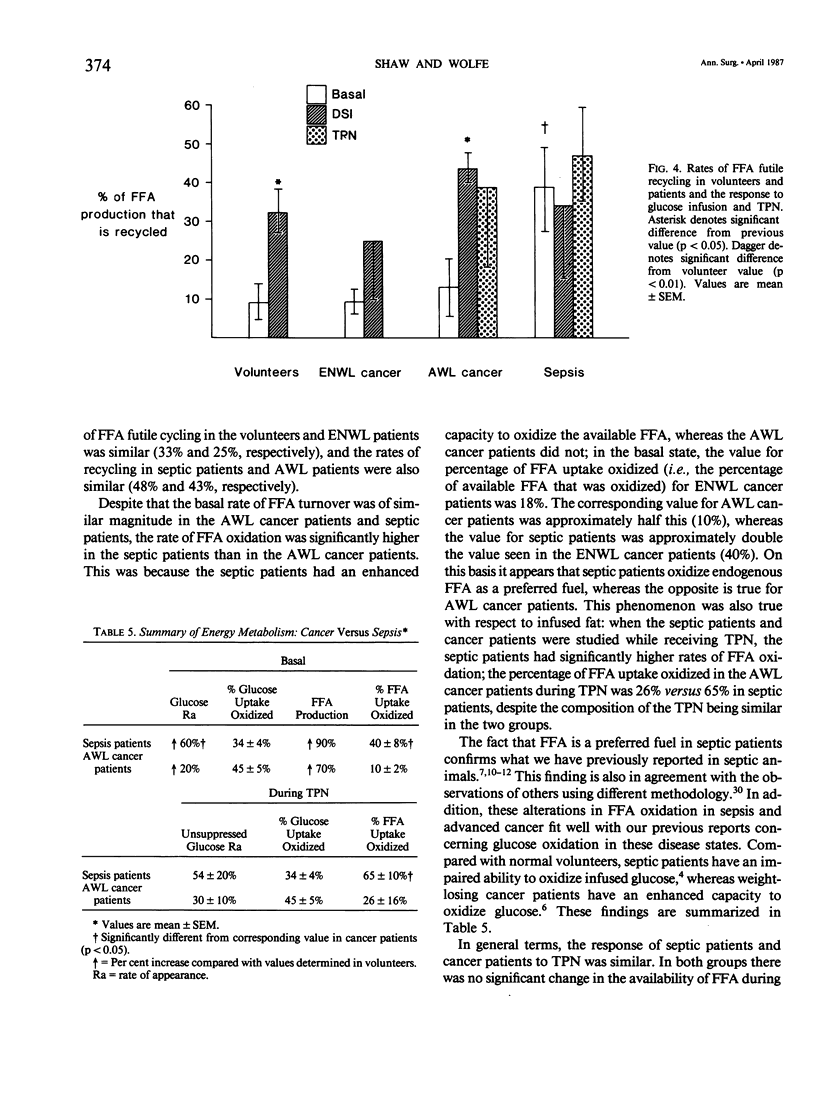
Abstract
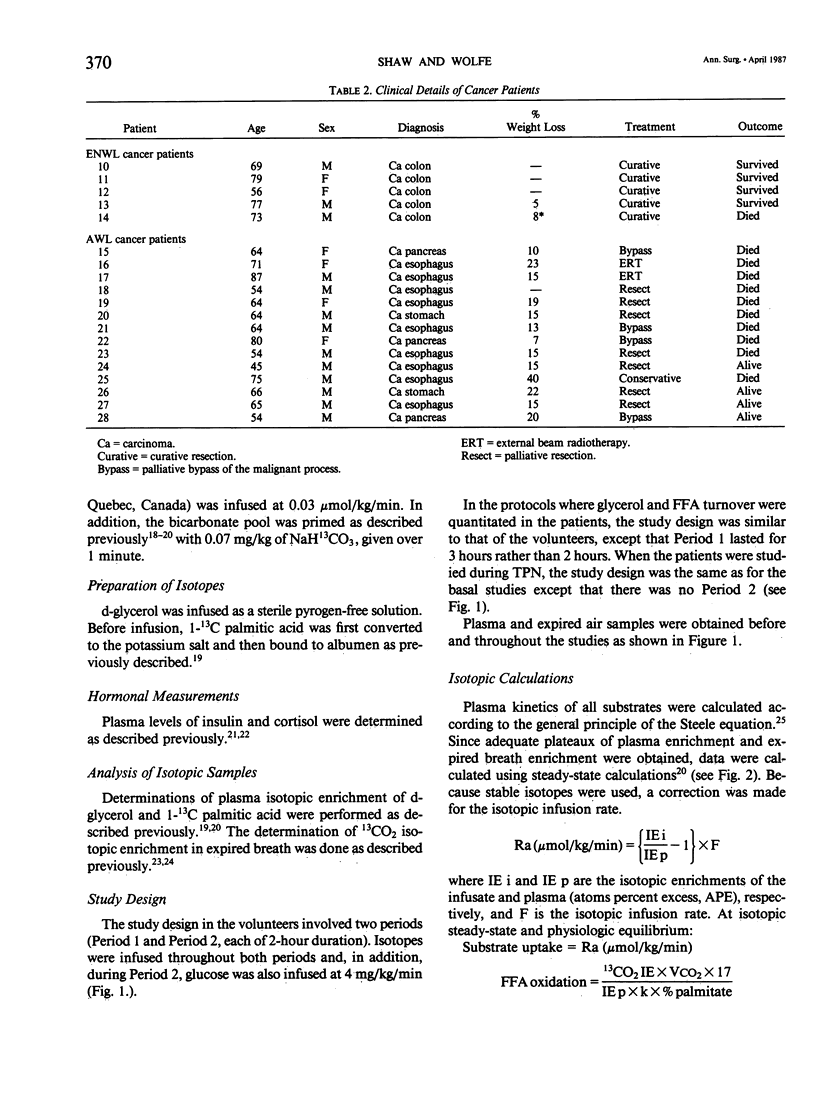
The rates of glycerol and free fatty acid (FFA) kinetics in normal volunteers (VOL), non-weight-losing (NWL) gastrointestinal cancer patients, weight-losing (WL) gastrointestinal cancer patients, and in severely septic patients, using constant infusions of d-glycerol and 1-13C palmitic acid; were determined. Rates of FFA oxidation have also been quantitated. Measurements were made in the basal state, during glucose infusion (4 mg/kg/min), and during total parenteral nutrition (TPN). Rates of glycerol and FFA appearance (Ra) in volunteers and NWL cancer patients were similar, and in both groups there was a significant suppression after glucose infusion. The basal Ra values for glycerol and FFA were 2.4 +/- 0.2 and 6.5 +/- 0.8 mumol/kg/min, respectively, in the volunteers, and in the NWL cancer patients the corresponding values were 2.7 +/- 0.4 and 7.1 +/- 1.1 mumol/kg/min (not significantly different). Compared with the volunteers, the rates of glycerol and FFA turnover were significantly elevated in both septic patients and WL cancer patients. The values for glycerol and FFA Ra were 6.3 +/- 1.1 and 13.1 +/- 3.0 mumol/kg/min, respectively, in the septic patients. The corresponding values were 4.1 +/- 0.4 and 11.7 +/- 1.6 mumol/kg/min in the WL cancer patients. In contrast to the response seen in the volunteers and NWL cancer patients, glucose infusion did not suppress lipolysis in either the septic or WL cancer patients. In all groups studied, glucose infusion resulted in an increase in FFA recycling. Despite the fact that the WL cancer patients had an increased FFA availability, they were significantly less able to oxidize either endogenous FFA or infused lipid when compared with NWL cancer patients (the basal % of FFA uptake oxidized in WL cancer patients was 10 +/- 2% vs. 18 +/- 3% in NWL cancer patients). In contrast, the septic patients had an enhanced capacity to oxidize either endogenous FFA or infused lipid (the basal % of FFA uptake oxidized was 40 +/- 8%, and during TPN this increased in 65 +/- 10%). From these studies the following was concluded: in terms of lipid kinetics, NWL cancer patients are not significantly different from volunteers; WL cancer patients and septic patients have elevated rates of lipolysis, and in contrast to what was seen in NWL cancer patients and in volunteers, glucose infusion in WL cancer patients and in septic patients does not result in a significant inhibition of lipolysis; and WL cancer patients have an impaired capacity to oxidize either endogenous FFA or infused lipid.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Allsop J. R., Wolfe R. R., Burke J. F. Tracer priming the bicarbonate pool. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):137–139. doi: 10.1152/jappl.1978.45.1.137. [DOI] [PubMed] [Google Scholar]
- Buzby G. P., Mullen J. L., Stein T. P., Miller E. E., Hobbs C. L., Rosato E. F. Host-tumor interaction and nutrient supply. Cancer. 1980 Jun 15;45(12):2940–2948. doi: 10.1002/1097-0142(19800615)45:12<2940::aid-cncr2820451208>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Carpentier Y. A., Askanazi J., Elwyn D. H., Jeevanandam M., Gump F. E., Hyman A. I., Burr R., Kinney J. M. Effects of hypercaloric glucose infusion on lipid metabolism in injury and sepsis. J Trauma. 1979 Sep;19(9):649–654. doi: 10.1097/00005373-197909000-00002. [DOI] [PubMed] [Google Scholar]
- Edmonson J. H. Fatty acid mobilization and glucose metabolism in patients with cancer. Cancer. 1966 Feb;19(2):277–280. doi: 10.1002/1097-0142(196602)19:2<277::aid-cncr2820190221>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kralovic R. C., Zepp F. A., Cenedella R. J. Studies of the mechanism of carcass fat depletion in experimental cancer. Eur J Cancer. 1977 Oct;13(10):1071–1079. doi: 10.1016/0014-2964(77)90003-2. [DOI] [PubMed] [Google Scholar]
- Levin L., Gevers W. Metabolic alterations in cancer. Part II. Protein and fat metabolism. S Afr Med J. 1981 Apr 11;59(16):553–556. [PubMed] [Google Scholar]
- Lundholm K., Bylund A. C., Holm J., Scherstén T. Skeletal muscle metabolism in patients with malignant tumor. Eur J Cancer. 1976 Jun;12(6):465–473. doi: 10.1016/0014-2964(76)90036-0. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Sender P. M., James W. P. "Catabolic" loss of body nitrogen in response to surgery. Lancet. 1974 Nov 2;2(7888):1035–1038. doi: 10.1016/s0140-6736(74)92149-7. [DOI] [PubMed] [Google Scholar]
- Robin A. P., Nordenström J., Askanazi J., Carpentier Y. A., Elwyn D. H., Kinney J. M. Influence of parenteral carbohydrate on fat oxidation in surgical patients. Surgery. 1984 May;95(5):608–618. [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Schein P. S., Kisner D., Haller D., Blecher M., Hamosh M. Cachexia of malignancy: potential role of insulin in nutritional management. Cancer. 1979 May;43(5 Suppl):2070–2076. doi: 10.1002/1097-0142(197905)43:5+<2070::aid-cncr2820430715>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Klein S., Wolfe R. R. Assessment of alanine, urea, and glucose interrelationships in normal subjects and in patients with sepsis with stable isotopic tracers. Surgery. 1985 May;97(5):557–568. [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. Response to glucose and lipid infusions in sepsis: a kinetic analysis. Metabolism. 1985 May;34(5):442–449. doi: 10.1016/0026-0495(85)90210-0. [DOI] [PubMed] [Google Scholar]
- Siegel J. H., Cerra F. B., Coleman B., Giovannini I., Shetye M., Border J. R., McMenamy R. H. Physiological and metabolic correlations in human sepsis. Invited commentary. Surgery. 1979 Aug;86(2):163–193. [PubMed] [Google Scholar]
- Waterhouse C., Kemperman J. H. Carbohydrate metabolism in subjects with cancer. Cancer Res. 1971 Sep;31(9):1273–1278. [PubMed] [Google Scholar]
- Wilmore D. W., Goodwin C. W., Aulick L. H., Powanda M. C., Mason A. D., Jr, Pruitt B. A., Jr Effect of injury and infection on visceral metabolism and circulation. Ann Surg. 1980;192(4):491–504. doi: 10.1097/00000658-198010000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. R., Allsop J. R., Burke J. F. Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism. 1979 Mar;28(3):210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Burke J. F. Glucose and lactate metabolism in experimental septic shock. Am J Physiol. 1978 Nov;235(5):R219–R227. doi: 10.1152/ajpregu.1978.235.5.R219. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Evans J. E., Mullany C. J., Burke J. F. Measurement of plasma free fatty acid turnover and oxidation using [1-13C]palmitic acid. Biomed Mass Spectrom. 1980 Apr;7(4):168–171. doi: 10.1002/bms.1200070407. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Shaw J. H., Durkot M. J. Effect of sepsis on VLDL kinetics: responses in basal state and during glucose infusion. Am J Physiol. 1985 Jun;248(6 Pt 1):E732–E740. doi: 10.1152/ajpendo.1985.248.6.E732. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Wolfe M. H., Nadel E. R., Shaw J. H. Isotopic determination of amino acid-urea interactions in exercise in humans. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jan;56(1):221–229. doi: 10.1152/jappl.1984.56.1.221. [DOI] [PubMed] [Google Scholar]



