Abstract
For children with ambulatory pneumonia, the World Health Organization (WHO) recommends oral amoxicillin (15 mg/kg of body weight/dose) thrice daily (t.i.d.) or oral cotrimoxazole (4 mg of trimethoprim/kg/dose) twice daily (b.i.d.). The more frequent amoxicillin dosing may lead to compliance problems. To compare the pharmacokinetics and levels of amoxicillin in plasma in the current WHO acute respiratory infection recommendations with the 25-mg/kg/dose b.i.d. regimen, we performed a two-group parallel study of 66 children ages 3 to 59 months with pneumonia. Amoxicillin was given orally at 25 mg/kg/dose b.i.d. or 15 mg/kg/dose t.i.d. Amoxicillin concentrations were determined by high-performance liquid chromatography after the first dose on days 1 and 3. After the first dose on day 1, the mean area under the concentration-time curve (AUC) for amoxicillin after the 25-mg/kg dose was 54.7 versus 24.9 μg · h/ml after the 15-mg/kg dose. After the first dose on day 3, the mean AUC was 44.1 versus 28.5 μg · h/ml. All but two children had plasma amoxicillin concentrations above 0.5 μg/ml for >50% of the dose interval on both days. Six children on day 1 and five children on day 3 had concentrations above 1.0 μg/ml for <50% of the dose interval. On day 1, 16 of 27 children in the b.i.d. group and 11 of 26 children in the t.i.d. group had concentrations that were above 2.0 μg/ml for <50% of the dose interval, and on day 3, 18 of 31 children in the b.i.d. group and 8 of 31 children in the t.i.d. group had concentrations that were above 2.0 μg/ml for <50% of the dose interval. Amoxicillin b.i.d. is a feasible alternative for t.i.d. dosing. To lengthen the time above the MIC at higher concentration levels, a 30- to 40-mg/kg/dose b.i.d. should be considered instead of the 25 mg/kg/dose used in this study.
Acute respiratory infection (ARI) is a major cause of childhood morbidity and mortality in developing countries (3; Anonymous, Editorial, Lancet 2:699-701, 1985). Streptococcus pneumoniae and Haemophilus influenzae cause most of the bacterial infections (10, 16). The ARI control program of the World Health Organization (WHO) has developed an improved case management strategy to reduce childhood mortality from pneumonia (14, 15). Oral cotrimoxazole and amoxicillin are recommended as the antimicrobial agents of choice in the outpatient management of children with ambulatory pneumonia in developing countries. These antibiotic alternatives are especially needed for treating cases of pneumonia as outpatient therapy. Oral medication should be used whenever possible for nonhospitalized patients in order to reduce the possibility of transmission of viruses (AIDS, hepatitis) through inadequate sterilization practices (unless the use of disposable syringes can be guaranteed for all the injections) (10). The use of oral penicillin is not recommended for the treatment of childhood pneumonia because it is not effective against H. influenzae. Oral cephalosporins and new macrolides have been used with good results for the treatment of pneumonia; cost is the main reason why they are not recommended as the standard treatment of pneumonia in developing countries.
Most countries have chosen oral cotrimoxazole over amoxicillin in their national ARI control programs because of its proven efficacy, lower cost, and twice-daily (b.i.d.) regimen. However, the discovery of cotrimoxazole resistance among both S. pneumoniae and H. influenzae isolates from children with ARI is cause for concern (9, 11, 13). Therefore, countries that have chosen oral cotrimoxazole are reviewing their treatment recommendations, as resistance to cotrimoxazole continues to increase.
Amoxicillin is active against most S. pneumoniae and H. influenzae strains and better absorbed than ampicillin and has been widely used for treating respiratory infections (including pneumonia) in children. According to the WHO ARI guidelines, the drug should be given orally at a dosage of approximately 15 mg/kg thrice daily (t.i.d.), which may lead to compliance problems compared to oral cotrimoxazole, which should be administered b.i.d. This problem could be reduced by dosing amoxicillin b.i.d., but experience is scanty (4, 12).
The objective of this study was to determine the pharmacokinetics of two oral amoxicillin treatment regimens in children between 3 months and 5 years of age who were diagnosed as having pneumonia. Specifically, we sought to compare the levels of amoxicillin in plasma when the drug was given following the current WHO ARI recommendation of 15 mg/kg every 8 h or b.i.d. at a higher dose to test whether b.i.d. dosing of 25 mg/kg/dose produces serum drug levels that are considered adequate for treating community-acquired pneumonia.
MATERIALS AND METHODS
The present study was carried out (from 12 September 1995 to 31 January 1996) in the tertiary level Albert Sabin Children's Hospital of Fortaleza in the state of Ceará, Brazil. Ceará is the third poorest state in the country, with a child mortality rate at the time of recruitment of over 80 per 1,000 newborns (live births). ARI is an important cause of child death, second to diarrhea, and mild to moderate malnutrition is common.
The study protocol was approved by the Committee on Medical Ethics of Albert Sabin Children's Hospital and the WHO Subcommittee on Research Involving Human Subjects.
Selection of patients.
Eligible patients 3 months to 5 years of age (inclusive) admitted to the hospital with nonsevere pneumonia were enrolled and hospitalized for the duration of the study. Nonsevere pneumonia was defined by the presence of a cough or difficult breathing and of fast breathing (≥50 breaths/min from 3 to 11 months of age; ≥40 breaths/min for 12 to 59 months of age), without lower chest indrawing and without any danger signs (inability to drink, central cyanosis, abnormal sleepiness, convulsions, or stridor at rest) (15). Patients were excluded if they had any of the following conditions: evidence of abnormal liver or kidney function, evidence of glucose-6-phosphate dehydrogenase deficiency, shock and severe dehydration, severe malnutrition, acute gastroenteritis, any history of receiving amoxicillin within the past 48 h, or any other severe life-threatening infection (such as septicemia or meningitis) requiring intravenous antimicrobial agents. The parent or guardian's informed consent to participate was required before inclusion.
Study protocol.
All patients attending the pediatric facility and fulfilling the clinical requirements were considered for enrollment. Patients were randomly assigned to receive amoxicillin orally at either 15 mg/kg/dose t.i.d. or 25 mg/kg/dose every 12 h. The allocations were determined in the coordination center by labeled envelopes taken sequentially. Amoxicillin (Amoxil; SmithKline Beecham, Rio de Janeiro, Brazil) dose (25- and 50-mg/ml suspensions) was measured with a gauged spoon and administered after 1 h of fasting with water. The children were allowed to eat normally after another hour. A trained nurse administered the drug, and the pediatrician who also performed clinical examinations of the child to look for possible adverse effects supervised the administration. The doctor was also present at the blood sampling to check the exact time of the blood drawing.
Blood sampling and analytical method.
On days 1 and 3 of the treatment, 2 ml of venous blood was drawn from an intravenous catheter. Sampling was done immediately before and 2, 5, and 8 h after the first dose of the day. An extra sample was obtained after 12 h in the b.i.d. group. The samples were collected in vacuum tubes (Vacutainer; Becton Dickinson, Plymouth, United Kingdom) containing EDTA as an anticoagulant and then centrifuged quickly (at 600 × g for 2 to 3 min). Two aliquots of the separated plasma were placed in 2-ml Eppendorf cryovials (Pro-Lab, Ontario, Canada) held on crushed ice before being frozen for 20 min after collection and stored at −70°C.
The samples were packed in a container with dry ice for 72 h and transported by a courier service to the laboratory of the Southwestern Medical Center, University of Texas, Dallas.
Amoxicillin concentrations were determined by reverse-phase high-performance liquid chromatography (7). Briefly described, plasma drug samples were filtered through Amicon (Danvers, Mass.) YMT membranes, and 25 μl was injected onto a 25-cm-long reverse-phase octyldecyl silane C18 column (Phenomenex, Torrance, Calif.). The mobile phase consisted of phosphate buffer (pH 3.0) in 6% methanol at a flow rate of 1.0 ml/min, with a retention time of less than 10 min. Eluent was monitored at 227 nm. Linearity was observed over the range of concentration expected, 10 to 0.5 μg/ml, with a regression coefficient of 0.990. Intra-assay precision was tested by the repeated determination of standard concentrations of 8.0, 4.0, 2.0, 1.0, and 0.5 μg/ml (n = 4) with coefficients of variation of 6.7, 8.0, 5.5, 8.3, and 9.4%, respectively. Unknown variables were determined by linear regression analysis of plasma drug standards and computer extrapolations.
Pharmacokinetic calculations.
The timing of the samples was planned to permit a rough estimation of peak concentration (sample at 2 h after the dose) and calculation of the elimination half-life (t1/2) with a minimum number of samples. The pharmacokinetic variables were calculated by standard noncompartmental methods with the WinNonlin computer program version 1.1. (Scientific Consulting, Inc.). The area under the blood drug concentration-time curve (AUC) extended to infinity on day 1 (after the first dose) was compared with the AUC for the dose interval on day 3 (steady state).
To estimate the relevance of the attained amoxicillin concentrations, the percentage of the dose interval when the concentration exceeded a given MIC was calculated. The time points when a given concentration was passed were determined with linear regression. MICs of 0.5, 1.0, and 2.0 μg/ml were chosen, as they are commonly used breakpoints for S. pneumoniae (1).
For some children, the elimination t1/2 and AUC (3 on day 1 and 10 on day 3) could not be calculated, as a linear elimination phase that both was postdistribution and included at least three data points could not be determined. In the case of the incomplete concentration data sets, the AUC was not calculated but the data for the concentration at 2 h were used if appropriate. The percentage of the dose interval when the concentration exceeded a given MIC could be calculated in many cases without the availability of a full concentration data set.
Sample size.
Based on the data available from previous amoxicillin pharmacokinetics studies showing a large variability difference and taking the most conservative approach, 30 children per group was considered adequate for detecting a difference between the two groups in the mean plasma amoxicillin level by using 95% confidence intervals (95% CI). In this study, we recruited and monitored 80 children. This larger sample size has taken into account the possible loss of children during the study and follow-up or exclusion during the hospital stay.
Data analysis.
Results are presented as the means, ranges, and 95% CI of the original data. Analysis of variance and analysis of covariance were used to compare data between the two dose groups and two study days and to look for possible age effects. Subsequent differences between groups were assessed with Scheffe's test. An unpaired t test, or nonparametric Fisher's exact test, and the Mann-Whitney test were used as appropriate. The level of significance used for all statistical tests was P < 0.05.
RESULTS
Patients.
A total of 80 children were admitted to the study. Fourteen children (seven in each group) did not have all of their blood samples collected for various reasons (parental refusal of hospitalization, refusal of serial blood drawing, inability to locate a vein, or development of pleural effusion). A single child died during hospitalization, after being withdrawn from the study with an erythema nodosum rash. The data from 66 children could be used for the analysis of at least one pharmacokinetic variable.
The two study groups did not differ in background variables (Table 1). In addition, the clinical variables at enrollment were similar (respiratory rate and body temperature) in both groups, except the pulse rate, which was slightly higher in the b.i.d. group (data not shown). All children had pneumonia, but some also had other problems. In the t.i.d. group, two infants had anemia, four had asthma, and one had Down's syndrome. In the b.i.d. group, one infant had anemia and one had asthma.
TABLE 1.
Background data for 66 infants receiving amoxicillin at either 15 mg/kg t.i.d. or 25 mg/kg b.i.d. orally
| Grouping based on background variable and dose | n | Median | Range | Mann-Whitney P |
|---|---|---|---|---|
| Age (mo) | ||||
| 15 mg/kg t.i.d. | 34 | 12.5 | 5.0-52.0 | |
| 25 mg/kg b.i.d. | 32 | 14.5 | 3.0-48.0 | 0.98 |
| Wt (kg) | ||||
| 15 mg/kg t.i.d. | 34 | 8.60 | 4.2-17.0 | |
| 25 mg/kg b.i.d. | 32 | 9.46 | 4.4-20.5 | 0.65 |
| Hta (cm) | ||||
| 15 mg/kg t.i.d. | 33 | 71.0 | 60-104 | |
| 25 mg/kg b.i.d. | 32 | 77.0 | 54-105 | 0.44 |
| Sex | ||||
| 15 mg/kg t.i.d. | ||||
| Girls | 14 | |||
| Boys | 20 | |||
| 25 mg/kg b.i.d. | ||||
| Girls | 16 | |||
| Boys | 16 |
Height for one patient is missing.
Pharmacokinetics.
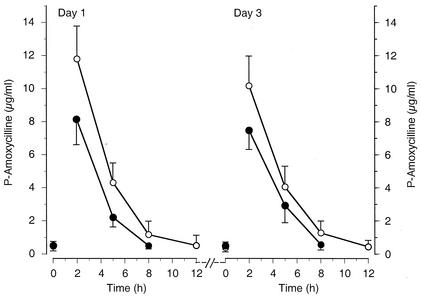
The amoxicillin concentration-time curvesare presented in Fig. 1. As expected, the concentrations were generally higher after the 25-mg/kg dose than after the 15-mg/kg dose. This was also true for the maximum observed concentration and the AUC (Table 2). The concentrations on day 3 were comparable to those after the first dose on day 1. This is well explained by the short elimination t1/2 of amoxicillin (Table 2), which was comparable in both groups and on both days. Within the subjects of this study, the age of the children had no effect on any of the pharmacokinetic variables (data not shown).
FIG. 1.
Means (± 95% CI) of plasma amoxicillin concentrations versus time in children after the first dose on day 1 and day 3 of treatment. The children were treated with either 15 mg/kg/dose t.i.d. (filled circles) (n = 34) or 25 mg/kg/dose b.i.d. (open circles) (n = 32) administered orally.
TABLE 2.
Pharmacokinetics of amoxycillin given to 66 infants at either 15 mg/kg t.i.d. or 25 mg/kg b.i.d. orallya
| Parameter, day, and dose | n | Mean | SD | Range | 95% CI | ANOVA P |
|---|---|---|---|---|---|---|
| Maximum concn (μg/ml) ob served at 2 h | ||||||
| Day 1 | ||||||
| 15 mg/kg t.i.d. | 27 | 6.9 | 3.0 | 2.9-14.0 | 5.7-8.1 | |
| 25 mg/kg b.i.d. | 23 | 10.5 | 4.9 | 3.6-19.5 | 8.3-12.6 | 0.0029 |
| Day 3 | ||||||
| 15 mg/kg t.i.d. | 28 | 7.9 | 3.5 | 2.4-13.8 | 6.6-9.3 | |
| 25 mg/kg b.i.d. | 31 | 10.6 | 5.1 | 2.3-20.9 | 8.7-12.4 | 0.027 |
| AUC (μg · h/ml) | ||||||
| Day 1 | ||||||
| 15 mg/kg t.i.d. | 26 | 24.9 | 9.6 | 11.1-50.8 | 21.0-28.8 | |
| 25 mg/kg b.i.d. | 22 | 54.7 | 60.2 | 14.4-310.5 | 28.0-81.4 | 0.016 |
| Day 3 | ||||||
| 15 mg/kg t.i.d. | 25 | 28.5 | 14.4 | 4.2-64.2 | 22.6-34.5 | |
| 25 mg/kg b.i.d. | 30 | 44.1 | 24.6 | 14.4-117.8 | 34.9-53.3 | 0.0075 |
| t1/2 (h) | ||||||
| Day 1 | ||||||
| 15 mg/kg t.i.d. | 26 | 1.5 | 0.6 | 0.8-2.7 | 1.2-1.7 | |
| 25 mg/kg b.i.d. | 22 | 2.0 | 1.9 | 0.8-10.0 | 1.2-2.9 | 0.15 |
| Day 3 | ||||||
| 15 mg/kg t.i.d. | 24 | 1.7 | 1.3 | 0.6-7.0 | 1.1-2.2 | |
| 25 mg/kg b.i.d. | 30 | 1.9 | 0.9 | 0.8-4.4 | 1.5-2.2 | 0.48 |
Although the data are available for 66 patients, the data points for each variable are not available for all patients.
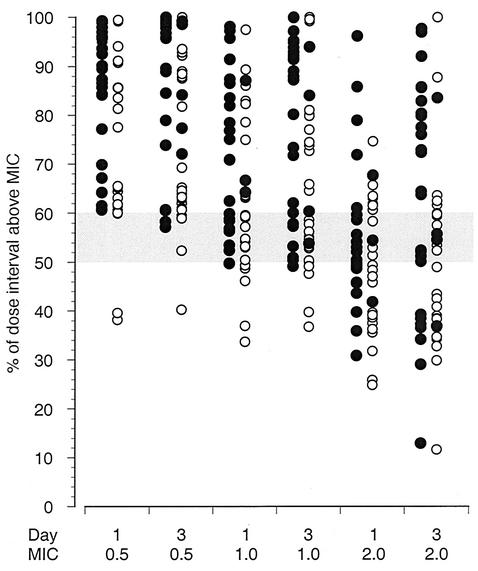
The mean percentage of the dose interval at which the amoxicillin concentration remained above a given MIC (0.5, 1.0, or 2.0 μg/ml) was higher in the children given 15 mg/kg t.i.d. than that in those given 25 mg/kg b.i.d. (Table 3). At 0.5 μg/ml, all but three of the children of the 25-mg/kg b.i.d. group had amoxicillin concentrations that were above the MIC for more than 50% of the dose interval (Fig. 2). At a MIC of 1.0 μg/ml, on day 1, 6 of 52 (11.5%) children had concentrations above the MIC for less than half of the dose interval whereas on day 3, only 5 of 61 (8.2%) did. Only one of them on each day belonged to the 15-mg/kg t.i.d. group (Fig. 2). At a MIC of 2.0 μg/ml on day 1, 16 of 27 children (59.3%) in the b.i.d. group and 11 of 26 children (42.3%) in the t.i.d. group had concentrations above the MIC for less than half of the dose interval (Fig. 2). On day 3 at 2.0 μg/ml, the difference between the groups was most evident, with concentrations from 18 of 31 (58.1%) and 8 of 31 (25.8%) children above the MIC for less than 50% of the dose interval (Fisher's P = 0.02).
TABLE 3.
Percentage of the dose interval plasma amoxycillin concentration remaining above the MIC in 66 infantsa
| MIC (μg/ml), day, and dose | No. | Mean | SD | Range | Mann-Whitney P |
|---|---|---|---|---|---|
| 0.5 | |||||
| Day 1 | |||||
| 15 mg/kg t.i.d. | 26 | 84.5 | 14.1 | 60.7-99.4 | |
| 25 mg/kg b.i.d. | 24 | 73.5 | 18.5 | 38.2-99.5 | 0.057 |
| Day 3 | |||||
| 15 mg/kg t.i.d. | 30 | 88.7 | 14.4 | 57.2-100.0 | |
| 25 mg/kg b.i.d. | 30 | 75.5 | 17.2 | 40.3-100.0 | 0.0076 |
| 1.0 | |||||
| Day 1 | |||||
| 15 mg/kg t.i.d. | 26 | 73.3 | 16.1 | 49.8-98.1 | |
| 25 mg/kg b.i.d. | 26 | 62.6 | 15.9 | 33.7-97.6 | 0.028 |
| Day 3 | |||||
| 15 mg/kg t.i.d. | 30 | 78.9 | 18.1 | 49.2-100.0 | |
| 25 mg/kg b.i.d. | 31 | 65.1 | 17.6 | 36.8-100.0 | 0.009 |
| 2.0 | |||||
| Day 1 | |||||
| 15 mg/kg t.i.d. | 26 | 56.4 | 16.1 | 30.9-96.2 | |
| 25 mg/kg b.i.d. | 27 | 48.2 | 12.8 | 24.9-74.7 | 0.088 |
| Day 3 | |||||
| 15 mg/kg t.i.d. | 31 | 62.2 | 22.4 | 13.0-97.8 | |
| 25 mg/kg b.i.d. | 31 | 50.7 | 20.4 | 11.7-100.0 | 0.06 |
Although the data are available for 66 patients, the data points for each variable are not available for all patients.
FIG. 2.
Percentage of the dose interval at which the plasma amoxicillin concentration remained above a predetermined level of 0.5, 1.0, or 2.0 μg/ml after the first dose on day 1 and day 3 of treatment. Amoxicillin was administered orally at 15 mg/kg/dose t.i.d. (filled circles) (n = 34) or 25 mg/kg/dose b.i.d. (open circles) (n = 32). The 50 to 60% dose interval is indicated by shading.
DISCUSSION
In children treated for pneumonia, increasing the amoxicillin dosing interval from 8 to 12 h and simultaneously, the dose from 15 to 25 mg/kg resulted in higher plasma drug concentrations at 2 h after the dose and an increased AUC. After both dosing regimens, the amoxicillin concentrations remained above a given MIC of 1.0 μg/ml for over 50% of the dose interval in the majority of the children. However, at a concentration of 2.0 μg/ml, the lower dose given more frequently gave better coverage of the dose interval.
Animal work confirmed by clinical studies has shown that for beta-lactam antimicrobial agents, the time that the serum drug concentration exceeds the MIC of the pathogen is a major factor in predicting successful clinical outcome (6). However, once a time level of 50 to 60% of the dosing interval above the MIC is achieved, there seems to be little more to gain in terms of efficacy (2). The peak concentration and the AUC have been less valuable as predictors of efficacy. The MICs for pathogens vary, and calculations have to be made accordingly. In this study, we used MICs of 0.5, 1.0, and 2.0 μg/ml, which are commonly used breakpoints for S. pneumoniae (1), a major pathogen causing pneumonia in children like the patients examined in this study.
The pharmacokinetics of amoxicillin in this study was comparable to the results of a previously published study of 24 infants and children of similar ages (4 to 45 months; 40% higher than the concentration at 2 h) (8).
Based on the results of this study, amoxicillin given in a sufficient dose b.i.d. seems to be a feasible alternative for giving the drug t.i.d. although there are very few supporting clinical data so far. Dachsner et al. studied 24 children with a mean age of 2.7 years. The children were given 50 mg/kg/day in two or four daily doses, with comparable efficacies and adverse effects (4). In a study of 367 children with a mean age of 2 years and 9 months who were treated for ARI, mainly otitis media, Valtonen et al. compared amoxicillin given at 40 mg/kg/day either b.i.d. or t.i.d. The results were comparable between the two groups (12).
As amoxicillin is generally well tolerated, we suggest that a larger single dose, perhaps 30 to 40 mg/kg, should be considered to lengthen the time above the MIC at a higher concentration level, like the 2.0 μg/ml used in this study. Recently even higher doses of 80 to 90 mg of amoxicillin/kg/day have been proposed for the treatment of acute otitis media to overcome the problem of possible penicillin-nonsusceptible S. pneumoniae strains (5).
Acknowledgments
This study project was supported by the Department of Child and Adolescent Health and Development, WHO, Geneva, Switzerland.
We acknowledge Anamaria C. Silva (Albert Sabin Children's Hospital) and Milson Gondim (Hoechst Marion Russel) in Fortaleza, Brazil, for their support in specimen collection and storage and Kurt Olsen (Southwestern Medical Center) for performing the drug assays.
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cars, O. 1997. Efficacy of beta-lactam antibiotics: integration of pharmacokinetics and pharmacodynamics. Diagn. Microbiol. Infect. Dis. 27:29-33. [DOI] [PubMed] [Google Scholar]
- 3.Chretien, J., W. Holland, P. Macklem, P. Murray, and A. Wollock. 1984. Acute respiratory tract infections in children: a global public-health problem. New Engl. J. Med. 310:982-984. [DOI] [PubMed] [Google Scholar]
- 4.Dachsner, F., U. Behre, and A. Dalhoff. 1981. Prospective clinical trial on the efficacy of amoxicillin administered twice or four times daily in children with respiratory tract infections. J. Int. Med. Res. 9:274-276. [DOI] [PubMed] [Google Scholar]
- 5.Deeks, S., R. Palacio, R. Ruvinsky, D. Kertesz, M. Hortal, A. Rossi, J. Spika, J. Di Fabio, et al. 1999. Risk factors and course of illness among children with invasive penicillin-resistant Streptococcus pneumoniae. Pediatrics 103:409-413. [DOI] [PubMed] [Google Scholar]
- 6.Drusano, G. L., and W. A. Craig. 1997. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J. Chemother. 9(Suppl. 3):38-44. [PubMed] [Google Scholar]
- 7.Foulstone, M., and C. Reading. 1982. Assay of amoxicillin and clavulanic acid, the components of Augmentin, in biological fluids with high-performance liquid chromatography. Antimicrob. Agents Chemother. 22:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsburg, C., G. J. McCracken, M. Thomas, and J. Clahsen. 1979. Comparative pharmacokinetics of amoxicillin and ampicillin in infants and children. Pediatrics 64:627-631. [PubMed] [Google Scholar]
- 9.Mastro, T., A. Ghafoor, N. Nomani, et al. 1991. Antimicrobial resistance of pneumococci in children with acute lower respiratory tract infection in Pakistan. Lancet 337:156-159. [DOI] [PubMed] [Google Scholar]
- 10.Shann, F. 1981. Use of antibiotics in Papua New Guinea (PNG). W. H. O./RSD/81.4. World Health Organization, Geneva, Switzerland.
- 11.Straus, W. L., S. A. Qazi, Z. Kundi, N. K. Nomani, B. Schwartz, et al. 1998. Antimicrobial resistance and clinical effectiveness of co-trimoxazole versus amoxicillin for pneumonia among children in Pakistan: randomised controlled trial. Lancet 352:270-274. [DOI] [PubMed] [Google Scholar]
- 12.Valtonen, M., T. Piippo, T. Pitkäjärvi, and M.-L. Pyykkönen. 1986. Comparison of amoxycillin given two and three times a day in acute respiratory tract infections in children. Scand. J. Prim. Health Care 4:201-204. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg, G., E. Spitzer, P. Murray, et al. 1990. Antimicrobial susceptibility patterns of Haemophilus isolates from children in eleven developing nations. Bull. W. H. O. 68:179-184. [PMC free article] [PubMed] [Google Scholar]
- 14.W. H. O. Program for the Control of Acute Respiratory Infections. 1990. Acute respiratory infections in children: case management in small hospitals in developing countries. A manual for doctors and other senior health workers. W. H. O./ARI/90.5. World Health Organization, Geneva, Switzerland.
- 15.W. H. O. Program for the Control of Acute Respiratory Infections. 1990. Management of the young child with acute respiratory infection. World Health Organization, Geneva, Switzerland.
- 16.W. H. O. Program for the Control of Acute Respiratory Infections. 1991. Technical bases for the W. H. O. recommendations on the management of pneumonia in children at first-level facilities. W. H. O./ARI/91.20. World Health Organization, Geneva, Switzerland.