Abstract
Like neuronal synaptic vesicles, intracellular GLUT4-containing vesicles must dock and fuse with the plasma membrane, thereby facilitating insulin-regulated glucose uptake into muscle and fat cells. GLUT4 colocalizes in part with the vesicle SNAREs VAMP2 and VAMP3. In this study, we used a single-cell fluorescence-based assay to compare the functional involvement of VAMP2 and VAMP3 in GLUT4 translocation. Transient transfection of proteolytically active tetanus toxin light chain cleaved both VAMP2 and VAMP3 proteins in L6 myoblasts stably expressing exofacially myc-tagged GLUT4 protein and inhibited insulin-stimulated GLUT4 translocation. Tetanus toxin also caused accumulation of the remaining C-terminal VAMP2 and VAMP3 portions in Golgi elements. This behavior was exclusive to these proteins, because the localization of intracellular myc-tagged GLUT4 protein was not affected by the toxin. Upon cotransfection of tetanus toxin with individual vesicle SNARE constructs, only toxin-resistant VAMP2 rescued the inhibition of insulin-dependent GLUT4 translocation by tetanus toxin. Moreover, insulin caused a cortical actin filament reorganization in which GLUT4 and VAMP2, but not VAMP3, were clustered. We propose that VAMP2 is a resident protein of the insulin-sensitive GLUT4 compartment and that the integrity of this protein is required for GLUT4 vesicle incorporation into the cell surface in response to insulin.
INTRODUCTION
Glucose enters cells by facilitated diffusion through intrinsic membrane proteins of the glucose transporter (GLUT) family. Insulin increases glucose uptake by mobilizing the GLUT4 isoform from intracellular compartments to the cell surface in fat (Cushman and Wardzala, 1980; Suzuki and Kono, 1980) and muscle tissues (Klip et al., 1987; Douen et al., 1990) as well as in cultured myotubes (Tsakiridis et al., 1994) and adipocytes (Robinson et al., 1992; Clarke et al., 1994). The incorporation of GLUT4-containing vesicles into the plasma membrane after insulin stimulation involves a membrane fusion step (Hashiramoto and James, 1998; Elmendorf and Pessin, 1999; Foster et al., 1999a). Several components of the fusion machinery have been described and are thought to be required for membrane fusion in both neuronal and nonneuronal cells (Rothman and Warren, 1994; Weber et al., 1998; Jahn and Südhof, 1999). The core proteins implicated in this process are termed SNAREs for SNAP (soluble N-ethylmaleimide–sensitive factor attachment protein) receptors (Jahn and Südhof, 1999). The SNARE proteins consist of the synaptobrevin/vesicle-associated membrane protein (VAMP), syntaxin, and SNAP-25 (synaptosome-associated protein of 25 kDa) families (Jahn and Südhof, 1999). These proteins interact through coiled-coil domains in which VAMP provides a single coiled coil and syntaxin and SNAP-25 collectively lend three coiled coils to the final fusion-competent SNARE complex (Fasshauer et al., 1998; Sutton et al., 1998).
Specific SNARE isoforms are expressed in muscle and fat cells, i.e., VAMP2 and VAMP3/cellubrevin (hereafter called VAMP3) (Cain et al., 1992; Volchuk et al., 1994, 1995), syntaxin4 (Sumitani et al., 1995; Volchuk et al., 1996), and SNAP-23 (SNAP-25–like protein of 23 kDa) (Wang et al., 1997; Wong et al., 1997; Rea et al., 1998). The participation of the target membrane (t)-SNAREs syntaxin4 (Cheatham et al., 1996; Olson et al., 1997) and SNAP-23 (Rea et al., 1998; Foran et al., 1999; Foster et al., 1999b) in GLUT4 translocation has been implicated with the use of various molecular and biochemical approaches, such as the introduction of botulinum toxins and neutralizing antibodies into 3T3-L1 adipocytes. There is also strong evidence for the participation of a vesicle (v)-SNARE in this process (Cheatham et al., 1996; Tamori et al., 1996; Chen et al., 1997; Olson et al., 1997; Foran et al., 1999; Millar et al., 1999). The clostridial tetanus and botulinum B or D neurotoxins specifically cleave and inactivate VAMP2 and VAMP3 (Schiavo et al., 1992; Niemann et al., 1994; Jahn et al., 1995; Montecucco and Schiavo, 1995; Tonello et al., 1996). Introduction of botulinum neurotoxin D into streptolysin O–permeabilized 3T3-L1 adipocytes (Cheatham et al., 1996) reduced the insulin-dependent gain in GLUT4 at the surface of 3T3-L1 adipocytes. A reduction in GLUT4 on isolated plasma membranes was also observed after microinjection of cytoplasmic VAMP2 soluble peptides and fusion proteins (Cheatham et al., 1996; Macaulay et al., 1997; Olson et al., 1997; Martin et al., 1998). Although these experiments support the notion of a need for VAMPs in GLUT4 translocation, they do not distinguish which one, VAMP2 or VAMP3, is the protein responsible for GLUT4 arrival at the plasma membrane. This question was raised in one study through the use of immunoglobulin (Ig) A protease, which cleaves several proteins, including VAMP2, but not VAMP3. The toxin caused a reduction of insulin-dependent GLUT4 appearance at the membrane (Cheatham et al., 1996). In a very recent study, fusion proteins containing the cytosolic tail of VAMP3 failed to inhibit GLUT4 translocation in 3T3-L1 adipocytes (Millar et al., 1999).
In contrast to this abundant literature on GLUT4 traffic in fat cells, the possible roles of VAMP2 and VAMP3 in muscle cells have not been explored, despite the importance of muscle to whole body glucose utilization. Recently, we reported that GLUT4 is recruited into a reorganized actin mesh in L6 myotubes exposed to insulin and that actin reorganization is required for the productive exposure of GLUT4 at the cell surface (Khayat et al., 2000). Cytochalasin D (CD)-mediated disruption of the actin cytoskeleton blocked the formation of the actin mesh along with the insulin-dependent stimulation of glucose transport and GLUT4 translocation in these cells (Tsakiridis et al., 1994; Khayat et al., 2000). The purpose of the present study was to determine if VAMPs facilitate GLUT4 externalization in intact muscle cells in culture and to discern the roles of VAMP2 and VAMP3 in GLUT4 translocation. By transient transfection of tetanus toxin alone or in combination with wild-type or toxin-resistant VAMP constructs, we show that only the toxin-resistant VAMP2 mutant restored the toxin-inhibited GLUT4 appearance at the cell surface after insulin stimulation in L6 muscle cells. Likewise, VAMP2, but not VAMP3, accompanied GLUT4 into the insulin-induced cortical actin mesh. Therefore, the integrity of VAMP2, likely a resident insulin-sensitive GLUT4 compartment protein, is required for GLUT4 vesicle incorporation into the muscle cell surface in response to insulin.
MATERIALS AND METHODS
Reagents, Constructs, and Cell Lines
α-MEM, FBS, and other tissue culture reagents were purchased from Life Technologies/GIBCO (Burlington, Ontario [ON]). Bicinchoninic acid reagent was purchased from Pierce (Rockford, IL). Bio-Rad protein assay reagent, all electrophoresis equipment, and polyvinylidene difluoride membranes were purchased from Bio-Rad Laboratories (Mississauga, ON). Brefeldin A was purchased from Sigma-Aldrich (Oakville, ON). Dynabeads M-500 subcellular were purchased from Dynal (Oslo, Norway). Enhanced chemiluminescence reagent was purchased from Amersham (Oakville, ON). Human insulin (Humulin R) was obtained from Eli Lilly Canada (Toronto, ON). pcDNA3 was purchased from Invitrogen (Carlsbad, CA). Indocarbocyanine (Cy3)-conjugated goat anti-mouse and Cy3-conjugated goat anti-rabbit IgGs and HRP-conjugated secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). Monoclonal anti-myc (9E10) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal mouse anti-rat glucose transporter GLUT4 (1F8) antibody was purchased from Genzyme Diagnostics (Cambridge, MA). Polyclonal anti-green fluorescent protein (GFP) antibody, a transferrin-tetramethylrhodamine conjugate, ProLong Antifade coverslip mounting solution, and Oregon green–conjugated phalloidin were purchased from Molecular Probes (Eugene, OR). Rabbit polyclonal antibodies, one raised to the N-terminal 20 amino acids of VAMP3 and the other raised to the cytosolic amino acids of a GST-VAMP2 fusion protein, were prepared as described (Volchuk et al., 1995). Sheep anti-mouse and nonimmune IgGs were purchased from ICN Biomedicals (Aurora, OH). A mAb to the light chain of tetanus toxin (TeTx) and TeTx cDNA in pcDNA3 were obtained from Dr. Heiner Niemann (Medizinische Hoshschule, Hannover, Germany). A mouse mAb to giantin was a gift from Dr. Hans-Peter Hauri (University of Basel, Basel, Switzerland). A rabbit polyclonal antibody to α-mannosidase II was a gift from Dr. Marilyn G. Farquhar (University of California, San Diego, CA).
Mammalian expression vectors for Enhanced Green Fluorescent Protein or Enhanced Green Fluorescent Protein fusion proteins, pEGFP and pEGFP-N1, were purchased from Clontech (Palo Alto, CA). Restriction enzymes, ligase, and polymerase were purchased from New England Biolabs (Mississauga, ON). Maxi-prep tip DNA purification columns and Effectene transfection kits were purchased from Qiagen (Mississauga, ON). GLUT4 protein with an exofacial myc epitope (GLUT4myc) cDNA was constructed by inserting the human c-myc epitope (14 amino acids) into the first ectodomain of GLUT4 and subcloned into the pCXN2 expression vector (Kanai et al., 1993). A clone of L6 skeletal muscle cells isolated for high fusion potential (Mitsumoto et al., 1991) was transfected with pCXN2-GLUT4myc to create a stable cell line, L6-GLUT4myc (L6 myoblasts stably expressing GLUT4myc protein) (Kishi et al., 1998). Constructs for expression of GFP fusion proteins of VAMP2 (V2-GFP) and VAMP3 (V3-GFP) were prepared with the use of the pEGFP-N1 vectors as described (Bajno et al., 2000). The toxin-resistant/insensitive (VW) mutants of the V2-GFP and V3-GFP chimeras were made by mutating the codons for Gln76Phe77 or Gln63Phe64 (CAGTTT), respectively, to encode for Val/Trp (GTGTGG) with the use of the Quickchange kit (Stratagene, La Jolla, CA) as described (Regazzi et al., 1996). All DNA constructs used in transfections were prepared with the use of Qiagen Maxi-prep columns according to the manufacturer's recommendations. For some experiments, L6-GLUT4myc myoblasts were differentiated into L6-GLUT4myc myotubes as described previously (Mitsumoto and Klip, 1992).
Cell Culture and Transfection of L6-GLUT4myc Cells
L6-GLUT4myc myoblasts were maintained in α-MEM culture medium supplemented with 10% (vol/vol) FBS in a humidified atmosphere containing 5% CO2 and 95% air at 37°C. Cells were seeded at a density of ∼2 × 105 cells/well onto 25-mm glass coverslips in six-well tissue culture plates or at a density of ∼2 × 106 cells per 10-cm dish for GLUT4 vesicle immunoadsorption. The next day, transfections were performed according to the Effectene product manual, with 6 μl of the Effectene reagent (or 25 μl for GLUT4 vesicle immunoadsorption) used per transfection condition (as indicated in figure legends). DNA was introduced to the cells for 5 h, and the cells were washed twice with PBS and maintained in culture medium for another 43 h until experimentation. These cells were deprived of serum in culture medium for 3 h at 37°C before processing for immunofluorescence, cell lysis, or GLUT4 vesicle immunoisolation.
Immunofluorescence
Indirect immunofluorescence for expression of cDNA constructs was performed as indicated (Piper et al., 1991) with slight modifications. The following steps were performed at room temperature unless indicated otherwise. Serum-starved cells were incubated with 10 μg/ml brefeldin A for 30 min at 37°C before the immunofluorescence assay described hereafter only for disruption of the Golgi complex as needed. After serum deprivation, cells were rinsed quickly three times with ice-cold PBS (100 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 50 mM NaH2PO4/Na2HPO4, pH 7.4) on ice and fixed with 4% (vol/vol) paraformaldehyde (PFA) in PBS for 30 min (initiated at 4°C and shifted immediately to room temperature). The cells were rinsed once with PBS, and unreacted fixative was quenched with 100 nM glycine in PBS for 10 min. Cells were permeabilized with 0.1% (vol/vol) Triton X-100 in PBS for 5 min, washed three times with PBS, and blocked with 5% (vol/vol) goat serum in PBS for 10 min. To detect the expression of various proteins, coverslips were incubated with primary antibody in 5% (vol/vol) goat serum in PBS (VAMP3 antiserum, 1:300; VAMP2 antiserum, 1:150; anti-myc 9E10, 1:100; anti-TeTx, 1:1000; anti-giantin, 1:750; anti-α-mannosidase II, 1:1000) for 1 h. Cells were rinsed three times with PBS and incubated with secondary antibody (Cy3-conjugated goat anti-mouse or anti-rabbit IgG, 1:1000) for 1 h in the dark. For labeling of actin filaments, fixed and permeabilized cells were incubated with Oregon green—-conjugated phalloidin (0.01 U) for 1 h during secondary antibody incubation. The cells were again washed three times with PBS for 5 min while shielded from light, rinsed twice with distilled water, and then mounted with 10 μl of Antifade solution. Mounted coverslips were stored at 4°C to set before analysis with Leica TCS (Leica Mikroscopie Systeme GmbH, Wetzlar, Germany) 4D fluorescence or confocal microscopes.
GLUT4myc Translocation Assay
After serum deprivation, cells were left untreated or treated with 100 nM insulin (20 min, 37°C). Indirect immunofluorescence for GLUT4myc translocation was carried out on intact cells as described (Kishi et al., 1998). The following steps were performed at 4°C unless indicated otherwise. Cells were quickly rinsed three times with ice-cold PBS before being incubated with 3% (vol/vol) PFA in PBS for 2 min, followed by 100 nM glycine in PBS for 10 min. The cells were blocked with 5% (vol/vol) goat serum in PBS for 10 min. To detect GLUT4myc, coverslips were incubated with anti-myc (9E10, 1:100) for 1 h, rinsed five times with PBS, and incubated in the dark with secondary antibody (Cy3-conjugated goat anti-mouse IgG, 1:1000) for 1 h. The cells were rinsed three times for 5 min each with PBS while shielded from light, fixed with 4% (vol/vol) PFA in PBS for 30 min, followed by quenching with 100 nM glycine, three PBS washes for 5 min each, and two distilled water rinses at room temperature. The coverslips were mounted, stored, and viewed as described above.
Transferrin-Rhodamine Endocytosis
Loading of cells with rhodamine-conjugated transferrin was performed as described (Siddhanta et al., 1998) with some modifications. The cells were incubated with 5 μg/ml rhodamine-conjugated transferrin for the last hour of serum starvation at 37°C. The cells were then washed three times for 5 min with ice-cold PBS on ice, followed by fixation with 4% (vol/vol) PFA for 30 min (initiated at 4°C and shifted immediately to room temperature), quenching with 100 nM glycine, three PBS washes for 5 min each, and two distilled water rinses. The coverslips were mounted, stored, and viewed as described above.
Lysate Preparation from Toxin-transfected Cells
On the day of processing, transfected cells were lysed with 1 ml of lysis buffer (150 mM NaCl, 0.25% [wt/vol] deoxycholate, 1% [vol/vol] NP-40, 0.1% [wt/vol] SDS, 2 μM pepstatin, 5 μM leupeptin, 2 mM PMSF, 50 mM Tris-HCl, pH 7.2). After protein analysis with the use of the bicinchoninic acid method, equal amounts of protein from each sample were precipitated and resuspended in Laemmli sample buffer. Samples were stored at −20°C until use.
Immunoadsorption of GLUT4 Vesicles
Magnetic Dynabeads (M-500) were conjugated to secondary and primary antibodies according to the Dynal product manual. Briefly, 107 magnetic beads were covalently bound to 10 μg of pure sheep anti-mouse IgG secondary antibody. Subsequently, 2 μg of monoclonal mouse anti-rat glucose transporter GLUT4 (1F8) or nonimmune mouse IgG was reacted to every 100 μl of washed secondary antibody–conjugated magnetic beads. Beads were stored at 4°C in PBS with 0.1% (vol/vol) BSA and 0.02% (vol/vol) sodium azide until use.
Cells were washed twice with PBS, collected in 3 ml of homogenization buffer (255 mM sucrose, 4 mM Na2EDTA, 20 mM HEPES, 1 μM pepstatin, 1 μM leupeptin, 200 μM PMSF, pH 7), and homogenized in a cell cracker (20 strokes). Cell homogenates were centrifuged for 5 min at 1500 × g at 4°C in an IEC HN SII centrifuge (International Equipment Company, Needham, MA) to remove nuclei and unbroken cells. Supernatants were collected and centrifuged in a Beckman Instruments (Fullerton, CA) ultracentrifuge for 20 min at 34,000 rpm at 4°C in a TLA 100.3 rotor to obtain pellets of crude plasma membrane and supernatants of light density microsomes with cytosol. The plasma membrane pellets were resuspended directly in Laemmli sample buffer. After protein analysis of the supernatants with the use of the Bio-Rad protein assay method, 800 μg of protein from each sample made up to 1 ml total volume with PBS and 100 mM Na2PO4 were added to 100 μl of antibody-conjugated magnetic beads for immunoprecipitation with rotation overnight at 4°C. Beads were collected by the magnet, and supernatants in addition to four subsequent washes with PBS were pooled and centrifuged for 60 min at 75,000 rpm at 4°C in a TLA 100.3 rotor to sediment light density microsome pellets devoid of GLUT4 vesicles. Total light density microsomes was also centrifuged for 60 min at 75,000 rpm at 4°C in a TLA 100.3 rotor to obtain light density microsome pellets. The light density microsomes and immune pellets after GLUT4 vesicle immunoprecipitation were resuspended directly in Laemmli sample buffer. Samples were stored at −20°C until use.
Electrophoresis and Immunoblotting
Protein samples were separated by 10 or 12% (vol/vol) SDS-PAGE as indicated and electrotransferred onto polyvinylidene difluoride membranes as described previously (Foster et al., 1998). Immunoreactive bands were visualized with HRP-conjugated goat anti-rabbit IgG for polyclonal antibodies, as indicated, by means of an enhanced chemiluminescence detection technique.
Quantitation and Statistical Analysis
Several representative images of either VAMP overexpression or GLUT4myc translocation from at least three separate experiments were quantitated with the use of NIH Image software (National Institutes of Health, Bethesda, MA). Raw data for VAMP overexpression were converted to fold expression relative to endogenous VAMP expression levels in untransfected cells. Raw data for GLUT4myc translocation were converted to fold stimulation by insulin above basal levels relative to surface GLUT4myc in untransfected cells. Statistical analyses were carried out with analysis of variance (Fisher, multiple comparisons).
RESULTS
VAMPs Partially Colocalize with GLUT4 in L6-GLUT4myc Myoblasts
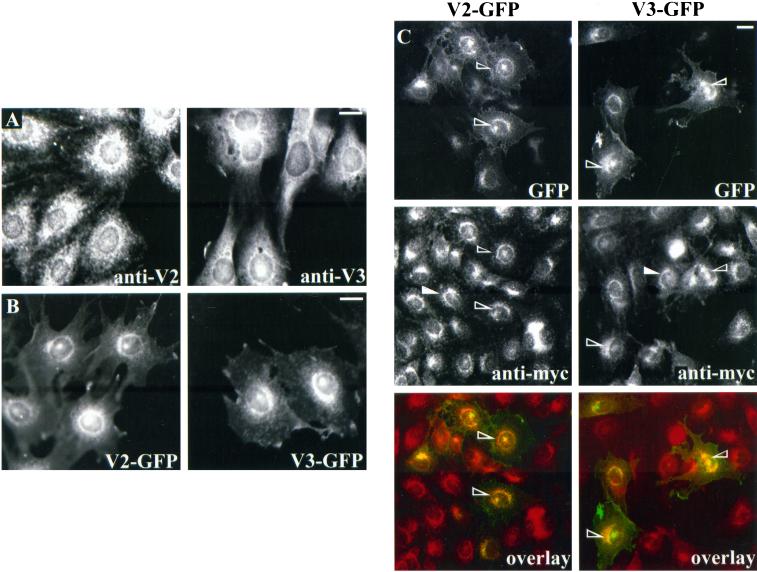
We and others have previously detected VAMP2 and VAMP3/cellubrevin in GLUT4-containing intracellular membranes in both 3T3-L1 adipocytes (Volchuk et al., 1995) and primary adipocytes (Cain et al., 1992). Such information was not available for L6 muscle cells. Hence, we first examined the spatial relationship between GLUT4 and VAMP2 or VAMP3 in L6-GLUT4myc myoblasts (L6 myoblasts stably expressing GLUT4myc protein). By immunofluorescence analysis of the endogenous proteins, both VAMP2 and VAMP3 were largely localized to the perinuclear region, but abundant punctate structures were also seen throughout the cytosol (Figure 1A). cDNAs encoding GFP fusion protein versions of VAMP2 or VAMP3 (V2-GFP or V3-GFP, respectively) were transiently transfected into L6-GLUT4myc cells, and the expression of the corresponding proteins was analyzed 48 h later by detecting the GFP signal. The transfected proteins showed distributions similar to those of their endogenous counterparts, i.e., a preferential perinuclear localization with additional punctate elements across the cytoplasm (Figure 1B). The level of expression of V2-GFP and V3-GFP was about twice that of the endogenous proteins, as detected by quantifying the immunofluorescence signal of anti-VAMP2 or anti-VAMP3 antibodies in transfected cells relative to untransfected cells. Together, these results suggest that the transfected GFP-v-SNAREs have similar subcellular localizations to their endogenous counterparts, and mild overexpression did not affect their sorting. The distribution of V2-GFP and V3-GFP was then compared with that of GLUT4myc by examining the GFP signals and the indirect immunofluorescence of the myc epitope. Both v-SNAREs colocalized in part with GLUT4myc, especially in the perinuclear region (Figure 1C, open arrowheads). By confocal microscopy of L6-GLUT4myc cells, the endogenous v-SNAREs colocalized with GLUT4myc within the perinuclear region and were also found in punctate structures throughout the cytoplasm (our unpublished results). Importantly, the intracellular distribution and intensity of GLUT4myc fluorescence was not altered by cotransfection of the GFP-v-SNAREs compared with L6 myoblasts expressing only GLUT4myc (Figure 1C, closed arrowheads).
Figure 1.
Subcellular distribution of VAMP2 and VAMP3 GFP chimeras in L6-GLUT4myc myoblasts. (A) Unstimulated L6-GLUT4myc myoblasts were prepared for indirect immunofluorescence to measure endogenous VAMP2 and VAMP3 expression with anti-VAMP2 (anti-V2) and anti-VAMP3 (anti-V3) IgGs, respectively. (B) L6-GLUT4myc myoblasts were transfected with 0.6 μg of V2-GFP or V3-GFP cDNA and processed for fluorescence microscopy. Shown is one representative image of the expression of each construct in unstimulated cells. (C) L6-GLUT4myc myoblasts were transfected with 0.6 μg of V2-GFP or V3-GFP cDNA and processed for indirect immunofluorescence with the use of anti-myc IgG. Shown is the green fluorescence of either chimera (top panels) or the anti-myc staining (middle panels) in the same field of view. The open arrowheads point to some V2-GFP (left) or V3-GFP (right) transfected cells, and the closed arrowheads point to some untransfected cells. The bottom panels show composite images of the GFP fluorescence with GLUT4myc staining. Bars, 5 μm.
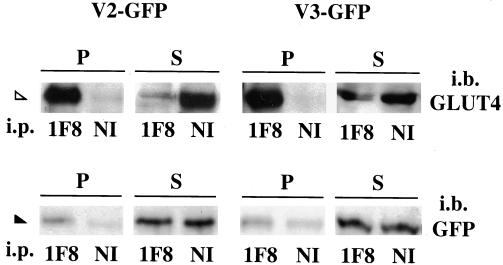
To complement the immunofluorescence comparison of the v-SNAREs and GLUT4, we used a biochemical approach. Intracellular GLUT4-containing compartments were immunopurified from L6-GLUT4myc myoblasts transiently transfected with V2-GFP or V3-GFP with the use of a mAb directed to the cytoplasmically exposed C terminus of GLUT4myc coupled to magnetic beads. By this method, all intracellular membranes containing GLUT4 were quantitatively sedimented, as revealed by immunodetection of GLUT4 in the pellet but not in the supernatant fractions, with the use of a polyclonal anti-GLUT4 antibody (Figure 2, top). The immune pellets and supernatants were probed for GFP to detect the transfected v-SNAREs. Small but detectable amounts of both V2-GFP and V3-GFP were found in the immune pellets and were absent from parallel pellets prepared with the use of nonimmune, irrelevant mouse IgG (Figure 2, bottom). Similar results were obtained with GLUT4-immunoadsorbed membranes from untransfected cells that were probed for the endogenous VAMP2 or VAMP3 (our unpublished results). These results suggest that both VAMP2 and VAMP3 are partially housed in some of the GLUT4 compartments and further highlight the need to distinguish their individual functional role in GLUT4 traffic.
Figure 2.
VAMP2 and VAMP3 GFP chimeras are found on intracellular GLUT4myc membrane compartments. L6-GLUT4myc myoblasts were transfected with 2 μg of V2-GFP (left) or V3-GFP (right) cDNA, and cells were fractionated to yield internal membranes plus cytosol. Intracellular GLUT4 vesicles were immunoadsorbed from the internal membrane-containing fractions with the use of monoclonal GLUT4 antibody (1F8) or nonimmune mouse IgG (NI). The resulting immune pellets (P) and supernatants (S) were separated by 12% SDS-PAGE and immunoblotted with polyclonal anti-GLUT4 (top panels) or anti-GFP (bottom panels) IgGs. Shown are results from one of four representative experiments. The open and closed arrowheads point to GLUT4 and the GFP-v-SNAREs, respectively.
Tetanus Toxin Efficiently Cleaves Wild-Type but Not Toxin-resistant VAMPs
Clostridial neurotoxins have been a useful molecular tool to examine the role of SNAREs in the control of exocytic events in various cell systems (Schiavo et al., 1992; Niemann et al., 1994; Jahn et al., 1995; Montecucco and Schiavo, 1995; Tonello et al., 1996). Here we used clostridial tetanus toxin, which inactivates VAMP2 and VAMP3 by cleaving at the bond between amino acids Gln76Phe77 and Gln63Phe64, respectively (Schiavo et al., 1992). Toxin-resistant mutants of these proteins containing amino acids Val and Trp for Gln and Phe in these positions (VW mutants) are relatively toxin-insensitive (Regazzi et al., 1996). L6-GLUT4myc myoblasts were transiently cotransfected with cDNAs encoding GFP and the proteolytically active TeTx, and the expression of TeTx protein was examined 48 h later. TeTx exhibited a rather diffuse cytoplasmic distribution as detected with anti-TeTx antibody. TeTx was also expressed almost exclusively in GFP-expressing myoblasts (99.6 ± 2.5%, n = 30 fields of transfected cells that expressed both constructs).
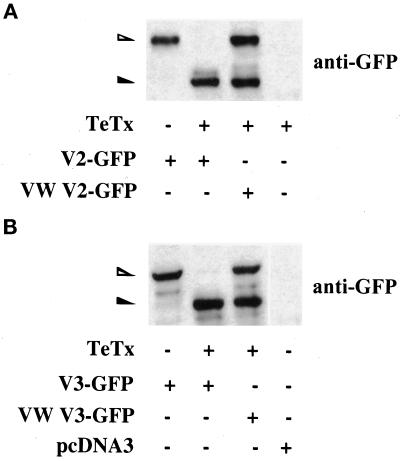
To detect the action of TeTx on VAMP integrity within the cells, we cotransfected the toxin along with either V2-GFP or V3-GFP and then analyzed cell lysates upon SDS-PAGE for immunoreactivity to anti-GFP antibody. Coexpression of TeTx effectively cleaved cotransfected V2-GFP and V3-GFP (Figure 3). TeTx did not cleave other proteins such as the t-SNAREs syntaxin4 or SNAP-23 (our unpublished results). In contrast to their endogenous counterparts, cotransfected VW mutants of V2-GFP and V3-GFP were significantly resistant to toxin cleavage, so that after 48 h of coexpression approximately half of the VW mutant proteins remained intact (Figure 3). Thus, under these conditions, abundant VW proteins were available and likely to carry out native VAMP functions.
Figure 3.
Tetanus toxin light chain can effectively cleave wild-type but not toxin-resistant VAMP2 or VAMP3 GFP chimeras in L6-GLUT4myc myoblasts. L6-GLUT4myc myoblasts were transfected with 0.6 μg of wild-type or toxin-resistant (VW) V2-GFP (A) or V3-GFP (B) cDNA in the absence (−) or presence (+) of 0.9 μg of TeTx as shown. In addition, L6-GLUT4myc myoblasts were transfected with TeTx (A) or pcDNA3 alone (B) as indicated. These latter samples showed no nonspecific cross-reactivity with the anti-GFP IgG. Total cell lysates from all samples were separated by 10% SDS-PAGE and immunoblotted with anti-GFP IgG. The open and closed arrowheads point to the uncleaved and cleaved fragments of the GFP-v-SNAREs, respectively.
2Tetanus Toxin Causes Redistribution of Wild-Type but Not of Toxin-resistant VAMPs
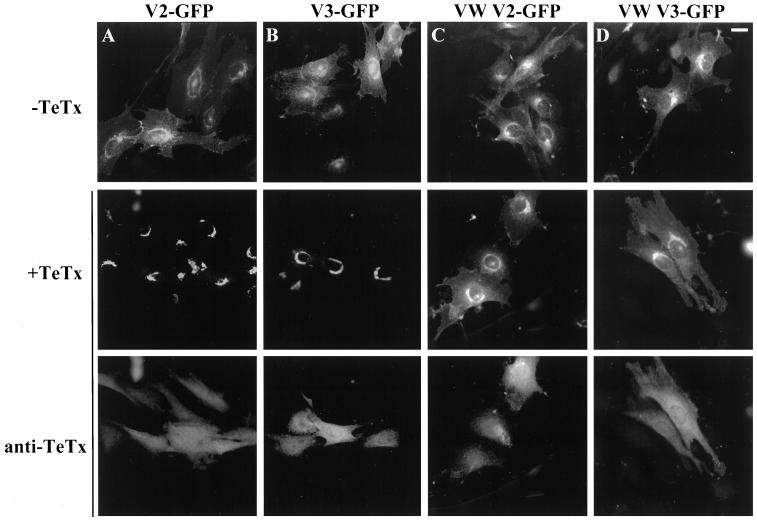
To begin to explore the functional consequence of TeTx action on v-SNAREs, we examined the localization of the V2-GFP and V3-GFP chimeras after toxin coexpression. Interestingly, the expression patterns of the remaining C-terminal portions of V2-GFP and V3-GFP (i.e., containing the C-terminal halves of the VAMPs linked to GFP) after TeTx cleavage were altered to form tight, almost circular perinuclear bands (Figure 4, A and B). This location is reminiscent of the position of Golgi elements and/or recycling endosomes. In contrast to the compacted distribution of the cleaved V2-GFP and V3-GFP proteins, the localization of the VW versions of these v-SNAREs was not appreciably changed by TeTx, showing only a slightly higher fluorescence intensity about the perinuclear region than the toxin-sensitive v-SNAREs (Figure 4, C and D). These observations suggest that the altered distribution of the V2-GFP and V3-GFP cleaved proteins generated by the toxin is related to its proteolytic action.
Figure 4.
Tetanus toxin causes the perinuclear clustering of wild-type but not toxin-resistant VAMP2 or VAMP3 GFP chimeras in L6-GLUT4myc myoblasts. L6-GLUT4myc myoblasts were transfected with 0.6 μg of wild-type or toxin-resistant (VW) V2-GFP (A and C) or V3-GFP (B and D) cDNA with (+) or without (−) 0.9 μg of TeTx as indicated. Shown is the green fluorescence of either chimera in the absence (top panels) or presence (middle panels) of TeTx. Toxin-transfected cells (middle panels) were also processed for indirect immunofluorescence with anti-TeTx IgG. The corresponding anti-TeTx staining of these cells is shown in the bottom panels from the same field of view, as indicated by the vertical line. Bar, 5 μm.
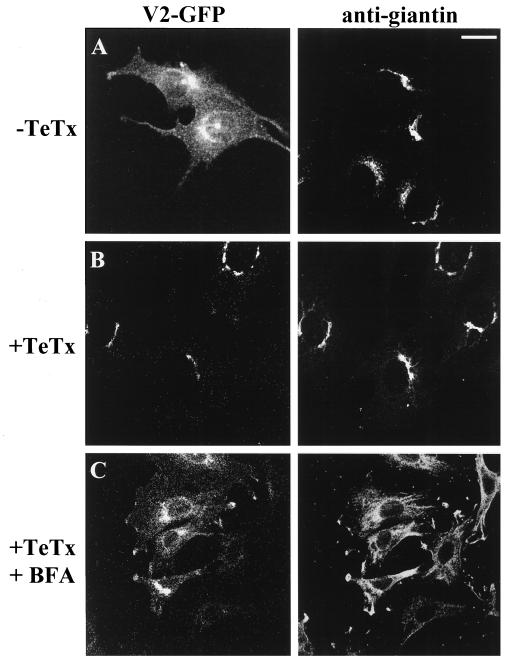
To identify the nature of the compartment housing the cleaved v-SNAREs, we compared the localization of V2-GFP with that of giantin and α-mannosidase II, both Golgi-specific markers. In the absence of TeTx treatment, there was only a small colocalization of the intact V2-GFP with giantin (Figure 5A) and α-mannosidase II (our unpublished results). However, upon TeTx transfection, the remaining C-terminal fragments of cleaved V2-GFP colocalized to a great extent in a tight perinuclear region with giantin (Figure 5B) and α-mannosidase II, as shown by confocal microscopy analysis. After treatment with brefeldin A, a drug that is known to cause the dispersal of the Golgi complex, the cleaved V2-GFP fragments generated by the toxin were dispersed into the cytoplasm away from the very tight perinuclear clustering along with giantin (Figure 5C) and α-mannosidase II. Similar behavior was displayed by the toxin-sensitive V3-GFP but not by the toxin-resistant versions of these v-SNAREs (our unpublished results). These results suggest that the proteolytic action of the toxin on the v-SNAREs precludes their exit from the Golgi during their biosynthesis.
Figure 5.
Tetanus toxin prevents exit of VAMP2 or VAMP3 GFP chimeras from the Golgi elements in L6-GLUT4myc myoblasts. L6-GLUT4myc myoblasts were transfected with 0.6 μg of V2-GFP cDNA in the absence (A) or presence (B and C) of 0.9 μg of TeTx and were subsequently left untreated (A and B) or treated with brefeldin A (C). All cells were processed for indirect immunofluorescence with the use of anti-giantin IgG. Shown is the green fluorescence of V2-GFP (left) or the giantin staining (right) in the same field of view without TeTx (top panels), with TeTx (middle panels), or with both TeTx and brefeldin A treatment (bottom panels). Bar, 5 μm.
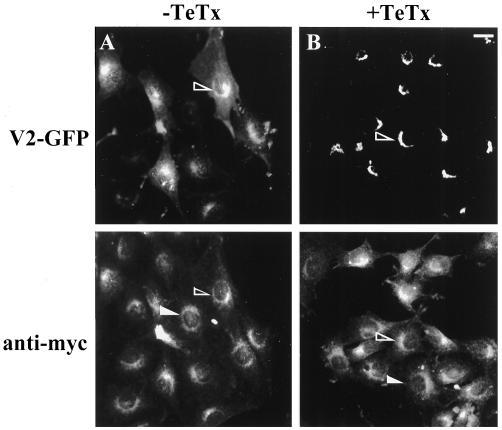
The retention of the V2-GFP and V3-GFP fragments at the Golgi by TeTx prompted us to examine whether the toxin affected the localization of other proteins, in particular GLUT4myc. Importantly, the toxin did not alter intracellular GLUT4myc distribution, which still showed a loose perinuclear localization and a presence in small cytoplasmic elements (Figure 6). In addition, to assess if the toxin had affected the recycling endosomal system, we examined the effect of TeTx expression on the distribution of rhodamine-labeled transferrin taken up by L6-GLUT4myc cells via the transferrin receptor (TfR) under steady-state conditions. The signal was observed mostly in a diffuse perinuclear region, and this pattern was not affected by TeTx transfection (our unpublished results). These results suggest that TeTx treatment did not prevent the endocytosis of proteins into the recycling endosomal system, nor did it affect the intracellular GLUT4myc and TfR-containing compartments. This allowed us to test the effect of TeTx on GLUT4myc translocation.
Figure 6.
Tetanus toxin does not alter GLUT4myc-containing compartments in L6-GLUT4myc myoblasts. L6-GLUT4myc myoblasts were transfected with 0.6 μg of V2-GFP cDNA in the absence (A) or presence (B) of 0.9 μg of TeTx and processed for indirect immunofluorescence with the use of anti-myc IgG. Shown is the green fluorescence of V2-GFP (top panels) and the immunofluorescence detected by the anti-myc IgG (bottom panels) in the same field of cells. The open and closed arrowheads point to some transfected and untransfected cells, respectively, in either panel. Bar, 5 μm.
Tetanus Toxin Inhibits Insulin-stimulated GLUT4myc Translocation
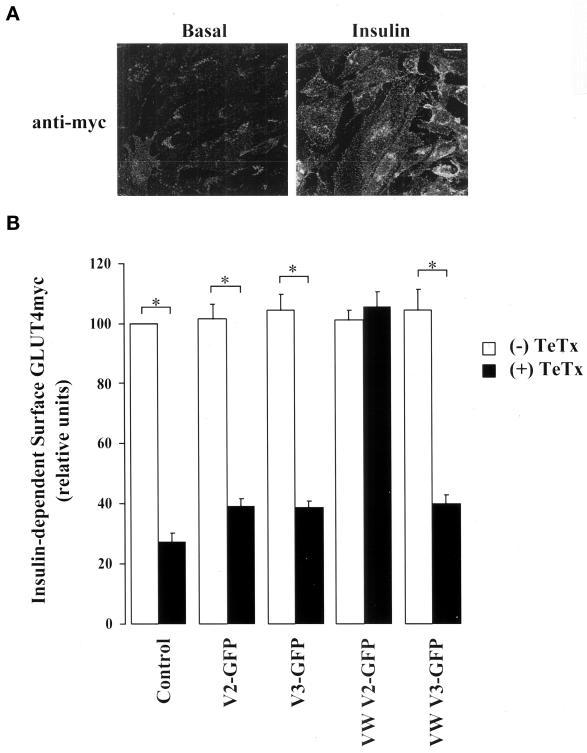
In L6-GLUT4myc myoblasts, the GLUT4myc protein segregates intracellularly along with the insulin-regulated aminopeptidase (IRAP) and away from GLUT1 into an insulin-sensitive compartment (Ueyama et al., 1999). In response to insulin, GLUT4myc is readily recruited to the plasma membrane, resulting in a twofold gain in surface-exposed GLUT4myc (Ueyama et al., 1999; Wang et al., 1999). The insulin effect is illustrated by immunofluorescence detection of the myc epitope in intact L6-GLUT4myc myoblasts (Figure 7A). Fluorescence quantification showed that insulin caused its typical twofold increase in cell surface GLUT4myc. Expression of V2-GFP or V3-GFP did not significantly alter the level of cell surface–exposed GLUT4myc in the presence of insulin (Figure 7B) or under basal conditions (our unpublished results). Importantly, the transient transfection of TeTx markedly reduced the insulin-stimulated incorporation of GLUT4myc to the cell surface (by ∼70%). However, the toxin did not alter the basal levels of surface GLUT4myc in unstimulated cells under parallel experimental conditions (our unpublished results). Coexpression of V2-GFP or V3-GFP did not improve the insulin response in cells expressing TeTx, because surface GLUT4myc levels were not statistically different from those in “control” cells transfected with toxin alone (Figure 7B). These results suggest a requirement for VAMPs in insulin-dependent GLUT4 traffic.
Figure 7.
Tetanus toxin inhibits insulin-stimulated GLUT4myc translocation in L6-GLUT4myc myoblasts. (A) Intact L6-GLUT4myc myoblasts under basal (left) or insulin-stimulated (right) conditions were processed for indirect surface immunofluorescence with anti-myc IgG. Bar, 5 μm. (B) L6-GLUT4myc myoblasts were transfected with 0.6 μg of wild-type or toxin-resistant (VW) V2-GFP or V3-GFP cDNA in conjunction with 0.9 μg of pcDNA3 (open bars) or TeTx cDNA (closed bars) as indicated. After 48 h, cells were serum-starved for 3 h and exposed to 100 nM insulin for 30 min. Surface GLUT4myc was detected as described above, and anti-myc surface staining in transfected and untransfected cells from 6–10 fields of view from at least three experiments was quantitated with the use of NIH Image software. A value of 100% was assigned to the insulin response above basal in untransfected cells treated with insulin in each field of view. The pixel intensity of the transfected cells in the same field of view was calculated as a fraction of this value for each experimental condition examined. Shown are the means ± SE of the insulin response above basal for each experimental condition; p < 0.01 relative to (*) untransfected cells or VAMP-transfected cells. Insulin caused an approximately twofold increase in surface GLUT4myc levels above basal in untransfected cells.
Toxin-resistant VAMP2 Rescues Cell Surface Incorporation of GLUT4myc
Because TeTx cleaved both VAMP2 and VAMP3 (Figure 3), the function of the individual VAMP isoforms in the fusion of GLUT4 vesicles was still unresolved. By cotransfecting each VW mutant into TeTx-transfected L6-GLUT4myc myoblasts, we sought to define which of the VAMPs was responsible for insulin-stimulated GLUT4myc arrival at the membrane. A complete rescue of GLUT4myc appearance at the cell surface was obtained in response to insulin in cells cotransfected with TeTx and VW V2-GFP (Figure 7B). This was not the case with VW V3-GFP, which failed to restore GLUT4myc surface labeling after insulin stimulation (Figure 7B). Neither VW V2-GFP nor VW V3-GFP on their own altered GLUT4myc levels at the cell surface in the presence (Figure 7B) or absence of insulin (our unpublished results). Collectively, these results suggest that VAMP2, but not VAMP3, participates in insulin-dependent GLUT4myc translocation.
VAMP2 Segregates Along with GLUT4myc into a Cortical Actin Mesh
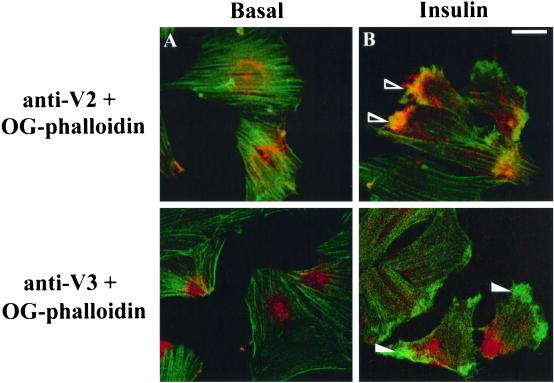
We have recently shown that insulin causes a rapid reorganization of cortical actin that appears to draw phosphatidylinositol 3-kinase (PI 3-kinase) to GLUT4myc-containing vesicles in L6 myotubes (Khayat et al., 2000). In that study, we hypothesized that the GLUT4myc vesicles gathered into the actin mesh represent the insulin-sensitive intracellular compartment. A prediction of the functional studies described above (Figure 7) would be that at least a fraction of the cellular complement of VAMP2 is present in this insulin-sensitive GLUT4myc-containing compartment. To further explore this possibility, we examined whether VAMP2 or VAMP3 is drawn into the cortical actin mesh induced by insulin. L6-GLUT4myc cells in the myoblast or myotube stage were untreated or stimulated for 10 min with 100 nM insulin, and subsequently, endogenous VAMP2 or VAMP3 as well as filamentous actin was examined by immunofluorescence or with the use of Oregon green–conjugated phalloidin, respectively. A marked cortical actin reorganization is shown to occur in both insulin-stimulated myoblasts (Figure 8B) and myotubes (Khayat et al., 2000). A striking difference in VAMP localization was also observed: whereas a distinct VAMP2 signal was detected in the actin mesh (Figure 8B, top), VAMP3 clearly appeared to escape this structure (Figure 8B, bottom). These results suggest that VAMP2 and VAMP3 may populate distinct vesicles and that only GLUT4 membranes containing VAMP2 gather into the actin mesh in response to insulin (Figure 9). Future studies should address a further biochemical characterization of the two pools.
Figure 8.
VAMP2, but not VAMP3, colocalizes with the dorsal actin-rich meshwork after insulin stimulation. L6-GLUT4myc myoblasts were left untreated (A) or stimulated with insulin (B) and processed for indirect immunofluorescence for endogenous VAMP2 or VAMP3 with anti-VAMP2 (top panels) or anti-VAMP3 (bottom panels) IgGs, respectively, along with F-actin staining with the use of Oregon green–conjugated phalloidin. Shown are composite images of the immunofluorescence of anti-VAMP2 (anti-V2) or anti-VAMP3 (anti-V3) staining with Oregon green–conjugated phalloidin labeling (OG-phalloidin). The arrowheads point to examples of the actin mesh structures induced by insulin in myoblasts, where the open and closed arrowheads show recruitment of VAMP2 or absence of VAMP3, respectively. Bar, 5 μm.
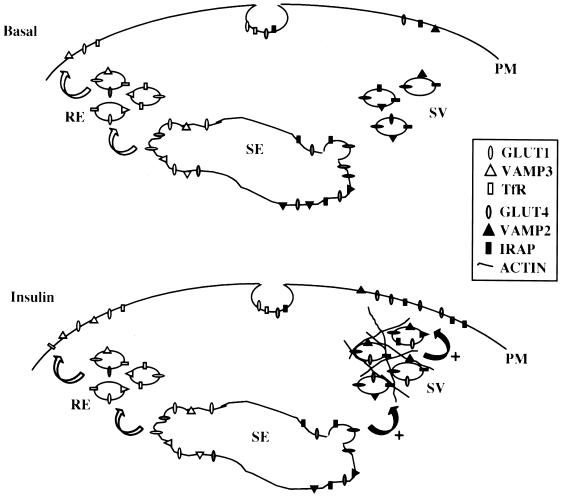
Figure 9.
A model of the intracellular GLUT4 compartment and insulin-stimulated GLUT4 traffic in L6 muscle cells. Under basal conditions, GLUT4 is continuously recycled to and from the plasma membrane (PM) but is largely sequestered intracellularly within the endosomal sorting system (SE) and a specialized GLUT4 vesicular (SV) pool. Other proteins, such as the transferrin receptor (TfR), recycle constitutively through the recycling endosome (RE). The mobilization of specific proteins out of both the RE and SV compartments can be modulated by insulin, possibly by differential protein sorting out of the SE. Insulin potentiates the exocytosis of GLUT4 from the SV pool by inducing the Rac-mediated formation of cortical actin mesh structures beneath the cell surface. This remodeled actin mesh recruits GLUT4-containing vesicles from the SV, allowing them to fuse with the PM. These insulin-responsive GLUT4 vesicles found in the actin mesh also comprise VAMP2, a vesicle SNARE that is essential for the incorporation of GLUT4 into the plasma membrane.
DISCUSSION
The aim of this study was to determine the singular roles of VAMP2 and VAMP3/cellubrevin in GLUT4 translocation to the cell surface, because both v-SNAREs are expressed in cells with regulated GLUT4 traffic (Cain et al., 1992; Volchuk et al., 1994, 1995) and are mobilized along with GLUT4 to the plasma membrane in response to insulin in 3T3-L1 adipocytes (James et al., 1988; Volchuk et al., 1995). To this end, we used L6 myoblasts that stably express an exofacially myc-tagged GLUT4 protein, allowing for the measurement of cell surface GLUT4myc incorporation in single cells. Previous work from our laboratory had shown that GLUT4myc is recruited to the myoblast surface in response to insulin (Ueyama et al., 1999). The extent and characteristics of this translocation are virtually identical to those of the endogenous GLUT4 that is expressed only in mature L6 myotubes (Ueyama et al., 1999). In both parental L6 myotubes and L6-GLUT4myc myoblasts, GLUT4 is concentrated in an intracellular membrane compartment that includes all of the IRAP and largely excludes GLUT1 (Ueyama et al., 1999). Given the amenability of myoblasts to transient transfection by various cDNA constructs, this experimental model is highly suitable to explore the translocation of GLUT4 vesicles to the plasma membrane. Indeed, we have recently shown that transient transfection of mutated enzymes thought to mediate the insulin signal (e.g., PI 3-kinase and Akt) abrogate GLUT4myc exposure at the cell surface (Wang et al., 1999). We now report that the intracellular GLUT4 compartments immunopurified from L6 myoblasts contain some VAMP2 and VAMP3, as seen previously in rat adipocytes (Cain et al., 1992) and 3T3-L1 adipocytes (Volchuk et al., 1995; Tamori et al., 1996). This observation in turn prompted us to investigate the possible roles of each of these v-SNAREs in insulin-dependent GLUT4 traffic, a question that had not been strictly answered for either cell type.
Cleavage of VAMP2 and VAMP3 by Tetanus Toxin Reduces GLUT4 Translocation
The individual participation of VAMP2 or VAMP3 was tested with the use of tetanus toxin to cleave the N termini of these proteins. Through transient transfection of the corresponding cDNAs into L6 myoblasts, the proteolytically active TeTx was expressed alone or in combination with wild-type or toxin-resistant (also termed VW) mutants of VAMP2 or VAMP3. After 48 h, TeTx cleaved both toxin-sensitive V2-GFP and V3-GFP but left a substantial amount of their VW counterparts intact. The use of VW mutants was pioneered by Regazzi et al. (1996) in a study of calcium-induced secretion in pancreatic β-cells. That study showed that both VW VAMP2 and VW VAMP3 were able to rescue the TeTx-inhibited fusion of insulin-containing secretory granules with the plasma membrane. This result suggested that both VW constructs generated proteins that are sorted to granules and that can sustain productive membrane fusion when interacting with surface SNARE molecules.
In the present study, we observed that TeTx reduced the insulin-dependent arrival of GLUT4myc at the surface of L6 myoblasts by ∼60–70% (Figure 7), even when an excess of wild-type VAMP2 or VAMP3 was coexpressed. However, cotransfection of TeTx along with VW VAMP2 completely rescued the insulin effect, with the levels of surface GLUT4myc reaching the levels in untransfected cells. In contrast, expression of VW VAMP3 at levels comparable to those of VW VAMP2 did not rescue the inhibition of GLUT4myc incorporation into the myoblast surface caused by the cotransfected TeTx.
Interestingly, TeTx also caused the collapse of the remaining C-terminal portions of V2-GFP and V3-GFP into a perinuclear compartment coincident with giantin and α-mannosidase II, both Golgi markers. In fact, disruption of the Golgi organelle with the use of brefeldin A did not perturb the extensive colocalization of either Golgi protein with the C-terminal VAMP fragments generated by TeTx, instead causing both to be dispersed into the cytoplasm. Hence, we hypothesize that the proteolytical cleavage of VAMP2 and VAMP3 not only affects the functional ability of these proteins to engage in SNARE complexes, as reported (Regazzi et al., 1996), but that during the 48 h of the transfection protocol it also results in trapping of the proteins within the Golgi complex, rendering them incapable of reaching the vesicles destined for fusion. It is possible that the cleaved VAMP portions, which still contain the endoplasmic reticulum targeting and insertion sequences (Kim et al., 1999), could become inserted in the endoplasmic reticulum membrane and make their way to the Golgi during their biosynthesis, where they remain trapped. These results also suggest a previously unrecognized function of the N termini of VAMP2 and VAMP3 in muscle cells, i.e., to allow exit of these proteins from the Golgi complex. The perinuclear collapse of the cleaved V2-GFP and V3-GFP fragments was specific to these proteins, because the basal steady-state distribution of intracellular GLUT4 was not affected by TeTx. This result implies that the GLUT4 compartments formed but were likely devoid of VAMP2 and VAMP3. Hence, the goal of eliminating VAMP2 and VAMP3 from the GLUT4 compartments to test their function was achieved. Both VW VAMP2 and VW VAMP3 escaped the complete perinuclear restriction in the presence of TeTx, suggesting that the integrity of some of their toxin-resistant polypeptides allowed them to reach their natural organellar destinations.
VAMP2 and VAMP3 Segregate into Distinct Intracellular Compartments
Two possible explanations can be offered to explain the differential behavior of VW VAMP2 and VW VAMP3 in insulin-dependent GLUT4 translocation. First, it is conceivable that VW VAMP3 may not be able to engage in productive SNARE complexes. However, this is unlikely given that: 1) VW VAMP3 restored the fusion of pancreatic insulin granules lost by TeTx action (Regazzi et al., 1996); and 2) VAMP3 was detected in SNARE complexes containing syntaxin4 (Timmers et al., 1996; St-Denis et al., 1999) and SNAP-23 (St-Denis et al., 1999) isolated from insulin-stimulated rat adipocytes. A second and more likely scenario to explain the inability of VW VAMP3 to rescue GLUT4 traffic is that VAMP3 does not normally populate the insulin-translocatable GLUT4 compartment. In support of this view, studies in other cells have suggested that VAMP2 is present on vesicles destined for regulated traffic (Cheatham et al., 1996; Martin et al., 1996, 1998; Malide et al., 1997), whereas VAMP3 has been detected in the recycling endosome and was in part responsible for TfR recycling (Galli et al., 1994; Martin et al., 1996).
In the present study, we lend further support to the segregation of VAMP3 away from the major insulin-regulated intracellular pool of GLUT4, because only VAMP2 but not VAMP3 was found to concentrate in the remodeled cortical actin mesh that forms in response to insulin. The cortical actin mesh recruits both the insulin-activated PI 3-kinase and GLUT4-containing membranes (Khayat et al., 2000), bringing these elements to the vicinity of the cell surface (Khayat et al., 2000). This step appears to be a prerequisite for GLUT4 externalization because CD- or latrunculin B–mediated disruption of the actin mesh prevents this phenomenon and the consequent increase in glucose uptake (Tsakiridis et al., 1994; Khayat et al., 2000). We have further shown that the formation of this actin mesh in L6 myotubes is regulated by the low-molecular-weight G protein Rac. Such remodeling is required for insulin-dependent exposure of GLUT4myc at the cell surface because a dominant negative mutant of Rac (RacN17) precluded actin reorganization as well as GLUT4 externalization (Khayat et al., 2000). In this study, we show that VAMP2 is present in the conglomerate at the actin mesh, whereas VAMP3 is excluded from it. This observation suggests that VAMP3 does not populate the same insulin-sensitive GLUT4 compartment as VAMP2. The VAMP2-containing GLUT4 compartment, therefore, is likely to be a specialized, cytoskeleton-linked vesicular pool. Because both the Rac mutant (Khayat et al., 2000) and TeTx (present study) separately reduced the insulin-dependent translocation of GLUT4, it is conceivable that VAMP2 mediates the fusion of this specialized insulin-regulated intracellular pool of GLUT4 with the plasma membrane. A corollary of this scenario is that VAMP3 does not populate the specialized insulin-regulated GLUT4 compartment. This specialized pool provides ∼70% of the GLUT4 that moves to the cell surface in response to insulin. Presumably, a recycling endosomal pool that contains GLUT4 is responsible for the small remainder of the insulin response. The recycling pool may contain VAMP3, but this protein does not appear to mediate the final fusion event because toxin-resistant VAMP3 did not rescue toxin-inhibited GLUT4myc translocation.
The Insulin-sensitive GLUT4 Intracellular Compartment in Adipose Cells
Studies with 3T3-L1 adipocytes have led to the suggestion that the intracellular GLUT4 distributes rather equally between two main intracellular compartments: the recycling endosome and a specialized vesicular pool (Martin et al., 1998; Foran et al., 1999; Millar et al., 1999). This model is based on the chemical ablation of the endosomal compartments through endocytosis of HRP-coupled transferrin via the TfR, followed by treatment with hydrogen peroxide and oxidation-prone diamide. Such endosomal ablation destroyed most of the VAMP3 but only 40–50% of the GLUT4 and an even smaller fraction of the VAMP2 in 3T3-L1 adipocytes (Martin et al., 1998; Millar et al., 1999). It has been argued that both the recycling endosomal and the specialized vesicular compartments furnish GLUT4 in response to insulin in 3T3-L1 adipocytes, the former by increasing its exocytic rate and the latter by acquiring exocytic capability (reviewed by Hashiramoto and James, 1998). That insulin potentiates exocytosis from the recycling endosomal compartment in 3T3-L1 adipocytes is suggested by the findings that: 1) insulin increases the exocytosis of GLUT1, VAMP3, and TfR, all proposed residents of the recycling endosome; and 2) ablation of the endocytic compartment reduced the rate, and more importantly, the extent of insulin-dependent GLUT4 exocytosis by 30% (Millar et al., 1999).
If insulin mobilizes GLUT4 out of two intracellular compartments in 3T3-L1 adipocytes, do SNARE proteins participate in the fusion of both types of incoming vesicles with the plasma membrane? The following observations suggest that fusion of only one of these GLUT4 compartments is toxin-sensitive SNARE-dependent: 1) microinjection of neutralizing antibodies to VAMP2 (Macaulay et al., 1997), syntaxin4 (Volchuk et al., 1996; Tellam et al., 1997), or SNAP-23 (Rea et al., 1998; Foster et al., 1999b) eliminated only ∼50% of the insulin-dependent arrival of GLUT4 at the surface of 3T3-L1 adipocytes; 2) introduction of botulinum toxin into streptolysin O–permeabilized 3T3-L1 adipocytes was also unable to eliminate >50% of the insulin-dependent GLUT4 externalization (Tamori et al., 1996); 3) a fusion protein encompassing the cytosolic tail of VAMP2 inhibited the insulin response of GLUT4 by ∼40% but spared the insulin-dependent translocation of the GLUT1 transporter (Millar et al., 1999); and 4) GLUT4 externalization provoked by transfected, constitutively active Akt (an enzyme activated by insulin) was fully inhibitable by botulinum toxin (Foran et al., 1999), suggesting that Akt produces exocytosis selectively from one pool. These authors implied that this is the insulin-sensitive vesicular pool and that the botulinum toxin–insensitive pool is the recycling endosome (Foran et al., 1999). Collectively, these results imply that VAMP2, syntaxin4, and SNAP-23 mediate fusion of half of the GLUT4 compartments mobilized in response to insulin. A corollary of the results described above is that VAMP2 is not the v-SNARE responsible for fusion of GLUT4 recruited from the recycling endosome compartment with the plasma membrane. However, those studies do not identify whether another neurotoxin-insensitive v-SNARE, such as TI-VAMP/VAMP7 (Galli et al., 1998), might fulfill this role.
However, it is also attractive to consider the possibility that VAMP3 may mediate fusion of the insulin-stimulated exocytosis of certain proteins out of the recycling endosome. Although the botulinum and tetanus toxin studies cited above do not support this route for GLUT4, a recent study showed that insulin-dependent GLUT1 translocation was partially inhibited by GST-VAMP3 but not by GST-VAMP2 (Millar et al., 1999). However, as stated above, GST-VAMP3 did not affect insulin-dependent GLUT4 exocytosis (Millar et al., 1999).
The Insulin-sensitive GLUT4 Intracellular Compartment in Muscle Cells
As in the case of 3T3-L1 adipocytes, the results of the present study suggest that insulin draws GLUT4myc from two compartments in L6 myoblasts. The major compartment contains VAMP2 but not VAMP3 and is recruited into a cortical actin mesh in response to the hormone (Figure 8). In keeping with this scenario, an elegant immunoelectron microscopy study showed that GLUT4 exists in large and small depots in isolated fibers from rat skeletal muscle tissue (Ploug et al., 1998). The small depots contained both TfR-positive and TfR-negative elements, and insulin appeared to tax predominantly the TfR-negative GLUT4 pool, whereas contraction appeared to draw GLUT4 from the TfR-positive pool. It is conceivable that the small TfR-negative and TfR-positive depots may represent the specialized vesicular and the recycling endosomal compartments, respectively, characterized in L6 myoblasts in the present study. In further support of this model, a dominant negative mutant of Akt reduced GLUT4myc translocation to the cell surface by ∼65% (Wang et al., 1999) in L6 myoblasts. Moreover, GLUT4myc segregates away from the intracellular GLUT1 and colocalizes with IRAP in these cells (Ueyama et al., 1999). These ideas are summarized in Figure 9.
In conclusion, the present study suggests that the specialized GLUT4 compartment contains VAMP2 and that its incorporation into the cell surface is fully dependent on this protein. Furthermore, our previous studies indicate that the insulin-dependent externalization of this specialized GLUT4 compartment requires the reorganization of cortical actin and the activity of the serine/threonine kinase Akt (Wang et al., 1999; Khayat et al., 2000). To the extent that observations made in cells in culture can be extrapolated to adult tissues, the results of this study should help us understand the regulation of GLUT4 traffic in skeletal muscle and its possible implications for insulin-resistant states.
ACKNOWLEDGMENTS
We thank Dr. Yousuke Ebina for introducing GLUT4myc into our L6 myoblasts, and Leonard Foster, Romel Somwar, and Celia Taha for advice and many useful discussions. We dedicate this article to the loving memory of Toolsie Ramlal. This work was supported by grants from the Juvenile Diabetes Foundation International to A.K. and W.S.T., from the Medical Research Council of Canada to A.K. (MT3701), and from the Swiss National Science Foundation to R.R. (31-050640.97).
Abbreviations used:
- CD
cytochalasin D
- Cy3
indocarbocyanine
- GFP
green fluorescent protein
- GLUT
glucose transporter
- GLUT4myc
GLUT4 protein with an exofacial myc epitope
- Ig
immunoglobulin
- IRAP
insulin-regulated aminopeptidase
- L6-GLUT4myc
L6 myoblasts stably expressing GLUT4myc protein
- PI 3-kinase
phosphatidylinositol 3-kinase
- SNAP-25
synaptosome-associated protein of 25 kDa
- SNAP-23
SNAP-25–like protein of 23 kDa
- SNARE
soluble N-ethylmaleimide-sensitive factor-attachment protein receptor
- t-SNARE
target membrane SNARE
- v-SNARE
vesicle SNARE
- TeTx
tetanus toxin light chain
- TfR
transferrin receptor
- VAMP
vesicle-associated membrane protein
- V2-GFP
VAMP2 GFP fusion protein
- V3-GFP
VAMP3 GFP fusion protein
- VW
toxin-resistant/insensitive
REFERENCES
- Bajno L, Peng X-R, Schreiber AD, Moore H-P, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149:1–9. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CC, Trimble WS, Lienhard GE. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992;267:11681–11684. [PubMed] [Google Scholar]
- Cheatham B, Volchuk A, Kahn CR, Wang L, Rhodes CJ, Klip A. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc Natl Acad Sci USA. 1996;93:15169–15173. doi: 10.1073/pnas.93.26.15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Foran P, Shone CC, Foster KA, Melling J, Dolly JO. Botulinum neurotoxin B inhibits insulin-stimulated glucose uptake into 3T3–L1 adipocytes and cleaves cellubrevin unlike type A toxin which failed to proteolyze the SNAP-23 present. Biochemistry. 1997;36:5719–5728. doi: 10.1021/bi962331n. [DOI] [PubMed] [Google Scholar]
- Clarke JF, Young PW, Yonezawa K, Kasuga M, Holman GD. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3–L1 cells by the PI 3-kinase inhibitor, wortmannin. Biochem J. 1994;300:631–635. doi: 10.1042/bj3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. J Biol Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- Douen AG, Ramlal T, Rastogi S, Bilan PJ, Cartee GD, Vranic M, Holloszy JO, Klip A. Exercise induces recruitment of the “insulin-responsive glucose transporter”: evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990;265:13427–13430. [PubMed] [Google Scholar]
- Elmendorf JS, Pessin JE. Insulin signaling regulating the trafficking and plasma membrane fusion of GLUT4-containing intracellular vesicles. Exp Cell Res. 1999;253:55–62. doi: 10.1006/excr.1999.4675. [DOI] [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran PG, Fletcher LM, Oatey PB, Mohammed N, Dolly JO, Tavare JM. Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3–L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin. J Biol Chem. 1999;274:28087–28095. doi: 10.1074/jbc.274.40.28087. [DOI] [PubMed] [Google Scholar]
- Foster LJ, Khayat ZA, Klip A. Insulin-dependent intracellular traffic of glucose transporters. In: Marshall SM, Home PD, Rizza RA, editors. The Diabetes Annual. Vol. 12. New York: Elsevier Science; 1999a. pp. 111–140. [Google Scholar]
- Foster LJ, Yaworsky K, Trimble WS, Klip A. SNAP23 promotes insulin-dependent glucose uptake in 3T3–L1 adipocytes: possible interaction with cytoskeleton. Am J Physiol. 1999b;276:C1108–C1114. doi: 10.1152/ajpcell.1999.276.5.C1108. [DOI] [PubMed] [Google Scholar]
- Foster LJ, Yeung B, Mohtashami M, Ross K, Trimble WS, Klip A. Binary interactions of the SNARE proteins syntaxin-4, SNAP23 and VAMP-2 and their regulation by phosphorylation. Biochemistry. 1998;37:11089–11096. doi: 10.1021/bi980253t. [DOI] [PubMed] [Google Scholar]
- Galli T, Chilcote T, Mundigl O, Binz T, Niemann H, De Camilli P. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J Cell Biol. 1994;125:1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiramoto M, James DE. SNAREing GLUT4 at the plasma membrane in muscle and fat. Adv Exp Med Biol. 1998;441:47–61. doi: 10.1007/978-1-4899-1928-1_5. [DOI] [PubMed] [Google Scholar]
- Jahn R, Hanson PI, Otto H, Ahnert-Hilger G. Botulinum and tetanus neurotoxins: emerging tools for the study of membrane fusion. Cold Spring Harbor Symp Quant Biol. 1995;60:329–335. doi: 10.1101/sqb.1995.060.01.037. [DOI] [PubMed] [Google Scholar]
- Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- James DE, Brown R, Navarro J, Pilch PF. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988;333:183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- Kanai F, Nishioka Y, Hayashi H, Kamohara S, Todaka M, Ebina Y. Direct demonstration of insulin-induced GLUT4 translocation to the surface of intact cells by insertion of a c-myc epitope into an exofacial GLUT4 domain. J Biol Chem. 1993;268:14523–14526. [PubMed] [Google Scholar]
- Khayat Z, Tong P, Yaworsky K, Bloch R, Klip A. Insulin-induced actin filament remodeling: colocalization with phosphatidylinositol 3-kinase and GLUT4 in L6 myotubes. J Cell Sci. 2000;113:279–290. doi: 10.1242/jcs.113.2.279. [DOI] [PubMed] [Google Scholar]
- Kim PK, Hollerbach C, Trimble WS, Leber B, Andrews DW. Identification of the endoplasmic reticulum targeting signal in vesicle-associated membrane proteins. J Biol Chem. 1999;274:36876–36882. doi: 10.1074/jbc.274.52.36876. [DOI] [PubMed] [Google Scholar]
- Kishi K, Muromoto N, Nakaya Y, Miyata I, Hagi A, Hayashi H, Ebina Y. Bradykinin directly triggers GLUT4 translocation via an insulin-independent pathway. Diabetes. 1998;47:550–558. doi: 10.2337/diabetes.47.4.550. [DOI] [PubMed] [Google Scholar]
- Klip A, Ramlal T, Young DA, Holloszy JO. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987;224:224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Macaulay SL, Hewish DR, Gough KH, Stoichevska V, MacPherson SF, Jagadish M, Ward CW. Functional studies in 3T3L1 cells support a role for SNARE proteins in insulin stimulation of GLUT4 translocation. Biochem J. 1997;324:217–224. doi: 10.1042/bj3240217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malide D, Dwyer NK, Blanchette-Mackie EJ, Cushman SW. Immunocytochemical evidence that GLUT4 resides in a specialized translocation post-endosomal VAMP2-positive compartment in rat adipose cells in the absence of insulin. J Histochem Cytochem. 1997;45:1083–1096. doi: 10.1177/002215549704500806. [DOI] [PubMed] [Google Scholar]
- Martin LB, Shewan A, Millar CA, Gould GW, James DE. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3–L1 adipocytes. J Biol Chem. 1998;273:1444–1452. doi: 10.1074/jbc.273.3.1444. [DOI] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar CA, Shewan A, Hickson GR, James DE, Gould GW. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3–L1 adipocytes. Mol Biol Cell. 1999;10:3675–3688. doi: 10.1091/mbc.10.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto Y, Burdett E, Grant A, Klip A. Differential expression of the GLUT1 and GLUT4 glucose transporters during differentiation of L6 muscle cells. Biochem Biophys Res Commun. 1991;175:652–659. doi: 10.1016/0006-291x(91)91615-j. [DOI] [PubMed] [Google Scholar]
- Mitsumoto Y, Klip A. Development regulation of the subcellular distribution and glycosylation of GLUT1 and GLUT4 glucose transporters during myogenesis of L6 muscle cells. J Biol Chem. 1992;267:4957–4962. [PubMed] [Google Scholar]
- Montecucco C, Schiavo G. Structure and function of tetanus and botulinum neurotoxins. Q Rev Biophys. 1995;28:423–472. doi: 10.1017/s0033583500003292. [DOI] [PubMed] [Google Scholar]
- Niemann H, Blasi J, Jahn R. Clostridial neurotoxins: new tools for dissecting exocytosis. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Olson AL, Knight JB, Pessin JE. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1997;17:2425–2435. doi: 10.1128/mcb.17.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Hess LJ, James DE. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am J Physiol. 1991;260:C570–C580. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- Ploug T, van Deurs B, Ai H, Cushman SW, Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J Cell Biol. 1998;142:1429–1446. doi: 10.1083/jcb.142.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, Martin LB, McIntosh S, Macaulay SL, Ramsdale T, Baldini G, James DE. Syndet, an adipocyte target SNARE involved in the insulin-induced translocation of GLUT4 to the cell surface. J Biol Chem. 1998;273:18784–18792. doi: 10.1074/jbc.273.30.18784. [DOI] [PubMed] [Google Scholar]
- Regazzi R, Sadoul K, Meda P, Kelly RB, Halban PA, Wollheim CB. Mutational analysis of VAMP domains implicated in Ca2+-induced insulin exocytosis. EMBO J. 1996;15:6951–6959. [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Pang S, Harris DS, Heuser J, James DE. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3–L1 adipocytes: effects of ATP, insulin, and GTPγS and localization of GLUT4 to clathrin lattices. J Cell Biol. 1992;117:1181–1196. doi: 10.1083/jcb.117.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Siddhanta U, McIlroy J, Shah A, Zhang Y, Backer JM. Distinct roles for the p110alpha and hVPS34 phosphatidylinositol 3′-kinases in vesicular trafficking, regulation of the actin cytoskeleton, and mitogenesis. J Cell Biol. 1998;143:1647–1659. doi: 10.1083/jcb.143.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Denis JF, Cabaniols JP, Cushman SW, Roche PA. SNAP-23 participates in SNARE complex assembly in rat adipose cells. Biochem J. 1999;338:709–715. [PMC free article] [PubMed] [Google Scholar]
- Sumitani S, Ramlal T, Liu Z, Klip A. Expression of syntaxin-4 in rat skeletal muscle and rat skeletal muscle cells in culture. Biochem Biophys Res Commun. 1995;213:462–468. doi: 10.1006/bbrc.1995.2154. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA. 1980;77:2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y, Hashiramoto M, Araki S, Kamata Y, Takahashi M, Kozaki S, Kasuga M. Cleavage of vesicle-associated membrane protein (VAMP)-2 and cellubrevin on GLUT-4-containing vesicles inhibits the translocation of GLUT4 in 3T3–L1 adipocytes. Biochem Biophys Res Commun. 1996;220:740–745. doi: 10.1006/bbrc.1996.0474. [DOI] [PubMed] [Google Scholar]
- Tellam JT, Macaulay SL, McIntosh S, Hewish DR, Ward CW, James DE. Characterization of Munc-18c and syntaxin-4 in 3T3–L1 adipocytes: putative role in insulin-dependent movement of GLUT-4. J Biol Chem. 1997;272:6179–6186. doi: 10.1074/jbc.272.10.6179. [DOI] [PubMed] [Google Scholar]
- Timmers KI, Clark AE, Omatsu-Kanbe M, Whiteheart SW, Bennett MK, Holman GD, Cushman SW. Identification of SNAP receptors in rat adipose cell membrane fractions and in SNARE complexes co-immunoprecipitated with epitope-tagged N-ethylmaleimide-sensitive fusion protein. Biochem J. 1996;320:429–436. doi: 10.1042/bj3200429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonello F, Morante S, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulism neurotoxins: a novel group of zinc-endopeptidases. Adv Exp Med Biol. 1996;389:251–260. [PubMed] [Google Scholar]
- Tsakiridis T, Vranic M, Klip A. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J Biol Chem. 1994;269:29934–29942. [PubMed] [Google Scholar]
- Ueyama A, Yaworsky KL, Wang Q, Ebina Y, Klip A. GLUT-4myc ectopic expression in L6 myoblasts generates a GLUT-4-specific pool conferring insulin sensitivity. Am J Physiol. 1999;277:E572–E578. doi: 10.1152/ajpendo.1999.277.3.E572. [DOI] [PubMed] [Google Scholar]
- Volchuk A, Mitsumoto Y, He L, Liu Z, Habermann E, Trimble W, Klip A. Expression of vesicle-associated membrane protein 2 (VAMP-2)/synaptobrevin II and cellubrevin in rat skeletal muscle and in a muscle cell line. Biochem J. 1994;304:139–145. doi: 10.1042/bj3040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchuk A, Sargeant R, Sumitani S, Liu Z, He L, Klip A. Cellubrevin is a resident protein of insulin-sensitive GLUT4 glucose transporter vesicles in 3T3–L1 adipocytes. J Biol Chem. 1995;270:8233–8240. doi: 10.1074/jbc.270.14.8233. [DOI] [PubMed] [Google Scholar]
- Volchuk A, Wang QH, Ewart HS, Liu Z, He LJ, Bennett MK, Klip A. Syntaxin 4 in 3T3–L1 adipocytes: regulation by insulin and participation in insulin-dependent glucose transport. Mol Biol Cell. 1996;7:1075–1082. doi: 10.1091/mbc.7.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Witkin JW, Hao G, Bankaitis VA, Scherer PE, Baldini G. Syndet is a novel SNAP25-related protein expressed in many tissues. J Cell Sci. 1997;110:505–513. doi: 10.1242/jcs.110.4.505. [DOI] [PubMed] [Google Scholar]
- Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wong PPC, Daneman N, Volchuk A, Lassam N, Wilson MC, Klip A, Trimble WS. Tissue distribution of SNAP-23 and its subcellular localization in 3T3–L1 cells. Biochem Biophys Res Commun. 1997;230:64–68. doi: 10.1006/bbrc.1996.5884. [DOI] [PubMed] [Google Scholar]