PREGNANCY
J. Baez-Escudero1; A. Samuels1; B. Taqui1. 1Temple University, Philadelphia, PA. (Tracking ID #116184)
LEARNING OBJECTIVES
1. Recognize clinical manifestations of Gitelman's Syndrome. 2. Learn to differentiate Bartter's Syndrome from Gitelman's Syndrome. 3. Recognize possible complications of severe electrolyte disturbances during late pregnancy.
CASE
A 26 year old G1P0A0 Hispanic female with childhood Bartter's syndrome presented in her 32nd week of gestation with severe premature uterine contractions and threatened preterm labor. Prior to her pregnancy she was chronically hypokalemic and mildly alkalotic, but was well controlled with potassium supplementation and spironolactone. Spironolactone was discontinued during pregnancy. On admission, she had increased uterine activity and contraction frequency with mild changes in fetal heart rate. She had minimal cervical dilatation and effacement. Urinary and vaginal infections and other triggers were excluded. Her serum electrolytes were: Na 137, K 2.6, Cl 104, Ca 9.8, magnesium 0.4, phosphorus 3.2. Her serum pH was 7.45, and creatinine 0.8 mg/dL. After rapid intravenous repletion of magnesium and potassium, her contractions ceased. She contined to require electrolyte repletion and was discharged four days later. Prior to her pregnancy, she had not been severely hypomagnesemic. Two weeks later she delivered a premature 34 week old infant who had no electrolyte disturbances. The patient now requires both oral potassium and magnesium to avoid other systemic manifestations. A diagnosis of Gitelman's syndrome was clinically confirmed.
DISCUSSION
Classic Bartter's syndrome is an autosomal recessive disorder characterized by sodium wasting, hypokalemic metabolic alkalosis, hyperreninemic hyperaldosteronism with normal or reduced blood pressure, urinary concentrating defect, and hypercalciuria. It usually presents in infancy or early childhood. Gitelman's syndrome is a variant of Bartter's syndrome that is distinguished primarily by hypocalciuria and hypomagnesemia. Patients with Gitelman's syndrome usually present later in life (after age 6) and have milder symptoms. The defect is due to inactivating mutations in the distal tubule sodium-chloride cotransporter. Hypomagnesemia due to renal wasting is universally found in patients with Gitelman's syndrome. Women of childbearing age with this disorder are at risk for preterm delivery secondary to severe hypomagnesemia and hypokalemia, well known triggers for premature uterine contractions. It is unclear whether pregnancy exacerbates electrolyte wasting. Treatment includes potassium and magnesium supplementation as well as spironolactone.
TB OR NOT TB? THAT IS THE QUESTION
H. Ly1; M. Rotblatt2. 1University of California, Los Angeles, Sylmar, CA; 2UCLA SFVP-Olive View Medical Center Department of Internal Medicine, Sylmar, CA. (Tracking ID #115714)
LEARNING OBJECTIVES
1. To recognize the presentation of Typhoid Fever. 2. To recognize the importance of this illness in undeveloped countries and returning travelers.
CASE
A 49 year old Hispanic male without significant medical history who returned from a 2 month field job in Mexico complaining of intermittent fevers and headache for 3 weeks. He also reported nightsweats for 1 week and a dry cough for 2 days. He had additionally lost 10 lbs from poor appetite over the past month. There were no GI complaints. He denied any sick contacts, TB exposure, unusual foods, smoking, or IV drug use. He did work near cattle, even witnessing the birth of a calf. His vital signs were T 38.9 C, BP 100/65, P 98, R 18, and O2 sat 98% on room air. He was diaphoretic and warm to touch on exam, but otherwise, his remaining physical examination was unremarkable. His WBC was 4.3 (N 83, L 14, M 3), Hb 12.1, Hct 35.8, and Plt 245. His chemistry panel was normal. His liver enzymes were: ALT 112, AST 97, Alk Phos 97, Tot bili 0.7. Chest X-ray was unremarkable. He was initially admitted to rule out TB and further evaluate this ill-defined illness. Considering his travel and exposure history, our differential included TB, community acquired pneumonia (CAP), malaria, hepatitis, brucellosis, and coccidiomycosis. Lymphoma was also considered. A battery of cultures and tests were sent. Meanwhile, he was isolated for TB and empirically treated with ceftriaxone for CAP. Over the next few days, he began to defervesce. Interestingly, he had a relative bradycardia for temperatures averaging over 39 C. His blood culture eventually grew Salmonella typhi, and his antibiotic was switched to levoquin. Within the next few days, he was discharged after full defervescence with marked improvement in symptoms and normalizing liver enzymes. His other tests were negative for TB, malaria, hepatitis, HIV, brucellosis, coccidiomycosis, and lymphoma.
DISCUSSION
Our case of Typhoid Fever exemplified the difficulty in a clinical diagnosis given its non-specific presentation. The symptomatology in our patient closely resembles the classic textbook description. Although our patient did not have the classic rose spot rash or hepatosplenomegaly, he did have relative bradycardia. Treatment of choice is a fluoroquinolone, but 3rd generation cephalosporins are also effective. While no longer common in developed countries, Typhoid fever is still prevalent in undeveloped countries where sanitation remains poor. Like TB, it is important to include in the differential diagnoses of unexplained fevers in travelers.
THE BLOOD CULTURE THAT ROTATED OFF SERVICE
S. Shaw1; M. Rotblatt1. 1UCLA/San Fernando Valley Program, Sylmar, CA. (Tracking ID #117005)
LEARNING OBJECTIVES
1) Recognize the prolonged diagnostic course for endocarditis due to HACEK organisms. 2) Recognize that discharging patients when all team members rotate off service can be hazardous. 3) Recognize the need for hospitals to have a back-up plan to follow-up positive blood cultures.
CASE
A 43 year old man with a history of DM presented to the ED complaining of left shoulder pain for two days after a fall. Vital signs were T 38.1, BP 102/42, RR 29, and HR 129. Physical exam was significant for rigors, right basilar rales, a 2/6 systolic murmur at the LUSB, 1+ pitting LEE, and generalized tenderness of the left shoulder. Labs were significant for WBC 18.6, Hb 6.2, Hct 17.9, Na 123, K 5.8, Cl 90, HCO3 21, Cr 1.1, glucose 503, and urinalysis with pH 5.0, 4 WBC and 5 RBC. The CXR demonstrated right hilar fullness. He was thought to have a UTI, a possible pneumonia, anemia of chronic disease based on previous workup, dehydration, possible type 4 RTA, and possible adrenal insufficiency…that is, until a TTE revealed a 2 × 2 cm vegetation on the right coronary cusp of the aortic valve with associated severe aortic insufficiency. Review of past laboratory results discovered a blood culture positive for Haemophilus aphrophilus taken two months earlier during hospitalization for new-onset diabetes, fever and elevated WBC. Repeat physical exam revealed embolic lesions on his toes and JVP 15 cm after hydration. Brain MRI showed evidence of septic emboli. He was diagnosed with infective endocarditis (IE) with embolic phenomena. He underwent valve replacement surgery and did well. On review of the previous admission, it was noted that the patient had been discharged at the end of June with blood cultures negative after 5 days; when the positive culture at 21 days was filed, the resident had graduated, the rotating intern had returned to his home hospital, and the attending had switched.
DISCUSSION
Prompt diagnosis of infective endocarditis (IE) is complicated by its myriad presentations. Of note, younger patients may present with heart failure with no prior cardiac disease; musculoskeletal complaints may be an early symptom in up to 40% of patients; and most patients with IE have an abnormal urinalysis. Furthermore, in 2-5% of patients with IE, no organism is isolated after three serial blood cultures. In cases with negative cultures, if there is high clinical suspicion, the HACEK organisms (H. aphrophilus, H. parainfluenzae, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella sp., and Kingella sp.) should be suspected. However, blood cultures may need to be incubated for prolonged periods. In academic centers with multiple rotating team members, there is often no straightforward follow-up for results that become available after all members are off service. In some centers, the Infectious Disease service takes responsibility. Each hospital should have a plan in place to follow up positive cultures.
THE CASE OF THE LITTLE OLD LADY WHO WASN'T QUITE SWEET ENOUGH
J. Beversdorf1; J.L. Sebastian1; D. Torre1. 1Medical College of Wisconsin, Milwaukee, WI. (Tracking ID #116777)
LEARNING OBJECTIVES
1) To recognize that insulinomas can present as a new diagnosis in elderly patients and 2) to recognize insulinoma as a cause of hypoglycemia even when sophisticated pancreatic imaging studies are normal.
CASE
An 82-year-old non-diabetic woman, a retired registered nurse, presented to the emergency room with symptoms of dizziness, cold sweats and visual changes. She was found to have a blood sugar of 42 mg/dl and administration of one amp of D50 promptly relieved her symptoms. The patient reported that she had experienced similar symptoms since 1998 and that her previous physician had prescribed treatment with prednisone to alleviate these spells. During the past year, her symptoms had increased in both frequency and severity. Upon admission to the hospital, the patient's vital signs and physical examination were essentially unremarkable. Initial laboratory studies revealed that the following tests were normal: urinalysis, blood urea nitrogen, serum creatinine and liver enzymes. Insulin and C-peptide levels were both elevated, glycated hemoglobin level was 5.5 and a sulfonylurea screen was negative. An abdominal MRI and endoscopic ultrasound to evaluate for the presence of an insulinoma did not reveal any pancreatic lesions. Early during the patient's hospital stay, she remained asymptomatic and her blood sugars ranged between 70 to 130 mg/dl. One morning, the patient suddenly experienced symptoms of sweating and palpitations. Her blood glucose dropped to 26 mg/dl and she became unresponsive. The patient promptly regained consciousness after receiving two amps of D50 and a continuous infusion of D10. Following this episode, the patient was scheduled for an exploratory laparotomy at which time a 1.6 cm lesion on the tail of the pancreas was found and removed. Histopathology revealed findings compatible with an insulin-secreting islet-cell tumor.
DISCUSSION
Severe hypoglycemia in the absence of diabetes, alcohol, exogenous administration of insulin or use of drugs which stimulate endogenous insulin secretion is thought to be quite uncommon. In the setting of spontaneous hypoglycemia, the diagnosis of insulinoma is highly suggested by the finding of elevated plasma insulin and C-peptide levels. Once an insulinoma is suspected, it is important to identify the tumor preoperatively as localization at the time of surgery may be quite difficult. Although the test characteristics of pancreatic imaging studies vary from institution to institution, the sensitivity of endoscopic ultrasound has been reported to be as high as 93%. This case emphasizes that clinical acumen and a high index of suspicion remain crucial to making an accurate diagnosis.
THE CASE OF THE STUBBORN SWOLLEN LEG
L.A. Blauwet1; A.K. Ghosh1. 1Mayo Clinic College of Medicine, Rochester, MN. (Tracking ID #115041)
LEARNING OBJECTIVES
1. Recognize May Thurner syndrome as an unusual etiology for deep venous thrombosis (DVT). 2. Discuss the approach to diagnosis and treatment of May Thurner syndrome.
CASE
A 71 year-old woman, status post repair of a left open tibiofibular fracture nine weeks previously, presented with a 2-day history of left lower extremity discoloration, pain and swelling. A duplex Doppler ultrasound revealed thrombus extending from the left external iliac vein proximally to the left femoral and popliteal veins distally. Physical examination revealed an extremely swollen and tender left leg that was dusky purple from the ankle to the groin. Pedal pulses were not palpable but were present using Doppler ultrasound. Unfractionated Heparin and Warfarin were initiated, and the patient was given narcotics for pain relief. Despite this, the patient's left leg showed no signs of improvement the following two days. Interventional radiology was then consulted. An inferior vena cavogram revealed findings suggestive of May-Thurner syndrome. Successful mechanical thrombectomy was performed and Urokinase was infused regionally. Follow-up venogram the next day revealed severe narrowing of the left common iliac vein and the left upper external iliac vein. Balloon dilation was performed and then a Wallstent was deployed in the external iliac vein. Symptoms quickly resolved. Plavix was given for one month, and then low dose aspirin was initiated. Warfarin was continued, with a target INR of 2.0–3.0. Hormone therapy was discontinued. Ongoing stent patency will be assessed by serial duplex Doppler sonography. Duration of anticoagulation treatment will be determined based upon sonographic findings.
DISCUSSION
May-Thurner syndrome (i.e., iliac compression syndrome) is the development of acute iliofemoral DVT by compression of the left common iliac vein against the spine and pelvic brim by the right common iliac artery. It occurs most commonly in 20–40 year-old women. Diagnostic ascending venography is essential to accurate diagnosis. Definitive treatment includes mechanical thrombectomy, catheter-directed thrombolytic therapy, and stent placement in the left common or external iliac vein. In our patient, additional risk factors for DVT included a 20-year history of hormone therapy and recent fracture. Poor resolution of leg swelling compelled us to look for an alternative etiology of DVT and seek thrombolytic therapy in this case.
THE CRIMSON LUNG: A CASE OF WEGENER's GRANULOMATOSIS
G. Agarwal1; R. Granieri1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115119)
LEARNING OBJECTIVES
1) Recognize the presentation of Wegener's Granulomatosis (WG) 2) Diagnose WG using clinical, laboratory, and histologic parameters 3) Recognize the treatment of WG
CASE
E.D. is a 23 y/o WM who was well until he noted diffuse myalgias and arthralgias two months prior to presentation. He subsequently developed discomfort and redness in both eyes and immeditaley prior to presentation, he noted a sore throat, rhinorrhea, and anorexia followed by dyspnea on exertion, fevers, and hemoptysis. He had no significant past medical history and was taking no medications. He was a ten pack year tobacco smoker and occasionally used cocaine and marijuana. Upon presentation, he was in respiratory distress and was intubated. His exam was notable for a temperature of 38.2(C), heart rate of 112, and respiratory rate of 22. He had diffuse coarse breath sounds but an otherwise unremarkable exam. His labs were notable for elevated creatinine, normochromic normocytic anemia, mild leukocytosis, markedly elevated sedimentation rate, and hematuria. A C-ANCA titer was 1:1280. The patient underwent a VATS procedure with biopsies revealing a granulomatous vasculitis consistent with Wegener's Granulomatosis (WG). He was begun on pulse IV steroids and cyclophosphamide, and subsequently tolerated extubation.
DISCUSSION
WG is a vasculitis of medium and small arteries that primarily involves the respiratory tracts and kidneys. Presenting symptoms include purulent/bloody nasal discharge, oral and/or nasal ulcers, polyarthralgias, and myalgias. Renal disease is common, being manifested by acute renal failure, hematuria, and proteinuria. The American College of Rheumatology proposed diagnostic criteria for WG (formulated prior to the availability of antineutrophil cytoplasmic antibody (ANCA) testing) which inlcude nasal/oral inflammation, abnormal CXR, abnormal urinary sediment, and granulomatous inflammation on biopsy of an artery. Two or more of these criteria yielded a sensitivity of 88% and a specificity of 92%. The diagnosis of WG is also suggested from circulating ANCA that are usually directed against proteinase 3 (C-ANCA). Nearly all patients with active WG have circulating ANCA. However, ANCA alone, including C-ANCA which is more specific for WG, does not appear to be sufficiently accurate to establish the diagnosis. The diagnosis is confirmed by tissue biopsy. Granulomatous inflammation and frank vasculitis are potential biopsy findings. Daily oral cyclophosphamide-corticosteroid therapy is the initial favored treatment. Once remission is induced, alternative regimens including methotrexate and azathioprine have been employed. Survival in untreated WG is poor, with up to 90% of patients dying within two years. Survival has significantly improved with the introduction of cyclophosphamide-corticosteroid therapy.
THE MAN WITH THE SWOLLEN LEG-A LESSON FROM A PRIMARY CARE HIV CLINIC IN UGANDA
A.E. Torreblanca1. 1University of California, San Francisco, San Francisco, CA. (Tracking ID #116547)
LEARNING OBJECTIVES
1) Recognize the causes of secondary lymphedema 2) Recognize Kaposi's sarcoma (KS) as a leading cause of cancer in sub-Saharan Africans 3) Recognize treatment limitations in sub-Saharan Africa.
CASE
A 42 year old male presented to the Reach Out Clinic in Kampala, Uganda complaining of swelling in his right leg. He first noted dark lesions on his feet 2 years prior to presentation. These lesions gradually spread to his groin and were associated with leg swelling. He did not know his HIV status but reported having 2 wives, one of whom died 2 years ago of tuberculosis. Examination of his right leg revealed non-pitting edema, numerous purple papules, and palpable inguinal nodes bilaterally. Serology was positive for HIV. He was sent to a public hospital where skin biopsy showed Kaposi's sarcoma. He was started on bleomycin and vincristine with minimal improvement. The clinic is currently trying to find a sponsor to pay for his antiretroviral therapy.
DISCUSSION
Non-pitting edema is generally due to lymphedema. Causes of secondary lymphedema includes lymph node trauma (surgery, radiation), malignancy (pelvic, KS), and infection (filariasis). KS is a common cause of lymphedema in sub-Saharan Africa. The four forms of KS are classic, endemic-African, organ transplant-associated and AIDS-related. In the era of AIDS there has been a 20-fold increase in the occurrence of KS in Uganda. Review of the cancer registry in Kampala from 1989–91 reported KS to be the leading cancer in males (48.6%) and the second most frequent (17.9%) in females. Treatment options for KS include local therapy (radiation, intralesional chemotherapy), and systemic chemotherapy (liposomal anthracyclines, bleomycin and vincristine). Although chemotherapy has proven effective in endemic-African KS, its effect on AIDS-related KS is limited. The widespread use of antiretrovirals (ARVs) in the western world has lead to a marked decline in new AIDS-related KS. In Uganda ARVs are only available to patients who can pay for the drugs or qualify for clinical trials. Patients with advanced KS are currently ineligible for the clinical trials underway in Uganda. The typical ARV regimen is Triommune (a generic combination of d4T/3TC/NVP). This regimen costs ∼$26 per month; the typical Ugandan lives on less than $30 a month.
Lymphedema secondary to Kaposi's sarcoma.
THE MARDI GRAS HEART SYNDROME
L. Quan1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117502)
LEARNING OBJECTIVES
1. To recognize the clinical presentation of atrial fibrillation in the holiday heart syndrome. 2. To emphasize the treatment of atrial fibrillation. 3. To identify the indications and contraindications for anticoagulation in atrial fibrillation.
CASE
A 29 year-old man presented with palpitations. He denied chest pain, dyspnea, or any other associated symptoms. During his vacation to New Orleans, he went on a binge of over twenty cans of beer. His cardiac enzymes were negative and his TSH was normal. An EKG showed atrial fibrillation with PVC's and a heart rate of 112. The heart rate was controlled with diltiazem. He was ruled out for cardiac thrombosis with a TEE and subsequently successfully cardioverted to a normal rhythm. Because he refused coumadin, he was discharged on aspirin.
DISCUSSION
Moderate alcohol consumtion (7 to 11 drinks per week) is associated with decreased cardiovascular mortality. Consuming all eleven drinks on the same occasion, however, may lead to an alcohol hangover associated with increased adrenergic tone, myocardial work, and cardiovascular morbidity. Atrial fibrillation is the most common arrhythmia associated with the hangover period, found in up to 60% of binge drinkers with or without underlying alcoholic cardiomyopathy. Treatment consists of rate control with calcium channel blockers, beta blockers, or digoxin. Recurrent or persistent atrial fibrillation may necessitate anti-arrythmic medications or cardioversion, and long-term anticoagulation. In addition to arrhythmias, the alcohol hangover is associated with an increased c-reactive peptide and thromboxane B2, both markers of systemic inflammation. This inflammation, in concert with increased myocardial work, may explain the two-fold increased risk of myocardial infarction in patients who are frequently hungover. Physicians should advise their patients that there are unique harms associated with the alcohol hangover.
THE CASE OF THE FEVERISH FRENZY
C. Weaver1; J. Hefner1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #116221)
LEARNING OBJECTIVES
1. Recognize a common presentation of Familial Mediterranean Fever (FMF) in an uncommon patient; 2. Diagnose, manage and treat FMF; 3. Recognize validation of a patient's symptoms is often of therapeutic significance.
CASE
A previously healthy 28 year-old female of Italian and Anglo-Saxon descent, presented to the outpatient setting complaining of intermittent fevers (37.8 to 39.5) and abdominal pain over 3 years. The patient frequently presented to the ED and was hospitalized 5 times. Emergent cholecystectomy and appendectomy were performed, with normal pathology. Six CT scans, one MRI, an angiogram, cystoscopy, CBC's, LFT's, blood and urine cultures, viral titers, lipase, porphyria evaluation, heavy metals, ANA, ds-DNA, complement levels and lipids were non-diagnostic. The only abnormalities were microscopic hematuria and beta-thalessemia minor. There was no pattern to the attacks and no exacerbating features. She had failed over-the-counter therapies and was taking no medications. She was well between attacks initially but became depressed over the past year. The frequency and severity of attacks and absence at work forced her to leave her job. She had been referred to psychiatric services on numerous occasions. Physical exam was unremarkable. Based on history, colchicine was started and she was instructed to obtain tests only during an attack. Several months later, after a tremendous response to colchicine, she had elevated LFT's, ESR, CRP and a leukocytosis upon presentation with abdominal pain and fever to 39.1.
DISCUSSION
FMF is an autosomal recessive condition manifesting in paroxysms of fever and severe abdominal pain. Primarily seen in persons of Mediterranean descent, it is also found in other groups, including Anglo-Saxons. Ten percent of patients present after the age of 20. The differential diagnosis includes acute surgical abdomen, acute intermittent porphyria, vasculitides/SLE, and relapsing pancreatitis/hypertriglyceridemia. Colchicine is the mainstay of treatment. A study published in 1991 determined that 72% of patients on colchicine averaged 1 attack in 6 months. Colchicine decreases the occurrence of amyloidosis, a serious consequence. FMF is primarily a clinical diagnosis; a reasonable evaluation includes a trial of colchicine and measurement of ESR, haptoglobin, CRP, and fibrinogen during an attack. FMF can be the cause of numerous ER visits, extensive laboratory testing and tremendous frustration for both patient and caregiver. High clinical suspicion is necessary and a PCP should handle management for optimal coordination of care. Validation of a patient's symptoms and distress can be of therapeutic significance and provide comfort in the setting of chronic disease.
THE SHOCKING CONSEQUENCE OF DISCONTINUING PHENYTOIN
B. Lee1; M. Rotblatt2. 1UCLA-SFVP, Sylmar, CA; 2UCLA/San Fernando Valley Program, Sylmar, CA. (Tracking ID #115654)
LEARNING OBJECTIVES
1) To recognize antiarrhythmic properties of phenytoin. 2) To review treatment of recurrent ventricular tachycardia (VT).
CASE
A 68 year-old male presented to cardiology clinic with a complaint of multiple ICD firings. He had a single-chamber ICD placed 9 years previously for sustained VT which had been well controlled on chronic sotalol therapy with no ICD firings for the past 4 years. The patient denied chest pain, lightheadedness, or shortness of breath prior to firings. On further questioning, the patient had been taking phenytoin for seizure prophylaxis for an intracranial hemorrhage 4 years prior to admission, and had just been tapered off 3 weeks prior. Despite an increase in his dose of sotalol from 80 mg QD to 120 mg BID, the patient continued to experience firings of his ICD. He was subsequently restarted on phenytoin with complete resolution of ICD firings.
DISCUSSION
Sustained VT is defined as VT that persists for greater than 30 seconds or requires termination because of hemodynamic collapse. VT generally accompanies structural heart disease such as coronary artery disease, cardiomyopathies, right ventricular dysplasia, valvular heart disease, and heart failure. VT may also occur in the absence of structural heart disease as seen with metabolic disorders, Brugada syndrome, prolonged QT syndrome, or idiopathic VT. The prevention of recurrent VT includes drug therapy (with or without selection through programmed stimulation) as well as devices combining antitachycardia pacing with ICD. In our patient, sotalol was started concomitantly with ICD placement in 1994 and phenytoin was started several years later for seizure prophylaxis. Though our patient had multiple risk factors for recurrent VT including a history of CAD s/p 4 vessel CABG, critical stenosis of his mechanical aortic valve, and heart failure (EF: 25%), the discontinuation of phenytoin appears to have caused the recurrent VT. Phenytoin is used mainly in the prophylactic management of tonic-clonic seizures and partial seizures with complex symptomatology. Moreover, phenytoin may be used for the prevention and treatment of seizures occurring during neurosurgery and in the treatment of status epilepticus. However, phenytoin is also a Class 1b antiarrhythmic, and an unlabelled use is in the treatment and maintenance of VT and paroxysmal atrial tachycardia, particularly in those patients who do not respond to conventional antiarrhythmic agents or cardioversion. Although phenytoin was started for seizure prophylaxis in our patient, it served a dual purpose as an antiarrhythmic as well. The VT was not well controlled despite increasing the sotalol dosage, and phenytoin was ultimately restarted with the desired effect.
THE UNFITTING PROSTHESIS
T. Tanabe1. 1University of Pennsylvania, Philadelphia, PA. (Tracking ID #117100)
LEARNING OBJECTIVES
1) Recognize the importance of carfeful stump examination in diabetic patients. 2) Suspect limb- or life-threatening infections early and facilitate the diagnostic workup in diabetic patients.
CASE
A 63-year-old man presented with a pain at the left stump for three days. Past medical history included chronic obstructive pulmonary disease, systolic dysfunction and diabetes. The patient underwent below knee amputation of left leg in 1998. He noticed a dull pain 3 days prior to presentation when he was walking on prosthesis, which he had used for 4 years. He denied any trauma, fall or manipulation to the stump. His finger stick was under control until the pain started, and remained above 300. On physical exam he was afebrile, not in acute distress with blood pressure of 128/64, heart rate 74, respiratory rate 16, 97% saturation on room air. The stump had a narrow ulcer of 2 cm in length without discharge. There was no erythema or fluctuation around the lesion. Cleansing and sterile gauzes were applied. He was instructed not to wear the prosthesis until further notice and to return to clinic in three days. Laboratory findings at the first visit were within normal limits. On a subsequent visit, the patient presented with a more severe pain and reported foul odor from the stump. The exam showed a 2 cm sinus-tract formation extending medially to the left knee and the gauze was blood-tinged. The odor was distinctive immideately after removal of the gauze, which he changed with normal saline wet-to-dry dressing daily. The patient was prescribed oral amoxicillin/clavulanic acid and evaluated by the surgery that day, who performed debridement. After 10 days into treatment, he was evaluated again, when he reported no foul odor but the same severe pain at the stump. Bone scan was obtained and the result came back positive for osteomyelitis in the remaining tibia extending to the proximal femor. The patient was admitted to the surgery service for above knee amputation.
DISCUSSION
The regular stump examination is not recommeded as opposed to foot examination in diabetic patients. Peripheral neuropathy is present in over 80% of diabetic patients with foot lesions and the prevalence of diabetic foot ulcers has been estimated to be 3–8%. However, the prevalence of ulcer formation after amputations is not known. After diabetic patients undergo amputation, their risk of requiring a second amputation increases dramatically. Fever, chills and leukocytosis are absent in two thirds of patients with limb-threatening infection. Hyperglycemia is a common sign of limb- or life-threatening infection. It is imperative that clinicians maintain a high index of suspicion for serious infection when evaluating diabetic patients with sudden onset of uncontrolled hyperglycemia. Clinicians should also pay a close attention to the fitting of prosthesis and examine a stump regularly.
THIS RASH HURTS!
J. Willis1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117522)
LEARNING OBJECTIVES
1. Identify the clinical presentation of herpes zoster. 2. Recognize the presenting symptoms that require intravenous therapy for herpes zoster.
CASE
A 26-year-old man presented with two days of a left-sided chest pain. The pain was constant, sharp and radiated to the left arm. He was admitted to the hospital for evaluation of acute coronary syndrome. His EKG's remained normal, as did serial troponin I's. The intense pain in the left lower chest continued with radiation now to the back. Two days following admission he noted an onset of the rash that he attributed to a recent contact with shrubbery. Over the next day, it progressed to a weeping, painful, pruritic rash along the left side of the lower chest. He noted associated fever and chills. His examination was normal with the exception of a vesicular rash along the T8 to T10 dermatome. There was exquisite tenderness to light touch along this same area. There were no ocular abnormalities and his lung examination was normal. His white cell count was 3,100 with 54% neutrophils; no bands. Remaining laboratory studies were normal.
DISCUSSION
Herpes zoster is a vesicular reactivation rash from prior exposure to the varicella virus. It frequently presents with a painful prodrome, with a subsequent vesicular rash that follows within the next forty-eight hours. Knowledge of the prodrome is important as other causes of chest pain can be falsely diagnosed. Excluding involvement of the ophthalmic branch of cranial nerve V is also important, as intravenous acyclovir is required to prevent corneal involvement and scarring. Hutchinson's sign is the appearance of vesicles on the tip of the nose, suggesting cranial nerve V involvement. The rash with herpes zoster is progressive, as if it is crawling along this skin (herpe- (L); to crawl). The dermatomal pattern is diagnostic and distinguishes zoster from other vesicular diseases (zoster- (G); girdle-like). The syndrome is also known as shingles, from the Latin, cingella; meaning girdle-like.
TOO YOUNG FOR AORTIC DISSECTION?
J.L. Oyler1. 1University of Chicago, Chicago, IL. (Tracking ID #115741)
LEARNING OBJECTIVES
1. Recognize and treat aortic dissection in atypical patients. 2. Diagnose bicuspid aorta and manage complications like aortic dilation/dissection.
CASE
A 30 year old graduate student presents to Student Care with chest pain. One day prior to admission he experienced chest pain while on the exercise bicycle at the gym. Chest pain was sharp, substernal, non-radiating, 7/10, lasting seconds, pleuritic and associated with SOB. He had 2 episodes of syncope lasting seconds, precipitated by N/V. He denied diaphoresis, F/C/S, or viral symptoms. On physical exam he was afebrile, blood pressure in each arm was 105/80, pulse 96, RA sat 98%. He was a pale appearing male in mild distress due to pain. Cardiovascular exam revealed nl S1,S2 no M/G/R, lungs were clear, abdomen was benign, pulses were equal. Initial labs revealed normal CBC, BMP, LFT's, CK, MB, troponin, urine toxicology, RPR, ANCA, ANA and ESR. Initial EKG showed diffuse ST segment elevation, PR elevation in AVR. CXR showed widened mediastinum. CT scan showed pericardial effusion, anuerysmal dilation of the aortic root. Final diagnosis was made by TEE revealing bicuspid aortic valve, aortic dissection 3 cm above aortic root. He was taken to surgery, but had crushing substernal chest pain in the pre-op area and died of pericardial tamponade.
DISCUSSION
Aortic dissection is an unlikely diagnosis in a young normotensive male presenting with chest pain. In young patients predisposing factors for aortic dissection include: vasculitis, collagen vascular disease, bicsupid aortic valve, aortic coarctation, crack cocaine, and trauma. Although most aortic dissections present as chest pain, when associated with syncope most patients have Daily type A dissections involving the ascending aorta and increased incidence of tamponade and worse outcomes. CT, MRI, and TEE are all recognized as effective imaging techniques for aortic dissection. Treatment of aortic dissection includes blood pressure control and surgery to excise the intimal tear. Bicuspid aortic valve (BAV) is the most common congenital cardiac malformation. Males are affected 4:1. BAV is primarily diagnosed by echocardiography after presence of aortic ejection click +/− systolic ejection murmur is detected. Serious complications of valvular stenosis, regurgitation, infective endocarditis, and aortic dilation and dissection occur in >33% of patients with BAV. Accelerated degeneration of the aortic media, not valvular dysfunction, causes the vascular complications of BAV. Even when BAV is replaced by prosthesis, abnormalities in aortic media can cause aortic dilation. Antibiotic prophylaxis and blood pressure control are mainstays of therapy. Patients with BAV should be monitored by echocardiography at regular intervals. Once AI/AS, dilated aorta >4cm, increased LV size or decreased LV function occurs BAV patients should undergo surgery.
TRANSIENT LEFT VENTRICULAR APICAL BALLOONING: A NOVEL HEART SYNDROME
H.L. Korlakunta1; S.K. Thambidorai1; S. Denney1; I. Khan1. 1Creighton University, Omaha, NE. (Tracking ID #115715)
LEARNING OBJECTIVES
To report a case of transient left ventricular apical ballooning without coronary artery stenosis, which mimics acute myocardial infarction with electrocardiographic changes and elevation of cardiac enzymes disproportionate to the extent of akinesia of left ventricle.
CASE
A 43-year-old white female with past medical history of hypertension and hyperlipidemia and family history of coronary artery disease presented with typical angina which occurred while she was giving a briefing to a large group. Her medications included hydrochlorothiazide and simvastatin. She was treated with sublingual nitroglycerin, morphine, and a beta-blocker with resolution of symptoms. Physical examination was unremarkable. Electrocardiogram showed minor non specific ST-T wasve changes and was negative for myocardial ischemia and injury. Laboratory workup revealed elevated serum cardiac troponin I (peak 2.03 ng/ml). Serum electrolytes were within normal limits. Coronary angiogram was performed which showed normal coronary arteries; severe distal anterior, apical, and distal basal hypokinesis; and ejection fraction of 25%. Echocardiogram reveled low ejection fraction, hypokinesis of apical segments of anterior, inferior, and lateral walls and distal segment of intraventricular septum. Repeat echocardiogram 3 days later showed improvement in regional wall motion abnormalities and ejection fraction rose to 45%. Subsequent electrocardiograms showed diffuse T-wave inversion with prolongation of QTc interval to 500 msec, which slowly reverted toward normal. The QTc interval before discharge was to 452 msec. The patient was discharged on a beta-blocker agent and an angiotensin-converting enzyme inhibitor and follow-up echocardiogram in 4 weeks was recommended.
DISCUSSION
A novel cardiac syndrome of left ventricular apical ballooning was recently described, which involves an acute onset of reversible left ventricular apical wall motion abnormalities with chest symptoms, electrocardiographic changes and minimal elevation of cardiac enzymes mimicking acute myocardial infarction. Patients have no angiographic evidence of coronary artery stenosis. There is complete recovery of left ventricular function in weeks. The precise etiologic basis of this syndrome is yet to be determined. Previous studies have indicated that triggering factors such as emotional exposure and physical stress may play a role in the pathophysiologic basis of this condition. This syndrome might be one of the clinical models of stress-related sudden death. Awareness of this syndrome is important because it mimics acute myocardial infarction and may inadvertently expose patients to futile administration of thrombolytic agents. In addition, since recurrence seems possible, there is need for prompt recognition and optimal treatment of this novel heart syndrome.
TRANSVERSE MYELITIS SECONDARY TO HERPES ZOSTER IN AN IMMUNOCOMPROMISED PATIENT
A. Halat1; J.T. Bates1. 1Baylor College of Medicine, Houston, TX. (Tracking ID #116903)
LEARNING OBJECTIVES
1. Recognize the clinical presentation of transverse myelitis. 2. Review the appropriate evaluation and treatment of transverse myelitis.
CASE
A 70 year-old man with myasthenia gravis, status post thymectomy and now treated with azathioprine and prednisone, presented with progressive leg weakness, bowel and bladder incontinence, and skin lesions. He described a two week history of burning pain down the back of his legs. Examination revealed vesicles on the roof of his mouth and on his lower extremities. He had decreased strength and sensation in his bilateral lower extremities, absent reflexes at the knees and ankles bilaterally, and a positive Babinski sign on the right. Neither magnetic resonance (MR) nor computerized tomography (CT) imaging could be obtained. A lumbar puncture was performed, and the CSF revealed a negative gram stain, 112 white blood cells with 56% lymphocytes, an elevated protein of 120, and a glucose 48% of the serum value. Given the strong suspicion of a viral etiology, CSF was sent for herpes simplex (HSV) and varicella zoster (VZV) analysis by polymerase chain reaction (PCR). PCR demonstrated the presence of varicella zoster. Given this finding and the patient's clinical picture, it was felt that the patient had a transverse myelitis secondary to varicella zoster. The combination of the patient's myasthenia gravis and his immunosuppressive regimen was felt to have predisposed him to this infection.
DISCUSSION
Transverse myelitis involves progressive limb weakness with loss of tendon reflexes and a sensory level that is typically sudden in onset. While infection can cause transverse myelitis, it is not the only cause. Imaging with either MR or CT should first exclude myelopathy from a structural cause, such as a herniated disk, vertebral fracture, or malignancy. In the absence of structural causes, transverse myelitis can result from multiple sclerosis, systemic diseases such as Sjogren's syndrome and systemic lupus erythematous, post radiation changes, infarction of the spinal cord, and infection. In the absence of structural lesions, lumbar puncture should be performed to assess the degree of inflammation, document any infection, and to ascertain the presence of oligoclonal bands to assess for multiple sclerosis. Treatment depends on the underlying etiology, but if VZV or HSV is suspected, then empiric treatment with acyclovir should be started immediately while definitive PCR testing is pending. Unfortunately, in cases of transverse myelitis secondary to VZV the response to treatment is limited, and most patients do not recover full neurological function.
TROPICAL SPLENOMEGALY
S.C. Reddymasu1; S. Alla1; S. Schlanger1. 1Creighton University, Omaha, NE. (Tracking ID #116488)
LEARNING OBJECTIVES
1. Describe the common causes of massive splenomegaly in the sub-Saharan African immigrant population 2. Outline a cost-effective clinical approach to massive splenomegaly. 3. Discuss the treatment of tropical splenomegaly
CASE
A 35-year-old Sudanese woman who immigrated to the US 6 months ago reported abdominal discomfort, fatigue, and low-grade intermittent fever, particularly at night. She had no dyspepsia, hematemesis, melena, dysuria, or hematuria. She reported malaria episodes, one requiring hospitalization in 1992, and a subsequent episode 7 months ago. She denied kala azar, schistosomiasis, inborn errors of metabolism, and malignancy. Family history for leukemias and lymphomas were negative. Examination was remarkable only for massive splenomegaly. The platelets were 95,000. The hemoglobin was 12.8 gm/dl and she had 6,000 white cells with 8% eosinophils and 18% lymphocytes. Peripheral smear was negative. Hepatitis B core antigen was present. Urinalysis and urine -human chorionic gonadotropin were negative. Chest film was negative but computed tomographic scan of the abdomen was remarkable only for splenomegaly measuring 16 cm. Urine and stool examination for ova and parasites was negative. Total serum IgM was normal. Treatment began with chloroquine for presumed tropical splenomegaly.
DISCUSSION
This 35-year-old Sudanese immigrant with recurrent malaria presented with massive splenomegaly. Given her age and geographic background as well as the absence of evidence of hematologic dyscrasias and malignancy, and inborn errors of metabolism, we considered infectious etiologies. Schistosomiasis and kala azar were unlikely based on her clinical picture. Her presentation was consistent with chronic malaria syndrome—caused by low-grade antigenemia—or tropical splenomegaly—or hyperreactive malaria syndrome (HMS), thought to be caused by hyperfunctioning B-lymphocytes in response to malaria antigen present in blood. There is no clear-cut difference in diagnostic criteria or treatment between these entities. In malaria-endemic regions, they are the leading cause of splenomegaly (followed by lymphoma) when other entities cannot be demonstrated. Common tests done for HMS are serum IgM and antimalarial antibodies, but they are insensitive, nonspecific, and costly. Malaria parasite cannot be found on peripheral smear in HMS, though peripheral smear done every 8 hours over 3 days can show malaria parasite in chronic malaria; this is expensive and impractical. Consequently, a trial of weekly chloroquine for 6 months, checking for splenic regression (40%)—which is neither costly nor toxic, is advocated for massive splenomegaly in malaria-endemic areas with no obvious cause. Persistence of splenomegaly justifies evaluation for splenic lymphoma as well as assay for anti-leishmanial antibodies. Tropical splenomegaly should be treated given its association with splenic lymphoma and risk of hypersplenism.
TUBERCULOSIS LYMPHADENITIS: A PERSISTENT PAIN IN THE NECK
M. Lee1; A. Kosmin1; B. Taqui1. 1Temple University, Philadelphia, PA. (Tracking ID #116178)
LEARNING OBJECTIVES
1. Recognize that tuberculosis (TB) lymphadenitis is still very common worldwide. 2. Recognize diagnostic and treatment strategies for TB lymphadenitis.
CASE
A 32 year old West African female with AIDS (CD4 334) and previously treated latent TB presented with four month history of progressive weakness and intermittent fevers/chills. She also complained of headaches, anorexia and weight loss, night sweats, nonproductive cough, nausea and post-tussive vomiting. One year prior, she had visited West Africa for several months. On exam, she had rectal temperature 103.5 and pulse 111. She had a 5 × 5 cm nontender mass at the anterior superior aspect of her right neck. She had 3/6 systolic murmur at the left apex. Lab data revealed WBC 5.4, Hgb 10.1, platelets 148. CXR, CT head and lumbar puncture were normal. Urine and blood cultures for bacteria, fungi and mycobacteria, serologies for Bartonella hensalae, and malarial smears were all negative. Echocardiogram and CT abdomen/pelvis were normal. Fine needle aspiration of the right neck mass revealed lymphoid cells (some atypical), epitheloid cells, RBCs and necrosis. Smears were negative for organisms, fungal elements and acid fast bacilli. After much debate, the patient was discharged on a four drug regimen for a presumptive diagnosis of TB lymphadenitis. Five weeks later, she was readmitted for enlarging painful neck mass. Surgical exploration revealed an underlying cervical abscess and lymph nodes that were positive for acid fast bacilli staining. Pathology showed granulomatous lymphadenitis with areas of necrosis. Cultures grew pan sensitive M. tuberculosis.
DISCUSSION
Tuberculous lymphadenitis remains a common cause of extrapulmonary TB worldwide. In developing nations, it causes 43% of peripheral lymphadenopathy. In the United States, 5.4% of TB is extrapulmonary, and 31% of these cases are lymphatic. Clinical presentation depends on the site of nodal involvement and the immune status of the patient. Immunocompetent patients present with an isolated chronic, nontender lymphadenopathy. Immunocompromised patients present with systemic symptoms and disseminated disease. The diagnosis of TB lymphadenitis is suggested histologically with necrotizing or caseating granulomata and confirmed with culture data from a lymph node biopsy. Fine needle aspiration (FNA) is safe and inexpensive but has sensitivity of 60–70% due to sample error. Excisional biopsy, the gold standard, is required if FNA is non-diagnostic. There is no consensus as to whether surgical excision is sufficient to treat TB lymphadenitis. Therefore, all patients are treated with a multidrug regimen, initiated prior to pathologic confirmation. Treatment regimen and duration are similar to that of pulmonary TB. Relapse rates of up to 3.5% have been reported.
WHY CAN'T MY PATIENT HEAR ME?
J.M. Weiss1; J.M. Sosman1. 1University of Wisconsin Medical School, Madison, WI. (Tracking ID #117050)
LEARNING OBJECTIVES
1. Identify extraintestinal manifestations of Ulcerative Colitis (UC). 2. Recognize that there is a well-documented association between sensorineural hearing loss and UC.
CASE
A 57 year-old man presented to his local MD with back pain. He was treated with Valdecoxib, but subsequently developed oral ulcers thought to be secondary to this medication. Two months later, he was found to have elevated liver function tests during a life insurance evaluation. Initial workup with viral hepatitis serologies was negative. Over the next three months, he developed a recurrent throbbing headache, jaw pain, and vertigo with nausea and vomiting. He was admitted to his local hospital. His workup included a normal head MRI/MRA but identified a microcytic anemia (Hct 35.5, MCV 80) with guaiac positive stools, a WBC 11.1 K, a Plt 440 K, an ESR of 116, and a CRP of 5.3. His temporal artery biopsy was negative. He began to complain of left sided earache and hearing loss and was transferred to our facility for evaluation. He was diagnosed with an acute idiopathic sensorineural hearing loss. He also complained of eye “floaters” and was found to have anterior iritis/uveitis. A liver biopsy to evaluate his abnormal LFTs (ALKPhos 523, GGT 1231, AST 23, ALT 37) revealed a possible small duct sclerosing cholangitis. Finally, his colonoscopy revealed quiescent colitis in the rectum, chronic inflammation in the left colon, and evidence of previous ulceration in the right colon. Although our patient never had problems with diarrhea—he was diagnosed with UC based on the above constellation of extraintestinal manifestations.
DISCUSSION
Ulcerative Colitis is an inflammatory bowel disease (IBD) involving the mucosal layer of the colon. UC is typically characterized by recurrent episodes of crampy abdominal pain and diarrhea (often bloody), however, patients may present in a variable manner. Extraintestinal manifestations are common and can occur in up to 25% of patients with UC or Crohn's IBD. These manifestations include reactive arthropathy (up to 20%), axial arthropathy, uveitis and episcleritis, skin lesions (erythema nodosum or pyoderma gangrenosum), and primary sclerosing cholangitis (2-5%). Of these, uveitis, axial arthropathy, and primary sclerosing cholangitis can occur at any time without active colitis. Sensorineural hearing loss has a well-documented association with UC and the relationship is speculated to be of autoimmune etiology (the prevalence of autoimmune disorders occur in up to 10% of UC patients). Once recognized, immediate treatment with steroids with or without immunosuppressive therapy is essential to prevent irreversible hearing loss. Our patient was started on high-dose oral steroids, but unfortunately continues to have significant hearing loss, tinnitus, and disequilibrium.
UNUSUAL NEUROLOGIC COMPLAINTS: CONSIDER MULTIPLE SCLEROSIS
N. Lischner1. 1University of California, San Francisco, San Francisco, CA. (Tracking ID #116995)
LEARNING OBJECTIVES
1) Recognize signs and symptoms of multiple sclerosis. 2) Diagnose multiple sclerosis using history, physical exam and central nervous system (CNS) imaging.
CASE
A 34 year old black male presented to his PCP with a six month history of progressively worsening diplopia, clumsy gait, and episodes of extreme fatigue. A few weeks prior to presentation, he also developed slurred speech. All symptoms seemed exacerbated by hot baths and physical exertion. He denied pain, paresthesias, dysesthesias, bowel or bladder changes, weakness, and vision loss. Remainder of ROS was negative. Physical exam was notable for slightly slurred speech, normal visual acuity, left eye adductor weakness, and bilateral hyperreflexic (3+) DTRs in the biceps, triceps, patella, and Achille's tendon without clonus. Exam also revealed a subtle gait disturbance, bilateral (L > R) dysmetria on finger-to-nose testing, and bilateral deficits on heel-to-shin testing. The remainder of his exam was normal, including negative Lhermitte's and Romberg signs. Brain MRI revealed extensive T2-weighted hyperintense lesions throughout the corpus callosum, periventricular white matter, deep and subcortical white matter, as well as left pons and middle cerebellar peduncle, consistent with a primary demyelinating disease such as multiple sclerosis. No gadolinium enhancement to suggest an acute demyelinating process was noted.
DISCUSSION
Multiple sclerosis (MS) is a chronic neurologic disease of autoimmune axonal demyelination. Symptoms include fatigue, bowel, bladder, or sexual dysfunction, motor weakness or spasticity, paresthesias, dysesthesias, ataxia, dysarthria, diplopia, vision loss, gait disturbance, balance problems, vertigo, and pain. Uhtoff's phenomenon, which is exacerbation of symptoms when the ambient body temperature is raised, is reported by some patients. Signs include optic neuritis, ataxia, dysarthria, dysmetria, internuclear ophthalmoplegia, clonus, dystonia, hyperreflexia, motor and sensory deficits. Lhermitte's sign, which is the sensation of electric shock in the extremities when the neck is flexed, can sometimes be elicited. Diagnosis of MS requires the presence of CNS lesions separated in time and site. Imaging the CNS can rule out alternate etiologies, such as infection or neoplasm. Brain or spine MRI is the usual modality. If MRI shows lesions consistent with demyelination, it can support the clinical diagnosis of MS. T2-weighted hyperdensities are typically found in the periventricular white matter, corpus callosum, centrum semiovale, and less commonly in the deep white matter structures and basal ganglia. Lesions are hypointense or not seen at all on T1-weighted imaging. Gadolinium enhancement of lesions indicates active inflammation; enhancement usually remains for 4–8 weeks after lesions become active.
URINARY URGENCY AS THE PREDOMINANT SYMPTOM OF NEURO. TB
S.G. Driscoll1; D.T. Fisk1; M. Schapira1. 1Medical College of Wisconsin, Milwaukee, WI. (Tracking ID #115957)
LEARNING OBJECTIVES
1. Recognize urinary urgency and intermittent back pain as indicators for CNS evaluation in a patient with disseminated TB. 2. Diagnose neuro TB utilizing the most appropriate radiological testing.
CASE
A 23 year-old, HIV negative man presented to the ER complaining of back pain and neck mass. The pain and neck mass began one month prior and were accompanied by malaise, drenching night sweats and an 8-pound weight loss. History was notable for immigration from Mexico two years prior. After admission, fluid aspiration from the neck mass grew pan-sensitive mycobacterium tuberculosis. Chest radiograph demonstrated a subtle right middle lobe infiltrate; chest CT showed a cavitary lesion within the area of the infiltrate. CT did not reveal any spine or bone pathology. The patient was initiated on isoniazid, rifampin, pyrazinamide, pyridoxine, and ethambutol. For the next six weeks, clinic visits documented complaints of urinary urgency that had predated anti-tubercular medication initiation. Post-void residual was 30 cc; prostate exam and multiple neurological exams were normal. Thoracic CT with contrast again detected no spine or cord pathology. MRI with gadolinium, however, noted enhancing basal cistern meningies, cortical lesions suggestive of tuberculomas, and evidence of epidural phlegmon and abscesses along the entire length of the thoracic cord to L1, deforming and displacing the cord, highly suggestive of neuro-TB. Steroids were initiated, isoniazid was doubled from 300 to 600 mg daily, ethambutol was discontinued, and his symptoms resolved within 2 weeks of therapy. At no time did the patient have altered mental status, seizures, or focal weakness.
DISCUSSION
This case demonstrates the importance of maintaining a high level of suspicion for neuro-TB in a patient with known or suspected TB. Suspicion for CNS involvement should be prompted not only by classically described neuro-TB symptoms of meningismis, mental status changes and focal neurological deficits, but also by mild symptoms such as urinary urgency and back pain. Urinary urgency has not been described as a presenting symptom of neuro-TB, though urinary retention is a recognized complication of spinal cord TB and meningeal TB has been implicated in diabetes insipidus development. These symptoms should be recognized as indicators of potential CNS pathology and appropriate diagnostic maneuvers pursued. This case was also remarkable in that MRI demonstrated extensive disease in the face of very mild symptomatology, confirming the great sensitivity of MRI for detecting neuro-TB. Given the growth of immunocompromised and immigrant populations in the United States, and the global ascent of multi-drug resistant tuberculosis, detection and treatment of TB will be an increasingly important aspect of health care in the future.
VENTRICULAR SEPTAL RUPTURE IN A PATIENT WITH COCAINE ABUSE
K. Dyehouse1; V.T. Martin1. 1University of Cincinnati, Cincinnati, OH. (Tracking ID #115676)
LEARNING OBJECTIVES
Recognize the clinic manifestations, diagnostic workup and management of ventricular septal rupture.
CASE
A 51-year-old male, with untreated diabetes and hypertension presented with dyspnea on exertion and lower extremity edema of two weeks duration. He also complained of PND and orthopnea. There was no history of chest discomfort, diaphoresis, nausea, vomiting, fever, chills or sweats. Physical exam revealed tachycardia, with a IV/VI systolic murmur best heard at left lower sternal border with radiation to the right sternal border, jugular venous distention, bilateral crackles to mid-lung fields, and pitting edema to the mid-thighs. Laboratory data included negative cardiac enzymes and troponin-T. The urine was negative for protein but positive for cocaine. Chest x-ray revealed cardiomegaly and pulmonary edema. His electrocardiogram demonstrated sinus tachycardia with right axis deviation, right bundle branch block and right ventricular hypertrophy. An echocardiogram showed a large pericardial effusion, a ventral septal defect and an apical ventricular aneurysm. Right heart catheterization revealed elevated pressures (81/13 mmHg) and an oxygen saturation step-up from 53% to 71% in the right ventricle. Coronary angiography revealed a total occlusion of the first marginal and right coronary arteries. These findings were presumed to be a result of a myocardial infarction possibly precipitated by recent cocaine abuse. The patient underwent a patch closure of his ventral septal defect and is doing well in follow-up.
DISCUSSION
Acute coronary syndrome is the most common cardiac pathology associated with cocaine abuse. Cocaine is attributable to approximately 25% of nonfatal myocardial infarction in adults ages 18–48. Ventricular septal rupture is a rare complication of myocardial infarction with an incidence of 1–3 percent. The incidence has decreased 10-fold with the advent of thrombolytics. Clinical manifestations include: chest pain, shortness of breath, hypotension, development of a holosystolic murmur at the lower left sternal border with a thrill. Rapid diagnosis is essential to optimize survival. Doppler echocardiogram is the diagnostic test of choice. Left ventriculography can also be diagnostic. Right heart catheterization is useful in differentiating between papillary muscle rupture and septal rupture. Treatment usually requires surgical intervention. Current guidelines of the American College of Cardiology—American Heart Association recommend immediate operative intervention on patients with septal rupture, regardless of their clinical status. Medical therapies in the interim consist of mechanical support with an intra-aortic balloon pump, afterload reduction, diuretics, inotropic agents and vasopressors. The mortality rate is extremely high. The 30-day survival rate is 47 percent versus 24 percent in surgically versus medically treated patients.
VERTIGO: THE IMPORTANCE OF THE PHYSICAL EXAMINATION. A CASE REPORT AND REVIEW OF THE LITERATURE
F.S. Drescher1; D. Berz1; R. Weiss1; K. Mark1. 1Norwalk Hospital, Norwalk, CT. (Tracking ID #117401)
LEARNING OBJECTIVES
Dizziness and vertigo are common symptoms of patients presenting to general internists. Though there are numerous etiologies for this complaint, a good history and physical can often identify the diagnosis.
CASE
63 yo white male with 3 days of right-sided earache and vertigo. He had a history of recurrent otitis media, hearing loss and bilateral mastoidectomy as well as diabetes. Initial otoscopic examination suggested severe right-sided otitis externa and a perforation of the right tympanic membrane. No nystagmus was noted. An ENT specialist was consulted and treatment with topical eardrops, intravenous quinolones and oral meclizine was begun. The patient's earache improved slightly but his vertigo persisted. On reevaluation the patient noted that his symptoms were worsened when pressure was applied to his right external meatus, which also resulted in the development of a profound nystagmus (see photographs). When this finding was described to the ENT consultant, he felt that it represented “the fistula sign” and recommended a CT-scan. This showed destruction of the horizontal semi-circular canal and a discontinuity of the petrous apex and tegmen tympani (figure 2). He was referred for surgical repair.
DISCUSSION
This case demonstrates the importance of the history and physical examination in the evaluation of vertigo. Though there are several useful clinical signs for evaluation of the vertiginous patient, in this case the fistula sign was most helpful. This test is useful in examining patients with recurrent vertigo. A finger is applied to the external meatus, which causes a pulse of air-transmitted pressure. If nystagmus is induced in association with symptoms of vertigo, bony destruction of the inner ear is likely. Demonstration of this sign as illustrated in this case, can result in prompt radiologic evaluation and surgical referral
WAITING FOR THE T IDE TO TURN: EISENMENGER's SYNDROME
D. Garrett1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117463)
LEARNING OBJECTIVES
1) Recognize the signs and symptoms of secondary polycythemia. 2) Recognize potential causes of secondary polycythemia. 3) Review effective treatment modalities for secondary polycythemia.
CASE
A 33 year-old man presented with four days of headache, dizziness, and shortness of breath. He had a history of double outlet right ventricle and surgical banding of the right pulmonary artery at six months of age. His exam was remarkable for a ruddy complexion, and a puritic rash on his head, neck, and trunk. His conjunctiva was bright red and he had cyanosis around the lips and ears. A holosystolic murmur was heard at the apex that decreased with hand-grip. His PMI was medially displaced. He had clubbing and cyanosis in all extremities. The hemoglobin was 19; the platelet count was 113; and the MCV was 77. By EKG, he had right-axis deviation with left and right ventricular enlargement. An Echo demonstrated a significant VSD.
DISCUSSION
As corrective surgery for congenital heart disease has become more successful, more and more patients are living long enough to present to the general internist's clinic. Physicians should recognize the signs of residual congenital heart disease, and know the appropriate time for referral and how to manage expected complications. This patient illustrates the importance of the physician's vigilance in detecting the reversal of flow through a VSD (Eisenmenger's syndrome). The signs of cyanosis and erythrocytosis suggest the beginning Eisenmenger's syndrome that results as the right ventricle increases in size and mass due to chronic pressure overload from the left-to-right VSD. The intracardiac shunt leads to hypoxia and a resultant secondary polycythemia. Unlike primary polycythemia, this is not associated with splenomegaly and thrombocytosis. Although both disorders have an increased RBC mass, mast cell proliferation, plethora, and microcytosis, patients with secondary polycythemia have suppressed erythropoietin and thus have thrombocytopenia as opposed to a thrombocytosis. Treatment by phlebotomy is based on symptoms, and an effort to maintain a hematocrit below 45. Iron supplementation is indicated in patients with secondary forms of polycythemia, where as it is contraindicated in primary forms. Oxygen supplementation for relief of hypoxia is a mainstay of treatment plans for these patients.
WEGENER's GRANULOMATOSIS: DIAGNOSTIC CHALLENGES IN EARLY STAGES
H. Khurana1; S. Chittivelu1; L. Cation1. 1University of Illinois at Peoria, Peoria, IL. (Tracking ID #115856)
LEARNING OBJECTIVES
To recognize chronic sinusitis and rhinitis as the most common presenting symptoms for Wegener's Granulomatosis (WG). To underscore that a strong index of suspicion is required for early diagnosis. To recognize that simple tests like C-ANCA and nasopharyngeal biopsy can help establish an early diagnosis of WG and that prompt treatment may potentially arrest disease progression.
CASE
An 18 year old white male was transferred to our institution for evaluation and management of acute renal failure and cavitating pulmonary nodules. He sought medical attention for a one-week history of nausea, vomiting, anorexia, arthralgias and non-productive cough. His medical history was significant for chronic rhinitis, sinusitis and repair of perforated nasal septum. Examination revealed pallor and saddle nose deformity. Laboratory abnormalities included mild leucocytosis, elevated BUN, creatinine and ESR. Urine analysis showed hematuria and proteinuria. ANA and anti GBM was negative. The diagnosis of Wegener's Granulomatosis (WG) was confirmed with a positive C-ANCA and lung biopsy. Patient responded to cyclophosphamide and prednisone therapy.
DISCUSSION
WG is an uncommon disease with an estimated occurrence of 3/100,000 population. The age of onset varies from 5 months to 60 years with a median of 40 years. Less than 15% of the cases occur below the age of 20. There is a female preponderance in younger age groups, as compared to adults where the gender difference is not marked. The “limited form” of WG is confined to upper and/or lower respiratory tract whereas the “classic form” also involves the kidneys. Most patients seek medical attention for symptoms like chronic sinusitis and rhinitis. However, there is progressive involvement of the lungs and kidneys in majority of patients by the time the diagnosis is established. Diagnosis in early stages of the disease can be established by keeping a high index of suspicion and simple tests like C-ANCA and nasopharyngeal biopsy. Early diagnosis and prompt use of appropriate therapy can slow down and may even arrest the progression of this potentially fatal disease
WEIGHT LOSS IN A PATIENT WITH METASTATIC MELANOMA
E.A. Sastre1; C. Bates1. 1Beth Israel Deaconess Medical Center, Boston, MA. (Tracking ID #116976)
LEARNING OBJECTIVES
1) Recognize that new symptoms in cancer survivors often indicate common benign conditions as opposed to recurrent malignancy; 2) List manifestations of hyperthyroidism; 3) Define the association between hyperthyroidism and hypercalcemia.
CASE
A 53 year-old female with history of hypertension and metastatic melanoma presents with weight loss and diarrhea. She was diagnosed with melanoma which metastasized to the lungs and liver in 1994. Treatment with interleukin 2 resulted in complete remission. On presentation, she described a 13 pound weight loss, abdominal cramping, non-bloody diarrhea, and fatigue. She denied fevers or chills but noted pruritus. Physical examination was notable for a normal thyroid gland, a normal cardiopulmonary exam, and mild left lower quadrant abdominal tenderness. Concern for recurrent melanoma was raised; her metoprolol was discontinued as she thought it was causing fatigue. The patient subsequently developed palpitations and dizziness. Laboratory values revealed normal liver function tests, calcium 10.9, an undetectable TSH, T4 of 5.2, and a T3 of 622. The patient restarted metoprolol; symptoms improved. Thyroid uptake scan demonstrated diffuse increased uptake consistent with Grave's disease. She was started on methimazole. Parathyroid hormone level was suppressed and both PTH-related protein and Vitamin D levels were normal. Treatment normalized thyroid function tests and serum calcium.
DISCUSSION
Physicians may assume that new symptoms in patients with prior malignancy are secondary to disease recurrence. However, these patients are also susceptible to common benign illnesses, such as hyperthyroidism, which may present similarly to metastatic disease. Symptoms include weight loss, diarrhea, palpitations, anxiety, and heat intolerance. Physical examination may reveal tachycardia, fever, moist skin, thyroid gland irregularities, arrythmias, and brisk reflexes. Suppressed TSH and elevated levels of T4 and T3 confirm the clinical suspicion. The most common etiology of hyperthyroidism, Grave's disease, is an autoimmune disorder; antibodies directed against TSH receptors on the thyroid gland increase thyroid hormone synthesis and gland size. The patient classically presents with diffuse goiter (90%), ophthalmopathy (50%), and dermopathy (<5%). Grave's disease is diagnosed by diffuse increased uptake on radioactive iodide uptake scan. One of the less common manifestations of hyperthyroidism is hypercalcemia. Thyroid hormone stimulates bone resorption through action on T3 receptors on osteoblasts and osteoclasts. In addition, elevated IL-6 levels also stimulate osteoclast activity. Approximately 8% of patients with clinical hyperthryroidism have hypercalcemia that usually resolves with treatment of hyperthyroidism.
WERNICKE ENCEPHALOPATHY
F. Nahab1; H. Limkemann2. 1University of California, Los Angeles, San Fernando Valley Program, Sylmar, CA; 2University of California, Los Angeles, Sylmar, CA. (Tracking ID #102043)
LEARNING OBJECTIVES
1. Recognize the clinical manifestations of Wernicke Encephalopathy (WE). 2. Consider WE as part of the differential diagnosis of any acute oculomotor dysfunction and/or ataxia. 3. Review the CT and MRI findings in WE.
CASE
A 53 year old Hispanic-German gentleman with a history of hypertension and hyperlipidemia presented with double vision and inability to walk without assistance for 12 hours. He denied any witnessed ALOC, headache, weakness, trauma or fever. The patient admitted to a 20+ year history of binge drinking with his last binge occurring 3 weeks prior. Family history was significant only for a grandfather who died of a stroke. On exam, the patient's blood pressure was 143/95. Although alert and oriented × 4, on neurological examination the patient was found to have an isolated right medial rectus palsy and an inability to converge. Otherwise, cranial nerves were intact. Strength was 5/5 in BUE and BLE with 2+ DTRs symmetrically. Vibratory sensation was intact. No ataxic extremities were noted. Patient's gait was broad-based but steady however on tandem gait marked ataxia was noted. Romberg test was negative. Hemoglobin was 13.1 with an MCV of 101.3. Total cholesterol was 239 and triglycerides were 613. Liver function tests and chemistry panel were normal. The differential diagnosis included stroke, WE and multiple sclerosis so the patient was started on IV hydration supplemented with thiamine, folate, and MVI while awaiting imaging. CT showed no evidence of mass, bleed, or midline shift. MRI findings on T1- and T2-weighted, FLAIR, and DW images showed no evidence of acute stroke but noted increased signal intensity of the mamillary bodies in the T2-weighted image and showed mild periventricular white matter changes consistent with microvascular ischemic disease. Within 12 hours of the patient's initial presentation, the medial rectus palsy had resolved, convergence was intact, ataxia on tandem gait had resolved, and patient was discharged home.
DISCUSSION
WE is a neurological syndrome that can include oculomotor dysfunction, ataxia and/or disturbances of consciousness that range from mild confusion to coma. Clinical features develop over a few days to weeks and result from a lack of thiamine. Neuroradiology findings on CT scan include hemorrhages of the mamillary bodies. MRI findings are best visualized on T2-weighted images and include hyperintensity of the mamillary bodies, periaqueductal area, hypothalamus, thalamus, cerebellum and/or cerebral cortex. Often, administration of high doses of thiamine may resolve symptoms though untreated WE may be fatal or result in permanent neurologic damage. Therefore, it is important to consider WE in the differential diagnosis of any acute oculomotor dysfunction, ALOC and/or ataxia so as to begin thiamine supplementation early.
WERNICKE's ENCEPHALOPATHY FOLLOWING 5-FLUROURACIL THERAPY IN A WOMAN WITH BREAST CANCER
A. Byrnes1; L. Coberly1. 1University of Cincinnati, Cincinnati, OH. (Tracking ID #115619)
LEARNING OBJECTIVES
1. Diagnose and act quickly to reverse Wernicke's Encephalopathy. 2. Recognize the potential side effects of chemotherapeutic agents such as 5-Fluorouracil and treat prophylactically or monitor closely to minimize complications.
CASE
Medication side effects are often overlooked when patients develop serious illness. We present an unusual case of a complicated Wernicke's Encephalopathy (WE) in a patient with recent neoadjuvant chemotherapy including 5-fluorouracil (5-FU). A 52-year-old female with a history of infiltrating ductal carcinoma (T2NXM0) presented with altered mental status. Her physical exam was normal except for a decreased level of alertness and poor cooperation. She had recently been admitted with similar symptoms, and had a normal head CT, a normal brain MRI with gadolinium, and three lumbar punctures, which were negative (including cytology) except for elevated protein. EEG showed moderate generalized slowing with epileptiform discharges.
DISCUSSION
This admission, repeat EEG on dilantin therapy revealed moderate to severe generalized slowing without epileptiform discharges. Head CT was normal. Suddenly, the patient became apneic (3 breaths/minute) and required intubation. She had a repeat MRI, revealing enhancement of the mamillary bodies consistent with WE, changes which, in retrospect, were present to a lesser degree on her prior MRI. She was started on intravenous thiamine and had an immediate increase in her spontaneous respiratory rate to normal. Her cognitive function returned. Unfortunately, she developed line sepsis with bacteremia and fungemia leading to a critical illness polyneuropathy, which prevented extubation. WE, caused by thiamine deficiency, is characterized by a constellation of neurologic abnormalities including a global confusional state, disorientation and lethargy, nystagmus and truncal ataxia. Respiratory depression is uncommon. Typically, EEG reveals diffuse slowing, and CSF is normal except for elevated protein, as in our patient. 5-FU therapy increases the risk of thiamine deficiency, and hence WE, as it blocks the conversion of thiamine to its active metabolite thiamine pyrophosphate (TPP). Patients with deficiency of dihydropyrimidine dehydrogenase (DPD, the enzyme responsible for 5-FU metabolism) are at greater risk of this adverse effect of 5-FU. Cancer patients appear to be at increased risk for thiamine deficiency. At baseline, they tend to have low levels of thiamine, as evidenced by liver biopsy and bioassay. Cancer patients also appear to have an increased incidence (3%) of DPD deficiency, thus making them more susceptible to the adverse effects of 5-FU. Careful monitoring, and prophylactic thiamine administration can be helpful.
WHAT CAUSED THE FEVER?
A. Agha1; A. Kolpakchi1. 1Baylor College of Medicine, Houston, TX. (Tracking ID #117405)
LEARNING OBJECTIVES
1. Review the definition of fever of unknown origin (FUO). 2. Construct a differential diagnosis of FUO. 3. Recognize that chronic bursitis can cause prolonged fever and constitutional symptoms.
CASE
A 68 year-old male without significant past medical history presented to his primary care provider with a one month history of malaise, anorexia, and 5lb weight loss. He was noted to have temperature up to 101 F on three consecutive clinic visits. The initial outpatient work up including CBC, liver function tests, chest x ray, urine and blood cultures were negative. He was then admitted to the hospital for work up of fever of unknown origin. Vital signs revealed temperature of 101 F, BP 140/80, RR 14, HR 80. Physical exam was unremarkable except for a thickened, non-tender left prepatellar bursa with small effusion. No warmth or erythema was noted. His labs revealed elevated sedimentation rate at 39 (0-20) and elevated C-reactive protein at 12 (normal being negative). The rest of the work up including RPR, MHA-TP, HIV, rheumatoid factor, ANA, hepatitis panel, urine and blood cultures, CXR, CT scan of thorax and abdomen was all negative. The patient continued to have elevated temperature up to 101.5 F while in the hospital, and the etiology of his fever remained unknown. The patient was reexamined again, and the previous non-tender thickened left prepatellar bursa was aspirated with 9cc of thick amber fluid obtained. Culture was positive for Staphylococcus Aureus sensitive to nafcillin. The patient was treated with nafcillin for 4 weeks with complete resolution of his symptoms.
DISCUSSION
The fever of unknown origin (FUO) is defined as fever >38.3 C on several occasions, duration of fever >3 weeks, and uncertain diagnosis after one week of study in the hospital. Differential diagnosis of FUO includes four major categories: infections (30%), malignancies (30%), collagen vascular diseases (10%) and unknown (30%). Interestingly, the FUO in our patient was from his chronic prepatellar bursitis. Literature search failed to produce a single case report of septic bursitis as the etiology of FUO. Prepatellar bursa is one of few bursas that can become infected, most commonly by Staphylococcus Aureus and other gram-positive organism (80%). Prepatellar bursitis becomes chronic in approximately 5% of patients. Bursa aspiration is indicated for diagnostic and therapeutic purposes. The treatment is with use of antibiotic for duration of 4 weeks.
WHEN A ROSE IS JUST A ROSE: A CASE OF MONONUCLEOSIS
R. Ashkenazy1; C. Bates1. 1Beth Israel Deaconess Medical Center, Boston, MA. (Tracking ID #116819)
LEARNING OBJECTIVES
1) Diagnose infectious mononucleosis (IM) using clinical and laboratory parameters; 2) Recognize the limitations of laboratory testing for IM.
CASE
A 37-year-old man had progressive fatigue and 5 days of temperature to 102.5 F, without sore throat. Physical exam was notable for temperature of 100 F, pulse of 100, pharyngeal erythema and cervical lymphadenopathy. The liver and spleen were not enlarged. WBC was 2.2 K with 28% PMN, 9% bands, 37% lymphs and 18% atypical lymphocytes. The platelet count was 97 K. Liver tests were elevated with AST of 373, ALT of 542 and alkaline phosphatase of 304. Monospot test was negative. EBV VCA-IgM, EBV VCA-IgG and EBV EBNA-IgG antibodies were negative. A throat culture was positive for rare group A strep; penicillin was prescribed. A rash developed 6 days later. Because the patient's wife was pregnant, the patient and his wife were tested for toxoplasmosis and CMV; serologies were negative. Over the next 8 days, he had increased anorexia and somnolence. Tests for HIV and hepatitis A, B and C were negative. He was referred to an infectious disease specialist. Four days later he was admitted for his deteriorating clinical state and uncertain diagnosis. Repeat monospot test and EBV VCA-IgM antibody were positive; EBV VCA-IgG and EBV EBNA-IgG antibodies were negative. Management was supportive and he recovered.
DISCUSSION
IM is most often caused by Epstein-Barr virus (EBV), a herpesvirus spread in saliva and, less commonly, through intimate contact or blood transfusions. Fever, pharyngitis and lymphadenopathy, with transient heterophile antibodies and atypical lymphocytes, characterize classic IM. Additional findings include fatigue, splenomegaly, rash (common after administration of ampicillin/amoxicillin) and various neurologic syndromes. EBV induces heterophile antibodies within 1 week of symptoms. The monospot test, a latex agglutination assay, and enzyme-linked immunosorbent assay (ELISA) are rapid diagnostic tests against these antibodies with a sensitivity of 86% and specificity of 100%. Heterophile antibodies peak between the second and fifth week of infection and may persist for up to a year. Epstein-Barr viral capsid antigen (VCA) antibodies are also diagnostic. IgM antibodies are expected at presentation, persist for 1 to 2 months and mark acute infection. IgG antibodies are expected at presentation, persist for life and mark acute or prior infection. IgG antibodies to EBV nuclear antigen (EBNA) appear 6 to 12 weeks after symptom onset and persist throughout life; they exclude acute infection early in an illness. This patient's initially negative EBV VCA IgM and IgG and concerns about perinatal transmission led to testing for other pathogens of mononuclosis-like syndromes. Of these, CMV is most common. Additional testing in EBV-negative patients depends on risk factors for, and clinical implications of, other infections.
WHEN ADDITIONAL HISTORY SOLVES THE MYSTERY: A CASE OF CAROTENOSIS CUTIS
M. Hadian1; B. Taqui1. 1Temple University, Philadelphia, PA. (Tracking ID #116190)
LEARNING OBJECTIVES
1. Recognize the clinical manifestations of beta-carotenemia. 2. Review the diagnostic and therapeutic approach for beta-carotenemia. 3. Recognize the importance of returning to the patient for additional history taking when diagnosis is unclear.
CASE
A 61year-old previously healthy Asian female presented complaining that she had become yellow, especially during the prior week. She denied nausea, vomiting, diarrhea, constipation, abdominal pain, anorexia, weight loss, fever, chills, change in urine/stool color. She said she felt completely healthy, but she and her family were concerned about her marked skin discoloration. She is a nurse, but denied needle sticks or hepatitis exposures. Her only medication was a daily multi-vitamin. She denied alcohol, tobacco or drug use. On exam, she had diffusely yellow skin with deeper discoloration of palms and soles. She did not have scleral icterus. Her chemistries, CBC, TSH, cholesterol, liver function tests were all normal. Her urine was microscopically normal, but had an orange-yellow discoloration. At this point, further questioning revealed that the patient was a vegetarian who ate a lot of green leafy vegetables and carrots. She had recently increased her carrot intake to 2–3 pounds/day. Subsequently, serum carotene was ordered. The patient had a value of 322 (normal 6–77 mg/dl). She was reassured that this condition is benign and related to the consumption of too many carrots and yellow vegetables. Patient was discharged with appropriate dietary instructions. One week later she called to inform us that her skin color was back to her normal.
DISCUSSION
Carotenosis cutis, yellow-orange coloring of the skin, results from excessive intake of vitamin A precursors in food, principally fruits and vegetables. Natural beta-carotene acts as an anti-oxidant, but in excess amounts can lead to skin discoloration. The palms and soles are predominantly involved. The yellowing of the skin is differentiated from jaundice in that there is no scleral icterus. Other than cosmetic changes, carotenemia is not harmful. Hypothyroidism and smoking makes patients particularly susceptible to carotenemia. The omission of carrots and other colored fruits and vegetables from the diet leads to the rapid disappearance of the hyperpigmentation. Our case illustrates the importance of returning to the patient for additional history taking, especially when the case does not follow a common, well-known pattern.
WHEN AMOXICILLIN ATTACKS: A CASE OF SERUM SICKNESS-LIKE REACTION
J.A. Chang1; C.M. McEvoy2. 1Creighton University, Omaha, NE; 2University of Nebraska Medical Center, Omaha, NE. (Tracking ID #115552)
LEARNING OBJECTIVES
1) Recognize the clinical manifestations of serum sickness 2) Diagnose serum sickness accurately and quickly 3) Treat serum sickness in an appropriate fashion.
CASE
Our patient is a 29-year-old Caucasian male. 15 days before the initial visit to allergy clinic, he had received Amoxicillin for sinusitis. 9 days later, he saw redness on his palm and the sides of his fingers; followed by progressive swelling, erythema, pruritis, and burning of his hands and feet, particularly on the dorsal surfaces. White rings appeared on his hands the next morning. He had blotchy erythema on his face, hands and feet. The hands and feet were swollen and very tender at the wrists and ankles. He was given a loading dose of prednisone (80 mg) by his PCP, followed with a Medrol Dose Pack for erythema multiforme. He initially improved, then developed worsening symptoms. 3 days before the allergy visit, he required subcutaneous epinephrine for acute angioedema. He presented to allergy clinic with severe, crippling joint pain. His temperature was 38.1. Diffuse, blotchy edema with linear wheals and flares over the face, trunk, and extremities was noted. ESR was 38, C3 was 161 and C4 was 33, with a CH50 of 199. Urinalysis showed protein of 30 mg/dL and blood of 25/uL. He was diagnosed with serum sickness secondary to amoxicillin and started on high-dose prednisone, which was slowly tapered over several weeks. His symptoms improved on this regimen. He has been symptom-free since about 4 weeks after the onset of symptoms, and has had no recurrences.
DISCUSSION
Serum sickness is the prototype of immune complex (type III) immune reactions. True serum sickness is caused by the administration of heterologous sera; serum sickness-like reaction results from non-protein drugs. Virtually all patients have fever and cutaneous reactions. Joint pain with or without swelling is present in about 2/3 of patients. Lymphadenopathy is also commonly seen. The pathophysiology is due to a combination of antigen with IgG or IgM immune complex deposition, leading to the release of vasoactive amines, which increase vascular permeability. The usual onset is 7–14 days after exposure to a trigger (commonly beta-lactam antibiotics); however, in a previously sensitized patient, symptoms may appear 2–4 days after exposure. The first line of treatment is to stop the suspected inciting agent; antihistamines and NSAIDS are used for pruritis and arthralgia. If severe symptoms exist, corticosteroids should be used for 10–14 days. Shorter courses are associated with recurrent symptoms which are more difficult to control. Serum sickness is likely to recur on reexposure and may not be predicted by skin testing.
WHEN GOOD INTENTIONS ARE NOT ENOUGH: A TRAGIC CASE OF I.V. PHENYTOIN USE
S. Daya1; S. Jagadeesh1; N. Shaikh2. 1York Hospital, York, PA; 2Mercy Catholic Medical Center, Lansdowne, PA. (Tracking ID #117245)
LEARNING OBJECTIVES
1) Recognize the increased risk for adverse events with phenytoin use in patients with multiple comorbidities. 2) Recognize the importance of the appropriate rate of parenteral phenytoin administration in high risk patients.
CASE
A 37 year old Afro-Caribbean male with a known history of End Stage Renal Disease and liver cirrhosis presented to the renal unit to undergo his scheduled dialysis. During dialysis he was noted to be diaphoretic and tachypneic, this was followed by an episode of grand mal seizure which was treated with lorazepam given intravenously. Patient had a second episode of grand mal seizure, given the recurrent nature phenytoin was administered with a loading dose followed by continuous infusion. The seizure episode was successfully terminated with the above treatment regime. Concurrent cardiac monitoring during this period showed an initial sinus rhythm quickly change to sinus bradycardia and second degree A-V block which deteriorated to asystole and cardiac arrest. Phenytoin infusion was discontinued and cardiopulmonary resuscitation (CPR) initiated. Patient was successfully resuscitated after 12 minutes with return to sinus rhythm and stabilization of hemodynamic parameters. He needed endotracheal intubation and mechanical ventilation for airway protection and oxygenation. Eventually the patient was extubated and a neurological evaluation revealed the patient to have significant cognitive and motor deficits which required long term nursing home care. An electroencephalographic study showed neurological damage consistent with hypoxic encephalopathy.
DISCUSSION
Phenytoin is a commonly prescribed anticonvulsant used to treat most types of seizure disorders and status epilepticus. Parenteral phenytoin administration is commonly undertaken with careful cardiac monitoring due to the risk of cardiac arrhythmias. The potential cardiac adverse effects include atrial and ventricular conduction disturbances, hypotension, ventricular fibrillation and reduced cardiac output. Cardiac effects are thought to be secondary to the propylene glycol diluent of the parenteral product. Phenytoin in its parenteral form is dissolved in 40% propylene glycol and 10% ethanol. Caution is advised with phenytoin use in any patient with cardiac disease because adverse effects may be potentiated or exacerbated. The drug is absolutely contra-indicated in patients with cardiac conduction abnormalities. Reactions to parenteral phenytoin occur more often in the elderly or in patients with other significant comorbidities such as renal or hepatic failure. The rate of intravenous administration of phenytoin is critically important to avoid or limit adverse cardiovascular events that could lead to serious long-term consequences.
WHEN TISSUE CAN'T ALWAYS BE THE ISSUE: USING CLINICAL CRITERIA TO DIAGNOSE CHRONIC NECROTIZING PULMONARY ASPERGILLOSIS
R. Pechulis1; B. Taqui1. 1Temple University, Philadelphia, PA. (Tracking ID #116208)
LEARNING OBJECTIVES
1. Recognize Chronic Necrotizing Pulmonary Aspergillosis (CNPA) as a clinical entity distinct from other forms of Aspergillus pulmonary disease. 2. Recognize risk factors and diagnostic criteria for CNPA.
CASE
A 55 year old African American female with sarcoidosis presented with one week of fever, nightsweats, anorexia and cough productive of dark sputum. She had a similar episode associated with hemoptysis 5 months prior. At that time, CXR revealed mycetoma and sputum grew Aspergillus fumigatus. On exam, she had T = 101.6, RR = 25. She had left apical bronchial breath sounds and right basilar rales. Lab data revealed Na 125, WBC 11. CXR showed a new thick walled left apical cavitary lesion with associated pleural thickening, parynchemal consolidation, diffuse reticulo-nodular interstital densities. The previously diagnosed right upper lobe mycetoma was not seen. The patient was treated for community acquired pneumonia, but remained febrile and symptomatic. Multiple sputum cultures grew Aspergilllus fumigatus and the patient had a bronchoscopy with trans-bronchial biopsy. The bronchoalveolar lavage culture was positive for Aspergillus fumigatus. Biospy specimen showed nonspecific inflammatory cells. After lengthy discussion with our consultants and patient, open lung biopsy was deferred and the patient was started on voraconazole for chronic necrotizing pulmonary aspergillosis. Since then, she has had sustained clinical and radiologic improvement.
DISCUSSION
Chronic necrotizing pulmonary aspergillosis (CNPA), also known as semi-invasive or chronic granulomatous aspergillosis, was first described in 1981. It is an indolant, locally invasive aspergillus infection without vascular invasion or dissemination, as seen in invasive pulmonary aspergillosis (IPA). It occurs in patients with underlying lung disease or mild immunocompromised states. This population is compromised enough to grow the fungus, but healthy enough to avoid the rapid demise that occurs in IPA patients. CNPA patients present with cough and constitutional symptoms. Hemoptysis occurs in 10% of cases. Radiologic studies reveal indolent upper lobe consolidation associated with pleural thickening. Cavitation and/or mycetoma occur in 50% of cases. The diagnosis is suggested by clinical presentation, isolation of Aspergillus from pulmonary secretions, and exclusion of other etiologies (anaerobes, mycobacteria, histoplasmosis, coccidiomycosis). The diagnosis is confirmed by pathologic evidence of tissue invasion or a response to specific antimycotic drugs. Transbronchial and percutaneous biopsies have low yield. Most patients have comorbid pulmonary conditions that make an open lung biopsy a high risk. The duration of therapy is not well established.
WHEN URGENCY IS MORE THAN AN OVERACTIVE BLADDER: INTERSTITIAL CYSTITIS
C.L. Spagnoletti1; M.A. McNeil1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #115016)
LEARNING OBJECTIVES
1) Recognize the clinical presentation of interstitial cystitis (IC) 2) Diagnose IC in the patient with urinary urgency and frequency 3) Review the therapeutic options for IC.
CASE
Ms. G. is a 50 year old female who presented with complaints of daily urinary frequency and urgency and intermittent bladder pain for 8 weeks. She reported voiding up to 15 times per day, without an increase in her fluid intake or change in medications. Her bladder pain improved with voiding. She denied dysuria, hematuria, recent UTI, history of radiation therapy or abdominal or pelvic surgery. Her past medical history was significant for chronic low back pain. Her medications included ibuprofen and cyclobenzaprine. She was in a same-sex relationship and denied a history of sexual abuse. On pelvic exam, she complained of bladder tenderness on palpation of the anterior vaginal wall. Vaginal wet prep, KOH slide, GC and chlamydia were negative. Urinalysis, culture, and cytology were unremarkable. Cystoscopy revealed a decreased bladder capacity and presence of glomerulations after hydrodistention. A diagnosis of IC was made. She was started on amytriptyline, hydroxyzine, and pentosan polysulfate sodium. Within three months, she noted significant improvement in her symptoms of urgency, frequency, and pain.
DISCUSSION
IC is a poorly understood condition affecting up to 700,000 people in the U.S. Most are Caucasian and women make up 90%. The median age of onset is 43. It is a severe and debilitating chronic pain syndrome that afflicts the bladder, and is characterized by urinary urgency, frequency and bladder pain in the absence of other definable pathology. Patients present with urinary urgency and frequency early in the disease and later develop pain symptoms including suprapubic or pelvic pain, or dyspareunia. Dysuria and incontinence are not typical. IC is also characterized by flare-ups and remissions. There are many proposed theories of pathogenesis, but the cause is not known. The differential diagnosis is broad and includes diseases which affect the urinary, gynecologic, and gastrointestinal systems. IC is mainly a diagnosis of exclusion. 95% of IC patients complain of a tender bladder base during pelvic exam, and the presence of this in a patient with classic symptoms is highly suggestive. Urinalysis, culture, and cytology are negative. An abnormal cystoscopy is diagnostic, but is only indicated in refractory cases. If performed, reduced bladder volumes, glomerulations after hydrodistention, and/or Hunner's ulcers are seen. Therapy is directed at symptom reduction and improved quality of life. The mainstay of therapy includes the combination of tricyclic antidepressants, antihistamines, and pentosan polysulfate sodium, plus dietary modification and pelvic floor physical therapy. For more severe cases, intravesicular therapy or cystectomy have been used.
WHERE's THE BEEF? AN INBORN ERROR OF METABOLISM PRESENTING AS A SEIZURE IN AN ADULT
S.D. Sisson1; D. Chaupin1; E. Schmidt1. 1Johns Hopkins University, Baltimore, MD. (Tracking ID #117081)
LEARNING OBJECTIVES
1) Recognize a rare cause of seizure in an adult 2) Understand the clinical sequelae of ornithine transcarbamylase deficiency.
CASE
A 53-year-old female with a history of achondroplasia was admitted for persistent abdominal pain, nausea, vomiting, and diarrhea. Past medical history was notable for chronic abdominal pain, attributed to chronic pancreatitis. In reviewing the patient's history of abdominal pain, she reported having abdominal pain and headaches since childhood, which she associated with eating meat. As a result, the patient had altered her diet to consist primarily of grains. Physical examination was notable for a cachectic, chronically ill-appearing female, lying still in bed. She had diffuse abdominal tenderness with rebound but without guarding. A CT scan of the abdomen showed acute pancreatitis with peripancreatic inflammation and multiple pseudocysts. As treatment, the patient was started on a standard parenteral solution of electrolytes, trace elements, glucose, amino acids, and lipids. Shortly after initiation of parenteral nutrition, the patient became somnolent and seized, confirmed by EEG. Benzodiazepines and phenytoin were administered, without a response. Parenteral nutrition was stopped, and on further evaluation an ammonia level came back profoundly elevated (190 mcg/dl). Liver function tests were normal. An elevated ammonia level in the setting of normal liver function suggested the diagnosis of a urea cycle disorder. An allopurinol challenge test was performed and confirmed the diagnosis of ornithine transcarbamylase deficiency.
DISCUSSION
In the normal host, ammonia produced by protein catabolism is converted to urea by a series of steps referred to as the urea cycle. Enzyme deficiencies in the urea cycle, including ornithine transcarbamylase deficiency, result in the accumulation of ammonia and precursor metabolites. This patient learned to avoid high protein foods in order to prevent precipitation of physical symptoms brought on by the accumulation of these toxic metabolites, resulting in protein malnutrition and cachexia. When parenteral nutrition (with amino acids) was administered to this patient as treatment for pancreatitis, accumulation of ammonia and other metabolites precipitated a seizure. An elevated ammonia level is found in all urea cycle enzyme deficiencies, including ornithine transcarbamylase deficiency. An ammonia level should be checked in all patients in whom a urea cycle enzyme deficiency is considered.
WOOSHING IN MY EAR: I THINK IT SOUNDS LIKE LUPUS
E. Choe1; H. Meatty1; D. Spruill1. 1Tulane Health Sciences Center, New Orleans, LA. (Tracking ID #117444)
LEARNING OBJECTIVES
1. Recognize the presentation of lupus nephritis 2. Recognize the clinical presentation of sagittal venous thrombosis.
CASE
A 19 year-old woman presented with two weeks of a headache associated with a “whooshing sound” in her left ear. She noted associated epistaxis, but no fever, neck stiffness or previous head trauma. She was afebrile and her vital signs were normal. She had a resolving discoid rash on her abdomen, but the remaining examination was normal. She had no focal neurologic deficits, and there were no carotid bruits. She had a history of renal insufficiency, and her baseline creatinine of 1.8 had increased on this admission to 6.4. Her hemoglobin was 4.0 g/dl; platelets 110. She had 250 blood and >100 RBC on microscopic examination of the urine. Her ANA was positive at 1:640 and she had low C3 levels with a false positive RPR. Her PTT was elevated. She was diagnosed with systemic lupus with renal failure secondary to lupus nephritis and was treated with high dose pulse steroids andcyclophosphamide. Although a CT of the head was normal, the persistent headache prompted an MRI with contrast that revealed a sagittal vein thrombosis.
DISCUSSION
Renal impairment is a common presenting complaint of lupus, affecting 90% of patients with lupus at some point in their course. Our patient had diffuse proliferative lupus nephritis, the most common and the most severe form of disease. Aggressive therapy with high dose pulse steroids and cyclophosphamide is indicated in these patients. Lupus patients are also at risk for anti-phospholipid antibody syndrome inducing a venous thrombosis. While deep venous thrombosis or stroke are the most common complications, a wooshing sound in the ear in the setting of a headache is suggestive of either severe carotid artery stenosis or venous thrombosis. In the latter diagnosis, the sound is induced from turbulence created from venous back-pressure from the thrombosis on the carotid artery flow. Fifty percent of lupus patients will have a CNS event of some type during the lifetime.
WWW.WPW: WHAT WOULD WOLFE-PARKINSON-WHITE DO?
S. Kahlon1; J. Wiese2. 1Tulane Health Sciences Center, New Orleans, LA; 2Tulane University, New Orleans, LA. (Tracking ID #117478)
LEARNING OBJECTIVES
1. Review the symptomatic presentation and treatment of Wolf-Parkinson-White Syndrome. 2. Recognize the medications contraindicated in Wolf-Parkinson-White.
CASE
A 35 year-old man presented after an episode of palpitations, shortness of breath, and an aching pain in the left upper chest and shoulder. He also noted lightheadedness that occurred suddenly and at rest, resolving spontaneously after twenty minutes. An ECG showed sinus rhythm with a rate of 83 BPM, left axis deviation, a QRS interval of 0.104 seconds, a PR interval of 0.126 seconds, and delta waves. Electrolytes and serial cardiac enzymes were normal. He was asymptomatic in the ER, and thus no treatment was given. He was discharged to clinic with a prescription for a calcium channel blocker to control the heart rate. At presentation in clinic, a repeat EKG was obtained demonstrating similar intervals and the delta waves noted above. Owing to the risk of accelerated conduction through the accessory pathway, the calcium channel blocker was held, and the patient was referred to cardiology for ablation.
DISCUSSION
Wolf-Parkinson-White Syndrome has a prevalence of 1/500 people, making it one of the more common congenital heart diseases. It is caused by an accessory electrical pathway between the atria and ventricles leading to conduction abnormalities that can cause arrhythmias. It is usually asymptomatic, discovered only by incidental EKG. When symptoms do present, they usually take the form of ventricular tachycardia (70%), atrial fibrillation (16%), or other arrhythmias. Recognizing the syndrome is important since treatment with Class I and III antiarrhythmics or radioablation is available. ECG changes of Wolf-Parkinson-White include the pathognemonic delta wave, a short PR interval (<0.12 seconds), and a widened QRS complex (>0.10 seconds) that are all manifestations of early ventricular activation through the accessory pathway. The EKG is also diagnostic of the location of the pathway: the left axis deviation in this case suggests that the pathway is posterio-septal, readily amenable to radioablation from a right heart cathertization. It is also important to recognize this syndrome to avoid administration of digoxin and Class II and IV antiarrhythmics that may increase AV nodal blockade, exacerbating the accessory pathway and potentially causing ventricular arrhythmias and sudden death.
YES, YOU CAN HAVE TOO MUCH OF A GOOD THING: A CASE OF THE MILK ALKALI SYNDROME
G. Ramani1; R. Granieri1. 1University of Pittsburgh, Pittsburgh, PA. (Tracking ID #116446)
LEARNING OBJECTIVES
1) Recognize milk alkali syndrome as an increasingly more common cause of hypercalcemia 2) Recognize the role of dietary calcium carbonate in the milk-alkali syndrome.
CASE
The patient is a 49 year old female, with past medical history of osteopenia with recent shoulder and spine fractures, who presented with a three day history of increased fatigue, nausea, and weakness. The patient had become very confused, fallen several times, and was brought to the ED by her husband, who provided much of the history. She had been very concerned about both her recent diagnosis of osteopenia and recent heartburn, had been taking 100–250 Rolaids® (calcium carbonate, magnesium hydroxide) tablets per week, as well as large quantities of baking soda (sodium bicarbonate). Physical examination was notable for the patient being oriented only to name, and for inappropriate speech. Reflexes, strength, cranial nerve testing, and sensation were intact. Laboratory testing revealed a creatinine of 3.8, bicarbonate of 64, potassium of 2.3, and a total serum calcium of 20. ABG demonstrated a pH of 7.55, pC02 of 60, and pO2 of 99. EKG revealed no abnormalities. The patient was admitted to the hospital and vigorously rehydrated with normal saline . Her electrolytes corrected over several days. Her creatinine stabilized at 1.5, and her mental status improved. The patient was educated about judicious calcium supplementation and discharged to home.
DISCUSSION
The milk-alkali syndrome is characterized by renal failure, hypercalcemia, and metabolic alkalosis. Although once considered rare, increased awareness and screening for osteoporosis has resulted in a marked rise in the number of patients taking calcium supplementation. The pathophysiology is related to consumption of large quantities of calcium, in the presence of an absorbable alkali. As calcium levels rise, serum levels of calcitriol fall, thereby decreasing renal absorption of calcium. However, the ingestion of greater then 10 grams of calcium can overwhelm this mechanism, and the presence of a metabolic alkalosis can enhance renal absorption of calcium. Furthermore, hypercalcemia frequently worsens the metabolic alkalosis by stimulating proton secretion and bicarbonate reabsorption within the kidney. The diagnosis can usually be obtained by the history along with documentation of electrolyte abnormalities. Treatment consists of cessation of the offending agents, and rapid hydration with normal saline in patients with renal impairment or severe hypercalcemia. Although renal function usually improves, some residual insufficiency may persist. Prevention of this condition focuses upon educating patients of the importance of limiting their supplemental calcium to no greater than 2 grams a day.