Abstract
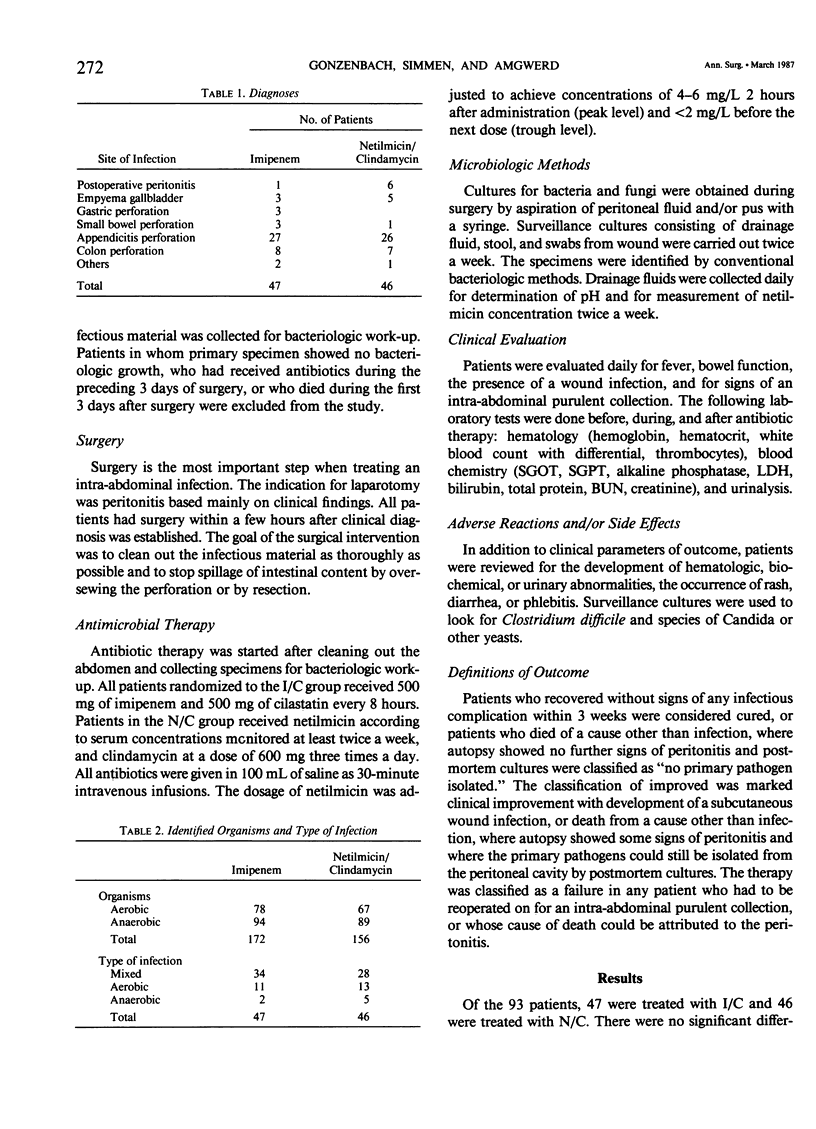
In a randomized study the clinical and bacteriologic effectiveness of imipenem was compared with the classical combination of netilmicin with clindamycin in patients who had surgery for an intraperitoneal infection, localized or generalized, with positive bacteriologic findings of the specimen taken at surgery. Excluded were all patients who received other antibiotics before surgery, or who died within 3 days after antibiotic therapy was started. Imipenem was given at a dose of 500 mg t.i.d., clindamycin 600 mg t.i.d., and netilmicin according to serum levels. The diagnoses ranged from postoperative peritonitis, gallbladder empyema, perforated gastroduodenal ulcer, small bowel perforation with and without obstruction, and perforated appendicitis to perforation of the colon. The bacteriologic work-up included examination of the primary specimen (aerobic and anaerobic), the urine, feces, and serologic testing for Candida albicans once or twice a week and after the course of antibiotic therapy. In addition, pH measurements of abscesses and drainage fluids were performed. Ninety-three patients entered the study. Forty-seven patients were treated with imipenem (test group), and 46 patients were treated with the combination therapy (control group). The two groups did not show significant differences in age, sex, diagnostic groups, risk factors, primary bacteriology, and duration of therapy (mean: 6.7 days). Thirty-eight patients (80.9%) treated with imipenem were cured, six patients (12.8%) were improved, and there were three (6.4%) failures. The respective numbers for the control group were 31 (67.4%), 10 (21.7%), and 5 (10.9%). The mean duration of hospitalization was 19 days for the test group and 24.5 days for the control group. There were four wound infections in the test group and 11 wound infections in the control group. Imipenem is at least as effective in the adjuvant therapy of intra-abdominal infections as the combination of netilmicin with clindamycin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eron L. J., Hixon D. L., Park C. H., Goldenberg R. I., Poretz D. M. Imipenem versus moxalactam in the treatment of serious infections. Antimicrob Agents Chemother. 1983 Dec;24(6):841–846. doi: 10.1128/aac.24.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimer E. H., Donabedian H., Raeder R., Ribner B. S. Empirical use of imipenem as the sole antibiotic in the treatment of serious infections. J Antimicrob Chemother. 1985 Oct;16(4):499–507. doi: 10.1093/jac/16.4.499. [DOI] [PubMed] [Google Scholar]
- Gonzenbach H. R., Sonnabend W. Moxalactam - ein beta-Lactam-Antibiotikum in Monotherapie bei schweren Infektionskrankheiten in der Chirurgie. Klinisch-bakteriologische Studie an 35 Patienten. Schweiz Med Wochenschr. 1981 Aug 4;111(31-32):1169–1183. [PubMed] [Google Scholar]
- Kahan F. M., Kropp H., Sundelof J. G., Birnbaum J. Thienamycin: development of imipenen-cilastatin. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):1–35. doi: 10.1093/jac/12.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- Kropp H., Sundelof J. G., Hajdu R., Kahan F. M. Metabolism of thienamycin and related carbapenem antibiotics by the renal dipeptidase, dehydropeptidase. Antimicrob Agents Chemother. 1982 Jul;22(1):62–70. doi: 10.1128/aac.22.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp H., Sundelof J. G., Kahan J. S., Kahan F. M., Birnbaum J. MK0787 (N-formimidoyl thienamycin): evaluation of in vitro and in vivo activities. Antimicrob Agents Chemother. 1980 Jun;17(6):993–1000. doi: 10.1128/aac.17.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Comparative in vitro activity of N-formimidoyl thienamycin against gram-positive and gram-negative aerobic and anaerobic species and its beta-lactamase stability. Antimicrob Agents Chemother. 1982 Jan;21(1):180–187. doi: 10.1128/aac.21.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Alestig K., Björnegård B., Burman L. A., Ferber F., Huber J. L., Jones K. H., Kahan F. M., Kahan J. S., Kropp H. Urinary recovery of N-formimidoyl thienamycin (MK0787) as affected by coadministration of N-formimidoyl thienamycin dehydropeptidase inhibitors. Antimicrob Agents Chemother. 1983 Feb;23(2):300–307. doi: 10.1128/aac.23.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Alestig K., Ferber F., Huber J. L., Jones K. H., Kahan F. M., Meisinger M. A., Rogers J. D. Pharmacokinetics and tolerance of N-formimidoyl thienamycin (MK0787) in humans. Antimicrob Agents Chemother. 1983 Feb;23(2):293–299. doi: 10.1128/aac.23.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomkin J. S., Meakins J. L., Jr, Allo M. D., Dellinger E. P., Simmons R. L. Antibiotic trials in intra-abdominal infections. A critical evaluation of study design and outcome reporting. Ann Surg. 1984 Jul;200(1):29–39. doi: 10.1097/00000658-198407000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Jacobus N. V., Gorbach S. L. In vitro activity of N-formimidoyl thienamycin (MK0787). Antimicrob Agents Chemother. 1980 Oct;18(4):642–644. doi: 10.1128/aac.18.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]


